Abstract
Background
Animal studies suggest that insulin-like growth factor 1 (IGF1) may influence the function of the hypothalamus–pituitary–testicular axis, especially in childhood, but the evidence in humans is scanty. Laron syndrome, a human model of IGF1 deficiency, may help to solve this issue.
Purpose
This systematic review aims to analyze puberty onset and progression, testicular volume, gonadotropin, and total testosterone serum levels, sperm parameters and fertility, and penile length in patients with Laron syndrome.
Methods
Specific keywords were used. All data on male patients with Laron syndrome were included.
Results
Seventeen articles matched the inclusion criteria and were entered in the analysis, for a total of 125 male patients. Puberty was absent in 8.9% and delayed in 35.6% of untreated patients of pubertal age. After onset, the duration of the pubertal process was prolonged in 76.9% of untreated patients. The growth spurt was absent in 52.6% and delayed in 31.6% of untreated patients. The testicular volume was small in the two patients who did not receive any treatment. Treatment with IGF1 increased gonadotropin and testosterone serum levels in five out of five patients of pubertal age. No effect was found in four out of four patients younger than 5 years. No study reported data on sperm parameters and fertility. Micropenis occurred in 67.2% of patients.
Conclusion and future perspectives
Delayed puberty is common in patients with Laron syndrome. The growth hormone–IGF1 axis may influence the time of puberty onset. Serum levels of IGF1 should be investigated in children with delayed puberty, scarce progression of testicular growth, and/or micropenis. IGF1 levels might be measured in children with delayed puberty, poor testicular growth, and/or micropenis.
Keywords: Laron syndrome, IGF1, oligozoospermia, puberty, testicular volume, micropenis
Introduction
The acromegaly levels of insulin-like growth factor 1 (IGF1) that occur in healthy children in pubertal age have led to the speculation that this hormone may stimulate the function of the hypothalamus–pituitary–testicular (HPT) axis in humans (1, 2, 3). In vitro studies and data carried out in the animal model corroborate this hypothesis. In particular, IGF1 stimulates the GnRH secretion from GT1-7 cell lines (4) and chemomigration of GnRH-secreting neurons in Zebrafish (5). Furthermore, we have recently shown that the growth hormone receptor (GHR) and the IGF1 receptor (IGF1R) are both expressed in the Gn11 and the GT1-7 cell lines. The incubation with either GH or IGF1 stimulated Gn11 cell migration and GnRH secretion from GT1-7 cells. Gn11 and GT1-7 cells represent in vitro models of immature and mature (GnRH secreting) neurons, respectively. Therefore, these findings suggest that GH and IGF1 may stimulate the GnRH neuron migration and secretion and that their deficiency may adversely affect the function of the GnRH neurons (6). Interestingly, in mouse models with a constitutive knockout for the IGF1R, IGF1 appears to be involved in testicular development and sex determination. In mammals, IGF1R mediates the proliferation and differentiation of Sertoli, germ, and Leydig cells and the mediation of follicle-stimulating hormone (FSH) effects through the common PI3K/AKT pathway (7).
This knowledge translated to humans would possibly highlight the role of IGF1 in the management of patients with delayed puberty or hypogonadotropic hypogonadism. It is concerning that the evidence in humans is scarce, as the IGF1R knockout is incompatible with life. The human phenotype that is closest to the animal model is that of the Laron dwarfism (7). Laron syndrome is a condition characterized by resistance to GH caused by mutations in its receptor. This results in a disease characterized by high levels of structurally normal GH, with low levels of IGF1. Phenotypically, these patients are characterized by dwarfism, obesity, severe hypoglycemia, typical head configuration with a small face and bulging forehead resulting in a saddle nose. Their voice is high-pitched and they have thinning hair. Finally, another feature common to male patients with Laron syndrome is poor genital development, which strengthens the correlation between IGF1 and the HPT axis function (8).
Since 1970, several studies have evaluated the andrological features of these patients. Therefore, the purpose of this systematic review is to analyze the evidence collected over the years on this topic in patients with Laron syndrome to highlight the effects of low IGF1 on the human HPT axis. Particularly, the following outcomes were analyzed: puberty onset and progression, testicular volume, gonadotropin, and total testosterone serum levels, sperm parameters and fertility, micropenis.
Methods
Sources
Data used to perform this systematic review were independently extracted by A C and R C A systematic search was performed through PubMed, MEDLINE, Cochrane, Academic One Files, Google Scholar, and Scopus databases, from each database inception to September 13, 2020.
The search strategy was based on the combination of the following medical subjects headings (MeSH) terms and keywords, by using 'AND' between each MeSH search term category: 'Laron syndrome,' 'Primary GH insensitivity,' 'GH resistance,' 'Growth hormone insensitivity,' 'testicular volume,' 'testis,' 'FSH,' 'LH,' 'puberty,' 'Leydig cell,' 'Sertoli cell,' 'fertility,' 'sperm,' 'testosterone,' 'GnRH,' 'sexual development,' 'micropenis.' Additional manual searches were made using the reference lists of relevant studies. No language restriction was used for any literature search. This study was performed using the preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) (7).
Study selection
All studies that analyzed the HPT axis of the patients with Laron syndrome were included in the study, comprising individual case reports. In particular, we focused on studies evaluating the onset of puberty, testicular volume, development of the external genitalia, fertility, and sex hormone levels of these patients. The studies on patients with GH deficiency, reviews, and experimental animals were excluded.
Results
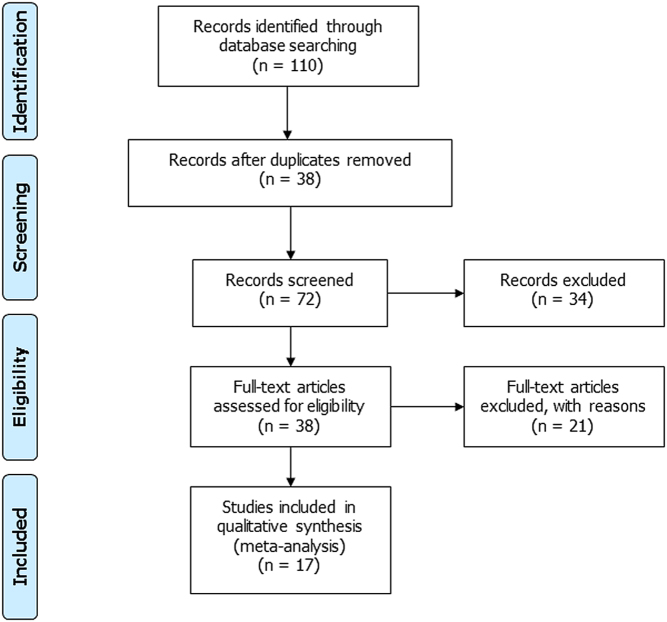
Using the above-mentioned search terms, 110 articles were selected. After the exclusion of 38 duplicated records, 72 articles were screened. Among these, 34 were excluded after having read their title and abstract, since they were not performed in patients with Laron syndrome. The remaining 38 full texts were carefully read. Among them, 10 studies were excluded as they were performed on animals, and 11 were excluded as they were review articles. Finally, 17 articles matched our inclusion criteria, for a total of 350 patients with Laron syndrome. Among these, 125 were males and were included in the analysis (Fig. 1). These patients are concentrated in the Mediterranean area, southern Ecuador, and South America, with a similar gender distribution (10). The main features of the included studies are summarized in Table 1.
Figure 1.
Flow chart of the studies included in the systematic review.
Table 1.
Summary of the included studies.
| Authors | Study design | Sample size (only boys) | Age of patientsa | Type of intervention | Follow-up | Duration of treatment | Outcomes |
|---|---|---|---|---|---|---|---|
| Laron & Sarel 1970 (12) | Observational | 6 | 1–11 | / | / | / | Penile and testicular size |
| Laron et al. 1980 (14) | Observational | 7 | 0–11 (at enrollment) | / | 15.8 years (age at least examination: M: 15–25) | / | Age, testicular volume, penile length and circumference, breast development, bone age, state of development |
| Guevara-Aguirre et al. 1993 (15) | Observational | 27 | NR | / | / | / | Auxological parameters, GH, IGF1, IGF2, IGFBP3 |
| Pertzelan et al. 1993 (16) | Observational | 6 | NR | / | / | / | Puberty onset and duration |
| Laron et al. 1993 (17) | Observational | 10 | NR | / | ~ 20 years (from infancy to puberty) | / | Pubertal development, growth curves |
| Savage et al. 1993 (18) | Observational | 12 | 3.6–22 | / | / | / | Height, pubertal development, bone age, GH, IGF1, IGF2, IGF1BP1, IGFBP2, IGFBP3, GHBP |
| Castilla-Cortazar et al. 2017 (19) | Case report | 1 | 14 and 10 months | Recombinant IGF1 (0.04–0.12 mg/kg twice daily) | 7 months | NR | Case description, including pubertal development, height, IGF1, glucose, shoe size increment |
| Laron & Klinger 1998 (20) | Longitudinal interventional | 7 (4 younger than 5 years, 2 of pubertal age, 1 adult) | 0.5–28 | Long-term treatment with recombinant IGF1 (150–120 µg/kg daily) | NR | Depend on the onset of side effects or the auxological parameters | Serum gonadotropin, androgens, insulin, IGF1, testicular volume, penile size |
| Ben-Amitai & Laron 2011 (21) | Longitudinal interventional | 10 (8 untreated, 2 treated) | 0–5 (at enrollment) | Long-term treatment with recombinant IGF1 (70–200 µg/Kg daily) | 28 years 8 years (age at least examination: M: 20–41) | M: 25.5–33.5 years | Serum gonadotropin, androgens, insulin, IGF1, acne |
| Phanse-Gupte et al. 2014 (22) | Observational | 7 | 7.8 ± 4.1b | / | / | / | Presence of high-pitched voice, sparse hair, micropenis, blue sclera, hypoglycemia, pubertal development, GH, IGF1, IGF2, IGFBP1, IGFBP3, GHBP |
| Burren et al. 2001 (23) | Observational | 24 | 8.6 ± 4.6b | / | / | / | Presence of high-pitched voice, sparse hair, micropenis, blue sclera, hypoglycemia, pubertal development, GH, IGF1, IGF2, IGFBP1, IGFBP3, GHBP |
| Ioimo et al. 2018 (24) | Case report | 1 | 2.5 years | Recombinant IGF1 0.20 mg/kg/day | NR | NR | Case description, including auxological parameters, micropenis, GH, GH peak, IGF1, IGF1 peak, IGFBP3 |
| Guleria et al. 2014 (27) | Case report | 2 | 7 years and 7 months 5.5 years |
NR | NR | NR | Case description, including auxological parameters, micropenis |
| Hopp et al. 1996 (28) | Case report | 1 | 11 years 9 months | NR | NR | NR | Case description, including auxological parameters, micropenis |
| Krzisnik et al. 1994 (25) | Case report | 1 | 6 | IGF1 treatment | NR | NR | Case description, including auxological parameters, and puberty onset |
| Rosenbloom et al. 1995 (26) | Case report | 2 | 3.7 1.9 |
/ | / | / | Case description, including auxological parameters, micropenis, GH, GHBP, IGF1, IGFBP3, LH, FSH, T |
| De Lima Jorge et al. 2008 (29) | Case report | 1 | 10.6 | / | / | / | Case description, including auxological parameters, micropenis, GH, GH peak, IGF1, IGF1 peak, IGFBP3 |
aWith the exception of the studies by Phanse-Gupte et al. 2014 and Burren et al. 2001, only the age of the male patients has been reported. bThe study reports the age of all (male and female) the included patients. The age of the male ones is not available.
FSH, follicle-stimulating hormone; GH, growth hormone; GHBP, GH binding protein; IGF1, insulin-like growth factor 1; IGF2, insulin-like growth factor 2; IGFBP1, IGF binding protein 1; IGFBP2, IGF binding protein 2; IGFBP3, IGF binding protein 3; LH, luteinizing hormone; T, testosterone.
Puberty onset and progression
In boys, delayed puberty is diagnosed when the testicular volume does not increase (<4 mL) despite coming of age or in case more than 4 years passed between the onset of testicular development and the completion of the puberty progression (11). Studies conducted on Laron’s patients suggest that these patients have a greater predisposition to delayed puberty, and the entire duration of the pubertal process appears to be longer. In the first study published in 1970, Laron and colleagues reported that three out of six boys with GH insensitivity syndrome (GHIS) and in pubertal age did not have any secondary sexual characteristics (12). Ten years later, they found these puberty alterations on seven boys with Laron syndrome. One of them, aged 20 years, did not show signs of puberty, the other six showed a delay in the pubertal progression with an increase in testicular volume starting between 12 and 14 years, the appearance of pubic hair between 13 and 16 years, and axillary hair later than 16 years old. The age of the first ejaculation, which is usually reported to be around 13.5 years old (13), was also reported delayed (after the age of 17 years) in three of them. In two patients, the entire pubertal process was completed at the age of 22 years. Finally, it was possible to evaluate the presence of pubertal growth spurt only in three of the seven boys (14). In another study carried out in 27 Ecuadorian children with Laron syndrome, puberty was delayed by up to 7 years in half of them (15). Also, Pertzelan and colleagues showed that puberty was delayed at the age of 16 and 21 years, respectively, in two out of six patients studied. Furthermore, this study showed that the onset of puberty was much more delayed in males than in females (15.6 ± 2.6 years vs 11.8 ± 0.2 years, respectively). Also, the duration of the pubertal process was much longer in patients with Laron syndrome (6.2 ± 0.6 years) than in normal subjects (4.5 years) and in patients with GH deficiency, compared to women with Laron syndrome (4.6 ± 0.5 years) (16). This supports the hypothesis that the GH–IGF1 axis stimulates the development of the male gonad, probably due to a mechanism of interdependence with the sex hormones (14). Finally, growth spurt was also reduced in patients with Laron syndrome of both sexes (16). The absence of the typical pubertal spurt was also shown by another study evaluating ten males with Laron syndrome from birth to adulthood. In all ten patients, the typical pubertal spurt was missing, and the mean onset age of the pubertal process was 15.6 years for men vs 10.7 years for women with the same disease (17). In another study including 12 males and 15 females with GHIS, puberty was absent in 2 boys aged 15 years and in 3 girls over 13 years (18). In a recent case report, a 14-year-old boy with laboratory tests suggestive of Laron syndrome (normal GH levels with low IGF-1 levels) presented a Tanner stage of 1 for genital development (absence of pubertal signs). In this patient, treatment with exogenous IGF1 led to the onset of the pubertal process (19).
Testicular volume
Few data have been published on the testicular volume. Albeit many studies report genital infantilism and microrchidism, few of them have evaluated testicular development in patients with Laron syndrome. Since his first study, Laron noted that the testes were smaller than normal in five out of the six patients with GHIS and remained small throughout the follow-up period (12). In Castillo-Cortazar’s case report, the patient's testicular volume at the age of 14 was 7 mL (19). In another study, Laron reported that the testes of these patients remained small even in the two patients who reached full sexual maturity with a volume of about 8 mL, as we have estimated from the graph reported in his article (14). GH and IGF1 may be able to stimulate the GnRH neuron migration and function, as in vitro data have recently shown (6). Therefore, their deficiency may adversely affect the function of the GnRH neurons, thus explaining the lower testicular volume of patients with Laron syndrome.
Gonadotropin and testosterone serum levels
Few studies have evaluated the association between the GH–IGF1 and the HPT axes. However, the association between the two is evidenced by the typical pubertal delay found in patients with Laron syndrome. Laron and colleagues (1998) have assessed the effect of IGF1 administration on gonadotropins and testosterone levels. While in four prepubertal patients, no effect was observed, in two pubertal patients, aged 10 and 14 years, the administration of IGF1 increased the levels of gonadotropins and testosterone at the beginning of the pubertal process. Finally, even in the 28-year-old patient whose sexual development reached completeness, the administration of IGF1 was associated with an increase of the testicular volume (from 13 to 18 mL) and in the length of the penis (from 12 to 13.5 cm). However, cessation of administration was associated with a return to pre-treatment testicular volume and penile dimensions. The study also assessed insulin levels before and during treatment, showing suppression of insulin secretion in patients receiving IGF1. These data further strengthen the direct relationship between IGF1 and sex hormones, excluding that the androgenizing action may be due to the relative hyperinsulinemia often present in these patients (20).
The evidence of an association between the GH–IGF1 axis and sexual hormones is further confirmed by another study showing that the administration of recombinant IGF1 to eight patients (two males and six females) with Laron syndrome resulted in increased gonadotropin and testosterone serum levels in both sexes. Interestingly, the women receiving IGF1 showed not only an increased gonadotropin serum levels but also the development of a polycystic ovary syndrome (PCOS)-like condition with oligomenorrhea, hirsutism, and acne (21).
Sperm parameters and fertility
No study to date has correlated GH and/or IGF1 levels with sperm parameters in patients with Laron syndrome. However, the information from patients who got married does not show a particular reduction in reproductive capacity despite the low testicular volume (12, 14, 19). This aspect certainly needs to be more deeply evaluated.
Micropenis
Micropenis is one of the main phenotypic features present at birth and during childhood in patients with Laron syndrome. In fact, its prevalence has been explored by several studies, although at the end of sexual development the size of the penis reaches the lower limit of the normal range (14). In the first 1970 Laron’s study, all six patients with GHIS had a penis length below the normal range (12). Phanse-Gupte and colleagues showed the presence of micropenis in three out of the seven patients they studied (22). In another study, micropenis was found in 19 out of 24 patients (23). Similarly, Savage and colleagues found micropenis in 7 out of 12 male patients with Laron syndrome (18). Finally, several case reports reported the presence of micropenis in these patients (19, 24, 25, 26, 27, 28, 29).
As regards the presence of genitourinary malformations, subcoronal hypospadias (19) and few cases of cryptorchidism have been described (22, 27, 30).
The presence of micropenis in patients with Laron syndrome might be firstly ascribable to the effects of the GH-IGF1 axis on the HPT one. In fact, low IGF1 levels might affect the GnRH neuron secretion and function, inducing hypogonadism (6). However, the direct effects of IGF1 on the penis may also be involved in pathogenesis. IGF1 indeed enhances penile smooth muscle cell proliferation (31), their relaxation (32), and fibroblast proliferation (33). Consequently, IGF1 has been speculated to be needed to achieve normal penile growth (33).
The prevalence of andrological abnormalities in patients with Laron syndrome is reported in Table 2.
Table 2.
Prevalence of andrological abnormalities in patients with Laron syndrome.
| Authors |
Sample size (only boys) |
Puberty onset |
Puberty progression |
Growth spurt |
Testicular volume |
Gonadotropins and total testosterone serum levels |
Sperm parameters and fertility |
Micropenis |
|---|---|---|---|---|---|---|---|---|
| Laron & Sarel 1970 (12) | 6 | Absent in 3 patients who reached pubertal age (50%) | Unavailable | Unavailable | Smaller than normal in 5 patients (83.3%) | Unavailable | Unavailable | 6 (100%) |
| Laron et al. 1980 (14) | 7 | Absent in 1 patient (14.3%) | Prolonged in the remaining 6 patients (85.7%) | Normal in the 3 patients in whom it was evaluated | Smaller than normal even at the end of the pubertal process in 2 patients The others were in prepubertal age |
Unavailable | Unavailable | 0 (0%) |
| Guevara-Aguirre et al. 1993 (15) | 27 | Delayed in half samplea | Unavailable | Unavailable | Unavailable | Unavailable | Unavailable | Unavailable |
| Pertzelan et al. 1993 (16) | 6 | Delayed in 2 patients (33.3%) | Prolonged in the remaining 4 patients (66.7%) | Delayed and reduced in all patients | Unavailable | Unavailable | Unavailable | Unavailable |
| Laron et al. 1993 (17) | 10 | Delayed (the number of patients is not reported) | Prolonged (the number of patients is not reported) | Absent in all patients | Unavailable | Unavailable | Unavailable | Unavailable |
| Savage et al. 1993 (18) | 12 | Absent in 2/4 patients aged 15 (50%) | Unavailable | Unavailable | Unavailable | Unavailable | Unavailable | 7 (58.3%) |
| Castilla-Cortazar et al. 2017 (19) | 1 | Delayed | Unavailable | Unavailable | Unavailable | Unavailable | Unavailable | 1 (100%) |
| Laron & Klinger 1998 (20) | 7 (4 younger than 5 years, 2 of pubertal age, 1 adult) | Unavailable | Unavailable | Unavailable | Unavailable | Rise in gonadotropin and testosterone levels in 3 patients of prepubertal or adult age who started IGF1 therapy (100%). No effect in patients of prepubertal age (100%). | Unavailable | Unavailable |
| Ben-Amitai & Laron 2011 (21) | 10 (8 untreated, 2 treated) | Unavailable | Unavailable | Unavailable | Unavailable | Rise in gonadotropin and testosterone levels in 2 patients treated with IGF1 (100%) | Unavailable | Unavailable |
| Phanse-Gupte et al. 2014 (22) | 7 | Unavailable | Unavailable | Unavailable | Unavailable | Unavailable | Unavailable | 3 (42.9%) |
| Burren et al. 2001 (23) | 24 | Unavailable | Unavailable | Unavailable | Unavailable | Unavailable | Unavailable | 19 (79.2%) |
| Ioimo et al. 2018 (24) | 1 | Unavailable | Unavailable | Unavailable | Unavailable | Unavailable | Unavailable | 1 (100%) |
| Guleira et al. 2014 (27) | 2 | Unavailable | Unavailable | Unavailable | Unavailable | Unavailable | Unavailable | 1 (100%) Unavailable in the other patient |
| Hopp et al. 1996 (28) | 1 | Unavailable | Unavailable | Unavailable | Unavailable | Unavailable | Unavailable | 1 (100%) |
| Krzisnik et al. 1994 (25) | 1 | Unavailable | Unavailable | Unavailable | Unavailable | Unavailable | Unavailable | 1 (100%) |
| Rosenbloom et al. 1995 (26) | 2 | Unavailable | Unavailable | Unavailable | Unavailable | Unavailable | Unavailable | 2 (100%) |
| De Lima Jorge et al. 2008 (29) | 1 | Unavailable | Unavailable | Unavailable | Unavailable | Unavailable | Unavailable | 1 (100%) |
| Total | 125 | Absent in 4/45 (8.9%) and delayed in 16/45 (35.6%) the untreated patients of pubertal agea | Prolonged in 10/13 (76.9%) of the untreated patientsa | Absent in 10/19 (52.6%) and delayed in 6/19 (31.6%) untreated patientsa | Smaller in 2/2 (100%) untreated patientsa | Rise in gonadotropin and testosterone levels in 5/5 patients (100%) of pubertal (or adult) age. No effect of treatment in 4/4 (100%) patients of prepubertal age | Unavailable | Present in 43/64 (67.2%) patients |
aThis was interpreted as 13 patients, since the exact number is not specified.
IGF1, insulin-like growth factor 1.
Discussion
The role of IGF1 on human HPT axis function is currently unknown. Evidence in animal models suggests that IGF1 stimulates GnRH secretion (4), GnRH neuron migration (5), and testicular differentiation and function (7), but whether this is also true for humans is a matter of debate. The model of Laron syndrome can offer important food for thought on the impact of IGF1 deficiency on the HPT function. This has prompted us to analyze the evidence collected over the years on this topic, mainly focusing on puberty onset and progression, testicular volume, gonadotropin and total testosterone serum levels, sperm parameters and fertility, and micropenis in male patients with Laron syndrome. We carefully reviewed all the literature in the attempt to calculate the prevalence of puberty absence and progression, delay of the growth spurt, microrchidism, abnormal sperm parameters or infertility, and micropenis in these patients. Some features could not be assessed due to the lack of information retrieved in the studies and, in some cases, the prevalence was estimated from a very low number of patients.
Puberty onset and progression
We found that puberty was absent in 8.9% and delayed in 35.6% of patients with Laron syndrome who were at a pubertal age and did not receive any treatment. Moreover, puberty progression was prolonged in 76.8% of them, and the growth spurt was absent in 52.6% and delayed in 31.6%. These data support that IGF1 may likely play an important role in puberty initiation and progression. Accordingly, untreated patients with growth hormone deficiency (GHD), a disease characterized by low serum IGF1 levels, commonly have delayed puberty, which has led to treatment with oxandrolone or testosterone in the past (34). In GHD patients, the onset of puberty has been reported to occur after 19.0 ± 3.5 months following the start of GH therapy (35). This may further confirm the possible role of IGF1 in puberty. Interestingly, an in vitro study performed in mice has shown that GnRH neurons express the IGF1R and that its expression varies during the developmental stage (36). Also, the incubation with IGF1 results in an increased GnRH secretion in GT1-7 GnRH neuronal cell line (37). Hence, the delay or absence of puberty in patients with Laron syndrome might hypothetically be ascribed to the lack of effect of IGF1 on the GnRH neurons. Nevertheless, it is currently unknown whether human GnRH neurons express the IGF1R and whether IGF1 stimulates GnRH release in humans. Recently, the 'acromegalic' levels of IGF1 occurring during the pubertal phase in healthy children seems to further suggest a role for IGF1 in puberty induction and sexual organs maturation (1).
Testicular volume
The vast majority of the studies on patients with Laron syndrome did not measure the testicular volume. When evaluated, it has been reported to be lower than normal in almost all the patients. This finding suggests that IGF1 may stimulates testicular growth during the prepubertal phase, which is in line with the testicular volume increase in boys with GHD once GH therapy is started (38). Testicular volume is an important indicator of fertility. In childhood, the testis is prevalently made of immature, actively proliferating Sertoli cells that secrete anti-Müllerian hormone (AMH) (39). When the pubertal process is completed, Sertoli cells reach a mature state, stop to secrete AMH, and initiate to produce trophic factors supporting spermatogenesis (39). From this moment on, testicular volume is prevalently given by the germ cell component. Accordingly, testicular volume is directly correlated with the sperm parameters in adulthood (40). Sertoli cells express IGF1R (7). The in vitro incubation of porcine prepubertal Sertoli cells with IGF1 results in increased cell proliferation (41). Therefore, low IGF1 levels in childhood may negatively impact the progression of testicular volume, explaining why patients with Laron syndrome have low testicular volumes. Accordingly, a study on 1030 healthy children reported a direct correlation between testicular volume and IGF1 (42).
These findings may explain the reduced testicular volume found in patients with Laron syndrome. In addition to its direct effects at the testicular level, IGF1 may also have an indirect impact on testicular function by interfering with the migration and function of GnRH neurons (6).
Gonadotropin and testosterone serum levels
The vast majority of the studies did not report the serum levels of gonadotropins and total testosterone in patients with Laron syndrome. Only two studies have assessed this aspect and reported an increase following IGF1 administration in all prepubertal patients older than 10 years of age but not in the very young boys (20, 21). In contrast, puberty was delayed and, hence, gonadotropins and testosterone remained low in the untreated patients (21).
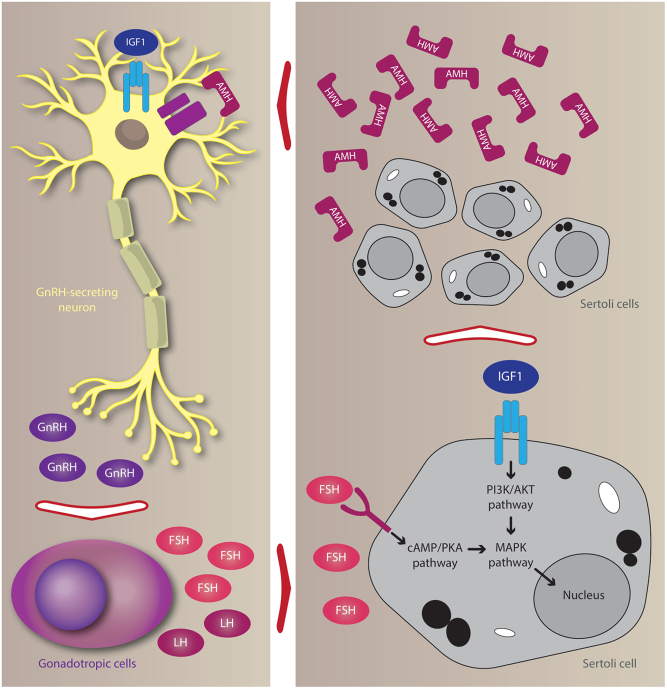
The IGF1-induced increase in gonadotropins and testosterone levels may relate to a direct and indirect effect of IGF1 on GnRH neurons, as supported by experimental data. Accordingly, as previously discussed, IGF1 is able to directly promote GnRH neuron migration (36) and GnRH secretion (37), this likely explaining the increase in gonadotropins and testosterone levels. The indirect effect may involve AMH. IGF1 promotes Sertoli cell proliferation and these cells, in turn, secrete AMH (41). Interestingly, AMH receptors (AMHR) have been reported in human GnRH neurons, where AMH stimulates neuron firing (43). Defective AMH signaling results in central hypogonadism (44), suggesting that normal Sertoli cell proliferation and AMH secretion in the prepubertal phase is needed for a physiological puberty onset and progression. Furthermore, insufficient Sertoli cell proliferation causes low circulating levels of AMH (39) which, in turn, could affect puberty onset (44). Hence, hypothetically, IGF1 may impact the firing of GnRH neurons with a direct or an indirect mechanism: the first consisting of the direct action of IGF1 on GnRH-secreting neurons; the second mechanism is AMH-mediated (Fig. 2).
Figure 2.
Effects of insulin-like growth factor 1 (IGF1) on gonadotropin-releasing hormone (GnRH)-secreting neurons and Sertoli cells. The IGF1 receptor (IGF1R) is expressed in mice GnRH neurons. Incubation with IGF1 enhances GnRH synthesis and release, which, in turn, stimulates the release of follicle-stimulating hormone (FSH) and luteinizing hormone (LH). IGF1R is also expressed in Sertoli cells. Incubation with IGF1 alone or in combination with FSH (but not FSH alone) results in Sertoli cell proliferation. Immature and proliferating Sertoli cells release high amounts of anti-Müllerian hormone (AMH). AMH receptors (AMHR) are expressed in human GnRH neurons. At this level, AMH is able to stimulate GnRH secretion.
Noteworthy, IGF1 can induce cellular proliferation in the adrenal cortex by triggering the MAPK/ERK signaling (45). In turn, evidence has shown that adrenal androgens could promote gonadal maturation (46). Hence, the GH/IGF1 axis may contribute to pubertal development by also stimulating adrenal function. However, more data are required on this topic before a conclusion can be achieved.
Sperm parameters and fertility
Another endpoint that we looked for in patients with Laron syndrome was sperm parameters/fertility. Unfortunately, to the best of our knowledge, no study has reported data on sperm parameters or fertility in these patients. In women, GH seems to influence fertility, as confirmed by the results of two meta-analyses, which have shown the efficacy of the treatment with GH to infertile women who did not respond to the administration of FSH in controlled ovarian hyperstimulation during cycles of assisted reproductive technique (47, 48). Few studies have investigated the effects of GH administration on sperm parameters in patients with idiopathic severe oligozoospermia (49, 50). Radicioni and colleagues treated ten patients with severe idiopathic oligozoospermia with GH. Five patients showed an increase in sperm concentration and total sperm count, whereas no difference was found in the others (49). The other study was undertaken in 12 patients with severe idiopathic oligozoospermia who were treated with GH administered daily for 5 months, but no significant effect was found on sperm count (50). On this account, this line of research was abandoned, mainly due to the lack of efficacy and to the side effects on the glycometabolic system. Due to the proliferative effect of IGF1 on Sertoli cells, the effectiveness of GH could likely be observed only before puberty when Sertoli cells are still able to proliferate and not in adulthood when they have reached maturity and are not able to divide any longer.
Micropenis
The last endpoint that could be evaluated in this systematic review was micropenis, which seems to occur in 67.2% of the patients with Laron syndrome. Penile length classically reflects the exposure to androgens. Accordingly, isolated micropenis is a clinical sign of androgen deficiency or insensitivity (51). However, as known, IGF1 mediates the action of GH and promotes tissue growth. Its role in the development of human sexual organs, including the penis, has already been suggested. Interestingly, treatment with GH has been proposed in pubertal children with hypogonadism and micropenis, in addition to sex steroids (1). On this account, the effectiveness of GH therapy combined with testosterone has been proven in a mice model with a micropenis (52). The results of this study showed that a complete restoration of penile length was achieved only with the combined therapy, whereas GH or testosterone alone were not effective (52).
Therapeutic implications
The role of GH administration in children with delayed puberty or central hypogonadism deserves to be studied as has already been suggested (1). From a theoretical point of view, treatment with GH (hence rising IGF1 serum levels) of a prepubertal child who does not reach IGF1 serum levels in the typical range for this age but that do not satisfy the diagnostic criteria of GHD may be preferable to gonadotropins because of future fertility prevention, due to the effect of IGF1 on Sertoli cell proliferation (41).
Since Sertoli cells can support a limited number of germ cells, the final number of Sertoli cells that are reached during the prepubertal phase limits the capacity of sperm production (53). Exposure to androgens block Sertoli cell proliferation, and they begin to maturate (53). Therefore, when puberty is induced, Sertoli cell proliferation should be supported and only afterward their maturation should be initiated with androgens. Indeed, the effects of FSH on Sertoli cells appear to be mediated by IGF1R (54), as supported by tyrosine-kinases receptor-associated second messengers, such as ERK1/2, PI3K/Akt, of the FSH signaling pathway (53). We have recently shown, using an experimental model on porcine prepubertal Sertoli cells, no effect of FSH alone on Sertoli cell proliferation. Instead, the proliferation was stimulated by incubation with IGF1 alone or FSH plus IGF1 (41). These findings highlight the importance of the presence of normal IGF1 levels for FSH to be fully effective.
Conclusion and future perspectives
The little available data on Laron syndrome show that these patients may have absence or delayed puberty and growth spurt, low testicular volume, and micropenis. These characteristics may be reasonably attributable to the lack of IGF1 effects, as supported by evidence in patients with GHD, animal models, and in vitro studies. Therefore, the 'lesson' that may be learned from Laron syndrome is that serum levels of IGF1 should be evaluated in children with delayed puberty, poor testicular growth, and/or micropenis.
In conclusion, prospective studies are needed to evaluate the effects of the GH/IGF1 axis for the induction of puberty in patients with delayed puberty, especially when these patients fail to reach the serum levels of IGF1 considered normal in the prepubertal period.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work did not receive any specific grant from funding agencies in the public, commercial, or not-profit sectors.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- 1.Juul A, Skakkebæk NE. Why do normal children have acromegalic levels of IGF-I during puberty? Journal of Clinical Endocrinology and Metabolism 20191042770–2776. ( 10.1210/jc.2018-02099) [DOI] [PubMed] [Google Scholar]
- 2.Chandrashekar V, Zaczek D, Bartke A. The consequences of altered somatotropic system on reproduction. Biology of Reproduction 20047117–27. ( 10.1095/biolreprod.103.027060) [DOI] [PubMed] [Google Scholar]
- 3.Tenuta M, Carlomagno F, Cangiano B, Kanakis G, Pozza C, Sbardella E, Isidori AM, Krausz C, Gianfrilli D, Axis S-T. Somatotropic-testicular axis: a crosstalk between GH/IGF-I and gonadal hormones during development, transition, and adult age. Andrology 20219168–184. ( 10.1111/andr.12918) [DOI] [PubMed] [Google Scholar]
- 4.Longo KM, Sun Y, Gore AC. Insulin-like growth factor-I effects on gonadotropin-releasing hormone biosynthesis in GT1-7 cells. Endocrinology 19981391125–1132. ( 10.1210/endo.139.3.5852) [DOI] [PubMed] [Google Scholar]
- 5.Onuma TA, Ding Y, Abraham E, Zohar Y, Ando H, Duan C. Regulation of temporal and spatial organization of newborn GnRH neurons by IGF signaling in zebrafish. Journal of Neuroscience 20113111814–11824. ( 10.1523/JNEUROSCI.6804-10.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cannarella R, Paganoni AJJ, Cicolari S, Oleari R, Condorelli RA, La Vignera S, Cariboni A, Calogero AE, Magni P. Anti-Müllerian hormone, growth hormone, and insulin-like growth factor 1 modulate the migratory and secretory patterns of GnRH neurons. International Journal of Molecular Sciences 202122 2445. ( 10.3390/ijms22052445) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cannarella R, Condorelli RA, La Vignera S, Calogero AE. Effects of the insulin-like growth factor system on testicular differentiation and function: a review of the literature. Andrology 201863–9. ( 10.1111/andr.12444) [DOI] [PubMed] [Google Scholar]
- 8.Laron Z, Kauli R. Fifty seven years of follow-up of the Israeli cohort of Laron syndrome patients-from discovery to treatment. Growth Hormone and IGF Research 20162853–56. ( 10.1016/j.ghir.2015.08.004) [DOI] [PubMed] [Google Scholar]
- 9.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. & PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015. 350 g7647. ( 10.1136/bmj.g7647) [DOI] [PubMed] [Google Scholar]
- 10.Villela TR, Freire BL, Braga NTP, Arantes RR, Funari MFA, Alexander JAL, Silva IN. Growth hormone insensitivity (Laron syndrome): report of a new family and review of Brazilian patients. Genetics and Molecular Biology 202042 e20180197. ( 10.1590/1678-4685-GMB-2018-0197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dye AM, Nelson GB, Diaz-Thomas A. Delayed puberty. Pediatric Annals 201847e16–e22. ( 10.3928/19382359-20171215-01) [DOI] [PubMed] [Google Scholar]
- 12.Laron Z, Sarel R. Penis and testicular size in patients with growth hormone insufficency. Acta Endocrinologica 197063625–633. ( 10.1530/acta.0.0630625) [DOI] [PubMed] [Google Scholar]
- 13.Dabaja AA, Wosnitzer MS, Bolyakov A, Schlegel PN, Paduch DA. When to ask male adolescents to provide semen sample for fertility preservation? Translational Andrology and Urology 201432–8. ( 10.3978/j.issn.2223-4683.2014.02.01) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laron Z, Sarel R, Pertzelan A. Puberty in Laron type dwarfism. European Journal of Pediatrics 198013479–83. ( 10.1007/BF00442408) [DOI] [PubMed] [Google Scholar]
- 15.Guevara-Aguirre J, Rosenbloom AL, Fielder PJ, Diamond Jr FB, Rosenfeld RG. Growth hormone receptor deficiency in Ecuador: clinical and biochemical phenotype in two populations. Journal of Clinical Endocrinology and Metabolism 199376417–423. ( 10.1210/jcem.76.2.7679400) [DOI] [PubMed] [Google Scholar]
- 16.Pertzelan A, Lazar L, Klinger B, Laron Z. Puberty in fifteen patients with Laron syndrome: a Longitudina Study. In Lessons from Laron syndrome (LS) 1966–1992: Pediatric and Adolescent Endocrinology, Vol. 24, pp. 27–33. Eds Laron Z, Parks JS. Basel: Karger, 1993. [Google Scholar]
- 17.Laron Z, Lilos P, Klinger B. Growth curves for Laron syndrome. Archives of Disease in Childhood 199368768–770. ( 10.1136/adc.68.6.768) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Savage MO, Blum WF, Ranke MB, Postel-Vinay MC, Cotterill AM, Hall K, Chatelain PG, Preece MA, Rosenfeld RG. Clinical features and endocrine status in patients with growth hormone insensitivity (Laron syndrome). Journal of Clinical Endocrinology and Metabolism 1993771465–1471. ( 10.1210/jcem.77.6.7505286) [DOI] [PubMed] [Google Scholar]
- 19.Castilla-Cortazar I, De Ita JR, Aguirre GA, García-Magariño M, Martín-Estal I, Lara-Diaz VJ, Elizondo MI. Growth hormone insensitivity: Mexican case report. Endocrinology, Diabetes and Metabolism Case Reports 2017201717–0126. ( 10.1530/EDM-17-0126) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laron Z, Klinger B. Effect of insulin-like growth factor-I treatment on serum androgens and testicular and penile size in males with Laron syndrome (primary growth hormone resistance). European Journal of Endocrinology 1998138176–180. ( 10.1530/eje.0.1380176) [DOI] [PubMed] [Google Scholar]
- 21.Ben-Amitai D, Laron Z. Effect of insulin-like growth factor-1 deficiency or administration on the occurrence of acne. Journal of the European Academy of Dermatology and Venereology 201125950–954. ( 10.1111/j.1468-3083.2010.03896.x) [DOI] [PubMed] [Google Scholar]
- 22.Phanse-Gupte SR, Khadilkar VV, Khadilkar AV. Clinical features and endocrine profile of Laron syndrome in Indian children. Indian Journal of Endocrinology and Metabolism 201418863–867. ( 10.4103/2230-8210.140236) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burren CP, Woods KA, Rose SJ, Tauber M, Price DA, Heinrich U, Gilli G, Razzaghy-Azar M, Al-Ashwal A, Crock PAet al. Clinical and endocrine characteristics in atypical and classical growth hormone insensitivity syndrome. Hormone Research 200155125–130. ( 10.1159/000049983) [DOI] [PubMed] [Google Scholar]
- 24.Ioimo I, Guarracino C, Meazza C, Domené HM, Bozzola M. Same phenotype in children with growth hormone deficiency and resistance. Case Reports in Pediatrics 201820185902835. ( 10.1155/2018/5902835) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krzisnik C, Silbergeld A, Laron Z. A case of Laron syndrome diagnosed in Slovenia. Journal of Pediatric Endocrinology 19947365–369. ( 10.1515/jpem.1994.7.4.365) [DOI] [PubMed] [Google Scholar]
- 26.Rosenbloom AL, Berg MA, Kasatkina EP, Volkova TN, Skorobogatova VF, Sokolovskaya VN, Francke U. Severe growth hormone insensitivity (Laron syndrome) due to nonsense mutation of the GH receptor in brothers from Russia. Journal of Pediatric Endocrinology and Metabolism 19958159–165. ( 10.1515/jpem.1995.8.3.159) [DOI] [PubMed] [Google Scholar]
- 27.Guleria S, Sharma J, Kaushik SL. Laron syndrome. Journal of Postgraduate Medicine 201460322–323. ( 10.4103/0022-3859.138816) [DOI] [PubMed] [Google Scholar]
- 28.Hopp M, Rosenbloom AL, Griffiths J, Kgwete S, Vaccarello MA. Growth hormone receptor deficiency (Laron syndrome) in black African siblings. South African Medical Journal 199686268–270. [PubMed] [Google Scholar]
- 29.De Lima Jorge AA.Short stature investigation: clinical, laboratorial and genetic aspects concerning the growth hormone insensitivity (GHI). Arquivos Brasileiros de Endocrinologia e Metabologia 2008521056–1065. ( 10.1590/s0004-27302008000600018) [DOI] [PubMed] [Google Scholar]
- 30.Laron Z.Laron syndrome (primary growth hormone resistance or insensitivity): the personal experience 1958–2003. Journal of Clinical Endocrinology and Metabolism 2004891031–10. [DOI] [PubMed] [Google Scholar]
- 31.Kim M, Hwang EC, Park IK, Park K. Insulin-like growth factor-1 gene delivery may enhance the proliferation of human corpus cavernosal smooth muscle cells. Urology 201076511.e5–511.e9. ( 10.1016/j.urology.2009.12.011) [DOI] [PubMed] [Google Scholar]
- 32.Xu ZP, Wang HP, Liu JM, Zheng XG, Wu D, Pu XY. Effects of insulin-like growth factor-1 on the relaxation responses of the cavernous smooth muscle from aged rats. Scandinavian Journal of Urology 201549260–266. ( 10.3109/21681805.2015.1021832) [DOI] [PubMed] [Google Scholar]
- 33.Dykstra KD, Payne AM, Abdelrahim M, Francis GL. Insulin-like growth factor 1, but not growth hormone, has in vitro proliferative effects on neonatal foreskin fibroblasts without affecting 5-alpha-reductase or androgen receptor activity. Journal of Andrology 19931473–78. [PubMed] [Google Scholar]
- 34.Albanese A, Stanhope R. Treatment of growth delay in boys with isolated growth hormone deficiency. European Journal of Endocrinology 199413065–69. ( 10.1530/eje.0.1300065) [DOI] [PubMed] [Google Scholar]
- 35.Tatò L, Zamboni G, Antoniazzi F, Piubello G. Gonadal function and response to growth hormone (GH) in boys with isolated GH deficiency and to GH and gonadotropins in boys with multiple pituitary hormone deficiencies. Fertility and Sterility 199665830–834. ( 10.1016/s0015-0282(1658222-3) [DOI] [PubMed] [Google Scholar]
- 36.Daftary SS, Gore AC. The hypothalamic insulin-like growth factor-1 receptor and its relationship to gonadotropin-releasing hormones neurones during postnatal development. Journal of Neuroendocrinology 200416160–169. ( 10.1111/j.0953-8194.2004.01149.x) [DOI] [PubMed] [Google Scholar]
- 37.Anderson RA, Zwain IH, Arroyo A, Mellon PL, Yen SS. The insulin-like growth factor system in the GT1-7 GnRH neuronal cell line. Neuroendocrinology 199970353–359. ( 10.1159/000054496) [DOI] [PubMed] [Google Scholar]
- 38.Lindgren AC, Chatelain P, Lindberg A, Price DA, Ranke MB, Reiter EO, Wilton P. & KIGS International Board. Normal progression of testicular size in boys with idiopathic short stature and isolated growth hormone deficiency treated with growth hormone: experience from the KIGS. Hormone Research 2002. 58 83–87. ( 10.1159/000064658) [DOI] [PubMed] [Google Scholar]
- 39.Condorelli RA, Cannarella R, Calogero AE, La Vignera S. Evaluation of testicular function in prepubertal children. Endocrine 201862274–280. ( 10.1007/s12020-018-1670-9) [DOI] [PubMed] [Google Scholar]
- 40.Condorelli R, Calogero AE, La Vignera S. Relationship between testicular volume and conventional or nonconventional sperm parameters. International Journal of Endocrinology 20132013145792. ( 10.1155/2013/145792) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cannarella R, Mancuso F, Condorelli RA, Arato I, Mongioì LM, Giacone F, Lilli C, Bellucci C, La Vignera S, Calafiore Ret al. Effects of GH and IGF1 on basal and FSH-modulated porcine Sertoli cells in-vitro. Journal of Clinical Medicine 20198 811. ( 10.3390/jcm8060811) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Juul A, Bang P, Hertel NT, Main K, Dalgaard P, Jørgensen K, Müller J, Hall K, Skakkebaek NE. Serum insulin-like growth factor-I in 1030 healthy children, adolescents, and adults: relation to age, sex, stage of puberty, testicular size, and body mass index. Journal of Clinical Endocrinology and Metabolism 199478744–752. ( 10.1210/jcem.78.3.8126152) [DOI] [PubMed] [Google Scholar]
- 43.Barbotin AL, Peigné M, Malone SA, Giacobini P. Emerging roles of anti-Müllerian hormone in hypothalamic-pituitary function. Neuroendocrinology 2019109218–229. ( 10.1159/000500689) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malone SA, Papadakis GE, Messina A, Mimouni NEH, Trova S, Imbernon M, Allet C, Cimino I, Acierno J, Cassatella Det al. Defective AMH signaling disrupts GnRH neuron development and function and contributes to hypogonadotropic hypogonadism. eLife 20198 e47198. ( 10.7554/eLife.47198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen G, Yuan C, Duan F, Liu Y, Zhang J, He Z, Huang H, He C, Wang H. IGF1/MAPK/ERK signaling pathway-mediated programming alterations of adrenal cortex cell proliferation by prenatal caffeine exposure in male offspring rats. Toxicology and Applied Pharmacology 201834164–76. ( 10.1016/j.taap.2018.01.008) [DOI] [PubMed] [Google Scholar]
- 46.Remer T, Shi L, Buyken AE, Maser-Gluth C, Hartmann MF, Wudy SA. Prepubertal adrenarchal androgens and animal protein intake independently and differentially influence pubertal timing. Journal of Clinical Endocrinology and Metabolism 2010953002–3009. ( 10.1210/jc.2009-2583) [DOI] [PubMed] [Google Scholar]
- 47.Duffy JM, Ahmad G, Mohiyiddeen L, Nardo LG, Watson A. Growth hormone for in vitro fertilization. Cochrane Database of Systematic Reviews 20102010CD000099. ( 10.1002/14651858.CD000099.pub3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li XL, Wang L, Lv F, Huang XM, Wang LP, Pan Y, Zhang XM. The influence of different growth hormone addition protocols to poor ovarian responders on clinical outcomes in controlled ovary stimulation cycles: a systematic review and meta-analysis. Medicine 201796 e6443. ( 10.1097/MD.0000000000006443) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Radicioni A, Paris E, Dondero F, Bonifacio V, Isidori A. Recombinant-growth hormone (rec-hGH) therapy in infertile men with idiopathic oligozoospermia. Acta Europaea Fertilitatis 199425311–317. [PubMed] [Google Scholar]
- 50.Bhangoo A, Paris F, Philibert P, Audran F, Ten S, Sultan C. Isolated micropenis reveals partial androgen insensitivity syndrome confirmed by molecular analysis. Asian Journal of Andrology 201012561–566. ( 10.1038/aja.2010.6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee KO, Ng SC, Lee PS, Bongso AT, Taylor EA, Lin TK, Ratnam SS. Effect of growth hormone therapy in men with severe idiopathic oligozoospermia. European Journal of Endocrinology 1995132159–162. ( 10.1530/eje.0.1320159) [DOI] [PubMed] [Google Scholar]
- 52.Oh JK, Im YJ, Park K, Paick JS. Effects of combined growth hormone and testosterone treatments in a rat model of micropenis. Endocrine Connections 201871150–1157. ( 10.1530/EC-18-0200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meroni SB, Galardo MN, Rindone G, Gorga A, Riera MF, Cigorraga SB. Molecular mechanisms and signaling pathways involved in Sertoli cell proliferation. Frontiers in Endocrinology 201910 224. ( 10.3389/fendo.2019.00224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cannarella R, Arato I, Condorelli RA, Luca G, Barbagallo F, Alamo A, Bellucci C, Lilli C, La Vignera S, Calafiore Ret al. The IGF1 receptor is involved in follicle-stimulating hormone signaling in porcine neonatal Sertoli cells. Journal of Clinical Medicine 20198 577. ( 10.3390/jcm8050577) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a