Abstract
There is a growing body of evidence demonstrating that Raf-1 is phosphorylated on tyrosines upon stimulation of a variety of receptors. Although detection of Raf-1 tyrosine phosphorylation has remained elusive, genetic analyses have demonstrated it to be important for Raf-1 activation. Here we report new findings which indicate that Raf-1 tyrosine phosphorylation is regulated in vivo. In both a mammalian and baculovirus expression system, a kinase-inactive allele of Raf-1 was found to be tyrosine phosphorylated at levels much greater than that of wild-type Raf-1. The level of tyrosine phosphate on Raf-1 was markedly increased upon treatment with phosphatase inhibitors either before or after cell lysis. Cdc25A was found to dephosphorylate Raf-1 on tyrosines that resulted in a significant decrease in Raf-1 kinase activity. In NIH 3T3 cells, coexpression of wild-type Raf-1 and phosphatase-inactive Cdc25A led to a marked increase in Raf-1 tyrosine phosphorylation in response to platelet-derived growth factor. These data suggest that the tyrosine phosphorylation of Raf-1 is regulated not only by itself but also by Cdc25A.
Phosphorylation and dephosphorylation of proteins provide an ideal regulatory mechanism to elicit major changes in cellular growth and metabolism. The observation that Raf-1, a serine/threonine kinase and the cellular homologue of the mouse sarcoma virus-encoded v-Raf, becomes hyperphosphorylated in response to many signaling events has long suggested that phosphorylation plays a role in regulating Raf-1 activity. The Raf-1 proto-oncoprotein is stimulated by the activation of many tyrosine kinases, including growth factor receptors, members of Janus kinases, and members of the Src kinase family (1, 11, 18, 19, 21, 23–25, 27, 32). Concomitant with activation, Raf-1 is phosphorylated on tyrosines. However, Raf-1 activation under these conditions is rapid and transient in nature, indicating the existence of activating and inactivating mechanisms. The significance of tyrosine phosphorylation of Raf-1 under physiological conditions has been an important issue. Reports in the literature have indicated that stimulation of either platelet-derived growth factor (PDGF), granulocyte-macrophage colony-stimulating factor or interleukin-2 receptors results in activation and tyrosine phosphorylation of Raf-1 in fibroblasts and hematopoietic cells (2, 21, 30). More recently, it was also found that Raf-1 becomes tyrosine phosphorylated and activated in CD4-cross-linked T cells (25), FcRI-cross-linked myeloid cells (23), and squamous carcinoma cells exposed to ionizing radiation (14), suggesting key roles of Raf-1 in activation of hematopoietic cells and in a DNA repair checkpoint. It has also been shown that in transient-expression systems, pp60c-src-mediated activation of Raf-1 involves the phosphorylation of tyrosine residues 340 and/or 341 (17). Mutational and biochemical analyses confirmed that phosphorylation of these two tyrosine residues is necessary to stimulate the catalytic activity of Raf-1 (7). The use of antisera specific for the tyrosine-phosphorylated state of Raf-1 has provided further evidence for the existence of tyrosine phosphorylated Raf-1 (16). Tyrosine kinases such as members of the Src kinase family, Janus kinases, and the PDGF receptor have been implicated in phosphorylating Raf-1 on these tyrosine residues and thereby enhancing its activity (17, 21, 32).
However, almost all reports have stated that the level of tyrosine phosphorylation of Raf-1 is low, though higher levels have been seen in rare cases (2, 30). The significance of tyrosine phosphorylation under physiological conditions remains controversial, in part because the levels of tyrosine phosphorylation of Raf-1 are near the limits of detection. The elusive nature of Raf-1 tyrosine phosphorylation in mammalian cells suggests that a rapid and active dephosphorylation process might counterbalance the phosphorylation process.
To investigate the mechanisms by which the tyrosine phosphorylation of Raf-1 is regulated, we used both a baculovirus system and PDGF-stimulated mammalian cell lines and found that not only Raf-1 itself but also the phosphatase Cdc25A is involved in this regulation.
MATERIALS AND METHODS
Cell cultures and reagents.
Spodoptera frugiperda Sf21 cells were grown at 27°C either in suspension or as a monolayer culture in Grace’s medium (GIBCO 350-1605AJ) supplemented with 10% fetal calf serum. NIH 3T3 cells were grown at 37°C in Dulbecco modified Eagle medium (GIBCO/BRL) supplemented with 10% calf serum. Rabbit polyclonal anti-Raf-1 serum was raised against a peptide corresponding to the C-terminal 12 residues of Raf-1 (CTLTTSPRLPVF). Mouse monoclonal antibody specific for phosphotyrosine (4G10) was also generated in our own laboratory. Rabbit polyclonal anti-Cdc25A, anti-Cdc25C, anti-SHP1, and anti-SHP2 antibodies were all purchased from either Upstate Biotechnology Inc. (Lake Placid, N.Y.) or Santa Cruz Biotechnology Inc. (Santa Cruz, Calif.).
Expression of Raf-1 protein and Cdc25A in NIH 3T3 cells.
The bacterial expression vectors carrying either the full-length wild-type Cdc25A or the phosphatase-inactive mutant (Cys→Ser) were kindly provided by Helen Piwnica-Worms (Washington University). For expression in mammalian cells, the coding regions (full length) of Cdc25A and its mutant were isolated from these glutathione S-transferase fusion constructs (pGex2T′-6) by digestion with NcoI and HindIII. The agarose-gel purified fragments were blunt ended with Klenow fragment. EcoRV-digested pcDNA3 vectors containing a cytomegalovirus promoter were ligated with the blunt-ended Cdc25A wild-type or mutant fragment and transformed into competent Escherichia coli JM109 cells. The resulting ampicillin-resistant colonies were isolated, and the inserts were checked for the proper orientation and size. These new constructs were then purified in quantity. For transfection, cells were plated onto P100 petri dishes at 50 to 70% confluence 1 day before transfection. Prior to transfection, the buffer (HEPES-buffered saline; 150 mM NaCl, 4 mM KCl, 10 mM HEPES, 15 mM Na2HPO4 [pH 7.05]) was mixed with 10 to 15 μg of appropriate plasmid DNA; 128 μl of 1 M CaCl2 was added dropwise, and the mixture was allowed to stay at room temperature for 10 min before being added to the cell layer gently. The transfected cells were incubated overnight, washed with phosphate-buffered saline (pH 7.4), and then incubated in appropriate media for the indicated time before either starving or lysis.
Sodium pervanadate treatment of cells.
Serum-starved confluent NIH 3T3 cells or Sf21 cells were treated with 0.5 ml of sodium pervanadate either alone or simultaneously with PDGF (NIH 3T3 cells only) for 12 to 15 min at 37°C. The cells were then washed with phosphate-buffered saline and lysed in the plates. Sodium pervanadate was prepared fresh; 200 mM Na3 VO4, 0.8 M 3% H2O2, and distilled H2O were added to 3 ml. The mixture was incubated at room temperature for 15 min, and then 60 μl of catalase (10 mg/ml) was added. The mixture was allowed to stand at room temperature for 1 min and then placed on ice until use.
Preparation of baculovirus-infected Sf21 cell lysates and PDGF-stimulated cell lysates.
Sf21 cells (2 × 106) were infected with the desired recombinant baculovirus or with combinations of the different recombinant baculoviruses in appropriate ratios. At 48 h postinfection, cell lysates were prepared in Nonidet P-40 (NP-40) lysis buffer (20 mM Tris [pH 8.0], 137 mM NaCl, 1 mM MgCl2, 10% [vol/vol] glycerol, 1% [vol/vol] NP-40) supplemented with phenylmethylsulfonyl fluoride (1 mM), aprotinin (0.15 U/ml), dithiothreitol (1 mM), and sodium orthovanadate (1 mM). Appropriately starved NIH 3T3 cells (5 × 106) were stimulated with PDGF (20 ng/ml) for 10 min at 37°C. Cell lysates were prepared essentially as described above.
Raf-1 immunoprecipitation and in vitro kinase assays.
The anti-Raf antibody was incubated with protein A-Sepharose beads and an appropriate amount of lysates for at least 4 h at 4°C. The beads bound to anti-Raf antibody were washed three times with modified NP-40 lysis buffer containing 0.2% NP-40 and once with kinase buffer (25 mM HEPES [pH 7.4], 1 mM dithiothreitol, 10 mM MgCl2, 10 mM MnCl2). For kinase reactions, washed immunoprecipitates were incubated in 40 μl of kinase buffer containing 15 μM nonradioactive ATP, 10 μCi (370 kBq) of [γ-32P]ATP (3,000 Ci/mmol), and 0.1 μg of 5′-p-fluorosulfonyl-benzoyladenosine-treated MEK-1 at room temperature for 30 min. The assays were terminated by addition of Laemmli sample buffer and resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The phosphoproteins were visualized by autoradiography.
RESULTS
Activated Raf-1 stimulates a tyrosine phosphatase.
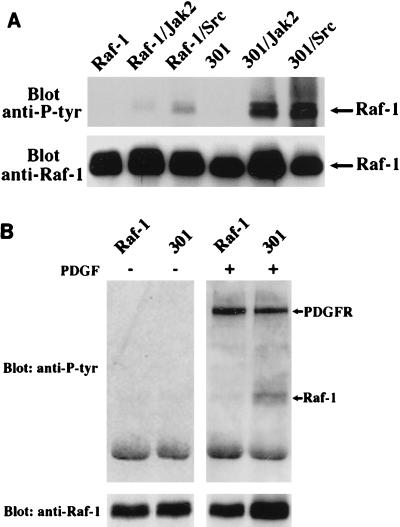
To investigate the elusive nature of tyrosine phosphorylation of Raf-1, we first examined the tyrosine phosphorylation states of different Raf-1 alleles in a baculovirus expression system. We obtained striking results with a kinase-inactive allele, termed R301 (or simply 301). The 301 allele of Raf-1 contains a single amino acid substitution of the catalytically important residue 375 (lysine to methionine) and functions as a dominant-negative mutant. Insect cells were infected with baculoviruses expressing mammalian Raf-1 or the 301 allele, either singly or in combination with Jak2 or pp60v-src, kinases that are known to phosphorylate Raf-1 on tyrosine residues (16, 29). Raf-1 immunoprecipitates were analyzed by Western blotting with an anti-phosphotyrosine antibody. Blots were subsequently stripped off and reprobed with the anti-Raf-1 antibody to ensure that comparable protein levels were used in the experiment (Fig. 1A). When expressed alone, Raf-1 in either its active or inactive state contained undetectable levels of tyrosine phosphates. Upon coexpression with either Jak2 or pp60v-src, a low level of tyrosine phosphorylation was seen reproducibly on wild-type Raf-1. However, a much (about 20- to 30-fold) higher level was seen on the kinase-inactive Raf-1. These results suggested that Raf-1 itself may regulate the level of its tyrosine phosphorylation.
FIG. 1.
Significant accumulation of tyrosine phosphates on kinase-inactive Raf-1. (A) Sf21 insect cells were infected with recombinant baculoviruses encoding either the wild-type Raf-1 (Raf-1) or kinase-inactive Raf-1 (301), alone or in combination with baculoviruses encoding Jak2 or pp60vsrc (Src). Anti-Raf-1 immunoprecipitates were analyzed by SDS-PAGE (8% gel), immunoblotted with antiphosphotyrosine (anti-P-tyr in all figures) antibody, and then reprobed with anti-Raf-1 antibody. The blots were developed by enhanced chemiluminescence. (B) NIH 3T3 cells were transfected with wild-type Raf-1 or with kinase-inactive mutant 301; 48 h posttransfection, cells were either unstimulated (−) or stimulated (+) with PDGF for 10 min. The expressed Raf-1 was immunoprecipitated and Western blotted with antiphosphotyrosine antibody 4G10 (top). Raf-1 expression was detected by probing the identical membrane with anti-Raf-1 antibody (bottom).
To examine whether this phenomenon also occurs in mammalian cells, we transfected the kinase-inactive mutant 301 and the kinase-active Raf-1 into NIH 3T3 cells. At 36 to 40 h posttransfection, cells were serum starved and stimulated with PDGF, and the Raf-1 immunoprecipitates were analyzed as described above. Again, the levels of phosphotyrosine on the kinase-inactive Raf-1 are significantly increased in response to PDGF stimulation (Fig. 1B). In contrast, without PDGF stimulation, neither the wild-type Raf-1 nor the kinase-inactive 301 showed detectable tyrosine phosphorylation. The two prominent tyrosine-phosphorylated bands in PDGF-stimulated lanes are presumably PDGF receptors as reported by Morrison et al. (20). They can be used as an internal control for proper stimulation by PDGF. These results not only further confirm that Raf-1 itself can regulate the levels of tyrosine phosphorylation but also raise the possibility that this regulation process depends on the Raf-1 activation state. That is, during the activation process, Raf-1 may be either inactivating a relevant tyrosine kinase or activating a tyrosine phosphatase, or both. We have measured pp60c-src activity by using enolase as a substrate and found it to be unaffected by Raf-1 (data not shown). Another possibility is that the tyrosine-phosphorylated proteins of roughly the same molecular weight as Raf-1 may be sequestered in the kinase-inactive 301 complex to enhance its apparent tyrosine phosphorylation. However, published data on the Raf-1 mutant YY340/341FF (6, 29) have shown that when these two major tyrosine phosphorylation sites are mutated, little tyrosine phosphorylation of Raf-1 is retained, indicating that the phosphotyrosine signal is Raf specific. Therefore, one plausible explanation of these data is that once activated, Raf-1 stimulates a tyrosine phosphatase.
Tyrosine phosphatase inhibitors enhance Raf-1 tyrosine phosphorylation.
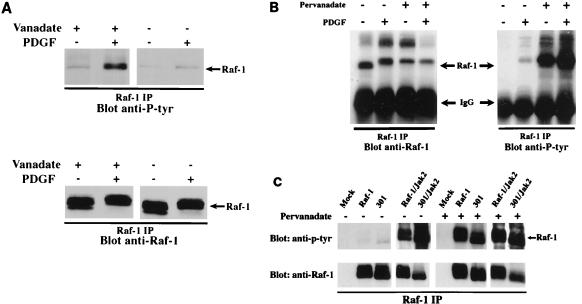
To test the above hypothesis further, we examined the effects of tyrosine phosphatase inhibitors on tyrosine phosphorylation of endogenous Raf-1. PDGF-stimulated NIH 3T3 cells were treated with tyrosine phosphatase inhibitors either in vitro (Fig. 2A) or in vivo (Fig. 2B). In the in vitro experiment, the NIH 3T3 cells were lysed in the presence or absence of 1 mM sodium vanadate. The levels of endogenous Raf-1 tyrosine phosphorylation were then measured via immunoprecipitation with the anti-Raf antibody followed by Western blotting with the antiphosphotyrosine antibody 4G10. In the in vivo experiment, PDGF-stimulated NIH 3T3 cells were simultaneously treated with 0.2 mM sodium pervanadate for 12 to 15 min prior to preparation of the cell lysates. As can be seen in Fig. 2A, treatment of cells with a tyrosine phosphatase inhibitor during cell lysis preserves tyrosine phosphates on Raf-1 in response to PDGF stimulation. In contrast, without the inhibition of tyrosine phosphatase activity, most of the Raf-1 tyrosine phosphates were rapidly removed. It is worth noting that under the latter condition, Raf-1 still maintains its mobility shift. Similarly, treating cells in vivo with the inhibitor sodium pervanadate leads to a dramatic increase in Raf-1 tyrosine phosphorylation (Fig. 2B). Consistent with the in vitro data, more tyrosine phosphorylation of Raf-1 does not appear to further retard the Raf-1 gel mobility, suggesting that serine/threonine phosphorylation may play a major role in its mobility shift. Both the in vitro and in vivo data strongly support the notion that a tyrosine phosphatase actively participates in the rapid dephosphorylation of Raf-1.
FIG. 2.
Effects of a tyrosine phosphatase inhibitor on Raf-1 tyrosine phosphorylation. (A) Serum-starved NIH 3T3 cells were either stimulated (+) or not stimulated (−) with PDGF (20 ng/ml) for 10 min. The cells were immediately washed and lysed in the presence or absence of 1 mM sodium vanadate. Raf-1 immunoprecipitated (IP) complexes were prepared and analyzed by Western blotting. The blot was first probed with an antiphosphotyrosine antibody (top) and then stripped and reprobed with an anti-Raf-1 antibody (bottom). (B) Similar to panel A except that the properly starved NIH 3T3 cells were either stimulated (+) or not stimulated (−) with PDGF (20 ng/ml) in the presence (+) or absence (−) of sodium pervanadate for 10 min. (C) Sf21 insect cells were infected with recombinant baculoviruses encoding the vector itself (Mock), wild-type Raf-1 (Raf-1), or kinase-inactive Raf-1 (301) with or without coexpression of Jak2; 48 h postinfection, cells were untreated or treated with sodium pervanadate for 10 min. Raf-1 immune complexes were prepared and analyzed by Western blotting as described for panel A.
Another formal explanation for our data is that the kinase-inactive Raf-1 is simply a better substrate for tyrosine kinases. To test this, we performed another in vivo experiment. Confluent Sf21 cells were first infected with baculovirus encoding either wild-type or kinase-inactive Raf-1 alone or in combination with Jak2; 46 to 48 h later, the infected cells were not treated or treated with sodium pervanadate. As shown in Fig. 2C, in the absence of sodium pervanadate treatment, phosphorylation of Raf-1, 301, Raf-1/Jak2, and 301/Jak2 was similar to that seen in Fig. 1A. However, with sodium pervanadate treatment, the tyrosine phosphorylation of wild-type Raf-1 was comparable to that of kinase-inactive Raf-1 with (lanes 9 and 10) or without (lanes 7 and 8) coexpression of Jak2. These results indicate that the two forms of Raf-1 are equally good substrates for tyrosine kinases. In general, this seems to be true for other kinases as well, since the two-dimensional phosphotryptic peptide mapping profiles of metabolically labeled wild-type Raf-1 and the kinase-inactive 301 were comparable upon coexpression with p21ras and pp60v-src (30a). These data also suggest that the in vivo phosphorylation sites of the kinase inactive Raf-1 are very similar to those of wild-type Raf-1.
Raf-1 tyrosine phosphorylation is also regulated by Cdc25A.
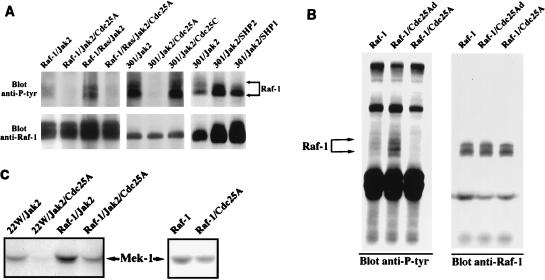
Recent literature has suggested several candidate tyrosine phosphatases that might act upon Raf-1, including the SH2-containing phosphatases SHP1, SHP2, SHP1B, and Cdc25A (3, 8). We tested the ability of each of these phosphatases to alter Raf-1 tyrosine phosphorylation and found that only Cdc25A had an effect. Cdc25 phosphatases are known to regulate the cell cycle. For instance, Cdc25C regulates the cyclin-dependent kinases by dephosphorylating the critical threonine and tyrosine residues (5, 7, 9, 14, 26). In humans, Cdc25 proteins are encoded by a multigene family consisting of three isoforms: Cdc25A, Cdc25B, and Cdc25C. These three proteins function at different phases of the cell cycle. Cdc25A and Cdc25B are expressed throughout the cell cycle, with peak expression in G1 for Cdc25A (11) and in both G1/S and G2 for Cdc25B (12). Cdc25C is predominantly expressed in G2 (24). Cdc25A has been shown to interact with both Raf-1 and 14-3-3 in mammalian cells (2, 8). Moreover, it has been demonstrated that Raf-1 participates in the activation of Cdc25A (8), but the significance of this interaction for Raf-1 has not been explored. To test whether Cdc25A is a phosphatase involved in the rapid dephosphorylation of Raf-1, we again used the baculovirus expression system. Insect cells were either doubly, triply, or quadruply infected with the indicated baculoviruses. As shown in Fig. 3A, coexpression of wild-type Raf-1 or the kinase-inactive 301 together with Jak2 or p21ras/Jak2 leads to Raf-1 tyrosine phosphorylation (lanes 1, 3, 5, 7, and 8). Interestingly, most of these tyrosine phosphates are removed upon coexpression with active Cdc25A (lanes 2, 4, 6) but not with active Cdc25C (lane 7), SHP2 (lane 9), or SHP1 (lane 10). Since antibodies used for Cdc25A, Cdc25C, SHP1, and SHP2 are different with distinct affinities, the amount of Cdc25A expressed in each sample was estimated to be either comparable to or less than the amount of Cdc25C, as confirmed by direct Coomassie staining of immunoprecipitated Cdc25A and Cdc25C proteins or Western blotting of SHP1 or SHP2 (data not shown). Although it might have been predicted that coexpression of any tyrosine phosphatase at the high levels seen in the baculovirus system would have led to dephosphorylation of Raf-1, this did not appear to be the case. Neither Cdc25C nor the SH2-containing phosphatases (SHP1 and SHP2) had any effect on tyrosine phosphorylation of Raf-1.
FIG. 3.
(A) Dephosphorylation of tyrosine-phosphorylated Raf-1 by Cdc25A. Anti-Raf-1 immune complexes were prepared from Sf21 cells coinfected with various combinations of baculoviruses encoding proteins indicated above the lanes. Raf-1 immune complexes were analyzed on a Western blot probed first with antiphosphotyrosine antibody (top), and then with anti-Raf-1 antibody. (B) Augmented tyrosine phosphorylation of ectopic Raf-1 in cells coexpressing the phosphatase-inactive Cdc25A. Exponentially growing NIH 3T3 cells at 50 to 75% confluence were transfected with plasmid pLGP3, encoding HA-tagged wild-type Raf-1, alone or in combination with plasmid pcDNA3, encoding either the active Cdc25A or the phosphatase-inactive Cdc25A (Cdc25Ad). Transfected cells were serum starved and stimulated with PDGF (20 ng/ml) for 10 min prior to lysis. Raf-1 immune complexes were prepared by using anti-HA monoclonal antibody and analyzed on a Western blot probed first with antiphosphotyrosine antibody (left) and then with anti-Raf-1 antibody (right). (C) Effect of dephosphorylation by Cdc25A on Raf-1 kinase activity. Anti-Raf-1 immunoprecipitates were prepared from Sf21 cells infected with the indicated baculoviruses. 22W is an N-terminally truncated and constitutively active form of Raf-1. Extensively washed immune complexes were subjected to an in vitro kinase assay using purified Mek-1 as a substrate. The resulting phosphorylated products were analyzed with a Molecular Dynamics PhosphorImager.
To gain further insight into Raf-1 dephosphorylation by Cdc25A, we investigated these effects in a mammalian cell expression system. In many mammalian cell systems, expression of a mutant enzyme can silence the endogenous active allele. Since the phosphatase-inactive Cdc25A may compete with the endogenous wild-type Cdc25A to bind Raf-1 and prevent dephosphorylation, we cotransfected NIH 3T3 cells with constructs expressing either a phosphatase-inactive Cdc25A mutant (Cdc25Ad) or an active Cdc25A together with a construct expressing hemagglutinin (HA)-tagged Raf-1. Transfected cells were stimulated with PDGF 48 to 60 h posttransfection. With comparable amounts of Raf-1 expressed (Fig. 3B, right), the tyrosine phosphorylation was significantly increased in cells coexpressing the phosphatase-inactive Cdc25Ad (Fig. 3B, left, lane 2) but decreased in cells coexpressing the phosphatase-active Cdc25A (lane 3). However, in the control experiments without PDGF stimulation, Raf-1 was not tyrosine phosphorylated, nor did transfection of NIH 3T3 cells with vector alone alter the tyrosine phosphorylation pattern of endogenous Raf-1 (data not shown). These results are consistent with the result shown in Fig. 3A and strongly support the idea that tyrosine phosphorylation of Raf-1 is regulated and Cdc25A is involved in this dephosphorylation process. Since it has been well documented that Raf-1 is a cytoplasmic protein, we also examined the localization of transfected and endogenous Cdc25A in PDGF-stimulated and unstimulated NIH 3T3 cells by indirect immunofluorescence. We found that Cdc25A localized predominantly to the cytoplasm and stimulation of cells with PDGF did not significantly alter its localization (data not shown). In separate experiments, we also determined that transfection of Cdc25A alleles had no apparent effect on cell cycle (data not shown).
To determine whether tyrosine dephosphorylation by Cdc25A is functionally significant, we analyzed the effects of Cdc25A on Raf-1 kinase activity in the baculovirus expression system. Active Raf-1 alleles were coinfected together with Jak2 and/or Cdc25A. 22W is a truncated, constitutively active Raf-1 mutant lacking the entire regulatory N-terminal domain. Raf-1 immunoprecipitates from Sf21 cells were measured for kinase activity in both its tyrosine-phosphorylated and Cdc25A-dephosphorylated forms (Fig. 3C). In all cases, removal of the tyrosine phosphate by Cdc25A resulted in a significant decrease in Raf-1 kinase activity.
Enhanced Raf-1 tyrosine phosphorylation after a kinase assay.
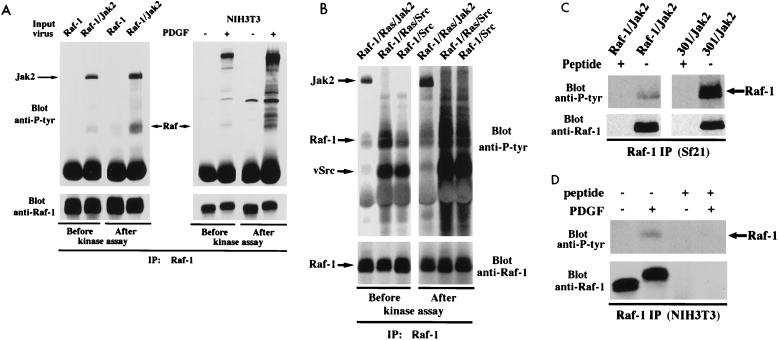
In addition to the role of phosphatases such as Cdc25A in removing tyrosine phosphates from Raf-1, there may be an additional factor contributing to difficulties in detecting Raf-1 tyrosine phosphorylation. Typically, the tyrosine phosphorylation state of Raf-1 from mammalian cells is measured before a kinase assay and not after it. Given that tyrosine kinases such as pp60c-src, Jak1, Jak2, and the PDGF receptor coimmunoprecipitate with Raf-1 (18, 20, 25, 27, 29), it is possible that during the course of the kinase assay, Raf-1 is phosphorylated on tyrosines. Figure 4 demonstrates that the levels of tyrosine phosphorylation on Raf-1 indeed increased in the course of a typical kinase assay. Anti-Raf-1 immunoprecipitates were prepared from either Sf21 cells infected with baculoviruses encoding Raf-1 alone or in combination with Jak2 (Fig. 4A, left) or NIH 3T3 cells in the presence or absence of PDGF stimulation (Fig. 4A, right). Half of the immunoprecipitates were subjected to an in vitro kinase assay in the absence of an exogenous substrate. As can be seen, with equal amounts of Raf-1 expressed, phosphorylation on tyrosine residues is significantly greater after the kinase assay than before the assay. Interestingly, when Raf-1 immune complexes were obtained from pp60vsrc-coinfected insect cells, a much higher level of tyrosine phosphorylation on Raf-1 was observed after a typical kinase assay (Fig. 4B). Although the indicated tyrosine-phosphorylated bands were immunoprecipitated by and Western blotted with Raf-1-specific antibody, it is still possible that other proteins comigrate with Raf-1. To ascertain that this is not the case, we performed peptide competition assays, using the synthetic peptide corresponding to the 12 amino acids from the C terminus of Raf-1 against which the antibody was raised. Again, anti-Raf-1 immunoprecipitates were prepared from either Sf21 cells infected with baculoviruses encoding Raf-1 and Jak2 (Fig. 4C) or NIH 3T3 cells in the presence or absence of PDGF stimulation (Fig. 4D). Half of the immunoprecipitates were incubated with the Raf-specific peptide, and the other half were immunoprecipitated with an unrelated peptide. The tyrosine-phosphorylated bands of the presumed Raf-1 were competed away with the peptide in both cases (Fig. 4C and D), indicating that the band in question is Raf-1 specific, i.e., either Raf-1 itself or a protein specifically bound to Raf-1. Therefore, Raf-1 seems to coprecipitate with one or more tyrosine kinases that in turn phosphorylate it in vitro. Because the activities of tyrosine phosphatases are inhibited in a typical kinase assay, a significant accumulation of tyrosine phosphates on Raf-1 can occur during this process.
FIG. 4.
Enhanced tyrosine phosphorylation of Raf-1 during a kinase assay. (A) Anti-Raf-1 immunoprecipitates (IP) were prepared from either Sf21 cells infected with baculoviruses encoding Raf-1 alone or in combination with Jak2 (top left) or NIH 3T3 cells in the presence or absence of PDGF stimulation (top right). Half of the immunoprecipitates were subjected to an in vitro kinase assay in the absence of an exogenous substrate. Samples before or after the kinase assay were immunoprecipitated with anti-Raf-1 antibody and analyzed by Western blotting with an antiphosphotyrosine antibody. The blot was stripped and reprobed with anti-Raf-1 antibody to indicate Raf-1 protein levels (bottom). (B) The same as panel A except that anti-Raf-1 immunoprecipitates were prepared from Sf21 cells infected with baculoviruses encoding Raf-1 and in combination with p21ras, Jak2, or pp60vsrc as indicated. (C and D) The same as panel A except that anti-Raf-1 immune complexes were prepared in the presence (+) or absence (−) of the synthetic Raf-1 peptide (10 nM).
DISCUSSION
Both baculovirus-Sf21 cell and mammalian cell systems have been used to study the mechanisms by which Raf-1 tyrosine phosphorylation is regulated. Although understanding how Raf-1 is activated represents a very important part in elucidating the mechanisms that govern cell growth and/or differentiation, constitutive activation of Raf-1 does result in cellular transformation and hence oncogenesis. Therefore, studying how Raf-1 becomes inactivated is also important. Recent studies by Dent et al. (4) have identified and partially purified a membrane-bound, GTP-dependent protein tyrosine phosphatase that seems to be involved in inactivating Raf-1. Our present investigation has found that another well-known cell cycle regulator, Cdc25A, may also participate in the down-regulation of Raf-1 kinase activity.
The significance of Raf-1 tyrosine phosphorylation has been controversial. The data presented here suggest that two mechanisms may cooperate to obscure the role of tyrosine phosphorylation of Raf-1. One mechanism is biological. Tyrosine dephosphorylation of Raf-1 appears to be a regulated process controlled at least in part by Raf-1 itself. The phosphatases involved in this process include, but are not necessarily limited to, Cdc25A. While the simplest interpretation of our results is that Cdc25A dephosphorylates Raf-1 directly, we have not been able to carry out this reaction in vitro. This leaves open the possibility that other tyrosine phosphatases may work either in concert with Cdc25A or separately to inactivate Raf-1. The second mechanism occurs in vitro and arises due to the presence of coprecipitated tyrosine kinases in Raf-1 immunoprecipitates. These kinases can clearly add phosphates to Raf-1 under conditions used in standard kinase assays. Several kinases, including PDGF receptor, Jak1, Jak2, and pp60c-src, have already been reported to coimmunoprecipitate with Raf-1; others may participate as well. We have not proved that this added phosphate activates Raf-1, but this is clearly a distinct possibility. If this is the case, it could lead to an underestimation of the importance of tyrosine phosphorylation to Raf-1 function.
Based on the data presented herein together with previously published studies, we propose the following model. A receptor or nonreceptor tyrosine kinase, when stimulated, activates an exchange factor for p21ras. The resulting GTP-loaded p21ras serves as a binding site for Raf-1, which is free to bind to the activated tyrosine kinase. Raf-1 brings with it an associated tyrosine phosphatase (Cdc25A, for instance). In the case of Cdc25A, a 14-3-3 dimer is thought to serve as the bridge, which anchors the phosphatase to Raf-1. Consistent with this idea, Raf-1 mutants that fail to bind 14-3-3 are transforming and display an elevated level of tyrosine phosphorylation in the baculovirus overexpression system (data not shown). Raf-1 tyrosine phosphorylation activates Raf-1, which in turn activates the associated tyrosine phosphatase, resulting in a rapid dephosphorylation of Raf-1. This model can explain why a high level of tyrosine phosphate on Raf-1 in cells is seldom seen. However, in this model we do not know the nature of the timing mechanism which allows Raf-1 to remain tyrosine phosphorylated long enough to relay the input signal, nor do we know if Raf-1 is the only molecule needed to activate the phosphatase. Thus, the molecular details which determine the exact kinetics of Raf-1 inactivation remain to be determined. Further elucidation of the mechanisms regulating the Raf protein family represents an exciting area of research that will undoubtedly contribute to our understanding of the complexity of signal transduction.
ACKNOWLEDGMENTS
We thank Helen Piwnica-Worms for providing plasmids encoding various forms of Cdc25A and baculoviruses encoding Cdc25A and Cdc25C; Anjana Rao for providing plasmid pLGP3, encoding HA-tagged wild-type Raf-1; Zhimin Zhu and Stephan Muhlebach for helpful technical advice; and Helen Piwnica-Worms, Kathy Campbell, Fred King, Joanne Chan, and Ibrahim Aksoy for critical reading of the manuscript.
This work was supported by NIH grants 2RO1CA43803-11A1 (to T.M.R.) and CA49152 (to B.G.N.).
REFERENCES
- 1.Barber D L, Corless C N, Xia K, Roberts T M, D’Andrea A D. Erythropoietin activates Raf1 by an Shc-independent pathway in CTLL-EPO-R cells. Blood. 1997;89:55–64. [PubMed] [Google Scholar]
- 2.Carroll M P, Clark-Lewis I, Rapp U R, May W S. Interleukin-3 and granulocyte-macrophage colony-stimulating factor mediate rapid phosphorylation and activation of cytosolic c-raf. J Biol Chem. 1990;265:19812–19817. [PubMed] [Google Scholar]
- 3.Conklin D S, Galaktionov K, Beach D. 14-3-3 proteins associate with cdc25 phosphatases. Proc Natl Acad Sci USA. 1995;92:7892–7896. doi: 10.1073/pnas.92.17.7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dent P, Jelinek T, Morrison D K, Weber M J, Sturgill T W. Reversal of Raf-1 activation by purified and membrane-associated protein phosphatases. Science. 1995;268:1902–1906. doi: 10.1126/science.7604263. . (Erratum, 269:1657.) [DOI] [PubMed] [Google Scholar]
- 5.Dent P, Reardon D B, Wood S L, Lindorfer M A, Graber S G, Garrison J C, Brautigan D L, Sturgill T W. Inactivation of raf-1 by a protein-tyrosine phosphatase stimulated by GTP and reconstituted by Galphai/o subunits. J Biol Chem. 1996;271:3119–3123. doi: 10.1074/jbc.271.6.3119. [DOI] [PubMed] [Google Scholar]
- 6.Dunphy W G, Kumagai A. The cdc25 protein contains an intrinsic phosphatase activity. Cell. 1991;67:189–196. doi: 10.1016/0092-8674(91)90582-j. [DOI] [PubMed] [Google Scholar]
- 7.Fabian J R, Daar I O, Morrison D K. Critical tyrosine residues regulate the enzymatic and biological activity of Raf-1 kinase. Mol Cell Biol. 1993;13:7170–7179. doi: 10.1128/mcb.13.11.7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galaktionov K, Beach D. Specific activation of cdc25 tyrosine phosphatases by B-type cyclins: evidence for multiple roles of mitotic cyclins. Cell. 1991;67:1181–1194. doi: 10.1016/0092-8674(91)90294-9. [DOI] [PubMed] [Google Scholar]
- 9.Galaktionov K, Jessus C, Beach D. Raf1 interaction with Cdc25 phosphatase ties mitogenic signal transduction to cell cycle activation. Genes Dev. 1995;9:1046–1058. doi: 10.1101/gad.9.9.1046. [DOI] [PubMed] [Google Scholar]
- 10.Gautier J, Solomon M J, Booher R N, Bazan J F, Kirschner M W. cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell. 1991;67:197–211. doi: 10.1016/0092-8674(91)90583-k. [DOI] [PubMed] [Google Scholar]
- 11.Jelinek T, Dent P, Sturgill T W, Weber M J. Ras-induced activation of Raf-1 is dependent on tyrosine phosphorylation. Mol Cell Biol. 1996;16:1027–1034. doi: 10.1128/mcb.16.3.1027. . (Erratum, 17:2971, 1997.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jinno S, Suto K, Nagata A, Igarashi M, Kanaoka Y, Nojima H, Okayama H. Cdc25A is a novel phosphatase functioning early in the cell cycle. EMBO J. 1994;13:1549–1556. doi: 10.1002/j.1460-2075.1994.tb06417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kakizuka A, Sebastian B, Borgmeyer U, Hermans-Borgmeyer I, Bolado J, Hunter T, Hoekstra M F, Evans R M. A mouse cdc25 homolog is differentially and developmentally expressed. Genes Dev. 1992;6:578–590. doi: 10.1101/gad.6.4.578. [DOI] [PubMed] [Google Scholar]
- 14.Kasid U, Suy S, Dent P, Ray S, Whiteside T L, Sturgill T W. Activation of Raf by ionizing radiation. Nature. 1996;382:813–816. doi: 10.1038/382813a0. [DOI] [PubMed] [Google Scholar]
- 15.Lee M S, Ogg S, Xu M, Parker L L, Donoghue D J, Maller J L, Piwnica-Worms H. cdc25+ encodes a protein phosphatase that dephosphorylates p34cdc2. Mol Biol Cell. 1992;3:73–84. doi: 10.1091/mbc.3.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marais, R. Personal communication.
- 17.Marais R, Light Y, Paterson H F, Marshall C J. Ras recruits Raf-1 to the plasma membrane for activation by tyrosine phosphorylation. EMBO J. 1995;14:3136–3145. doi: 10.1002/j.1460-2075.1995.tb07316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marshall C J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. . (Review.) [DOI] [PubMed] [Google Scholar]
- 19.Morrison D K, Cutler R E. The complexity of Raf-1 regulation. Curr Opin Cell Biol. 1997;9:174–179. doi: 10.1016/s0955-0674(97)80060-9. . (Review.) [DOI] [PubMed] [Google Scholar]
- 20.Morrison D K, Heidecker G, Rapp U R, Copeland T D. Identification of the major phosphorylation sites of the Raf-1 kinase. J Biol Chem. 1993;268:17309–17316. [PubMed] [Google Scholar]
- 21.Morrison D K, Kaplan D R, Escobedo J A, Rapp U R, Roberts T M, Williams L T. Direct activation of the serine/threonine kinase activity of Raf-1 through tyrosine phosphorylation by the PDGF beta-receptor. Cell. 1989;58:649–657. doi: 10.1016/0092-8674(89)90100-1. [DOI] [PubMed] [Google Scholar]
- 22.Muslin A J, Tanner J W, Allen P M, Shaw A S. Interaction of 14-3-3 with signaling proteins is mediated by the recognition of phosphoserine. Cell. 1996;84:889–897. doi: 10.1016/s0092-8674(00)81067-3. [DOI] [PubMed] [Google Scholar]
- 23.Park R K, Liu Y, Durden D L. A role for Shc, Grb2, and Raf-1 in FcgammaRI signal relay. J Biol Chem. 1996;271:13342–13348. doi: 10.1074/jbc.271.23.13342. [DOI] [PubMed] [Google Scholar]
- 24.Roberts T M. Cell biology. A signal chain of events. Nature. 1992;360:534–535. doi: 10.1038/360534a0. . (News; comment.) [DOI] [PubMed] [Google Scholar]
- 25.Popik W, Pitha P M. Binding of human immunodeficiency virus type 1 to CD4 induces association of Lck and Raf-1 and activates Raf-1 by a Ras-independent pathway. Mol Cell Biol. 1996;16:6532–6541. doi: 10.1128/mcb.16.11.6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sadhu K, Reed S I, Richardson H, Russell P. Human homolog of fission yeast cdc25 mitotic inducer is predominantly expressed in G2. Proc Natl Acad Sci USA. 1990;87:5139–5143. doi: 10.1073/pnas.87.13.5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakatsume M, Stancato L F, David M, Silvennoinen O, Saharinen P, Pierce J, Larner A C, Finbloom D S. Interferon gamma activation of Raf-1 is Jak1-dependent and p21ras-independent. J Biol Chem. 1998;273:3021–3026. doi: 10.1074/jbc.273.5.3021. [DOI] [PubMed] [Google Scholar]
- 28.Sebastian B, Kakizuka A, Hunter T. Cdc25M2 activation of cyclin-dependent kinases by dephosphorylation of threonine-14 and tyrosine-15. Proc Natl Acad Sci USA. 1993;90:3521–3524. doi: 10.1073/pnas.90.8.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stancato L F, Sakatsume M, David M, Dent P, Dong F, Petricoin E F, Krolewski J J, Silvennoinen O, Saharinen P, Pierce J, Marshall C J, Sturgill T, Finbloom D S, Larner A C. Beta interferon and oncostatin M activate Raf-1 and mitogen-activated protein kinase through a JAK1-dependent pathway. Mol Cell Biol. 1997;17:3833–3840. doi: 10.1128/mcb.17.7.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turner B, Rapp U, App H, Greene M, Dobashi K, Reed J. Interleukin 2 induces tyrosine phosphorylation and activation of p72-74 Raf-1 kinase in a T-cell line. Proc Natl Acad Sci USA. 1991;88:1227–1231. doi: 10.1073/pnas.88.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams, N. G., and T. M. Roberts. Unpublished data.
- 32.Xia K, Mukhopadhyay N K, Inhorn R C, Barber D L, Rose P E, Lee R S, Narsimhan R P, D’Andrea A D, Griffin J D, Roberts T M. The cytokine-activated tyrosine kinase JAK2 activates Raf-1 in a p21ras-dependent manner. Proc Natl Acad Sci USA. 1996;93:11681–11686. doi: 10.1073/pnas.93.21.11681. [DOI] [PMC free article] [PubMed] [Google Scholar]