Introduction
The etiology of amyotrophic lateral sclerosis (ALS) remains unknown but is considered to be an interplay of environmental exposures and genetic predisposition (van Es et al. 2017). Few epidemiological studies have examined the association between ambient air pollution and ALS. We previously reported an increased risk of developing ALS for long-term exposure to traffic-related air pollution in a Dutch case–control study (917 cases and 2,662 controls) (Seelen et al. 2017). Increased knowledge about the possible associations between particulate matter (PM) and its constituents and ALS will provide additional insight into the potential pathophysiology of ALS. We aimed to extend on our previous analyses by including 2,081 more cases and controls and by extending the exposure assessment to a broader range of air pollutants [ultrafine particles ( in aerodynamic diameter or UFPs), PM elemental components, and oxidative potentials (OPs)].
Methods
Present analyses were based on ALS patients and controls enrolled in the Prospective ALS in the Netherlands (PAN) study (Huisman et al. 2011) from 1 January 2006 to 31 December 2018. All patients with a diagnosis of possible, probable, or definite ALS according to the revised El Escorial criteria (Brooks et al. 2000) were included. Population-based controls selected from the registers of the patients’ general practitioners were frequency matched by sex and age (). Information including sex, date of birth, education level, body mass index, smoking, alcohol consumption, residential history, and area-level socioeconomic status (SES) was collected. Annual concentrations of air pollution constituents were estimated at the geocoded residential addresses of each participant based on land-use regression (LUR) models developed within the European Study of Cohorts for Air Pollution Effects (ESCAPE) and Exposomics projects (Beelen et al. 2013; de Hoogh et al. 2013; Eeftens et al. 2012; van Nunen et al. 2017) (see supporting information at https://github.com/kevininef/Airpollution-ALS). We averaged the air pollutant concentrations from 1992 to the date of onset for cases or recruitment for controls as the main exposure.
Unconditional logistic regression models were used to estimate the association between exposure to air pollution and ALS in single-pollutant models. Two-pollutant models were performed for each air pollutant by additionally adjusting for the other pollutants one by one. All analyses were performed within R software (version 3.6.1; R Development Core Team). Supporting information is available at https://github.com/kevininef/Airpollution-ALS. The PAN study was approved by the institutional review board of the University Medical Center Utrecht.
Results and Discussion
A total of 1,636 patients with ALS and 4,024 controls were included (see supporting information at https://github.com/kevininef/Airpollution-ALS), covering all of the Netherlands. We observed increased odds ratios (ORs) for ALS in association with most air pollutants, with the strongest associations for () { [95% confidence interval (CI): 1.10, 1.28], nitrogen dioxide [] [ (95% CI: 1.15, 1.34)], and nitrogen oxides [] [ (95% CI: 1.07, 1.22)]} (Table 1). For UFPs, an elevated OR of 1.11 (95% CI: 1.05, 1.16) was observed. For particle elements, road traffic non-tailpipe emissions of copper (Cu), iron (Fe), nickel (Ni), sulfur (S), silicon (Si), and vanadium (V) were associated with significantly higher ORs for ALS in both and fractions. Marginal effects for all air pollutants are presented in the supporting information at https://github.com/kevininef/Airpollution-ALS.
Table 1.
Association between long-term exposure to air pollution and ALS in single-pollutant models.
| Exposure (IQR)a | Average exposure levela | OR (95% CI)b | Valuec | |
|---|---|---|---|---|
| Case () | Control () | |||
| (2.0) | 1.10 (1.04, 1.16) | 0.001 | ||
| (1.5) | 1.05 (0.92, 1.10) | 0.153 | ||
| (0.9) | 1.06 (1.00, 1.12) | |||
| absorbance (0.3) | 1.19 (1.10, 1.28) | |||
| (7.4) | 1.25 (1.15, 1.34) | |||
| (10.7) | 1.14 (1.07, 1.22) | |||
| UFPs (1,240) | 1.11 (1.05, 1.16) | |||
| Cu (1.1) | 1.18 (1.10, 1.27) | |||
| Cu (3.6) | 1.08 (1.02, 1.15) | 0.019 | ||
| Fe (27.1) | 1.22 (1.13, 1.31) | |||
| Fe (125.0) | 1.16 (1.09, 1.24) | |||
| K (13.3) | 0.98 (0.90, 1.07) | 0.764 | ||
| K (17.3) | 1.09 (1.02, 1.17) | 0.008 | ||
| Ni (1.0) | 1.15 (1.05, 1.25) | 0.004 | ||
| Ni (1.1) | 1.17 (1.07, 1.28) | 0.001 | ||
| S (63.8) | 1.10 (1.02, 1.18) | 0.021 | ||
| S (47.3) | 1.08 (1.01, 1.15) | 0.034 | ||
| (12.2) | 1.12 (1.05, 1.19) | 0.003 | ||
| (80.7) | 1.18 (1.11, 1.25) | |||
| V (1.5) | 1.15 (1.05, 1.25) | 0.004 | ||
| V (1.6) | 1.14 (1.05, 1.23) | 0.004 | ||
| Zn (18.8) | 0.96 (0.88, 1.04) | 0.315 | ||
| Zn (25.8) | 0.99 (0.91, 1.08) | 0.857 | ||
| OP ESR (171.9) | 1.14 (1.06, 1.23) | 0.032 | ||
| OP DTT (0.2) | 1.01 (0.93, 1.09) | 0.343 | ||
Note: ALS, amyotrophic lateral sclerosis; CI, confidence interval; Cu, copper; Fe, iron; IQR, interquartile range; K, potassium; Ni, nickel; , nitrogen dioxide; , nitrogen oxides; , particulate matter with aerodynamic ; , particulate matter with aerodynamic ; , ; , particulate matter with aerodynamic diameter between and ; OP DTT, oxidative potential metric with dithiothreitol; OP ESR, oxidative potential metric with electron spin resonance; OR, odds ratio; S, sulfur; SES, socioeconomic status; Si, silicon; UFPs, ultrafine particles; V, vanadium; Zn, zinc.
Units are for , , , and ; for absorbance; particle for UFPs; for all PM elemental constituents; atomic units for OP ESR; and mol for OP DTT.
Results were adjusted for sex, age (age in y at diagnosis for cases and at recruitment for controls), education level, body mass index, smoking status, alcohol consumption, and area SES using unconditional logistic regression models; ORs are presented as per IQR increment.
-Values corrected for multiple testing using Benjamini and Hochberg method (Benjamini and Hochberg 1995) are presented.
In two-pollutant models adjusted for PM mass, the associations of most air pollutant elements with ALS remained positive, whereas the association of PM mass became null (Figure 1). In two-pollutant models corrected for , the associations of most air pollutants were reduced toward the null, except for Si in the Si fraction (), whereas the estimated positive association for remained, indicating independent associations between , , and the risk of ALS. Sensitivity analyses showed the associations of and with ALS were robust (see supporting information at https://github.com/kevininef/Airpollution-ALS).
Figure 1.
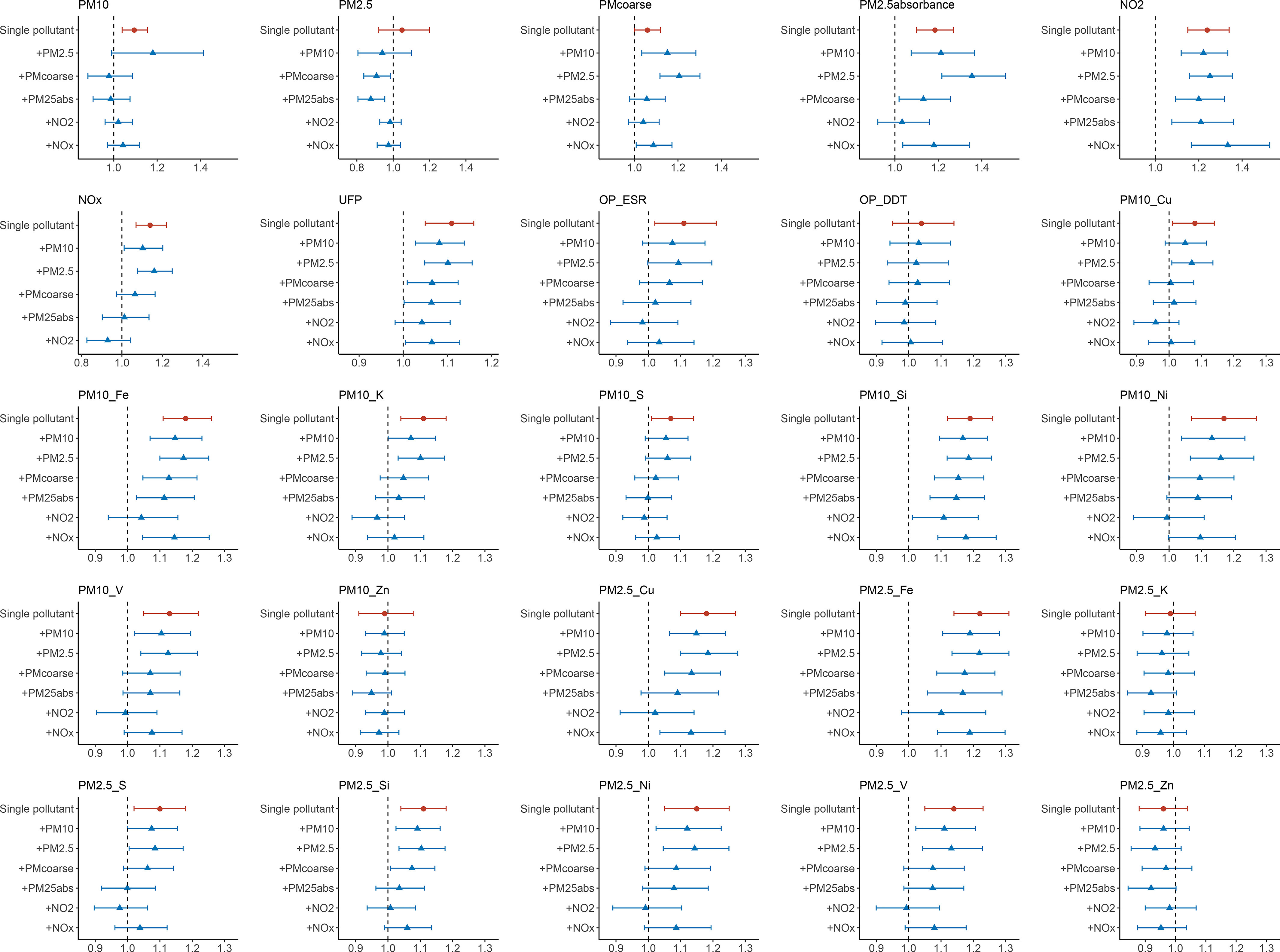
Two-pollutant model with the main effects of PM mass, absorbance, , , UFPs, PM OP, and PM elemental compositions. The x-axis represents the estimate of certain air pollution constituents, the y-axis represents the pollutants adjusted in the two-pollutant models. All results were adjusted for sex, age, education level, body mass index, smoking status, alcohol consumption, and area SES using unconditional logistic regression models. The model adjusted for and is difficult to interpret because is the sum of these two. The models including both and are also difficult to interpret because is included in . Red dots stand for single-pollutant models; blue triangles stand for two-pollutant models. Numeric data of this figure are presented in supporting information at https://github.com/kevininef/Airpollution-ALS. Note: Cu, copper; Fe, iron; K, potassium; Ni, nickel; , nitrogen dioxide; , nitrogen oxides; OP DTT, oxidative potential metric with dithiothreitol; OP ESR, oxidative potential metric with electron spin resonance; PM, particulate matter; , particulate matter with aerodynamic ; , particulate matter with aerodynamic ; , ; , particulate matter with aerodynamic diameter between and ; PM OP, particulate matter oxidative potential; S, sulfur; SES, socioeconomic status; Si, silicon; UFPs, ultrafine particles; V, vanadium; Zn, zinc.
With an extended sample [nearly twice the size of the previous analyses by Seelen et al. (2017)], we here confirm the positive associations for (see supporting information at https://github.com/kevininef/Airpollution-ALS). Moreover, restricting the analysis to the participants who were recruited after the previous publication showed consistent associations for air pollution and ALS, speaking to the robustness of the associations (see supporting information at https://github.com/kevininef/Airpollution-ALS). We also broadened our previously published analyses to a wider range of air pollutants and found that the association between long-term air pollution exposure and ALS, as previously hypothesized (Seelen et al. 2017), is mainly driven by local traffic-related constituents. primarily comes from tailpipe emissions and predictors in the Si LUR models were also traffic variables. The concentrations were already below the current World Health Organization air quality guidelines (), suggesting potential benefits of tightening the guidelines and regulatory limits of (World Health Organisation Fact Sheet 2018).
A potential limitation might be that we used the disease onset date for cases in calculating the exposure period, subsequently resulting in a slightly different etiological time window for cases than controls. However, we reestimated the average concentrations for controls from 1992 to 1 y prior to the recruitment date (see supporting information at https://github.com/kevininef/Airpollution-ALS) and generated essentially the same exposure values.
Using the air pollution models developed in 2010 for PM elements and in 2014 for UFPs to predict historical exposure might also be a concern, but this is supported by previous studies that reported that the spatial contrasts in measured and modeled annual average levels were stable over time (Eeftens et al. 2011; Downward et al. 2018). Sensitivity analysis of the present study using concentrations without back- extrapolation rendered essentially similar results (see supporting information at https://github.com/kevininef/Airpollution-ALS). Possible residual confounding cannot be excluded given that data regarding medical comorbidities, for example, were not included in the present analysis.
Overall, we found that long-term exposures to and were independently associated with ALS in a large population-based case–control study. These associations hint toward the potential health relevance of both tailpipe and non-tailpipe emissions of motorized traffic contributing to ALS risk.
Acknowledgments
S.P., L.v.B., L.v.d.B., and R.V. contributed to the study concept and design and participated in data collection and processing. S.P., G.D., G.H., M.-A.K., M.G.W., J.H., and R.V. contributed to the analyses plan. Z.Y. performed the statistical analyses. S.P., G.D., G.H., M.-A.K., M.G.W., J.H., and R.V. contributed to the analyses and interpretation of data. Z.Y. drafted the manuscript. S.P., L.v.B., G.D., G.H., M.-A.K., M.G.W., J.H., L.v.d.B., and R.V. revised the manuscript for intellectual content. L.v.d.B. obtained the funding for the case–control study.
This case–control study was funded by the ALS Foundation Netherlands; Prinses Beatrix Spierfonds; the European Community’s Health Seventh Framework Programme (grant agreements 259867 and 211250); Netherlands Organisation for Health Research and Development (ZonMW) under the frame of E-Rare-2, the European Research Area Network on Rare Diseases; EU Joint Programme Neurodegenerative Disease Research project [Sampling and biomarker OPtimization and Harmonization In ALS and other motor neuron diseases (SOPHIA) and Survival, Trigger and Risk, Epigenetic, eNvironmental and Genetic Targets for motor neuron Health (STRENGTH) projects]; and the ZonMW Vici scheme to L.v.d.B. Z.Y. was supported by a scholarship from the Chinese Scholarship Council. M.-A.K. was supported by the National Institutes of Health/National Institute of Environmental Health Science (R21 ES028472 and R01 ES028805).
Supporting information can be found at the GitHub online repository at https://github.com/kevininef/Airpollution-ALS.
References
- Beelen R, Hoek G, Vienneau D, Eeftens M, Dimakopoulou K, Pedeli X, et al. 2013. Development of NO2 and NOx land use regression models for estimating air pollution exposure in 36 study areas in Europe—the ESCAPE project. Atmos Environ 72:10–23, 10.1016/j.atmosenv.2013.02.037. [DOI] [Google Scholar]
- Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 57(1):289–300, 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- Brooks BR, Miller RG, Swash M, Munsat TL, World Federation of Neurology Research Group on Motor Neuron Diseases. 2000. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 1(5):293–299, PMID: 11464847, 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- de Hoogh K, Wang M, Adam M, Badaloni C, Beelen R, Birk M, et al. 2013. Development of land use regression models for particle composition in twenty study areas in Europe. Environ Sci Technol 47(11):5778–5786, PMID: 23651082, 10.1021/es400156t. [DOI] [PubMed] [Google Scholar]
- Downward GS, van Nunen EJHM, Kerckhoffs J, Vineis P, Brunekreef B, Boer JMA, et al. 2018. Long-term exposure to ultrafine particles and incidence of cardiovascular and cerebrovascular disease in a prospective study of a Dutch cohort. Environ Health Perspect 126(12):127007, PMID: 30566375, 10.1289/EHP3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeftens M, Beelen R, de Hoogh K, Bellander T, Cesaroni G, Cirach M, et al. 2012. Development of land use regression models for PM2.5, PM2.5 absorbance, PM10 and PMcoarse in 20 European study areas; results of the ESCAPE project. Environ Sci Technol 46(20):11195–11205, PMID: 22963366, 10.1021/es301948k. [DOI] [PubMed] [Google Scholar]
- Eeftens M, Beelen R, Fischer P, Brunekreef B, Meliefste K, Hoek G. 2011. Stability of measured and modelled spatial contrasts in NO2 over time. Occup Environ Med 68(10):765–770, PMID: 21285243, 10.1136/oem.2010.061135. [DOI] [PubMed] [Google Scholar]
- Huisman MHB, de Jong SW, van Doormaal PTC, Weinreich SS, Schelhaas HJ, van der Kooi AJ, et al. 2011. Population based epidemiology of amyotrophic lateral sclerosis using capture–recapture methodology. J Neurol Neurosurg Psychiatry 82(10):1165–1170, PMID: 21622937, 10.1136/jnnp.2011.244939. [DOI] [PubMed] [Google Scholar]
- Seelen M, Toro Campos RA, Veldink JH, Visser AE, Hoek G, Brunekreef B, et al. 2017. Long-term air pollution exposure and amyotrophic lateral sclerosis in Netherlands: a population-based case–control study. Environ Health Perspect 125(9):097023, PMID: 29989551, 10.1289/EHP1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Es MA, Hardiman O, Chio A, Al-Chalabi A, Pasterkamp RJ, Veldink JH, et al. 2017. Amyotrophic lateral sclerosis. Lancet 390(10107):2084–2098, PMID: 28552366, 10.1016/S0140-6736(17)31287-4. [DOI] [PubMed] [Google Scholar]
- van Nunen E, Vermeulen R, Tsai M-Y, Probst-Hensch N, Ineichen A, Davey M, et al. 2017. Land use regression models for ultrafine particles in six European areas. Environ Sci Technol 51(6):3336–3345, PMID: 28244744, 10.1021/acs.est.6b05920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organisation Fact Sheet. 2018. Ambient (outdoor) air pollution. Published in 2018. https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health [accessed 30 August 2021]. [Google Scholar]


