Abstract
The corneal epithelium is continuously renewed by limbal stem/progenitor cells (LSCs), a cell population harbored in a highly regulated niche located at the limbus. Dysfunction and/or loss of LSCs and their niche cause limbal stem cell deficiency (LSCD), a disease that is marked by invasion of conjunctival epithelium into the cornea and results in failure of epithelial wound healing. Corneal opacity, pain, loss of vision, and blindness are the consequences of LSCD. Successful treatment of LSCD depends on accurate diagnosis and staging of the disease and requires restoration of functional LSCs and their niche. This review highlights the major advances in the identification of potential LSC biomarkers and components of the LSC niche, understanding of LSC regulation, methods and regulatory standards in bioengineering of LSCs, and diagnosis and staging of LSCD. Overall, this review presents key points for researchers and clinicians alike to consider in deepening the understanding of LSC biology and improving LSCD therapies.
Keywords: Limbal stem cell, limbal stem cell deficiency, Wnt signaling pathway, Notch signaling pathway, cell therapy, in vivo laser scanning confocal microscopy, anterior segment coherence tomography, small molecules
1. Introduction and Background
The cornea and conjunctiva, which are separated by the junctional zone called the limbus, are the main tissues of the ocular surface. The limbus contains a population of self-renewing stem cells called limbal epithelial stem/progenitor cells (LSCs) that are responsible for maintenance of the integrity of the corneal surface and continuous renewal of the corneal epithelium (Tseng, 1989). Under homeostatic conditions, LSCs maintain a slow cell cycle and can increase in proliferative capacity in the event of injury (Cotsarelis et al., 1989). LSCs can undergo symmetric division that produces 2 daughter stem cells or asymmetric division that produces one daughter stem cell to replenish the stem cell pool and a progenitor cell that is still undifferentiated but has finite proliferative potential (Di Girolamo, 2015; Guo et al., 2018; Tseng, 1989). The progenitor cells proliferate and migrate toward the central cornea, giving rise to terminally differentiated cells. Terminally differentiated cells are eventually shed from the corneal surface as a result of normal functions such as blinking.
LSCs are supported and regulated by the LSC niche, a highly controlled microenvironment in the limbus specifically tailored for harboring and maintaining LSCs. Most reside in limbal regions that are highly protective, such as the limbal crypts, limbal lacunae, and palisades of Vogt (POV) (Davanger and Evensen, 1971; Dua et al., 2005; Zarei-Ghanavati et al., 2011). An alternative corneal epithelial maintenance model has been proposed, specifically that the corneal epithelium-regenerating stem cells could be present throughout the ocular surface, as observed in rodents and pigs (Majo et al., 2008). While the central corneal epithelial cells of rodents and pigs form holoclones indicating the presence of stem cells, human central corneal epithelial cells cannot form holoclones (Bojic et al., 2018; Majo et al., 2008; Pellegrini et al., 1999). This debunks the notion that stem cells exist in the central cornea in humans, and supports the model that corneal epithelial stem cells reside exclusively in the limbus. The limbus forms a barrier to prevent the conjunctival epithelium from extending onto the corneal surface. Loss of LSC function by either a loss of LSCs or the destruction of the limbal niche may lead to disruption of the limbal barrier, impaired epithelial wound healing, and corneal neovascularization, inflammation, and scarring (Deng et al., 2019; Sejpal et al., 2013b). Clinically, the disease is defined as limbal stem cell deficiency (LSCD), which can compromise visual acuity over time and cause blindness in severe cases. LSCs can be replenished by transplantation of limbal tissue or cultivated LSCs (Bonnet et al., 2021; Deng et al., 2020a).
The field has faced distinct challenges in translating LSCD treatment strategies from bench to bedside. Advances in the understanding of LSC biology, LSC regulation, and limbal niche function have opened the possibility of novel LSC therapies via bioengineering of the stem cells. These investigations include the use of small molecules to enrich the stem cell population in culture (Gonzalez et al., 2019a; Zhang et al., 2020), a 3-dimensional (3D) culture that maintains the polarity of the epithelium, xenobiotic-free and/or feeder-free culture system (Gonzalez et al., 2017; Gonzalez et al., 2016; Mei et al., 2014a), and synthetic biocompatible culture substrates (Levis and Daniels, 2016; Nguyen et al., 2018). Although the methods employed for the cultivation of LSCs vary among laboratories worldwide, there is strong evidence supporting the effectiveness of the LSC bioengineering techniques described in this review. More clinically stringent strategies to enrich and cultivate LSCs, i.e., quality controls and release criteria are also being developed to ensure the success of corneal surface reconstruction (Kureshi et al., 2014; Pellegrini et al., 2016). Additionally, significant developments have been made in the diagnostic and staging strategies of LSCD to mitigate misdiagnosis and misinterpreted severity of the disease (Deng et al., 2019; Le et al., 2020a).
In this review, we highlight these recent advances in the understanding of LSC biology, bioengineering of LSCs, and the clinical diagnosis and treatment of LSCD. Specifically, we focus on how recent work enhances LSC identification and cultivation, and the clinical diagnostic parameters to confirm and stage LSCD. These advances will permit standardized clinical outcome measures of LSC treatment, and promote the development of novel in vivo strategies to repopulate LSCs and restore eyesight to patients. Ultimately, these advances will increase the efficacy of current LSCD therapies. Lastly, we discuss the future directions of the field, in particular the use of technologic advances to better understand LSCs and LSCD, and to improve therapeutic outcomes.
2. Limbal Stem/Progenitor Cells and Their Niche
2.1. Molecular and phenotypic markers of limbal stem cells
The search for a marker(s) of LSCs has been an intense area of research since the introduction of the concept of LSCs in the early 1980s. Many proteins are preferentially expressed in basal limbal epithelial cells (Chee et al., 2006; Chen et al., 2004; Schlotzer-Schrehardt and Kruse, 2005) as identified through differential transcriptome and immunohistochemistry studies in both mouse (Sartaj et al., 2017) and human (Collin et al., 2020). These putative markers have been reviewed extensively (Collin et al., 2020; Ebrahimi et al., 2009; Guo et al., 2018; Sartaj et al., 2017).
One widely used marker is ΔNp63α, an isoform of the transcription factor p63. A member of the p53 gene family, p63 has been implicated in stem cell regulation as a factor that promotes cell senescence and genomic stability (Yang et al., 1998; Yang et al., 2009). The p63 gene is normally expressed in the nuclei of keratinocytes with proliferative potential in different tissue types, including skin, cervix, prostate, and cornea (Bergholz and Xiao, 2012; Kawasaki et al., 2006). In addition, p63 is essential for epithelial development and regeneration (Pellegrini et al., 2001). There are 6 p63 isoforms, three of which are considered full length (TAp63) and three of which lack the N-terminal domain (ΔNp63). The 3 ΔNp63 isoforms are most highly expressed in the basal cells of human epithelial tissues. The ΔNp63α isoform is the most highly associated with LSCs, although other ΔNp63 isoforms are expressed in ocular keratinocytes (Kawasaki et al., 2006). Detection of ΔNp63α in LSCs is challenging because the majority of commercially available antibodies lack specificity and recognize both TAp63α and ΔNp63α. Given that the truncated ΔNp63α is the most abundant isoform in the limbus (Di Iorio et al., 2005; Kawasaki et al., 2006; Pellegrini et al., 2001), a pattern of high level expression detected by the antibody recognizing p63α is considered to mainly represent the pattern of ΔNp63α expression. Moreover, a high proportion of cells (>3%) expressing a high level of p63α (classified as p63αbright cells) among cultured limbal epithelial cells has been shown to positively correlate with the clinical success of LSC transplantation (Rama et al., 2010). This points to the critical role of ΔNp63α in LSC self-renewal. Thus, the expression level of p63α indicated by the intensity of fluorescent antibody staining is used to estimate the proportion of LSCs in the culture. An antibody (p40) has been used recently to detect the truncated ΔNp63α in carcinoma cells (Bishop et al., 2012; Liu et al., 2020), however its specificity in identifying LSCs needs further validation.
Nevertheless, since p63α is also a proliferation marker, it is too ubiquitous to be classified as an LSC-specific marker.
Cytokeratins (K) have been used to distinguish corneal epithelial cells of differing maturity. Specifically, K5, K14, K15 and K17 are associated with undifferentiated epithelial cells in the human limbal epithelium and are thus considered markers of undifferentiated corneal epithelial cells, including LSCs and progenitor cells (Kasper et al., 1988; Lyngholm et al., 2008; Merjava et al., 2011). K14 expression is detected in corneal epithelial cells during development but is mainly found in the basal and suprabasal layers of the limbus in adults (Richardson et al., 2017). Of note, K14+ basal cells are shown to migrate during corneal epithelial repair (Park et al., 2019). K5 and K14 pairs are expressed specifically in basal cells of the limbal epithelium (Zhao et al., 2008). K15 is found at the basal layer of the limbus and conjunctiva (Yoshida et al., 2006). K17 is expressed in basal and suprabasal layers of developing epithelium. Upon migration to the cornea, expression of K5, K14, and K17 are lost and K3 and K12 are expressed (Pearton et al., 2005). K19, while found in the basal limbal epithelia, can also be found in the conjunctiva and cornea and is thus not a good marker for undifferentiated cells (Ramirez-Miranda et al., 2011). Additionally, LSCs lack K3 and K12, 2 cytokeratins that mark differentiation seen in more mature cells. Because K3 expression has been found in conjunctival epithelium (Barbaro et al., 2010), K12 is a more specific marker of corneal epithelium than K3. Therefore, during LSC expansion, the percentage of undifferentiated cells (e.g., K14+ cells) and mature corneal epithelial cells (e.g., K12+ cells) could be broadly distinguished by an examination of cytokeratin expression.
Other markers used to identify LSCs include N-cadherin, a cell adhesion molecule found in both basal limbal epithelial cells and melanocytes (Hayashi et al., 2007), and ABCG2, an ATP-binding cassette transporter sub-family G2 protein that is expressed on the cell surface in a specific, small subset of limbal basal epithelial cells (Watanabe et al., 2004). Although promising in its specificity, ABCG2 has not been shown to be associated with increased colony-forming efficiency (CFE) in culture, a functional assessment of clonogenic ability and growth capacity (Umemoto et al., 2006). Whereas ABCG2 may not be a specific marker on its own, the combination of ABCG2+ and ABCB5+ (an ATP-binding cassette, subfamily B, member 5 protein that is also preferentially expressed in p63α+ LSCs) (Ksander et al., 2014) indicates cells that have a high proliferative potential and an ability to differentiate into corneal epithelial cells (Kim et al., 2017). ABCB5+ human or mouse LSCs are also shown to fully restore the cornea in an LSCD mouse model (Ksander et al., 2014). A later study suggested that the level of cell pigmentation is correlated with a higher stemness hierarchy, where the ABCB5+ population identifies more committed progenitor cells and p63+ cells are indicative of less mature stem cells (Liu et al., 2018). This hierarchy of stemness is still being investigated.
We have identified 2 additional putative markers, Frizzled (Fzd) 7 and stage-specific embryonic antigen-4 (SSEA4) (Mei et al., 2014b; Truong et al., 2011). Fzd proteins are cell membrane receptors of the Wnt signaling pathway which plays a critical role in stem cell renewal, proliferation, and differentiation (Nusse and Clevers, 2017). Ten Fzd isoforms have been identified in mammals. Of the 4 Fzd proteins that have a higher expression level in the limbus than in the cornea, only Fzd7 is preferentially expressed at the basal layer of the limbal epithelium and colocalized with K14+/p63αbright cells (Mei et al., 2014b). The presence of high levels of p63α and K14 is indicative of an undifferentiated status. The absence of differentiation marker K12 in the basal limbal epithelium where Fzd7 is highly expressed further supports the hypothesis that Fzd7 is a marker of LSCs (Mei et al., 2014b). These findings suggest that preferential expression of Fzd7 may serve as a marker of LSCs and as an important regulatory niche factor to maintain LSC stemness.
SSEA4 is a glycoprotein with a carbohydrate epitope that is expressed during early development and is a marker of human embryonic stem cells (Henderson et al., 2002). High levels of SSEA4 protein have been detected in all layers of the corneal epithelium (Truong et al., 2011). Examination of the expression of the putative LSC markers ABCG2, ΔNp63α, and K14 in the SSEA4− population confirmed their higher expression levels in these cells than in SSEA4+ cells. When the clonogenicity of the SSEA4+ and SSEA4− populations was examined, the SSEA4− cells formed 25.2% more colonies than the unsorted population did. SSEA4− cells were also smaller than SSEA4+ cells, confirming that SSEA4− cells were less differentiated. The SSEA4− population also was correlated with significantly higher expression of the putative markers Fzd7 and N-cadherin (Mei et al., 2014b; Truong et al., 2011). Taken together, these findings support the notion that the SSEA4− population contains a higher proportion of LSCs and that SSEA4 may be used as a negative marker to enrich the LSC population in culture (Nakatsu and Deng, 2013).
Stem cells tend to be small with a high nucleus-to-cytoplasm ratio. The size of the cell has been used to distinguish stem/progenitor cells from mature corneal epithelial cells (Romano et al., 2003). The smaller cells (diameter ≤12 μm) display measurable LSC phenotypic characteristics such as higher proliferation capacity, increased CFE, and high percentage of correlation with ΔNp63α+, SSEA4− and ABCG2+ expression (De Paiva et al., 2006; Truong et al., 2011).
A common limitation of the LSC markers discussed is that their preferential but nonexclusive expression in LSCs. Additionally, the observed variability in the staining pattern of some proteins such as ABCG2 and ABCB5 may be due to the enzymatic digestion process necessary to isolate LSCs and the fixation step required for immunostaining, both of which have been shown to alter surface antigens. Thus, a definitive LSC-specific marker remains elusive, and it may not be feasible to define LSCs by using a single marker. A set of markers and phenotypic descriptions to define a cell population that exhibits stem cell characteristics is likely necessary. These markers include but are not limited to p63αbright, Fzd7+, SSEA4−, K14+, K12−, ABCG2+, ABCB5+, and N-cadherin+, and a small cell size (diameter ≤12 μm).
2.2. Limbal stem cell niche
The fate of LSCs is governed by their niche, which is a highly complex microenvironment that maintains the homeostasis of the human corneal epithelium. The LSC niche is characterized by the sum of molecular and cellular components that surround and communicate with LSCs to ensure proper function of the corneal surface. A better understanding of the composition and function of the niche is essential to advance our knowledge of LSCs and improve treatments for LSCD. Given the complexity of stem cell niches, LSCs need to be evaluated in the context of their environment rather than as an isolated cell entity or a predestinated anatomical location.
2.2.1. Differential gene expression in the limbal stem cell niche
The identification of specific genes expressed in the human limbal niche is important in understanding LSC regulation in normal conditions. Previous studies have aimed to identify these specific genes by differential gene profiling of the limbus and central cornea in adult human (Chen et al., 2004; Kulkarni et al., 2010; Takacs et al., 2011), and during development (Figueira et al., 2007). However, only comparing the differentially expressed genes identified in the limbus and central cornea does not ensure limbal specificity, as many genes are also expressed in the conjunctiva. To decipher LSC biology in a more comprehensive way and to identify potential LSC markers that remain elusive, we compared gene expression in the central cornea, limbus, and conjunctiva of vervet monkey (Ding et al., 2008) and human eyes by microarray analysis (Nakatsu et al., 2013). As the interaction between the epithelium and stroma is essential, especially in the context of the LSC niche, the underlying stroma was included in the analysis of each tissue type. A total of 186 transcripts were preferentially expressed (>2-fold increase) in the monkey limbus and 216 transcripts in the human limbus when compared with the cornea and conjunctiva. In both monkey and human eyes, the most upregulated biological processes in the limbus were melanin biosynthesis, cell adhesion, and extracellular matrix (ECM) synthesis. Wnt signaling and TGFβ/BMP (Transforming Growth Factor Beta/Bone morphogenetic protein) signaling were 2 major regulatory pathways that were upregulated in the human limbus (Table 1). The expression of 3 molecules involved in the Wnt signaling pathways, i.e., FZD7, FRZB (Frizzled-related protein B), and PITX2 (Paired Like Homeodomain 2), was confirmed at the mRNA or/and the protein level (Mei et al., 2014b; Nakatsu et al., 2013). Additionally, Kulkarni et al. identified several genes of the Notch signaling pathway upregulated in the limbal epithelial crypt (Kulkarni et al., 2010).
Table 1:
Wnt molecules and receptors differentially expressed in the limbus or cornea.
| Category | Gene | Limbus | Cornea |
|---|---|---|---|
| Wnt ligands | WNT2 | + | − |
| WNT3 | + | ++ | |
| WNT6 | ++ | + | |
| WNT7A | + | ++ | |
| WNT7B | + | ++ | |
| WNT10A | + | ++ | |
| WNT11 | ++ | − | |
| WNT16 | ++ | − | |
| Canonical Wnt/β-catenin inhibitors | DKK1 | ++ | − |
| WIF1 | ++ | − | |
| FRZB | ++ | + | |
| SFRP-5 | ++ | − | |
| Frizzled receptors | FZD1 | ++ | − |
| FZD4 | ++ | − | |
| FZD7 | ++ | + | |
| FZD8 | + | ++ | |
| FZD10 | ++ | + |
The finding of preferential gene expression in the limbus reinforces the notion that the LSC niche is tailored to harbor LSCs. Upregulation of tyrosinase proteins in the limbus is consistent with the presence of melanocytes. Although melanin biosynthesis occurs in melanocytes, pigment abundance has been correlated with the differentiation status of corneal epithelium progenitor cells (Liu et al., 2018). Specifically, high pigmentation is associated with stemness, whereas low pigmentation is observed in greater differentiation. This relationship is in contrast to what is observed in the central cornea where no pigments are found. Factors such as pigmentation may provide a more protective environment to prevent UV damage of stem cells.
2.2.2. Structure of limbal stem cell niche
In normal eyes without LSCD, LSCs localize in the limbus. The structure of the limbal region is characterized by a thicker epithelium that contributes to LSC protection because LSCs localize at the basal layer. The nonlinear epithelium-to-stroma junction in the limbus and differences in cellular junction composition might contribute to LSC protection and maintenance by decreasing shear forces (Foster et al., 2014). Basal epithelial cells connect with the basal membrane through hemi-desmosomes and focal complexes (Figure 1). The structural protein content of this junction in the limbus is different from that in the central cornea (Mei et al., 2012).
Figure 1. Schematic of limbal epithelial stem cell niche.
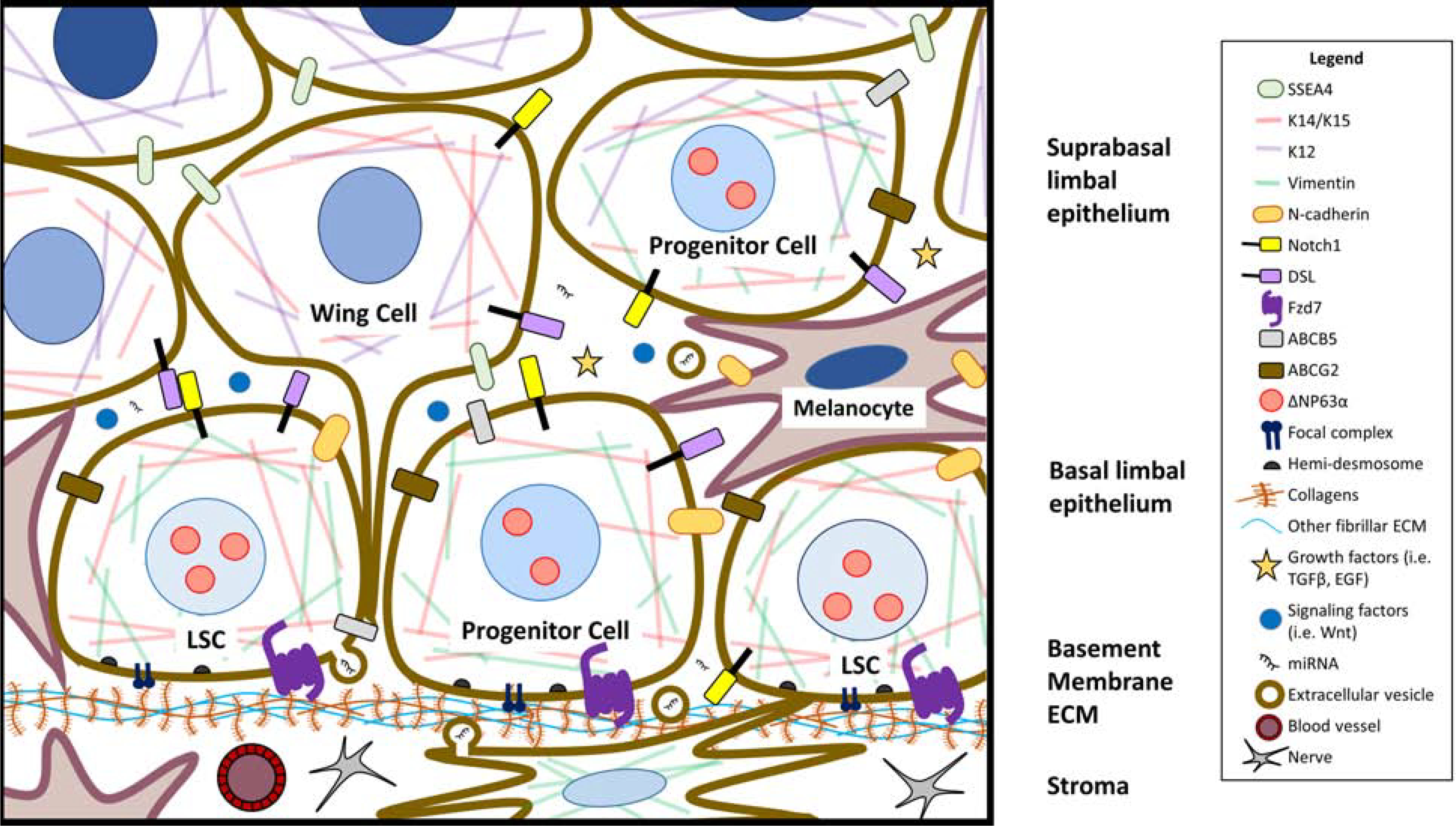
This schematic of the limbal niche highlights currently known markers of limbal stem/progenitor cells (LSCs), the molecular signaling factors that regulate LSC maintenance, and the different niche cells that support LSC function. Lighter blue nuclei represent less differentiated epithelial LSCs and progenitor cells, while the darker nuclei cells in the suprabasal epithelium are more differentiated. Wing cells of the suprabasal epithelium are so named because of their wing-like protrusions. The basal cell layer harbors LSCs, melanocytes, and other basal cells. Structural factors such as cell-to-cell adhesion, signaling through the components of the extracellular matrix, and interaction between basal epithelial cells and niche cells, as well as soluble factors coordinate to regulate LSCs. Limbal stromal cells and epithelial cells produce exosomes that can regulate limbal homeostasis through their cargo, which includes miRNA. The limbus is also highly innervated and vascularized, which are crucial for the support of the limbal niche.
Contrary to the avascular central cornea, the limbal area is densely vascularized, and this vascularization provides a source of nutrients for LSCs and ensures a growth factor-enriched supply to their niche (Notara et al., 2018). In addition, the limbal region is highly innervated (Lawrenson and Ruskell, 1991), which provides LSCs with neurotrophic factors necessary for their maintenance (Kolli et al., 2019).
Although POV have been historically considered the main structure harboring LSCs (Davanger and Evensen, 1971; Goldberg and Bron, 1982), the entire corneoscleral rim represents a potential niche, as POV are not detected in 20% of the population (Townsend, 1991). The interpalisade epithelial crypts contain LSCs, and a positive correlation exists between their presence and crypt volume. Crypts also exhibit intraindividual and interindividual patterns (Grieve et al., 2015). Limbal lacunae, unique structures consisting of cords of limbal epithelial cells in the deep limbal stroma have greater depth than crypts and serve as another niche location for LSCs. The lacunae deeply project into the stroma (>100 μm) but are separated by an acellular ECM layer (Zarei-Ghanavati et al., 2011). Such a deep location into the stroma further protects LSCs from trauma and insult.
In human eyes with pathologic conditions in which the normal limbal structure is altered or absent, LSCs might locate ectopically outside of the limbal region or deeper in the limbal stroma underneath the pannus or corneal neovascularization (Chan et al., 2016; Dua et al., 2009; Zarei-Ghanavati et al., 2011). It is likely that LSCs remodel their immediate surrounding ECM to recreate a suitable niche in pathologic conditions. A lack of POV or presence of corneal neovascularization does not necessarily indicate a lack of LSCs (Deng et al., 2019; Le et al., 2018b). Comprehensive evaluation of the ocular surface structures using in vivo confocal microscopy (IVCM) in normal and pathologic conditions has been informative in the understanding of LSC biology, and LSCD pathophysiology and diagnosis (Le et al., 2018c).
2.2.3. Limbal niche cells
The identification of homeobox genes such as HOP (Homeodomain-only protein) in our microarray gene profiling data suggests the presence of LSC differentiation in the limbus, as HOP is known to regulate differentiation in other cell types such as cardiomyocytes (Chen et al., 2002). The proximity between LSCs and more differentiated progenitors within the niche is an early concept described by the x, y, z model that explains corneal epithelial maintenance (Thoft and Friend, 1983). The more differentiated progenitor cells might provide external cue that regulates proliferation and differentiation of LSCs. Additionally, other surrounding cells such as mesenchymal cells are found in the stroma, which is beneath the basal membrane (Figure 1). The spatial proximity between LSCs and stromal cells might be indicative of a paracrine interaction between those two cell types. Indeed, it has been showed that limbal stromal cells cocultured with LSCs in vitro are able to maintain LSCs in a progenitor-like state by secreting elevated levels of IL6 (Notara et al., 2010). Interestingly, stromal cells located directly beneath the limbus have a greater ability to support LSCs growth as compared to cells located in the deeper limbal stroma (Li et al., 2014). Melanocytes are present in the epithelial layer and in the stroma (Figure 1). Interactions between melanocytes and K19 positive cells located in the niche have been observed (Higa et al., 2005). The ability of melanocytes to support LSC expansion in vitro has also been recently demonstrated (Polisetti et al., 2020). Both mesenchymal cells and melanocytes interact with LSCs and play a role in maintaining niche homeostasis (Dziasko et al., 2014; Polisetti et al., 2016; Xie et al., 2011). The importance of the limbal microvascular net in supporting LSCs is demonstrated in a rabbit model (Huang et al., 2015). Additionally, the presence of B and T lymphocytes, as well as Langerhans cells, in the limbal region was first observed in 1985 (Vantrappen et al., 1985). The interaction between immune cells and LSCs expressing upregulated levels of ICAM-1 and VCAM1 has recently been demonstrated (Polisetti et al., 2016).
The interaction between the niche cells and LSCs can be maintained in cultures by using methods such as explant cultivation. Interestingly, mesenchymal/stromal cells from different sources including the limbal stroma (Nakatsu et al., 2014), adipose tissue, and bone marrow (Gonzalez et al., 2016; Mei et al., 2017) are able to support LSC growth. These mesenchymal cells may produce similar factors that are favorable for maintaining the phenotype of LSCs. However, the efficiency of each type of mesenchymal cells in supporting the expansion of LSCs varies. The selection of mesenchymal cells to serve as feeder cells for clinical application will depend on their availability and capacity to support the growth of the stem cell population. This topic is further discussed in Section 4.
Another important aspect that regulates LSC proliferation and maintains their stemness is cell-to-cell interaction. The yield of the stem cell population in culture is higher when cells are cultured in clusters (i.e., the trypsinization step is omitted during isolation) rather than in single cells (Gonzalez and Deng, 2013). Additionally, when grown as colonies, limbal epithelial cells located in the center of the colony tend to be more differentiated than those at the edge, which are in direct contact with the feeder cells (Mei et al., 2014a). The signals provided by the cell-cell contact in the regulation of LSCs remain to be elucidated.
2.2.4. Extracellular matrix
One critical component of the LSC niche is the ECM, which is a physical scaffold mainly composed of water, collagens, proteins, and polysaccharides. The ECM mediates intercellular communication, signal transduction and provides a 3D structure that contributes to tissue organization, mechanical strength, and biomechanical transduction. As previously mentioned, the protein content of the ECM of the limbus differs from that of the central cornea and conjunctiva (Mei et al., 2012; Schlotzer-Schrehardt et al., 2007). Differential gene profiling of human eye tissues found that the expression of fibronectin, tenascin, and several members of the cadherin and collagen families is upregulated in limbus compared to those of the cornea and conjunctiva (Ding et al., 2008; Nakatsu et al., 2013). Immunohistochemistry studies have also highlighted differences in the ECM composition between basement membrane at the limbus and the cornea.
Collagens, which includes 28 members with varying subunits, exhibit differential expression patterns between the cornea, limbus and conjunctiva. Collagen V and IVα3 are exclusively detected in the cornea while the α1 chain of collagen IV is preferentially expressed in the limbus and conjunctiva. Collagen IVα2, although detected in both cornea and limbus, is enriched in the limbus (Ljubimov et al., 1995). Collagen IVα5–6 and VII, XV, XVII, XVIII are found in both limbus and cornea. Collagen IV coating has been used to selectively enrich limbal epithelial cells with a stem cell phenotype due to their rapid adhesion properties (Li et al., 2005). Additionally, collagen I has been used in tissue engineering approaches as well as hydrogel composition to provide a support for LSC growth. In both cases, LSCs expressed p63α, produced ECM proteins such as collagen IV, and deposited basement membrane proteins such as laminin (Haagdorens et al., 2019; Levis and Daniels, 2016).
Laminins are composed of a heterotrimer of α, β and γ chains and represent a major component of the basement membrane in adult stem cell niches. They can influence cell processes such as adhesion, differentiation and phenotype stability. In the eye, laminins also exhibit a specific expression pattern. Laminin α2 chain is detected in the limbus (Kabosova et al., 2007; Schlotzer-Schrehardt et al., 2007), as well as β2 chain (Schlotzer-Schrehardt et al., 2007; Tuori et al., 1996). Laminins α5β1γ1 and α5β2γ1 based matrices have been showed to increase adhesion, migration and proliferation of LSC in vitro, while maintaining their phenotype (Polisetti et al., 2017).
Other ECM components such as glycoproteins and proteoglycans have differential expression pattern in the limbus and cornea. Tenascin C, a glycoprotein also identified by gene profiling, is mainly detected in the limbus (Ding et al., 2008). Similarly, versican is a basement membrane proteoglycan specific to the limbus. In the LSC niche, proteoglycans mediate cell-basement membrane adhesion (Gattazzo et al., 2014; Polisetti et al., 2016).
Biomechanical properties of ECM such as stiffness also provide external cues in modulating the phenotype of stem cells via multiple regulatory pathways including YAP/TAZ (Yes-Associated Protein/ Transcriptional Co-activator with PDZ-binding Motif), ΔNp63, and β-catenin signaling pathways (Gouveia et al., 2019). Similarly, the upregulated members of the TGF-β/BMP, TNF (tumor necrosis factor), and FGF (fibroblast growth factor) pathways found in the limbus are likely to be influenced by the surrounding ECM (Nakatsu et al., 2013). Additionally, the arrangement and topography of the limbus ECM differ from that of the central cornea ECM (Gipson, 1989). In this early ultrastructural study, the author used electron microscopy to visualize anchoring fibrils projection into the flat basement membrane and deeper stroma to form parallel plaques in the central cornea. On the contrary, the limbal region is characterized by a more complex anchoring fibril network that terminates in undulating plaques at varying depth within the stroma. This observation is also linked to the far more irregular surface of the basal membrane due to POV folds in the limbal region. The convoluted geometric surface could further increase the compactness of basal limbal epithelial cells compared to the superficial epithelial layer. This increased pressure from the adjacent cells might provide a second mechanical signal in the regulation of LSC fate.
2.2.5. Other niche factors
Along with genomic profiling studies, micro RNAs (miRNAs) have been investigated to understand their functions in LSCs and their environment. miRNA represents master regulators of post-transcriptional gene expression (Lee et al., 1993) that are able to uniquely target and silence up to 200 different mRNAs (Krek et al., 2005). In a recent study, 34 miRNAs were found to be differentially expressed in the limbus compared with the central cornea (Kulkarni et al., 2017). Expression of one particular miRNA, miR10b, was 92-fold higher in limbal epithelial cells than in central corneal cells. Additionally, miR10b increased epithelial basal cell layer proliferation through Wnt upregulation. This finding highlights the importance of Wnt signaling in LSC biology. Interestingly, several miRNAs such as miR31, miR184, and miR205 have been associated with corneal epithelial cell differentiation and metabolism regulation (Peng et al., 2012b; Peng et al., 2015; Yu et al., 2008). Mounting evidence supports a role for miRNAs in LSC niche regulation. Because of their association with argonaute proteins that usually mediate miRNA function intracellularly or their function as cargo of extracellular vesicles, miRNAs can mediate cell-cell communication and regulation (McKay et al., 2020; Shojaati et al., 2019; Turchinovich et al., 2011). Further identification of LSC-specific miRNAs and their function will advance our understanding of LSC biology.
Recently, single-cell sequencing has permitted the identification of different cell populations in the limbus of mice. Ten distinct subpopulations were segregated into stem/early transient amplifying cells, mature transient amplifying cells, and differentiated corneal epithelial cells (Kaplan et al., 2019). Another study combined single-cell and ATAC sequencing methods to generate an atlas of corneal cell types from development to adulthood in human (Collin et al., 2020). The study highlighted the close interactions between quiescent LSCs and other niche components such as immune cells, blood cells, corneal nerves as well as limbal fibroblasts and stroma. These interactions were mediated by the expression of upstream regulators involved in inflammatory response, angiogenesis and growth factors. These transcriptome analyses are informative in dissecting different cell populations based on gene expression. However, further phenotypic and functional analyses of the sequenced cells to confirm their identity are not yet feasible. Nevertheless, these transcriptome studies serve as a foundation for future investigation that could provide an overall landscape of the LSC biological processes and niche components in human.
Extracellular vesicles (EVs), in particular exosomes and microvesicles are found to be another important mechanism of cell-cell communication without direct cell-cell contact. (Mathieu et al., 2019). These EVs contain small RNAs and proteins that can be delivered into target cells and regulate the function of target cells. (Qiu et al., 2018) The packaging of the RNAs and proteins of EVs are highly regulated, and hence the functions of EVs (Temoche-Diaz et al., 2019). Recent findings suggest that EVs of stem cells exert effects on target cells/tissues similar to those exerted by their parental stem cells, i.e., effects resulting from paracrine signaling and modification of the host’s microenvironment (Camussi et al., 2013; Deng et al., 2020b). EVs secreted from limbal stromal cells are shown to regulate migration, proliferation and marker expression of limbal epithelial cells (Leszczynska et al., 2018). Corneal stromal stems cells are also shown to prevent scarring of the stroma and restoring transparency (Basu et al., 2014). When the production of EVs is inhibited, the scar reduction ability of the corneal stromal stem cells is abrogated (Shojaati et al., 2019). This finding demonstrates that the corneal stromal scar reduction capability of the corneal stromal stem cells actually is mediated via their EVs. It is likely that EVs secreted in the limbal niche also play an important role in LSC regulation. Identification and characterization of these limbal EVs are necessary to elucidate their roles in corneal wound healing.
2.2.6. Summary of limbal stem cell niche
Altogether, the niche constitutes a complex 3D structure specifically tailored to harbor LSCs. Innervation maintains niche homeostasis, and vasculature provides nutrients to the tissue. The ECM not only anchors the basal epithelium but also mediates intercellular communication and provides distinct mechanical properties that influence their phenotype. Additionally, surrounding niche cells regulate the hemostasis of LSCs by modulating signaling pathways such as Wnt, Notch, TGFβ and BMP signaling. LSCs integrate all the cues from the niche to maintain a progenitor state characterized by the expression of stem cell markers (Figure 1).
3. Regulatory Pathways in Human Limbal Stem/Progenitor Cells
Our initial microarray data identified cell adhesion, wound healing, cell proliferation, cell migration, and cell differentiation as the top differentially expressed biological functions in the limbus versus the cornea and conjunctiva (Nakatsu et al., 2013). This section will highlight past and ongoing studies of the roles of Wnt and Notch signaling, which are integrally involved in these biological functions, in LSC regulation by using primary human LSCs cultivated on mouse NIH-3T3-J2 (3T3 cells) feeder cells as the model system.
3.1. Wnt signaling pathway
Wnt signaling is ubiquitously involved in several stem cell processes, including migration, differentiation, quiescence, self-renewal, proliferation, migration, and polarity (Clevers et al., 2014; Nusse, 2008; Yang and Mlodzik, 2015). Nineteen Wnt ligands, 10 Fzd receptors, 4 Dickkopf (DKK) inhibitors, and 5 inhibitory secreted Fzd-related proteins (SFRPs) have been reported in humans. As depicted in Figure 2, Wnt ligands are secreted growth factors that can activate 3 major Wnt pathways: the canonical Wnt/β-catenin pathway, the noncanonical Wnt/planar cell polarity (PCP) pathway, and the noncanonical Wnt/Ca2+ pathway. β-catenin is a major intracellular transducer of the canonical Wnt signal. In the inactive canonical Wnt/β-catenin state, cytosolic β-catenin is associated with its destruction complex, so named because the proteins in the destruction complex ubiquitinate β-catenin to be targeted for proteosomal degradation. β-catenin may also be associated with α-catenin and cadherin at the membrane, where they form an adhesion complex to mediate cell-to-cell interactions and cytoskeletal arrangement (Figure 2A) (Nelson and Nusse, 2004; van Noort et al., 2002). When the canonical Wnt/β-catenin pathway is activated, Wnt ligand oligomerizes its membrane co-receptors LRP5/6 and Fzd. This oligomerization allows β-catenin to dissociate from its destruction complex, translocate to the nucleus, and activate the transcription of target genes under the TCF/LEF (T-cell factor/lymphoid enhancer factor) family of transcription factors (Figure 2B). Conversely, both noncanonical Wnt pathways are β-catenin–independent. The Wnt/Ca2+ pathway involves the release of calcium stores from the endoplasmic reticulum leading to the downstream activation of the nuclear factor of activated T cells (NFATc) transcription factor (Figure 2C). The Wnt/planar cell polarity (PCP) pathway involves the oligomerization of Fzd with the receptor ROR (tyrosine kinase-like orphan receptor) or RYK (receptor-like tyrosine kinase) coreceptors, which activate JNK (c-Jun N-terminal kinase) and RhoA (ras homolog family member A) downstream (Figure 2D).
Figure 2. Wnt signaling regulation in human limbal stem/progenitor cells.
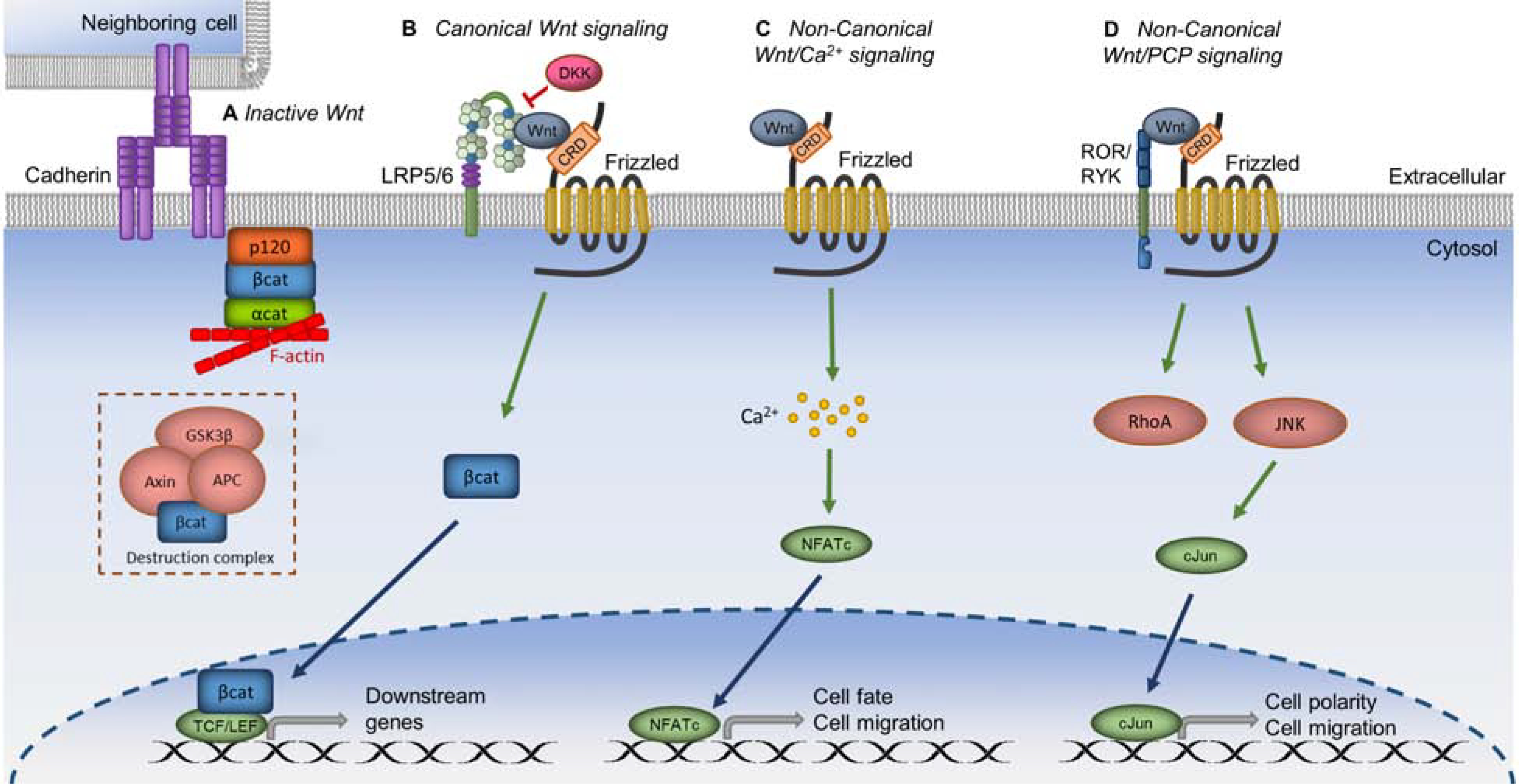
A. In inactive Wnt signaling, β-catenin (βcat) is associated either with cadherin at the membrane or with its destruction complex, which targets βcat for proteosomal degradation. B. When canonical Wnt signaling is activated, Wnt ligand binds with LRP5/6 and Frizzled coreceptors; βcat is released from the membrane or destruction complex and translocated into the nucleus to activate transcription of downstream genes. DKK inhibits canonical Wnt signaling by preventing Wnt binding to LRP5/6. C. In the noncanonical Wnt/Ca2+ pathway, Wnt activation leads to calcium influx into the cell. This action leads to the transcription of downstream genes involved in cell fate and cell migration. D. In the noncanonical Wnt/PCP pathway, binding of Wnt ligand to ROR or RYK with Frizzled causes downstream signaling that leads to cell polarity and cell migration. Green arrows represent activation, whereas red connectors represent inhibition.
Early studies of Wnt signaling pathways in the cornea revealed their role in corneal epithelial wound healing. Nuclear localization of β-catenin in the corneal epithelial cells was detected immediately adjacent to wounds in rat corneas (Lyu and Joo, 2006). Gene expression of Wnt4, Wnt5b, Wnt7a, and proliferating cell nuclear antigen (a proliferation marker) was upregulated in these wounded corneas. In addition, Wnt7a increased proliferation of human corneal epithelial cells in culture (Lyu and Joo, 2006). Although this result suggests that canonical Wnt/β-catenin accelerates corneal epithelial cell proliferation and wound closure, overactive canonical Wnt/β-catenin signaling through loss of the Wnt inhibitor DKK2 leads to hyperplasia and epidermal differentiation of corneal epithelial cells during corneal development in mice (Mukhopadhyay et al., 2006). This study provides evidence that Wnt signaling in the limbal stroma influences LSC differentiation during development.
It was originally believed that the canonical and noncanonical Wnt pathways operate antagonistically, but many components of the noncanonical Wnt pathway are associated with cadherins and the nuclear translocation of β-catenin; both findings reveal a possible interdependency between the canonical and noncanonical pathways (Arnsdorf et al., 2009; Nelson and Nusse, 2004; Thrasivoulou et al., 2013). When the canonical pathway is activated in cultivated LSCs, the noncanonical pathways are subsequently activated and might exert an inhibitory effect on the canonical pathway (unpublished data). These interdependency between the Wnt signaling pathways tightly regulates the proliferation and differentiation of LSCs.
3.1.1. Differential expression of components of the Wnt signaling pathways in the human limbus and central cornea
To characterize the possible function of Wnt signaling pathways in the regulation of human LSCs, investigators have conducted differential profiling of genes involved in the Wnt pathways in the human and monkey limbus and cornea (Ding et al., 2008; Nakatsu et al., 2011; Nakatsu et al., 2013). The specific Wnt ligands, receptors, and inhibitors differentially expressed in the limbus relative to that in the central cornea are summarized in Table 2. Notably, of the 19 mammalian Wnt ligands, Wnt2, Wnt6, Wnt11, and Wnt16b were more highly expressed in the limbus than in the central cornea, whereas Wnt3, Wnt7a, Wnt7b, and Wnt10a were more highly expressed in the central cornea than in the limbus. Of the 10 mammalian Fzd co-receptors, only Fzd1, Fzd4, Fzd7, and Fzd10 expression was upregulated in the limbus, whereas Fzd8 expression was upregulated in the cornea as shown in Figure 3 (Mei et al., 2014b). Among the canonical Wnt/β-catenin inhibitors, DKK1, Wnt inhibitory factor-1, Frizzled-related protein B, and secreted frizzled-related protein 5 expression was upregulated in the limbus, but none of the Wnt inhibitors was differentially upregulated in the central cornea (Nakatsu et al., 2011). These findings suggest that the careful balance of Wnt signaling activators and inhibitors in the limbal niche is crucial in harmonizing the quiescence, self-renewal, asymmetric division, and differentiation of the LSCs. Moreover, Wnt inhibitors were present in the limbus and not in the cornea, a difference that suggests Wnt inhibitors maintain LSC quiescence even in the presence of Wnt-activating ligands.
Table 2.
Preferentially expressed genes in limbus grouped by biological function
| Gene Category | List Hits | Genes | P-value |
|---|---|---|---|
| Cell adhesion | 24 | ACTN1; AEBP1; CD93; CDH11; CDH19; CNTN3; COL12A1; COL13A1; COL16A1; COL24A1; EMCN; ECM2; FERMT2; FBLN5; FN1; ITGA10; POSTN; SPP1; SRPX; SVEP1; THBS4; TNC; TNN; VCAM1 | 2.20E-08 |
| Wound healing | 15 | DARC; CFH; CDO1; EPHA3; F2R; F2RL2; FGF2; FBLN5; FN1; MAP1B; SERPINA3; SERPING1; SPP1; TIMP3; TNC | 1.70e-04 |
| Cell proliferation | 15 | AXIN2; BMP4; CCL14/15; EDNRA; F2R; FGF18; FGF2; ID4; PRRX1; PTGER2; PTN; RBP4; TIMP2; VCAM1; ZEB1 | 7.40E-03 |
| Cell migration | 9 | DCLK1; ENPEP; FGF2; FN1; TNN; THBS4; TNN; TWIST1; VCAM1 | 2.60E-03 |
| Cell differentiation | 9 | ANGPT1; BMP4; COL13A1; DCLK1; FGF2; FHL1; FRZB; NTRK3; TWIST1 | 1.90E-02 |
| TGF-β signaling pathway | 5 | BMP4; FMOD; ID4; LTBP2; THBS4 | 3.00E-02 |
| WNT signaling pathway | 4 | AXIN2; FRZB; FZD7; PITX2 | 5.90E-02 |
Figure 3. Preferential expression of Frizzled receptors in human cornea and limbus.
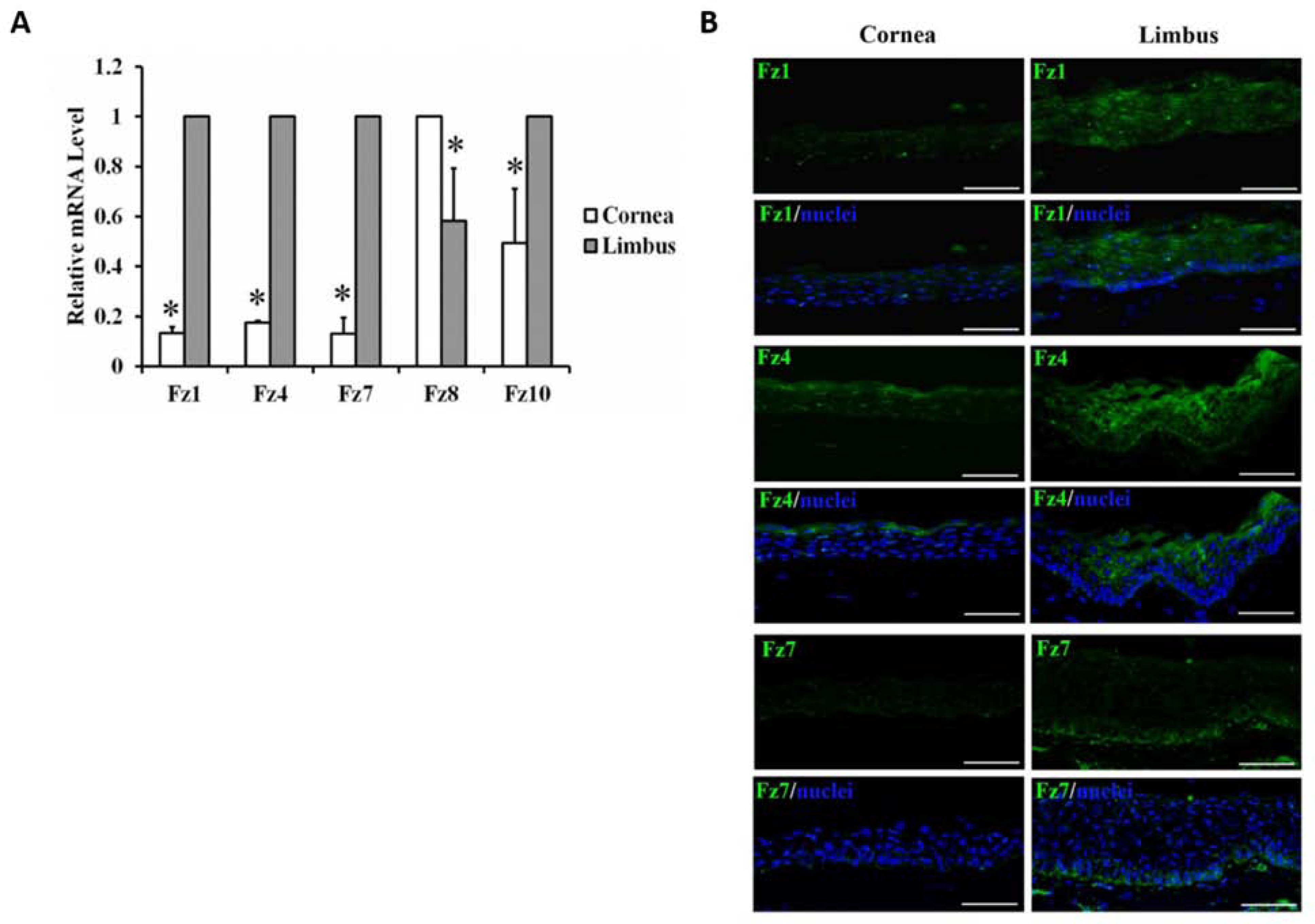
A. The mRNA expression levels of Frizzled (Fz), Fz1, 4, 7, 8 and 10 in the limbus and cornea through qRT-PCR. Fz1, 4, 7 and 10 had significantly higher mRNA level in the limbus than in the cornea whereas Fz8 had a higher expression in the cornea. Error bar represents S.E.M. *: p<0.05. B. Protein expression patterns of Fz1, 4 and 7 in human cornea and limbus by immunohistochemistry. Only Fz7 (green) was preferentially expressed at the basal limbal epithelium. The nuclei were co-stained with Hoechst (blue). Scale bar represents 50 μm. This figure has been adapted from a Stem Cells article (Mei et al., 2014a).
Because LSCs reside in the basal limbal epithelium, characterizing the spatial expression pattern of Wnt molecules and inhibitors in the basal limbal epithelium would provide additional information about the involvement of these molecules in LSC regulation. Our immunolocalization studies in the normal human cornea revealed β-catenin is localized primarily to the membrane, presumably incorporated in cadherin/catenin adhesion complexes. A few cells at the limbal basal epithelial layer showed nuclear and cytoplasmic β-catenin localization, suggesting active Wnt/β-catenin signaling in these cells. Wnt2 localized primarily to the superficial and suprabasal layers of the limbal epithelium, whereas Wnt16 localized to the basal and suprabasal layers of the limbal epithelium and the superficial layer of the corneal epithelium. In addition to the basal epithelial localization of Fzd7, the expression of Fzd4 in the basal limbal epithelium suggests that Fzd4 may also play a role in the regulation of LSCs (Mei et al., 2014b) and warrants further investigation.
3.1.2. Wnt signaling pathways maintain the stem/progenitor cell phenotype
To characterize the role of Wnt signaling on the maintenance of the stem/progenitor cell phenotype in culture, the role of Fzd7 was investigated in LSCs. As mentioned previously, Fzd7 expression was preferentially expressed in the K14+ basal epithelial cells with high level of p63α (Figure 4A). When the expression of Fzd7 was knocked down by shRNA in primary human LSCs, the mRNA expression of putative LSC markers K14, ABCG2, and ΔNp63α was reduced whereas the expression of differentiation marker K12 was increased by 25% (Figure 4B). The reduction of Ki67 expression also suggests a decrease in cell proliferative capacity. Moreover, Fzd7-deficient cells demonstrated reduced CFE (Figure 4C). In a subgroup of basal limbal epithelial cells, Fzd7 was found colocalized with syndecan-4, especially at the junction between adjacent basal epithelial cells (Mei et al., 2014b). Fzd7 forms a complex with syndecan-4, and the binding of fibronectin to this complex induces symmetric division of muscle satellite stem cells stimulated by Wnt7a (Bentzinger et al., 2013). Whether Fzd7 maintains the undifferentiated state and promotes proliferation of LSCs through a similar mechanism is yet to be elucidated.
Figure 4. Frizzled 7 expression localization correlates with putative LSC markers.

A. Micrographs of human sclerolimbal tissue immunostained for Frizzled 7 (Fzd7), p63α, N-cad, K14, and K12. White arrowheads mark cells that show high Fzd7 expression but low levels of other cell markers. Conversely, yellow arrowheads mark cells that express high putative LSC markers but low Fzd expression. White arrows mark basal epithelial cells that highly express both Fzd7 and putative LSC markers. Data indicates that majority of the cells expressing high Fzd7 colocalized with p63α, N-cadherin (N-cad), and K14. Scale bar = 50 μm. B. mRNA expressions of putative LSC markers and proliferation marker, Ki67, were decreased in Fzd7-deficient LSCs; determined by qRT-PCR. C. Fzd-deficient LSCs displayed a reduced colony forming efficiency. P1 indicates colonies were analyzed at passage 1 after transfection with Fzd7 shRNA. P2 indicates the transfected cells were passaged twice before colony analysis. *p<0.05 Error bar ±SEM. This figure has been adapted with permission from a Stem Cells article (Mei et al., 2014a).
The effect of canonical Wnt/β-catenin signaling activation was first investigated by supplementing lithium chloride (LiCl) to human LSC cultures. LiCl inhibits glycogen synthase kinase 3β (GSK3β), a member of the β-catenin destruction complex (Clement-Lacroix et al., 2005; Hedgepeth et al., 1997). Increased nuclear localization of β-catenin indicated an activation of canonical Wnt/β-catenin signaling in the cultivated LSCs following LiCl treatment (Figure 5A&B). LiCl improved CFE (Figure 5C) and proliferation of cultivated cells. LiCl also increased the expression of the stem/progenitor cell markers ABCG2 and ΔNp63α and decreased the expression of the differentiation marker K12 (Figure 5D). Collectively, this study demonstrates that the activation of the canonical Wnt pathway increases the proliferation of LSCs (Nakatsu et al., 2011). However, commonly used GSK3β inhibitors such as LiCl and CHIR99021 also affect other kinases that could also influence stem cell marker expression, CFE, and proliferation (Blagg and Workman, 2017; Coghlan et al., 2000). Therefore, specific activators of Wnt signaling would aid in further dissecting the role of canonical Wnt/β-catenin signaling in regulating LSC proliferation and differentiation.
Figure 5. Activation of canonical Wnt/β-catenin signaling improves progenitor cell phenotype, while inhibition of canonical Wnt/β-catenin signaling causes loss of the stem/progenitor cell population in cultivated LSCs.
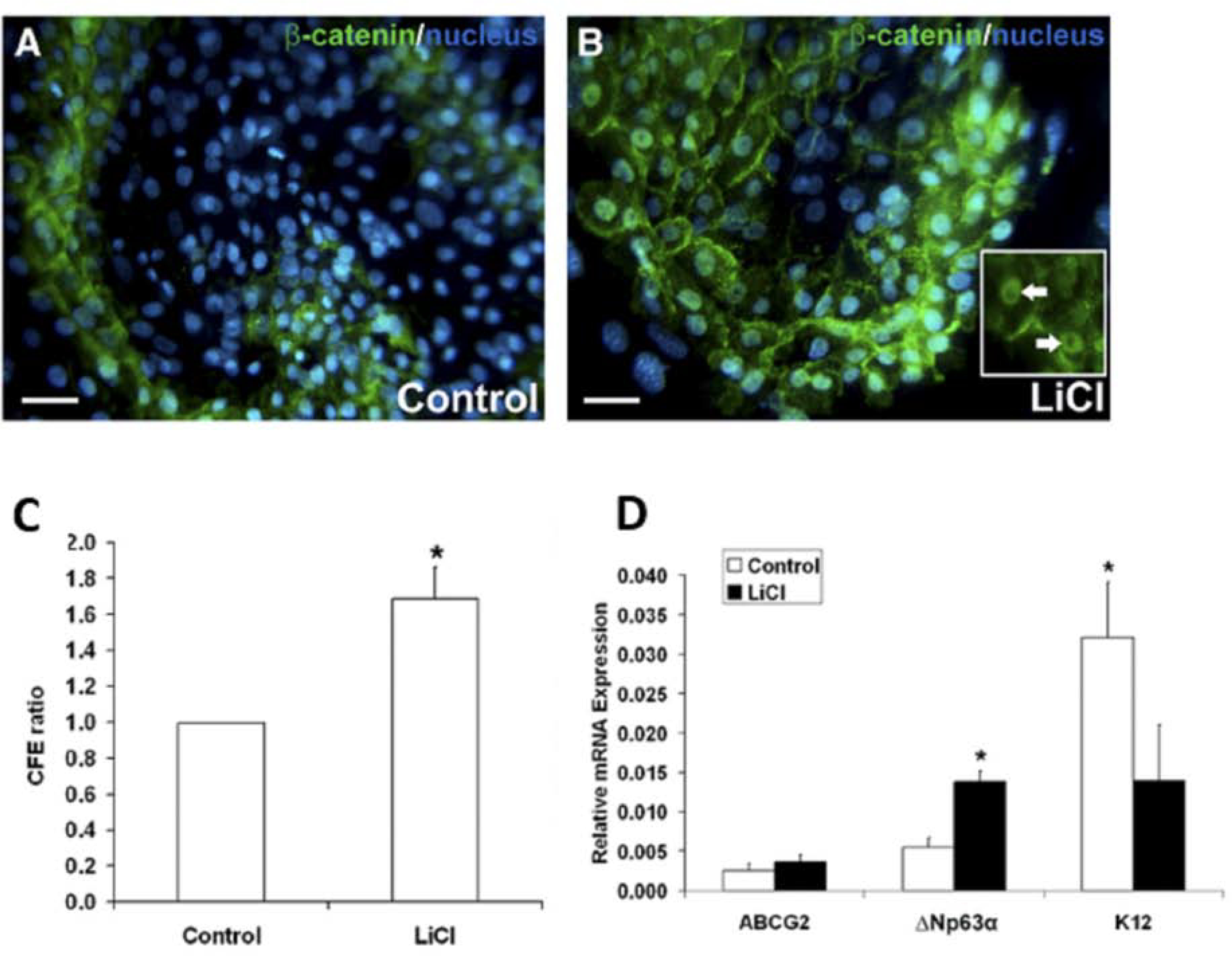
A. and B. β-catenin (green) immunofluorescent staining and Hoescht (blue) nuclear staining of LiCl-treated LSC colonies compared to control. In the LiCl-treated LSC colonies, nuclear β-catenin was observed (white arrows) that was not present in the control colonies. C. Quantification of CFE of LSC colonies as a ratio of LiCl-treated LSC cultures relative to their donor-matched control. D. Quantitative real-time PCR measure analysis of the progenitor cell markers ABCG2 and ΔNP63α, and the differentiated cell marker K12. White bars: control cultivated LSCs. Black bars: LiCl-treated cultivated LSCs. Data are represented as mean ± SEM, where *p < 0.05 was considered significant. This figure has been adapted with permission from an IOVS article (Nakatsu et al., 2011).
Wnt ligands and many proteins involved in the activation of the pathway are not readily available for research or for clinical development because of the difficulty in purification, large-scale production, and unintended effects (Janda et al., 2017). LiCl is not specific for GSK3β, demonstrated by its ability to induce expression of TCF/LEF-independent genes (Coghlan et al., 2000). The effect of LiCl on cultivated LSCs may be partially separate from Wnt signaling. Therefore, designing specific small molecules that operate at the level of the membrane coreceptors LRP5/6 and Fzd and their regulatory molecules is important to accurately evaluate the direct effect of Wnt activity on LSCs (Ahadome et al., 2017; Chen et al., 2020; Gonzalez et al., 2019a; Janda et al., 2017; Tran and Zheng, 2017). The advent of small molecules that interact specifically with Wnt membrane coreceptors or soluble inhibitors has allowed further investigation into the role of different Wnt signaling pathways in the regulation of LSCs. Four small-molecule inhibitors have been designed to target different molecules involved in Wnt signaling (Figure 6). Small molecule IIIC3 acts as a Wnt agonist by preventing the binding of the Wnt inhibitor DKK to LRP5/6 (Bao et al., 2012; Gonzalez et al., 2019a). Small molecules IC15 and ND both antagonize the canonical Wnt signaling pathway by inhibiting Wnt binding to LRP5/6 (Bao et al., 2012; Zhang et al., 2020). The small molecule MFH inhibits both the canonical and noncanonical pathways by occupying the cysteine-rich Wnt binding domain of Fzd and thus preventing the binding of Wnt to Fzd (Figure 6) (Zhang et al., 2020).
Figure 6. Mechanism of action of small-molecule Wnt modulators.

IIIC3 acts as a canonical Wnt/β-catenin signaling agonist by preventing the binding of Wnt inhibitor DKK to LRP5/6. ND and IC15 both inhibit canonical Wnt/β-catenin signaling by inhibiting the Wnt binding to LRP5/6. MFH inhibits all Wnt signaling by inhibiting the Wnt binding site, or CRD domain, of Frizzled. When MFH and ND are linked together, the resulting molecule MFH-ND acts as a Wnt mimic that activates canonical Wnt/β-catenin signaling by biding to the LRP5/6 and Frizzed co-receptors. Green arrows represent activation, whereas red connectors represent inhibition.
Although canonical Wnt signaling enhancement using IIIC3 improves the stem/progenitor cell phenotype of cultivated LSCs, IIIC3 at a higher concentration slightly decreases the stem/progenitor cell phenotype measured by decreased CFE and p63αbright cell percentage, and increased K12+ cell percentage (Figure 7); this finding suggests that overactivation of canonical Wnt/β-catenin signaling is detrimental to the cultivated LSCs, or that because the DKK-binding site of LRP5/6 shares homology with the Wnt-binding site of LRP5/6, IIIC3 may bind to the Wnt-binding site of LRP5/6 at high concentrations and thus inhibits Wnt signaling. Inhibition of canonical Wnt signaling by IC15 (Figure 7) and ND (Figure 8) showed a similar degree of loss in the stem/progenitor cell population, as both decreased p63αbright cell percentage and increased K12+ cell percentage (Gonzalez et al., 2019a; Zhang et al., 2020). In the presence of MFH, both canonical and noncanonical Wnt pathways were inhibited, and both the LSCs and 3T3 cells were unable to maintain their viability (Figure 8) (Zhang et al., 2020). These findings demonstrate the critical role of Wnt signaling in the survival and self-renewal of human LSCs.
Figure 7. Canonical Wnt signaling modulation affects stem/progenitor cell phenotype.
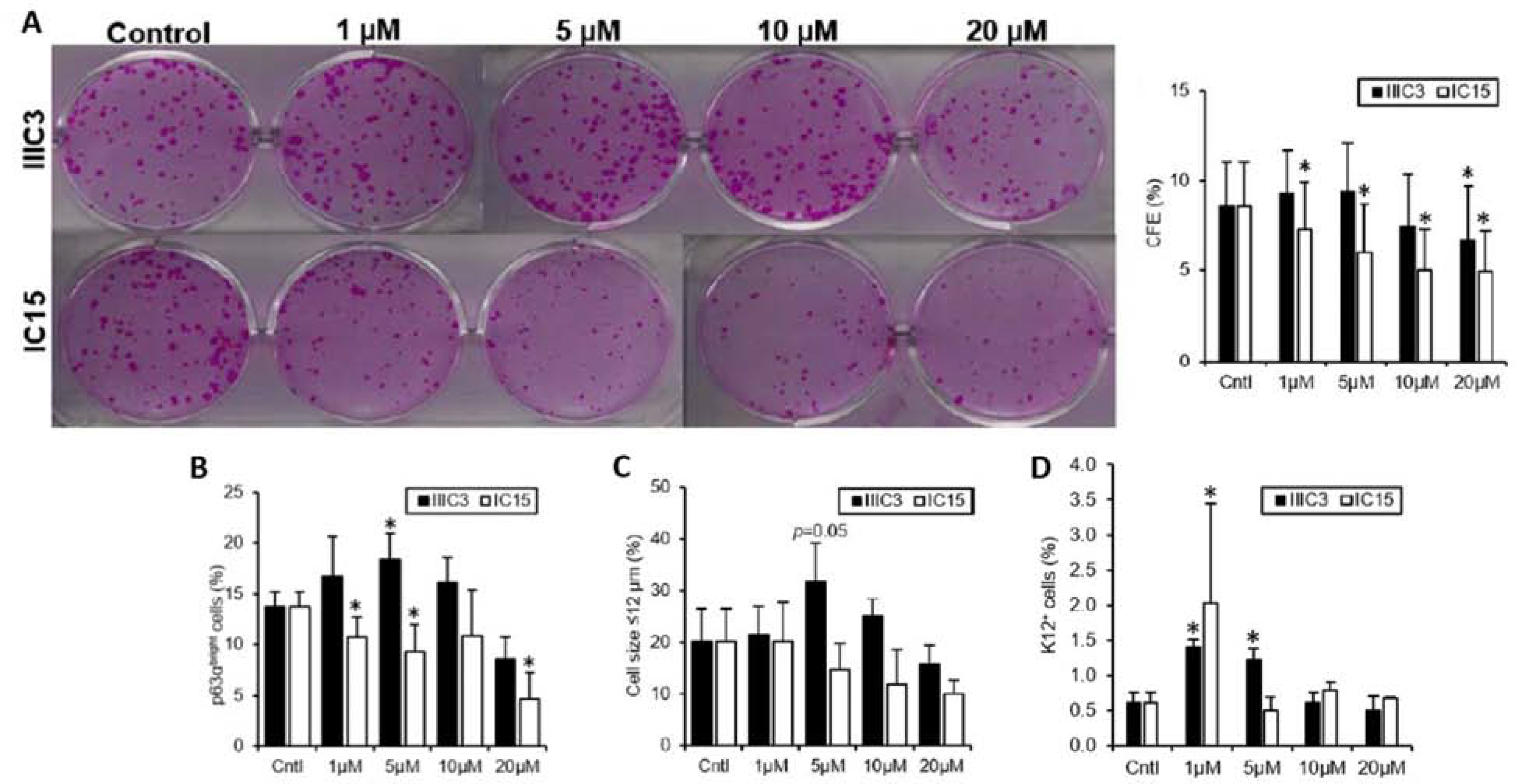
A. Quantification CFE of LSC colonies as a percentage of the number of cells seeded. Black bars: cultivated LSCs treated with IIIC3, a DKK inhibitor. White bars: cultivated LSCs treated with IC15, an LRP5/6 inhibitor. B. Quantification of the percentage of p63αbright cells, a measure of progenitor cells, in cultivated LSCs after treatment with IIIC3 (black bars) or IC15 (white bars). C. Quantification of the percentage of small cells (< 12 μm), which is a quality of LSCs. D. Quantification of the percentage of cells positive for K12 protein expression, a marker of differentiated cells, in cultivated LSCs after treatment with IIIC3 (black bars) or IC15 (white bars). Data are represented as mean ± SEM, where *p < 0.05 was considered significant. This figure has been adapted from an IOVS article under a Creative Commons License. http://creativecommons.org/licenses/by/4.0/. Three figures have been combined from the original article.
Figure 8. The canonical Wnt mimic MFH-ND improves stem/progenitor cell properties of expanded LSCs in vitro.
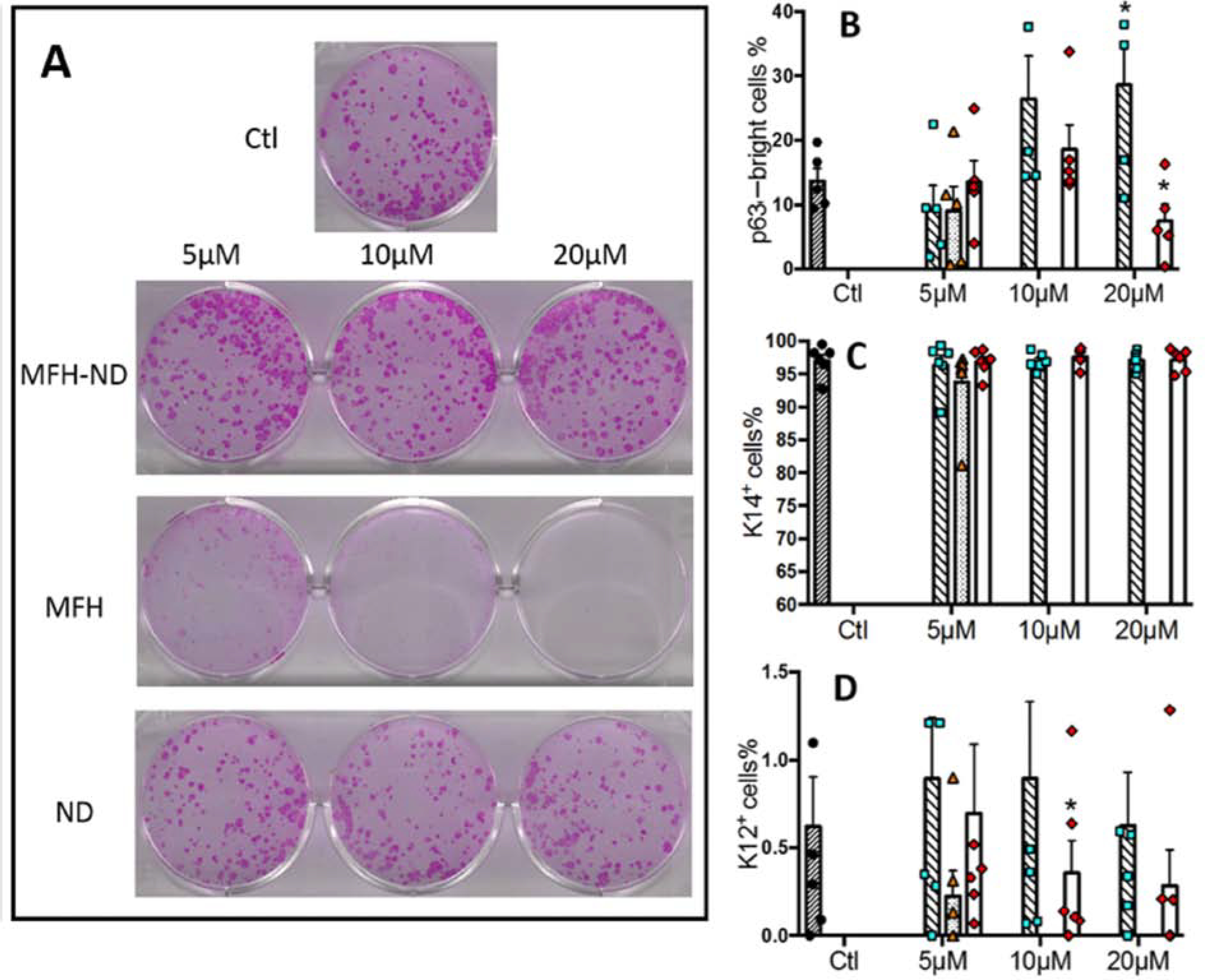
A. Rhodamine B staining of cultivated LSC colonies measures CFE. B. Quantification of the percentage of p63αbright cells, a measure of progenitor cells. C. Quantification of the percentage of cells expressing K14 protein, a marker of undifferentiated cells. D. Quantification of the percentage K12+ cells. Data are represented as mean ± SEM, where *p < 0.05 was considered significant. This figure has been adapted from an iScience article under a Creative Commons License. http://creativecommons.org/licenses/by/4.0/. Two figures have been combined from the original article.
To bring the membrane co-receptors LRP5/6 and Fzd together, we developed a Wnt-mimic small molecule by linking the LRP5/6 inhibitor ND to the Fzd inhibitor MFH (Figure 6). The Wnt mimic, MFH-ND, improved the stem/progenitor cell phenotype of LSCs in vitro, shown by increased CFE and p63αbright cell percentage (Figure 8). Interestingly MFH-ND had limited ability to activate Wnt/β-catenin on its own but showed substantial ability to activate Wnt/β-catenin when added in combination with recombinant human Wnt3a in TopFlash assays (Zhang et al., 2020). This finding suggests that this Wnt mimic might oligomerize Fzd and LRP5/6 on the cell membrane. The amount of active β-catenin significantly decreased in LSCs treated with MFH-ND; this reduction suggests that a negative feedback loop might be in place when Wnt/β-catenin signaling is activated in LSCs (unpublished data).
The balance between the canonical Wnt/β-catenin pathway and the noncanonical Wnt/PCP and Wnt/Ca2+ pathways in LSC regulation is poorly understood. The broad Wnt inhibitor MFH abolished both the LSC population and the 3T3 cells, but the LSCs and 3T3 cells survived when only canonical Wnt/β-catenin signaling was inhibited (Zhang et al., 2020). This result suggests that noncanonical Wnt signaling likely contributes to cell survival. Additional studies in cultivated human limbal stromal cells showed that HC-HA/PTX3 (Heavy chain-Hyaluronan/Pentraxin 3), which is a component of human amniotic membrane (AM) that is frequently used to culture LSCs, promotes expression of quiescence markers and activation of the noncanonical Wnt/PCP pathway (Chen et al., 2015). MicroRNAs 103 and 107, which participate in cell-to-cell communication and adhesion, also exert their function via inhibiting canonical Wnt3a activity in cultivated human limbal epithelial cells, likely through the Wnt/Ca2+ pathway. These miRNAs improve LSC holoclone formation (Peng et al., 2015).
Currently, we are investigating the effect of 2 upregulated Wnt molecules in the human limbus, Wnt6 and Wnt16b on the cultivated LSCs. Wnt16 activates the canonical Wnt/β-catenin pathway in mouse keratinocytes and the noncanonical Wnt/PCP pathway in primary human keratinocytes, whereas Wnt6 activates the canonical pathway in mouse cardiac progenitor cells and the noncanonical Wnt/PCP signaling in smooth muscle tumors (Mendoza-Reinoso and Beverdam, 2018; Schmeckpeper et al., 2015; Teh et al., 2007; Teiken et al., 2018). Our preliminary results suggest that Wnt6 and Wnt16b may maintain a balance between the canonical and noncanonical pathways in LSCs in vitro. Additionally, combinatorial effects of the multiple Wnt ligands expressed in the limbus (Alok et al., 2017; Nakatsu et al., 2011), and crosstalk between Wnt signaling and other signaling pathways, e.g., the BMP, Notch, and Sonic Hedgehog signaling pathways, need to be characterized in the regulation of LSCs.
Wnt signaling modulators affect the function of the LSC niche as discussed above. Given that the niche is a key factor in the maintenance of LSCs, it is important to consider the influence of small molecules on the niche in vitro, which then affects the LSC population. Both Wnt canonical and noncanonical signaling are necessary for the proliferation, differentiation, and migration of limbal epithelial cells in vitro (Gonzalez et al., 2019a; Lee et al., 2017). Understanding the response of LSCs to pharmacologic Wnt activation will help in characterizing the underlying regulatory Wnt signaling cascade, and in translation of MFH-ND and other Wnt mimic small molecules to treat LSCD.
3.2. Notch signaling pathway
Notch signaling is an evolutionarily conserved signaling pathway that controls multiple cellular processes such as cell proliferation, differentiation, and survival to regulate stem cell maintenance during tissue homeostasis and injury (Andersson et al., 2011; Weinmaster, 1997). The Notch family of receptors (Notch 1–4) are heterodimeric transmembrane proteins, activated by direct cell-to-cell interaction with Delta-Serrate-Lag-type canonical ligands: Jagged 1, Jagged 2, and Delta-like 1, 3, and 4 (D’Souza et al., 2010). Once bound by a ligand, the Notch receptor undergoes a series of proteolytic processing events and is cleaved into an intracellular and extracellular domain (Figure 9). The Notch extracellular domain is trans-endocytosed into the cell expressing the ligand. The Notch intracellular domain (NICD) traffics to the nucleus of the cell and acts as a biologically active signal transducer (Kopan and Ilagan, 2009). Assays to assess expression of the HES/HEY (Hairy and enhancer-of-split/Hairy/enhancer-of-split related with YRPW motif protein 1) family of downstream target genes are often used as the indicators of Notch activation (Andersson et al., 2011).
Figure 9. Notch signaling cascade.
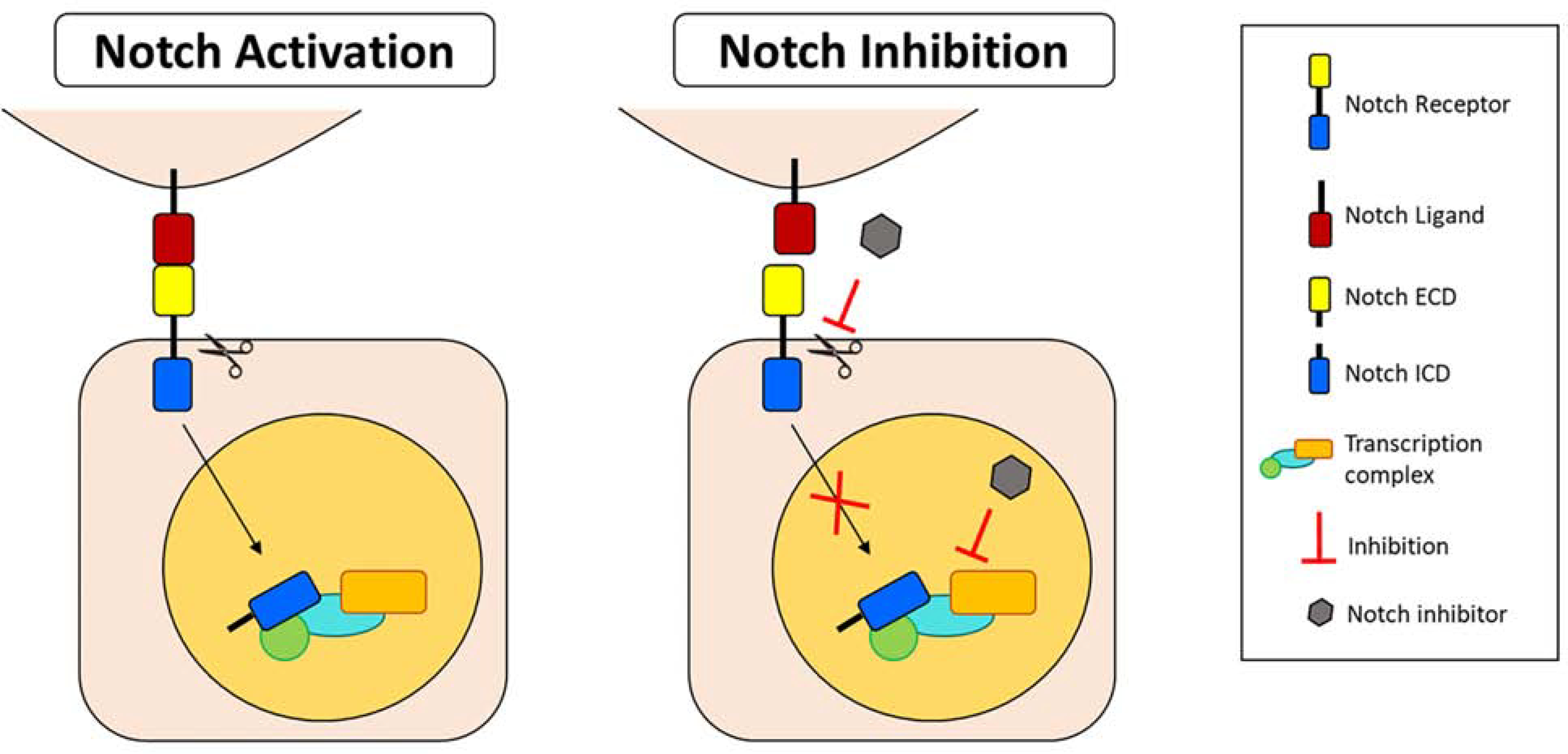
Upon binding of the Notch ligand to the receptor, the Notch intracellular domain (ICD) is cleaved from the extracellular domain (ECD) and translocates to the nucleus where it binds to the downstream transcription complex and activates target gene expression. Notch activation induces differentiation of limbal stem/progenitor cells (LSCs) and decreases proliferation and stratification. Notch signaling inhibition in LSCs by small molecule inhibitors maintains LSC phenotype and induces proliferation and stratification. Abbreviations: ECD: Extracellular domain; ICD: Intracellular domain.
3.2.1. Considerations in understanding Notch signaling in the regulation of limbal stem cells
Although simple in design, Notch signaling is a very complex and versatile signaling pathway based on the different cell responses it triggers. The diversity in Notch-induced cell responses is largely due to complex Notch-ligand interactions, varied expression patterns of the receptor and ligand, and post-translational modifications during signaling. These complexities hinder the understanding of the Notch signaling role in the regulation of the corneal and limbal epithelium.
The type of Notch-ligand interaction directly affects whether the output of the signaling cascade will be activating or inhibitory. In the LSC niche, receptor-ligand interaction from opposing cells (trans-interaction) results in activation, whereas autonomous interaction (cis-interaction) in the same cell promotes Notch inhibition (D’Souza et al., 2010; del Alamo et al., 2011; Palmer et al., 2014; Sprinzak et al., 2010). In the human corneal epithelium, the expression of the Notch ligands and receptors are widely distributed across the epithelium layers (Djalilian et al., 2008; Kulkarni et al., 2010; Ma et al., 2007; Thomas et al., 2007). Notch ligands with different expression patterns in the limbal and corneal epithelium might exert different effects in the regulation of the corneal epithelium regeneration. Notch activation also requires immobilization of the ligand (Varnum-Finney et al., 2000). However, a ligand that is not immobilized may have an inhibitory effect on Notch signaling. For example, a soluble form of Jagged 1 was previously reported to have an inhibitory effect on Jagged 1–induced Notch signaling in 3T3 cells (Small et al., 2001). The direction and the strength of ligand interaction(s) can also potentially affect target gene expression and, hence, cell-type lineage specification (Andersson et al., 2011; Fortini, 2009). For this reason, the immobilization of the ligand is important to activate Notch signaling and should be standardized across the studies. Also, the selection of the reagents and species in which Notch signaling is studied may increase variability in the results.
Diversity in Notch signaling responses could also originate from the downstream Notch response at different steps in the signal transduction process after ligand binding due to post-translational modifications (Andersson et al., 2011). These modifications may be important for Notch function in different epithelial cell contexts in the cornea and limbus. Another major source of diversity comes from the interactions with other signaling pathways. For example, crosstalk between Notch and Wnt signaling has been characterized in other epithelia such as those in the intestine (Fre et al., 2009; Nakamura et al., 2007) and cochlea (Munnamalai and Fekete, 2016; Ni et al., 2016). It is likely that Notch and Wnt crosstalk plays a crucial role in LSC regulation and thus warrants further investigation.
3.2.2. Notch signaling-mediated regulation of cornea and limbal epithelial cell differentiation and stratification
Disruption of Notch signaling has been shown to interfere with cell proliferation (Djalilian et al., 2008), cell differentiation, and junctional specialization in the developing corneal epithelium (Nakamura et al., 2008). Moreover, Notch signaling is involved in corneal epithelium homeostasis (Ma et al., 2007; Peng et al., 2012a) and wound healing (Djalilian et al., 2008; Lu et al., 2012; Ma et al., 2011; Movahedan et al., 2012; Vauclair et al., 2007). There is conflicting information as to how Notch signaling affects these cell processes during corneal epithelium homeostasis and wound healing. Overall, expression of Notch signaling molecules in the mouse (Nakamura et al., 2008) and human (Djalilian et al., 2008; Gonzalez et al., 2019b; Kulkarni et al., 2010; Ma et al., 2007; Thomas et al., 2007) corneal and limbal epithelium suggests a role for this signaling pathway in LSC regulation.
We confirmed the presence of Notch signaling proteins, including the Notch 1 receptor and the HES1/HEY1 target genes, at the basal layer of the human limbal epithelium (Gonzalez et al., 2019b). The cleaved intracellular domain of Notch 1, which is indicative of Notch signaling activation, was detected in the basal and suprabasal layers of the limbal epithelium in normal human sclerocorneal tissues. Therefore, Notch signaling might be necessary for the regeneration of the corneal epithelium during normal homeostasis. The effect of Notch signaling in human LSCs was elucidated by using 2 small-molecule Notch inhibitors, DAPT and SAHM1 (Gonzalez et al., 2019b). DAPT is a small molecule that targets γ-secretase, an integral membrane protein that cleaves Notch receptors, thereby blocking Notch activation (Olsauskas-Kuprys et al., 2013). SAHM1 inhibits Notch signaling by preventing the assembly of the transcription complex, NICD bound to transcription factor CSL (Moellering et al., 2009). SAHM1 was chosen to specifically block Notch signaling and avoid the nonspecific effects induced by DAPT on other signaling pathways. Proliferation of LSCs is slightly reduced after Notch inhibition (Figure 10A) while notch signaling inhibition, in the presence of either small molecule, led to an increase in the population of LSCs characterized by the cell morphology, increase in CFE, and increase in p63αbright, K14+ and small size cell population (Figure 10B–E). A decrease in the population of differentiated K12+ cells is also observed (Figure 10F). A similar effect after Notch inhibition has also been reported in rats (Li et al., 2017) and confirmed in humans by another groups (Dhamodaran et al., 2019).
Figure 10. Inhibition of Notch signaling by DAPT and SAHM1 preserves the LSC phenotype.
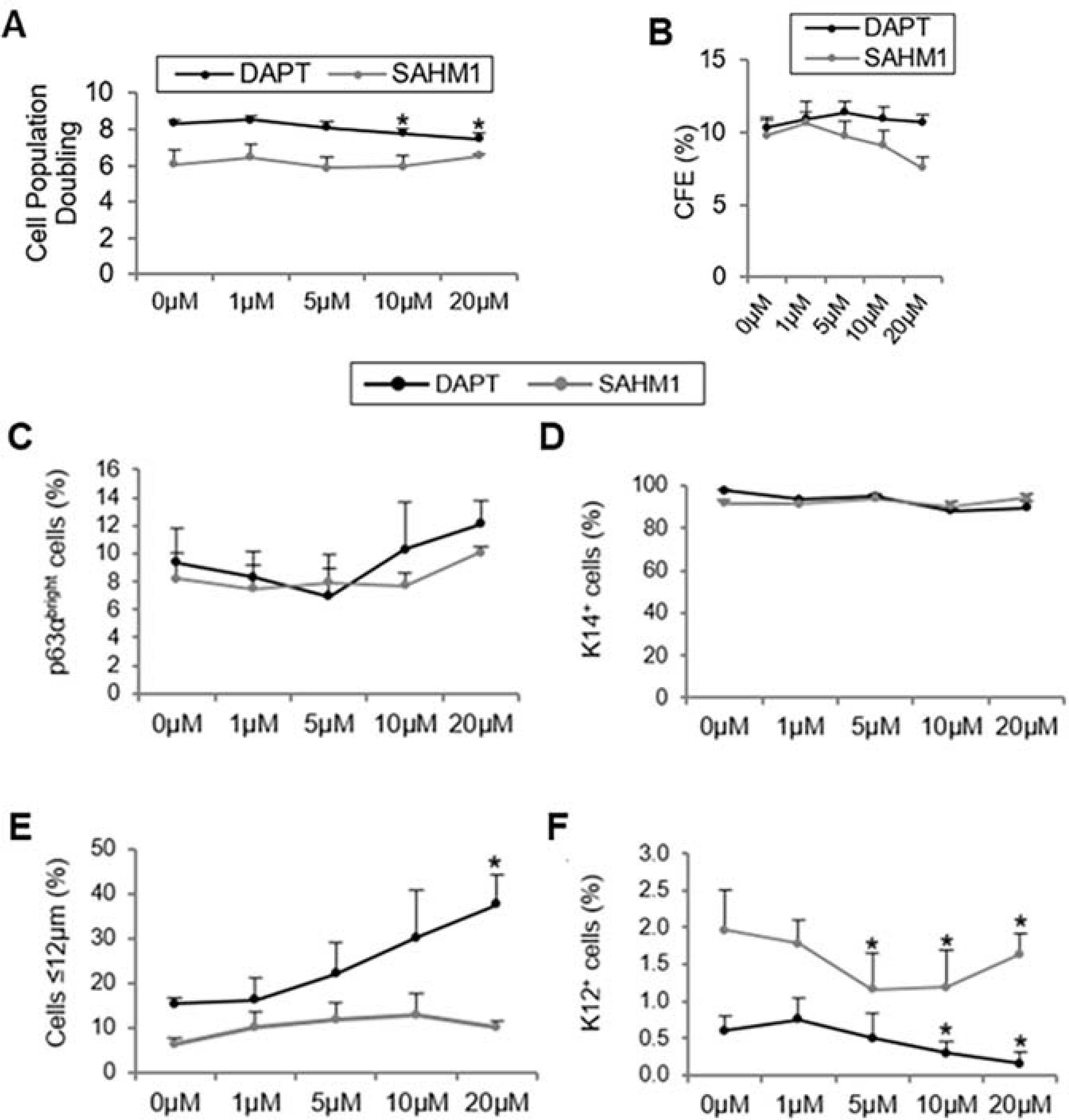
A. Cell population doubling with DAPT/SAHM1. B. CFE quantification on the Rhodamine B-stained plates. C. Quantification of p63αbright cells at the protein level. D. Quantification of K14+ cells at the protein level. E. Percentage of small (≤12 μm) LSC-like cells. F. Quantification of K12+ cells at the protein level. Data are represented as mean ± SEM. *p < 0.05 were considered significant. Data are statistically analyzed by using the pairwise t-test and represented as mean ± SEM. Abbreviations: CFE: Colony Forming Efficiency; K12: cytokeratin 12; K14: cytokeratin 14; LSCs: limbal stem/progenitor cells. This figure has been adapted from a Scientific Reports article (Gonzalez et al., 2019) under a Creative Commons License. http://creativecommons.org/licenses/by/4.0/. Two figures were combined from the original article.
Effects of Notch activation in human LSCs were investigated by activation using an immobilized Jagged 1 (González et al., 2020). Notch activation which was confirmed by the level of cleaved intracellular Notch 1, reduces the amount of the LSC population and proliferation, and induces differentiation of LSCs. The percentage of p63αbright cells was reduced significantly in the presence of Jagged 1 and the percentage of K12+ cells was increased. Notch activation also changed the cellular architecture by decreasing the stratification of the limbal epithelium as evidenced by the reduced cell layers (Figure 11A&B). The K12+ population was increased (Figure 11B) while the p63αbright stem cell population was reduced (Figure 11C&D). Interestingly, in the presence of Jagged 1, the number of dividing cells indicated by the cellular location of pericentrin was dramatically reduced (Figure 12A&B). In the controls, N1ICD (Notch 1 intracellular domain) was expressed in the nucleus of basal cells that were actively dividing asymmetrically; cells dividing symmetrically had mostly cytoplasmic expression of N1ICD. In contrast, upon activation with Jagged 1, cells dividing symmetrically expressed nuclear N1ICD (Figure 12C). Asymmetric divisions of basal limbal epithelial cells were reduced, and the expression pattern of the polarity protein Par3, normally present at the apical-lateral membrane of basal cells, was dispersed in the cells (Figure 12C–E).
Figure 11. Jagl-mediated Notch activation reduced stratification and promoted differentiation of LECs.
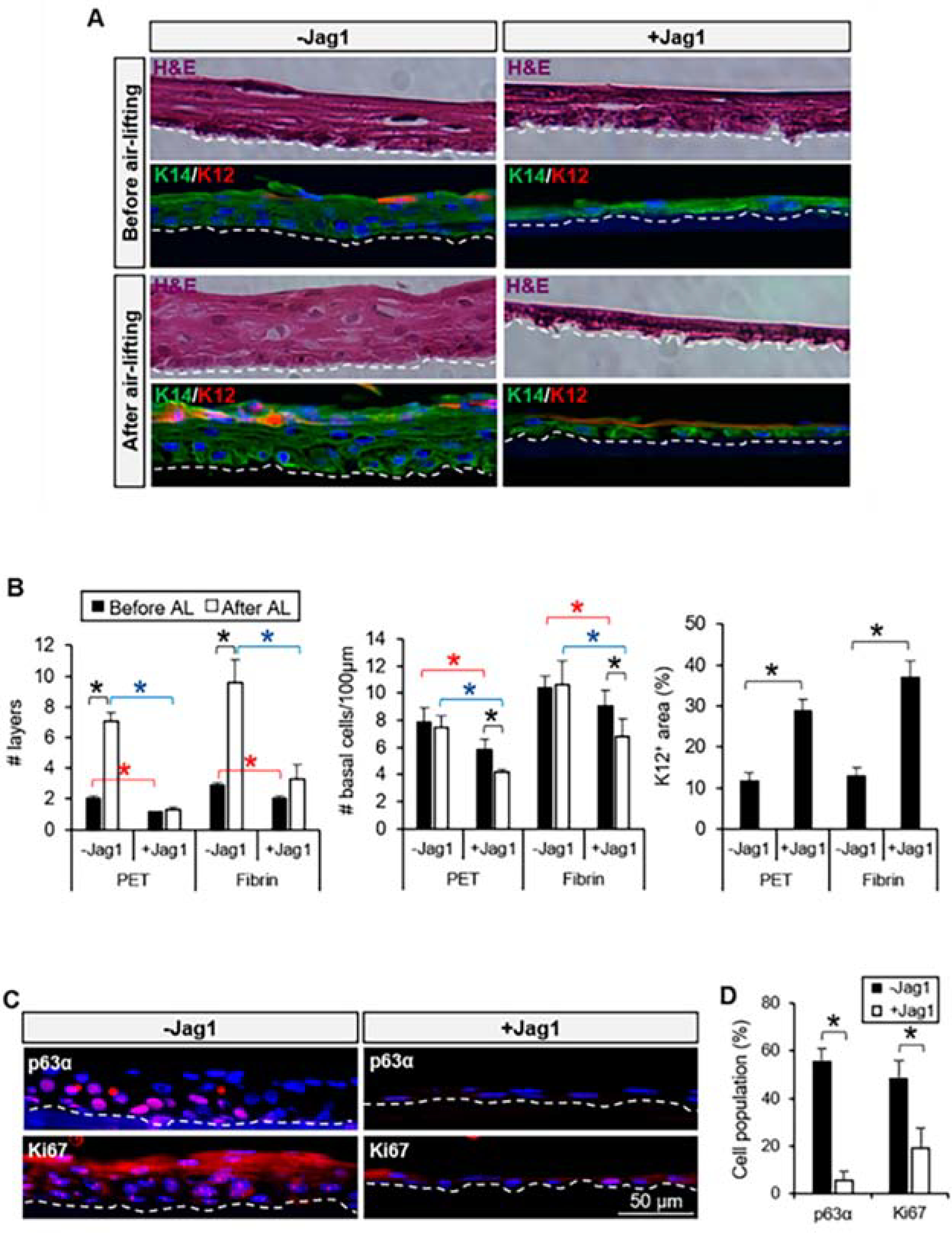
A. Stratification of the cultivated LECs in the presence of Jag1 was reduced before and after air-lifting induction. Differentiation was maintained after air-lifting in the Jag1 group at the superficial layer(s). B. The number of layers and number of cells per μm at the basal layer were reduced in the Jag1 cultures; the K12+ area in the presence of Jag1 was increased compared to the control. C. Expression of p63α and Ki67 was reduced at the basal layer of the cultivated LECs with Jag1. D. Quantification of the percentage of cells positive for p63α and Ki67 showed a significant reduction in the cultivated LECs with Jagl. The dotted line in A and C panels delineates the BM. In panels B and D, *p < 0.05 were considered significant. Data were statistically analyzed by using the Student’s t-test and represented as mean ± SEM. Abbreviations: BM: basement membrane; H&E: hematoxylin and eosin; Jagl: Jagged 1; K12: cytokeratin 12; K14: cytokeratin 14; PET: Polyethylene Terephthalate. This figure has been adapted from a Cells article (Gonzalez et al., 2020) under a Creative Commons License. http://creativecommons.org/licenses/by/4.0/.
Figure 12. Jag1 decreased asymmetric divisions in basal limbal epithelial cells.
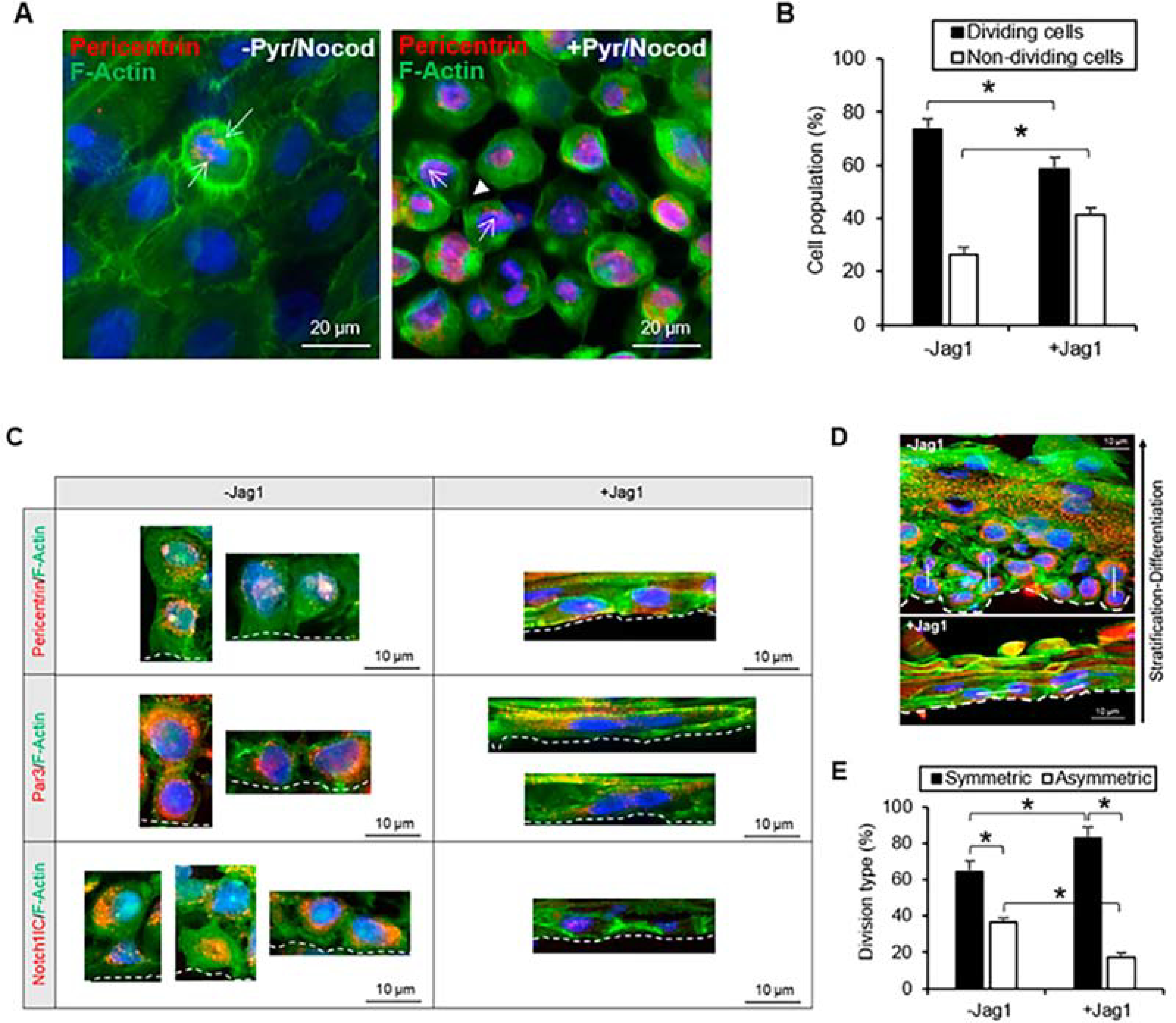
A. Pericentrin stained both poles of the mitotic spindle in cells undergoing mitosis before and after a combined treatment of nocodazole and pyrimydine-7. Arrows indicate pericentrin staining. Arrowhead indicates cleavage furrow and contractile ring stained with F-actin. B. The percentage of dividing cells was significantly reduced in the presence of Jag1. C. Both in the presence and absence of Jag1, pericentrin was identified in the daughter cells of symmetric and asymmetric divisions, and together with F-actin helped identify the orientation of the mitotic spindle. In control cultures without Jag1, Par3 was expressed at the apical-lateral membrane of cells; in the Jag1 cultures, the expression of Jag1 was more delocalized and scattered in the cells. In control cultures, N1IC was expressed in the nucleus of basal cells dividing asymmetrically; cells dividing symmetrically had mostly cytoplasmic expression of N1IC; in Jag1 cultures, cells dividing symmetrically expressed nuclear N1IC. D. The plane of asymmetric and symmetric divisions (represented by the white line) is shown in the presence and absence of Jag1 in cross-sections of cultivated LECs. A decrease in the number of asymmetric divisions was observed in LECs cultivated with Jag1. E. The percentage of asymmetric divisions was reduced in the presence of Jag1. In panels C and D, the BM is delineated by the dotted line. *p < 0.05 were considered significant. Data were statistically analyzed by using the Student’s t-test and represented as mean ± SEM. Abbreviations: BM: Basement membrane; Jag1: Jagged 1; Nocod: Nocodazole; NotchllC: Notch 1 intracellular domain. Par3: Partitioning defective protein 3; Pyr: Pyrimidyn-7. This figure has been adapted from a Cells article (Gonzalez et al., 2020) under a Creative Commons License. http://creativecommons.org/licenses/by/4.0/.
Notch signaling and p63 crosstalk have been demonstrated in the literature to be directly involved in the stratification of epithelial tissues and maintaining a balance between keratinocyte self-renewal and differentiation (Koster et al., 2004; Nguyen et al., 2006; Truong et al., 2006). Therefore, Notch-p63 crosstalk may be directly responsible for the loss of the progenitor population among the LSCs and the reduction of the limbal epithelium stratification as a result of reduced asymmetric division. A working model of the Notch regulation of LSCs differentiation and stratification is proposed in Figure 13.
Figure 13. Proposed model of stratification-differentiation of the human limbal epithelium.
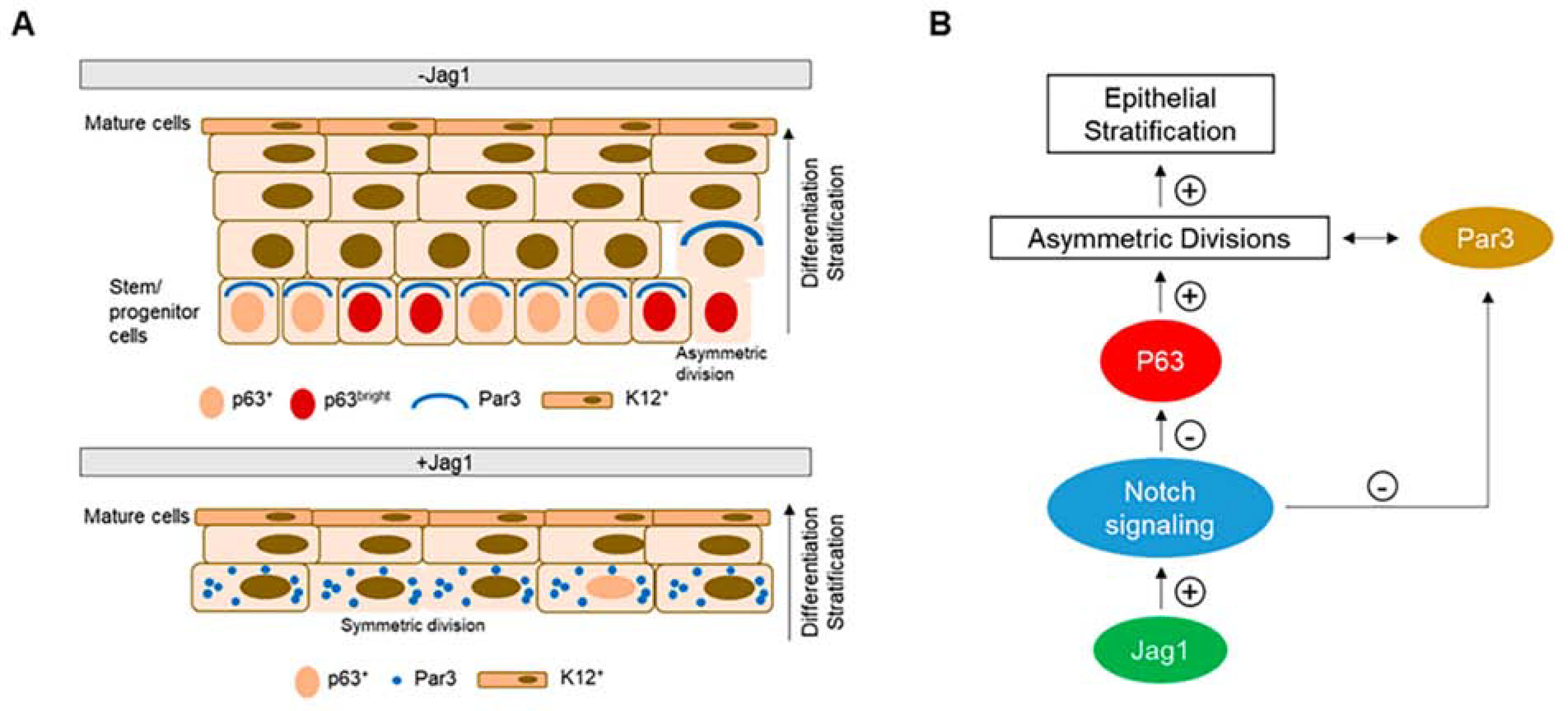
A. In the absence of Jagl (control), basal stem cells expressing p63 retain the capacity to divide asymmetrically generating two daughter cells, a new stem cell and a suprabasal more differentiated cell. The orientation of the mitotic spindle is controlled by the polarity proteins such as Par3 that are distributed on the apical-lateral membrane of polarized basal cells. Differentiated K12+ cells are present at the superficial layer(s). In the presence of Jagl, basal cells in direct contact with Jag1 have a scattered Par3 distribution, p63 expression is low, and there is a decrease in the proportion of asymmetric divisions. As a consequence, the stratification of the epithelium is reduced. Differentiated K12+ cells are still present at the superficial layer(s). B. Schematic diagram showing that upon Jag1-mediated Notch activation, the expression of p63 is downregulated. P63 is the main driver of epithelial stratification. High levels of p63 promote asymmetric divisions, which in turns increases the stratification of the epithelium. Also, Notch signaling directly affects the expression of Par3. Overexpression of Notch signaling dysregulates Par3 expression and decreases asymmetric divisions. This figure has been adapted from a Cells article (Gonzalez et al., 2020) under a Creative Commons License. http://creativecommons.org/licenses/by/4.0/.
Understanding the temporal and spatial regulation of Notch signaling in the LSCs will help further elucidate the role of Notch signaling in the machinery responsible for corneal epithelial cell differentiation. The core molecular components of the Notch signaling pathway seem to have specific expression patterns in the limbus, which may potentially label the LSC population in that region. Finally, similar to our goal in understanding the Wnt pathways, unraveling the complexities of Notch signaling in LSC regulation is a key to designing cell therapies or small molecules that modulate specific components of Notch signaling in disease.
3.3. Other signaling pathways
In addition to Wnt and Notch signaling pathways, TGF-β/BMP (Hu et al., 2019; Kawakita et al., 2013), Sonic Hedgehog (Fan et al., 2019), YAP/TAZ (Gouveia et al., 2019), integrin (Ma et al., 2016), and cadherin-mediated signaling pathways (Hayashi et al., 2007) have all been shown to regulate the function and phenotype of LSCs. YAP/TAZ signaling is regulated by the stiffness of external ECM as discussed in Section 2.2.4. In addition, argin promotes LSCs proliferation via the Hippo-YAP signaling (Hou et al., 2020). Inhibition of Sonic Hedgehog signaling suppresses rabbit LSC proliferation and holoclone formation in culture, and limbal wound healing in mice (Fan et al., 2019). Integrin signaling could activate canonical Wnt/β-catenin signaling (Ma et al., 2016). TGFβ, BMP, and noncanonical Wnt signaling might mediate LSC quiescence. Indeed, symmetric and asymmetric division of LSCs to maintain the LSC pool while replenish corneal epithelium likely involve a cooperation between many key signaling pathways. Therefore, further investigation into the mechanism of each signaling pathway and the crosstalk among these pathways in maintaining a balance between LSC proliferation, differentiation and quiescence is necessary.
4. Bioengineering of Limbal Stem/Progenitor Cells
Transplantation of LSCs is an effective treatment to restore a normal corneal epithelial surface in eyes that lack LSCs. LSCs can be transplanted by using tissues or cultivated LSCs from the patient (autograft) (Deng et al., 2020a; Holland, 2015; Kolli et al., 2010; Rama et al., 2010; Sangwan et al., 2011) or a donor (allograft) (Borderie et al., 2019; Shortt et al., 2014; Zakaria et al., 2014). LSC therapies have a lower risk of complications than keratoprostheses, and autologous transplants have superior clinical outcomes to allogeneic transplants (Le et al., 2020b).
Significant progress has been made in the treatment of LSCD, particularly in cell-based therapies over the last 2 decades since the first report of this treatment approach in 1997 (Pellegrini et al., 1997). Holoclar was the first stem cell treatment for LSCD approved in 2005 in Europe. The goal of cell-based therapy is to expand a sufficient quantity of LSCs in ex vivo culture derived from the smallest amount of donor tissue that could result in long-term successful restoration of a normal corneal epithelial surface. When LSCs are cultivated ex vivo, their successful expansion often requires a surrogate niche composed of extracellular matrices and niche cells. Many attempts have been focusing on preserving the native niche or reconstituting a surrogate niche during LSC cultivation (Gonzalez et al., 2016; Levis and Daniels, 2016; Mei et al., 2017; Mei et al., 2014a; Nakatsu et al., 2014).
Despite good clinical success, most cell-based LSC therapies varied greatly in the cultivation process and were not Good Manufacturing Practices (cGMP) compliant. Nonhuman products such as mouse 3T3 feeder cells, bovine serum and cholera toxin were used in the cultivation. For the last decade, standardizing LSC therapies and increasing the safety of the cultivated LSCs have been high priorities in the field; therefore, significant effort has focused on developing a cultivation system that is feasible, efficient, GMP-compliant, and, if possible, xenobiotic-free. Several aspects of the process including the starting LSC material, feeder cells, substrate, and culture medium have been targeted for optimization.
4.1. Selection and processing of starting material
The first step in the LSC cultivation process is the selection of the starting material. LSCs can be cultivated from a small limbal biopsy, i.e., an explant (usually between 1 × 2 mm to 2 × 2 mm), or cell clusters or single cells after enzymatic digestion from the limbal tissue. The process of dissociating limbal epithelial cells from limbal tissue is time-consuming and requires enzymes, which could damage the LSC population. Moreover, dissociated cells need feeder cells that serve as the surrogate niche cells by providing the necessary niche factors for the proliferation of LSCs. The importance of the niche cells, cell-to-cell interactions, and paracrine trophic factors in the cultivation of LSCs has been previously demonstrated (Chen et al., 2011; Gonzalez and Deng, 2013). When LSCs are cultured as cell clusters, i.e. the trypsinization step is omitted after dispase digestion, the number of stem/progenitor cells obtained from the culture is higher than that obtained when LSCs are cultured as fully dissociated single cells on 3T3 cells or human mesenchymal stem cells (MSCs) (Gonzalez and Deng, 2013; Kawakita et al., 2009). Therefore, maintaining cell-cell contact could prevent the loss of the stem/progenitor cell population during the LSC cultivation process.
Although the limbal explant culture method could induce epithelial-to-mesenchymal transition (Li et al., 2007), this method offers several advantages. First, a limbal explant culture does not need feeder cells to maintain LSC growth because the niche is provided by the limbal tissue. Although explants can introduce limbal stromal cells in the final cell product, stromal cells are needed to support the growth of LSCs in vitro (Gonzalez and Deng, 2013) and have the added benefit of reconstructing the LSC niche once they are transplanted onto the diseased eye. Second, a limbal explant can be easily procured with minimal manipulation and used for LSC cultivation without the need for isolating and damaging the LSCs by enzymatic treatments. By using the limbal explant, LSCs can be directly cultivated on the cell carrier for transplantation and thus avoid the need of passaging the cells, which could induce differentiation and a change in phenotype. Finally, the growth of LSCs from the explant is robust, and the number of p63αbright cells often meets the criteria needed for a successful outcome (Rama et al., 2010). All of these properties are advantages in regards to the regulatory requirement.
4.2. Culture configuration
Another important aspect in the optimization and standardization of the LSC cultivation process is the development of a structural configuration that maintains the niche factors, cell-to-cell interactions, cell polarity of the LSCs, supporting cells (if present), and substrate. A novel 3-dimensional (3D) culture system (Figure 14A) has been developed to mimic the in vivo niche by culturing the LSCs on one side of the culture porous membrane insert while the feeder cells are seeded on the other side of the membrane (Mei et al., 2014a). The 3D culture system increased the growth of the LSCs in the presence of 3T3 feeder cells without affecting the LSC phenotype (Figure 14B&C). Moreover, the 3D system was able to expand the LSC population by using human MSCs derived from multiple tissues including bone marrow (Figure 14D–G) and adipose tissues more efficiently than did the 2D method. This 3D configuration allows LSCs to grow in close proximity with the feeder cells while remaining separated. Other advantages of the 3D system are its ability to provide an even diffusion of growth factors and cytokines and to allow possible direct cell-to-cell interactions between LSCs and feeder cells via the pores (Figure 14H–K) (Nakatsu et al., 2014). This 3D method is more efficient and consistent in supporting the growth of single LSCs than is the traditional 2D method in which single LSCs are cultured directly on a layer of feeder cells.
Figure 14. The 3-dimensional limbal stem/progenitor cell cultivation method resembles the in vivo environment.
A. Diagram of the standard 2-dimensional (2D) and sandwich 3-dimensional (3D) culture methods. In the 2D method, LSCs are cultured directly on feeder cells. In 3D method, LSCs and feeder cells are cultured on the opposite sites of the PET membrane. B. Morphology of the LSC sheet in the 2D and 3D methods with 3T3-J2 feeder cells. C. Cell population doubling of LSCs in the 2D and 3D methods with 3T3-J2 feeder cells. D. Cell population doubling of LSCs in the 2D and 3D methods with BMs as feeder cells. E. Percentage of p63αbright cells in the 2D and 3D methods with BMs as feeder cells. F. Percentage of K14+ cells in the 2D and 3D methods with BMs as feeder cells. G. Percentage of K12+ cells in the 2D and 3D methods with BMs as feeder cells. H, I, J. Structure of the LEC sheet and BMs on both sides of the PET membrane in the 3D method. Possible cell-to-cell contact between LSCs and BMs (arrow) in the 3D method. In panels H, I and J, scale bar represents a distance of 10 μm. K. Quantitation of PET membrane pores with and without possible cell contact. *p < 0.05 were considered significant. Data were statistically analyzed by using the Student’s t-test and represented as mean ± SEM. Abbreviations: BM: Bone Marrow-Derived Mesenchymal Stem Cells; 2D-SC: single LSCs in the standard or 2D method; 2D-CC: LSC clusters in the standard or 2D method; 3D-CC: LSC clusters in the sandwich or 3D method; K12: cytokeratin 12; K14: cytokeratin 14; Epi: epithelium; PET: polyethylene terephthalate. This figure has been adapted from a Tissue Engineering Part C: Methods article (Mei et al., 2013) and a Stem Cell Research article (Gonzalez et al., 2016) under a Creative Commons License. http://creativecommons.org/licenses/by/4.0/. Four figures were combined from the original articles.
Interestingly, when a limbal explant is cultivated without feeder cells by using the 2D method, the LSC expansion efficiency is comparable to that achieved by the 3D method (unpublished data). This finding further supports the importance of preserving the native LSC niche during the growth of LSCs ex vivo.
4.3. Feeder cells
The choice of the supporting cells, i.e., feeder cells in the cultivation of the LSCs, is another important aspect in the optimization of a xenobiotic-free cultivation protocol for the manufacturing of LSCs. The first report of successful transplantation of cultivated LSCs in 1997 used fetal bovine serum (FBS) and mouse 3T3 cells (Pellegrini et al., 1997). Since then, many groups have tried to develop LSC cultivation methods that avoid the use of animal-derived components to eliminate the risk of cross-contamination. Several groups have developed xenobiotic-free LSC expansion methods that resulted in good clinical success (Kolli et al., 2010; Sangwan et al., 2011; Zakaria et al., 2014). Nonetheless, the best long-term clinical outcome of cultivated LSCs over a 10-year follow-up period are reported when mouse 3T3 cells and FBS are utilized in LSC cultivation (Rama et al., 2010). Several studies, including ours, have demonstrated that 3T3 cells can be replaced with human MSCs sourced from different tissues, including the limbal stroma (Nakatsu et al., 2014), bone marrow (Gonzalez et al., 2016), and adipose tissue (Mei et al., 2017). When single LSCs are cultivated by the 2D method, human limbal MSCs do not efficiently support LSC growth, and the stem/progenitor phenotype is lost during cultivation. However, when clusters of LSCs are cultured indirectly on limbal MSCs using the 3D method, the yield of LSCs (i.e., the percentage of p63αbright cells) is comparable to that obtained by using 3T3 feeder cells (Nakatsu et al., 2014). A similar finding was observed when adipose-derived MSCs and bone marrow–derived MSCs were used as the feeder cells (Gonzalez et al., 2016; Mei et al., 2017). The combination of factors such as paracrine signaling, cell-to-cell interactions, density of the feeder cells, and cell polarity is key to the success of single LSC expansion.
4.4. Culture substrate
Culture substrate provides another external cue that influences the phenotype of LSCs and can serve as the carrier for transplantation. The most frequently used substrate-carrier in LSC therapies is the human AM, which has been widely used in ocular surface reconstruction for many years (Azuara-Blanco et al., 1999). Human AM is a very attractive LSC culture substrate-carrier as it is fairly translucent, avascular, and poorly immunogenic; has anti-inflammatory and anti-scarring properties; and promotes wound healing. These properties make the AM a suitable material for the expansion and transplantation of LSCs (Tseng et al., 2004), although the advantage of AM in limbal tissue transplantation has not been validated in high level clinical studies (Le and Deng, 2019).
Good clinical outcome of LSCs cultivated on AM has been reported with a mean follow up of 3 years (Sangwan et al., 2011). One challenge of processing AM for the cultivation of LSCs is the variation of the tissue quality and epithelial debridement. We have demonstrated consistency when the manufacturing process is standardized to reduce donor-to-donor variability with AM (Gonzalez et al., 2017). However, the manufacturing of AM for LSC cultivation is costly and labor-intensive.
Fibrin, a substrate used extensively in ophthalmology as a glue, has been used in the cultivation of LSCs (Rama et al., 2001). Fibrin gel is easily degraded by the LSCs in culture. To preserve the fibrin membrane, aprotinin needs to be added to the culture medium for the duration of the cultivation. Importantly, the efficiency of this fibrin membrane in the clinical setting has been shown only in combination with 3T3 feeder cells and FBS (Rama et al., 2010).
Because of the disadvantages of AM and fibrin, synthetic hydrogels have been tested in LSC cultivation. Siloxane hydrogel in combination with a contact lens was tested in a clinical trial (Di Girolamo et al., 2009). Most hydrogels still are in development and have not been studied in clinical trials. In recent years, biosynthetic collagen scaffolds have been developed to mimic the biomechanical properties of the native LSC niche, which contains different types of collagen in vivo (Mei et al., 2012; Nakatsu et al., 2013). Preclinical studies of Real Architecture for 3D Tissue (RAFT) are promising in creating a 3D corneal equivalent that composed of compressed collagens, corneal stromal keratocytes and engineered limbal epithelial cells (Massie et al., 2015). In addition, the mechanical properties of synthetic hydrogels and ECM scaffold can be tailored to mimic that in the limbal niche to maximize the efficiency of LSCs expansion in vitro. Although the efficiency and feasibility of these synthetic hydrogels and collagen scaffolds for LSC cultivation need to be validated in clinical study, their consistency and predictability pose them as a preferred substrate-carrier of LSCs in the foreseeable future.
4.5. Culture medium
To increase the safety of LSC therapy, many groups have either eliminated or substituted FBS and 3T3 cells in LSC cultivation (Behaegel et al., 2019; Sangwan et al., 2011). The conventional supplemented hormonal epithelium medium (SHEM) contains other components such as dimethyl sulfoxide (DMSO) and cholera toxin, which could be toxic in humans. Moreover, high concentrations of DMSO increase differentiation of LSCs in culture. Recently, we developed a xenobiotic-free culture medium in which FBS, cholera toxin, and DMSO were eliminated (Gonzalez et al., 2017). Three different formulations of a xenobiotic-free culture medium were evaluated to assess their ability to support the expansion of LSCs. Of these 3 formulations of culture medium, a modified SHEM formulation supported similar cell growth and yielded a similar proportion of p63αbright stem/progenitor cells to the conventional SHEM when limbal explants were cultivated on denuded human AM. Moreover, LSCs cultivated according to this protocol successfully reconstructed the ocular surface of a rabbit model of total LSCD (unpublished data); this successful reconstruction indicates that these cultivated LSCs are functional.
The choice of each individual component for the LSC expansion, i.e., the starting LSC material, feeder cells, cell substrate, cultivation configuration, and culture medium, and the various combinations of these individual components, is a key step during the therapeutic discovery process. The combination of all these parameters will determine the most feasible, safe, and efficient LSC cultivation method to use in the clinical setting and the particular method that is compatible with regulatory standards.
4.6. Considerations in cGMP manufacturing of limbal stem/progenitor cells
A robust, reproducible, and standardized manufacturing process is critical in the development of LSC therapies (Pellegrini et al., 2016). Successful cell product development requires controlling product quality in compliance with cGMP, the main regulatory standards for ensuring pharmaceutical quality.
In-process controls (IPCs) are routine checks performed during manufacturing to ensure that the product conforms to its specifications, and quality is met throughout the manufacturing process. Release criteria (RC) are a set of specifications that the final product must meet in order to be released for patient treatment (Eichler et al., 2013). These quality checks are essential to ensure the safety and purity of the product and to demonstrate lot-to-lot consistency (Riviere and Roy, 2017). Another aspect to consider is the biological activity of the LSC product. Potency assays determine the relationship between the specifications of the product and the safety and efficacy of the product (clinical outcomes).
The establishment of quality controls during the manufacturing process is one of the major challenges that must be overcome in cell therapy using cultivated LSCs (de Almeida Fuzeta et al., 2020). Indeed, Haagdorens et al. reported that of 1164 transplants using cultivated LSCs, only 147 of them were produced under cGMP conditions (Haagdorens et al., 2016). The vast majority of studies failed to document any kind of quality control measures (Ang et al., 2007; Sejpal et al., 2013a). One challenge in the characterization of the cultivated LSC product is the limited cell availability. Generally, the cultivated LSC sheet remains untouched for transplantation to avoid compromising its quality and integrity. Cultivated LSCs cells are no longer suitable for cell therapy after undergoing phenotyping assays. Alternative options include a parallel culture derived from a second biopsy or a biopsy from cadaveric donors. Obtaining a second biopsy from the donor eye may be unacceptable for some patients as their ocular surface might not tolerate the procedure. Cadaveric donor could serve as a control for the LSC manufacturing process. However, donor differences associated with this approach do not ensure a clear correlation between the product that is transplanted and clinical outcome (Fatima et al., 2007). Another strategy is to correlate LSC transplant quality with that of previous LSC cultures using different donor developed with the same manufacturing process. The weaknesses of this approach again arise from low reproducibility between experiments and donor variability.
To overcome these limitations, many laboratories, including ours, have contributed to the establishment of a set of specifications used as the IPCs and RC to standardize and measure the quality of cultivated LSCs. Di Iorio et al. (2010) suggested that a small proportion of cultivated cells could be used for analysis before transplantation. CFE testing was done as an IPC and repeated later as RC (Di Iorio et al., 2010). Gram staining, endotoxin concentrations, and detection of mycoplasma contamination by qRT-PCR in addition to the use of specific virus detection kits were also suggested to ensure treatment safety. The US Food and Drug Administration (FDA) recommends that the basic product characterization of the IPCs and RC include sterility testing, endotoxin testing, mycoplasma testing, contaminating virus testing, and dose and identity assays. One limitation of fresh cell products is the fact that not all the RC testing can be finalized before transplantation. Cryopreservation allows for longer storage of the LSC product and completion of all necessary assays prior to release of the product before transplantation.
A robust LSC potency assay must be developed to correlate the quality of the LSC product and the clinical outcomes associated with its use. As mentioned previously, Rama et al. (2010) evaluated the expression of p63αbright cells in subconfluent primary cultures by quantifying the fluorescence intensity after immunostaining (Rama et al., 2010). In this study the percentage of p63αbright cells was correlated with the clinical success rate. However, this method requires a portion of LSCs for this assay. The most desirable approach would be one that does not require scarifying any LSC product for quality control. We are investigating the possibility of a nondestructive assay to evaluate the quality of the final LSC product. By implementing standardized IPCs and RC, the efficacy of different cultivation protocols can then be compared.
5. Evaluation of in vivo LSC Function
The function of LSCs in vivo is represented by the phenotype of epithelial cells and epithelial wound healing capacity on the corneal surface, i.e., the degree of LSCD. The initial diagnosis of LSCD can be made by slit-lamp examination when the clinical history is evocative and the typical stippled fluorescein staining in a vortex pattern is detected (Figure 15, upper 2 rows). These findings suggest a conjunctival epithelium phenotype on the cornea, the hallmark of LSCD (Deng et al., 2019). However, this clinical sign is subtle and not always detectable. The diagnosis of LSCD remains challenging if clinical signs alone are used. In partial LSCD, slit-lamp findings can be tenuous and subjective, e.g., abnormalities in the regularity and thickness of the epithelium may not be apparent, and signs of partial LSCD can be missed by clinical examination. The lack of POV can guide the diagnosis of LSCD. However, age and anatomical variation are confounding factors: POV tends to become less prominent or absent in older populations, thereby limiting its usefulness. Recurrent or persistent epithelial defects, corneal neovascularization, and corneal scarring can be seen in severe and total LSCD, but these signs are not unique to LSCD. The presence of one or more of these clinical signs is not sufficient for an LSCD diagnosis and can lead to a wrong diagnosis of LSCD, which could pose serious consequences as a result of unnecessary surgeries (Le et al., 2018b).
Figure 15. Clinical presentation and in vivo images of eyes with different stages of limbal stem cell deficiency.
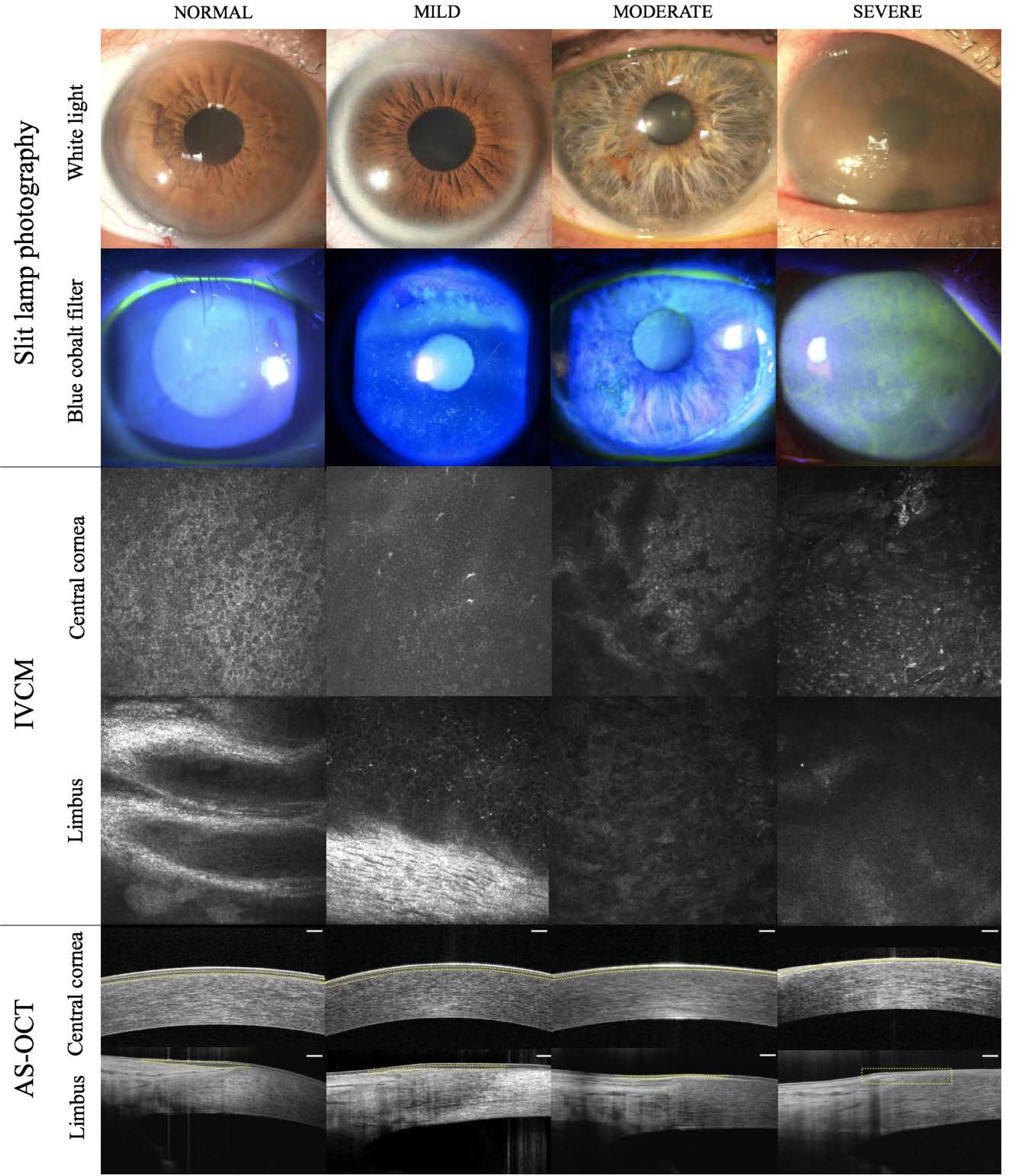
Slit-lamp photography under white light (first row) and blue cobalt light (second row) show a clear cornea in normal eye (left column) and abnormal fluorescein staining pattern in eyes with different severity of limbal stem cell deficiency (LSCD, right 3 columns). Images obtained from in vivo confocal microscopy (IVCM) of basal central corneal epithelial cells (third row) and limbus (fourth row) show palisade of Vogt in normal eye (left image) and different morphologic changes in the LSCD (right 3 images). Images from anterior segment optical coherence tomography (AS-OCT, bottom row) show normal epithelial thickness in normal eye (left image) and progressive epithelial thinning in LSCD (right 3 images).
In addition, identification of conjunctival goblet cells among the corneal epithelial cells by impression cytology, although widely used to confirm an LSCD diagnosis, has limitations. Its sensitivity is affected by several factors, including concurrent goblet cell deficiency and sampling error. The presence of goblet cells is not quantitative and is not indicative of the degree of LSCD. For example, a patient could present with clinical signs suggestive of total LSCD, and goblet cells could be detected on the corneal surface by impression cytology. Normal limbal epithelial cells could still be present as detected by IVCM, a finding suggestive of partial LSCD (Chan et al., 2016). However, less than 25% of eyes underwent LSC transplants between 2003 and 2019 had additional diagnostic tests to confirm the diagnosis of LSCD prior to surgery (Le et al., 2020a).
The severity of LSCD and amount of host residual LSCs likely affect clinical outcomes of LSC transplantation. Several studies have showed that no donor cells or a mixture of donor and recipient cells were detected in eyes with successful restoration of a stable corneal surface after allogeneic LSC transplants (Daya et al., 2005; Shimazaki et al., 1999; Williams et al., 1995). This observation suggests that the transplanted donor LSCs might provide necessary trophic factors to restore the function or pool of residual recipient LSCs in addition to serving as LSCs for the maintenance of corneal epithelium. Furthermore, medical treatments of other ocular surface comorbidities such as dry eye disease, ocular surface inflammation and drug toxicity can reverse the disease severity in partial LSCD (Deng et al., 2020a). Taken together, pharmacologic agents that restore the pool of residual functional LSCs might be sufficient to treat partial LSCD without the need of LSC transplantation. The ideal patient population of each treatment approach, i.e., medical treatment vs. surgical LSC transplantation needs to be delineated based on the severity of LSCD.
5.1. In vivo imaging
Until recently, there was no agreement on the disease entity, making it impossible to compare clinical outcomes across different studies. The recent global consensus on the definition, classification, diagnosis, and staging of LSCD fulfilled the need for an international agreement on objective criteria to define the disease and to provide a clinical grading system (Deng et al., 2019). The global consensus recommends that additional diagnostic tests should be performed to confirm the diagnosis, if possible, prior to surgical intervention. These tests include impression cytology to detect goblet cells or conjunctival epithelial cells on the cornea, and in vivo imaging such as IVCM and/or anterior segment optical coherence tomography (AS-OCT) to detect abnormal corneal epithelium and limbal structures. This section will describe the diagnostic tools developed recently to objectively evaluate the function of LSCs in vivo.
The use of IVCM in the diagnosis of LSCD has expanded exponentially over the last decade because this noninvasive method visualizes the microstructures of the cornea, limbus, and conjunctiva at the single-cell level (Figure 15, middle 2 rows) (Chan et al., 2015a; Deng et al., 2012; Lagali et al., 2013; Le et al., 2013; Le et al., 2010; Miri et al., 2012; Nubile et al., 2013). The Heidelberg Retina Tomograph (HRT) with Rostock Cornea module (Heidelberg Engineering, GmbH, Heidelberg, Germany) can image the limbus because HRT is a laser confocal device that uses a 670-nm wavelength red diode laser, whereas other slit scanning confocal devices cannot because of the back scattering of light (Tavakoli et al., 2008). Images of the central cornea and 4 areas of the limbus (superior, inferior, nasal, and temporal) are acquired by a minimum of 3 high-quality Z-scans (volume scans) of each area. To obtain images of the limbus, positioning of the eye is critical. The location of the limbus is confirmed visually by the red aiming beam through the side camera of the HRT and is based on cell morphology. Because the typical area of an HRT image is 0.4 × 0.4 mm2 (0.15% of the total cornea area), careful slit-lamp examination is necessary to determine the location of disease in patients with sectoral LSCD. Conversely, a 360° sequence scan can be performed to quickly look for residual LSCs in severe to total LSCD. Interpretation of the scans can be difficult; therefore, trained and independent examiners are required (Le et al., 2018c).
Because of its high resolution, IVCM is most informative in analyzing the microstructural changes that occur in the corneal and limbal epithelium of patients with LSCD, in addition to the detection of goblet cells (Aravena et al., 2019; Le et al., 2010). Criteria that were developed as in vivo markers of LSC function are the corneal epithelial thickness, cell morphology, and basal epithelial cell density and subbasal nerve density. They all have been correlated with the severity of LSCD graded by clinical examination (Chan et al., 2015a; Chuephanich et al., 2017; Deng et al., 2012). In mild stage of LSCD, basal epithelial cells border become less distinct. In moderate stage, the nuclei of these cells become more prominent. the cells become enlarged and metaplastic, and in severe stage cells resemble corneal epithelium are absent as well as very low density of subbasal nerve plexus (Deng et al., 2012). The cutoff values for normal basal cell and sub-basal nerve densities are >8107 cells/mm2 and >937.5 μm/area, respectively (Chuephanich et al., 2017). A basal cell density <4575 cells/mm2 and a sub-basal nerve density <114 μm/area are suggestive of severe LSCD. The cutoff value for a normal epithelium thickness is evaluated at >45.5 μm in the central cornea (sensitivity 67%, specificity 100%) and >51.0 μm in the limbus (sensitivity 81%, specificity 100%) (Chan et al., 2015a). The central basal epithelial cell density, the sub-basal nerve density, and the central epithelial thickness are much more reduced in LSCD than those in other corneal diseases presenting with decreased basal cell density. Changes of these in vivo markers in the central cornea are correlated with those in the limbus (Aravena et al., 2019; Chan et al., 2015a; Chan et al., 2015b; Chuephanich et al., 2017). Therefore, the changes in the central cornea represent the global LSC function.
IVCM provides very detailed information of the cornea and limbus, but the test is time-consuming and requires skilled technicians. AS-OCT is a quick, noninvasive, and no-contact imaging technique based on low coherence interferometry. This method compares the time delay and intensity of infrared light reflected from the tissue structures against a reference beam. AS-OCT can yield images of ocular elements of the anterior segment, including the cornea, limbus, anterior segment, and irido-corneal angle, and does not require the use of topical anesthesia. With the development of spectral-domain OCT, which provides an axial resolution of 3 μm, the corneal and limbal structures can be better visualized by this high resolution OCT than by time-domain devices (Feng and Simpson, 2005).
The anatomic structure and landmarks of the normal limbus need to be defined by AS-OCT so that the pathologic conditions can be detected and evaluated. The limbal area visualized by AS-OCT is defined as the region between a virtual line perpendicular to the scleral spur posteriorly and a virtual line between the end of the Bowman’s membrane and Descemet’s membrane anteriorly (Le et al., 2018a). The limbal epithelium has different thicknesses in different locations: the limbal epithelium is thickest in the superior quadrant followed by the inferior, temporal, and nasal quadrants. In addition, identification of normal variations in the limbal structures due to aging and differences among races is essential to set the appropriate normal standards. For example, Caucasian eyes have larger limbal areas than Chinese eyes. The distance from the limbus to the scleral spur is also greater in Caucasian eyes than in Chinese eyes (Le et al., 2018a). Using the AS-OCT enface mode, the POV and limbal crypts can also be delineated in normal eyes and used as a criterion to rule out LSCD (Banayan et al., 2018).
Progressive epithelial thinning of the corneal epithelium has been confirmed using AS-OCT (Figure 15, bottom 2 rows). Automated measurements of the central epithelial thickness using the software provided by the manufacturer have been reported in normal eyes and in eyes with keratoconus or dry eye diseases (Cui et al., 2014; Francoz et al., 2011). However, in eyes with LSCD, manual measurements are preferred as the automatic program is not validated for this pathologic condition. Indeed, common characteristics of eyes with LSCD such as irregular and epithelium thickness, hyperreflectivity of subepithelial scars, and stromal opacities can interfere with automated epithelial thickness measurement. On the highest quality cross-sectional images, the central epithelial thickness and the maximal limbal epithelial thicknesses in each quadrant can be assessed by averaging 3 manual measurements (Le et al., 2018a; Liang et al., 2020). Epithelial thickness obtained using this 3-point measurement protocol from AS-OCT has a better correlation with that obtained using IVCM. The positive correlation between epithelial thinning and the severity of LSCD clinical grading has been confirmed using AS-OCT (Liang et al., 2020), which supports the use of AS-OCT to measure epithelial thickness in lieu of IVCM.
Because each individual in vivo parameter, i.e., decreased corneal basal cell density, epithelial thinning and subbasal nerve plexus density reduction is observed in other diseases, a diagnosis of LSCD can’t be reached by using only one parameter. A combination of these in vivo parameters are necessary to confirm a LSCD diagnosis and stage the disease severity.
5.2. Molecular diagnostic tests of limbal stem cell deficiency
Impression cytology to detect goblet cells on the cornea was the gold-standard diagnostic test for LSCD until recently. In impression cytology, cells on the ocular surface are sampled by using a filter membrane with a small pore size. The absence of tears increases the quantity of cells sampled (Doughty, 2012; Singh et al., 2005). To ensure the collection of conjunctival epithelial cells in sectoral LSCD, identifying the location of the abnormal epithelium by careful slit-lamp examination is critical. One method to reduce the chance of sampling error is to sample the entire corneal surface using a 13-mm round membrane. A gentle pressure is applied for 5–15 seconds on the membrane before peeling it off of the cornea. The cell sample is then subjected to histochemical staining, e.g., Papanicolaou or periodic acid Schiff staining, to detect goblet cells. A sample from the nasal or inferior bulbar conjunctiva serves as control to ensure the histochemical staining process is successful.
As mentioned previously, goblet cell detection has low sensitivity. To overcome this shortcoming, immunohistochemical analysis of impression cytology specimens is used to identify the specific type of cytokeratin and thus the type of epithelium (Le et al., 2018c). Cytokeratin profiles have become available markers of epithelial origins and have been used to confirm the diagnosis of LSCD. K12 is a specific marker of differentiated corneal epithelial cells, whereas K13 and K7 are reportedly specific conjunctival epithelial cell markers (Merjava et al., 2011; Ramirez-Miranda et al., 2011). K19 is also expressed in conjunctival epithelial cells but is less specific than K13 (Ramirez-Miranda et al., 2011). Mucin 5AC is a marker of goblet cell. RT-PCR to detect the mRNA of these conjunctival markers is an alternative method to confirm the presence of conjunctival epithelial cells on the corneal surface. Because impression cytology tends to be more effective in collecting the conjunctival epithelial cells than corneal epithelial cells, this sampling error renders impression cytology a nonquantitative test to measure LSCD severity. Moreover, the presence of goblet cells on the corneal surface confirms the presence of LSCD but does not imply that the patient has total LSCD.
5.3. A quantitative system to stage limbal stem cell function
The global consensus has provided a useful grading system that can be adapted by general ophthalmologists worldwide to stage LSCD (Deng et al., 2019). The grading system is based on the extent of corneal and limbal involvement of the clinical presentation. The 2 most important factors to consider are whether the visual axis is involved (it is not involved in stage I) and whether more than 50% of the limbus is affected (stage IIC and III). The final stage—stage III—corresponds to total LSCD with involvement of the entire corneal surface.
To quantify the degree of LSCD, a numeric clinical grading system based on 3 criteria includes the extent of limbal and corneal involvement (Figure 16) (Aravena et al., 2019). A numeric grade is assigned on the basis of the clock hours involved as follows: 1) the number of clock hours of limbal involvement (0–4 points), 2) the number of quadrants of cornea involvement (0–4 points), and 3) the pupil (central 4 mm) involvement (0 point if not involved, 2 points if involved). LSCD is then classified as mild, moderate, or severe stages when the point total is ≤ 4, 5–7, or ≥ 8 points, respectively. This clinical score is positively correlated with the degree of reduction in visual acuity, density of central corneal basal cells and of central sub-basal nerves, as well as with the reduction of central corneal epithelial thickness measured by AS-OCT (Figure 17). This clinical grading system allows a more precise staging of the corneal involvement and grading combined with in vivo parameters.
Figure 16. Clinical grading of limbal stem cell deficiency.

Limbus involvement in clock hours (top panel), corneal surface area (middle panel), and visual axis involvement (bottom panel) criteria are shown. Slit-lamp photograph of an eye with a total score of 6 points, classified as the moderate stage of LSCD (far right panel). Reprinted from Aravena et al. (Aravena et al., 2019) with permission from Wolters Kluwer Health, Inc.
Figure 17. Correlation between clinical score, in vivo parameters in patients with limbal stem cell deficiency.
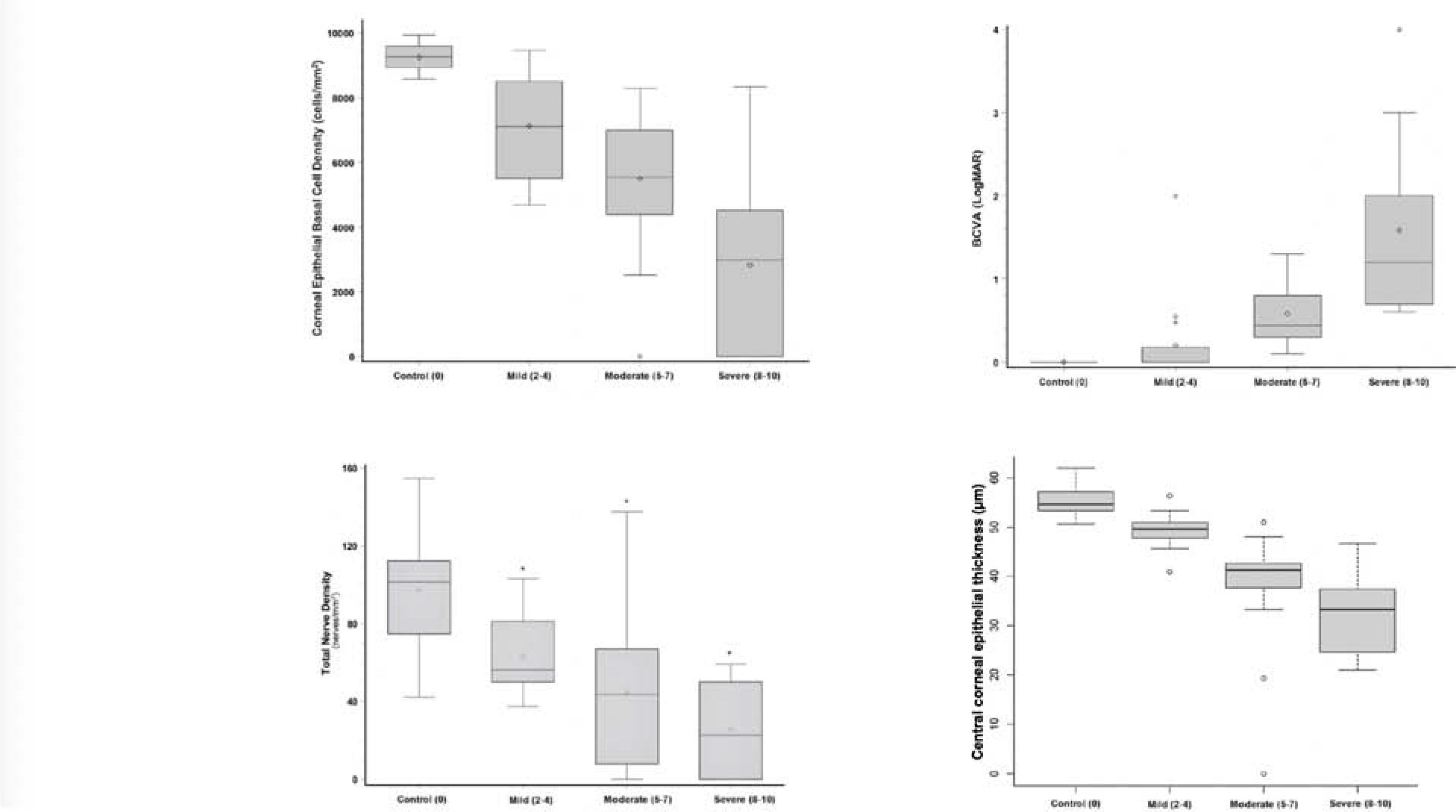
Box and whisker plots of the central corneal basal cell density (top left panel), sub-basal nerve density (bottom left panel), best corrected distance visual acuity (BCVA, top right panel) and central corneal epithelial thickness measured by AS-OCT (bottom right panel) in control groups and in patients with mild, moderate and severe limbal stem cell deficiency. The clinical score positively correlated with the degree of reduction in visual acuity and all in vivo parameters. Statistical analyses were performed with Kruskall-Wallis test and Pearson correlation coefficient. Reprinted from Chan et al. (Chan et al., 2015b), Aravena et al. (Aravena et al., 2019) with permission from Wolters Kluwer Health, Inc., Chuephanich et al. (Chuephanich et al., 2017), Liang et al. (Liang et al., 2020).
Improvement in image resolution and analysis by deep machine learning will optimize the quantification of in vivo parameters and will increase the standardization of the measurements to allow a faster and more reliable assessment. The development of limbal 3D imaging, reconstruction, and analysis is also being investigated. The 3D limbal structure can be mapped by IVCM and AS-OCT, and results of such mapping could provide structural information about the LSC niche that potentially allows precise localization and quantitation of residual functional LSCs (Bizheva et al., 2011; Grieve et al., 2015; Lathrop et al., 2012; Li et al., 2011). These imaging techniques will enable precise targeted biopsy in eyes with partial LSCD for expansion of LSCs in culture for autologous therapy in unilateral and bilateral LSCD. A recent meta-analysis and the global consensus of the management of LSCD reported that autologous LSC therapy results in higher success rates and fewer complications than allogeneic LSC therapy; therefore, autologous LSC therapy should be preferred is all possible even in eyes with bilateral LSCD (Deng et al., 2020a; Le et al., 2020b). If residual limbal epithelial cells could be located in severe LSCD using IVCM, targeted biopsy of these residual limbal epithelial cells followed by expansion of LSCs in culture could allow autologous LSC treatment of these bilateral LSCD.
An accurate diagnosis and precise staging of LSCD requires clinical experience and the availability of the imaging devices and a laboratory to process the impression cytology samples. IVCM, experience to analyze the IVCM images, and a laboratory are usually available only in selected tertiary eye care centers. However, the recent establishment of a diagnostic method using AS-OCT could overcome these challenges because AS-OCT is available in most ophthalmology clinics (Liang et al., 2020). AS-OCT can be used by most general ophthalmologists to measure central epithelial thickness when LSCD is suspected. In any case of uncertainty, referral of the patient to a tertiary eye care center for IVCM and detection of molecular biomarkers would be an appropriate subsequent step to confirm or rule out the diagnosis, and stage LSCD. Co-management between local comprehensive ophthalmologist and corneal specialists would benefit patients with complex LSCD.
In summary, recent advances in the anterior segment imaging and development of conjunctival molecular biomarkers have greatly aided in the accurate diagnosis and staging of LSCD. The same grading method could be used to evaluate outcomes after medical treatment and LSC therapies. However, the degree of LSCD severity that benefits most from LSC transplantation remains to be established. It is likely that the benefits of LSC transplantation are reserved for severe to total LSCD, whereas medical treatment to optimize the ocular surface is sufficient for treatment of partial LSCD. Cultivated LSCs is likely more effective to treat severe LSCD than simple limbal epithelial transplantation does. An internationally standardized LSCD staging criteria is important in assessing the indication and efficacy of different therapies.
6. Conclusion and Future Directions
Significant advances have been made in the understanding of LSC biology, and LSCD pathophysiology and treatment over the last several decades. The advancements in the field of LSC research has been built on the studies of corneal epithelial homeostasis and wound healing, LSC maintenance and niche regulation, and biomarker identification. The first global consensus on the definition, diagnosis, classification, staging, and management of LSCD has set general guidelines for ophthalmologists and corneal specialists. Newly developed diagnostic tools such as IVCM, AS-OCT, and detection of specific conjunctival epithelial biomarkers have permitted an accurate diagnosis and precise staging of LSCD. Despite these advancements, LSCD management still faces significant hurdles in translating bench to bedside. This is largely due to a lack of standardized criteria of clinical outcome measures to compare different LSC therapies globally, and the lack of standardized quality controls for LSC cultivation, in the case of LSC treatment using cultivated LSCs.
Further understanding of the molecular biology of LSC regulation, improvement of LSC bioengineering, and refinement of LSCD diagnostic tools will be of paramount in improving clinical outcomes of LSC treatments. First, a critical need is the further elucidation of the regulation of LSC self-renewal, differentiation, and proliferation by different signaling pathways and via the crosstalk among these pathways such as Wnt, Notch, TGF-β/BMP, and Sonic hedgehog, and by cell-to-cell contact and ECM-mediated signaling pathways. Small molecules that modulate the fate of LSCs could enhance the efficiency of LSC expansion in culture and enable medical treatment for partial LSCD by repopulating the residual LSC pool, thereby obviating the need for LSC transplantation in this subset of patients. Additionally, a more complete understanding of LSC regulation will improve the use of other stem cell sources such as oral mucosal stem cells, induced pluripotent stem cells, and mesenchymal stem cells for the treatment for LSCD. Second, novel bioengineering approaches could incorporate new technology such as 3D printing, hydrogels, nanotechnology, and matrix biology to reconstruct the LSC niche to improve the long-term outcome of LSC transplantation. Third, a standardized and streamlined reading of in vivo images could be achieved by employing machine learning and artificial intelligence. This advancement will allow ophthalmologists across the world to accurately diagnose and stage LSCD, which is critical in the management of the disease.
This is an exciting time in the field of LSC biology and LSCD management. Stem cell therapy for LSCD has pioneered regenerative medicine in ophthalmology. The field will continue to lead the way in regenerative medicine by taking advantage of the next generation of imaging, artificial intelligence, and molecular technologies for the development of novel LSC therapies.
Highlights.
The limbal niche consists of unique cellular and molecular components.
Wnt signaling pathways play a critical role in the survival and regulation of LSCs.
Notch signaling regulates the differentiation and stratification of corneal epithelia.
Xenobiotic-free cGMP LSC cultivation could raise the safety standards.
Standardized LSCD diagnosis and staging criteria are necessary.
8. Acknowledgments
Funding source: SXD received grant support from the National Eye Institute (NEI, 2R01 EY021797 and R01 EY028557) and the California Institute for Regenerative Medicine (TR2–01768, CLIN1–08686, CLIN2–11650). JZ received grant support from the NEI (R01 EY028557). JSR received a Diversity Supplement from the NEI (R01 EY028557). This work is supported in part by an unrestricted grant from Research to Prevent Blindness to the Department of Ophthalmology at the University of California, Los Angeles. Funding sources have no role in the design of the work reported in this manuscript.
We would like to thank Ms. Alis Balayan and Ms. Jennifer Sunga for their assistance in compiling the manuscript. Editing service was provided by Julia C. Jones, PharmD, PhD.
Abbreviations used in the manuscript:
- ABCG2
ATP-binding cassette super-family G member 2
- ABCB5
ATP-binding cassette, sub-family B, member 5
- AM
amniotic membrane
- AS-OCT
anterior segment optical coherence tomography
- BCVA
best corrected visual acuity
- CFE
colony forming efficiency
- cGMP
current good manufacturing practices
- DKK
dickkopf
- ECM
extracellular Matrix
- FBS
fetal bovine serum
- FGF
fibroblast Growth Factor
- FRZB
frizzled-related protein B
- Fzd
frizzled
- HOP
homeodomain-only protein
- HRT
Heidelberg retina tomography
- IPC
in-process controls
- IVCM
in vivo confocal microscopy
- K
cytokeratin
- LiCl
lithium chloride
- LSC
limbal stem cell
- LSCD
limbal stem cell deficiency
- miRNA
micro RNA
- MSC
mesenchymal stem cells
- NICD
Notch intracellular domain
- N1ICD
Notch 1 intracellular domain
- PCP
planar cell polarity
- POV
palisades of Vogt
- SFRP
secreted frizzled-related protein
- SHEM/mSHEM
supplemented hormonal epithelium medium/modified SHEM
- SSEA4
stage-specific embryonic antigen-4
- TCF/LEF
T-cell factor/lymphoid enhancer factor
- TGF-β/BMP
transforming growth factor beta/bone morphogenetic protein
- YAP/TAZ
yes-associated protein/ transcriptional co-activator with PDZ-binding motif
Footnotes
Disclosure: SXD is a consultant for Dompe US. All other authors: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
9. References:
- Ahadome SD, Zhang C, Tannous E, Shen J, Zheng JJ, 2017. Small-molecule inhibition of Wnt signaling abrogates dexamethasone-induced phenotype of primary human trabecular meshwork cells. Exp Cell Res 357, 116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alok A, Lei Z, Jagannathan NS, Kaur S, Harmston N, Rozen SG, Tucker-Kellogg L, Virshup DM, 2017. Wnt proteins synergize to activate beta-catenin signaling. J Cell Sci 130, 1532–1544. [DOI] [PubMed] [Google Scholar]
- Andersson ER, Sandberg R, Lendahl U, 2011. Notch signaling: simplicity in design, versatility in function. Development 138, 3593–3612. [DOI] [PubMed] [Google Scholar]
- Ang LP, Sotozono C, Koizumi N, Suzuki T, Inatomi T, Kinoshita S, 2007. A comparison between cultivated and conventional limbal stem cell transplantation for Stevens-Johnson syndrome. Am J Ophthalmol 143, 178–180. [DOI] [PubMed] [Google Scholar]
- Aravena C, Bozkurt K, Chuephanich P, Supiyaphun C, Yu F, Deng SX, 2019. Classification of Limbal Stem Cell Deficiency Using Clinical and Confocal Grading. Cornea 38, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsdorf EJ, Tummala P, Jacobs CR, 2009. Non-canonical Wnt signaling and N-cadherin related beta-catenin signaling play a role in mechanically induced osteogenic cell fate. PLoS One 4, e5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuara-Blanco A, Pillai CT, Dua HS, 1999. Amniotic membrane transplantation for ocular surface reconstruction. Br J Ophthalmol 83, 399–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banayan N, Georgeon C, Grieve K, Borderie VM, 2018. Spectral Domain Optical Coherence Tomography in Limbal Stem Cell Deficiency. A Case Control Study. Am J Ophthalmol. [DOI] [PubMed] [Google Scholar]
- Bao J, Zheng JJ, Wu D, 2012. The structural basis of DKK-mediated inhibition of Wnt/LRP signaling. Sci Signal 5, pe22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaro V, Ferrari S, Fasolo A, Pedrotti E, Marchini G, Sbabo A, Nettis N, Ponzin D, Di Iorio E, 2010. Evaluation of ocular surface disorders: a new diagnostic tool based on impression cytology and confocal laser scanning microscopy. Br J Ophthalmol 94, 926–932. [DOI] [PubMed] [Google Scholar]
- Basu S, Hertsenberg AJ, Funderburgh ML, Burrow MK, Mann MM, Du Y, Lathrop KL, Syed-Picard FN, Adams SM, Birk DE, Funderburgh JL, 2014. Human limbal biopsy-derived stromal stem cells prevent corneal scarring. Sci Transl Med 6, 266ra172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behaegel J, Zakaria N, Tassignon MJ, Leysen I, Bock F, Koppen C, Ni Dhubhghaill S, 2019. Short- and Long-Term Results of Xenogeneic-Free Cultivated Autologous and Allogeneic Limbal Epithelial Stem Cell Transplantations. Cornea 38, 1543–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzinger CF, Wang YX, von Maltzahn J, Soleimani VD, Yin H, Rudnicki MA, 2013. Fibronectin regulates Wnt7a signaling and satellite cell expansion. Cell Stem Cell 12, 75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergholz J, Xiao ZX, 2012. Role of p63 in Development, Tumorigenesis and Cancer Progression. Cancer Microenviron 5, 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop JA, Teruya-Feldstein J, Westra WH, Pelosi G, Travis WD, Rekhtman N, 2012. p40 (DeltaNp63) is superior to p63 for the diagnosis of pulmonary squamous cell carcinoma. Mod Pathol 25, 405–415. [DOI] [PubMed] [Google Scholar]
- Bizheva K, Hutchings N, Sorbara L, Moayed AA, Simpson T, 2011. In vivo volumetric imaging of the human corneo-scleral limbus with spectral domain OCT. Biomed Opt Express 2, 1794–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagg J, Workman P, 2017. Choose and Use Your Chemical Probe Wisely to Explore Cancer Biology. Cancer Cell 32, 9–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojic S, Hallam D, Alcada N, Ghareeb A, Queen R, Pervinder S, Buck H, Amitai Lange A, Figueiredo G, Rooney P, Stojkovic M, Shortt A, Figueiredo FC, Lako M, 2018. CD200 Expression Marks a Population of Quiescent Limbal Epithelial Stem Cells with Holoclone Forming Ability. Stem cells (Dayton, Ohio) 36, 1723–1735. [DOI] [PubMed] [Google Scholar]
- Bonnet C, Roberts JS, Deng SX, 2021. Limbal stem cell diseases. Experimental eye research, 108437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borderie VM, Ghoubay D, Georgeon C, Borderie M, de Sousa C, Legendre A, Rouard H, 2019. Long-Term Results of Cultured Limbal Stem Cell Versus Limbal Tissue Transplantation in Stage III Limbal Deficiency. Stem Cells Transl Med 8, 1230–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camussi G, Deregibus MC, Cantaluppi V, 2013. Role of stem-cell-derived microvesicles in the paracrine action of stem cells. Biochem Soc Trans 41, 283–287. [DOI] [PubMed] [Google Scholar]
- Chan E, Le Q, Codriansky A, Hong J, Xu J, Deng SX, 2016. Existence of Normal Limbal Epithelium in Eyes With Clinical Signs of Total Limbal Stem Cell Deficiency. Cornea 35, 1483–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan EH, Chen L, Rao JY, Yu F, Deng SX, 2015a. Limbal Basal Cell Density Decreases in Limbal Stem Cell Deficiency. Am J Ophthalmol 160, 678–684 e674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan EH, Chen L, Yu F, Deng SX, 2015b. Epithelial Thinning in Limbal Stem Cell Deficiency. Am J Ophthalmol 160, 669–677 e664. [DOI] [PubMed] [Google Scholar]
- Chee KY, Kicic A, Wiffen SJ, 2006. Limbal stem cells: the search for a marker. Clin Experiment Ophthalmol 34, 64–73. [DOI] [PubMed] [Google Scholar]
- Chen F, Kook H, Milewski R, Gitler AD, Lu MM, Li J, Nazarian R, Schnepp R, Jen K, Biben C, Runke G, Mackay JP, Novotny J, Schwartz RJ, Harvey RP, Mullins MC, Epstein JA, 2002. Hop is an unusual homeobox gene that modulates cardiac development. Cell 110, 713–723. [DOI] [PubMed] [Google Scholar]
- Chen H, Lu C, Ouyang B, Zhang H, Huang Z, Bhatia D, Lee SJ, Shah D, Sura A, Yeh WC, Li Y, 2020. Development of Potent, Selective Surrogate WNT Molecules and Their Application in Defining Frizzled Requirements. Cell Chem Biol. [DOI] [PubMed] [Google Scholar]
- Chen SY, Han B, Zhu YT, Mahabole M, Huang J, Beebe DC, Tseng SC, 2015. HC-HA/PTX3 Purified From Amniotic Membrane Promotes BMP Signaling in Limbal Niche Cells to Maintain Quiescence of Limbal Epithelial Progenitor/Stem Cells. Stem cells 33, 3341–3355. [DOI] [PubMed] [Google Scholar]
- Chen SY, Hayashida Y, Chen MY, Xie HT, Tseng SC, 2011. A new isolation method of human limbal progenitor cells by maintaining close association with their niche cells. Tissue Eng Part C Methods 17, 537–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, de Paiva CS, Luo L, Kretzer FL, Pflugfelder SC, Li DQ, 2004. Characterization of putative stem cell phenotype in human limbal epithelia. Stem cells 22, 355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuephanich P, Supiyaphun C, Aravena C, Bozkurt TK, Yu F, Deng SX, 2017. Characterization of the Corneal Subbasal Nerve Plexus in Limbal Stem Cell Deficiency. Cornea 36, 347–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement-Lacroix P, Ai M, Morvan F, Roman-Roman S, Vayssiere B, Belleville C, Estrera K, Warman ML, Baron R, Rawadi G, 2005. Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc Natl Acad Sci U S A 102, 17406–17411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H, Loh KM, Nusse R, 2014. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 346, 1248012. [DOI] [PubMed] [Google Scholar]
- Coghlan MP, Culbert AA, Cross DA, Corcoran SL, Yates JW, Pearce NJ, Rausch OL, Murphy GJ, Carter PS, Roxbee Cox L, Mills D, Brown MJ, Haigh D, Ward RW, Smith DG, Murray KJ, Reith AD, Holder JC, 2000. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem Biol 7, 793–803. [DOI] [PubMed] [Google Scholar]
- Collin J, Queen R, Zerti D, Bojic S, Moyse N, Molina MM, Yang C, Reynolds G, Hussain R, Coxhead JM, Lisgo S, Henderson D, Joseph A, Rooney P, Ghosh S, Connon C, Haniffa M, Figueiredo F, Armstrong L, Lako M, 2020. A single cell atlas of human cornea that defines its development, limbal stem and progenitor cells and the interactions with the limbal niche. bioRxiv, 2020.2007.2009.195438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotsarelis G, Cheng SZ, Dong G, Sun TT, Lavker RM, 1989. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell 57, 201–209. [DOI] [PubMed] [Google Scholar]
- Cui X, Hong J, Wang F, Deng SX, Yang Y, Zhu X, Wu D, Zhao Y, Xu J, 2014. Assessment of corneal epithelial thickness in dry eye patients. Optom Vis Sci 91, 1446–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza B, Meloty-Kapella L, Weinmaster G, 2010. Canonical and non-canonical Notch ligands. Current topics in developmental biology 92, 73–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davanger M, Evensen A, 1971. Role of the pericorneal papillary structure in renewal of corneal epithelium. Nature 229, 560–561. [DOI] [PubMed] [Google Scholar]
- Daya SM, Watson A, Sharpe JR, Giledi O, Rowe A, Martin R, James SE, 2005. Outcomes and DNA analysis of ex vivo expanded stem cell allograft for ocular surface reconstruction. Ophthalmology 112, 470–477. [DOI] [PubMed] [Google Scholar]
- de Almeida Fuzeta M, de Matos Branco AD, Fernandes-Platzgummer A, da Silva CL, Cabral JMS, 2020. Addressing the Manufacturing Challenges of Cell-Based Therapies. Advances in biochemical engineering/biotechnology 171, 225–278. [DOI] [PubMed] [Google Scholar]
- De Paiva CS, Pflugfelder SC, Li DQ, 2006. Cell size correlates with phenotype and proliferative capacity in human corneal epithelial cells. Stem cells 24, 368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Alamo D, Rouault H, Schweisguth F, 2011. Mechanism and significance of cis-inhibition in Notch signalling. Current biology : CB 21, R40–47. [DOI] [PubMed] [Google Scholar]
- Deng S, Kruse F, Gomes J, Chan C, Daya S, Dana R, Figueiredo F, Kinoshita S, Rama P, Sangwan V, Slomovic A, Tan D, 2020a. Global Consensus on the Management of Limbal Stem Cell Deficiency. Cornea. [DOI] [PubMed] [Google Scholar]
- Deng SX, Borderie V, Chan CC, Dana R, Figueiredo FC, Gomes JAP, Pellegrini G, Shimmura S, Kruse FE, and The International Limbal Stem Cell Deficiency Working, G., 2019. Global Consensus on Definition, Classification, Diagnosis, and Staging of Limbal Stem Cell Deficiency. Cornea 38, 364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng SX, Dos Santos A, Gee S, 2020b. Therapeutic Potential of Extracellular Vesicles for the Treatment of Corneal Injuries and Scars. Transl Vis Sci Technol 9, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng SX, Sejpal KD, Tang Q, Aldave AJ, Lee OL, Yu F, 2012. Characterization of limbal stem cell deficiency by in vivo laser scanning confocal microscopy: a microstructural approach. Arch Ophthalmol 130, 440–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhamodaran K, Subramani M, Krishna L, Matalia H, Jayadev C, Chinnappaiah N, Shetty R, Das D, 2019. Temporal Regulation of Notch Signaling and Its Influence on the Differentiation of Ex Vivo Cultured Limbal Epithelial Cells. Curr Eye Res, 1–12. [DOI] [PubMed] [Google Scholar]
- Di Girolamo N, 2015. Moving epithelia: Tracking the fate of mammalian limbal epithelial stem cells. Prog Retin Eye Res 48, 203–225. [DOI] [PubMed] [Google Scholar]
- Di Girolamo N, Bosch M, Zamora K, Coroneo MT, Wakefield D, Watson SL, 2009. A contact lens-based technique for expansion and transplantation of autologous epithelial progenitors for ocular surface reconstruction. Transplantation 87, 1571–1578. [DOI] [PubMed] [Google Scholar]
- Di Iorio E, Barbaro V, Ruzza A, Ponzin D, Pellegrini G, De Luca M, 2005. Isoforms of DeltaNp63 and the migration of ocular limbal cells in human corneal regeneration. Proc Natl Acad Sci U S A 102, 9523–9528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Iorio E, Ferrari S, Fasolo A, Bohm E, Ponzin D, Barbaro V, 2010. Techniques for culture and assessment of limbal stem cell grafts. Ocul Surf 8, 146–153. [DOI] [PubMed] [Google Scholar]
- Ding Z, Dong J, Liu J, Deng SX, 2008. Preferential gene expression in the limbus of the vervet monkey. Mol Vis 14, 2031–2041. [PMC free article] [PubMed] [Google Scholar]
- Djalilian AR, Namavari A, Ito A, Balali S, Afshar A, Lavker RM, Yue BY, 2008. Down-regulation of Notch signaling during corneal epithelial proliferation. Mol Vis 14, 1041–1049. [PMC free article] [PubMed] [Google Scholar]
- Doughty MJ, 2012. Goblet cells of the normal human bulbar conjunctiva and their assessment by impression cytology sampling. Ocul Surf 10, 149–169. [DOI] [PubMed] [Google Scholar]
- Dua HS, Miri A, Alomar T, Yeung AM, Said DG, 2009. The role of limbal stem cells in corneal epithelial maintenance: testing the dogma. Ophthalmology 116, 856–863. [DOI] [PubMed] [Google Scholar]
- Dua HS, Shanmuganathan VA, Powell-Richards AO, Tighe PJ, Joseph A, 2005. Limbal epithelial crypts: a novel anatomical structure and a putative limbal stem cell niche. Br J Ophthalmol 89, 529–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziasko MA, Armer HE, Levis HJ, Shortt AJ, Tuft S, Daniels JT, 2014. Localisation of epithelial cells capable of holoclone formation in vitro and direct interaction with stromal cells in the native human limbal crypt. PLoS One 9, e94283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimi M, Taghi-Abadi E, Baharvand H, 2009. Limbal stem cells in review. J Ophthalmic Vis Res 4, 40–58. [PMC free article] [PubMed] [Google Scholar]
- Eichler H, Schrezenmeier H, Schallmoser K, Strunk D, Nystedt J, Kaartinen T, Korhonen M, Fleury-Cappellesso S, Sensebe L, Bonig H, Rebulla P, Giordano R, Lecchi L, Takanashi M, Watt SM, Austin EB, Guttridge M, McLaughlin LS, Panzer S, Reesink HW, 2013. Donor selection and release criteria of cellular therapy products. Vox sanguinis 104, 67–91. [DOI] [PubMed] [Google Scholar]
- Fan NW, Ho TC, Wu CW, Tsao YP, 2019. Pigment epithelium-derived factor peptide promotes limbal stem cell proliferation through hedgehog pathway. J Cell Mol Med 23, 4759–4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatima A, Vemuganti GK, Iftekhar G, Rao GN, Sangwan VS, 2007. In vivo survival and stratification of cultured limbal epithelium. Clin Exp Ophthalmol 35, 96–98. [DOI] [PubMed] [Google Scholar]
- Feng Y, Simpson TL, 2005. Comparison of human central cornea and limbus in vivo using optical coherence tomography. Optom Vis Sci 82, 416–419. [DOI] [PubMed] [Google Scholar]
- Figueira EC, Di Girolamo N, Coroneo MT, Wakefield D, 2007. The phenotype of limbal epithelial stem cells. Investigative ophthalmology & visual science 48, 144–156. [DOI] [PubMed] [Google Scholar]
- Fortini ME, 2009. Notch signaling: the core pathway and its posttranslational regulation. Dev Cell 16, 633–647. [DOI] [PubMed] [Google Scholar]
- Foster JW, Jones RR, Bippes CA, Gouveia RM, Connon CJ, 2014. Differential nuclear expression of Yap in basal epithelial cells across the cornea and substrates of differing stiffness. Experimental eye research 127, 37–41. [DOI] [PubMed] [Google Scholar]
- Francoz M, Karamoko I, Baudouin C, Labbe A, 2011. Ocular surface epithelial thickness evaluation with spectral-domain optical coherence tomography. Investigative ophthalmology & visual science 52, 9116–9123. [DOI] [PubMed] [Google Scholar]
- Fre S, Pallavi SK, Huyghe M, Lae M, Janssen KP, Robine S, Artavanis-Tsakonas S, Louvard D, 2009. Notch and Wnt signals cooperatively control cell proliferation and tumorigenesis in the intestine. Proc Natl Acad Sci U S A 106, 6309–6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattazzo F, Urciuolo A, Bonaldo P, 2014. Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochim Biophys Acta 1840, 2506–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson IK, 1989. The epithelial basement membrane zone of the limbus. Eye (Lond) 3 ( Pt 2), 132–140. [DOI] [PubMed] [Google Scholar]
- Goldberg MF, Bron AJ, 1982. Limbal palisades of Vogt. Trans Am Ophthalmol Soc 80, 155–171. [PMC free article] [PubMed] [Google Scholar]
- Gonzalez S, Chen L, Deng SX, 2017. Comparative Study of Xenobiotic-Free Media for the Cultivation of Human Limbal Epithelial Stem/Progenitor Cells. Tissue Eng Part C Methods 23, 219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez S, Deng SX, 2013. Presence of native limbal stromal cells increases the expansion efficiency of limbal stem/progenitor cells in culture. Experimental eye research 116, 169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González S, Halabi M, Ju D, Tsai M, Deng X, S., 2020. Role of Jagged1-mediated Notch Signaling Activation in the Differentiation and Stratification of the Human Limbal EpitheliumRole of Jagged1-mediated Notch Signaling Activation in the Differentiation and Stratification of the Human Limbal Epithelium. Cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez S, Mei H, Nakatsu MN, Baclagon ER, Deng SX, 2016. A 3D culture system enhances the ability of human bone marrow stromal cells to support the growth of limbal stem/progenitor cells. Stem Cell Res 16, 358–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez S, Oh D, Baclagon ER, Zheng JJ, Deng SX, 2019a. Wnt Signaling Is Required for the Maintenance of Human Limbal Stem/Progenitor Cells In Vitro. Investigative ophthalmology & visual science 60, 107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez S, Uhm H, Deng SX, 2019b. Notch Inhibition Prevents Differentiation of Human Limbal Stem/Progenitor Cells in vitro. Sci Rep 9, 10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouveia RM, Vajda F, Wibowo JA, Figueiredo F, Connon CJ, 2019. YAP, DeltaNp63, and beta-Catenin Signaling Pathways Are Involved in the Modulation of Corneal Epithelial Stem Cell Phenotype Induced by Substrate Stiffness. Cells 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve K, Ghoubay D, Georgeon C, Thouvenin O, Bouheraoua N, Paques M, Borderie VM, 2015. Three-dimensional structure of the mammalian limbal stem cell niche. Experimental eye research 140, 75–84. [DOI] [PubMed] [Google Scholar]
- Guo ZH, Zhang W, Jia YYS, Liu QX, Li ZF, Lin JS, 2018. An Insight into the Difficulties in the Discovery of Specific Biomarkers of Limbal Stem Cells. Int J Mol Sci 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haagdorens M, Cepla V, Melsbach E, Koivusalo L, Skottman H, Griffith M, Valiokas R, Zakaria N, Pintelon I, Tassignon MJ, 2019. In Vitro Cultivation of Limbal Epithelial Stem Cells on Surface-Modified Crosslinked Collagen Scaffolds. Stem Cells Int 2019, 7867613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haagdorens M, Van Acker SI, Van Gerwen V, Ni Dhubhghaill S, Koppen C, Tassignon MJ, Zakaria N, 2016. Limbal Stem Cell Deficiency: Current Treatment Options and Emerging Therapies. Stem Cells Int 2016, 9798374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi R, Yamato M, Sugiyama H, Sumide T, Yang J, Okano T, Tano Y, Nishida K, 2007. N-Cadherin is expressed by putative stem/progenitor cells and melanocytes in the human limbal epithelial stem cell niche. Stem cells 25, 289–296. [DOI] [PubMed] [Google Scholar]
- Hedgepeth CM, Conrad LJ, Zhang J, Huang HC, Lee VM, Klein PS, 1997. Activation of the Wnt signaling pathway: a molecular mechanism for lithium action. Dev Biol 185, 82–91. [DOI] [PubMed] [Google Scholar]
- Henderson JK, Draper JS, Baillie HS, Fishel S, Thomson JA, Moore H, Andrews PW, 2002. Preimplantation human embryos and embryonic stem cells show comparable expression of stage-specific embryonic antigens. Stem cells 20, 329–337. [DOI] [PubMed] [Google Scholar]
- Higa K, Shimmura S, Miyashita H, Shimazaki J, Tsubota K, 2005. Melanocytes in the corneal limbus interact with K19-positive basal epithelial cells. Experimental eye research 81, 218–223. [DOI] [PubMed] [Google Scholar]
- Holland EJ, 2015. Management of Limbal Stem Cell Deficiency: A Historical Perspective, Past, Present, and Future. Cornea 34 Suppl 10, S9–15. [DOI] [PubMed] [Google Scholar]
- Hou L, Fu W, Liu Y, Wang Q, Wang L, Huang Y, 2020. Agrin Promotes Limbal Stem Cell Proliferation and Corneal Wound Healing Through Hippo-Yap Signaling Pathway. Investigative ophthalmology & visual science 61, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Pu Q, Zhang Y, Ma Q, Li G, Li X, 2019. Expansion and maintenance of primary corneal epithelial stem/progenitor cells by inhibition of TGFbeta receptor I-mediated signaling. Experimental eye research 182, 44–56. [DOI] [PubMed] [Google Scholar]
- Huang M, Wang B, Wan P, Liang X, Wang X, Liu Y, Zhou Q, Wang Z, 2015. Roles of limbal microvascular net and limbal stroma in regulating maintenance of limbal epithelial stem cells. Cell Tissue Res 359, 547–563. [DOI] [PubMed] [Google Scholar]
- Janda CY, Dang LT, You C, Chang J, de Lau W, Zhong ZA, Yan KS, Marecic O, Siepe D, Li X, Moody JD, Williams BO, Clevers H, Piehler J, Baker D, Kuo CJ, Garcia KC, 2017. Surrogate Wnt agonists that phenocopy canonical Wnt and beta-catenin signalling. Nature 545, 234–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabosova A, Azar DT, Bannikov GA, Campbell KP, Durbeej M, Ghohestani RF, Jones JC, Kenney MC, Koch M, Ninomiya Y, Patton BL, Paulsson M, Sado Y, Sage EH, Sasaki T, Sorokin LM, Steiner-Champliaud MF, Sun TT, Sundarraj N, Timpl R, Virtanen I, Ljubimov AV, 2007. Compositional differences between infant and adult human corneal basement membranes. Invest Ophthalmol Vis Sci 48, 4989–4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan N, Wang J, Wray B, Patel P, Yang W, Peng H, Lavker RM, 2019. Single-Cell RNA Transcriptome Helps Define the Limbal/Corneal Epithelial Stem/Early Transit Amplifying Cells and How Autophagy Affects This Population. Investigative ophthalmology & visual science 60, 3570–3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper M, Moll R, Stosiek P, Karsten U, 1988. Patterns of cytokeratin and vimentin expression in the human eye. Histochemistry 89, 369–377. [DOI] [PubMed] [Google Scholar]
- Kawakita T, Espana EM, Higa K, Kato N, Li W, Tseng SC, 2013. Activation of Smad-mediated TGF-beta signaling triggers epithelial-mesenchymal transitions in murine cloned corneal progenitor cells. J Cell Physiol 228, 225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakita T, Shimmura S, Higa K, Espana EM, He H, Shimazaki J, Tsubota K, Tseng SC, 2009. Greater growth potential of p63-positive epithelial cell clusters maintained in human limbal epithelial sheets. Investigative ophthalmology & visual science 50, 4611–4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki S, Tanioka H, Yamasaki K, Connon CJ, Kinoshita S, 2006. Expression and tissue distribution of p63 isoforms in human ocular surface epithelia. Experimental eye research 82, 293–299. [DOI] [PubMed] [Google Scholar]
- Kim EK, Lee GH, Lee B, Maeng YS, 2017. Establishment of Novel Limbus-Derived, Highly Proliferative ABCG2(+)/ABCB5(+) Limbal Epithelial Stem Cell Cultures. Stem Cells Int 2017, 7678637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolli S, Ahmad S, Lako M, Figueiredo F, 2010. Successful clinical implementation of corneal epithelial stem cell therapy for treatment of unilateral limbal stem cell deficiency. Stem cells 28, 597–610. [DOI] [PubMed] [Google Scholar]
- Kolli S, Bojic S, Ghareeb AE, Kurzawa-Akanbi M, Figueiredo FC, Lako M, 2019. The Role of Nerve Growth Factor in Maintaining Proliferative Capacity, Colony-Forming Efficiency, and the Limbal Stem Cell Phenotype. Stem cells 37, 139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopan R, Ilagan MX, 2009. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 137, 216–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster MI, Kim S, Mills AA, DeMayo FJ, Roop DR, 2004. p63 is the molecular switch for initiation of an epithelial stratification program. Genes Dev 18, 126–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krek A, Grun D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, MacMenamin P, da Piedade I, Gunsalus KC, Stoffel M, Rajewsky N, 2005. Combinatorial microRNA target predictions. Nat Genet 37, 495–500. [DOI] [PubMed] [Google Scholar]
- Ksander BR, Kolovou PE, Wilson BJ, Saab KR, Guo Q, Ma J, McGuire SP, Gregory MS, Vincent WJ, Perez VL, Cruz-Guilloty F, Kao WW, Call MK, Tucker BA, Zhan Q, Murphy GF, Lathrop KL, Alt C, Mortensen LJ, Lin CP, Zieske JD, Frank MH, Frank NY, 2014. ABCB5 is a limbal stem cell gene required for corneal development and repair. Nature 511, 353–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni BB, Tighe PJ, Mohammed I, Yeung AM, Powe DG, Hopkinson A, Shanmuganathan VA, Dua HS, 2010. Comparative transcriptional profiling of the limbal epithelial crypt demonstrates its putative stem cell niche characteristics. BMC Genomics 11, 526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni M, Leszczynska A, Wei G, Winkler MA, Tang J, Funari VA, Deng N, Liu Z, Punj V, Deng SX, Ljubimov AV, Saghizadeh M, 2017. Genome-wide analysis suggests a differential microRNA signature associated with normal and diabetic human corneal limbus. Sci Rep 7, 3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kureshi AK, Drake RA, Daniels JT, 2014. Challenges in the development of a reference standard and potency assay for the clinical production of RAFT tissue equivalents for the cornea. Regen Med 9, 167–177. [DOI] [PubMed] [Google Scholar]
- Lagali N, Eden U, Utheim TP, Chen X, Riise R, Dellby A, Fagerholm P, 2013. In vivo morphology of the limbal palisades of vogt correlates with progressive stem cell deficiency in aniridia-related keratopathy. Investigative ophthalmology & visual science 54, 5333–5342. [DOI] [PubMed] [Google Scholar]
- Lathrop KL, Gupta D, Kagemann L, Schuman JS, Sundarraj N, 2012. Optical coherence tomography as a rapid, accurate, noncontact method of visualizing the palisades of Vogt. Investigative ophthalmology & visual science 53, 1381–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrenson JG, Ruskell GL, 1991. The structure of corpuscular nerve endings in the limbal conjunctiva of the human eye. J Anat 177, 75–84. [PMC free article] [PubMed] [Google Scholar]
- Le Q, Chauhan T, Deng SX, 2020a. Diagnostic criteria for limbal stem cell deficiency before surgical intervention-A systematic literature review and analysis. Surv Ophthalmol 65, 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Q, Chauhan T, Yung M, Tseng CH, Deng SX, 2020b. Outcomes of Limbal Stem Cell Transplant: A Meta-analysis. JAMA Ophthalmol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Q, Cordova D, Xu J, Deng SX, 2018a. In Vivo Evaluation of the Limbus Using Anterior Segment Optical Coherence Tomography. Transl Vis Sci Technol 7, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Q, Deng SX, 2019. The application of human amniotic membrane in the surgical management of limbal stem cell deficiency. Ocul Surf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Q, Deng SX, Xu J, 2013. In vivo confocal microscopy of congenital aniridia-associated keratopathy. Eye (Lond) 27, 763–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Q, Samson CM, Deng SX, 2018b. A Case of Corneal Neovascularization Misdiagnosed as Total Limbal Stem Cell Deficiency. Cornea 37, 1067–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Q, Xu J, Deng SX, 2018c. The diagnosis of limbal stem cell deficiency. Ocul Surf 16, 58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le QH, Wang WT, Hong JX, Sun XH, Zheng TY, Zhu WQ, Xu JJ, 2010. An in vivo confocal microscopy and impression cytology analysis of goblet cells in patients with chemical burns. Investigative ophthalmology & visual science 51, 1397–1400. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Wolosin JM, Chung SH, 2017. Divergent effects of Wnt/beta-catenin signaling modifiers on the preservation of human limbal epithelial progenitors according to culture condition. Sci Rep 7, 15241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RC, Feinbaum RL, Ambros V, 1993. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75, 843–854. [DOI] [PubMed] [Google Scholar]
- Leszczynska A, Kulkarni M, Ljubimov AV, Saghizadeh M, 2018. Exosomes from normal and diabetic human corneolimbal keratocytes differentially regulate migration, proliferation and marker expression of limbal epithelial cells. Sci Rep 8, 15173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levis HJ, Daniels JT, 2016. Recreating the Human Limbal Epithelial Stem Cell Niche with Bioengineered Limbal Crypts. Curr Eye Res 41, 1153–1160. [DOI] [PubMed] [Google Scholar]
- Li DQ, Chen Z, Song XJ, de Paiva CS, Kim HS, Pflugfelder SC, 2005. Partial enrichment of a population of human limbal epithelial cells with putative stem cell properties based on collagen type IV adhesiveness. Exp Eye Res 80, 581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Chen SY, Zhao XY, Zhang MC, Xie HT, 2017. Rat Limbal Niche Cells Prevent Epithelial Stem/Progenitor Cells From Differentiation and Proliferation by Inhibiting Notch Signaling Pathway In Vitro. Investigative ophthalmology & visual science 58, 2968–2976. [DOI] [PubMed] [Google Scholar]
- Li P, An L, Reif R, Shen TT, Johnstone M, Wang RK, 2011. In vivo microstructural and microvascular imaging of the human corneo-scleral limbus using optical coherence tomography. Biomed Opt Express 2, 3109–3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Hayashida Y, He H, Kuo CL, Tseng SC, 2007. The fate of limbal epithelial progenitor cells during explant culture on intact amniotic membrane. Investigative ophthalmology & visual science 48, 605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Inoue T, Takamatsu F, Kobayashi T, Shiraishi A, Maeda N, Ohashi Y, Nishida K, 2014. Differences between niche cells and limbal stromal cells in maintenance of corneal limbal stem cells. Invest Ophthalmol Vis Sci 55, 1453–1462. [DOI] [PubMed] [Google Scholar]
- Liang Q, Le Q, Cordova DW, Tseng CH, Deng SX, 2020. Corneal Epithelial Thickness Measured Using AS-OCT as a Diagnostic Parameter for Limbal Stem Cell Deficiency. Am J Ophthalmol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Nielsen FM, Emmersen J, Bath C, Ostergaard Hjortdal J, Riis S, Fink T, Pennisi CP, Zachar V, 2018. Pigmentation Is Associated with Stemness Hierarchy of Progenitor Cells Within Cultured Limbal Epithelial Cells. Stem cells 36, 1411–1420. [DOI] [PubMed] [Google Scholar]
- Liu Y, Nekulova M, Nenutil R, Horakova I, Appleyard MV, Murray K, Holcakova J, Galoczova M, Quinlan P, Jordan LB, Purdie CA, Vojtesek B, Thompson AM, Coates PJ, 2020. Np63/p40 correlates with the location and phenotype of basal/mesenchymal cancer stem-like cells in human ER(+) and HER2(+) breast cancers. J Pathol Clin Res 6, 83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljubimov AV, Burgeson RE, Butkowski RJ, Michael AF, Sun TT, Kenney MC, 1995. Human corneal basement membrane heterogeneity: topographical differences in the expression of type IV collagen and laminin isoforms. Lab Invest 72, 461–473. [PubMed] [Google Scholar]
- Lu H, Lu Q, Zheng Y, Li Q, 2012. Notch signaling promotes the corneal epithelium wound healing. Mol Vis 18, 403–411. [PMC free article] [PubMed] [Google Scholar]
- Lyngholm M, Vorum H, Nielsen K, Ostergaard M, Honore B, Ehlers N, 2008. Differences in the protein expression in limbal versus central human corneal epithelium--a search for stem cell markers. Exp Eye Res 87, 96–105. [DOI] [PubMed] [Google Scholar]
- Lyu J, Joo CK, 2006. Expression of Wnt and MMP in epithelial cells during corneal wound healing. Cornea 25, S24–28. [DOI] [PubMed] [Google Scholar]
- Ma A, Boulton M, Zhao B, Connon C, Cai J, Albon J, 2007. A role for notch signaling in human corneal epithelial cell differentiation and proliferation. Investigative ophthalmology & visual science 48, 3576–3585. [DOI] [PubMed] [Google Scholar]
- Ma A, Zhao B, Boulton M, Albon J, 2011. A role for Notch signaling in corneal wound healing. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society 19, 98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma DH, Chen HC, Ma KS, Lai JY, Yang U, Yeh LK, Hsueh YJ, Chu WK, Lai CH, Chen JK, 2016. Preservation of human limbal epithelial progenitor cells on carbodiimide cross-linked amniotic membrane via integrin-linked kinase-mediated Wnt activation. Acta Biomater 31, 144–155. [DOI] [PubMed] [Google Scholar]
- Majo F, Rochat A, Nicolas M, Jaoude GA, Barrandon Y, 2008. Oligopotent stem cells are distributed throughout the mammalian ocular surface. Nature 456, 250–254. [DOI] [PubMed] [Google Scholar]
- Massie I, Kureshi AK, Schrader S, Shortt AJ, Daniels JT, 2015. Optimization of optical and mechanical properties of real architecture for 3-dimensional tissue equivalents: Towards treatment of limbal epithelial stem cell deficiency. Acta Biomater 24, 241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu M, Martin-Jaular L, Lavieu G, Thery C, 2019. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol 21, 9–17. [DOI] [PubMed] [Google Scholar]
- McKay BT, Hutcheon EKA, Zieske DJ, Ciolino BJ, 2020. Extracellular Vesicles Secreted by Corneal Epithelial Cells Promote Myofibroblast Differentiation. Cells 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei H, Gonzalez S, Deng SX, 2012. Extracellular Matrix is an Important Component of Limbal Stem Cell Niche. Journal of functional biomaterials 3, 879–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei H, Gonzalez S, Nakatsu MN, Baclagon ER, Chen FV, Deng SX, 2017. Human adipose-derived stem cells support the growth of limbal stem/progenitor cells. PLoS One 12, e0186238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei H, Gonzalez S, Nakatsu MN, Baclagon ER, Lopes VS, Williams DS, Deng SX, 2014a. A three-dimensional culture method to expand limbal stem/progenitor cells. Tissue Eng Part C Methods 20, 393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei H, Nakatsu MN, Baclagon ER, Deng SX, 2014b. Frizzled 7 maintains the undifferentiated state of human limbal stem/progenitor cells. Stem cells 32, 938–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza-Reinoso V, Beverdam A, 2018. Epidermal YAP activity drives canonical WNT16/beta-catenin signaling to promote keratinocyte proliferation in vitro and in the murine skin. Stem Cell Res 29, 15–23. [DOI] [PubMed] [Google Scholar]
- Merjava S, Neuwirth A, Tanzerova M, Jirsova K, 2011. The spectrum of cytokeratins expressed in the adult human cornea, limbus and perilimbal conjunctiva. Histol Histopathol 26, 323–331. [DOI] [PubMed] [Google Scholar]
- Miri A, Alomar T, Nubile M, Al-Aqaba M, Lanzini M, Fares U, Said DG, Lowe J, Dua HS, 2012. In vivo confocal microscopic findings in patients with limbal stem cell deficiency. Br J Ophthalmol 96, 523–529. [DOI] [PubMed] [Google Scholar]
- Moellering RE, Cornejo M, Davis TN, Del Bianco C, Aster JC, Blacklow SC, Kung AL, Gilliland DG, Verdine GL, Bradner JE, 2009. Direct inhibition of the NOTCH transcription factor complex. Nature 462, 182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movahedan A, Majdi M, Afsharkhamseh N, Sagha HM, Saadat NS, Shalileh K, Milani BY, Ying H, Djalilian AR, 2012. Notch inhibition during corneal epithelial wound healing promotes migration. Investigative ophthalmology & visual science 53, 7476–7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay M, Gorivodsky M, Shtrom S, Grinberg A, Niehrs C, Morasso MI, Westphal H, 2006. Dkk2 plays an essential role in the corneal fate of the ocular surface epithelium. Development 133, 2149–2154. [DOI] [PubMed] [Google Scholar]
- Munnamalai V, Fekete DM, 2016. Notch-Wnt-Bmp crosstalk regulates radial patterning in the mouse cochlea in a spatiotemporal manner. Development 143, 4003–4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Ohtsuka T, Sekiyama E, Cooper LJ, Kokubu H, Fullwood NJ, Barrandon Y, Kageyama R, Kinoshita S, 2008. Hes1 regulates corneal development and the function of corneal epithelial stem/progenitor cells. Stem cells 26, 1265–1274. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Tsuchiya K, Watanabe M, 2007. Crosstalk between Wnt and Notch signaling in intestinal epithelial cell fate decision. J Gastroenterol 42, 705–710. [DOI] [PubMed] [Google Scholar]
- Nakatsu MN, Deng SX, 2013. Enrichment of human corneal epithelial stem/progenitor cells by magnetic bead sorting using SSEA4 as a negative marker. Methods Mol Biol 1014, 71–77. [DOI] [PubMed] [Google Scholar]
- Nakatsu MN, Ding Z, Ng MY, Truong TT, Yu F, Deng SX, 2011. Wnt/beta-catenin signaling regulates proliferation of human cornea epithelial stem/progenitor cells. Investigative ophthalmology & visual science 52, 4734–4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsu MN, Gonzalez S, Mei H, Deng SX, 2014. Human limbal mesenchymal cells support the growth of human corneal epithelial stem/progenitor cells. Investigative ophthalmology & visual science 55, 6953–6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsu MN, Vartanyan L, Vu DM, Ng MY, Li X, Deng SX, 2013. Preferential biological processes in the human limbus by differential gene profiling. PLoS One 8, e61833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WJ, Nusse R, 2004. Convergence of Wnt, beta-catenin, and cadherin pathways. Science 303, 1483–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen BC, Lefort K, Mandinova A, Antonini D, Devgan V, Della Gatta G, Koster MI, Zhang Z, Wang J, Tommasi di Vignano A, Kitajewski J, Chiorino G, Roop DR, Missero C, Dotto GP, 2006. Cross-regulation between Notch and p63 in keratinocyte commitment to differentiation. Genes Dev 20, 1028–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KN, Bobba S, Richardson A, Park M, Watson SL, Wakefield D, Di Girolamo N, 2018. Native and synthetic scaffolds for limbal epithelial stem cell transplantation. Acta Biomater 65, 21–35. [DOI] [PubMed] [Google Scholar]
- Ni W, Zeng S, Li W, Chen Y, Zhang S, Tang M, Sun S, Chai R, Li H, 2016. Wnt activation followed by Notch inhibition promotes mitotic hair cell regeneration in the postnatal mouse cochlea. Oncotarget 7, 66754–66768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notara M, Lentzsch A, Coroneo M, Cursiefen C, 2018. The Role of Limbal Epithelial Stem Cells in Regulating Corneal (Lymph)angiogenic Privilege and the Micromilieu of the Limbal Niche following UV Exposure. Stem Cells Int 2018, 8620172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notara M, Shortt AJ, Galatowicz G, Calder V, Daniels JT, 2010. IL6 and the human limbal stem cell niche: a mediator of epithelial-stromal interaction. Stem Cell Res 5, 188–200. [DOI] [PubMed] [Google Scholar]
- Nubile M, Lanzini M, Miri A, Pocobelli A, Calienno R, Curcio C, Mastropasqua R, Dua HS, Mastropasqua L, 2013. In vivo confocal microscopy in diagnosis of limbal stem cell deficiency. Am J Ophthalmol 155, 220–232. [DOI] [PubMed] [Google Scholar]
- Nusse R, 2008. Wnt signaling and stem cell control. Cell Res 18, 523–527. [DOI] [PubMed] [Google Scholar]
- Nusse R, Clevers H, 2017. Wnt/beta-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 169, 985–999. [DOI] [PubMed] [Google Scholar]
- Olsauskas-Kuprys R, Zlobin A, Osipo C, 2013. Gamma secretase inhibitors of Notch signaling. OncoTargets and therapy 6, 943–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer WH, Jia D, Deng WM, 2014. Cis-interactions between Notch and its ligands block ligand-independent Notch activity. Elife 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, Richardson A, Pandzic E, Lobo EP, Whan R, Watson SL, Lyons JG, Wakefield D, Di Girolamo N, 2019. Visualizing the Contribution of Keratin-14(+) Limbal Epithelial Precursors in Corneal Wound Healing. Stem Cell Reports 12, 14–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearton DJ, Yang Y, Dhouailly D, 2005. Transdifferentiation of corneal epithelium into epidermis occurs by means of a multistep process triggered by dermal developmental signals. Proc Natl Acad Sci U S A 102, 3714–3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini G, Dellambra E, Golisano O, Martinelli E, Fantozzi I, Bondanza S, Ponzin D, McKeon F, De Luca M, 2001. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci U S A 98, 3156–3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini G, Golisano O, Paterna P, Lambiase A, Bonini S, Rama P, De Luca M, 1999. Location and clonal analysis of stem cells and their differentiated progeny in the human ocular surface. J Cell Biol 145, 769–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini G, Lambiase A, Macaluso C, Pocobelli A, Deng S, Cavallini GM, Esteki R, Rama P, 2016. From discovery to approval of an advanced therapy medicinal product-containing stem cells, in the EU. Regen Med 11, 407–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini G, Traverso CE, Franzi AT, Zingirian M, Cancedda R, De Luca M, 1997. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet 349, 990–993. [DOI] [PubMed] [Google Scholar]
- Peng H, Hamanaka RB, Katsnelson J, Hao LL, Yang W, Chandel NS, Lavker RM, 2012a. MicroRNA-31 targets FIH-1 to positively regulate corneal epithelial glycogen metabolism. FASEB J 26, 3140–3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H, Kaplan N, Hamanaka RB, Katsnelson J, Blatt H, Yang W, Hao L, Bryar PJ, Johnson RS, Getsios S, Chandel NS, Lavker RM, 2012b. microRNA-31/factor-inhibiting hypoxia-inducible factor 1 nexus regulates keratinocyte differentiation. Proc Natl Acad Sci U S A 109, 14030–14034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H, Park JK, Katsnelson J, Kaplan N, Yang W, Getsios S, Lavker RM, 2015. microRNA-103/107 Family Regulates Multiple Epithelial Stem Cell Characteristics. Stem cells 33, 1642–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polisetti N, Giessl A, Li S, Sorokin L, Kruse FE, Schlotzer-Schrehardt U, 2020. Laminin-511-E8 promotes efficient in vitro expansion of human limbal melanocytes. Sci Rep 10, 11074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polisetti N, Sorokin L, Okumura N, Koizumi N, Kinoshita S, Kruse FE, Schlotzer-Schrehardt U, 2017. Laminin-511 and −521-based matrices for efficient ex vivo-expansion of human limbal epithelial progenitor cells. Sci Rep 7, 5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polisetti N, Zenkel M, Menzel-Severing J, Kruse FE, Schlotzer-Schrehardt U, 2016. Cell Adhesion Molecules and Stem Cell-Niche-Interactions in the Limbal Stem Cell Niche. Stem cells 34, 203–219. [DOI] [PubMed] [Google Scholar]
- Qiu G, Zheng G, Ge M, Wang J, Huang R, Shu Q, Xu J, 2018. Mesenchymal stem cell-derived extracellular vesicles affect disease outcomes via transfer of microRNAs. Stem Cell Res Ther 9, 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rama P, Bonini S, Lambiase A, Golisano O, Paterna P, De Luca M, Pellegrini G, 2001. Autologous fibrin-cultured limbal stem cells permanently restore the corneal surface of patients with total limbal stem cell deficiency. Transplantation 72, 1478–1485. [DOI] [PubMed] [Google Scholar]
- Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, Pellegrini G, 2010. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med 363, 147–155. [DOI] [PubMed] [Google Scholar]
- Ramirez-Miranda A, Nakatsu MN, Zarei-Ghanavati S, Nguyen CV, Deng SX, 2011. Keratin 13 is a more specific marker of conjunctival epithelium than keratin 19. Mol Vis 17, 1652–1661. [PMC free article] [PubMed] [Google Scholar]
- Richardson A, Lobo EP, Delic NC, Myerscough MR, Lyons JG, Wakefield D, Di Girolamo N, 2017. Keratin-14-Positive Precursor Cells Spawn a Population of Migratory Corneal Epithelia that Maintain Tissue Mass throughout Life. Stem Cell Reports 9, 1081–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riviere I, Roy K, 2017. Perspectives on Manufacturing of High-Quality Cell Therapies. Mol Ther 25, 1067–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano AC, Espana EM, Yoo SH, Budak MT, Wolosin JM, Tseng SC, 2003. Different cell sizes in human limbal and central corneal basal epithelia measured by confocal microscopy and flow cytometry. Investigative ophthalmology & visual science 44, 5125–5129. [DOI] [PubMed] [Google Scholar]
- Sangwan VS, Basu S, Vemuganti GK, Sejpal K, Subramaniam SV, Bandyopadhyay S, Krishnaiah S, Gaddipati S, Tiwari S, Balasubramanian D, 2011. Clinical outcomes of xeno-free autologous cultivated limbal epithelial transplantation: a 10-year study. Br J Ophthalmol 95, 1525–1529. [DOI] [PubMed] [Google Scholar]
- Sartaj R, Zhang C, Wan P, Pasha Z, Guaiquil V, Liu A, Liu J, Luo Y, Fuchs E, Rosenblatt MI, 2017. Characterization of slow cycling corneal limbal epithelial cells identifies putative stem cell markers. Sci Rep 7, 3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlotzer-Schrehardt U, Dietrich T, Saito K, Sorokin L, Sasaki T, Paulsson M, Kruse FE, 2007. Characterization of extracellular matrix components in the limbal epithelial stem cell compartment. Experimental eye research 85, 845–860. [DOI] [PubMed] [Google Scholar]
- Schlotzer-Schrehardt U, Kruse FE, 2005. Identification and characterization of limbal stem cells. Experimental eye research 81, 247–264. [DOI] [PubMed] [Google Scholar]
- Schmeckpeper J, Verma A, Yin L, Beigi F, Zhang L, Payne A, Zhang Z, Pratt RE, Dzau VJ, Mirotsou M, 2015. Inhibition of Wnt6 by Sfrp2 regulates adult cardiac progenitor cell differentiation by differential modulation of Wnt pathways. J Mol Cell Cardiol 85, 215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sejpal K, Ali MH, Maddileti S, Basu S, Ramappa M, Kekunnaya R, Vemuganti GK, Sangwan VS, 2013a. Cultivated limbal epithelial transplantation in children with ocular surface burns. JAMA Ophthalmol 131, 731–736. [DOI] [PubMed] [Google Scholar]
- Sejpal K, Bakhtiari P, Deng SX, 2013b. Presentation, diagnosis and management of limbal stem cell deficiency. Middle East Afr J Ophthalmol 20, 5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki J, Kaido M, Shinozaki N, Shimmura S, Munkhbat B, Hagihara M, Tsuji K, Tsubota K, 1999. Evidence of long-term survival of donor-derived cells after limbal allograft transplantation. Investigative ophthalmology & visual science 40, 1664–1668. [PubMed] [Google Scholar]
- Shojaati G, Khandaker I, Funderburgh ML, Mann MM, Basu R, Stolz DB, Geary ML, Dos Santos A, Deng SX, Funderburgh JL, 2019. Mesenchymal Stem Cells Reduce Corneal Fibrosis and Inflammation via Extracellular Vesicle-Mediated Delivery of miRNA. STEM CELLS Translational Medicine 8, 1192–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortt AJ, Bunce C, Levis HJ, Blows P, Dore CJ, Vernon A, Secker GA, Tuft SJ, Daniels JT, 2014. Three-year outcomes of cultured limbal epithelial allografts in aniridia and Stevens-Johnson syndrome evaluated using the Clinical Outcome Assessment in Surgical Trials assessment tool. Stem Cells Transl Med 3, 265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Joseph A, Umapathy T, Tint NL, Dua HS, 2005. Impression cytology of the ocular surface. Br J Ophthalmol 89, 1655–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small D, Kovalenko D, Kacer D, Liaw L, Landriscina M, Di Serio C, Prudovsky I, Maciag T, 2001. Soluble Jagged 1 represses the function of its transmembrane form to induce the formation of the Src-dependent chord-like phenotype. J Biol Chem 276, 32022–32030. [DOI] [PubMed] [Google Scholar]
- Sprinzak D, Lakhanpal A, LeBon L, Santat LA, Fontes ME, Anderson GA, Garcia-Ojalvo J, Elowitz MB, 2010. Cis-interactions between Notch and Delta generate mutually exclusive signalling states. Nature 465, 86–U95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takacs L, Toth E, Losonczy G, Szanto A, Bahr-Ivacevic T, Benes V, Berta A, Vereb G, 2011. Differentially expressed genes associated with human limbal epithelial phenotypes: new molecules that potentially facilitate selection of stem cell-enriched populations. Investigative ophthalmology & visual science 52, 1252–1260. [DOI] [PubMed] [Google Scholar]
- Tavakoli M, Hossain P, Malik RA, 2008. Clinical applications of corneal confocal microscopy, Clin Ophthalmol, pp. 435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teh MT, Blaydon D, Ghali LR, Briggs V, Edmunds S, Pantazi E, Barnes MR, Leigh IM, Kelsell DP, Philpott MP, 2007. Role for WNT16B in human epidermal keratinocyte proliferation and differentiation. J Cell Sci 120, 330–339. [DOI] [PubMed] [Google Scholar]
- Teiken K, Kuehnel M, Rehkaemper J, Kreipe H, Laenger F, Hussein K, Jonigk D, 2018. Non-canonical WNT6/WNT10A signal factor expression in EBV+ post-transplant smooth muscle tumors. Clin Sarcoma Res 8, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temoche-Diaz MM, Shurtleff MJ, Nottingham RM, Yao J, Fadadu RP, Lambowitz AM, Schekman R, 2019. Distinct mechanisms of microRNA sorting into cancer cell-derived extracellular vesicle subtypes. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoft RA, Friend J, 1983. The X, Y, Z hypothesis of corneal epithelial maintenance. Investigative ophthalmology & visual science 24, 1442–1443. [PubMed] [Google Scholar]
- Thomas PB, Liu YH, Zhuang FF, Selvam S, Song SW, Smith RE, Trousdale MD, Yiu SC, 2007. Identification of Notch-1 expression in the limbal basal epithelium. Mol Vis 13, 337–344. [PMC free article] [PubMed] [Google Scholar]
- Thrasivoulou C, Millar M, Ahmed A, 2013. Activation of intracellular calcium by multiple Wnt ligands and translocation of beta-catenin into the nucleus: a convergent model of Wnt/Ca2+ and Wnt/beta-catenin pathways. J Biol Chem 288, 35651–35659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend WM, 1991. The limbal palisades of Vogt. Trans Am Ophthalmol Soc 89, 721–756. [PMC free article] [PubMed] [Google Scholar]
- Tran FH, Zheng JJ, 2017. Modulating the wnt signaling pathway with small molecules. Protein Sci 26, 650–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong AB, Kretz M, Ridky TW, Kimmel R, Khavari PA, 2006. p63 regulates proliferation and differentiation of developmentally mature keratinocytes. Genes Dev 20, 3185–3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong TT, Huynh K, Nakatsu MN, Deng SX, 2011. SSEA4 is a potential negative marker for the enrichment of human corneal epithelial stem/progenitor cells. Investigative ophthalmology & visual science 52, 6315–6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng SC, 1989. Concept and application of limbal stem cells. Eye (Lond) 3 ( Pt 2), 141–157. [DOI] [PubMed] [Google Scholar]
- Tseng SC, Espana EM, Kawakita T, Di Pascuale MA, Li W, He H, Liu TS, Cho TH, Gao YY, Yeh LK, Liu CY, 2004. How does amniotic membrane work? Ocul Surf 2, 177–187. [DOI] [PubMed] [Google Scholar]
- Tuori A, Uusitalo H, Burgeson RE, Terttunen J, Virtanen I, 1996. The immunohistochemical composition of the human corneal basement membrane. Cornea 15, 286–294. [DOI] [PubMed] [Google Scholar]
- Turchinovich A, Weiz L, Langheinz A, Burwinkel B, 2011. Characterization of extracellular circulating microRNA. Nucleic Acids Res 39, 7223–7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemoto T, Yamato M, Nishida K, Yang J, Tano Y, Okano T, 2006. Limbal epithelial side-population cells have stem cell-like properties, including quiescent state. Stem cells 24, 86–94. [DOI] [PubMed] [Google Scholar]
- van Noort M, Meeldijk J, van der Zee R, Destree O, Clevers H, 2002. Wnt signaling controls the phosphorylation status of beta-catenin. J Biol Chem 277, 17901–17905. [DOI] [PubMed] [Google Scholar]
- Vantrappen L, Geboes K, Missotten L, Maudgal PC, Desmet V, 1985. Lymphocytes and Langerhans cells in the normal human cornea. Invest Ophthalmol Vis Sci 26, 220–225. [PubMed] [Google Scholar]
- Varnum-Finney B, Wu L, Yu M, Brashem-Stein C, Staats S, Flowers D, Griffin JD, Bernstein ID, 2000. Immobilization of Notch ligand, Delta-1, is required for induction of notch signaling. J Cell Sci 113 Pt 23, 4313–4318. [DOI] [PubMed] [Google Scholar]
- Vauclair S, Majo F, Durham AD, Ghyselinck NB, Barrandon Y, Radtke F, 2007. Corneal epithelial cell fate is maintained during repair by Notch1 signaling via the regulation of vitamin A metabolism. Dev Cell 13, 242–253. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Nishida K, Yamato M, Umemoto T, Sumide T, Yamamoto K, Maeda N, Watanabe H, Okano T, Tano Y, 2004. Human limbal epithelium contains side population cells expressing the ATP-binding cassette transporter ABCG2. FEBS Lett 565, 6–10. [DOI] [PubMed] [Google Scholar]
- Weinmaster G, 1997. The ins and outs of notch signaling. Molecular and cellular neurosciences 9, 91–102. [DOI] [PubMed] [Google Scholar]
- Williams KA, Brereton HM, Aggarwal R, Sykes PJ, Turner DR, Russ GR, Coster DJ, 1995. Use of DNA polymorphisms and the polymerase chain reaction to examine the survival of a human limbal stem cell allograft. Am J Ophthalmol 120, 342–350. [DOI] [PubMed] [Google Scholar]
- Xie HT, Chen SY, Li GG, Tseng SC, 2011. Limbal epithelial stem/progenitor cells attract stromal niche cells by SDF-1/CXCR4 signaling to prevent differentiation. Stem cells 29, 1874–1885. [DOI] [PubMed] [Google Scholar]
- Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dotsch V, Andrews NC, Caput D, McKeon F, 1998. p63, a p53 homolog at 3q27–29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell 2, 305–316. [DOI] [PubMed] [Google Scholar]
- Yang K, Jiang Z, Wang D, Lian X, Yang T, 2009. Corneal epithelial-like transdifferentiation of hair follicle stem cells is mediated by pax6 and beta-catenin/Lef-1. Cell Biol Int 33, 861–866. [DOI] [PubMed] [Google Scholar]
- Yang Y, Mlodzik M, 2015. Wnt-Frizzled/planar cell polarity signaling: cellular orientation by facing the wind (Wnt). Annu Rev Cell Dev Biol 31, 623–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Shimmura S, Kawakita T, Miyashita H, Den S, Shimazaki J, Tsubota K, 2006. Cytokeratin 15 can be used to identify the limbal phenotype in normal and diseased ocular surfaces. Investigative ophthalmology & visual science 47, 4780–4786. [DOI] [PubMed] [Google Scholar]
- Yu J, Ryan DG, Getsios S, Oliveira-Fernandes M, Fatima A, Lavker RM, 2008. MicroRNA-184 antagonizes microRNA-205 to maintain SHIP2 levels in epithelia. Proc Natl Acad Sci U S A 105, 19300–19305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakaria N, Possemiers T, Dhubhghaill SN, Leysen I, Rozema J, Koppen C, Timmermans JP, Berneman Z, Tassignon MJ, 2014. Results of a phase I/II clinical trial: standardized, non-xenogenic, cultivated limbal stem cell transplantation. J Transl Med 12, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarei-Ghanavati S, Ramirez-Miranda A, Deng SX, 2011. Limbal lacuna: a novel limbal structure detected by in vivo laser scanning confocal microscopy. Ophthalmic Surg Lasers Imaging 42 Online, e129–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Mei H, Robertson SYT, Lee HJ, Deng SX, Zheng JJ, 2020. A Small-Molecule Wnt Mimic Improves Human Limbal Stem Cell Ex Vivo Expansion. iScience 23, 101075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Allinson SL, Ma A, Bentley AJ, Martin FL, Fullwood NJ, 2008. Targeted cornea limbal stem/progenitor cell transfection in an organ culture model. Invest Ophthalmol Vis Sci 49, 3395–3401. [DOI] [PubMed] [Google Scholar]


