Abstract
Background:
Radium-223 and enzalutamide are approved agents for patients with metastatic castration-resistant prostate cancer (mCRPC). Combining radium-223 and enzalutamide to improve outcomes is of clinical interest due to their differing modes of action and non-overlapping toxicity profiles.
Methods:
This phase II study enrolled patients with mCRPC and bone metastases. Patients received six cycles of radium-223 in combination with enzalutamide, followed by enzalutamide alone. The primary endpoint was safety for the combination; secondary endpoints included radiographic/clinical progression-free survival (PFS), PSA PFS, overall survival (OS), change in alkaline phosphatase, patient-reported pain outcomes and skeletal related events.
Results:
Forty-five patients received the combination treatment: 42 patients (93.3%) received all six cycles. Fourteen patients (31.1%) developed grade 3 or 4 toxicities, most commonly fatigue and neutropaenia. Fractures during the combination period occurred in four patients (8.9%). A further 13 patients (28.9%) developed fractures after completing combination treatment, giving a total of 17 patients (37.8%) who developed a fracture at any time on study. The median time to fracture was greater than 17.2 months [95% confidence interval (CI), 17.2–not estimable]. The median time to PSA progression was 18.1 months (95% CI, 12.68–22.60) and the median time to radiological/clinical progression was 28.0 months (95% CI, 22.54–not reached). At the primary analysis, 19 (42.2%) out of 45 patients had died with a median OS not reached (mean 34.8 months, standard error 1.4).
Conclusion:
In men with progressive mCRPC and bone metastases, the combination of radium-223 and enzalutamide was tolerable with the majority of patients completing the combination treatment. Bone fractures during the combination period were uncommon; however, we did identify a higher incidence of fractures occurring in patients after completing combination treatment. Bone health agents should be administered and bone health should be closely monitored following treatment with radium-223 and enzalutamide.
Keywords: enzalutamide, prostate cancer, radium-223
Introduction
Prostate cancer is a leading cause of male cancer mortality in Western Europe and the United States.1 Androgen deprivation therapy (ADT) is the mainstay of treatment; however, resistance inevitably develops, leading to castration-resistant prostate cancer (CRPC) that most commonly metastasises to bone.2 Enzalutamide is a potent androgen receptor inhibitor that acts on multiple parts of the androgen receptor signalling pathway within the tumour cell.3 The efficacy and safety of enzalutamide has been demonstrated across the spectrum of CRPC in a number of large randomised controlled trials.4–6
Radium-223 is a therapeutic alpha particle-emitting pharmaceutical approved to treat patients with CRPC and symptomatic bone metastases.7 Its active agent mimics calcium and selectively targets bone, specifically areas of high turnover in bone metastases, by forming complexes with the bone mineral hydroxyapatite.8–10 In the phase III ALSYMPCA trial, radium-223 increased overall survival (OS), delayed the onset of symptomatic skeletal related events (SREs) and improved quality of life (QoL) outcomes compared with placebo in men with metastatic CRPC (mCRPC) and bone metastases who had received prior docetaxel.11
Combining therapeutic agents with differing modes of action is a strategy that has been explored successfully in many solid tumour types, with the aim of delaying the development of resistance and improving outcomes for patients.12,13 In prior early access programmes involving radium-223, there was no apparent increase in toxicity in patients who received concomitant enzalutamide with radium-223.14–16
After recruitment commenced, the ERA-223 trial exploring the combination of radium-223 and abiraterone acetate reported an unexpected increase in fractures, leading to early unblinding of the trial.17 Outcomes were inferior in patients who did not receive a bone health agent (BHA) compared with those who did.18
We hypothesised that combining radium-223 and enzalutamide in patients with mCRPC would be safe, tolerable and improve disease control and survival. In light of the ERA-223 findings, a protocol amendment was introduced mandating the use of BHAs in all patients. Furthermore, we report here the fracture risk occurring during combination treatment and those occurring after completion of radium-223 and enzalutamide.
Methods
Study design
This was an open-label phase II multi-centre single arm study of the combination of radium-223 and enzalutamide in mCRPC with bone metastases who had progressed on ADT. Inclusion criteria included age ⩾18 years, an Eastern Cooperative Oncology Group (ECOG) performance status ⩽2 and histologically confirmed adenocarcinoma of the prostate without neuroendocrine differentiation. A signed, written informed consent form was obtained prior to any study-related assessments and procedures. Patients must have had confirmed metastatic disease and documented progressive disease either by radiographic progression according to RECIST version 1.1 or PSA criteria as per the Prostate Cancer Working Group 2 (PCWG2) criteria.19,20 Patients with visceral metastases were eligible. BHAs were permitted at any time and initiated at the discretion of the investigator. A subsequent protocol amendment (November 2018) mandated the use of BHAs in all patients. Prior docetaxel chemotherapy for metastatic castration-sensitive disease was allowed. Full inclusion and exclusion criteria are contained in the protocol.
Treatment
Radium-223 dichloride was administered as a dose of 55 kBq/kg (slow bolus intravenous injection) on day 1 of a four-week cycle, for a maximum of six doses. Enzalutamide was administered daily as an oral dose of 160 mg continually. Patients continued on enzalutamide after completing treatment with radium-223.
Assessments
Adverse events were graded 1–4 as per the Common Terminology Criteria for Adverse Events version 4.0. The grade 3 or 4 toxicity rate is presented as the percentage of patients in the safety population who experienced a grade 3 or higher toxicity during combination. The combination treatment period extended from initiation of treatment until 4 weeks after the last administration of enzalutamide, unless a new systemic anti-cancer therapy was initiated, at which point the patient entered long-term follow-up. At the end of the combined treatment period, patients who had no clinical or radiographic progression and had not begun a new anti-cancer therapy continued on enzalutamide and entered an active follow-up period. This period extended until the patient had clinical or radiographic progression, initiated a new anti-cancer therapy or died. Radiological assessments (technetium-99 m bone scans and computed tomography scans) were performed at baseline and subsequently every 12 weeks. PSA was measured at every cycle visit (every 4 weeks during the combination period and then every 6 weeks after).
SREs were defined as new pathologic vertebral or non-vertebral bone fractures, spinal cord compression, the first use of external-beam radiation therapy (EBRT) to relieve skeletal symptoms or tumour-related orthopaedic surgery to bone. SRE data was collected from the date of consent to the end of the 2 year follow-up period. A subsequent post hoc retrospective analysis of all reported adverse events for first bone fracture was performed from the date of registration until the data analysis cut-off date.
QoL assessments were performed using the Brief Pain Inventory Short Form for patient-reported pain. Pain response was determined by calculating the degree of change in worst pain relative to baseline. This was completed monthly throughout the study, commencing from the first cycle of treatment until patient withdrawal from study.
Outcomes
The primary endpoint was to determine the safety of radium-223 when administered in combination with enzalutamide. Secondary endpoints were radiological and/or clinical progression-free survival (PFS) (time from commencing radium-223 to radiological progression or clinical progression), PSA PFS (as per PCWG2), OS (which was defined as time from commencing radium-223 to death from any cause), change in alkaline phosphatase (ALP), time to first SRE, and QoL assessments.
Statistical analysis
For the primary endpoint, the incidence of grade 3 or 4 toxicity rate for the combination therapy was not expected to exceed 20%. Using the normal approximation, a sample size of 45 patients would be sufficient to estimate a two-sided 90% (one-sided 95%) confidence interval (CI) of width 20% for an expected grade 3 or 4 toxicity rate of 20%. A median of 16.5 months for radiographic PFS would indicate efficacy of treatment, and less than 8 months would indicate futility. A protocol amendment allowed for either radiographic or clinical PFS to be included in the event analysis. Similarly, a median of 11 months for PSA PFS would indicate efficacy, and less than 5.6 months would indicate futility.
Oversight
The trial was registered with Cancer Trials Ireland (CTRIAL-IE 13-21) and with ClinicalTrials.gov (ClinicalTrials.gov identifier: NCT02225704) before the first patient was enrolled. This study received ethical approval by the Clinical Research Ethics Committee of the Cork Teaching Hospitals on 21 July 2014, REF: ECM51 D1/07/14. An enrolment period of 12 months from initiation was expected; however, an extension was granted to meet the defined target accrual. The first patient signed the study informed consent form on 20 July 2015 and was registered on study 4 August 2015. Data analysis cut-off date was April 7 2020.
Results
Patients
Between July 2015 and July 2017, 47 patients were enrolled in this study. Forty-five patients proceeded to receive the combination treatment (two patients were deemed not suitable for the trial). The mean age was 68 (range 51–79) years (Table 1). The majority of patients (98%) had an ECOG performance status of ⩽1. In total, 42 patients (93%) received all six cycles of combination therapy with radium-223 and enzalutamide. Twenty-six patients (57.8%) were in receipt of a BHA at study entry with either a bisphosphonate (42.2%) or a RANK-L inhibitor (15.6%).
Table 1.
Baseline characteristics of all patients receiving study treatment.
| Characteristic | Value |
|---|---|
| Age, years | |
| Mean | 68 |
| Range | 51–79 |
| ECOG, number (%) | |
| 0 | 17 (38) |
| 1 | 27 (60) |
| 2 | 1 (2) |
| Total alkaline phosphatase | |
| Median, IU/l | 99.0 |
| Range, IU/l | 39–964 |
| Haemoglobin | |
| Median, g/dl | 13.3 |
| Range, g/dl | 9.7–15.9 |
| PSA | |
| Median, ng/ml | 16.8 |
| Range, ng/ml | 2.1–907.3 |
| Extent of disease, bone metastasesa (%) | |
| <6 | 23 (51.1) |
| 6–20 | 15 (33.3) |
| >20 | 9 (19.1) |
| Duration of ADT, years | |
| Median | 4.3 |
| Range | (0.5–18.8) |
| Bone health agentsb, number (%) | |
| Denosumab | 7 (15.6) |
| Zoledronic acid | 19 (42.2) |
Two patients did not receive study treatment.
Received prior to study entry.
ADT, androgen deprivation therapy; ECOG, Eastern Cooperative Oncology Group; PSA, prostate specific antigen.
Safety
Fatigue (55.5%) followed by nausea (46.7%) were the most common adverse events and were typically mild with the majority of events reported as either grade 1 or 2 (Table 2). A total of 14 patients (31.1%) developed grade 3 or 4 toxicities (90% CI, 19.8–42.5%). Of these, 11 patients (24.4%) had grade 3 or 4 toxicities that were considered to be combination therapy related. The most common grade 3 or 4 adverse events were fatigue (6.7%) and neutropaenia (6.7%). Dose reduction of enzalutamide for toxicity occurred in four patients (8.9%). There were no therapy-related deaths during the combination treatment period.
Table 2.
Combination therapy related adverse eventsa.
| Adverse event | Grade 1–2 | Grade 3 | Grade 4 | All grades |
|---|---|---|---|---|
| Number (%) | ||||
| Fatigue | 22 (48.9) | 3 (6.7) | 0 | 25 (55.5) |
| Nausea | 20 (44.4) | 1 (2.2) | 0 | 21 (46.7) |
| Diarrhoea | 16 (35.6) | 0 | 0 | 16 (35.6) |
| Back pain | 10 (22.2) | 1 (2.2) | 0 | 11 (24.4) |
| Neutropaenia | 8 (17.8) | 2 (4.4) | 1 (2.2) | 11 (24.4) |
| Decreased appetite | 9 (20) | 0 | 0 | 9 (20) |
| Arthralgia | 8 (17.8) | 0 | 0 | 8 (17.8) |
| Anaemia | 7 (15.6) | 0 | 0 | 7 (15.6) |
| Pain in extremity | 7 (15.6) | 0 | 0 | 7 (15.6) |
| Restless legs syndrome | 7 (15.6) | 0 | 0 | 7 (15.6) |
| Headache | 5 (11.1) | 1 (2.2) | 0 | 6 (13.3) |
| Weight decreased | 6 (13.3) | 0 | 0 | 6 (13.3) |
| Dizziness | 5 (11.1) | 0 | 0 | 5 (11.1) |
| Hypertension | 4 (8.9) | 1 (2.2) | 0 | 5 (11.1) |
| Lymphocyte count decreased | 4 (8.9) | 1 (2.2) | 0 | 5 (11.1) |
| White blood cell count decreased | 4 (8.9) | 1 (2.2) | 0 | 5 (11.1) |
| Gastro-oesophageal reflux disease | 5 (11.1) | 0 | 0 | 5 (11.1) |
| Fracturesb | 4 (8.9) | 0 | 0 | 4 (8.9) |
| Hot flush | 4 (8.9) | 0 | 0 | 4 (8.9) |
| Constipation | 4 (8.9) | 0 | 0 | 4 (8.9) |
| Gynaecomastia | 4 (8.9) | 0 | 0 | 4 (8.9) |
| Depressed mood | 4 (8.9) | 0 | 0 | 4 (8.9) |
| Lower respiratory tract infection | 3 (6.7) | 1 (2.2) | 0 | 4 (8.9) |
| Hyperkalaemia | 0 | 1 (2.2) | 0 | 1 (2.2) |
| Lymphopaenia | 0 | 0 | 1 (2.2) | 1 (2.2) |
| Urticaria | 0 | 1 (2.2) | 0 | 1 (2.2) |
| Hypokalaemia | 0 | 1 (2.2) | 0 | 1 (2.2) |
| Hyponatraemia | 0 | 1 (2.2) | 0 | 1 (2.2) |
| Syncope | 0 | 1 (2.2) | 0 | 1 (2.2) |
Adverse events listed here were reported in more than 8% of patients and all grade 3 or 4 events regardless of the relationship to the study drug.
Compound term for adverse events inclusive of fracture: pathological, traumatic, osteoporotic and stress fractures.
At the primary analysis, a total of eight patients (17.8%) developed an SRE occurring within a 2-year period of follow-up. Seven patients required EBRT. No patient required surgery and there were no cases of spinal cord compression. The median time to SRE could not be calculated with all SREs occurring after completing radium-223, with an estimated mean of 31.1 months (SD of 1.0 months). All eight patients were in receipt of a BHA. There were no deaths as a result of an SRE.
When all first fracture events that occurred in the study are included, treatment-emergent fractures occurred in four patients (8.9%) during the combination period with radium-223 and enzalutamide and in 13 patients (28.9%) in the post-combination treatment follow-up period, giving a total of 17 patients (37.8%) who developed fractures (Table 3). Grade 1 first fractures (asymptomatic) occurred in 15 patients (33.3%) and grade 2 first fractures (symptomatic but non-displaced) occurred in two patients (4.4%). A median time to fracture could not be estimated; however, the lower bound was greater than 17.2 months (95% CI, 17.2–not estimable), suggesting a late onset to these fractures. In total nine patients (52.9%) who developed a fracture were in receipt of a BHA at study registration. The most common type of fractures were stress fractures, followed by traumatic, osteoporotic and pathological fractures.
Table 3.
First fracture events.
| Fracture type (n = 17) | Grade | Time to eventa (months) |
|---|---|---|
| Stress fracture | 2 | 40.0 |
| Traumatic fracture | 1 | 18.0 |
| Traumatic fracture | 1 | 17.0 |
| Stress fracture | 1 | 16.0 |
| Stress fracture | 1 | 16.0 |
| Traumatic fracture | 1 | 12.0 |
| Stress fracture | 1 | 11.0 |
| Pathological fracture | 2 | 11.0 |
| Stress fracture | 1 | 9.0 |
| Osteoporotic fracture | 1 | 8.0 |
| Traumatic fracture | 1 | 8.0 |
| Stress fracture | 1 | 8.0 |
| Stress fracture | 1 | 6.0 |
| Traumatic fracture | 1 | 6.0 |
| Osteoporotic fracture | 1 | 5.0 |
| Stress fracture | 1 | 5.0 |
| Traumatic fracture | 1 | 3.0 |
Time to event was calculated from date of start of treatment to the beginning of fracture event.
Pain
QoL assessments were performed using the Brief Pain Inventory Short Form detailing the patient’s worst pain in the last 24 h. A baseline median pain level of 3.0 was reported for all patients (range 0–9). Pain outcomes were initially improved with a reduction in pain reported after three cycles of combination treatment, with a median change from baseline of −1.0 (range −9 to +2). After completing all six cycles of combination treatment, pain remained at baseline with no change from baseline (range −5 to +5).
Efficacy
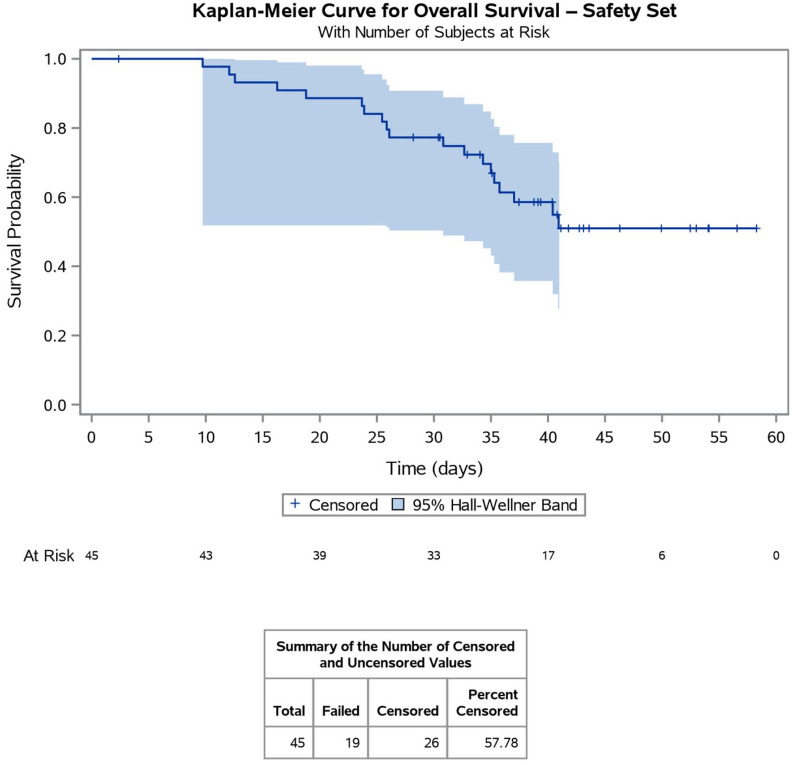
The median time to PSA progression among all 45 patients who received combination therapy was 18.1 months (95% CI, 12.68–22.60), which exceeded the acceptable median of 11 months as stated in the sample size calculation (Figure 1). The median time to radiological or clinical progression was 28.0 months (95% CI, 22.54 –not reached), which again exceeded the acceptable median of 16.5 months, as stated in the sample size calculation (Table 4). The mean time for OS among all 45 patients was 34.8 months (median not reached), which was underestimated due to the low number of events at time of censoring the analysis (Figure 2). ALP levels decreased after six cycles of combination treatment by a median of −25.4% (range −93.8% to −28.8%, p-value 0.01), with a median of 99.0 at baseline (range 39–964) compared with 66.5 (range 30–107) after treatment.
Figure 1.
Kaplan–Meier curve for PSA progression. The median time to PSA progression among all 45 patients who received radium-223 and enzalutamide was 18.14 months (95% confidence interval, 12.68–22.60).
ITT, intention to treat.
Table 4.
Summary of progression-free survival: time to radiographic/clinical – ITTa.
| All patients N = 44 |
|
|---|---|
| Number of patients with event | 22 |
| Number of patients censored | 22 |
| Median time to event, months (95% CI) | 28.0 (22.54–not reached) |
One patient was evaluable for PSA but not radiographic/clinical.
ITT, intention to treat.
Figure 2.
Kaplan–Meier Curve for overall survival. The mean overall survival time among all 45 patients was 34.8 months (median not reached), which was underestimated due to the low number of events at time of censoring.
Discussion
This open-label phase II multi-centre single arm study of radium-223 in combination with enzalutamide was designed primarily to assess the safety of combining the two agents in patients with mCRPC and bone metastases who had progressed on ADT. Concurrent treatment with radium-223 and enzalutamide was tolerable, with the vast majority of patients completing all six cycles. The side effect profile was consistent with previous studies examining these agents as single therapies.4,5,11 The majority of reported adverse events were mild and, importantly, there were no therapy-related deaths. Combining radium-223 and enzalutamide demonstrated anti-tumour activity with promising PSA PFS and a trend towards improved radiographic/clinical PFS and OS, though the median was not reached. Our findings compared favourably with prior studies analysing these agents as single therapies in the mCRPC setting.5,6,21 A median time to SRE could not be reported in our study; however, the mean time to SRE reported (31.1 months) suggests a late onset of these events. The majority of these patients required palliative radiotherapy for symptom management. Pain reported using the Brief Pain Inventory Short Form was initially improved during the combination period; however, this response was not maintained after completing the combination treatment.
Fractures occurring during treatment with radium-223 and enzalutamide were infrequent; however, we did identify a higher proportion of patients who developed fractures after completing their combination treatment. The reason for this is unclear; however, patients with mCRPC and bone metastases are at higher risk of developing fractures.22,23 A median time to fracture could not be estimated; however, a lower bound of 17.2 months suggests a late onset to these fractures and therefore may be more likely attributable to underlying bone health or progressive cancer. Not all patients received a BHA, which may have contributed to the higher rate of fractures. A retrospective analysis could not identify any patient factor that may have contributed to the increased risk. The previously reported ERA-223 was a prospective phase III trial combining radium-223 with abiraterone acetate in men with mCRPC and bone metastases.17 The trial was unblinded prematurely, when an increase in fractures of all types was noted in patients treated with the combination of radium-223 and abiraterone acetate. A number of factors may have contributed to this increased risk including the lack of use of BHAs, the concomitant use of steroids which can increase bone fragility and depleted androgen levels associated with the combined use of ADT and abiraterone acetate, which can further increase the risk of osteoporosis.18,24–26
Our study had several limitations, including a small patient cohort size and the lack of a control arm. Determining skeletal events and their aetiology can be difficult in patients with metastatic prostate cancer due to the long natural history of the disease, multiple therapies including corticosteroid use and the associated risk of osteoporosis with ADT. The ongoing phase III PEACE III trial (NCT02194842) is investigating combining radium-223 with enzalutamide versus enzalutamide alone. This trial now mandates the use of BHAs in all patients and preliminary data has demonstrated a significant decrease in the risk of fracture as a result.27
In conclusion, this phase II trial confirmed the combination of radium-223 and enzalutamide to be well tolerated, with the majority of patients completing all planned cycles. Adverse events were in keeping with previously reported data for these therapies as single agents. The promising efficacy results reported here demonstrate significant anti-tumour activity with this combination. Bone fractures are a known complication of mCRPC and, as survival improves, bone health is becoming increasingly important. BHAs should be administered in patients receiving radium-223 and enzalutamide and bone health should be closely monitored after completing treatment. In this context we await the results of the PEACE III trial with interest.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was funded by Bayer Pharmaceuticals and Astellas Pharma.
ORCID iD: John Greene  https://orcid.org/0000-0002-3341-6841
https://orcid.org/0000-0002-3341-6841
Contributor Information
Raymond S. McDermott, Cancer Trials Ireland, Dublin, Ireland Tallaght University Hospital, Dublin, Ireland; St. Vincent’s University Hospital, Dublin, Ireland.
John Greene, Cancer Trials Ireland, Innovation House, Glasnevin, Dublin 8, IrelandTallaght University Hospital, Dublin, Ireland Trinity College Dublin, Ireland.
John McCaffrey, Cancer Trials Ireland, Dublin, Ireland; Mater Misericordiae University Hospital, Dublin, Ireland.
Imelda Parker, Cancer Trials Ireland, Dublin, Ireland.
Sylva Helanova, Cancer Trials Ireland, Dublin, Ireland.
Anne-Marie Baird, Cancer Trials Ireland, Dublin, Ireland; Trinity College Dublin, Ireland.
Ausra Teiserskiene, Cancer Trials Ireland, Dublin, Ireland.
Marvin Lim, Cancer Trials Ireland, Dublin, Ireland; Tallaght University Hospital, Dublin, Ireland; Trinity College Dublin, Ireland.
Helen Matthews, Cancer Trials Ireland, Dublin, Ireland.
Olwyn Deignan, Cancer Trials Ireland, Dublin, Ireland.
John Feeney, Cancer Trials Ireland, Dublin, Ireland; Tallaght University Hospital, Dublin, Ireland.
Pierre G. Thirion, Cancer Trials Ireland, Dublin, Ireland Saint Luke’s Hospital, Dublin, Ireland.
Stephen P. Finn, Cancer Trials Ireland, Dublin, Ireland Trinity College Dublin, Ireland; St. James’s Hospital, Dublin, Ireland.
Paul J. Kelly, Cancer Trials Ireland, Dublin, Ireland Cork University Hospital, Cork, Ireland.
References
- 1.Sartor O, de Bono JS. Metastatic prostate cancer. N Engl J Med 2018; 378: 645–657. [DOI] [PubMed] [Google Scholar]
- 2.Watson PA, Arora VK, Sawyers CL. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat Rev Cancer 2015; 15: 701–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scher HI, Beer TM, Higano CS, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1-2 study. Lancet 2010; 375: 1437–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012; 367: 1187–1197. [DOI] [PubMed] [Google Scholar]
- 5.Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 2014; 371: 424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armstrong AJ, Lin P, Tombal B, et al. Five-year survival prediction and safety outcomes with enzalutamide in men with chemotherapy-naïve metastatic castration-resistant prostate cancer from the PREVAIL trial. Eur Urol 2020; 78: 347–357. [DOI] [PubMed] [Google Scholar]
- 7.Henriksen G, Breistol K, Bruland OS, et al. Significant antitumor effect from bone-seeking, alpha-particle-emitting 223Ra demonstrated in an experimental skeletal metastases model. Cancer Res 2002; 62: 3120–3125. [PubMed] [Google Scholar]
- 8.Morris MJ, Corey E, Guise TA, et al. Radium-223 mechanism of action: implications for use in treatment combinations. Nat Rev Urol 2019; 16: 745–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suominen MI, Fagerlund KM, Rissanen JP, et al. Radium-223 inhibits osseous prostate cancer growth by dual targeting of cancer cells and bone microenvironment in mouse models. Clin Cancer Res 2017; 23: 4335–4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruland ØS, Nilsson S, Fisher DR, et al. High-linear energy transfer irradiation targeted to skeletal metastases by the α-emitter 223Ra: adjuvant or alternative to conventional modalities? Clin Cancer Res 2006; 12: 6250s–6257s. [DOI] [PubMed] [Google Scholar]
- 11.Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013; 369: 213–223. [DOI] [PubMed] [Google Scholar]
- 12.Grassberger C, Ellsworth SG, Wilks MQ, et al. Assessing the interactions between radiotherapy and antitumour immunity. Nat Rev Clin Oncol 2019; 16: 729–745. [DOI] [PubMed] [Google Scholar]
- 13.Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med 2019; 381: 121–131. [DOI] [PubMed] [Google Scholar]
- 14.Saad F, Carles J, Gillessen S, et al. Radium-223 and concomitant therapies in patients with metastatic castration-resistant prostate cancer: an international, early access, open-label, single-arm phase 3b trial. Lancet Oncol 2016; 17: 1306–1316. [DOI] [PubMed] [Google Scholar]
- 15.Sartor O, Vogelzang NJ, Sweeney C, et al. Radium-223 safety, efficacy, and concurrent use with abiraterone or enzalutamide: first U.S. experience from an expanded access program. Oncologist 2018; 23: 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shore N, Higano CS, George DJ, et al. Concurrent or layered treatment with radium-223 and enzalutamide or abiraterone/prednisone: real-world clinical outcomes in patients with metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis 2020; 23: 680–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith M, Parker C, Saad F, et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019; 20: 408–419. [DOI] [PubMed] [Google Scholar]
- 18.Sedhom R, Antonarakis ES. Radium-223 plus abiraterone in metastatic castration-resistant prostate cancer: a cautionary tale. Transl Androl Urol 2019; 8(Suppl. 3): S341–S345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol 2008; 26: 1148–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 21.Beer TM, Armstrong AJ, Rathkopf D, et al. Enzalutamide in men with chemotherapy-naïve metastatic castration-resistant prostate cancer: extended analysis of the phase 3 PREVAIL study. Eur Urol 2017; 71: 151–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith MR, Lee WC, Brandman J, et al. Gonadotropin-releasing hormone agonists and fracture risk: a claims-based cohort study of men with nonmetastatic prostate cancer. J Clin Oncol 2005; 23: 7897–7903. [DOI] [PubMed] [Google Scholar]
- 23.Graff JN, Baciarello G, Armstrong AJ, et al. Efficacy and safety of enzalutamide in patients 75 years or older with chemotherapy-naive metastatic castration-resistant prostate cancer: results from PREVAIL. Ann Oncol 2016; 27: 286–294. [DOI] [PubMed] [Google Scholar]
- 24.Dalla Volta A, Formenti AM, Berruti A. Higher risk of fragility fractures in prostate cancer patients treated with combined radium-223 and abiraterone: prednisone may be the culprit. Eur Urol 2019; 75: 894–895. [DOI] [PubMed] [Google Scholar]
- 25.Cursano MC, Iuliani M, Casadei C, et al. Combination radium-223 therapies in patients with bone metastases from castration-resistant prostate cancer: a review. Crit Rev Oncol Hematol 2020; 146: 102864. [DOI] [PubMed] [Google Scholar]
- 26.Farooki A, Scher HI. Maintaining bone health during hormonal therapy for prostate cancer. Ann Intern Med 2017; 167: 357–358. [DOI] [PubMed] [Google Scholar]
- 27.Tombal BF, Loriot Y, Saad F, et al. Decreased fracture rate by mandating bone-protecting agents in the EORTC 1333/PEACE III trial comparing enzalutamide and Ra223 versus enzalutamide alone: an interim safety analysis. J Clin Oncol 2019; 37(Suppl. 15): 5007. [Google Scholar]