Abstract
Objective
Little is known regarding the functional role of microRNA-193-3p (miR-193-3p) in sepsis. Hence, the aim of the present study was to investigate the effect of miR-193-3p on myocardial injury in mice with sepsis and its mechanism through the regulation of signal transducers and activators of transcription 3 (STAT3).
Methods
The mice model of sepsis was established by cecal ligation and puncture (CLP), septic mice were injected with miR-193-3p agomir, miR-193-3p antagomir or siRNA-STAT3. The expression of miR-193-3p, STAT3 and HMGB1 in the myocardial tissue of septic mice were detected. Cardiac ultrasound, hemodynamics, myocardial injury markers, inflammatory factors and cardiomyocyte apoptosis in septic mice were measured.
Results
MiR-193-3p expression was reduced while STAT3 expression was increased in septic mice. Down-regulated STAT3 or up-regulated miR-193-3p improved cardiac function, attenuated myocardial injury, inflammation and cardiomyocyte apoptosis in septic mice. Knockdown STAT3 reversed the role of inhibited miR-193-3p for mice with sepsis. miR-193-3p targeted STAT3, thereby inhibiting HMGB1 expression.
Conclusion
This study provides evidence that miR-193-3p targets STAT3 expression to reduce HMGB1 expression, thereby reducing septic myocardial damage. MiR-193-3p might be a potential candidate marker and therapeutic target for sepsis.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12967-021-03022-x.
Keywords: Sepsis, MicroRNA-193-3p, Signal transducers and activators of transcription 3, High-mobility group box 1 protein, Cecal ligation and puncture, Inflammatory reaction
Introduction
Sepsis is a serious clinical symptom induced by a deregulated systemic host response to infection contributing to tissue damage and organ dysfunction [1]. It is one of the main causes of infection death and intensive care unit death, and the prognosis has not ameliorated dramatically in the past few years [2]. The common clinical manifestations of sepsis are associated with organ dysfunction and systemic inflammatory response syndrome, including hypoxemia, hemodynamic instability and intestinal barrier dysfunction [3]. The effective sepsis treatments are antimicrobial therapy and early drainage or excision of the infected site [4]. Sepsis can occur after serious trauma, multiple injuries, shock, burns or after major surgery, as well as it develops swiftly from bacteremia to vital organ failure and even death [5]. The severe situation asks for more exploration of potential molecular mechanisms for sepsis treatment. Cardiac dysfunction caused by sepsis is closely related to increased mortality [6, 7]. The mortality rate of sepsis patients with cardiac dysfunction is 70–90%, while the mortality rate of patients without cardiac dysfunction is only 20% [8, 9]. Therefore, it is very important to explore how to improve cardiac dysfunction caused by sepsis. In addition, the modeling method by cecal ligation and puncture (CLP) is a commonly used to simulate septic myocardial injury [10, 11]. The main purpose of this study was to explore the myocardial injury induced by sepsis rather than just heart failure.
MicroRNAs (miRs) are a species of non-coding RNAs with short ribonucleic acid molecules of about 17–25 nucleotides in length and typically bind to the 3′untranslated region (3′UTR) of target mRNAs to modulate their expression levels [12]. A study has reported that overexpression of miR-135a can increase inflammation and myocardial dysfunction induce by sepsis [13]. Another study has revealed that miR-23b mediates the activation of cardiac fibrosis of late sepsis myocardial dysfunction [14]. A previous study has identified that the miR-193 family is involved in the pathological mechanisms (including diffuse myocardial fibrosis and electrical/ionic remodeling processes) of cardiovascular diseases [15–17]. Wang et al. have found that expression levels of miR-193b* in patients with mild sepsis, severe sepsis and septic shock are significantly lower than in normal controls [18]. It is presented that miR-193 binds to signal transducer and activator of transcription 3 (STAT3) [19]. STAT3 is a highly modulated transcription factor which serves a critical role in cell growth and inflammation, as well as survival [20]. Moreover, it is revealed that miR-29a suppresses sepsis by inhibition of STAT3 induced by IL-10 in human monocytes [21]. There is a study reports that myocardial injury in obese mice with sepsis may occur by changing the STAT3 pathway [22]. STAT3 is involved in modification of high-mobility group box 1 protein (HMGB1) to mediate inflammatory response [23]. HMGB1 is a multifunctional nuclear protein that has pro-inflammatory property during sepsis [24]. Movement of HMGB1 between cell compartments is a dynamic process caused by disease processes including sepsis [25]. In this research, we speculated that miR-193-3p could regulate the uncontrolled inflammatory response in sepsis via modulating STAT3.
Materials and methods
Compliance with ethical standards
All animal experiments were conducted in line with the Guide for the Care and Use of Laboratory Animal of the National Institutes of Health. The protocol was permitted by the Committee on the Ethics of Animal Experiments of Mannheim Medical Faculty Heidelberg University Center for Biomedicine and Medical Technology Mannheim.
Animals
Male C57BL/6 mice (the Experimental Animal Center of Heidelberg University, Mannheim, Germany), aging 10–12 w, were housed in a special aseptic device which kept constant temperature and humidity and had sufficient food and water with natural light–dark cycle [26, 27]. Through a priori analysis of G*Power software, we calculated that the total sample size required was 120 [effect size f = 0.4; α err prob = 0.05; Power (1-β erro prob) = 0.89] and divided it into eight groups [28]. The mice were randomly assigned into 8 groups (n = 15/group): (1) sham group (without any treatment); (2) CLP group (modeled with CLP); (3) CLP + miRagomir negative control (NC) group (tail vein injection with miR-193-3p agomir NC at 30 mg/kg for 3 d and modeled with CLP on the 4th d); (4) CLP + miR-193-3p agomir group (tail vein injection with miR-193-3p agomir at 30 mg/kg for 3 d and modeled with CLP on the 4th d); (5) CLP + small interfering RNA (siRNA)-NC group (tail vein injection with 100 ng siRNA-STAT3 NC for 3 d and modeled with CLP on the 4th d); (6) CLP + siRNA-STAT3 group (tail vein injection with 100 ng siRNA-STAT3 for 3 d and modeled with CLP on the 4th d); (7) CLP + miR-193-3p antagomir + siRNA-NC group (tail vein injection with miR-193-3p antagomir at 30 mg/kg and 100 ng siRNA-STAT3 NC for 3 d and modeled with CLP on the 4th d); (8) CLP + miR-193-3p antagomir + siRNA-STAT3 group (tail vein injection with miR-193-3p antagomir at 30 mg/kg and 100 ng siRNA-STAT3 for 3 d and modeled with CLP on the 4th d) [10, 29]. miR-193-3p agomir/antagomir, miR agomir NC, siRNA-NC and siRNA-STAT3 were synthesized by Guangzhou RiboBio Co., Ltd. (Guangdong, China).
Preparation of mice models of CLP
All mice were fasting for 12 h before operation and then anaesthetized with 1% pentobarbital sodium (80 μg/g, Sigma-Aldrich, SF, CA, USA) through intraperitoneal injection. A long 1.5 cm longitudinal incision along the midline of abdomen was made to separate the skin and subcutaneous tissue layer by layer. The rectus abdominis and peritoneum were cut open in the abdominal white line, the cecum and its surrounding intestine were fully exposed with sterile forceps, then the cecum was gently pulled out, and next the cecum and mesenteric vessel were ligated with the No. 4 surgical line at the end of the cecum approximately 1/3 to 1/2 of the length of the cecum. Then 23 G needle was used to puncture the ligated part of cecum, and a little intestinal content was extruded slightly to ensure that the puncture hole was unobstructed. Then all the intestines were brought back into the abdominal cavity and the incision was sutured layer by layer with No. 4 surgical line. In the sham group, the cecum was pulled out from the abdominal cavity by laparotomy, and the cecum was returned without puncture and ligation. The animal experiment flow was shown in Additional file 1: Figure S1.
Cardiac ultrasound detection
The mice cardiac function was measured by high resolution transthoracic color echocardiography Vevo 2100 (Visualsonics, Toronto, Canada) after modeling 48 h. Mice were pre-inhaled 2% isoflurane (A506592, Sangon, Shanghai, China) and 1 L/min 100% O2 for anesthesia and fixed on the ventral side of the heating platform to maintain body temperature at 37 °C ± 0.5 °C. Heart rate (HR) and respiratory physiology were continuously monitored by electrocardiogram (ECG) electrodes. ECG was performed with Vevo 2100 system with 40 MHz sensor (Visualsonics). The ECG probe was placed in front of the left chest of mice. The short axis of left ventricle near sternum was taken for 2D ultrasound. The left ventricular motion was recorded at papillary muscle level by M ultrasound, and the left ventricular internal dimension at diastole (LVIDd) and systole (LVIDs), and shortening fraction (FS) was gauged. FS = (LVIDd − LVIDs)/LVIDd × 100%.
Hemodynamic detection
Hemodynamic detection was performed by Millar Pressure–Volume System (MPVS-300, Millar Instrument Powerla, Houston, Texas, USA). The mice were anesthetized for tracheotomy and mechanical ventilation, the right common carotid artery was separated and incised, the micro-catheter was inserted through the incision and the mean arterial pressure (MAP) was recorded. The maximum rate of rise of left ventricular pressure increase (+ dp/dt max)/decrease (− dp/dt max), left ventricular systolic pressure (LVSP) and left ventricular end-diastolic pressure (LVEDP) were tested.
Collection of blood and myocardial tissue samples
The blood was drawn from the aorta abdominalis, placed for 2 h and centrifuged for 20 min with 3000 r/min and the serum was stored at − 80℃. After that, the mice were euthanized by cervical dislocation. The heart tissue was fixed in 4% paraformaldehyde (A500684, Sangon), and the remaining tissue was stored at − 80 °C.
Detection of various indices in serum of mice
The contents of brain natriuretic peptide (BNP), cardiac troponinI (cTnI), HMGB1, lactic dehydrogenase (LDH), creatine kinase (CK), creatine kinase isozyme (CK-MB), tumor necrosis factor-a (TNF-α), interleukin-6 (IL-6) and interleukin-1β (IL-1β) were measured by enzyme-linked immunosorbent assay (ELISA). BNP (E-EL-M0204c), cTnI (E-EL-M1203c), LDH (E-EL-M0419c), CK (E-EL-H1433c), CK-MB (E-EL-M0355c, all from Elabscience, Hubei, China), TNF-α (ab208348), IL-6 (ab100713), IL-1β (ab197742, all from abcam, UK), and HMGB1 (MOFI00232, Beijing Lebo Biotechnology Co., Ltd. (Beijing, China) were applied. The serum was remelted at 4 °C. The optical density (OD) value was measured at the 450 nm of a Sunrise microplate reader (Tecan Group Ltd., Männedorf, Switzerland).
Detection of myeloperoxidase (MPO) activity
The myocardial tissue was homogenized with pre-cooled phosphate buffered saline. The tissue homogenate (0.9 mL) was mixed with 0.1 mL MPO, and tested by the kit (A044-1-1, NanJing JianCheng Bioengineering Institute, Nanjing, China). The mixture were bathed for 15 min, and then supplemented to hydrogen peroxide and placed in a colorimetric plate, and cultured at 60 °C with water bathing for 10 min, and the OD value was tested at the 460 nm.
Hematoxylin–eosin (HE) staining
The heart tissue was fastened with 4% paraformaldehyde (A500684, Sangon), dehydrated with gradient alcohol (70%, 80%, 90%, 95%, 100%) and cleared with xylene (A530011, Sangon). The coronal section of the middle left ventricle was embedded in paraffin, cut into 5 μm and treated with HE staining kit (E607318, Sangon). The tissues were dyed with hematoxylin, differentiated with hydrochloric acid alcohol and counterstained with eosin. Finally, the tissues were dehydrated with alcohol, cleared with xylene, sealed with neutral gum and dried. The pathological changes of myocardial tissues were observed and photographed under a light microscope (Leica Microsystems, Wetzlar, Germany).
TdT-mediated dUTP-biotin nick end-labeling (TUNEL) assay
The paraffin samples of the myocardium were prepared and tested by TUNEL kit (MK1025, Boster Biological Technology Co., Ltd., Hubei, China). Apoptotic cells were those with brown or brown-yellow particles. A total of five high-power visual fields were randomly taken from each section. ImageJ software was utilized to calculate the number of TUNEL-positive cells in each group. Apoptosis rate = (number of positive cells/total number of counted cells) × 100%.
Masson staining
The paraffin sections were heated at 65 °C for 3 h, routinely deparaffinized and dehydrated and placed in 10% trichloroacetic acid and 10% potassium dichromate liquid. Then, the sectons were processed with hematoxylin (PT001, Bogoo, Shanghai, China), put in 1% Ponceau (HL12202, Shanghai Haling Biotechnology Co., Ltd., Shanghai, China) and 1% eosin (HPBIO-SJ820, Shanghai Hepeng Biological Co., Ltd., Shanghai, China) for 40 min, and added with 1% glacial acetic acid (first) and 1% molybdic acid solution (after) to terminate the reaction. Followed by conventional dehydration, the sections were permeabilized and resin-sealed. Positive result: basement membrane and collagen fibers were stained blue or green, immune complexes were stained red, and cell nuclei were stained blue-brown. Images were captured under a polarized light microscope in 5 fields of view. Collagen volume fraction was calculated by Image-Pro plus 5.1 image analysis software (Media Cybernetics, Rockville, MD, USA). Collagen volume fraction (%) = collagen area/full field area × 100%.
Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
Tissue total RNA was extracted with Trizol reagent (Invitrogen, CA, USA). Reverse transcription was executed using the PrimeScriptTM RT Master Mix (RR036A, TaKaRa, Kusatsu, Japan) for mRNA and the miRcute Plus miRNA First-Strand cDNA Kit (KR211, Tiangen, Beijing, China) for miRNA. The primer sequences of miR-193-3p, U6, STAT3, HMGB1 and glyceraldehyde phosphate dehydrogenase (GAPDH) were attained by Genbank and the primer sequences were composed by Invitrogen (Shanghai, China) (Table 1). The relative expression of miR-193-3p was standardized by U6, and STAT3 and HMGB1 were standardized by GAPDH. The relative expression of target gene in myocardial tissue was computed by 2−ΔΔCt.
Table 1.
Primer sequence
| Gene | Sequence (5′ → 3′) |
|---|---|
| miR-193-3p | F: 5′-AACTGGCCTACAAAGTCCCAGT-3′ |
| R: universal primers | |
| U6 | F: 5′-GCTTCGGCAGCACATATACTAAAAT-3′ |
| R: 5′-CGCTTCACGAATTTGCGTGTCAT-3′ | |
| STAT3 | F: 5′-CAATACCATTGACCTGCCGAT-3′ |
| R: 5′-GAGCGACTCAAACTGCCCT-3′ | |
| HMGB1 | F: 5′-TGGCAAAGGCTGACAAGGCTC-3′ |
| R: 5′-GGATGCTCGCCTTTGATTTTGG-3′ | |
| GADPH | F: 5′-TGATGACATCAAGAAGGTGGTGAA-3′ |
| R: 5′-TCCTTGGAGGCCATGTAGGCCAT-3′ |
F forward, R reverse, miR-193-3p microRNA-193-3p, STAT3, signal transducers and activators of transcription 3, HMGB1 high-mobility group box 1 protein, GADPH glyceraldehyde phosphate dehydrogenase
Western blot analysis
The myocardial tissue was lysed, and the protein was extracted and quantified. The protein (50 μg) was separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis, transferred to membrane by polyvinylidene fluoride membrane, and then blocked on a 5% skim milk diluted by Tris-buffered saline with Tween 20 (TBST) for 2 h. The membrane was probed with rabbit anti-primary antibodies STAT3 (ab68153, 1:1000), HMGB1 (ab18256, 1:1000) and GAPDH (ab9485, 1:1000, all from Abcam), overnight, and then reprobed with secondary antibody labeled by horseradish peroxidase (1:4000) for 1 h. Band exposure and analyses were finally conducted.
Co-immunoprecipitation (co-IP) assay
The tissue was lysed on ice to extract the protein. The protein extract was centrifuged at 13,200 rpm and the supernatant was collected, and incubated with 50 μL magnetic beads on ice. Next, HGMB1 or STAT3 antibody was added to the supernatant, incubated overnight at 4 °C, and tested by Western blot [31, 32].
Dual luciferase reporter gene assay
The biological information software website https://cm.jefferson.edu/rna22/Precomputed/ predicted the potential downstream target gene STAT3 of miR-193-3p. The wild type (pGL3-STAT3-3′UTR WT) and mutant type (pGL3-STAT3-3′UTR MUT) dual luciferase reporter plasmids containing miR-193-3p binding sites (Shanghai Genechem Co., Ltd., Shanghai, China) were constructed, then mixed with miR-193-3p mimic and mimic NC to co-transfect with 293 T cells for 48 h, respectively. According to the instructions of Dual-Luciferase ®Reporter Assay System (E1910, promega, MI, USA), the cells were added with 30 μL 1× Passive Lysis Buffer and reacted. After absorbing 40 μL cell lysate to a Lockwell maxisorp detection plate, 20 μL luciferase assay reagent was added in, then the fluorescence value of firefly luminescence was tested by the microplate reader immediately. Next, the fluorescence value of renilla luminescence was detected. The relative fluorescence value was calculated as the fluorescent value of firefly luminescence/fluorescent value of renilla luminescence.
Statistical analysis
All data were analyzed by SPSS 19.0 (IBM, NY, USA) software and GraghPad Prism 6 statistical software. G*Power 3.1 software was used to perform the power calculation to determine the required sample size. The measurement data were conveyed by mean ± standard deviation. Comparison between two groups was conducted by independent sample t test. Comparisons among multiple groups were assessed by one-way analysis of variance (ANOVA), and Tukey's post hoc test was used for pairwise comparison after ANOVA analysis. P value < 0.05 was indicative of statistically significant difference.
Results
MiR-193-3p expression is reduced in myocardial tissues of mice with sepsis
To explore the effect of miR-193-3p on myocardial injury in septic mice, we constructed a sepsis mouse model and used cardiac ultrasound, hemodynamics and measurements of serum factors to identify the model. It was found that in relation to the sham group, LVIDs and HR level enhanced while FS, LVSP, LVEDP, + dp/dt max, − dp/dt max and MAP level depressed in the CLP group (all P < 0.05) (Fig. 1A–F). ELISA measured that BNP, cTnI, HMGB1, LDH, CK, CK-MB, TNF-α, IL-6 and IL-1β levels enhanced in the CLP group relative to the sham group (all P < 0.05) (Fig. 1G–O). Thus, the success of sepsis induction was validated.
Fig. 1.
Myocardial injury of mice with sepsis. A–C Echocardiographic parameters comparison of mice; D–F Hemodynamic indexes of mice; G–I ELISA detected cTnI, BNP and HMGB1 in the serum; J–L ELISA detected LDH, CK and CK-MB expression levels; M–O ELISA detected TNF-α, IL-6 and IL-1β levels; n = 15; *P < 0.05 vs. the sham group
Also, we used HE staining and Masson staining to detect the pathological condition of myocardial tissue. It was displayed that in the sham group, the myocardial fibers were clear, and the interval of cardiomyocytes was uniform and clear without degeneration and necrosis. In the CLP group, cardiomyocytes were obviously denatured, necrotic, nucleus was swollen, some cells were broken and dissolved, some myocardial fibers were broken, denatured and dissolved, inflammatory cell infiltration could be seen between cells, and the fibrosis degree was enhanced. The increased collagen volume fraction was found in the CLP group in contrast to the sham group (P < 0.05) (Fig. 2A, B).
Fig. 2.
miR-193-3p is degraded in mice with sepsis. A HE staining detected myocardial tissue pathology (×400); B Masson staining detected myocardial tissue fibrosis (×400); C TUNEL staining detected myocardial tissue apoptosis (×400); D ELISA detected MPO activity; E RT-qPCR detected miR-193-3p expression; n = 15; *P < 0.05 vs. the sham group
TUNEL staining observed apoptosis, MPO detection measured myocardial neutrophil infiltration and RT-qPCR examined miR-193-3p expression in the myocardial tissue. The results displayed that by comparison to the sham group, apoptosis rate of cardiomyocytes increased, MPO activity promoted and miR-193-3p level reduced in the CLP group (all P < 0.05) (Fig. 2C–E).
Restored miR-193-3p inhibits myocardial injury in mice with sepsis
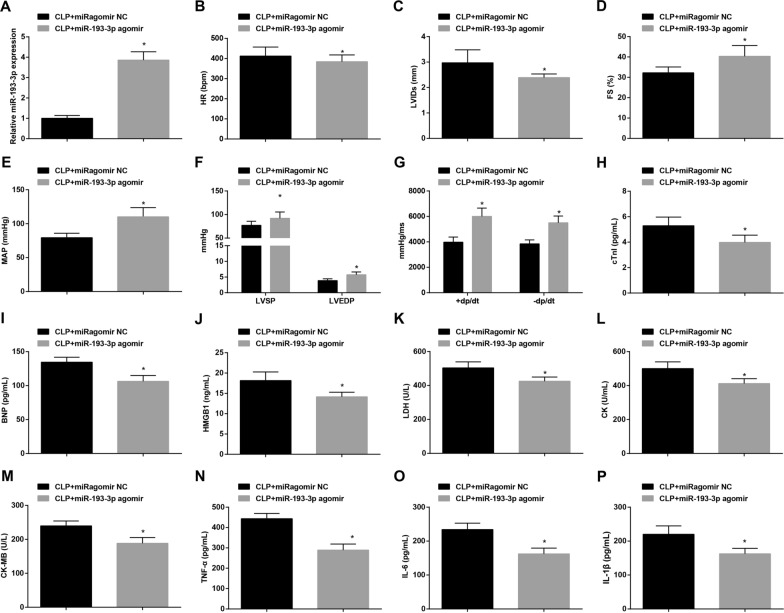
To explore the effect of miR-193-3p on myocardial injury in septic mice, we up-regulated miR-193-3p in septic mice, and used RT-qPCR to detect miR-193-3p level. It was manifested that in contrast with the CLP + miRagomir NC group, miR-193-3p expression elevated in the CLP + miR-193-3p agomir group (P < 0.05) (Fig. 3A). Then, the function of restored miR-193-3p was further navigated with the results highlighting that by comparison with the CLP + miRagomir NC group, LVIDs and HR level declined while FS, LVSP, LVEDP, + dp/dt max, − dp/dt max and MAP level elevated in the CLP + miR-193-3p agomir group (all P < 0.05) (Fig. 3B–G). Also, versus the CLP + miRagomir NC group, the CLP + miR-193-3p agomir group displayed reduced BNP, cTnI, HMGB1, LDH, CK, CK-MB, TNF-α, IL-6 and IL-1β contents (all P < 0.05) (Fig. 3H–P).
Fig. 3.
Restored miR-193-3p inhibits myocardial injury in mice with sepsis. A RT-qPCR detected miR-193-3p expression; B–D Echocardiographic parameters comparison of mice; E–G hemodynamic indexes of mice; H–J ELISA detected cTnI, BNP and HMGB1 in the serum; K–M ELISA detected LDH, CK and CK-MB expression levels; N–P ELISA detected TNF-α, IL-6 and IL-1β levels; n = 15; *P < 0.05 vs. the CLP + miRagomir NC group
HE staining, Masson staining, TUNEL staining and MPO activity detection demonstrated that with respect to the CLP + miRagomir NC group, the myocardial tissue damage and the level of fibrosis were reduced, collagen volume fraction was reduced, apoptosis was inhibited and MPO activity was impaired in the CLP + miR-193-3p agomir group (all P < 0.05) (Fig. 4A–D).
Fig. 4.
Restored miR-193-3p inhibits myocardial injury in mice with sepsis. A HE staining detected myocardial tissue pathology (×400); B Masson staining detected myocardial tissue fibrosis (×400); C TUNEL staining detected myocardial tissue apoptosis (×400); D ELISA detected MPO activity; n = 15; *P < 0.05 vs. the CLP + miRagomir NC group
MiR-193-3p directly targets STAT3
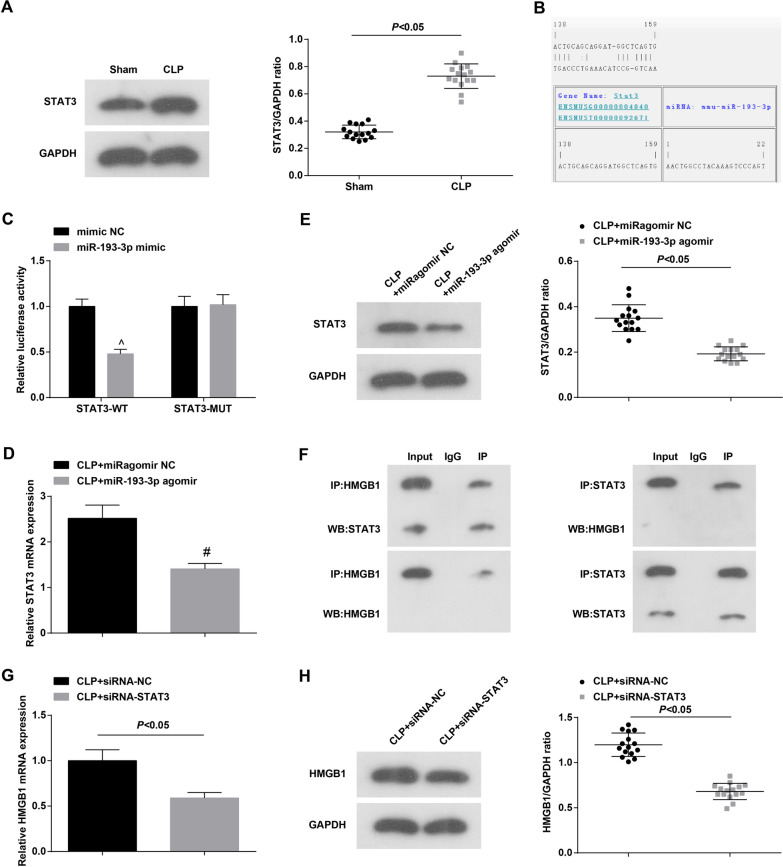
STAT3 is associated with sepsis [33]. To understand the role of STAT3 in septic mice, Western blot was applied to measure STAT3 level in myocardial tissue. The results showed (Fig. 5A) that contrasted to the Sham group, STAT3 protein expression elevated in the CLP group (P < 0.05). Through bioinformatics software analysis, it was found that there was a miR-193-3p binding site in the 3’UTR of STAT3 gene (Fig. 5B). The results of dual luciferase reporter gene assay showed that when co-transfected with pGL3-STAT3-3′UTR WT, the luciferase activity in the miR-193-3p mimic group was dramatically lower than that in the mimic NC group (P < 0.05) (Fig. 5C). Also, RT-qPCR and Western blot detected STAT3 level changes and found that versus the CLP + miRagomir NC group, the CLP + miR-193-3p agomir group showed reduced STAT3 expression (Fig. 5D, E). These results suggested that miR-193-3p could inhibit STAT3 expression.
Fig. 5.
STAT3 is the target gene of miR-193-3p. A Western blot detected STAT3 protein expression; B online software to predict the binding site of miR-193-3p to STAT3; C the targeting relationship between miR-193-3p and STAT3 was verified by luciferase activity test (N = 3); D RT-qPCR detected STAT3 expression; E Western blot detected STAT3 protein expression; F Co-IP detected the binding relationship between HMGB1 and STAT3 (N = 3); G, H RT-qPCR and Western blot detected the expression level of HMGB1; n = 15
STAT3 inhibition can reduce the increase in HMGB1 expression in CLP mice, and inhibit the excessive increase in serum HMGB1 levels in CLP mice [34, 35]. Using co-IP analysis, it was found that HMGB1 can bind to STAT3 to form a precipitated complex (Fig. 5F). RT-qPCR and Western blot detection showed that depleting STAT3 repressed HMGB1 expression (Fig. 5G, H).
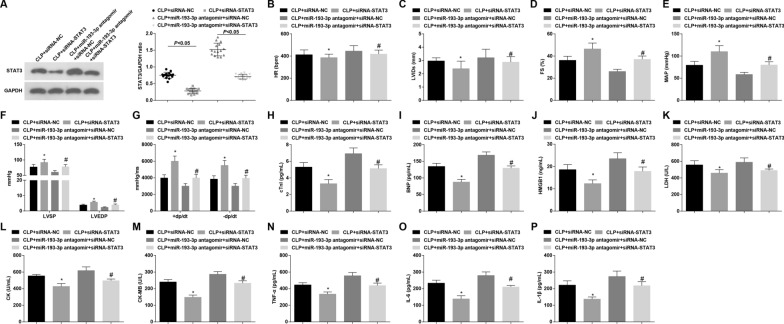
Loss of STAT3 attenuates myocardial injury in mice with sepsis and reverses the effect of miR-193-3p inhibition on septic mice
To explore the effect of miR-193-3p/STAT3 on myocardial injury in septic mice, we silenced STAT3 and/or miR-193-3p. It was measured that compared to the CLP + siRNA-NC group and the CLP + miR-193-3p antagomir + siRNA-NC group, respectively, STAT3 expression dropped in the CLP + siRNA-STAT3 group and the CLP + miR-193-3p antagomir + siRNA-STAT3 group (both P < 0.05) (Fig. 6A). Then, we noticed that versus the CLP + siRNA-NC group and the CLP + miR-193-3p antagomir + siRNA-NC group, respectively, LVIDs and HR level decreased while FS, LVSP, LVEDP, + dp/dt max, − dp/dt max and MAP level elevated in the CLP + siRNA-STAT3 group and the CLP + miR-193-3p antagomir + siRNA-STAT3 group (all P < 0.05) (Fig. 6B–G). Moreover, with respect to the CLP + siRNA-NC group and the CLP + miR-193-3p antagomir + siRNA-NC group, respectively, reduced BNP, cTnI, HMGB1, LDH, CK, CK-MB, TNF-α, IL-6 and IL-1β contents were measured in the CLP + siRNA-STAT3 group and the CLP + miR-193-3p antagomir + siRNA-STAT3 group (all P < 0.05) (Fig. 6I–P).
Fig. 6.
Loss of STAT3 attenuates myocardial injury in mice with sepsis and reverses the effect of miR-193-3p inhibition on septic mice. A Western blot detected STAT3 protein expression. B–G Echocardiographic parameters and hemodynamic indexes of mice; H–J ELISA detected cTnI, BNP and HMGB1 in the serum; K–M ELISA detected LDH, CK and CK-MB expression levels; N–P ELISA detected TNF-α, IL-6 and IL-1β levels; n = 15; *P < 0.05 vs. the CLP + siRNA-NC group; #P < 0.05 vs. the CLP + miR-193-3p antagomir + siRNA-NC group
Further tests revealed that in comparison to the CLP + siRNA-NC group and CLP + miR-193-3p antagomir + siRNA-NC group, respectively, myocardial tissue damage was alleviated, fibrosis, collagen volume fraction, apoptosis and MPO activity were reduced in the CLP + siRNA-STAT3 group and the CLP + miR-193-3p antagomir + siRNA-STAT3 group (all P < 0.05) (Fig. 7A–D).
Fig. 7.
Loss of STAT3 attenuates myocardial injury in mice with sepsis and reverses the effect of miR-193-3p inhibition on septic mice. A HE staining detected myocardial tissue pathology (×400); B Masson staining detected myocardial tissue fibrosis (×400); C TUNEL staining detected myocardial tissue apoptosis (×400); D ELISA detected MPO activity; n = 15; *P < 0.05 vs. the CLP + siRNA-NC group; #P < 0.05 vs. the CLP + miR-193-3p antagomir + siRNA-NC group
Discussion
Sepsis is a life-threatening organ dysfunction induced by a dysregulated host response to infection [36]. A previous study has verified that miRNAs play an important biological process in the progression of sepsis by targeting specific genes [37]. Another study has reported that andrographolide sulfonate exerts anti-sepsis action in mice by suppressing STAT3 pathways [38]. As the related mechanisms of miR-193-3p in sepsis still remained enigmatic, our study was to inquire the effect of miR-193-3p in sepsis and its inner mechanisms.
Our study has provided evidence that miR-193-3p expression degraded and STAT3 expression raised in myocardial tissues of mice with sepsis. Recent study has presented that miR-193 expression is specifically degraded not only in human liver fibrosis but also in experimental hepatofibrogenesis [39]. Another study has presented that miR-193 expression is declined in congenital diaphragmatic hernia induced by nitrofen [40]. It is reported that cardiac phosphorylation of STAT3 is elevated at baseline in obese mice and enhanced further only in obese septic mice [22]. Similarly, a previous study has proved that the expression of STAT3 is heightened in patients with sepsis [41]. Furthermore, our study has proven that miR-193-3p directly targeted STAT3. Consistent with our study, it is showed that miR-193 is bind the 3′UTR of a number of genes which are either participated in the stringent modulation of inflammation or factors that cause it to deteriorate, such as STAT3 [19].
In addition, it was revealed in our study that down-regulated STAT3 and up-regulated miR-193-3p decreased LVIDs, HR, BNP, LDH, CK, CK-MB, cTnI, TNF-α, IL-6, IL-1β and HMGB1 levels, MPO activity, and enhanced FS, LVSP, LVEDP, + dp/dt max, − dp/dt max and MAP levels, as well as attenuated apoptosis of cardiomyocytes. It has been suggested previously that CLP-induced sepsis leads to cardiac dysfunction, inflammatory response and apoptosis [42]. Another study has verified that LVIDd and LVIDs are markedly enhanced in the acute myocardial infarction model group, while the number of apoptotic cardiomyocytes is notably raised in the model group [43]. Additionally, an experiment presents that in relation to the sham group, LVIDs and LVIDd are significantly raised in the myocardial ischemia–reperfusion injury model group [44]. A study reveals that following cardiopulmonary bypass, inflammatory cytokines (IL‑1β, IL‑6 and TNF‑α), myocardial injury markers (cTnI, LDH, CK‑MB and BNP) and MDA levels are dramatically raised [45]. LDH is an important enzyme to bring energy in hypoxia via anaerobic glycolysis [46]. In addition, it is displayed that corydalis hendersonii hemsl suppresses the serum levels of LDH and CK-MB as well as depresses expressions of TNF-α, IL-6 and IL-1β in plasma and down-regulates p-STAT3 expressions in acute myocardial infarction mice [47]. It has been documented that plasma levels of pro-inflammatory cytokines such as IL-6 and TNF-α enhances markedly in sepsis patients [48]. MPO is a kind of peroxidase enzyme induced by activated leukocytes which plays a pathogenic role in cardiovascular disease, mostly through initiating endothelial dysfunction [49]. A previous study contends that STAT3 expression is higher in CD4+ T cells of acute graft-versus-host disease (aGVHD) patients in contrast to those without aGVHD [50]. Ren et al. notes that elevated miR-193a attenuates cell apoptosis in I/R injury H9c2 cells [51]. Furthermore, it is reported that overexpression of miR-193a-5p inhibits the apoptosis of hepatocellular carcinoma cells [52]. A study also showes that LC28 and its analogs inhibits survival of cisplatin-resistant ovarian cancer cells via suppressing STAT3 signaling and promoting apoptosis [53]. Moreover, another study reports a protective effect of STATTIC suppresses STAT3 activity on cardiomyocytes apoptosis [54]. STAT3 inhibition can reduce the increase in HMGB1 expression in CLP mice, and inhibit the excessive increase in serum HMGB1 levels in CLP mice [34, 35].
Altogether, investigation revealed that up-regulating miR-193-3p alleviated myocardial injury in mice with sepsis by inhibition of STAT3/HMGB1 (Additional file 2: Figure S2). MiR-193-3p might be a potential candidate marker and therapeutic target for sepsis. However, clinical researches might be further carried out for the treatment of sepsis. Additionally, the survival analysis experiment has not been carried out for the time being. In future studies, we will further explore this aspect.
Supplementary Information
Additional file 1: Figure S1. The specific process of animal experiment.
Additional file 2: Figure S2. miR-193-3p affects myocardial injury in septic mice by mediating the STAT3/HMGB1 axis.
Acknowledgements
We would like to give our sincere gratitude to the reviewers for their constructive comments.
Authors’ contributions
YT, BA and DD contributed to study design; JP and DD contributed to manuscript editing; CB and LIMC contributed to experimental studies; CB and JP contributed to data analysis. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
All animal experiments were conducted in line with the Guide for the Care and Use of Laboratory Animal of the National Institutes of Health. The protocol was permitted by the Committee on the Ethics of Animal Experiments of Mannheim Medical Faculty Heidelberg University Center for Biomedicine and Medical Technology Mannheim.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1186/s12967-024-05807-2"
Change history
2/24/2022
This article has been updated to correct the funding information.
Change history
10/31/2024
This article has been retracted. Please see the Retraction Notice for more detail: 10.1186/s12967-024-05807-2
References
- 1.Heun Y, et al. Inactivation of the tyrosine phosphatase SHP-2 drives vascular dysfunction in Sepsis. EBioMedicine. 2019;42:120–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahmel T, et al. Increased circulating microRNA-122 is a biomarker for discrimination and risk stratification in patients defined by sepsis-3 criteria. PLoS ONE. 2018;13(5):e0197637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhan CY, et al. Protective role of down-regulated microRNA-31 on intestinal barrier dysfunction through inhibition of NF-kappaB/HIF-1alpha pathway by binding to HMOX1 in rats with sepsis. Mol Med. 2018;24(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graetz TJ, Hotchkiss RS. Sepsis: preventing organ failure in sepsis—the search continues. Nat Rev Nephrol. 2017;13(1):5–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang S, et al. Diagnostic value of the lncRNA NEAT1 in peripheral blood mononuclear cells of patients with sepsis. Dis Markers. 2017;2017:7962836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung HY, et al. Adjustment of dysregulated ceramide metabolism in a murine model of sepsis-induced cardiac dysfunction. Int J Mol Sci. 2017;18(4):839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landesberg G, et al. Diastolic dysfunction and mortality in severe sepsis and septic shock. Eur Heart J. 2012;33(7):895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fattahi F, Ward PA. Complement and sepsis-induced heart dysfunction. Mol Immunol. 2017;84:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merx MW, Weber C. Sepsis and the heart. Circulation. 2007;116(7):793–802. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Yu Y. MiR-146b protect against sepsis induced mice myocardial injury through inhibition of Notch1. J Mol Histol. 2018;49(4):411–7. [DOI] [PubMed] [Google Scholar]
- 11.Zhang XM, Li LB, Sun CH. The effect of myocardial infarction-associated transcript 2 (Mirt2) and miR-101 on sepsis-induced myocardial injury in rats. Eur Rev Med Pharmacol Sci. 2020;24(11):6299–310. [DOI] [PubMed] [Google Scholar]
- 12.Han YL, Yin JJ, Cong JJ. Downregulation of microRNA-193-3p inhibits the progression of intrahepatic cholangiocarcinoma cells by upregulating TGFBR3. Exp Ther Med. 2018;15(5):4508–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng G, et al. MicroRNA-135a is up-regulated and aggravates myocardial depression in sepsis via regulating p38 MAPK/NF-kappaB pathway. Int Immunopharmacol. 2017;45:6–12. [DOI] [PubMed] [Google Scholar]
- 14.Zhang H, et al. Inhibition of microRNA-23b prevents polymicrobial sepsis-induced cardiac dysfunction by modulating TGIF1 and PTEN. Biomed Pharmacother. 2018;103:869–78. [DOI] [PubMed] [Google Scholar]
- 15.Chang KP, et al. MicroRNA signatures in ischemia–reperfusion injury. Ann Plast Surg. 2012;69(6):668–71. [DOI] [PubMed] [Google Scholar]
- 16.Fang L, et al. Circulating microRNAs as biomarkers for diffuse myocardial fibrosis in patients with hypertrophic cardiomyopathy. J Transl Med. 2015;13:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo X, et al. Regulation of human cardiac ion channel genes by microRNAs: theoretical perspective and pathophysiological implications. Cell Physiol Biochem. 2010;25(6):571–86. [DOI] [PubMed] [Google Scholar]
- 18.Wang HJ, et al. Four serum microRNAs identified as diagnostic biomarkers of sepsis. J Trauma Acute Care Surg. 2012;73(4):850–4. [DOI] [PubMed] [Google Scholar]
- 19.Rao R, Nagarkatti P, Nagarkatti M. Role of miRNA in the regulation of inflammatory genes in staphylococcal enterotoxin B-induced acute inflammatory lung injury and mortality. Toxicol Sci. 2015;144(2):284–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee DS, et al. Biochemical properties of a decoy oligodeoxynucleotide inhibitor of STAT3 transcription factor. Int J Mol Sci. 2018;19(6):1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song X, Wang CT, Geng XH. MicroRNA-29a promotes apoptosis of monocytes by targeting STAT3 during sepsis. Genet Mol Res. 2015;14(4):13746–53. [DOI] [PubMed] [Google Scholar]
- 22.DeMartini T, et al. High fat diet-induced obesity increases myocardial injury and alters cardiac STAT3 signaling in mice after polymicrobial sepsis. Biochim Biophys Acta Mol Basis Dis. 2017;1863(10 Pt B):2654–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.An JY, et al. AG490 ameliorates early brain injury via inhibition of JAK2/STAT3-mediated regulation of HMGB1 in subarachnoid hemorrhage. Exp Ther Med. 2018;15(2):1330–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdulmahdi W, et al. HMGB1 redox during sepsis. Redox Biol. 2017;13:600–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng M, et al. Location is the key to function: HMGB1 in sepsis and trauma-induced inflammation. J Leukoc Biol. 2019;106(1):161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yin J, et al. Knockdown of long non-coding RNA SOX2OT downregulates SOX2 to improve hippocampal neurogenesis and cognitive function in a mouse model of sepsis-associated encephalopathy. J Neuroinflammation. 2020;17(1):320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Z, et al. Kcnh2 mediates FAK/AKT-FOXO3A pathway to attenuate sepsis-induced cardiac dysfunction. Cell Prolif. 2021;54(2):e12962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ozgur BC, et al. The effect of sildenafil and udenafil on testicular damage following ischemia–reperfusion injury in rats. J Urol. 2014;192(4):1272–7. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, et al. Up-regulation of miR-146b-3p protects septic mice with acute respiratory distress syndrome by inhibiting PI3K/AKT signaling pathway. J Bioenergy Biomembr. 2020;52(4):229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qin X, et al. Exosomal miR-196a derived from cancer-associated fibroblasts confers cisplatin resistance in head and neck cancer through targeting CDKN1B and ING5. Genome Biol. 2019;20(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J, et al. MicroRNA-200a inhibits inflammation and atherosclerotic lesion formation by disrupting EZH2-mediated methylation of STAT3. Front Immunol. 2020;11:907. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Xu X, et al. HMGB1 recruits TET2/AID/TDG to induce DNA demethylation in STAT3 promoter in CD4(+) T cells from aGVHD patients. J Immunol Res. 2020;2020:7165230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuan FH, et al. microRNA-30a inhibits the liver cell proliferation and promotes cell apoptosis through the JAK/STAT signaling pathway by targeting SOCS-1 in rats with sepsis. J Cell Physiol. 2019;234(10):17839–53. [DOI] [PubMed] [Google Scholar]
- 34.Imbaby S, et al. Beneficial effect of STAT3 decoy oligodeoxynucleotide transfection on organ injury and mortality in mice with cecal ligation and puncture-induced sepsis. Sci Rep. 2020;10(1):15316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang S, et al. STAT3-dependent CXC chemokine formation and neutrophil migration in streptococcal M1 protein-induced acute lung inflammation. Am J Physiol Lung Cell Mol Physiol. 2015;308(11):L1159–67. [DOI] [PubMed] [Google Scholar]
- 36.Kim H, et al. Circulating biologically active adrenomedullin predicts organ failure and mortality in sepsis. Ann Lab Med. 2019;39(5):454–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang J, et al. Identification of microRNA as sepsis biomarker based on miRNAs regulatory network analysis. Biomed Res Int. 2014;2014:594350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo W, et al. Water-soluble andrographolide sulfonate exerts anti-sepsis action in mice through down-regulating p38 MAPK, STAT3 and NF-kappaB pathways. Int Immunopharmacol. 2012;14(4):613–9. [DOI] [PubMed] [Google Scholar]
- 39.Roy S, et al. miR-30c and miR-193 are a part of the TGF-beta-dependent regulatory network controlling extracellular matrix genes in liver fibrosis. J Dig Dis. 2015;16(9):513–24. [DOI] [PubMed] [Google Scholar]
- 40.Zhu S, et al. Decreased expression of miR-33 in fetal lungs of nitrofen-induced congenital diaphragmatic hernia rat model. J Pediatr Surg. 2016;51(7):1096–100. [DOI] [PubMed] [Google Scholar]
- 41.Zheng S, et al. Blood purification treatment initiated at the time of sepsis diagnosis effectively attenuates serum HMGB1 upregulation and improves patient prognosis. Exp Ther Med. 2017;14(4):3029–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu T, et al. Dexmedetomidine prevents septic myocardial dysfunction in rats via activation of alpha7nAChR and PI3K/Akt-mediated autophagy. Biomed Pharmacother. 2019;120:109231. [DOI] [PubMed] [Google Scholar]
- 43.Sun HY, et al. Influence of MiR-154 on myocardial apoptosis in rats with acute myocardial infarction through Wnt/beta-catenin signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23(2):818–25. [DOI] [PubMed] [Google Scholar]
- 44.Li J, et al. Influences of remifentanil on myocardial ischemia–reperfusion injury and the expressions of Bax and Bcl-2 in rats. Eur Rev Med Pharmacol Sci. 2018;22(24):8951–60. [DOI] [PubMed] [Google Scholar]
- 45.Song D, et al. Hydrogenrich solution against myocardial injury and aquaporin expression via the PI3K/Akt signaling pathway during cardiopulmonary bypass in rats. Mol Med Rep. 2018;18(2):1925–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leyva-Carrillo L, et al. Purification and partial biochemical characterization of recombinant lactate dehydrogenase 1 (LDH-1) of the white shrimp Litopenaeus vannamei. Protein Expr Purif. 2019;164:105461. [DOI] [PubMed] [Google Scholar]
- 47.Bai R, et al. Corydalis hendersonii Hemsl. protects against myocardial injury by attenuating inflammation and fibrosis via NF-kappaB and JAK2-STAT3 signaling pathways. J Ethnopharmacol. 2017;207:174–83. [DOI] [PubMed] [Google Scholar]
- 48.Zhou J, et al. Dysregulation in microRNA expression in peripheral blood mononuclear cells of sepsis patients is associated with immunopathology. Cytokine. 2015;71(1):89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Etwebi Z, et al. Mechanistic role of the calcium-dependent protease calpain in the endothelial dysfunction induced by MPO (myeloperoxidase). Hypertension. 2018;71(4):761–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu YJ, et al. Role of HMGB1 in regulation of STAT3 expression in CD4(+) T cells from patients with aGVHD after allogeneic hematopoietic stem cell transplantation. Clin Immunol. 2015;161(2):278–83. [DOI] [PubMed] [Google Scholar]
- 51.Ren L, et al. Downregulation of long non-coding RNA nuclear enriched abundant transcript 1 promotes cell proliferation and inhibits cell apoptosis by targeting miR-193a in myocardial ischemia/reperfusion injury. BMC Cardiovasc Disord. 2019;19(1):192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang JT, Wang ZH. Role of miR-193a-5p in the proliferation and apoptosis of hepatocellular carcinoma. Eur Rev Med Pharmacol Sci. 2018;22(21):7233–9. [DOI] [PubMed] [Google Scholar]
- 53.Huang W, et al. Small-molecule compounds targeting the STAT3 DNA-binding domain suppress survival of cisplatin-resistant human ovarian cancer cells by inducing apoptosis. Eur J Med Chem. 2018;157:887–97. [DOI] [PubMed] [Google Scholar]
- 54.He F, et al. Inhibition of microRNA-124 reduces cardiomyocyte apoptosis following myocardial infarction via targeting STAT3. Cell Physiol Biochem. 2018;51(1):186–200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. The specific process of animal experiment.
Additional file 2: Figure S2. miR-193-3p affects myocardial injury in septic mice by mediating the STAT3/HMGB1 axis.
Data Availability Statement
Not applicable.