Abstract
Background:
Only a few controversial studies have assessed the repair bond strength of a fresh composite to aged composite. Moreover, no studies exist on repair bond strength of fresh composites to bleached composites. Therefore, this preliminary study was conducted to assess repair shear bond strength (SBS) of three composites bonded to nonbleached and at-home and in-office bleached composites.
Materials and Methods:
In this experimental in vitro study, 108 disks (36 specimens per composite) of hybrid, microhybrid, and nanofilled composites were divided into three subgroups of three bleaching treatments: no bleaching (control), at-home bleaching, and in-office bleaching. Composite disks were incubated for 4 weeks in artificial saliva (also dipped in tea and coffee for 3 h a day). They were then thermocycled (5000 cycles). Afterward, the control group remained unbleached, while the other groups were bleached according to office and home bleaching methods. They were repaired with the same composite type. Their repair SBS and mode of failure were measured and analyzed using two-way ANOVA, Tukey, one-sample t-test, and Chi-square tests (α = 0.05, β = 0.2).
Results:
The mean (standard deviation) SBS values of hybrid, microhybrid, and nanofilled composites were 20.71 ± 5.99, 21.06 ± 6.68, and 9.46 ± 4.32 MPa, respectively. The mean SBS values of the bleaching techniques “home bleaching, office bleaching, and no bleaching (control)” were, respectively, 16.35 ± 7.13, 16.39 ± 8.07, and 18.49 ± 8.35 MPa. There was a significant difference among composites (two-way ANOVA P = 0.000) but not among nonbleaching/bleaching methods (P = 0.176). Their interaction was significant (P = 0.017). The difference between hybrid and microhybrid was not significant. Nevertheless, nanofilled had significantly poorer results compared to both hybrid and microhybrid composites (Tukey P = 0.000). Both hybrid and microhybrid were capable of producing satisfactory clinical repair bond strengths (above 20 MPa) regardless of bleaching or lack of it. Nanofilled composite failed to provide proper repair SBS values, even in the control (no-bleaching) group. By moving from Z100 or from Z250 to Z350, modes of failure shifted from mostly cohesive to mostly adhesive (P < 0.05).
Conclusion:
Bleaching of an aged composite might not affect the repair bond strength. Hybrid and microhybrid composites can provide clinically acceptable repair bond strengths, regardless of bleaching. Nonetheless, nanofilled composite is inferior to them and cannot provide appropriate repair bond strengths (regardless of bleaching).
Key Words: Dental materials, composite resins, light-curing of dental adhesives, tooth bleaching
INTRODUCTION
The discoloration of teeth can disrupt facial beauty; hence, numerous methods are used to treat it. Compared to various restorative techniques such as crowns or veneers, dental bleaching is a good way to improve the esthetics of discolored teeth.[1] Bleaching is the least invasive method with a long and successful history. This technique is used to whiten and brighten teeth because of its benefits such as material availability, low cost, and high application safety.[2] Dental bleaching can be done under the supervision of a dentist or in the dental office using gel or paste containing high concentrations of hydrogen peroxide (30%–35%) or carbamide peroxide 35% for a faster effect or can be done at home by the patient and using a specialized tray and gel usually containing up to 10% hydrogen peroxide or up to 16% carbamide peroxide or higher concentrations.[3,4,5,6] Bleaching agents whiten dental structures by breaking peroxides into free radicals and oxidizing large pigment molecules.[7,8] Of course, light and heat can be used in the office to break hydrogen peroxide into active oxygen faster and more effectively.[9]
Various factors including staining or fractures can disrupt the esthetic and/or function of composite resin restorations.[10,11,12,13] Esthetic restorations change in appearance over time due to factors including light, moisture, oral habits such as tobacco use, specific dietary habits such as daily consumption of caffeine, alcohol, or tea on the external color change of restorative materials.[14,15] The color change of the restoration can also be due to internal factors when the composite is aged in the oral environment and under specific physical and thermal conditions such as high temperature changes and high humidity, hydrolyzed polymer matrix becomes hydrolyzed, and fillers with defective silane can be separated from the composite.[16]
Such compromised esthetic restorations can be either fully replaced or repaired.[12,13] Albeit the most frequent practice is currently complete substitution, it is overtreatment while restorations can be fixed usually with partial replacements; and it can remove intact dental structures and etched enamel, endanger tooth or pulpal tissues, and enlarge the cavities.[13,17,18] Thus, it seems that repair can be a better option in many conditions.[12,13,17,18,19,20] Nowadays, the use of esthetic restorative materials, especially composite resins, for the purpose of repairing and restoring, old composites are an important part of modern dentistry.[21]
Nevertheless, repair has its own drawback: it can deteriorate the bonding potential of the fresh composite to the older one.[12,13,21,22,23,24] A nonpolymerized oxygen-inhibited layer of resin is needed for the proper adhesion between fresh and old composite surfaces.[12,13,19,25,26] This nonpolymerized film can be compromised by water sorption and aging, which can also diminish the amount of unsaturated double carbon = carbon bonds, can cause water infiltration into the junction of fillers and matrix, deplete monomers, and wear of the surface.[12,13,25,27,28,29]
A way of extra aging and chemical manipulation of composite structures can be bleaching: organic matrix of composite resins is more susceptible to chemical modifications as a result of bleaching and may affect the hardness of the restorative materials and the clinical durability of esthetic restorations.[30] In many patients who require tooth whitening, there may be previous composite restorations; and bleaching treatment may influence these restorations and restoration-tooth junctions. An example of such adverse effect is the microleakage of bleached old composite restorations that cause the recurrence of caries, pulpitis, discoloration, tooth sensitivity, and reduction of shear bond strength (SBS) to new restorative materials.[31,32,33]
Research on repair bond strength of composites to aged composites is a few and controversial and limited to few types and brands of composites.[13] Moreover, there is no research on repair bond strength of new composites bonded to bleached older composites. Since this matter is of clinical significance and yet not studied, this preliminary study was conducted.
MATERIALS AND METHODS
In this in vitro experimental study, 108 composite samples were made and tested [Table 1]. All of these composites are manufactured by 3M Company (3M ESPE, USA) and include the Filtek Z250 (micro hybrid), Filtek Z350 (nanofield), and Filtek Z100 (hybrid).
Table 1.
Materials used in this study
| Brand | Ingredients | Type |
|---|---|---|
| Z100 | Matrix: Bis-GMA and TEGDMA; Filler: single filler 100% zirconia/silica (0.01-3.5 lm); Filler volume: 66% | Hybrid |
| Z250 | Matrix: Bis-GMA, UDMA, and Bis-EMA; Filler: zirconia/silica (0.01-3.5 lm); Filler by volume: 60% | Microhybrid |
| Z350 | Matrix: Bis-GMA, UDMA, TEGDMA, and Bis-EMA; Filler: combination of aggregated zirconia/silica cluster filler (0.6-1.4 μm) and nonaggregated 20-nm silica filler; Filler volume: 59.5% | Nanofilled |
Each composite specimen consisted of 36 disk-like specimens with a diameter of 4 mm and a thickness of 3 mm. All A3 shades were selected for uniform shading. The disks were mounted on a glass plate of Mylar tape, and a 3 mm × 8 mm plastic mold was placed on it. The composite was placed inside the mold and covered with a Mylar strip (Sina/Iran).[34] Prior to curing, a glass slab was placed on the surface of the composite to remove its excesses and reduce porosity. Slabs were removed, and samples were cured for 40 s with a light cure LED (Demi/Kerr/USA) and 800 mw/cm2 power. All samples were finished using silicon carbide polishing disks (SofLex/3M/USA) in the order of medium, fine, and superfine disks, respectively. All samples were washed with water for 2 min to remove surface debris and then kept in distilled water at 25°C for 24 h to complete the polymerization process.[34]
Aging
To simulate aged composite specimens in the mouth, the specimens were kept in artificial saliva (Hypozalix/France) for 4 weeks.[35] Samples were also incubated at room temperature for 3 h in tea (Ahmad/England) and coffee (Nestle/Brazil) staining solution, and this solution was replaced daily.[16] After this period, the samples were placed in a thermocycling device for 5000 cycles at temperatures of 5°C–55°C to simulate the thermal aging process.[36]
Bleaching
Then, the samples of each composite group were randomly divided into three subgroups of n = 12 each:[34] Group A (control group): the specimens in this group were kept in artificial saliva for 14 days and were not bleached. Group B (in-office bleaching): samples were bleached for three periods of 30 min with 35% hydrogen peroxide gel (FGM/Brazil). The interval between each two treatment periods was 1 week. Group C (at-home bleaching): samples were bleached for 14 days and daily for 4 h with 16% carbamide peroxide gel (FGM/Brazil). During testing, the samples were kept at room temperature, and after each treatment phase, the samples were washed with water syringe to remove bleaching materials from the surface. Samples were kept in artificial saliva between treatment periods.[8]
Shear bond strength
To evaluate the SBS, a cylinder 2 mm high and 4 mm in diameter from the same-name composite was bonded to each composite disk. For bonding the new composite, the surface of the composite disk was first roughened with diamond bur (Tizkavan-Iran) and surface etching with 35% phosphoric acid (Voco, Cuxhaven, Germany) for 20 s and washing for 15 s and then drying for 10 s. Then, silane was applied on the surface of aged composite (Monobond-S, Ivoclar Vivadent) and we waited for 4 min for the solvent to evaporate. Then, using a microbrush, two layers of fifth-generation dentin bonding agent (3M ESPE, Adper Single Bond 2, USA) was applied on the surface and dried for 5 s by air spray and light cured for 20 s at a distance of 1 mm. Finally, one layer of new, same-name composite 2 mm thick was placed on the surface and cured for 40 s.
SBS testing was performed using a universal testing machine, set at 500 kg of force and a crosshead speed of 1 mm/min. The breakage force (Newton) was divided by the surface area (12.566 mm2) to calculate SBS in mega Pascal (MPa).
Detached specimens were examined under stereomicroscopy at ×20 and divided into three types of adhesive, cohesive, or hybrid fractures.
Statistical analysis
To obtain a power of 80% at a 0.05 level of significance and according to a previous study on microhardness by bleaching (since there was no study on our own subject)[34] with the standard deviation (SD) of 0.75 and to reach a significant difference of at least 1.2 units of difference in the groups, 12 samples per group were required. This meant nine groups of 12 specimens each (3 composite types ×3 bleaching methods). Descriptive statistics were calculated for the groups. Kolmogorov–Smirnov test confirmed the normality of SBS sample. Two-way analysis of variance was used to assess the bleaching and composite types. Tukey post hoc test was performed for pairwise comparisons. All SBS values were compared with the constant value 20 MPa as a clinically acceptable repair bond strength.[12,37,38,39] Modes of failure were assessed using a Chi-square test. The software in use was SPSS 25 (IBM, Armonk, NY, USA). The level of significance was predetermined as 0.05.
RESULTS
Descriptive statistics and 95% confidence interval for SBS values of the subgroups are presented in Table 2 and Figure 1. Overall, the mean (SD) SBS values of Z100, Z250, and Z350 were 20.71 ± 5.99, 21.06 ± 6.68, and 9.46 ± 4.32 MPa, respectively (n = 36 per composite). The mean SBS values of the bleaching techniques “home bleaching, office bleaching, and no bleaching (control)” were, respectively, 16.35 ± 7.13, 16.39 ± 8.07, and 18.49 ± 8.35 MPa (n = 36 per bleaching method). The two-way ANOVA showed that there was a significant difference among three composite types (P = 0.000). However, bleaching (or lack of it) did not have a significant effect on SBS values (P = 0.176). The Tukey test showed that there was not a significant difference between Z100 and Z250 (P = 0.961). However, Z100 was significantly superior to Z350 (P = 0.000). Similarly, Z250 was as well significantly better than Z350 (P = 0.000). The interaction of composite and bleaching methods was significant (P = 0.017), meaning that different composites had different patterns of SBS trends under various bleaching conditions [Figure 1]: Z100 showed no considerable difference in three bleaching methods; Z250 was most vulnerable to home bleaching technique, while Z350 showed the least SBS values in the case of office bleaching.
Table 2.
Descriptive statistics and 95% confidence interval for repair shear bond strength values (MPa, number of each row=12)
| Bleaching | Composite | Mean±SD | Minimum | Maximum | Percentiles | 95% CI | P | ||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| 25th | Med | 75th | |||||||
| None (control) | Z100 | 20.84±7.04 | 14.72 | 40.75 | 15.84 | 19.07 | 23.04 | 16.85-24.82 | 0.688 |
| Z250 | 24.52±6.01 | 16.76 | 38.56 | 19.73 | 23.64 | 27.53 | 21.12-27.92 | 0.025 | |
| Z350 | 10.10±3.72 | 3.77 | 16.75 | 8.19 | 9.57 | 12.69 | 8.00-12.21 | 0.000 | |
| Home bleaching | Z100 | 21.51±6.10 | 12.55 | 34.00 | 17.45 | 20.75 | 22.83 | 18.05-24.96 | 0.411 |
| Z250 | 16.65±6.76 | 5.31 | 26.70 | 10.59 | 17.68 | 22.48 | 12.83-20.48 | 0.114 | |
| Z350 | 10.90±4.14 | 4.08 | 18.74 | 7.74 | 11.85 | 13.58 | 8.56-13.25 | 0.000 | |
| Office bleaching | Z100 | 19.80±5.07 | 11.16 | 27.69 | 15.92 | 20.00 | 24.32 | 16.93-22.67 | 0.893 |
| Z250 | 22.01±4.97 | 13.54 | 29.90 | 17.43 | 23.47 | 25.86 | 19.20-24.82 | 0.189 | |
| Z350 | 7.37±4.56 | 0.26 | 14.54 | 2.61 | 8.16 | 11.02 | 4.79-9.95 | 0.000 | |
The P value is calculated using the one-sample t-test comparing mean shear bond strength values with the value 20 MPa. SD: Standard deviation; CI: Confidence interval
Figure 1.
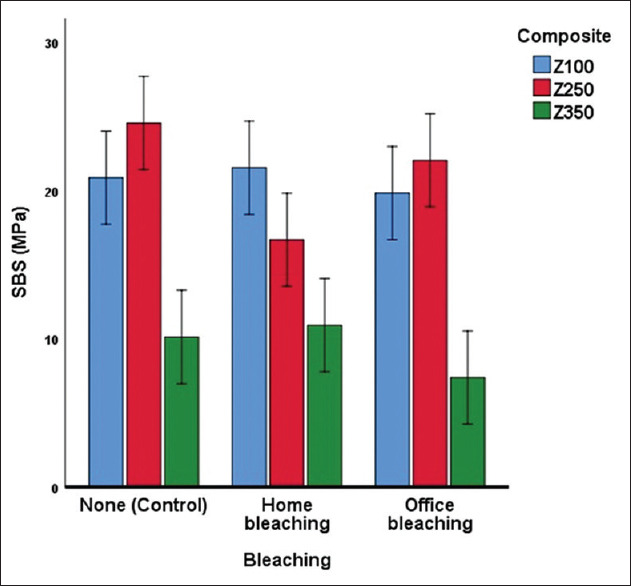
Estimated marginal means and 95% confidence interval for repair shear bond strength (MPa) of different subgroups.
The one-sample t-test showed that, compared to the value 20 MPa, only nanofilled composite had significantly lower repair SBS values [Table 2]. The hybrid and microhybrid composites had SBS values either significantly >20 MPa value, or not significantly smaller than it.
The mode of failure showed that, by moving from Z100 or from Z250 to Z350, modes of breakage shift from mostly cohesive to mostly adhesive. The Chi-square test detected a significant trend in this regard [P < 0.05, Table 3]. The mode of failure also changed across bleaching methods, shifting from mostly cohesive detachments in the control group (no bleaching) to a considerable number of adhesive and mixed failures [P = 0.031, Table 3].
Table 3.
Net (%) distributions of specimens with cohesive, mixed, and adhesive modes of failure in different subgroups and groups, and results of the Chi-square test
| Bleaching | Composites | Cohesive | Mixed | Adhesive | P |
|---|---|---|---|---|---|
| None (control) | Z100 | 12 (33.3) | 0 | 0 | 0.020 |
| Z250 | 11 (30.6) | 0 | 1 (2.8) | ||
| Z350 | 6 (16.7) | 3 (8.3) | 3 (8.3) | ||
| Home bleaching | Z100 | 11 (30.6) | 1 (2.8) | 0 | 0.000 |
| Z250 | 4 (11.1) | 4 (11.1) | 4 (11.1) | ||
| Z350 | 1 (2.8) | 2 (5.6) | 9 (25) | ||
| Office bleaching | Z100 | 9 (25) | 2 (5.6) | 1 (2.8) | 0.000 |
| Z250 | 12 (33.3) | 0 | 0 | ||
| Z350 | 0 | 5 (13.9) | 7 (19.4) | ||
| All bleaching methods | Z100 | 32 (29.6) | 3 (2.8) | 1 (0.9) | 0.000 |
| Z250 | 27 (25) | 4 (3.7) | 5 (4.6) | ||
| Z350 | 7 (6.5) | 10 (9.3) | 19 (17.6) | ||
| None (control) | All composites | 29 (26.9) | 3 (2.8) | 4 (3.7) | 0.031 |
| Home bleaching | 16 (14.8) | 7 (6.5) | 13 (12) | ||
| Office bleaching | 21 (19.4) | 7 (6.5) | 8 (7.4) |
DISCUSSION
Although an acceptable repair bond strength is not estimated in clinical studies, it is suggested that SBS values about at least 15–30 MPa can properly attach composites to enamel.[12,13,38] Similar SBS values can be considered for repair bond strengths of fresh composites to old composites.[12,13,40] Some investigators have suggested that a clinically satisfactory repair bond strength needs to be above 18 MPa or at least between 20 and 25 MPa.[12,13,37,38,39] We compared our results with the value 20 MPa and noted that only the nanofilled composite failed to reach clinically sufficient bond strengths (even when bleaching had not been done). The other two groups succeeded to produce high-enough repair SBSs (either significantly above 20 MPa or not significantly different from it) either in the absence of bleaching or in its presence. This was similar to another study, in which the nanofilled composite had provided lower repair bond strengths.[13] However, our finding was in contrast to another one that showed nanofilled composite to have better repair bond strengths.[41] The reasons for controversy can be various methodological differences (such as methods of aging, brands of composite in use, and different types of composite tested).[12,26,42]
Bleaching materials in contact with composite restorations can affect the organic and inorganic structure of the composite and cause chemical, superficial, and physical alterations that may affect the clinical durability of the restoration.[43] Therefore, in such cases, the effect of bleaching on the properties of existing composite restorations with different adhesive materials is of clinical importance.[44] The bleaching changes surface properties of the composite such as its microhardness.[43] If the composite contains a dense polymeric network with heavy molecules, the bleaching agent needs more time to penetrate it.[45] Until now, some studies have been performed on the effect of bleaching agents on the microhardness of various composite types, with controversial results: some studies have reported a decrease, some an increase, and others reported no significant change in the hardness of the composite.[35,46,47] Ferrari et al.[1] assessed the effect of internal bleaching on SBS of composite-to-composite bond. They reported that some bleaching regiments (those involve the use of sodium perborate mixed with water or 3% hydrogen peroxide) increased the bond strength. Their results might be attributed to the use of specific bleaching agents as well as the method of surface treatment.
The repair bond of the new composite to the old composite can be assessed in vitro by the essential restoration tests being repair bond strength and mode of failure.[12,13,48] Failure modes can be interpreted in a way that materials with high SBSs will demonstrate cohesive failure through the composite. Whereas materials that have low bond strengths can show more adhesive failures rather than cohesive failures. Therefore, in a study on modes of failure, failures through the composite resin (cohesive failure) can be more desirable for tolerating occlusal loads.[12,48,49] In the current study, the nanofilled composite showed mostly adhesive and mixed failures, indicating less proper bearing of occlusal loads in the oral environment. However, the hybrid and microhybrid composites showed mostly cohesive failures which are favorable.
The need for bonding new composites to repair old composites has always been felt in the clinic, and several factors, including surface preparation, affect the strength of the intermediate bond in repaired composite restorations. Mechanical stress and surface roughness are important factors in establishing a proper bond between the previous restoration and the new restorative material.[30] Accordingly, one of the major problems in repairing old composite restorations is to create a strong bond between the new and old composites. Since removing and completely replacing the old restoration weakens the tooth structure and removes the healthy tooth tissue, repair of the old restoration seems more reasonable than its total replacement, as the risk of damage to the pulp is also reduced.[48,50] Rueggeberg and Margeson[51] as well as Dishman et al.[52] believe that the lack of SBS of resin composites to the bleached old composite after bleaching can be due to the production of free radicals and oxygen residues derived from hydrogen peroxide, which could inhibit polymerization and break down the polymer chain and chemical double bonds.[53] Surface properties of composites have an influence on the bond between new composite and the old composite. A considerable part of repair bond is chemical and caused by monomers of the fresh composite and monomers in the oxygen-inhibited layer of the old composite.[13,25,26,29] Many factors determine how the repair will succeed such as surface characteristics of the old composite like its wettability and smoothness besides the surface treatments applied.[13,17,48,54,55,56,57] Such treatments include washing, etching, applying silane or bonding agent, abrading using disks or burs, sandblasting, or laser irradiation.[12,13,26,48,57,58,59] These treatments are highly controversial[27] reporting success for acid etching using hydrofluoric and phosphoric acids and surface treatment with burs or sandblasting[13,23,24,56] versus lack of such successes.[10,60] Not only surface roughening is necessary, it is one of the most important procedures to improve repair bond strength, and this is because of broadening the surface and creating more microinterlocking and macrointerlocking.[12,13,20,25,26,29,48,57,58,61,62] Besides, trimming a layer of composite can expose the underlying fresh composite which might contribute to increased bond strength.[13,26,58] Still, repair bond strength might not reach an original bond between two fresh composites, because of the lack of the oxygen-inhibited layer, reduced monomers, and water sorption that can reduce silane among other effects.[17,26,29,62,63,64,65] Moreover, thermal fluctuations can cause microcracks through the interface of resin with fillers or through the resin itself.[13,17,63,66] This is why thermocycling reduces SBS,[49,67,42] and why we thermocycled our specimens for 5000 cycles, instead of placing them in citric acid or water.[13,27,56] We also used artificial saliva incubation in order to simulate oral condition better. The roughening technique can matter; different methods of surface roughening can affect the overall SBS, but since they were the same for all groups, their effects on the SBS would be less visible.
Surface topology can also be influenced by the ratio and composition of fillers.[13,17] Nanocomposites are said to have a high proportion of filler particles and therefore might have superior physicomechanical properties. Interestingly, in spite of their vast usage in esthetic dentistry, their repair bond strengths are not examined apart from merely a few controversial researches.[13,17,24] A previous study showed that nanocomposites had the lowest repair bond strengths while microhybrid composites might have a better repair bond strength,[13] consistent with our findings. In our study, nanocomposite failed to produce proper repair bond strengths in the presence or absence of bleaching. Clinical performance of dental composites is affected by a low degree of conversion (DC).[68,69,70,71] Monomers might exhibit considerable residual unsaturation in the final product so that the DC might range from 55 to 75%.[29,72,73,74,75] An inadequate rate of polymerization might lead to weakened bonding strengths, low physicomechanical properties, the release of toxic materials such as monomers and initiators.[29,72,73,74,75] After polymerization, BA films – particularly simplified ones – might behave as permeable membranes, allowing water to flow from the dentin substrate to the top of the adhesive layer, weakening the coupling with resin-based restorative materials, plasticizing polymers, degrading the bonded interface, and forming a permeable interface along the margin of the restoration which can negatively affect the longevity of the bonded restoration.[76] Monomer trapped in the restoration can decrease the clinical serviceability of composite through oxidation and hydrolytic degradation, leading to discoloration of the fillings and accelerated wear. These unfavorable changes may lead to restoration detachment, and caries formation, or discoloration around the adhesive, which are of great clinical concern.[77] Therefore, assessment of the degree of polymerization of various dental monomer systems is of significant value.[78]
CONCLUSION
The findings of this study suggest that, overall, bleaching of an aged composite might not affect the repair bond strength of a fresh same-name composite to the older one. However, different composites had different profiles of SBS trends under various bleaching conditions: Z100 showed no considerable difference in three bleaching methods; Z250 was most vulnerable to home bleaching technique, while Z350 showed the least SBS values in the case of office bleaching. The hybrid and microhybrid types can produce clinically acceptable repair bond strengths, either in the absence of bleaching or in its presence (regardless of the method of bleaching). However, nanofilled composite cannot produce proper repair bond strengths no matter if bleaching has been done or not.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or nonfinancial in this article.
Acknowledgment
The present study is the result of a research project (Registration No: U-98169). The authors would like to express sincere gratitude to Vice Chancellor for Research and Technology, Ahvaz Jundishapur University of Medical Sciences (AJUMS) for the technical and financial support for this study.
REFERENCES
- 1.Ferrari R, Attin T, Wegehaupt FJ, Stawarczyk B, Tauböck TT. The effects of internal tooth bleaching regimens on composite-to-composite bond strength. J Am Dent Assoc. 2012;143:1324–31. doi: 10.14219/jada.archive.2012.0095. [DOI] [PubMed] [Google Scholar]
- 2.Joiner A, Luo W. Tooth colour and whiteness: A review. J Dent. 2017;67S:S3–10. doi: 10.1016/j.jdent.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Tredwin CJ, Naik S, Lewis NJ, Scully C. Hydrogen peroxide tooth-whitening (bleaching) products: Review of adverse effects and safety issues. Br Dent J. 2006;200:371–6. doi: 10.1038/sj.bdj.4813423. [DOI] [PubMed] [Google Scholar]
- 4.Rezende M, Ferri L, Kossatz S, Loguercio AD, Reis A. Combined bleaching technique using low and high hydrogen peroxide in-office bleaching gel. Oper Dent. 2016;41:388–96. doi: 10.2341/15-266-C. [DOI] [PubMed] [Google Scholar]
- 5.Cvikl B, Lussi A, Moritz A, Flury S. Enamel surface changes after exposure to bleaching gels containing carbamide peroxide or hydrogen peroxide. Oper Dent. 2016;41:E39–47. doi: 10.2341/15-010-L. [DOI] [PubMed] [Google Scholar]
- 6.Malkondu Ö, Yurdagüven H, Say EC, Kazazoğlu E, Soyman M. Effect of bleaching on microhardness of esthetic restorative materials. Oper Dent. 2011;36:177–86. doi: 10.2341/10-078-L. [DOI] [PubMed] [Google Scholar]
- 7.Esmaeili B, Abolghasemzade F, Gholampour A. Comparing the effect of different bleaching regims of carbamide peroxide on microhardness of Z250 composite. SSU J. 2015;23:799–805. [Google Scholar]
- 8.Silva Costa SX, Becker AB, de Souza Rastelli AN, de Castro Monteiro Loffredo L, de Andrade MF, Bagnato VS. Effect of four bleaching regimens on color changes and microhardness of dental nanofilled composite. Int J Dent 2009. 2009 doi: 10.1155/2009/313845. 313845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plotino G, Buono L, Grande NM, Pameijer CH, Somma F. Nonvital tooth bleaching: A review of the literature and clinical procedures. J Endod. 2008;34:394–407. doi: 10.1016/j.joen.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 10.Lucena-Martín C, González-López S, Navajas-Rodríguez de Mondelo JM. The effect of various surface treatments and bonding agents on the repaired strength of heat-treated composites. J Prosthet Dent. 2001;86:481–8. doi: 10.1067/mpr.2001.116775. [DOI] [PubMed] [Google Scholar]
- 11.Oztas N, Alaçam A, Bardakçy Y. The effect of air abrasion with two new bonding agents on composite repair. Oper Dent. 2003;28:149–54. [PubMed] [Google Scholar]
- 12.Jafarzadeh Kashi TS, Erfan M, Rakhshan V, Aghabaigi N, Tabatabaei FS. An in vitro assessment of the effects of three surface treatments on repair bond strength of aged composites. Oper Dent. 2011;36:608–17. doi: 10.2341/10-386-L. [DOI] [PubMed] [Google Scholar]
- 13.Nassoohi N, Kazemi H, Sadaghiani M, Mansouri M, Rakhshan V. Effects of three surface conditioning techniques on repair bond strength of nanohybrid and nanofilled composites. Dent Res J (Isfahan) 2015;12:554–61. doi: 10.4103/1735-3327.170575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Nahedh HN, Awliya WY. The effectiveness of four methods for stain removal from direct resin-based composite restorative materials. Saudi Dent J. 2013;25:61–7. doi: 10.1016/j.sdentj.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kazemi AD, Johar N. The color effect of bleaching agent on different composite restoration materials after aging. J Int Oral Health. 2016;8:697. [Google Scholar]
- 16.Villalta P, Lu H, Okte Z, Garcia-Godoy F, Powers JM. Effects of staining and bleaching on color change of dental composite resins. J Prosthet Dent. 2006;95:137–42. doi: 10.1016/j.prosdent.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 17.Rinastiti M, Özcan M, Siswomihardjo W, Busscher HJ. Effects of surface conditioning on repair bond strengths of non-aged and aged microhybrid, nanohybrid, and nanofilled composite resins. Clin Oral Investig. 2011;15:625–33. doi: 10.1007/s00784-010-0426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mjör IA, Shen C, Eliasson ST, Richter S. Placement and replacement of restorations in general dental practice in Iceland. Oper Dent. 2002;27:117–23. [PubMed] [Google Scholar]
- 19.Gordan VV, Mondragon E, Shen C. Replacement of resin-based composite: Evaluation of cavity design, cavity depth, and shade matching. Quintessence Int. 2002;33:273–8. [PubMed] [Google Scholar]
- 20.Söderholm KJ, Roberts MJ. Variables influencing the repair strength of dental composites. Scand J Dent Res. 1991;99:173–80. doi: 10.1111/j.1600-0722.1991.tb01881.x. [DOI] [PubMed] [Google Scholar]
- 21.Padipatvuthikul P, Mair LH. Bonding of composite to water aged composite with surface treatments. Dent Mater. 2007;23:519–25. doi: 10.1016/j.dental.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Yesilyurt C, Kusgoz A, Bayram M, Ulker M. Initial repair bond strength of a nano-filled hybrid resin: Effect of surface treatments and bonding agents. J Esthet Restor Dent. 2009;21:251–60. doi: 10.1111/j.1708-8240.2009.00271.x. [DOI] [PubMed] [Google Scholar]
- 23.Rodrigues SA, Jr, Ferracane JL, Della Bona A. Influence of surface treatments on the bond strength of repaired resin composite restorative materials. Dent Mater. 2009;25:442–51. doi: 10.1016/j.dental.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Rinastiti M, Ozcan M, Siswomihardjo W, Busscher HJ. Immediate repair bond strengths of microhybrid, nanohybrid and nanofilled composites after different surface treatments. J Dent. 2010;38:29–38. doi: 10.1016/j.jdent.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 25.Cavalcanti AN, De Lima AF, Peris AR, Mitsui FH, Marchi GM. Effect of surface treatments and bonding agents on the bond strength of repaired composites. J Esthet Restor Dent. 2007;19:90–8. doi: 10.1111/j.1708-8240.2007.00073.x. [DOI] [PubMed] [Google Scholar]
- 26.Khosravanifard B, Nemati-Anaraki S, Faraghat S, Sajjadi SH, Rakhshan H, Rakhshan V. Efficacy of 4 surface treatments in increasing the shear bond strength of orthodontic brackets bonded to saliva-contaminated direct composites. Orthodontic Waves. 2011;70:65–70. [Google Scholar]
- 27.Joulaei M, Bahari M, Ahmadi A, Savadi Oskoee S. Effect of different surface treatments on repair micro-shear bond strength of silica- and zirconia-filled composite resins. J Dent Res Dent Clin Dent Prospects. 2012;6:131–7. doi: 10.5681/joddd.2012.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finer Y, Santerre JP. Salivary esterase activity and its association with the biodegradation of dental composites. J Dent Res. 2004;83:22–6. doi: 10.1177/154405910408300105. [DOI] [PubMed] [Google Scholar]
- 29.Powers J, Sakaguchi R, Craig R. Craig's Restorative Dental Materials. St. Louis, MO, United States: Mosby Elsevier; 2006. [Google Scholar]
- 30.AlJehani YA, Baskaradoss JK, Geevarghese A, AlShehry MA, Vallittu PK. Shear bond strength between fiber-reinforced composite and veneering resin composites with various adhesive resin systems. J Prosthodont. 2016;25:392–401. doi: 10.1111/jopr.12315. [DOI] [PubMed] [Google Scholar]
- 31.Kuga MC, dos Santos Nunes Reis JM, Fabrício S, Bonetti-Filho I, de Campos EA, Faria G. Fracture strength of incisor crowns after intracoronal bleaching with sodium percarbonate. Dent Traumatol. 2012;28:238–42. doi: 10.1111/j.1600-9657.2011.01077.x. [DOI] [PubMed] [Google Scholar]
- 32.Deliperi S. Clinical evaluation of nonvital tooth whitening and composite resin restorations: five-year results. Eur J Esthet Dent. 2008;3:148–59. [PubMed] [Google Scholar]
- 33.Dahl JE, Pallesen U. Tooth bleaching – A critical review of the biological aspects. Crit Rev Oral Biol Med. 2003;14:292–304. doi: 10.1177/154411130301400406. [DOI] [PubMed] [Google Scholar]
- 34.AlQahtani MQ. The effect of a 10% carbamide peroxide bleaching agent on the microhardness of four types of direct resin-based restorative materials. Oper Dent. 2013;38:316–23. doi: 10.2341/12-224-L. [DOI] [PubMed] [Google Scholar]
- 35.Türker SB, Biskin T. The effect of bleaching agents on the microhardness of dental aesthetic restorative materials. J Oral Rehabil. 2002;29:657–61. doi: 10.1046/j.1365-2842.2002.00896.x. [DOI] [PubMed] [Google Scholar]
- 36.Bauer H, Ilie N. Effects of aging and irradiation time on the properties of a highly translucent resin-based composite. Dent Mater J. 2013;32:592–9. doi: 10.4012/dmj.2012-309. [DOI] [PubMed] [Google Scholar]
- 37.Puckett AD, Holder R, O’Hara JW. Strength of posterior composite repairs using different composite/bonding agent combinations. Oper Dent. 1991;16:136–40. [PubMed] [Google Scholar]
- 38.Turner CW, Meiers JC. Repair of an aged, contaminated indirect composite resin with a direct, visible-light-cured composite resin. Oper Dent. 1993;18:187–94. [PubMed] [Google Scholar]
- 39.Kupiec KA, Barkmeier WW. Laboratory evaluation of surface treatments for composite repair. Oper Dent. 1996;21:59–62. [PubMed] [Google Scholar]
- 40.Teixeira EC, Bayne SC, Thompson JY, Ritter AV, Swift EJ. Shear bond strength of self-etching bonding systems in combination with various composites used for repairing aged composites. J Adhes Dent. 2005;7:159–64. [PubMed] [Google Scholar]
- 41.Özcan M, Corazza PH, Marocho SM, Barbosa SH, Bottino MA. Repair bond strength of microhybrid, nanohybrid and nanofilled resin composites: Effect of substrate resin type, surface conditioning and ageing. Clin Oral Investig. 2013;17:1751–8. doi: 10.1007/s00784-012-0863-5. [DOI] [PubMed] [Google Scholar]
- 42.Khosravanifard B, Rakhshan V, Saadatmand A. Effects of blood and saliva contamination on shear bond strength of metal orthodontic brackets and evaluating certain methods for reversing the effect of contamination. Orthod Waves. 2010;69:156–63. [Google Scholar]
- 43.Yu H, Li Q, Hussain M, Wang Y. Effects of bleaching gels on the surface microhardness of tooth-colored restorative materials in situ. J Dent. 2008;36:261–7. doi: 10.1016/j.jdent.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 44.Attin T, Hannig C, Wiegand A, Attin R. Effect of bleaching on restorative materials and restorations – A systematic review. Dent Mater. 2004;20:852–61. doi: 10.1016/j.dental.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 45.Li Q, Yu H, Wang Y. Colour and surface analysis of carbamide peroxide bleaching effects on the dental restorative materials in situ. J Dent. 2009;37:348–56. doi: 10.1016/j.jdent.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 46.Esmaeili B, Zenouz GA, Khazaei F, Daryakenari G, Bizhani A. Effect of different concentrations of carbamide peroxide on the staining susceptibility of resin composites. J Conserv Dent. 2018;21:500–4. doi: 10.4103/JCD.JCD_59_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takesh T, Sargsyan A, Lee M, Anbarani A, Ho J, Wilder-Smith P. Evaluating the whitening and microstructural effects of a novel whitening strip on porcelain and composite dental materials. Dentistry (Sunnyvale) 2017;7:448. doi: 10.4172/2161-1122.1000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bouschlicher MR, Reinhardt JW, Vargas MA. Surface treatment techniques for resin composite repair. Am J Dent. 1997;10:279–83. [PubMed] [Google Scholar]
- 49.Erfan M, Jafarzadeh-Kashi TS, Ghadiri M, Rakhshan V. The effects of dentin bonding agent formulas on their polymerization quality, and together with tooth tissues on their microleakage and shear bond strength: An explorative 3-step experiment. J Adv Prosthodont. 2014;6:333–45. doi: 10.4047/jap.2014.6.5.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sung EC, Chan SM, Mito R, Caputo AA. Effect of carbamide peroxide bleaching on the shear bond strength of composite to dental bonding agent enhanced enamel. J Prosthet Dent. 1999;82:595–9. doi: 10.1016/s0022-3913(99)70060-0. [DOI] [PubMed] [Google Scholar]
- 51.Rueggeberg FA, Margeson DH. The effect of oxygen inhibition on an unfilled/filled composite system. J Dent Res. 1990;69:1652–8. doi: 10.1177/00220345900690100501. [DOI] [PubMed] [Google Scholar]
- 52.Dishman MV, Covey DA, Baughan LW. The effects of peroxide bleaching on composite to enamel bond strength. Dent Mater. 1994;10:33–6. doi: 10.1016/0109-5641(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 53.Atali PY, Buuml F. The effect of different bleaching methods on the surface roughness and hardness of resin composites. J Dent Oral Hyg. 2011;3:10–7. [Google Scholar]
- 54.Papacchini F, Dall’Oca S, Chieffi N, Goracci C, Sadek FT, Suh BI, et al. Composite-to-composite microtensile bond strength in the repair of a microfilled hybrid resin: Effect of surface treatment and oxygen inhibition. J Adhes Dent. 2007;9:25–31. [PubMed] [Google Scholar]
- 55.Chiba K, Hosoda H, Fusayama T. The addition of an adhesive composite resin to the same material: Bond strength and clinical techniques. J Prosthet Dent. 1989;61:669–75. doi: 10.1016/s0022-3913(89)80039-3. [DOI] [PubMed] [Google Scholar]
- 56.Ozcan M, Barbosa SH, Melo RM, Galhano GA, Bottino MA. Effect of surface conditioning methods on the microtensile bond strength of resin composite to composite after aging conditions. Dent Mater. 2007;23:1276–82. doi: 10.1016/j.dental.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 57.Shahdad SA, Kennedy JG. Bond strength of repaired anterior composite resins: An in vitro study. J Dent. 1998;26:685–94. doi: 10.1016/s0300-5712(97)00044-4. [DOI] [PubMed] [Google Scholar]
- 58.Furuse AY, da Cunha LF, Benetti AR, Mondelli J. Bond strength of resin-resin interfaces contaminated with saliva and submitted to different surface treatments. J Appl Oral Sci. 2007;15:501–5. doi: 10.1590/S1678-77572007000600009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sobouti F, Dadgar S, Sanikhaatam Z, Nateghian N, Saravi MG. Effects of two erbium-doped yttrium aluminum garnet lasers and conventional treatments as composite surface abrasives on the shear bond strength of metal brackets bonded to composite resins. J Orthod Sci. 2016;5:18–24. doi: 10.4103/2278-0203.176654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brosh T, Pilo R, Bichacho N, Blutstein R. Effect of combinations of surface treatments and bonding agents on the bond strength of repaired composites. J Prosthet Dent. 1997;77:122–6. doi: 10.1016/s0022-3913(97)70224-5. [DOI] [PubMed] [Google Scholar]
- 61.Bonstein T, Garlapo D, Donarummo J, Jr, Bush PJ. Evaluation of varied repair protocols applied to aged composite resin. J Adhes Dent. 2005;7:41–9. [PubMed] [Google Scholar]
- 62.Fritz UB, Finger WJ, Stean H. Salivary contamination during bonding procedures with a one-bottle adhesive system. Quintessence Int. 1998;29:567–72. [PubMed] [Google Scholar]
- 63.Kawano F, Ohguri T, Ichikawa T, Matsumoto N. Influence of thermal cycles in water on flexural strength of laboratory-processed composite resin. J Oral Rehabil. 2001;28:703–7. doi: 10.1046/j.1365-2842.2001.00724.x. [DOI] [PubMed] [Google Scholar]
- 64.Ortengren U, Andersson F, Elgh U, Terselius B, Karlsson S. Influence of pH and storage time on the sorption and solubility behaviour of three composite resin materials. J Dent. 2001;29:35–41. doi: 10.1016/s0300-5712(00)00055-5. [DOI] [PubMed] [Google Scholar]
- 65.McCabe JF, Rusby S. Water absorption, dimensional change and radial pressure in resin matrix dental restorative materials. Biomaterials. 2004;25:4001–7. doi: 10.1016/j.biomaterials.2003.10.088. [DOI] [PubMed] [Google Scholar]
- 66.Hakimeh S, Vaidyanathan J, Houpt ML, Vaidyanathan TK, Von Hagen S. Microleakage of compomer class V restorations: effect of load cycling, thermal cycling, and cavity shape differences. J Prosthet Dent. 2000;83:194–203. doi: 10.1016/s0022-3913(00)80012-8. [DOI] [PubMed] [Google Scholar]
- 67.Diaz-Arnold AM, Aquilino SA. An evaluation of the bond strengths of four organosilane materials in response to thermal stress. J Prosthet Dent. 1989;62:257–60. doi: 10.1016/0022-3913(89)90327-2. [DOI] [PubMed] [Google Scholar]
- 68.Cotti E, Scungio P, Dettori C, Ennas G. Comparison of the degree of conversion of resin based endodontic sealers using the DSC technique. Eur J Dent. 2011;5:131–8. [PMC free article] [PubMed] [Google Scholar]
- 69.Jafarzadeh-Kashi TS, Mirzaii M, Erfan M, Fazel A, Eskandarion S, Rakhshan V. Polymerization behavior and thermal characteristics of two new composites at five temperatures: Refrigeration to preheating. J Adv Prosthodont. 2011;3:216–20. doi: 10.4047/jap.2011.3.4.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Daronch M, Rueggeberg FA, De Goes MF. Monomer conversion of pre-heated composite. J Dent Res. 2005;84:663–7. doi: 10.1177/154405910508400716. [DOI] [PubMed] [Google Scholar]
- 71.Prasanna N, Pallavi Reddy Y, Kavitha S, Lakshmi Narayanan L. Degree of conversion and residual stress of preheated and room-temperature composites. Indian J Dent Res. 2007;18:173–6. doi: 10.4103/0970-9290.35827. [DOI] [PubMed] [Google Scholar]
- 72.Shen C, Anusavice K. Phillip's Science of Dental Materials. St. Louis, MO, United States: Mosby Elsevier Location; 2003. [Google Scholar]
- 73.William Joseph OB. Dental Materials and Their Selection. Batavia, IL, USA: Quintessence Pub. Co; 2008. [Google Scholar]
- 74.Van Noort R. Introduction to dental materials. Recherche. 2007;67:02. [Google Scholar]
- 75.Roberson T, Heymann H, Swift E. Sturdevant’s Art and Science of Operative Dentistry. St. Louis, MO, United States: Mosby; 2006. pp. 381–95. [Google Scholar]
- 76.De Munck J, Van Meerbeek B, Yoshida Y, Inoue S, Vargas M, Suzuki K, et al. Four-year water degradation of total-etch adhesives bonded to dentin. J Dent Res. 2003;82:136–40. doi: 10.1177/154405910308200212. [DOI] [PubMed] [Google Scholar]
- 77.Usümez S, Büyükyilmaz T, Karaman AI, Gündüz B. Degree of conversion of two lingual retainer adhesives cured with different light sources. Eur J Orthod. 2005;27:173–9. doi: 10.1093/ejo/cjh085. [DOI] [PubMed] [Google Scholar]
- 78.Antonucci JM, Toth EE. Extent of polymerization of dental resins by differential scanning calorimetry. J Dent Res. 1983;62:121–5. doi: 10.1177/00220345830620020701. [DOI] [PubMed] [Google Scholar]


