Abstract
Acetaminophen (APAP) was first synthesized in the 1800s, and came on the market approximately 65 years ago. Since then, it has become one of the most used drugs in the world. However, it is also a major cause of acute liver failure. Early investigations of the mechanisms of toxicity revealed that cytochrome P450 enzymes catalyze formation of a reactive metabolite in the liver that depletes glutathione and covalently binds to proteins. That work led to the introduction of N-acetylcysteine (NAC) as an antidote for APAP overdose. Subsequent studies identified the reactive metabolite N-acetyl-p-benzoquinone imine, specific P450 enzymes involved, the mechanism of P450-mediated oxidation, and major adducted proteins. The mechanisms downstream of metabolism remain poorly understood but several events appear critical. These events include development of an initial oxidative stress, reactive nitrogen formation, altered calcium flux, JNK activation and mitochondrial translocation, inhibition of mitochondrial respiration, the mitochondrial permeability transition, and nuclear DNA fragmentation. Additional research is necessary to fill remaining gaps in our knowledge of the toxicity, such as the source of the initial oxidative stress, and to improve our understanding liver regeneration after APAP overdose. A better understanding of these mechanisms may lead to additional treatment options. Even though NAC is an excellent antidote, its effectiveness is limited to the first 16 hours following overdose.
Keywords: Acetaminophen overdose, APAP-protein adducts, biomarkers, drug-induced liver injury, drug metabolism, liver injury, liver regeneration, mitochondria, oxidative stress, reactive nitrogen
Introduction
Acetaminophen (N-acetyl-p-aminophenol; APAP), or paracetamol as it is known in Europe, is a popular over-the-counter drug with analgesic and antipyretic properties similar to aspirin. However, unlike aspirin and other non-steroidal anti-inflammatory drugs (NSAIDs), it has only weak anti-inflammatory effects. At therapeutic doses, it is believed to be safe. In fact, it is one of the most commonly used drugs in the United States (Kaufman et al. 2002). Unfortunately, overdose can cause severe liver injury.
The development of APAP as a drug began with a happy accident. In the 1880’s, two medical interns working under German physician Adolf Kussmaul at the University of Strassburg were given the wrong compound by a pharmacist who thought he was dispensing naphthalene to treat a patient’s intestinal worms. While the compound was ineffective against the parasite, it did reduce the patient’s fever (Cahn and Hepp 1886). Chemists soon identified the new drug as acetanilide and it was soon marketed by the German company Kalle under the brand name Antifebrin (Cahn and Hepp 1886). The analgesic effects of acetanilide were initially attributed to the acetyl moiety that distinguished it from its precursor aniline, resulting in an acetylation craze that likely led to the development of phenacetin (N-acetyl-p-ethoxyacetanilide), the immediate precursor of APAP. Bayer chemists seeking a use for the unwanted by-product of dye synthesis, p-aminophenol, decided to acetylate it. Like acetanilide, the product phenacetin exhibited strong analgesic and antipyretic effects and quickly became the analgesic of choice. However, acetanilide and phenacetin both produced serious adverse effects, leading to the search for a safer alternative. In 1893, clinical pharmacologist Joseph Baron von Mering was the first to administer APAP to humans (Von Mering 1893). However, Von Mering claimed that APAP produced side effects similar to acetanilide and phenacetin, most notably methemoglobinemia. APAP was subsequently discarded in favor of phenacetin, which was taken either alone or in combination with aspirin and caffeine. Phenacetin remained the preferred mild analgesic until approximately 1970.
Only in the past 50 years has APAP become the analgesic drug of choice. This occurred for a few major reasons. First, Greenberg and Lester demonstrated that APAP is a metabolite of acetanilide, and that the methemoglobinemia observed by Von Mering was probably not due to APAP itself (Greenberg and Lester 1947). Shortly thereafter, Brodie and Axelrod found that APAP is also the major metabolite of phenacetin (Brodie and Axelrod 1949), and indeed that it is the pharmacologically active product (Flinn and Brodie 1948). Second, phenacetin was acutely and chronically nephrotoxic. Long-term abuse of phenacetin was associated with kidney damage, renal carcinoma, and tumors of the bladder. It was also carcinogenic in long-term feeding studies in both mice and rats and induced tumors of the kidney and lower urinary tract. Thus, the US FDA removed it from the market in 1983. Although phenacetin is to APAP, nephrotoxicity was not associated with APAP early on. Seeing an opportunity, McNeil Laboratories introduced APAP to the market in a barbiturate combination called Algoson, then later as a stand-alone product under the brand name Tylenol. Although the NSAID aspirin was also available as an analgesic by this time, APAP became more popular than aspirin due to the absence of GI toxicity. Aspirin causes GI bleeding and has also been associated with Reye’s syndrome (Schror 2007), a rare condition that may occur in children that causes swelling of the liver and brain. Furthermore, in contrast to aspirin, APAP does not prevent blood from clotting, so it can be used in patients who have concerns with blood coagulation such as during and following surgery.
First Reports of Hepatoxicity and Description of Pathology
The first reports that APAP is hepatotoxic were published by Boyd and Bereczky (Boyd and Bereczky 1966) and by Davidson and Eastham (Davidson and Eastham 1966) in 1966. Boyd and Bereczky found that acute doses of APAP in rats produced hepatotoxicity. Davidson and Eastham reported on two individuals who developed hepatotoxicity following overdose. Both individuals died on the third day following the overdose. Microscopic examination of sections from their livers revealed fulminating hepatic necrosis. The necrosis was primarily in the centrilobular areas but extended through the midzonal areas to the periportal areas. High power examination revealed eosinophilic degeneration of the cells with pyknosis of the nuclear material within the centrilobular areas. Vacuolization and early degenerative changes were observed in the more peripheral cells surrounding the portal tracts. A mild polymorphonuclear leukocytic infiltration had also occurred in both cases. These features indicated an acute fulminating hepatic necrosis of the centrilobular type.
Subsequently there were a number of reports of APAP toxicity in overdose patients (Thomson and Prescott 1966; Rose 1969; Proudfoot and Wright 1970; Boyer and Rouff 1971; Prescott et al. 1971). The clinical features of the toxicity were described by Boyer and Rouff (Boyer and Rouff 1971): nausea and vomiting within two to three hours followed by abdominal pain, chiefly in the upper right quadrant. Liver dysfunction is present within approximately 24 hours and peaks at four days after the overdose. The clinical biochemistry was summarized by Prescott et al. (Prescott et al. 1971): dramatic increases of circulating alanine aminotransferase (ALT), aspartate aminotransferase (AST), and lactate dehydrogenase (LDH); mild hyperbilirubinaemia; increases in prothrombin time; and increased APAP half-life to greater than 4 hours. When the half-life was greater than 12 hours, hepatic coma frequently occurred.
Dixon et al. (Dixon et al. 1971) subsequently described the pathology of APAP in more detail. There was a high incidence of lethality. In rats surviving at 24 hours there was hepatic congestion, dilation of central veins and surrounding sinusoids, and accumulation of red blood cells. Necrosis of the hepatocytes was observed. The nuclei of the necrotic cells showed characteristic signs of pyknosis and karyorrhexis. The necrotic zones contained neutrophils but no inflammatory cell reactions were observed. The endothelial and Kupffer cells were primarily in the necrotic zones.
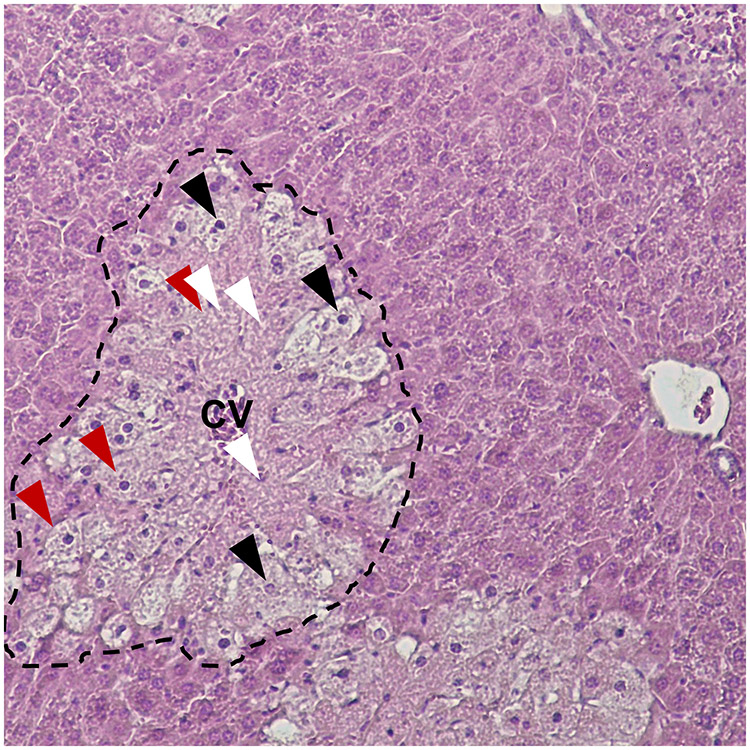
Researchers later discovered that mice are much more sensitive to APAP than rats, and develop hepatotoxicity at doses that are quite similar to humans. As a result, mice have become the primary animal to investigate mechanisms of APAP hepatotoxicity. In mice, toxicity can occur at doses as low as 200 mg APAP per kg bodyweight (mg/kg), or an order of magnitude lower than that which occurs in the rat (Mitchell et al. 1973, 1973a). Histologically, glycogen depletion and vacuolization of centrilobular hepatocytes are observed by 2 hours, resulting in a clear demarcation of the hepatic centrilobular areas. By 3 hours, nuclear changes are observed in hepatocytes of the centrilobular areas with single cell necrosis and pyknotic cells showing eosinophilic degeneration. By 6 hours, gross necrosis of the centrilobular zones is evidenced by nuclear pyknosis, karyorrhexis, karyolysis and eosinophilia of the necrotic hepatocytes. A more detailed description of the pathological changes in the liver over time with photographs was later published in the American Journal of Pathology in a manuscript by Roberts et al. (Roberts et al. 1991), with similar results. A representative image of APAP-induced liver necrosis is shown in figure 1.
Figure 1. Histopathology of APAP-induced liver injury.
A mouse was treated with 300 mg/kg APAP. The liver was collected 24 h later. Liver tissue was fixed in 10% phosphate-buffered formalin and embedded in paraffin wax. 5 μm sections were cut, mounted on glass slides, and stained with hematoxylin and eoson. The dotted line indicates an area of hepatocellular necrosis displaying eosinophilia and loss of basophilic nuclei. Black arrowheads: Pyknosis and karyorrhexis. White arrowheads: Karyolysis. Red arrowheads: Cell swelling.
Walker et al. performed scanning electron microscopic analysis of the liver of APAP-treated mice (Walker et al. 1983). They reported endocytic vacuolation at lateral and sinusoidal margins of the centrilobular hepatocytes, sparing periportal areas. Associated with the changes in the centrilobular areas, there was loss of microvilli, Space of Disse enlargement with the presence of erythrocytes, dilation of bile canaliculi, and alterations of cell surfaces. Congestion abated by 24 hours.
In humans, APAP-induced hepatotoxicity occurs with hepatic congestion in overdose victims (Thompson et al. 1972). Hepatic congestion was also observed in rodents (Dixon et al. 1971; Walker et al. 1980; Walker et al. 1985). In mice, hepatic congestion occurs early and before necrosis. Walker et al. (Walker et al. 1980; Walker et al. 1983, 1985) also reported accumulation of erythrocytes within endocytic vacuoles and the Space of Disse with collapse of the sinusoidal lumens. At 1.5 hours after administration of a toxic dose of APAP to mice, liver weights significantly increased and doubled by 6 hours. At 24 hours, the weights had decreased. Increased liver weights occurred with a very large increase in hepatic hemoglobin levels (4-fold at 6 hours) and a subsequent decrease by 24 hours. The decrease in liver weights has been attributed to hepatocyte lysis and leakage with large increases in serum ALT and AST as a result (Roberts et al. 1991).
In 1995, Lim and coworkers (Lim et al. 1995), using a vascular casting technique, reported that APAP toxicity in the centrilobular areas of the rat occurred with microvascular injury. Subsequently, McCuskey and coworkers examined APAP-induced microvascular injury in more detail in the mouse (Ito et al. 2003; McCuskey 2006). They reported hepatocellular injury to the sinusoidal endothelial cells, preceding hepatocellular injury. Swelling of the endothelial cells and penetration of erythrocytes into the extrasinusoidal Space of Disse was observed (Ito et al. 2003). Blood flow in the sinusoids by 2 h was significantly decreased (Ito et al. 2003). The functional integrity of the endothelial cells was impaired in the centrilobular regions but not in the periportal regions. It was postulated that endothelial damage may play a role in the reduced sinusoidal perfusion (Ito et al. 2003).
Metabolism in APAP Toxicity: Early Research Findings
In a series of four manuscripts, Mitchell, Jollow, Potter, Gillette, and Brodie examined the role of metabolism in the hepatotoxicity of APAP (Jollow et al. 1973; Mitchell et al. 1973; Potter et al. 1973; Mitchell et al. 1973a). These are now classic, highly cited manuscripts that have contributed significantly to our understanding mechanisms of APAP-induced hepatotoxicity.
The first manuscript described the toxicity and showed the importance of drug metabolism (Mitchell et al. 1973). Importantly, the group found that mice are more susceptible to APAP hepatotoxicity than rats. Whereas a dose of 300 mg/kg was toxic to the mouse, a dose eight to ten times higher was required to produce a similar hepatotoxic effect in the rat. Moreover, they observed frequent lethality at these very high doses in the rat. They demonstrated the role of drug metabolism by pre-treating mice with inducers and inhibitors of drug metabolizing enzymes and then histologically evaluating the incidence of hepatic necrosis at 24 hours. Thus, following a dose of 375 mg/kg of APAP, saline pre-treated mice had a 46% incidence of hepatic necrosis, whereas pre-treatment of mice with the cytochrome P450 inducer phenobarbital increased the incidence of hepatic necrosis to 90%. In contrast, pre-treatment with piperonyl butoxide or cobaltous chloride, two inhibitors of drug metabolizing enzymes, decreased the incidence of hepatic necrosis to 0%. These results pointed to a cytochrome P450-dependent mechanism of hepatotoxicity.
In the second manuscript, following toxic doses of radiolabeled APAP to mice, radiolabel was associated with proteins (covalent binding) (Jollow et al. 1973). Pre-treatment of mice with phenobarbital increased both the incidence of hepatic necrosis and the amount of radiolabel associated with proteins, while pre-treatment with the inhibitors of drug metabolism greatly decreased both. Importantly, the relative amount of radiolabel associated with protein preceded hepatic necrosis and was dose dependent. Autoradiographs also indicated that the radiolabel was localized in the necrotic hepatocytes. The nature of the association was then investigated. The radiolabel was firmly attached to proteins and could not be removed by extraction with organic solvents. Moreover, treatment of the protein with pronase released a product with the characteristics of an amino acid. These data were interpreted to indicate that APAP had been metabolized to a reactive metabolite (metabolic activation) that had covalently bound to proteins (protein adduct formation). Important to this mechanism is that APAP does not act through hepatic lipid peroxidation (Knight et al. 2003), a mechanism of hepatotoxicity believed to be mechanistically important in carbon tetrachloride-induced liver injury (Koster et al. 1977).
In the third manuscript, they studied the covalent binding of APAP to proteins in vitro in microsomal incubation mixtures (Potter et al. 1973). Microsomes from mice pre-treated with phenobarbital had increased velocities of covalent binding whereas microsomes from mice pre-treated with inhibitors of drug metabolism had decreased velocities of covalent binding. Covalent binding was shown to require NADPH and oxygen, and was inhibited in an oxygen/carbon monoxide atmosphere. These data were consistent with the involvement of cytochrome P450 enzymes in the metabolism to a reactive metabolite. N-Hydroxylation was postulated to be the metabolic step.
Finally, the fourth manuscript reported the importance of glutathione (GSH) in preventing the APAP-induced hepatotoxicity (Mitchell et al. 1973). Thus, a dose of 375 mg/kg of APAP produced approximately a 50% incidence of hepatotoxicity. Pre-treatment with the nontoxic compound diethylmaleate to deplete hepatic GSH increased the incidence to 100%. Pre-treatment with cysteine decreased the incidence of hepatotoxicity in a dose dependent manner. Hepatic GSH was depleted following a toxic dose of APAP in a time and dose dependent manner. By 2 hours following a very hepatotoxic dose of APAP (400 mg/kg), total hepatic GSH was depleted by more than 80%. Pre-treatments that altered drug metabolism and toxicity (increased or decreased) similarly altered hepatic GSH depletion. Importantly, GSH depletion preceded covalent binding and, as discussed above, covalent binding preceded the development of hepatotoxicity (Jollow et al. 1973). These data indicated that GSH is important to prevent covalent binding and development of hepatotoxicity.
Overall, these manuscripts showed that APAP is converted by drug metabolizing enzymes to a reactive metabolite that covalently binds to proteins. At nontoxic doses, the metabolite is efficiently detoxified by GSH, forming an APAP-GS conjugate. A small amount binds proteins, but it is not sufficient to cause toxicity (McGill et al. 2013). However, at toxic doses, the metabolite depletes hepatic GSH by as much as 80-90% and binds extensively to protein. Finally, the amount of protein binding correlates with the relative hepatotoxicity.
Introduction of N-acetylcysteine as an Antidote
In the 1970’s, there was an intense effort by various clinical research groups to develop an effective antidote that could be used in APAP overdose patients. Three major drugs were considered: N-acetylcysteine (NAC), methionine, and cysteamine. All three are sulfur-containing drugs. NAC may be deacetylated to cysteine and cysteine may then be metabolized to GSH. Thus, the reactive metabolite may be detoxified by either NAC, cysteine, or GSH. Methionine may also be metabolized to cysteine. On the other hand, cysteamine (2-methylthioethylamine) is a small molecular thiol derivative which was envisioned to directly detoxify the reactive metabolite (Mitchell et al. 1974).
Ultimately, NAC was determined to be the antidote of choice (Rumack and Peterson 1978; Prescott et al. 1979; Smilkstein et al. 1988; Bateman and Dear 2019). Most importantly, it was found to be more effective than cysteamine or methionine and was already FDA approved to treat bronchitis. Moreover, it was free from serious adverse effects, whereas cysteamine causes distressing off-target effects and methionine may itself be toxic as some doses (Prescott et al. 1979). However, for superior antidotal properties, NAC treatment should begin within approximately 10 hours following APAP ingestion (Prescott et al. 1979).
The currently used decision for utilization of NAC in APAP overdose is based on a graph of APAP concentration versus time, the so called Rumack-Matthew Nomogram (Smilkstein et al. 1988; Rumack 2002) (Figure 2). The nomogram plots a line at times from 4 to 24 hours. If the APAP plasma concentration is above this line at a given time after overdose, then NAC treatment is recommended. If the APAP concentration is below this line, NAC treatment is not recommended.
Figure 2. The Rumack-Matthew nomogram.
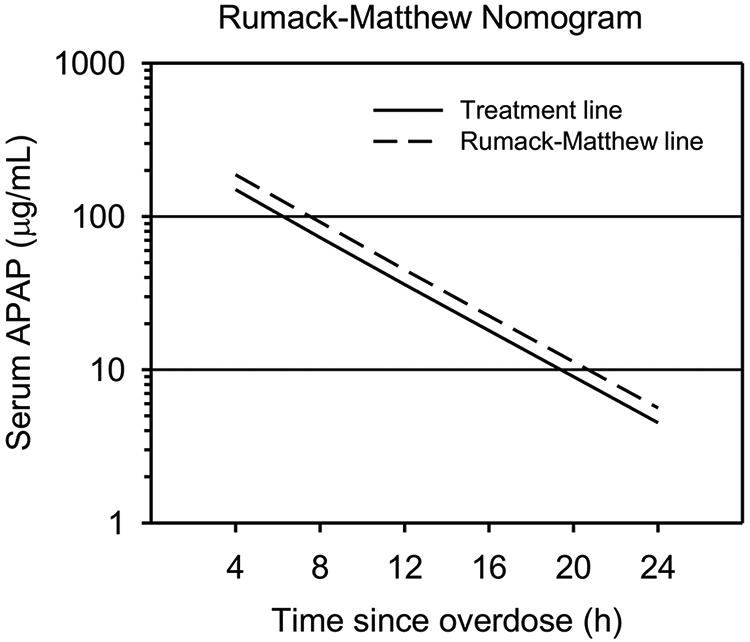
When measured between 4 and 24 hours after APAP overdose, values above the dashed line (Rumack-Matthew line) are likely to result in hepatotoxicity, values between the dashed line and the solid line (Treatment line) may or may not lead to hepatotoxicity, and values below the solid line are unlikely to cause hepatotoxicity. All patients with values above the Treatment line should receive NAC.
Lauterburg and coworkers (Lauterburg et al. 1983) examined the question if NAC detoxified the reactive metabolite of APAP directly or via increased GSH synthesis, and therefore if increased GSH was responsible for the hepatoprotective effects of NAC. Utilizing [35S]-NAC, it was shown that following a toxic dose of APAP (1 g/kg) and radiolabeled NAC (1.2 g/kg) to the rat, the specific activity of the resulting NAC-APAP in the urine averaged 76% of the specific activity of the APAP-GS conjugate in the bile. These data were interpreted to indicate that virtually all of the NAC-APAP originated from the APAP-GS conjugate. Moreover, following APAP treatment to rats, NAC increased GSH synthesis. These data suggest that the mechanism by which NAC prevents APAP hepatotoxicity is by an increase in GSH synthesis and efficient detoxification of the toxic metabolite.
Studies on the Mechanism of Metabolic Activation
In the initial research on mechanisms, metabolic activation by N-hydroxylation was postulated to be the mechanism of activation of APAP to a reactive toxic metabolite (Potter et al. 1973). N-Hydroxylation was known to be important in the hepatocarcinogenesis of 2-acetylaminofluorene, another N-acetyl aromatic amine (Miller JA and Miller 1975). Subsequently, both p-chloroacetanilide and phenacetin (p-ethoxyacetanilide), two other APAP analogs, were shown to be N-hydroxylated (Hinson and Mitchell 1976; Hinson et al. 1976).
This mechanism was subsequently modified following reports by Dr. Ian Calder’s laboratory in Australia that N-acetyl-p-benzoquinone imine (NAPQI) was an electrophilic species (Calder et al. 1973; Calder et al. 1974; Calder et al. 1981). They were able to synthesize it by oxidation with lead tetra-acetate, but it rapidly decomposed. Identification was indirect as a Diels-Alder adduct (Calder et al. 1973). Based on their findings that it was a reactive species, Mitchell and coworkers subsequently postulated that APAP was initially N-hydroxylated by cytochrome P450 to form N-hydroxy-APAP, which then spontaneously dehydrated to form NAPQI (Fig. 3). Thus, the reactive toxic metabolite that covalently bound to protein was hypothesized to be NAPQI, and the binding site in proteins was postulated to be cysteine (Hinson 1980; Streeter et al. 1984).
Figure 3. Proposed mechanisms of NAPQI formation.
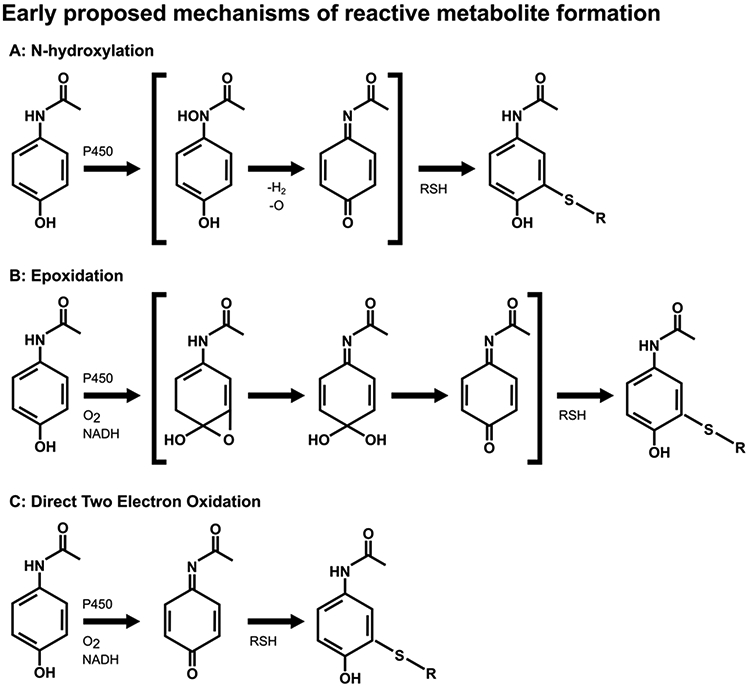
Initially, it was thought that formation of the reactive metabolite of APAP may precede through N-hydroxylation (A) or epoxidation (B), as shown. These mechanisms were proven to be incorrect. (C) It is now thought that APAP is directly oxidized by a two-electron oxidation, a previously unrecognized cytochrome P-450 mechanism. Once formed, NAPQI readily reacts with sulfhydryl groups, such as on GSH or cysteine residues in proteins.
Dr. Calder’s group was able to synthesize N-hydroxy-APAP and test that hypothesis (Calder et al. 1981). They found that it spontaneously dehydrated to NAPQI with a half-life of 80 minutes. Hinson et al. obtained a sample of the N-hydroxy-APAP from Dr. Calder and using that sample they developed a HPLC assay to determine if NAPQI was a metabolite of APAP (Hinson, Pohl, Gillette 1980). In a microsomal incubation mixture, they showed that N-hydroxy-APAP was a metabolite of APAP. However, N-hydroxyphenacetin (N-hydroxy-p-ethoxyacetanilide) was metabolized to N-hydroxy-APAP (a deethylation reaction) (Hinson, Pohl, et al. 1979), but there was more covalent binding of radiolabeled APAP to protein than covalent binding of radiolabeled N-hydroxyphenacetin to protein (Hinson, Pohl, et al. 1979). Also, Dr. Nelson obtained a sample of the proposed metabolite from Dr. Calder and using an isotope dilution approach they were unable to find N-hydroxy-APAP as a metabolite. Thus, N-hydroxy-APAP is apparently not a metabolite of APAP. However, as reported by Corcoran and coworkers, N-hydroxy-APAP led to a reactive metabolite that had metabolic properties similar to the reactive metabolite of APAP: both formed conjugates with cysteine and were reducible by ascorbate (Corcoran et al. 1980). Taken as a whole, these data indicated that APAP is converted directly to NAPQI by a two electron oxidation. However, this mechanism had not yet been described as a P450 mechanism. Thus, at that time, the mechanism of APAP metabolic activation to NAPQI remained unclear.
APAP epoxidation had been suggested by a number of investigators as another possible toxic mechanism. Epoxidation was known to be important in the carcinogenicity of polycyclic aromatic hydrocarbons (Nebert et al. 1976) and the hepatotoxicity of bromobenzene (Jollow et al. 1974). Thus, the potential 3,4-epoxidation of APAP was evaluated. By this mechanism, the 3,4-epoxide could react directly with a nucleophile, or the ring could open to yield the hydrated NAPQI and dehydration would yield NAPQI. This mechanism was investigated by incubating APAP in a microsomal incubation mixture in the presence of GSH and an 18O2 atmosphere. If 3,4-epoxidation were the mechanism of metabolic activation of APAP to yield a GSH conjugate, then 18O would be incorporated into the APAP-GS metabolite at a 50% level. Isolation of the APAP-GS metabolite from this experiment and analysis by mass spectrometry indicated no incorporation of 18O (Hinson et al. 1977). Also, the p-18O-APAP was synthesized and incubated with GSH in a microsomal incubation mixture and 18O was not lost in the isolated 18O-APAP-GS metabolite. Finally, p-18O-APAP was administered to hamsters and the urinary APAP mercapturate (N-acetylcysteine-APAP) metabolite isolated (Hinson, Nelson, et al. 1979). The 18O content in the mercapturate was the same as that in the administered p-18O-APAP. Thus, 3,4-epoxidation was not the mechanism of metabolic activation of APAP to a reactive metabolite (Figure 3B).
2,3-Epoxidation was also investigated as a mechanism of APAP metabolic activation (Hinson, Pohl, Monks, et al. 1980). Since epoxides may break down to phenols, it seemed possible that 3-hydroxy-APAP may be a metabolite. In a microsomal incubation, the 3-hydroxy-APAP was found to be a significant metabolite. However, addition of epoxide hydrolase to the microsomal incubation mixture did not affect the rate of its formation. Moreover, addition of GSH to the incubation did not alter the amount of the 3-hydroxy metabolite. Also, it was shown that ascorbic acid almost completely inhibited covalent binding but had no effect on the formation of the 3-hydroxy-APAP formation. Finally, the finding that ascorbic acid inhibited covalent binding was consistent with the postulation that a readily reducible product such as NAPQI was the reactive metabolite (Hinson 1980). Subsequent studies showed that in mice the 3-hydroxy-APAP was not a major in vivo metabolite and also was not very toxic (Forte et al. 1984).
Even though the data of Calder and co-workers implicated NAPQI as a reactive metabolite, they were unable to purify it and thus identified it by an indirect technique, as a Diels-Alder adduct. Since they were unable purify it they were not able to perform detailed studies on its chemical properties (Calder et al. 1973). Nelson and co-workers were able to synthesize pure NAPQI by oxidation of APAP with silver oxide (AgO) in chloroform and study its chemical reactivity (Dahlin and Nelson 1982). The compound was unstable in aqueous buffer with a half-life of approximately 11 minutes. It decomposed to a number of products including hydrolysis to benzoquinone. In isolated liver cells, it was about ten times more toxic than APAP and was also toxic in vivo (Dahlin and Nelson 1982). Subsequently, using purified cytochromes P450, radiolabeled APAP and cumene hydroxide HPLC analysis indicated a radiolabeled metabolite with the retention time of NAPQI. In the presence of NADPH and NADPH-cytochrome P450 reductase, steady-state levels of NAPQI were below their detection limits of 6.7 X 10-8 M. Thus, they were forced to use cumene hydroperoxide in the incubation mixture. In the presence of GSH, an APAP-glutathione conjugate was formed (Dahlin et al. 1984). Overall, these data support the hypothesis that NAPQI is the major reactive metabolite of APAP (Figure 3).
Studies on APAP Peroxidation
N-Acetyl-p-benzosemiquinone imine (NAPSQI) has also been evaluated as a reactive toxic metabolite of APAP. This is the free radical of APAP that may be formed by one electron oxidation, a peroxidation reaction. Nelson and coworkers (Nelson et al. 1981) showed that incubation of APAP with protein, horseradish peroxidase, hydrogen peroxide, and radiolabeled APAP resulted in covalent binding of APAP to protein. Using a spin trap technique, further evidence was found for peroxidation of APAP to a phenoxy free radical (Nelson et al. 1981; West et al. 1984). The free radical and covalent binding were eliminated by addition of ascorbic acid. These data indicated that APAP was indeed metabolized to the free radical NAPSQI. Moreover, this metabolic pathway led to a reactive metabolite that covalently bound to protein.
Potter et al. (Potter et al. 1985) examined metabolic products formed by incubation of APAP with horse radish peroxidase and hydrogen peroxide. They identified a number of polymerization products: two dimers, three trimers, and a tetramer. The primary product was a 3,3’-dimer where the carbon ortho to the hydroxy group was bonded to the same carbon of the other APAP group in the dimer. Also, there was a 3,3’,3’’-trimer, a 3,3’’,3’’’,3’’’’-tetramer, a dimer bonded from the 3 position of one APAP to the nitrogen of another APAP, a trimer containing a N- to 3 linkage, and a tetramer containing a N- to 3 linkage. Dimers were the major products at high APAP concentrations or low horseradish concentrations, whereas trimers and tetramers predominated at low APAP concentrations or high horseradish peroxidase concentrations. The reaction was very fast. Approximately one mole of hydrogen peroxide was utilized per two moles of free radical, a result consistent with a free radical termination reaction. 99% of polymerization products were quenched by 2 mM ascorbate (Potter et al. 1985).
In subsequent work, it was shown that addition of GSH to an incubation mixture containing APAP, horseradish peroxidase and hydrogen peroxide resulted in the formation of an APAP-GS conjugate and a GSH conjugate of the 3,3’-dimer (Potter and Hinson 1987b). Increasing concentrations of GSH decreased polymer formation and formation of the GSH conjugates. Ascorbate and NADPH were extremely effective in decreasing both polymers and GSH conjugates. NAPQI was detected in the horseradish peroxidase, APAP, and hydroperoxide incubation mixtures. Maximal NAPQI formation was at 15 seconds followed by a decrease with a half-life of approximately 1 minute. The NAPQI could be trapped by addition of GSH with formation of an APAP-GS conjugate. These data indicated that APAP was metabolized by peroxidation to form NAPSQI. This species can react with another free radical to form a dimer or higher polymers, or disproportionate to form NAPQI plus APAP. The NAPQI rapidly conjugated with GSH to form an APAP-GSH conjugate. The NAPQSI was very easily reduced by ascorbate, GSH, or NADPH but there was no evidence it could conjugate with GSH (Potter and Hinson 1987b).
Polymer formation was not observed when rat liver microsomes from phenobarbital treated rats were incubated with APAP and NADPH (Potter and Hinson 1987b). When GSH was added, the APAP-GS conjugate was the major product. Incubation of the microsomes with APAP and cumene hydroperoxide resulted in polymer formation, and addition of GSH resulted in formation of an APAP-GS conjugate and a decrease in polymer formation. These data indicate that P450 enzymes metabolize APAP by both one and two electron oxidation. Two electron oxidation results in formation of NAPQI, which rapidly reacts with GSH to form an APAP-GS conjugate or covalently bind to protein. One electron oxidation forms NAPQSI which polymerizes or disproportionates to form polymers or NAPQI and APAP, respectively. It is easily reduced by GSH or NADPH back to APAP (Potter DW and Hinson 1987b).
APAP metabolism studies were also performed using purified prostaglandin H synthase (Potter and Hinson 1987a). In the presence of arachidonic acid, APAP was metabolized to polymers. Metabolism was not observed with hydrogen peroxide. Addition of GSH resulted in formation of an APAP-GS conjugate and a decrease in polymerization. Addition of GSH plus ascorbic acid, or NADPH, only slightly decreased APAP-GS formation, but almost completely inhibited polymer formation. It was concluded that prostaglandin H synthase metabolized APAP by both one electron oxidation leading to NAPSQI and formation of polymers, and two electron oxidation to NAPQI which conjugated with GSH (Potter and Hinson 1987a).
Overall, by the mid-1980s, it was firmly established that the reactive metabolite that covalently bound to protein was NAPQI. The mechanism was postulated to be a direct two-electron oxidation of APAP, a previously unrecognized mechanism for cytochrome P450 enzymes (Figure 3C). This mechanism has been postulated to occur by interaction of APAP with P450 by an initial one-electron abstraction from the amide nitrogen, followed by a rapid recombination of the radical pair to form a P450 heme bound N-hydroxy-Fe intermediate. As a result of a p-phenolic group on the APAP moiety, the enzyme-substrate intermediate dehydrates to generate NAPQI and a ferric heme (Harvison et al. 1988).
Mechanisms of NAPQI Detoxification
Structural identification of the APAP-GS conjugate was determined by NMR spectrometry on a biliary metabolite isolated from the bile of APAP treated rats. These data firmly established that the sulfur of GSH was attached to the ring of APAP at the carbon 3 position (3-glutathion-S-ylAPAP), ortho to the phenolic group (Hinson et al. 1982). Subsequently, Nelson and coworkers isolated and identified an additional GSH conjugate, a thiohemiketal ipso adduct (Chen et al. 1999). The sulfur of this adduct was attached to APAP at the carbon 1 position. Once formed, the ipso adduct was reversible to regenerate NAPQI and GSH, or it could react with another GSH to yield APAP and GSSG. Thus, a mechanism for how GSH reduced NAPQI was found. It was postulated that the ipso adduct could migrate and subsequently rearrange to yield NAPQI at a point distant from its formation. In the presence of acid, the ipso adduct decomposed to a GSH conjugate attached to the APAP ring in the carbon 2 position. The mechanism of reaction of GSH with NAPQI to yield 3-cys-APAP was postulated to be an initial attack on the carbonyl carbon in the 4 position to yield a conjugate which rearranged with sulfur migration to the 3 position to form 3-cys-APAP (Chen WQ et al. 1999). However, Bessems and Vermeulen have postulated a more traditional Michael Addition reaction where the sulfur attacks carbon 3 of NAPQI (Bessems and Vermeulen 2001).
The reaction of GSH with NAPQI was determined to be very rapid. The spontaneous reaction was first order in NAPQI and GSH, and inversely proportional to the H+ ion concentration. At pH 7.0 and 25°C, the second-order rate constant was 3.2 X 104 M−1 s−1 (Coles et al. 1988). Generation of NAPQI in a microsomal incubation mixture and using a crude GSH transferase preparation revealed that the metabolite was enzymatically detoxified (Buckpitt et al. 1979). Determination of the conjugation of various purified human glutathione-S-transferases (GST) showed that GST-Pi was a major detoxification enzyme, but the reaction could be catalyzed by GST delta and mu as well (Coles et al. 1988). Surprisingly, GST-Pi knockout mice were not as susceptible to APAP hepatotoxicity as wildtype mice (Henderson CJ et al. 2000). However, depletion of hepatic GSH was significantly greater at 0.5, 1 and 5 hours in the wildtype mice than in the knockout mice post dosing. At 1 hour, hepatic GSH depletion was approximately 95% in the wildtype mice compared to approximately 70% in the knockout mice (Henderson et al. 2000). These data suggest that toxicity may be more complex than the initially proposed in the covalent binding hypothesis, where covalent binding to a critical protein is postulated to kill the hepatocyte.
NAPQI is readily detoxified not only by GSH but by ascorbic acid. Whereas the detoxification of NAPQI is by conjugation to form 3-glutathion-S-ylAPAP, the detoxification by ascorbic acid is by reduction back to APAP. Thus, if GSH is a critical detoxification mechanism in vivo, it could be asked how important ascorbic acid is in vivo. Miller and Jollow examined the effect of this compound on APAP hepatotoxicity in the hamster. A large dose of ascorbic acid (400 mg/kg, ip), which doubled hepatic ascorbic acid levels, did not alter the hepatotoxicity of APAP or decrease APAP covalent binding to protein (Miller and Jollow 1984). These data are consistent with the finding that the conjugation of NAPQI with GSH is much more rapid than the reduction of NAPQI by ascorbic acid or other reducing agents (Potter and Hinson 1987b). Thus, NAPQI preferentially reacts with GSH or cysteine groups in proteins.
Cytochrome P450 Enzymes in NAPQI Formation
In the third of the initial manuscripts by Mitchell, Jollow and co-workers on APAP metabolism described above, the importance of a reactive metabolite in APAP toxicity and covalent binding of APAP to proteins was studied in microsomal incubation mixtures (Potter et al. 1973). NADPH and oxygen were necessary for covalent binding. Pre-treatments which were known to alter P450 levels similarly altered in vitro covalent binding. From these data, it was concluded that APAP was metabolically activated to the reactive metabolite by P450.
Purification of the cytochromes P450 led to the determination of specific isoforms important in the metabolic activation of APAP. In 1983, Morgan et al. (Morgan et al. 1983) examined the ability of six purified rabbit P450s to oxidize APAP to the reactive metabolite (trapped as a GSH conjugate). They found that Cyp2e1, Cyp1a2 and Cyp1a1 readily activated APAP (Morgan et al. 1983). Subsequently, Harvison and coworkers reported that rat Cyp1a1, Cyp1a2 and Cyp2c11 metabolically activated APAP (Harvison et al. 1988). Using isolated human P450s, Patten and coworkers (Patten et al. 1993) found that the major isoforms that metabolically activated were CYP2E1, CYP1A2, and CYP3A4 (Patten et al. 1993; Sinclair et al. 1998). CYP2E1 is the ethanol inducible form, CYP1A2 is inducible by polycyclic aromatic hydrocarbons, and CYP3A4 is not inducible but is the major constitutive P450 isoform important in drug metabolism. Moreover, animals pre-treated with ethanol to induce Cyp2e1 are more susceptible to APAP hepatotoxicity (McClain et al. 1980; Peterson et al. 1980). Interestingly, animals that are treated with ethanol at the same time as APAP are less susceptible because both are metabolized by Cyp2e1 (Lee et al. 1999).
Cyp2e1 is of major interest in human toxicity because of the consumption of alcoholic beverages. In 1986, Seef and coworkers (Seeff et al. 1986) reported on six alcoholics who developed toxicity after taking APAP and reviewed nineteen similar case reports. In 1995, Zimmerman and Maddrey (Zimmerman and Maddrey 1995) reported on an additional 67 alcoholic patients who developed liver toxicity after taking moderate doses of APAP. The increased toxicity of APAP was attributed to induction of CYP2E1 in alcoholics and increased production of the toxic metabolite as a result (Zimmerman and Maddrey 1995). However, the possibility that APAP may be toxic in alcoholics at moderate doses has been disputed by Prescott (Prescott 2000) and by Rumack (Rumack 2002, 2004). The problems in obtaining proper controls makes it difficult to know if alcoholics are more susceptible to APAP hepatotoxicity. Nevertheless, it is considered a risk factor when decisions are made whether to treat with NAC (Schmidt et al. 2002).
The importance of Cyp2e1 and Cyp1a2 in hepatotoxicity of APAP was clearly shown in knockout mice. In 1996, Lee and coworkers found that serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were significantly increased in wildtype but not knockout mice at an APAP dose of 200 mg/kg. However, serum ALT and AST did increase in the knockouts at a dose of 600 mg/kg (Lee et al. 1996), demonstrating that other P450 enzymes may compensate for loss of Cyp2e1 at very large doses. APAP hepatotoxicity was subsequently studied in mice where both Cyp2e1 and Cyp1a2 had been deleted. In the wildtype control mice, serum ALT levels increased at doses of 400 mg/kg, 600 mg/kg and 800 mg/kg in a dose-responsive manner. Even following doses a high as 1200 mg/kg, serum ALT levels did not increase in the double knockout mice (Zaher et al. 1998). These data demonstrate that Cyp1a2 becomes important for APAP bioactivation at very high doses that may saturate Cyp2e1. Altogether, it appears that the major P450 enzymes involved in APAP metabolism of CYP2E1, CYP1A2, and CYP3A4.
APAP-Protein Adducts
In the mid-1980s, two research groups raised antibodies in rabbits that were used to investigate APAP covalently bound to proteins (Bartolone et al. 1987; Roberts et al. 1987). Whereas the antiserum developed by Roberts and coworkers was specific for APAP covalently bound to cysteine (Roberts et al. 1987), the antiserum developed by Cohen and coworkers was specific for APAP itself (Bartolone et al. 1987). Nevertheless, the results obtained by the two groups were comparable. Three different immunochemical approaches were utilized: immunohistochemical analyses to determine specific hepatocyte targets (Roberts et al. 1991; Hart et al. 1995), competitive ELISA to quantify adducts in subcellular fractions (Pumford et al. 1989), and immunoblot for analysis of protein targets of NAPQI (Bartolone et al. 1987; Bartolone et al. 1988; Pumford et al. 1990; Matthews et al. 1996). These data have been reviewed (Hinson et al. 1995; Cohen and Khairallah 1997).
APAP-protein adduct formation is specific for proteins in liver. Adduct formation occurs before toxicity and a number of specific proteins are primary targets. Figure 4 is an immunoblot of the liver cytosolic fraction from a mouse 2 h after a hepatotoxic dose of APAP, taken from the first study to identify a specific target of APAP binding (Cohen and Khairallah 1997). Note a major protein adduct at approximately 58 kDa. This protein was isolated and identified as the Selenium Binding Protein (Pumford et al. 1992) (Hoivik et al. 1996), which had been previously shown to associate with selenium following a tracer dose of the metal. A large number of other proteins have been subsequently identified, but the relevance of APAP binding of the Selenium Binding Protein or any other protein relative to toxicity has never been defined (James, Mayeux, et al. 2003).
Figure 4. Anti-APAP immunoblot.
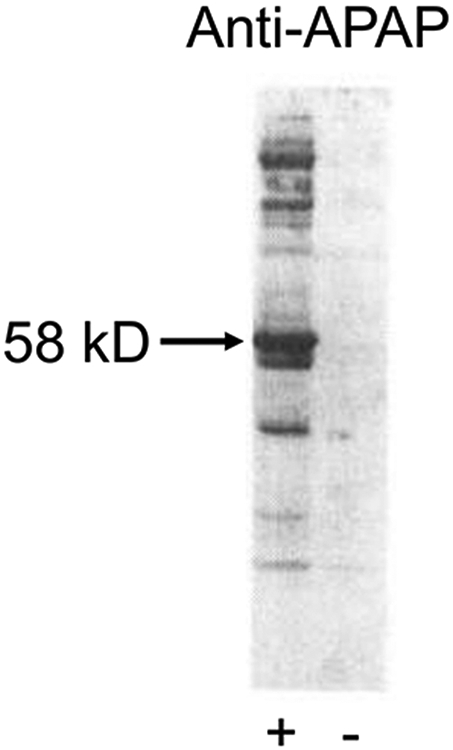
Cytosolic fractions from the livers of control (−) and 600 mg/kg APAP-treated (+) mice, isolated 2 hours after treatment. Western blotting was performed using an anti-APAP antibody. A dominant band is visible around 58 kD. Adapted from(Cohen and Khairallah 1997), with permission from the publisher.
Although the significance of covalent binding to specific proteins is unknown, it is clear that the mitochondrion is a critical target. The regioisomer of APAP, m-aminophenol (AMAP), is toxic to primary human hepatocytes but not to primary mouse hepatocytes, and it has been demonstrated that AMAP binds to mitochondrial proteins in the former but not the latter (Xie, McGill, et al. 2015). Furthermore, APAP-protein adduct levels are lower in mitochondria from rat liver than mouse liver after treatment with APAP despite prolonged GSH depletion in rats, and it is thought that this may partially explain the resistance of rats to APAP toxicity (McGill, Williams, et al. 2012).
APAP-protein adduct formation is an excellent biomarker of toxicity. Immunohistochemical analysis for APAP protein binding in histological sections of livers from mice treated with APAP shows protein binding occurs only in the necrotic centrilobular hepatocytes. Moreover, binding and toxicity co-localized in hepatocytes high in cyp2e1 (Hart et al. 1995). This is additional evidence of the importance of metabolic activation in toxicity and the role of Cyp2e1. Figure 5A is a time course for the immunohistochemical detection of APAP-protein adducts in liver sections of mice treated with a hepatotoxic dose of APAP (400 mg/kg) (Roberts et al. 1991). Adducts are stained red. By 1 and 4 hours after APAP, adducts are present in the centrilobular area, the site of the ensuing necrosis. Adducts are not detectable in the periportal areas. By 6 hours, hepatic necrosis is maximal, with loss of adducts from the centrilobular areas as a result of hepatocyte lysis. These data clearly indicate the importance of metabolic activation in APAP toxicity and that APAP-protein adducts are an excellent biomarker of toxicity. Although it cannot be concluded that adducts per se caused the toxicity, necrosis was not observed in any hepatocytes that did not contain adducts. This strongly argues against a mechanism proposed by Mehendale (Mehendale 2012) where lysing cells release proteolytic enzymes which cause necrosis in surrounding cells, a phenomenon called toxic expansion. If this mechanism occurred with APAP, there would not be a 100% correlation between protein adduct formation and necrosis. Furthermore, it is inconsistent with the hypothesis that sterile inflammation is a significant contributor to APAP-induced liver injury.
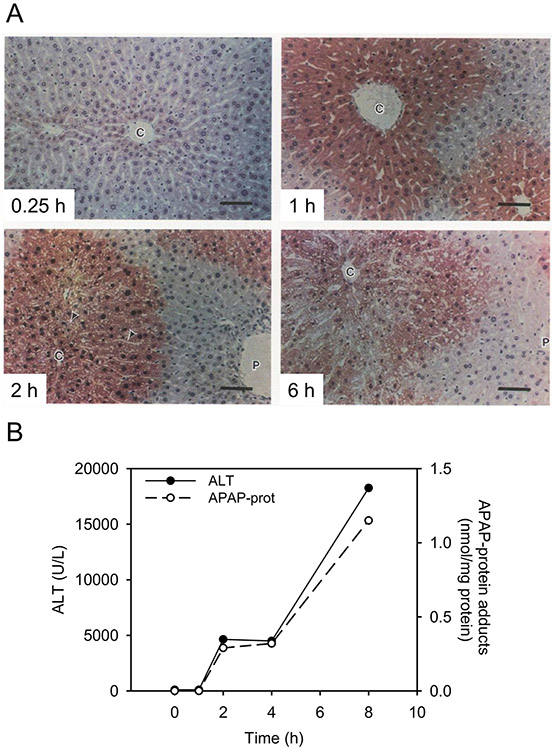
Figure 5. Measurement of covalent APAP-protein adducts in the liver.
Mice were treated with a hepatotoxic dose of acetaminophen (APAP). Livers and serum were collected at multiple time points after APAP overdose. (A) Immunohistochemistry for APAP. (B) Serum alanine aminotransferase (ALT) activity (left y-axis) and serum APAP-protein adducts (right y-axis) over time. Adapted from (Roberts D. W. et al. 1991) and (McGill et al. 2013) with permission.
A time course for APAP-protein adduct formation was quantified by competitive ELISA (Pumford et al. 1989). Mice were treated with a hepatotoxic dose of APAP and were subsequently sacrificed at various times. Serum levels of ALT and AST peaked at 4 hours and remained high for the duration (Figure 5B). APAP-protein adducts in liver peaked at 2 hours and subsequently decreased with a concomitant rise in adducts in serum. Thus, APAP-protein adducts accumulate in liver between 0 and 2 hours. By 4 hours, adducts were released into the blood along with ALT and AST as a result of hepatocyte lysis. Interestingly, experiments with primary mouse hepatocytes have demonstrated that APAP-protein adducts are released in part by mechanisms other than necrosis (McGill et al. 2013). However, it is likely that the dominant mechanism in vivo is passive release due to hepatocyte death because induction of hepatocyte necrosis by ischemia-reperfusion after a nontoxic dose of APAP dramatically increased APAP-protein adduct levels in serum compared to sham surgery (McGill et al. 2013). Altogether, these data provide support for the importance of metabolic activation in APAP toxicity and show that adducts in serum are a biomarker of hepatotoxicity. This was further demonstrated by showing APAP-protein adducts in serum from APAP overdose patients (Hinson et al. 1990).
In 2002, a new chromatographic assay was developed to quantify APAP-protein adducts (Muldrew et al. 2002). In this assay, a protein sample is first gel filtered to remove any low molecular weight metabolites and then hydrolyzed with protease to release the APAP-cysteine conjugate (3-cys-APAP). The 3-cys-APAP is then separated by HPLC and quantified by electrochemical detection (HPLC-EC). The assay has proven to be excellent for analyzing serum samples of APAP overdose victims (Davern et al. 2006; James et al. 2007). The APAP-protein adducts are long-lived in serum, with a half-life of approximately 2 days (James et al. 2009). Of particular note, in 2006 Davern and coworkers analyzed 36 patients who had severe liver toxicity of indeterminate cause. Seven of these patients (19%) had APAP protein adducts in their sera (Davern et al. 2006). Thus, detection of APAP-protein adducts in serum may be invaluable in helping identify APAP as a cause of liver toxicity.
Nitration of Proteins
Whereas APAP-protein adducts have proven to be an excellent biomarker of toxicity, more recent data have indicated that toxicity is much more complex than simply NAPQI covalent binding to a critical protein which then loses activity or function, resulting in cell death. Immunohistochemical analysis of liver sections from APAP-intoxicated mice using an anti-3-nitrotyrosine antibody indicated tyrosine nitration in the proteins of the centrilobular cells (Hinson et al. 1998). Tyrosine nitration has been shown to occur by peroxynitrite, a reactive intermediate formed by an extremely rapid reaction of nitric oxide and superoxide (Beckman and Koppenol 1996; Reiter et al. 2000; Beckman 2009). Analysis of liver sections using an APAP antiserum indicated the centrilobular cells also contained APAP-protein adducts (Hinson et al. 1998) (Figure 6). Peroxynitrite is an intermediate that is detoxified by GSH (Radi et al. 1991; Lizasoain et al. 1996), which is depleted in APAP toxicity (Radi et al. 1991). Importantly, blocking protein nitration by peroxynitrite protects against APAP-induced liver injury. Knight et al. (Knight et al. 2002) demonstrated that 1.5 and 2.25 hour post-treatment with exogenous glutathione after APAP overdose does not affect reactive metabolite formation or glutathione oxidation, but does reduce nitrotyrosine formation and liver injury. They also found that it can enhance liver regeneration (Bajt et al. 2003). Altogether, these data are consistent with APAP metabolic activation leading to increased synthesis of nitric oxide and superoxide and to peroxynitrite as an important intermediate in the toxicity.
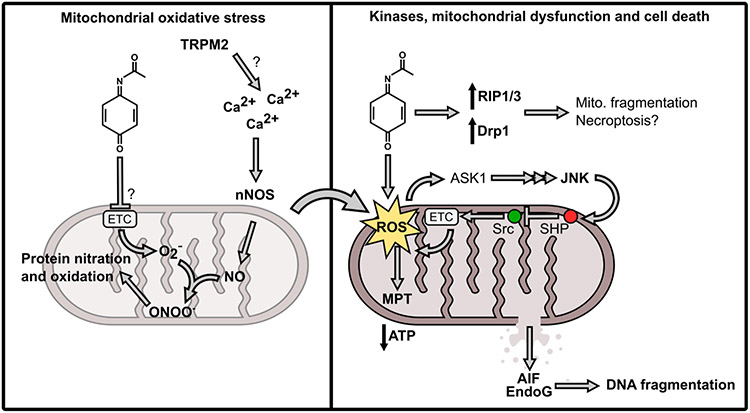
Figure 6. Mechanisms of APAP hepatotoxicity.
Acetaminophen (APAP) hepatotoxicity begins with formation of a reactive metabolite, N-acetyl-p-benzoquinone imine (NAPQI). Left side: NAPQI depletes hepatic glutathione (GSH) and binds to proteins. The initial protein binding leads to oxidative stress through an unknown mechanism that may involve covalent modification and inhibition of electron transport chain (ETC) complex proteins leading to increased superoxide [O2−]. At the same time, activation of neuronal nitric oxide synthase (nNOS)/NOS1 occurs, possibly through redox modification or covalent modification of the transient receptor potential melastatin 2 (TRPM2) which increases intracellular calcium (Ca2+). Nitric oxide (NO) from nNOS then reacts with O2− to form peroxynitrite (ONOO−), which then reacts with tyrosine and other residues on intracellular proteins. Right side: The initial reactive oxygen species (ROS) also activate apoptosis-signaling kinase 1 (ASK1), ultimately leading to activation of the c-Jun N-terminal kinases 1 and 2 (JNK). JNK then translocates to mitochondria, binding to the protein Sab in the outer mitochondrial membrane (OMM). This interaction causes release of the protein tyrosine phosphatase, nonreceptor type 6 (SHP) from Sab on intermembrane side of the OMM. SHP then translocates to the inner mitochondrial membrane and inhibits the ETC enhancer Src, resulting in reduced ETC activity and therefore increased ROS production. This forms a feed-forward loop in which ROS activate JNK, and JNK increases ROS. Eventually, the mitochondrial membrane permeability transition (MPT) occurs with loss of ATP production. The mitochondria swell and the membranes rupture, liberating the endonucleases apoptosis-inducing factor (AIF) and endonuclease G. The free nucleases can then fragment nuclear DNA. In parallel, expression and activity of the receptor-interacting protein (RIP) kinases 1 and 3 increase, along with Drp1-mediated mitochondrial fragmentation, through underexplored mechanisms. Ultimately, the hepatocyte dies by oncotic necrosis.
Immunoblot analysis using anti-nitrotyrosine antibody indicated nitration affected a number of specific proteins (Hinson et al. 2000) and later subcellular fractionation revealed that nitration occurs primarily on mitochondrial proteins (Cover et al. 2005). Since nitration had been reported to occur specifically on the mitochondrial protein manganese superoxide dismutase (MnSOD) in renal cells (MacMillan-Crow et al. 1996), the possible nitration of this protein in the liver was investigated in mice treated with APAP (Agarwal, MacMillan-Crow, et al. 2010). MnSOD is a critical mitochondrial antioxidant enzyme that detoxifies superoxide and thus prevents peroxynitrite formation within the mitochondria (Macmillan-Crow and Cruthirds 2001). A dose-responsive decrease in MnSOD activity was observed after treatment with APAP at 100, 200, and 300 mg/kg. Immunoprecipitation of MnSOD from livers of APAP-treated mice followed by Western blot analysis revealed nitrated MnSOD. APAP-MnSOD adducts were not detected (Agarwal, MacMillan-Crow, et al. 2010). Thus, a vital enzyme preventing peroxynitrite formation in APAP toxicity was inhibited. In addition to MnSOD, other nitrated mitochondrial and cytosolic proteins have been more recently identified: mitochondrial aldehyde dehydrogenase, glutathione peroxidase, ATP synthase, and 3-ketoacyl-CoA thiolase (Abdelmegeed et al. 2013).
A critical role was ascribed to neuronal nitric oxide synthase (nNOS, NOS1). Knockout of one isoform of this enzyme decreased toxicity but did not eliminate it (Agarwal et al. 2012). Subsequently, two different inhibitors of nNOS were studied in freshly isolated mouse hepatocytes: NANT (10 μM)(N-[(4S)-4-amino-5-[(2-aminoethyl)amino]pentyl]-N'-nitroguanidinetris-trifluoroacetate) (Banerjee et al. 2015) and Trifluoperazine (10 μM) (Banerjee et al. 2017). Incubation of freshly isolated hepatocytes with APAP (1 mM) resulted in toxicity (release of lactate dehydrogenase into the media). Both nNOS inhibitors eliminated the toxicity without altering the depletion of GSH or covalent binding of APAP to proteins. These data suggest that a nNOS species is important in APAP toxicity in the hepatocytes. Moreover, trifluoperazine inhibited APAP toxicity in the mouse (Chaudhuri et al. 2012). Importantly, trifluoperazine inhibits nNOS by associating with the calcium binding protein calmodulin, which is required for nNOS activation (Vandonselaar et al. 1994). Thus, it appears that altered calcium homeostasis and peroxynitrite may play mechanistic roles in APAP hepatotoxicity.
Mitochondrial Dysfunction
A number of studies have examined the importance of mitochondrial dysfunction in APAP toxicity. As early as 1980, electron microscopic examination of livers from APAP treated mice revealed alterations in mitochondrial morphology (Walker et al. 1980). Jollow et al. reported that mitochondria were a covalent adduct target (Jollow et al. 1973) and a number of APAP-protein adducts have been reported in mitochondria (Pumford et al. 1990; Bulera et al. 1996). Calcium alterations and oxidative stress in mitochondria have also been reported (Tirmenstein and Nelson 1989; Jaeschke 1990). Moreover, inhibition of activity at complexes I and II, but not at complex III, were reported in isolated rat hepatocytes (Burcham and Harman 1991) and in vivo (Donnelly et al. 1994). Also, ATP levels decrease in vivo and in treated hepatocytes (Burcham and Harman 1991; Halmes et al. 1995; Vendemiale et al. 1996; Banerjee et al. 2015; Banerjee et al. 2017). Similar changes have been shown by adding NAPQI to hepatocytes (Andersson et al. 1990). Also, in isolated rat liver hepatocytes NAPQI caused release of sequestered calcium (Weis et al. 1992; Weis et al. 1994).
The mitochondrial permeability transition (MPT) may be an important mechanism in APAP toxicity (Kon et al. 2004; Masubuchi et al. 2005; Reid et al. 2005). The MPT occurs with depolarization of the inner mitochondrial membrane, uncoupling of oxidative phosphorylation, release of intra-mitochondrial ions and metabolic intermediates, mitochondrial swelling, and decreased ATP synthesis. Blockade of APAP toxicity both in vitro and in vivo by MPT inhibitors has been reported. Kon and coworkers (Kon et al. 2004; Kon et al. 2007) showed that APAP toxicity in cultured mouse hepatocytes was inhibited by cyclosporine A and by the non-immunosuppressive Cyclosporine A analog NM811. Cyclosporine A did not alter APAP-induced glutathione depletion, which suggests that the prevention of toxicity did not occur by inhibition of metabolism of APAP to NAPQI. Reid et al. (Reid et al. 2005) examined the effect of MPT inhibitors in freshly isolated mouse hepatocytes using the approach of Boobis (Boobis et al. 1986; Tee et al. 1986). In these studies, APAP toxicity was inhibited by the MPT inhibitors Cyclosporine A, trifluoperazine, or dithiothreitol. Subsequently, Banerjee and coworkers (Banerjee et al. 2017) examined APAP toxicity and the effect of trifluoperazine in freshly isolated hepatocytes in greater detail. They reported that toxicity occurs with increased NO and superoxide formation, increased protein nitration, loss of mitochondrial membrane potential, decreased ATP levels, decreased oxygen consumption rate, and decreased NADH levels. All of these parameters were reversed in the presence of trifluoperazine without altering GSH depletion or APAP protein adduct formation. Finally, Ramachandran et al. (Ramachandran et al. 2011) reported that mice deficient for cyclophilin D (CypD), thought by some to be a component of the MPT pore, are less susceptible to APAP hepatotoxicity than wildtype mice. Together, these data have been interpreted as demonstrating that the MPT is a critical step in APAP hepatotoxicity, though the exact composition of the MPT pore and the role of CypD remain highly controversial (Baines and Gutiérrez-Aguilar 2018).
Reactive nitrogen may be important in the APAP-induced MPT. Cover and coworkers found that nitration, a biomarker of peroxynitrite, was predominantly in mitochondria of APAP-treated mice (Cover et al. 2005). LoGuidice and Boelsterli (LoGuidice and Boelsterli 2011) reported that CypD knock out and CypD inhibitors did not protect against a very large dose of APAP (600 mg/kg), but a peroxynitrite decomposition catalyst did protect. These data indicate that peroxynitrite may facilitiate MPT pore-independent mitochondrial depolarization and damage at especially large doses of APAP. In addition, iron may promote the oxidative stress and protein nitration that leads to the MPT. Dr. Lemasters’s group has demonstrated that iron chelation prevents loss of mitochondrial membrane potential and reduces toxicity due to APAP in primary hepatocytes, but does not prevent lysosomal iron release (Kon et al. 2010; Hu et al. 2016). These data indicate that iron translocation from lysosomes to mitochondria, where ferrous iron can contribute to ROS formation, may precipitate the MPT. Importantly, recent in vivo data also support that conclusion (Hu and Lemasters 2020).
Furthermore, Lizasoain and coworkers (Lizasoain et al. 1996) examined the effect of nitric oxide and peroxynitrite on respiration by brain submitochondrial particles. They found that nitric oxide potently inhibited respiration reversibly at cytochrome oxidase. Peroxynitrite inhibited NADH-dependent respiration and succinate dependent respiration. Thus, the transfer of electrons from Complex 1 to complex 3 (NADH dependent) and from Complex 2 to Complex 3 (succinate dependent) was inhibited. These electron transfers depend on the ubiquinone derivatives. Addition of GSH, which detoxifies peroxynitrite, prevented the inhibition of respiration. Importantly, mitochondrial GSH is depleted in APAP hepatotoxicity (Zhao and Slattery 2002). Schopfer and coworkers have shown that peroxynitrite will oxidize ubihydroquinone to the semiquinone. The semiquinone may subsequently react with molecular oxygen to produce superoxide followed by reaction of the superoxide with additional nitric oxide to regenerate peroxynitrite. The net result is oxidation of ubihydroquinone to ubiquinone with production of no apparent biomarker (Schopfer et al. 2000). If this occurred in APAP toxicity, it would explain why determining the mechanism of toxicity has been so elusive.
Calcium Flux in APAP Toxicity
The role of altered calcium flux has been postulated to be important in various drug-induced toxicities (Kaplowitz et al. 1986; Nicotera et al. 1992). A number of manuscripts have described altered calcium metabolism in APAP toxicity (Corcoran et al. 1987; Burcham and Harman 1988; Tirmenstein and Nelson 1989; Tsokos 1989). Increased mitochondrial calcium levels following a toxic dose of APAP were reported by Burcham and Harmon (Burcham and Harman 1988). They found no significant increases in cytosolic or microsomal calcium levels. Timerstein and Nelson (Tirmenstein and Nelson 1989) found that following a toxic dose of APAP to mice there was approximately a two-fold increase in mitochondrial calcium at 2 hours and a four-fold increase at 6 hours. In addition, a large decrease in plasma membrane calcium-ATPase activity has been reported (Tsokos-Kuhn et al. 1988; Tirmenstein and Nelson 1989). This enzyme is important in control of calcium levels in the cell, and inhibition of the enzyme results in calcium accumulation (Strehler and Zacharias 2001). It was suggested that protein adduction was responsible for the altered activity (Tsokos-Kuhn et al. 1988; Tirmenstein and Nelson 1989).
Boobis and coworkers examined APAP toxicity in freshly isolated hamster hepatocytes. They found that toxicity occurred in two phases (Boobis et al. 1986). In the first phase, GSH depletion occurred; in the second phase, toxicity occurred. Addition of the cell-permeable Ca2+ binder Quin 2-AM in the second phase prevented the loss of viability (Boobis et al. 1990). Subsequently, however, they reported that the alteration in calcium accompanied the loss of viability and suggested that it may not be mechanistically important (Hardwick et al. 1992).
Corcoran and coworkers reported nuclear DNA damage in APAP toxicity. Following a toxic dose of APAP to mice, there was an increase in hepatic nuclear calcium levels that coincided in time with the development of toxicity. Electrophoretic analyses indicated a decline in large DNA accompanied by accumulation of small DNA fragments. It was suggested that calcium-activated endonuclease digestion of the DNA had occurred (Ray et al. 1990; Ray et al. 1993). Similar results were obtained using cultured hepatocytes (Shen et al. 1992). The calcium ion chelator EGTA blocked toxic cell death which suggested that altered calcium metabolism was important in the cell death. These data suggest a role for extracellular calcium in APAP toxicity since EGTA is ionized and does not enter the cells (Shen et al. 1992). The effects of various other calcium antagonists on APAP induced hepatotoxicity have been examined in experimental animals by a number of other investigators (Yamamoto 1990; Ray et al. 1993; Dimova et al. 1995). Generally, these drugs inhibit APAP toxicity.
It has been recently reported that Transient Receptor Potential Melastatin 2 (TRPM2) channels play a critical role in APAP toxicity (Ehsan Kheradpezhouha 2014). The TRPM2 channels are an important calcium ion (Ca2+) signaling mechanism in a variety of cells, contributing to cellular functions. Importantly, TRPM2 channels may be activated by oxidative stress, leading to influx of calcium ions into the cell (Sumoza-Toledo and Penner 2011) (Miller BA and Cheung 2016). Kheradpezhouha and coworkers used patch clamp methods to show that APAP treatment of rat hepatocytes resulted in activation of a cation current similar to that observed with hydrogen peroxide. siRNA knockdown of TRPM2 in hepatocytes inhibits activation of the current by either APAP or hydrogen peroxide. In TRPM2 knockout mice, APAP-induced liver toxicity, as assessed by the blood concentrations of liver enzymes and liver histology, was significantly decreased compared with wildtype mice (Ehsan Kheradpezhouha 2014). These data suggest that TRPM2 channels are critical for development of hepatotoxicity by APAP. In subsequent work, Kheradpezhouha and coworkers found that curcumin, a natural plant obtained polyphenol from tumeric was an inhibitor of the TRPM2 channel. It prevented the hydrogen peroxide or APAP induced rise in calcium in rat hepatocytes (Kheradpezhouh et al. 2016). However, Curcumin has been reported to inhibit Cyp3a4, an enzyme that activates APAP, but it did not inhibit Cyp1a2 or Cyp2e1, other enzymes important in APAP metabolic activation (Volak et al. 2008). Thus, even the curcumin data are encourging, though additional work is needed to fully understand any antidotal potential that curcumin may have.
The involvement of TRPM2 channels in APAP toxicity would be consistent with a mechanism where metabolic activation by P450 enzymes to NAPQI results in GSH depletion which causes increased oxidative stress and, as a result, TRPM2 channels are activated, leading to an influx of calcium ions. The increased calcium ions could then activate NOS1 via a mechanism involving calmodulin. Increased NOS1 activity could then lead to increased NO which then reacts with the superoxide, occurring as a result of increased oxidative stress, to form peroxynitrite. Peroxynitrite may then nitrate MnSOD, resulting in its inhibition which leads to augmented levels of superoxide. The increased peroxynitrite could also react at Complex 1 and Complex 2 to disrupt mitochondrial electron flow with mitochondrial dysfunction and cell death. This proposed mechanism when applied to APAP toxicity pieces together large amounts of what previously appeared to be disparate data. However, more research is needed to explore that model.
Role of the c-Jun N-terminal Kinases
There is strong evidence that mitogen-activated protein kinases (MAPKs) are also important mediators of APAP-induced liver injury. Phosphorylation of the c-Jun N-terminal kinase (JNK) during APAP hepatotoxicity in mice was first reported in 2003 (Elsby et al. 2003). Shortly thereafter, Neil Kaplowitz’s research group used multiple approaches to demonstrate that blocking JNK protects against the injury. Using both mice and primary mouse hepatocytes, they found that the JNK inhibitor SP600125, antisense oligonucleotides against JNK2, and JNK2 knockout all reduced APAP hepatotoxicity (Gunawan et al. 2006). Although the specific isoform of JNK of greatest significance was initially controversial, with multiple other groups reporting conflicting data on the role of JNK2 (Bourdi et al. 2008; Saito et al. 2010), that issue was resolved when it was discovered that some investigators had accidentally used JNK2−/− mice on a different background strain than their control groups (Bourdi et al. 2011). More recent studies using JNK1 knockout and double JNK1/2 knockout mice provided further conflicting data, as the double knockout mice had much worse injury (Cubero et al. 2016). Furthermore, they found that SP600125 was still protective in the JNK1/2 knockouts. However, it appears that those investigators may have used the same problematic JNK2 knockout mice from the previous conflicting studies to generate their double knockout, and it is not clear if the results were properly controlled using mice of the correct genetic background. Furthermore, there was a clear pattern of reduced serum ALT in the JNK1 knockout mice after APAP overdose. Although the differences between wildtype and JNK1 knockout mice were apparently not statistically significant, it is not obvious in the methods description whether or not the experiments were powered to detect those differences (Cubero et al. 2016). Finally, even if SP600125 has off-target effects, many other JNK inhibitors have also been shown to reduce liver injury after APAP overdose in mice, including leflunomide and D-JNKI1 (Henderson et al. 2007; Latchoumycandane et al. 2007), and blockade of apoptosis signal-regulating kinase 1 (ASK1), mixed-lineage kinase 3 (MLK3) or protein kinase C (PKC) upstream of JNK is also protective (Nakagawa et al. 2008; Sharma et al. 2012; Xie, Ramachandran, et al. 2015). Overall, most investigators agree that JNK contributes to APAP hepatotoxicity (Du et al. 2015). Importantly, the mechanism of JNK activation likely involves oxidative stress, as ASK1 is normally sequestered in an inactive state by thioredoxin, but dissociates after APAP overdose (Nakagawa et al. 2008).
The mechanism by which JNK exacerbates APAP toxicity is now coming into focus. We know that JNK can translocate into mitochondria after APAP overdose, and experiments with mitochondria isolated from livers of APAP-treated mice revealed that JNK can directly inhibit respiration and cause the MPT (Hanawa et al. 2008). These effects require the SH3 domain-binding protein (Sab), as knockdown of Sab expression with adenoviral shRNA and Sab knockout both prevent the JNK translocation and later injury (Win et al. 2011; Win et al. 2016). Furthermore, female mice have reduced Sab expression compared to males and are also less susceptible to APAP hepatotoxicity (Win et al. 2019). Additional studies have also revealed that JNK works through other mediators in addition to Sab to ultimately inactivate mitochondrial Src kinase (Win et al. 2016), which normally promotes electron transport chain activity. The end result is that JNK inhibits mitochondrial respiration and increases ROS production, which could lead to a self-sustaining feed-forward loop wherein JNK activation is maintained by the additional oxidative stress.
Cell Death Mechanisms
The first reports of APAP hepatotoxicity in humans and rodents included descriptions of histopathological features characteristic of oncotic necrosis, including eosinophilic degeneration and pyknosis (Davidson and Eastham 1966). However, the later discovery that hepatocyte nuclear DNA is extensively fragmented after APAP overdose in mice (Ray et al. 1991; Ray et al. 1993) led to the hypothesis that apoptosis may be a major mode of cell death (Ray et al. 1996). At the time, DNA fragmentation with laddering in gel electrophoresis was thought to be specific for apoptotic cell death. However, rigorous studies by Gujral and Jaeschke in which they quantified apoptotic hepatocytes by morphology and using biochemical methods such as caspase 3 activity at multiple time points after APAP overdose revealed that apoptosis contributes very little to APAP hepatotoxicity (Gujral et al. 2002). Instead, the DNA fragmentation has been attributed to release of endonucleases from mitochondria due to the mitochondrial damage. Endonuclease G (Endo G) and apoptosis-inducing factor (AIF) are normally present within mitochondrial intermembrane space. Immunofluorescence staining of primary mouse hepatocytes treated with APAP revealed that both EndoG and AIF translocate to the nucleus during APAP toxicity (Bajt et al. 2006), and AIF-deficient mice were found to have reduced liver injury as a result of both lower oxidative stress and reduced DNA fragmentation (Bajt et al. 2011). There is also evidence that DNase I is released by necrotic hepatocytes and promotes the DNA fragmentation in neighboring cells (Napirei et al. 2006), though, as stated above, the evidence for propagation of injury by toxic expansion is questionable.
For many years, the lack of apoptosis during APAP toxicity despite release of Smac/DIABLO and cytochrome c from the damaged mitochondria, was assumed to be due to loss of ATP, which is necessary for activation of the apoptosome. However, Ramachandran et al. (Ramachandran et al. 2013) proposed a new explanation. They suggested that the major mode of cell death in APAP-induced liver injury is necroptosis, a specific type of programmed necrosis. Consistent with that, they found that the receptor-interacting protein kinase 3 (RIP3), a critical mediator of necroptosis, was increased in the liver after APAP overdose in mice, and that blocking RIP3 by knockdown with morpholinos or genetic knockout is protective (Ramachandran et al. 2013). It was also reported that the inhibitor of RIP1, necrostatin-1, is protective in primary mouse hepatocytes and in mice in vivo (Sharma et al. 2012; Ramachandran et al. 2013; Zhang et al. 2014). However, other groups quickly provided conflicting data on the involvement of both RIP1 and RIP3 in APAP hepatotoxicity using their own genetic and pharmacologic approaches (Dara et al. 2015; Deutsch et al. 2015). Currently, most researchers accept that necroptosis has a role in APAP hepatotoxicity, but the controversy regarding specific mediators is unresolved. Moreover, the overall progression of APAP toxicity described in this review, beginning with protein binding, progressing through mitochondrial damage and JNK activation, and ending with nuclear DNA fragmentation and cell death, has itself been referred to as a form of programmed necrosis (Jaeschke et al. 2019). Thus, programmed necrosis is certainly important in APAP hepatotoxicity, despite some confusion concerning the details.
Endogenous Protective Mechanisms and Liver Regeneration
Over the last several years, it has become apparent that APAP overdose also results in activation of endogenous protective mechanisms in order to limit the extent of injury. Nuclear factor erythroid 2-related factor 2 (Nrf2) is a major regulator of antioxidant response genes in the liver. Normally, Nrf2 is held inactive in the liver by Kelch like-ECH-associated protein 1 (KEAP1). However, ROS can oxidize sulfhydryl groups in KEAP1, resulting in release of Nrf2. The latter then translocates to the nucleus and upregulates expression of antioxidant defense enzymes. Thus, Nrf2 is an important endogenous means to limit oxidative stress. It has been demonstrated that Nrf2 knockout mice are highly susceptible to APAP hepatotoxicity (Chan et al. 2001; Enomoto et al. 2001). Nrf2 KO mice have dramatically higher plasma ALT activity and increased mortality compared to WT mice. Measurement of non-protein sulfhydryls and anti-APAP reactivity in tissue indicated that this was likely due to reduced glutathione synthesis. Indeed, Nrf2 KO mice consistently have lower expression of glutamate-cysteine ligase and other enzymes involved in glutathione synthesis, and therefore greater APAP-protein binding and increased oxidative stress (Chan et al. 2001; Enomoto et al. 2001; Goldring et al. 2004). Similarly, mice with constitutive Nrf2 activation due to KEAP1 deficiency are resistant to APAP hepatotoxicity (Okawa et al. 2006). It is generally thought that Nrf2 activation after APAP overdose in mice is due to oxidation of cysteine sulfhydryl groups on KEAP1. However, there is also evidence that the reactive metabolite of APAP can directly modify KEAP1. Copple et al. (Copple et al. 2008) reported that treatment with NAPQI increases nuclear translocation of Nrf2 and Nrf2 transcriptional activity in a liver cell line, and further demonstrated that NAPQI can alkylate several cysteine residues in KEAP1 in cell lines. Since then, numerous studies have demonstrated that any stimulus that activates Nrf2 can protect against APAP-induced liver injury. For example, some genetic knockout models display off-target constitutive activation of Nrf2 and similar pre-conditioning effects, leading to protection (Ni, Boggess, et al. 2012; Williams et al. 2013). Interestingly, compelling recent data have demonstrated that the protective effects of Nrf2 are limited by JNK. JNK appears to phosphorylate Nrf2 and reduce its activity after APAP overdose in mice, and that inhibiting JNK may protect in part by reducing that effect (Chen Y et al. 2020). However, it should be noted that these data have recently been challenged due to the delayed effects of Nrf2 phosphorylation on target genes in the paper and the fact that JNK inhibitors protect even when administered after the time point at which Nrf2 phosphorylation is observed (Jaeschke and Ramachandran 2020). Nevertheless, overall, it is quite clear that Nrf2 is an important mediator of the endogenous protective response in APAP hepatotoxicity.
Autophagy is also thought to be critical to limit APAP-induced liver injury. Ni et al. (Ni, Bockus, et al. 2012) reported that autophagic flux increases in hepatocytes after APAP treatment, and that inhibition of autophagy enhances APAP-induced injury. Around the same time, Igusa et al. (Igusa et al. 2012) reported that Atg7-deficient mice have greater APAP hepatotoxicity than control animals. Extending these observations, Ni et al. recently proposed a novel mechanism whereby APAP-protein binding contributes to APAP hepatotoxicity. They treated primary mouse hepatocytes with 5 mM APAP and observed colocalization of immunofluorescence-labeled APAP-protein adducts with markers of autophagosomes and lysosomes (Ni et al. 2016). They also reported that blocking autophagy using both pharmacologic and genetic approaches increases APAP-protein adducts in serum, while stimulation of autophagy results in lower serum adducts and less injury (Ni et al. 2016). Based on these observations, the researchers proposed that APAP-protein binding leads to accumulation of damaged proteins that must be removed through either autophagy or transport out to circulation in order to minimize cell damage. The same authors have further demonstrated that combined deletion of two proteins necessary for autophagic degradation of damaged mitochondria (mitophagy) also worsens APAP hepatotoxicity (Wang et al. 2019). Thus, autophagy appears to protect against APAP-induced liver injury by removing NAPQI-modified proteins and damaged mitochondria.
There is strong evidence that the outcome of liver injury depends upon a balance of the damage and repair (Mehendale 2005). Furthermore, the liver has an incredible capacity for regeneration, and acute liver failure patients with greater expression of regeneration biomarkers like proliferating cell nuclear antigen (PCNA) in tissue (Kayano et al. 1992) and alpha-fetoprotein in circulation are more likely to survive (Schmidt and Dalhoff 2005). However, until recently, little effort was made to explore the mechanisms and importance of liver regeneration and repair after APAP hepatotoxicity. Many researchers have assumed that the mechanisms resemble those seen in the partial hepatectomy model, but recent data have challenged that notion. For example, a recent transcriptomics study revealed that activation of both epidermal growth factor and IL-6 signaling is stronger after APAP hepatotoxicity (Bhushan et al. 2020), while other data may indicate that HGF signaling is less important (Clemens, McGill, et al. 2019). Consistent with a major role for IL-6, James et al. (James, Lamps, et al. 2003) demonstrated that IL-6 KO mice have reduced PCNA expression in the liver at 48-72 h after APAP overdose, indicating the IL-6/STAT3 signaling is important in repair after APAP overdose similar to partial hepatectomy. The same group demonstrated that treatment with recombinant vascular endothelial growth factor (VEGF) stimulates regeneration (Donahower et al. 2010). Around the same time, Apte et al. (Apte et al. 2009) demonstrated that ablation of β-catenin decreases markers of liver regeneration at 24 h after APAP overdose in mice. The role of Wnt/β-catenin signaling is further supported by the observation that β-catenin signaling is only increased after APAP overdoses from which mice recover, not after lethal doses (Bhushan et al. 2014). In addition, overexpression of stable β-catenin enhances liver regeneration after APAP (Bhushan et al. 2014), and treatment with an inhibitor of glycogen synthase kinase 3 β (GSK3β), which itself inhibits β-catenin signaling, has the same effect (Bhushan et al. 2017). More recently, a role for the phospholipid phosphatidic acid (PA) in Wnt/β-catenin signaling after APAP overdose has been demonstrated. Tissue and circulating levels of PA dramatically and persistently increase during APAP hepatotoxicity and recovery in mice (Lutkewitte et al. 2018). Importantly, inhibition of PA synthesis decreases GSK3β phosphorylation, β-catenin signaling and PCNA expression (Lutkewitte et al. 2018; Clemens, Kennon-McGill, et al. 2019), which is consistent with a role of PA in regulation of GSK3β, while addition of a GSK3 inhibitor blocks those effects (Clemens, Kennon-McGill, et al. 2019). Overall, it is clear the IL-6, EGFR and Wnt/β-catenin signaling are critical for proper repair and regeneration after APAP overdose.
Finally, there is also some evidence that mitochondrial biogenesis is important for liver repair after APAP overdose. It has been observed that proliferating hepatocytes surrounding areas of tissue necrosis have more mitochondria, indicating increased mitochondrial biogenesis in those cells (Ni et al. 2013). Increased expression of PPARγ co-activator 1α (Pgc-1α), nuclear respiratory factor 1 (Nrf1), and dynamin-related protein 1 (Drp1) has also been observed with time courses that resemble liver regeneration (Du et al. 2017). Most importantly, treatment with SRT1720 increased markers of mitochondrial biogenesis and accelerated regeneration after APAP overdose in mice (Du et al. 2017). Together, these data indicate that mitochondrial biogenesis is likely important to provide energy for liver repair after APAP hepatotoxicity.
Role of Inflammation
The significance of sterile inflammation in APAP hepatotoxicity is controversial. Numerous reviews on this topic have been published in the last several years (Woolbright and Jaeschke 2017, 2018), so we will describe these issues only briefly. Laskin et al. observed infiltration and activation of monocytes in the liver in rats treated with large doses of APAP (Laskin and Pilaro 1986; Laskin et al. 1986). Based on that, they hypothesized that macrophages in the liver contribute to APAP hepatotoxicity. Consistent with their early results, they later discovered that treatment with the Kupffer cell inactivators gadolinium chloride (GdCl) and dextran sulfate significantly reduced APAP hepatotoxicity (Laskin et al. 1995) and that was reproduced in mice (Michael et al. 1999). However, it was later discovered that complete ablation of Kupffer cells using liposome-entrapped clodronate actually aggravated APAP-induced liver injury (Ju et al. 2002), indicating that there may be another mechanism to explain the earlier results. Furthermore, NADPH oxidase KO mice were not protected against APAP hepatotoxicity, and displayed similar evidence of oxidative stress compared to wildtype controls, demonstrating that macrophages do not contribute to the oxidative stress or injury (James LP, McCullough, et al. 2003). Finally, CCR2 knockout mice were not protected against APAP, and in fact had delayed resolution of injury (Holt et al. 2008). Taken together, these and other data strongly indicate that macrophages do not contribute to APAP toxicity and may even be beneficial for repair. For example, it was recently demonstrated that netrin-1 promotes regeneration and recovery after APAP hepatotoxicity in mice through a mechanism involving Kupffer cells (Duan et al. 2020).
Another inflammatory cell type often implicated in APAP hepatotoxicity is the neutrophil. Mitchell, Jollow, Potter, Gillette and Brodie, described neutrophil accumulation in the liver after APAP overdose in mice in their original 1973 reports and, we now know that they are an important driver of injury in rodent ischemia-reperfusion models of acute liver injury. One of the first studies to directly test the role of neutrophils in APAP toxicity revealed that neutrophil antiserum reduced APAP-induced injury in rats (Smith et al. 1998). However, the use of polyclonal serum may have confounded the results. Indeed, later experiments revealed that treatment with a specific CD18-neutrophil antibody had no effect on APAP hepatotoxicity in mice (Lawson et al. 2000). Since then, numerous studies have been published presenting wildly conflicting data. For example, some research groups have found that knockout of inflammasome components reduces APAP-induced liver injury (Imaeda et al. 2009), while others have found no effect (Williams et al. 2011). Overall, the role of neutrophils in APAP hepatotoxicity remains controversial.
Several groups have reported important roles for cytokines in APAP-induced liver injury. Cytokines clearly increase after APAP overdose, but again their effects are controversial. For example, interleukin-22 (IL-22) binding protein (IL22BP) knockout mice have increased IL-22 signaling and more severe injury than controls (Kleinschmidt et al. 2017), but treatment with exogenous IL-22 is protective against APAP (Feng et al. 2014). Similarly, there is some evidence that IL-6 can exacerbate the liver injury in certain conditions (Woolbright and Jaeschke 2018), but treatment with exogenous IL-6 is protective (Gao et al. 2019) and IL-6 KO mice have worse outcomes and delayed injury resolution {James et al. 2003}. Additional research is needed to fully understand the importance of cytokines in APAP hepatotoxicity.
Translation of APAP Hepatotoxicity Mechanisms to Humans
Despite decades of research using animal models and cell culture models, the mechanisms of APAP hepatotoxicity have only recently been fully translated to humans. Davis et al. (Davis et al. 1975) demonstrated that rats had impaired bromosulphthalein glutathionylation after APAP overdose due to hepatic glutathione depletion, and they observed a similar reduction of bromosulphthalein metabolism in APAP overdose patients, indicating that APAP depletes glutathione in humans similar to rodents. Lauterburg and Mitchell later confirmed that by demonstrating that increasing therapeutic doses of APAP result in greater incorporation of cysteine derived from radiolabeled cystine into the NAC-APAP metabolite in humans (Lauterburg and Mitchell 1987). A few years later, the first evidence of APAP-protein binding in APAP overdose patients was published (Hinson et al. 1990), later confirmed in larger studies (Webster et al. 1996; James et al. 2001; Davern et al. 2006; James et al. 2007). Finally, it was recently shown that serum values for APAP-protein adducts in early-presenting overdose patients can predict development of severe liver injury (Chiew et al. 2020), which is consistent with the idea that protein binding is a driver of injury in humans like mice. Taken together, those data demonstrate that glutathione depletion and protein binding are also critical in the mechanism of toxicity in humans.
More recently, using furosemide as a negative control for mitochondrial dysfunction in hepatotoxicity, it was reported that glutamate dehydrogenase (GLDH), mitochondrial DNA (mtDNA) and long-chain acylcarnitines in plasma are specific biomarkers for mitochondrial damage in the context of liver injury (McGill, Sharpe, et al. 2012; McGill et al. 2014). Importantly, both GLDH and mtDNA were found to be elevated in APAP overdose patients with liver injury (peak ALT≥1,000 U/L) (McGill, Sharpe, et al. 2012). In addition, long-chain acylcarnitine values were greater in blood from patients with delayed NAC treatment than from those who received NAC early (Bhattacharyya et al. 2013; Bhattacharyya et al. 2014). Furthermore, nuclear DNA fragments have also been reported in plasma after APAP overdose in humans (Craig et al. 2011; McGill, Sharpe, et al. 2012; McGill et al. 2014), and nuclear DNA fragmentation is mediated by endonucleases released from damaged mitochondria (discussed above). Altogether, these data clearly demonstrate that APAP hepatotoxicity causes mitochondrial damage and DNA fragmentation in humans in addition to mice. Importantly, these appear to be critical events, as serum values for GLDH, mtDNA, and nuclear DNA fragments are at least modestly predictive of death in APAP-induced acute liver failure patients (McGill et al. 2014). Finally, caspase 3 activity as a biomarker of apoptosis was not detectable in plasma from APAP overdose patients, despite being elevated in plasma from mice with liver apoptosis due to LPS/Galactosamine treatment, indicating that apoptosis is not a major mechanism of cell death in humans (McGill, Sharpe, et al. 2012). Overall, these data indicate that the mechanisms of APAP-induced liver injury are similar in humans and mice.
Additional translational data have come from recent studies using cultured human hepatocytes. HepaRG is a unique bipotential human liver cell line that can differentiate into hepatocytes and bile duct epithelium-like cells. Importantly, HepaRG cells express most major drug metabolizing enzymes and transporters at levels similar to primary human hepatocytes. It has been demonstrated that APAP is toxic specifically to hepatocytes within HepaRG cultures, with a time course of injury that resembles APAP overdose patients (McGill et al. 2011). APAP also causes glutathione depletion, protein binding, mitochondrial depolarization and oxidative stress in HepaRG cells, and each of those precedes the cell death, indicating that they may be important for the mechanism (McGill et al. 2011). More recently, the mechanisms of APAP toxicity were explored in primary human hepatocytes (PHH) freshly isolated from resected liver tissue. Like HepaRG cells, fresh PHH develop toxicity with a time course that resembles humans (Xie et al. 2014). And, again, mitochondrial depolarization and oxidative stress precede the cell death. Importantly, JNK activation was also observed in PHH after APAP treatment, and the JNK inhibitor SP600125 reduced APAP toxicity in that model (Xie et al. 2014). Finally, there is evidence that 4-methylpyrazole protects PHH against APAP toxicity by inhibiting cytochromes P450 and reducing protein alkylation (Akakpo et al. 2018). Collectively, the data from APAP overdose patients and cultured human hepatocytes strongly indicate that the basic mechanisms of APAP hepatotoxicity in humans and mice are at least similar, if not identical.
Novel Treatments for APAP Overdose
New treatments for APAP overdose could be beneficial for some patients. Although NAC is highly protective when administered early, it loses efficacy in patients ≥10 h after APAP overdose (Rumack et al. 1981) and recent data indicate that most APAP overdose patients present later than that (Craig et al. 2012). However, life-threatening acute liver injury is relatively rare compared to other conditions, and there is a general perception that NAC is good enough. As a result, few pharmaceutical companies have demonstrated interest in development of new drugs to treat APAP hepatotoxicity. To address that, some research groups have focused on re-purposing existing drugs.
Currently, two drugs are the focus of clinical investigation. Mangifodopir is a manganese-containing compound that approved in many countries as an imaging contrast agent. The manganese ions dissociate and enter normal tissues, which makes them appear brighter by MRI. It has also been tested as a MnSOD mimetic, due to the presence of manganese. In the latter case, dissociation is undesirable. Calmangifodopir (CMF) was developed to address that. CMF is a mangifodopir derivative in which all but one manganese is replaced by calcium, which prevents release of the single remaining manganese and facilitates its function as an antioxidant. Mangifodopir itself has been shown to be hepatoprotective after APAP overdose and ischemia-reperfusion injury in mice (Bedda et al. 2003; Coriat et al. 2011). Importantly, a recent phase I clinical trial in which CMF treatment was combined with a truncated NAC regimen revealed that addition of CMF modestly decreased the serum liver injury biomarkers keratin 18 and caspase-cleaved keratin 18 in overdose patients with no effect on serious adverse event reporting (Morrison et al. 2019). These data demonstrate that CMF is a safe and potentially efficacious adjunct to NAC.
Some have promoted the drug 4-methylpyrazole (4MP; a.k.a. fomepizole) as another novel treatment for APAP overdose patients. 4MP is widely used to treat ethylene glycol and alcohol poisoning because it is an alcohol dehydrogenase inhibitor. It has been known for some time that 4MP can also reduce bioactivation of APAP (Thummel et al. 1989) and protect against APAP hepatotoxicity in rats (Burk et al. 1990; Brennan et al. 1994). In 2013, a case report appeared describing a patient who had apparently consumed an extremely large dose of APAP, with a peak serum APAP concentration in a range which few people have been reported to survive (Zell-Kanter et al. 2013). The authors treated the patient with NAC, along with a single dose of 4MP due to suspected alcohol co-ingestion, and the patient recovered. This led to speculation that 4MP could be a useful adjunct to NAC (Yip and Heard 2016). Akakpo et al. subsequently demonstrated that 4MP reduces APAP-induced liver injury in mice by blocking reactive metabolite formation as a pre-treatment and reducing JNK activation as a post-treatment (Akakpo et al. 2018; Akakpo et al. 2019). Most recently, a small crossover trial in volunteer subjects revealed that 4MP also reduces APAP reactive metabolite formation in humans (Kang et al. 2020). These data indicate that 4MP is another promising adjunct to NAC therapy, though we need additional clinical trials to confirm that.
Summary
In the past 65 years, we have seen the introduction of APAP as a commercially viable analgesic and antipyretic, the rise of APAP overdose to both a popular drug and the primary cause of acute liver failure in several countries, the introduction of NAC as an antidote, tremendous advances in understanding of the fundamental toxicity mechanisms, and now the emergence of a new generation of therapeutics for overdose patients. Along the way, we have learned an incredible amount about the basic mechanisms of xenobiotic hepatotoxicity, as well as liver injury more generally, by studying the pathophysiology of APAP hepatotoxicity in rodents and humans. In the future, we hope to see the widespread adoption of new treatments for APAP overdose patients, and we will continue to explore the mechanisms of APAP-induced liver injury and subsequent repair in mice as a clinically relevant model of liver disease.
Funding
This work was supported by the Association for the Study of Liver Diseases Foundation (AASLD Foundation) under a 2018 Pinnacle Research Award (MRM) and the National Institutes of Health under grant NIDDK 5R42 DK079387 (JAH; PI: Laura P. James, MD).
Footnotes
Disclosure Statement
JAH is Chief Scientific Officer of Acetaminophen Toxicity Diagnostics, LLC (ATD). MRM serves as a consultant for ATD. The company had no role in the funding, preparation, or submission of this manuscript.
References
- Abdelmegeed MA, Jang S, Banerjee A, Hardwick JP, Song BJ. 2013. Robust protein nitration contributes to acetaminophen-induced mitochondrial dysfunction and acute liver injury. Free Radic Biol Med. 60:211–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal R, Hennings L, Rafferty TM, Letzig LG, McCullough S, James LP, MacMillan-Crow LA, Hinson JA. 2012. Acetaminophen-induced hepatotoxicity and protein nitration in neuronal nitric-oxide synthase knockout mice. J Pharmacol Exp Ther. 340(1):134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal R, MacMillan-Crow LA, Rafferty T, Saba H, and Hinson JA 2010. Acetaminophen-induced alterations in hepatic mitochondrial manganese superoxide dismutase MnSOD; SOD2) activity in mice. Experimental Biology 2010 Abstracts. C45:225. [Google Scholar]
- Akakpo JY, Ramachandran A, Duan L, Schaich MA, Jaeschke MW, Freudenthal BD, Ding WX, Rumack BH, Jaeschke H. 2019. Delayed Treatment With 4-Methylpyrazole Protects Against Acetaminophen Hepatotoxicity in Mice by Inhibition of c-Jun n-Terminal Kinase. Toxicol Sci. 170(1):57–68. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akakpo JY, Ramachandran A, Kandel SE, Ni HM, Kumer SC, Rumack BH, Jaeschke H. 2018. 4-Methylpyrazole protects against acetaminophen hepatotoxicity in mice and in primary human hepatocytes. Hum Exp Toxicol. 37(12):1310–1322. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson BS, Rundgren M, Nelson SD, Harder S. 1990. N-acetyl-p-benzoquinone imine-induced changes in the energy metabolism in hepatocytes. Chem Biol Interact. 75(2):201–211. [DOI] [PubMed] [Google Scholar]
- Apte U, Singh S, Zeng G, Cieply B, Virji MA, Wu T, Monga SP. 2009. Beta-catenin activation promotes liver regeneration after acetaminophen-induced injury. Am J Pathol. 175(3):1056–1065. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines CP, Gutiérrez-Aguilar M. 2018. The still uncertain identity of the channel-forming unit(s) of the mitochondrial permeability transition pore. Cell Calcium. 73:121–130. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajt ML, Cover C, Lemasters JJ, Jaeschke H. 2006. Nuclear translocation of endonuclease G and apoptosis-inducing factor during acetaminophen-induced liver cell injury. Toxicol Sci. 94(1):217–225. eng. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Knight TR, Farhood A, Jaeschke H. 2003. Scavenging peroxynitrite with glutathione promotes regeneration and enhances survival during acetaminophen-induced liver injury in mice. J Pharmacol Exp Ther. 307(1):67–73. eng. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Ramachandran A, Yan HM, Lebofsky M, Farhood A, Lemasters JJ, Jaeschke H. 2011. Apoptosis-inducing factor modulates mitochondrial oxidant stress in acetaminophen hepatotoxicity. Toxicol Sci. 122(2):598–605. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Melnyk SB, Krager KJ, Aykin-Burns N, Letzig LG, James LP, Hinson JA. 2015. The neuronal nitric oxide synthase inhibitor NANT blocks acetaminophen toxicity and protein nitration in freshly isolated hepatocytes. Free Radic Biol Med. 89:750–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Melnyk SB, Krager KJ, Aykin-Burns N, McCullough SS, James LP, Hinson JA. 2017. Trifluoperazine inhibits acetaminophen-induced hepatotoxicity and hepatic reactive nitrogen formation in mice and in freshly isolated hepatocytes. Toxicol Rep. 4:134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolone JB, Birge RB, Sparks K, Cohen SD, Khairallah EA. 1988. IMMUNOCHEMICAL ANALYSIS OF ACETAMINOPHEN COVALENT BINDING TO PROTEINS - PARTIAL CHARACTERIZATION OF THE MAJOR ACETAMINOPHEN-BINDING LIVER PROTEINS [Article]. Biochem Pharmacol. 37(24):4763–4774. English. [DOI] [PubMed] [Google Scholar]
- Bartolone JB, Sparks K, Cohen SD, Khairallah EA. 1987. IMMUNOCHEMICAL DETECTION OF ACETAMINOPHEN-BOUND LIVER PROTEINS [Note]. Biochem Pharmacol. 36(8):1193–1196. English. [DOI] [PubMed] [Google Scholar]
- Bateman DN, Dear JW. 2019. Acetylcysteine in paracetamol poisoning: a perspective of 45 years of use [Review]. Toxicol Res. 8(4):489–498. English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman JS. 2009. Understanding peroxynitrite biochemistry and its potential for treating human diseases [Article]. Arch Biochem Biophys. 484(2):114–116. English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman JS, Koppenol WH. 1996. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 271(5 Pt 1):C1424–1437. [DOI] [PubMed] [Google Scholar]
- Bedda S, Laurent A, Conti F, Chéreau C, Tran A, Tran-Van Nhieu J, Jaffray P, Soubrane O, Goulvestre C, Calmus Y et al. 2003. Mangafodipir prevents liver injury induced by acetaminophen in the mouse. J Hepatol. 39(5):765–772. eng. [DOI] [PubMed] [Google Scholar]
- Bessems JGM, Vermeulen NPE. 2001. Paracetamol (acetaminophen)-induced toxicity: Molecular and biochemical mechanisms, analogues and protective approaches [Review]. Crit Rev Toxicol. 31(1):55–138. English. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Pence L, Beger R, Chaudhuri S, McCullough S, Yan K, Simpson P, Hennings L, Hinson J, James L. 2013. Acylcarnitine profiles in acetaminophen toxicity in the mouse: comparison to toxicity, metabolism and hepatocyte regeneration. Metabolites. 3(3):606–622. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Yan K, Pence L, Simpson PM, Gill P, Letzig LG, Beger RD, Sullivan JE, Kearns GL, Reed MD et al. 2014. Targeted liquid chromatography-mass spectrometry analysis of serum acylcarnitines in acetaminophen toxicity in children. Biomark Med. 8(2):147–159. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhushan B, Gunewardena S, Edwards G, Apte U. 2020. Comparison of liver regeneration after partial hepatectomy and acetaminophen-induced acute liver failure: A global picture based on transcriptome analysis. Food Chem Toxicol. 139:111186. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhushan B, Poudel S, Manley MW Jr., Roy N, Apte U. 2017. Inhibition of Glycogen Synthase Kinase 3 Accelerated Liver Regeneration after Acetaminophen-Induced Hepatotoxicity in Mice. Am J Pathol. 187(3):543–552. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhushan B, Walesky C, Manley M, Gallagher T, Borude P, Edwards G, Monga SP, Apte U. 2014. Pro-regenerative signaling after acetaminophen-induced acute liver injury in mice identified using a novel incremental dose model. Am J Pathol. 184(11):3013–3025. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boobis AR, Seddon CE, Nasseri-Sina P, Davies DS. 1990. Evidence for a direct role of intracellular calcium in paracetamol toxicity. Biochem Pharmacol. 39(8):1277–1281. [DOI] [PubMed] [Google Scholar]
- Boobis AR, Tee LB, Hampden CE, Davies DS. 1986. Freshly isolated hepatocytes as a model for studying the toxicity of paracetamol. Food Chem Toxicol. 24(6-7):731–736. [DOI] [PubMed] [Google Scholar]
- Bourdi M, Davies JS, Pohl LR. 2011. Mispairing C57BL/6 substrains of genetically engineered mice and wild-type controls can lead to confounding results as it did in studies of JNK2 in acetaminophen and concanavalin A liver injury. Chem Res Toxicol. 24(6):794–796. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdi M, Korrapati MC, Chakraborty M, Yee SB, Pohl LR. 2008. Protective role of c-Jun N-terminal kinase 2 in acetaminophen-induced liver injury. Biochem Biophys Res Commun. 374(1):6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd EM, Bereczky GM. 1966. Liver necrosis from paracetamol. Br J Pharmacol Chemother. 26(3):606–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer TD, Rouff SL. 1971. Acetaminophen-induced hepatic necrosis and renal failure. Jama. 218(3):440–441. [PubMed] [Google Scholar]
- Brennan RJ, Mankes RF, Lefevre R, Raccio-Robak N, Baevsky RH, DelVecchio JA, Zink BJ. 1994. 4-Methylpyrazole blocks acetaminophen hepatotoxicity in the rat. Ann Emerg Med. 23(3):487–494. eng. [DOI] [PubMed] [Google Scholar]
- Brodie BB, Axelrod J. 1949. The fate of acetophenetidin in man and methods for the estimation of acetophenetidin and its metabolites in biological material. J Pharmacol Exp Ther. 97(1):58–67. eng. [PubMed] [Google Scholar]
- Buckpitt AR, Rollins DE, Mitchell JR. 1979. VARYING EFFECTS OF SULFHYDRYL NUCLEOPHILES ON ACETAMINOPHEN OXIDATION AND SULFHYDRYL ADDUCT FORMATION [Article]. Biochem Pharmacol. 28(19):2941–2946. English. [DOI] [PubMed] [Google Scholar]
- Bulera SJ, Cohen SD, Khairallah EA. 1996. Acetaminophen-arylated proteins are detected in hepatic subcellular fractions and numerous extra-hepatic tissues in CD-1 and C57B1/6J mice. Toxicology. 109(2-3):85–99. [DOI] [PubMed] [Google Scholar]
- Burcham PC, Harman AW. 1988. Effect of acetaminophen hepatotoxicity on hepatic mitochondrial and microsomal calcium contents in mice. Toxicol Lett. 44(1-2):91–99. [DOI] [PubMed] [Google Scholar]
- Burcham PC, Harman AW. 1991. Acetaminophen toxicity results in site-specific mitochondrial damage in isolated mouse hepatocytes. J Biol Chem. 266(8):5049–5054. [PubMed] [Google Scholar]
- Burk RF, Hill KE, Hunt RJ, Martin AE. 1990. Isoniazid potentiation of acetaminophen hepatotoxicity in the rat and 4-methylpyrazole inhibition of it. Res Commun Chem Pathol Pharmacol. 69(1):115–118. [PubMed] [Google Scholar]
- Cahn A, Hepp P. 1886. Das Antifebrin, ein neues Fiebermittel. Zentralblatt fur klinische Medizin. 7:561–564. [Google Scholar]
- Calder IC, Creek MJ, Williams PJ. 1974. N-HYDROXYPHENACETIN AS A PRECURSOR OF 3-SUBSTITUTED 4-HYDROXYACETANILIDE METABOLITES OF PHENACETIN [Article]. Chemico-Biological Interactions. 8(2):87–90. English. [DOI] [PubMed] [Google Scholar]
- Calder IC, Creek MJ, Williams PJ, Funder CC, Green CR, Ham KN, Tange JD. 1973. N-HYDROXYLATION OF PARA-ACETOPHENETIDE AS A FACTOR IN NEPHROTOXICITY [Article]. Journal of Medicinal Chemistry. 16(5):499–502. English. [DOI] [PubMed] [Google Scholar]
- Calder IC, Hart SJ, Healey K, Ham KN. 1981. N-HYDROXYACETAMINOPHEN - A POSTULATED TOXIC METABOLITE OF ACETAMINOPHEN [Article]. J Med Chem. 24(8):988–993. English. [DOI] [PubMed] [Google Scholar]
- Chan K, Han XD, Kan YW. 2001. An important function of Nrf2 in combating oxidative stress: detoxification of acetaminophen. Proc Natl Acad Sci U S A. 98(8):4611–4616. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri S, McCullough SS, Hennings L, Brown AT, Li SH, Simpson PM, Hinson JA, James LP. 2012. Effect of trifluoperazine on toxicity, HIF-1alpha induction and hepatocyte regeneration in acetaminophen toxicity in mice. Toxicol Appl Pharmacol. 264(2):192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WQ, Shockcor JP, Tonge R, Hunter A, Gartner C, Nelson SD. 1999. Protein and nonprotein cysteinyl thiol modification by N-acetyl-p-benzoquinone imine via a novel Ipso adduct [Article]. Biochemistry. 38(25):8159–8166. English. [DOI] [PubMed] [Google Scholar]
- Chen Y, Liu K, Zhang J, Hai Y, Wang P, Wang H, Liu Q, Wong CC, Yao J, Gao Y et al. 2020. JNK Phosphorylates The Neh6 Domain Of Nrf2 And Downregulates Cytoprotective Genes In Acetaminophen-Induced Liver Injury. Hepatology. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiew AL, James LP, Isbister GK, Pickering JW, McArdle K, Chan BSH, Buckley NA. 2020. Early acetaminophen-protein adducts predict hepatotoxicity following overdose (ATOM-5). J Hepatol. 72(3):450–462. eng. [DOI] [PubMed] [Google Scholar]
- Clemens MM, Kennon-McGill S, Apte U, James LP, Finck BN, McGill MR. 2019. The inhibitor of glycerol 3-phosphate acyltransferase FSG67 blunts liver regeneration after acetaminophen overdose by altering GSK3β and Wnt/β-catenin signaling. Food Chem Toxicol. 125:279–288. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens MM, McGill MR, Apte U. 2019. Mechanisms and biomarkers of liver regeneration after drug-induced liver injury. Adv Pharmacol. 85:241–262. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SD, Khairallah EA. 1997. Selective protein arylation and acetaminophen-induced hepatotoxicity. Drug Metab Rev. 29(1-2):59–77. [DOI] [PubMed] [Google Scholar]
- Coles B, Wilson I, Wardman P, Hinson JA, Nelson SD, Ketterer B. 1988. The spontaneous and enzymatic reaction of N-acetyl-p-benzoquinonimine with glutathione: a stopped-flow kinetic study. Arch Biochem Biophys. 264(1):253–260. [DOI] [PubMed] [Google Scholar]
- Copple IM, Goldring CE, Jenkins RE, Chia AJ, Randle LE, Hayes JD, Kitteringham NR, Park BK. 2008. The hepatotoxic metabolite of acetaminophen directly activates the Keap1-Nrf2 cell defense system. Hepatology. 48(4):1292–1301. eng. [DOI] [PubMed] [Google Scholar]
- Corcoran GB, Mitchell JR, Vaishnav YN, Horning EC. 1980. EVIDENCE THAT ACETAMINOPHEN AND N-HYDROXYACETAMINOPHEN FORM A COMMON ARYLATING INTERMEDIATE, N-ACETYL-PARA-BENZOQUINONEIMINE [Article]. Mol Pharmacol. 18(3):536–542. English. [PubMed] [Google Scholar]
- Corcoran GB, Wong BK, Neese BL. 1987. Early sustained rise in total liver calcium during acetaminophen hepatotoxicity in mice. Res Commun Chem Pathol Pharmacol. 58(3):291–305. [PubMed] [Google Scholar]
- Coriat R, Leconte M, Kavian N, Bedda S, Nicco C, Chereau C, Goulvestre C, Weill B, Laurent A, Batteux F. 2011. Mangafodipir protects against hepatic ischemia-reperfusion injury in mice. PLoS One. 6(11):e27005. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover C, Mansouri A, Knight TR, Bajt ML, Lemasters JJ, Pessayre D, Jaeschke H. 2005. Peroxynitrite-Induced Mitochondrial and Endonuclease-Mediated Nuclear DNA Damage in Acetaminophen Hepatotoxicity. J Pharmacol Exp Ther. [DOI] [PubMed] [Google Scholar]
- Craig DG, Bates CM, Davidson JS, Martin KG, Hayes PC, Simpson KJ. 2012. Staggered overdose pattern and delay to hospital presentation are associated with adverse outcomes following paracetamol-induced hepatotoxicity. Br J Clin Pharmacol. 73(2):285–294. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig DG, Lee P, Pryde EA, Masterton GS, Hayes PC, Simpson KJ. 2011. Circulating apoptotic and necrotic cell death markers in patients with acute liver injury. Liver Int. 31(8):1127–1136. eng. [DOI] [PubMed] [Google Scholar]
- Cubero FJ, Zoubek ME, Hu W, Peng J, Zhao G, Nevzorova YA, Al Masaoudi M, Bechmann LP, Boekschoten MV, Muller M et al. 2016. Combined Activities of JNK1 and JNK2 in Hepatocytes Protect Against Toxic Liver Injury. Gastroenterology. 150(4):968–981. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlin DC, Miwa GT, Lu AYH, Nelson SD. 1984. N-ACETYL-PARA-BENZOQUINONE IMINE - A CYTOCHROME-P-450-MEDIATED OXIDATION-PRODUCT OF ACETAMINOPHEN [Article]. Proceedings of the National Academy of Sciences of the United States of America-Biological Sciences. 81(5):1327–1331. English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlin DC, Nelson SD. 1982. Synthesis, decomposition kinetics, and preliminary toxicological studies of pure N-acetyl-p-benzoquinone imine, a proposed toxic metabolite of acetaminophen. J Med Chem. 25(8):885–886. [DOI] [PubMed] [Google Scholar]
- Dara L, Johnson H, Suda J, Win S, Gaarde W, Han D, Kaplowitz N. 2015. Receptor interacting protein kinase 1 mediates murine acetaminophen toxicity independent of the necrosome and not through necroptosis. Hepatology. 62(6):1847–1857. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davern TJ 2nd, James LP, Hinson JA, Polson J, Larson AM, Fontana RJ, Lalani E, Munoz S, Shakil AO, Lee WM. 2006. Measurement of serum acetaminophen-protein adducts in patients with acute liver failure. Gastroenterology. 130(3):687–694. [DOI] [PubMed] [Google Scholar]
- Davidson DG, Eastham WN. 1966. Acute liver necrosis following overdose of paracetamol. Br Med J. 5512:497–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Ideo G, Harrison NG, Williams R. 1975. Hepatic glutathione depletion and impaired bromosulphthalein clearance early after paracetamol overdose in man and the rat. Clin Sci Mol Med. 49(5):495–502. eng. [DOI] [PubMed] [Google Scholar]
- Deutsch M, Graffeo CS, Rokosh R, Pansari M, Ochi A, Levie EM, Van Heerden E, Tippens DM, Greco S, Barilla R et al. 2015. Divergent effects of RIP1 or RIP3 blockade in murine models of acute liver injury. Cell Death Dis. 6(5):e1759. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimova S, Koleva M, Rangelova D, Stoythchev T. 1995. Effect of nifedipine, verapamil, diltiazem and trifluoperazine on acetaminophen toxicity in mice. Arch Toxicol. 70(2):112–118. [DOI] [PubMed] [Google Scholar]
- Dixon MF, Nimmo J, Prescott LF. 1971. Experimental paracetamol-induced hepatic necrosis: a histopathological study. J Pathol. 103(4):225–229. [DOI] [PubMed] [Google Scholar]
- Donahower BC, McCullough SS, Hennings L, Simpson PM, Stowe CD, Saad AG, Kurten RC, Hinson JA, James LP. 2010. Human recombinant vascular endothelial growth factor reduces necrosis and enhances hepatocyte regeneration in a mouse model of acetaminophen toxicity. J Pharmacol Exp Ther. 334(1):33–43. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly PJ, Walker RM, Racz WJ. 1994. Inhibition of mitochondrial respiration in vivo is an early event in acetaminophen-induced hepatotoxicity. Arch Toxicol. 68:110–118. [DOI] [PubMed] [Google Scholar]
- Du K, Ramachandran A, McGill MR, Mansouri A, Asselah T, Farhood A, Woolbright BL, Ding WX, Jaeschke H. 2017. Induction of mitochondrial biogenesis protects against acetaminophen hepatotoxicity. Food Chem Toxicol. 108(Pt A):339–350. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du K, Xie Y, McGill MR, Jaeschke H. 2015. Pathophysiological significance of c-jun N-terminal kinase in acetaminophen hepatotoxicity. Expert Opin Drug Metab Toxicol. 11(11):1769–1779. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan L, Woolbright BL, Jaeschke H, Ramachandran A. 2020. Late Protective Effect of Netrin-1 in the Murine Acetaminophen Hepatotoxicity Model. Toxicol Sci. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehsan Kheradpezhouha LM, Arthur Morphettb, Barrittb Greg J., and Rychkova Grigori Y.,1. 2014. TRPM2 channels mediate acetaminophen-induced liver damage Proc Natl Acad Sci. 111(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsby R, Kitteringham NR, Goldring CE, Lovatt CA, Chamberlain M, Henderson CJ, Wolf CR, Park BK. 2003. Increased constitutive c-Jun N-terminal kinase signaling in mice lacking glutathione S-transferase Pi. J Biol Chem. 278(25):22243–22249. [DOI] [PubMed] [Google Scholar]
- Enomoto A, Itoh K, Nagayoshi E, Haruta J, Kimura T, O'Connor T, Harada T, Yamamoto M. 2001. High sensitivity of Nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicol Sci. 59(1):169–177. eng. [DOI] [PubMed] [Google Scholar]
- Feng D, Wang Y, Wang H, Weng H, Kong X, Martin-Murphy BV, Li Y, Park O, Dooley S, Ju C et al. 2014. Acute and chronic effects of IL-22 on acetaminophen-induced liver injury. J Immunol. 193(5):2512–2518. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flinn FB, Brodie BB. 1948. The effect on the pain threshold of N-acetyl p-aminophenol, a product derived in the body from acetanilide. J Pharmacol Exp Ther. 94(1):76. eng. [PubMed] [Google Scholar]
- Forte AJ, Wilson JM, Slattery JT, Nelson SD. 1984. The formation and toxicity of catechol metabolites of acetaminophen in mice. Drug Metab Dispos. 12(4):484–491. eng. [PubMed] [Google Scholar]
- Gao RY, Wang M, Liu Q, Feng D, Wen Y, Xia Y, Colgan SP, Eltzschig HK, Ju C. 2019. Hypoxia-Inducible Factor-2α Reprograms Liver Macrophages to Protect Against Acute Liver Injury Through the Production of Interleukin-6. Hepatology. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldring CE, Kitteringham NR, Elsby R, Randle LE, Clement YN, Williams DP, McMahon M, Hayes JD, Itoh K, Yamamoto M et al. 2004. Activation of hepatic Nrf2 in vivo by acetaminophen in CD-1 mice. Hepatology. 39(5):1267–1276. eng. [DOI] [PubMed] [Google Scholar]
- Greenberg LA, Lester D. 1947. The metabolic fate of acetanilid and other aniline derivatives; the role of p-aminophenol in the production of methemoglobinemia after acetanilid. J Pharmacol Exp Ther. 90(2):150–153. eng. [PubMed] [Google Scholar]
- Gujral JS, Knight TR, Farhood A, Bajt ML, Jaeschke H. 2002. Mode of cell death after acetaminophen overdose in mice: apoptosis or oncotic necrosis? Toxicol Sci. 67(2):322–328. [DOI] [PubMed] [Google Scholar]
- Gunawan BK, Liu ZX, Han D, Hanawa N, Gaarde WA, Kaplowitz N. 2006. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology. 131(1):165–178. eng. [DOI] [PubMed] [Google Scholar]
- Halmes NC, Hinson JA, Martin BA, Pumford NR. 1995. Purification and characterization of a 50 kDa hepatic mitochondrial protein which covalently binds to a reactive metabolite of acetaminophen. ISSX Proceedings. [Google Scholar]
- Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. 2008. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick SJ, Wilson JW, Fawthrop DJ, Boobis AR, Davies DS. 1992. Paracetamol toxicity in hamster isolated hepatocytes: the increase in cytosolic calcium accompanies, rather than precedes, loss of viability. Arch Toxicol. 66(6):408–412. [DOI] [PubMed] [Google Scholar]
- Hart SG, Cartun RW, Wyand DS, Khairallah EA, Cohen SD. 1995. Immunohistochemical localization of acetaminophen in target tissues of the CD-1 mouse: correspondence of covalent binding with toxicity. Fundam Appl Toxicol. 24(2):260–274. eng. [DOI] [PubMed] [Google Scholar]
- Harvison PJ, Guengerich FP, Rashed MS, Nelson SD. 1988. CYTOCHROME-P-450 ISOZYME SELECTIVITY IN THE OXIDATION OF ACETAMINOPHEN [Article]. Chem Res Toxicol. 1(1):47–52. English. [DOI] [PubMed] [Google Scholar]
- Henderson CJ, Wolf CR, Kitteringham N, Powell H, Otto D, Park BK. 2000. Increased resistance to acetaminophen hepatotoxicity in mice lacking glutathione S-transferase Pi. Proc Natl Acad Sci U S A. 97(23):12741–12745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson NC, Pollock KJ, Frew J, Mackinnon AC, Flavell RA, Davis RJ, Sethi T, Simpson KJ. 2007. Critical role of c-jun (NH2) terminal kinase in paracetamol- induced acute liver failure. Gut. 56(7):982–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinson JA. 1980. Biochemical toxicology of acetaminophen. Rev Biochem Toxicol. 2:103–129. [Google Scholar]
- Hinson JA, Michael SL, Ault SG, Pumford NR. 2000. Western blot analysis for nitrotyrosine protein adducts in livers of saline-treated and acetaminophen-treated mice [In Process Citation]. Toxicol Sci. 53(2):467–473. [DOI] [PubMed] [Google Scholar]
- Hinson JA, Mitchell JR. 1976. N-Hydroxylation of phenacetin by hamster liver microsomes. Drug Metab Dispos. 4(5):430–435. [PubMed] [Google Scholar]
- Hinson JA, Mitchell JR, Jollow DJ. 1976. N-hydroxylation of p-chloroacetanilde in hamsters. Biochem Pharmacol. 25(5):599–601. [DOI] [PubMed] [Google Scholar]
- Hinson JA, Monks TJ, Hong M, Highet RJ, Pohl LR. 1982. 3-(glutathion-S-yl)acetaminophen: a biliary metabolite of acetaminophen. Drug Metab Dispos. 10(1):47–50. [PubMed] [Google Scholar]
- Hinson JA, Nelson SD, Gillette JR. 1979. Metabolism of [p-18O]-phenacetin: the mechanism of activation of phenacetin to reactive metabolites in hamsters. Mol Pharmacol. 15(2):419–427. [PubMed] [Google Scholar]
- Hinson JA, Nelson SD, Mitchell JR. 1977. Studies on the microsomal formation of arylating metabolites of acetaminophen and phenacetin. Mol Pharmacol. 13(4):625–633. [PubMed] [Google Scholar]
- Hinson JA, Pike SL, Pumford NR, Mayeux PR. 1998. Nitrotyrosine-protein adducts in hepatic centrilobular areas following toxic doses of acetaminophen in mice. Chem Res Toxicol. 11(6):604–607. [DOI] [PubMed] [Google Scholar]
- Hinson JA, Pohl LR, Gillette JR. 1979. N-Hydroxyacetaminophen: a microsomal metabolite of N-hydroxyphenacetin but apparently not of acetaminophen. Life Sci. 24(23):2133–2138. [DOI] [PubMed] [Google Scholar]
- Hinson JA, Pohl LR, Gillette JR. 1980. A simple high-pressure liquid chromatographic assay for the N-hydroxy derivatives of phenacetin, acetaminophen, 2-acetylaminofluorene, and other hydroxamic acids. Anal Biochem. 101(2):462–467. [DOI] [PubMed] [Google Scholar]
- Hinson JA, Pohl LR, Monks TJ, Gillette JR, Guengerich FP. 1980. 3-Hydroxyacetaminophen: a microsomal metabolite of acetaminophen. Evidence against an epoxide as the reactive metabolite of acetaminophen. Drug Metab Dispos. 8(5):289–294. [PubMed] [Google Scholar]
- Hinson JA, Pumford NR, Roberts DW. 1995. Mechanisms of acetaminophen toxicity: immunochemical detection of drug-protein adducts. Drug Metab Rev. 27(1-2):73–92. [DOI] [PubMed] [Google Scholar]
- Hinson JA, Roberts DW, Benson RW, Dalhoff K, Loft S, Poulsen HE. 1990. Mechanism of paracetamol toxicity. Lancet. 335(8691). [DOI] [PubMed] [Google Scholar]
- Hoivik DJ, Manautou JE, Tveit A, Mankowski DC, Khairallah EA, Cohen SD. 1996. Evidence suggesting the 58-kDa acetaminophen binding protein is a preferential target for acetaminophen electrophile. Fundam Appl Toxicol. 32(1):79–86. [DOI] [PubMed] [Google Scholar]
- Holt MP, Cheng L, Ju C. 2008. Identification and characterization of infiltrating macrophages in acetaminophen-induced liver injury. J Leukoc Biol. 84(6):1410–1421. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Kholmukhamedov A, Lindsey CC, Beeson CC, Jaeschke H, Lemasters JJ. 2016. Translocation of iron from lysosomes to mitochondria during acetaminophen-induced hepatocellular injury: Protection by starch-desferal and minocycline. Free Radic Biol Med. 97:418–426. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Lemasters JJ. 2020. Suppression of iron mobilization from lysosomes to mitochondria attenuates liver injury after acetaminophen overdose in vivo in mice: Protection by minocycline. Toxicol Appl Pharmacol. 392:114930. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igusa Y, Yamashina S, Izumi K, Inami Y, Fukada H, Komatsu M, Tanaka K, Ikejima K, Watanabe S. 2012. Loss of autophagy promotes murine acetaminophen hepatotoxicity. J Gastroenterol. 47(4):433–443. eng. [DOI] [PubMed] [Google Scholar]
- Imaeda AB, Watanabe A, Sohail MA, Mahmood S, Mohamadnejad M, Sutterwala FS, Flavell RA, Mehal WZ. 2009. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest. 119(2):305–314. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y, Bethea NW, Abril ER, McCuskey RS. 2003. Early hepatic microvascular injury in response to acetaminophen toxicity. Microcirculation. 10(5):391–400. [DOI] [PubMed] [Google Scholar]
- Jaeschke H 1990. Glutathione disulfide formation and oxidant stress during acetaminophen-induced hepatotoxicity in mice in vivo: the protective effect of allopurinol. J Pharmacol Exp Ther. 255(3):935–941. [PubMed] [Google Scholar]
- Jaeschke H, Ramachandran A. 2020. Does JNK Regulate Acetaminophen Hepatotoxicity by Modulating Nrf2-dependent Genes or Mitochondrial Oxidant Stress? Hepatology. eng. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Ramachandran A, Chao X, Ding WX. 2019. Emerging and established modes of cell death during acetaminophen-induced liver injury. Arch Toxicol. 93(12):3491–3502. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James L, Simon M, Capparelli E, Hinson J, Davern T, Lee W. 2007. Pharmacokinetic analysis of acetaminophen protein adducts in adults with acute liver failure. Clinical Pharmacology & Therapeutics. 81((Supplement 1)):2. [Google Scholar]
- James LP, Farrar HC, Sullivan JE, Givens TG, Kearns GL, Wasserman GS, Walson PD, Hinson JA, Pumford NR. 2001. Measurement of acetaminophen-protein adducts in children and adolescents with acetaminophen overdoses. Pediatric Pharmacology Research Unit Network, NICHD. J Clin Pharmacol. 41(8):846–851. [DOI] [PubMed] [Google Scholar]
- James LP, Lamps LW, McCullough S, Hinson JA. 2003. Interleukin 6 and hepatocyte regeneration in acetaminophen toxicity in the mouse. Biochem Biophys Res Commun. 309(4):857–863. [DOI] [PubMed] [Google Scholar]
- James LP, Letzig L, Simpson PM, Capparelli E, Roberts DW, Hinson JA, Davern TJ, Lee WM. 2009. Pharmacokinetics of Acetaminophen-Protein Adducts in Adults with Acetaminophen Overdose and Acute Liver Failure [Article; Proceedings Paper]. Drug Metab Dispos. 37(8):1779–1784. English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James LP, Mayeux PR, Hinson JA. 2003. Acetaminophen-induced hepatotoxicity. Drug Metab Dispos. 31(12):1499–1506. [DOI] [PubMed] [Google Scholar]
- James LP, McCullough SS, Knight TR, Jaeschke H, Hinson JA. 2003. Acetaminophen toxicity in mice lacking NADPH oxidase activity: role of peroxynitrite formation and mitochondrial oxidant stress. Free Radic Res. 37(12):1289–1297. [DOI] [PubMed] [Google Scholar]
- Jollow DJ, Mitchell JR, Potter WZ, Davis DC, Gillette JR, Brodie BB. 1973. Acetaminophen-induced hepatic necrosis. II. Role of covalent binding in vivo. J Pharmacol ExpTher. 187(1):195–202. [PubMed] [Google Scholar]
- Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR. 1974. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 11(3):151–169. [DOI] [PubMed] [Google Scholar]
- Ju C, Reilly TP, Bourdi M, Radonovich MF, Brady JN, George JW, Pohl LR. 2002. Protective role of Kupffer cells in acetaminophen-induced hepatic injury in mice. Chem Res Toxicol. 15(12):1504–1513. [DOI] [PubMed] [Google Scholar]
- Kang AM, Padilla-Jones A, Fisher ES, Akakpo JY, Jaeschke H, Rumack BH, Gerkin RD, Curry SC. 2020. The Effect of 4-Methylpyrazole on Oxidative Metabolism of Acetaminophen in Human Volunteers. J Med Toxicol. 16(2):169–176. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplowitz N, Aw TY, Simon FR, Stolz A. 1986. Drug-induced hepatotoxicity. Ann Intern Med. 104(6):826–839. eng. [DOI] [PubMed] [Google Scholar]
- Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. 2002. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. Jama. 287(3):337–344. eng. [DOI] [PubMed] [Google Scholar]
- Kayano K, Yasunaga M, Kubota M, Takenaka K, Mori K, Yamashita A, Kubo Y, Sakaida I, Okita K, Sanuki K. 1992. Detection of proliferating hepatocytes by immunohistochemical staining for proliferating cell nuclear antigen (PCNA) in patients with acute hepatic failure. Liver. 12(3):132–136. eng. [DOI] [PubMed] [Google Scholar]
- Kheradpezhouh E, Barritt GJ, Rychkov GY. 2016. Curcumin inhibits activation of TRPM2 channels in rat hepatocytes. Redox Biol. 7:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt D, Giannou AD, McGee HM, Kempski J, Steglich B, Huber FJ, Ernst TM, Shiri AM, Wegscheid C, Tasika E et al. 2017. A Protective Function of IL-22BP in Ischemia Reperfusion and Acetaminophen-Induced Liver Injury. J Immunol. 199(12):4078–4090. eng. [DOI] [PubMed] [Google Scholar]
- Knight TR, Fariss MW, Farhood A, Jaeschke H. 2003. Role of lipid peroxidation as a mechanism of liver injury after acetaminophen overdose in mice. Toxicol Sci. 76(1):229–236. eng. [DOI] [PubMed] [Google Scholar]
- Knight TR, Ho YS, Farhood A, Jaeschke H. 2002. Peroxynitrite is a critical mediator of acetaminophen hepatotoxicity in murine livers: protection by glutathione. J Pharmacol Exp Ther. 303(2):468–475. eng. [DOI] [PubMed] [Google Scholar]
- Kon K, Ikejima K, Okumura K, Aoyama T, Arai K, Takei Y, Lemasters JJ, Sato N. 2007. Role of apoptosis in acetaminophen hepatotoxicity. J Gastroenterol Hepatol. 22Suppl 1:S49–52. [DOI] [PubMed] [Google Scholar]
- Kon K, Kim JS, Jaeschke H, Lemasters JJ. 2004. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 40(5):1170–1179. [DOI] [PubMed] [Google Scholar]
- Kon K, Kim JS, Uchiyama A, Jaeschke H, Lemasters JJ. 2010. Lysosomal iron mobilization and induction of the mitochondrial permeability transition in acetaminophen-induced toxicity to mouse hepatocytes. Toxicol Sci. 117(1):101–108. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster U, Albrecht D, Kappus H. 1977. EVIDENCE FOR CARBON TETRACHLORIDE-INDUCED AND ETHANOL-INDUCED LIPID PEROXIDATION INVIVO DEMONSTRATED BY ETHANE PRODUCTION IN MICE AND RATS [Article]. Toxicol Appl Pharmacol. 41(3):639–648. English. [DOI] [PubMed] [Google Scholar]
- Laskin DL, Gardner CR, Price VF, Jollow DJ. 1995. Modulation of macrophage functioning abrogates the acute hepatotoxicity of acetaminophen. Hepatology. 21(4):1045–1050. [PubMed] [Google Scholar]
- Laskin DL, Pilaro AM. 1986. Potential role of activated macrophages in acetaminophen hepatotoxicity. I. Isolation and characterization of activated macrophages from rat liver. Toxicol Appl Pharmacol. 86(2):204–215. [DOI] [PubMed] [Google Scholar]
- Laskin DL, Pilaro AM, Ji S. 1986. Potential role of activated macrophages in acetaminophen hepatotoxicity. II. Mechanism of macrophage accumulation and activation. Toxicol Appl Pharmacol. 86(2):216–226. [DOI] [PubMed] [Google Scholar]
- Latchoumycandane C, Goh CW, Ong MM, Boelsterli UA. 2007. Mitochondrial protection by the JNK inhibitor leflunomide rescues mice from acetaminophen-induced liver injury. Hepatology. 45(2):412–421. [DOI] [PubMed] [Google Scholar]
- Lauterburg BH, Corcoran GB, Mitchell JR. 1983. Mechanism of Action of N-Acetylcysteine in the Protection Against the Hepatotoxicity of Acetaminophen in Rats In Vivo. 71(4):980–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterburg BH, Mitchell JR. 1987. Therapeutic doses of acetaminophen stimulate the turnover of cysteine and glutathione in man. J Hepatol. 4(2):206–211. eng. [DOI] [PubMed] [Google Scholar]
- Lawson JA, Farhood A, Hopper RD, Bajt ML, Jaeschke H. 2000. The hepatic inflammatory response after acetaminophen overdose: role of neutrophils. Toxicol Sci. 54(2):509–516. [DOI] [PubMed] [Google Scholar]
- Lee SM, Cho TS, Kim DJ, Cha YN. 1999. Protective effect of ethanol against acetaminophen-induced hepatotoxicity in mice - Role of NADH : quinone reductase [Article]. Biochem Pharmacol. 58(10):1547–1555. English. [DOI] [PubMed] [Google Scholar]
- Lee SS, Buters JT, Pineau T, Fernandez-Salguero P, Gonzalez FJ. 1996. Role of CYP2E1 in the hepatotoxicity of acetaminophen. J Biol Chem. 271(20):12063–12067. eng. [DOI] [PubMed] [Google Scholar]
- Lim SP, Andrews FJ, O'Brien PE. 1995. Acetaminophen-induced microvascular injury in the rat liver: protection with misoprostol. Hepatology. 22(6):1776–1781. [PubMed] [Google Scholar]
- Lizasoain I, Moro MA, Knowles RG, DarleyUsmar V, Moncada S. 1996. Nitric oxide and peroxynitrite exert distinct effects on mitochondrial respiration which are differentially blocked by glutathione or glucose [Article]. Biochem J. 314:877–880. English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoGuidice A, Boelsterli UA. 2011. Acetaminophen overdose-induced liver injury in mice is mediated by peroxynitrite independently of the cyclophilin D-regulated permeability transition. Hepatology. 54(3):969–978. eng. [DOI] [PubMed] [Google Scholar]
- Lutkewitte AJ, Schweitzer GG, Kennon-McGill S, Clemens MM, James LP, Jaeschke H, Finck BN, McGill MR. 2018. Lipin deactivation after acetaminophen overdose causes phosphatidic acid accumulation in liver and plasma in mice and humans and enhances liver regeneration. Food Chem Toxicol. 115:273–283. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan-Crow LA, Crow JP, Kerby JD, Beckman JS, Thompson JA. 1996. Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allografts. Proc Natl Acad Sci U S A. 93(21):11853–11858. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmillan-Crow LA, Cruthirds DL. 2001. Invited review: manganese superoxide dismutase in disease. Free Radic Res. 34(4):325–336. [DOI] [PubMed] [Google Scholar]
- Masubuchi Y, Suda C, Horie T. 2005. Involvement of mitochondrial permeability transition in acetaminophen-induced liver injury in mice. J Hepatol. 42(1):110–116. [DOI] [PubMed] [Google Scholar]
- Matthews AM, Roberts DW, Hinson JA, Pumford NR. 1996. Acetaminophen-induced hepatotoxicity. Analysis of total covalent binding vs. specific binding to cysteine. Drug Metab Dispos. 24(11):1192–1196. [PubMed] [Google Scholar]
- McClain CJ, Kromhout JP, Peterson FJ, Holtzman JL. 1980. POTENTIATION OF ACETAMINOPHEN HEPATOTOXICITY BY ALCOHOL [Article]. JAMA-J Am Med Assoc. 244(3):251–253. English. [PubMed] [Google Scholar]
- McCuskey RS. 2006. Sinusoidal endothelial cells as an early target for hepatic toxicants. Clin Hemorheol Microcirc. 34(1-2):5–10. [PubMed] [Google Scholar]
- McGill MR, Lebofsky M, Norris HR, Slawson MH, Bajt ML, Xie Y, Williams CD, Wilkins DG, Rollins DE, Jaeschke H. 2013. Plasma and liver acetaminophen-protein adduct levels in mice after acetaminophen treatment: dose-response, mechanisms, and clinical implications. Toxicol Appl Pharmacol. 269(3):240–249. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H. 2012. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest. 122(4):1574–1583. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Staggs VS, Sharpe MR, Lee WM, Jaeschke H. 2014. Serum mitochondrial biomarkers and damage-associated molecular patterns are higher in acetaminophen overdose patients with poor outcome. Hepatology. 60(4):1336–1345. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Williams CD, Xie Y, Ramachandran A, Jaeschke H. 2012. Acetaminophen-induced liver injury in rats and mice: comparison of protein adducts, mitochondrial dysfunction, and oxidative stress in the mechanism of toxicity. Toxicol Appl Pharmacol. 264(3):387–394. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Yan HM, Ramachandran A, Murray GJ, Rollins DE, Jaeschke H. 2011. HepaRG cells: a human model to study mechanisms of acetaminophen hepatotoxicity. Hepatology. 53(3):974–982. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehendale HM. 2005. Tissue repair: an important determinant of final outcome of toxicant-induced injury. Toxicol Pathol. 33(1):41–51. eng. [DOI] [PubMed] [Google Scholar]
- Mehendale HM. 2012. Once initiated, how does toxic tissue injury expand? [Review]. Trends Pharmacol Sci. 33(4):200–206. English. [DOI] [PubMed] [Google Scholar]
- Michael SL, Pumford NR, Mayeux PR, Niesman MR, Hinson JA. 1999. Pretreatment of mice with macrophage inactivators decreases acetaminophen hepatotoxicity and the formation of reactive oxygen and nitrogen species. Hepatology. 30(1):186–195. [DOI] [PubMed] [Google Scholar]
- Miller BA, Cheung JY. 2016. TRPM2 protects against tissue damage following oxidative stress and ischaemia-reperfusion. J Physiol. 594(15):4181–4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JA, Miller EC. 1975. Metabolic activation and reactivity of chemical carcinogens. Mutat Res. 33(1 Spec No):25–26. [DOI] [PubMed] [Google Scholar]
- Miller MG, Jollow DJ. 1984. Effect of L-ascorbic acid on acetaminophen-induced hepatotoxicity and covalent binding in hamsters. Evidence that in vitro covalent binding differs from that in vivo. Drug Metab Dispos. 12(3):271–279. [PubMed] [Google Scholar]
- Mitchell JR, Jollow DJ, Potter WZ, Davis DC, Gillette JR, Brodie BB. 1973. Acetaminophen-induced hepatic necrosis. I. Role of drug metabolism. J Pharmcol Exp Ther. 187:185–194. [PubMed] [Google Scholar]
- Mitchell JR, Jollow DJ, Potter WZ, Davis DC, Gillette JR, Brodie BB. 1973a. Acetaminophen-induced hepatic necrosis. I. Role of drug metabolism. J Pharmcol Exp Ther. 187:185–194. [PubMed] [Google Scholar]
- Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BB. 1973. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther. 187(1):211–217. [PubMed] [Google Scholar]
- Mitchell JR, Thorgeirsson SS, Potter WZ, Jollow DJ, Keiser H. 1974. Acetaminophen-induced hepatic injury: protective role of glutathione in man and rationale for therapy. Clin Pharmacol Ther. 16(4):676–684. [DOI] [PubMed] [Google Scholar]
- Morgan ET, Koop DR, Coon MJ. 1983. Comparison of six rabbit liver cytochrome P-450 isozymes in formation of a reactive metabolite of acetaminophen. Biochem Biophys Res Commun. 112(1):8–13. eng. [DOI] [PubMed] [Google Scholar]
- Morrison EE, Oatey K, Gallagher B, Grahamslaw J, O'Brien R, Black P, Oosthuyzen W, Lee RJ, Weir CJ, Henriksen D et al. 2019. Principal results of a randomised open label exploratory, safety and tolerability study with calmangafodipir in patients treated with a 12 h regimen of N-acetylcysteine for paracetamol overdose (POP trial). EBioMedicine. 46:423–430. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muldrew KL, James LP, Coop L, McCullough SS, Hendrickson HP, Hinson JA, Mayeux PR. 2002. Determination of acetaminophen-protein adducts in mouse liver and serum and human serum after hepatotoxic doses of acetaminophen using high-performance liquid chromatography with electrochemical detection. Drug Metab Dispos. 30(4):446–451. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Maeda S, Hikiba Y, Ohmae T, Shibata W, Yanai A, Sakamoto K, Ogura K, Noguchi T, Karin M et al. 2008. Deletion of apoptosis signal-regulating kinase 1 attenuates acetaminophen-induced liver injury by inhibiting c-Jun N-terminal kinase activation. Gastroenterology. 135(4):1311–1321. eng. [DOI] [PubMed] [Google Scholar]
- Napirei M, Basnakian AG, Apostolov EO, Mannherz HG. 2006. Deoxyribonuclease 1 aggravates acetaminophen-induced liver necrosis in male CD-1 mice. Hepatology. 43(2):297–305. [DOI] [PubMed] [Google Scholar]
- Nebert DW, Thorgeirsson SS, Lambert GH. 1976. Genetic aspects of toxicity during development. Environ Health Perspect. 18:35–45. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SD, Dahlin DC, Rauckman EJ, Rosen GM. 1981. PEROXIDASE-MEDIATED FORMATION OF REACTIVE METABOLITES OF ACETAMINOPHEN [Article]. Mol Pharmacol. 20(1):195–199. English. [PubMed] [Google Scholar]
- Ni HM, Bockus A, Boggess N, Jaeschke H, Ding WX. 2012. Activation of autophagy protects against acetaminophen-induced hepatotoxicity. Hepatology. 55(1):222–232. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni HM, Boggess N, McGill MR, Lebofsky M, Borude P, Apte U, Jaeschke H, Ding WX. 2012. Liver-specific loss of Atg5 causes persistent activation of Nrf2 and protects against acetaminophen-induced liver injury. Toxicol Sci. 127(2):438–450. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni HM, McGill MR, Chao X, Du K, Williams JA, Xie Y, Jaeschke H, Ding WX. 2016. Removal of acetaminophen protein adducts by autophagy protects against acetaminophen-induced liver injury in mice. J Hepatol. 65(2):354–362. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni HM, Williams JA, Jaeschke H, Ding WX. 2013. Zonated induction of autophagy and mitochondrial spheroids limits acetaminophen-induced necrosis in the liver. Redox Biol. 1(1):427–432. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicotera P, Bellomo G, Orrenius S. 1992. Calcium-mediated mechanisms in chemically induced cell death. Annu Rev Pharmacol Toxicol. 32:449–470. eng. [DOI] [PubMed] [Google Scholar]
- Okawa H, Motohashi H, Kobayashi A, Aburatani H, Kensler TW, Yamamoto M. 2006. Hepatocyte-specific deletion of the keap1 gene activates Nrf2 and confers potent resistance against acute drug toxicity. Biochem Biophys Res Commun. 339(1):79–88. eng. [DOI] [PubMed] [Google Scholar]
- Patten CJ, Thomas PE, Guy RL, Lee M, Gonzalez FJ, Guengerich FP, Yang CS. 1993. Cytochrome P450 enzymes involved in acetaminophen activation by rat and human liver microsomes and their kinetics. Chem Res Toxicol. 6(4):511–518. eng. [DOI] [PubMed] [Google Scholar]
- Peterson FJ, Holloway DE, Erickson RR, Duquette PH, McClain CJ, Holtzman JL. 1980. ETHANOL INDUCTION OF ACETAMINOPHEN TOXICITY AND METABOLISM [Article]. Life Sci. 27(18):1705–1711. English. [DOI] [PubMed] [Google Scholar]
- Potter DW, Hinson JA. 1987a. THE 1-ELECTRON AND 2-ELECTRON OXIDATION OF ACETAMINOPHEN CATALYZED BY PROSTAGLANDIN-H SYNTHASE [Article]. J Biol Chem. 262(3):974–980. English. [PubMed] [Google Scholar]
- Potter DW, Hinson JA. 1987b. MECHANISMS OF ACETAMINOPHEN OXIDATION TO N-ACETYL-P-BENZOQUINONE IMINE BY HORSERADISH-PEROXIDASE AND CYTOCHROME-P-450 [Article]. J Biol Chem. 262(3):966–973. English. [PubMed] [Google Scholar]
- Potter DW, Miller DW, Hinson JA. 1985. IDENTIFICATION OF ACETAMINOPHEN POLYMERIZATION PRODUCTS CATALYZED BY HORSERADISH-PEROXIDASE [Article]. J Biol Chem. 260(22):2174–2180. English. [PubMed] [Google Scholar]
- Potter WZ, Davis DC, Mitchell JR, Jollow DJ, Gillette JR, Brodie BB. 1973. Acetaminophen-induced hepatic necrosis. 3. Cytochrome P-450-mediated covalent binding in vitro. J Pharmacol Exp Ther. 187(1):203–210. [PubMed] [Google Scholar]
- Prescott LF. 2000. Paracetamol, alcohol and the liver. Br J Clin Pharmacol. 49(4):291–301. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott LF, Illingworth RN, Critchley JA, Stewart MJ, Adam RD, Proudfoot AT. 1979. Intravenous N-acetylcystine: the treatment of choice for paracetamol poisoning. BMJ. 2(6198):1097–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott LF, Roscoe P, Wright N, Brown SS. 1971. Plasma-paracetamol half-life and hepatic necrosis in patients with paracetamol overdosage. Lancet. 1(7698):519–522. [DOI] [PubMed] [Google Scholar]
- Proudfoot AT, Wright N. 1970. Acute paracetamol poisoning. Br Med J. 3(5722):557–558. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumford NR, Hinson JA, Benson RW, Roberts DW. 1990. Immunoblot analysis of protein containing 3-(cystein-S-yl)acetaminophen adducts in serum and subcellular liver fractions from acetaminophen-treated mice. Toxicol Appl Pharmaco. 104(3):521–532. [DOI] [PubMed] [Google Scholar]
- Pumford NR, Hinson JA, Potter DW, Rowland KL, Benson RW, Roberts DW. 1989. Immunochemical quantitation of 3-(cystein-S-yl)acetaminophen adducts in serum and liver proteins of acetaminophen-treated mice. J Pharmacol Exp Ther. 248(1):190–196. [PubMed] [Google Scholar]
- Pumford NR, Martin BM, Hinson JA. 1992. A metabolite of acetaminophen covalently binds to the 56 kDa selenium binding protein. Biochemical and Biophysical Research Communications. 182(3):1348–1355. [DOI] [PubMed] [Google Scholar]
- Radi R, Beckman JS, Bush KM, Freeman BA. 1991. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem. 266(7):4244–4250. [PubMed] [Google Scholar]
- Ramachandran A, Lebofsky M, Baines CP, Lemasters JJ, Jaeschke H. 2011. Cyclophilin D deficiency protects against acetaminophen-induced oxidant stress and liver injury. Free Radic Res. 45(2):156–164. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, McGill MR, Xie Y, Ni HM, Ding WX, Jaeschke H. 2013. Receptor interacting protein kinase 3 is a critical early mediator of acetaminophen-induced hepatocyte necrosis in mice. Hepatology. 58(6):2099–2108. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray SD, Kamendulis LM, Gurule MW, Yorkin RD, Corcoran GB. 1993. Ca2+ antagonists inhibit DNA fragmentation and toxic cell death induced by acetaminophen. Faseb j. 7(5):453–463. eng. [DOI] [PubMed] [Google Scholar]
- Ray SD, Mumaw VR, Raje RR, Fariss MW. 1996. Protection of acetaminophen-induced hepatocellular apoptosis and necrosis by cholesteryl hemisuccinate pretreatment. J Pharmacol Exp Ther. 279(3):1470–1483. [PubMed] [Google Scholar]
- Ray SD, Sorge CL, Raucy JL, Corcoran GB. 1990. Early loss of large genomic DNA in vivo with accumulation of Ca2+ in the nucleus during acetaminophen-induced liver injury. Toxicol Appl Pharmacol. 106(2):346–351. [DOI] [PubMed] [Google Scholar]
- Ray SD, Sorge CL, Tavacoli A, Raucy JL, Corcoran GB. 1991. Extensive alteration of genomic DNA and rise in nuclear Ca2+ in vivo early after hepatotoxic acetaminophen overdose in mice. Adv Exp Med Biol. 283(699):699–705. [DOI] [PubMed] [Google Scholar]
- Reid AB, Kurten RC, McCullough SS, Brock RW, Hinson JA. 2005. Mechanisms of acetaminophen-induced hepatotoxicity: role of oxidative stress and mitochondrial permeability transition in freshly isolated mouse hepatocytes. J Pharmacol Exp Ther. 312(2):509–516. [DOI] [PubMed] [Google Scholar]
- Reiter CD, Teng RJ, Beckman JS. 2000. Superoxide reacts with nitric oxide to nitrate tyrosine at physiological pH via peroxynitrite. J Biol Chem. 275(42):32460–32466. [DOI] [PubMed] [Google Scholar]
- Roberts DW, Benson RW, Pumford NR, Potter DW, Poulsen HE, Hinson JA. 1991. Sensitive immunochemical assays for monitoring acetaminophen toxicity in humans. In: Vanderlaan M, Stanker LH, Watkins BE et al. , editors. Immunoassays for trace chemical analysis: Monitoring toxic chemicals in humans, food and the environment. Washington, DC: American Chemical Society; p. 314–326. [Google Scholar]
- Roberts DW, Bucci TJ, Benson RW, Warbritton AR, McRae TA, Pumford NR, Hinson JA. 1991. Immunohistochemical localization and quantification of the 3-(cystein-S-yl)-acetaminophen protein adduct in acetaminophen hepatotoxicity. Am J Pathol. 138(2):359–371. [PMC free article] [PubMed] [Google Scholar]
- Roberts DW, Pumford NR, Potter DW, Benson RW, Hinson JA. 1987. A sensitive immunochemical assay for acetaminophen-protein adducts. J Pharmacol Exp Ther. 241(2):527–533. [PubMed] [Google Scholar]
- Rose PG. 1969. Paracetamol overdose and liver damage. Br Med J. 1(5640):381–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumack BH. 2002. Acetaminophen hepatotoxicity: the first 35 years. J Toxicol Clin Toxicol. 40(1):3–20. eng. [DOI] [PubMed] [Google Scholar]
- Rumack BH. 2004. Acetaminophen misconceptions. Hepatology. 40(1):10–15. eng. [DOI] [PubMed] [Google Scholar]
- Rumack BH, Peterson RC, Koch GG, Amara IA. 1981. Acetaminophen overdose. 662 cases with evaluation of oral acetylcysteine treatment. Arch Intern Med. 141(3 Spec No):380–385. [DOI] [PubMed] [Google Scholar]
- Rumack BH, Peterson RG. 1978. Acetaminophen overdose: incidence, diagnosis, and management in 416 patients. Pediatrics. 62(5 Pt 2 Suppl):898–903. [PubMed] [Google Scholar]
- Saito C, Lemasters JJ, Jaeschke H. 2010. c-Jun N-terminal kinase modulates oxidant stress and peroxynitrite formation independent of inducible nitric oxide synthase in acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 246(1-2):8–17. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt LE, Dalhoff K. 2005. Alpha-fetoprotein is a predictor of outcome in acetaminophen-induced liver injury. Hepatology. 41(1):26–31. eng. [DOI] [PubMed] [Google Scholar]
- Schmidt LE, Dalhoff K, Poulsen HE. 2002. Acute versus chronic alcohol consumption in acetaminophen-induced hepatotoxicity [Article]. Hepatology. 35(4):876–882. English. [DOI] [PubMed] [Google Scholar]
- Schopfer F, Riobo N, Carreras MC, Alvarez B, Radi R, Boveris A, Cadenas E, Poderoso JJ. 2000. Oxidation of ubiquinol by peroxynitrite: implications for protection of mitochondria against nitrosative damage. Biochem J. 349(Pt 1):35–42. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schror K 2007. Aspirin and Reye syndrome: a review of the evidence. Paediatr Drugs. 9(3):195–204. eng. [DOI] [PubMed] [Google Scholar]
- Seeff LB, Cuccherini BA, Zimmerman HJ, Adler E, Benjamin SB. 1986. ACETAMINOPHEN HEPATOTOXICITY IN ALCOHOLICS - A THERAPEUTIC MISADVENTURE [Review]. Ann Intern Med. 104(3):399–404. English. [DOI] [PubMed] [Google Scholar]
- Sharma M, Gadang V, Jaeschke A. 2012. Critical role for mixed-lineage kinase 3 in acetaminophen-induced hepatotoxicity. Mol Pharmacol. 82(5):1001–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Kamendulis LM, Ray SD, Corcoran GB. 1992. Acetaminophen-induced cytotoxicity in cultured mouse hepatocytes: effects of Ca(2+)-endonuclease, DNA repair, and glutathione depletion inhibitors on DNA fragmentation and cell death. Toxicol Appl Pharmacol. 112(1):32–40. [DOI] [PubMed] [Google Scholar]
- Sinclair J, Jeffery E, Wrighton S, Kostrubsky V, Szakacs J, Wood S, Sinclair P. 1998. Alcohol-mediated increases in acetaminophen hepatotoxicity - Role of CYP2E and CYP3A [Article]. Biochem Pharmacol. 55(10):1557–1565. English. [DOI] [PubMed] [Google Scholar]
- Smilkstein MJ, Knapp GL, Kulig KW, Rumack BH. 1988. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985) [see comments]. N Engl J Med. 319(24):1557–1562. [DOI] [PubMed] [Google Scholar]
- Smith GS, Nadig DE, Kokoska ER, Solomon H, Tiniakos DG, Miller TA. 1998. Role of neutrophils in hepatotoxicity induced by oral acetaminophen administration in rats. J Surg Res. 80(2):252–258. eng. [DOI] [PubMed] [Google Scholar]
- Streeter AJ, Dahlin DC, Nelson SD, Baillie TA. 1984. The covalent binding of acetaminophen to protein. Evidence for cysteine residues as major sites of arylation in vitro. Chem Biol Interact. 48(3):349–366. [DOI] [PubMed] [Google Scholar]
- Strehler EE, Zacharias DA. 2001. Role of alternative splicing in generating isoform diversity among plasma membrane calcium pumps. Physiol Rev. 81(1):21–50. eng. [DOI] [PubMed] [Google Scholar]
- Sumoza-Toledo A, Penner R. 2011. TRPM2: a multifunctional ion channel for calcium signalling [Review]. J Physiol-London. 589(7):1515–1525. English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tee LB, Boobis AR, Huggett AC, Davies DS. 1986. Reversal of acetaminophen toxicity in isolated hamster hepatocytes by dithiothreitol. Toxicol Appl Pharmacol. 83(2):294–314. [DOI] [PubMed] [Google Scholar]
- Thompson RP, Clark R, Willson RA, Borirakchanyavat V, Widdop B, Goulding R, Williams R. 1972. Hepatic damage from overdose of paracetamol. Gut. 13(10):836. [PMC free article] [PubMed] [Google Scholar]
- Thomson JS, Prescott LF. 1966. Liver damage and impaired glucose tolerance after paracetamol overdosage. Br Med J. 2(5512):506–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thummel KE, Slattery JT, Nelson SD, Lee CA, Pearson PG. 1989. Effect of ethanol on hepatotoxicity of acetaminophen in mice and on reactive metabolite formation by mouse and human liver microsomes. Toxicol Appl Pharmacol. 100(3):391–397. eng. [DOI] [PubMed] [Google Scholar]
- Tirmenstein MA, Nelson SD. 1989. Subcellular binding and effects on calcium homeostasis produced by acetaminophen and a nonhepatotoxic regioisomer, 3'-hydroxyacetanilide, in mouse liver. J Biol Chem. 264(17):9814–9819. [PubMed] [Google Scholar]
- Tsokos-Kuhn JO, Hughes H, Smith CV, Mitchell JR. 1988. Alkylation of the liver plasma membrane and inhibition of the Ca2+ ATPase by acetaminophen. Biochem Pharmacol. 37(11):2125–2131. [DOI] [PubMed] [Google Scholar]
- Tsokos KJ. 1989. Evidence in vivo for elevation of intracellular free Ca2+ in the liver after diquat, acetaminophen, and CCl4. Biochem Pharmacol. 38(18):3061–3065. [DOI] [PubMed] [Google Scholar]
- Vandonselaar M, Hickie RA, Quail JW, Delbaere LTJ. 1994. TRIFLUOPERAZINE-INDUCED CONFORMATIONAL CHANGE IN CA2+-CALMODULIN [Article]. Nat Struct Biol. 1(11):795–801. English. [DOI] [PubMed] [Google Scholar]
- Vendemiale G, Grattagliano I, Altomare E, Turturro N, Guerrieri F. 1996. Effect of acetaminophen administration on hepatic glutathione compartmentation and mitochondrial energy metabolism in the rat. Biochem Pharmacol. 52(8):1147–1154. [DOI] [PubMed] [Google Scholar]
- Volak LP, Ghirmai S, Cashman JR, Court MH. 2008. Curcuminoids inhibit multiple human cytochromes P450, UDP-glucuronosyltransferase, and sulfotransferase enzymes, whereas piperine is a relatively selective CYP3A4 inhibitor. Drug Metab Dispos. 36(8):1594–1605. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Mering J 1893. Beitrage zur kenntniss der Antipyretica. Therapeutische Monatshefte. 7:577–587. [Google Scholar]
- Walker RM, Racz WJ, McElligott TF. 1980. Acetaminophen-induced hepatotoxicity in mice. Lab Invest. 42(2):181–189. [PubMed] [Google Scholar]
- Walker RM, Racz WJ, McElligott TF. 1983. Scanning electron microscopic examination of acetaminophen-induced hepatotoxicity and congestion in mice. Am J Pathol. 113(3):321–330. [PMC free article] [PubMed] [Google Scholar]
- Walker RM, Racz WJ, McElligott TF. 1985. Acetaminophen-induced hepatotoxic congestion in mice. Hepatology. 5(2):233–240. [DOI] [PubMed] [Google Scholar]
- Wang H, Ni HM, Chao X, Ma X, Rodriguez YA, Chavan H, Wang S, Krishnamurthy P, Dobrowsky R, Xu DX et al. 2019. Double deletion of PINK1 and Parkin impairs hepatic mitophagy and exacerbates acetaminophen-induced liver injury in mice. Redox Biol. 22:101148. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster PA, Roberts DW, Benson RW, Kearns GL. 1996. Acetaminophen toxicity in children: diagnostic confirmation using a specific antigenic biomarker. J Clin Pharmacol. 36(5):397–402. [DOI] [PubMed] [Google Scholar]
- Weis M, Kass GE, Orrenius S. 1994. Further characterization of the events involved in mitochondrial Ca2+ release and pore formation by prooxidants. Biochem Pharmacol. 47(12):2147–2156. [DOI] [PubMed] [Google Scholar]
- Weis M, Kass GEN, Orrenius S, Moldeus P. 1992. N-acetyl-p-benzoquinone imine induces Ca2+ release from mitochondria by stimulating pyridine nucleotide hydrolysis. J Biol Chem. 267(2):804–809. [PubMed] [Google Scholar]
- West PR, Harman LS, Josephy PD, Mason RP. 1984. ACETAMINOPHEN - ENZYMATIC FORMATION OF A TRANSIENT PHENOXYL FREE-RADICAL [Note]. Biochem Pharmacol. 33(18):2933–2936. English. [DOI] [PubMed] [Google Scholar]
- Williams CD, Antoine DJ, Shaw PJ, Benson C, Farhood A, Williams DP, Kanneganti TD, Park BK, Jaeschke H. 2011. Role of the Nalp3 inflammasome in acetaminophen-induced sterile inflammation and liver injury. Toxicol Appl Pharmacol. 252(3):289–297. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CD, McGill MR, Farhood A, Jaeschke H. 2013. Fas receptor-deficient lpr mice are protected against acetaminophen hepatotoxicity due to higher glutathione synthesis and enhanced detoxification of oxidant stress. Food Chem Toxicol. 58:228–235. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Win S, Min RW, Chen CQ, Zhang J, Chen Y, Li M, Suzuki A, Abdelmalek MF, Wang Y, Aghajan M et al. 2019. Expression of mitochondrial membrane-linked SAB determines severity of sex-dependent acute liver injury. J Clin Invest. 129(12):5278–5293. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Win S, Than TA, Han D, Petrovic LM, Kaplowitz N. 2011. c-Jun N-terminal kinase (JNK)-dependent acute liver injury from acetaminophen or tumor necrosis factor (TNF) requires mitochondrial Sab protein expression in mice. J Biol Chem. 286(40):35071–35078. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Win S, Than TA, Min RW, Aghajan M, Kaplowitz N. 2016. c-Jun N-terminal kinase mediates mouse liver injury through a novel Sab (SH3BP5)-dependent pathway leading to inactivation of intramitochondrial Src. Hepatology. 63(6):1987–2003. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolbright BL, Jaeschke H. 2017. Role of the inflammasome in acetaminophen-induced liver injury and acute liver failure. J Hepatol. 66(4):836–848. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolbright BL, Jaeschke H. 2018. Mechanisms of Inflammatory Liver Injury and Drug-Induced Hepatotoxicity. Curr Pharmacol Rep. 4(5):346–357. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, McGill MR, Dorko K, Kumer SC, Schmitt TM, Forster J, Jaeschke H. 2014. Mechanisms of acetaminophen-induced cell death in primary human hepatocytes. Toxicol Appl Pharmacol. 279(3):266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, McGill MR, Du K, Dorko K, Kumer SC, Schmitt TM, Ding WX, Jaeschke H. 2015. Mitochondrial protein adducts formation and mitochondrial dysfunction during N-acetyl-m-aminophenol (AMAP)-induced hepatotoxicity in primary human hepatocytes. Toxicol Appl Pharmacol. 289(2):213–222. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Ramachandran A, Breckenridge DG, Liles JT, Lebofsky M, Farhood A, Jaeschke H. 2015. Inhibitor of apoptosis signal-regulating kinase 1 protects against acetaminophen-induced liver injury. Toxicol Appl Pharmacol. 286(1):1–9. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H 1990. Antagonism of acetaminophen-induced hepatocellular destruction by trifluoperazine in mice. Pharmacol Toxicol. 67(2):115–119. [DOI] [PubMed] [Google Scholar]
- Yip L, Heard K. 2016. Potential adjunct treatment for high-risk acetaminophen overdose. Clin Toxicol (Phila). 54(5):459. eng. [DOI] [PubMed] [Google Scholar]
- Zaher H, Buters JT, Ward JM, Bruno MK, Lucas AM, Stern ST, Cohen SD, Gonzalez FJ. 1998. Protection against acetaminophen toxicity in CYP1A2 and CYP2E1 double-null mice. Toxicol Appl Pharmacol. 152(1):193–199. eng. [DOI] [PubMed] [Google Scholar]
- Zell-Kanter M, Coleman P, Whiteley PM, Leikin JB. 2013. A gargantuan acetaminophen level in an acidemic patient treated solely with intravenous N-acetylcysteine. Am J Ther. 20(1):104–106. eng. [DOI] [PubMed] [Google Scholar]
- Zhang YF, He W, Zhang C, Liu XJ, Lu Y, Wang H, Zhang ZH, Chen X, Xu DX. 2014. Role of receptor interacting protein (RIP)1 on apoptosis-inducing factor-mediated necroptosis during acetaminophen-evoked acute liver failure in mice. Toxicol Lett. 225(3):445–453. eng. [DOI] [PubMed] [Google Scholar]
- Zhao P, Slattery JT. 2002. Effects of ethanol dose and ethanol withdrawal on rat liver mitochondrial glutathione: Implication of potentiated acetaminophen toxicity in alcoholics [Article]. Drug Metab Dispos. 30(12):1413–1417. English. [DOI] [PubMed] [Google Scholar]
- Zimmerman HJ, Maddrey WC. 1995. Acetaminophen (paracetamol) hepatotoxicity with regular intake of alcohol: analysis of instances of therapeutic misadventure [published erratum appears in Hepatology 1995 Dec;22(6):1898]. Hepatology. 22(3):767–773. [PubMed] [Google Scholar]