Abstract
Purpose
Pseudomonas aeruginosa is a common cause of nosocomial infections with associated morbidity and mortality because the organism is unresponsive to commonly available antimicrobials. This study was undertaken to determine the multiple drug-resistant (MDR), extensive drug-resistant (XDR) and pan drug-resistant (PDR) phenotype of P. aeruginosa and its carbapenemase production rate from presumptive isolates stored in the biobank at the Ethiopian Public Health Institute (EPHI).
Methods
A cross-sectional study was conducted at the EPHI laboratory, Addis Ababa, Ethiopia from March to June 2021. Stored isolates of Pseudomonas spp. which had been characterized by manual identification methods were further processed for species-level identification (ID) and antimicrobial susceptibility testing (AST) using a Becton Dickinson (BD) Phoenix automated system. The isolates were analyzed for carbapenemase enzyme production using the modified Carbapenem Inactivation Method (mCIM). The data analysis was done using SPSS version 20 software.
Results
In this study, 110 presumptive Pseudomonas isolates from a biobank were re-analyzed, 100 of them were found to be Pseudomonas and among these P. aeruginosa accounted for 98% and P. putida accounted for 2%. The majority of isolates were recovered from wound (46%) specimens followed by ear swabs (18%). The highest level of resistance was observed against ceftazidime (35%) and the lowest level of resistance was observed against amikacin (2%). Twenty-seven isolates were identified as candidates for carbapenemase enzyme production testing, of which only 3/27 (11%) isolates were detected as carbapenemase enzyme producers.
Conclusion
This study shows an increasing rate of MDR and XDR isolates and the appearance of PDR in P. aeruginosa strains; this is a serious problem in Ethiopia. The lack of newer anti-pseudomonal antibiotics adds to the problem. In order to alleviate this, infection prevention activities should be promoted, and treatment of bacterial infections should be guided by antibiotic susceptibility test results.
Keywords: P. aeruginosa, antimicrobial resistance, carbapenemase enzyme, Ethiopia
Background
Pseudomonas species are gram-negative, rod-shaped, aerobic, non-spore-forming, polar-flagellated organisms belonging to the family Pseudomonadaceae, which includes over 202 species in the current molecular classification. Among them, Pseudomonas aeruginosa is the most common medically important bacterial species that causes nosocomial infection in clinical settings; it is a ubiquitous microorganism found in the environment including water, soil, animals and plants.1–3
Infection by P. aeruginosa is associated with mortality and morbidity, particularly in immunocompromised patients. It causes infections in wounds (especially in burn patients), the urinary tract, bloodstream, surgical sites, eye, external ear, and the respiratory tract.1,2,4
The treatment of multidrug-resistant P. aeruginosa has become a great challenge. The organism is intrinsically resistant to many commonly available antimicrobials and has extraordinary adaptive mechanisms like; upregulation of efflux pumping genes, down regulation of outer membrane proteins, mutations in chromosomal genes, and horizontal acquisition of transferable resistant genes encoding β-lactamase production. These are predominantly carbapenemases, 16S rRNA methylases, and aminoglycoside-modifying enzymes.5–7
This has led the World Health Organization to categorize P. aeruginosa in the first list of ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) as a top priority (critical) organism for research, discovery, and new-drug development because of its high level of resistance to carbapenems.8
In developing countries like Ethiopia, the clinical outcome of such infections are not being studied, in fact, the burden is expected to be higher and spreading faster because of poor health facilities, insufficient microbiology testing laboratories, widely practiced empirical use of antibiotics, unregulated distribution of the drugs, poor sanitation, and infection prevention approaches.
Thus, to contain the spreading of such nosocomial infection urgent focused interventions are required. This study aimed to determine the rate of MDR, XDR, and PDR phenotypes of P. aeruginosa. In addition, we have assessed the carbapenemase enzyme production rate from clinical isolates stored at the Ethiopian Public Health Institute, Addis Ababa.
Methods
Study Design, Period, and Setting
A laboratory-based, cross-sectional study was conducted between March to June, 2021 at the EPHI, National Clinical Bacteriology and Mycology Reference Laboratory (NCBMRL), which is located in Gulele sub-city, Addis Ababa, Ethiopia. The EPHI is recognized as a key wing of the Ministry of Health for covering most aspects of public health-related issues. The NCBMRL is one of the full-scope laboratories accredited by the Ethiopian national accreditation office since 2016. Accreditation was achieved after fulfillment of ISO/IEC15189:12 requirements by providing bacterial culture and sensitivity testing from different clinical samples.
The clinical specimens were submitted from different referring health facilities in Addis Ababa, and other parts of the country. Thus, all patient specimens referred to the EPHI, NCBMRL for routine culture, and sensitivity tests during the study period were the source isolates. In this study, we re-analyzed all presumptive (manually characterized) Pseudomonas isolates, and stored at biobank between January 2017 up to March 2021 as the study isolates. The demographic data of the isolates were assessed retrospectively.
Sample Size and Sampling
We have included all presumptive Pseudomonas isolates stored at the EPHI biobank from January 2017 to March 2021, and a non-probability sampling method was employed to consider all manually identified Pseudomonas isolates.
Variables
Antimicrobial resistance pattern of each Pseudomonas isolate, the extent of carbapenemase enzyme production, and species of Pseudomonas isolates were the dependent variables while age, sex, type of specimen, and year of bacterial isolation were the independent variables of the study.
Inclusion and Exclusion Criteria
All non-duplicate presumptive Pseudomonas isolates which have the required clinical information; age, sex, type of specimen submitted, and isolation date were included in this study. Contaminated isolates and those with incomplete information were excluded.
Stored Clinical Isolates and Sub-Culturing
As per the NCBMRL protocol, all pathogenic bacteria identified using manual identification methods from clinical samples were stored at −80°C in Tryptic Soy Broth (TSB) with 5% glycerol. The vials were labeled with the name of the organism, specimen type, date of identification, and its unique laboratory identification number. For this study, only presumptive Pseudomonas isolates were used. The isolates were sub-cultured into Blood agar plate (BAP), Cetrimide agar plate, and MacConkey agar plates (MAC), and incubated for 24 h at 35–37°C. Identification and AST were performed on the subcultures.
Bacterial Identification
The bacterial identification was done using the BD Phoenix M50 automated system, which utilizes a series of conventional, fluorogenic, and chromogenic biochemical tests to determine species-level identification of the organism. The Phoenix identification panel has 51 wells with dried biochemical substrates and 2-fluorescent control wells.9
Both enzymatic and growth-based substrates are employed to show a variety of reactivity in the range of taxa. Therefore, bacterial identification is based on microbial utilization and degradation of specific substrates detected by various color indicator systems. When the testing organism utilizes the carbohydrate substrate, the pH drops because of acid formation which is indicated by the phenol red indicator, in addition upon enzymatic hydrolysis of either p-nitroanilide or p-nitrophenyl compounds the chromogenic substrate forms a yellow color.9
There were also additional tests that can detect the ability of the organism to degrade, hydrolyze, reduce, or otherwise utilize a substrate. Finally, results from each substrate were displayed as +, -, V, or X for each reaction, then the organism identification was reported with the probability percentage, compared from the Phoenix updated database version V6.81A.9
Antimicrobial Susceptibility Testing
AST was performed simultaneously with the BD Phoenix M50 automated system using NMIC/ID-431 combination panels according to the manufacturer’s instructions. The AST panels have 84 wells with dried antimicrobial agents and 1 growth control well. Principally, the system is a broth-based micro-dilution using a redox indicator solution for the detection of testing bacterial growth in the existence of an antimicrobial agent.9,10 Side by side, once the organism identification is done, the MIC values of each antibiotic are generated and interpreted as Susceptible, Intermediate, or Resistant based on the most recent CLSI M100 performance standard guideline for possible phenotypes for microorganism antimicrobial agent combination with the internal database.7,9
NMIC/ID-431 panels are intended for use with the BD Phoenix M50 automated system to determine the susceptibility of the clinically relevant aerobic gram-negative rod to the antimicrobial agents; in addition, it indicates resistance markers like carbapenemase. NMIC/ID-431 panels are composed of all recommended antimicrobial agents for P. aeruginosa such as amikacin, cefepime, ceftazidime, ceftolozane-tazobactam, ciprofloxacin, colistin, gentamicin, imipenem, levofloxacin, meropenem, piperacillin-tazobactam, and other intrinsically resistant antimicrobials.7,9
Test for Carbapenemase Production
After AST tests, candidate (non-susceptible) isolates having ≥ 4 µg/mL MIC for imipenem and/or meropenem were selected for further carbapenemase production testing. The carbapenemase production test was performed according to CLSI 2020 recommendations using the modified Carbapenem Inactivation Method (mCIM).7
For each tested isolate, a 10-μL loopful of colonies was emulsified in 2 mL TSB and a 10 μg meropenem disk added, then incubated for a minimum of 4 h at 35–37°C. Following completion of incubation, 0.5 McFarland suspension of American Type Culture Collection (ATCC) 25922 E. coli was prepared in saline. Then a Mueller-Hinton agar (MHA) plate was inoculated with the prepared E. coli ATCC 25922 suspensions as the routine disk diffusion procedure and the meropenem disk taken from the TSB-meropenem suspension was placed in it, then after labeling this was incubated at 35–37°C in ambient air for 18–24 h.7
Following incubation, the measured inhibition zone diameter of 6–15 mm or pinpoint colonies within 16–18 mm was determined as positive for carbapenemase enzyme production, and a zone of inhibition ≥19 mm was considered to be negative for carbapenemase enzyme production according to CLSI guideline. The procedure was monitored using Quality Control (QC) strains; K. pneumoniae ATCC BAA-1706 as negative and K. pneumoniae ATCC BAA-1705 as the positive control.7
Quality Assurance
All stored presumptive Pseudomonas spp. isolates were checked for proper labeling with the name of the organism, type of the specimen, date of identification, and its unique laboratory identification number before sub-culturing. All culture media were prepared according to the manufacturer’s instructions; the sterility of prepared media was verified by overnight incubation at 35–37°C. The performance of culture media was checked using the following ATCC control strains: P. aeruginosa 27853, E. coli 25922, and S. aureus 25923.
The phoenix system was verified using standard strains; P. aeruginosa ATCC 27853, E. coli ATCC 25922, and Klebsiella pneumonia ATCC 700603 as per the manufacturer’s instructions. Each new lot ID and AST panel was also verified using the above QC strains and the results were compared against the given expected results of each QC organism on the package inserts to ensure the appropriate setup procedure and acceptable performance of the system.
Data Analysis
The data entry and analysis were done using SPSS version 20. The analysis of descriptive statistics was used to see the relationship between the dependent variable and independent variables. Then the determined frequencies of different variables were compared. Lastly, the results were presented in words, tables, and figures.
Results
General Description of the Pseudomonas Isolates
Altogether 110 presumptive Pseudomonas spp. isolates were tested, and 100 isolates were found to be Pseudomonas spp. The remaining isolates belonged to other gram-negative species. Among the 100 isolates 98% were P. aeruginosa, and P. putida was found in 2%, with the mean confidence value of 97% according to the BD Phoenix identification system. The maximum numbers of isolates were detected among 21–30 years age groups and male study subjects were most commonly affected by Pseudomonas infection with a rate of 63%.
Most Pseudomonas spp. were identified from wounds (46%), followed by ear swab (18%), urine (15%), sputum (8%), tracheal aspirate (6%), blood (5%), CSF, and other body fluid (2%). MDR and XDR isolates were recovered in all specimen types, and PDR isolates were particularly identified from tracheal aspirate and urine samples. From the total number of isolates the highest percentage (34%) were identified in 2018 and the lowest (7%) were identified in the first three months of 2021.
Antimicrobial Susceptibility Profile
The overall resistance rate of P. aeruginosa was higher against cephalosporins, i.e. ceftazidime (35%) and cefepime (31%), followed by fluoroquinolones, i.e. levofloxacin (24%) and ciprofloxacin (18%), then carbapenems, i.e. imipenem (18%) and meropenem (13%). Combination antimicrobials were more active than the above antibiotics on their own; the resistance rate was ceftolozane-tazobactam (9%) and piperacillin-tazobactam (16%).
Sensitivity to amikacin was the highest (98%) followed by colistin (94%) and gentamicin (93%). Resistance to levofloxacin and imipenem was higher than to ciprofloxacin and meropenem, respectively. Among the 18 imipenem-resistant isolates, 16 (89%) were susceptible to amikacin, 15 (83%), 12 (67%), and 11 (61%) were susceptible to gentamicin, ceftolozane-tazobactam, and piperacillin-tazobactam, respectively. The proportional level of resistance was increased from year to year for all antimicrobials except colistin. In contrast, from the total of 100 Pseudomonas isolates 34% were susceptible to all tested antibiotics (Table 1).
Table 1.
Antibiotic Susceptibility Pattern of P. aeruginosa at National Reference Laboratory Ethiopia, January 2017 to March 2021
| Antimicrobial Category | Antibiotics | 2017 | 2018 | 2019 | 2020 | 2021 | Total | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N = 26 | N=34 | N=21 | N=12 | N=7 | N=100 | ||||||||||||||
| S | I | R | S | I | R | S | I | R | S | I | R | S | I | R | %S | %I | %R | ||
| Aminoglycosides | Amikacin | 26 | 0 | 0 | 34 | 0 | 0 | 20 | 1 | 0 | 11 | 0 | 1 | 6 | 0 | 1 | 97 | 1 | 2 |
| Gentamicin | 25 | 0 | 1 | 31 | 2 | 1 | 19 | 0 | 2 | 11 | 0 | 1 | 5 | 0 | 2 | 91 | 2 | 7 | |
| Cephalosporins | Cefepime | 21 | 0 | 5 | 25 | 0 | 9 | 12 | 0 | 9 | 7 | 0 | 5 | 4 | 0 | 3 | 69 | 0 | 31 |
| Ceftazidime | 19 | 1 | 6 | 24 | 0 | 10 | 9 | 0 | 12 | 8 | 1 | 3 | 3 | 0 | 4 | 63 | 2 | 35 | |
| Fluoroquinolones | Ciprofloxacin | 23 | 2 | 1 | 29 | 0 | 5 | 12 | 3 | 6 | 9 | 0 | 3 | 4 | 0 | 3 | 77 | 5 | 18 |
| Levofloxacin | 22 | 1 | 3 | 23 | 3 | 8 | 11 | 3 | 7 | 9 | 0 | 3 | 4 | 0 | 3 | 69 | 7 | 24 | |
| Carbapenems | Imipenem | 24 | 2 | 0 | 26 | 2 | 6 | 15 | 0 | 6 | 6 | 2 | 4 | 4 | 1 | 2 | 75 | 7 | 18 |
| Meropenem | 26 | 0 | 0 | 27 | 4 | 3 | 15 | 0 | 6 | 8 | 1 | 3 | 5 | 1 | 1 | 81 | 6 | 13 | |
| Polymyxin B | Colistin | 20 | 4 | 2 | 33 | 0 | 1 | 18 | 0 | 3 | 10 | 2 | 0 | 6 | 1 | 0 | 87 | 7 | 6 |
| β-Lactam combinations | Ceftolozane-tazobactam | 25 | 0 | 1 | 32 | 0 | 2 | 18 | 0 | 3 | 10 | 0 | 2 | 5 | 1 | 1 | 90 | 1 | 9 |
| Piperacillin-tazobactam | 20 | 2 | 4 | 26 | 2 | 6 | 12 | 7 | 2 | 9 | 1 | 2 | 4 | 1 | 2 | 71 | 13 | 16 | |
Abbreviations: S, sensitive; I, intermediate; R, Resistance.
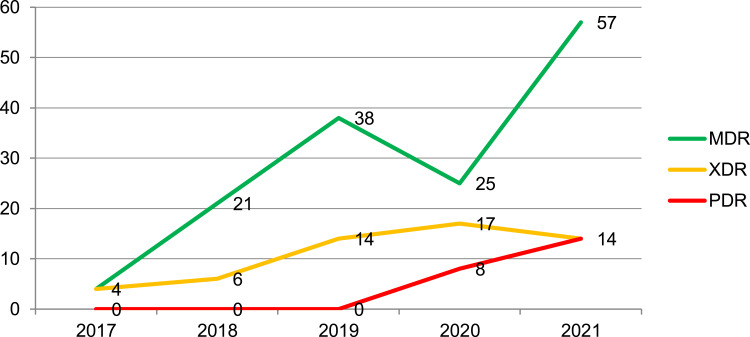
The overall MDR, XDR, and PDR rate of P. aeruginosa was found to be 23%, 9%, and 2% respectively from the total tested isolates. The proportions of MDR isolates per year showed an increasing pattern for five consecutive years except in 2020. Likewise, the proportion of XDR isolates increased from 4% to 17% from the year 2017 to 2020, rising to 14% in the first three months of 2021. In the latter years, one isolate from 2020 and another isolate from 2021, proportionally 8% and 14% of PDR isolates were identified (Figure 1).
Figure 1.
Proportions of MDR, XDR, and PDR P. aeruginosa at national reference laboratory Ethiopia, January 2017 to March 2021.
Among the total of nine XDR isolates, 6 (67%) of them were resistant to both carbapenem drugs and all 9 were resistant to both cephalosporin drugs. On the other hand, of the 23 MDR isolates, 10 (43%) were resistant to both carbapenem and cephalosporin drugs, and 18 (78%) of them were resistant to both cephalosporin drugss.
Carbapenemase Producing P. aeruginosa
Among 100 identified isolates, 27 isolates were identified as a candidate (non-susceptible to imipenem and/or meropenem) for carbapenemase enzyme production testing. Of these 10/27 (37%) were detected as carbapenemase producers by the BD Phoenix automated system; from those isolates identified as carbapenemase producers by the system, one isolate was susceptible to both carbapenem drugs, which means that it was not a candidate for carbapenemase testing, Therefore, as per the manufacturer’s recommendation, an additional confirmatory test (mCIM) was performed. Of the 27 isolates tested with the mCIM only 3/27 (11%) were positive, they were also positive by the BD Phoenix system, and the remaining seven isolates were negative by this method. Hence, the reason for the carbapenem resistance phenotype of P. aeruginosa might not always be a production of carbapenemase enzymes. One of the three carbapenemase positive isolates by mCIM was PDR and the remaining two isolates were XDR. The mCIM results are shown below in Figure 2.
Figure 2.
Modified carbapenem inactivation method results for tested P. aeruginosa at national reference laboratory Ethiopia.
In this study, the most commonly used/prescribed antimicrobials such as amoxicillin-clavulanate, ampicillin, cefazolin, ceftriaxone, cefuroxime, ertapenem, tigecycline, and trimethoprim-sulfamethoxazole were 100% confirmed as resistant.
Discussion
In this study, we have described the antimicrobial resistance profile and carbapenemase production rate of P. aeruginosa isolated from referral samples in the EPHI. The isolates were characterized to species level using a BD Phoenix automated system. In this study, 9% (10 out of 110) isolates had been misidentified as Pseudomonas spp. by the manual method. They were identified as; Stenotrophomonas maltophilia (2), Burkholderia cepacia, Alcaligenes faecalis (2), Pantoea agglomerans, Serratia marcescens, Achromobacter sp., Citrobacter farmer, and Providencia rustigianii. This misidentification could be due to the limited biochemical tests available in the laboratory and the overlapping phenotypic nature of the isolates.
In this study, P. aeruginosa (98%) was the most prevalent species and the remaining was P. putida (2%) identified as the common cause of human pathogen among the genus Pseudomonas. Similar results were reported in Ethiopia and elsewhere in the world;11–13 these reports included a few uncommon species such as P. luteola, P. stutzeri, P. mendocina, P. pseudoalcaligenes, and P. fluorescens which were not seen in this study.
P. aeruginosa is an important cause of nosocomial infection and may be isolated from any clinical specimen. In this study the majority of the isolates were recovered from wound and ear infections (46% and 18%, respectively); this finding is similar to that of another study conducted in Ethiopia.14 This might be due to the environmental spread of P. aeruginosa in the health facilities, and contaminated surgical instruments. In addition, the prevalence of this organism in ear infections might be due to its ability to survive in competition over other bacteria.15
In the present study, P. aeruginosa showed higher resistance to cephalosporins particularly to ceftazidime (35%) and cefepime (31%). This is in agreement with study findings in Ethiopia (28% and 20%)11 and in Iran (35% and 38%)16 for both drugs, respectively. In contrast, this number is reported as higher (69% and 55%) in Uganda,17 (63% and 62%) in Egypt,18 (65% and 55%) in Mexico,19 and (66% and 63%) in India12 for ceftazidime and cefepime respectively. Unnecessary prescription of cephalosporins leads to genetic alteration of the pathogen; particularly over-production of β–lactamases could be the possible reason for the increased resistance seen in those countries.
The fluoroquinolones ciprofloxacin (18%), and levofloxacin (24%) were more effective than cephalosporins. This is consistent with the previous finding in Ethiopia that reported 19.7% and 23% resistance to ciprofloxacin and levofloxacin, respectively11 and 19% average resistance for both drugs were reported in 2019 Europe AMR surveillance.20 In contrast, Ethiopian researchers reported 35–61%,14,21,22 Uganda 64%,17 and India 67%12 resistance rates for ciprofloxacin. The reason for this discrepancy could be due to merged analysis of intermediate and resistant categories, lower isolates or variation in sample size, different study settings and patient conditions, and considerable geographical difference.
In this study, the resistance rate for carbapenems, imipenem (18%), and meropenem (13%) were lower than for cephalosporins and fluoroquinolones. Thus, those drugs are considered to be good anti-pseudomonal drugs. Even though carbapenemase-producing strains may limit their use17 these agents are the last-resort drugs for gram-negative bacterial infections. Our findings were consistent with other reports from Ethiopia,22 Uganda,17 and Europe.20 However, higher levels of resistance were reported in Mexico (70% and 54%)19 and in India (53% and 63%)12 for imipenem and meropenem, respectively. The comparatively lower resistance rate to those carbapenems in this study might be because of lower prescription practice in addition to higher cost for those drugs in Ethiopia.
In this study, the imipenem resistance rate was higher than for meropenem. This could be because of molecular structure variability between the two drugs; meropenem is more potent against P. aeruginosa because it passes more quickly through the outer membrane porin-D (OprD).5 In contrast, imipenem has been less active because it is associated with a higher risk of membrane selection.23 This finding was similar to a study conducted in Uganda17 which reported 19% and 14% resistance for imipenem and meropenem, respectively.
This study revealed better antimicrobial activities in combination drugs, i.e. ceftolozane-tazobactam (9%) and piperacillin-tazobactam (16%) than cephalosporins, fluoroquinolones, and carbapenems alone. Among 18% of imipenem-resistant strains tested for combinations, 67% and 61% of them were susceptible to ceftolozane-tazobactam and piperacillin-tazobactam combinations, respectively. Therefore, combination drugs are effective agents in the treatment of cases of infections caused by carbapenem-resistant P. aeruginosa. In the same way, utilization of these combinations in clinical practice could reserve carbapenems, although inherent side effects from usage of multiple agents should be considered.23
Experimental research using rabbits demonstrated that ceftolozane-tazobactam was a highly potent anti-pseudomonal drug that can eradicate genetically distinctive Pseudomonas strains having excellent host survival with effective clearance from tissue.24 Various surveillance-based studies also showed greater than 90% susceptibility results on this drug,25 which is in agreement with our finding. Nowadays, this drug is approved by the United States Food and Drug Administration as it is a confirmed novel anti-pseudomonal β-lactam/β-lactamase inhibitor combination.26 In Ethiopia, there are no available data regarding combination drugs, particularly on ceftolozane-tazobactam.
Aminoglycosides were the most potent anti-pseudomonal drugs in this study having only a 2% resistance rate for amikacin and 7% resistance rate for gentamicin. Those drugs remain the most powerful therapeutic options for carbapenem-resistant Pseudomonas strains. Among 18 imipenem-resistant strains tested for aminoglycosides, 16 (89%) and 15 (83%) of the isolates were susceptible to amikacin and gentamicin, respectively. Our finding on aminoglycosides was comparable with the European surveillance report20 and relatively lower than the reports in Ethiopia (13–28%) for gentamicin,11,22 and the resistance rates in Uganda (31% and 69%),17 India (58% and 68%),12 and Mexico (58% and 52%)19 for amikacin and gentamicin, respectively. Amikacin is reserved for use in specialized centers for the treatment of multidrug-resistant tuberculosis in Ethiopia27 and physicians are not able to prescribe this drug as it is not available in local pharmacies; this might be the reason for the lower resistance rate reported in this study. Moreover, the report done by Ethiopian scholars seems relatively higher; this could be because of the lower sample size and merged analysis of intermediate and resistant results.
In this study, colistin was the only treatment of choice for PDR P. aeruginosa, despite having a 6% overall resistant rate. This drug was previously not used by clinicians, due to its nephrotoxicity and neurotoxicity but nowadays it is being prescribed, and international consensus is that the optimal use of this drug would be for the treatment of infections with MDR gram-negative bacilli, particularly for P. aeruginosa and Acinetobacter spp.28,29 As of 2020, colistin is on the Ethiopian essential medicines list; it is reserved for the treatment of confirmed or suspected infections caused by MDR organisms.27 Our finding supports the introduction of colistin into clinical practice for the treatment of infections caused by PDR P. aeruginosa. This has also been seen with clinical research studies, with the clinical outcome of patients. This is needed in the Ethiopian context.
The colistin-resistance rate was similar to that seen in studies conducted by Saderi and Owlia in Iran16 which reported a rate of 9%, the rate was lower than that reported in Egypt (23%).18 The disparity might be because of method variability and/or prevalence of colistin-resistant strains due to misuse of this drug in veterinary medicine, since it has been widely used for growth promotion in food-producing animals.30 The emergence of colistin-resistant strains of P. aeruginosa is highly alarming and it is a serious global problem because there are no other drugs that can be used. In Ethiopia, there are limited data on colistin resistance status, therefore research should be encouraged to identify the sequence type and resistance determinants so that there will be detailed information on such strains.
The overall proportion of MDR, XDR, and PDR isolates of P. aeruginosa in this study was 23%, 9%, and 2% respectively. The definition of those abbreviated words was done according to the international standard document for P. aeruginosa31 with the exception of colistin in the case of PDR isolates. Our findings on MDR, XDR, and PDR Pseudomonas isolates were comparable with the five years European antimicrobial resistance surveillance report20 which was 10% for MDR, 6.2% for XDR, and 3.4% for resistance to five antimicrobial groups. Whereas from 55% for MDR, 33% for XDR, and 0% for PDR were reported in Iran.16 The proportion of MDR and XDR isolates increased from year to year in our study, this showed that antimicrobial resistance is progressive. The occurrence of PDR isolates from the latter two years is highly alarming, and prompt action is needed in order to curtail the development of resistance.
P. aeruginosa has different acquired resistance mechanisms,5,6 the production of carbapenemase enzyme is the most serious one as this enzyme can hydrolyze all penicillins, cephalosporins, and carbapenem drugs. In our finding, the rate of carbapenemase enzyme was found to be 10% from all isolates and 37% (10/27) from candidate isolates by the BD Phoenix system, while it was 3% from all isolates and 11% (3/27) from candidates using mCIM. This method was investigated by CLSI and demonstrated 100% specificity and >97% sensitivity for the detection of carbapenemases among P. aeruginosa.7 Therefore, even though automated systems are efficient for bacterial ID and AST testing, the software in the BD Phoenix system will not always precisely identify the presence of this enzyme; similar results were reported in Uganda.17 In this regard, we can conclude that the majority of carbapenem-resistant P. aeruginosa isolates in this study were not associated with the production of the carbapenemase enzyme.
The total referral samples and the number of Pseudomonas isolates had decreased in 2020 because of the Covid-19 pandemic, and only three month’s data were taken from 2021, which makes it difficult to identify a trend or association between the study period and resistance pattern of this organism, in addition, this could be the possible reason for the decrement of MDR isolates in 2020. Moreover, because of inconsistent results (except resistant ones) in the BD Phoenix system, the MIC value of colistin was interpreted manually referring to the most recent CLSI guideline, and two anti-pseudomonal drugs, piperacillin and aztreonam, were not tested because those drugs are not incorporated in the AST panel. The other major limitation of this study was that molecular characterization of resistant strains and carbapenemases could not be performed.
Conclusions
In this study, the increasing rate of MDR and XDR isolates and the appearance of PDR P. aeruginosa strains in Ethiopia is a serious problem. This is compounded by the lack of newer alternative anti-pseudomonal drugs. Utilization of colistin in clinical practice in cases of infection by PDR P. aeruginosa strains has a life-saving advantage. In the same way, aminoglycosides and combination drugs particularly ceftolozane-tazobactam can be used in the treatment of infection by carbapenem resistance P. aeruginosa strains.
Antimicrobials have different efficacy in various geographical locations, therefore updating the treatment guidelines in the local setting using such kinds of studies is very crucial. Treatment of bacterial infections should be guided by antibiotic susceptibility test results.
Acknowledgments
Addis Ababa University, College of Health Science Department of Medical Laboratory Sciences.
Ethiopian Public Health Institute.
We sincerely express our heartfelt gratitude to all staff members of the NCBMRL case team; particularly Mr. Estifanos Tsige, Mr. Dawit Assefa, and Zelalem Tazu.
Funding Statement
This research project was sponsored by the Department of Medical Laboratory Sciences, College of Health Sciences, Addis Ababa University, and the Ethiopian Public Health Institute. The fund was used for purchasing Identification and AST panels, supplies, data analysis, and interpretation.
Abbreviations
AST, Antimicrobial Susceptibility Testing; ATCC, American Type Culture Collection; BAP, Blood Agar Plate; BD, Becton and Dickinson; CLSI, Clinical and Laboratory Standard Institute; EPHI, Ethiopian Public Health Institute; ID, Identification; mCIM, Modified Carbapenem Inactivation Method; MDR, Multiple Drug Resistance; NCBMRL, National Clinical Bacteriology and Mycology Reference Laboratory; PDR, Pan-Drug Resistant; QC, Quality Control; TSB, Tryptic Soy Broth; XDR, Extensive Drug Resistance.
Data Sharing Statement
All the necessary data is presented in this manuscript and there are no supplementary files. The original data that we used in this report can be provided upon request.
Ethics Approval and Consent to Participate
The study was carried out after the approval of the research and ethics review committee of the Department of Medical Laboratory Sciences, College of Health Sciences, Addis Ababa University (DRERC/566/20/MLS), and permission was given by the EPHI institutional review board and NCBMRL. All the information gathered from the study subjects was coded to maintain confidentiality.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, agreed to the submitted journal, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
- 1.Fujitani S, Moffett KS, Yu V. Pseudomonas aeruginosa. Antimicrobe: infectious Disease & Antimicrobial Agents; 2017. Available from:http://www.antimicrobe.org/new/b112.asp. Accessed August19, 2021
- 2.Karami P, Mohajeri P, Mashouf RY, et al. Molecular characterization of clinical and environmental Pseudomonas aeruginosa isolated in a burn center. Saudi J Biol Sci. 2019;26(7):1731–1736. doi: 10.1016/j.sjbs.2018.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tindall BJ, Kämpfer P, Euzéby JP, Oren A. Valid publication of names of prokaryotes according to the rules of nomenclature: past history and current practice. Int J Syst Evol Microbiol. 2006;56(11):2715–2720. doi: 10.1099/ijs.0.64780-0 [DOI] [PubMed] [Google Scholar]
- 4.Moore NM, Flaws ML. Introduction: pseudomonas aeruginosa. Clin Lab Sci. 2011;24(1):41. doi: 10.29074/ascls.24.1.41 [DOI] [PubMed] [Google Scholar]
- 5.Meletis G, Exindari M, Vavatsi N, Sofianou D, Diza E. Mechanisms responsible for the emergence of carbapenem resistance in Pseudomonas aeruginosa. Hippokratia. 2012;16(4):303. [PMC free article] [PubMed] [Google Scholar]
- 6.Oliver A, Mulet X, López-Causapé C, Juan C. The increasing threat of Pseudomonas aeruginosa high-risk clones. Drug Resistance Updates. 2015;21:41–59. doi: 10.1016/j.drup.2015.08.002 [DOI] [PubMed] [Google Scholar]
- 7.CLSI. Performance standards for antimicrobial susceptibility testing.30th ed. CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standard Institute; 2020. [Google Scholar]
- 8.World Health Organization. Prioritization of pathogens to guide discovery, research and development of new antibiotics for drug-resistant bacterial infections, including tuberculosis; 2017. Available from:www.who.int/medicines/areas/rational_use/PPLreport_2017_09_19.pdf?ua=1. Accessed August19, 2021.
- 9.Phoenix BD. Laboratory procedure. latest released user manual; 2020:13–19.
- 10.Lancaster MV, Fields RD. Antibiotic and cytotoxic drug susceptibility assays using resazurin and poising agents. Google Patents; 1996.
- 11.Bitew A. High Prevalence of Multi-Drug Resistance and Extended Spectrum Beta Lactamase Production in Non-Fermenting Gram-Negative Bacilli in Ethiopia. Infect Dis. 2019;12:1178633719884951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumari M, Khurana S, Bhardwaj N, Malhotra R, Mathur P. Pathogen burden & associated antibiogram of Pseudomonas spp. in a tertiary care hospital of India. Indian J Med Res. 2019;149(2):295. doi: 10.4103/ijmr.IJMR_14_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.England PH. Identification of Pseudomonas species and other NonGlucose Fermenters. UK Standards for Microbiology Investigations. ID 17 Issue 3; 2015. Available from:https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/422699/ID_17i3.pdf. Accessed August19, 2021.
- 14.Mulu W, Abera B, Yimer M, Hailu T, Ayele H, Abate D. Bacterial agents and antibiotic resistance profiles of infections from different sites that occurred among patients at Debre Markos Referral Hospital, Ethiopia: a cross-sectional study. BMC Res Notes. 2017;10(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davane M, Suryawanshi N, Pichare A, Nagoba BS. Pseudomonas aeruginosa from hospital environment. J Microbiol Infect Dis. 2014;4:01. doi: 10.5799/ahinjs.02.2014.01.0124 [DOI] [Google Scholar]
- 16.Saderi H, Owlia P. Detection of multidrug resistant (MDR) and extremely drug resistant (XDR) P. aeruginosa isolated from patients in Tehran, Iran. Iranian j Pathol. 2015;10(4):265. [PMC free article] [PubMed] [Google Scholar]
- 17.Kateete DP, Nakanjako R, Namugenyi J, Erume J, Joloba ML, Najjuka CF. Carbapenem resistant Pseudomonas aeruginosa and Acinetobacter baumannii at Mulago Hospital in Kampala, Uganda (2007–2009). Springerplus. 2016;5(1):1–11. doi: 10.1186/s40064-016-2986-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abd El-Baky RM, Masoud SM, Mohamed DS, et al. Prevalence and some possible mechanisms of colistin resistance among multidrug-resistant and extensively drug-resistant Pseudomonas aeruginosa. Infect Drug Resist. 2020;13:323. doi: 10.2147/IDR.S238811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uc-Cachón AH, Gracida-Osorno C, Luna-Chi IG, Jiménez-Guillermo JG, Molina-Salinas GM. High prevalence of antimicrobial resistance among gram-negative isolated Bacilli in intensive care units at a tertiary-care hospital in Yucatan Mexico. Medicina. 2019;55(9):588. doi: 10.3390/medicina55090588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.European Centre for Disease Prevention and Control. Antimicrobial resistance in the EU/ EEA (EARS-Net) - Annual Epidemiological Report 2019. Stockholm: ECDC; 2020. Available from:https://www.ecdc.europa.eu/sites/default/files/documents/surveillance-antimicrobial-resistance-Europe-2019.pdf. Accessed August19, 2021. [Google Scholar]
- 21.Motbainor H, Bereded F, Mulu W. Multi-drug resistance of blood stream, urinary tract and surgical site nosocomial infections of Acinetobacter baumannii and Pseudomonas aeruginosa among patients hospitalized at Felegehiwot referral hospital, Northwest Ethiopia: a cross-sectional study. BMC Infect Dis. 2020;20(1):92. doi: 10.1186/s12879-020-4811-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mekonnen H, Seid A, Molla G, Gebrecherkos T. Antimicrobial Resistance Profiles and associated factors of Acinetobacter and Pseudomonas aeruginosa nosocomial infection among patients admitted at Dessie comprehensive specialized Hospital, North-East Ethiopia. A cross sectional study; 2021. [DOI] [PMC free article] [PubMed]
- 23.Kanj SS, Kanafani ZA, editors. Current concepts in antimicrobial therapy against resistant gram-negative organisms: extended-spectrum β-lactamase–producing enterobacteriaceae, carbapenem-resistant enterobacteriaceae, and multidrug-resistant Pseudomonas aeruginosa. Mayo Clinic Proceedings. Elsevier; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petraitis V, Petraitiene R, Naing E, et al. Ceftolozane-tazobactam in the treatment of experimental Pseudomonas aeruginosa pneumonia in persistently neutropenic rabbits: impact on strains with genetically defined mechanisms of resistance. Antimicrob Agents Chemother. 2019;63(9):344–319. doi: 10.1128/AAC.00344-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farrell DJ, Flamm RK, Sader HS, Jones RN. Antimicrobial activity of ceftolozane-tazobactam tested against Enterobacteriaceae and Pseudomonas aeruginosa with various resistance patterns isolated in US hospitals (2011–2012). Antimicrob Agents Chemother. 2013;57(12):6305. doi: 10.1128/AAC.01802-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho JC, Fiorenza MA, Estrada SJ. Ceftolozane/Tazobactam: a Novel Cephalosporin/β‐Lactamase Inhibitor Combination. Pharmacotherapy. 2015;35(7):701–715. doi: 10.1002/phar.1609 [DOI] [PubMed] [Google Scholar]
- 27.Authority M. Ethiopian Essential Medicines List; 2020. Available from:http://www.fmhaca.gov.et/wp-content/uploads/2020/12/EML-sixth-edition.pdf. Accessed August19, 2021.
- 28.Dijkmans AC, Wilms EB, Kamerling IM, et al. Colistin: revival of an old polymyxin antibiotic. Ther Drug Monit. 2015;37(4):419–427. doi: 10.1097/FTD.0000000000000172 [DOI] [PubMed] [Google Scholar]
- 29.Tsuji BT, Pogue JM, Zavascki AP, et al. International Consensus Guidelines for the Optimal Use of the Polymyxins: endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti‐infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy. 2019;39(1):10–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization. The detection and reporting of colistin resistance; 2018. Available from:https://apps.who.int/iris/bitstream/handle/10665/277175/WHO-WSI-AMR-2018.4-eng.pdf?sequence=1&isAllowed=y. Accessed August19, 2021.
- 31.Magiorakos A-P, Srinivasan A, Carey R, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi: 10.1111/j.1469-0691.2011.03570.x [DOI] [PubMed] [Google Scholar]