Abstract
Introduction:
Pancreaticoduodenectomy is historically associated with incisional surgical site infection (iSSI) rates between 15-20%. Prospective studies have been mixed with respect to the benefit of individual interventions directed at decreasing iSSI. We hypothesized that the application of a perioperative bundle during pancreaticoduodenectomy would significantly decrease the rate of iSSI.
Methods:
An initial cohort of 150 consecutive post-pancreaticoduodenectomy patients were assessed within 2-4 weeks of operation to determine baseline iSSI rates. The Centers for Disease Control definition of iSSI was utilized. A four-part perioperative bundle was then instituted for the second cohort of 150 patients. This bundle consisted of a double ring wound protector, gown/glove and drape change prior to fascial closure, irrigation of the wound with bacitracin solution, and a negative pressure wound dressing which was left in place until postoperative day 7, or day of discharge. 300 patients provided 80% power to detect a 50% risk reduction in iSSI.
Results:
Cohorts 1 and 2 were similar with respect to age (68 vs 69 yrs, p=0.92), gender (male, 51% vs 55%, p=0.644), BMI (26 vs 26, p=0.928), use of neoadjuvant therapy (32% vs 25%, p=0.377), median operative time (222 vs 215 min, p=0.366) and the presence of a preoperative stent (53% vs 41%, p=0.064). The iSSI rate was 22.3% in the initial cohort. This rate was higher than both our institutional database (13%) and NSQIP reporting (11%). Within the second cohort, the iSSI rate decreased significantly to 10.7% (n=16; p=0.012). All four components of the bundle were utilized in 91% of cohort 2 patients.
Conclusion:
In this cohort study of 300 consecutive patients who underwent pancreaticoduodenectomy, the implementation of a four-part bundle decreased iSSI from 22% to 11%.
Keywords: surgical site infection, wound infection, pancreaticoduodenectomy
Precis:
The implementation of a four-part perioperative bundle was associated with a significant reduction in incisional surgical site infection (iSSI) in patients undergoing pancreaticoduodenectomy. Patients who received the perioperative bundle were 50% less likely to develop iSSI than those who did not.
Introduction
Surgical site infections (SSI) account for approximately 20% of all hospital acquired infections (HAI), and will occur in 2-5% of surgical patients each year.1, 2 Categorized as superficial incisional, deep incisional, and organ/space based on the depth of the infection,3 SSI can increase hospital costs by approximately $20,000 per admission,4 and add an average of 10 days to inpatient length-of-stay.2 Given this effect on patient outcomes and healthcare costs, much work has been done to develop interventions that can decrease the rate of SSI.5 The most robust data have been produced in the fields of colorectal, gynecology, and urology which involve manipulation of the gastrointestinal or genitourinary systems; or in fields such as orthopedics, neurosurgery, and cardiothoracic, in which placement of prostheses require all effort to reduce SSI.
Efforts at reducing SSI in patients undergoing pancreaticoduodenectomy (PD) may have received less attention because postoperative pancreatic fistula is a unique and challenging morbidity associated with this procedure. Previous data however, have noted overall SSI rates in pancreatic surgery to be as high as 30%6, 7 and specifically, incisional SSI (iSSI) rates as high as 20%.7-9 A previously published retrospective study demonstrated that the implementation of a 12-part surgical care bundle led to a significant decrease in the rate of wound infection in patients undergoing PD.10 Prior studies that have evaluated single interventions to lower iSSI rates in abdominal surgery have been mixed. For example, two separate prospective randomized trials have evaluated negative pressure wound therapy. In a study by Shen et al, this intervention did not decrease wound infection rates in patients undergoing major abdominal surgery (PD included),11 whereas a more recent trial from Johns Hopkins University suggested a decrease in wound infection rates with this approach.12
We designed this study to evaluate the impact of a four-part perioperative bundle aimed at reducing iSSI following PD. Because the literature suggested that this would decrease iSSI, we designed the trial as a prospective cohort study, rather than a randomized trial. Additionally, we sought to define patient and operative variables that are associated with an increased risk of iSSI in our cohort.
Methods
Patient selection
Two consecutive cohorts of patients who underwent PD at Memorial Sloan Kettering (MSK) between September 2016 and June 2018 were included. Approval for this study was obtained from MSK’s Institutional Review Board. Patients underwent PD by one of seven hepatopancreatobiliary surgeons in the Department without any change in their standard practice between September 2016 and June 2017 (cohort 1), followed by implementation of a perioperative bundle between July 2017 and June 2018 (cohort 2).
All patients were prospectively identified from a weekly review of the operative schedule. Demographic, clinical, laboratory and pathologic data were collected in a prospectively maintained database from the electronic medical record. Operative records were reviewed to ensure PD was completed in all patients, and patients were excluded if their PD was aborted or converted to another procedure.
The primary endpoint was development of a superficial or deep iSSI within 30 days of operation. Organ/space SSI and iSSI that were associated with organ/space infections were not included in the final analysis. Secondary endpoints included the evaluation of clinical factors associated with iSSI.
Evaluation of SSI
A questionnaire was developed based on the Centers for Disease Control and Prevention (CDC) and American College of Surgeons National Surgical Quality Improvement Program (NSQIP) guidelines for superficial and deep iSSI.13, 14 This questionnaire was used by office staff and clinicians to evaluate all PD patients during outpatient visits in the 30 days following the operation, with day one being counted as the day of the operation as per CDC/NSQIP. iSSI were also identified through review of the patient’s postoperative inpatient progress notes. The same evaluation system was used to identify iSSI both before and after implementation of the perioperative bundle.
The iSSI data obtained from our questionnaire was compared to the iSSI rates that are captured by larger national and institutional databases. To do this, data from the NSQIP database was obtained for patients who underwent PD at our institution between September 2016 and June 2017. Additionally, our Departmental database was reviewed for documentation of iSSI during this time period. Again, only iSSI without associated organ/space infections were included in this analysis.
Development and Implementation of the Perioperative Bundle
At the time of study design, review of the literature identified three interventions with varying levels of evidence supporting their role in reducing SSI rates individually in non-pancreatic surgery - use of a wound protector,15-18 antibiotic irrigation,19 and incisional negative pressure dressing.20, 21 In addition, a fourth intervention, gown and glove change with new sterile instruments for fascial closure, was noted to be a common practice in SSI reduction bundles both at our institution and in the literature.10, 22
The perioperative bundle implemented consisted of four components: 1. double-ring wound protector (Alexis Wound Protector/Retractor, Applied Medical, Rancho Santa Margarita, CA); 2. 500cc of bacitracin/saline irrigation of incision prior to skin closure; 3. gown/glove change and sterile instruments for fascial and skin closure; 4. negative pressure incisional dressing (PICO System, Smith & Nephew, St. Petersburg, Fl) placed over the closed surgical wound until postoperative day 7 or the day of discharge, whichever occurred first. The size of the wound protector and PICO dressing varied based on incision size, and was left to the discretion of the operating surgeon. Of note, all patients received perioperative antibiotics per institutional protocol, and our standard approach to operative fluid management and blood sugar control was utilized as previously published.23
This four-step perioperative bundle was implemented by all attending surgeons as a change-of-practice for all patients who underwent PD on or after July 2017. Prior to implementation, in-service presentations were provided to all physicians, nurse practitioners, operating room and ward nurses, and research study assistants within the hepatopancreatobiliary division. All patients who had at least half of the bundle were included as an intention to treat analysis.
Statistical Analysis
Sample size was calculated to provide 80% power to detect a 50% reduction in iSSI. It was determined that the study population should consist of 300 patients, and there would be 150 patients in each of the pre- and post-bundle cohorts.
Continuous data were expressed as median and range, and categorical variables were expressed as frequency and percentage. Fisher’s exact test and Wilcoxon rank sum test were used to compare differences in parameters between bundle and SSI groupings. All tests were two-sided and p<0.05 was considered significant. Multivariable logistic regression was used to build a model to control for covariates that were significant at p<0.05 in the univariate analysis. SAS version 9.4 SAS institute Inc., Cary, NC was used for all analysis.
Results
This study included a total of 300 patients who underwent PD; the first cohort consisted of 150 patients prior to implementation of the bundle and the second cohort was comprised of 150 patients who received the perioperative bundle (Figure 1). Demographic, clinical and operative characteristics of the study cohort are listed in Table 1. Of note, approximately half of patients were jaundiced at presentation (52%, n=156) and 47% (n=141) received a preoperative biliary stent. Of all patients included, 19% (n=57) received neoadjuvant therapy. Final pathologic diagnosis was: pancreatic ductal adenocarcinoma (PDAC) in 60% (n=180), ampullary or duodenal adenocarcinoma in 10% (n=31), non-invasive intraductal papillary mucinous neoplasm (IPMN) in 10% (n=31) and cholangiocarcinoma in 4% (n=13). The remainder of pathologic diagnoses varied at a frequency <4%.
Figure 1.
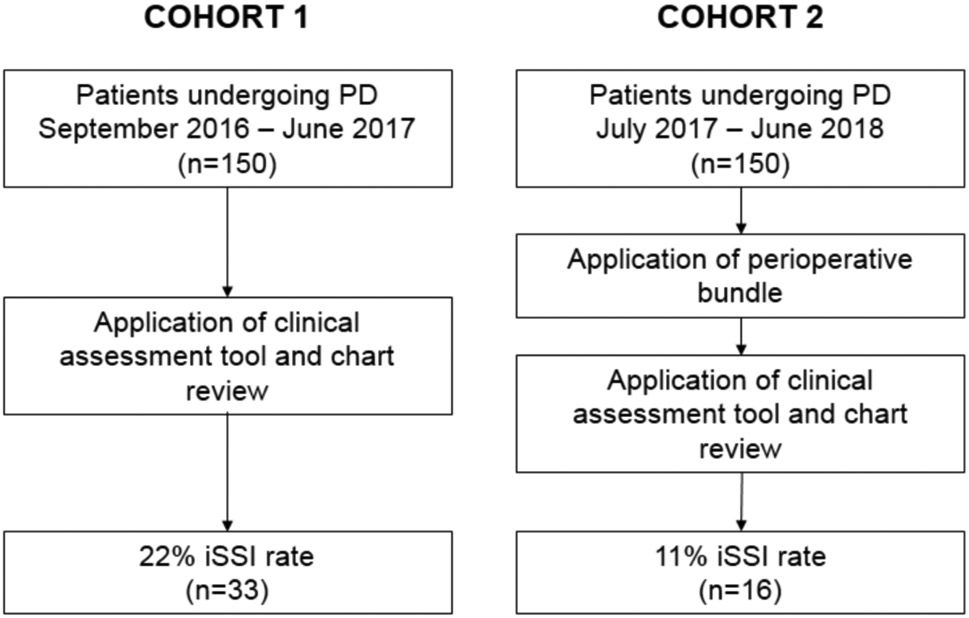
Study Workflow
Table 1.
Patient demographic, clinical, and operative characteristics in the pre- and post-bundle cohorts. Median (range) or n (%).
| Variable | All Patients (n=300) |
Pre-Bundle (n=150) |
Post-Bundle (n=150) |
p-value |
|---|---|---|---|---|
| Male gender | 159 (53) | 77 (51) | 82 (55) | 0.644 |
| Age, years | 68 (19-94) | 68 (24-90) | 69 (19-94) | 0.918 |
| Body mass index | 26 (17-78) | 26 (18-78) | 26 (17-47) | 0.928 |
| Weight loss | 118 (39) | 64 (43) | 54 (36) | 0.287 |
| Smoking history | 164 (55) | 89 (59) | 75 (50) | 0.131 |
| Diabetes | 65 (22) | 38 (25) | 27 (18) | 0.161 |
| Immunosuppressed | 10 (3) | 3 (2) | 7 (5) | 0.335 |
| Preoperative stent | 141 (47) | 79 (53) | 62 (41) | 0.064 |
| Preoperative jaundice | 156 (52) | 82 (55) | 74 (49) | 0.419 |
| Neoadjuvant therapy | 57 (19) | 32 (21) | 25 (17) | 0.377 |
| Pylorus preserving | 100 (33) | 52 (35) | 48 (32) | 0.713 |
| Operative time, minutes | 216 (100-640) | 222 (112-480) | 215 (100-640) | 0.366 |
| Estimated blood loss, cc | 250 (10.0-4600) | 250 (25.0-4600) | 200 (10.0-2000) | 0.086 |
The rates of iSSI prior to implementation of the bundle were captured using the questionnaire developed for the study, and subsequently compared to the rates of iSSI as noted by NSQIP and institutional data. Using our questionnaire, the incidence of iSSI was 22% in the first cohort of patients. In contrast, the iSSI rate for this cohort was calculated to be 11% using NSQIP data and 13% based on the information recorded in our departmental database.
Patients in both cohorts were similar with regards to baseline demographic and clinical variables (Table 1). Additionally, operative variables, including pylorus-preserving PD, length of operation and estimated blood loss (EBL) were similar between the two groups. The incidence of iSSI prior to the use of the perioperative bundle was 22% (n=33), and was reduced to 11% (n=16) following bundle implementation (p=0.012). Time between operation and development of iSSI was similar in both groups (median (range), pre-bundle 12 days (4-29) and post-bundle 12.5 days (7-24), p=0.422).
Table 2 lists demographic and clinical variables based on the presence or absence of iSSI. Neoadjuvant therapy (31% vs 17%, p=0.029), jaundice (74% vs 48%, p<0.001), and preoperative biliary stent placement (76% vs 41%, p<0.001) were more common in patients who developed iSSI. Furthermore, patients who developed iSSI were more likely to have a longer operation (238 vs 214 minutes, p=0.028) and greater EBL (300 vs 250cc, p=0.017). Body mass index (BMI), weight loss, smoking history, and diabetes were not significantly different between those who did and did not develop an iSSI.
Table 2.
Patient demographic, clinical, and operative characteristics in patients who did and not develop iSSI. Median (range) or n (%).
| Variable | All Patients (n=300) |
iSSI absent (n=251) |
iSSI present (n=49) |
p-value |
|---|---|---|---|---|
| Male gender | 159 (53) | 127 (51) | 32 (65) | 0.062 |
| Age, years | 68 (19-94) | 69 (19-94) | 67 (35-89) | 0.691 |
| Body mass index | 26 (17-78) | 26 (17-78) | 27 (19-38) | 0.086 |
| Weight loss | 118 (39) | 93 (37) | 25 (51) | 0.079 |
| Smoking history | 164 (55) | 134 (53) | 30 (61) | 0.349 |
| Diabetes | 65 (22) | 53 (21) | 12 (25) | 0.575 |
| Immunosuppressed | 10 (3) | 10 (4) | 0 (0) | 0.376 |
| Preoperative stent | 141 (47) | 104 (41) | 37 (76) | <0.001 |
| Preoperative jaundice | 156 (52) | 120 (48) | 36 (74) | <0.001 |
| Neoadjuvant therapy | 57 (19) | 42 (17) | 15 (31) | 0.029 |
| Pylorus preserving | 100 (33) | 80 (32) | 20 (41) | 0.248 |
| Operative time, minutes | 216 (100-640) | 214 (100-480) | 238 (128-640) | 0.028 |
| Estimated blood loss, cc | 250 (10-4600) | 250 (10-3500) | 300 (50-4600) | 0.017 |
Univariate analysis of these variables demonstrated a significant association between development of iSSI and receiving neoadjuvant therapy, jaundice at presentation, preoperative biliary stent placement, longer operative time, and increased blood loss (Table 3). Use of the perioperative bundle was associated with a decreased rate of iSSI on univariate analysis (OR 0.42, 95%CI 0.22-0.81, p=0.009). Using multivariate logistic regression to control for confounding variables, use of the perioperative bundle was found to be independently associated with the rate of iSSI (OR 0.50, 95% CI 0.25-0.99, p=0.046). Conversely, the presence of a preoperative biliary stent was independently associated with an increased rate of iSSI (OR 3.13, 95% CI 1.05, p=0.041).
Table 3.
Univariate and multivariate analysis of demographic, clinical and operative variables based on presence of SSI.
| Variable | Univariate analysis |
Multivariate Analysis |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Male gender | 1.84 | 0.97-3.48 | 0.062 | |||
| Age | 1.00 | 0.97-1.03 | >0.95 | |||
| Body mass index | 1.02 | 0.98-1.07 | 0.320 | |||
| Weight loss | 1.77 | 0.96-3.28 | 0.069 | |||
| Smoking history | 1.38 | 0.74-2.58 | 0.315 | |||
| Diabetes | 1.21 | 0.59-2.48 | 0.600 | |||
| Immunosuppressed | 0.00 | 0.00 | >0.95 | |||
| Preoperative stent | 4.36 | 2.17-8.76 | <0.001 | 3.13 | 1.05-9.34 | 0.041 |
| Preoperative jaundice | 3.02 | 1.53-5.97 | 0.001 | 1.24 | 0.42-3.71 | 0.698 |
| Neoadjuvant therapy | 2.20 | 1.10-4.39 | 0.026 | 1.76 | 0.81-3.82 | 0.152 |
| Pylorus preserving | 1.47 | 0.79-2.76 | 0.226 | |||
| Operative time | 2.33 | 1.13-4.80 | 0.022 | 1.16 | 0.89-1.52 | 0.258 |
| Estimated blood loss | 1.40 | 1.09-1.78 | 0.007 | |||
| Use of perioperative bundle | 0.42 | 0.22-0.81 | 0.009 | 0.50 | 0.25-0.99 | 0.046 |
In the pre-bundle cohort, the complete bundle was never utilized, however, individual components were used based on the preference of the operating surgeon. A wound protector was used in 14% (n=21) of cases, bacitracin irrigation in 13% (n=19), and gown/glove and instrument change for fascial closure in 8% (n=12). Negative pressure incisional therapy was not used. In the second cohort, all four components of the bundle were used 91% of the time.
Discussion
SSIs are seen in 2-5% of all surgical patients,1 and can significantly contribute to postoperative morbidity and increased healthcare costs. Multiple studies have evaluated the use of perioperative interventions that decrease iSSI rates in clean-contaminated cases, including PD. A prior retrospective cohort study by Lavu et al. demonstrated that use of a 12-part perioperative bundle for patients undergoing PD significantly reduced the incidence of wound infection.10
In this prospective cohort study, we demonstrate that the implementation of a four-part bundle, consisting of a plastic wound protector, antibiotic irrigation, gown/glove and instrument change for facial closure and negative pressure incisional dressing, significantly reduced the incidence of iSSI following PD. The perioperative bundle was chosen from review of available literature at the time of study design, which supported the use of wound protectors, antibiotic irrigation and negative pressure incisional dressings in clean-contaminated cases. The rationale for these interventions include physical barrier to contamination of the abdominal wall provided by the wound protector, reduction in bacterial load with the use of antibiotic irrigation, and negative pressure wound therapy to remove dead space and suction fluid. Changing of gown/gloves and the use of new sterile instruments were included in our bundle as they are affordable interventions, and have been components of successful perioperative bundles, although there was a lack of specific data supporting their use.
Subsequent to the start of our study, multiple studies have been published that report on the use of individual interventions for reduction of iSSI specifically in patients undergoing pancreatic resection. Several studies demonstrated a decrease in iSSI rates with the use of incisional negative pressure therapy.12, 24, 25 Additionally, two separate studies have demonstrated that the use of wound protectors in PD is associated with fewer iSSIs.26, 27 One of these studies was a randomized control trial that included only patients with preoperative biliary stents, a high-risk population.26 Finally, a single study demonstrated a lower iSSI rate with instrument and drape change in all pancreatic resections.28 However, not all studies have shown a benefit. A randomized control trial by Shen et al. noted no difference in ISSI following pancreatic resection, however, this study included included both proximal and distal pancreatectomies, as well as other major abdominal operations.11 Given the totality of the literature, we believe that all of these interventions have a degree of protective benefit, and that application of multiple interventions (perioperative bundle) is justified.
The data from the current study also confirm that neoadjuvant therapy, preoperative biliary stenting, increased operative time, and greater blood loss are factors associated with iSSI. The strongest of these was preoperative biliary stenting, as it was independently associated with an approximate 3-fold risk in iSSI. This is consistent with previous studies that have evaluated risk factors for postoperative wound infection following pancreatic resection.7, 8, 29-31 Although some of these factors are recognized prior to operation, and interventions for reduction of iSSI could target high-risk patients, we would favor routine use of a perioperative bundle given that operative variables can be unpredictable.
Furthermore, our proposed interventions should be viewed as affordable, and do not increase operative time in a clinically meaningful manner. Estimating costs with readily available data, total cost of the perioperative bundle is under $600 (retail cost of the Alexis wound protector is approximately $200, bacitracin irrigation $30, surgical gown/gloves $10 and PICO system $320). These estimates are retail, and thus we anticipate the true cost to be even less given the significant discount received through hospital contracts. The estimated reported cost of a single SSI is generally approximated at $15,000 in recent studies.4, 32, 33 Therefore, for this bundle to be “cost-neutral” the number needed to treat to prevent one wound infection would be approximately 25. The data from our study suggest that only patients need to be treated with the bundle to prevent one SSI. PLEASE CHECK WITH KEN FOR THAT NUMBER
The true incidence of SSI can be difficult to capture through conventional methods, including evaluation of ICD9 codes and NSQIP reporting. We calculated the incidence of iSSI to be 11-13% in the first cohort of patients using NSQIP and institutional databases. However, after implementing a more structured tool for prospectively evaluating iSSI in postoperative patients, the measured rate was higher at 22%. This suggests that conventional methods may underestimate true iSSI rates, and that the magnitude of the problem may be larger than has been generally reported.
There are several limitations to this study. One is the inconsistent use of the perioperative bundle in cohort 2, and the limited use of the individual components in cohort 1. During the time period of cohort 2, components of the bundle were occasionally omitted secondary to operating room and patient factors. All patients who had at least half of the bundle were included as an intention to treat analysis, as these variations in practice are to be expected in a standard surgical practice. With 91% compliance, we were able to demonstrate a 50% reduction in iSSI during the study time. Another limitation of this study is our inability to pinpoint which aspect of the bundle provides the greatest amount of protection against surgical site infection. Although we believe that in comparison to the cost of management of SSI, the cost of the bundle is acceptable, it would be valuable to know which components are most important, and in which patients. Finally, as this was not a randomized trial, our attention to the problem of SSI increased with time, and thus our reported decrease in SSI may be secondary to Hawthorne effect rather than a true causal relationship to the implementation of a perioperative bundle. The randomized trials noted above, however, do demonstrate a causal relationship and thus we should assume that there is a true protective effect of perioperative bundle implementation.
Conclusion
In this study, the implementation of a four-part bundle was associated with a decrease in the rate of iSSI from 22% to 11% in patients undergoing PD. Furthermore, after controlling for neoadjuvant therapy, preoperative biliary stent, operative time and EBL, perioperative bundle use was independently associated with improved outcome for SSI. Patients who received the perioperative bundle had a 50% decreased risk of iSSI as compared to those who did not. Further randomized studies should evaluate this bundle in other clean-contaminated cases to validate its use for reduction of iSSI.
References
- 1.Anderson DJ, Podgorny K, Berrios-Torres SI, et al. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol 2014; 35(6):605–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ban KA, Minei JP, Laronga C, et al. American College of Surgeons and Surgical Infection Society: Surgical Site Infection Guidelines, 2016 Update. J Am Coll Surg 2017; 224(1):59–74. [DOI] [PubMed] [Google Scholar]
- 3.Horan TC, Gaynes RP, Martone WJ, et al. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol 1992; 13(10):606–8. [PubMed] [Google Scholar]
- 4.Zimlichman E, Henderson D, Tamir O, et al. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med 2013; 173(22):2039–46. [DOI] [PubMed] [Google Scholar]
- 5.Berrios-Torres SI, Umscheid CA, Bratzler DW, et al. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg 2017; 152(8):784–791. [DOI] [PubMed] [Google Scholar]
- 6.Ceppa EP, Pitt HA, House MG, et al. Reducing surgical site infections in hepatopancreatobiliary surgery. HPB (Oxford) 2013; 15(5):384–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elliott IA, Chan C, Russell TA, et al. Distinction of Risk Factors for Superficial vs Organ-Space Surgical Site Infections After Pancreatic Surgery. JAMA Surg 2017; 152(11):1023–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fadayomi AB, Kasumova GG, Tabatabaie O, et al. Unique predictors and economic burden of superficial and deep/organ space surgical site infections following pancreatectomy. HPB (Oxford) 2018; 20(7):658–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fong ZV, McMillan MT, Marchegiani G, et al. Discordance Between Perioperative Antibiotic Prophylaxis and Wound Infection Cultures in Patients Undergoing Pancreaticoduodenectomy. JAMA Surg 2016; 151(5):432–9. [DOI] [PubMed] [Google Scholar]
- 10.Lavu H, Klinge MJ, Nowcid LJ, et al. Perioperative surgical care bundle reduces pancreaticoduodenectomy wound infections. J Surg Res 2012; 174(2):215–21. [DOI] [PubMed] [Google Scholar]
- 11.Shen P, Blackham AU, Lewis S, et al. Phase II Randomized Trial of Negative-Pressure Wound Therapy to Decrease Surgical Site Infection in Patients Undergoing Laparotomy for Gastrointestinal, Pancreatic, and Peritoneal Surface Malignancies. J Am Coll Surg 2017; 224(4):726–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Javed AA, Teinor J, Wright M, et al. Negative Pressure Wound Therapy for Surgical-site Infections: A Randomized Trial. Ann Surg 2018. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention/ National Healthcare Safety Network. Identifying Healthcare-associated Infections (HAI) for NHSN Surveillance January2017. Available at: https://www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf.
- 14.American College of Surgeons National Surgical Quality Improvement Program. User Guide for the 2014 ACS NSQIP Participant Use Data File. Available at: https://www.facs.org/~/media/files/quality%20programs/nsqip/nsqip_puf_userguide_2014.ashx.
- 15.Mihaljevic AL, Muller TC, Kehl V, et al. Wound edge protectors in open abdominal surgery to reduce surgical site infections: a systematic review and meta-analysis. PLoS One 2015; 10(3):e0121187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mihaljevic AL, Schirren R, Ozer M, et al. Multicenter double-blinded randomized controlled trial of standard abdominal wound edge protection with surgical dressings versus coverage with a sterile circular polyethylene drape for prevention of surgical site infections: a CHIR-Net trial (BaFO; NCT01181206). Ann Surg 2014; 260(5):730–7; discussion 737-9. [DOI] [PubMed] [Google Scholar]
- 17.Sookhai S, Redmond HP, Deasy JM. Impervious wound-edge protector to reduce postoperative wound infection: a randomised, controlled trial. Lancet 1999; 353(9164):1585. [DOI] [PubMed] [Google Scholar]
- 18.Edwards JP, Ho AL, Tee MC, et al. Wound protectors reduce surgical site infection: a meta-analysis of randomized controlled trials. Ann Surg 2012; 256(1):53–9. [DOI] [PubMed] [Google Scholar]
- 19.Mueller TC, Loos M, Haller B, et al. Intra-operative wound irrigation to reduce surgical site infections after abdominal surgery: a systematic review and meta-analysis. Langenbecks Arch Surg 2015; 400(2):167–81. [DOI] [PubMed] [Google Scholar]
- 20.Hyldig N, Birke-Sorensen H, Kruse M, et al. Meta-analysis of negative-pressure wound therapy for closed surgical incisions. Br J Surg 2016; 103(5):477–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaidi A, El-Masry S. Closed-incision negative-pressure therapy in high-risk general surgery patients following laparotomy: a retrospective study. Colorectal Dis 2017; 19(3):283–287. [DOI] [PubMed] [Google Scholar]
- 22.Cima R, Dankbar E, Lovely J, et al. Colorectal surgery surgical site infection reduction program: a national surgical quality improvement program--driven multidisciplinary single-institution experience. J Am Coll Surg 2013; 216(1):23–33. [DOI] [PubMed] [Google Scholar]
- 23.Grant F, Brennan MF, Allen PJ, et al. Prospective Randomized Controlled Trial of Liberal Vs Restricted Perioperative Fluid Management in Patients Undergoing Pancreatectomy. Ann Surg 2016; 264(4):591–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burkhart RA, Javed AA, Ronnekleiv-Kelly S, et al. The use of negative pressure wound therapy to prevent post-operative surgical site infections following pancreaticoduodenectomy. HPB (Oxford) 2017; 19(9):825–831. [DOI] [PubMed] [Google Scholar]
- 25.Gupta R, Darby GC, Imagawa DK. Efficacy of Negative Pressure Wound Treatment in Preventing Surgical Site Infections after Whipple Procedures. Am Surg 2017; 83(10):1166–1169. [PubMed] [Google Scholar]
- 26.Bressan AK, Aubin JM, Martel G, et al. Efficacy of a Dual-ring Wound Protector for Prevention of Surgical Site Infections After Pancreaticoduodenectomy in Patients With Intrabiliary Stents: A Randomized Clinical Trial. Ann Surg 2018; 268(1):35–40. [DOI] [PubMed] [Google Scholar]
- 27.Liu JB, Baker MS, Thompson VM, et al. Wound protectors mitigate superficial surgical site infections after pancreatoduodenectomy. HPB (Oxford) 2018. [DOI] [PubMed] [Google Scholar]
- 28.Hashimoto D, Chikamoto A, Arima K, et al. Unused sterile instruments for closure prevent wound surgical site infection after pancreatic surgery. J Surg Res 2016; 205(1):38–42. [DOI] [PubMed] [Google Scholar]
- 29.Gavazzi F, Ridolfi C, Capretti G, et al. Role of preoperative biliary stents, bile contamination and antibiotic prophylaxis in surgical site infections after pancreaticoduodenectomy. BMC Gastroenterol 2016; 16:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pisters PW, Hudec WA, Hess KR, et al. Effect of preoperative biliary decompression on pancreaticoduodenectomy-associated morbidity in 300 consecutive patients. Ann Surg 2001; 234(1):47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poruk KE, Lin JA, Cooper MA, et al. A novel, validated risk score to predict surgical site infection after pancreaticoduodenectomy. HPB (Oxford) 2016; 18(11):893–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boltz MM, Hollenbeak CS, Julian KG, et al. Hospital costs associated with surgical site infections in general and vascular surgery patients. Surgery 2011; 150(5):934–42. [DOI] [PubMed] [Google Scholar]
- 33.Schweizer ML, Cullen JJ, Perencevich EN, et al. Costs Associated With Surgical Site Infections in Veterans Affairs Hospitals. JAMA Surg 2014; 149(6):575–81. [DOI] [PMC free article] [PubMed] [Google Scholar]


