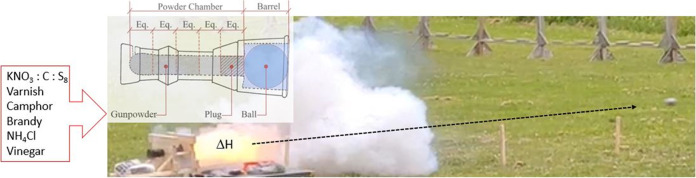
Abstract
Medieval gunpowder recipes of potassium nitrate (KNO3), charcoal (C), and sulfur (S8) were investigated by bomb calorimetry to determine their enthalpies of combustion and by differential scanning calorimetry (DSC) to determine their pre-ignition and propagative ignition enthalpies. Various sample preparation methods and several additional ingredients were also tested to determine any effects on the thermodynamic values. Gunpowder recipes were prepared and used in a replica cannon that was manufactured and operated according to medieval records. Post-firing residues were collected from the bomb calorimeter and the cannon in efforts to further characterize recipe energetics using DSC. In general, during the period of 1338–1400, the %KNO3 increased, and heats of combustion decreased, while between 1400 and 1460, the %KNO3 decreased, and heats of combustion increased. However, since KNO3 was usually found in the post-bomb calorimetry and post-cannon firing residues, it was not the limiting reactant. The highest pre-ignition and propagative ignition energies occurred when the KNO3:S8 ratio was 3:1 as determined by DSC, and the highest enthalpies of combustion were measured for recipes where the KNO3:C ratio was 1:1 as determined by bomb calorimetry.
Introduction
Gunpowder, also known as black powder, is only humanity’s second great experiment (after fire) with harnessing chemical energy, so naturally, it has long attracted the attention of both historians and chemists. Historians have focused on what it could do and what impact it had on society, while chemists have worked to explain the science and the molecular-level interactions that produced its practical effects. Black powder is a combination of potassium nitrate (also called saltpeter), sulfur, and charcoal (which will be represented by “C”) and is used today primarily in historical weapons, fireworks, and pyrotechnics. Modern composition ratios are typically 75:10:15 (KNO3:S8:C). Medieval recipes were developed by trial and error of varying composition ratios and sometimes included interesting additives that modern historians and chemists have generally found puzzling or presumed to be worthless.1,2 The purpose of this study is to analyze gunpowder recipes to aid historians in their interpretation of medieval texts and to determine whether there was intent in the creation of these recipes by master gunners. Additionally, understanding the energetics of the recipes provides important technical information on the early manufacturing of gunpowder.
It is clear that medieval master gunners had developed, at least in some respect, a solid practical understanding of the variables that affected the effective power output obtainable from gunpowder charges, including the purity of ingredients, varieties of charcoal, grain size, and methods of mixing.3 They understood, for example, that a cannonball was thrown by gas pressure, not flame, and that willow charcoal prepared in a closed container was far superior to oak charcoal made in a traditional pit. Nonetheless, it seems from records of recipes used at different times that progress toward the “ideal” ratio was slow and indeed often retrograde.4 This could be due to physical changes in artillery occurring at the same time.
Figure 1A illustrates the changes made to the sizes of the guns, the shot, and the powder charge used during the period of 1341 to 1450, showing how the largest-recorded artillery pieces rapidly grew more powerful over time.5 Recent historical work has reinterpreted some of the small number of known 14th century recipes and brought to light some additional recipes, giving a better picture of change over time in the formulation of the powders used in these guns. Figure 1B shows the mass percentages of KNO3 (green), S8 (yellow), and C (black) of various recipes used during the same period of time. Clearly earlier on was a time of greater fluctuation in recipe ratios; during mid-1300s to early 1400s, KNO3:S8 varied from 2:1 all the way to 16:1, while the KNO3:C ratios varied from 1:1 to 8:1. By 1900, a mass percent ratio of 75:10:15 (KNO3:S8:C) became the standard that continues today. Except for a single outlier, medieval recipes were generally lower in saltpeter and higher in sulfur than the modern formulation.
Figure 1.
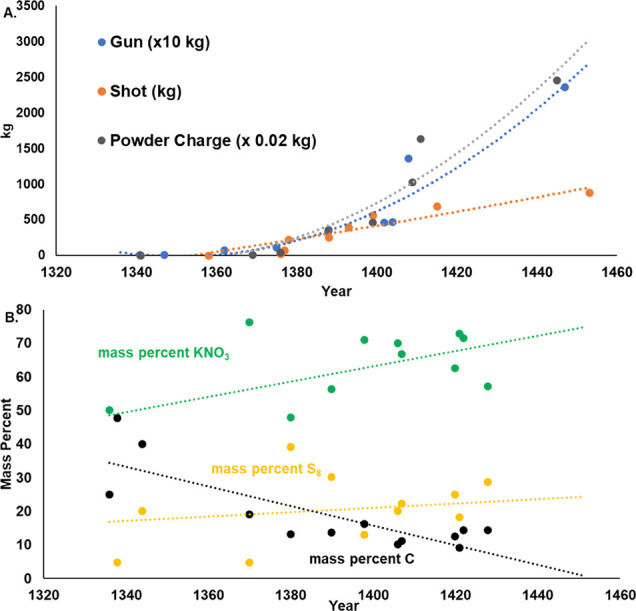
(A) Medieval gun, shot, and powder charge masses; (B) gunpowder KNO3, S8, and C mass percentages.
Thermodynamic studies of black powder have been conducted to experimentally examine the dynamic interactions involved in the processes of pre-ignition, ignition, and propagative reactions.6−8 These studies were performed on modern gunpowder composition ratios yet made it possible to see that some medieval practices, such as the use of a void space in the powder chamber to allow the creation of a high-pressure environment at an early stage of the combustion, made sense scientifically. That raised the question of whether some other seemingly useless or even “backward” choices of the master gunners, such as the use of mixtures with relatively low saltpeter:sulfur ratios, might have been equally sensible.
For this study, a sample set (Table 1) of different medieval recipes, ranging from the earliest known (1336) to a group from circa 1420, was examined by performing thermodynamic evaluations of pure oxygen combustion, via bomb calorimetry, as well as of pre-combustion energies via differential scanning calorimetry (DSC) in an inert nitrogen atmosphere. The intent of the bomb calorimetry data is to provide a theoretical value for the amount of energy available from “perfect” combustion of each black powder recipe as well as the relative rates of combustion. The intent of the DSC data is to study the pre-combustion steps (pre-ignition, propagative ignition, and propagative combustion) to better understand how the individual components of the recipes affect the energy output (example can be seen in Figure S3).6,7
Table 1. Gunpowder Recipe Ingredient Ratios and Additives.
| recipe |
ratios
by weight |
additive | ||||
|---|---|---|---|---|---|---|
| dates | designation | KNO3 | S8 | C | ingredients | |
| 1380–1395 | group 1 | A | 3.67 | 3 | 1 | |
| 1389–1405 | B | 4.15 | 2.22 | 1 | ||
| 1405 | C | 4 | 2 | 1 | ||
| 1420–1429 | C-i | 4 | 2 | 1 | vinegar | |
| 1336 | D | 2 | 1 | 1 | ||
| (1338–1350) | Ea | 2 | 1 | 2 | ||
| 1338–1350 | E-i | 2 | 1 | 2 | varnish | |
| 1420–1429 | group 2 | A | 5 | 2 | 1 | |
| (1420–1429) | Ba | 6 | 2 | 1 | ||
| 1420–1429 | B-i | 6 | 2 | 1 | vinegar | |
| (1400–1411) | Ca | 7 | 2 | 1 | ||
| 1400–1411 | C-i | 7 | 2 | 1 | brandy | |
| 1420–1449 | group 3 | A | 8 | 2 | 1 | |
| 1405 | A-i | 8 | 2 | 1 | water | |
| 1400–1411 | A-ii | 8 | 2 | 1 | brandy, NH4Cl, and camphor | |
| 1400 | B | 5 | 1 | 1 | ||
| 1420–1429 | B-i | 5 | 1 | 1 | camphor and quicklime | |
| 1390–1410 | group 4 | A | 22 | 4 | 5 | |
| 1900 | Ba | 15 | 2 | 3 | ||
| 1900 | B-ia | 15 | 2 | 3 | water | |
| 1338–1350 | Cb | 10 | 1 | 10 | ||
| 1338–1350 | C-ib | 10 | 1 | 10 | varnish | |
| 1370–1389 | D | 16 | 1 | 4 | ||
Control recipes.
Confirmed as not actual medieval recipes.
In addition to using DSC to study the various gunpowder recipes, it was also used to evaluate the residues left behind from the oxygen combustion reactions in the bomb calorimeter. The goal was to see if complete combustion occurred or if any starting material from the recipe could be considered to be in excess. Finally, a few recipes were scaled up and tested at a West Point firing range using a replica of a short-barreled Steinbüchse stone-throwing gun of circa 1400, with internal dimensions closely matching a gun (Inventory Number H10688) in the collection of the Bernisches Historisches Museum. Of those, post-blast residues were collected from the mouth of the gun for evaluation by DSC, in recognition that combustion of the powder might be significantly different in the field than in the oxygen-saturated environment of the bomb calorimeter.
To provide insight into the development of gunpowder technology in its crucial first century of use in European artillery, the researchers began with the earliest known recipes (1336 and 1338 to ca. 1350) and identified for analysis a series of well-documented recipes culminating with the set of basic formulations, identified as “common”, “better”, and “still better”, contained within the German Feuerwerkbuch (FWB) manuscripts, which probably date back to around 1420, although the earliest dated manuscript is from 1429.9 Some compositions were prepared both dry-mixed (as the early recipes call for) and, like modern gunpowder, wet-mixed. The technique of mixing the three main ingredients along with some liquid (the most common being water, vinegar, or brandy) was introduced probably in the late 14th century; the first text to describe it was likely composed around 1400, although the earliest extant manuscript copy is dated 1411. According to various medieval texts, powder mixed in this fashion was supposed to be substantially more powerful than dry-mixed powder, and the researchers wanted to see if this claim was borne out by testing.4
Materials and Methods
Laboratory Testing
Samples were prepared using mass ratios where the three main components (potassium nitrate, sulfur, and charcoal) were combined in a mortar and crushed for uniformity. Approximately 0.5 g of the mixtures was used for bomb calorimetry as a serpentine (dry-mixed) powder, as a moistened, then dried, and crumbled “corned” sample, or as a moistened and pressed pellet. Using a Parr pellet press, about 0.5 g of serpentine gunpowder and 100 μL of deionized water were combined, pressed three times, and allowed to air dry at room temperature for a minimum of 48 h before being tested in the bomb calorimeter.
Mixed hardwood air float charcoal with a 99.5% purity was purchased from the Skylighter company, KNO3 was from Carolina (ACS Grade), and sulfur was from Ward Science (lab grade). All reagents were used without further purification. Table 1 indicates the specific mass percent ratios of the ingredients and the years when those recipes were recorded. The group # increases from 1 to 4, indicating a binning of the amount of oxidant (KNO3) with respect to sulfur from approximately 1:1 up to 16:1. The letters used within the groups indicate specific increases in the KNO3 mass percentage relative to S8 and/or C. Recipes with additional ingredients are indicated with lowercase Roman numerals.
Field Testing
Commercial grade KNO3 with a purity of 99.8% and sulfur with a purity of 99.5% were obtained from the company Seed Ranch, an agricultural supplier. Charcoal for the testing was mixed hardwood airfloat charcoal with a 99.5% purity acquired from the Skylighter company. NH4Cl was of ACS grade from Fisher Scientific. The brandy used was Paul Masson 40% alcohol Grande Amber. The vinegar was Heinz all-natural distilled vinegar with a 5% acidity; the varnish used was Zinsser’s Bulls Eye Shellac. The camphor was ordered from Aspi-Care.
Preparation of Cannon Samples
Potassium nitrate, sulfur, and charcoal were combined in a plastic container and mixed before the addition of the minor additives that were called for in some recipes (if needed). Specific mixtures were then either left dry or wetted with vinegar, water, or brandy. Cakes of gunpowder were produced and allowed to dry in the hood for at least 48 h and then crushed through a 2 mm sieve. This produced a coarse-grained powder with grains no larger than 2 mm. This coarse powder (a rough form of “corned” powder) was then stored in its corresponding plastic container and allowed to continue drying for at least 48 h before being sealed for transport the day of the range tests.
Range Tests
The cannon used was a reproduction Steinbüchse (stone-throwing cannon) copied in most respects from an extant gun (Bernisches Historisches Museum, Inv. No. 10688) that dates to the turn of the 15th century. For safety, the replica gun was milled from a solid steel billet, with thicker walls than the original and with a rounded floor of the barrel, but none of those changes should have significantly affected the shots. In accordance with medieval gunnery procedures, approximately 200 g of gunpowder (one-ninth the mass of the cannonballs used) was poured down the barrel of the gun into the narrower powder chamber at the back and then tamped into the rear 3/5 of the chamber using a dowel and a rubber mallet. A wooden plug one-fifth the length of the powder chamber was inserted and hammered into place and flushed with the edge of the mouth of the powder chamber. Next, a 4 in.-diameter marble cannonball was placed on top of the plug and wedged in place by hammering in two hardwood shims. Finally, the touch hole was primed with priming powder of medieval specifications, and an electronic ignition system ignitor was emplaced.
Results and Discussion
Bomb Calorimetry: Heats of Combustion and Reaction Rates
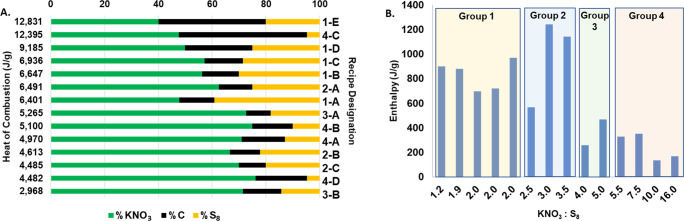
The average thermodynamic potential, or heat of combustion (J/g), was calculated for each recipe using eq S2, and a minimum of three trials was averaged for each recipe. Table S1 shows the average heat of combustion for each recipe, and the recipes are ranked in order of highest to lowest heat of combustion based on the serpentine form of each recipe.
Figure 2 shows the thermodynamic potential of the gunpowder recipes in chronological order. The earliest recipe (1-D) dates back to 1336, while the latest medieval recipe tested (1-C) was used from 1420 to 1460. Recipes 4-B and 4-B-i are variations of the modern gunpowder recipe that is utilized today. Recipe 4-C is an alternative interpretation of the same medieval German text as 1-E. It is hypothesized that 4-C was the incorrect interpretation and 1-E was the correct reading. Testing both in the bomb calorimeter and comparing the two results did not show a large difference between the two, but (as will be discussed below) the DSC tests seem to validate that hypothesis.
Figure 2.
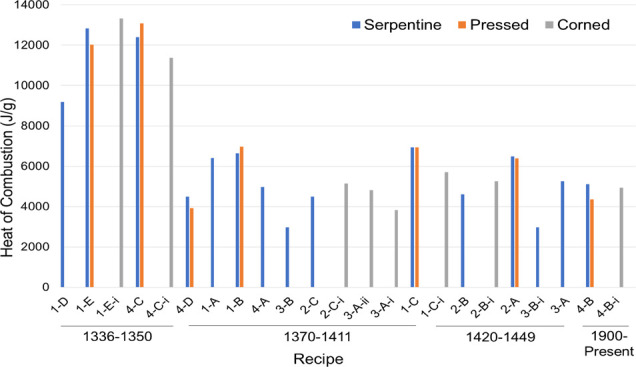
Heats of combustion for each recipe in chronological order.
It has been suggested that one reason gunpowder recipes changed over time is the need for safer recipes that did not put medieval gunners at risk or cause damage to cannons.4 This idea is supported by the fact that the two oldest recipes, 1-D and 1-E, had two of the highest heats of combustion. Gunners may have stopped using these recipes because they had such high levels of thermodynamic activity. Although the modern gunpowder recipe heat of combustion was less than half of that of 1-D, it is likely that such a high level of potential is no longer needed due to advances in weaponry and understanding of the mechanics of artillery.
When analyzing the relative reaction rates (Table S1 and Figure S1a–c), some recipes (no matter how they were prepared) tended to have more temperature fluctuations than others. Recipes 1-E, 1-B, and 4-D are examples of recipes with high, medium, and low temperature fluctuations, respectively. The low (0–0.05 °C), medium (0.051–0.125 °C), or high (≥0.126 °C) fluctuation ranking, in Table S1, is based on the average of the largest peak-to-peak temperature spike in each bomb calorimetry experiment within the first 100 s after ignition.
Trends were observed for the heats of combustion and relative reaction rates. As the KNO3:C ratio increased, these physical properties decreased. For example, the closer this ratio (i.e., 1:1 or 2:1), the higher the heat of combustion and rate of reaction.
Differential Scanning Calorimetry: Enthalpies of Pre-combustion Events
Analysis of the gunpowder recipes using DSC allowed for the observation of pre-ignition, propagative ignition, and propagative combustion. Figure S3 shows a typical first heat curve.6,7
The individual components (charcoal, sulfur, and KNO3) were first analyzed under the same temperature ramps to serve as controls (Figure S4a,b). As reported in the literature, sulfur and KNO3 exhibited melting points at 119.32 and 339.17 °C, respectively. The temperature ramp also allowed the visualization of KNO3’s rhombic-to-trigonal transition in the first heating cycle (134.92 °C). Upon analyzing the second heating cycle (post cooling cycling), two distinct peaks appeared, which likely captured the transformation between two known rhombic forms (131.36 and 132.91 °C).15
When comparing the serpentine recipes, many of the first heat curves aligned with the stepwise process for ignition as described by Campbell and Weingarten.7 Pre-ignition for the medieval samples started around 232 °C with a gradual increase in the heat flow until a rapid increase in the heat flow, which occurred around 330 °C, which corresponds to the melting point of KNO3, culminating with the ignition of the mixture, often represented by another large exotherm (see Figure S5a,b for an example). The onset of pre-ignition for the modern recipe deviated from the medieval recipes in its onset temperature being almost 12 °C higher (247 °C). Depending on the recipe ratio of the mixture, the ignition step post KNO3 melting was sometimes smaller than the first large exotherm or even non-existent. The mechanics of igniting a gun with a hot iron placed in the touch hole (the medieval method) offer limited opportunity for heat transfer, so the lower pre-ignition temperature characteristic of medieval powders (likely resulting from higher sulfur content due to its low melting point, which facilitates better mixing between components) may have been practically advantageous and may be a possible explanation for this difference seen in the ignition temperatures.
Effect of Gunpowder Recipe Composition (Serpentine)
Changes in gunpowder composition affected the thermodynamic potential determined by bomb calorimetry as well as the enthalpies of pre-ignition and propagative ignition obtained with DSC. Heats of combustion for various serpentine samples determined by bomb calorimetry are shown in Figure 3A as a function of the three gunpowder components. Figure 3B shows the enthalpies determined by the DSC data. The DSC data is shown as a ratio of KNO3:S8 because complete combustion (oxidation of the charcoal) is not observed at the temperatures available in the instrument.
Figure 3.
Effect of serpentine recipe compositions on (A) bomb calorimetry heats of combustion and (B) DSC enthalpies from the first heat cycle.
The bomb calorimetry data shows that increasing the percent of charcoal leads to higher heats of combustion. The two highest values are from recipes 1-E and 4-C, where KNO3:C ratios are 1:1. As the sulfur content goes from approximately 20% to 5% (from 1-E to 4-C), the heat of combustion decreases by about 3.5%. This shows that even with complete combustion of the charcoal (the fuel of the recipe) in the oxygen-rich environment, sulfur still plays a role, especially in its molten state where it is known to lower the activation energy of the combustion.11 The role of KNO3 is harder to define in an oxygen-rich environment. It is evident that as KNO3 is increased to greater than 60% of the recipe mixture, the heat of combustion decreases by about 50%. This decrease is likely due to the decrease in the amount of charcoal to 20% and lower. Later in the article, results will be presented where DSC was used to evaluate the residues from bomb calorimetry to study the efficiency of the combustion of the various recipes.
While the bomb calorimeter was used to determine the overall thermodynamic potential, DSC provides information in an inert environment prior to combustion. Enthalpy was calculated using the pre-ignition and propagative ignition exothermic events. As seen in Figure 3B, groups 1 and 2 have the highest enthalpy values with a maximum at the 3:1 ratio (KNO3:S8) from recipe 2-B, which was used circa 1405–1460. This recipe was considered “best” by the medieval author of the FWB, and as he thought, higher saltpeter content, at least up to a point, produced more energy for ignition.9Figure 3B presents data that supports the medieval author’s conclusion and determines that point. Recipes in groups 3 and 4 with higher KNO3:S8 ratios clearly yielded smaller enthalpies during pre-ignition. In fact, as the ratio changes from 3:1 to 3.5:1, there is an 8% decrease in enthalpy. Then, when the ratio goes to 4:1 (recipe 3-A), there is almost an 80% decrease when compared to 3:1.
Figure 4 shows the DSC curves for recipes 2-B and 3-A. The 80% decrease in enthalpy measured for 3-A is seen in the much smaller exothermic rise during pre-ignition, and there is only one peak during propagative ignition. Going from the 2-B recipe to the 3-A recipe, there is a 6% increase in KNO3, a 4% decrease in S8, and a 2% decrease in charcoal. The decrease in organic material (charcoal), which is normally oxidized via reaction between solid components during pre-ignition12 (280–300 °C), yields less exothermic energy available for the subsequent reactions. The exothermic peak prior to 339 °C is likely due to the oxidation of sulfur, which starts to vaporize at 200 °C and is usually oxidized by molten KNO3 during propagative ignition.13 The result is that the smaller amount of sulfur is consumed prior to the fusion of KNO3 at 339 °C, thus leaving no reactant for the molten KNO3 to oxidize since the high temperatures to oxidize the charcoal are not achieved in this DSC.
Figure 4.
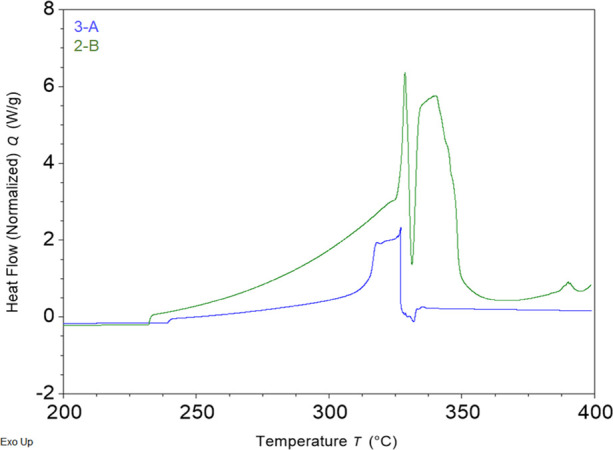
First heat DSC overlays for recipes 2B and 3A.
A similar behavior was observed for samples 4-C and 4-D, which correspond to 10:1 and 16:1 KNO3:S8, respectively. When looking at the DSC curves, the heat flow profiles for these samples did not exhibit any large exotherms including the one generally seen near the melting point of KNO3 in the other samples (Figure S5a,b). Instead, after the initial pre-ignition broad exotherm, there was a broad endotherm and then a slow increase in heat flow that could correspond to a propagative combustion, but a maximum was not achieved within the same temperature range as the other samples.
Comparing Sample Preparation Methods (Serpentine and Pressed)
Examining Table S1 and Figure 2 (and Figure S2), it can be seen that in general, when analyzed by bomb calorimetry, most samples that were pressed yielded slightly lower thermodynamic potential than the serpentine values. This could be accounted for, in part, by the lack of oxygen permeation to the entire combustible materials due to the lack of spacing between the particles in the pressed sample. Incomplete combustion would be the result and would explain the smaller enthalpies observed for the pressed samples. Additionally, in the pressed samples, there is less space for any hot particles to propagate combustion to unignited regions of the pellet. Williams and Brown et al. saw this while studying the impact of porosity on flame spread through black powder.11,10 While DSC was not used to evaluate pressed samples, it was used to analyze the residues from the bomb calorimeter. This data will be discussed later and can provide further evidence for incomplete combustion of the pressed samples.
The relative rates of combustion for the two sample preparations also provide an interesting comparison. Typically, the serpentine and pressed samples do not have statistically different relative rates of combustion (Table S1). The exceptions are the 1-E and 4-C recipes, which both show that the serpentine sample combustion rates are approximately 1.2 times that of the pressed sample rates of combustion. These recipes have the highest relative ratio of charcoal, the highest fluctuation in temperatures during combustion, and the highest overall heats of combustion. The relatively large amounts of charcoal present in these recipes not only provide the fuel for the reaction, but also, the significantly higher surface area would allow for more combustion product gases to form and to escape, which accelerates the reaction.11
Comparing Historically Significant Recipes with and without Additives
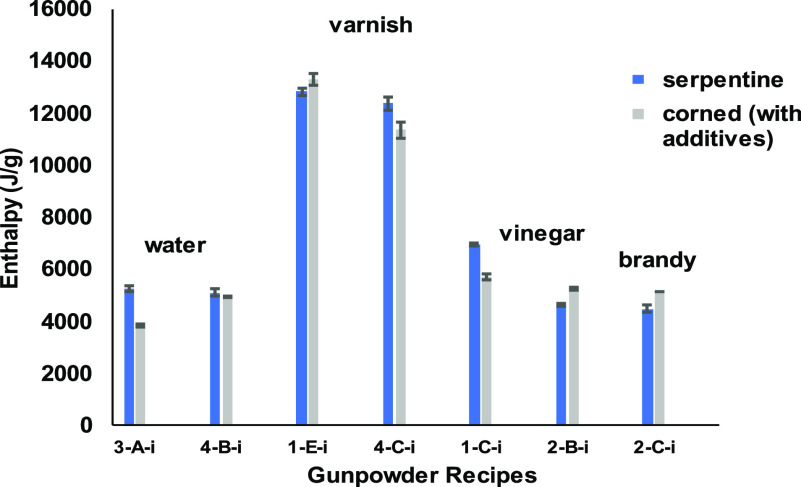
Several medieval recipes that were tested included additional ingredients beyond potassium nitrate (saltpeter), sulfur, and charcoal (Table 1). To determine whether the additives contributed significantly to the thermodynamic potential, the heats of combustions and DSC enthalpies of these recipes were compared to serpentine control recipes made of the same primary component ratios but without additives. The first additives to be discussed are the ones used during the corning step of sample preparation: water, varnish, vinegar, and brandy. Wet-mixing bound the saltpeter and sulfur into the fibrous structure of the charcoal, creating grains that were less prone to absorption of atmospheric moisture and that kept the ingredients in close contact during an explosion.16,17 This study evaluated the effects of wet-mixing from an energetic perspective.
Water
Figure 5 shows the heats of combustion (from bomb calorimetry) of various recipes in their serpentine form and corned with the indicated additive. Corning with water caused the 8:2:1 (3-A) recipe to have a significantly lower heat of combustion; however, it did not significantly lower the value of the 15:2:3 (4-B) recipe. These results would suggest that the impact of using water for corning is very specific to the ratio of the recipe components. When the amount of KNO3 is lower, its dissolution in the added water may assist in distributing it throughout the mixture, but it lowers the thermodynamic potential. This could be due to non-uniform distribution of salt as water dries, and thus, incomplete reaction occurs.11 When a higher ratio of KNO3 is corned with water, there is no realized benefit or detriment to the thermodynamic potential. The higher ratio of KNO3 likely produces a more uniform distribution of salt as water dries, yielding a more complete reaction similar to that of the serpentine mix.
Figure 5.
Comparison of combustion enthalpies when various corning additives are used.
The results from DSC show an increase of approximately 51% when the 3-A recipe is corned with water (3-A-i) and an insignificant decrease for 4-B when corned with water (4-B-i). This confirms that the impact of adding water is specific to the recipe. The same sample that showed a decrease in the heat of combustion in the bomb calorimetry data shows an increase in enthalpy from the DSC data. This is likely due to the slower rate of heating and smaller sample size used during the DSC event, thus allowing for the more even distribution of KNO3 in 3-A-i compared to the rapid event that occurs during combustion in the oxygen-rich bomb calorimeter. Recipe 4-B-i (which is modern gunpowder) does not show a significant impact of added water when using either bomb calorimetry or DSC. If the historic purpose of the water added during corning was to form incorporated grains of powder that were less prone to spoilage, then the variability in energy output could have been acceptable due to this gain in stability.
Varnish
Although varnish is not a common ingredient in medieval cannon-powder recipes, it is a component of the second-oldest formulation (1-E). This is not surprising because it was often included in recipes for incendiaries (“Greek fire)” or fireworks.1 Since it was not included in later recipes, it was not expected to be highly beneficial. The bomb calorimetry data reveals that adding varnish causes an increase in the measured enthalpy of combustion of the 2:1:2 (1-E-i) mix yet a statistically significant decrease in the measured energy of the 10:1:10 (4-C-i) mix. As mentioned earlier, the ratios in recipe 4-C reflect a likely misinterpretation of the text for 1-E by previous scholars; however, from a chemist’s perspective, it is interesting to note that the addition of varnish to a recipe with high charcoal content and relatively low sulfur content actually decreases the potential energy measured by the bomb calorimeter. This could be due to the varnish causing clumping of the ingredients and preventing the small amount of sulfur from thoroughly mixing and/or diminishing the surface area of the charcoal. This would be less noticeable in the 2:1:2 recipe as the relative amounts of active ingredients are much closer. Future studies using SEM and porosimetry will help confirm the particle distribution and surface area differences between the various recipes. If medieval gunners added varnish as a means of increasing the available energy of their recipe, then recipe 1-E confirms that notion, at least in an oxygen-rich environment like the bomb calorimeter.
It is interesting to consider the relative rates of reaction for 1-E-i and the serpentine and pressed forms of the 1-E recipes, because medieval gunners believed that varnish was an accelerant.1 The reaction rate for the 1-E-i recipe is significantly higher than that of the pressed 1-E but not significantly higher than the serpentine recipe. This observation is more likely due to the difference in preparation (pressed versus corned) where propagation of combustion in the pressed sample is hampered by lack of space between the reagents.
Enthalpies determined from DSC curves result in insignificant changes when varnish is added. When the heat cycles for the recipes with and without varnish were overlaid, they demonstrated nearly identical pre-ignition profiles, as seen in Figure 6. Given these two examples, it seems as if the varnish did not provide any detectable benefits as seen by DSC.
Figure 6.
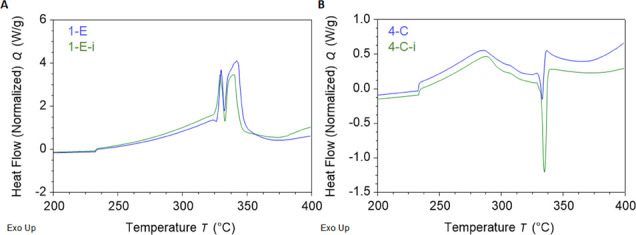
First heat DSC overlays of (A) 1-E and (B) 4-C recipes without varnish and the same recipes with varnish (1-E-i and 4-C-i).
Brandy
The addition of brandy as the corning agent to recipe 2-C (7:2:1) did not show a statistically significant increase in the heat of combustion when compared to the serpentine recipe. It is assumed that brandy was used to “quench fresh charcoal” and to provide any missing organic compounds for better quality burning.3 The bomb calorimetry results do not verify this; however, the quality of the charcoal used in this experiment was already high and so it did not require additional organics as a lower quality charcoal might need. This same recipe with brandy (2-C-i) did yield a substantially lower enthalpy of pre-ignition and propagative ignition via DSC (Figure 7A). It appears that the pre-ignition heat flow is reduced by the presence of the brandy, potentially saving the exothermic energy for future thermodynamic events as seen by the rapid increase in heat flow around 390 °C.
Figure 7.

First heat DSC overlays indicating effects of adding (A) brandy to recipe 2-C and adding (B) vinegar to recipe 2-B.
Vinegar
Vinegar was used by gunners to recrystallize the KNO3 and to enhance mixing of the dry ingredients so that they do not separate during transport (as stated earlier). Recipes that were corned with vinegar, 1-C-i and 2-B-i, yielded variable results, similar to the addition of water. The 1-C-i recipe yielded a statistically significant lower enthalpy with vinegar, whereas 2-B-i yielded a higher (yet not statistically significant) enthalpy value than 2-B. The DSC results yield an insignificant increase in enthalpy for the 1-C-i recipe compared to 1-C and a substantially lower (approximately 50%) enthalpy for 2-B-i when compared to 2-B. Looking at Figure 7B, it is apparent that both the pre-ignition and propagative ignition parts of the curves have different shapes, overall heat flow, and a shift toward lower temperatures for the events in 2-B-i. These effects could be due to either the pH shift caused by the vinegar or to the recrystallization of KNO3 into a different form. Further studies are required to determine if these hypotheses are correct.
Solid Additives
Bomb Calorimetry Results
In addition to the liquids added during the corning process, medieval gunners sometimes added ammonium chloride, camphor, and quicklime. A Student’s t-test was performed to determine whether there was a statistically significant difference between the medieval recipes with these additives and their control recipes. Of the recipes tested, none with these additives showed a statistically significant increase in thermodynamic potential compared to the serpentine control recipes.
However, there was a statistically significant increase in potential when the 3-A recipe was mixed with camphor, ammonium chloride, and salpractium (a mixture of additional KNO3 and ammonium chloride) and then corned with brandy (3-A-ii) when compared to the same recipe with no additives that was corned with water (3-A-i).
Thus, with respect to the thermodynamic potential, medieval master gunners apparently did not, on average, achieve a gain in power by employing additives in their gunpowder recipes. However, these results show only the potential energy of the recipes combusted in a pure oxygen environment. In an actual air environment, the additives may have contributed more to the overall energy of the gunpowder reaction; thus, the DSC results can be used to further understand whether there was any energetic benefit with these additives.
DSC Results with Camphor and Ammonium Chloride
Camphor in all recipes tested was used in conjunction with another additive. The first recipe evaluated used camphor and ammonium chloride (NH4Cl). Like varnish, camphor was a common ingredient for incendiary mixtures; unlike varnish, camphor appeared in multiple recipes and was continued to be used into the 15th century and even the 16th century. One 15th century text notes that it strengthens all powders when it is added.14 Indeed, as late as 1917, John Buxbaum filed a U.S. patent for mixing black gunpowder with spirits of camphor, presumably unaware that he was reinventing a medieval technique.18 In a gunner’s handbook from the turn of the 15th century, ammonium chloride is praised as a preservative: “it is good in powder that will be stored for a long time”.16
When comparing the difference in enthalpy upon addition of these components, 3-A-ii was 184.91 J/g higher than 3-A. This difference is higher than the instrument variability between two replicate runs of 3-A-ii (145.68 J/g), which indicates that this combination of additives did make the gunpowder stronger. When the two heat cycles were overlaid (Figure 8A), there was a difference in the profiles. Recall that recipe 3-A is 8:2:1 and has a much lower exothermic pre-ignition event than recipes from groups 1 and 2. It appears that adding camphor and NH4Cl and corning with brandy yield a lower temperature for pre-ignition (by about 7 °C), a much larger exothermic event at 330 °C (propagative ignition), and an additional exothermic event starting at 375 °C, increasing beyond the temperatures available in the DSC used for this study.
Figure 8.
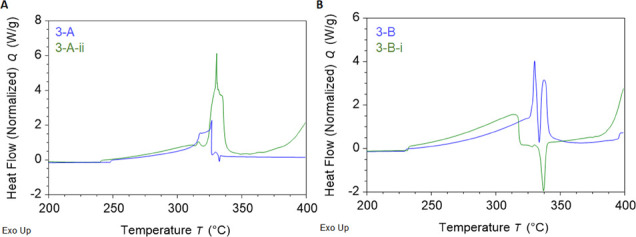
First heat DSC overlays indicating effects of adding (A) camphor and ammonium chloride to recipe 3-A and adding (B) camphor and quicklime to recipe 3-B.
DSC Results with Camphor and Quicklime
Camphor and ammonium chloride, as seen with 3-A-ii and 3-A, demonstrated an influence on not only the exothermic enthalpy seen during pre-ignition and propagative ignition but also on the heating profile. Figure 8B shows the DSC curves for 3-B and 3-B-i (a 5:1:1 ratio where 3-B-i was the formulation that included the camphor and quicklime additives). A recipe incorporating quicklime and camphor was included in the FWB as a special formulation that would ignite on contact with water (calcium carbonate and water reacting in a highly exothermic way).9 Although the nature of the DSC test made it not very useful in assessing that historical purpose, the results were interesting from a chemical perspective. When comparing the difference in enthalpy, 3-B was 56.77 J/g higher compared to 3-B-i. When the two heat cycles were overlaid, there was little to no similarity in the heat profiles in that 3-B-i demonstrated a more gradual climb to the peak exothermic temperature and then an endotherm at the KNO3 melting point with no further propagation of the ignition process. In the case of 3-B, there was a sharp increase right before the KNO3 melting likely due to the melting of a few crystals, an endotherm for the bulk melt, and then another exothermic event. Given this, it appears that whatever benefits gained from camphor and NH4Cl are not seen with camphor and quicklime as measured by DSC.
Residue Analysis Determined by DSC
As mentioned earlier, DSC was used not only to gather data on the pre-burned gunpowder recipes but also to evaluate the residues left behind in the bomb calorimeter and after firing the 14th century replica cannon. This was done primarily to evaluate whether the gunpowder recipes underwent complete combustion efficiently and, if there was any leftover starting material, to evaluate which of the three main ingredients were in excess. An efficient combustion would be one that does not have any (or has very little) starting material left over. Additionally, this analysis will help determine how the laboratory data can be used to predict gunpowder performance in a cannon. The general temperature range near the KNO3 melting was analyzed for either exo- or endothermic event enthalpies. An exothermic peak seems to indicate that there was a sufficient ratio of components left over to have another pre-ignition/propagative ignition, whereas an endothermic peak seems to indicate that the remaining components were not in a ratio suitable for starting the pre-ignition.
Bomb Calorimetry Residue
When analyzing the bomb calorimetry residues and generating Table 2, it was apparent that all residues that exhibited exothermic peaks were clearly pre-ignition and occurred before the endothermic KNO3 molten state, indicating that these recipes were inefficient. Additionally, every sample (with the exception of 1-D and 2-B) had an endotherm present for the melting of KNO3, indicating that there was always leftover KNO3 or it was never the limiting reactant. The only trend that was present was the fact that groups 3 and 4 only had endotherms present. Other than these observations, there is not a clear trend in terms of which recipe ratios yielded residues with exotherms present.
Table 2. Summary of DSC Data from Bomb Calorimetry Residue Analysis.
| recipe | exotherm presenta | enthalpy exo (J/g) | endotherm presentb | enthalpy endo (J/g) |
|---|---|---|---|---|
| 1-A | yes | 43.51 | yes | 3.61 |
| 1-B | no | yes | 19.99 | |
| 1-C | no | yes | 15.98 | |
| 1-D | yes | 64.88 | no | |
| 1-E | yes | 51.19 | yes | 1.67 |
| 1-E-i | yes | 33.56 | yes | 6.03 |
| 2-A | no | yes | 16.12 | |
| 2-B | yes | 5.03 | no | |
| 2-C | yes | 24.25 | yes | 1.59 |
| 3-A | no | yes | 2.14 | |
| 3-A-i | no | yes | 4.55 | |
| 3-A-ii | no | yes | 3.55 | |
| 3-B | no | yes | 1.54 | |
| 4-A | no | yes | 2.63 | |
| 4-C | no | yes | 6.70 |
Pre-ignition.
KNO3 melting.
DSC data of the bomb calorimetry residue for recipe 4-C showed that the pellet residue had twice the endothermic enthalpy when compared to the serpentine residue (Table 3). The endothermic response indicates that there are still starting materials left over in both the pellet and serpentine samples. This is not surprising given the large ratio of KNO3 to sulfur, where sulfur would be the limiting reagent in the 4-C recipe. Looking at the residue from the pressed and serpentine samples of the 1-E recipe, similar exothermic responses were observed. The serpentine sample also has an endothermic peak, indicating that starting materials remain after combustion.
Table 3. DSC Data of Bomb Calorimetry Residues from Different Sample Preparations.
| sample | preparation method | enthalpy (J/g) | endo/exo |
|---|---|---|---|
| 1E | pellet | 42.05 | exo |
| serpentine | 47.03 | exo | |
| 2.36 | endo | ||
| 4C | pellet | 15.24 | endo |
| serpentine | 6.74 | endo |
Comparison of Residue Types
Table S2 shows data from four specific recipes tested in the replica cannon. The cannon residues, bomb calorimetry residues, and the DSC “residues” (second heats of the gunpowder recipes) are compared. When looking at 3-A-i and 3-A-ii, the samples both had a 4:1 KNO3:S8 ratio but presented an endotherm in the bomb residue and not in the cannon residue. The researchers hypothesize that since the bomb calorimeter is an oxygen-rich environment, it is possible that the amount of KNO3 needed in an atmospheric environment (i.e., in the cannon) is more than what is used in the bomb.
The DSC “residues” seemed to indicate the most “efficient” burn (i.e., whatever components that were left over were not in such a ratio as to undergo a second pre-ignition). This could be due to the controlled temperature ramp, which provides an opportunity for adequate sample mixing from the molten components. To evaluate formulation efficiency, despite the controlled heating (which is not present within the cannon), DSC is a better tool to use because it more closely resembles an atmospheric environment. That being said, formulation efficiency does not necessarily translate into greater kinetic energy being transferred to the cannonball. In both cases, the 3-A-i shot had a lower enthalpy for both bomb calorimetry and DSC, yet the cannonball speed was calculated as being close to double of what was seen from the 2-B-i shot. However, the field testing as a whole suggested that the difference might well have more to do with variations in the loading or the random variation of the early ignition than with the powder formulation. More field work must be conducted to evaluate which formulation would have performed the “best” in historical contexts.
Conclusions and Future Works
Figure 2 illustrates that the evolution of the adopted ratios of the three main ingredients of gunpowder seemed to have developed as a trial-and-error process. As time progressed (1340–1440), the gunners created recipe ratios that produced lower heats of combustion. Figure 9A indicates that prior to 1400 (before recipes were used as corned powders), there was an increase in the amount of KNO3, while S8 and C were decreased. During this time period, the %KNO3 increased (in general) by approximately 20% (from 50% to 70%), while the heat of combustion generally declined by approximately 50%, from 10 kJ/g to 5 kJ/g. This was likely due to the decrease in fuel (charcoal) and decrease in sulfur, which is now known to lower the activation energy of combustion.7
Figure 9.

(A) Pre- and (B) post-1400 ingredient ratios and their impact on heats of combustion.
After 1400, gunners began corning their recipes. Corning with water lowered the enthalpy of combustion in the bomb calorimeter, but the impact of vinegar, varnish, and brandy is inconclusive. In the DSC results, only the varnish produced a measurable difference in pre-ignition enthalpy and that was to decrease it. Other additives yield inconclusive results as well, with only certain combinations yielding increases in available energy.
In addition to corning the powders, the gunners adjusted their recipe compositions (Figure 9B) so that they decreased the mass percentage of KNO3 by about 15% while raising that of S8 by 10% and C by about 5%. This led to an increase in the measured heats of combustion from approximately 5 kJ/g up to 7 kJ/g. It is interesting that even in the oxygen-rich bomb calorimeter, the amount of the oxidizer, KNO3, made a difference in the potential energy available. Since saltpeter (KNO3) was the costliest of ingredients, decreasing its percentage would have been a desirable saving without sacrificing the thermodynamic potential. The optimal ratio of saltpeter to sulfur for maximum pre-ignition enthalpy was determined to be 3:1 in the serpentine (dry) formulation (Figure 3B). However, in the pure oxygen environment of the bomb calorimeter, it is the saltpeter-to-charcoal ratio that determined the maximum enthalpy and it is optimized at 1:1 (Figure 3A).
As mentioned earlier, future studies will include using porosimetry and SEM to compare the surface areas and spacing between the ingredients of the recipes to better understand the impact of corning. Additionally, since the DSC used for this study had an upper temperature limit of 400 °C, the researchers could not probe beyond that into the temperatures needed for combustion of the charcoal. Thus, future work will include using thermogravimetry with mass spectrometric detection to provide an upper temperature limit of 1000 °C and information that will help identify product gases formed during combustion.
Acknowledgments
We thank the Stevens Institute of Technology’s Pinnacle Scholars program for supporting K.E.R. We also thank the Omar N. Bradley Foundation for providing the funding to manufacture the cannon and supporting R.J.S. We also like to thank Julia Coon and Joshua Mooney for the preliminary method development for pellet preparation and bomb calorimetry analysis and Chloe F. Deutschman for contributing to the graphical abstract artwork.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c03380.
Instrumental methods for bomb calorimetry and DSC, along with additional data (PDF)
The opinions expressed herein are those of the authors and not necessarily representative of those of the Department of the Army, Department of Defense (DoD), or U.S. Government.
The authors declare no competing financial interest.
Supplementary Material
References
- Partington J. R.A History of Greek Fire and Gunpowder; 2nd. Ed. The Johns Hopkins University Press: 1999, 152, 155. [Google Scholar]
- Kramer G. W.The Firework Book: Gunpowder in Medieval Germany; Arms and Armour Society: 2001, 17, (1), . [Google Scholar]
- Smith G. Medieval Gunpowder Chemistry: A Commentary on the Firework Book. Icon 2015, 21, 147. [Google Scholar]
- Rogers C. J. The Military Revolutions of the Hundred Years’ War. J. Mil. History 1993, 57, 270–272. [Google Scholar]
- Adapted from; Rogers C. J.Gunpowder Artillery in Europe, 1326-1500: Innovation and Impact, Technology, Violence and War. Essays in Honor of John F. Guilmartin, Jr.; Brill: Leiden, 2019, 39–71. [Google Scholar]; Figure 2.1
- Blackwood J. D.; Bowden F. P. The Initiation, Burning and Thermal Decomposition of Gunpowder. Proc. R. Soc. London, Ser. A 1952, 213, 285. [Google Scholar]
- Campbell C.; Weingarten G. A Thermoanalytical Study of the Ignition and Combustion Reactions of Black Powder. Trans. Faraday Soc. 1959, 55, 2221. 10.1039/tf9595502221. [DOI] [Google Scholar]
- Turcotte R.; Fouchard R. C.; Turcotte A.-M.; Jones D. E. G. Thermal Analysis of Black Powder. J. Therm. Anal. Calorim. 2003, 73, 105. 10.1023/A:1025181424038. [DOI] [Google Scholar]
- Hassenstein W.Das Feuerwerkbuch von 1420; Verlag der Deutschen Technik: Munich, 1941. [Google Scholar]
- Rao C. N. R., Prakash B., Natarajan M.. Crystal Structure Transformation in Inorganic Nitrates, Nitrites, and Carbonates. In National Standards Reference Data System; 1975. https://nvlpubs.nist.gov/nistpubs/Legacy/NSRDS/nbsnsrds53.pdf (accessed 2021-04-26).
- Brown M. E.; Rugunanan R. A. A Temperature-Profile Study of the Combustion of Black Powder and its Constituent Binary Mixtures. Propellants, Explos., Pyrotech. 1989, 14, 69–75. 10.1002/prep.19890140205. [DOI] [Google Scholar]
- Husain G.; Rees G. J. Combustion of Black Powder Part1: Thermo-Analytical Studies. Propellants, Explos., Pyrotech. 1990, 15, 43–47. 10.1002/prep.19900150202. [DOI] [Google Scholar]
- Conkling J. A. Chemistry of Fireworks. Chem. Eng. News 1981, 29, 24–32. [Google Scholar]
- Williams F. A. Observations on Burning and Flame-Spread of Black Powder. AIAA J. 1976, 14, 637. 10.2514/3.7131. [DOI] [Google Scholar]
- Hall B. S.Weapons and Warfare in Renaissance Europe; The Johns Hopkins University Press, 1997. [Google Scholar]
- Guilmartin J. F.Ballistics in the Black Powder Era. In Brit. Naval Armaments, Royal Armories Conf. Proceeding , London 1989.
- Leng R.; ed. Anleitung Schieβpulver zu bereiten; Büchsen zu laden und zu beschieβen; Wiesbaden, 2000. [Google Scholar]
- Official Gazette of the United States Patent Office 259. Patent no. 1,293,326 (Feb. 1919), p. 82.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.