Abstract
We aim to study kinetics of anti-SARS-CoV-2 IgG antibody levels in subjects with COVID-19 for up to 11 months and the potential influential factors. The study was a prospective longitudinal study. The analyses were based on 77 serum/plasma samples with a mean of 4 samples per participant (range 1 – 18) in 20 participants with at least one positive Polymerase Chain Reaction testing result from 19 March 2020 up to 10 February 2021. Among the subjects (median age 34.5 years, 65% male), IgG level declined with the follow-up time (per month; geometric mean ratio [GMR] 0.73; 95% CI, 0.72 – 0.74). In a small sample of subjects from the general population with COVID-19, IgG levels declined non-linearly from month 2 to 11 with individual heterogeneity in quantity and changing speed and may be associated with gender, race and the loss of smell and taste.
Keywords: COVID-19, SARS-CoV-2, coronavirus, antibody, IgG, kinetics
1. Introduction
As the infectious disease COVID-19 continues to spread, it is vitally important to understand well the pattern of immune response and its influential factors. Anti-SARS-CoV-2 humoral response kinetics can aid in COVID-19 diagnosis, vaccine development, therapeutic immune plasma studies, and epidemiologic studies including prevalence, exposure, and immunity. Decrease in antibody levels is likely to indicate a lack of protective immunity (Bauer et al., 2021). Most COVID-19 patients develop detectable immunoglobulin M (IgM) and immunoglobulin G (IgG) antibodies targeting the nucleocapsid (N) or the spike (S) protein of SARS-CoV-2 within several weeks post infection (Lynch et al., 2021, Legros et al., 2021).
Previous studies have shown that IgG responses against SARS-CoV-2 infection can persist for 3 to 8 months post-symptom onset (Jiang et al., 2021, Dan et al., 2021). But longer-term kinetics of IgG antibodies remain to be investigated. In addition, previous studies mostly included limited sample sizes and narrow spectrums of disease severity (Vogelzang et al., 2020, Semmler et al., 2021, To et al., 2020, Sakhi et al., 2021). More data from asymptomatic and mild COVID-19 cases is necessary to better understand anti-SARS-CoV-2 IgG antibody detectable/positive rate and IgG level kinetics in the general population screened for SARS-CoV-2 infection. Previous reports have examined the associations between IgG antibody response against SARS‐CoV‐2 and potential influential factors including disease severity (Vogelzang et al., 2020, Semmler et al., 2021), comorbidities (Lee et al., 2020), and immunocompromised status (Sakhi et al., 2021), but the evidence on predictive factors of IgG levels was still limited.
Hence, we aimed to provide more information on the IgG detectable/positive rate and the IgG level changes over time after SARS-CoV-2 infection for up to 11 months and identify the potential influential factors associated with IgG levels in the general population screened for SARS-CoV-2 infection.
2. Material and methods
2.1. Study design and participants
The study was a prospective longitudinal study conducted at Richmond Pharmacology Ltd, London, UK and the Richmond Research Institute, St George's University of London. The participant inclusion criteria were (1) male or female aged 5 and older, (2) an understanding, ability, and willingness to fully comply with the project procedures and restrictions and (3) consent from a parent/legal guardian for participants aged 5 to 15 years. Informed written consent was obtained from each participant/guardian. The study complied with the principles of the World Medical Assembly (Helsinki 1964) and subsequent amendments.
Questionnaires were used to collect participant baseline characteristics. Polymerase Chain Reaction (PCR) testing of throat swab specimens for SARS-CoV-2-specific RNA were performed repeatedly per participant to confirm the status of SARS-CoV-2 infection. The Abbott Laboratories (Illinois, USA) chemiluminescent microparticle immunoassay (CMIA) against the nucleocapsid protein (N) of SARS-CoV-2 was used to assess the anti-SARS-CoV-2 antibody IgG levels and IgG statuses (detectable/positive or undetectable/negative) of serum/plasma samples. The cut-off value of Abbott CMIA for SARS-CoV-2 positive has been set at 1.4 signal/cut-off (S/CO) units (Bryan et al., 2020), which was calculated to maximise positive predictive values and minimise false positives, according to the manufacturer. Public Health England assessed that the assay had a specificity of 100% but sensitivity of 93% (Evaluation of the Abbott SARS-CoV-2 2021).
Variables
The primary outcome was the IgG level measured repeatedly during the follow up. The secondary outcome was the IgG status (detectable/positive or undetectable/negative). Predictive variables measured at screening included time, age, gender, race, fever, and loss of smell and taste (loss of smell and taste, loss of smell only, loss of taste only, neither loss of smell nor taste). Race was classified as Caucasian, Black African, and other races (Hispanic, Indian, Pakistani, other Asian than Chinese and Japanese).
2.2. Statistical analysis
Characteristics of subjects with at least one positive PCR result were summarised as n, median (interquartile range [IQR]) and minimum-maximum or frequency (percentage). IgG levels and the statuses of whether IgG was detectable or positive were recorded by day, but to make the trend information more concise, we summarised them by month. The IgG statuses (detectable/positive or undetectable/negative) were described as frequency and percentage, and IgG levels were as n, median (IQR), and minimum-maximum.
To explore potential factors associated with IgG levels in COVID-19, the generalized linear mixed models (GLMMs) with normal distribution and identity link function, predictive variables as fixed effects, and subject as random effect were employed. The natural logarithm of IgG level was the dependent variable. Time (month), age (year), gender, race, fever, and loss of smell and taste were predictive variables. All predictive variables were included in univariate GLMMs separately and in multivariate GLMM simultaneously. Geometric mean ratios (GMRs) and 95% confidence intervals (CIs) were estimated by taking an antilog transformation of estimates coming from the GLMM. The half-life was calculated from the GLMM using the formula where was the coefficient of day. The half-life was defined as the time elapsed (days) for the IgG level to reduce to half of its initial level. The graph comprised of the daily change of IgG levels since positive PCR and the fit curve for the predicted day effect from the GLMM was presented. Missing data of baseline characteristics were imputed by median (continuous variables) and category which occupies the majority (categorical variables) in the GLMM.
Statistical analyses were performed using SAS 9.4 software (SAS Institute).
2.3. Ethical approval
The study was approved by the Committee of National Research Ethics Service (NRES) (West Midlands - Edgbaston) (IRAS ID: 281788).
3. Results
3.1. Participants included in the analysis
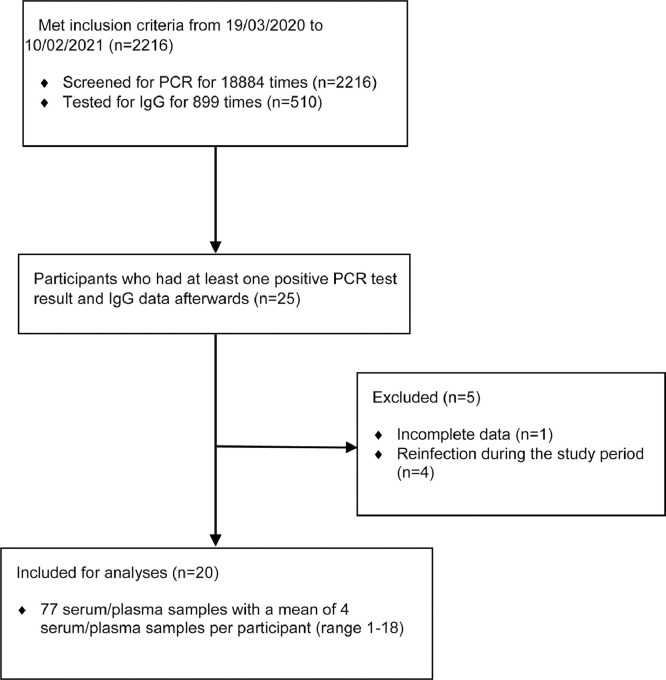
From 19 March 2020 up to 10 February 2021, 2216 participants were screened for PCR for 18884 times; 510 participants were tested for IgG for 899 times (Fig. 1 ). 25 participants had at least one positive PCR testing results and IgG data afterwards, 1 participant was excluded from the analyses due to incomplete data, 4 participants were excluded due to reinfection during the study period (who may have different patterns of IgG kinetics), and finally 20 participants were included. The analyses were based on 77 serum/plasma samples with a mean of 4 serum/plasma samples per participant (range 1 – 18).
Fig. 1.
Consort diagram.
3.2. Characteristics of participants
Median age in the study sample was 34.5 years (IQR 28.5 – 52.0), and most of the subjects were male (65.0%) (Table 1 ). Approximately half of the subjects were Caucasian (52.6%), 15.8% were Black African, and 31.6% were other races (including Hispanic, Indian, Pakistani, other Asian than Chinese and Japanese). Around half of the subjects (47.4%) had fever; the majority of subjects (68.4%) had lost their smell and taste, and one third of subjects had neither lost smell nor taste (31.6%). The median follow-up time post initial positive PCR testing was 2 months (IQR 1-2).
Table 1.
Demographic characteristics of subjects with at least one positive PCR result.
| Characteristics | Statistics | All |
|---|---|---|
| Age (year) | n | 20 |
| Median (IQR) | 34.5 (28.5 – 52.0) | |
| Min-Max | 24.0 – 66.0 | |
| Gender (n/N [%]) | Female | 7/20 (35.0%) |
| Male | 13/20 (65.0%) | |
| Race (n/N [%]) | Caucasian | 10/19 (52.6%) |
| Black African | 3/19 (15.8%) | |
| Other races* | 6/19 (31.6%) | |
| Fever (n/N [%]) | Yes | 9/19 (47.4%) |
| No | 10/19 (52.6%) | |
| Loss smell taste (n/N [%])⁎⁎ | Loss of smell and taste | 13/19 (68.4%) |
| Neither loss of smell nor taste | 6/19 (31.6%) | |
| Time (month) | n | 20 |
| Median (IQR) | 2.0(1.0 – 2.0) | |
| Min-Max | 1.0 – 11.0 |
Including Hispanic, Indian, Pakistani and other Asian than Chinese and Japanese.
No participant in the study only lost smell or only lost taste.
Abbreviation: IQR = interquartile range.
3.3. Percentage of participants with detectable or positive IgG
The percentage of the subjects who had detectable or positive IgG decreased over time. At month 1 post initial positive PCR testing, 75.0% (9 subjects) of the subjects had detectable or positive IgG, while 25.0% (3) had not (Table 2 ). At month 2, 70.0% (14) of the subjects still had detectable or positive IgG. At month 3, the percent dropped to only 42.9% (3); from month 4 to 7, only 10% to 20% (1); from month 8 to 11, our data did not show any subjects who had detectable or positive IgG.
Table 2.
Percent of participants with detectable or positive IgG since positive PCR by month.
| Month | Detectable or positive, n/N(%)* |
|---|---|
| 1 | 9/12 (75.0%) |
| 2 | 14/20 (70.0%) |
| 3 | 3/7 (42.9%) |
| 4 | 1/6 (16.7%) |
| 1-4 | 27/45 (60%) |
| 5 | 1/5 (20.0%) |
| 6 | 1/7 (14.3%) |
| 7 | 1/5 (20.0%) |
| 8 | 0/5 (0%) |
| 5-8 | 3/22 (13.6%) |
| 9 | 0/5 (0%) |
| 10 | 0/4 (0%) |
| 11 | 0/1 (0%) |
| 9-11 | 0/10 (0%) |
n, numbers of participants with detectable or positive IgG since positive PCR; N, numbers of participants tested IgG status since positive PCR; %, percent of participants with detectable or positive IgG since positive PCR.
3.4. IgG kinetics and potential influential factors
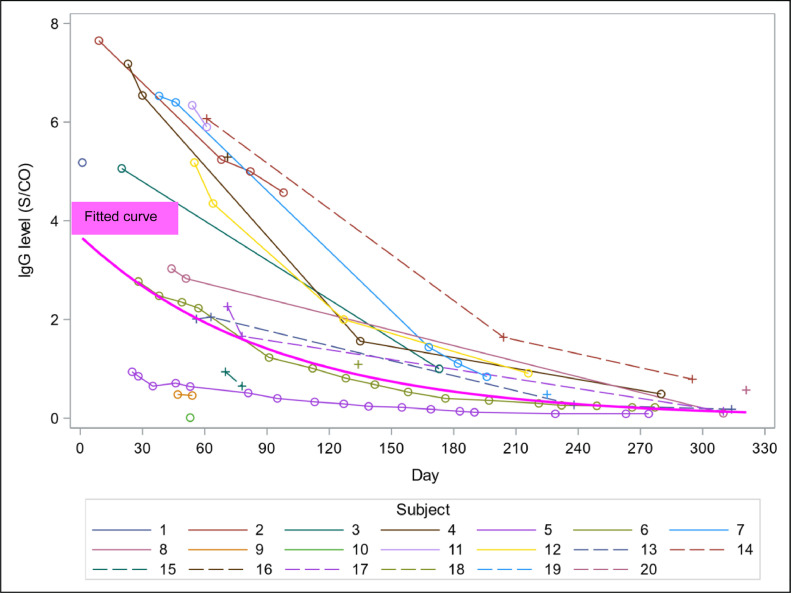
IgG levels showed a decreasing pattern over time within 11 months with an individual heterogeneity in quantity and speed (Fig. 2 ). The median IgG level at month 1 was 4.05 S/CO (IQR 1.71 – 6.54), then decreased to 2.31 (IQR 0.83 – 5.27) at month 2, 1.23 (IQR 0.51 – 4.57) at month 3, and then below 1 from month 4 to month 11 (Table 3 ).
Fig. 2.
Daily change of IgG levels since positive PCR per subject and fitted curve of IgG levels from the generalized linear mixed model (thick magenta curve).
Table 3.
IgG levels (S/CO) since positive PCR by month.
| Month | Statistics | All |
|---|---|---|
| 1 | n | 12 |
| Median (IQR) | 4.05 (1.71 – 6.54) | |
| Min-Max | 0.65-7.65 | |
| 2 | n | 20 |
| Median (IQR) | 2.31 (0.83 – 5.27) | |
| Min-Max | 0.01 – 6.40 | |
| 3 | n | 7 |
| Median (IQR) | 1.23 (0.51 – 4.57) | |
| Min-Max | 0.40-5.00 | |
| 4 | n | 6 |
| Median (IQR) | 0.91 (0.33 – 1.09) | |
| Min-Max | 0.29-2.00 | |
| 1-4 | n | 45 |
| Median (IQR) | 2.23(0.81 – 5.18) | |
| Min-Max | 0.01-7.65 | |
| 5 | n | 5 |
| Median (IQR) | 0.53 (0.24 – 0.68) | |
| Min-Max | 0.22-1.56 | |
| 6 | n | 7 |
| Median (IQR) | 0.40 (0.14 – 1.11) | |
| Min-Max | 0.12 – 1.44 | |
| 7 | n | 5 |
| Median (IQR) | 0.84 (0.36 – 0.91) | |
| Min-Max | 0.30-1.64 | |
| 8 | n | 5 |
| Median (IQR) | 0.26 (0.25 – 0.27) | |
| Min-Max | 0.09 – 0.48 | |
| 5-8 | n | 22 |
| Median (IQR) | 0.38(0.24 – 0.91) | |
| Min-Max | 0.09 – 1.64 | |
| 9 | n | 5 |
| Median (IQR) | 0.22 (0.09 – 0.22) | |
| Min-Max | 0.09 – 0.49 | |
| 10 | n | 4 |
| Median (IQR) | 0.16 (0.12 – 0.49) | |
| Min-Max | 0.10 – 0.79 | |
| 11 | n | 1 |
| Median (IQR) | 0.57 (0.57 – 0.57) | |
| Min-Max | 0.57 – 0.57 | |
| 9-11 | n | 10 |
| Median (IQR) | 0.20(0.10 – 0.49) | |
| Min-Max | 0.09 – 0.79 |
Abbreviation: IQR = interquartile range.
IgG level declined non-linearly with the follow-up time (per month; GMR 0.73; 95% CI, 0.72 – 0.74; Table 4 ). There was some evidence on the association between IgG level and loss of smell and taste (GMR 9.40; 95% CI, 1.12 – 78.97) but weak evidence on the associations between IgG level and gender and race: female vs male (GMR 4.78; 95% CI, 0.99 – 22.98), Caucasian vs. other races (including Hispanic, Indian, Pakistani, other Asian than Chinese and Japanese; GMR 0.19; 95% CI, 0.03 – 1.02). There was insufficient evidence on the associations between IgG level and age or fever. In addition, the calculated IgG half-life was 65 days (95% CI, 62 – 68). The fit curve of IgG levels from the generalized linear mixed model fitted the data well, showing a non-linear decreasing trend (Fig. 2).
Table 4.
Estimates of geometric mean ratios and 95% CI of IgG from the univariate linear mixed models and multivariate linear mixed model.
| Characteristics* | Crude GMR (95% CI) | P-value | Adjusted GMR (95% CI) | P-value |
|---|---|---|---|---|
| Time (month) | 0.73(0.72,0.74) | <.0001 | 0.73(0.72,0.74) | <.0001 |
| Age (per 5 years) | 1.01(0.81,1.25) | 0.95 | 0.83(0.60,1.15) | 0.25 |
| Female vs Male | 1.15(0.34,3.89) | 0.82 | 4.78(0.99,22.98) | 0.05 |
| Caucasian vs Other races† | 0.33(0.09,1.12) | 0.07 | 0.19(0.03,1.02) | 0.05 |
| Black African vs Other races† | 0.41(0.08,2.19) | 0.29 | 0.12(0.01,1.22) | 0.07 |
| Fever vs No fever | 0.88(0.28,2.81) | 0.83 | 0.57(0.12,2.61) | 0.46 |
| Loss of smell and taste vs | ||||
| Neither loss of smell nor taste†† | 3.38(0.95,12.00) | 0.06 | 9.40(1.12,78.97) | 0.04 |
Missing data of categorical variables of baseline characteristics were imputed by the category which occupies the majority, and continuous variables had no missing data: race: 1 missing data was replaced by Caucasian; fever: 1 missing data was replaced by no; loss of smell and taste: 1 missing data was replaced by loss of smell and taste.
Including Hispanic, Indian, Pakistani and other Asian than Chinese and Japanese.
No participant in the study only lost smell or only lost taste.
Abbreviation: GM = geometric mean ratio.
4. Discussion
We longitudinally characterized the detectable/positive rate of IgG antibody and the dynamic changes of IgG level over time after the onset (positive PCR for SARS-CoV-2), allowing a better understanding of the immune response in the general population with SARS-CoV-2 infection. Our study showed that IgG antibodies could be detected in up to 70% of infections in the first 2 months after a positive PCR, and the detectable/positive rate of IgG antibody responses in subjects gradually decreased within 3-7 months. IgG antibody levels continued to wane from the second month to the eleventh month with an individual heterogeneity in quantity and speed. Gender, race and loss of smell and taste may be associated with IgG levels.
The IgG detectable/positive rate in the PCR positive population can help estimate the proportion of individuals that has antibodies against SARS-CoV-2. Here we report that among 20 subjects with noncritical disease, a high proportion of individuals had detectable or positive IgG in the first 2 months while a growing proportion of individuals lost their detectable or positive IgG from month 3. Previous studies have shown high rates of seroconversion of IgG to detectable or positive levels between 4 and 14 days after symptoms onset in SARS-CoV-2-infected patients (Lynch et al., 2021, Vogelzang et al., 2020, Cameron et al., 2021, Bavaro et al., 2021, Suthar et al., 2020). A study described that substantial amounts of IgG antibody in hospitalized and non-hospitalized patients with COVID-19 were detectable up to 60 days after symptom onset (Vogelzang et al., 2020). Similar results were reported in another serological study showing that except for the patients who failed to produce detectable levels of IgG with commercial assays, irrespective of the severity of symptoms, other patients still had detectable IgG levels >75 days post symptom onset (Marklund et al., 2020). A longer-term study of anti-SARS-CoV-2 IgG levels reported that IgG can be detected in most recovered patients at 3 – 4 months after infection (Jiang et al., 2021). Another study detected a high percentage of subjects with seropositive IgG at 6 to 8 months post-symptom onset (Dan et al., 2021). By contrast, for the SARS-CoV-1 infection that occurred in 2003, previous studies have shown that a high proportion (>70%) of patients’ IgG levels were detectable after 1, 2, and 3 years (Cao et al., 2007, Wu et al., 2007). However, to understand the IgG detectable/positive rate and kinetics, the performance of the serological tests used (e.g. sensitivity to detect IgG) needs to be taken into consideration (Tuaillon et al., 2020). In addition, the specific positive proportion values in our study need to be interpreted with caution and may be underestimated, because validation of the assay we used may have been performed in COVID-19 patients with severe symptoms and the fixed cut-off for a positive diagnosis may be set too high for the general population, which is also a problem previously encountered in the SARS-CoV-2 antibody tests (Deeks et al., 2020).
On the other hand, our study found 4 reinfections among 25 PCR-positive participants within the 11 months study period. This may suggest immunity can rapidly decline over time and improving immune persistence through vaccines is necessary. The declined immunity may be due to the wane antibody response which represents part of the immune system, or the falling T cell response which is the other part (Jiang et al., 2021, Dan et al., 2021). In addition, some SARS-CoV-2 variants, such as B.1.617, may evade antibodies induced by prior infections and lead to reinfections (Hoffmann et al., 2021).
The daily change plot of IgG levels showed extensive individual heterogeneity in quantity and changing speed over time in COVID-19 positive subjects, so we used a generalised linear mixed model in which random effects were fitted to handle with between-subject and within-subject variabilities. We demonstrated a decreasing tendency of IgG antibody levels from the second month to the eleventh month. Previous reports presented that antibody response peaked between the 2 – 5 weeks after infection and declined afterwards (Korte et al., 2021, Zhao et al., 2020, Long et al., 2020). A study observed no drastic decline in IgG levels 3 – 4 months after infection (Jiang et al., 2021). Nevertheless, our results are in line with previous studies indicating the decline for IgG was statistically significant at month 2 – 3 (Korte et al., 2021), most patients showed a variable degree of reduction in antibody levels within 6 months post-illness onset (Zhang et al., 2020), and a progressive decline of IgG values was observed at about 6 months later (Legros et al., 2021). In addition, the calculated IgG half-life in our data was 65 days post positive PCR (95% CI, 62-68), which was similar to a previous study of 68 days, suggesting that IgG may wane from 2 month post-infection (Dan et al., 2021).
Our study provided some evidence on the association between higher IgG levels and loss of smell and taste in subjects with SARS-CoV-2 infection but insufficient evidence on the association between IgG levels and fever. To the best of our knowledge, the studies on the association between immune responses and loss of smell and taste are currently rare, highlighting the novelty and impact of the present study. A study showed that among patients with COVID-19, those reporting loss of smell and taste developed higher antibody titers (Dehgani-Mobaraki et al., 2021); another study demonstrated that among patients with upper respiratory tract infection, COVID-19 IgG antibody titers were higher in patients with olfactory disorders than those without (Taziki Balajelini et al., 2020); but both studies did not further discuss the potential mechanisms. De Melo et al. investigated the interaction between SARS-CoV-2 and the olfactory system and its pathophysiological mechanisms based on patients and animal models with SARS-CoV-2 related anosmia/ageusia (de Melo et al., 2021). They observed the expression of cleaved caspase-3 in the olfactory mucosa, indicating cell damage and death caused by SARS-CoV-2 infection. They found the cleaved caspase-3 in both infected and uninfected cells, suggesting that cell damage and death are not only caused by cytopathic effects of SARS-CoV-2, but also possibly by the inflammation and immune responses to infection, and observed some up-regulated genes which were mainly involved in inflammatory and immune responses and functions associated with chemokine signalling. In addition, they did not observe cell death or immune cells in the olfactory mucosa in a COVID-19 patient without loss of smell, suggesting the importance of assessing the associations between inflammation, immune responses, and cell and tissue damage and smell loss using larger cohorts to validate their observations. However, since different variants of the SARS-CoV-2 may have different symptoms, loss of smell and taste may not always be a dominant feature and associated with IgG levels. A previous study showed that in several asymptomatic cases, the antibody levels were lower, and the IgG seroconversion was delayed compared to the symptomatic cases (Zhang et al., 2020). Among studies exploring the relationship between disease severity and humoral immunity against SARS-CoV-2, some studies reported IgG seroconversion time, positive rates, and levels were associated with more severe forms of the disease (Vogelzang et al., 2020, Semmler et al., 2021, Zhao et al., 2020, Hartog et al., 2020, Okba et al., 2020, Long et al., 2020) but others did not (To et al., 2020, Sakhi et al., 2021, Hu et al., 2020, Gudbjartsson et al., 2020). Some publications proposed that higher IgG levels in patients with more severe disease may be due to the high amounts of SARS-CoV-2 RNA (Kwon et al., 2020), and a strong and uncontrolled humoral response may be a feature of over-activation of the immune system in patients with severe disease and may contribute to the disease pathogenesis of a severe systemic inflammatory response (called “cytokine storm”) and organ damage (Legros et al., 2021, Qin et al., 2021). On the other hand, another study stated that the IgG levels in critically ill patients were lower than moderate and severe patients, which may be the result of longer virus exposure or a severely impaired immune response in these patients (Zhang et al., 2021).
We found weak evidence on the association between IgG levels and gender. Caution needs to be taken when interpreting the result and further studies are warranted to verify the association. Legros et al.’s longitudinal study of 140 COVID-19 patients revealed that the IgG response can be used as a marker for neutralizing antibody activity and found that gender was not associated with neutralizing antibody activity (Legros et al., 2021). In agreement with Legros et al., other studies did not show gender differences in the antibody response (Cameron et al., 2021, Graham et al., 2020, Zeng et al., 2020). By contrast, a study observed gender differences on anti-nucleocapsid IgG antibody response at weeks 6 – 7 during a 10-week follow-up, but did not test the gender differences on the overall trend of IgG (Korte et al., 2021).
In addition, our study looked at whether there was a difference in the generation of antibodies against SARS-CoV-2 infection in individuals from different ethnicities. We provided weak evidence on the difference on IgG levels between Caucasian and other races (including Hispanic, Indian, Pakistani, other Asian than Chinese and Japanese) but insufficient evidence on the difference between Black African and other races. However, currently the studies exploring this question are rare.
Our study provided insufficient evidence on differences in immune response in relation to age. However, a study covering COVID-19 patients from 16 to over 65 years old found that antibody levels were age-related, showing that higher antibody levels correlated with older patients (Ojeda et al., 2021). Another study detected a moderate association between age and neutralizing activity (Wu et al., 2020). However, Legros et al.’s study found no association when examining whether age was related to neutralizing antibody activity in the same disease severity group of COVID-19 patients, indicating that disease severity may be the main factor explaining the neutralizing activity (Legros et al., 2021). Other studies did not find a clear correlation between IgG levels and age (Cameron et al., 2021, Graham et al., 2020, Wang et al., 2020).
This study has several limitations. First, although the study provided insight into the IgG response and potential influential factors in PCR-confirmed COVID-19 subjects, the sample size of this study is still modest and the study findings need to be corroborated by larger studies. But the generalised mixed model we employed allowed us to efficiently use the information by combining measurements from different subjects. Second, while our study described the longer-term kinetics of IgG up to 11 months, we only characterized the decreasing phase and did not have enough data to model the early growth phase and peaking point which was supposed to happen around the first month. Third, due to lack of data, we did not analyse the impact of other potential factors on antibody kinetics, e.g. Asian race including Chinese and Japanese, disease severity, comorbidities (Lee et al., 2020), laboratory features such as C-reactive protein (Sun et al., 2020), and virus neutralization titre (To et al., 2020). For the same reasons, we were unable to investigate the kinetics of IgG responses to the spike protein of coronavirus.
5. Conclusion
This study demonstrated that in the general population confirmed with SARS-CoV-2 infection, a high proportion of individuals had detectable or positive IgG antibody levels in the first 2 months while a growing proportion of individuals lost their detectable or positive IgG after that. IgG levels declined non-linearly from month 2 to 11 with individual heterogeneity in quantity and changing speed and tended to be associated with gender, race, and the loss of smell and taste.
Authors’ contributions
Huanyuan Luo: Conceptualization, Formal analysis, Methodology, Writing - original draft, Writing - review & editing, Software. Dorothée Camilleri: Methodology, Data Curation, Investigation, Writing - review & editing. Ibon Garitaonandia: Methodology, Investigation, Writing - review & editing. Dilshat Djumanov: Data Management, Quality Assurance, Investigation, Writing - review & editing. Tao Chen: Methodology, Supervision, Writing - review & editing. Ulrike Lorch: Resources, Methodology, Data curation, Investigation, Writing - review & editing. Jörg Täubel: Resources, Conceptualization, Supervision, Validation, Writing - review & editing. Duolao Wang: Resources, Conceptualization, Software, Methodology, Supervision, Writing - review & editing.
Declaration of competing interests
The authors declare that they have no competing interests.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Bauer G, Struck F, Schreiner P, Staschik E, Soutschek E, Motz M. The challenge of avidity determination in SARS-CoV-2 serology. J Med Virol. 2021;93:3092–3104. doi: 10.1002/jmv.26863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavaro DF, Laghetti P, Milano E, Brindicci G, Volpe A, Lagioia A, et al. Anti-spike S1 receptor-binding domain antibodies against SARS-CoV-2 persist several months after infection regardless of disease severity. J Med Virol. 2021;93:3158–3164. doi: 10.1002/jmv.26878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan A, Pepper G, Wener MH, Fink SL, Morishima C, Chaudhary A, et al. Performance characteristics of the Abbott architect SARS-CoV-2 IgG assay and seroprevalence in Boise, Idaho. J Clin Microbiol. 2020;58 doi: 10.1128/JCM.00941-20. e00941-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron A, Porterfield CA, Byron LD, Wang J, Pearson Z, Bohrhunter JL, et al. A multiplex microsphere IgG assay for SARS-CoV-2 using ACE2-mediated inhibition as a surrogate for neutralization. J Clin Microbiol. 2021;59 doi: 10.1128/JCM.02489-20. e02489-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao WC, Liu W, Zhang PH, Zhang F, Richardus JH. Disappearance of antibodies to SARS-associated coronavirus after recovery. N Engl J Med. 2007;357:1162–1163. doi: 10.1056/NEJMc070348. [DOI] [PubMed] [Google Scholar]
- Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371 doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Spijker R, Taylor-Phillips S, et al. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst Rev. 2020;6 doi: 10.1002/14651858.CD013652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehgani-Mobaraki P, Zaidi AK, Yadav N, Floridi A, Floridi E. Longitudinal observation of antibody responses for 14 months after SARS-CoV-2 infection [published online ahead of print, 2021 Jul 31] Clin Immunol. 2021;230 doi: 10.1016/j.clim.2021.108814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Melo GD, Lazarini F, Levallois S, Hautefort C, Michel V, Larrous F, et al. COVID-19-related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci Transl Med. 2021;13 doi: 10.1126/scitranslmed.abf8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evaluation of the Abbott SARS-CoV-2 . Public Health England; 2021. IgG for the detection of anti-SARS-CoV-2 antibodies.https://www.gov.uk/government/publications/covid-19-laboratory-evaluations-of-serologicalassays Accessed: June. [Google Scholar]
- Graham NR, Whitaker AN, Strother CA, Miles AK, Grier D, McElvany BD, et al. Kinetics and isotype assessment of antibodies targeting the spike protein receptor-binding domain of severe acute respiratory syndrome-coronavirus-2 in COVID-19 patients as a function of age, biological sex and disease severity. Clin Transl Immunol. 2020;9 doi: 10.1002/cti2.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudbjartsson DF, Norddahl GL, Melsted P, Gunnarsdottir K, Holm H, Eythorsson E, et al. Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med. 2020;383:1724–1734. doi: 10.1056/NEJMoa2026116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartog G, Schepp RM, Kuijer M, GeurtsvanKessel C, van Beek J, Rots N, et al. SARS-CoV-2-specific antibody detection for seroepidemiology: a multiplex analysis approach accounting for accurate seroprevalence. J Infect Dis. 2020;222:1452–1461. doi: 10.1093/infdis/jiaa479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M, Hofmann-Winkler H, Krüger N, Kempf A, Nehlmeier I, Graichen L, et al. SARS-CoV-2 variant B.1.617 is resistant to bamlanivimab and evades antibodies induced by infection and vaccination. Cell Rep. 2021;36 doi: 10.1016/j.celrep.2021.109415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu WT, Howell JC, Ozturk T, Benameur K, Bassit LC, Ramonell R, et al. Antibody Profiles According to Mild or Severe SARS-CoV-2 Infection, Atlanta, Georgia, USA, 2020. Emerg Infect Dis. 2020;26:2974–2978. doi: 10.3201/eid2612.203334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang XL, Wang GL, Zhao XN, Yan FH, Yao L, Kou ZQ, et al. Lasting antibody and T cell responses to SARS-CoV-2 in COVID-19 patients 3 months after infection. Nat Commun. 2021;12:897. doi: 10.1038/s41467-021-21155-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte W, Buljan M, Rösslein M, Wick P, Golubov V, Jentsch J, et al. SARS-CoV-2 IgG and IgA antibody response is gender dependent; and IgG antibodies rapidly decline early on. J Infect. 2021;82:e11–e14. doi: 10.1016/j.jinf.2020.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon JS, Kim JY, Kim MC, Park SY, Kim BN, Bae S, et al. Factors of severity in patients with COVID-19: cytokine/chemokine concentrations, viral load, and antibody responses. Am J Trop Med Hyg. 2020;103:2412–2418. doi: 10.4269/ajtmh.20-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YL, Liao CH, Liu PY, Cheng CY, Chung MY, Liu CE, et al. Dynamics of anti-SARS-Cov-2 IgM and IgG antibodies among COVID-19 patients. J Infect. 2020;81:e55–e58. doi: 10.1016/j.jinf.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legros V, Denolly S, Vogrig M, Boson B, Siret E, Rigaill J, et al. A longitudinal study of SARS-CoV-2-infected patients reveals a high correlation between neutralizing antibodies and COVID-19 severity. Cell Mol Immunol. 2021;18:318–327. doi: 10.1038/s41423-020-00588-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long QX, Liu BZ, Deng HJ, Wu GC, Deng K, Chen YK, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26:845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- Long QX, Tang XJ, Shi QL, Li Q, Deng HJ, Yuan J, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- Lynch KL, Whitman JD, Lacanienta NP, Beckerdite EW, Kastner SA, Shy BR, et al. Magnitude and kinetics of anti-severe acute respiratory syndrome coronavirus 2 antibody responses and their relationship to disease severity. Clin Infect Dis. 2021;72:301–308. doi: 10.1093/cid/ciaa979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund E, Leach S, Axelsson H, Nyström K, Norder H, Bemark M, et al. Serum-IgG responses to SARS-CoV-2 after mild and severe COVID-19 infection and analysis of IgG non-responders. PLoS One. 2020;15 doi: 10.1371/journal.pone.0241104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda DS, Gonzalez Lopez Ledesma MM, Pallarés HM, Costa Navarro GS, Sanchez L, Perazzi B, et al. Emergency response for evaluating SARS-CoV-2 immune status, seroprevalence and convalescent plasma in Argentina. PLoS Pathog. 2021;17 doi: 10.1371/journal.ppat.1009161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okba NMA, Müller MA, Li W, Wang C, GeurtsvanKessel CH, Corman VM, et al. Severe acute respiratory syndrome coronavirus 2-specific antibody responses in coronavirus disease patients. Emerg Infect Dis. 2020;26:1478–1488. doi: 10.3201/eid2607.200841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X, Shen J, Dai E, Li H, Tang G, Zhang L, et al. The seroprevalence and kinetics of IgM and IgG in the progression of COVID-19. BMC Immunol. 2021;22:14. doi: 10.1186/s12865-021-00404-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakhi H, Dahmane D, Attias P, Kofman T, Bouvier M, Lapidus N, et al. Kinetics of anti-SARS-CoV-2 IgG antibodies in hemodialysis patients 6 months after infection. J Am Soc Nephrol. 2021;32:1033–1036. doi: 10.1681/ASN.2020111618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semmler G, Traugott MT, Graninger M, Hoepler W, Seitz T, Kelani H, et al. Assessment of S1-, S2-, and NCP-specific IgM, IgA, and IgG antibody kinetics in acute SARS-CoV-2 infection by a microarray and 12 other immunoassays. J Clin Microbiol. 2021;59 doi: 10.1128/JCM.02890-20. e02890-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Feng Y, Mo X, Zheng P, Wang Q, Li P, et al. Kinetics of SARS-CoV-2 specific IgM and IgG responses in COVID-19 patients. Emerg Microbes Infect. 2020;9:940–948. doi: 10.1080/22221751.2020.1762515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthar MS, Zimmerman MG, Kauffman RC, Mantus G, Linderman SL, Hudson WH, et al. Rapid generation of neutralizing antibody responses in COVID-19 patients. Cell Rep Med. 2020;1 doi: 10.1016/j.xcrm.2020.100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taziki Balajelini MH, Vakili MA, Saeidi M, Tabarraei A, Hosseini SM. Using Anti-SARS-CoV-2 IgG and IgM Antibodies to Detect Outpatient Cases with Olfactory and Taste Disorders Suspected as Mild Form of COVID-19: a Retrospective Survey. SN Compr Clin Med. 2020 doi: 10.1007/s42399-020-00623-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To KK, Tsang OT, Leung WS, Tam AR, Wu TC, Lung DC, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20:565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuaillon E, Bolloré K, Pisoni A, Debiesse S, Renault C, Marie S, et al. Detection of SARS-CoV-2 antibodies using commercial assays and seroconversion patterns in hospitalized patients. J Infect. 2020;81:e39–e45. doi: 10.1016/j.jinf.2020.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelzang EH, Loeff FC, Derksen NIL, Kruithof S, Ooijevaar-de Heer P, van Mierlo G, et al. Development of a SARS-CoV-2 total antibody assay and the dynamics of antibody response over time in hospitalized and nonhospitalized patients with COVID-19. J Immunol. 2020;205:3491–3499. doi: 10.4049/jimmunol.2000767. [DOI] [PubMed] [Google Scholar]
- Wang X, Guo X, Xin Q, Pan Y, Hu Y, Li J, et al. Neutralizing antibody responses to severe acute respiratory syndrome coronavirus 2 in coronavirus disease 2019 inpatients and convalescent patients. Clin Infect Dis. 2020;71:2688–2694. doi: 10.1093/cid/ciaa721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LP, Wang NC, Chang YH, Tian XY, Na DY, Zhang LY, et al. Duration of antibody responses after severe acute respiratory syndrome. Emerg Infect Dis. 2007;13:1562–1564. doi: 10.3201/eid1310.070576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Liu M, Wang A, Lu L, Wang Q, Gu C, et al. Evaluating the association of clinical characteristics with neutralizing antibody levels in patients who have recovered from mild COVID-19 in Shanghai, China. JAMA Intern Med. 2020;180:1356–1362. doi: 10.1001/jamainternmed.2020.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng F, Dai C, Cai P, Wang J, Xu L, Li J, et al. A comparison study of SARS-CoV-2 IgG antibody between male and female COVID-19 patients: a possible reason underlying different outcome between sex. J Med Virol. 2020;92:2050–2054. doi: 10.1002/jmv.25989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Yuan Q, Wang H, Liu W, Liao X, Su Y, et al. Antibody responses to SARS-CoV-2 in patients with novel coronavirus disease 2019. Clin Infect Dis. 2020;71:2027–2034. doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Lu S, Li H, Wang Y, Lu Z, Liu Z, et al. Viral and antibody kinetics of COVID-19 patients with different disease severities in acute and convalescent phases: a 6-month follow-up study. Virol Sin. 2020;35:820–829. doi: 10.1007/s12250-020-00329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Yue D, Wang Y, Wang F, Wu S, Hou H. The dynamics of immune response in COVID-19 patients with different illness severity. J Med Virol. 2021;93:1070–1077. doi: 10.1002/jmv.26504. [DOI] [PubMed] [Google Scholar]