Abstract
Background:
Although the short-term neurological complications of Shigella spp. are well described, potential neuropsychiatric outcomes have not been studied yet. We investigated the association between early childhood shigellosis and subsequent ADHD.
Methods:
This is a retrospective population-based cohort. Using a large Health Maintenance Organization database, the prevalence of ADHD was investigated among children aged 5–18 years who underwent stool culture prior to the age of 3 years.
Results:
Of 52,761 children with a stool culture examined, 5,269 (9.98%) had Shigella-positive results. The rate of ADHD was 10.6% and 8.6% among children with Shigella-positive and Shigella-negative stool cultures, respectively (p < .001). Adjusted odds ratio for ADHD after controlling for gender and socioeconomic status was 1.21 (CI 1.13–1.29, p < .001). The younger the child was during Shigella gastroenteritis, the higher was the association with ADHD (p < .001).
Conclusion:
Early childhood shigellosis is associated with an increased rate of long-term ADHD.
Keywords: ADHD, childhood shigellosis, gastroenteritis
Introduction
Shigella spp. causes acute gastrointestinal infection with a presentation ranging from watery or loose stools with minimal constitutional symptoms to severe disease, including high fever, abdominal cramps, tenesmus, and mucous and/or bloody stools (Kotloff et al., 2018). The pathogenesis of shigellosis includes invasion of the intestinal mucosa, toxin production and induction of inflammation (Ashkenazi, 2004; Kotloff et al., 2018). It is estimated that there are annually 188 to 269 million new cases of shigellosis globally, with 164,300 to 212,440 deaths and a peak incidence in children aged 1 to 4 years (Behar et al., 2018; Cohen et al., 2014; Khalil et al., 2018). Shigellosis is common in developing countries, especially in sun-Saharan Africa and south Asia; although it is uncommon in most industrialized countries, it is still endemic in Israel (Adamker et al., 2018; Kotloff et al., 2018)).
Neurological symptoms are the most common extraintestinal manifestations of shigellosis, reported in up to 53% of hospitalized children (Avital et al., 1982; Ephros et al., 1996). The most common neurological symptom is generalized seizures (Ashkenazi et al., 1987; Khan et al., 1999), followed by various presentations of encephalopathy (Mulligan et al., 1992). In cases of fatal encephalopathy, cerebral edema has been documented (Perles et al., 1995).
Although short-term neurological symptoms of shigellosis are well established, possible long-term neurobehavioral effects of childhood shigellosis have not been evaluated yet. This study aims to examine whether early childhood shigellosis during the critical period of brain development plays a role in long-term neuropsychiatric and developmental outcomes.
ADHD is one of the most common childhood neuropsychiatric conditions. Its prevalence among children and adolescents is variable and estimated at about 7.2% (Thomas et al., 2015). It is usually first diagnosed during childhood and in 40% to 50% of the cases lasts into adulthood, resulting in long-term consequences, such as poor school achievements, unemployment, frequent injuries, higher rates of psychiatric disorders and a greater healthcare utilization (Danielson et al., 2018).
ADHD may be a final common pathway for a variety of disturbances in brain developmental processes. Mothers of children with ADHD are more likely to experience birth complications, such as toxemia, lengthy labor, and complicated delivery. Maternal drug use, smoking and alcohol consumption during pregnancy are commonly linked to attentional difficulties associated with the development of ADHD (Gutvirtz et al., 2019). These conditions reflect damage caused to the evolving brain in the critical prenatal and postnatal periods.
We examined whether Shigella infection during infancy and early childhood might be associated with an increased rate of ADHD.
Methods
This retrospective cohort study included all children aged 5 to 18 years who were registered at Leumit Health Services (LHS) between January 1, 2000 and December 31, 2018. LHS is a large Health Maintenance Organization (HMO) in Israel serving 724,129 persons. LHS has a comprehensive computerized database, continuously updated with patients’ demographics, medical visits, laboratory tests and medication prescriptions. Prescription records are available since 1998 and include those filled and purchased per patient. All study participants have similar health insurance and similar access to health services. During each physician visit, a diagnosis is entered according to the International Classification of Diseases-9 (ICD-9). The validity of diagnoses in the registry was previously examined and confirmed as high (Rennert & Peterburg, 2001).
The study population consisted of all 52,761 children aged 5 to 18 years who were LHS registries during the study period, suffered from acute gastroenteritis and underwent at least one stool culture examination prior to the age of 3 years. The referral of the children for stool culturing was according to their pediatrician’s decision, usually in cases of moderate-severe gastroenteritis or clinical dysentery, according to the Israeli guidelines. In LHS Central Laboratories, stool cultures were examined on Shigella/Salmonella agar with selenite enrichment at 37°C. Four age groups were defined: early infancy (0–3 months), late infancy (4–12 months), second year (13–24 months) and third year (25–36 months).
ADHD diagnosis was identified by ICD-9 codes (314.00–314.9). According to the Israeli Ministry of Health directive, ADHD diagnosis is and treatment made only by child or adult psychiatrists, child or adult neurologists, or pediatricians and family physicians with a certified ADHD training; grading is usually not given. The diagnosis involves information retrieved from several sources, including schools, caregivers, and parents; and is based on the American Psychiatric Association’s Diagnostic and Statistical Manual (DSM) criteria.
Since at times ICD9 “ADHD” is given temporally with referral of the patient to ADHD clinic, to confirm the diagnosis of ADHD only patients that purchased at least three prescriptions of Anatomical Therapeutic Chemical (ATC) code N06B (Psychostimulant agents and Nootropics), used for ADHD treatment, were included in the final analysis. The follow up period was defined as time from the first documented stool culture to the event (ADHD diagnosis), or until censored at age 18 years or upon ending LHS membership.
Data were collected from the LHS computerized database and included demographics for the entire cohort. Socioeconomic status (SES) was defined according to the child’s home address, using the Israeli Central Bureau of Statistics classification that includes 20 subgroups. Classifications one to seven were considered low SES, eight to thirteen middle SES and fourteen to twenty were considered high SES.
Ethnicity- was also defined according to the child’s home address, and into three largest axis of ethno-cultural origin: secular Jewish, Orthodox Jews and Israeli Arabs.
Salmonella and ADHD
To better understand if an association found between Shigella gastroenteritis and ADHD is specific to Shigella infection and its possible effect on the developing brain, a separate similar analysis of covariance was conducted to examine the correlation between Salmonella-positive stool culture and ADHD, since Salmonella gastroenteritis is usually not associated with neurologic symptoms.
The study protocol was approved by the Helsinki Institutional Review Board of Shamir Medical Center and Research Committee of LHS.
Statistical Analysis
Statistical analysis was performed using STATA 12.0. Assumptions were two sided with an α of.05. Initial analysis compared demographic characteristics between the study groups (positive vs. negative stool culture for Shigella spp.), using t-test and Fischer exact χ2 test for continuous and categorical variables, respectively, based on normal distribution and variable characteristics. Categorical data are shown in counts and percentages. Data on continuous variables with normal distribution are presented as mean and 95% confidence interval (CI).
Preliminary evaluation of risk estimates was conducted by stratified analyses. Subsequently, we used multivariate logistic regression to estimate the odds ratios (OR) and 95% CI for the independent association between positive stool culture for Shigella spp. and ADHD while controlling for potential confounders.
We hypothesized that being exposed to Shigella infection in early childhood may affect not only the risk of having ADHD, but also the likelihood that ADHD symptoms will be more profound and diagnosed at an earlier age, and therefore carried out a survival analysis of time to ADHD diagnosis. Participants were defined as being at risk from 3 years of age and were followed to the time of ADHD diagnosis or censored at December 31, 2018. Survival was analyzed using the Kaplan-Meier estimate and Nelson Aalen model to compare the cumulative ADHD prevalence.
To adjust for confounders, such as gender and socioeconomic status that have been recognized as potential risk factors for ADHD (Arnold, 1996; Russell et al., 2016), multivariate Cox proportional hazard regression models analysis were applied.
Assuming that the brain might be more vulnerable during early life, we conducted separate Cox proportional hazards regression models for each age stratum: early infancy, late infancy, second year and third year of life. For each analysis, the unexposed reference group was defined as having negative Shigella stool results during the study period.
Results
Of the 52,761 children aged 5 to 18 years who were LHS members during the study period and underwent a stool culture for Shigella/Salmonella spp. prior to 3 years of age, 5,269 (9.98%) had at least one positive stool culture for Shigella spp., 766 had a positive stool culture for Salmonella spp. and 46,726 (89.87%) had negative stool cultures (Figure 1). Among the Shigella isolates, 4894 (92.9%) were Shigella sonnei, 215 (4.1%) Shigella flexneri, 149 (2.8%) Shigella boydii and 11 (0.2%) Shigella dysenteriae. Table 1 compares the demographic data of the study groups. Differences in age, age at the time of stool culture examination, gender, and SES were all not statistically significant. In addition, there were no significant differences in the proportion of children born in August-September (the beginning of the academic year for young children) between culture-negative and Shigella/Salmonella-positive groups.
Figure 1.
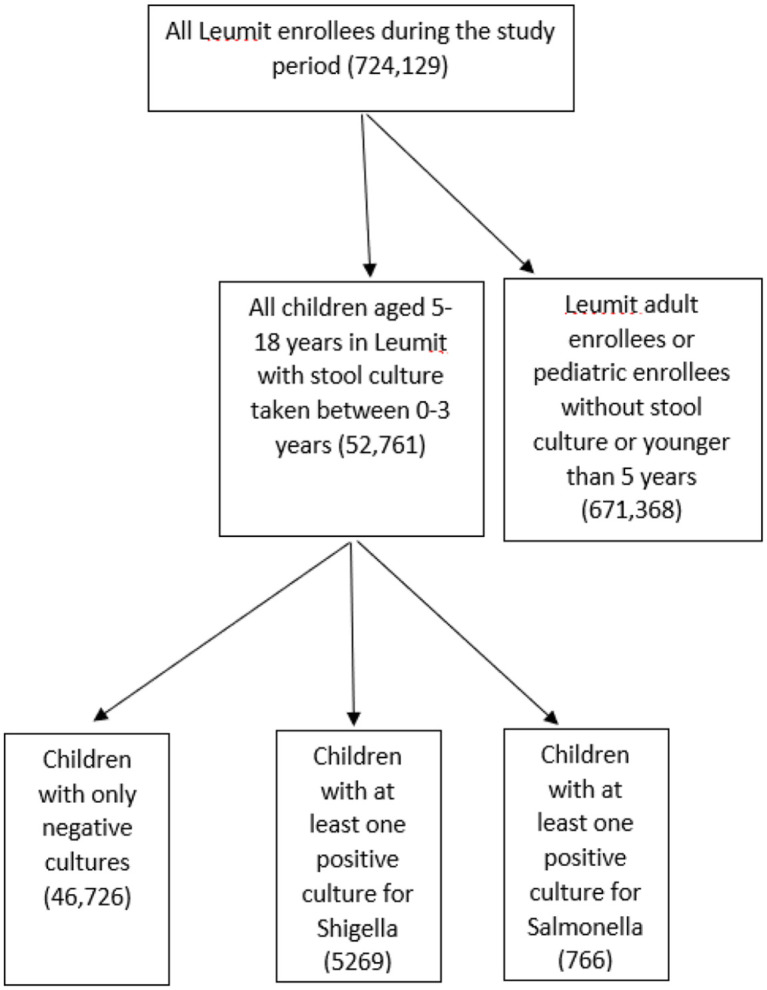
Flow chart of the study cohort.
Table 1.
Demographic Characteristics of the Study Groups Stratified by Shigella Culture Results.
| Variable | Shigella-positive stool cultures n = 5,269 (10.13%) |
Shigella-negative stool cultures n = 46,726 (89.87%) |
p-value | |
|---|---|---|---|---|
| Mean age at stool culture examination, (years, CI) | 2.50 (2.49–2.51) | 2.59 (2.56–2.61) | .644 | |
| Age categories at stool culture examination n (%) | 0–3 months | 1,059 (20.1) | 8,993 (19.4) | .579 |
| >3-12 months | 1,190 (22.7) | 10,293 (23.1) | .416 | |
| 1–2 years | 2,075 (39.3) | 21,137 (45.3) | .115 | |
| >2–3 years | 945 (17.9) | 5,673 (12.2) | .01 | |
| Mean age at censoring (years, CI) | 12.2 (6.7–17.6) | 11.8 (6.5–17.9) | .584 | |
| Gender n (%) | Male n (%) | 2,887 (54.8) | 25,857 (55.3) | .935 |
| Female n (%) | 2,382 (45.2) | 20,869 (44.7) | ||
| Socio-economic status (SES) | Low | 2,173( 41.2) | 20,226(43.3) | .505 |
| Middle | 2,115 (40.1) | 18,553(39.7) | ||
| High | 424 (8.2) | 3,628 (7.7) | ||
| Missing | 557 (10.5) | 4,319 (9.3) | ||
| Ethnic group | Secular Jewish | 3,296 (62.6) | 27,226 (58.3) | .001 |
| Orthodox Jews | 1,523 (28.9) | 12,997 (27.8) | ||
| Israeli Arabs | 450 (8.5) | 6,503 (13.9) | ||
| No ADHD (n, %) | 4,033 (76.5) | 37,508 (80.3) | .001 | |
| Suspected ADHD cases (n, %) | 680 (12.9) | 5,173 (11.1) | .001 | |
| Confirmed ADHD cases (n, %) | 556 (10.6) | 4,045 (8.6) | .001 | |
| Born on August-September (n%) | 954 (18.1) | 8559 (18.3) | .427 | |
Univariate and Multivariate Analyses
ADHD diagnoses up to 18 years of age were recorded, with a total of 4,601 events in all study groups during the follow up period. In the Shigella-positive culture group, 556 (10.6%) were diagnosed with ADHD, as compared to 4,045 (8.6%) in the stool-negative culture group (p < .001). Because of the low numbers of the not S. sonnei isolates, the species-specific association rates of ADHD were not evaluated.
Our primary outcome was the OR for ADHD amongst Shigella-positive group as compared to stool-negative culture group. Shigella infection in early childhood was associated with an increased ADHD morbidity with a crude OR of 1.27 (95% CI = 1.16–1.40, p < .001). After controlling for the variables of age, gender and SES, the adjusted OR was 1.21 (1.13–1.29, p < .001). Children who had more than one positive Shigella culture had higher rates ADHD: the ORs of two and three positive cultures were 1.54 (1.13–2.09) and 1.73 (1.39–2.09), respectively (p = .0033 and <.0001, respectively).
Further survival analysis was conducted using Cox proportional hazards model: children exposed to early childhood shigellosis had an increased unadjusted hazard rate of 1.34 (95% CI = 1.16–1.46, p < .001) for ADHD. After controlling for the covariates age, gender and SES, childhood shigellosis was found to be a significant and independent associate of ADHD diagnosis with an adjusted hazard ratio of 1.24 (95% CI = 1.12–1.34, p < .001, Table 2).
Table 2.
Univariate and Multivariate Analyses of the Association Between Childhood Shigellosis and ADHD.
| Variable | Crude HR (95% CI) | p value | Adjusted HR (95% CI) * | p value |
|---|---|---|---|---|
| Positive Shigella stool culture | 1.34 (1.16–1.46) | <.001 | 1.26 (1.11–1.34) | <.001 |
| Male gender | 2.32 (2.17–2.47) | <.001 | 2.33 (2.18–2.49) | <.001 |
| High SES** | 1.44 (1.31–1.53) | <.001 | 1.46 (1.37–1.56) | <.001 |
| Age | 1.08 (1.07–1.09) | <.001 | 1.06 (1.05–1.07) | <.001 |
| Non-Jewish ethnicity | 0.17 (0.14–0.21) | <.001 | 0.16 (0.13–0.19) | <.001 |
Adjusted for age, gender, ethnicity and SES.
SES- socioeconomic status.
The younger the child was during the documented Shigella infection, the higher was the risk of the child to be diagnosed with ADHD (Table 3). Shigellosis in early infancy, late infancy and second year of life was associated with 36%, 33% and 18% higher rates of ADHD, respectively, but with no significant increased rate when the infection occurred during the third year of life [early infancy: HR = 1.36 (95% CI = 1.18–1.71), late infancy: HR = 1.24 (95% CI = 1.12–1.36), second year: HR = 1.18 (95% CI = 1.08–1.28), third year: HR = 1.09 (95% CI = 0.88–1.35)].
Table 3.
The Risk of ADHD Stratified by Age at the Time of Shigella Infection.
| Variable | Crude HR (95% CI) | Adjusted HR (95% CI) * | p value |
|---|---|---|---|
| Early infancy (0–3 months) | 1.56 (1.25–1.94) | 1.36 (1.18–1.71) | <.001 |
| Late infancy (>3–12 months) | 1.33 (1.15–1.51) | 1.24 (1.12–1.36) | <.001 |
| 2nd year (13–24 months) | 1.29 (1.09–1.53) | 1.18 (1.08–1.28) | <.001 |
| 3rd year (25–36 months) | 1.08 (0.87–1.34) | 1.09 (0.88–1.35) | .46 |
Adjusted for gender, and SES.
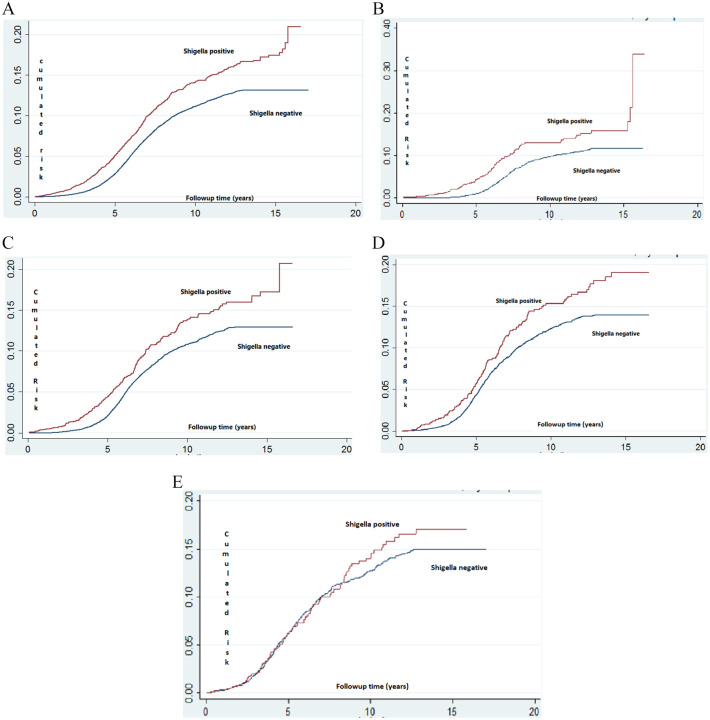
Kaplan–Meier estimate and Nelson Aalen models are presented in Figure 2. A significantly higher cumulative incidence of ADHD was found in the Shigella-positive stool culture group (log-rank p value <.001) in all age subgroups except for the 3rd-year subgroup. The significantly greater rate for ADHD diagnosis started at approximately 4 years of age (just following the beginning of compulsory education) with the difference being more obvious at the beginning of high school (approximately 15 years of age, Figure 2).
Figure 2.
(A) Kaplan–Meier survival plot estimating the risk of ADHD among children with Shigella-positive versus Shigella-negative stool cultures. (B) Kaplan–Meier survival plot estimating the risk of ADHD among children with Shigella-positive versus Shigella-negative stool cultures obtained during early infancy. (C) Kaplan–Meier survival plot estimating the risk of ADHD among children with Shigella-positive versus Shigella-negative stool cultures obtained during the first year of life. (D) Kaplan–Meier survival plot estimating the risk of ADHD among children with Shigella-positive versus Shigella-negative stool cultures obtained during the second year of life. (E) Kaplan–Meier survival plot estimating the risk of ADHD among children with Shigella-positive versus Shigella-negative stool cultures obtained during the third year of life.*
*Log- rank, Mantel-Cox: p < .001.
Salmonella Infection and ADHD
In a sensitivity analysis performed to assess possible confounding by infectious diarrhea, we examined the association between Salmonella gastroenteritis and ADHD (Table 4). Of the 766 children with Salmonella gastroenteritis, 55 (7.2%) were diagnosed with ADHD, as compared to 8.6% (4371/49970) in the stool culture-negative group (p = .12). Salmonella infection was not associated with increased rate of ADHD with a crude OR of 0.81 (95% CI = 0.64–1.09) and an adjusted OR of 0.84 (95% CI = 0.60–1.06) after controlling for the same variables as in the Shigella analysis (Table 5). No significant association was found between Salmonella-positive stool cultures and the risk of ADHD morbidity after applying survival analysis (Figure 3). When we examined the 6,034 children who had positive stool cultures either with Shigella spp or with Salmonella spp, no increased rate of ADHD was detected (adjusted OR 1.024, 95% CI = 0.93–1.13, p = .643).
Table 4.
Demographic Characteristics of Children with Salmonella Positive versus Salmonella Negative Stool Cultures.
| Variable |
Salmonella-positive stool culture n = 766 (1.61%) |
Salmonella Negative Stool culture n = 46,726 (89.87%) |
p value | |
|---|---|---|---|---|
| Mean age at stool culture examination, (years, CI) | 2.81 (2.64, 2.91) | 2.59 (2.56, 2.61) | .64 | |
| Age categories at stool culture examination | 0–3 months | 181 (23.6) | 8,993 (19.4) | .46 |
| >3–12 months | 167 (21.8) | 10,923 (23.1) | .06 | |
| 1–2 years | 329 (45.2) | 21,137 (45.3) | .784 | |
| >2–3 years | 89 (12.1) | 5,673 (12.2) | .759 | |
| Mean age at censoring (years, CI) | 12.8 (7.1–17.9) | 11.8 (6.5–17.9) | .554 | |
| Gender, n (%) | Male | 422 (55.1) | 25,857 (55.3) | .925 |
| Socio-economic status (SES) | Low | 310 (38.7) | 20,226 (43.3 ) | .025 |
| Middle | 337 (43.9) | 18,553 (39.7) | ||
| High | 49 (6.4) | 3,628 (7.7) | ||
| Missing | 70 (9.1) | 4,319 (9.3) | ||
| Ethnic group | Secular Jewish | 402 (52.5) | 27,226 (58.3) | .279 |
| Orthodox Jews | 247 (32.2) | 12,997 (27.8) | ||
| Israeli Arabs | 117 (15.3) | 6,503 (13.9) | ||
| No ADHD (n, %) | 630 (82.2) | 37,508 (80.3) | .784 | |
| Suspected ADHD cases (n, %) | 77 (10.1) | 5,173 (11.1) | .193 | |
| Confirmed ADHD cases (n, %) | 59 (7.7%) | 4,045 (8.6) | .14 5 | |
| Born on August-September (n, %) | 142 (18.5) | 8559 (18.3) | .568 | |
Table 5.
The Risk of ADHD Diagnosis by Salmonella-positive Gastroenteritis.
| Variable | Crude HR (95% CI) | p value | Adjusted HR (95% CI)* | p value |
|---|---|---|---|---|
| Positive Salmonella stool culture | 0.85 (0.64–1.09) | <.001 | 0.82 (0.59–1.08) | .155 |
| Male gender | 2.32 (2.17–2.47) | <.001 | 2.19 (2.04–2.35) | <.001 |
| -High SES** | 1.44 (1.31–1.53) | <.001 | 1.23 (1.11–1.38) | <.001 |
| Age | 1.08 (1.07–1.09) | <.001 | 1.04 (1.01–1.09) | <.001 |
| Non-Jewish ethnicity | 0.17 (0.14; 0.21) | <.001 | 0.16 (0.13–0.19) | <.001 |
Adjusted for age, gender, and SES.
SES- socioeconomic status.
Figure 3.
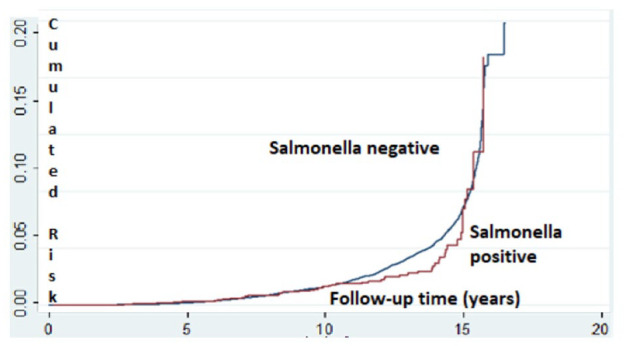
Kaplan–Meier survival plot estimating the risk of ADHD among children with Salmonella-positive versus Salmonella-negative stool cultures.*
*Log- rank, Mantel-Cox: p < .001.
Discussion
The short-term neurological manifestations of childhood shigellosis are well established. This study examined possible long-term neuropsychiatric associations of childhood shigellosis during the first 3 years of life, the critical period of brain development. In this population-based cohort study, children with gastroenteritis and a positive stool culture for Shigella spp. during early childhood were at greater risk for ADHD morbidity (10.6%) compared to children who had negative stool cultures (8.6%), independently of SES and gender. Our study also found that amongst children with Shigella-positive stool culture, ADHD was diagnosed at an earlier age, possibly because ADHD symptoms were more profound. The younger was the child during the Shigella infection, the higher was the association with ADHD.
ADHD is one of the most prevalent chronic neuropsychiatric disorders in the pediatric population. Its prevalence in Israeli children ranges between 7.4% and 9.5% (Ornoy et al., 2016). In our study, the prevalence of ADHD among children with Shigella-positive stool cultures was 10.6%, higher than the Israeli and global rates. It is well accepted that ADHD is highly heritable, with more than 70% attributed to genetic factors (Banerjee et al., 2007). However, it is estimated that 10% to 40% of the variance associated with ADHD is attributed to environmental factors, including food additives, nutrition, lead contamination, and low birth weight (Matsuzawa et al., 2001).
Early childhood, particularly the first 2 years of life, may be the most dynamic and important phase of postnatal brain development in humans. There is a dramatic increase in grey matter volume and overall brain size during this period, with the brain reaching 80% to 90% of adult volume by the age of 2 years (Pfefferbaum et al., 1994; Huttenlocher and Dabholkar (1997). There is also elaboration of new synapses in the first two years of life (Dubois et al., 2014; Glantz et al., 2007) and myelination of white matter proceeds rapidly after birth and reaches the adult pattern of myelination by the end of the second year (Kohler-Forsberg et al., 2014).
Concurrent with the rapid pace of structural brain development, are rapid achievements of a wide range of cognitive and motor functions. Thus, theoretically, acute distress of any kind to the central nervous system during this critical period may lead to varied neurobehavioral outcomes. Our study showed that the earlier the infection was encountered, the greater was the risk for ADHD morbidity.
Plausible biological mechanisms for the association between Shigella gastroenteritis in early childhood and ADHD could be ascribed to systemic inflammatory processes, brain inflammation, and damage that occurs during shigellosis. A recent nationwide study has demonstrated an association between treated infections and future risk of treated mental disorders in children and adolescents (Kohler-Forsberg et al., 2019). This study has shown a correlation between various bacterial and viral infections and a wide range of mental disorders. This could imply that the infectious process with the inflammatory response induced by it, rather than the specific pathogen, may be the cause for the increased neuropsychiatric morbidity rates. However, our study suggests that the specific pathogen causing the infection is also of importance, since increased rates of ADHD were associated significantly and specifically with Shigella gastroenteritis, but not with Salmonella gastroenteritis.
Previous human observations have shown that Shigella gastroenteritis in children is more commonly associated with seizures and other neurologic manifestations than other causes of gastroenteritis (Ashkenazi et al., 1987; Avital et al., 1982; Khan et al., 1999). To the best of our knowledge, the late neuropsychiatric outcome has not been evaluated. In a previous study, we have followed our selected cohort of 111 children with Shigella-associated seizures for 3 to 18 years. A poor coordination of fine hand movements was found in 3.3%, as well as a single case (0.9%) of epilepsy; ADHD was not evaluated in this study (Zvulonov et al., 1990).
In a mouse model of Shigella-related seizures, we have demonstrated that administration of Shigella sonicate induced seizures that correlated with increased serum levels of inflammatory markers and cytokines, such as tumor necrosis factor alpha (TNFα), interleukin-1beta (IL-1β) and nitric oxide (NO) and that those seizures could be prevented by monoclonal antibodies to TNFα and IL-1β and by inhibitors of inducible NO synthase (Yuhas et al., 1999). Using the same model, the involvement of the neuropeptide corticotropin-releasing hormone (CRH) in the pathogenesis of Shigella-related seizures was documented (Yuhas et al., 2004). CRH is prominent in the prefrontal cortex (PFC) and could be related to PFC-based psychopathologies such as ADHD. However, further studies are required to indicate whether Shigella infection is indeed a causal factor for ADHD and to elucidate these and other plausible mechanisms.
Our study holds inherent limitations. First, this is a retrospective database study. However, using patient data enabled us to create a large cohort with a prolonged follow-up, and to detect the association. Being insured by the LHS throughout the study period, it is very unlikely that the children could have tested for stool culture elsewhere. Second, our analysis lacked variables such as family history of ADHD, maternal smoking and birth weight that might all have affected ADHD morbidity rates. In addition, we had neither data regarding the clinical presentation of shigellosis, nor did we consider antimicrobial treatment during the course of the disease. Antibiotic use could have influenced the outcome, for example by affecting human microbiome, and should be further investigated in the future.
Conclusion
Our study shows that Shigella gastroenteritis during early childhood is associated with an increased ADHD rates, independently of SES and gender, and that the younger the child is during infection, the higher is the association with future ADHD morbidity. Since Shigella infection is treatable, we believe it is important to further investigate its relationship with potential long-term neuro-developmental outcomes. Presumably, interventions such as early antimicrobial therapy, and preventative measures such as sanitation and a preventive vaccine (Ashkenazi et al., 2013), may theoretically reduce ADHD morbidity, thereby improve lives of millions of children and their families worldwide.
Author Biographies
Eugene Merzon is a senior family physician and an assistant professor at the Department of Family Medicine, Sackler School of Medicine, Tel Aviv University. He is head of the Department of Managed Care in Leumit Health Services (LHS) in Israel. Dr. Merzon is an Israeli Ministry of Health certified ADHD diagnostician and executive board member of the Israeli Society of ADHD (ISA).
Yuval Gutbir has earned his MD at Ben Gurion University of the Negev in Israel in 2018. He completed his internship year in Sheba-Tel Hashomer Medical Center with honors, and is a resident in pediatrics in Hadassah Ein-Karem Hospital in Jerusalem. Being a younger brother for a sister with CP, and as a young physician, he devotes his research to both clinical as well as epidemiological and basic science aspects of pregnancy, delivery and early childhood pathologies and their potential long-term effects.
Shlomo Vinker is full professor in Family Medicine, Vice Dean for community teaching, and Chair of the Department of Family Medicine (between 2006 and 2011, and since 2016) at the Sackler School of Medicine, Tel Aviv University. He is Chief Medical Director of LHS.
Prof. Vinker established a research institute in LHS and has been its chair since 2018. He is an active family physician, working in an urban clinic in the city of Ashdod, Israel.
Prof. Vinker was the chairman of the Israeli Association of Family Physicians between 2009 and 2018 and Treasurer since 2018. He is an executive board member of EGPRN (European General Practice Research Network) and of WONCA (world organization of family physicians) EUROPE. He published about 200 research articles in peer review medical journals.
Avivit Golan Cohen is chief of the Department of Health Care Quality in the Medical Division at LHS since 2017. Until recently – Chief Physician of the Central District of LHS. An active family physician, working in an urban clinic in the city of Tel Aviv, Israel. A lecturer at Tel Aviv University, performing a variety of senior academic positions at the Tel Aviv University and in LHS.
Dana Horwitz is a resident in family medicine in Maccabi HMO. Dana finished with honors her medical studies at the Ben-Gurion University of the Negev in 2018, including an internship in the Soroka medical center.
Shai Ashkenazi completed his medical education and residency in pediatrics in Israel and a fellowship in pediatric infectious diseases in Houston, Texas, USA (1987-90). He is currently Professor of Pediatrics and Dean at the Adelson School of Medicine, Ariel University; Chairman of the Israel Pediatric Association, member of the National Council for Child Health and member of the Education Committee of the World Society for Pediatric Infectious Diseases.
Ashkenazi is a member of the Editorial Boards of several national and international medical journals, a co-author of over 270 medical publications, over 30 chapters in books and the Editor of the Hebrew Textbook of Pediatrics (9 editions). He has received dozens of research grants, including from the NIH (USA), European Union, Chief Scientist, the USA-Israel Bi-national Science Foundation (BSF) and the Education Award of the European Society for Paediatric Infectious Diseases.
Yair Sadaka is a pediatric neurologist and developmental pediatrician. Currently head of the Negev Child Development Center of the Ministry of Health. Dr. Sadaka holds a PhD in neurosciences and has experience in behavioral sciences and data analysis.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics approval: The research has received permission from The Leumit Healthcare Institutional ethics committee.
ORCID iD: Dana Horwitz  https://orcid.org/0000-0001-9329-0833
https://orcid.org/0000-0001-9329-0833
Data Access: All authors confirm full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- Adamker G., Holzer T., Karakis I., Amitay M., Anis E., Singer S. R., Barnett-Itzhaki Z. (2018). Prediction of Shigellosis outcomes in Israel using machine learning classifiers. Epidemiology and Infection, 146(11), 1445–1451. 10.1017/S0950268818001498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold L. E. (1996). Sex differences in ADHD: Conference summary. Journal of Abnormal Child Psychology, 24(5), 555–569. [DOI] [PubMed] [Google Scholar]
- Ashkenazi S, Dinari G., Zevulunov A., Nitzan M. (1987). Convulsions in childhood shigellosis. Clinical and laboratory features in 153 children. American Journal of Diseases of Children (1960), 141(2), 208–210. [DOI] [PubMed] [Google Scholar]
- Ashkenazi S. (2004). Shigella infections in children: New insights. Seminars in Pediatric Infectious Diseases, 15(4), 246–252. [DOI] [PubMed] [Google Scholar]
- Ashkenazi S., Cohen D. (2013). An update on vaccines against Shigella. Therapeutic Advances in Vaccines, 1(3), 113–123. 10.1177/2051013613500428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avital A., Maayan C., Goitein K. J. (1982). Incidence of convulsions and encephalopathy in childhood Shigella infections. Survey of 117 hospitalized patients. Clinical Pediatrics, 21(11), 645–648. 10.1177/000992288202101101 [DOI] [PubMed] [Google Scholar]
- Banerjee T. D., Middleton F., Faraone S. V. (2007). Environmental risk factors for attention-deficit hyperactivity disorder. Acta Paediatrica, 6(9), 1269–1274. [DOI] [PubMed] [Google Scholar]
- Behar A., Baker K. S., Bassal R., Ezernitchi A., Valinsky L., Thomson N. R., Cohen D. (2018). Microevolution and patterns of transmission of Shigella sonnei within cyclic outbreaks Shigellosis, Israel. Emerging Infectious Diseases, 24(7), 1335–1339. 10.3201/eid2407.171313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D., Bassal R., Goren S., Rouach T., Taran D., Schemberg B., Peled N., Keness Y., Ken-Dror S., Vasilev V., Nissan I., Agmon V., Shohat T. (2014). Recent trends in the epidemiology of shigellosis in Israel. Epidemiology and Infection, 142(12), 2583–2594. 10.1017/S0950268814000260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson M. L., Visser S. N., Chronis-Tuscano A., DuPaul G. J. (2018). A National Description of treatment among United States children and adolescents with attention-deficit/hyperactivity disorder. The Journal of Pediatrics, 192, 240–246.e1. 10.1016/j.jpeds.2017.08.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J., Dehaene-Lambertz G., Kulikova S., Poupon C., Huppi P. S., Hertz-Pannier L. (2014). The early development of brain white matter: A review of imaging studies in fetuses, newborns and infants. Neuroscience, 276, 48–71. 10.1016/j.neuroscience.2013.12.044 [DOI] [PubMed] [Google Scholar]
- Ephros M., Cohen D., Yavzori M., Rotman N., Novic B., Ashkenazi S. (1996). Encephalopathy associated with enteroinvasive Escherichia coli 0144:NM infection. Journal of Clinical Microbiology, 34(10), 2432–2434. https://www.ncbi.nlm.nih.gov/pubmed/8880494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz L. A., Gilmore J. H., Hamer R. M., Lieberman J. A., Jarskog L. F. (2007). Synaptophysin and postsynaptic density protein 95 in the human prefrontal cortex from mid-gestation into early adulthood. Neuroscience, 149(3), 582–591. 10.1016/j.neuroscience.2007.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutvirtz G., Wainstock T., Landau D., Sheiner E. (2019). Maternal smoking during pregnancy and long-term neurological morbidity of the offspring. Addictive Behaviors, 88, 86–91. 10.1016/j.addbeh.2018.08.013 [DOI] [PubMed] [Google Scholar]
- Huttenlocher P. R., Dabholkar A. S. (1997). Regional differences in synaptogenesis in human cerebral cortex. The Journal of Comparative Neurology, 387(2), 167–178. [DOI] [PubMed] [Google Scholar]
- Khalil I. A., Troeger C., Blacker B. F., Rao P. C., Brown A., Atherly D. E., Brewer T.G., Engmann C. M., Houpt E. R., Kang G., Kotloff K. L., Levine M. M., Luby S. P., MacLennan C. A., Pan W. K., Pavlinac P. B., Platts-Mills J. A., Qadri F., Riddle M. S., Ryan E. T., … Reiner R. C. J. (2018). Morbidity and mortality due to shigella and enterotoxigenic Escherichia coli diarrhoea: The Global Burden of Disease Study 1990-2016. The Lancet. Infectious Diseases, 18(11), 1229–1240. 10.1016/S1473-3099(18)30475-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan W. A., Dhar U., Salam M. A., Griffiths J. K., Rand W., Bennish M. L. (1999). Central nervous system manifestations of childhood shigellosis: Prevalence, risk factors, and outcome. Pediatrics, 103(2), E18. 10.1542/peds.103.2.e18 [DOI] [PubMed] [Google Scholar]
- Kohler-Forsberg O., Petersen L., Gasse C., Mortensen P. B., Dalsgaard S., Yolken R. H., Mors O., Benros M. E. (2019). A Nationwide Study in Denmark of the association between treated infections and the subsequent risk of treated mental disorders in children and adolescents. JAMA Psychiatry, 76(3), 271–279. 10.1001/jamapsychiatry.2018.3428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotloff K. L., Riddle M. S., Platts-Mills J. A., Pavlinac P., Zaidi A. K. M. (2018). Shigellosis. Lancet (London, England), 391(10122), 801–812. 10.1016/S0140-6736(17)33296-8 [DOI] [PubMed] [Google Scholar]
- Matsuzawa J., Matsui M., Konishi T., Noguchi K., Gur R. C., Bilker W., Miyawaki T. (2001). Age-related volumetric changes of brain gray and white matter in healthy infants and children. Cerebral Cortex (New York, N.Y. : 1991), 11(4), 335–342. 10.1093/cercor/11.4.335 [DOI] [PubMed] [Google Scholar]
- Mulligan K., Nelson S., Friedman H. S., Andrews P. I. (1992). Shigellosis-associated encephalopathy. The Pediatric Infectious Disease Journal, 11(10), 889–890. [DOI] [PubMed] [Google Scholar]
- Ornoy A., Ovadia M., Rivkin D., Milshtein E., Barlev L. (2016). Prevalence of ADHD among 7-9-year-old children in Israel. A comparison between jewish and Arab populations. The Israel Journal of Psychiatry and Related Sciences, 53(2), 3–8. [PubMed] [Google Scholar]
- Perles Z., Bar-Ziv J., Granot E. (1995). Brain edema: An underdiagnosed complication of Shigella infection. The Pediatric Infectious Disease Journal, 14(12), 1114–1115. [PubMed] [Google Scholar]
- Pfefferbaum A., Mathalon D. H., Sullivan E. V, Rawles J. M., Zipursky R. B., Lim K. O. (1994). A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Archives of Neurology, 51(9), 874–887. [DOI] [PubMed] [Google Scholar]
- Rennert G., Peterburg Y. (2001). Prevalence of selected chronic diseases in Israel. The Israel Medical Association Journal : IMAJ, 3(6), 404–408. [PubMed] [Google Scholar]
- Russell A. E., Ford T., Williams R., Russell G. (2016). The association between socioeconomic disadvantage and attention deficit/hyperactivity disorder (ADHD): A systematic review. Child Psychiatry and Human Development, 47(3), 440–458. 10.1007/s10578-015-0578-3 [DOI] [PubMed] [Google Scholar]
- Thomas R., Sanders S., Doust J., Beller E., Glasziou P. (2015). Prevalence of attention-deficit/hyperactivity disorder: A systematic review and meta-analysis. Pediatrics, 135(4), e994–e1001. 10.1542/peds.2014-3482 [DOI] [PubMed] [Google Scholar]
- Yuhas Y., Shulman L., Weizman A., Kaminsky E., Vanichkin A., Ashkenazi S. (1999). Involvement of tumor necrosis factor alpha and interleukin-1beta in enhancement of pentylenetetrazole-induced seizures caused by Shigella dysenteriae. Infection and Immunity, 67(3), 1455–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuhas Y., Weizman A., Chrousos G. P., Ovadia H., Ashkenazi S. (2004). Involvement of the neuropeptide corticotropin-releasing hormone in an animal model of Shigella-related seizures. Journal of Neuroimmunology, 153(1–2), 36–39. 10.1016/j.jneuroim.2004.04.009 [DOI] [PubMed] [Google Scholar]
- Zvulonov A., Lerman M., Ashkenazi S., Weitz R., Nitzan M., Dinari G. (1990). The prognosis of convulsions during childhood shigellosis. Eurpean Journal of Pediatrics, 149(3), 293–294. [DOI] [PubMed] [Google Scholar]