Abstract
Focal adhesion kinase (FAK) is a nonreceptor protein tyrosine kinase involved in integrin-mediated control of cell behavior. Following cell adhesion to components of the extracellular matrix, FAK becomes phosphorylated at multiple sites, including tyrosines 397, 576, and 577. Tyr-397 is an autophosphorylation site that promotes interaction with c-Src or Fyn. Tyr-576 and Tyr-577 lie in the putative activation loop of the kinase domain, and FAK catalytic activity may be elevated through phosphorylation of these residues by associated Src family kinase. Recent studies have implicated FAK as a positive regulator of cell spreading and migration. To further study the mechanism of adhesion-induced FAK activation and the possible role and signaling requirements for FAK in cell spreading and migration, we utilized the tetracycline repression system to achieve inducible expression of either wild-type FAK or phosphorylation site mutants in fibroblasts derived from FAK-null mouse embryos. Using these Tet-FAK cells, we demonstrated that both the FAK autophosphorylation and activation loop sites are critical for maximum adhesion-induced FAK activation and FAK-enhanced cell spreading and migration responses. Negative effects on cell spreading and migration, as well as decreased phosphorylation of the substrate p130Cas, were observed upon induced expression of the FAK autophosphorylation site mutant. These negative effects appear to result from an inhibition of integrin-mediated signaling by the FAK-related kinase Pyk2/CAKβ/RAFTK/CadTK.
FAK (focal adhesion kinase) is a widely expressed nonreceptor protein tyrosine kinase found in focal adhesions of cultured cells (23, 61). FAK becomes activated by tyrosine phosphorylation in response to integrin clustering achieved by cell adhesion or antibody cross-linking (5, 19, 23, 34, 40). FAK Tyr-397 is an autophosphorylation site and a high-affinity binding site for Src homology 2 (SH2) domains of Src family kinases, including c-Src and Fyn (48, 62, 80). This interaction could contribute both to the recruitment of Src family kinases to sites of cell adhesion and to their catalytic activation through C-terminal tail displacement. Other adhesion-regulated sites of FAK phosphorylation are tyrosines 407, 576, 577, 861, and 925 (7, 8, 65). These tyrosines do not appear to be autophosphorylation sites but are efficiently phosphorylated by c-Src in vitro and elevated in Src-transformed cells (7, 8, 66). Tyr-397 can also be phosphorylated by c-Src (7); hence, it is not strictly an autophosphorylation site. Tyr-576 and Tyr-577 lie in the putative activation loop of the kinase domain, and mutation of these residues reduces FAK catalytic activity (7, 42). The potential for reciprocal activation of FAK and Src family kinases suggests a mechanism for signal amplification following an initial integrin-induced FAK autophosphorylation event. Other sites of FAK tyrosine phosphorylation are likely to participate in downstream signaling through recruitment of additional SH2-containing proteins. Indeed, phosphorylation of Tyr-925 creates a binding site for the Grb2 SH2 domain (65), and this interaction contributes to integrin-stimulated activation of the Ras-ERK2/mitogen-activated protein kinase pathway (69). Phosphorylated Tyr-397 may have signaling roles in addition to recruitment and activation of Src family kinases, since phosphatidylinositol-3-kinase (PI3K) (11) and phospholipase C-γ (PLC-γ) (82) also appear to interact with this site in vivo.
In addition to integrin-mediated cell adhesion, increased FAK tyrosine phosphorylation ensues from stimulating cells with a variety of soluble growth factors, neuropeptides, and bioactive lipids (reviewed in reference 56). These responses likely arise through integrin clustering achieved from within the cell as a result of Rho-mediated actomyosin contraction (6).
FAK directly interacts with paxillin and Crk-associated substrate p130Cas (Cas), and FAK-promoted tyrosine phosphorylation of these substrates with subsequent recruitment of additional effector proteins, including c-Crk, are likely critical signaling events downstream of FAK (reviewed in reference 24). The paxillin binding site on FAK overlaps extensively with the focal adhesion targeting (FAT) domain found near the C terminus (27, 72). Two distinct proline-rich sites C-terminal to the FAK kinase domain interact with the SH3 domain of Cas (26, 48, 49). A role for FAK in the tyrosine phosphorylation of paxillin and Cas is supported by observations that all three proteins are localized in focal adhesions and undergo adhesion-induced tyrosine phosphorylation with similar kinetics (5, 45, 47, 75). Moreover, transfection experiments have linked FAK expression with tyrosine phosphorylation of paxillin (63) and Cas (73, 77). By interacting with Src family kinases and thereby positioning them to phosphorylate the FAK-associated substrates, FAK may play primarily a scaffolding role in paxillin and Cas phosphorylation. This view is supported by observations that FAK Tyr-397 is required for efficient stimulation of paxillin and Cas phosphorylation (63, 77). Also, studies examining paxillin and Cas tyrosine phosphorylation in cells lacking FAK, Src, Fyn, or C-terminal Src kinase (Csk) indicate that Src family kinases are primarily responsible for phosphorylating these substrates (21, 58, 77). In vitro kinase reactions suggest that FAK may also directly phosphorylate Cas (67, 77), and an alternative mechanism has been proposed involving FAK phosphorylation of the Src-binding domain near the Cas C terminus, followed by Src recruitment to this site to achieve further Cas phosphorylation (73). Another possible substrate for the FAK-Src complex is the actin-binding protein tensin (4), and phosphorylation of this protein may be important for proper integrin linkages to the actin cytoskeleton.
From the time of its discovery, FAK has been considered a candidate molecule for the regulation of cellular attributes or functions known to be influenced by integrin-ligand engagement. Much evidence has pointed to a positive role for FAK in cell migration. FAK expression is upregulated in the rapidly migrating basal epidermal keratinocytes in the repair of burn wounds (16). Mesodermal cells derived from FAK-knockout mouse embryos migrate at a reduced rate relative to cells obtained from same-stage normal embryos (29). Overexpression of a C-terminal region of FAK containing the FAT domain, which acts in a dominant-negative manner, reduces migration rates of human endothelial (17) and chicken embryo (52) cells. Finally, overexpression of epitope-tagged FAK in CHO cells enhances cell migration relative to that of cells expressing only endogenous levels of FAK (9). FAK has also been implicated in integrin-mediated cell spreading, although the evidence for this is more equivocal. Expression of the FAK C-terminal region inhibits the rate of chicken embryo cell spreading on fibronectin, an effect which can be rescued by coexpression of FAK (52, 53). However, FAK overexpression does not appear to enhance the rate of CHO cell spreading (9).
FAK has one known mammalian family member, variously known as Pyk2 (37), CAKβ (60), RAFTK (3), and CadTK (81) (for convenience, here referred to as Pyk2). FAK and Pyk2 have 45% overall amino acid identity, with the greatest similarities in the kinase domain and C-terminal region corresponding to the FAK FAT domain. Also conserved in Pyk2 are tyrosines corresponding to FAK tyrosine −397, −576, −577, and −925. As in FAK, Pyk2 signaling appears to involve SH2-mediated interactions with Src family kinases and Grb2 (14, 37). Also like FAK, Pyk2 directly interacts with paxillin and Cas and can act to promote their tyrosine phosphorylation (1, 2, 39, 46, 59, 64). Nonetheless, Pyk2 has other properties that distinguish it from FAK. Pyk2 expression is more restricted than the nearly ubiquitous FAK, being notably absent in cultured fibroblasts (60), where FAK has been extensively studied. Moreover, Pyk2 and FAK are differentially activated. Unlike FAK, Pyk2 is activated by elevation of intracellular calcium (37, 81), and although Pyk2 can undergo integrin-dependent tyrosine phosphorylation (38, 71, 76), in cells where Pyk2 and FAK are coexpressed the response is much weaker for Pyk2 than for FAK (83).
Here, we have further studied FAK signaling mechanism and function by utilizing the tetracycline repression system to achieve inducible expression of wild-type versus phosphorylation site FAK mutants in fibroblasts derived from FAK-null mouse embryos. With this experimental system we examined the mechanism of FAK activation in response to cell adhesion and further investigated the possible role and signaling requirements for FAK in cell spreading and migration. We found that both the FAK autophosphorylation site (Tyr-397) and activation loop phosphorylation sites (Tyr-576 and Tyr-577) are critical for adhesion-induced FAK activation and for FAK-enhanced cell spreading and migration responses. Negative effects on cell spreading and migration were observed upon induced expression of the FAK autophosphorylation site mutant, which may be due to FAK inhibition of Pyk2 adhesion-induced signaling.
MATERIALS AND METHODS
Antibodies.
Anti-FAK rabbit polyclonal antibody C-20 was from Santa Cruz Biotechnology (Santa Cruz, Calif.). FAK polyclonal antibody 331 was described in the work of Hanks et al. (23). Monoclonal antibodies 2A7 against FAK (30), 327 against c-Src (41), and 15D2 against p120ctn (79) were all generously provided by Al Reynolds, Vanderbilt University. Monoclonal antibodies against Pyk2, Cas, and phosphotyrosine (PY20) were from Transduction Laboratories (Lexington, Ky.). Antiphosphotyrosine monoclonal antibody 4G10 was from Upstate Biotechnology (Lake Placid, N.Y.). Monoclonal antibody 8d4 against talin was from Sigma. Texas red-conjugated donkey anti-rabbit immunoglobulin G (IgG) and fluorescein isothiocyanate (FITC)-conjugated donkey anti-mouse IgG were from Jackson Immunolabs (West Grove, Pa.).
Cells and cell culture.
FAK-null cells derived from mouse embryos homozygous for a disrupted fadk allele (29) and their wild-type counterparts were generously provided by Dusko Ilic, (University of California at San Francisco). Both FAK-null and wild-type cells exhibited morphological variability, ranging from a well-spread polarized fibroblast-like shape to a distinct rounded shape more closely resembling the FAK-null cell phenotype previously described (29). A clonal derivative of FAK-null cells (termed R1) with a uniform fibroblast-like morphology was obtained by serial dilution and served as the parental line for establishment of cells for inducible FAK expression (see below). All cells were maintained at 37°C in a humidified 5% CO2 incubator in Dulbecco’s modified Eagle medium (DMEM) containing 4,500 mg of d-glucose/ml and 584 mg of glutamine/liter and further supplemented with 1 mM sodium pyruvate, 100 μg of streptomycin/ml, 100 U of penicillin/ml, 0.25 μg of Amphotericin B/ml, 1 mM nonessential amino acids (all from GIBCO/BRL), and 10% fetal bovine serum (FBS) (Atlanta Biologicals, Norcross, Ga.).
Plasmid construction and selection of Tet-FAK cells inducibly expressing FAK.
A cDNA encoding full-length wild-type mouse FAK (23) was inserted into the XbaI site of the tetracycline repression vector pTRE (Clontech, Palo Alto, Calif.) to yield plasmid pTRE-FAK(WT). Plasmids pTRE-FAK(F397) and pTRE-FAK(F576/F577), expressing FAK phosphorylation site mutants, were constructed by substituting appropriate regions from the previously described plasmids pRC/CMV-FAK-F397-HA and pRC/CMV-FAK-F576/F577-HA (7). All new constructs were verified by DNA sequencing. Plasmids were transfected by using Lipofectamine (GIBCO/BRL). Three plasmids were transfected concurrently, the expression plasmid pTRE [either vector only, -FAK(WT), -FAK(F397), or -FAK(F576/F577)], the tetracycline-controlled transactivator plasmid pTet-tTAk (GIBCO/BRL), and the selectable marker plasmid pBABE-Puro (44). FAK-null R1 cells were transfected at a 50:50:1 molar ratio of pTRE–pTet-tTAk–pBABE-Puro. After selection in 1.4 μg of puromycin (Calbiochem, La Jolla, Calif.)/ml and 1 μg of tetracycline (Calbiochem)/ml for 4 weeks, isolated colonies were picked and grown to confluency. Potential inducible FAK clones, maintained in 1 μg of tetracycline/ml, were replated in the absence of tetracycline and cultured for 48 h to test for inducible expression. A parallel plate was maintained with tetracycline as a noninduced control. Cells were lysed in radioimmunoprecipitation (RIPA) buffer (50 mM Tris-Cl [pH 7.4], 150 mM NaCl, 5 mM EDTA, 1% Nonidet P-40 [NP-40], 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 50 mM NaF, 1% aprotinin, and 0.1 mM Na3VO4), and FAK expression was determined by immunoblot analysis (23). By this screening procedure, two cell clones (termed Tet-FAK) were selected for inducible expression of each FAK variant, wild-type FAK [Tet-FAK(WT)-46 and Tet-FAK(WT)-70], F397-FAK [Tet-FAK(F397)-18 and Tet-FAK(F397)-21], and F576/F577-FAK [Tet-FAK(F576/F577)-16 and Tet-FAK(F576/F577)-29]. Control cells expressing pTet-tTAk and the pTRE vector without insert (Tet-tTA-3 and Tet-tTA-13) were also obtained and selected for inducible expression of the tTA transactivator by luciferase assay (18).
Tet-FAK cells were induced to permit FAK expression by replating in media lacking tetracycline, and levels of expression were controlled by varying the duration of time cells were maintained in the absence of tetracycline. Cells were typically induced for 2 to 4 days to achieve maximal expression, but in some experiments Tet-FAK(F397) cells were induced for only 12 to 14 h to achieve F397-FAK expression to levels comparable to those of WT- and F576/F577-FAK. For control noninduced cells, parallel cultures were maintained in the presence of 1 μg of tetracycline/ml.
Fibronectin-replating and analysis of FAK, Pyk2, and Cas phosphotyrosine levels.
Fibronectin replating experiments were as performed previously (23) with induced versus noninduced Tet-FAK cells that had been serum starved for 2 or 14 h, as indicated. Attached cells were obtained by rinsing the adherent cells in phosphate-buffered saline (PBS) and then lysing in RIPA buffer. Suspended cells were detached with trypsin, washed with PBS containing 1 mg of chicken egg white trypsin inhibitor (Sigma)/ml, and then held in suspension in serum-free DMEM for 30 min at 37°C/5% CO2, before pelleting by gentle centrifugation, washing again in PBS, and lysing in RIPA buffer. Fibronectin-replated cells were detached and treated with trypsin inhibitor as described above and then replated onto dishes coated with 5 μg of fibronectin (Sigma)/ml and allowed to attach and spread for 30 or 60 min before being rinsed in PBS and lysed in RIPA buffer. For experiments in which FAK-associated kinase activity was also measured, cells were lysed in NP-40 buffer (50 mM Tris-Cl [pH 7.4], 150 mM NaCl, 5 mM EDTA, 1% NP-40, 1% aprotinin, 50 mM NaF, and 0.1 mM Na3VO4). The bicinchoninic acid protein assay (Pierce, Rockford, Ill.) was used to determine protein concentrations, and equal protein amounts from each lysate were used for immunoprecipitation.
For analysis of FAK phosphotyrosine levels, FAK was immunoprecipitated (23, 48) with C-20 antibody, and proteins in the immunoprecipitates were resolved by SDS–7% polyacrylamide gel electrophoresis (PAGE) and subjected to immunoblot analysis with either antiphosphotyrosine monoclonal antibody 4G10 or FAK antibody C-20 or 331. For Pyk2 analysis, the lysates were immunoprecipitated with anti-Pyk2 monoclonal antibody, and immunoblots were prepared and probed with either 4G10 or anti-Pyk2 antibody. For Cas analysis, lysates were immunoprecipitated with PY20 antiphosphotyrosine antibody, and immunoblots were probed with anti-Cas antibody. To demonstrate FAK and Cas levels in the lysates used for Cas phosphotyrosine analysis, 20 μg of total protein was used for immunoblot analysis.
Characterization of FAK versus Src inhibition by PD161430.
The pyrido[2,3-d] pyrimidine tyrosine kinase inhibitor PD161430 (compound 4f in the work of Hamby et al. [22]) was assessed for its efficacy in inhibiting the kinase activities of Src and FAK. Previously, PD161430 was shown to inhibit c-Src activity toward poly(GluTyr) with a 50% inhibitory concentration of 0.22 μM (22). To measure Src activity toward p120ctn, NIH 3T3 cells stably expressing the F527 activating mutant of chicken c-Src were lysed in NP-40 buffer, and Src and p120ctn were immunoprecipitated together on protein A-Sepharose beads with a mixture of monoclonal antibodies 327 and 15D2, followed by rabbit anti-mouse IgG. The beads were washed extensively in NP-40 buffer followed by kinase assay buffer [50 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES) (pH 7.0), 10 mM MnCl2, 1 mM dithiothreitol], and then equally divided and incubated for 30 min at room temperature with intermittent agitation in kinase assay buffer containing 0.25 μCi of [γ-32P]ATP/μl (4,500 Ci/mmol; ICN, Irvine, Calif.) and various concentrations (0 to 220 μM) of PD161430. Reactions were stopped by adding an equal volume of 2× SDS-PAGE sample buffer and boiling for 5 min. Samples were then separated by SDS–7% PAGE, and dried gels were exposed to X-ray film for 5 min. Relative kinase activity was quantitated by phosphorimage analysis of the p120ctn band. To assess the ability of PD161430 to inhibit FAK, kinase assays were carried out with 100 ng of baculoviral FAK (78), and relative autophosphorylation activity was measured in the presence of increasing concentrations of PD161430.
In vitro kinase assays of FAK immunoprecipitates from Tet-FAK cells.
FAK was immunoprecipitated with C-20 antibody from NP-40 buffer lysates of induced Tet-FAK cells harvested under either attached, suspended, or fibronectin-replated conditions, and the immunoprecipitates were washed and divided equally for separate analysis of either FAK recovery and phosphotyrosine levels (as described above), or kinase activity. The kinase reactions were carried out as described above in the presence or absence of 22 μM PD161430 and stopped by the addition of an equal volume of 2× SDS-PAGE sample buffer. The samples were then separated by SDS–7% PAGE, and in vitro FAK phosphorylation was visualized by autoradiography of the dried gel. FAK phosphorylation was quantitated by phosphorimage analysis from three independent experiments and expressed as mean phosphorylation (+ standard error of the mean [SEM]) relative to attached WT-FAK samples assayed in the absence of PD161430. Assays assessing phosphorylation of the exogenous substrate poly(GluTyr) were carried out essentially as described above, except 0.25 μg of poly(GluTyr) (4:1; Fluka, Buchs, Switzerland) was included in the kinase reaction mixtures and samples were separated by SDS–15% PAGE for quantitation of phosphorylated substrate by phosphorimage analysis.
Analysis of cell spreading on fibronectin.
Induced or noninduced Tet-FAK and Tet-tTA cells were collected by trypsinization, washed in PBS containing 1 μg of chicken egg white trypsin inhibitor/ml, and then resuspended in serum-free DMEM. Two million cells were plated into 60-mm-diameter tissue culture dishes precoated with 0.1 μg of fibronectin/cm2. After 30 min or 20 h of incubation in DMEM (serum free or containing 10% FBS, respectively), adherent cells were photographed by phase-contrast microscopy. For the 30-min samples, cells in each of six random fields were scored under investigator-blind conditions as being either spread (defined as phase-dark cells with extended processes) or unspread (defined as round phase-bright cells). Data were averaged from results of three to four independent experiments.
Boyden chamber cell migration analysis.
Migration of Tet-FAK and Tet-tTA cells was assessed by using the modified Boyden chamber (Neuro-Probe, Cabin John, Md.) with 8-μm polycarbonate membrane and DMEM containing 10% FBS in the lower chamber as the attractant. The analysis was carried out simultaneously on induced versus noninduced cells. Thirty thousand cells, suspended in DMEM containing 200 μg of bovine serum albumin/ml, were seeded into each of six wells of the upper chamber. The cells were allowed to migrate for 5 h at 37°C, and then adherent cells on the upper surface of the membrane were removed by scraping. Migratory cells attached to the bottom of the membrane were fixed in methanol and stained with hematoxylin. Membranes were mounted on glass slides, and images of migrating cells were recorded by using a charge-coupled-device camera mounted on a Zeiss Axiophot photomicroscope. For each experimental variable, cells were counted from random microscopic fields from four to six wells under investigator-blind conditions. The average number of cells/field migrating in the induced state was divided by the average number of cells/field migrating in the noninduced state to obtain a percentage of change in cell migration. For each cell type, three independent experiments were carried out, and final data are expressed as the average percentages of change in cell migration (±SEM) of induced relative to noninduced cells.
Immunofluorescence microscopy.
Double-label immunofluorescent costaining of cells was carried out by using antibody C-20 against FAK (10 μg/ml) together with monoclonal antibody against talin (8d4; 5.0 μg/ml). Tet-FAK cells were induced and then plated onto coverslips coated with 5 μg of fibronectin (Sigma)/ml. Cells were allowed to adhere and spread overnight and then fixed in 4% paraformaldehyde and permeabilized by incubating with −20°C acetone. The fixed cells were blocked in 10% donkey serum for 1 h at 37°C, and then primary antibodies were applied for 45 min. After repeated washes in Hanks’ buffered saline solution, secondary antibodies (7.0 μg/ml) Texas red-conjugated donkey anti-rabbit IgG and FITC-conjugated donkey anti-mouse IgG were applied for 30 min and washed as described above. Stained cells were viewed with a Zeiss Axiophot photomicroscope.
RESULTS
Inducible FAK expression by using the tetracycline repression system.
A modified tetracycline repression system which permits tightly regulated gene expression was implemented in a fibroblast-like derivative of FAK-null cells isolated from knockout mouse embryos (see Materials and Methods). Upon tetracycline withdrawal, Tet-FAK(WT), Tet-FAK(F397), and Tet-FAK(F576/577) cells express either WT-FAK, the autophosphorylation site mutant F397-FAK, or the activation loop site double-mutant F576/F577-FAK, respectively. In the studies reported here, two distinct clonal isolates were employed for expression of each FAK variant. Tet-tTA cells, expressing only the tTA transactivator protein, were also generated from the FAK-null fibroblasts for use as an additional control.
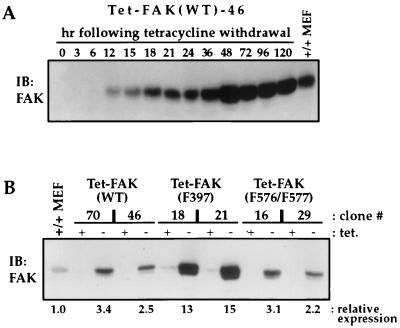
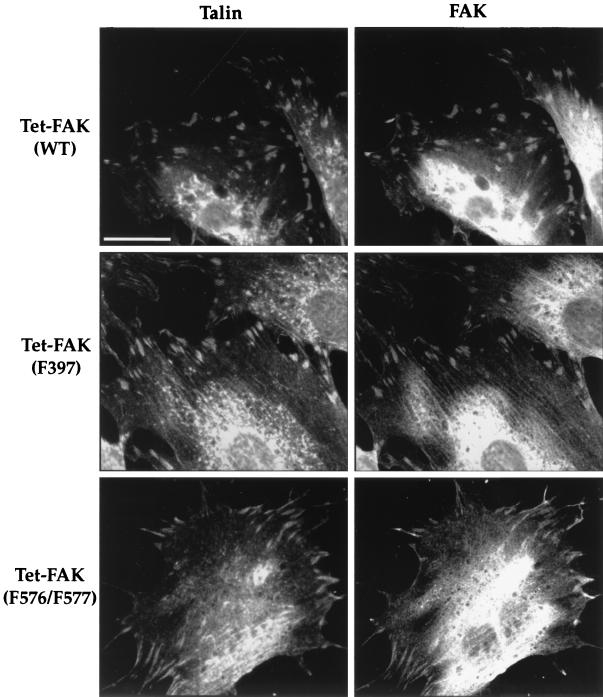
Figure 1A shows the time course of FAK expression following tetracycline withdrawal from Tet-FAK(WT)-46 cells. FAK protein is detected as early as 6 h and reaches maximum levels between 36 and 48 h. Upon full induction, WT-FAK is expressed to a level about two- to threefold greater than that of endogenous FAK from normal mouse embryo fibroblasts (+/+ MEF) (Fig. 1). Similar time courses (data not shown) and maximal levels of induction (Fig. 1B) were observed for the other Tet-FAK(WT) and Tet-FAK(F576/F577) clones. The Tet-FAK(F397) clones induced with similar kinetics but maximal expression of F397-FAK clones were ∼four- to fivefold higher than that of WT-FAK (Fig. 1B). The inducibly expressed WT-FAK, F397-FAK, and F576/F577-FAK mutants all become prominently localized to focal adhesions, as demonstrated by colocalization with talin (Fig. 2).
FIG. 1.
Inducible FAK expression in Tet-FAK cells. (A) Induction time course. Parallel cultures of exponentially growing Tet-FAK(WT)-46 cells were induced by tetracycline withdrawal (0 h), and cell lysates were prepared at the times indicated for analysis of FAK expression. (B) Expression levels. The indicated Tet-FAK clones were either maintained in the presence of tetracycline (+ tet.) or induced by tetracycline withdrawal for 2 days (− tet.), and then total cell lysates were prepared and analyzed for relative FAK expression levels. For both panels A and B, cells were lysed in RIPA buffer, and 30 μg of total protein was loaded per lane for assessment of FAK levels by immunoblotting (IB) with C-20 antibody and detection with 125I-labeled protein A. Control samples (MEF +/+ lanes) contained 30 μg of total protein prepared from normal mouse embryo fibroblasts. Relative expression levels were quantitated by phosphorimage volume integration.
FIG. 2.
Immunolocalization of FAK in induced Tet-FAK cells. Tet-FAK(WT)-46, Tet-FAK(F397)-21, and Tet-FAK(F576/F577)-16 cells were induced for 2 days and then plated overnight on fibronectin-coated coverslips. Double-label indirect immunofluorescence was carried out by using monoclonal antibody 8d4 against talin (left panels) and polyclonal antibody C-20 against FAK (right panels). Bar = 30 μm.
Both autophosphorylation and activation loop phosphorylation sites are required for maximal FAK tyrosine phosphorylation and adhesion-induced catalytic activation.
The Tet-FAK cells were used to examine the requirements for the auto- and activation loop phosphorylation sites in integrin-stimulated FAK tyrosine phosphorylation and catalytic activation. Of particular interest was whether F576/F577-FAK exhibited impaired signaling responses, as would be predicted from a model in which phosphorylation of the activation loop in turn stimulated autophosphorylation as part of a signal amplification mechanism. Initially, the relative phosphotyrosine levels of WT-, F397-, and F576/F577-FAK were examined. RIPA buffer lysates of the respective induced Tet-FAK cells growing at subconfluent density in the presence of 10% FBS were adjusted to contain near-equal amounts of FAK protein, and then FAK phosphotyrosine levels were assessed by subjecting FAK immunoprecipitates prepared from the lysates to antiphosphotyrosine immunoblot analysis. The results revealed that both F397- and F576/F577-FAK have greatly reduced phosphotyrosine levels (Fig. 3). F397-FAK phosphotyrosine was virtually absent, while F576/F577-FAK phosphotyrosine was reduced by about 75% relative to WT-FAK. We have also recently demonstrated reduced Tyr-397 phosphorylation in F576/F577-FAK, using a phosphospecific antibody against the autophosphorylation site (57).
FIG. 3.
Relative tyrosine phosphorylation of WT-, F397-, and F576/F577-FAK in Tet-FAK cells growing under normal culture conditions. Tet-FAK cells were induced for either 2 days [Tet-FAK(WT)-46 and Tet-FAK(F576/F577)-16] or 12 h [Tet-FAK(F397)-21] to obtain near-equal levels of expression of the FAK variants, and then FAK was immunoprecipitated (IP) from RIPA buffer lysates containing 650 μg of total protein. The immunoprecipitates were then divided equally for immunoblot (IB) analysis by using either antiphosphotyrosine antibody 4G10 (top panel) or anti-FAK antibody C-20 (bottom panel). Immunoblots were developed by enhanced chemiluminescence, and signals were quantitated from digitized images.
The above-described results are consistent with the notion that F576/F577-FAK has reduced autophosphorylation activity relative to that of WT-FAK. This issue was addressed more directly by kinase assays of FAK immunoprecipitates. To eliminate activity of coprecipitating Src family kinases, the assays were carried out in the presence of 22 μM PD161430, a pyrido[2,3-d] pyrimidine that potently inhibits c-Src kinase activity at this concentration while having no effect on FAK autophosphorylation activity (Fig. 4A). WT- and F576/F577-FAK immunoprecipitates were prepared from NP-40 buffer lysates of induced Tet-FAK cells, either attached (serum starved), suspended, or fibronectin replated, and then divided to permit both kinase assays and immunoblot analyses. A representative experiment is shown in Fig. 4B, and kinase assay data from three independent experiments are quantitatively represented in Fig. 4C. In vitro phosphorylation of F576/F577-FAK was reduced by 50 to 75% (greatest for the fibronectin-replated samples), relative to that of WT-FAK, as measured in the presence of PD161430 (Fig. 4B, bottom panel, and 4C). In the absence of PD161430, the total activity associated with F576/F577-FAK was also much reduced relative to that of WT-FAK, and an adhesion-dependent increase in activity for both FAK variants was evident (Fig. 4B and C). Again, the in vivo tyrosine phosphorylation of F576/F577-FAK in the attached and fibronectin-replated samples is greatly reduced relative to that of WT-FAK (Fig. 4B, top panels; compare to Fig. 3). Together, these data further indicate that both FAK autophosphorylation activity and association with Src family kinases is significantly reduced in the F576/F577-FAK mutant.
FIG. 4.
In vitro kinase assays. (A) Inhibition of c-Src but not FAK by PD161430. Increasing concentrations of PD161430 (0 to 220 μM) were added to mixtures for kinase reactions carried out on either coimmunoprecipitates of c-Src and p120ctn (top panel) or baculovirus-expressed FAK (bottom panel). PD161430 inhibited c-Src phosphorylation of p120ctn with a 50% inhibitory concentration of ∼0.2 μM but had little or no effect on FAK autophosphorylation at concentrations up to 220 μM. (B and C) F576/F577-FAK shows reduced adhesion-dependent tyrosine phosphorylation and in vitro autophosphorylation activity. WT-FAK or F576/F577-FAK was immunoprecipitated (IP) from NP-40 buffer lysates of induced Tet-FAK(WT)-46 or Tet-FAK(F576/F577)-16 cells, respectively, under either attached (Att) (serum-starved 14 h), suspended (Sus), or fibronectin-replated (Fn) conditions. The immunoprecipitates were then divided equally for assessment of in vivo FAK tyrosine phosphorylation by immunoblotting (IB) with antiphosphotyrosine antibody 4G10 (top panel), FAK recovery by immunoblotting with anti-FAK antibody C-20 (middle panel), and in vitro FAK phosphorylation from kinase assays carried out either in the absence or presence of 22 μM PD161430 (bottom panel). In panel C, in vitro FAK phosphorylation from three independent kinase assays is plotted as mean activity (+SEM) relative to that of WT-FAK assayed from attached cells in the absence of PD161430. (D) F397-FAK and F576/F577-FAK show reduced phosphorylation of poly(GluTyr). WT, -F397, and F576/F577-FAK were immunoprecipitated from NP-40 buffer lysates prepared from induced Tet-FAK(WT)-46, Tet-FAK(F397)-21, or Tet-FAK(F576/F577)-16 cells, respectively, and used to assess vitro kinase phosphorylation of poly(GluTyr) in kinase assays carried out either in the absence or presence of 22 μM PD161430. Data from three independent assays are plotted as mean activities (+SEM) relative to that of WT-FAK assayed from attached (Att) cells in the absence of PD161430.
Additional evidence for impaired adhesion-stimulated activation of F576/F577-FAK, as well as F397-FAK, was obtained by in vitro kinase assays measuring phosphorylation of an exogenous substrate, poly(GluTyr). When immunoprecipitated from attached or fibronectin-replated cells and assayed in the presence of PD161430, both F576/F577-FAK and F397-FAK phosphorylated poly(GluTyr) only to about 20 to 30% of the level achieved by WT-FAK (Fig. 4D). Neither FAK mutant exhibited elevated activity when isolated from adherent cells, relative to suspended cells, as was observed for WT-FAK. The kinase activity of WT-FAK toward poly(GluTyr), measured in the presence of PD161430, is clearly elevated under cell-adherent conditions (attached and fibronectin replated) compared to that under suspended-cell conditions (Fig. 4D). The lack of a similar adhesion-dependent elevation of WT-FAK autophosphorylation (Fig. 4C, +PD161430) may reflect the fact that FAK is already highly tyrosine phosphorylated in the lysates prepared from attached and fibronectin-replated cells (Fig. 4B, top panels) and thus provides less available substrate for autophosphorylation.
Both autophosphorylation and activation loop phosphorylation sites are required for FAK-enhanced cell spreading.
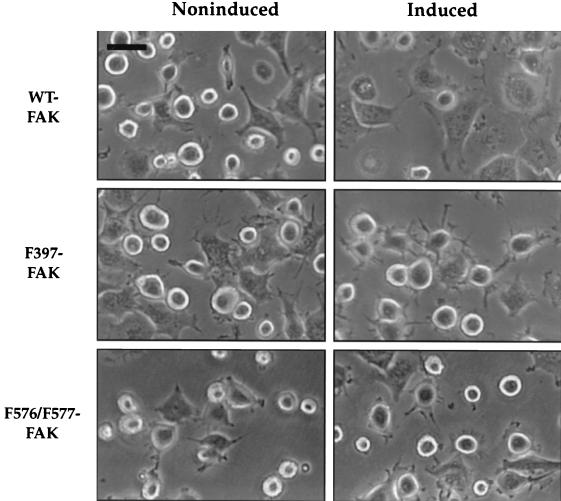
The Tet-FAK cells were next used to examine the role of FAK in cell spreading. Expression of WT-FAK led to a significant enhancement in cell spreading, relative to that of noninduced cells, observed soon after suspended cells were replated onto fibronectin-coated dishes (Fig. 5, top panels). After 30 min on fibronectin, about 70% of the induced cells were judged to be spread, based on loss of the rounded phase-bright appearance, while only about 40% of the noninduced control cells were spread after this time. Moreover, the lamellipodia of the spread cells expressing WT-FAK appeared more fully extended than those of noninduced spread cells. These spreading differences were not observed when control Tet-tTA cells were analyzed under induced versus noninduced conditions (data not shown), indicating that this enhanced spreading response was due to FAK expression and not to either tetracycline withdrawal or expression of the tTA transactivator. Moreover, expression of either F397-FAK or F576/F577-FAK did not obviously enhance this cell-spreading response (Fig. 5, middle and bottom panels). However, spreading cells expressing F397-FAK are characterized by notable filopodium-like extensions, while the same noninduced cells display a more typical spreading morphology reflected by both filopodial and lamellipodial extensions (Fig. 5, compare middle panels). The spreading morphology of cells expressing F576/F577-FAK was not readily distinguishable from that of the control noninduced cells (Fig. 5, bottom panels). These data support a positive role for FAK in promoting the initial rate and extent of cell spreading on fibronectin, requiring both the autophosphorylation and activation loop sites. Moreover, F397-FAK appears to have a negative effect on lamellipodial extension.
FIG. 5.
Early spreading analysis of Tet-FAK cells. Induced or noninduced Tet-FAK(WT)-46, Tet-FAK(F397)-21, or Tet-FAK(F576/F577)-16 cells were plated onto fibronectin-coated tissue culture dishes and, after 30 min, phase-contrast light microscopic images were captured for spreading analysis. Note full lamellipod extension of induced WT-FAK cells and more apparent filopod extension of induced F397-FAK cells. Bar = 37.5 μm.
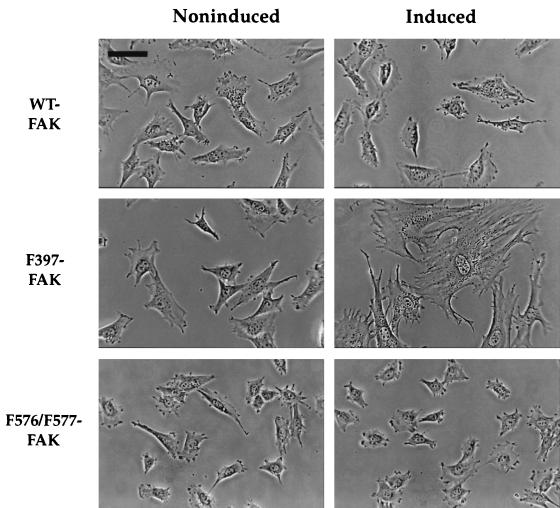
Cells expressing F397-FAK become unusually well spread after an extended period of time on fibronectin.
The appearance of the various Tet-FAK cells under induced (2 days) versus noninduced conditions was also examined 20 h after replating on fibronectin. In contrast to the results obtained after 30 min on fibronectin, there were no obvious morphological differences between cells expressing WT-FAK and their noninduced counterparts after this prolonged exposure to fibronectin (Fig. 6, top panels). Induced versus noninduced Tet-FAK(F576/F577) cells (Fig. 6, bottom panels) and Tet-tTA cells (data not shown) were also morphologically indistinguishable after 20 h on fibronectin. However, cells expressing F397-FAK exhibited an unusually well-spread morphology after 20 h on fibronectin that was not apparent in the noninduced cells (Fig. 6, middle panels). The ultraspread appearance of Tet-FAK(F397) cells was also observed under normal cell culture conditions, becoming evident after 4 to 5 days of induction and then imperceptible 2 to 3 days after the readdition of tetracycline to the culture media (data not shown).
FIG. 6.
Late spreading analysis of Tet-FAK cells. Induced and noninduced Tet-FAK(WT)-46, Tet-FAK(F397)-21, and Tet-FAK(F576/F577)-16 cells were plated onto fibronectin-coated glass coverslips and, after 20 h, phase-contrast light microscopic images were captured for spreading analysis. Note the highly spread morphology of induced F397-FAK cells. Bar = 75 μm.
Both autophosphorylation and activation loop phosphorylation sites are required for a FAK-enhanced cell migration response.
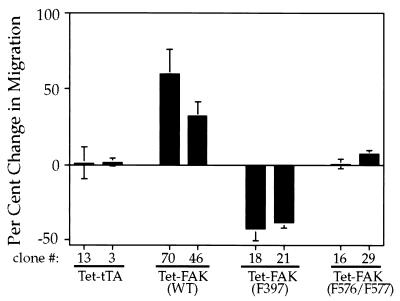
To further examine the role of FAK in cell migration, modified Boyden chamber assays were performed with the Tet-FAK and Tet-tTA cell clones induced for 2 days. Induced expression of WT-FAK resulted in a significant increase (30 to 60%) in the number of migrating cells compared to that of the noninduced controls [Fig. 7, Tet-FAK(WT)], while induction of the control Tet-tTA cells had no effect on cell migration (Fig. 7, Tet-tTA). As with the early fibronectin spreading response, expression of F576/F577-FAK had little or no effect on cell migration [Fig. 7, Tet-FAK(F576/F577)]. However, expression of F397-FAK to maximal levels led to a significant decrease (∼40%) in cell migration [Fig. 7, Tet-FAK(F397)]. A similar migration decrease was observed when F397-FAK was induced for a shorter period of time (12 h) to achieve a level of expression comparable to that of WT- and F576/F577-FAK (data not shown). These results indicate that both the autophosphorylation site and activation loop phosphorylation sites are required for a FAK-enhanced migration response, while F397-FAK has a negative effect on cell migration.
FIG. 7.
Analysis of cell migration changes following induced expression of wild-type FAK versus phosphorylation site FAK mutants. Cell migration was determined by using the modified Boyden chamber assay for noninduced versus induced cell clones as follows: Tet-tTA (clones 13 and 3), Tet-FAK(WT) (clones 70 and 46), Tet-FAK(F397) (clones 18 and 21), and Tet-FAK(F576/F577) (clones 16 and 29). The assay was carried out for 5 h, with 10% FBS as the attractant, and migrating cells were counted and averaged from four or more representative wells. The percentage of change in migration was determined by dividing the number of cells migrating under induced (without tetracycline) conditions by the number of cells migrating under noninduced (with tetracycline) conditions. Three independent experiments (inductions) were carried out for each cell clone, and the average percentages of change in migration (±SEM) are shown.
Pyk2 is upregulated in FAK-null cells, and expression of FAK inhibits adhesion-dependent Pyk2 tyrosine phosphorylation.
Our cell spreading and migration results indicated that F397-FAK has properties indicative of a dominant negative. Since the Tet-FAK(F397) cells do not express endogenous FAK, we considered the possibility that the FAK-related Pyk2 kinase is expressed in these cells, although it is not normally expressed in fibroblasts derived from mouse embryos (60). Pyk2 levels were assessed in the parental FAK-null cell population versus control cells from normal mouse embryos by immunoblot analysis of total cell lysates. Results indicate that Pyk2 levels are elevated ∼10-fold in FAK-null cells relative to that of the control (Fig. 8A). Thus, cells expressing high levels of Pyk2 may have been selected during the initial establishment of the FAK-null cells and could be compensating partially for the loss of FAK in signaling to promote cell spreading and migration.
FIG. 8.
Pyk2 adhesion-dependent tyrosine phosphorylation is impaired by FAK expression. (A) Relative Pyk2 levels were determined by immunoblot (IB) analysis of total cell lysates (30 μg of total protein) from FAK-null cells (parental population to Tet-FAK clones) versus normal mouse embryo fibroblasts. (B) Induced or noninduced Tet-FAK(F397)-21 cells were serum starved for 2 h and then lysed when either attached (Att.), trypsinized and held in suspension for 30 min (Susp.), or replated onto fibronectin for either 30 min (Fn-30′) or 60 min (Fn-60′). Either FAK or Pyk2 was immunoprecipitated (IP) from 300 μg of total protein lysates and then divided equally for immunoblot (IB) detection of either FAK, Pyk2, or phosphotyrosine (pTyr).
A fibronectin replating experiment was performed in order to determine if Pyk2 undergoes integrin-mediated tyrosine phosphorylation in Tet-FAK cells and if this is affected by expression of F397-FAK. In the absence of FAK expression, Pyk2 contains readily detectable phosphotyrosine in stably adherent cells and within 30 min following fibronectin replating (Fig. 8B, bottom right panels). However, when F397-FAK is expressed, Pyk2 phosphotyrosine is greatly reduced in both the stably adherent and fibronectin-replated cells, even though Pyk2 protein levels are unaltered (Fig. 8B, bottom left panels). Similar results were obtained from expression of WT-FAK (data not shown). Thus, FAK expression markedly inhibits the ability of Pyk2 to undergo an integrin-dependent signaling response.
Tyrosine phosphorylation of Cas correlates with FAK-enhanced cell spreading and migration.
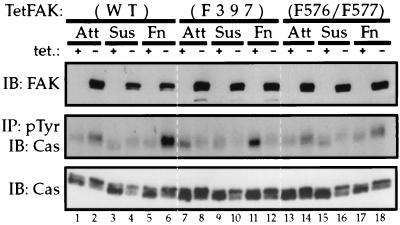
To determine if FAK-regulated changes in cell spreading and migration properties correlate with substrate phosphorylation, we examined Cas tyrosine phosphorylation in induced versus that in noninduced Tet-FAK cells. Cells from attached, suspended, or fibronectin-replated conditions were prepared, and RIPA buffer lysates were analyzed. Tet-FAK(WT) and Tet-FAK(F576/F577) cells were induced for 2 days, and Tet-FAK(F397) cells were induced for 12 h to achieve near-equal expression of the FAK variants, as demonstrated by immunoblot analysis of total protein from the cell lysates (Fig. 9, top panel). The relative phosphotyrosine content of Cas was assessed by anti-Cas immunoblot analysis of proteins immunoprecipitated from the same lysates with an antiphosphotyrosine antibody. Expression of both WT- and F576/F577-FAK resulted in elevated Cas phosphotyrosine levels in adherent (attached and fibronectin-replated) cells (Fig. 9, middle panel, lanes 1 to 6 and 13 to 18). This was most evident in the fibronectin-replated cells and was much more striking for cells induced to express WT-FAK (2.2-fold increase, comparing lanes 5 and 6) as opposed to F576/F577-FAK (0.5-fold increase, comparing lanes 17 and 18). In contrast, expression of F397-FAK led to a significant decrease in Cas tyrosine phosphorylation, relative to the level in uninduced cells (Fig. 9, middle panel, lanes 7 to 12). Again, this effect was most pronounced in the fibronectin-replated cells, where F397-FAK expression resulted in a ∼35% decrease in the amount of Cas recovered in the antiphosphotyrosine immunoprecipitates (compare lanes 11 and 12). Total Cas levels were not affected by FAK expression, although induction of each FAK variant correlated with a shift in the electrophoretic mobility of Cas such that slower-migrating forms became predominant (Fig. 9, lower panel). The nature of this mobility shift is presently unknown. Coimmunoprecipitation analyses revealed no apparent differences in the stable associations of Cas with the FAK variants (data not shown). Together, these results indicate that FAK autophosphorylation is an important event leading to Cas phosphorylation and correlate changes in Cas phosphotyrosine content with cell migration and early spreading responses observed following induced expression of WT-, F397-, or F576/F577-FAK.
FIG. 9.
Induced FAK expression alters Cas phosphotyrosine levels. Tet-FAK(WT)-46, Tet-FAK(F397)-21, and Tet-FAK(F576/F577)-29 cells were either maintained in the presence of tetracycline or induced to express near-equal amounts of WT-, F397, and F576/F577-FAK protein, respectively, and then cells from either attached (Att) (serum starved for 14 h), suspended (Sus), or fibronectin-replated (Fn) (30 min) conditions were lysed in RIPA buffer. FAK (top panel) and Cas (bottom panel) levels were assessed by immunoblot (IB) analysis of 20 μg of total protein from the lysates. Two hundred eighty micrograms of total protein from the same lysates was subjected to immunoprecipitation (IP) by using PY20 antiphosphotyrosine (pTyr) antibody, followed by Cas immunoblot analysis, to determine relative levels of tyrosine phosphorylation of Cas (middle panel).
DISCUSSION
In this study we examined the mechanism of adhesion-induced FAK activation and FAK signaling requirements for cell spreading and migration responses. The tetracycline repression system was used to achieve inducible expression of either WT-FAK or phosphorylation site mutants in cells derived from FAK-deficient mouse embryos. This experimental system has several favorable attributes for the analysis of FAK function. First, a positive expression system avoids potential problems associated with nonspecificity of inhibitory agents such as dominant negative proteins, injected antibodies, and antisense oligonucleotides. Second, inducibility of expression provides an ideal negative control consisting of the identical, but noninduced, cell population. This ensures that any observed changes in cell behavior are indeed due to FAK expression and not to a manifestation of phenotypic variation of clonally selected cells. Finally, induced FAK expression in a null background eliminates problems in data interpretation due to the presence of an endogenous FAK protein. The null background also eliminates the need to epitope tag the expressed FAK, which may alter its functional properties, in order to distinguish it from endogenous FAK.
WT-FAK.
Induced WT-FAK was prominently localized in focal adhesions and exhibited adhesion-induced elevation of both phosphotyrosine content and associated kinase activity. Thus, induced FAK behaves essentially like the normal, endogenously expressed protein. Immune complex kinase assays carried out in the presence of the Src-selective inhibitor PD161430 indicated that the increase in FAK-associated kinase activity brought about by cell adhesion is due in part to increased association with c-Src or another Src family kinase. Under the conditions of our assay, we did not obtain evidence for a dramatic increase in FAK-associated Src activity in fibronectin-replated cells relative to that in the attached, serum-starved cells, as reported by Schlaepfer et al. (67). This apparent conflict may reflect differences in cell lysis conditions. Indeed, Schlaepfer et al. noted that the large increase in FAK/c-Src coimmunoprecipitation they observed upon fibronectin replating is not apparent when cells are lysed in a buffer lacking SDS, similar to that employed in our analysis. Nevertheless, the pool of FAK that we assayed clearly demonstrates an adhesion-dependent increase in tyrosine phosphorylation (Fig. 4B, top panels) and is thus relevant for assessing the requirement of FAK phosphorylation in catalytic activation.
Expression of WT-FAK significantly enhanced the rate and extent of cell spreading observed soon after cells were replated onto fibronectin and also enhanced the migration of cells toward 10% FBS in the modified Boyden chamber assay. Previously Ilic et al. (29) showed that FAK-null mouse embryo cells are more rounded and exhibit a diminished rate of migration relative to that of control cells prepared from same-stage normal embryos. Our findings of enhanced spreading and migration by WT-FAK expression in FAK-null cells can be viewed as a rescue of this cellular deficiency. Similarly, Sieg et al. (71) recently reported increased migration of mouse embryo FAK-null cells following transient expression of an epitope-tagged FAK. These studies support previous conclusions, made by using different experimental systems, of a positive role for FAK in cell spreading (53) and migration (9, 17).
F397-FAK.
As expected, F397-FAK is deficient in its ability to become activated in response to cell adhesion as determined by reduced tyrosine phosphorylation and associated kinase activity. Unlike with WT-FAK, expression of F397-FAK failed to stimulate either the initial rate of cell spreading on fibronectin or cell migration to FBS. In fact, as if a dominant-negative mutant, F397-FAK expression had an inhibitory effect on cell spreading (lamellipodial extension) and cell migration. Cary et al. (9) also examined F397-FAK by using their CHO cell overexpression system and observed a failure of this mutant to promote cell spreading and migration, although they did not observe a negative effect of F397-FAK on these processes. A requirement for FAK Tyr-397 in a cell spreading response was also indicated by the finding of Richardson et al. (52) that inhibition of spreading by the FAK C-terminal domain cannot be rescued by coexpression of F397-FAK. Although our Tet-FAK(F397) cells display a retarded lamellipodial extension during early cell spreading on fibronectin, these cells eventually become unusually well spread on fibronectin. This ultraspread morphology of Tet-FAK(F397) cells was also observed under normal cell culture conditions several days after induction. A possible explanation is that expression of F397-FAK results in unusually stable adhesive contacts that are not readily released as the cells locomote across the substratum. This explanation supports the notion that FAK may promote focal adhesion turnover as first suggested by Ilic et al. (29) and is consistent with the mechanism for cell motility proposed by Fincham and Frame (15) which involves FAK phosphorylation by associated Src family kinases promoting FAK degradation and subsequent weakening of adhesive sites.
Taken together, our findings and those of Cary et al. (9) and Richardson et al. (52) emphasize the critical importance of FAK Tyr-397 in signaling to promote cell spreading and migration and suggest that the interaction of FAK with effector molecules that bind to this site is a key signaling step promoting these events. The best-characterized molecular activity of FAK phosphoTyr-397 is the interaction with Src family kinases. Thus, substrate phosphorylation by FAK-associated Src family kinases may contribute to FAK-enhanced cell spreading and migration. In this regard it is notable that other studies have demonstrated a requirement for Src kinase activity in promoting cell spreading (52) and motility (15, 20). Also, impairing Src family kinases by overexpression of Csk can interfere with cell spreading (74). In contrast, Kaplan et al. (31) observed that expression in src−/− fibroblasts of a truncated c-Src lacking the kinase domain can promote cell spreading through a mechanism requiring both Src SH2 and SH3 domains. The mechanism of enhanced spreading by this truncated Src is uncertain, but one possibility is that the SH2 and SH3 domains of the truncated mutant interact with and promote catalytic activation of other Src family kinases expressed in these cells. It has also been suggested that c-Src SH2 and SH3 domains may act as a bridge linking FAK to Cas to promote FAK phosphorylation of Cas (67), although other studies indicate that the interaction between FAK and Cas depends upon the Cas SH3 domain binding to FAK (49). Finally, FAK phosphoTyr-397 interactions with other signaling proteins, including PI3K (11) and PLC-γ (82), could contribute to cell spreading and motility responses.
F576/F577-FAK.
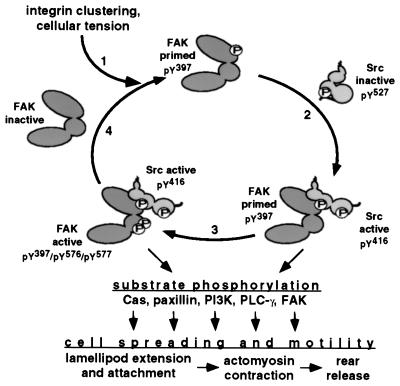
Activation loop phosphorylation is a common mechanism for stimulating the catalytic activity of protein kinases (25). We have shown previously, using a COS-7 cell transient expression system, that FAK activation loop Tyr-576 and Tyr-577 are required for maximal FAK-associated kinase activity toward the artificial substrate poly(GluTyr) (7). Our new findings of impaired adhesion-induced tyrosine phosphorylation and associated kinase activity of F576/F577-FAK, expressed to near-normal levels in a fibroblast cell type, expand upon these early results and suggest that FAK activation loop phosphorylation plays an important role in integrin-mediated activation of the FAK-Src complex by stimulating intermolecular FAK autophosphorylation following an integrin stimulus. Further supporting this notion are our other recent findings that F576/F577-FAK shows substantially reduced Tyr-397 phosphorylation, relative to that of WT-FAK, as assessed by a phosphospecific antibody against the Tyr-397 site (57). Other recent evidence implicating FAK activation loop phosphorylation as an important factor in FAK catalytic activation consists of reports showing that inhibition of Src kinase activity with another selective Src inhibitor PP-1 did not fully block bombesin-stimulated FAK-associated kinase activity (54) and that Tyr-576 and Tyr-577 are required for vanadate-induced FAK activation in chicken embryo cells (42). Taken together, these observations support a model of reciprocal activation of FAK and Src family kinases at new sites of integrin-mediated cell adhesion, with the potential for signal amplification achieved through FAK intermolecular autophosphorylation (Fig. 10).
FIG. 10.
Model for reciprocal catalytic activation by FAK and c-Src (or Fyn) with potential signal amplification loop and possible downstream signaling events promoting cell spreading and motility. See text for details.
The importance of the FAK activation loop phosphorylation sites in FAK signaling was further indicated by our failure to observe enhanced cell spreading or migration following induced expression of F576/F577-FAK. The requirement for the FAK Tyr-576 and Tyr-577 sites in these cellular responses had not been previously investigated. Since these tyrosines are required for maximal FAK kinase activity, our finding that expression of the F576/F577-FAK mutant is unable to effectively promote cell spreading and migration could indicate that FAK’s own kinase activity plays an important signaling role in promoting these events. This possibility is in apparent conflict with the findings of Richardson et al. (52) and Cary et al. (9) that overexpression of FAK mutants with severely impaired kinase activity are able to rescue inhibition of cell spreading and promote cell migration, respectively. However, as pointed out by these investigators, the kinase-deficient FAK mutants still appear to achieve near-normal levels of Tyr-397 phosphorylation, due perhaps to the association with the endogenous WT-FAK in the cells employed. It is thus notable that F576/F577-FAK expressed in FAK-null cells is poorly phosphorylated on Tyr-397 (Fig. 3 and 4) (57), reflecting the weak autophosphorylation activity of this mutant and absence of a compensatory endogenous WT-FAK. Although it seems most likely that the negative effects on FAK catalytic activity we observed for F576/F577-FAK are due to the inability of this mutant to become phosphorylated on the activation loop, we cannot rule out the possibility that these phenylalanine substitutions have other inhibitory effects on activity related to structural alterations of the kinase domain active site. It should also be noted that Src-mediated phosphorylation of the FAK activation loop tyrosines has been shown to stabilize the interaction with Cas (49), and this may also contribute to downstream events promoting cell spreading and/or migration.
Pyk2.
The negative effects on cell spreading and migration we observed upon induced expression of F397-FAK is most likely due to an inhibition of Pyk2 signaling. Confirming other recent reports (71, 76), we found that Pyk2 is expressed to relatively high levels in FAK-null cells, where it undergoes adhesion-dependent tyrosine phosphorylation. We speculate that Pyk2 compensates, in part, for the lack of FAK expression by replacing FAK in a signaling pathway leading to enhanced cell spreading and motility. The possibility that Pyk2 functions instead of FAK in this pathway is supported by the fact that these two proteins are well conserved in a number of important functional/signaling features (see the introduction). Despite these similarities, our results indicate that Pyk2 is relatively inefficient at promoting cell spreading and migration, since induced expression of wild-type FAK enhances these events relative to uninduced cells. Indeed, Sieg et al. (71) recently showed that FAK is more effective than Pyk2 in promoting cell migration toward fibronectin following transient expression of the epitope-tagged proteins in FAK-null cells.
We observed that induced expression of FAK results in the inability of Pyk2 to achieve adhesion-dependent tyrosine phosphorylation. The mechanism by which FAK expression inhibits Pyk2 phosphorylation is presently unclear. An attractive possibility is that FAK physically displaces Pyk2 from binding sites in the focal adhesion complex because its FAT domain has a higher affinity for these sites (83). Thus, the lower affinity of Pyk2 for the focal adhesion complex may contribute to weaker spreading and migration promoting activities relative to those of FAK. Expression of F397-FAK would impair cell spreading and migration due to the displacement of Pyk2, which would be coupled with the inability of this mutant to effectively signal to promote these cellular activities. The lack of a negative effect on cell spreading and migration upon expression of F576/F577-FAK may be due to the limited degree of Tyr-397 phosphorylation achieved by this mutant, thus allowing limited signaling, comparable to that achieved by Pyk2 in the uninduced cells. Despite its attractiveness, we have been unable to obtain experimental support for this Pyk2 focal adhesion displacement model. By immunofluorescence microscopy Pyk2 appears to be prominently localized to the perinuclear region in both induced and uninduced Tet-FAK cells (data not shown), consistent with the results of other researchers (71). We detect slight Pyk2 immunoreactivity at the cell periphery of Tet-FAK cells, overlapping focal adhesions, but this is evident in both induced and uninduced cells, and the weakness of the signal prevents quantitative measurements.
Possible downstream events in FAK-enhanced cell spreading and motility.
The forward movement of fibroblast-like cells on an extracellular matrix (ECM) substratum is an integrated, multistep process (reviewed in references 36, 43, and 70) involving (i) extension of actin-rich lamellipodia and filopodia at the leading edge, (ii) formation and stabilization of extracellular matrix attachments at the newly extended cell periphery involving transmembrane integrin linkages between ECM ligands and the actin cytoskeleton, (iii) contraction of actin filaments connecting the cell-substratum adhesion sites with intracellular structures, and (iv) detachment of the cell rear brought about by the contractile forces acting on weakened adhesion sites at the rear of the cell. Likely substrates of the FAK-Src complex have been implicated in each of these events (Fig. 10).
Supporting a role for Cas signaling in FAK-enhanced cell spreading and migration, we observed increased Cas tyrosine phosphorylation upon expression of WT-FAK and decreased Cas tyrosine phosphorylation upon expression of F397-FAK, correlating well with the spreading and migration effects of these FAK variants. Cary et al. (10) first implicated Cas as a downstream target in the FAK signaling pathway promoting cell migration by showing that expression of a FAK mutant impaired in its ability to bind Cas is deficient in migration-promoting activity. Further supporting a role for Cas tyrosine phosphorylation in cell migration is the demonstration by Klemke et al. (33) that Cas and its interacting partner, c-Crk, which binds to multiple Cas phosphotyrosines, have an additive effect in promoting cell migration when expressed in COS-7 cells. Klemke et al. (33) also observed that Cas/Crk-enhanced migration is blocked by a dominant-negative Rac. Since Rac and Cdc42 play a role in integrin-mediated membrane ruffling and lamellipod extension during cell spreading (13, 50), a possible pathway linking FAK/Src to Cas/Crk to Rac/Cdc42 in a cell spreading response is suggested. Cas signaling could contribute to cell spreading by anchoring the actin cytoskeleton to sites of cell-ECM adhesion, as suggested by recent findings that disruption of the Cas-encoding gene in mice is associated with a disorganization of both myofibrils and Z discs in cardiocytes and stress fibers and focal adhesions in cultured fibroblasts (28). Paxillin has also been implicated in FAK-enhanced cell spreading. Richardson et al. (52) showed that inhibition of spreading by the FAK C-terminal domain cannot be rescued by coexpression of a FAK mutant impaired in its ability to bind paxillin. Paxillin phosphorylation and interaction with c-Crk could conceivably promote lamellipodial extension through Rac activation as proposed above for Cas.
The SH2-mediated interactions of PI3K (11) and PLC-γ1 (82) with FAK phosphoTyr-397, and subsequent catalytic activation of these enzymes, may also play a role in FAK-enhanced spreading and motility. Increased production of D-3 phosphoinositides by PI3K contributes to an integrin-mediated pathway activating Rac to promote cell spreading (13, 50, 51, 55), and both PI3K and PLC-γ1 have been implicated as major effector molecules promoting cell motility following activation of receptor protein-tyrosine kinases (12, 35). Finally, Src phosphorylation of FAK itself, in addition to promoting focal adhesion turnover through FAK degradation as discussed above, could also conceivably stimulate cell migration through FAK Tyr-925 phosphorylation. This could recruit Grb2, leading to activation of the Ras-MAP kinase cascade (68), with subsequent stimulation of actomyosin contraction by ERK1/2 phosphorylation of myosin light chain (32). However, the results of Cary et al. (10) argue against a role for mitogen-activated protein kinase activity contributing to FAK-enhanced cell migration.
In summary, our results add to a growing body of evidence implicating FAK as a positive regulator of cell spreading and migration. In addition to confirming the essential role of the FAK autophosphorylation site Tyr-397, we have shown that the FAK activation loop phosphoacceptors Tyr-576 and Tyr-577 contribute to the FAK signaling events promoting cell spreading and migration. Our finding that expression of the F397-FAK mutant has a negative effect on cell spreading and cell migration while disrupting Pyk2 adhesion-dependent tyrosine phosphorylation suggests that Pyk2 may also signal to stimulate these events in cells where FAK is not expressed.
ACKNOWLEDGMENTS
The first two authors contributed equally to this work.
We thank Samyukta Reddy and Yuki Ohi for excellent technical assistance and Peter Dempsey, Steve Hann, Gene Oltz, Tom Polte, and Al Reynolds for providing useful reagents.
This work was supported by Public Health Service grant R01-GM49882 from the National Institute of General Medical Sciences (to S.K.H.). J.D.O. was supported by training grant F31-AA05408 from the National Institute on Alcohol Abuse and Alcoholism. P.J.R. was supported by training grant T32-CA78136 from the National Cancer Institute Training Program in Breast Cancer Research.
REFERENCES
- 1.Astier A, Avraham H, Manie S N, Groopman J, Canty T, Avraham S, Freedman A S. The related adhesion focal tyrosine kinase is tyrosine-phosphorylated after beta1-integrin stimulation in B cells and binds to p130cas. J Biol Chem. 1997;272:228–232. doi: 10.1074/jbc.272.1.228. [DOI] [PubMed] [Google Scholar]
- 2.Astier A, Manie S N, Avraham H, Hirai H, Law S F, Zhang Y, Golemis E A, Fu Y, Druker B J, Haghayeghi N, Freedman A S, Avraham S. The related adhesion focal tyrosine kinase differentially phosphorylates p130Cas and the Cas-like protein, p105HEF1. J Biol Chem. 1997;272:19719–19724. doi: 10.1074/jbc.272.32.19719. [DOI] [PubMed] [Google Scholar]
- 3.Avraham S, London R, Fu Y, Ota S, Hiregowdara D, Li J, Jiang S, Pasztor L M, White R A, Groopman J E, Avraham H. Identification and characterization of a novel related adhesion focal tyrosine kinase (RAFTK) from megakaryocytes and brain. J Biol Chem. 1995;270:27742–27751. doi: 10.1074/jbc.270.46.27742. [DOI] [PubMed] [Google Scholar]
- 4.Bockholt S M, Burridge K. Cell spreading on extracellular matrix proteins induces tyrosine phosphorylation of tensin. J Biol Chem. 1993;268:14565–14567. [PubMed] [Google Scholar]
- 5.Burridge K, Turner C E, Romer L H. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J Cell Biol. 1992;119:893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol. 1996;12:463–518. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- 7.Calalb M B, Polte T R, Hanks S K. Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol Cell Biol. 1995;15:954–963. doi: 10.1128/mcb.15.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calalb M B, Zhang X, Polte T R, Hanks S K. Focal adhesion kinase tyrosine-861 is a major site of phosphorylation by Src. Biochem Biophys Res Commun. 1996;228:662–668. doi: 10.1006/bbrc.1996.1714. [DOI] [PubMed] [Google Scholar]
- 9.Cary L A, Chang J F, Guan J-L. Stimulation of cell migration by overexpression of focal adhesion kinase and its association with Src and Fyn. J Cell Sci. 1996;109:1787–1794. doi: 10.1242/jcs.109.7.1787. [DOI] [PubMed] [Google Scholar]
- 10.Cary L A, Han D C, Polte T R, Hanks S K, Guan J-L. Identification of p130Cas as a mediator of focal adhesion kinase-promoted cell migration. J Cell Biol. 1998;140:211–221. doi: 10.1083/jcb.140.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen H-C, Appeddu P A, Isoda H, Guan J-L. Phosphorylation of tyrosine 397 in focal adhesion kinase is required for binding phosphatidylinositol 3-kinase. J Biol Chem. 1996;271:26329–26334. doi: 10.1074/jbc.271.42.26329. [DOI] [PubMed] [Google Scholar]
- 12.Chen P, Xie H, Sekar M C, Gupta K, Wells A. Epidermal growth factor receptor-mediated cell motility: phospholipase C activity is required, but mitogen-activated protein kinase activity is not sufficient for induced cell movement. J Cell Biol. 1994;127:847–857. doi: 10.1083/jcb.127.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark E A, King W G, Brugge J S, Symons M, Hynes R O. Integrin-mediated signals regulated by members of the Rho family of GTPases. J Cell Biol. 1998;142:573–586. doi: 10.1083/jcb.142.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dikic I, Tokiwa G, Lev S, Courtneidge S A, Schlessinger J. A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature. 1996;383:547–550. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- 15.Fincham V J, Frame M C. The catalytic activity of Src is dispensable for translocation to focal adhesions but controls the turnover of these structures during cell motility. EMBO J. 1998;17:81–92. doi: 10.1093/emboj/17.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gates R E, King L E, Jr, Hanks S K, Nanney L B. Potential role for focal adhesion kinase in migrating and proliferating keratinocytes near epidermal wounds and in culture. Cell Growth Differ. 1994;5:891–899. [PubMed] [Google Scholar]
- 17.Gilmore A P, Romer L H. Inhibition of focal adhesion kinase (FAK) signaling in focal adhesions decreases cell motility and proliferation. Mol Biol Cell. 1996;7:1209–1224. doi: 10.1091/mbc.7.8.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guan J-L, Shalloway D. Regulation of focal adhesion-associated protein tyrosine kinase by both cellular adhesion and oncogenic transformation. Nature. 1992;358:690–692. doi: 10.1038/358690a0. [DOI] [PubMed] [Google Scholar]
- 20.Hall C L, Lange L A, Prober D A, Zhang S, Turley E A. pp60c-src is required for cell locomotion regulated by the hyaluronanreceptor RHAMM. Oncogene. 1996;13:2213–2224. [PubMed] [Google Scholar]
- 21.Hamasaki K, Mimura T, Morino N, Furuya H, Nakamoto T, Aizawa S I, Morimoto C, Yazaki Y, Hirai H, Nojima Y. Src kinase plays an essential role in integrin-mediated tyrosine phosphorylation of Crk-associated substrate p130Cas. Biochem Biophys Res Commun. 1996;222:338–343. doi: 10.1006/bbrc.1996.0745. [DOI] [PubMed] [Google Scholar]
- 22.Hamby J M, Connolly C J, Schroeder M C, Winters R T, Showalter H D, Panek R L, Major T C, Olsewski B, Ryan M J, Dahring T, Lu G H, Keiser J, Amar A, Shen C, Kraker A J, Slintak V, Nelson J M, Fry D W, Bradford L, Hallak H, Doherty A M. Structure-activity relationships for a novel series of pyrido[2,3-d]pyrimidine tyrosine kinase inhibitors. J Med Chem. 1997;40:2296–2303. doi: 10.1021/jm970367n. [DOI] [PubMed] [Google Scholar]
- 23.Hanks S K, Calalb M B, Harper M C, Patel S K. Focal adhesion protein-tyrosine kinase phosphorylated in response to cell attachment to fibronectin. Proc Natl Acad Sci USA. 1992;89:8487–8491. doi: 10.1073/pnas.89.18.8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanks S K, Polte T R. Signaling through focal adhesion kinase. BioEssays. 1997;19:137–145. doi: 10.1002/bies.950190208. [DOI] [PubMed] [Google Scholar]
- 25.Hardie G, Hanks S, editors. The protein kinase factsbook, vol. 1 and 2. London, England: Academic Press; 1995. [Google Scholar]
- 26.Harte M T, Hildebrand J D, Burnham M R, Bouton A H, Parsons J T. p130Cas, a substrate associated with v-Src and v-Crk, localizes to focal adhesions and binds to focal adhesion kinase. J Biol Chem. 1996;271:13649–13655. doi: 10.1074/jbc.271.23.13649. [DOI] [PubMed] [Google Scholar]
- 27.Hildebrand J D, Schaller M D, Parsons J T. Paxillin, a tyrosine phosphorylated focal adhesion-associated protein binds to the carboxyl terminal domain of focal adhesion kinase. Mol Biol Cell. 1995;6:637–647. doi: 10.1091/mbc.6.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Honda H, Oda H, Nakamoto T, Honda Z, Sakai R, Suzuki T, Saito T, Nakamura K, Nakao K, Ishiwawa T, Katsuki M, Yazaki Y, Hirai H. Cardiovascular anomaly, impaired actin bundling and resistance to Src-induced transformation in mice lacking p130Cas. Nat Genet. 1998;19:361–365. doi: 10.1038/1246. [DOI] [PubMed] [Google Scholar]
- 29.Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T, Aizawa S. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- 30.Kanner S B, Reynolds A B, Vines R R, Parsons J T. Monoclonal antibodies to individual tyrosine-phosphorylated protein substrates of oncogene-encoded tyrosine kinases. Proc Natl Acad Sci USA. 1990;87:3328–3332. doi: 10.1073/pnas.87.9.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaplan K B, Swedlow J R, Morgan D O, Varmus H E. c-Src enhances the spreading of src−/− fibroblasts on fibronectin by a kinase-independent mechanism. Genes Dev. 1995;9:1505–1517. doi: 10.1101/gad.9.12.1505. [DOI] [PubMed] [Google Scholar]
- 32.Klemke R L, Cai S, Giannini A L, Gallagher P J, de Lanerolle P, Cheresh D A. Regulation of cell motility by mitogen-activated protein kinase. J Cell Biol. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klemke R L, Leng J, Molander R, Brooks P C, Vuori K, Cheresh D A. CAS/Crk coupling serves as a “molecular switch” for induction of cell migration. J Cell Biol. 1998;140:961–972. doi: 10.1083/jcb.140.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kornberg L J, Earp H S, Parsons J T, Schaller M, Juliano R L. Cell adhesion or integrin clustering increases phosphorylation of a focal adhesion-associated tyrosine kinase. J Biol Chem. 1992;267:23439–23442. [PubMed] [Google Scholar]
- 35.Kundra V, Escobedo J A, Kazlauskas A, Kim H K, Rhee S G, Williams L T, Zetter B R. Regulation of chemotaxis by the platelet-derived growth factor receptor-β. Nature. 1994;367:474–476. doi: 10.1038/367474a0. [DOI] [PubMed] [Google Scholar]
- 36.Lauffenburger D A, Horwitz A F. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 37.Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio J M, Plowman G D, Rudy B, Schlessinger J. Protein tyrosine kinase PYK2 involved in Ca2+-induced regulation of ion channel and MAP kinase functions. Nature. 1995;376:737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- 38.Li J, Avraham H, Rogers R A, Raja S, Avraham S. Characterization of RAFTK, a novel focal adhesion kinase, and its integrin-dependent phosphorylation and activation in megakaryocytes. Blood. 1996;88:417–428. [PubMed] [Google Scholar]
- 39.Li X, Earp H S. Paxillin is tyrosine-phosphorylated by and preferentially associates with the calcium-dependent tyrosine kinase in rat liver epithelial cells. J Biol Chem. 1997;272:14341–14348. doi: 10.1074/jbc.272.22.14341. [DOI] [PubMed] [Google Scholar]
- 40.Lipfert L, Haimovich B, Schaller M D, Cobb B S, Parsons J T, Brugge J S. Integrin-dependent phosphorylation and activation of the protein tyrosine kinase pp125FAK in platelets. J Cell Biol. 1992;119:905–912. doi: 10.1083/jcb.119.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lipsich L A, Lewis A J, Brugge J S. Isolation of monoclonal antibodies that recognize the transforming proteins of avian sarcoma viruses. J Virol. 1983;48:352–360. doi: 10.1128/jvi.48.2.352-360.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maa M-C, Leu T-H. Vanadate-dependent FAK activation is accomplished by the sustained FAK Tyr-576/577 phosphorylation. Biochem Biophys Res Commun. 1998;251:344–349. doi: 10.1006/bbrc.1998.9464. [DOI] [PubMed] [Google Scholar]
- 43.Mitchison T J, Cramer L P. Actin-based cell motility and locomotion. Cell. 1996;84:371–379. doi: 10.1016/s0092-8674(00)81281-7. [DOI] [PubMed] [Google Scholar]
- 44.Morgenstern J P, Land H. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nojima Y, Morino N, Mimura T, Hamasaki K, Furuya H, Sakai R, Sato T, Tachibana K, Morimoto C, Yazaki Y, Hirai H. Integrin-mediated cell adhesion promotes tyrosine phosphorylation of p130Cas, a Src homology 3-containing molecule having multiple Src homology 2-binding motifs. J Biol Chem. 1995;270:15398–15402. doi: 10.1074/jbc.270.25.15398. [DOI] [PubMed] [Google Scholar]
- 46.Ohba T, Ishino M, Aoto H, Sasaki T. Interaction of two proline-rich sequences of cell adhesion kinase beta with SH3 domains of p130Cas-related proteins and a GTPase-activating protein, Graf. Biochem J. 1998;330:1249–1254. doi: 10.1042/bj3301249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petch L A, Bockholt S M, Bouton A, Parsons J T, Burridge K. Adhesion-induced tyrosine phosphorylation of the p130 SRC substrate. J Cell Sci. 1995;108:1371–1379. doi: 10.1242/jcs.108.4.1371. [DOI] [PubMed] [Google Scholar]
- 48.Polte T R, Hanks S K. Interaction between focal adhesion kinase and Crk-associated tyrosine kinase substrate p130Cas. Proc Natl Acad Sci USA. 1995;92:10678–10682. doi: 10.1073/pnas.92.23.10678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Polte T R, Hanks S K. Complexes of focal adhesion kinase (FAK) and Crk-associated substrate (p130Cas) are elevated in cytoskeletal-associated fractions following adhesion and Src transformation: requirements for Src kinase activity and FAK proline-rich motifs. J Biol Chem. 1997;272:5501–5509. doi: 10.1074/jbc.272.9.5501. [DOI] [PubMed] [Google Scholar]
- 50.Price L S, Leng J, Schwartz M A, Bokoch G M. Activation of Rac and Cdc42 by integrins mediates cell spreading. Mol Biol Cell. 1998;9:1863–1871. doi: 10.1091/mbc.9.7.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reif K, Nobes C D, Thomas G, Hall A, Cantrell D A. Phosphatidylinositol 3-kinase signals activate a selective subset of Rac/Rho-dependent effector pathways. Curr Biol. 1996;6:1445–1455. doi: 10.1016/s0960-9822(96)00749-x. [DOI] [PubMed] [Google Scholar]
- 52.Richardson A, Malik R K, Hildebrand J D, Parsons J T. Inhibition of cell spreading by expression of the C-terminal domain of focal adhesion kinase (FAK) is rescued by coexpression of Src or catalytically inactive FAK: a role for paxillin tyrosine phosphorylation. Mol Cell Biol. 1997;17:6906–6914. doi: 10.1128/mcb.17.12.6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Richardson A, Parsons J T. A mechanism for regulation of the adhesion-associated protein tyrosine kinase pp125FAK. Nature. 1996;380:538–540. doi: 10.1038/380538a0. [DOI] [PubMed] [Google Scholar]
- 54.Rodriquez-Fernandez J L, Rozengurt E. Bombesin, vasopressin, lysophosphatidic acid, and sphingosylphosphorylcholine induce focal adhesion kinase activation in intact Swiss 3T3 cells. J Biol Chem. 1998;273:19321–19328. doi: 10.1074/jbc.273.30.19321. [DOI] [PubMed] [Google Scholar]
- 55.Rodriguez-Viciana P, Warne P H, Khwaja A, Marte B M, Pappin D, Das P, Waterfield M D, Ridley A, Downward J. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- 56.Rozengurt E, Rodriguez-Fernandez J L. Tyrosine phosphorylation in the action of neuropeptides and growth factors. Essays Biochem. 1997;32:73–86. [PubMed] [Google Scholar]
- 57.Ruest, P. J., S. Roy, J. D. Owen, E. Schaefer, and S. K. Hanks. Unpublished data.
- 58.Sakai R, Nakamoto T, Ozawa K, Aizawa S, Hirai H. Characterization of the kinase activity essential for tyrosine phosphorylation of p130Cas in fibroblasts. Oncogene. 1997;14:1419–1426. doi: 10.1038/sj.onc.1200954. [DOI] [PubMed] [Google Scholar]
- 59.Salgia R, Avraham S, Pisick E, Li J L, Raja S, Greenfield E A, Sattler M, Avraham H, Griffin J D. The related adhesion focal tyrosine kinase forms a complex with paxillin in hematopoietic cells. J Biol Chem. 1996;271:31222–31226. doi: 10.1074/jbc.271.49.31222. [DOI] [PubMed] [Google Scholar]
- 60.Sasaki H, Nagura K, Ishino M, Tobioka H, Kotani K, Sasaki T. Cloning and characterization of cell adhesion kinase β, a novel protein-tyrosine kinase of the focal adhesion kinase subfamily. J Biol Chem. 1995;270:21206–21219. doi: 10.1074/jbc.270.36.21206. [DOI] [PubMed] [Google Scholar]
- 61.Schaller M D, Borgman C A, Cobb B S, Vines R R, Reynolds A B, Parsons J T. PP125FAK, a structurally unique protein tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci USA. 1992;89:5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schaller M D, Hildebrand J D, Shannon J D, Fox J W, Vines R R, Parsons J T. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol Cell Biol. 1994;14:1680–1688. doi: 10.1128/mcb.14.3.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schaller M D, Parsons J T. pp125FAK-dependent tyrosine phosphorylation of paxillin creates a high-affinity binding site for Crk. Mol Cell Biol. 1995;15:2635–2645. doi: 10.1128/mcb.15.5.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schaller M D, Sasaki T. Differential signaling by the focal adhesion kinase and cell adhesion kinase beta. J Biol Chem. 1997;272:25319–25325. doi: 10.1074/jbc.272.40.25319. [DOI] [PubMed] [Google Scholar]
- 65.Schlaepfer D D, Hanks S K, Hunter T, van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- 66.Schlaepfer D D, Hunter T. Evidence for in vivo phosphorylation of the Grb2 SH2-domain binding site on focal adhesion kinase by Src-family protein-tyrosine kinases. Mol Cell Biol. 1996;16:5623–5633. doi: 10.1128/mcb.16.10.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schlaepfer D D, Broome M A, Hunter T. Fibronectin-stimulated signaling from a focal adhesion kinase–c-Src complex: involvement of the Grb2, p130cas, and Nck adapter proteins. Mol Cell Biol. 1997;17:1702–1713. doi: 10.1128/mcb.17.3.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schlaepfer D D, Hunter T. Focal adhesion kinase overexpression enhances Ras-dependent integrin signaling to ERK2/mitogen-activated protein kinase through interactions with and activation of c-Src. J Biol Chem. 1997;272:13189–13195. doi: 10.1074/jbc.272.20.13189. [DOI] [PubMed] [Google Scholar]
- 69.Schlaepfer D D, Jones K C, Hunter T. Multiple Grb2-mediated integrin-stimulated signaling pathways to ERK2 mitogen-activated protein kinase: summation of both c-Src- and focal adhesion kinase-initiated tyrosine phosphorylation events. Mol Cell Biol. 1998;18:2571–2585. doi: 10.1128/mcb.18.5.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sheets M P, Felsenfeld D P, Galbraith C G. Cell migration: regulation of force on extracellular matrix-integrin complexes. Trends Cell Biol. 1998;81:51–54. doi: 10.1016/s0962-8924(98)80005-6. [DOI] [PubMed] [Google Scholar]
- 71.Sieg D J, Ilic D, Jones K C, Damsky C H, Hunter T, Schlaepfer D D. Pyk2 and Src-family protein-tyrosine kinases compensate for the loss of FAK in fibronectin-stimulated signaling events but Pyk2 does not fully function to enhance FAK- cell migration. EMBO J. 1998;17:5933–5947. doi: 10.1093/emboj/17.20.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tachibana K, Sato T, D’Avirro N, Morimoto C. Direct association of pp125FAK with paxillin, the focal adhesion targeting mechanism of pp125FAK. J Exp Med. 1995;182:1089–1100. doi: 10.1084/jem.182.4.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tachibana K, Urano T, Fujita H, Ohashi Y, Kamiguchi K, Iwata S, Hirai H, Morimoto C. Tyrosine phosphorylation of Crk-associated substrates by focal adhesion kinase—a putative mechanism for the integrin-mediated tyrosine phosphorylation of Crk-associated substrates. J Biol Chem. 1997;272:29083–29090. doi: 10.1074/jbc.272.46.29083. [DOI] [PubMed] [Google Scholar]
- 74.Takayama T, Tanaka S, Nagai K, Okada M. Adenovirus-mediated overexpression of C-terminal Src kinase (Csk) in type I astrocytes interferes with cell spreading and attachment to fibronectin. J Biol Chem. 1999;274:2291–2297. doi: 10.1074/jbc.274.4.2291. [DOI] [PubMed] [Google Scholar]
- 75.Turner C E, Glenney J R, Burridge K. Paxillin: a new vinculin-binding protein present in focal adhesions. J Cell Biol. 1990;111:1059–1068. doi: 10.1083/jcb.111.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ueki K, Mimura T, Nakamoto T, Sasaki T, Aizawa S, Hirai H, Yano S, Naruse T, Nojima Y. Integrin-mediated signal transduction in cells lacking focal adhesion kinase p125FAK. FEBS Lett. 1998;432:197–201. doi: 10.1016/s0014-5793(98)00862-x. [DOI] [PubMed] [Google Scholar]
- 77.Vuori K, Hirai H, Aizawa S, Ruoslahti E. Induction of p130cas signaling complex formation upon integrin-mediated cell adhesion: a role for Src family kinases. Mol Cell Biol. 1996;16:2606–2613. doi: 10.1128/mcb.16.6.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Withers B E, Keller P R, Fry D W. Expression, purification, and characterization of focal adhesion kinase using a baculovirus system. Protein Expr Purif. 1996;7:12–18. doi: 10.1006/prep.1996.0002. [DOI] [PubMed] [Google Scholar]
- 79.Wu J, Mariner D J, Thoreson M A, Reynolds A B. Production and characterization of monoclonal antibodies to the catenin p120ctn. Hybridoma. 1998;17:175–183. doi: 10.1089/hyb.1998.17.175. [DOI] [PubMed] [Google Scholar]
- 80.Xing Z, Chen H-C, Nowlen J K, Taylor S J, Shalloway D, Guan J-L. Direct interaction of v-Src with the focal adhesion kinase mediated by the Src SH2 domain. Mol Biol Cell. 1994;5:413–421. doi: 10.1091/mbc.5.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yu H, Li X, Marchetto G S, Dy R, Hunter D, Calvo B, Dawson T L, Wilm M, Anderegg R J, Graves L M, Earp H S. Activation of a novel calcium-dependent protein-tyrosine kinase. Correlation with c-Jun N-terminal kinase but not mitogen-activated protein kinase activation. J Biol Chem. 1996;271:29993–29998. doi: 10.1074/jbc.271.47.29993. [DOI] [PubMed] [Google Scholar]
- 82.Zhang, X., A. Chattopadhyay, Q.-S. Ji, J. D. Owen, P. J. Ruest, G. Carpenter, and S. K. Hanks. Proc. Natl. Acad. Sci. USA, in press. [DOI] [PMC free article] [PubMed]
- 83.Zheng C, Xing Z, Bian Z C, Guo C, Akbay A, Warner L, Guan J-L. Differential regulation of Pyk2 and focal adhesion kinase (FAK). The C-terminal domain of FAK confers response to cell adhesion. J Biol Chem. 1998;273:2384–2389. doi: 10.1074/jbc.273.4.2384. [DOI] [PubMed] [Google Scholar]