Abstract
The most distinctive pathological characteristics of diabetes mellitus induced by various stressors or immune-mediated injuries are reductions of pancreatic islet β-cell populations and activity. Existing treatment strategies cannot slow disease progression; consequently, research to genetically engineer β-cell mimetics through bi-directional plasticity is ongoing. The current consensus implicates β-cell dedifferentiation as the primary etiology of reduced β-cell mass and activity. This review aims to summarize the etiology and proposed mechanisms of β-cell dedifferentiation and to explore the possibility that there might be a time interval from the onset of β-cell dysfunction caused by dedifferentiation to the development of diabetes, which may offer a therapeutic window to reduce β-cell injury and to stabilize functionality. In addition, to investigate β-cell plasticity, we review strategies for β-cell regeneration utilizing genetic programming, small molecules, cytokines, and bioengineering to transdifferentiate other cell types into β-cells; the development of biomimetic acellular constructs to generate fully functional β-cell-mimetics. However, the maturation of regenerated β-cells is currently limited. Further studies are needed to develop simple and efficient reprogramming methods for assembling perfectly functional β-cells. Future investigations are necessary to transform diabetes into a potentially curable disease.
Keywords: dedifferentiation, transdifferentiation, β-cell, diabetes
Introduction
Diabetes mellitus (DM) is an epidemic chronic disease characterized by impaired glucose homeostasis, leading to hyperglycemia and multiple complications such as cardiopathy, neuropathy, nephropathy, and retinopathy. The prevalence of DM has increased dramatically in recent decades and is projected to rise to 642 million people by 2040 (1), with profound impacts on quality of life, demands for health services, and economic costs (2). There are two common syndromes, type 1 (T1D) and type 2 diabetes (T2D), characterized by an absolute or relative deficiency of β-cells, respectively (3). A previously held belief stipulated that the fate of fully differentiated β-cells is fixed and that gradual β-cell death due to glucotoxicity was a final outcome of DM (4). Treatment ultimately depended on supplemental insulin and islet transplantation, which alleviated disease severity by reducing or normalizing glycemic levels without curing DM (4). Long-term insulin treatment carries risks of hypoglycemic episodes, weight gain, and an increased incidence of cancer (5). The effectiveness of islet transplantation is limited by a shortage of donor islets and immune rejection (6). Therefore, new therapeutic strategies are urgently needed to prevent and treat this highly prevalent metabolic disorder.
The study of β-cell maturation and physiology demonstrates the heterogeneity and plasticity of mature β-cell phenotypes and function (7). The three main β-cell phenotypes in diabetes are dedifferentiated, senescent, and transdifferentiated types (8). Glucotoxicity leads to β-cell dedifferentiation during hyperglycemia and reduces the expression of β-cell enrichment genes such as key transcription factors and genes that encode insulin; glucose metabolism, protein processing, and secretory pathways; as well as upregulation of genes that are suppressed or expressed at low levels in normal β-cells, including forbidden and progenitor cell genes (9). Under various stress conditions, mature β-cells may lose their differentiated phenotypes and return to a less differentiated or even a progenitor cell state. β-cell dedifferentiation is a potential adaptive mechanism to escape cell death during physiologic stress (10). Exploring the mechanisms of dedifferentiation may inform new strategies for the reversal of dedifferentiation and the restoration of β-cell functionality. We focus on the possible mechanisms of hyperglycemia-induced dedifferentiation that may result from the cascade of metabolic, oxidative, and endoplasmic reticulum stresses; and on epigenetic changes due to chronic stress. We also review current antidiabetic strategies and potential future research directions that may identify a time window for alleviating the β-cell stress response; thus preserving β-cell mass, regaining cell maturation, improving cellular function, and delaying disease progression.
Current research is focused on methods to increase β-cell numbers, maturity, function, and post-transplantation survival in addition to protecting existing β-cells, thus providing potential breakthroughs in the treatment of DM (11). β-Cell deficiency could be reversed by promoting cellular replication and redifferentiation during the early stages of DM (12). However, the application of these methods has been limited due to low β-cell proliferation rates, instability, and high heterogeneity. Transdifferentiation (13), defined as the phenotype switch between different cell types, obviates the shortcomings of the aforementioned methods and provides a safe and efficient approach to regeneration. The focus herein is on the eventual differentiation of non-β-cells into a β-cell phenotype. At present, most studies focus on the transdifferentiation of pancreatic non-β-cells; hepatic and biliary cells; gastrointestinal cells into β-cells through genetic programming; cytokines; and small molecules. These cells comprise the leading candidates because of their common endodermal origin with β-cells, abundant populations, and high conversion efficiency. The development of bionic technology offers an expanding range of options for β-cell regeneration.
Benefiting from excellent reviews of this field, we focus on the state of knowledge of β-cell dedifferentiation and transdifferentiation, as well as the highlights of exciting new research. Optimized protocols to augment functional mature β-cells will guide future precision medicine studies of improved treatment strategies for patients with DM and may even result in potential cures.
β-Cell dedifferentiation
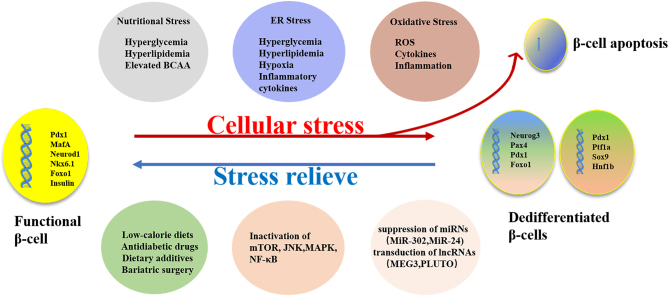
Stress response is an adaptation to environmental changes. Moderate stress responses can induce effective adaptation strategies to improve survival, while an excessive stress response will cause stress injury, leading to the onset and development of a variety of organic and psychological diseases, including DM. A growing body of evidence has shown that the onset and development of DM is closely related to metabolic, oxidative, and endoplasmic reticulum stresses and to epigenetic changes caused by chronic stress. Both T1D and T2D present with a loss of β-cell mass and identity that ultimately impair insulin secretion. The current view is that β-cell loss during the development of DM may be related to β-cell dedifferentiation rather than apoptosis, because the relatively low rate of apoptosis may not fully explain the loss of β-cell mass. β-cell dedifferentiation is characterized by decreased expression of specific genes that maintain the characteristics and function of mature β-cells and by the increased expression of endocrine precursor cell genes and additional genes that are expressed at low levels in normal β-cells. In this section, we discuss the various stresses and mechanisms that trigger dedifferentiation, as well as the currently available methods to inhibit β-cell dedifferentiation (Figs 1 and 2).
Figure 1.
β-Cells lost the mature phenotype under various stressors and dedifferentiated as an adaptive response to avoid apoptosis.
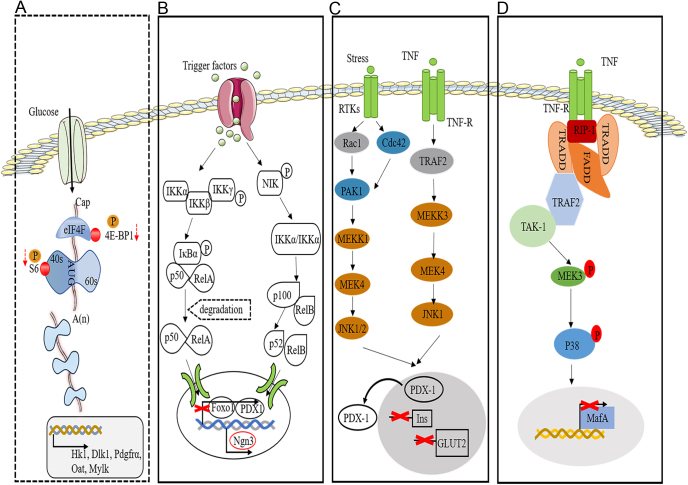
Figure 2.
Mechanisms of β-cell dedifferentiation. (A) Reduced expression of raptor-induced suppression of the mTORC1 signaling pathway increased the expressions of β-cell-specific disallowed genes (e.g. Hk1, Dlk1, Pdgfra, Oat, and Mylk). (B) Stress is a major contributor to β-cell dedifferentiation via activating Nf-κB signaling, which compromises β-cell identity and thus decreases insulin secretion. (C) The JNK pathway is activated under diabetic conditions such as stress and cytokine release, accompanied by Pdx1 nuclear translocation and suppression of insulin and GLUT2 gene expression. (D) The p38 MAPK pathway mediates the degradation of endogenous MafA during hyperglycemia. The dotted and solid boxes represent signaling pathway inhibition and activation, respectively. 4E-BP1, 4E binding protein 1; eIF4F, eukaryotic initiation factor 4F; Hk1, hexokinase 1; Oat:,ornithine aminotransferase; Pdgfrα, platelet-derived growth factor receptor α; Mylk, myosin light chain kinase; IKK, IκB kinase; IκB, nuclear factor-kappaB inhibitor alpha; NIK, Nf-κB inducing kinase; RTK, receptor tyrosine kinases; TNF, tumor necrosis factor; TRAF2, TNF receptor-associated factor 2; RIP1, receptor-interacting protein1; MEK, MAPK kinase; MEKK, MEK kinase; TRADD, TNFR1-associated death domain-containing protein; FADD, Fas-associated death domain-containing protein; TAK-1, TGF-beta-activating kinase 1.
Nutritional stress
The effects of glucose, lipid, and amino acid metabolism on pancreatic β-cells have received increasing attention (14). β-Cells gradually change from their initial adaptive stage to develop metabolic disorders and apoptosis (15). This process was previously ascribed to glucotoxicity, lipotoxicity, glucolipotoxicity, and metabolic stress; however, these proposed etiologies could not accurately reflect the state changes of β-cells in the processes of mixed-nutrient energy balance and imbalance. At present, nutritional stress is considered the most appropriate terminology (16). Transitory metabolic imbalances stimulate insulin synthesis and release (17). Once the balance is broken, usually in the context of a poor lifestyle, long-term overnutrition, and aging, β-cells confront chronic and persistent insults that are aggravated by the individual’s genetic and epigenetic composition (16). Chronic metabolic stress can inhibit transcription factors such as MAF BZIP transcription factor A (MafA), pancreatic and duodenal homeobox 1 (PDX-1), neuronal differentiation 1 (NeuroD1), and insulin gene expression, thus causing β-cell dysfunction and failure, leading to T2DM (18).
In rodent and human pancreatic islets, β-cells induced by high-glucose concentrations exhibited cellular dysfunction, decreased insulin secretion, glucose-stimulated insulin secretion (GSIS), and expression of mature β-cell genes; while expressing progenitor or precursor cell-related genes (19). Elevated glucose levels and the duration of hyperglycemia are the main factors affecting β-cell dedifferentiation (20). Zinc deficiency induced by hyperglycemia may reduce the expression of key β-cell transcription factors MafA, paired box 6 (Pax6), and NK2 homeobox 2 (NKX2.2) and promote β-cell dedifferentiation (21). β-cells (MIN6) cultured in a high-glucose environment exhibited reduced expression of vitamin D receptor (VDR). Vitamin D3 treatment can prevent β-cell dedifferentiation and increase the expression of genes encoding essential transcription factors such as Pdx1, MafA, and VDR. Notably, the expressions of insulin 1(Ins1) and insulin 2 (Ins2) are also increased (22). β-Cell absorbed free fatty acids (FFA) (including triglyceride hydrolyzed by lipoprotein lipase), very low-density lipoprotein, and low-density lipoprotein produced by endocytosis attenuate glucose toxicity (22). Thus, circulating lipid levels appear to influence not only glucose toxicity but also β-cell adaptation to hyperglycemia. Hyperglycemia and hyperlipidemia interact to impair β-cell function (23, 24). Circulating glucose and lipid levels are elevated prior to the onset of obesity-related T2D, so a reasonable suggestion is that excessive levels of these two nutrients are pathogenic. In the context of overnutrition associated with T2D, disorders of lipid homeostasis associated with hyperlipidemia and hypercholesterolemia, and often elevated plasma FFA, precede the onset of T2D in both human clinical experience and in rodent models. Multiple in vitro studies using β-cell lines (INS, MIN6, or HIT cells) or isolated rodent and human islets have shown synergistic toxicity of elevated glucose and FFA on β-cell function and survival (25, 26). A recent 6-year Canadian follow-up study showed a strong negative association between total plasma FFA levels and β-cell function (27). Similarly, another study showed that elevated plasma FFA is strongly associated with decreased β-cell function in children and adults and impaired insulin secretion rather than insulin sensitivity (28). Reduced in vivo and in vitro GSIS is associated with changes in the glyceride/fatty acid cycle (29, 30, 31, 32, 33, 34). In lipid-treated MIN6 cells, inhibition of ID1 expression can reduce the expression of islet stress genes and increase insulin secretion. ID1 is a negative regulator of insulin secretion, and its expression plays a crucial role in the etiology of β-cell dedifferentiation under conditions of glucose intolerance, insulin secretion dysfunction, and increased lipid load, providing a molecular link between chronic lipid-induced damage and β-cell dedifferentiation and dysfunction (35). None of these models demonstrated β-cell apoptosis or reduced cell mass, suggesting that β-cell dysfunction, rather than cell death, promotes the onset of DM (36). The effects of glucose and lipids on β-cells in different experiments were discriminating. Another study suggested that β-cell dedifferentiation could be induced in lipotoxic conditions with or without hyperglycemia (37). This may be related to the composition and quantity of FFA. Nutritional stress in β-cells may result not only from glucose and lipids but also from amino acids, especially branched-chain amino acids (BCAA), including leucine, isoleucine, and valine. These three amino acids, as well as tyrosine and phenylalanine, were increased while glycine was decreased in obese hyperinsulinemic patients (14, 38, 39). Elevated plasma levels of BCAA and aromatic amino acids (tyrosine and tryptophan) are associated with obesity, insulin resistance, and susceptibility to T2D. The β-cell dysfunction induced by disordered amino acid metabolism is possibly mediated through the continued activation of the mammalian target of rapamycin (mTOR) signaling and consequent mitochondrial dysfunction (40).
An increasing body of evidence suggests that reduced nutritional stress can improve β-cell function (37). Low-calorie diets (41), hypoglycemic drugs (42), dietary additives (43), or bariatric surgery (44, 45) have led to diabetic remission and/or improved insulin secretion in a significant proportion of T2D patients. We suggest that in most cases, these benefits are derived from the reversal of pancreatic β-cells dedifferentiation (37, 46).
Endoplasmic reticulum stress
Endoplasmic reticulum (ER) stress is caused primarily by reactive oxygen species (ROS) accumulation, toxic substances, and genetic mutations. Stressors usually originate from changes in the cellular internal environment and include protein misfolding, aggregation of misfolded proteins, calcium homeostasis disorders, and other ER dysfunctions (47). In prediabetes, complexes of misfolded proteins accumulate in the ER of β-cells, suggesting that the disruption of ER balance is an early event in the development of T2D (48). There is solid evidence that ER stress contributes to β-cell dysfunction in both T1D and T2D (49, 50, 51). In the context of DM, chronic ER stressors such as hyperglycemia, hyperlipidemia, hypoxia, and pro-inflammatory cytokines (TNF-α, IL-1) lead to the gradual loss of β-cell-specific transcription factors, including Pdx1, MafA, and forkhead box O1 (FoxO1), and the acquisition of endocrine progenitor cell markers such as neurogenin3 (Ngn3) and octamer-binding transcription factor 4 (Oct4) (52, 53, 54). In this setting, ER homeostasis will collapse and initiate the unfolded protein response (UPR). The UPR is a protective mechanism that maintains the balance between synthesis and degradation; supports correct folding and function; sustains protein homeostasis, or proteostasis (55). Many factors in pancreatic β-cells can disrupt the UPR balance to trigger ER stress. These include genetic mutations, cytokines, infections, excess nutrients, islet amyloid polypeptide (IAPP), and insulin resistance (IR) and can result in DM (50, 56, 57, 58). This disruption of the adaptive UPR promotes diabetic progression and β-cell dedifferentiation (59). Combinatorial signals from the three core components of the UPR (protein kinase R-like endoplasmic reticulum kinase (PERK), inositol-requiring enzyme 1 (IRE1), and activating transcription factor 6 (ATF6)) initially trigger transcriptional programs that upregulate genes encoding many of the aforementioned ER-resident protein-folding machines that play important roles in insulin biosynthesis (60, 61). Consequently, further research is needed to clarify which steps of the ER stress process are easily targeted, because activation of key proteins of the UPR may be beneficial in the treatment of T2D (62). In fact, some of the therapeutic effects of drugs currently used to treat DM may stem from their ability to regulate ER stress, target protein folding, and modulate the UPR signaling pathway. These processes may provide targets for future drug candidates such as chemical chaperone molecules 4-phenylbutyric acid and taurine-deoxycholic acid to promote correct protein folding and cellular function (63, 64).
Oxidative stress
Oxidative stress and metabolic disorders are considered the two most important pathogenic factors of insulin secretion disorder (65). The development of T2D results from a variety of cellular changes associated with oxidative stress and impaired redox signaling, usually caused by lipotoxicity and glucotoxicity due to continued overfeeding (66). Oxidative stress is not only closely related to lipotoxicity and glucotoxicity but is also associated with inflammation, ER stress, hypoxia, and mitochondrial damage in the promotion of β-cell dedifferentiation (66, 67, 68). Oxidative stress impairs pancreatic β-cell maintenance and function (69), which is widely believed to be associated with the onset of DM and diabetic complications. The consequent increase of oxidized biological components leads directly not only to pathologies such as inhibited insulin secretion but also to the induction of new cellular responses, namely programmed cell death, i.e., apoptosis. These phenomena occur in parallel after cumulative oxidative stress reaches a certain threshold (66). In T1D, ROS promote autoimmune responses, cytokine release, and inflammation-induced impairment of β-cell function (70). Drews et al. suggested that oxidative stress-mediated loss of cellular function (e.g. impaired secretion and increased insulin resistance) plays an important role in the pathogenesis of both T1D and T2D (36). Oxidative stress-related β-cell dysfunction induces cytoplasmic transposition and inactivation of transcription factors MafA, NKX6.1, and Pdx1 in the islets of T2D patients (54, 71). The expression of oxidative stress-related genes increased the release of pro-inflammatory cytokines and upregulated the expressions of pancreatic progenitor cell-specific transcription factors such as SRY-Box transcription factor 4 (SOX4), SRY-Box transcription factor 9 (SOX9), inhibitor of DNA binding (ID2), and vimentin in islet cells cultured for 3 days. These findings indicated that cells dedifferentiated under oxidative stress in vitro (72). Oxidative stress leads to dephosphorylation and inactivation of these pathways by stimulating the activity of phosphatases such as protein tyrosine phosphatase 1B (PTP1B) and SH2-containing tyrosine-protein phosphatase, thus inhibiting insulin effect (73). Free radicals not only have the aforesaid direct effects but can also indirectly activate various intracellular signaling pathways such as mTOR, nuclear factor-kappa b (Nf-κB), p38 mitogen-activated protein kinases (p38 MAPK), stress-activated protein kinase/c-Jun NH (2)-terminal kinase (JNK/SAPK), hexosamine pathways, protein kinase C (PKC), and advanced glycation end product/receptor for AGE (AGE/RAGE) interaction (74). The inactivation of mTOR signaling reduced β-cell mass and insulin secretion. The mTOR complex 1(mTORC1) was suppressed under oxidative stress, suggesting that the mTOR pathway has a potential role in β-cell dedifferentiation under oxidative stress (75, 76). p38 inhibition can reduce β-cell apoptosis and protect cellular function (77). Administration of antioxidants to β-cells may restore free radical scavenging potential and reduce oxidative stress, thereby increasing PDX-1, Ins-1, ngn3, GLUT, and IRS-1 expressions, thus promoting β-cell regeneration and subsequent pancreatic insulin release (78). Oxidative stress in the setting of diabetes activates the JNK pathway, which impairs insulin signaling, thereby increasing IRS1 serine phosphorylation and decreasing both IRS1 tyrosine phosphorylation and IRS1-associated PI3K activity. JNK activation reduces the phosphorylation of FoxO1, which in turn inhibits the expression of the insulin transcription factor Pdx1, thus lowering insulin levels and ultimately impairing β-cell function (79, 80). Activation of the p38 MAPK and JNK signaling pathways contributes to β-cell dysfunction in the pathogenesis of T2DM (81).
Commonly used antidiabetic drugs include metformin, thiazolidinediones, α-glucosidase inhibitors, insulin, glucagon-like peptide-1 (GLP-1) receptor agonists, dipeptidyl peptidase-4 inhibitors, and sodium-glucose cotransporter type 2 inhibitors (SGLT2is); which may enhance the regulation of adaptive responses to multiple stressors. Metformin has direct and indirect antioxidant and anti-inflammatory properties, inhibits the PERK/CHOP signaling pathway, reduces the ER stress response, and also prevents lipotoxic β-cell apoptosis (82, 83). Pioglitazone attenuates β-cell oxidative stress, inflammation, and ER stress by inhibiting Nf-κB activation (84). Rosiglitazone prevents oxidative stress by regulating Nf-κB activity through a PPARα-dependent mechanism (85). Exendin-4 augments cellular defenses by inducing the ER chaperone BIP and the anti-apoptotic protein JUNB (86), and also prevents β-cell dysfunction and apoptosis by inhibiting the activation of JNK and p38 MAPK signaling (87, 88). SGLT2i attenuates the oxidative stress mediated by AGE-RAGE (89). Drugs that are currently used to treat diabetes exhibit excellent performance in stabilizing glycemic levels; however, the morbidity caused by diabetic complications obliges the development of novel alternatives and the creation of new preventive protocols for patients at high risk of insulin resistance (24, 63).
The discovery of safe and effective antioxidants could yield novel therapeutic options for the treatment of DM. Significantly, vitamin E, contained in nuts, reduces cellular oxidation by reacting with lipid radicals produced in the lipid peroxidation chain. Vitamin C, obtained from green leafy vegetables and fruits, acts synergistically with vitamin E to quench ROS through antioxidant activity (90). Several herbal derivatives (i.e. curcumin, cinnamon, garlic, and resveratrol) may have potential roles in maintaining β-cell function and inhibiting oxidative injury through their antioxidant properties (90).
In addition, the interaction of multiple cellular stressors induces epigenetic changes that can disrupt cellular function and trigger β-cell dedifferentiation. DNA methylation, histone modification, and noncoding RNA (ncRNA)-mediated gene regulation are examples of epigenetic mechanisms (91). A role of ncRNAs in epigenetic inheritance has been suggested recently (91). The function of ncRNAs, including miRNAs and long noncoding RNAs (lncRNAs), in β-cell dedifferentiation, has attracted increasing attention (91). lncRNA is abundant in β-cells and plays a vital role in differentiation (92). β-cell-specific lncRNAs interact with transcription factors to orchestrate transcription networks. lncRNAs could represent therapeutic targets to mitigate β-cell dysfunction. MiR-24 may trigger β-cell dedifferentiation (93). Ectopic MiR-24 expression in Min6 cells and primary islets increases Ngn3 and SOX9 expression and also inhibits its direct target Ire1α, which consequently reduces XBP1 and ATF4 expressions. MiR-302 upregulation simultaneously suppresses the expression of several β-cell identity genes such as NeuroD1, peroxisome proliferator-activated receptor α (PPARα), and lysine acetyltransferase 2B (Kat2B), suggesting a role of MiR-302 as a therapeutic target to prevent β-cell dedifferentiation (94). Defects in β-cell-specific lncRNAs cause DM in humans (95). MEG3, an lncRNA associated with normal β-cell function, acts as a unique controller to decrease MafA and Pdx-1 expressions. A crucial mechanistic role of PLUTO in preventing β-cell dedifferentiation is highly likely (9). PLUTO promotes Pdx1 expression by facilitating binding between the Pdx1 promoter and its enhancer cluster (95). Novel lncRNAs will probably be characterized in the near future to further elucidate their regulation of pancreatic development and β-cell function (96). The role of lncRNAs in β-cell dedifferentiation is a fascinating research topic that deserves further exploration.
Although existing therapies improve glycemic control effectively, the increasing prevalence of serious diabetic complications suggests that targeting existing β-cell populations is inadequate. The exploitation of new resources to produce β-like cells may generate solutions to replenish depleted β-cells. Transdifferentiation offers a promising option.
β-Cell transdifferentiation
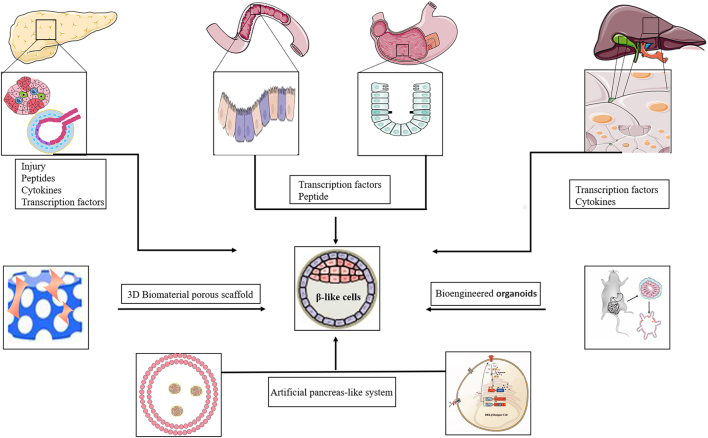
Cellular transdifferentiation, also known as lineage reprogramming, can be used to regenerate β-cells (97). Since hepatic, gastrointestinal, and pancreatic exocrine cells are derived from common endodermal progenitor cells, the transdifferentiation of developmentally related cells into β-cells can be easily accomplished. Because of identical developmental transcription mechanisms, similar epigenetic landscapes, and unique locations of endogenous cells, only a small portion of the epigenome needs to be rearranged, thus providing an attractive process for cellular reprogramming (98). The efficacy of particular technical strategies and the feasibility of using specific cell types for transdifferentiation are key questions that have been explored in exciting new studies of pancreatic β-cell regeneration (11) (Fig. 3 and Table 1).
Figure 3.
Alternative sources of β-cells by transdifferentiation from other cell types.
Table 1.
Promising strategies for pancreatic β-cell regeneration.
| Cell types | Instructive strategies | Advantages | Limitations and challenges | Functioning validity (weeks) | Involved mechanisms | Refs |
|---|---|---|---|---|---|---|
| Pancreatic non-β-cells | ||||||
| Acinar cells | Injury (PDL, PPX); Transduction of pancreatic transcription factors MafA, PDX1 and Ngn3/ NeuroD1; Administration of cytokines EGF, CNTF, nicotinamide, LIF; Peptide (GLP-1) | Similar developmental background to β-cells in vivo; Large proportion and flexible plasticity; Share quantity of transcription factors; Corresponding metabolic mechanism and hormone secretion | Low conversion rate; Weak proliferative capacity and stability; Immature morphology and biological function; The reprogramming effectiveness and efficiency with or without gene manipulation need to be improved | 2 | PI3K/Akt pathways; MAPK/STAT3 signaling pathways; Erk1/2 signaling pathways | (101, 111, 112, 113) |
| Duct cells | Injury (PDL, PPX); Ectopic overexpression of MafA, PDX1 and Ngn3/ NeuroD1, Pax6 needed for human ductal reprogramming; Administration of cytokines TGFα, DNA methyltransferase inhibitor; Peptide (GLP-1, gastrin) | 5–12 | (100, 107, 114, 115) | |||
| α and δ cells | Almost complete ablation of β-cells (PDL, PPX, diphtheria toxin); Overexpression of Pdx1, PAX4, MafA; Suppression of Arx; Peptides (GLP-1, GABA, artemisinin) | 4–16 | (98, 99, 102, 103, 104, 108, 109, 110) | |||
| Liver cells | Ectopically overexpressing PDX1 and NeuroD1; Down-regulating the expression of HNF1α and HNF4α; Specific factors (GLP-1R, Notch inhibitors, TGF-β inhibitors) | Conveniently accessible; Sufficient source and cultivate easily; Great regeneration and conversion ability; Share common characteristics including responsiveness to glucose, and mass of specific transcription factors | Efficient viral transfection strategies accompanied with safety concerns; Exploring cytokine or chemical induced safe and effective transition is prospective | 4–8 | Wnt signaling pathway | (119, 120, 121) |
| Biliary cells | Transduction of pancreatic transcription factors Pdx1, NeuroD1, Ngn3, MafA or Pdx1/VP16; Excision of Hes1 | Conveniently accessible | Limited source of cells; Transient transformation; Expand cell source and optimize reprogramming protocol need to be investigated | 3–4 | Notch signaling pathway | (122, 123, 124, 125) |
| Gastrointestinal cells | ||||||
| Intestinal cells | Ectopic expression of Pdx1, MafA, and Ngn3; Excision of transcription factor FoxO1; Peptide (GLP-1); ‘Small intestinal organ’ | Simple, non-invasive, easy to access; Sufficient source and cultivate easily; Great regeneration and conversion ability; Similar glucose sensitive system and secretion mechanism with islet cells | Low conversion rate | 1–3 | PI3K/Akt/FoxO1 pathways | (126, 128, 129) |
| Glandular cells | Ectopic expression of Pdx1, MafA, and Ngn3; ‘Stomach mini-organs’ | 3–6 | (134, 136, 138) |
Transdifferentiation of pancreatic non-β-cells to β-cells
Pancreatic non-β-cells, such as α, δ, acinar, and duct cells, share developmental histories and have similar epigenetic profiles. Because they may share common pathways during transdifferentiation into β-cells (97), we summarize their processes here. α-cell to β-cell transdifferentiation was observed upon β-cell loss in mice from puberty to adulthood (13). Near-total in vivo ablation of β-cells can be induced by diphtheria toxin (99), pancreatic duct ligation (PDL), and partial pancreatectomy (PPX) (98). PDL and PPX promote β-cell transdifferentiation not only from α-cells but also from duct (100) and acinar cells (101) in murine models. However, cell reprogramming is technically difficult. Current transdifferentiation strategies lack uniform methods. Efficiencies are usually low, and reprogrammed cells may exhibit unstable or immature phenotypes (97). Regulation of transcription factor expression and the use of several drugs offer more promising options. MafA and Pdx1 are essential transcription factors that interact with other factors to regulate transdifferentiation. The ablation of the Aristaless-related homeobox gene (Arx) (102) or the overexpression of PAX4 (103), MafA, and Pdx1 (104) promotes the transdifferentiation of β-cells from α-cells. Transdifferentiation from adult murine pancreatic duct (105) and acinar cells (106) to β-cells can be induced by MafA, Pdx1, and Ngn3/NeuroD, while induction of Pax6 was also needed to reprogram human ductal cells into β-cells (107). GLP-1 (108) may transform α-, duct, and acinar cells into cells with a β-cell-like phenotype. GABA (109) and artemisinins (110) promote the transdifferentiation of α-cells into β-cells. EGF in combination with ciliary neurotrophic factor (CNTF), nicotinamide, and leukemic inhibitory factor (LIF) (111, 112, 113) can also induce transdifferentiation of pancreatic acinar cells to insulin-producing cells in culture. Other growth factors such as gastrin and transforming growth factor-α (TGF-α) (114) as well as the DNA methyltransferase inhibitor 5-aza-2ʹ-deoxycytidine (115) induced Pdx1 expression in ductal cells to promote endocrine differentiation. These processes may be related to the PI3K/AKT/FOXO1 (116) and MAPK/STAT3 signaling pathways (117). The aforementioned results indicate that overexpression of endocrine genes, inhibition of exocrine genes, and treatment with cytokines or small molecules are promising strategies for β-cell regeneration. Results are variable due to the different models and methods; conversion rates are often low. More data are needed to identify safe and effective transcription factors and drugs to promote transdifferentiation for clinical use.
Transdifferentiation of hepatocytes and biliary cells to β-cells
Due to the proliferative ability and tissue specificity of liver tissue, as well as the common endodermal origin shared between hepatocytes and pancreatic cells, the clinical manipulation of genetic factors in combination with small molecules targeting specific pathways could render the human liver an ideal source of functional insulin-producing cells (118). Some hepatocytes display ectopic expression of Pdx1. These hepatocytes are typically located near central veins and seem predisposed to transdifferentiation into β-cells (119). Ectopic overexpression of Pdx1 and NeuroD1, downregulation of the expressions of hepatic transcription factors HNF1α and HNF4α, the reprogramming of hepatocytes into insulin-producing cells, and activation of the Wnt signaling pathway are necessary conditions for maintaining this plasticity (119, 120). Other factors such as GLP-1R agonists, Notch inhibitors, and transforming growth factor-β (TGF-β) inhibitors could enhance hepatocyte transdifferentiation (121). A notable finding is that the plasticity of the extrahepatic biliary tree enables intrahepatic biliary epithelial cells to express Pdx1, NeuroD, Pdx1/VP16, the insulin gene Ins, and Glut2 (122). In Hes1 knockout models, biliary epithelium differentiated into pancreatic exocrine and endocrine cells that formed acinar and islet-like structures in bile ducts with upregulated expression of Ngn3 (123, 124). Murine gallbladder epithelial cells were reprogrammed into β-like cells through the overexpression Pdx1, Ngn3, and MafA, that led to increased expressions of pancreatic endocrine genes (insulin, NeuroD1, Nkx2.2, and Isl1) (125). The overexpression of critical transcription factors combined with suppression of inhibitory factors may potentially enhance the efficiency of cell reprogramming.
Transdifferentiation of gastrointestinal cells to β-cells
The gastrointestinal tract is a highly regenerative organ system rich in endocrine cells that are highly similar to pancreatic β-cells and is also an immune-privileged site. Based on these advantages, the gastrointestinal tract can be used as a site for either transdifferentiation to produce β-like cells or for the engraftment of regenerated cells that mimic β-cell function (126, 127).
By screening adult cell types capable of becoming insulin-producing cells in vivo, we found that due to the ectopic expression of Pdx1, MafA, and Ngn3 in the intestinal crypts, intestinal cells can form β-like cells and may represent an accessible and abundant source of functional insulin-producing cells (128). Under the influence of Pdx1, MafA, and Ngn3 (PMN), enterocytes are capable of acquiring β-like characteristics that include the abilities to process preproinsulin into its mature form (with the release of C-peptide); to upregulate genes encoding the β-cell KATP channel subunits Kir6.2 and Sur1, and to display distinctive β-granules (128). Ablation of the FoxO1 transcription factor in enteroendocrine cells produces functional β-like cells (126). GLP-1 treatment induces insulin production in developing enterocytes, and to a lesser extent, in adult enterocytes both in vitro and in vivo; this process is mediated by the activation of Ngn3 and its downstream genes (128, 129).
However, the success rate of intestinal epithelial transdifferentiation is low, the transformation of enterocytes is relatively incomplete and the lifespan of intestinal insulin+ cells is shorter than that of sinus insulin+ cells due to CDX2, the intestine-specific cell surface marker, which prevents enterocytes from reprogramming into effective β-cells (130). Although these studies have revealed the feasibility of producing β-like cells from the intestinal epithelium, intestinal insulin+ cells cannot be regarded as completely regenerated β-cells because of their deficient NKX6.1 expression and unstable phenotype (131, 132, 133). A new framework that promotes a complete reprogramming of intestinal epithelial cells to become fully functioning β-cells is urgently needed.
However, the stomach represents a potential source of reprogrammable cells that may be transdifferentiated to produce insulin-secreting cells (134). The transformation mechanisms of different gastric cell types are varied. Based on the regenerative capacity of the antrum, native stem cells can supplement the increased demand for antral insulin+ cells and provide an excellent source of functional β-like cells. Antral endocrine cells mediated by PMN can be effectively reprogrammed into insulin+ cells, with robust expressions of key β-cell genes such as Sur1, and Glp1R, resulting in substantially improved glucose responsiveness. The gastric corpus contains a small amount of Ngn3-derived endocrine cells which differ from those of the antrum or intestinal tract and are not derived through PMN-mediated β-cell transformation (134, 135, 136). To summarize, cell plasticity and mechanisms of transdifferentiation differ in various segments of the gastrointestinal tract. The advantages of easy accessibility and transformability of gastrointestinal cells have attracted increasing attention. Further developments in biotechnology are expected to yield functional β-cells.
Bioengineered approaches
The in situ induction of β-cells from the native gastrointestinal tract may be limited due to a physiological environment that may disrupt normal endocrine homeostasis. In addition, the functionality of reprogrammed gastrointestinal cells remains to be determined. Unresolved issues include whether they express all key beta cytokines, whether their physiological function is complete, and whether they can reliably control insulin production in response to various physiologic stimuli. The development of regenerative engineering offers a potential replacement option described as organoids or functional 3D structures assembled with cell types from different sources. This strategy is widely used in regenerative medicine for tissue replacement or repair (137). However, the tissue-engineered stomach represents a versatile in vivo tool. ‘Stomach mini-organs’ that contain genetically engineered antral tissue enable both the formation and protection of transformed cells to constitute a new β-cell reservoir (134). Bioengineered gastric spheres isolate newly derived β-mimetic cells from the native organ, maintaining the physiological stability of the endocrine cell population in the intestinal tract, while on the other hand protecting the deposited β-mimetic cells from inappropriate glucose responses under stress (138). Recent advances in genetic engineering have expanded the accessibility of gastric organoids. The potential research (139) and therapeutic applications of genetically engineered organoids are substantial.
Emerging advances in synthetic biology have enabled the construction of specialized cells capable of performing vital functions. Conditioned media and the addition of a glucose sensing medium have been employed to synthesize β-cell-mimetic designer cells (140). We engineered a glucose-inducible transcriptional system by coupling a β-cell-mimetic cascade of glycolysis-mediated calcium entry to a synthetic excitation transcription coupling system in human embryonic kidney 293 (HEK-293) cells. These engineered cells are capable of glucose sensing and concentration-dependent expression of insulin and GLP-1. Injection of microencapsulated cells increased insulin secretion and improved hyperglycemia in diabetic mice (141). A semi-autonomous light control system stimulated the secretion of glucose-lowering hormones by photoactivated HEK293 cells implanted in diabetic mice, thus restoring glucose homeostasis (142, 143). Glucose-sensing devices loaded with biomimetic cells displayed glucose-induced insulin release comparable to endogenous β-cells; these constructs may be modified to secrete therapeutic proteins such as GLP-1 required for T2D therapy. However, the elucidation of the long-term in vivo effects of biomimetic cells will require additional studies.
Future research could explore the potential advantages of combining bioengineering (utilizing polymers such as PTFE or polycaprolactone, and/or microencapsulation of β-cells in materials such as alginate, polyacrylate, collagen or agarose) (144); gene-editing tools (CRISPR-Cas9) (145, 146); immune tolerance induction (147, 148); the adaptation of cell lines for enhanced mass production in bioreactors; optimization of biological process parameters and bioreactor environments; the promotion of cell growth and differentiation to augment biological function. The efficient mass production of cells that are stable, functionally mature, and that can mitigate functional deficiencies will accelerate the development of long-term cell replacement therapy for DM.
Acellular bioengineered constructs may offer alternatives to avoid design complexities and bioincompatabilities of reprogrammed cells. These non-living biomimetic assemblies include vesicles that carry drug payloads in cell membrane-cloaked nanoparticles that deliver insulin in a dynamic response to hyperglycemia and could theoretically act as β-cell surrogates for DM therapy (149). A disadvantage of acellular constructs is that they only provide basic β-cell functions such as insulin secretion. Capacities for insulin synthesis, its modulation through amplifying signal pathways, and the fine control of relative insulin content in response to external stimuli are absent (13). However, these constructs represent an exciting advance in the search for β-cell alternatives.
Conclusion
Dedifferentiation is a key mechanism of pancreatic β-cell failure. β-cell dysfunction induced by stress is driven by a complex set of reversible environmental factors. Improved understanding of the mechanisms of β-cell dedifferentiation will inform its reversal. Augmentation of β-cell volume and mass is essential to maintain normal glucose homeostasis and treat DM.
Recent studies have shown that supplementation of endogenous β-cells by transdifferentiation of other cell types may be a better approach than the differentiation of pluripotent stem cells and induced pluripotent stem cells with reduced proliferative capacity. The transdifferentiation of endogenous cells to produce β-cell mimetics is considered a safer approach. Recent studies have reported different conversion efficiencies of various cell types. Conversion rates range from 10 to 20% in pancreatic ductal cells; 0.2 to 70% (typically 20 to 30%) in acinar cells; 30% in gastrointestinal cells; 5 to 20% in biliary cells; 10 to 30% in hepatocytes. In addition, the different combinations of islet transcription factors such as Pdx1, Ngn3, MafA, and NeuroD1 affect the efficiency of transdifferentiation, and the co-expression of islet transcription factors and EGF/TGFβ growth factors promotes insulin gene expression. Studies of the effects of GABA, artemisinin, and GLP-1 on β-cell transdifferentiation have yielded contradictory results. Drug effects need further validation because of the different cell types, animal models, study intervals, and methods used in previous studies. Numerous strategies have been developed to improve the efficiency of cellular transformation, but there are also significant challenges to be addressed. Although there are obstacles to the reconstitution of β-cell populations through dedifferentiation and transdifferentiation, the development and application of regenerative medicine may provide a wide range of options for the generation of targeted and effective DM treatments.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Funding
This work was supported by 2015 National Natural Science Foundation of China (81471028).
Availability of data and materials
The datasets used during the current study are available from the corresponding author on reasonable request.
Author contribution statement
Literature collection and writing: W R; revision: C Z. All authors have read and agreed to the published version of the manuscript.
References
- 1.Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nature Reviews: Endocrinology 20181488–98. ( 10.1038/nrendo.2017.151) [DOI] [PubMed] [Google Scholar]
- 2.Harding JL, Pavkov ME, Magliano DJ, Shaw JE, Gregg EW. Global trends in diabetes complications: a review of current evidence. Diabetologia 2019623–16. ( 10.1007/s00125-018-4711-2) [DOI] [PubMed] [Google Scholar]
- 3.Nasteska D, Viloria K, Everett L, Hodson DJ. Informing β-cell regeneration strategies using studies of heterogeneity. Molecular Metabolism 201927SS49–S59. ( 10.1016/j.molmet.2019.06.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brereton MF, Rohm M, Ashcroft FM. β-Cell dysfunction in diabetes: a crisis of identity? Diabetes, Obesity and Metabolism 201618 (Supplement 1) 102–109. ( 10.1111/dom.12732) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mannucci E.Insulin therapy and cancer in type 2 diabetes. ISRN Endocrinology 20122012240634. ( 10.5402/2012/240634) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rother KI, Harlan DM. Challenges facing islet transplantation for the treatment of type 1 diabetes mellitus. Journal of Clinical Investigation 2004114877–883. ( 10.1172/JCI23235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Remedi MS, Emfinger C. Pancreatic β-cell identity in diabetes. Diabetes, Obesity and Metabolism 201618 (Supplement 1) 110–116. ( 10.1111/dom.12727) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salinno C, Cota P, Bastidas-Ponce A, Tarquis-Medina M, Lickert H, Bakhti M. β-Cell maturation and identity in health and disease. International Journal of Molecular Sciences 2019205417. ( 10.3390/ijms20215417) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bensellam M, Jonas JC, Laybutt DR. Mechanisms of β-cell dedifferentiation in diabetes: recent findings and future research directions. Journal of Endocrinology 2018236 R109–R143. ( 10.1530/JOE-17-0516) [DOI] [PubMed] [Google Scholar]
- 10.Efrat S.Beta-cell dedifferentiation in type 2 diabetes: concise review. Stem Cells 2019371267–1272. ( 10.1002/stem.3059) [DOI] [PubMed] [Google Scholar]
- 11.Aguayo-Mazzucato C, Bonner-Weir S. Pancreatic β cell regeneration as a possible therapy for diabetes. Cell Metabolism 20182757–67. ( 10.1016/j.cmet.2017.08.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang P, Fiaschi-Taesch NM, Vasavada RC, Scott DK, García-Ocaña A, Stewart AF. Diabetes mellitus – advances and challenges in human β-cell proliferation. Nature Reviews: Endocrinology 201511201–212. ( 10.1038/nrendo.2015.9) [DOI] [PubMed] [Google Scholar]
- 13.Nair GG, Tzanakakis ES, Hebrok M. Emerging routes to the generation of functional β-cells for diabetes mellitus cell therapy. Nature Reviews: Endocrinology 202016506–518. ( 10.1038/s41574-020-0375-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moullé VS, Ghislain J, Poitout V. Nutrient regulation of pancreatic β-cell proliferation. Biochimie 201714310–17. ( 10.1016/j.biochi.2017.09.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brun T, Jiménez-Sánchez C, Madsen JGS, Hadadi N, Duhamel D, Bartley C, Oberhauser L, Trajkovski M, Mandrup S, Maechler P. AMPK profiling in rodent and human pancreatic beta-cells under nutrient-rich metabolic stress. International Journal of Molecular Sciences 2020213982. ( 10.3390/ijms21113982) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prentki M, Peyot ML, Masiello P, Madiraju SRM. Nutrient-induced metabolic stress, adaptation, detoxification, and toxicity in the pancreatic β-cell. Diabetes 202069279–290. ( 10.2337/dbi19-0014) [DOI] [PubMed] [Google Scholar]
- 17.Flamez D, Berger V, Kruhøffer M, Orntoft T, Pipeleers D, Schuit FC. Critical role for cataplerosis via citrate in glucose-regulated insulin release. Diabetes 2002512018–2024. ( 10.2337/diabetes.51.7.2018) [DOI] [PubMed] [Google Scholar]
- 18.van der Meulen T, Huising MO. Role of transcription factors in the transdifferentiation of pancreatic islet cells. Journal of Molecular Endocrinology 201554R103–R. ( 10.1530/JME-14-0290) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weir GC, Gaglia J, Bonner-Weir S. Inadequate β-cell mass is essential for the pathogenesis of type 2 diabetes. Lancet: Diabetes and Endocrinology 20208249–256. ( 10.1016/S2213-8587(2030022-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moin ASM, Butler AE. Alterations in beta cell identity in type 1 and type 2 diabetes. Current Diabetes Reports 201919 83. ( 10.1007/s11892-019-1194-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawson R, Maret W, Hogstrand C. Prolonged stimulation of insulin release from min6 cells causes zinc depletion and loss of β-cell markers. Journal of Trace Elements in Medicine and Biology 20184951–59. ( 10.1016/j.jtemb.2018.04.020) [DOI] [PubMed] [Google Scholar]
- 22.Tong X, Dai C, Walker JT, Nair GG, Kennedy A, Carr RM, Hebrok M, Powers AC, Stein R. Lipid droplet accumulation in human pancreatic islets is dependent on both donor age and health. Diabetes 202069342–354. ( 10.2337/db19-0281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oh YS, Bae GD, Baek DJ, Park EY, Jun HS. Fatty acid-induced lipotoxicity in pancreatic beta-cells during development of type 2 diabetes. Frontiers in Endocrinology 20189 384. ( 10.3389/fendo.2018.00384) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ye R, Onodera T, Scherer PE. Lipotoxicity and β cell maintenance in obesity and type 2 diabetes. Journal of the Endocrine Society 20193617–631. ( 10.1210/js.2018-00372) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Unger RH.Lipotoxicity in the pathogenesis of obesity-dependent NIDDM. Genetic and clinical implications. Diabetes 199544863–870. ( 10.2337/diab.44.8.863) [DOI] [PubMed] [Google Scholar]
- 26.Lee Y, Hirose H, Ohneda M, Johnson JH, McGarry JD, Unger RH. Beta-cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: impairment in adipocyte-beta-cell relationships. PNAS 19949110878–10882. ( 10.1073/pnas.91.23.10878) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnston LW, Harris SB, Retnakaran R, Giacca A, Liu Z, Bazinet RP, Hanley AJ. Association of NEFA composition with insulin sensitivity and beta cell function in the prospective metabolism and islet cell evaluation (PROMISE) cohort. Diabetologia 201861821–830. ( 10.1007/s00125-017-4534-6) [DOI] [PubMed] [Google Scholar]
- 28.Salgin B, Ong KK, Thankamony A, Emmett P, Wareham NJ, Dunger DB. Higher fasting plasma free fatty acid levels are associated with lower insulin secretion in children and adults and a higher incidence of type 2 diabetes. Journal of Clinical Endocrinology and Metabolism 2012973302–3309. ( 10.1210/jc.2012-1428) [DOI] [PubMed] [Google Scholar]
- 29.Delghingaro-Augusto V, Nolan CJ, Gupta D, Jetton TL, Latour MG, Peshavaria M, Madiraju SR, Joly E, Peyot ML, Prentki Met al. Islet beta cell failure in the 60% pancreatectomised obese hyperlipidaemic Zucker fatty rat: severe dysfunction with altered glycerolipid metabolism without steatosis or a falling beta cell mass. Diabetologia 2009521122–1132. ( 10.1007/s00125-009-1317-8) [DOI] [PubMed] [Google Scholar]
- 30.Nolan CJ, Leahy JL, Delghingaro-Augusto V, Moibi J, Soni K, Peyot ML, Fortier M, Guay C, Lamontagne J, Barbeau Aet al. Beta cell compensation for insulin resistance in Zucker fatty rats: increased lipolysis and fatty acid signalling. Diabetologia 2006492120–2130. ( 10.1007/s00125-006-0305-5) [DOI] [PubMed] [Google Scholar]
- 31.Delghingaro-Augusto V, Décary S, Peyot ML, Latour MG, Lamontagne J, Paradis-Isler N, Lacharité-Lemieux M, Akakpo H, Birot O, Nolan CJet al. Voluntary running exercise prevents β-cell failure in susceptible islets of the Zucker diabetic fatty rat. American Journal of Physiology: Endocrinology and Metabolism 2012302E254–E. ( 10.1152/ajpendo.00360.2011) [DOI] [PubMed] [Google Scholar]
- 32.Fontés G, Zarrouki B, Hagman DK, Latour MG, Semache M, Roskens V, Moore PC, Prentki M, Rhodes CJ, Jetton TLet al. Glucolipotoxicity age-dependently impairs beta cell function in rats despite a marked increase in beta cell mass. Diabetologia 2010532369–2379. ( 10.1007/s00125-010-1850-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peyot ML, Pepin E, Lamontagne J, Latour MG, Zarrouki B, Lussier R, Pineda M, Jetton TL, Madiraju SR, Joly Eet al. Beta-cell failure in diet-induced obese mice stratified according to body weight gain: secretory dysfunction and altered islet lipid metabolism without steatosis or reduced beta-cell mass. Diabetes 2010592178–2187. ( 10.2337/db09-1452) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tiano JP, Delghingaro-Augusto V, Le May C, Liu S, Kaw MK, Khuder SS, Latour MG, Bhatt SA, Korach KS, Najjar SMet al. Estrogen receptor activation reduces lipid synthesis in pancreatic islets and prevents β cell failure in rodent models of type 2 diabetes. Journal of Clinical Investigation 20111213331–3342. ( 10.1172/JCI44564) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akerfeldt MC, Laybutt DR. Inhibition of Id1 augments insulin secretion and protects against high-fat diet-induced glucose intolerance. Diabetes 2011602506–2514. ( 10.2337/db11-0083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun T, Han X. Death versus dedifferentiation: the molecular bases of beta cell mass reduction in type 2 diabetes. Seminars in Cell and Developmental Biology 202010376–82. ( 10.1016/j.semcdb.2019.12.002) [DOI] [PubMed] [Google Scholar]
- 37.White MG, Shaw JA, Taylor R. Type 2 diabetes: the pathologic basis of reversible β-cell dysfunction. Diabetes Care 2016392080–2088. ( 10.2337/dc16-0619) [DOI] [PubMed] [Google Scholar]
- 38.Lynch CJ, Adams SH. Branched-chain amino acids in metabolic signalling and insulin resistance. Nature Reviews: Endocrinology 201410723–736. ( 10.1038/nrendo.2014.171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Owei I, Umekwe N, Stentz F, Wan J, Dagogo-Jack S. Amino acid signature predictive of incident prediabetes: a case-control study nested within the longitudinal pathobiology of prediabetes in a biracial cohort. Metabolism: Clinical and Experimental 20199876–83. ( 10.1016/j.metabol.2019.06.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takahara T, Amemiya Y, Sugiyama R, Maki M, Shibata H. Amino acid-dependent control of mTORC1 signaling: a variety of regulatory modes. Journal of Biomedical Science 202027 87. ( 10.1186/s12929-020-00679-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor R.Calorie restriction for long-term remission of type 2 diabetes. Clinical Medicine 20191937–42. ( 10.7861/clinmedicine.19-1-37) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rathwa N, Patel R, Palit SP, Parmar N, Rana S, Ansari MI, Ramachandran AV, Begum R. β-Cell replenishment: possible curative approaches for diabetes mellitus. Nutrition, Metabolism, and Cardiovascular Diseases 2020301870–1881. ( 10.1016/j.numecd.2020.08.006) [DOI] [PubMed] [Google Scholar]
- 43.Jiang H, Tong Y, Yan D, Jia S, Ostenson CG, Chen Z. The soybean peptide vglycin preserves the diabetic β-cells through improvement of proliferation and inhibition of apoptosis. Scientific Reports 20155 15599. ( 10.1038/srep15599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor R, Al-Mrabeh A, Sattar N. Understanding the mechanisms of reversal of type 2 diabetes. Lancet: Diabetes and Endocrinology 20197726–736. ( 10.1016/S2213-8587(1930076-2) [DOI] [PubMed] [Google Scholar]
- 45.Taylor R, Al-Mrabeh A, Zhyzhneuskaya S, Peters C, Barnes AC, Aribisala BS, Hollingsworth KG, Mathers JC, Sattar N, Lean MEJ. Remission of human Type 2 diabetes requires decrease in liver and pancreas fat content but is dependent upon capacity for β cell recovery. Cell Metabolism 201828547.e543–556.e543.( 10.1016/j.cmet.2018.07.003) [DOI] [PubMed] [Google Scholar]
- 46.Marselli L, Piron A, Suleiman M, Colli ML, Yi X, Khamis A, Carrat GR, Rutter GA, Bugliani M, Giusti Let al. Persistent or transient human β cell dysfunction induced by metabolic stress: specific signatures and shared gene expression with Type 2 diabetes. Cell Reports 202033108466. ( 10.1016/j.celrep.2020.108466) [DOI] [PubMed] [Google Scholar]
- 47.Song M, Cubillos-Ruiz JR. Endoplasmic reticulum stress responses in intratumoral immune cells: implications for cancer immunotherapy. Trends in Immunology 201940128–141. ( 10.1016/j.it.2018.12.001) [DOI] [PubMed] [Google Scholar]
- 48.Arunagiri A, Haataja L, Pottekat A, Pamenan F, Kim S, Zeltser LM, Paton AW, Paton JC, Tsai B, Itkin-Ansari Pet al. Proinsulin misfolding is an early event in the progression to type 2 diabetes. eLife 20198e44532. ( 10.7554/eLife.44532) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cnop M, Toivonen S, Igoillo-Esteve M, Salpea P.Endoplasmic reticulum stress and eIF2α phosphorylation: the Achilles heel of pancreatic β cells. Molecular Metabolism 201761024–1039. ( 10.1016/j.molmet.2017.06.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocrine Reviews 20082942–61. ( 10.1210/er.2007-0015) [DOI] [PubMed] [Google Scholar]
- 51.Cnop M, Foufelle F, Velloso LA. Endoplasmic reticulum stress, obesity and diabetes. Trends in Molecular Medicine 20121859–68. ( 10.1016/j.molmed.2011.07.010) [DOI] [PubMed] [Google Scholar]
- 52.Sachdeva MM, Claiborn KC, Khoo C, Yang J, Groff DN, Mirmira RG, Stoffers DA. Pdx1 (MODY4) regulates pancreatic beta cell susceptibility to ER stress. PNAS 200910619090–19095. ( 10.1073/pnas.0904849106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kluth O, Mirhashemi F, Scherneck S, Kaiser D, Kluge R, Neschen S, Joost HG, Schürmann A. Dissociation of lipotoxicity and glucotoxicity in a mouse model of obesity associated diabetes: role of forkhead box O1 (FOXO1) in glucose-induced beta cell failure. Diabetologia 201154605–616. ( 10.1007/s00125-010-1973-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guo S, Dai C, Guo M, Taylor B, Harmon JS, Sander M, Robertson RP, Powers AC, Stein R. Inactivation of specific β cell transcription factors in type 2 diabetes. Journal of Clinical Investigation 20131233305–3316. ( 10.1172/JCI65390) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salvadó L, Palomer X, Barroso E, Vázquez-Carrera M. Targeting endoplasmic reticulum stress in insulin resistance. Trends in Endocrinology and Metabolism 201526438–448. ( 10.1016/j.tem.2015.05.007) [DOI] [PubMed] [Google Scholar]
- 56.Demine S, Schiavo AA, Marín-Cañas S, Marchetti P, Cnop M, Eizirik DL. Pro-inflammatory cytokines induce cell death, inflammatory responses, and endoplasmic reticulum stress in human iPSC-derived beta cells. Stem Cell Research and Therapy 202011 7. ( 10.1186/s13287-019-1523-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nemecz M, Constantin A, Dumitrescu M, Alexandru N, Filippi A, Tanko G, Georgescu A. The distinct effects of palmitic and oleic acid on pancreatic beta cell function: the elucidation of associated mechanisms and effector molecules. Frontiers in Pharmacology 20189 1554. ( 10.3389/fphar.2018.01554) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marré ML, Piganelli JD. Environmental factors contribute to β cell endoplasmic reticulum stress and neo-antigen formation in Type 1 diabetes. Frontiers in Endocrinology 20178 262. ( 10.3389/fendo.2017.00262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chan JY, Luzuriaga J, Bensellam M, Biden TJ, Laybutt DR. Failure of the adaptive unfolded protein response in islets of obese mice is linked with abnormalities in β-cell gene expression and progression to diabetes. Diabetes 2013621557–1568. ( 10.2337/db12-0701) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thomas SE, Dalton LE, Daly ML, Malzer E, Marciniak SJ. Diabetes as a disease of endoplasmic reticulum stress. Diabetes/Metabolism Research and Reviews 201026611–621. ( 10.1002/dmrr.1132) [DOI] [PubMed] [Google Scholar]
- 61.Szabat M, Page MM, Panzhinskiy E, Skovsø S, Mojibian M, Fernandez-Tajes J, Bruin JE, Bround MJ, Lee JT, Xu EEet al. Reduced insulin production relieves endoplasmic reticulum stress and induces β cell proliferation. Cell Metabolism 201623179–193. ( 10.1016/j.cmet.2015.10.016) [DOI] [PubMed] [Google Scholar]
- 62.Das I, Krzyzosiak A, Schneider K, Wrabetz L, D'Antonio M, Barry N, Sigurdardottir A, Bertolotti A. Preventing proteostasis diseases by selective inhibition of a phosphatase regulatory subunit. Science 2015348239–242. ( 10.1126/science.aaa4484) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hasnain SZ, Prins JB, McGuckin MA. Oxidative and endoplasmic reticulum stress in β-cell dysfunction in diabetes. Journal of Molecular Endocrinology 201656R33–R. ( 10.1530/JME-15-0232) [DOI] [PubMed] [Google Scholar]
- 64.Mohan S, R PRM, Brown L, Ayyappan P, G RK. Endoplasmic reticulum stress: a master regulator of metabolic syndrome. European Journal of Pharmacology 2019860172553. ( 10.1016/j.ejphar.2019.172553) [DOI] [PubMed] [Google Scholar]
- 65.Rani V, Deep G, Singh RK, Palle K, Yadav UC. Oxidative stress and metabolic disorders: pathogenesis and therapeutic strategies. Life Sciences 2016148183–193. ( 10.1016/j.lfs.2016.02.002) [DOI] [PubMed] [Google Scholar]
- 66.Ježek P, Jabůrek M, Plecitá-Hlavatá L. Contribution of oxidative stress and impaired biogenesis of pancreatic β-cells to type 2 diabetes. Antioxidants and Redox Signaling 201931722–751. ( 10.1089/ars.2018.7656) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tan BL, Norhaizan ME, Liew WP. Nutrients and oxidative stress: friend or foe? Oxidative Medicine and Cellular Longevity 201820189719584. ( 10.1155/2018/9719584) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eguchi N, Vaziri ND, Dafoe DC, Ichii H. The role of oxidative stress in pancreatic β cell dysfunction in diabetes. International Journal of Molecular Sciences 202122 1509. ( 10.3390/ijms22041509) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Prattichizzo F, De Nigris V, Mancuso E, Spiga R, Giuliani A, Matacchione G, Lazzarini R, Marcheselli F, Recchioni R, Testa Ret al. Short-term sustained hyperglycaemia fosters an archetypal senescence-associated secretory phenotype in endothelial cells and macrophages. Redox Biology 201815170–181. ( 10.1016/j.redox.2017.12.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lou Y, Kong M, Li L, Hu Y, Zhai W, Qi X, Liu Z, Wu J. Inhibition of the Keap1/Nrf2 signaling pathway significantly promotes the progression of type 1 diabetes mellitus. Oxidative Medicine and Cellular Longevity 202120217866720. ( 10.1155/2021/7866720) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yin Q, Ni Q, Wang Y, Zhang H, Li W, Nie A, Wang S, Gu Y, Wang Q, Ning G. Raptor determines β-cell identity and plasticity independent of hyperglycemia in mice. Nature Communications 202011 2538. ( 10.1038/s41467-020-15935-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Negi S, Jetha A, Aikin R, Hasilo C, Sladek R, Paraskevas S. Analysis of beta-cell gene expression reveals inflammatory signaling and evidence of dedifferentiation following human islet isolation and culture. PLoS ONE 20127 e30415. ( 10.1371/journal.pone.0030415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nandi S, Saxena M. Potential inhibitors of protein tyrosine phosphatase (PTP1B) enzyme: promising target for Type-II diabetes mellitus. Current Topics in Medicinal Chemistry 2020202692–2707. ( 10.2174/1568026620999200904121432) [DOI] [PubMed] [Google Scholar]
- 74.Yaribeygi H, Sathyapalan T, Atkin SL, Sahebkar A. Molecular mechanisms linking oxidative stress and diabetes mellitus. Oxidative Medicine and Cellular Longevity 202020208609213. ( 10.1155/2020/8609213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang J, Yang X, Zhang J. Bridges between mitochondrial oxidative stress, ER stress and mTOR signaling in pancreatic β cells. Cellular Signalling 2016281099–1104. ( 10.1016/j.cellsig.2016.05.007) [DOI] [PubMed] [Google Scholar]
- 76.Ardestani A, Lupse B, Kido Y, Leibowitz G, Maedler K. mTORC1 signaling: a double-edged sword in diabetic β cells. Cell Metabolism 201827314–331. ( 10.1016/j.cmet.2017.11.004) [DOI] [PubMed] [Google Scholar]
- 77.Wei X, Gu N, Feng N, Guo X, Ma X. Inhibition of p38 mitogen-activated protein kinase exerts a hypoglycemic effect by improving β cell function via inhibition of β cell apoptosis in db/db mice. Journal of Enzyme Inhibition and Medicinal Chemistry 2018331494–1500. ( 10.1080/14756366.2018.1477138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Iftikhar A, Aslam B, Iftikhar M, Majeed W, Batool M, Zahoor B, Amna N, Gohar H, Latif I. Effect of Caesalpinia bonduc polyphenol extract on alloxan-induced diabetic rats in attenuating hyperglycemia by upregulating insulin secretion and inhibiting JNK signaling pathway. Oxidative Medicine and Cellular Longevity 202020209020219. ( 10.1155/2020/9020219) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ji JJ, Qian LL, Zhu Y, Wu YP, Guo JQ, Ma GS, Yao YY. Serpina3c protects against high-fat diet-induced pancreatic dysfunction through the JNK-related pathway. Cellular Signalling 202075109745. ( 10.1016/j.cellsig.2020.109745) [DOI] [PubMed] [Google Scholar]
- 80.Ding M, Fang QH, Cui YT, Shen QL, Liu Q, Wang PH, Yu DM, Li CJ. Liraglutide prevents β-cell apoptosis via inactivation of NOX2 and its related signaling pathway. Journal of Diabetes and Its Complications 201933267–277. ( 10.1016/j.jdiacomp.2018.12.013) [DOI] [PubMed] [Google Scholar]
- 81.Liu Y, Han J, Zhou Z, Li D. Paeoniflorin protects pancreatic β cells from STZ-induced damage through inhibition of the p38 MAPK and JNK signaling pathways. European Journal of Pharmacology 201985318–24. ( 10.1016/j.ejphar.2019.03.025) [DOI] [PubMed] [Google Scholar]
- 82.Griffin SJ, Leaver JK, Irving GJ. Impact of metformin on cardiovascular disease: a meta-analysis of randomised trials among people with type 2 diabetes. Diabetologia 2017601620–1629. ( 10.1007/s00125-017-4337-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lupi R, Del Guerra S, Fierabracci V, Marselli L, Novelli M, Patanè G, Boggi U, Mosca F, Piro S, Del Prato Set al. Lipotoxicity in human pancreatic islets and the protective effect of metformin. Diabetes 200251 (Supplement 1) S134–S. ( 10.2337/diabetes.51.2007.s134) [DOI] [PubMed] [Google Scholar]
- 84.Erdmann E, Dormandy J, Wilcox R, Massi-Benedetti M, Charbonnel B. PROactive 07: pioglitazone in the treatment of type 2 diabetes: results of the PROactive study. Vascular Health and Risk Management 20073355–370. [PMC free article] [PubMed] [Google Scholar]
- 85.Lupi R, Del Guerra S, Marselli L, Bugliani M, Boggi U, Mosca F, Marchetti P, Del Prato S. Rosiglitazone prevents the impairment of human islet function induced by fatty acids: evidence for a role of PPARgamma2 in the modulation of insulin secretion. American Journal of Physiology: Endocrinology and Metabolism 2004286E560–E567. ( 10.1152/ajpendo.00561.2002) [DOI] [PubMed] [Google Scholar]
- 86.Shiraki A, Oyama J, Komoda H, Asaka M, Komatsu A, Sakuma M, Kodama K, Sakamoto Y, Kotooka N, Hirase Tet al. The glucagon-like peptide 1 analog liraglutide reduces TNF-α-induced oxidative stress and inflammation in endothelial cells. Atherosclerosis 2012221375–382. ( 10.1016/j.atherosclerosis.2011.12.039) [DOI] [PubMed] [Google Scholar]
- 87.Natalicchio A, Labarbuta R, Tortosa F, Biondi G, Marrano N, Peschechera A, Carchia E, Orlando MR, Leonardini A, Cignarelli Aet al. Exendin-4 protects pancreatic beta cells from palmitate-induced apoptosis by interfering with GPR40 and the MKK4/7 stress kinase signalling pathway. Diabetologia 2013562456–2466. ( 10.1007/s00125-013-3028-4) [DOI] [PubMed] [Google Scholar]
- 88.Cunha DA, Ladrière L, Ortis F, Igoillo-Esteve M, Gurzov EN, Lupi R, Marchetti P, Eizirik DL, Cnop M. Glucagon-like peptide-1 agonists protect pancreatic beta-cells from lipotoxic endoplasmic reticulum stress through upregulation of BiP and JunB. Diabetes 2009582851–2862. ( 10.2337/db09-0685) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ojima A, Matsui T, Nishino Y, Nakamura N, Yamagishi S. Empagliflozin, an inhibitor of sodium-glucose cotransporter 2 exerts anti-inflammatory and antifibrotic effects on experimental diabetic nephropathy partly by suppressing AGEs-receptor axis. Hormone and Metabolic Research 201547686–692. ( 10.1055/s-0034-1395609) [DOI] [PubMed] [Google Scholar]
- 90.Papachristoforou E, Lambadiari V, Maratou E, Makrilakis K. Association of glycemic indices (hyperglycemia, glucose variability, and hypoglycemia) with oxidative stress and diabetic complications. Journal of Diabetes Research 202020207489795. ( 10.1155/2020/7489795) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang H, Pollin TI. Epigenetics variation and pathogenesis in diabetes. Current Diabetes Reports 201818 121. ( 10.1007/s11892-018-1091-4) [DOI] [PubMed] [Google Scholar]
- 92.You L, Wang N, Yin D, Wang L, Jin F, Zhu Y, Yuan Q, De W. Downregulation of long noncoding RNA Meg3 affects insulin synthesis and secretion in mouse pancreatic beta cells. Journal of Cellular Physiology 2016231852–862. ( 10.1002/jcp.25175) [DOI] [PubMed] [Google Scholar]
- 93.Zhu Y, Sun Y, Zhou Y, Zhang Y, Zhang T, Li Y, You W, Chang X, Yuan L, Han X. MicroRNA-24 promotes pancreatic beta cells toward dedifferentiation to avoid endoplasmic reticulum stress-induced apoptosis. Journal of Molecular Cell Biology 201911747–760. ( 10.1093/jmcb/mjz004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sebastiani G, Grieco GE, Brusco N, Ventriglia G, Formichi C, Marselli L, Marchetti P, Dotta F. MicroRNA expression analysis of in vitro dedifferentiated human pancreatic islet cells reveals the activation of the pluripotency-related microRNA cluster miR-302s. International Journal of Molecular Sciences 2018191170. ( 10.3390/ijms19041170) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Akerman I, Tu Z, Beucher A, Rolando DMY, Sauty-Colace C, Benazra M, Nakic N, Yang J, Wang H, Pasquali Let al. Human pancreatic β cell lncRNAs control cell-specific regulatory networks. Cell Metabolism 201725400–411. ( 10.1016/j.cmet.2016.11.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Arnes L, Sussel L. Epigenetic modifications and long noncoding RNAs influence pancreas development and function. Trends in Genetics 201531290–299. ( 10.1016/j.tig.2015.02.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wei R, Hong T. Lineage reprogramming: a promising road for pancreatic β cell regeneration. Trends in Endocrinology and Metabolism 201627163–176. ( 10.1016/j.tem.2016.01.002) [DOI] [PubMed] [Google Scholar]
- 98.Zhang J, Liu F. The de-, re-, and trans-differentiation of β-cells: regulation and function. Seminars in Cell and Developmental Biology 202010368–75. ( 10.1016/j.semcdb.2020.01.003) [DOI] [PubMed] [Google Scholar]
- 99.Thorel F, Népote V, Avril I, Kohno K, Desgraz R, Chera S, Herrera PL. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature 20104641149–1154. ( 10.1038/nature08894) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang RN, Klöppel G, Bouwens L. Duct- to islet-cell differentiation and islet growth in the pancreas of duct-ligated adult rats. Diabetologia 1995381405–1411. ( 10.1007/BF00400600) [DOI] [PubMed] [Google Scholar]
- 101.Pan FC, Bankaitis ED, Boyer D, Xu X, Van de Casteele M, Magnuson MA, Heimberg H, Wright CV. Spatiotemporal patterns of multipotentiality in Ptf1a-expressing cells during pancreas organogenesis and injury-induced facultative restoration. Development 2013140751–764. ( 10.1242/dev.090159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chakravarthy H, Gu X, Enge M, Dai X, Wang Y, Damond N, Downie C, Liu K, Wang J, Xing Yet al. Converting adult pancreatic islet α cells into β cells by targeting both Dnmt1 and Arx. Cell Metabolism 201725622–634. ( 10.1016/j.cmet.2017.01.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Collombat P, Xu X, Ravassard P, Sosa-Pineda B, Dussaud S, Billestrup N, Madsen OD, Serup P, Heimberg H, Mansouri A. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into alpha and subsequently beta cells. Cell 2009138449–462. ( 10.1016/j.cell.2009.05.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xiao X, Guo P, Shiota C, Zhang T, Coudriet GM, Fischbach S, Prasadan K, Fusco J, Ramachandran S, Witkowski Pet al. Endogenous reprogramming of alpha cells into beta cells, induced by viral gene therapy, reverses autoimmune diabetes. Cell Stem Cell 20182278, .e4–90.e4. ( 10.1016/j.stem.2017.11.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Assouline-Thomas B, Ellis D, Petropavlovskaia M, Makhlin J, Ding J, Rosenberg L. Islet neogenesis associated protein (INGAP) induces the differentiation of an adult human pancreatic ductal cell line into insulin-expressing cells through stepwise activation of key transcription factors for embryonic beta cell development. Differentiation: Research in Biological Diversity 20159077–90. ( 10.1016/j.diff.2015.10.008) [DOI] [PubMed] [Google Scholar]
- 106.Zhu Y, Liu Q, Zhou Z, Ikeda Y. PDX1, neurogenin-3, and MAFA: critical transcription regulators for beta cell development and regeneration. Stem Cell Research and Therapy 20178 240. ( 10.1186/s13287-017-0694-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee J, Sugiyama T, Liu Y, Wang J, Gu X, Lei J, Markmann JF, Miyazaki S, Miyazaki J, Szot GLet al. Expansion and conversion of human pancreatic ductal cells into insulin-secreting endocrine cells. eLife 20132 e00940. ( 10.7554/eLife.00940) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee YS, Lee C, Choung JS, Jung HS, Jun HS. Glucagon-like peptide 1 increases β-cell regeneration by promoting α- to β-cell transdifferentiation. Diabetes 2018672601–2614. ( 10.2337/db18-0155) [DOI] [PubMed] [Google Scholar]
- 109.Ben-Othman N, Vieira A, Courtney M, Record F, Gjernes E, Avolio F, Hadzic B, Druelle N, Napolitano T, Navarro-Sanz Set al. Long-term GABA administration induces alpha cell-mediated beta-like cell neogenesis. Cell 201716873, .e11–85.e11. ( 10.1016/j.cell.2016.11.002) [DOI] [PubMed] [Google Scholar]
- 110.Li J, Casteels T, Frogne T, Ingvorsen C, Honoré C, Courtney M, Huber KVM, Schmitner N, Kimmel RA, Romanov RAet al. Artemisinins target GABA(A) receptor signaling and impair α cell identity. Cell 201716886.e115–100.e115.( 10.1016/j.cell.2016.11.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Minami K, Okuno M, Miyawaki K, Okumachi A, Ishizaki K, Oyama K, Kawaguchi M, Ishizuka N, Iwanaga T, Seino S. Lineage tracing and characterization of insulin-secreting cells generated from adult pancreatic acinar cells. PNAS 200510215116–15121. ( 10.1073/pnas.0507567102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lemper M, De Groef S, Stangé G, Baeyens L, Heimberg H. A combination of cytokines EGF and CNTF protects the functional beta cell mass in mice with short-term hyperglycaemia. Diabetologia 2016591948–1958. ( 10.1007/s00125-016-4023-3) [DOI] [PubMed] [Google Scholar]
- 113.Baeyens L, De Breuck S, Lardon J, Mfopou JK, Rooman I, Bouwens L. In vitro generation of insulin-producing beta cells from adult exocrine pancreatic cells. Diabetologia 20054849–57. ( 10.1007/s00125-004-1606-1) [DOI] [PubMed] [Google Scholar]
- 114.Wang TC, Bonner-Weir S, Oates PS, Chulak M, Simon B, Merlino GT, Schmidt EV, Brand SJ. Pancreatic gastrin stimulates islet differentiation of transforming growth factor alpha-induced ductular precursor cells. Journal of Clinical Investigation 1993921349–1356. ( 10.1172/JCI116708) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lefebvre B, Belaich S, Longue J, Vandewalle B, Oberholzer J, Gmyr V, Pattou F, Kerr-Conte J. 5′-AZA induces Ngn3 expression and endocrine differentiation in the PANC-1 human ductal cell line. Biochemical and Biophysical Research Communications 2010391305–309. ( 10.1016/j.bbrc.2009.11.054) [DOI] [PubMed] [Google Scholar]
- 116.Zhang Z, Hu Y, Xu N, Zhou W, Yang L, Chen R, Yang R, Sun J, Chen H. A new way for beta cell Neogenesis: transdifferentiation from alpha cells induced by glucagon-like peptide 1. Journal of Diabetes Research 201920192583047. ( 10.1155/2019/2583047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lemper M, Leuckx G, Heremans Y, German MS, Heimberg H, Bouwens L, Baeyens L. Reprogramming of human pancreatic exocrine cells to β-like cells. Cell Death and Differentiation 2015221117–1130. ( 10.1038/cdd.2014.193) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Motoyama H, Kobayashi A, Yokoyama T, Shimizu A, Sakai H, Notake T, Fukushima K, Miyagawa SI. Treatment with specific soluble factors promotes the functional maturation of transcription factor-mediated, pancreatic transdifferentiated cells. PLoS ONE 201813 e0197175. ( 10.1371/journal.pone.0197175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Meivar-Levy I, Ferber S. Liver to pancreas transdifferentiation. Current Diabetes Reports 201919 76. ( 10.1007/s11892-019-1198-2) [DOI] [PubMed] [Google Scholar]
- 120.Cohen H, Barash H, Meivar-Levy I, Molakandov K, Ben-Shimon M, Gurevich M, Zoabi F, Har-Zahav A, Gebhardt R, Gaunitz Fet al. The Wnt/β-catenin pathway determines the predisposition and efficiency of liver-to-pancreas reprogramming. Hepatology 2018681589–1603. ( 10.1002/hep.29827) [DOI] [PubMed] [Google Scholar]
- 121.Chang FP, Cho CH, Shen CR, Chien CY, Ting LW, Lee HS, Shen CN. PDGF facilitates direct lineage reprogramming of hepatocytes to functional β-like cells induced by Pdx1 and Ngn3. Cell Transplantation 2016251893–1909 ( 10.3727/096368916X691439) [DOI] [PubMed] [Google Scholar]
- 122.Nagaya M, Katsuta H, Kaneto H, Bonner-Weir S, Weir GC. Adult mouse intrahepatic biliary epithelial cells induced in vitro to become insulin-producing cells. Journal of Endocrinology 200920137–47. ( 10.1677/JOE-08-0482) [DOI] [PubMed] [Google Scholar]
- 123.Sumazaki R, Shiojiri N, Isoyama S, Masu M, Keino-Masu K, Osawa M, Nakauchi H, Kageyama R, Matsui A. Conversion of biliary system to pancreatic tissue in Hes1-deficient mice. Nature Genetics 20043683–87. ( 10.1038/ng1273) [DOI] [PubMed] [Google Scholar]
- 124.Coad RA, Dutton JR, Tosh D, Slack JM. Inhibition of Hes1 activity in gall bladder epithelial cells promotes insulin expression and glucose responsiveness. Biochemistry and Cell Biology 200987975–987. ( 10.1139/o09-063) [DOI] [PubMed] [Google Scholar]
- 125.Galivo F, Benedetti E, Wang Y, Pelz C, Schug J, Kaestner KH, Grompe M. Reprogramming human gallbladder cells into insulin-producing β-like cells. PLoS ONE 201712 e0181812. ( 10.1371/journal.pone.0181812) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Talchai C, Xuan S, Kitamura T, DePinho RA, Accili D. Generation of functional insulin-producing cells in the gut by FoxO1 ablation. Nature Genetics 201244406–41, S1. ( 10.1038/ng.2215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Spadoni I, Fornasa G, Rescigno M. Organ-specific protection mediated by cooperation between vascular and epithelial barriers. Nature Reviews: Immunology 201717761–773. ( 10.1038/nri.2017.100) [DOI] [PubMed] [Google Scholar]
- 128.Chen YJ, Finkbeiner SR, Weinblatt D, Emmett MJ, Tameire F, Yousefi M, Yang C, Maehr R, Zhou Q, Shemer Ret al. De novo formation of insulin-producing ‘neo-β cell islets’ from intestinal crypts. Cell Reports 201461046–1058. ( 10.1016/j.celrep.2014.02.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Suzuki A, Nakauchi H, Taniguchi H. Glucagon-like peptide 1 (1–37) converts intestinal epithelial cells into insulin-producing cells. PNAS 20031005034–5039. ( 10.1073/pnas.0936260100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Verzi MP, Shin H, San Roman AK, Liu XS, Shivdasani RA. Intestinal master transcription factor CDX2 controls chromatin access for partner transcription factor binding. Molecular and Cellular Biology 201333281–292. ( 10.1128/MCB.01185-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schaffer AE, Taylor BL, Benthuysen JR, Liu J, Thorel F, Yuan W, Jiao Y, Kaestner KH, Herrera PL, Magnuson MAet al. Nkx6.1 controls a gene regulatory network required for establishing and maintaining pancreatic beta cell identity. PLoS Genetics 20139 e1003274. ( 10.1371/journal.pgen.1003274) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rezania A, Bruin JE, Xu J, Narayan K, Fox JK, O'Neil JJ, Kieffer TJ. Enrichment of human embryonic stem cell-derived NKX6.1-expressing pancreatic progenitor cells accelerates the maturation of insulin-secreting cells in vivo. Stem Cells 2013312432–2442. ( 10.1002/stem.1489) [DOI] [PubMed] [Google Scholar]
- 133.Taylor BL, Liu FF, Sander M. Nkx6.1 is essential for maintaining the functional state of pancreatic beta cells. Cell Reports 201341262–1275. ( 10.1016/j.celrep.2013.08.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ariyachet C, Tovaglieri A, Xiang G, Lu J, Shah MS, Richmond CA, Verbeke C, Melton DA, Stanger BZ, Mooney Det al. Reprogrammed stomach tissue as a renewable source of functional β cells for blood glucose regulation. Cell Stem Cell 201618410–421. ( 10.1016/j.stem.2016.01.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Choi E, Roland JT, Barlow BJ, O'Neal R, Rich AE, Nam KT, Shi C, Goldenring JR. Cell lineage distribution atlas of the human stomach reveals heterogeneous gland populations in the gastric antrum. Gut 2014631711–1720. ( 10.1136/gutjnl-2013-305964) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li HJ, Johnston B, Aiello D, Caffrey DR, Giel-Moloney M, Rindi G, Leiter AB. Distinct cellular origins for serotonin-expressing and enterochromaffin-like cells in the gastric corpus. Gastroenterology 2014146754, .e3–764.e3. ( 10.1053/j.gastro.2013.11.048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wassmer CH, Lebreton F, Bellofatto K, Bosco D, Berney T, Berishvili E. Generation of insulin-secreting organoids: a step toward engineering and transplanting the bioartificial pancreas. Transplant International 2020331577–1588. ( 10.1111/tri.13721) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell 20121501223–1234. ( 10.1016/j.cell.2012.07.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.McCracken KW, Catá EM, Crawford CM, Sinagoga KL, Schumacher M, Rockich BE, Tsai YH, Mayhew CN, Spence JR, Zavros Yet al. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 2014516400–404. ( 10.1038/nature13863) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ramasubramanian L, Kumar P, Wang A. Engineering extracellular vesicles as nanotherapeutics for regenerative medicine. Biomolecules 2019104, 8. ( 10.3390/biom10010048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xie M, Ye H, Wang H, Charpin-El Hamri G, Lormeau C, Saxena P, Stelling J, Fussenegger M. β-Cell-mimetic designer cells provide closed-loop glycemic control. Science 20163541296–1301. ( 10.1126/science.aaf4006) [DOI] [PubMed] [Google Scholar]
- 142.Ye H, Daoud-El Baba M, Peng RW, Fussenegger M. A synthetic optogenetic transcription device enhances blood-glucose homeostasis in mice. Science 20113321565–1568. ( 10.1126/science.1203535) [DOI] [PubMed] [Google Scholar]
- 143.Shao J, Xue S, Yu G, Yu Y, Yang X, Bai Y, Zhu S, Yang L, Yin J, Wang Yet al. Smartphone-controlled optogenetically engineered cells enable semiautomatic glucose homeostasis in diabetic mice. Science Translational Medicine 20179eaal2298. ( 10.1126/scitranslmed.aal2298) [DOI] [PubMed] [Google Scholar]
- 144.Desai T, Shea LD. Advances in islet encapsulation technologies. Nature Reviews: Drug Discovery 201716338–350. ( 10.1038/nrd.2016.232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Balboa D, Prasad RB, Groop L, Otonkoski T. Genome editing of human pancreatic beta cell models: problems, possibilities and outlook. Diabetologia 2019621329–1336. ( 10.1007/s00125-019-4908-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mourad NI, Gianello P. Gene editing, gene therapy, and cell xenotransplantation: cell transplantation across species. Current Transplantation Reports 20174193–200. ( 10.1007/s40472-017-0157-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ferreira LMR, Muller YD, Bluestone JA, Tang Q. Next-generation regulatory T cell therapy. Nature Reviews: Drug Discovery 201918749–769. ( 10.1038/s41573-019-0041-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Li D, Zhao B, Luo Y, Limbara S, Zhao B, Zou X, Yang W, Li Y. Transplantation of aire-overexpressing bone marrow-derived dendritic cells delays the onset of type 1 diabetes. International Immunopharmacology 20174913–20. ( 10.1016/j.intimp.2017.05.023) [DOI] [PubMed] [Google Scholar]
- 149.Chen Z, Wang J, Sun W, Archibong E, Kahkoska AR, Zhang X, Lu Y, Ligler FS, Buse JB, Gu Z. Synthetic beta cells for fusion-mediated dynamic insulin secretion. Nature Chemical Biology 20181486–93. ( 10.1038/nchembio.2511) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used during the current study are available from the corresponding author on reasonable request.



 This work is licensed under a
This work is licensed under a