Abstract
Background
Portal vein thrombosis (PVT) is a poorly described complication of inflammatory bowel disease (IBD). We sought to better characterize presentations, compare treatments, and assess outcomes in IBD-related PVT.
Methods
We conducted a retrospective investigation of IBD-related PVT at our institution. Multivariable Cox proportional hazards modeling was used to estimate adjusted hazard ratios across treatments.
Results
Sixty-three patients with IBD-related PVT (26 with Crohn disease, 37 with ulcerative colitis) were followed for a median 21 months (interquartile ratio [IQR] = 9-52). Major risk factors included intra-abdominal surgery (60%), IBD flare (33%), and intra-abdominal infection (13%). Primary hematologic thrombophilias were rare and did not impact management. Presentations were generally nonspecific, and diagnosis was incidental. Ninety-two percent of patients (58/63) received anticoagulation (AC), including 23 who received direct oral anticoagulants (DOACs), 22 who received warfarin, and 13 who received enoxaparin. All anticoagulated patients started AC within 3 days of diagnosis. Complete radiographic resolution (CRR) of PVT occurred in 71% of patients. We found that DOACs were associated with higher CRR rates (22/23; 96%) relative to warfarin (12/22; 55%): the hazard ratio of DOACs to warfarin was 4.04 (1.83-8.93; P = 0.0006)). Patients receiving DOACs required shorter courses of AC (median 3.9 months; IQR = 2.7-6.1) than those receiving warfarin (median 8.5 months; IQR = 3.9-NA; P = 0.0190). Incidence of gut ischemia (n = 3), symptomatic portal hypertension (n = 3), major bleeding (n = 4), and death (n = 2) were rare, and no patients receiving DOACs experienced these adverse outcomes.
Conclusions
We show that early and aggressive use of AC can lead to excellent outcomes in IBD-associated PVT and that DOACs are associated with particularly favorable outcomes in this setting.
Keywords: inflammatory bowel disease, portal vein thrombosis, splanchnic vein thrombosis, anticoagulation, direct oral anticoagulants
BACKGROUND
Portal vein thrombosis (PVT) is most commonly encountered in the context of cirrhosis or can occasionally be the result of primary hematologic disorders (most notably JAK2V617F-positive myeloproliferative neoplasms). Alternatively, PVT may be a potential consequence of any inflammatory intra-abdominal process (including but not limited to intra-abdominal infection, intra-abdominal surgery, pancreatitis, or inflammatory bowel disease [IBD]).1 Among patients with IBD, the baseline intra-abdominal thrombophilia imparted by chronic inflammation is often and repeatedly heightened in the setting of acute flares, colorectal surgeries, and infectious complications.2, 3 Indeed, PVT in IBD is prone to occur in these settings, when inflammation and therefore thrombotic tendency are at their greatest. Diagnosis of PVT among such patients is challenging in that its highly nonspecific symptoms (typically abdominal pain) may also commonly occur as the result of any of its provoking factors (IBD flare, intra-abdominal infection, postsurgical complication).1, 2 It is therefore not surprising that the diagnosis is most often made incidentally, when imaging is performed to evaluate for one of these provoking processes.3
The most concerning short-term complication of PVT is rapid clot extension into the adjoining splanchnic vasculature (usually the superior mesenteric vein [SMV]), causing intestinal ischemia and necrosis.3, 4 The long-term concern is that failure to adequately recanalize the portal vein may lead to chronic thrombosis, resulting in chronic noncirrhotic portal hypertension.5 The mainstay of treatment is anticoagulation (AC), the prompt initiation of which is intended to prevent acute clot extension and chronic thrombosis.1 Although AC is essential, the ideal anticoagulant and the required duration of treatment are uncertain, and outcomes among these patients remain poorly described. Herein we present the largest series of IBD-related PVT in the literature to date, and the first to examine and compare treatments and assess long-term outcomes.
METHODS
Patients and Outcomes
We searched the medical records of the Mount Sinai Hospital, a large tertiary care IBD center, for patients with an ICD code for PVT seen from January 2000 through January 2019. All of these patients with a concurrent history of IBD were identified. Patient medical records were then reviewed to confirm a history of acute PVT, with or without concurrent thrombosis in additional splanchnic vessels. Each patient’s PVT was confirmed via the radiology report at diagnosis, and the subsequent course of each PVT was tracked via succeeding radiology reports. Criteria for exclusion were tumor thrombus, receipt of thrombolysis/thrombectomy, absence of baseline imaging at PVT diagnosis, absence of subsequent follow-up imaging 3 or more months after diagnosis, and evidence of chronic as opposed to acute PVT at diagnosis (eg, known clinical history of long-standing PVT, or presence of portal cavernoma, portal collaterals, or other radiographic findings suggestive of chronic PVT at diagnosis).
At the time of PVT diagnosis the patients’ age, sex, etiology of PVT, location of PVT, and degree of PVT occlusion were assessed. Location of PVT was defined as being only in the main portal vein (PV), in the left or right PV only, or involving any portion of the PV with concurrent involvement of additional splanchnic vessels. Degree of PV occlusion (either occlusive or nonocclusive) was determined by the radiology report at diagnosis. The initial long-term AC used in each instance was recorded and formed the basis for comparison across patients. In many patients intravenous heparin was used as initial short-term (or bridging) AC, and in these instances the first long-term AC transitioned to thereafter was considered. Because a large proportion of patients with PVT were diagnosed shortly following intra-abdominal surgery, comparisons of postsurgical and nonsurgical patients were also carried out. Many patients received extensive thrombophilia testing, and results of these workups were recorded when available.
The primary outcome of interest was complete radiographic resolution (CRR) of PVT. Secondary outcomes were recanalization (RC) of occlusive PVT, development of symptomatic portal hypertension (SPH, defined as new varices documented on esophagogastroduodenoscopy, or new ascites requiring diuretics), and major (World Health Organization grade 3 or 4) bleeding.
Statistical Analysis
Continuous variables were summarized by the median and interquartile range (IQR), while categorical variables were summarized by N (%). Distributions of continuous and categorical variables were compared across etiologic categories of PVT using the Kruskal-Wallis and Fisher exact tests, respectively. The Kaplan-Meier method was used to estimate the median times to event for all outcomes, with corresponding 95% confidence intervals (CIs) constructed based on the Brookmeyer-Crowley method.6 Univariable comparisons of time-to-event outcomes were made with the log-rank test. Multivariable Cox proportional hazards models were run to estimate adjusted hazard ratios (HRs) with their corresponding 95% CI. The multivariable models controlled for age, sex, location/extent of PVT, occlusivity of PVT, and type of anticoagulant used. We performed pairwise comparisons among the different anticoagulants for the primary outcome (CRR) and secondary outcome (RC). Because of small numbers, we were not able to perform multivariable analyses on the outcomes of SPH and major bleeding events. All hypothesis testing was 2-sided, with the type-1 error rate fixed at 5% for the determination of factors associated with time-to-event outcomes. All statistical analyses were conducted using SAS v9.4 (SAS Institute, Cary, NC).
ETHICAL CONSIDERATIONS
This retrospective study was approved by our institutional internal review board and program for the protection of human subjects.
RESULTS
Baseline Characteristics
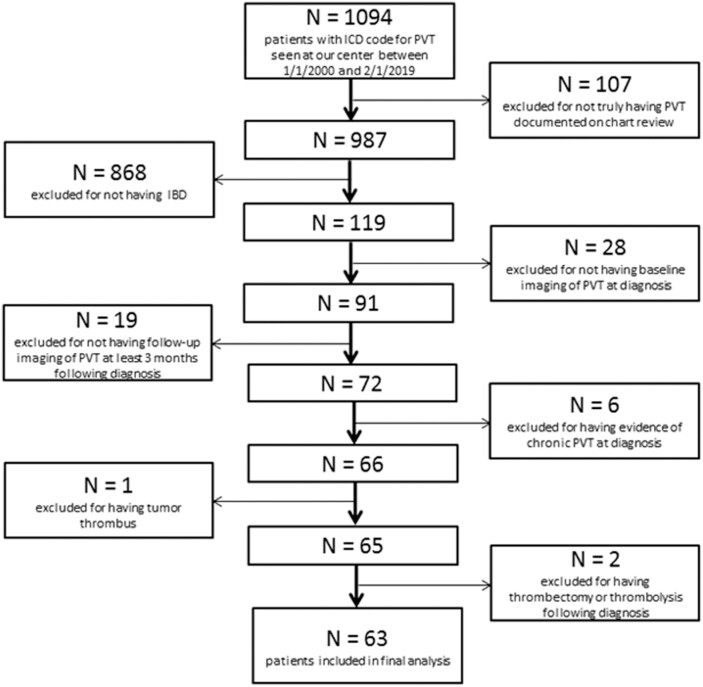
A total of 1094 patients bearing an ICD code for PVT were identified, of whom 119 had concurrent IBD. Upon chart review, 63 of these 119 patients met all inclusion criteria (26 Crohn disease [CD], 37 ulcerative colitis [UC]) (Fig. 1). Patients were followed for a median 21 months (IQR = 9-52). The baseline characteristics of this cohort are described in Table 1. Of note, only 8% of patients (5/63) had any prior history of venous thromboembolism (VTE). Seventy-five percent of patients (47/63) had an additional clinically evident provoking factor for PVT besides IBD, most often recent intra-abdominal surgery or intra-abdominal infection. Because recent intra-abdominal surgery was particularly common among our patients, additional detail specifically regarding the postsurgical cohort is provided in Supplementary Table 1. Among the CD patients, 54% (14/26) had stricturing or penetrating disease (Montreal Classification B2 or B3). Among the UC patients, 81% (30/37) had moderate or severe disease (Montreal Classification S2 or S3), including 38% (14/37) who met the criteria for acute severe UC (Montreal Classification S3). Additional details regarding the baseline characteristics of our cohort are presented in Supplementary Table 2. We found that IBD therapies received within 3 months of PVT diagnosis included steroids (62%; 40/63), biologics (60%; 38/63), and immunomodulators (37%; 23/63). The majority of biologic agents used (25/38; 66%) were inhibitors of tumor necrosis factor alpha. No patients had received tofacitinib.
FIGURE 1.
A CONSORT diagram summarizing reasons for exclusion.
TABLE 1.
Characteristics of IBD Patients With PVT
| Median age (IQR) | 42 (29-55) |
| Percent male | 63% (40/63) |
| Type of IBD | CD: 41% (26/63) UC: 59% (37/63) |
| IBD therapies near time of PVT* | Steroids: 63% (40/63) Biologics: 60% (38/63) Immunomodulators: 37% (23/63) |
| Prior VTE history | 8% (5/63) |
| Additional risk factor for PVT | 75% (47/63)† Intra-abdominal surgery: 60% (n = 38)‡ Intra-abdominal infection: 13% (n = 8)‡ Cirrhosis: 6% (n = 4) Myeloproliferative neoplasm: 2% (n = 1) Pancreatitis: 2% (n = 1) |
| IBD flare at PVT diagnosis | 33% (21/63)§ |
| Presenting signs/symptoms | abdominal pain: 71% (45/63) diarrhea or increased ostomy output: 38% (24/63) Constipation or ileus: 11% (7/63) Back pain: 8% (5/63) Nausea and/or vomiting: 8% (5/63) Fever: 6% (4/63) Rectal bleeding: 6% (4/63) |
| Laboratory findings | Leukocytosis: 83% (52/63) Elevated inflammatory markers: 83% (34/41)¶ Hyperbilirubinemia: 16% (10/63) Elevated aminotransferases: 11% (7/63) |
| Vessel involvement | Left and/or right PV only: 32% (20/63) Main PV +/- left and/or right PV: 14% (9/63) Main PV + SMV: 38% (24/63) Main PV + SMV + splenic: 10% (6/63) Main PV + splenic: 6% (4/63) |
| Occlusivity of thrombus | Occlusive: 33% (21/63) Nonocclusive: 67% (42/63) |
The baseline characteristics of all patients included in the study cohort are described above.
*All IBD therapies received within 3 months before PVT diagnosis. Steroids included nonabsorbable oral steroids. The majority of biologic agents used (25/38; 66%) were inhibitors of tumor necrosis factor alpha. No patients had received tofacitinib.
†Five patients had both recent intra-abdominal surgery and recent intra-abdominal infection.
‡All intra-abdominal surgeries and infections occurred within 3 months before PVT diagnosis.
§Presence or absence of IBD flare was determined from the notes of the documenting gastroenterologist(s).
¶Inflammatory markers included erythrocyte sedimentation rate and C-reactive protein.
Presenting Characteristics
Presenting signs, symptoms, and laboratory findings among our cohort are summarized in Table 1. Of note, 86% of patients (54/63) were diagnosed in an acute care setting (either during an inpatient hospitalization or an emergency department visit) with the remainder diagnosed on outpatient imaging. Initial presentations were generally nonspecific, with the most common presenting complaint being abdominal pain. One-third of patients (21/63) were felt to be in acute IBD flare by a documenting gastroenterologist. Laboratory results were nonspecific as well, with the most common abnormal findings being leukocytosis and elevated inflammatory markers. Liver function test abnormalities were infrequent. Fecal calprotectin levels were not often sent at presentation but were elevated in a large proportion of the few evaluated patients (68%; 13/19). Sixty percent (38/63) received intra-abdominal surgery within 3 months before presentation. All of these patients received bowel surgeries of some variation specifically for the treatment of IBD and/or an associated complication. Patients were diagnosed with PVT a median 11 days following surgery (range = 4-78; IQR = 7-19). Nearly all patients (37/38) were on appropriate postoperative VTE prophylaxis.
Diagnosis and Workup
In each instance the diagnosis of PVT was made on imaging (CT in 79%, MRI in 21%). In each case, PVT appeared acute (no prior history of PVT, no evidence of cavernous transformation or other radiographic features to suggest chronic PVT). One-third of patients (21/63) had completely occlusive thrombosis of the PV. More than half of patients (34/63) had PVT extending into a PV tributary (most often the SMV) (Table 1). In no instance was imaging undertaken to specifically assess for PVT. Rather, the impetus for imaging was typically to evaluate for potential IBD complication (eg, obstruction, fistula), intra-abdominal infection, and/or postsurgical complication. Thus PVT was generally an unexpected or incidental finding. Forty-three percent of patients (27/63) had evidence of active bowel inflammation on imaging. Regions of radiographically involved bowel at PVT diagnosis included rectum (n = 16; 25%), sigmoid colon (n = 15; 24%), descending colon (n = 7; 11%), transverse colon (n = 7; 11%), ileum (n = 6; 10%), ascending colon (n = 5; 8%), jejunum (n = 1; 2%), and duodenum (n = 1; 2%).
Forty-three percent of patients (27/63) received thrombophilia testing. Overall, the incidence of clinically meaningful positive test results was exceedingly low (Table 2). On chart review, there was no instance of either acute or long-term management being changed by the results of thrombophilia testing (ie, no instances of selection of specific AC, alteration of duration of AC, or changes in future thrombophylaxis practices based on the results of testing).
TABLE 2.
Thrombophilia Testing
| Test | Percentage Testing Positive |
|---|---|
| Factor V Leiden mutation | 7% (2/27)* |
| Prothrombin gene mutation | 4% (1/27)† |
| APLS testing‡ | 4% (1/27) |
| Protein C deficiency§ | 0% (0/24) |
| Protein S deficiency§ | 0% (0/24) |
| Antithrombin III deficiency§ | 0% (0/20) |
| JAK2V617F mutation | 0% (0/16) |
| Paroxysmal nocturnal hemoglobinuria | 0% (0/6) |
The results of thrombophilia testing among our cohort are described above.
*One patient was heterozygous and 1 patient was homozygous.
†This patient was heterozygous.
‡Testing included assessment for anticardiolipin antibodies, beta-2 glycoprotein I antibodies, and lupus anticoagulant. The 1 positive patient was subsequently found to be negative on repeat testing.
§Tests were excluded if sent in the context of acute thrombosis or while a patient was on warfarin (for proteins C and S).
Management
Ninety-two percent of patients (58/63) received AC. In 3 instances the decision not to anticoagulate was made because of concern regarding bleeding risk, and in the remaining 2 instances the reason for this decision was not specified in the medical record. The initial anticoagulant used in nearly all cases (51/58; 88%) was intravenous heparin. All anticoagulated patients were eventually started on or transitioned to an oral or subcutaneous anticoagulant (including direct oral anticoagulant [DOAC], n = 23; warfarin, n = 22; and enoxaparin, n = 13). The DOACs used included apixaban (n = 4), dabigatran (n = 4), and rivaroxaban (n = 15). All anticoagulated patients started AC within 3 days of PVT diagnosis (with the majority starting on the day of diagnosis). Transition from intravenous heparin to an oral or subcutaneous anticoagulant occurred a median of 3 days (IQR = 2-4) after starting intravenous heparin (this was identical among the DOAC, enoxaparin, and warfarin groups, with the transition day for warfarin taken as the day on which bridging began). All anticoagulated patients received at least 3 months of AC. Median duration of AC was 5.2 months (IQR = 3.1-9.9). Patients who received DOACs required shorter courses of AC (median 3.9 months; IQR = 2.7-6.1) than those who received warfarin (median 8.5 months; IQR = 3.9-NA; P = 0.0190). The characteristics of all patients stratified by AC used are described in Table 3. Baseline characteristics were generally similar across treatment groups.
TABLE 3.
Patient Characteristics by AC Used
| All Patients | Warfarin | Enoxaparin | DOAC | No Anticoagulant | P-value | |
|---|---|---|---|---|---|---|
| n = 63 | n = 22 | n =13 | n = 23 | n = 5 | ||
| Median age (IQR) | 42 (29-55) | 43 (33-54) | 44 (32-53) | 42 (29-53) | 58 (38-61) | 0.7558 |
| Sex | 0.4251 | |||||
| Male | 40 (63.5%) | 14 (63.6%) | 6 (46.2%) | 17 (73.9%) | 3 (60.0%) | |
| Female | 23 (36.5%) | 8 (36.4%) | 7 (53.8%) | 6 (26.1%) | 2 (40.0%) | |
| Median time to anticoagulant start-days (IQR) | 0 (0-2) | 0 (0-1) | 0 (0-1) | 0 (0-1) | - | - |
| Location of PVT | 0.3037 | |||||
| Main PV only | 9 (14.3%) | 4 (18.2%) | 1 (7.7%) | 2 (8.7%) | 2 (40.0%) | |
| Left or right PV only | 20 (31.7%) | 8 (36.4%) | 5 (38.5%) | 5 (21.7%) | 2 (40.0%) | |
| PV and additional SV | 34 (54.0%) | 10 (45.5%) | 7 (53.8%) | 16 (69.6%) | 1 (20.0%) | |
| Degree of PV occlusion | 0.9061 | |||||
| Occlusive | 21 (33.3%) | 8 (36.4%) | 5 (38.5%) | 7 (30.4%) | 1 (20.0%) | |
| Nonocclusive | 42 (66.7%) | 14 (63.6%) | 8 (61.5%) | 16 (69.6%) | 4 (80.0%) | |
| Type of IBD | 0.2666 | |||||
| CD | 26 (41.3%) | 10 (45.5%) | 4 (30.8%) | 8 (34.8%) | 4 (80.0%) | |
| UC | 37 (58.7%) | 12 (54.5%) | 9 (69.2%) | 15 (65.2%) | 1 (20.0%) | |
| Recent abdominal surgery | 38 (60.3%) | 14 (63.6%) | 7 (53.8%) | 15 (65.2%) | 2 (40.0%) | 0.7092 |
| Median follow-up time in months (IQR) | 21 (9-52) | 43 (9-80) | 23 (10-58) | 12 (6-35) | 32 (29-39) | 0.0053 |
The characteristics of all patients by anticoagulant used are described above.
SV indicates splanchnic vein.
Outcomes
Two patients died during follow-up, with 1 death a direct complication of PVT. This patient experienced rapid clot extension into the mesenteric vasculature in spite of treatment with intravenous heparin and then warfarin, resulting in small bowel ischemia and septic shock. Small bowel ischemia because of mesenteric clot extension occurred in a total of 3 patients (5%). In the 2 remaining instances there was evidence of small bowel ischemia at presentation that resolved following initiation of AC.
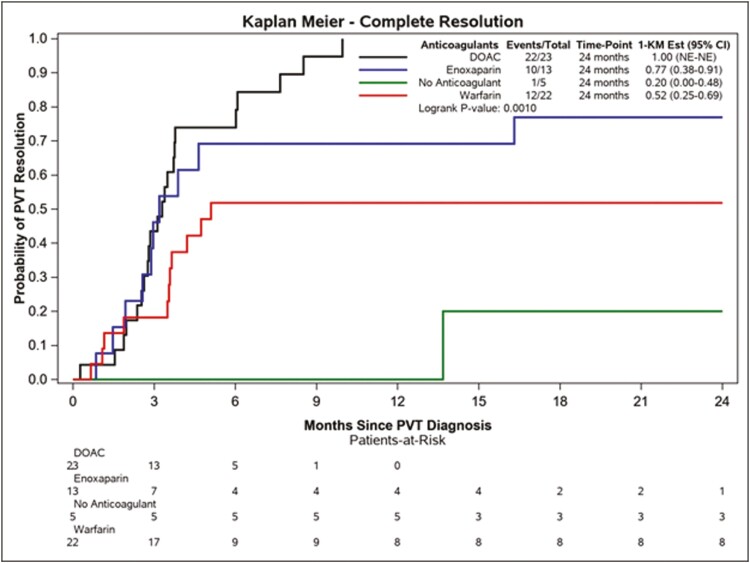
Seventy-one percent of patients (45/63) achieved the primary outcome of CRR of PVT. All instances of CRR of PVT were associated with concurrent CRR in any other involved splanchnic vessels. Kaplan-Meier curves depicting CRR by AC used are depicted in Fig. 2. Among ACs used, DOACs had the highest rate of CRR (22/23; 96%), and warfarin the lowest (12/22; 55%). Patients who received no AC were unlikely to experience CRR (1/5; 20%). Multivariable analysis of potential determinants of CRR among our IBD cohort is shown in Table 4. The only factor significantly associated with CRR on multivariable analysis was the type of AC used, with an adjusted HR for CRR for DOACs relative to that for warfarin of 4.04 (1.83-8.93; P = 0.0006). Note that in this instance an HR > 1 was favorable, as CRR is a favorable outcome.
FIGURE 2.
Kaplan-Meier curves for the primary outcome of CRR of PVT by AC used.
TABLE 4.
Multivariable Analysis for CRR of PVT
| Reference | HR (95% CI) | P | |
|---|---|---|---|
| Age | 1.00 (0.98-1.03) | 0.8837 | |
| Sex | |||
| Male | Female | 0.47 (0.23-0.94) | 0.0319 |
| Anticoagulant | |||
| None | Warfarin | 0.17 (0.02-1.34) | 0.0917 |
| DOAC | Warfarin | 4.04 (1.83-8.93) | 0.0006 |
| Enoxaparin | Warfarin | 1.51 (0.62-3.66) | 0.3665 |
| Location | |||
| Left or right PV | Main PV | 3.48 (0.97-12.42) | 0.0550 |
| PV + other SV | Main PV | 1.13 (0.36-3.57) | 0.8410 |
| Baseline occlusion | |||
| Occlusive | Nonocclusive | 1.25 (0.56-2.77) | 0.5842 |
| Type of IBD | |||
| CD | UC | 0.96 (0.51-1.81) | 0.8977 |
| Recent abdominal surgery | |||
| Yes | No | 0.97 (0.45-2.09) | 0.9382 |
Multivariable analysis of potential determinants of CRR among our IBD cohort is shown above. Note that in this instance an HR >1 was favorable, because CRR is a favorable outcome.
SV indicates splanchnic vein.
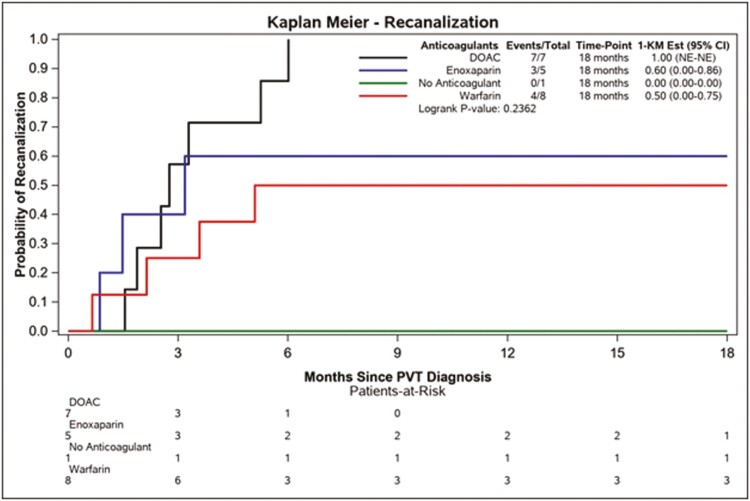
In addition, DOACs yielded the highest rate of RC of occlusive PVT, with warfarin again yielding the lowest such rates among ACs (depicted in Fig. 3). On multivariable analysis no particular factor was significantly associated with RC, likely because of the relatively small number of patients with fully occlusive PVT in our cohort. Among patients who had thrombophilia testing, those who were positive for factor V Leiden mutation, prothrombin gene mutation, and antiphospholipid syndrome (APLS) testing all experienced CRR, while the patient who tested positive for JAK2V617F did not. Outcomes did not differ significantly among the patients deemed to be in acute IBD flare and the remainder of the cohort, nor did they differ significantly between the CD patients with stricturing/penetrating disease and the remainder of the CD cohort, nor between the UC patients meeting the criteria for moderate or acute severe disease and the remainder of the UC cohort. However, this study was likely underpowered to make such comparisons.
FIGURE 3.
Kaplan-Meier curves for the secondary outcome of RC of occlusive PVT by AC used.
Three of the 18 patients (17%) who failed to achieve CRR of PVT went on to develop SPH. Two of these patients had been treated with enoxaparin and 1 had been treated with warfarin. None of the patients who received DOACs, and none of the patients who achieved CRR, developed SPH. One of the 3 patients who developed SPH went on to receive a transjugular intrahepatic portosystemic shunt with subsequent improvement in portal hypertensive symptoms. Major (World Health Organization grade 3 or 4) bleeding occurred in 4 patients, 3 of whom were receiving warfarin and 1 of whom was receiving enoxaparin at that time. None of these bleeding events were fatal. No patients receiving DOACs experienced major bleeding. Two of the 45 patients (4%) who achieved CRR experienced recurrent PVT during follow-up. Both of these patients had discontinued AC before recurrence. One of these 2 patients had cirrhosis as an additional persistent risk factor for PVT, and the other had recurrence following intra-abdominal surgery (these recurrences were diagnosed 8 months and 23 months after discontinuing AC, respectively).
DISCUSSION
Research has found that PVT is a relatively underrecognized and poorly described complication of IBD.7 Prevalence rates ranging from 0.17% to 1.7% of IBD patients have been reported in large retrospective cohorts.8-10 Among our cohort the most prominent risk factor for PVT was intra-abdominal surgery, a finding that seems to be borne out in a number of other institutional experiences.11-14 Additional notable risk factors in our cohort included intra-abdominal infection and acute IBD flare.
Primary hematologic thrombophilias were rare among our patients, as has been shown in other types of provoked splanchnic vein thrombosis (SVT).15, 16 Given that laboratory thrombophilia evaluations do not seem to impact management or outcomes (both in this cohort and in others), they likely do not merit routine use in cases of clearly provoked PVT.16, 17 We would recommend that in instances of clearly provoked PVT (such as following recent surgery or in the context of recent/active intra-abdominal infection or recent/active flare), no thrombophilia testing need be sent. However, if PVT is not clearly provoked (ie, a spontaneous PVT in an otherwise stable and inactive IBD patient), thrombophilia testing should be considered. Thrombophilia testing should also be considered in patients with history of other VTE or unexplained blood count abnormalities. The most crucial thrombophilia test to send in such instances is an assessment for JAK2V617F, as it is fairly common among patients with otherwise unprovoked SVT and can have a major impact on further workup and management.18, 19 In addition, APLS and paroxysmal nocturnal hemoglobinuria testing may impact management and thus can be considered in some specific circumstances (history of autoimmune disease and/or arterial thrombosis for APLS or unexplained cytopenias and/or evidence of intravascular hemolysis for paroxysmal nocturnal hemoglobinuria). Other thrombophilia testing generally does not merit sending because results do not impact management.17, 19 Consultation with a hematologist is reasonable when thrombophilia testing is being considered.
Presenting signs, symptoms, and laboratory results are highly nonspecific, and a PVT diagnosis is nearly always made incidentally, when contrast imaging to evaluate abdominal complaints reveals non-opacification or other abnormalities in the portal vasculature.20 It is notable that some professional society guidelines advise that patients with incidentally detected SVT receive no AC.21, 22 If these guidelines were truly incorporated into clinical practice, few if any IBD patients would receive AC for PVT. At our institution we routinely anticoagulate patients with incidentally detected SVT. This is because spontaneous PVT resolution in the absence of AC is unlikely (20% in our cohort), the potential consequences of persistent thrombosis are dire (gut ischemia and chronic portal hypertension), and major bleeding rates on AC are low (6.8% in our cohort and 2.7% when we excluded warfarin).
Seventy-one percent of patients in our cohort achieved CRR of PVT. No other studies currently report long-term outcomes of IBD-specific PVT; however, this rate is notably higher than those reported in studies of all PVT, in which resolution and RC rates are usually <50% in large cohorts.23 We note that in our institution, the CRR rate of all acute noncirrhotic PVT is 43%.24 Similarly, RC rates of occlusive PVT were higher, and rates of portal hypertensive complications were lower, in this IBD-specific cohort than in nonspecific PVT cohorts (both those described in the literature and those in our institutional experience).23, 25 The relatively excellent outcomes in this cohort may result from some intrinsically benign property of IBD-related PVT (indeed, non-IBD-specific cohorts may be enriched with patients who have unprovoked PVT or PVT related to underlying hematologic disorders, both of which are likely more recalcitrant). Alternatively, or concurrently, the excellent outcomes in this cohort may be the result of the high rates of AC (92%) or the prompt initiation of AC (all anticoagulated patients started AC within 3 days of PVT diagnosis, with the majority starting on the day of diagnosis). Indeed, prior studies have demonstrated that delays in AC as short as days to 2 weeks may lead to inferior outcomes.25 Prompt AC may be particularly important among those patients with concurrent SMV involvement because complete occlusion of this vessel may lead to mesenteric ischemia and, if complicated by portal Hypertension the need for multivisceral transplantation. In our series, all patients who had gut necrosis or died had evidence of SMV extension at diagnosis or upon reimaging.4 It is unclear why men were less likely to achieve CRR in this cohort than women (HR = 0.47; 95% CI, 0.23-0.94; P = 0.0319); such a difference in outcomes has not been reported previously in the PVT literature. In the wider VTE literature men are known to have a higher risk for first and recurrent venous thrombosis; however, the specific factors underlying this phenomenon are unknown.26
Professional society guidelines and published expert opinions currently recommend vitamin K antagonists (such as warfarin) or low-molecular-weight heparins (such as enoxaparin) as the standard anticoagulants for SVT.21, 27, 28 This is because data (particularly prospective data) to support the use of DOACs in this setting are currently lacking.29 Retrospective data supporting the safety and efficacy of DOACs in such cases are emerging, and these drugs are coming into increasing real-world use in the treatment of PVT.29, 30 There is a growing body of literature supporting the safety and efficacy of DOACs in PVT-complicating cirrhosis, leading to their inclusion in at least 1 consensus statement on PVT in liver disease patients.29, 31 At our institution we now routinely use DOACs in the management of PVT and are finding outcomes and safety profiles that may be superior to those of warfarin.32These findings were clearly exemplified in our IBD-specific PVT cohort in which the CRR rate with DOACs was an unprecedented 96%, significantly better than the 55% rate we saw with warfarin. In addition, DOACs showed trends toward an improved RC rate, reduced portal hypertensive complications, and reduced bleeding risk, relative to the warfarin standard in our cohort, all while requiring shorter courses of AC. Based on these findings, we would regard DOACs as the preferred AC for IBD-associated PVT.
It is unclear why warfarin should be inferior to DOACs among IBD patients; however, impaired absorption, frequent antibiotic use, and interaction with some IBD medications, particularly thiopurines, may play a role.33-35 In addition, it is known from prior studies that earlier initiation of therapeutic AC may improve outcomes in PVT.25 Warfarin blood levels tend to be at their most labile during the initial weeks of therapy, while dosage is most frequently being adjusted.36 Therefore, patients receiving warfarin may be suboptimally anticoagulated during the early phase of therapy, the phase that may be the most crucial in ensuring desirable long-term outcomes. In contrast, DOACs tend to reach therapeutic blood levels early, reliably, and typically without the need for dose adjustment. However, although serious complications of PVT or of the use of AC for PVT were not seen among those receiving DOACs, this study was not sufficiently powered to determine the strength of the association between this therapy and such fortunately rare events.
It is difficult for us to comment on the ideal duration of AC because the timing of follow-up imaging was not standardized across our patients, with some patients receiving follow-up scans at frequent and regular intervals and others waiting a long time before their first follow-up imaging. Although this issue somewhat limits any conclusions that may be drawn about the time-to-resolution of PVT, it did seem that the majority of clots resolved after 3 to 6 months of AC. As such, it may be reasonable to treat for at least 3 to 6 months and then repeat imaging with a plan to discontinue AC if CRR has occurred, or to continue and repeat interval imaging if it has not. Among our DOAC cohort, patients received AC for a median 3.9 months (IQR = 2.7-6.1). Patients receiving warfarin stayed on AC longer (median 8.5 months; IQR = 3.9-NA) likely because their PVT took longer to resolve or was less likely to resolve, prompting clinicians to extend their courses in hopes of achieving response. Safe discontinuation of AC was supported by the low rate of recurrent thrombosis in this cohort and by current guidelines regarding provoked deep vein thrombosis.37
Limitations of this study include its retrospective design, which may be associated with a number of biases, most notably that the clinician choice of AC could have been influenced by unknown or uncontrolled variables. Specifically, it is possible that the superior outcome seen with DOACs may have resulted from some inherent selection bias. Although patients treated with DOACs did not differ significantly from the other treatment groups with respect to several key baseline characteristics (Table 3), it is possible that our limited sample size prevented the detection of relevant associations. Similarly, our limited sample size should prompt moderation when one assesses the positive associations observed between therapies and outcomes. Indeed, perhaps treatment with DOACs is a surrogate for a subpopulation that will have a better course and resolution of their clot because of some unforeseen clinical variables. In addition, DOACs were introduced into practice during the latter portion of the study period, which could have coincided or have had temporal associations with other changes in practice that may have favorably altered the rate of study endpoints. Thus, the only way to confirm the provocative findings in this study would be via a prospective randomized trial. Notably, all patients were diagnosed and managed at a tertiary IBD referral center and may therefore not be representative of the general IBD population. Finally, although this is currently the largest reported cohort of IBD-associated PVT, it is still underpowered to examine relatively rare outcomes such as gut ischemia, development of portal hypertension, major bleeding, and recurrent thrombosis.
CONCLUSION
This study demonstrates that the use of AC may potentially lead to excellent outcomes in IBD-associated PVT. Administered DOACs were associated with particularly favorable outcomes among such patients in this retrospective study and were in particular associated with favorable efficacy and safety profiles relative to warfarin. Rates of recurrent VTE were low, and such patients may likely discontinue AC following at least 3 months of treatment, assuming that follow-up imaging shows resolution of PVT. The incidence of clinically meaningful results in thrombophilia testing was low, and such testing need not be routinely sent among this patient population. Further studies, both prospective and retrospective, would be helpful to confirm these findings.
Supplementary Material
ACKNOWLEDGMENTS
The authors wish to acknowledge the administrative support of the Biostatistics Shared Resource Facility, Icahn School of Medicine at Mount Sinai, and NCI Cancer Center Support Grant P30 CA196521-01.
Supported by: The authors have no financial disclosures.
Conflicts of interest: None of the authors have any relevant conflicts of interest to report.
REFERENCES
- 1.Intagliata NM, Caldwell SH, Tripodi A. Diagnosis, development, and treatment of portal vein thrombosis in patients with and without cirrhosis. Gastroenterology. 2019;156:1582–1599.e1. [DOI] [PubMed] [Google Scholar]
- 2.Zezos P, Kouklakis G, Saibil F. Inflammatory bowel disease and thromboembolism. World J Gastroenterol. 2014;20:13863–13878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Landman C, Nahon S, Cosnes J, et al. ; Groupe d’Etude Thérapeutique des Affections Inflammatoires du Tube Digestif. Portomesenteric vein thrombosis in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:582–589. [DOI] [PubMed] [Google Scholar]
- 4.Russell CE, Wadhera RK, Piazza G. Mesenteric venous thrombosis. Circulation. 2015;131:1599–1603. [DOI] [PubMed] [Google Scholar]
- 5.Harmanci O, Bayraktar Y. Portal hypertension due to portal venous thrombosis: etiology, clinical outcomes. World J Gastroenterol. 2007;13:2535–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics. 1982;38:29–41. [Google Scholar]
- 7.Ott C, Schölmerich J. Extraintestinal manifestations and complications in IBD. Nat Rev Gastroenterol Hepatol. 2013;10:585–595. [DOI] [PubMed] [Google Scholar]
- 8.Maconi G, Bolzacchini E, Dell’Era A, et al. Portal vein thrombosis in inflammatory bowel diseases: a single-center case series. J Crohns Colitis. 2012;6:362–367. [DOI] [PubMed] [Google Scholar]
- 9.Talbot RW, Heppell J, Dozois RR, Beart RW Jr. Vascular complications of inflammatory bowel disease. Mayo Clin Proc. 1986;61:140–145. [DOI] [PubMed] [Google Scholar]
- 10.Bruining DH, Siddiki HA, Fletcher JG, et al. Prevalence of penetrating disease and extraintestinal manifestations of Crohn’s disease detected with CT enterography. Inflamm Bowel Dis. 2008;14:1701–1706. [DOI] [PubMed] [Google Scholar]
- 11.Kayal M, Radcliffe M, Plietz M, et al. Portomesenteric venous thrombosis in patients undergoing surgery for medically refractory ulcerative colitis. Inflamm Bowel Dis. 2019;141:e419S–e496S. [DOI] [PubMed] [Google Scholar]
- 12.Baker ME, Remzi F, Einstein D, et al. CT depiction of portal vein thrombi after creation of ileal pouch-anal anastomosis. Radiology. 2003;227:73–79. [DOI] [PubMed] [Google Scholar]
- 13.Remzi FH, Fazio VW, Oncel M, et al. Portal vein thrombi after restorative proctocolectomy. Surgery. 2002;132:655–61; discussion: 661. [DOI] [PubMed] [Google Scholar]
- 14.Ball CG, MacLean AR, Buie WD, et al. Portal vein thrombi after ileal pouch-anal anastomosis: its incidence and association with pouchitis. Surg Today. 2007;37:552–557. [DOI] [PubMed] [Google Scholar]
- 15.Zarrouk M, Salim S, Elf J, et al. Testing for thrombophilia in mesenteric venous thrombosis—retrospective original study and systematic review. Best Pract Res Clin Gastroenterol. 2017;31:39–48. [DOI] [PubMed] [Google Scholar]
- 16.Naymagon L, Tremblay D, Schiano T, et al. The role of anticoagulation in pylephlebitis: a retrospective examination of characteristics and outcomes. J Thromb Thrombolysis. 2020;49:325–331. [DOI] [PubMed] [Google Scholar]
- 17.Connors JM. Thrombophilia testing and venous thrombosis. N Engl J Med. 2017;377:2298. [DOI] [PubMed] [Google Scholar]
- 18.Regina S, Herault O, D’Alteroche L, et al. JAK2 V617F is specifically associated with idiopathic splanchnic vein thrombosis. J Thromb Haemost. 2007;5:859–861. [DOI] [PubMed] [Google Scholar]
- 19.Tremblay D, Naymagon L, Troy K, et al. The utility of thrombophilia testing in patients with newly diagnosed portal vein thrombosis [published online ahead of print February 25, 2020]. Blood Coagul Fibrinolysis. 2020. In press. [DOI] [PubMed] [Google Scholar]
- 20.Margini C, Berzigotti A. Portal vein thrombosis: the role of imaging in the clinical setting. Dig Liver Dis. 2017;49:113–120. [DOI] [PubMed] [Google Scholar]
- 21.Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e419S–e496S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen GC, Bernstein CN, Bitton A, et al. Consensus statements on the risk, prevention, and treatment of venous thromboembolism in inflammatory bowel disease: Canadian Association of Gastroenterology. Gastroenterology. 2014;146:835–848.e6. [DOI] [PubMed] [Google Scholar]
- 23.Plessier A, Darwish-Murad S, Hernandez-Guerra M, et al. ; European Network for Vascular Disorders of the Liver (EN-Vie). Acute portal vein thrombosis unrelated to cirrhosis: a prospective multicenter follow-up study. Hepatology. 2010;51:210–218. [DOI] [PubMed] [Google Scholar]
- 24.Naymagon L, Tremblay D, Zubizarreta N, et al. Portal vein thrombosis patients harboring JAK2V617F have poor long-term outcomes despite anticoagulation. J Thromb Thrombolysis. 2020. In press. [DOI] [PubMed] [Google Scholar]
- 25.Turnes J, García-Pagán JC, González M, et al. Portal hypertension-related complications after acute portal vein thrombosis: impact of early anticoagulation. Clin Gastroenterol Hepatol. 2008;6:1412–1417. [DOI] [PubMed] [Google Scholar]
- 26.Roach RE, Cannegieter SC, Lijfering WM. Differential risks in men and women for first and recurrent venous thrombosis: the role of genes and environment. J Thromb Haemost. 2014;12:1593–1600. [DOI] [PubMed] [Google Scholar]
- 27.Ageno W, Beyer-Westendorf J, Garcia DA, et al. Guidance for the management of venous thrombosis in unusual sites. J Thromb Thrombolysis. 2016;41:129–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ageno W, Dentali F, Squizzato A. How I treat splanchnic vein thrombosis. Blood. 2014;124:3685–3691. [DOI] [PubMed] [Google Scholar]
- 29.Priyanka P, Kupec JT, Krafft M, et al. Newer oral anticoagulants in the treatment of acute portal vein thrombosis in patients with and without cirrhosis. Int J Hepatol. 2018;2018:8432781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janczak DT, Mimier MK, McBane RD, et al. Rivaroxaban and apixaban for initial treatment of acute venous thromboembolism of atypical location. Mayo Clin Proc. 2018;93:40–47. [DOI] [PubMed] [Google Scholar]
- 31.Intagliata NM, Argo CK, Stine JG, et al. ; Faculty of the 7th International Coagulation in Liver Disease. Concepts and controversies in haemostasis and thrombosis associated with liver disease: proceedings of the 7th International Coagulation in Liver Disease Conference. Thromb Haemost. 2018;118:1491–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naymagon L, Tremblay D, Zubizarreta, N, et al. The efficacy and safety of direct oral anticoagulants in noncirrhotic portal vein thrombosis. Blood Adv. 2020;4:655–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sabol BJ, Basa RR, Wilkins CE. Malabsorption-associated warfarin resistance. Am J Health Syst Pharm. 2009;66:1548–1553. [DOI] [PubMed] [Google Scholar]
- 34.Gisbert JP, Chaparro M. Systematic review with meta-analysis: inflammatory bowel disease in the elderly. Aliment Pharmacol Ther. 2014;39:459–477. [DOI] [PubMed] [Google Scholar]
- 35.Konidari A, Matary WE. Use of thiopurines in inflammatory bowel disease: Safety issues. World J Gastrointest Pharmacol Ther. 2014;5:63–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Urbonas G, Valius L, Sakalyte G, et al. The quality of anticoagulation therapy among warfarin-treated patients with atrial fibrillation in a primary health care setting. Medicina (Kaunas). 2019;55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kearon C, Akl EA. Duration of anticoagulant therapy for deep vein thrombosis and pulmonary embolism. Blood. 2014;123:1794–1801. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.