Abstract
This review examines the prevalence, aetiology, pathophysiology, prognostic value, and investigation of dysnatraemia in hospitalised COVID-19 patients, taking into account all relevant studies published in PubMed and Cochrane Library studies until March 2021. Hyponatraemia is commonly observed in patients with bacterial pneumonia and is an independent predictor for excess mortality and morbidity. However, it remains unknown whether this association applies to coronavirus disease-2019 (COVID-19). Several studies reported a 20–35% prevalence for hyponatraemia and 2–5% for hypernatraemia in patients admitted with COVID-19. In addition, hyponatraemia on admission was a risk factor for progression to severe disease, being associated with an increased likelihood for the need for invasive mechanical ventilation, with an odds ratio (OR) of 1.83–3.30. Hyponatraemia seems to be an independent risk factor for mortality, with an OR of 1.40–1.50 compared to normonatraemia, while hypernatraemia is related to even worse outcomes than hyponatraemia. Furthermore, preliminary data show an inverse association between serum sodium and interleukin-6 levels, suggesting that hyponatraemia might be used as a surrogate marker for the risk of a cytokine storm and the need for treatment with interleukin antagonists. In conclusion, dysnatraemia is common and carries a poor prognosis in COVID-19 patients, indicating that it may play a future role in risk stratification and individualising therapy.
Introduction
Numerous studies have demonstrated a U-shaped relationship between serum sodium concentration and in-patient mortality in general hospital populations, with both hyponatraemia and hypernatraemia (defined as serum sodium levels below 135 mmol/L and above 145 mmol/L, respectively) being independent risk factors for mortality (1, 2). Many studies in patients with community-acquired pneumonia (CAP) have reported that hyponatraemia, mainly attributable to the syndrome of inappropriate antidiuretic hormone secretion (SIADH) or hypovolaemic hyponatraemia (3), is highly prevalent on admission and is associated with both an excess of in-hospital mortality and an increase in the length of hospital stay (3, 4, 5).
More than 150 million people have been infected by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), rendering the coronavirus disease-2019 (COVID-19) pandemic the greatest global public health crisis of this generation. A significant proportion of patients with COVID-19 are hospitalised with viral pneumonia which can progress to severe disease, characterised by various types of organ dysfunction, including septic shock, acute respiratory distress syndrome (ARDS), acute kidney injury (AKI), acute cardiac injury, neurological complications (6), and disseminated intravascular coagulation (DIC) (7). A significant association of adverse outcomes in patients with COVID-19 with several laboratory abnormalities has been shown, including lymphocytopenia, anaemia, thrombocytopenia, hypoalbuminaemia, increased neutrophil count, lactic dehydrogenase (LDH), C-reactive protein (CRP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), urea, creatinine, creatine kinase (CK), erythrocyte sedimentation rate (ESR), ferritin, troponin I, D-dimer, interleukin 6 (IL-6), and interleukin 10 (IL-10) levels (8, 9, 10, 11, 12, 13). In addition, fasting blood glucose concentration on admission above 126 mg/dL (7 mmol/L) (14) and a serum cortisol concentration above 27 mcg/dL (744 nmol/L) (15) are independent predictors for mortality in patients with COVID-19. Regarding the prevalence and prognostic impact of dysnatraemia (abnormal serum sodium levels) in patients with COVID-19, some important publications have appeared in this area since September 2020. In this review, we summarise the literature to date with regards to the prevalence, aetiology, and prognostic value of alterations in serum sodium levels in hospitalised patients with COVID-19. We also provide an overview of the potential pathophysiological mechanisms of hyponatraemia, underpinning the association between sodium values and the magnitude of the inflammatory response, supplemented by recommendations for the optimal investigation and management of hyponatraemia in this context.
Methods
We undertook a search via PubMed and the Cochrane Library until 16th March 2021 of all studies which included the key words 'COVID-19', 'SARS-CoV-2', 'hyponatraemia', 'hypernatraemia', 'sodium', and 'syndrome of inappropriate antidiuretic hormone secretion (SIADH)'. However, of the numerous studies flagged up, only six were found to have examined the prevalence of sodium abnormalities, and their association with clinical outcomes in COVID-19 patients (Table 1), which are reviewed in detail below.
Table 1.
Prevalence of sodium abnormalities on admission in main observational studies.
| Study | COVID-19 patients, n | Location | Sodium levels (nmol/L) on admission | ||
|---|---|---|---|---|---|
| <135* (%) | <130 (%) | >145† (%) | |||
| Frontera et al. (1) | 4645 | 4 hospitals, New York | 30 | 7.3 | 4.1 |
| Hu et al. (2) | 1254 | 3 hospitals, China | 9.9 | NR | 2.4 |
| Tezcan et al. (3) | 408 | 1 hospital, Turkey | 35.8 | NR | 0 |
| HOPE-COVID-19 (4) | 4664 | 37 hospitals, 7 countries‡ | 20.5 | 3.8 | 3.7 |
| Atila et al. (5) | 172 | 1 hospital, Switzerland | 29.1 | NR | 2.9 |
| Tzoulis et al. (6) | 488 | 2 hospitals, London | 24.6 | 6.2 | 5.3 |
*Hyponatraemia; †Hypernatraemia; ‡Spain (81%) and Italy (10%).
NR, not reported.
Prevalence, risk factors, and aetiology of dysnatraemia in COVID-19
The emerging data from six recent observational studies (16, 17, 18, 19, 20, 21) confirm that, in line with other respiratory infections, alterations of serum sodium levels, mainly hyponatraemia, are common in COVID-19 (Table 1). A study, including 4645 hospitalised patients with confirmed SARS-CoV-2 admitted to four New York City hospitals, showed a 30% prevalence of hyponatraemia on admission (16). Older age and lower BMI were independent risk factors for hyponatraemia, but only a small cohort of 36 patients with a serum sodium ≤ 120 mmol/L had data regarding its aetiology (16). In this subgroup, hypovolaemic hyponatraemia and SIADH were equally prevalent, affecting 36% of cases each, in addition, 22% were classified as euvolaemic hyponatraemia owing to low solute intake and 6% as hypervolaemic hyponatraemia (16). Another study of 1254 patients in China showed a 9.9% prevalence of hyponatraemia on admission, although without stating the aetiology of hyponatraemia (17). Hyponatraemia was associated with older age, more co-morbidities, more severe radiological lung findings, and higher levels of neutrophils and CRP (17). Analysis of the international HOPE-COVID-19 registry (Health Outcome Predictive Evaluation for COVID-19), including 4664 hospitalised adults with pneumonia and a positive reverse-transcriptase PCR (RT-PCR) for SARS-CoV-2 (19), reported a prevalence for mild/moderate to severe hyponatraemia (serum sodium 130–134 mmol/L and <130 mmol/L) of 16.7 and 3.8%, respectively (19). Age ≥ 70 years, male gender, chronic kidney disease, tachypnoea, and bilateral pneumonia were risk factors for hyponatraemia (19). An observational cohort study, comparing the prevalence of dysnatraemia and its prognostic impact between cases of COVD-19 and a control group of patients with suspected COVID-19 and similar symptoms, but negative SARS-CoV-2 PCR testing, showed that patients with COVID-19 had a two-fold higher rate of hyponatraemia on admission (20). The only longitudinal study of sodium abnormalities in patients with COVID-19 has reported that almost two-thirds of inpatients experienced dysnatraemia, with 36.9% of patients developing hyponatremia, 10.9% hypernatraemia, and 14.3% both hypernatremia and hyponatremia (21). Using a plasma urea concentration of 5mmol/L as the cut-off value to differentiate euvo- from hypovolaemic hyponatraemia, 56% of cases were classified as probable hypovolaemic and 44% as non-hypovolemic hyponatremia (21). However, there are only limited data regarding the different causes of hyponatraemia, suggesting that SIADH and hypovolaemic hyponatraemia are the commonest causes in COVID-19 (16, 21).
Association of sodium with disease severity, need for ventilation, and mortality
Many studies have demonstrated the link between serum sodium values on admission with the likelihood of progression to severe illness. Three meta-analyses have reported that patients who develop severe or critical COVID-19 have significantly lower serum sodium concentrations compared to those who do not develop severe COVID-19 (12, 13, 22). These studies indicated a significant association between severe COVID-19 and lower serum sodium concentrations on admission, but the clinical relevance of these small differences of sodium levels, in the range of 0.91–1.97 mmol/L, between patients with severe and non-severe illness, remains questionable.
Table 2 summarises the key findings regarding the association of hyponatraemia with key clinical outcomes in COVID-19 from the six main observational studies (16, 17, 18, 19, 20, 21). It appears that hyponatraemia is associated with a greater need for any form of respiratory support (17, 21), increased likelihood of invasive mechanical ventilation (IMV) with an odds ratio (OR) from 1.83 to 3.30 (16, 18, 20), higher ICU admission rates (OR 2.80–3.73) (18, 20), and the more frequent development of severe sepsis (19) compared to normonatraemic patients.
Table 2.
Association of hyponatraemia on admission with ICU admission, need for mechanical ventilation, and mortality rate in main observational studies.
| Study | ICU admission rate | Applies to | Need for IMV | Mortality | |||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | Cases vs normal Na | OR (95% CI) | P | Cases vs normal Na | OR (95% CI) | P | ||
| Frontera et al. (1) | N/A | Na <130 mmol/L | 46.6% vs 19.4% | 1.83 (1.50–2.25 | <0.001 | 26.6% vs 13.2% | 1.43 (1.08–1.88) | 0.012 | |
| Hu et al. (2) | N/A | Any form of respiratory support‡ | 87.1% vs 60.1% | <0.001 | 16.1% vs 6.3% | <0.001 | |||
| Tezcan et al. (3) | 3.73 (1.93–7.21) | <0.001 | 3.20 (1.47– 6.99) | 0.003 | 10.33 (1.62–65.62) | 0.01 | |||
| HOPE-COVID-19 (4) | Composite endpoint of ICU, IMV | 1.35 (1.02–1.78) | 0.035 | 1.73 (1.28–2.34) | <0.001 | ||||
| Atila et al. (5) | 2.80 (1.64–4.88) | <0.001 | 3.30 (1.67– 6.63)* | <0.001 | 1.40 (1.10–16.62)† | 0.05 | |||
| Tzoulis et al. (6) | N/A | NIV or IMV | 2.18 (1.34– 3.46) | 0.0011 | NS | ||||
‡Oxygen, NIV, IMV; *MV only; †Value is HR (95% CI).
ICU, intensive care unit; IMV, invasive mechanical ventilation; NIV, non-invasive ventilation; N/A, not applicable; NS, no significant association.
Several studies have also identified hyponatraemia on admission as an independent risk factor for mortality, with ORs of 1.40 (20), 1.43 (16), 1.73 (19), and 10.33 (18). Despite the higher mortality rate in hypovolaemic or hypervolaemic hyponatraemia compared to that related to SIADH in general hospital populations (23), most studies in the context of COVID-19 have not classified hyponatraemia into subtypes according to volume status. The only study which differentiated hyponatraemia according to volume status did not find an association for hyponatraemia with excess mortality but reported that an increased mortality rate was found only in patients with hypovolaemic hyponatraemia that was independent of acute kidney injury (21). This raises the question as to whether all types of hyponatraemia are linked with excess mortality, and whether the relative risks differ.
Hypernatraemia is also associated with poor prognosis, such as excess in-hospital mortality, ORs of 2.38 (19), 3.05 (21), and 11.50 (20) and seems to be a worse prognostic factor than hyponatraemia in patients with COVID-19 (20) (21). A plausible explanation could be that hypernatraemia affects disproportionally the oldest and most frail individuals who are at the highest risk of death due to COVID-19.
Association of hyponatraemia with the length of stay and acute kidney injury
Contrary to the well-established significant association of hyponatraemia with the length of hospitalisation in patients with CAP (4), there have been mixed data regarding the relationship of sodium serum abnormalities with the length of hospital stay in patients with COVID-19. Two studies have found no association between sodium levels and the length of stay in patients with COVID-19 (16, 19), whereas three studies (17, 18, 20) have reported a significantly longer duration of hospitalisation in patients with COVID-19 and baseline hyponatraemia compared to those with normal sodium levels (median length of stay 20 vs 17 days, P = 0.022 (17); mean length of stay 8.7 vs 7.2 days, P = 0.001 (18). This was probably related to the varying criteria determining the length of hospitalisation in various countries and facilities.
Acute kidney injury is common in hospitalised patients with COVID-19, with a prevalence of around 37% (24). Studies have shown contradictory results about a possible link of hyponatraemia with AKI, with some reporting no association (16, 21), whereas others have documented a higher frequency of acute kidney injury (AKI) in relation to hyponatraemia (17). The distribution of types of hyponatraemia in those with AKI is similar to that in those without AKI (21).
Risk calculators
As hospitals around the world have been facing an increased influx of patients with COVID-19, there has been an urgent need to develop pragmatic risk stratification tools, incorporating demographic, clinical, radiological, and laboratory parameters, to detect patients at high risk of severe illness and thus optimise resource allocation (25). A critical appraisal of 50 prognostic models showed that the most frequently used prognostic factors are age, gender, number of co-morbidities, imaging features, lymphocyte count, serum CRP, and creatinine, with only two including sodium levels (26). Serum sodium levels are not incorporated in most risk stratification tools and do not currently influence real-life decision-making about the therapeutic strategy in COVID-19 patients.
Pathophysiology of hyponatraemia
The main aetiology of euvolaemic hyponatraemia in patients with COVID-19 is SIADH via four potential mechanisms. First, increased levels of cytokines, such as IL-6, can directly stimulate the non-osmotic release of arginine vasopressin (AVP) (27, 28). Secondly, the injury to lung tissue and alveolar cells can result in a ventilation-perfusion mismatch and compensatory hypoxic pulmonary vasoconstriction leading to inadequate filling of the left atrium, decreased left atrial stretch, and increased AVP secretion (29). Thirdly, patients with COVID-19 pneumonia, through various stimuli, such as pain, nausea, and medications, can stimulate the direct release of AVP. Fourthly, patients receiving positive pressure ventilation (PPV) can have non-osmotic stimulation of AVP secretion, as pulmonary baroreceptors respond to a reduction in effective arterial blood volume (29).
Euvolaemic hyponatraemia can also be attributed to low solute intake, as reported in 22% of COVID-19 patients with severe hyponatraemia (16). An example is the so-called 'tea and toast' diet, commonly experienced by elderly people who are unable to prepare meals at home and eat simple foods with poor protein and salt content. It is characterised by minimal oral solute intake and subsequent low urinary solute excretion, limiting the nephrons’ capacity for solute-free water excretion and resulting in dilutional hyponatraemia (30). Another cause is the development of the adrenal crisis in patients with secondary or tertiary adrenal insufficiency without adequate glucocorticoid treatment during acute COVID-19 infection (31). Observational data suggest that the normal stress response in hospitalised patients with COVID-19 consists of a marked cortisol release, higher than that observed in individuals with similar symptoms but without COVID-19 (15). In light of the COVID-19-related persistent and significant inflammatory response, patients with a suppressed hypothalamo–pituitary–adrenal axis have a high risk of glucocorticoid deficiency, which may lead to 'inappropriate' AVP release and hyponatraemia (31).
Hypovolaemic hyponatraemia is also commonly observed in COVID-19, characterised by depletion of circulating volume triggering baroreceptor-mediated non-osmotic AVP release (32). The high frequency of volume depletion in COVID-19 illness may be explained by an increase in insensible fluid losses due to pyrexia and tachypnoea, limited oral intake, and possible gastrointestinal manifestations of COVID-19, such as vomiting or diarrhoea (16), the use of diuretics, or overly conservative i.v. fluid administration (33). Finally, patients with COVID-19 may have hypervolaemic hyponatraemia, due to pre-existing conditions such as heart failure, cirrhosis, or nephrotic syndrome.
Link of sodium with inflammation
The main underlying mechanism involved in the development of hyponatraemia associated with inflammatory conditions is that pro-inflammatory cytokines induce the non-osmotic release of AVP, with IL-6 levels being inversely correlated with the sodium concentration (28, 34). Therefore, hyponatraemia is a good surrogate marker of the degree of inflammatory response and tends to reflect the severity of various infections, including pneumonia, tuberculosis, meningitis, encephalitis, HIV infection, and malaria (35).
A small retrospective study of 29 patients with COVID-19 showed that individuals with high serum IL-6 levels had a significantly lower median serum sodium of 133.1 mmol/L vs 139.6 mmol/L compared to those with normal IL-6 levels, while serum sodium concentration was inversely related to IL-6 levels (36). In addition, administration of tocilizumab, an IL-6 antagonist, to hyponatraemic patients with abnormal IL-6 levels led to a significant 48-h increase in serum sodium from 132.4 mmol/L to 139.6 mmol/L (36). Another larger study showed that the higher the IL-6 levels, the lower the serum sodium concentration, confirming this significant inverse relationship between serum sodium and IL-6 levels (16).
High levels of IL-6, and other cytokines such as IL-1β, IL-10, and interferon γ, in the context of COVID-19 lead to a hyperactive immune response state, called the ‘cytokine release syndrome’ (CRS) or cytokine storm, characterised by elevated cytokine levels in the circulation, acute systemic inflammatory symptoms, and secondary organ dysfunction (37, 38). Potent immunomodulatory medications, such as dexamethasone and IL-6 antagonists, have been effectively used in patients with COVID-19 and severe respiratory complications (39, 40).
Based on the hypothesis that IL-6 receptor blockade may be able to improve patient prognosis through disruption of the cytokine storm, tocilizumab has been evaluated in several studies, with mixed negative (41, 42, 43) and positive results, but overall showing better outcomes and lower mortality rates (44, 45, 46).
Evaluation and treatment of hyponatraemia in COVID-19
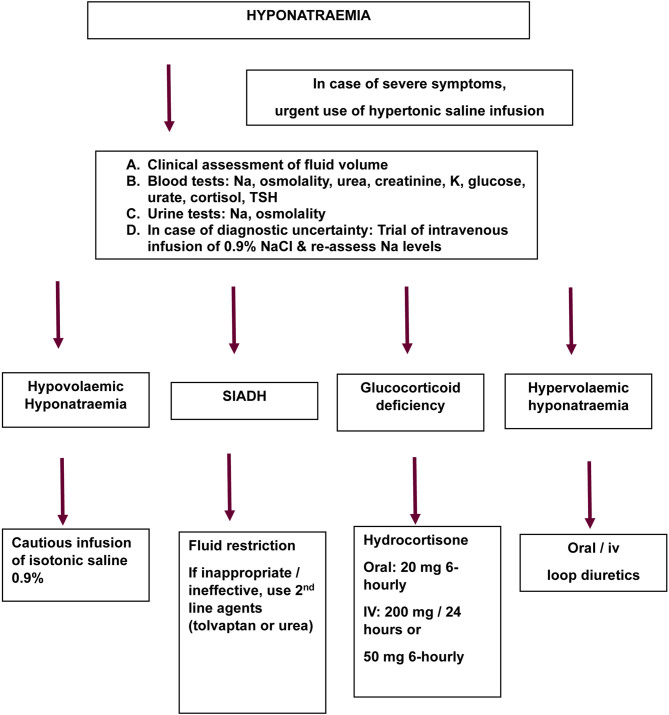
The most appropriate treatment of hyponatraemia necessitates prompt identification of its aetiology, with the therapeutic approach following the principles of managing hyponatraemia in general (Fig. 1) (30, 32). Laboratory evaluation is of paramount importance in view of the low sensitivity and specificity of clinical assessment of volume status in discriminating euvolaemic from hypovolaemic hyponatraemia, the commonest types in the context of COVID-19 (32). Endocrine workup for patients with sodium levels of <130 mmol/L should include measurement of paired serum and urine osmolality and sodium as well as serum glucose, urea, creatinine, urate, cortisol, thyroxine, and thyroid-stimulating hormone (TSH) concentrations (30, 32, 47). Serum urate levels can be useful in distinguishing SIADH from hypovolaemic hyponatraemia, while in cases of diagnostic uncertainty, a trial of volume expansion with i.v. administration of isotonic saline can be a valuable diagnostic and, sometimes in cases of volume depletion, therapeutic tool (30, 47). Although random serum cortisol should be measured to detect new cases of primary or secondary adrenal insufficiency, the serum cortisol concentration cannot be interpreted in patients who are receiving exogenous glucocorticoids, either as part of treatment for acute COVID-19 infection (39) or for pre-existing conditions
Figure 1.
Algorithm for investigation and management of hyponatraemia in patients with COVID-19.
Regardless of aetiology, patients with severe symptoms of hyponatraemia, such as seizures, coma, or reduced Glasgow Coma Score, should be promptly treated with hypertonic saline in order to prevent brain herniation, or even, death (30, 32). Evidence and clinical experience are lacking about the optimal form of hypertonic saline treatment in patients with COVID-19. In contrast to contemporary guidelines (30, 32) and recent studies (48) favouring the use of hypertonic saline boluses, continuous infusion of hypertonic saline might be preferable in the context of COVID-19 in order to minimise the risk of pulmonary oedema due to the sudden volume increase (33).
A significant proportion of patients with COVID-19 might not be suitable candidates for fluid restriction, the mainstay of treatment in the general hospital population (30, 32, 47), due to malnutrition, impaired sense of taste, and increased fluid losses due to pyrexia, tachypnoea, and, sometimes, vomiting and diarrhoea (49). In these patients, pharmacotherapy, either tolvaptan or possibly urea, should be considered (30, 47); however, there is very little clinical experience in the use of these agents for the treatment of SIADH patients with COVID-19.
Future studies
The high prevalence of hyponatraemia and association with poor outcomes in patients with COVID-19 highlight the need for prospective intervention studies in order to determine whether correction of sodium abnormalities might improve clinical outcomes. Specifically, the effectiveness and safety of therapeutic strategies, such as fluid restriction, tolvaptan, and urea, should be evaluated in the context of COVID-19-related SIADH. Studies are needed to explore the pathophysiological basis of hyponatraemia in patients with COVID-19, the frequency of different types of hyponatraemia and their prognostic impact. In order to advance our understanding of the link between inflammatory response and hyponatraemia, prospective studies are warranted to examine longitudinally the serum IL-6 levels, serum sodium concentration, inflammatory markers, and severity of the clinical condition. In addition, the added value of incorporating serum sodium in current risk stratification scores needs to be further explored. These studies will be of value not only in the context of COVID-19 but may also be of value in future pandemics with related viruses.
Conclusions
Hyponatraemia is highly prevalent at hospital admission in patients with COVID-19 and presents an independent predictor for severe disease, mortality, need for ICU admission, and IMV. In most patients, hyponatraemia is either euvolaemic due to SIADH, mainly owing to IL-6-induced AVP release, or hypovolaemic due to significant insensible fluid loss. Hypernatraemia is also associated with poor clinical outcomes and seems to be an even worse prognostic factor than hyponatraemia.
There is an inverse correlation between sodium and IL-6 levels, suggesting a role for serum sodium as a marker of the inflammatory response. It remains to be seen whether serum sodium should be incorporated in risk stratification scores to enhance their prognostic performance and might also be used for the prompt identification of patients at risk of cytokine storm who may benefit from the early initiation of immunomodulatory agents, such as IL-6 antagonists. It still remains unknown whether correction of hyponatraemia may improve clinical outcomes in patients with COVID-19.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
References
- 1. Wald R, Jaber BL, Price LL, Upadhyay A, Madias NE. Impact of hospital-associated hyponatremia on selected outcomes. Archives of Internal Medicine 2010170294–302. ( 10.1001/archinternmed.2009.513) [DOI] [PubMed] [Google Scholar]
- 2. Tzoulis P, Bagkeris E, Bouloux PM. A case-control study of hyponatraemia as an independent risk factor for inpatient mortality. Clinical Endocrinology 201481401–407. ( 10.1111/cen.12429) [DOI] [PubMed] [Google Scholar]
- 3. Cuesta M, Slattery D, Goulden EL, Gupta S, Tatro E, Sherlock M, Tormey W, O’Neill S, Thompson CJ. Hyponatraemia in patients with community-acquired pneumonia; prevalence and aetiology, and natural history of SIAD. Clinical Endocrinology 201990744–752. ( 10.1111/cen.13937) [DOI] [PubMed] [Google Scholar]
- 4. Kruger S, Ewig S, Giersdorf S, Hartmann O, Frechen D, Rohde G, Suttorp N, Welte T & CAPNETZ Study Group. Dysnatremia, vasopressin, atrial natriuretic peptide and mortality in patients with community-acquired pneumonia: results from the German Competence Network CAPNETZ. Respiratory Medicine 20141081696–1705. ( 10.1016/j.rmed.2014.09.014) [DOI] [PubMed] [Google Scholar]
- 5. Muller M, Schefold JC, Guignard V, Exadaktylos AK, Pfortmueller CA. Hyponatraemia is independently associated with in-hospital mortality in patients with pneumonia. European Journal of Internal Medicine 20185446–52. ( 10.1016/j.ejim.2018.04.008) [DOI] [PubMed] [Google Scholar]
- 6. Paterson RW, Brown RL, Benjamin L, Nortley R, Wiethoff S, Bharucha T, Jayaseelan DL, Kumar G, Raftopoulos RE, Zambreanu L et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain 20201433104–3120. ( 10.1093/brain/awaa240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 20203231239–1242. ( 10.1001/jama.2020.2648) [DOI] [PubMed] [Google Scholar]
- 8. Petrilli CM, Jones SA, Yang J, Rajagopalan H, O’Donnell L, Chernyak Y, Tobin KA, Certfolio RJ, Francois F, Horwitz LI. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ 2020369 m1966. ( 10.1136/bmj.m1966) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liao D, Zhou F, Luo L, Xu M, Wang H, Xia J, Gao Y, Cai L, Wang Z, Yin P et al. Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: a retrospective cohort study. Lancet: Haematology 20207e671–e678. ( 10.1016/S2352-3026(20)30217-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Du RH, Liang LR, Yang CQ, Wang W, Cao TZ, Li M, Guo GY, Du J, Zheng CL, Zhu Q et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. European Respiratory Journal 202055 2000524. ( 10.1183/13993003.00524-2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lippi G, Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clinical Chemistry and Laboratory Medicine 2020581131–1134. ( 10.1515/cclm-2020-0198) [DOI] [PubMed] [Google Scholar]
- 12. Mudatsir M, Fajar JK, Wulandari L, Soegiarto G, Ilmawan M, Purnamasari Y, Mahdi BA, Jayanto GD, Suhendra S, Setianingsih YA et al. Predictors of COVID-19 severity: a systematic review and meta-analysis. F1000Research 20209 1107. ( 10.12688/f1000research.26186.2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moutchia J, Pokharel P, Kerri A, McGaw K, Uchai S, Nji M, Goodman M. Clinical Laboratory parameters associated with severe or critical novel coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. PLoS ONE 202015 e0239802. ( 10.1371/journal.pone.0239802) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang S, Ma P, Zhang S, Song S, Wang Z, Ma Y, Xu J, Wu F, Duan L, Yin Z et al. Fasting blood glucose at admission is an independent predictor for 28-day mortality in patients with COVID-19 without previous diagnosis of diabetes: a multi-centre retrospective study. Diabetologia 2020632102–2111. ( 10.1007/s00125-020-05209-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tan T, Khoo B, Mills EG, Phylactou M, Patel B, Eng PC, Thurston L, Muzi B, Meeran K, Prevost AT et al. Association between high serum total cortisol concentrations and mortality from COVID-19. Lancet: Diabetes and Endocrinology 20208659–660. ( 10.1016/S2213-8587(20)30216-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Frontera JA, Valdes E, Huang J, Lewis A, Lord AS, Zhou T, Kahn DE, Melmed K, Czeisler BM, Yaghi S et al. Prevalence and impact of hyponatremia in patients with coronavirus disease 2019 in New York City. Critical Care Medicine 202048e1211–e1217. ( 10.1097/CCM.0000000000004605) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hu W, Lv X, Li C, Xu Y, Qi Y, Zhang Z, Li M, Cai F, Liu D, Yue J et al. Disorders of sodium balance and its clinical implications in COVID-19 patients: a multicenter retrospective study. Internal and Emergency Medicine 202116853–862. ( 10.1007/s11739-020-02515-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tezcan ME, Dogan Gokce G, Sen N, Zorlutuna Kaymak N, Ozer RS. Baseline electrolyte abnormalities would be related to poor prognosis in hospitalized COVID-19 patients. New Microbes and New Infections 202037100753. ( 10.1016/j.nmni.2020.100753) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ruiz-Sanchez JG, Nunez-Gil IJ, Cuesta M, Rubio MA, Maroun-Eid C, Arroyo-Espliguero R, Romero R, Beccera-Munoz VM, Uribarri A, Feltes G et al. Prognostic impact of hyponatremia and hypernatremia in COVID-19 pneumonia: a HOPE-COVID-19 (health outcome predictive evaluation for COVID-19) registry analysis. Frontiers in Endocrinology 202011599255. ( 10.3389/fendo.2020.599255) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Atila C, Sailer CO, Bassetti S, Tschudin-Sutter S, Bingisser R, Siegemund M, Osswald S, Rentsch K, Ruegg M, Schaerli S et al. Prevalence and outcome of dysnatremia in patients with COVID-19 compared to controls. European Journal of Endocrinology 2021184409–418. ( 10.1530/EJE-20-1374) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tzoulis P, Waung JA, Bagkeris E, Hussein Z, Biddanda A, Cousins J, Dewsnip A, Falayi K, McCaughran W, Mullins C et al. Dysnatremia is a predictor for morbidity and mortality in hospitalized patients with COVID-19. Journal of Clinical Endocrinology and Metabolism 20211061637–1648. ( 10.1210/clinem/dgab107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lippi G, South AM, Henry BM. Electrolyte imbalances in patients with severe coronavirus disease 2019 (COVID-19). Annals of Clinical Biochemistry 202057262–265. ( 10.1177/0004563220922255) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cuesta M, Garrahy A, Slattery D, Gupta S, Hannon AM, McGurren K, Sherlock M, Tormey W, Thompson CJ. Mortality rates are lower in siad, than in hypervolaemic or hypovolaemic hyponatraemia; results of a prospective observational study. Clinical Endocrinology 201787400–406. ( 10.1111/cen.13388) [DOI] [PubMed] [Google Scholar]
- 24. Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, Hazzan AD, Fishbane S, Jhaveri KDNorthwell Covid-Research Consortium et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney International 202098209–218. ( 10.1016/j.kint.2020.05.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liang W, Liang H, Ou L, Chen B, Chen A, Li C, Li Y, Guan W, Sang L, Lu J et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Internal Medicine 20201801081–1089. ( 10.1001/jamainternmed.2020.2033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wynants L, Van Calster B, Collins GS, Riley RD, Heinze G, Schuit E, Bonten MMJ, Damen JAA, Debray TPA, De Vos M et al. Prediction models for diagnosis and prognosis of covid-19 infection: systematic review and critical appraisal. BMJ 2020369 m1328. ( 10.1136/bmj.m1328) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yasin SA, Costa A, Forsling ML, Grossman A. Interleukin-1 beta and interleukin-6 stimulate neurohypophysial hormone release in vitro. Journal of Neuroendocrinology 19946179–184. ( 10.1111/j.1365-2826.1994.tb00570.x) [DOI] [PubMed] [Google Scholar]
- 28. Park SJ, Shin JI. Inflammation and hyponatremia: an underrecognized condition? Korean Journal of Pediatrics 201356519–522. ( 10.3345/kjp.2013.56.12.519) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yousaf Z, Al-Shokri SD, Al-Soub H, Mohamed MFH. Covid-19 associated SIADH: a clue in the times of pandemic! American Journal of Physiology, Endocrinology and Metabolism 2020318E882–E885. ( 10.1152/ajpendo.00178.2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Verbalis JG, Goldsmith SR, Greenberg A, Korzelius C, Schrier RW, Sterns RH, Thompson CJ. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. American Journal of Medicine 2013126 (Supplement 1) S1–S42. ( 10.1016/j.amjmed.2013.07.006) [DOI] [PubMed] [Google Scholar]
- 31. Arlt W, Baldeweg SE, Pearce SHS, Simpson HL. Endocrinology in the time of COVID-19: management of adrenal insufficiency. European Journal of Endocrinology 2020183G25–G32. ( 10.1530/EJE-20-0361) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Spasovski G, Vanholder R, Allolio B, Annane D, Ball S, Bichet D, Decaux G, Fenske W, Hoorn EJ, Ichai C et al. Clinical practice guideline on diagnosis and treatment of hyponatraemia. European Journal of Endocrinology 2014170G1–G47. ( 10.1530/EJE-13-1020) [DOI] [PubMed] [Google Scholar]
- 33. Christ Crain M, Hoorn EJ, Sherlock M, Thompson CJ, Wass JAH. Endocrinology in the time of COVID-19: management of hyponatraemia and diabetes insipidus. European Journal of Endocrinology 2020183G9–G15. ( 10.1530/EJE-20-0338) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Park SJ, Oh YS, Choi MJ, Shin JI, Kim KH. Hyponatremia may reflect severe inflammation in children with febrile urinary tract infection. Pediatric Nephrology 2012272261–2267. ( 10.1007/s00467-012-2267-9) [DOI] [PubMed] [Google Scholar]
- 35. Swart RM, Hoorn EJ, Betjes MG, Zietse R. Hyponatremia and inflammation: the emerging role of interleukin-6 in osmoregulation. Nephron Physiology 201111845–51. ( 10.1159/000322238) [DOI] [PubMed] [Google Scholar]
- 36. Berni A, Malandrino D, Parenti G, Maggi M, Poggesi L, Peri A. Hyponatremia, IL-6, and SARS-CoV-2 (COVID-19) infection: may all fit together? Journal of Endocrinological Investigation 2020431137–1139. ( 10.1007/s40618-020-01301-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sinha P, Matthay MA, Calfee CS. Is a ‘cytokine storm’ relevant to COVID-19? JAMA Internal Medicine 20201801152–1154. ( 10.1001/jamainternmed.2020.3313) [DOI] [PubMed] [Google Scholar]
- 38. Fajgenbaum DC, June CH. Cytokine storm. New England Journal of Medicine 20203832255–2273. ( 10.1056/NEJMra2026131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski Aet al. Dexamethasone in hospitalized patients with Covid-19. New England Journal of Medicine 2021384693–704. ( 10.1056/NEJMoa2021436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Parr JB Time to reassess tocilizumab’s role in COVID-19 pneumonia. JAMA Internal Medicine 202118112–15. ( 10.1001/jamainternmed.2020.6557) [DOI] [PubMed] [Google Scholar]
- 41. Stone JH, Frigault MJ, Serling-Boyd NJ, Fernandes AD, Harvey L, Foulkes AS, Horick NK, Healy BC, Shah R, Bensaci AM et al. Efficacy of tocilizumab in patients hospitalized with Covid-19. New England Journal of Medicine 20203832333–2344. ( 10.1056/NEJMoa2028836) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Salama C, Han J, Yau L, Reiss WG, Kramer B, Neidhart JD, Criner GJ, Kaplan-Lewis E, Baden R, Pandit L et al. Tocilizumab in patients hospitalized with Covid-19 pneumonia. New England Journal of Medicine 202138420–30. ( 10.1056/NEJMoa2030340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rosas IO, Brau N, Waters M, Go RC, Hunter BD, Bhagani S, Skiest D, Aziz MS, Cooper N, Douglas IS et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. New England Journal of Medicine 20213841503–1516. ( 10.1056/NEJMoa2028700) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gupta S, Wang W, Hayek SS, Chan L, Mathews KS, Melamed ML, Brenner SK, Leonberg-Yoo A, Schenck EJ, Radbel J et al. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Internal Medicine 202118141–51. ( 10.1001/jamainternmed.2020.6252) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.The REMAP-CAP Investigators, Gordon AC, Mouncey PR, Al-Beidh F, Rowan KM, Nichol AD, Arabi YM, Annane D, Beane A, Van Bentum-Puijk Wet al. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. New England Journal of Medicine 2021. ( 10.1056/NEJMoa2100433) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rubin EJ, Longo DL, Baden LR. Interleukin-6 receptor inhibition in Covid-19 – cooling the inflammatory soup. New England Journal of Medicine 20213841564–1565. ( 10.1056/NEJMe2103108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Grant P, Ayuk J, Bouloux PM, Cohen M, Cranston I, Murray RD, Rees A, Thatcher N, Grossman A. The diagnosis and management of inpatient hyponatraemia and SIADH. European Journal of Clinical Investigation 201545888–894. ( 10.1111/eci.12465) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Garrahy A, Dineen R, Hannon AM, Cuesta M, Tormey W, Sherlock M, Thompson CJ. Continuous versus bolus infusion of hypertonic saline in the treatment of symptomatic hyponatremia caused by SIAD. Journal of Clinical Endocrinology and Metabolism 20191043595–3602. ( 10.1210/jc.2019-00044) [DOI] [PubMed] [Google Scholar]
- 49. Fernandez Martinez A, Barajas Galindo D, Ruiz Sanchez J. Management of hyponatraemia and hypernatraemia during the Covid-19 pandemic: a consensus statement of the Spanish Society for Endocrinology (Acqua Neuroendocrinology Group). Reviews in Endocrine and Metabolic Disorders 202122317–324. ( 10.1007/s11154-021-09627-3) [DOI] [PMC free article] [PubMed] [Google Scholar]