Abstract
BRCA1 is a cell cycle-regulated nuclear protein that is phosphorylated mainly on serine and to a lesser extent on threonine residues. Changes in phosphorylation occur in response to cell cycle progression and DNA damage. Specifically, BRCA1 undergoes hyperphosphorylation during late G1 and S phases of the cell cycle. Here we report that BRCA1 is phosphorylated in vivo at serine 1497 (S1497), which is part of a cyclin-dependent kinase (CDK) consensus site. S1497 can be phosphorylated in vitro by CDK2-cyclin A or E. BRCA1 coimmunoprecipitates with an endogenous serine-threonine protein kinase activity that phosphorylates S1497 in vitro. This cellular kinase activity is sensitive to transfection of a dominant negative form of CDK2 as well as the application of the CDK inhibitors p21 and butyrolactone I but not p16. Furthermore, BRCA1 coimmunoprecipitates with CDK2 and cyclin A. These results suggest that the endogenous kinase activity is composed of CDK2-cyclin complexes, at least in part, concordant with the G1/S-specific increase in BRCA1 phosphorylation.
While breast cancer occurs mostly as a sporadic disease, genetic predisposition accounts for 5 to 10% of all breast cancer cases. However, the contribution of hereditary factors to breast cancer in women under 30 years of age may be as high as 25%, since familial breast cancer often occurs at an early age (17). Mutations in the BRCA1 gene account for about half of the families with high breast cancer incidence and at least 80% of families predisposed to both early-onset breast and ovarian cancer (18). It has been proposed that the BRCA1 gene encodes a tumor suppressor protein, since tumors from BRCA1 mutation carriers display loss or inactivation of the remaining wild-type allele (32, 47). Although somatic mutations in the BRCA1 gene are rarely found in sporadic breast and ovarian cancers, BRCA1 may still play a role in these forms of cancer (20, 21, 25, 38, 44, 55). Loss of heterozygosity is frequently observed in the region of chromosome 17q that harbors the BRCA1 gene (20, 33, 34), and recent evidence suggests that BRCA1 levels are reduced in sporadic breast cancers (53, 65, 77). It also has been proposed that BRCA1 is aberrantly localized in sporadic breast cancers (14).
The BRCA1 gene encodes a 1,863-amino-acid (aa) protein whose primary sequence offers few clues about its function (45). It contains a RING finger at its amino (N)-terminal region and a domain termed BRCT at its carboxy (C) terminus (36). Proteins harboring BRCT domains have been implicated to participate in DNA damage-responsive checkpoint and cell cycle control functions (6, 10, 36). BRCA1 is a nuclear protein whose RNA and protein levels are cell cycle regulated (14, 15, 26, 57, 62, 68, 73, 76). Moreover, BRCA1 is phosphorylated, and its phosphorylation state also undergoes cell cycle-specific alterations (16, 57, 60, 69).
The molecular function of BRCA1 has not yet been determined. Evidence that the C-terminal domain of BRCA1 (aa 1528 to 1863) has transcription activation activity and that BRCA1 is linked to the RNA polymerase II holoenzyme via RNA helicase A has been presented (3, 11, 46, 59, 64). Furthermore, BRCA1 can regulate gene expression in concert with p53 or CBP/p300 (52, 54, 78). The finding that BRCA1 associates with Rad51 in mitotic and meiotic cells implies a role for BRCA1 in the control of recombination and genome integrity and underlines the proposed function of BRCA1 as a caretaker (35, 61). Moreover, the subnuclear localization and the phosphorylation state of BRCA1 change in response to DNA damage, suggesting that BRCA1 participates in a DNA damage-dependent replication checkpoint response (60, 69). A recent report demonstrates a role for BRCA1 in transcription-coupled repair of oxidative DNA damage (23). BRCA1 has also been proposed to regulate cell proliferation, differentiation, and apoptosis (24, 27, 37, 41, 42, 63, 70).
Since BRCA1 phosphorylation responds to cell cycle progression and DNA damage, one can assume that phosphorylation regulates the activity of the protein, as is the case for other tumor suppressors, e.g., p53 and Rb (4, 22). It is therefore crucial to understanding the biology of BRCA1 that the specifics of BRCA1 phosphorylation be investigated. This includes the identification of the sites of phosphorylation and the responsible protein kinase(s). Such information would link BRCA1 to a cellular pathway(s) and facilitate investigation of its function at the molecular level. Candidate protein kinases that may be responsible at least in part for the cell cycle-specific phosphorylation changes of BRCA1 include the cyclin-dependent kinases (CDKs).
Members of the CDK family are important regulators of the eukaryotic cell cycle (29, 50, 56). The activity of CDKs is tightly regulated by association with other polypeptides and by addition or removal of phosphate moieties. The intrinsically inactive CDK catalytic subunit requires association with a positive regulatory cyclin partner, and multiple regulatory phosphorylation and dephosphorylation events occur on both CDK and cyclin subunits. The activity of the CDK-cyclin complexes can be further modulated by association with other polypeptides, such as inhibitors of CDKs (CDIs) (19, 39, 49). Vertebrates possess multiple CDK and cyclin subunits. D-type cyclins in association with CDK4 and CDK6 function early in the cell cycle, during G1-phase progression, whereas CDK2-cyclin E and CDK2-cyclin A act later on, at the G1/S transition and during S-phase progression, respectively. CDC2-cyclin B1 functions at the G2/M transition. The CDIs are divided into two categories, based on differences in structure, mechanism of inhibition, and specificity. Members of the p21 family (comprising p21, p27, and p57) preferentially inhibit CDKs of the G1 and S phases (CDK2, -3, -4, and -6), whereas members of the INK4 (inhibitor of CDK4) family (comprising p15, p16, p18, and p19) are selective for CDK4 and CDK6 (28). The CDKs display substrate specificity toward proteins containing the motif S/T-P-(X)-K/R (S, serine; T, threonine; P, proline; X, any amino acid; K, lysine; R, arginine) or minimally S/T-P (followed by K/R) (40, 48).
Here we show that serine residue 1497 (S1497), which constitutes one of the four CDK consensus sites in BRCA1, is phosphorylated in vivo and can be phosphorylated by recombinant CDK2-cyclin complexes in vitro. BRCA1 coimmunoprecipitates with an endogenous serine-threonine protein kinase activity that phosphorylates this particular serine residue in a manner sensitive to p21 and butyrolactone I but not to p16. Moreover, a dominant negative CDK2 mutant (CDK2 dn) inhibits BRCA1 phosphorylation in vivo. We propose that cellular CDK2 is responsible, at least in part, for the G1/S-dependent increase in BRCA1 phosphorylation.
MATERIALS AND METHODS
Plasmids.
To make subsequent cloning easier, full-length BRCA1 cDNA derived from pCL-MFG-BRCA1 (57) was engineered in two consecutive steps into pCL-MFG-MCS (64a) to generate pCL-MFGΔ-BRCA1. BRCA1Δ772-1050 was generated in pCL-MFGΔ-BRCA1 by ligation of KpnI (blunted by 3′ overhang removal) to ScaI-cut cDNA in a three-way ligation. BRCA1 1051-1863 was generated by inserting a composite of ScaI-ApaI and ApaI-BamHI from pUHD-P1-BRCA1 (57) into NcoI (blunted by fill in) and BamHI-cut pCL-MFG-MCS. To generate Myc-tagged, full-length wild-type BRCA1 (Myc-BRCA1 wt), a tag containing five Myc epitopes derived by PCR from 6x myc BRCA1 1314-1863 (54) was inserted into the NcoI site (at the start methionine) of pCL-MFGΔ-BRCA1. BRCA1 1314-1652 was cloned by deleting aa 1653 to 1863 by PCR in 6x myc BRCA1 1314-1863. Mutants S1497A and S1497T were generated first in BRCA1 1051-1863, using a Clontech transformer site-directed mutagenesis kit, and then lifted into Myc-BRCA1 (yielding Myc-BRCA1 S1497A and S1497T); mutants T967S and T967D were first generated in pCL-MFGΔ-BRCA1 lacking aa 2 to 473 before being cloned into Myc-BRCA1 (yielding Myc-BRCA1 T967S and T967D). Mutagenic primers for S1497A, S1497T, T967S, and T967D were GCATTTAGAAGGGGCTGACCTTTCCACTCC, GGCATTTAGAAGGGGTTGACCTTTCCACTCCTG, GTTTATTTGGAGAAATGAGTCCAG, and GTTTATTTGGATCAATGAGTCCAG, respectively.
In addition, we used plasmids expressing CDK2 and CDK2 dn (71), cyclin E (29a), and cyclin A (25a).
Cell culture, transfections, and in vivo labeling.
293T and HBL-100 cells were cultivated in Dulbecco modified Eagle medium (DMEM) (Cellgro; Mediatech) containing 10% fetal bovine serum (FBS) (HyClone) at 37°C in 10% CO2 and in McCoy’s 5A medium (Gibco BRL) containing 10% FBS at 37°C in 5% CO2, respectively.
Transfections of 293T cells were performed by an adaptation of the calcium phosphate method described by Wigler et al. (75). We usually obtained transfection efficiencies of 70 to 100%, as determined by green fluorescent protein staining due to cotransfection of trace amounts of a green fluorescent protein-expressing plasmid in some experiments (data not shown).
For 32P labeling, cells were washed twice in phosphate-free DMEM and incubated in phosphate-free DMEM containing 10% dialyzed FBS (Gibco BRL), 0.1 mM MEM nonessential amino acids (Gibco), 2 mM l-glutamine (Gibco), and 1 mM MEM-sodium pyruvate (Gibco) for about 45 min at 37°C before addition of 0.7 mCi of 32P (H3PO4; ICN Pharmaceuticals, Inc.) per ml. After 4 h of labeling, cells were washed with phosphate-buffered saline (without Ca2+ and Mg2+), scraped from the plates, pelleted by centrifugation, and frozen on dry ice.
Western blot analysis.
Western blot analysis was performed as described elsewhere (57) except that cells were lysed under slightly modified inhibitor conditions: 50 mM HEPES (pH 7.4)–150 mM NaCl–10% glycerol–1% Triton X-100–15 mM MgCl2–10 mM EGTA–1 mM dithiothreitol containing inhibitors pepstatin A (1 μg/ml), phenylmethylsulfonyl fluoride (1 mM), leupeptin (10 μg/ml), aprotinin (21 μg/ml), 20 mM NaF, 10 mM 4-nitrophenyl phosphate, 1 mM Na3VO4, 20 mM β-glycerophosphate, 10 mM Na2MoO4, and 100 nM calyculin A. The following antibodies were used: BRCA1 Ab-D (57), Myc-specific antibody 9E10 (Jill Meisenhelder, The Salk Institute), and mouse CDK2-specific monoclonal antibody (D-12; Santa Cruz).
Immunoprecipitations.
For immunoprecipitations of in vivo-labeled overexpressed and endogenous proteins and of unlabeled BRCA1, cells were lysed in radioimmunoprecipitation assay (RIPA) buffer (57) containing 2 mM EDTA and the proteinase/phosphatase inhibitors mentioned above in the presence of 0.5% sodium dodecyl sulfate (SDS) before being heated for 6 to 10 min at 99°C. After the SDS concentration was adjusted to 0.1% by addition of 4 volumes of the above buffer without SDS (and addition of RNase A to 100 μg/ml in the case of labeled proteins), the lysates were passed 10 times through a 22G1 1/2 needle (Becton Dickinson & Co.) and cleared by centrifugation at 4°C and 12,000 rpm for 30 min. To reduce nonspecific binding, the supernatants were incubated with protein A-Sepharose (Pharmacia) for 1 h at 4°C while rotating; after removal of the beads by centrifugation, the lysates were incubated with BRCA1 antibody Ab-D or Ab-C plus Ab-D (57) at a total of 5 μl/ml for at least 2 h at 4°C while rotating. Finally, protein A-Sepharose beads were added for 1 h while tumbling at 4°C before the beads were washed five to seven times with RIPA buffer–0.1% SDS. The immunoprecipitates were dissociated from the beads by heating at 99°C for 6 to 8 min and then separated on SDS-polyacrylamide gels (5% gels for full-length BRCA1 and 8% for the fragment spanning aa 1051 to 1863 [BRCA1 1051-1863]) before they were transferred to polyvinylidene difluoride membranes (Immobilon-P; Millipore) and visualized by autoradiography or subjected to Western blot analysis.
For in vitro kinase assays of Myc-tagged BRCA1, cells were lysed and processed in RIPA buffer as described above, without phosphatase inhibitors, SDS, and heat denaturation, using 10 μg of antibody 9E10 per ml for immunoprecipitation in the presence of protein G-Sepharose (Pharmacia). After precipitation, beads were washed twice with RIPA buffer and three times with 50 mM HEPES (pH 7.4)–10 mM MgCl2. In some experiments, bacterial alkaline phosphatase (BAP) (BAPF; Worthington) was added to the immunoprecipitation reactions (together with the first addition of protein G-Sepharose) in order to efficiently dephosphorylate BRCA1 before performance of in vitro kinase reactions; however, tryptic phosphopeptide maps of BAP-treated and untreated BRCA1 were similar (data not shown).
For coimmunoprecipitations, lysates were prepared and processed as for in vitro kinase assays, using the following rabbit antibodies (Santa Cruz) in the presence of protein A-Sepharose: anti-CDK2 (M2; where indicated, the antibody was preincubated with an equal amount of the corresponding blocking peptide on ice for 35 min), anti-cyclin A (H-432), anti-cyclin E (C-19), and anti-NF-κB p65 (A). The immunocomplexes were washed six times with RIPA buffer containing 2 mM EDTA and proteinase inhibitors before analysis.
In vitro kinase assays, phosphopeptide mapping, and phosphoamino acid analysis (PAA).
In vitro kinase assays were performed on the immunoprecipitates bound to the beads in 25 μl of a mixture containing 50 mM HEPES (pH 7.4), 10 mM MgCl2, 1 mM dithiothreitol, 25 μM ATP, and 10 μCi of [γ-32P]ATP at 30°C for 30 min. Where indicated, the corresponding baculovirus-expressed, purified CDK-cyclin complexes (50 to 100 ng/reaction [30]) were added to the reactions; for the inhibition experiments, 5 μg of either recombinant p16 or p21 (Tim Mayall, The Salk Institute) or 62 μM butyrolactone I (Calbiochem) (31) was added. The reaction products were separated on SDS–5% polyacrylamide gels before autoradiography.
Two-dimensional phosphopeptide mapping and PAA were performed as previously described (57, 72).
Mass spectrometric analysis.
Matrix-assisted laser desorption measurements were carried out on a Bruker Reflex (Bruker Instruments Inc., Manning Park, Billerica, Mass.) reflectron time-of-flight mass spectrometer utilizing a nitrogen UV laser. The instrument was operated with an accelerating voltage of +31 kV and a reflector potential of +30 kV. The mass spectrum represents the accumulation of approximately 20 laser shots. The mass accuracy of the instrument was typically ±200 ppm. Micro-high-performance liquid chromatography analysis was performed on a UMA (Michrom Bioresources, Auburn, Calif.) using a 0.5- by 150-mm column (Michrom Bioresources) packed with 5-μm (300-Å) C18 reverse-phase material (Vydac, Hesperia, Calif.) to separate the Lys-C (Boehringer Mannheim, Mannheim, Germany) digest.
RESULTS
Phosphorylation pattern of endogenous and overexpressed BRCA1.
BRCA1 is a cell cycle-regulated nuclear phosphoprotein that is phosphorylated at multiple amino acids, mainly on serine and to a lesser extent on threonine residues (57). Because human BRCA1 contains 224 serine and 111 threonine residues (corresponding to 12 and 6%, respectively, of the entire protein [45]), we decided to narrow our search to regions of the protein that are phosphorylated before identifying individual phosphorylated residues. To investigate the phosphorylation of BRCA1, we set out to characterize the phosphorylation of the transiently overexpressed protein, which would allow us to introduce mutations and test their effects on phosphorylation. We transiently transfected a series of overlapping BRCA1 fragments encompassing the entire BRCA1 protein into human embryonic kidney cells (293T) that were then labeled with [32P]phosphoric acid. The tryptic phosphopeptide maps generated from the immunopurified fragments after separation on SDS-polyacrylamide gels were then compared to the map of full-length BRCA1 (72). The presence of the same phosphopeptide in the maps derived from a fragment and wild-type protein indicated that the phosphorylated amino acid is located within that particular fragment. BRCA1 was found to be phosphorylated in several regions, and some were located within the C-terminal half of the protein (57a). We then investigated candidate consensus phosphorylation sites within these phosphorylated regions, with particular emphasis on CDK consensus sites, because of the cell cycle-specific changes in phosphorylation of BRCA1.
Human BRCA1 contains four CDK consensus sites; three [S(896)PK, T(967)PNK, and S(1009)PER] are located within exon 11, and one [S(1497)PSK] is located close to the C terminus. All of these sites could be target sites in vivo for CDKs based on their primary sequences. To determine whether BRCA1 is phosphorylated at these sites, we transiently expressed in 293T cells either full-length BRCA1 protein or fragments harboring mutations at the CDK sites and compared their resulting phosphopeptide maps to that of wild-type full-length BRCA1 protein. A difference in the map of a particular mutant indicated that the sequence surrounding that amino acid played a role in phosphorylation in vivo.
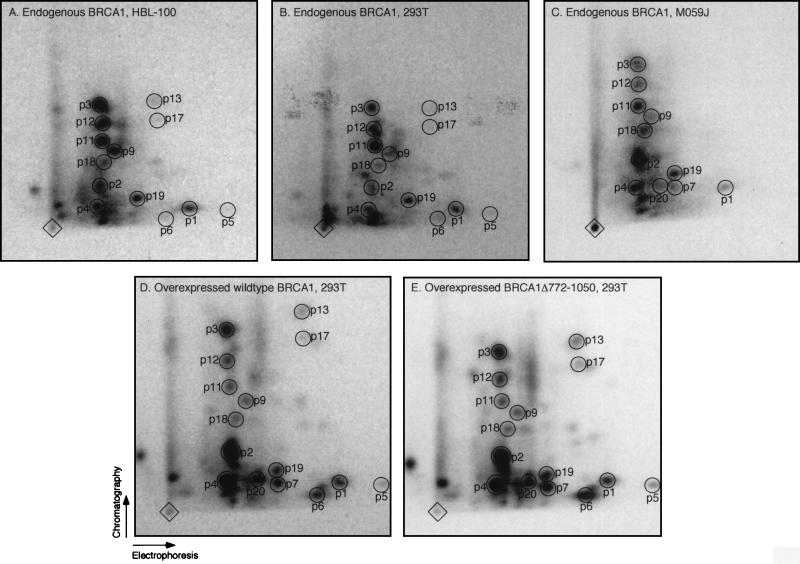
To demonstrate that in vivo-overexpressed BRCA1 is indeed correctly phosphorylated and reflects the phosphorylation of the endogenous protein, we compared the tryptic phosphopeptide maps of endogenous BRCA1 derived from human breast epithelial (HBL-100 [Fig. 1A]), 293T (Fig. 1B), and malignant glioma (M059J [Fig. 1C]) cells as well as from cervical carcinoma cells (HeLa [data not shown]) to the map of overexpressed protein (pCL-MFGΔ-BRCA1 [Fig. 1D; see also reference 57]). The majority of tryptic phosphopeptides present in the map of the endogenous protein are identical to those present in the map of the overexpressed protein (p1 to p4, p9, p11, p12, p18, and p19), verifying that the phosphorylation of the overexpressed protein occurs at physiological sites. Phosphopeptides p7 and p20 found in the map of overexpressed BRCA1 (Fig. 1D), although not detected in the maps of endogenous BRCA1 from HBL-100 and 293T cells (Fig. 1A and B), are present in the map of endogenous BRCA1 from M059J cells (Fig. 1C). Phosphopeptides p5, p6, p13, and p17 are present in the maps of endogenous BRCA1 from HBL-100 and 293T cells, although at relatively low levels (Fig. 1A and B), and of overexpressed BRCA1 (Fig. 1D), but they are below detection levels in the map of endogenous BRCA1 from M059J cells (Fig. 1C). We note that some of the spots differ in relative intensities between (i) endogenous BRCA1 from different cell lines and (ii) endogenous and overexpressed protein. The former may reflect differences in phosphorylation characteristic for each cell line. The latter observation may be due to an alteration in the ratio of protein kinase(s) to BRCA1 and/or improper subcellular localization of overexpressed BRCA1, since it is expressed in large excess compared to the endogenous protein. Immunofluorescence analysis of transiently overexpressed BRCA1 protein revealed nuclear and cytoplasmic staining (data not shown). Alternatively, overexpressed BRCA1 could have an effect on the cellular machinery which leads to an imbalanced phosphorylation of BRCA1. There are a few additional phosphopeptides detected in the maps of endogenous and overexpressed BRCA1, e.g., the two spots below p4 (Fig. 1; spots are not numbered). The variation of their relative abundance among different experiments as well as the fact that these spots are also present in the tryptic peptide map of the BRCA1 fragment lacking aa 772 to 1050 (Fig. 1E [see below]), which has a different mobility on a SDS-polyacrylamide gel compared to wild-type protein, suggests that they are partially digested BRCA1 phosphopeptides. We conclude that we are able to study the characteristics of BRCA1 phosphorylation by using the transiently overexpressed protein.
FIG. 1.
Endogenous and overexpressed BRCA1 reveal similar phosphorylation patterns in vivo. Shown are the two-dimensional tryptic phosphopeptide maps of endogenous BRCA1 from HBL-100 (A), 293T (B), and M059J (C) cells and of overexpressed full-length (D) and truncated BRCA1 lacking aa 772 to 1050 (E) from transiently transfected 293T cells. BRCA1 was immunoprecipitated with Ab-D (for maps B to E) or Ab-C plus Ab-D (for map A) from lysates of [32P]phosphoric acid-labeled cells. The immunoprecipitates were separated by SDS-polyacrylamide gel electrophoresis, and the labeled BRCA1 protein species were enzymatically hydrolyzed with trypsin. The resulting peptides were separated in the first and second dimensions by electrophoresis and chromatography, respectively, as indicated by the arrows. Diamonds mark sample origins; circles (labeled p1 to p7, p9, p11 to p13 [57], and p17 to p20) indicate phosphopeptides detected in the individual maps. Map D represents a combination of phosphopeptide maps of endogenous and overexpressed BRCA1 protein from 293T cells because Ab-D immunoprecipitated both proteins, which have similar mobilities on SDS-polyacrylamide gels (unlike the faster-running truncated BRCA1 lacking aa 772 to 1050 [data not shown]); however, since overexpressed BRCA1 is far more abundant than the endogenous protein (data not shown), the contribution of endogenous BRCA1 in map D is negligible.
BRCA1 is phosphorylated at S1497 in vivo.
We then compared the phosphopeptide map of an in vivo-labeled overexpressed fragment of BRCA1 protein from which aa 772 to 1050, encompassing the three CDK sites in exon 11, were deleted (Fig. 1E) to the map of the full-length protein (Fig. 1D). The two maps are virtually identical, revealing that no tryptic phosphopeptides were derived from the region between aa 772 and 1050. Furthermore, a Myc-tagged, full-length BRCA1 protein in which T967 was replaced by aspartic acid (see below) revealed the same tryptic phosphopeptide map as the corresponding wild-type protein (data not shown). Therefore, the three CDK sites in exon 11 are not major phosphorylation sites in vivo (see Discussion).
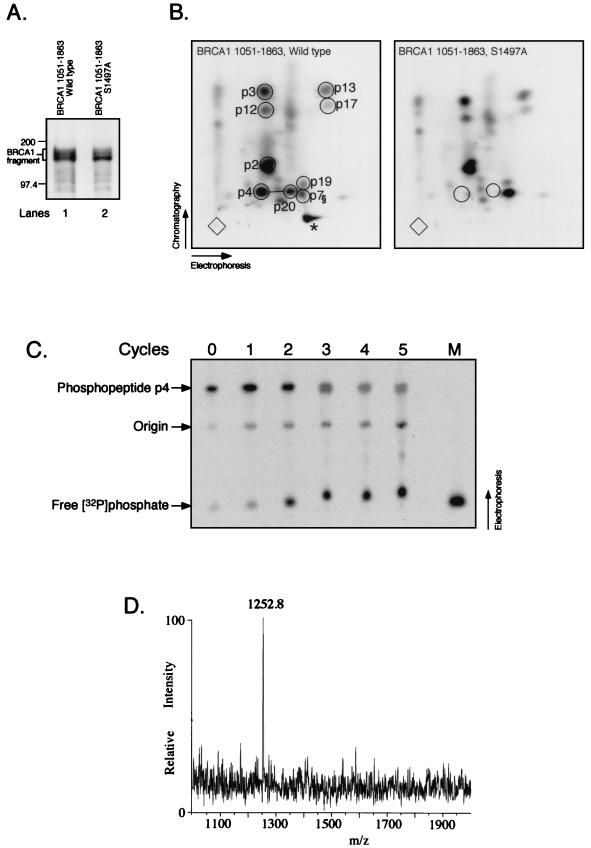
To check whether the C terminus of BRCA1 containing the fourth CDK consensus site (S1497) is phosphorylated, we overexpressed the C-terminal half of BRCA1 (aa 1051 to 1863, untagged) in 293T cells followed by immunoprecipitation using Ab-D (Fig. 2A, lane 1) (57) and performed tryptic phosphopeptide analysis as described above. Several phosphopeptides in full-length BRCA1 are derived from this fragment (Fig. 2B, left; compare to Fig. 1D), in accordance with this fragment appearing as several closely migrating bands that probably represent distinctly phosphorylated forms (Fig. 2A, lane 1). Substituting alanine for S1497 (S1497A) results in reduced phosphorylation of the fragment, as evidenced by a decrease in the slower-migrating species compared to the fastest-migrating phosphorylated form (Fig. 2A; compare lanes 1 and 2). This reduction is due to the loss of phosphopeptides p4 and p20 (Fig. 2B, right), suggesting that this CDK site of BRCA1 is phosphorylated in vivo. The fact that two phosphopeptides, of which at least p4 is detected in maps of endogenous BRCA1 protein (Fig. 1A to C), disappear in the two-dimensional peptide map upon mutation of one amino acid implies that these two peptides are related, suggesting that peptide p20 is a partial tryptic hydrolysis fragment encompassing peptide p4. This result is also obtained in the context of full-length BRCA1 protein, both untagged and Myc tagged (data not shown). BRCA1 fragment 1051-1863 described above lacks the BRCA1 nuclear localization signals (12, 67, 76) and localizes primarily to the cytoplasm, as determined by indirect immunofluorescence analysis and biochemical fractionation (data not shown). Since CDK2-cyclins are predominantly nuclear, a cytoplasmic localization might be expected to preclude phosphorylation of this fragment. However, it has been reported that in transformed cells, including 293 cells, a significant fraction of CDK-cyclin complexes including cyclin A, cyclin E, and CDK2 are present in the cytoplasm (51). Such cytoplasmic CDK-cyclin complexes could phosphorylate the 1051-1863 fragment localized in the cytoplasm.
FIG. 2.
BRCA1 serine residue 1497 is phosphorylated in vivo. (A) The C-terminal half of BRCA1 (aa 1051 to 1863), either wild type (lane 1) or with the S1497A substitution (lane 2), was expressed in 293T cells that were labeled with [32P]phosphoric acid. The fragments were immunoprecipitated with Ab-D and separated on SDS-polyacrylamide gels. Protein marker bands (200 and 97.4 kDa) are indicated on the left. (B) Two-dimensional tryptic phosphopeptide maps of the BRCA1 fragments shown in panel A. The asterisk (*) in the left panel denotes a fraction of phosphopeptide p7 (§) having an abnormal mobility in the chromatographic dimension, possibly due to a thin-layer chromatography plate artifact. (C) Manual Edman degradation of phosphopeptide p4. The procedure was performed as described elsewhere (72), and the reaction products were analyzed on a PhosphorImager (Molecular Dynamics). Numbers above the panel indicate cycles of degradation (0 denotes starting material). M, free [32P]phosphate applied as a marker. The ratio of free [32P]phosphate to phosphopeptide p4 was determined by using the ImageQuaNT software (Molecular Dynamics). About 50% of [32P]phosphate was liberated after the second cycle (compared to the fifth cycle), whereas most of the remaining [32P]phosphate was gradually released over cycles 3 to 5, probably due to incomplete reactions. (D) Matrix-assisted laser desorption mass spectrum of the purified Lys-C peptide 1490-1500 derived from BRCA1 aa 1314 to 1652. x axis, mass-to-charge ratio (m/z); y axis, relative intensity.
Based on the results described above and the predicted relative phosphopeptide mobilities in the electrophoresis and chromatographic dimensions (reference 7 and data not shown), we propose that the amino acid sequences of peptides p4 and p20 are S(1496)SPSK(1500) and N(1488)KEPGVERSSPSK(1500), respectively (the phosphorylated serine residue is underlined; trypsin does not efficiently cleave the sequences K-E and R-X-phospho-S [7]).
We note that it is formally possible that rather than S1497, a neighboring residue is the phosphorylated amino acid within peptide p4, in which case the S1497A substitution inhibits the phosphorylation of the neighboring residue. This amino acid would have to be a serine residue, since phosphopeptide p4 contains only phosphoserine (57). To identify S1497 as the phosphorylated amino acid, we carried out the following biochemical characterization. First, we showed that the radiolabeled amino acid within phosphopeptide p4 is the second residue, as predicted for the peptide SSPSK (aa 1496 to 1500). A Myc-tagged BRCA1 fragment consisting of aa 1314 to 1652 was transiently transfected into 293T cells and subsequently labeled with [32P]phosphoric acid. The fragment was immunoprecipitated with the Myc-specific antibody 9E10, separated by SDS-polyacrylamide gel electrophoresis, and subjected to two-dimensional tryptic peptide mapping, revealing the presence of phosphopeptide p4 within this fragment (data not shown). The phosphopeptide was isolated from the thin-layer chromatography plate and subjected to manual Edman degradation (72). The major release of radiolabeled phosphate occurred after the second cycle (Fig. 2C), consistent with the hypothesis that phosphopeptide p4 is phosphorylated at S1497. Second, mass spectrometric analysis was performed on peptides derived from the same in vivo-labeled C-terminal BRCA1 fragment described above. The fragment was enzymatically hydrolyzed, purified by micro-high-performance liquid chromatography, and subjected to mass spectrometric analysis. To optimize the analysis, we chose to hydrolyze the BRCA1 fragment with endoproteinase Lys-C rather than trypsin, generating a peptide fragment larger than the 5-aa peptide p4. A peptide with a mass-to-charge ratio of 1,252.8 was observed (Fig. 2D), consistent with the expected monoisotopic [M+H]+ of 1,252.56 Da for the singly phosphorylated form of the Lys-C peptide aa 1490 to 1500 (EPGVERSSPSK). Thus, considering the results described above combined with the Edman degradation data and mass spectrometric analysis, we conclude that S1497 is the phosphorylated serine residue.
BRCA1 can be phosphorylated in vitro by CDK2-cyclin A and CDK2-cyclin E.
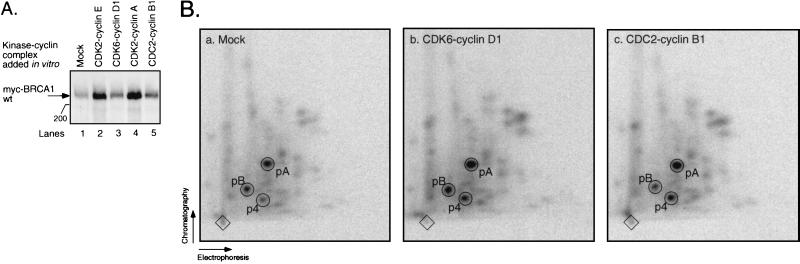
To investigate which cyclin-dependent protein kinase might be responsible for phosphorylating BRCA1 at aa S1497, we performed in vitro kinase reactions on BRCA1 protein, using four different recombinant CDK-cyclin complexes. Myc-BRCA1 wild type (wt) was transiently overexpressed in 293T cells and subsequently immunoprecipitated under native conditions using antibody 9E10. The immunoprecipitate was incubated with baculovirus-expressed, purified CDK2-cyclin A, CDK2-cyclin E, CDK6-cyclin D1, or CDC2-cyclin B1 in the presence of [γ-32P]ATP (30). The reaction products were separated on SDS-polyacrylamide gels and transferred to a membrane before autoradiography. Figure 3A shows that both CDK2-cyclin E (lane 2) and CDK2-cyclin A (lane 4) were able to phosphorylate BRCA1 in vitro, as evidenced by increased incorporation of radioactivity compared to the reaction where no CDK-cyclin was added (lane 1). In contrast, BRCA1 was a poor substrate for CDK6-cyclin D1 and CDC2-cyclin B1 in vitro (Fig. 3A, lanes 3 and 5, respectively). All four CDK-cyclin complexes efficiently phosphorylated glutathione S-transferase–Rb in vitro (30). Two-dimensional tryptic peptide maps of CDK2-cyclin A-treated BRCA1 (see below [Fig. 4B, map d]) and CDK2-cyclin E-treated BRCA1 (data not shown) were very similar, both showing highly increased phosphorylation of peptides p4 and p20, compared to the map of untreated BRCA1 (see below [Fig. 4B, map a]). Both CDK6-cyclin D1 and CDC2-cyclin B1 treatment led to a comparably modest increase in phosphorylation of peptides p4 and p20 (Fig. 3B). In conclusion, BRCA1 is a good substrate for CDK2-cyclin A and CDK2-cyclin E in vitro.
FIG. 3.
BRCA1 is phosphorylated by CDK2-cyclin complexes in vitro. (A) Myc-BRCA1 wt was transiently transfected into 293T cells and immunoprecipitated with antibody 9E10. The immunoprecipitate was phosphorylated in vitro in the absence (lane 1) or presence of a recombinant CDK-cyclin complex: CDK2-cyclin E (lane 2), CDK6-cyclin D1 (lane 3), CDK2-cyclin A (lane 4), or CDC2-cyclin B1 (lane 5). The reaction products were visualized by autoradiography following SDS-polyacrylamide gel electrophoresis and transfer to a membrane. The 200-kDa protein marker is indicated on the left. (B) Two-dimensional tryptic phosphopeptide maps derived from BRCA1 protein depicted in panel A. a, b, and c, maps of BRCA1 from lanes 1, 3, and 5, respectively. Phosphopeptides p4, pA, and pB are depicted in Fig. 4B.
FIG. 4.
An endogenous protein kinase activity as well as recombinant CDK2-cyclin complexes phosphorylate BRCA1 residues T967 and S1497 in vitro. (A) 293T cells were transiently transfected with Myc-BRCA1 that was either wild type (Wt; lanes 1 and 2) or mutated as indicated. The different BRCA1 species were immunoprecipitated with antibody 9E10 and subjected to in vitro kinase reactions in the absence (lanes 1, 3, 5, 7, and 9) or presence (lanes 2, 4, 6, 8, and 10) of the recombinant CDK2-cyclin A complex. The 200-kDa protein marker is shown on the left. (B) Two-dimensional tryptic phosphopeptide maps derived from the overexpressed BRCA1 protein species depicted in panel A. a to j, maps of BRCA1 from lanes 1, 3, 5, 2, 4, 6, 7, 9, 8, and 10, respectively. (C) Two-dimensional maps of mixtures of tryptic phosphopeptides derived from in vitro-phosphorylated and in vivo-labeled BRCA1. a, in vitro-phosphorylated BRCA1 alone; b, in vivo-labeled endogenous BRCA1 from HBL-100 cells alone; c, mixture of in vitro-phosphorylated BRCA1 and in vivo-labeled endogenous BRCA1 from HBL-100 cells; d, in vivo-labeled overexpressed BRCA1 in 293T cells alone; e, mixture of in vitro-phosphorylated BRCA1 and in vivo-labeled overexpressed BRCA1 in 293T cells. Numbered circles indicate phosphopeptides p3, p4, p9, p11, p18, p20, pA, and pB (for simplicity, only a few phosphopeptides were circled); unlabeled circles indicate the lack of the respective peptides; circles labeled by an asterisk mark phosphopeptides related to pA (see text); T (threonine) and S (serine) indicate the phosphoamino acid content of the corresponding phosphopeptide.
S1497 and T967 are in vitro substrates for an endogenous protein kinase activity and for CDK2.
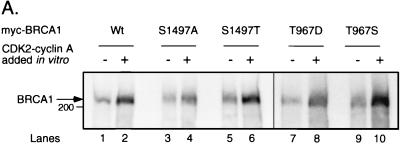
Since we have shown above by in vivo labeling experiments that the C-terminal CDK site of BRCA1 is phosphorylated, and since BRCA1 can be phosphorylated by CDK2 in vitro, we examined whether CDK2 can specifically phosphorylate S1497 in vitro. We replaced S1497 with alanine (S1497A) or threonine (S1497T) in the context of Myc-BRCA1 wt and transfected each construct into 293T cells. The Myc tag allows immunoprecipitation of the overexpressed but not endogenous BRCA1, allowing us to investigate an individual mutant without interference of the endogenous wild-type protein. The overexpressed proteins were immunoprecipitated from cell lysates by using antibody 9E10 under native conditions as mentioned above in order to coimmunoprecipitate associated protein kinases. The immunoprecipitates were subjected to in vitro kinase reactions, both in the presence and in the absence of CDK2-cyclin A. All three forms of the protein (wild type, S1497A, and S1497T) were phosphorylated by CDK2-cyclin A in vitro (Fig. 4A, lanes 1 to 6). The labeled BRCA1 species were then subjected to tryptic phosphopeptide analysis. The majority of phosphopeptides derived from in vivo-labeled BRCA1 were also detected in the case of in vitro-labeled untreated wild-type BRCA1 (data not shown), among them peptides p4 (as judged by the same relative migration of phosphopeptide p4 derived from both sources [Fig. 4C]; see below) and p20 (detected on longer autoradiographic exposures [data not shown]). For the S1497A mutant, the peptide map obtained was like that for wild-type BRCA1 except that phosphopeptides p4 and p20 were absent (Fig. 4B, map b). In the case of the S1497T mutation, PAA (72) on phosphopeptide p4 revealed that its identity had changed accordingly from phosphoserine (wild type; Fig. 4B, map a) to phosphothreonine (Fig. 4B, map c). These results demonstrate that an endogenous serine-threonine protein kinase activity coimmunoprecipitates with and phosphorylates BRCA1 at S1497 in vitro.
On the other hand, in vitro-phosphorylated BRCA1 revealed a few phosphopeptides that were not detected in a map of in vivo-labeled protein, including peptide pA (Fig. 4B, maps a to c). Tryptic hydrolysates of in vitro-phosphorylated Myc-tagged BRCA1 and in vivo-labeled endogenous BRCA1 from HBL-100 cells or overexpressed BRCA1 from 293T cells were compared by two-dimensional phosphopeptide analysis either individually (Fig. 4C, maps a, b, and d) or in combination (Fig. 4C, maps c and e). Whereas phosphopeptides p4 derived from in vitro-phosphorylated and in vivo-labeled BRCA1 run identically, pA is unique to in vitro-phosphorylated BRCA1 and runs slightly distinct from p18 derived from in vivo-labeled BRCA1 (Fig. 4C, maps c and e; note the ellipse-like spot resulting from partial overlap of pA and p18 and its migration relative to other phosphopeptides, e.g., p3, p11, and p9). As was the case for wild-type BRCA1, peptide pA derived from the S1497A and S1497T mutants consisted of phosphothreonine (Fig. 4B, maps a to c). Another phosphopeptide that appears only in maps of in vitro-phosphorylated but not in vivo-labeled BRCA1 is peptide pB (Fig. 4B and C). It contains phosphoserine (Fig. 4B, maps a and h) and is probably derived from the BRCA1 sequence from aa 772 to 1050, since pB is not present in a tryptic phosphopeptide map of an in vitro-phosphorylated BRCA1 fragment lacking this region (data not shown). To locate pA within BRCA1, we divided BRCA1 into four consecutive fragments, each of them Myc tagged (54). These fragments were expressed in 293T cells, immunoprecipitated with antibody 9E10, and subjected to in vitro kinase reactions. Phosphopeptide mapping revealed that phosphopeptide pA is derived from a BRCA1 fragment spanning aa 772 to 1314 (data not shown). Since this sequence contains the threonine CDK consensus site T(967)PNK, we hypothesized that T967 may be the phosphorylated residue that gives rise to phosphopeptide pA. We therefore mutated T967 to aspartic acid (T967D) or to serine (T967S) in the context of Myc-BRCA1 wt and analyzed these mutants as described above (Fig. 4A, lanes 7 to 10). As predicted, the T967D mutation abolished phosphopeptide pA (Fig. 4B, map g), whereas its phosphoamino acid content changed accordingly to phosphoserine in the case of the T967S mutant (Fig. 4B, map h). These results demonstrate that T967 of BRCA1 is phosphorylated in vitro (but not in vivo) by a serine-threonine protein kinase activity that coimmunoprecipitates with BRCA1.
In the case of wild-type BRCA1, treatment with CDK2-cyclin A led to an increased phosphorylation of several peptides, such as phosphopeptides p4 and p20 (Fig. 4B, map d). As for untreated BRCA1, both phosphopeptides were abolished by the S1497A mutation (Fig. 4B, map e), whereas the S1497T mutation led to a change in their phosphoamino acid identities from phosphoserine (Fig. 4B, map d) to phosphothreonine (Fig. 4B, map f). Peptide pA also displayed increased phosphorylation due to CDK2 treatment in vitro (Fig. 4B, maps d to f), but this effect was abrogated as a result of the T967D mutation (Fig. 4B, map i). Moreover, pA of the CDK2-cyclin A-treated T967S mutant consisted of phosphoserine (Fig. 4B, map j). The three spots marked with an asterisk in Fig. 4B, maps d to f, which probably represent incompletely digested tryptic fragments containing phosphopeptide pA [predicted sequence of pA is G(960)NETGLITPNK(970), where the phosphorylated T967 is underlined], since they all contain phosphothreonine (as determined for CDK2-cyclin A-treated wild-type BRCA1 [Fig. 4B, map d]), were eliminated by the T967D mutation (Fig. 4B, map i), and at least the two peptides that migrate faster in the chromatography dimension were converted to phosphoserine as a consequence of the T967S mutation (Fig. 4B, map j) (the phosphoamino acid identity of the third peptide was not determined). In conclusion, S1497 and T967 of BRCA1 are phosphorylated by CDK2-cyclin A or E in vitro.
Is CDK2 responsible for phosphorylation of BRCA1 at S1497 in vivo?
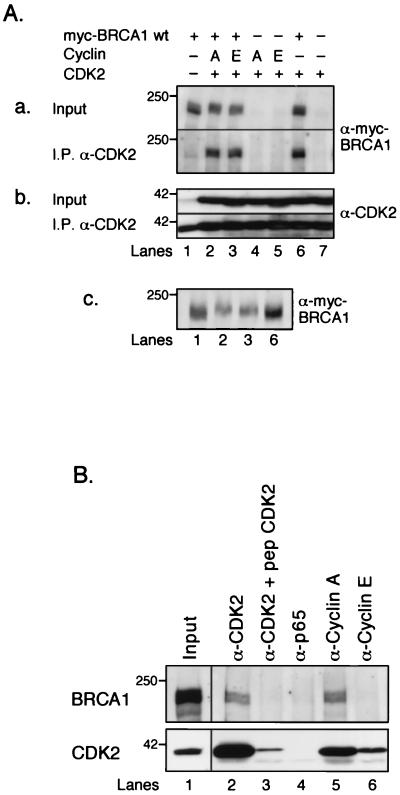
As described above, S1497 of BRCA1 is phosphorylated in vivo and can be phosphorylated by CDK2 in vitro. Is the in vivo phosphorylation due to CDK2 action? To address this question, we first tested whether BRCA1 and CDK2 interact in vivo. Myc-BRCA1 wt was overexpressed alone or in combination with CDK2 in the presence or absence of cyclin A or cyclin E in 293T cells, and cell lysates were subjected to anti-CDK2 immunoprecipitations under native conditions. As shown in Fig. 5A, parts a and b, Myc-BRCA1 wt coimmunoprecipitated with CDK2 in a manner proportional to the amount of CDK2 (e.g., more Myc-BRCA1 wt was immunoprecipitated from lysates where CDK2 was overexpressed [Fig. 5A, parts a and b, lanes 2, 3, and 6] than from a lysate where only endogenous CDK2 was present [lane 1]). These results suggest that BRCA1 and CDK2 interact. This interaction was not dependent on cotransfected cyclin A or E, presumably because the levels of endogenous cyclin A and/or E are sufficient to mediate interaction. Interestingly, when cyclin A or E was cotransfected with CDK2 (Fig. 5A, part c, lanes 2 and 3), BRCA1 migrated slightly slower than BRCA1 expressed alone or in combination with only CDK2 (lanes 1 and 6), suggesting that overexpression of CDK2-cyclin A or CDK-cyclin E led to increased phosphorylation of BRCA1. Furthermore, BRCA1 and CDK2 were coimmunoprecipitated from HeLa cells that stably express untagged, full-length wild-type BRCA1 protein (57a). As shown in Fig. 5B, BRCA1 was coimmunoprecipitated by a CDK2-specific antibody (lane 2) but not by an antibody that had been preincubated with the cognate CDK2 blocking peptide (lane 3). A p65-specific control antibody was not able to precipitate BRCA1 (lane 4). Similarly, a cyclin A-specific antibody coimmunoprecipitated BRCA1 as well as CDK2 (lane 5), suggesting that the CDK2-cyclin A complex interacts with BRCA1 in vivo. Under our experimental conditions, a cyclin E-specific antibody was able to coimmunoprecipitate CDK2 but not BRCA1 (lane 6). Whether this means that CDK2-cyclin E does not interact with BRCA1 in vivo remains to be investigated.
FIG. 5.
BRCA1 coimmunoprecipitates with CDK2 and cyclin A. (A) Myc-BRCA1 wt coimmunoprecipitates with CDK2. 293T cells were transfected with expression plasmids for the following proteins: lane 1, Myc-BRCA1 wt; lane 2, Myc-BRCA1 wt, cyclin A, and CDK2; lane 3, Myc-BRCA1 wt, cyclin E, and CDK2; lane 4, cyclin A and CDK2; lane 5, cyclin E and CDK2; lane 6, Myc-BRCA1 wt and CDK2; and lane 7, CDK2. CDK2-containing complexes were immunoprecipitated (I.P.) from each sample with a rabbit CDK2 antibody (α-CDK2) and analyzed after separation on SDS-polyacrylamide gels by Western blot analysis for the presence of Myc-BRCA1 wt, using antibody 9E10 (a, bottom row), and CDK2, using a mouse anti-CDK2 monoclonal antibody (b, bottom row). The two top rows in panels a and b (Input) represent the relative amounts of Myc-BRCA1 wt and CDK2 in the cell lysates before immunoprecipitation. Panel c shows that in a duplicate experiment, Myc-BRCA1 wt from cell lysates overexpressing proteins as indicated in lanes 1, 2, 3, and 6 (in panels, a and b) was further resolved on an SDS-polyacrylamide gel (compared to panel a, top row) in order to increase resolution. (B) Untagged, full-length wild-type BRCA1 coimmunoprecipitates with CDK2 and cyclin A. Lysates from a HeLa cell line stably overexpressing BRCA1 (57a) were subjected to immunoprecipitations using the following antibodies: lane 1, an aliquot before immunoprecipitation; lane 2, anti-CDK2; lane 3, anti-CDK2, after preincubation with the blocking peptide; lane 4, anti-NF-κB p65 as a control; lane 5, anti-cyclin A; and lane 6, anti-cyclin E. Western blot analysis revealed the presence of BRCA1 and CDK2, as shown in the upper and lower panels, respectively. The 250- and 42-kDa markers are indicated on the left.
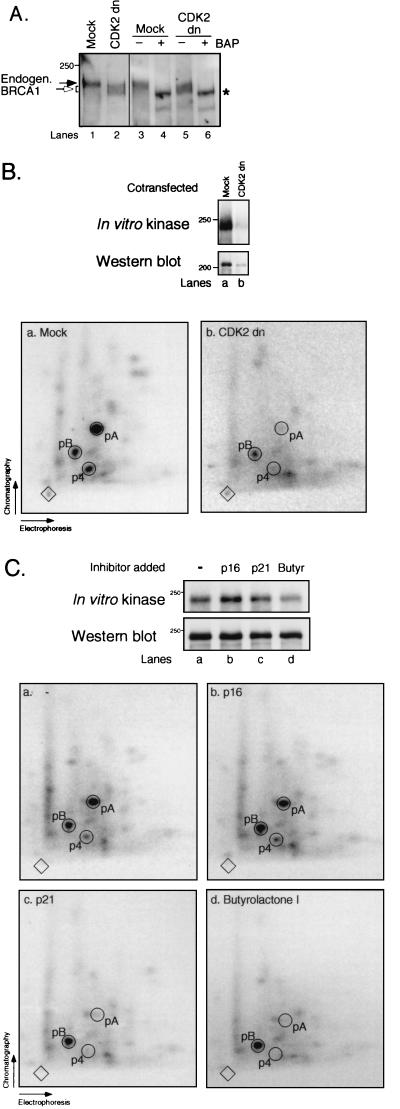
To test the effect of CDK2 on BRCA1 phosphorylation in vivo, we transiently overexpressed CDK2 dn (with an Asp145→Asn mutation that renders the kinase inactive but does not affect its ability to associate with cyclins [71]) in 293T cells and measured the phosphorylation state of endogenous BRCA1 by Western blot analysis. CDK2 dn caused a decrease in BRCA1 phosphorylation compared to the mock-transfected sample, as judged by an increase in BRCA1 mobility (Fig. 6A, lanes 1 and 2). BRCA1 from both sources was then immunoprecipitated and treated with BAP as previously described (57). Phosphatase treatment led to a BRCA1 species with increased mobility in both cases (compare lanes 3 and 4 and lanes 5 and 6), showing that the CDK2 dn had caused partial BRCA1 dephosphorylation. These results demonstrate that inhibition of CDK2 activity leads to a decrease in BRCA1 phosphorylation. Furthermore, we cotransfected a Myc-tagged BRCA1 expression plasmid with either CDK2 dn or a control plasmid into 293T cells. Antibody 9E10 was used to immunoprecipitate BRCA1, and the immunoprecipitates were subjected to in vitro kinase reactions as described above. Expression of CDK2 dn led to decreased levels of cotransfected Myc-BRCA1 wt protein compared to expression of Myc-BRCA1 wt alone (Fig. 6B, top, lanes a and b). Importantly, peptide mapping revealed that the intensities of spots p4 and pA were markedly reduced relative to other phosphopeptides by the CDK2 dn, e.g., pB (Fig. 6B, maps a and b), suggesting that the level of the associated in vitro protein kinase activity was decreased by the CDK2 dn.
FIG. 6.
Phosphorylation of BRCA1 is decreased by inhibition of CDK2. (A) CDK2 dn decreases the phosphorylation state of endogenous BRCA1. 293T cells were transiently transfected with a control plasmid (lane 1) or a plasmid expressing CDK2 dn (lane 2). Endogenous BRCA1 from both sources was immunoprecipitated with Ab-D prior to treatment with (lanes 4 and 6) or without (lanes 3 and 5) BAP before analysis by immunoblotting with Ab-D. Filled arrow, BRCA1 from mock-transfected cells; open arrow, faster-migrating BRCA1 species due to expression of CDK2 dn; asterisk, BAP-treated BRCA1. (B) BRCA1 is phosphorylated in vitro by a cellular kinase activity that is sensitive to CDK2 dn. 293T cells were cotransfected with a plasmid expressing Myc-BRCA1 wt and either a control plasmid (lane a) or CDK2 dn (lane b). BRCA1 was immunoprecipitated under native conditions with antibody 9E10, and in vitro kinase reactions were performed on the immunoprecipitates. a and b, two-dimensional tryptic phosphopeptide maps of the BRCA1 protein species shown in lanes a and b, respectively, from a duplicate experiment. (C) p21 and butyrolactone I but not p16 inhibit the endogenous protein kinase activity that phosphorylates BRCA1 at T967 and S1497 in vitro. Myc-BRCA1 wt was subjected to in vitro kinase reactions as described above. Lane a, no CDI added; lanes b, c, and d, p16, p21, and butyrolactone I (Butyr), respectively, added. a to d, phosphopeptide maps of BRCA1 protein shown in lanes a to d, respectively. The top portions of panels B and C represent the amount of (Western blot analysis using antibody 9E10 [bottom rows]) and the in vitro kinase activity on (radioactive incorporation onto BRCA1 [top rows]) BRCA1. The 250- and 200-kDa markers are shown on the left.
The above results are consistent with a role of CDK2 in phosphorylating BRCA1 in vivo. However, they do not prove that CDK2 directly phosphorylates BRCA1, since CDK2 dn blocks cells in the G1 phase of the cell cycle (71). Therefore, the decrease in BRCA1 phosphorylation could be due to an effect of the cell cycle block on BRCA1 rather than to the mutant CDK2 directly. We therefore performed in vitro kinase reactions on Myc-BRCA1 wt immunoprecipitated from lysates of transiently transfected 293T cells, in the presence of the CDI p16 or p21 or in the presence of butyrolactone I, which is an inhibitor of CDC2, CDK2, and CDK5 but not CDK4 and CDK6 (43). As shown in Fig. 6C, p21 and butyrolactone I but not p16 decreased the relative amount of phosphopeptides p4 and pA compared to mock-treated BRCA1. These results suggest that the cellular protein kinase activity that phosphorylates BRCA1 at S1497 and T967 in vitro is in fact CDK2 and that CDK2 phosphorylates BRCA1 at S1497 in vivo.
DISCUSSION
The results presented here demonstrate that S1497 of BRCA1 is phosphorylated in vivo. They further establish BRCA1 as an in vivo substrate of CDK2-cyclin complexes, by virtue of at least partial fulfillment of the following criteria, as proposed elsewhere (48). First, BRCA1 is phosphorylated by CDK2-cyclin A or CDK2-cyclin E in vitro. Second, as previously reported by us and others, BRCA1 is phosphorylated in vivo, and the G1/S-specific increase in phosphorylation is consistent with the cell cycle-dependent activation of CDK2-cyclin complexes. Third, S1497 is phosphorylated in vivo and by CDK2-cyclin A or E in vitro. An endogenous serine-threonine protein kinase activity that is sensitive to p21, to butyrolactone I, and to a dominant negative form of CDK2 phosphorylates BRCA1 at S1497 in vitro. The fourth condition, that S1497 phosphorylation should change the properties of BRCA1 in a way consistent with a corresponding G1/S-phase event, cannot be addressed yet since the molecular function of BRCA1 is unknown. However, determining the cellular effects of expressing the mutant forms S1497A (which cannot be phosphorylated) and S1497D or S1497E (the negative charge may mimic the phosphate moiety on the serine residue) may provide insights into normal BRCA1 function during cell cycle progression. We are currently investigating these mutants toward this end.
Phosphorylation of S1497 by CDK2-cyclin complexes is concordant with increased phosphorylation of BRCA1 in late G1 and S phases. We propose that CDK2-cyclin E and/or CDK2-cyclin A phosphorylate BRCA1 during late G1 and/or S phase, respectively. However, we cannot rule out the possibility that other protein kinases also phosphorylate BRCA1 at the G1/S transition, since we have previously detected that BRCA1 undergoes extensive hyperphosphorylation during this cell cycle phase (57). For S1497 to be solely responsible for the observed BRCA1 hyperphosphorylation at G1/S, one would expect that the sum of phosphorylation at all the other phosphorylated sites is comparably marginal. We have not formally demonstrated by 32P metabolic labeling experiments that phosphorylation of S1497 increases relative to that of other phosphorylation sites during this phase, since our standard labeling conditions themselves induce increased phosphorylation of BRCA1 (possibly due to 32P-induced DNA damage) and would therefore interfere with cell cycle-specific phosphorylation events (57a). Alternatively, we could generate antibodies that specifically recognize phospho-S1497 or phospho-T967 to study cell cycle-specific phosphorylation by using nonradioactive methods. We are currently generating inducible cell lines that express the full-length BRCA1 mutants S1497A and T967D in a regulated manner; these cell lines will be useful to study the effect of the lack of these phosphorylation sites on the overall G1/S-specific phosphorylation of BRCA1.
Two previous reports had shown that BRCA1 associates with endogenous CDK2 and cyclin A but not with cyclin E in lysates of HBL-100 or CAL-51 (a human breast cancer cell line) cells (16, 74), in agreement with our data obtained from the BRCA1-overexpressing HeLa cell line. These findings and our in vitro phosphorylation studies suggest that BRCA1 is a substrate for CDK2-cyclin A (but possibly not for CDK2-cyclin E) in vivo. Moreover, both groups provided data showing that BRCA1 also interacts with cyclin D (cyclin D1), suggesting that BRCA1 is phosphorylated by a kinase(s) associated with cyclin D. At least in vitro, CDK6-cyclin D1 was able to only weakly phosphorylate BRCA1 (Fig. 3). The same was true for CDC2-cyclin B1, in agreement with the notion that BRCA1 is not phosphorylated by CDC2 or kinases associated with cyclin B in lysates from HBL-100 cells (16). Nevertheless, CDK6-cyclin D1, CDC2-cyclin B1, and other CDK-cyclin complexes could possibly phosphorylate BRCA1 in vivo, at least in certain cell types. For example, in lysates of CAL-51 cells, BRCA1 has been reported to associate with cyclin B1 and CDC2 (74). Other kinases may also be involved in phosphorylating BRCA1. Two groups have reported that BRCA1 is a tyrosine phosphoprotein (74, 79). However, our previous studies revealed that BRCA1 is predominantly phosphorylated on serine and threonine residues, at least in the cell lines analyzed (57). Furthermore, a kinase activity that associates with and phosphorylates a BRCA1 fragment containing aa 329 to 435 in vitro was identified (9).
What is the consequence of phosphorylating S1497 on BRCA1? The addition or removal of the phosphate group may affect the interaction with other proteins. So far, no protein that interacts with BRCA1 in the domain encompassing S1497 has been identified. Two proteins that may be considered as candidates are CBP and BRCA2, which interact with BRCA1 sequences from aa 1314 to 1863 and 1314 to 1756, respectively (13, 54). However, in vitro CBP binding occurs irrespective of BRCA1 phosphorylation (54). Whether the same is true for BRCA2 is now under investigation, in particular with regard to S1497 phosphorylation. It is also possible that a change in phosphorylation leads to a change in BRCA1’s subnuclear localization, which changes in response to cell cycle progression (from G1 to S phase) and DNA damage, two events that cause phosphorylation of BRCA1. Alternatively, the S1497 phosphorylation state may regulate a so far undetected intrinsic activity of BRCA1, possibly by modulating the protein’s secondary structure.
The fact that the SPXK motif (where the S is S1497 in human BRCA1) is conserved among human, mouse, and rat BRCA1 proteins underlines the functional importance of this motif (1, 5) (in dogs, SP but not the K is conserved [66]). However, no tumor-associated mutation has been found at either of these residues (8). A mutation in close proximity has been compiled as an unclassified variant, where a conserved arginine is changed to methionine (R1495M [8]). If this mutation turns out to be associated with cancer development, one could investigate whether R1495 influences phosphorylation of the neighboring CDK site, such as by determining the specificity for CDK2-cyclin complexes. On the other hand, the TPNK motif (where the T is T967 in human BRCA1) is not conserved, and no mutations have yet been identified either within this motif or at conserved amino acids in the vicinity (1, 5, 8, 66). Consistently, in vivo labeling has not revealed T967 phosphorylation of either endogenous or overexpressed BRCA1 (Fig. 1 and 4C). However, T967 is phosphorylated in vitro by a cellular serine-threonine protein kinase activity. Why is T967 phosphorylation detected in vitro but not in vivo? One explanation is that T967 is not in fact phosphorylated in vivo but rather is phosphorylated in vitro by the same protein kinase activity that is associated with S1497. This scenario would require that phosphorylation of T967 in vivo is impaired by BRCA1’s secondary structure, or a protein that masks this site, and that these constraints are lost upon cell lysis and/or immunoprecipitation in vitro kinase procedures. Consequently, both S1497 and T967 are excellent substrates for CDK2-cyclin complexes in vitro. Alternatively, phosphorylation of T967 in vivo could occur very transiently or at a low stoichiometric level or turnover rate and would therefore escape detection by in vivo labeling.
The results presented above are consistent with a direct action of CDK2-cyclin complexes on BRCA1. We note, however, that it cannot be ruled out that CDK2 and BRCA1 exist in a complex with another serine-threonine protein kinase which is phosphorylated by CDK2 and in turn phosphorylates BRCA1, as opposed to a direct action of CDK2 on BRCA1. A subset of CDK-cyclin substrates contain an RXL (L designates leucine) motif that is required for docking of the CDK-cyclin complex via the cyclin and for phosphorylation (2, 58). BRCA1 contains nine such motifs, seven in the N-terminal half and two at the C terminus (aa 1699 to 1701 and 1762 to 1764), which may be critical for CDK2-cyclin A or E-mediated phosphorylation of endogenous BRCA1. The fact that S1497 was also phosphorylated in the overexpressed BRCA1 fragment 1314-1652 (which contains no RXL motif) suggests either that S1497 phosphorylation of endogenous BRCA1 occurs in an RXL-independent manner or that overexpressed BRCA1 does not rely on the RXL motif due to an increased substrate to CDK2-cyclin ratio. Furthermore, it is possible that other protein kinases contribute to the phosphorylation of S1497 in vivo, for example, mitogen-activated protein (MAP) kinases. MAP kinases and CDKs display similar proline-directed substrate specificities (48), although MAP kinases generally require an L or P and an intervening residue preceding S/T-P (consensus L/P-X-S/T-P) and are therefore probably not involved in S1497 phosphorylation.
In summary, we present evidence that BRCA1 is a physiological substrate of CDK2-cyclin complexes. Elucidation of the functional consequences of S1497 phosphorylation will shed light on the role that BRCA1 plays during cell cycle progression and may help in understanding why cells become cancerous in the absence of the functional protein.
ACKNOWLEDGMENTS
We thank Tim Mayall for providing recombinant p16 and p21, Nik Somia for providing pCL-MFG-MCS and for excellent suggestions, Jill Meisenhelder for providing 9E10 antibody and for technical advice, Mirta Grifman and Matthew Weitzman for the pRK5-cyclin A expression construct, Lamya Shihabuddin for technical advice, and Brian Spain, Chris Larson, Tal Kafri, and other members of the Verma laboratory for valuable discussions. We thank Jean E. Rivier for his interest in our work.
H.R. is supported by consecutive funds from the Schweizerische Nationalfonds für wissenschaftliche Forschung, grant 823A-046698, and the California Breast Cancer Research Program of the University of California, grant 4FB-0102. W.J. is supported by a postdoctoral fellowship from the American Cancer Society. I.M.V., T.H., and A.G.C. are supported by grants from the National Institutes of Health. I.M.V. is an American Cancer Society Professor of Molecular Biology, and he is also supported by the Elsa Pardee Foundation. T.H. is a Frank and Else Schilling American Cancer Society Research Professor.
REFERENCES
- 1.Abel K J, Xu J, Yin G Y, Lyons R H, Meisler M H, Weber B L. Mouse Brca1: localization, sequence analysis and identification of evolutionarily conserved domains. Hum Mol Genet. 1995;4:2265–2273. doi: 10.1093/hmg/4.12.2265. [DOI] [PubMed] [Google Scholar]
- 2.Adams P D, Sellers W R, Sharma S K, Wu A D, Nalin C M, Kaelin W G., Jr Identification of a cyclin-cdk2 recognition motif present in substrates and p21-like cyclin-dependent kinase inhibitors. Mol Cell Biol. 1996;16:6623–6633. doi: 10.1128/mcb.16.12.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson S F, Schlegel B P, Nakajima T, Wolpin E S, Parvin J D. BRCA1 protein is linked to the RNA polymerase II holoenzyme complex via RNA helicase A. Nat Genet. 1998;19:254–256. doi: 10.1038/930. [DOI] [PubMed] [Google Scholar]
- 4.Beijersbergen R L, Bernards R. Cell cycle regulation by the retinoblastoma family of growth inhibitory proteins. Biochim Biophys Acta. 1996;1287:103–120. doi: 10.1016/0304-419x(96)00002-9. [DOI] [PubMed] [Google Scholar]
- 5.Bennett L M, Brownlee H A, Hagevik S, Haugen-Strano A, Wiseman R W. Evolutionarily conserved domains of the rat BRCA1 protein. Proc Annu Meet Am Assoc Cancer Res. 1996;37:514–515. [Google Scholar]
- 6.Bork P, Hofmann K, Bucher P, Neuwald A F, Altschul S F, Koonin E V. A superfamily of conserved domains in DNA damage-responsive cell cycle checkpoint proteins. FASEB J. 1997;11:68–76. [PubMed] [Google Scholar]
- 7.Boyle W J, van der Geer P, Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 1991;201:110–149. doi: 10.1016/0076-6879(91)01013-r. [DOI] [PubMed] [Google Scholar]
- 8.Breast cancer information core. 12 March 1999, revision date. www.nhgri.nih.gov/Intramural_research/Lab_transfer/bic/. [Online.] [5 May 1999, last date accessed.]
- 9.Burke T F, Cocke K S, Lemke S J, Angleton E, Becker G W, Beckmann R P. Identification of a BRCA1-associated kinase with potential biological relevance. Oncogene. 1998;16:1031–1040. doi: 10.1038/sj.onc.1201623. [DOI] [PubMed] [Google Scholar]
- 10.Callebaut I, Mornon J P. From BRCA1 to RAP1: a widespread BRCT module closely associated with DNA repair. FEBS Lett. 1997;400:25–30. doi: 10.1016/s0014-5793(96)01312-9. [DOI] [PubMed] [Google Scholar]
- 11.Chapman M S, Verma I M. Transcriptional activation by BRCA1. Nature. 1996;382:678–679. doi: 10.1038/382678a0. [DOI] [PubMed] [Google Scholar]
- 12.Chen C F, Li S, Chen Y, Chen P L, Sharp Z D, Lee W H. The nuclear localization sequences of the BRCA1 protein interact with the importin-alpha subunit of the nuclear transport signal receptor. J Biol Chem. 1996;271:32863–32868. doi: 10.1074/jbc.271.51.32863. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Silver D P, Walpita D, Cantor S B, Gazdar A F, Tomlinson G, Couch F J, Weber B L, Ashley T, Livingston D M, Scully R. Stable interaction between the products of the BRCA1 and BRCA2 tumor suppressor genes in mitotic and meiotic cells. Mol Cell. 1998;2:317–328. doi: 10.1016/s1097-2765(00)80276-2. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Chen C F, Riley D J, Allred D C, Chen P L, Von Hoff D, Osborne C K, Lee W H. Aberrant subcellular localization of BRCA1 in breast cancer. Science. 1995;270:789–791. doi: 10.1126/science.270.5237.789. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Chen P-L, Riley D J, Lee W-H, Allred D C, Osborne C K. Location of BRCA1 in human breast and ovarian cancer cells. Science. 1996;272:125–126. doi: 10.1126/science.272.5258.125. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Farmer A A, Chen C F, Jones D C, Chen P L, Lee W H. BRCA1 is a 220-kDa nuclear phosphoprotein that is expressed and phosphorylated in a cell cycle-dependent manner. Cancer Res. 1996;56:3168–3172. [PubMed] [Google Scholar]
- 17.Claus E, Risch N, Thompson W. Genetic analysis of breast cancer in the cancer and steroid hormone study. Am J Hum Genet. 1991;48:232–242. [PMC free article] [PubMed] [Google Scholar]
- 18.Easton D F, Bishop D T, Ford D, Crockford G P The Breast Cancer Linkage Consortium. Genetic linkage analysis in familial breast and ovarian cancer: results from 214 families. Am J Hum Genet. 1993;52:678–701. [PMC free article] [PubMed] [Google Scholar]
- 19.Fisher R P. CDKs and cyclins in transition(s) Curr Opin Genet Dev. 1997;7:32–38. doi: 10.1016/s0959-437x(97)80106-2. [DOI] [PubMed] [Google Scholar]
- 20.Futreal P A, Liu Q, Shattuck-Eidens D, Cochran C, Harshman K, Tavtigian S, Bennett L M, Haugen-Strano A, Swensen J, Miki Y, et al. BRCA1 mutations in primary breast and ovarian carcinomas. Science. 1994;266:120–122. doi: 10.1126/science.7939630. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Patino E, Gomendio B, Provencio M, Silva J M, Garcia J M, Espana P, Bonilla F. Germ-line BRCA1 mutations in women with sporadic breast cancer: clinical correlations. J Clin Oncol. 1998;16:115–120. doi: 10.1200/JCO.1998.16.1.115. [DOI] [PubMed] [Google Scholar]
- 22.Gottlieb T M, Oren M. p53 in growth control and neoplasia. Biochim Biophys Acta. 1996;1287:77–102. doi: 10.1016/0304-419x(95)00019-c. [DOI] [PubMed] [Google Scholar]
- 23.Gowen L C, Avrutskaya A V, Latour A M, Koller B H, Leadon S A. BRCA1 required for transcription-coupled repair of oxidative DNA damage. Science. 1998;281:1009–1012. doi: 10.1126/science.281.5379.1009. [DOI] [PubMed] [Google Scholar]
- 24.Gowen L C, Johnson B L, Latour A M, Sulik K K, Koller B H. Brca1 deficiency results in early embryonic lethality characterized by neuroepithelial abnormalities. Nat Genet. 1996;12:191–194. doi: 10.1038/ng0296-191. [DOI] [PubMed] [Google Scholar]
- 25.Greenman J, Mohammed S, Ellis D, Watts S, Scott G, Izatt L, Barnes D, Solomon E, Hodgson S, Mathew C. Identification of missense and truncating mutations in the BRCA1 gene in sporadic and familial breast and ovarian cancer. Genes Chromosomes Cancer. 1998;21:244–249. [PubMed] [Google Scholar]
- 25a.Grifman, M., and M. Weitzman (The Salk Institute). Unpublished data.
- 26.Gudas J M, Li T, Nguyen H, Jensen D, Rauscher III F J, Cowan K H. Cell cycle regulation of BRCA1 messenger RNA in human breast epithelial cells. Cell Growth Differ. 1996;7:717–723. [PubMed] [Google Scholar]
- 27.Hakem R, de la Pompa J L, Sirard C, Mo R, Woo M, Hakem A, Wakeham A, Potter J, Reitmair A, Billia F, Firpo E, Hui C C, Roberts J, Rossant J, Mak T W. The tumor suppressor gene Brca1 is required for embryonic cellular proliferation in the mouse. Cell. 1996;85:1009–1023. doi: 10.1016/s0092-8674(00)81302-1. [DOI] [PubMed] [Google Scholar]
- 28.Harper J W, Elledge S J. Cdk inhibitors in development and cancer. Curr Opin Genet Dev. 1996;6:56–64. doi: 10.1016/s0959-437x(96)90011-8. [DOI] [PubMed] [Google Scholar]
- 29.Hunter T, Pines J. Cyclins and cancer II: cyclin D and CDK inhibitors come of age. Cell. 1994;79:573–582. doi: 10.1016/0092-8674(94)90543-6. [DOI] [PubMed] [Google Scholar]
- 29a.Jiang, W. Unpublished data.
- 30.Jiang W, Jimenez G, Wells N J, Hope T J, Wahl G M, Hunter T, Fukunaga R. PRC1: a human mitotic spindle-associated CDK substrate protein required for cytokinesis. Mol Cell. 1998;2:877–885. doi: 10.1016/s1097-2765(00)80302-0. [DOI] [PubMed] [Google Scholar]
- 31.Kanemitsu M Y, Jiang W, Eckhart W. Cdc2-mediated phosphorylation of the gap junction protein, connexin43, during mitosis. Cell Growth Differ. 1998;9:13–21. [PubMed] [Google Scholar]
- 32.Kelsell D P, Black D M, Bishop D T, Spurr N K. Genetic analysis of the BRCA1 region in a large breast/ovarian family: refinement of the minimal region containing BRCA1. Hum Mol Genet. 1993;2:1823–1828. doi: 10.1093/hmg/2.11.1823. [DOI] [PubMed] [Google Scholar]
- 33.Kelsell D P, Spurr N K, Barnes D M, Gusterson B, Bishop D T. Combined loss of BRCA1/BRCA2 in grade 3 breast carcinomas. Lancet. 1996;347:1554–1555. doi: 10.1016/s0140-6736(96)90707-2. [DOI] [PubMed] [Google Scholar]
- 34.Kerangueven F, Eisinger F, Noguchi T, Allione F, Wargniez V, Eng C, Padberg G, Theillet C, Jacquemier J, Longy M, Sobol H, Birnbaum D. Loss of heterozygosity in human breast carcinomas in the ataxia telangiectasia, Cowden disease and BRCA1 gene regions. Oncogene. 1997;14:339–347. doi: 10.1038/sj.onc.1200818. [DOI] [PubMed] [Google Scholar]
- 35.Kinzler K W, Vogelstein B. Cancer-susceptibility genes. Gatekeepers and caretakers. Nature. 1997;386:761–763. doi: 10.1038/386761a0. [DOI] [PubMed] [Google Scholar]
- 36.Koonin E V, Altschul S F, Bork P. BRCA1 protein products... functional motifs.. Nat Genet. 1996;13:266–268. doi: 10.1038/ng0796-266. [DOI] [PubMed] [Google Scholar]
- 37.Lane T F, Deng C, Elson A, Lyu M S, Kozak C A, Leder P. Expression of Brca1 is associated with terminal differentiation of ectodermally and mesodermally derived tissues in mice. Genes Dev. 1995;9:2712–2722. doi: 10.1101/gad.9.21.2712. [DOI] [PubMed] [Google Scholar]
- 38.Langston A A, Malone K E, Thompson J D, Daling J R, Ostrander E A. BRCA1 mutations in a population-based sample of young women with breast cancer. N Engl J Med. 1996;334:137–142. doi: 10.1056/NEJM199601183340301. [DOI] [PubMed] [Google Scholar]
- 39.Lees E. Cyclin dependent kinase regulation. Curr Opin Cell Biol. 1995;7:773–780. doi: 10.1016/0955-0674(95)80060-3. [DOI] [PubMed] [Google Scholar]
- 40.Lewin B. Driving the cell cycle: M phase kinase, its partners, and substrates. Cell. 1990;61:743–752. doi: 10.1016/0092-8674(90)90181-d. [DOI] [PubMed] [Google Scholar]
- 41.Liu C Y, Flesken-Nikitin A, Li S, Zeng Y, Lee W H. Inactivation of the mouse Brca1 gene leads to failure in the morphogenesis of the egg cylinder in early postimplantation development. Genes Dev. 1996;10:1835–1843. doi: 10.1101/gad.10.14.1835. [DOI] [PubMed] [Google Scholar]
- 42.Marquis S T, Rajan J V, Wynshaw-Boris A, Xu J, Yin G Y, Abel K J, Weber B L, Chodosh L A. The developmental pattern of Brca1 expression implies a role in differentiation of the breast and other tissues. Nat Genet. 1995;11:17–26. doi: 10.1038/ng0995-17. [DOI] [PubMed] [Google Scholar]
- 43.Meijer L. Chemical inhibitors of cyclin-dependent kinases. Trends Cell Biol. 1996;6:393–397. doi: 10.1016/0962-8924(96)10034-9. [DOI] [PubMed] [Google Scholar]
- 44.Merajver S D, Pham T M, Caduff R F, Chen M, Poy E L, Cooney K A, Weber B L, Collins F S, Johnston C, Frank T S. Somatic mutations in the BRCA1 gene in sporadic ovarian tumours. Nat Genet. 1995;9:439–443. doi: 10.1038/ng0495-439. [DOI] [PubMed] [Google Scholar]
- 45.Miki Y, Swensen J, Shattuck-Eidens D, Futreal P A, Harshman K, Tavtigian S, Liu Q, Cochran C, Bennett L M, Ding W, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- 46.Monteiro A N, August A, Hanafusa H. Evidence for a transcriptional activation function of BRCA1 C-terminal region. Proc Natl Acad Sci USA. 1996;93:13595–13599. doi: 10.1073/pnas.93.24.13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neuhausen S L, Marshall C J. Loss of heterozygosity in familial tumors from three BRCA1-linked kindreds. Cancer Res. 1994;54:6069–6072. [PubMed] [Google Scholar]
- 48.Nigg E A. Cellular substrates of p34cdc2 and its companion cyclin-dependent kinases. Trends Cell Biol. 1993;3:296–301. doi: 10.1016/0962-8924(93)90011-o. [DOI] [PubMed] [Google Scholar]
- 49.Nigg E A. Cyclin-dependent protein kinases: key regulators of the eukaryotic cell cycle. Bioessays. 1995;17:471–480. doi: 10.1002/bies.950170603. [DOI] [PubMed] [Google Scholar]
- 50.Norbury C, Nurse P. Animal cell cycles and their control. Annu Rev Biochem. 1992;61:441–470. doi: 10.1146/annurev.bi.61.070192.002301. [DOI] [PubMed] [Google Scholar]
- 51.Orend G, Hunter T, Ruoslahti E. Cytoplasmic displacement of cyclin E-cdk2 inhibitors p21Cip1 and p27Kip1 in anchorage-independent cells. Oncogene. 1998;16:2575–2583. doi: 10.1038/sj.onc.1201791. [DOI] [PubMed] [Google Scholar]
- 52.Ouchi T, Monteiro A N, August A, Aaronson S A, Hanafusa H. BRCA1 regulates p53-dependent gene expression. Proc Natl Acad Sci USA. 1998;95:2302–2306. doi: 10.1073/pnas.95.5.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ozcelik H, To M D, Couture J, Bull S B, Andrulis I L. Preferential allelic expression can lead to reduced expression of BRCA1 in sporadic breast cancers. Int J Cancer. 1998;77:1–6. doi: 10.1002/(sici)1097-0215(19980703)77:1<1::aid-ijc1>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 54.Pao, G., R. Janknecht, H. Ruffner, T. Hunter, and I. M. Verma. CBP/p300 interact functionally with BRCA1 as transcriptional co-activators. Submitted for publication.
- 55.Papa S, Seripa D, Merla G, Gravina C, Giai M, Sismondi P, Rinaldi M, Serra A, Saglio G, Fazio V M. Identification of a possible somatic BRCA1 mutation affecting translation efficiency in an early-onset sporadic breast cancer patient. J Natl Cancer Inst. 1998;90:1011–1012. doi: 10.1093/jnci/90.13.1011. [DOI] [PubMed] [Google Scholar]
- 56.Pines J. Cyclins and cyclin-dependent kinases: take your partners. Trends Biochem Sci. 1993;18:195–197. doi: 10.1016/0968-0004(93)90185-p. [DOI] [PubMed] [Google Scholar]
- 57.Ruffner H, Verma I M. BRCA1 is a cell cycle-regulated nuclear phosphoprotein. Proc Natl Acad Sci USA. 1997;94:7138–7143. doi: 10.1073/pnas.94.14.7138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57a.Ruffner, H. Unpublished data.
- 58.Schulman B A, Lindstrom D L, Harlow E. Substrate recruitment to cyclin-dependent kinase 2 by a multipurpose docking site on cyclin A. Proc Natl Acad Sci USA. 1998;95:10453–10458. doi: 10.1073/pnas.95.18.10453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scully R, Anderson S F, Chao D M, Wei W, Ye L, Young R A, Livingston D M, Parvin J D. BRCA1 is a component of the RNA polymerase II holoenzyme. Proc Natl Acad Sci USA. 1997;94:5605–5610. doi: 10.1073/pnas.94.11.5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scully R, Chen J, Ochs R L, Keegan K, Hoekstra M, Feunteun J, Livingston D M. Dynamic changes of BRCA1 subnuclear location and phosphorylation state are initiated by DNA damage. Cell. 1997;90:425–435. doi: 10.1016/s0092-8674(00)80503-6. [DOI] [PubMed] [Google Scholar]
- 61.Scully R, Chen J, Plug A, Xiao Y, Weaver D, Feunteun J, Ashley T, Livingston D M. Association of BRCA1 with Rad51 in mitotic and meiotic cells. Cell. 1997;88:265–275. doi: 10.1016/s0092-8674(00)81847-4. [DOI] [PubMed] [Google Scholar]
- 62.Scully R, Ganesan S, Brown M, De Caprio J A, Cannistra S A, Feunteun J, Schnitt S, Livingston D M. Location of BRCA1 in human breast and ovarian cancer cells. Science. 1996;272:123–125. doi: 10.1126/science.272.5258.123. [DOI] [PubMed] [Google Scholar]
- 63.Shao N, Chai Y L, Shyam E, Reddy P, Rao V N. Induction of apoptosis by the tumor suppressor protein BRCA1. Oncogene. 1996;13:1–7. [PubMed] [Google Scholar]
- 64.Somasundaram K, Zhang H, Zeng Y X, Houvras Y, Peng Y, Wu G S, Licht J D, Weber B L, El-Deiry W S. Arrest of the cell cycle by the tumour-suppressor BRCA1 requires the CDK-inhibitor p21WAF1/CiP1. Nature. 1997;389:187–190. doi: 10.1038/38291. [DOI] [PubMed] [Google Scholar]
- 64a.Somia, N. Unpublished data.
- 65.Sourvinos G, Spandidos D A. Decreased BRCA1 expression levels may arrest the cell cycle through activation of p53 checkpoint in human sporadic breast tumors. Biochem Biophys Res Commun. 1998;245:75–80. doi: 10.1006/bbrc.1998.8379. [DOI] [PubMed] [Google Scholar]
- 66.Szabo C I, Wagner L A, Francisco L V, Roach J C, Argonza R, King M C, Ostrander E A. Human, canine and murine BRCA1 genes: sequence comparison among species. Hum Mol Genet. 1996;5:1289–1298. doi: 10.1093/hmg/5.9.1289. [DOI] [PubMed] [Google Scholar]
- 67.Thakur S, Zhang H B, Peng Y, Le H, Carroll B, Ward T, Yao J, Farid L M, Couch F J, Wilson R B, Weber B L. Localization of BRCA1 and a splice variant identifies the nuclear localization signal. Mol Cell Biol. 1997;17:444–452. doi: 10.1128/mcb.17.1.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thomas J E, Smith M, Rubinfeld B, Gutowski M, Beckmann R P, Polakis P. Subcellular localization and analysis of apparent 180-kDa and 220-kDa proteins of the breast cancer susceptibility gene, BRCA1. J Biol Chem. 1996;271:28630–28635. doi: 10.1074/jbc.271.45.28630. [DOI] [PubMed] [Google Scholar]
- 69.Thomas J E, Smith M, Tonkinson J L, Rubinfeld B, Polakis P. Induction of phosphorylation on BRCA1 during the cell cycle and after DNA damage. Cell Growth Differ. 1997;8:801–809. [PubMed] [Google Scholar]
- 70.Thompson M E, Jensen R A, Obermiller P S, Page D L, Holt J T. Decreased expression of BRCA1 accelerates growth and is often present during sporadic breast cancer progression. Nat Genet. 1995;9:444–450. doi: 10.1038/ng0495-444. [DOI] [PubMed] [Google Scholar]
- 71.van den Heuvel S, Harlow E. Distinct roles for cyclin-dependent kinases in cell cycle control. Science. 1993;262:2050–2054. doi: 10.1126/science.8266103. [DOI] [PubMed] [Google Scholar]
- 72.van der Geer P, Luo K, Sefton B M, Hunter T. Phosphopeptide mapping and phosphoamino acid analysis on cellulose thin-layer plates. In: Celis J E, editor. Cell biology: a laboratory handbook. New York, N.Y: Academic Press; 1994. pp. 422–448. [Google Scholar]
- 73.Vaughn J P, Davis P L, Jarboe M D, Huper G, Evans A C, Wiseman R W, Berchuck A, Iglehart J D, Futreal P A, Marks J R. BRCA1 expression is induced before DNA synthesis in both normal and tumor-derived breast cells. Cell Growth Differ. 1996;7:711–715. [PubMed] [Google Scholar]
- 74.Wang H, Shao N, Ding Q M, Cui J, Reddy E S, Rao V N. BRCA1 proteins are transported to the nucleus in the absence of serum and splice variants BRCA1a, BRCA1b are tyrosine phosphoproteins that associate with E2F, cyclins and cyclin dependent kinases. Oncogene. 1997;15:143–157. doi: 10.1038/sj.onc.1201252. [DOI] [PubMed] [Google Scholar]
- 75.Wigler M, Pellicer A, Silverstein S, Axel R, Urlaub G, Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci USA. 1979;76:1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wilson C A, Payton M N, Elliott G S, Buaas F W, Cajulis E E, Grosshans D, Ramos L, Reese D M, Slamon D J, Calzone F J. Differential subcellular localization, expression and biological toxicity of BRCA1 and the splice variant BRCA1-delta11b. Oncogene. 1997;14:1–16. doi: 10.1038/sj.onc.1200924. [DOI] [PubMed] [Google Scholar]
- 77.Wilson C A, Ramos L, Villasenor M R, Anders K H, Press M F, Clarke K, Karlan B, Chen J J, Scully R, Livingston D, Zuch R H, Kanter M H, Cohen S, Calzone F J, Slamon D J. Localization of human BRCA1 and its loss in high-grade, non-inherited breast carcinomas. Nat Genet. 1999;21:236–240. doi: 10.1038/6029. [DOI] [PubMed] [Google Scholar]
- 78.Zhang H, Somasundaram K, Peng Y, Tian H, Bi D, Weber B L, El-Deiry W S. BRCA1 physically associates with p53 and stimulates its transcriptional activity. Oncogene. 1998;16:1713–1721. doi: 10.1038/sj.onc.1201932. [DOI] [PubMed] [Google Scholar]
- 79.Zhang H T, Zhang X, Zhao H Z, Kajino Y, Weber B L, Davis J G, Wang Q, O’Rourke D M, Zhang H B, Kajino K, Greene M I. Relationship of p215BRCA1 to tyrosine kinase signaling pathways and the cell cycle in normal and transformed cells. Oncogene. 1997;14:2863–2869. doi: 10.1038/sj.onc.1201140. [DOI] [PubMed] [Google Scholar]