Abstract
Background & Aims
Detailed information on the immune response after second vaccination of cirrhotic patients and liver transplant (LT) recipients against severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) is largely missing. We aimed at comparing the vaccine-induced humoral and T-cell responses of these vulnerable patient groups.
Methods
In this prospective cohort study, anti-SARS-CoV-2 spike-protein titers were determined using the DiaSorin LIAISON (anti-S trimer) and Roche Elecsys (anti-S RBD) immunoassays in 194 patients (141 LT, 53 cirrhosis Child-Pugh A-C) and 56 healthy controls before and 10 to 84 days after second vaccination. The spike-specific T-cell response was assessed using an interferon-gamma release assay (EUROIMMUN). A logistic regression analysis was performed to identify predictors of low response.
Results
After the second vaccination, seroconversion was achieved in 63% of LT recipients and 100% of cirrhotic patients and controls using the anti-S trimer assay. Median anti-SARS-CoV-2 titers of responding LT recipients were lower compared with cirrhotic patients and controls (P < .001). Spike-specific T-cell response rates were 36.6%, 65.4%, and 100% in LT, cirrhosis, and controls, respectively. Altogether, 28% of LT recipients did neither develop a humoral nor a T-cell response after second vaccination. In LT recipients, significant predictors of absent or low humoral response were age >65 years (odds ratio [OR], 4.57; 95% confidence interval [CI], 1.48-14.05) and arterial hypertension (OR, 2.50; 95% CI, 1.10-5.68), whereas vaccination failure was less likely with calcineurin inhibitor monotherapy than with other immunosuppressive regimens (OR, 0.36; 95% CI, 0.13-0.99).
Conclusion
Routine serological testing of the vaccination response and a third vaccination in patients with low or absent response seem advisable. These vulnerable cohorts need further research on the effects of heterologous vaccination and intermittent reduction of immunosuppression before booster vaccinations.
Keywords: Immunosuppression, Liver Cirrhosis, Liver Transplant Recipients, SARS-CoV-2 Vaccination
Abbreviations used in this paper: Anti-S RBD, anti-SARS-CoV-2 antibodies in Roche Elecsys immunoassay; anti-S trimer, anti-SARS-CoV-2 antibodies in DiaSorin LIAISON immunoassay; BAU, binding antibody units; CI, confidence interval; CNI, calcineurin inhibitor; COVID-19, Coronavirus disease 2019; eGFR, estimated glomerular filtration rate; IGRA, interferon gamma release assay; IFN-γ, interferon-gamma; IQR, interquartile range; LC, liver cirrhosis; LT, liver transplant; MMF, mycophenolate mofetil; mTORi, mammalian target of rapamycin inhibitors; OR, odds ratio; RBD, receptor binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus type 2; SD, standard deviation; SOT, solid-organ transplantation; TIPS, transjugular intrahepatic portosystemic stent-shunt
What You Need to Know.
Background
After vaccination against severe acute respiratory syndrome coronavirus type 2, the immune response is reduced in organ transplant recipients as compared with the healthy population.
Findings
Older age, arterial hypertension, and immunosuppression other than calcineurin inhibitor monotherapy predict vaccination failure in liver transplant recipients. In decompensated liver cirrhosis patients, the humoral immune response is comparable to healthy controls.
Implications for patient care
Identification of predictors of no or low immune response after initial vaccinations will help to decide on further booster strategies. Patients with liver cirrhosis should be vaccinated pre transplantation.
In the initial clinical trials investigating the efficacy and safety of severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) vaccines, various immunocompromised or immunosuppressed patient populations (ie, patients with liver cirrhosis [LC] or liver transplant [LT] recipients) were not included.1 , 2 However, a markedly increased mortality due to Coronavirus disease 2019 (COVID-19) has been described for both patient groups compared with the healthy population, indicating the need for SARS-CoV-2 vaccination.3 , 4
Preliminary data showed that LT recipients might be less likely to reach seroconversion after SARS-CoV-2 vaccination,5 , 6 but up to now, few detailed data are available on patients with cirrhosis. Also, individual risk factors for an inadequate vaccination response have not been studied comprehensively in these populations so far. An ongoing trial found an overall seroconversion rate of 89% in immunocompromised patients and the highest risk of non-seroconversion in patients with vasculitis and B-cell depletion.7
This prospective observational study explores the humoral and T-cell response to SARS-CoV-2 vaccination in a large cohort of patients with compensated and decompensated LC and LT recipients. Also, predictors of low response to vaccination were identified in this highly vulnerable patient population.
Methods
Study population and data collection
Non-pregnant patients ≥18 years with LC presenting for LT or patients post LT were enrolled in this prospective observational cohort study at the University Medical Center Hamburg-Eppendorf in case of SARS-CoV-2 vaccination with a 2-dose regimen, consisting of an mRNA (BNT162b2; BioNTech SE/Pfizer or mRNA-1273; Moderna Biotech) or vector-based vaccine (AZD1222; AstraZeneca). LT recipients receiving a combined transplantation and cirrhotic patients under immunosuppression were excluded. Clinical data were obtained from the patients’ electronic medical records. In addition, control subjects matched for age and vaccination regimen were included. In all participants, the immune response was determined 10 to 84 days after the second vaccination, and in a subgroup also directly before the first and second vaccination. The study was approved by the local Ethics Committee of Hamburg, Germany (Reg. number PV7103) and the Paul Ehrlich Institute, the German Federal Institute for Vaccines and Biomedicines (Reg. number NIS508). All participants signed written informed consent, and all authors had access to the study data and reviewed and approved the final manuscript.
Investigation of the vaccine-specific humoral and T-cell response
The vaccine-specific humoral immune response was quantitatively determined by 2 different anti-SARS-CoV-2 spike immunoassays in parallel: The DiaSorin LIAISON XL anti-SARS-CoV-2 TrimericS IgG ChemiLuminescent ImmunoAssay (sensitivity, 99.4%; specificity, 99.8%; cutoff, 33.8 binding antibody units [BAU]/mL8), with spike S1 glycoproteins assembled as trimers allowing to detect a broad range of antibodies including responses to the N terminal regions of the spike protein (anti-S trimer) and the Roche Elecsys anti-SARS-CoV-2 S Ig ElectroChemiLuminescent ImmunoAssay (sensitivity, 93.9%; specificity, 99.6%; cutoff, 0.8 U/mL9) with a receptor-binding domain protein (RBD) sandwich assay design (anti-S RBD). For both assays, a low positive response was defined from 33.8 to 100 BAU/mL and from 0.8 to 100 U/mL, respectively, based on thresholds of validating studies and on cutoffs used in randomized trials.10 , 11
The SARS-CoV-2 spike protein-specific T-cell response was determined by a commercial, standardized interferon-gamma (IFN-γ) release assay (IGRA) using the EUROIMMUN SARS-CoV-2 IGRA stimulation tube set (product No. ET 2606-3003) and EUROIMMUN IFN-γ enzyme-linked immunosorbent assay (product No. EQ 6841-960). The specific T-cell response was quantified according to the manufacturer’s instructions and values >100 mIU/mL were interpreted as low positive, values >200 mIU/mL as positive.12
Statistical analysis
Epidemiologic data and test results are displayed as mean and standard deviation (SD) for normally distributed, or as median and interquartile range (IQR) for non-normally distributed continuous variables and as number of patients and percentage for categorical variables, respectively. The Pearson χ2 test was used to test the difference in dichotomous variables between 2 or more groups. If test assumptions were not fulfilled, the Fisher exact test was used instead. Normally and abnormally distributed continuous variables were compared by the t test and Mann-Whitney U test when comparing 2 groups or the Kruskal-Wallis test when comparing more than 2 groups, respectively. Differences of dependent variables were evaluated by the McNemar (categorical) and Wilcoxon (continuous) tests. The correlation of humoral and T-cell immune response was calculated using the Spearman rank test. A binary logistic regression model was constructed based on rational assumptions to predict a positive immune response. Significance was expected for P-values smaller than .05. SPSS Statistics Version 26 for Mac (IBM Corp, Armonk, NY) and GraphPad Prism version 8.0.0 for Mac (GraphPad Software, San Diego, CA) were used for statistical analyses and to create figures, respectively.
Results
Patient characteristics
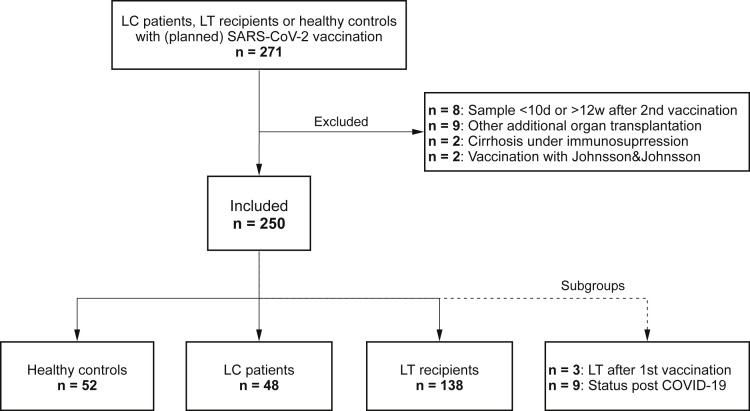
Altogether, 194 patients (53 cirrhotic patients with Child-Pugh class A to C and 141 LT recipients) and 56 controls were enrolled in this study (Figure 1 ). Nine convalescents recovered from COVID-19 (2 LC, 3 LT) were analyzed separately.
Figure 1.
Flowchart of study cohort.
The clinical characteristics of the patients and controls included in the main analysis are shown in Table 1 . All patients with liver cirrhosis were presenting for evaluation of liver transplantation or check-up investigations on the waiting list. Of those, 32 patients (66.7%) had a decompensated Child B or C cirrhosis, and 9 (18.8%) had undergone implantation of a transjugular intrahepatic portosystemic stent-shunt (TIPS). Most LT recipients were long-term recipients (median time since LT, 7 years), whereas 17 (12.3%) were vaccinated within 1 year post LT. Altogether, fewer patients with cirrhosis than LT recipients had arterial hypertension (37.5% vs 61.6%) or suffered from chronic kidney disease (16.7% vs. 37.3%).
Table 1.
Baseline Characteristics and Laboratory Values Before the First SARS-CoV-2 Vaccination
| LC patients (n = 48) | LT recipients (n = 138) | Controls (n = 52) | P | |
|---|---|---|---|---|
| Age, y | 53.8 (9.5) | 55.0 (13.19) | 50.9 (11.6) | .095 |
| Females | 19 (39.6) | 59 (42.8) | 33 (63.5) | .021 |
| Vaccine regimen | .068 | |||
| mRNA/mRNA | 44 (91.6) | 121 (87.7) | 39 (75.0) | |
| BNT162b2 | 38 (79.2) | 110 (79.7) | 36 (69.2) | |
| mRNA-1273 | 6 (12.4) | 11 (8.0) | 3 (5.8) | |
| AZD1222/AZD1222 | 1 (2.1) | 6 (4.3) | 2 (3.8) | |
| AZD1222/mRNA | 3 (6.3) | 11 (8.0) | 11 (21.2) | |
| Days 1st to 2nd vaccine | 42 (41–43) | 42 (40–42) | 36 (22–63) | .054 |
| Days 2nd vaccine to follow-up | 28 (21–41) | 29 (25–39) | 49 (28–74) | < .001 |
| BMI, kg/m2 | 26.3 (23.4–29.8) | 24.8 (22.4–28.5) | .069 | |
| Diabetes | 12 (25.0) | 29 (21.0) | .566 | |
| Arterial hypertension | 18 (37.5) | 85 (61.6) | .004 | |
| CKD with eGFR 30–59 mL/min | 6 (16.7) | 38 (37.3) | .026 | |
| eGFR <30 mL/min | 1 (2.8) | 8 (7.8) | .446 | |
| Etiology of liver disease | .006 | |||
| ALD | 23 (47.9) | 28 (20.3) | ||
| Viral | 3 (6.3) | 17 (12.3) | ||
| AILD | 11 (22.9) | 40 (29.0) | ||
| NASH | 4 (8.3) | 7 (5.1) | ||
| Pediatric | – | 5 (3.6) | ||
| Cryptogenic | 5 (10.4) | 13 (9.4) | ||
| ALF | 1 (2.1) | 5 (3.6) | ||
| Other | 1 (2.1) | 23 (16.7) | ||
| HCC | 5 (10.4) | 25 (18.1) | .212 | |
| Child-Pugh class | ||||
| A | 16 (33.3) | |||
| B | 18 (37.5) | |||
| C | 14 (29.2) | |||
| TIPS | 9 (18.9) | |||
| Time from 1st LT, y | 7 (2–17) | |||
| Vaccination < 1 y post LT | 17 (12.3) | |||
| Prednisone | 43 (31.2) | |||
| CNI | 128 (92.8) | |||
| Tacrolimus | 95 (68.8) | |||
| Cyclosporin | 33 (23.9) | |||
| CNI monotherapy | 33 (23.9) | |||
| CNI + prednisone | 19 (13.8) | |||
| CNI + mTORi | 17 (12.3) | |||
| CNI + MMF | 48 (34.8) | |||
| CNI + azathioprine | 9 (6.5) | |||
| Biologicals | 8 (5.8) | |||
| ≥3 Immunosuppressants | 18 (13.0) |
| LCa | LTb | P | ||
|---|---|---|---|---|
| HbA1c, % (ref. 4.8-5.6) | 4.4 (4.1–5.9) | 5.7 (5.1–6.0) | .080 | |
| Creatinine, mg/dL (ref. 0.55-1.02) | 0.9 (0.8–1.1) | 1.1 (0.9–1.5) | < .001 | |
| eGFR, mL/min | 80.5 (66.0–102.8) | 64.0 (43.5–82.3) | .001 | |
| MELD | 14 (10–19) | – | ||
| IgG, g/L (ref. 6.5-16.0) | 14.7 (11.9–19.1) | 10.6 (8.4–12.0) | < .001 | |
| IgA, g/L (ref. 0.4-3.5) | 4.2 (3.6–5.6) | 1.7 (1.3–2.6) | < .001 | |
| IgM, g/L (ref. 0.5-3.0) | 1.8 (1.0–2.7) | 1.1 (0.7–1.7) | .053 | |
| Lymphocytes, /μl (ref. 1000-3600) | 994 (757–1422) | 950 (667–1404) | .786 | |
| T-lymphocytes, /μl (ref. 900-2900) | 626 (406–933) | 746 (391–1044) | .488 | |
| B-lymphocytes, /μl (ref. 80-500) | 128 (84–252) | 92 (56–130) | .100 | |
| CD4-helper cells, /μl (ref. 500-1350) | 504 (264–682) | 413 (225–572) | .447 | |
| CD8-cytotoxic cells, /μl (ref. 290-930) | 112 (86–240) | 242 (158–440) | .004 | |
| NK-cells, /μl (ref. 35-350) | 174 (67–326) | 122 (63–207) | .263 | |
| CD4/CD8 ratio (ref. 0.6-3.6) | 3.3 (2.1–5.1) | 1.5 (1.1–2.2) | < .001 |
Note: Data are presented as number (%), mean (standard deviation), or median (interquartile range).
Note: Boldface P values indicate statistical significance.
AILD, Autoimmune liver disease; ALD, alcoholic liver disease; ALF, acute liver failure; BMI, body mass index; CKD, chronic kidney disease; CNI, calcineurin inhibitor; eGFR, estimated glomerular filtration rate; HC, healthy control; HCC, hepatocellular carcinoma; IQR, interquartile range; LC, liver cirrhosis; LT, liver transplant; MELD, Model for End-Stage Liver Disease; MMF, mycophenolate mofetil; mTORi, mammalian target of rapamycin inhibitors; NASH, nonalcoholic steatohepatitis; SARS-CoV-2, severe acute respiratory syndrome coronavirus type 2; SD, standard deviation; TIPS, transjugular intrahepatic portosystemic stent-shunt.
n ranges from 17 to 36.
n ranges from 42 to 102.
Calcineurin inhibitor (CNI) therapy was used in almost all patients (92.8%), with 23.9% receiving a CNI monotherapy, and additional mycophenolate mofetil (MMF), mammalian target of rapamycin inhibitors (mTORis), or prednisone in the remaining cases. Laboratory values are shown in Table 1.
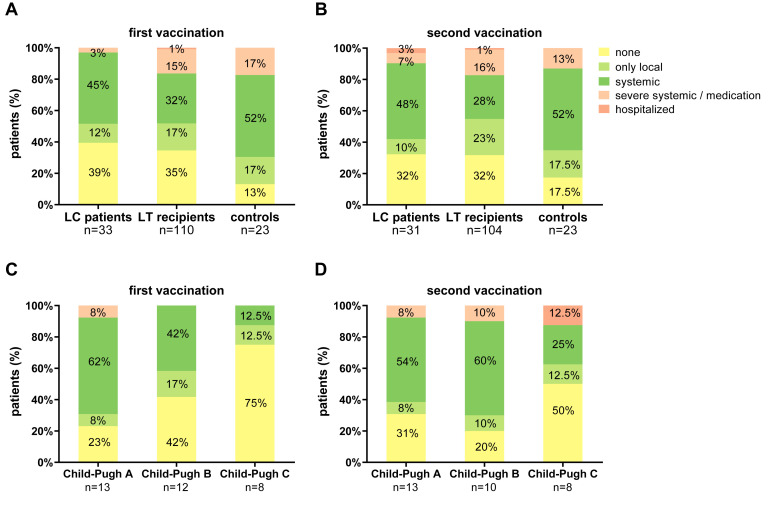
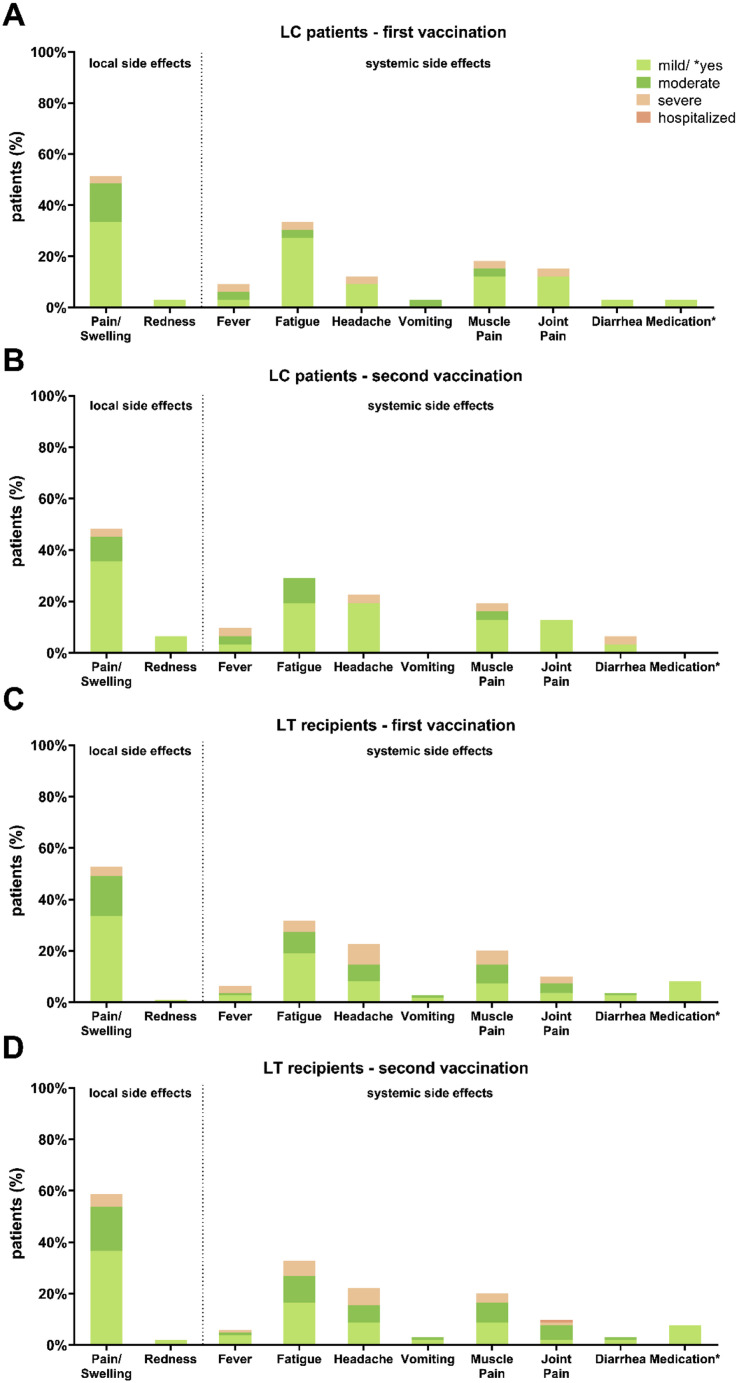
The vaccination regimen used (Table 1) as well as vaccination side effects (Supplementary Figures 1 and 2) did not differ between the groups.
Supplementary Figure 1.
Comparison of side effects after first and second SARS-CoV-2 vaccination in all patients and in cirrhotic patients with different Child-Pugh classes. Side effects were classified into 5 groups: none, only local, systemic, severe systemic/medication, and hospitalized. (A) Side effects after first vaccination in LC patients, LT recipients, and controls. (B) Side effects after second vaccination in the 3 groups. (C) Side effects after first vaccination in LC patients with different Child-Pugh classes. (D) Side effects after second vaccination in LC patients with different Child-Pugh classes. Statistical analysis was performed by Wilcoxon matched pairs rank test. All P-values were > .05.
Supplementary Figure 2.
Comparison of local and systemic side effects after first and second vaccination in cirrhotic patients and LT recipients. Detailed comparison of local and systemic side effects in LC patients and LT recipients. Side effects were classified into mild, moderate, severe, and hospitalized. Medications have been classified only into yes (light green) or no. (A) Side effects after first vaccination in LC patients. (B) Side effects after second vaccination in LC patients. (C) Side effects after first vaccination in LT recipients. (D) Side effects after second vaccination in LT recipients.
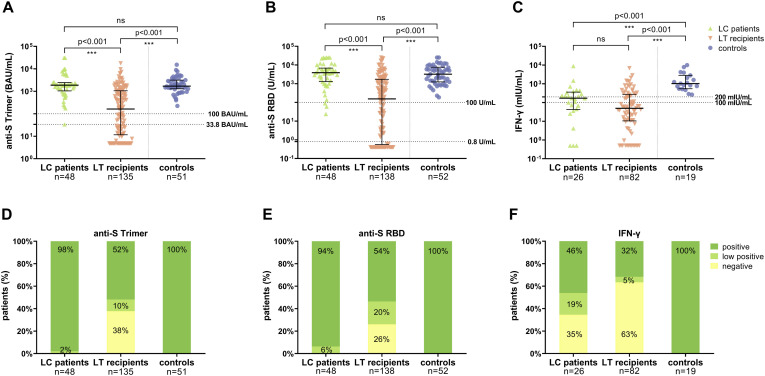
The humoral immune response after the second vaccination
After the second vaccination (median, 29 days), significantly fewer LT recipients tested positive for anti-SARS-CoV-2 Ig compared with cirrhotic patients and controls using the anti-S RBD (73.9% vs 100% vs 100%, respectively) or the anti-S trimer assay (63.0% vs 97.9% vs 100%, respectively). A negative or weak anti-SARS-CoV-2 response was seen in 2% (anti-S RBD) and 6% (anti-S trimer) of the cirrhotic patients and 46% (anti-S RBD) and 48% (anti-S trimer) of the LT recipients, respectively (Figure 2D-F). Furthermore, the median titers of anti-SARS-CoV-2 Ig were significantly lower in patients post LT as compared with patients with liver cirrhosis (Figure 2A-C). Thus, in contrast to LT recipients, cirrhotic patients were not found to have an impaired humoral immune response compared with controls based on concerning seroconversion rate and median antibody titers of responding patients (Supplementary Table 1).
Figure 2.
Serological and T-cell response after second SARS-CoV-2 vaccination in cirrhotic patients, LT recipients, and healthy controls. (A) Anti-S Trimer; (B) anti-S RBD; (C) IFN-γ release. Statistical analysis was performed by Mann-Whitney test. Solid horizontal lines indicate medians and interquartile range; dotted horizontal lines indicate cutoff values for no response, low positive, and positive response. The respective proportions are provided as bar graphs. (D) Anti-S Trimer; (E) anti-S RBD ; (F) IFN-γ release.
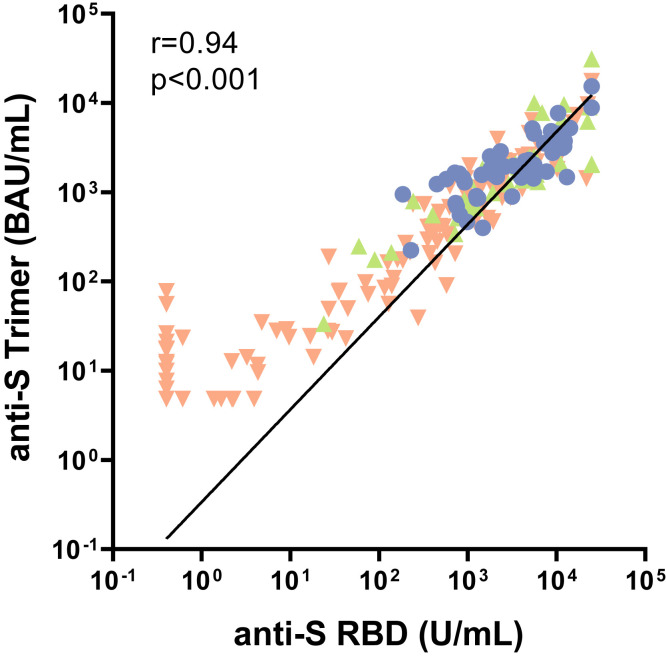
Of note, there was a high concordance between both immunoassays (Supplementary Figure 3). Therefore, for all subsequent analyses the results of the trimer assay are shown. Additionally, the results of the RBD assay are provided as numerical values in the corresponding tables and as additional figures in the supplementary.
Supplementary Figure 3.
Correlation of anti-SARS-CoV-2 spike RBD and spike trimer. Correlation between anti-S RBD (U/mL) and anti-S trimer (BAU/mL) for cirrhotic patients (green ascending triangles), LT recipients (orange descending triangles), and controls (blue dots). Statistical analysis was performed by Spearman r with a 95% CI.
Development of anti-SARS-CoV-2 Ig titers after the first and second vaccination
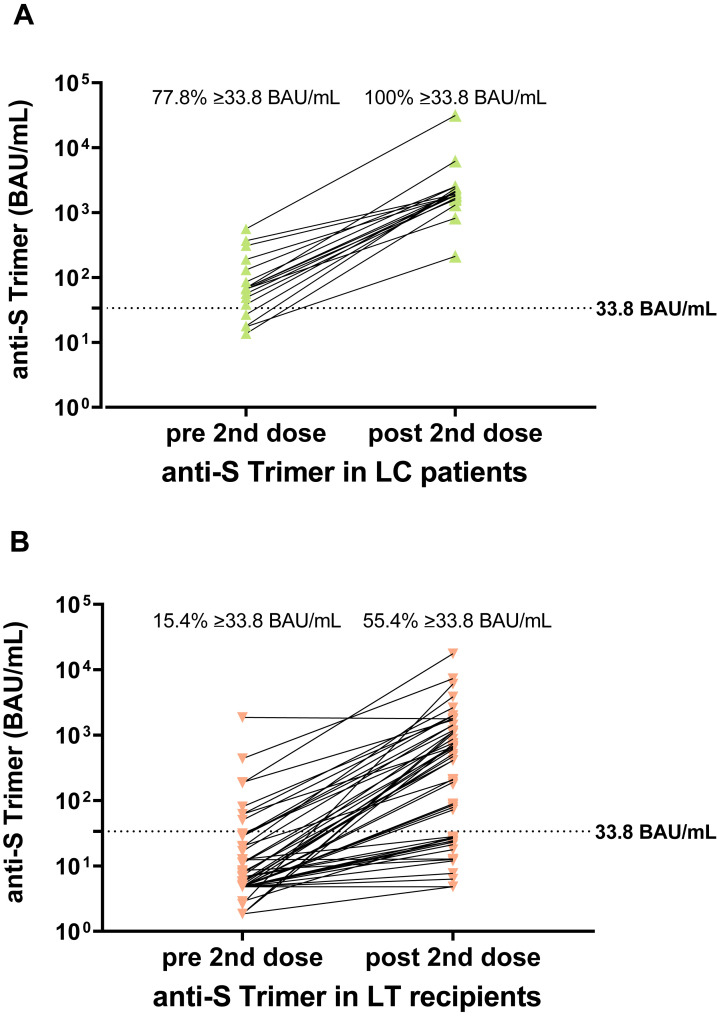
The anti-SARS-CoV-2 Ig titers after the first and second vaccination (19 LC, 88 LT) are shown in Supplementary Figures 4A and B. The seroconversion rate markedly increased in cirrhotic patients (from 77.8% to 100%) and LT recipients (from 15.4% to 55.4%). In patients who did not develop a detectable humoral response after the first vaccination, the probability of seroconversion after the second vaccination was 100% for cirrhotic patients and 43.6% for LT recipients. Also, there was a significant 28- and 19-fold increase of the median anti-SARS-CoV-2 Ig titers in cirrhotic patients and LT recipients, respectively, at last follow-up 5 ± 3 weeks after vaccination.
Supplementary Figure 4.
Comparison of antibody titers after first and second SARS-CoV-2 vaccination. Comparison of anti-S trimer (BAU/mL) titers after first and second SARS-CoV-2 vaccination in cirrhotic patients (green) and LT recipients (orange). (A) Anti-S trimer in cirrhotic patients. (B) Anti-S Trimer in LT recipients. Statistical analysis was performed by Wilcoxon matched pairs rank test. Percentages indicate the seroconversion rate; dotted horizontal lines indicate cutoff values.
The T-cell response after the second vaccination
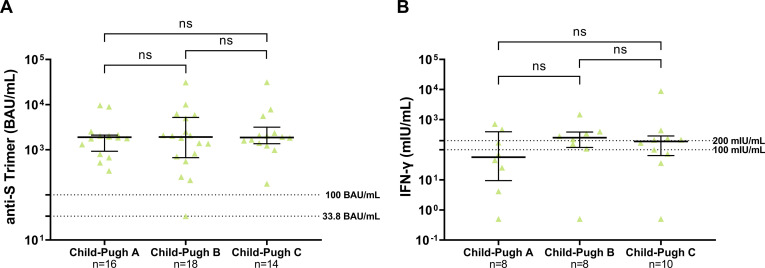
The cellular immune response assessed by semiquantitative analysis of IFN-γ release after spike-specific stimulation of T-cells was determined in a subgroup of 26 cirrhotic patients, 82 LT recipients, and 19 controls. Overall, after the second vaccination, a T-cell response (cutoff >100 mIU/mL) was less frequently detectable in LT recipients (37%) and cirrhotic patients (65%) compared with controls (100%) (Figure 2F). Only 32% of LT recipients and 46% of cirrhotic patients showed a strong response (cutoff >200 mIU/mL) as compared with 100% of controls. Also, the median concentration of IFN-γ release was significantly lower in patients with cirrhosis and LT recipients compared with controls (Figure 2C, Supplementary Table 1).
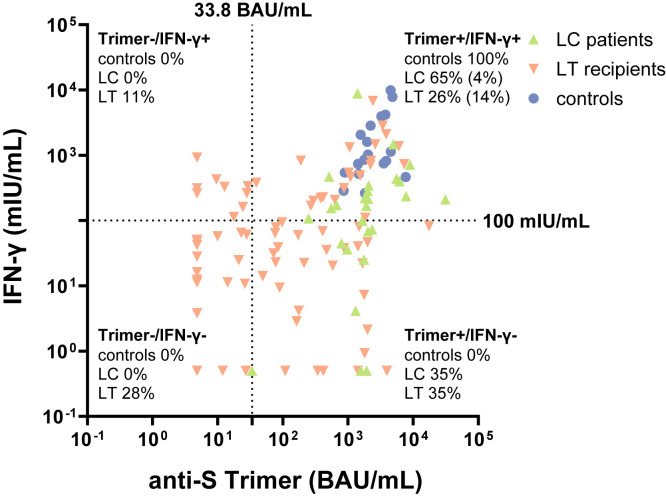
Correlation between the vaccine-induced humoral and T-cell response
Although in controls positive and negative test results correlated in 100% of cases between the trimer immunoassay and the IGRA, there was a high discordance in cirrhotic and LT patients (Supplementary Figure 5). Nine of 32 LT patients (27%) without detectable antibody response had a positive T-cell response. On the other hand, 29 of 50 LT patients (58%) with a negative IGRA result tested positive for anti-S trimer antibodies. Altogether, 23 of 82 LT recipients (28%) did show neither a detectable spike-specific humoral nor a T-cell response, and even 40% showed no or a low humoral and T-cell response. Also, 35% of patients with cirrhosis with a positive antibody response tested negative for a T-cell response.
Supplementary Figure 5.
Correlation between humoral and T-cell immune response. Correlation between humoral and T-cell immune response for cirrhotic patients (green ascending triangles), LT recipients (red descending triangles), and controls (blue dots). Humoral response measured with anti-S trimer (BAU/mL); T-cell response measured with IFN-γ release (mIU/mL). Dotted lines indicate cutoff values. Percentages indicate proportions of values for every patient group. In addition, low positive responses (cutoff values 100 BAU/mL and 200 mIU/mL) are shown in brackets.
Risk factors for no or a low humoral immune response in LT recipients
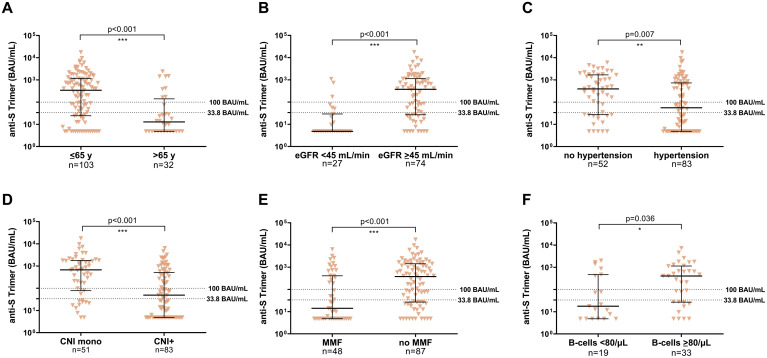
Parameters investigated by univariate and multivariate binary logistic regression analysis as potential predictors for a low immune response after the second SARS-CoV-2 vaccination in LT recipients are given in Table 2 and Supplementary Table 2. Independent prognostic factors for no or only a weak antibody response were age >65 years (odds ratio [OR], 4.57; 95% confidence interval [CI], 1.48-14.05) and arterial hypertension (OR, 2.50; 95% CI, 1.10-5.68), whereas calcineurin inhibitor monotherapy was a positive prognostic factor for a response as compared with other immunosuppressive regimens (OR, 0.36; 95% CI, 0.13-0.99) (Figure 3 ). In the LT cohort, only 19.2% of patients were >65 years, but 59.6% of patients <65 years attained anti-S trimer titers above 100 BAU/mL (Supplementary Table 3). Of note, laboratory values were not considered for multivariate analysis because of limited baseline values (n = 42). However, the seroconversion rate (31.6% vs 60.6%; P = .044) and median antibody titers (P = .039) significantly differed between LT recipients with B-lymphocytes below and above the reference value (80/μl) (Figure 3F). For easy clinical use, we added a table to estimate the relative risk of no or low immune response in case of multiple risk factors (Supplementary Table 4).
Table 2.
Risk of LT Recipients of No or Only a Low Humoral Immune Response After Second SARS-CoV-2 Vaccination Based on the Trimer Immunoassay
| Univariate OR (95% CI) | P | Multivariate OR (95% CI) | P | |
|---|---|---|---|---|
| Age >65 y | 6.21 (2.18–17.69) | .001 | 4.57 (1.48–14.05) | .008 |
| Sex (male) | 2.10 (1.04–4.22) | .038 | ||
| Vaccination regimen | 1.47 (0.71–3.04) | .300 | ||
| LT <1 y before vaccination | 2.94 (0.98–8.88) | .055 | ||
| Obesity (BMI ≥30 kg/m2) | 0.74 (0.28–2.01) | .557 | ||
| eGFR <45 mL/min | 11.10 (3.07–40.15) | < .001 | N/A | |
| Arterial hypertension | 2.82 (1.37–5.83) | .005 | 2.50 (1.10–5.68) | .028 |
| Diabetes | 2.48 (1.05–5.84) | .038 | 1.78 (0.67–4.77) | .251 |
| CNI monotherapy | 0.26 (0.11–0.63) | .003 | 0.36 (0.13–0.99) | .049 |
| CNI + MMF | 3.08 (1.47–6.45) | .003 | 1.78 (0.74–4.30) | .198 |
| CNI + mTORi | 1.94 (0.66–5.68) | .227 | ||
| CNI + azathioprine | 0.29 (0.06–1.43) | .127 | ||
| CNI + prednisone | 0.49 (0.17–1.40) | .183 | ||
| No CNI | 4.77 (0.97–23.38) | .054 | ||
| Prednisone >5 mg | 1.32 (0.38–4.56) | .659 | ||
| Triple immunosuppression | 2.42 (0.85–6.87) | .098 | ||
| Biological | 3.46 (0.67–17.79) | .138 | ||
| Re-cirrhosis | 0.38 (0.10–1.48) | .161 | ||
| IgG <13.8 g/L | N/A | |||
| IgA <3.9 g/L | N/A | |||
| IgM <1.8 g/L | 3.71 (0.35–38.93) | .275 | ||
| Lymphocytes <1000/μl | 1.36 (0.46–4.07) | .578 | ||
| B-lymphocytes <80/μl | 3.33 (1.01–10.99) | .048 | N/A | |
| T-lymphocytes <900/μl | 1.07 (0.35–3.28) | .910 | ||
| NK-cells <35/μl | 0.46 (0.04–5.42) | .537 | ||
| CD4+ cells <400/μl | 0.80 (0.27–2.42) | .695 | ||
| CD8+ cells <290/μl | 1.09 (0.37–3.24) | .875 | ||
| CD4/CD8 ratio <0.6 | N/A |
Note: For laboratory values, the lower levels of normal were set as thresholds.
Note: Bold values indicate statistical significance.
BMI, Body mass index; CI, confidence interval; CNI, calcineurin inhibitor; eGFR, estimated glomular filtration rate; LT, liver transplantation; MMF, mycophenolate mofetil; mTORi, mTOR inhibitor; N/A, not applicable: laboratory values that were not considered for univariate or multivariate analysis because of insufficient baseline values; OR, odds ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus type 2.
Figure 3.
All values were measured with anti-S trimer (BAU/mL). (A) Age groups ≤65 years and >65 years. (B) Normal blood pressure vs arterial hypertension. (C) eGFR <45 mL/min vs ≥45 mL/min. (D) CNI mono vs CNI plus additional immunosuppressive drugs. (E) MMF vs no MMF as an additional drug to CNI. (F) Baseline B-lymphocytes <80 and ≥80 per μl. Statistical analysis was performed by Mann-Whitney test. Solid horizontal lines indicate medians and interquartile range; dotted horizontal lines indicate cutoff values.
Special patient groups
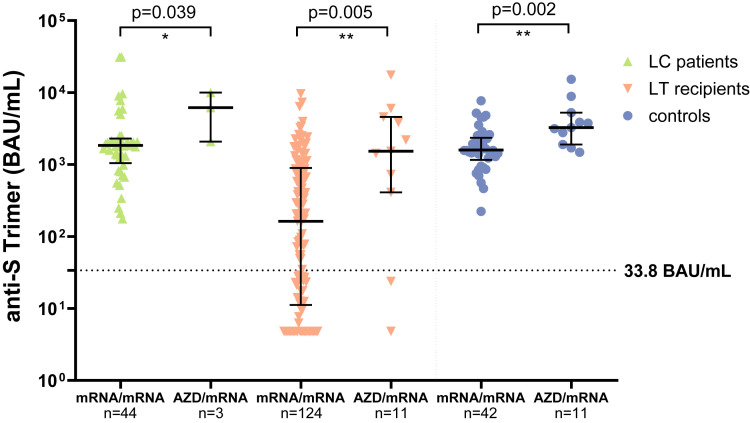
LT recipients who obtained heterologous vaccination. A significantly better immune response was found in LT recipients with mixed (AZD1222/mRNA, n = 11) as compared with homologous mRNA vaccination (n = 121) in terms of the level of antibody titers and IFN-γ titers (Supplementary Figure 6, Supplementary Table 5). Similarly, significantly higher anti-SARS-CoV-2 antibody responses were detectable in cirrhotic patients and controls after heterologous vaccination.
Supplementary Figure 6.
Humoral immune response for homologous vs heterologous vaccination regimens. Comparison of homologous (mRNA/mRNA) and heterologous (mRNA/AZD1222) vaccination regimens by detection of anti-S trimer (BAU/mL) in cirrhotic patients (green ascending triangles), LT recipients (orange descending triangles), and controls (blue dots). Statistical analysis was performed by Mann-Whitney test. Solid horizontal lines indicate medians and interquartile ranges; dotted horizontal lines indicate cutoff values.
Immune response according to Child-Pugh class. There were no differences between patients with varying Child-Pugh classes with regard to the humoral and cellular immune response based on the seroconversion rate (Supplementary Table 6) and the level of antibody titers or IFN-γ production (Figure 4A and B), and vaccination side effects (Supplementary Figure 1C and D), respectively. Compared with LT recipients, higher antibody titers were found in both patients with compensated (Child-Pugh class A) and decompensated liver cirrhosis (Child-Pugh class B and C). Also, no differences in the antibody titers were found in the subgroup of cirrhotic patients after TIPS implantation (n = 9; 18.9%) compared with patients without TIPS (Supplementary Table 6).
Figure 4.
Comparison of humoral and cellular immune responses in cirrhotic patients. (A) Anti-S Trimer (BAU/mL). (B) IFN-γ release (mIU/mL). Statistical analysis was performed by Mann-Whitney test. Solid horizontal lines indicate medians and interquartile ranges; dotted horizontal lines indicate cutoff values.
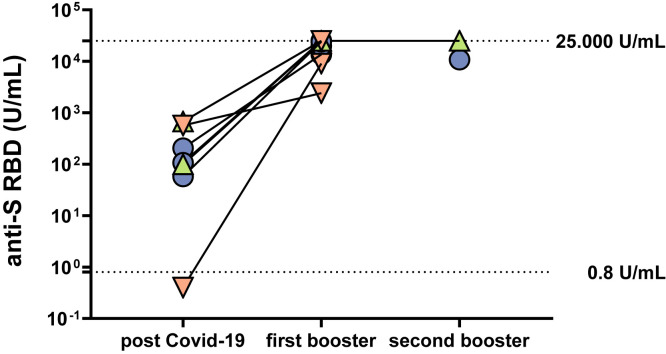
Convalescents with a booster vaccination. All nine convalescents (2 LC, 3 LT, 4 controls) received an mRNA-based vaccine. All subjects developed very high anti-SARS-CoV-2 titers with a 29- to 76-fold increase (Supplementary Figure 7) and additionally showed high titers of IFN-γ in the IGRA (1 LC, 1 LT, and 3 controls) of 2127 and 9738 mIU/mL.
Supplementary Figure 7.
Humoral immune response in convalescents after 1 or 2 booster vaccinations. Humoral immune response in patients with previous SARS-CoV-2 infection and 1 or 2 booster mRNA vaccinations by detection of anti-S RBD (U/mL). Cirrhotic patients (green), LT recipients (orange), and controls (blue). Lower dotted horizontal line indicates cutoff value; upper dotted horizontal line indicates maximum value.
Discussion
This prospective study analyzed the humoral and cellular immune response in patients with different stages of LC and LT recipients. Cirrhotic patients, including patients with decompensated Child-Pugh class B or C or after TIPS implantation, had an overall serological response comparable to healthy controls. In contrast, almost one-half of LT recipients showed no or only a low spike-specific antibody response after the second vaccination. Moreover, there was no evidence of a spike-specific T-cell response in the majority of LT recipients without any detectable antibody response. More than one-quarter of the LT recipients were left potentially immunologically unprotected against SARS-CoV-2 infection, and an additional 20% of patients were considered to have only a suboptimal immune response.
In this study, we used 2 different immunoassays to determine the humoral immune response: the more commonly used anti-S RBD assay (Roche Elecsys) and the anti-S trimer assay (DiaSorin LIAISON). The latter assay was previously shown to correlate highly with the current gold standard for detection of neutralizing antibodies.8 Here, both assays showed a very high correlation in patients and controls (r = 0.94).
In addition, we also evaluated the T-cell immune response by assessing the ex vivo IFN-γ release after spike-specific stimulation of T-cells in a commercial, standardized IGRA assay. It has been suggested that the vaccine-induced T-cell response may have a protective effect even in the absence of a detectable vaccine-induced B-cell response by limiting the extent of viral replication and by supporting long-term immunological memory.13 Therefore, solid organ transplant (SOT) recipients with a strong T-cell response may be protected against a severe course of SARS-CoV-2 infection even in the absence of a seroconversion.14 Our results demonstrate a spike-specific T-cell response only in around one-fifth (22%) of LT recipients without seroconversion, being in line with previously reported results in cardiothoracic (20%), and kidney transplant recipients (29.8%).15 , 16 Furthermore, in 54% of the patients with liver cirrhosis who attained seroconversion, the IFN-γ release in the IGRA was below the cutoff. Whether this correlates with a lower protection against COVID-19 disease has to be further investigated in larger prospective studies.
Previously, Rabinowich et al identified age, low estimated glomular filtration rate (eGFR), high-dose prednisone, triple immunosuppression, and MMF use as predictors of a missing immune response after vaccination in a cohort of 80 LT recipients.6 In patients with autoimmune rheumatic diseases receiving immunosuppression lymphopenia and B-cell depletion, among other factors, were associated with lack of seroconversion after the second vaccination.17 In our cohort, we confirmed older age and low eGFR as predictors for a low or negative humoral immune response. Additionally, a pre-vaccination B-lymphocyte count below the reference value of 80 μl was a negative predictive factor, whereas CNI monotherapy was a predictor for a positive humoral response. In principle, our results are in line with those of Rabinowich et al, revealing that higher immunosuppression reduces the response to vaccination6 and adds to the knowledge that LT recipients with low B-lymphocytes and arterial hypertension are at increased risk of failure to SARS-CoV-2 vaccination.
In our small group of LT recipients who received prime AZD1222 and second mRNA vaccination (n = 11), a seroconversion rate of 81.8% and high titers were found. This extends the recently published data describing significantly higher titers of neutralizing antibodies after heterologous vaccination of this highly vulnerable population.16 New approaches to improve the immune response of LT recipients are urgently needed because breakthrough infections have been reported to occur more frequently in SOT recipients than in the healthy population.18 Although recently a randomized trial found significantly higher antibody titers in this population after a third homologous vaccination, the seroconversion rate remained low with only 50% of previously nonresponsive SOT recipients attaining anti-S RBD titer ≥100 U/mL.11 Therefore, before the third vaccination, temporary discontinuation of the antiproliferative immunosuppressive medication and the preference of a heterologous vaccination scheme should be considered in older, long-term LT recipients with a stable graft function and no history of a recent rejection episode but with risk factors for vaccination failure.
Our data also indicate a high probability of vaccine response even in patients with decompensated Child-Pugh class C liver cirrhosis without an increase of side effects. This is of great importance due to the markedly increased mortality of cirrhotic patients infected with COVID-19, in particular in case of decompensated patients.19 , 20 Our data are in line with another retrospective study, which showed that for fully immunized patients with cirrhosis, the risk of COVID-19 disease and hospitalization is reduced by 78.6% and 100%, respectively.21 These results underline the relevance of immunizing cirrhotic patients as proposed by interim recommendations.22
In comparison to previous studies,5 , 6 this study included different vaccination regimens, measured immune response in more detail, and also investigated pre-transplant patients with decompensated cirrhosis.
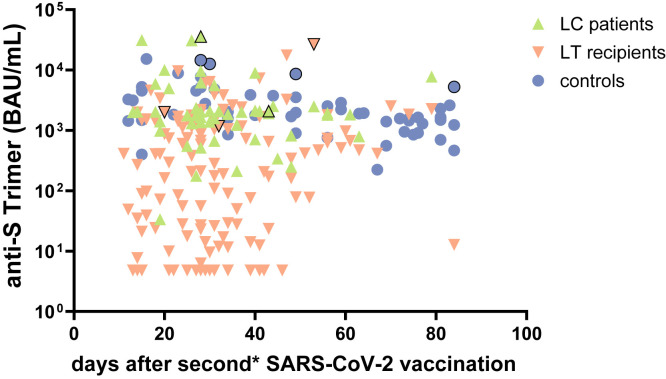
Nonetheless, the study is not without limitations. First, due to the study design, the time interval between the second vaccination and blood sample varied between 10 and 84 days in patients and was longer in the controls. However, the data presented here and those of previous studies of our group23 did not show a significant increase or decrease of antibody titers over the defined inclusion period (Supplementary Figure 8). Second, different time intervals between the 2 vaccinations may impact the immune response. Third, sample sizes were too small to detect potential inherent effects of TIPS placement on the vaccination response. Finally, it should be kept in mind that the level of circulating SARS-CoV-2 antibodies that renders sterile protection against infection has not been established yet. So, the chosen cutoffs, in particular those for suboptimal titers, should only be regarded as estimates based on the current data in the healthy population and based on correlation with neutralizing antibody assays. According to the literature, titers above the cutoff should provide protection against severe COVID-19 disease for most individuals, but not against asymptomatic SARS-CoV-2 infection.14 , 24 , 25 Case reports also demonstrated that even high antibody titers did not render protection in individual constellations. Therefore, it can be assumed that protective titers may vary between patient populations and different SARS-CoV-2 variants.24
Supplementary Figure 8.
Humoral immune response over time after second vaccination. Comparison of humoral immune response according to the time point after second SARS-CoV-2 vaccination in cirrhotic patients (green ascending triangles), LT recipients (orange descending triangles), and controls (blue dots). Convalescents were indicated with a black border line and have received 1∗ mRNA booster vaccination. Detection by anti-S trimer titers (BAU/mL).
This study has important clinical implications. Due to the preserved vaccine-induced humoral immune response of cirrhotic patients,14 this group of patients, including those with decompensated cirrhosis, should be vaccinated before transplantation. However, the clinical significance of the low cellular vaccine response in approximately one-third of the cirrhotic patients is currently unknown and should be carefully monitored concerning the extent and duration of vaccination protection.
On the other hand, a considerable proportion of LT recipients, and the majority of older or lymphopenic patients, showed an absent or reduced humoral and T-cell immune response to SARS-CoV-2 vaccination, which correlated with the intensity of immunosuppression.
Therefore, we suggest a third or even a fourth booster vaccination in all LT recipients and cirrhotic patients with low or missing antibody titers. Further prospective studies are needed to establish an effective vaccination strategy for non-responders. This may include intermittent reduction of the immunosuppression to a CNI monotherapy, or in older patients heterologous vaccination schemes.
Acknowledgment
The authors wish to thank all study participants and contributing departments of the University Medical Center Hamburg-Eppendorf for their active participation in the study. The authors would like to thank Elaine Hussey for her critical review of the manuscript.
CRediT Authorship Contributions
Dr Darius Ferenc Ruether (Data curation: Equal; Formal analysis: Lead; Visualization: Equal; Writing – original draft: Lead; Writing – review & editing: Equal)
Golda Meline Schaub (Data curation: Equal; Formal analysis: Supporting; Investigation: Equal; Methodology: Supporting; Visualization: Lead; Writing – original draft: Supporting; Writing – review & editing: Supporting)
Paul-Maria Duengelhoef (Data curation: Equal; Investigation: Supporting; Validation: Supporting; Visualization: Supporting)
Prof Dr Friedrich Haag (Supervision: Supporting; Writing – review & editing: Supporting)
Dr Thomas Theo Brehm (Investigation: Supporting; Resources: Supporting; Writing – review & editing: Supporting)
Dr Anahita Fathi (Conceptualization: Supporting; Funding acquisition: Equal; Writing – review & editing: Supporting)
Dr Malte Wehmeyer (Resources: Supporting; Writing – review & editing: Supporting)
Jacqueline Jahnke-Triankowski (Data curation: Supporting; Investigation: Supporting; Project administration: Supporting)
Leonie Mayer (Methodology: Supporting)
Armin Hoffmann (Investigation: Supporting)
Prof Dr Lutz Fischer (Resources: Supporting; Writing – review & editing: Supporting)
Prof Dr Marylyn Martina Addo (Conceptualization: Equal; Funding acquisition: Equal; Project administration: Supporting; Resources: Supporting; Writing – review & editing: Supporting)
Dr Marc Luetgehetmann (Investigation: Equal; Resources: Equal; Supervision: Supporting; Writing – review & editing: Supporting)
Prof Dr Ansgar Wilhelm Lohse (Conceptualization: Supporting; Funding acquisition: Supporting; Resources: Supporting; Supervision: Supporting; Writing – review & editing: Equal)
Prof Dr Julian Schulze zur Wiesch, MD (Conceptualization: Supporting; Funding acquisition: Supporting; Resources: Supporting; Supervision: Equal; Visualization: Supporting; Writing – original draft: Equal; Writing – review & editing: Equal)
Prof Dr Martina Sterneck (Conceptualization: Lead; Funding acquisition: Lead; Project administration: Lead; Resources: Supporting; Supervision: Lead; Writing – original draft: Equal; Writing – review & editing: Lead)
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding Friedrich Haag and Julian Schulze zur Wiesch report research funding from SFB1328, project A12; Golda Meline Schaub was supported by a DZIF MD Stipend through the DZIF academy. Anahita Fahti, Marylyn Martina Addo, and Leonie Mayer report funding from the DZIF TTU 01709. No further funding was used for the research reported.
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at http://doi.org/10.1016/j.cgh.2021.09.003.
Supplementary Materials
Supplementary Table 1.
Humoral and T-cell Immune Response of All and Only Responding Patients 10 to 84 Days After Second SARS-CoV-2 Vaccination
| LC median (IQR) | LT median (IQR) | P | |
|---|---|---|---|
| Anti-S trimer titer, BAU/mL | 1880 (1044–2455) | 163 (12–1060) | < .001 |
| Only responders (≥33.8 BAU/mL) | 1910 (1230–2490) | 678 (197–1735) | < .001 |
| Anti-S RBD titer, U/mL | 3883 (1295–6791) | 154 (1–1723) | < .001 |
| Only responders (≥0.8 U/mL) | – | 555 (64–2477) | < .001 |
| IFN-γ release titer, mIU/mL | 170 (43–359) | 49 (10–274) | .086 |
| Only responders (≥100 mIU/mL) | 236 (170–454) | 404 (226–853) | .111 |
Note: Boldface P values indicate statistical significance.
Anti-S RBD, anti-SARS-CoV-2 antibodies in Roche Elecsys immunoassay; anti-S trimer, anti-SARS-CoV-2 antibodies in DiaSorin LIAISON immunoassay; BAU, binding antibody units; IFN-γ, interferon gamma; IQR, interquartile range; LC, liver cirrhosis patients; LT, liver transplant recipients; RBD, receptor binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus type 2.
Supplementary Table 2.
Risk of LT Recipients for No or Only a Low Humoral Immune Response After Second SARS-CoV-2 Vaccination Based on the RBD Immunoassay
| Univariate OR (95% CI) | P | Multivariate OR (95% CI) | P | |
|---|---|---|---|---|
| Age >65 y | 7.23 (2.55–20.53) | < .001 | 5.79 (1.90–17.70) | .002 |
| Sex (male) | 1.91 (0.96–3.80) | .066 | ||
| Vaccination regimen | 0.92 (0.45–1.86) | .811 | ||
| LT <1 y before vaccination | 2.35 (0.82–6.78) | .113 | ||
| Obesity (BMI ≥30 kg/m2) | 0.82 (0.30–2.22) | .699 | ||
| eGFR <45 mL/min | 9.12 (2.86–29.07) | < .001 | N/A | |
| Arterial hypertension | 2.62 (1.28–5.37) | .009 | 2.53 (1.13–5.65) | .024 |
| Diabetes | 2.24 (0.97–5.20) | .060 | ||
| CNI monotherapy | 0.23 (0.09–0.58) | .002 | 0.37 (0.13–1.05) | .063 |
| CNI + MMF | 3.63 (1.73–7.59) | .001 | 2.19 (0.92–5.19) | .077 |
| CNI + mTORi | 1.35 (0.49–3.73) | .563 | ||
| CNI + azathioprine | 0.13 (0.02–1.08) | .059 | ||
| CNI + prednisone | 0.64 (0.23–1.72) | .372 | ||
| No CNI | 5.14 (1.05–25.18) | .043 | ||
| Prednisone >5 mg | 0.96 (0.28–3.31) | .949 | ||
| Triple immunosuppression | 1.53 (0.56–4.14) | .405 | ||
| Biological | 3.72 (0.72–19.15) | .115 | ||
| Re-cirrhosis | 0.41 (0.10–1.60) | .197 | ||
| IgG <13.8 g/L | N/A | |||
| IgA <3.9 g/L | N/A | |||
| IgM <1.8 g/L | 4.13 (0.39–43.38) | .238 | ||
| Lymphocytes <1000/μl | 1.40 (0.47–4.20) | .548 | ||
| B-lymphocytes <80/μl | 4.33 (1.30–14.51) | .017 | N/A | |
| T-lymphocytes <900/μl | 0.82 (0.27–2.54) | .735 | ||
| NK-cells <35/μl | 0.54 (0.05–6.40) | .628 | ||
| CD4+ cells <400/μl | 0.86 (0.28–2.58) | .782 | ||
| CD8+ cells <290/μl | 0.83 (0.28–2.48) | .745 | ||
| CD4/CD8 ratio <0.6 | N/A | N/A |
Note: For laboratory values, the lower levels of normal were set as thresholds.
Note: Bold values indicate statistical significance.
BMI, Body mass index; CI, confidence interval; CNI, calcineurin inhibitor; eGFR, estimated glomular filtration rate; LT, liver transplantation; MMF, mycophenolate mofetil; mTORi, mTOR inhibitor; N/A, not applicable: laboratory values that were not considered for univariate or multivariate analysis because of insufficient baseline values; OR, odds ratio; RBD, receptor binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus type 2.
Supplementary Table 3.
Predictors for No or Only a Low Humoral Immune Response in LT Recipients After Second SARS-CoV-2 Vaccination Based on the Trimer Immunoassay (Cutoff 33.8 BAU/mL)
| Anti-S trimer, BAU/mL n, % within group |
P | Median anti-S trimer (IQR), BAU/mL | ||
|---|---|---|---|---|
| < 33.8 | ≥ 33.8 | |||
| Age >65 y | 21 (80.8) | 5 (19.2) | < .001 | 10 (5–41) |
| Age ≤65 y | 44 (40.4) | 65 (59.6) | 294 (24–1245) | |
| Arterial hypertension | 48 (57.8) | 35 (42.2) | .004 | 56 (5–732) |
| No arterial hypertension | 17 (32.7) | 48 (57.8) | 394 (27–1693) | |
| CNI mono | 8 (24.2) | 25 (75.8) | .002 | 753 (137–2020) |
| CNI + other IS | 56 (55.4) | 45 (44.6) | 72 (5–664) | |
| B-lymphocytes <80/μl | 13 (68.4) | 6 (31.6) | .044 | 18 (5–471) |
| B-lymphocytes ≥80/μl | 13 (39.4) | 20 (60.6) | 407 (27–1145) | |
Note: Boldface P values indicate statistical significance.
Anti-S trimer, Anti-SARS-CoV-2 antibodies in DiaSorin LIAISON immunoassay; BAU, binding antibody units; CNI, calcineurin inhibitor; IQR, interquartile range; IS, immunosuppression; LT, liver transplant; SARS-CoV-2, severe acute respiratory syndrome coronavirus type 2.
Supplementary Table 4.
Risk of Vaccination Failure in LT Recipients With More Than One Negative Prognostic Predictor
| Proportion of patientswith anti-S trimer <100 BAU/mL, % (n/N) | Ratio of relative risk | |||
|---|---|---|---|---|
| CNI and other | Age >65 y | eGFR <45 mL/min | 100 (7/7) | 6.25 |
| eGFR ≥45 mL/min | 100 (6/6) | 6.25 | ||
| Age ≤65 y | eGFR <45 mL/min | 87 (13/15) | 5.44 | |
| eGFR ≥45 mL/min | 45 (22/49) | 2.81 | ||
| CNI mono | Age ≤65 y | eGFR ≥45 mL/min | 16 (3/19) | 1 |
| eGFR <45 mL/min | 75 (3/4) | 4.69 | ||
| Age >65 y | eGFR ≥45 mL/min | 1/1 | ||
| eGFR <45 mL/min | 0 |
Anti-S trimer, Anti-SARS-CoV-2 antibodies in DiaSorin LIAISON immunoassay; BAU, binding antibody units; CNI, calcineurin inhibitor; eGFR, estimated glomerular filtration rate; LT, liver transplant.
The table illustrates the risk of vaccination failure, defined by anti-S trimer <100 BAU/mL, in LT recipients with a combination of several risk factors. The percentage of patients with vaccination failure in the respective patient group is indicated. Also, the relative risk compared with LT patients with optimal prognostic parameters (ie, CNI monotherapy plus ≤65 years plus eGFR ≥45 mL/min) is given.
Colors visualize the relative risk: green 1.0, yellow >1 and ≤4, orange >4 and ≤6, red >6, grey: not available due to low number of subjects)
Supplementary Table 5.
Humoral and T-cell immune response after second SARS-CoV-2 vaccination with mRNA/mRNA vs. AZD1222/mRNA
| mRNA/mRNA, median (IQR) |
AZD1222/mRNA, median (IQR) | P | |
|---|---|---|---|
| LC (n = 44 vs 3) | |||
| Anti-S trimer titer, BAU/mL | 1840 (1044–2295) | 6180 (2080–x) | .043 |
| Anti-S RBD titer, U/mL | 3798 (1295–6456) | 22422 (5595–x) | .043 |
| IFN-γ release titer, mIU/mL | N/A | N/A | N/A |
| LT (n = 121 vs 11) | |||
| Anti-S trimer titer, BAU/mL | 163 (11–895) | 1530 (411–4590) | .006 |
| Only responders (≥33.8 BAU/mL) | 641 (186–1535) | 2200 (1081–5310) | .004 |
| Anti-S RBD titer, U/mL | 128 (1–1411) | 4892 (323–15,503) | .004 |
| Only responders (≥0.8 U/mL) | 474 (43–2179) | 5448 (482–17,072) | .007 |
| IFN-γ release titer, mIU/mL | 44 (10–223) | 926 (288–1738) | .004 |
| Only responders (≥100 mIU/mL) | 328 (214–778) | 1151 (602–1919) | .043 |
| Controls (n = 39 vs 11) | |||
| Anti-S trimer titer, BAU/mL | 1610 (1230–2520) | 3260 (1900–5240) | .004 |
| Anti-S RBD titer, U/mL | 2079 (888–5503) | 12194 (8840–14,245) | < .001 |
| IFN-γ release titer, mIU/mL | N/A | N/A | |
Note: Boldface P values indicate statistical significance.
Anti-S RBD, anti-SARS-CoV-2 antibodies in Roche Elecsys immunoassay; anti-S trimer, anti-SARS-CoV-2 antibodies in DiaSorin LIAISON immunoassay; BAU, binding antibody units; IFN-γ, interferon gamma; LT, liver transplant; N/A, not available; RBD, receptor binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus type 2.
Supplementary Table 6.
Comparison of Response Between Different C-P Classes in Cirrhotic Patients and Between Decompensated Patients With l and LT Recipients After Second SARS-CoV-2 Vaccination
| C-P class |
||||
|---|---|---|---|---|
| A (n = 16), median (IQR) | B (n = 18), median (IQR) | C (n = 14), median (IQR) | P | |
| Anti-S trimer titer, BAU/mL | 1890 (925–2105) | 1915 (668–5205) | 1870 (1358–3155) | .923 |
| Anti-S RBD titer, U/mL | 4124 (1542–6456) | 2951 (786–10307) | 4134 (2645–7849) | .576 |
| IFN-γ release titer, mIU/mL | 56 (9–396) | 252 (119–386) | 189 (64–286) | .423 |
| Patients with decompensated LC vs LT recipients |
|||||
|---|---|---|---|---|---|
| CP B+C (n = 34), median(IQR) | TIPS (n = 9), median(IQR) | LT (n = 82), median(IQR) | CP B+C vs LT | TIPS vs LT | |
| Anti-S trimer titer, BAU/mL | 1870 (1044–4350) | 1910 (1053–3990) | 163 (12–1060) | < .001 | .001 |
| Anti-S RBD titer, U/mL | 3812 (1128–9230) | 3693 (2186–4966) | 154 (1–1723) | < .001 | .002 |
| IFN-γ release titer, mIU/mL | 212 (93–359) | 168 (66–4532) | 49 (10–275) | .040 | .176 |
Note: Boldface P values indicate statistical significance.
Anti-S RBD, anti-SARS-CoV-2 antibodies in Roche Elecsys immunoassay; anti-S trimer, anti-SARS-CoV-2 antibodies in DiaSorin LIAISON immunoassay; BAU, binding antibody units; CP, Child-Pugh class; IFN-γ, interferon gamma; IQR, interquartile range; LC, liver cirrhosis patients; LT, liver transplant recipients; RBD, receptor binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus type 2; TIPS, transjugular intrahepatic portosystemic stent-shunt.
References
- 1.Baden L.R., El Sahly H.M., Essink B., et al. COVE Study Group Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack F.P., Thomas S.J., Kitchin N., et al. C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marjot T., Moon A.M., Cook J.A., et al. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: an international registry study. J Hepatol. 2021;74:567–577. doi: 10.1016/j.jhep.2020.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Webb G.J., Marjot T., Cook J.A., et al. Outcomes following SARS-CoV-2 infection in liver transplant recipients: an international registry study. Lancet Gastroenterol Hepatol. 2020;5:1008–1016. doi: 10.1016/S2468-1253(20)30271-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyarsky B.J., Werbel W.A., Avery R.K., et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rabinowich L., Grupper A., Baruch R., et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J Hepatol. 2021;75:435–438. doi: 10.1016/j.jhep.2021.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kearns P., Siebert S., Willicombe M., et al. Examining the immunological effects of COVID-19 vaccination in patients with conditions potentially leading to diminished immune response capacity – the OCTAVE trial. 2021. https://ssrn.com/abstract=3910058 Available at:
- 8.Bonelli F., Blocki F.A., Bunnell T., et al. Evaluation of the automated LIAISON((R)) SARS-CoV-2 TrimericS IgG assay for the detection of circulating antibodies. Clin Chem Lab Med. 2021;59:1463–1467. doi: 10.1515/cclm-2021-0023. [DOI] [PubMed] [Google Scholar]
- 9.Patel E.U., Bloch E.M., Clarke W., et al. Comparative performance of five commercially available serologic assays to detect antibodies to SARS-CoV-2 and identify individuals with high neutralizing titers. J Clin Microbiol. 2021;59 doi: 10.1128/JCM.02257-20. e02257-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Resman Rus K., Korva M., Knap N., et al. Performance of the rapid high-throughput automated electrochemiluminescence immunoassay targeting total antibodies to the SARS-CoV-2 spike protein receptor binding domain in comparison to the neutralization assay. J Clin Virol. 2021;139:104820. doi: 10.1016/j.jcv.2021.104820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall V.G., Ferreira V.H., Ku T., et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med. 2021;385:1244–1246. doi: 10.1056/NEJMc2111462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huzly D., Panning M., Smely F., et al. Validation and performance evaluation of a novel interferon-γ release assay for the detection of SARS-CoV-2 specific T-cell response. medRxiv. 2021 doi: 10.1016/j.jcv.2022.105098. 2021.07.17.21260316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le Bert N., Tan A.T., Kunasegaran K., et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584:457–462. doi: 10.1038/s41586-020-2550-z. [DOI] [PubMed] [Google Scholar]
- 14.Khoury D.S., Cromer D., Reynaldi A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 15.Schramm R., Costard-Jackle A., Rivinius R., et al. Poor humoral and T-cell response to two-dose SARS-CoV-2 messenger RNA vaccine BNT162b2 in cardiothoracic transplant recipients. Clin Res Cardiol. 2021;110:1142–1149. doi: 10.1007/s00392-021-01880-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt T., Klemis V., Schub D., et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat Med. 2021;27:1530–1535. doi: 10.1038/s41591-021-01464-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prendecki M., Clarke C., Edwards H., et al. Humoral and T-cell responses to SARS-CoV-2 vaccination in patients receiving immunosuppression. Ann Rheum Dis. 2021;80:1322–1329. doi: 10.1136/annrheumdis-2021-220626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qin C.X., Moore L.W., Anjan S., et al. Risk of breakthrough SARS-CoV-2 infections in adult transplant recipients. Transplantation. 2021 doi: 10.1097/TP.0000000000003907. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim D., Bonham C.A., Konyn P., et al. Mortality trends in chronic liver disease and cirrhosis in the united states, before and during COVID-19 pandemic. Clin Gastroenterol Hepatol. 2021 doi: 10.1016/j.cgh.2021.07.009. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim D., Adeniji N., Latt N., et al. Predictors of outcomes of COVID-19 in patients with chronic liver disease: US multi-center study. Clin Gastroenterol Hepatol. 2021;19:1469–1479.e19. doi: 10.1016/j.cgh.2020.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.John B.V., Deng Y., Scheinberg A., et al. Association of BNT162b2 mRNA and mRNA-1273 vaccines with COVID-19 infection and hospitalization among patients with cirrhosis. JAMA Intern Med. 2021;181:1306–1314. doi: 10.1001/jamainternmed.2021.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rolak S., Hayney M.S., Farraye F.A., et al. What gastroenterologists should know about COVID-19 vaccines. Clin Gastroenterol Hepatol. 2021;19:657–661. doi: 10.1016/j.cgh.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brehm T.T., Thompson M., Ullrich F., et al. Low SARS-CoV-2 infection rate and high vaccine-induced immunity among German healthcare workers at the end of the third wave of the COVID-19 pandemic. medRxiv. 2021 doi: 10.1016/j.ijheh.2021.113851. 2021.08.02.21260667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng S., Phillips D.J., White T., et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. medRxiv. 2021 doi: 10.1038/s41591-021-01540-1. 2021.06.21.21258528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilbert P.B., Montefiori D.C., McDermott A., et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy trial. medRxiv. 2021 doi: 10.1126/science.abm3425. 2021.08.09.21261290. [DOI] [PMC free article] [PubMed] [Google Scholar]