Abstract
Introduction
Total body irradiation is an effective conditioning regimen for allogeneic stem cell transplantation in pediatric and adult patients with high risk or relapsed/refractory leukemia. The most common adverse effect is pulmonary toxicity including idiopathic pneumonia syndrome (IPS). As centers adopt more advanced treatment planning techniques for TBI, total marrow irradiation (TMI), or total marrow and lymphoid irradiation (TMLI) there is a greater need to understand treatment-related risks for IPS for patients treated with conventional TBI. However, definitions of IPS as well as risk factors for IPS remain poorly characterized. In this study, we perform a critical review to further evaluate the literature describing pulmonary outcomes after TBI.
Materials and Methods
A search of publications from 1960-2020 was undertaken in PubMed, Embase, and Cochrane Library. Search terms included “total body irradiation”, “whole body radiation”, “radiation pneumonias”, “interstitial pneumonia”, and “bone marrow transplantation”. Demographic and treatment-related data was abstracted and evidence quality supporting risk factors for pulmonary toxicity was evaluated.
Results
Of an initial 119,686 publications, 118 met inclusion criteria. Forty-six (39%) studies included a definition for pulmonary toxicity. A grading scale was provided in 20 studies (17%). In 42% of studies the lungs were shielded to a set mean dose of 800cGy. Fourteen (12%) reported toxicity outcomes by patient age. Reported pulmonary toxicity ranged from 0-71% of patients treated with TBI, and IPS ranged from 1-60%. The most common risk factors for IPS were receipt of a TBI containing regimen, increasing dose rate, and lack of pulmonary shielding. Four studies found an increasing risk of pulmonary toxicity with increasing age.
Conclusions
Definitions of IPS as well as demographic and treatment-related risk factors remain poorly characterized in the literature. We recommend routine adoption of the diagnostic workup and the definition of IPS proposed by the American Thoracic Society. Additional study is required to determine differences in clinical and treatment-related risk between pediatric and adult patients. Further study using 3D treatment planning is warranted to enhance dosimetric precision and correlation of dose volume histograms with toxicities.
Keywords: radiation pneumonitis, pulmonary toxicity, allogeneic stem cell transplantation, total body irradiation, total body irradiation complications
Introduction
Acute leukemia is the most common cancer in children and adolescents, and exhibits a bimodal distribution with an initial peak among infants and exponential rise in adulthood (1, 2). Between 2001-2007, 29,682 individuals were diagnosed with acute leukemia, with an incidence ratio of 57.2 per 100,000 person years (2). Overall, acute myeloid leukemia (AML) accounted for 65.7% of cases, acute lymphoblastic leukemia or lymphoma (ALL/L) 31.0% of cases, and acute leukemia of ambiguous lineage 3.4% of cases (2).
Allogeneic stem cell transplant is used in a subset of patients with high risk or relapsed/refractory disease. In pediatric patients with ALL, myeloablative regimens containing total body irradiation (TBI) have demonstrated improvement in event free survival from 29-35% without TBI as compared to 50-58% with and remain the standard of care (3–5). In the adult setting, myeloablative regimens have demonstrated improvements in recurrence free survival at the cost of increased transplant related mortality compared to reduced intensity conditioning regimens (6, 7). The use of reduced-intensity conditioning regimens has therefore increased over the last decade, particularly in patients 50 years of age and older (8).
Transplant-related morbidity and mortality following a myeloablative transplant is significant. In particular, pulmonary toxicity and mortality has been reported in up to 60% of patients (9). Historically, approximately half of all pneumonias following stem cell transplant were secondary to infection, but use of prophylaxis has resulted in a relatively greater risk from noninfectious etiologies (10). In 1993 the National Institutes of Health (NIH) defined idiopathic pneumonia syndrome (IPS) as widespread alveolar injury without evidence of active lower tract infection or cardiogenic cause after transplant (11). Updated definitions now include newly described pathogens as determined on bronchoalveolar lavage (BAL) or lung biopsy (Table 1) (10).
Table 1.
Definitions of pulmonary toxicity.
| National Institutes of Health, 1993 (1) |
|
| American Thoracic Society (2) |
|
As centers adopt more advanced treatment planning techniques for TBI or total marrow irradiation (TMI), there is a greater need to understand the patient and treatment-related risks for IPS. In addition, standardized evaluation and reporting of IPS is crucial to compare outcomes between treatment techniques. Therefore, in this report, we critically evaluate the literature with the goal of characterizing the workup and definitions of pulmonary toxicity as well as levels of evidence in support of risk factors for IPS following TBI-based myeloablative stem cell transplant.
Materials and Methods
A search was undertaken in PubMed, Embase, and Cochrane Library. Articles from 1960-2020 were searched using terms including “total body irradiation”, or “whole body radiation” and “radiation pneumonias” or “interstitial pneumonia” and “bone marrow transplantation” (Supplemental Table 1). Only English language reports of myeloablative transplant regimens were included. Studies in which the dose of TBI was not reported or intensity modulated techniques were used were omitted. Studies in which the incidence or risk factors for pneumonitis from TBI based regimens were not separately reported from those using chemotherapy alone were omitted.
Abstracted data included patient clinical characteristics such as age and disease; treatment-related characteristics including conditioning regimen, donor source, and graft versus host disease (GVHD) prophylaxis; TBI parameters including dose, dose rate, lung shielding, and beam arrangement; and outcomes including rates of acute GVHD.
We evaluated definitions of pulmonary complications in each publication. Any pulmonary complication was classified as pulmonary toxicity (PT). Pulmonary complications specifically reported as idiopathic were classified as IPS. Evidence reported to support these diagnoses including radiographic criteria, infectious workup, and change in pulmonary function tests was documented. Distinctions between acute and late toxicity, grading scales, and rates and mortality from acute PT and IPS were abstracted.
Evidence quality supporting risk factors for PT and IPS were categorized as: level Ia (evidence from meta-analyses of multiple randomized controlled trials), level Ib (evidence from ≥1 randomized controlled trial), level IIA (evidence from ≥1 controlled study without randomization), level IIb (evidence from ≥ other quasi experimental study), level III (evidence from non-experimental descriptive studies such as comparative studies, correlation studies, and case-control studies), and level IV (evidence from expert committee reports or opinions and/or clinical experience of respected authorities).
Results
Clinical Characteristics
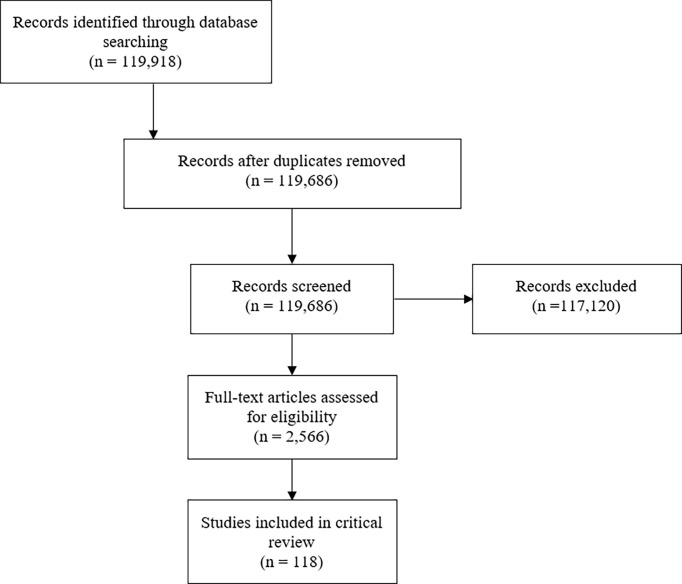
Of an initial 119,686 publications, 118 met inclusion criteria and were included for review (9, 12–127) (Figure 1). Studies were published between 1961-2020 and included patients from less than one year of age to 68 years of age (Table 2). Ten studies (8%) included patients with benign hematologic conditions and 17 included patients who received autologous stem cell transplants (16%). Most conditioning regimens were cyclophosphamide-based (88%) and most studies used methotrexate (MTX) for GVHD prophylaxis (57%). Rates of grade II-IV acute GVHD, when reported as such, ranged from 6-65%.
Figure 1.
PRISMA diagram.
Table 2.
Study characteristics.
| Year (range) | 1961-2020 | |
| Age (range, years) | <1-68 | |
| Conditions Included | ||
| Benign | 10 (10%) | |
| Malignant | 102 (99%) | |
| Transplant Type | ||
| Autologous | 17 (16%) | |
| Allogeneic | 98 (95%) | |
| Chemotherapeutic Backbone | ||
| Cyclophosphamide | 91 (88%) | |
| Other/NR | 12 (12%) | |
| GVHD Prophylaxis | ||
| MTX | 62 (60%) | |
| Other | 14 (13%) | |
| NR | 19 (18%) | |
| NA | 5 (5%) | |
| Rate of Acute Gr II-IV GVHD | 7-65% | |
| Definition of Pulmonary Toxicity | ||
| Interstitial pneumonitis | 47 (45%) | |
| Definition of Idiopathic Pulmonary Toxicity | ||
| Idiopathic interstitial pneumonitis | 24 (23%) | |
| Radiographic Criteria | ||
| Yes | 46 (44%) | |
| No | 57 (55%) | |
| Infectious Workup | ||
| Yes | 40 (38%) | |
| No | 63 (61%) | |
| Defined Acute versus Late | ||
| Yes | 9 (9%) | |
| No | 94 (91%) | |
| Toxicity Grading Scale | ||
| None | 84 (81%) | |
| CTCAE | 7 (7%) | |
| RTOG | 2 (2%) | |
| Individualized | 10 (10%) |
GVHD, graft versus host disease; MTX, methotrexate; NR, not reported; NA, not applicable; Gr, grade.
Definitions
Separate definitions for PT and IPS were reported in 59 studies (50%). The most common definition for pulmonary toxicity was “interstitial pneumonitis” (45%) and for IPS was “idiopathic interstitial pneumonitis” (21%) (Table 2).
Forty-six (39%) of studies included or referenced a definition including radiographic criteria and 40 (34%) described an infectious workup including blood, sputum, BAL, or biopsy. Ten studies (8%) reported changes in pulmonary function tests.
A minority of studies differentiated between acute and late pulmonary toxicities (9%). Of these, 38% used a cutoff of 90 days, 31% a cutoff of 100 days, and 31% a cutoff greater than 6 months.
A grading scale for PT was provided in 20 studies (17%). Of these, 37% used Common Terminology for Adverse Events (CTCAE), 16% used Radiation Therapy Oncology Group (RTOG), and 47% used an individualized definition proposed by the study.
Radiation Treatment Parameters
Of the studies evaluated, 59% reported the TBI source, 35% reported the beam arrangement, and 72% reported the dose rate. Of those that reported the source, 53% utilized Co-60 and of those that reported the beam arrangement 50% utilized anterior-posterior posterio-anterior (AP-PA) fields. Dose rate ranged from 1.2-30.0cGy/min.
Treatment fractionation was not described in 15% (Table 3). In 40% of studies patients were treated with a single fraction, most commonly to a total dose of 1000cGy (range 400-1754cGy). In 13% patients were treated with single daily fractions, most commonly to a total dose of 1200cGy (range 800-1575cGy). In 49% of studies patients were treated with twice daily fractions, most commonly to a total dose of 1200cGy (range 1020-1530cGy). In 4% of studies patients were treated three times per day, to a range of 1200-1610cGy.
Table 3.
Dose and fractionation.
| Fractionation | Dose (cGy) | N (%) |
|---|---|---|
| Fractionation not reported | 19 (15) | |
| 700-1440 | 1 (1) | |
| ≥800 | 1 (1) | |
| ≥900 | 1 (1) | |
| 990-1600 | 1 (1) | |
| 1000 | 1 (1) | |
| 1000-1200 | 1 (1) | |
| 1000-1440 | 1 (1) | |
| 1000-1500 | 1 (1) | |
| 1100-1400 | 1 (1) | |
| 1125-1400 | 1 (1) | |
| 1200 | 3 (3) | |
| 1200-1500 | 1 (1) | |
| 1200-1575 | 2 (2) | |
| 1300-1375 | 1 (1) | |
| Single treatment | 47 (40) | |
| 400-1505 | 1 (1) | |
| 550 | 1 (1) | |
| 550-900 | 1 (1) | |
| 600 | 1 (1) | |
| 700-850 | 1 (1) | |
| 750 | 1 (1) | |
| 750-900 | 1 (1) | |
| 800 | 2 (2) | |
| 800-1000 | 1 (1) | |
| 950-1300 | 1 (1) | |
| 900 | 1 (1) | |
| 920 | 2 (2) | |
| 1000 | 33 (28) | |
| 1228-1754 | 1 (1) | |
| Daily | 15 (13) | |
| 800 | 2 (2) | |
| 900 | 1 (1) | |
| 990 | 1 (1) | |
| 1200 | 11 (9) | |
| 1200-1500 | 1 (1) | |
| 1400 | 1 (1) | |
| 1575 | 3 (3) | |
| Twice daily | 58 (49) | |
| 600-1200 | 1 (1) | |
| 700-1100 | 1 (1) | |
| 800-1200 | 1 (1) | |
| 900-1200 | 1 (1) | |
| 1000-1200 | 1 (1) | |
| 1000-1320 | 1 (1) | |
| 1000-1350 | 1 (1) | |
| 1050-1400 | 1 (1) | |
| 1100 | 2 (2) | |
| 1100-1350 | 1 (1) | |
| 1100-1320 | 1 (1) | |
| 1200 | 34 (29) | |
| 1200-1320 | 2 (2) | |
| 1200-1360 | 2 (2) | |
| 1200-1400 | 1 (1) | |
| 1200-1700 | 1 (1) | |
| 1320 | 7 (6) | |
| 1320-1440 | 1 (1) | |
| 1350 | 4 (3) | |
| 1360 | 2 (2) | |
| 1400 | 1 (1) | |
| 1440 | 5 (4) | |
| 1485 | 1 (1) | |
| 1530 | 1 (1) | |
| Three times daily | 5 (4) | |
| 1200-1610 | 1 (1) | |
| 1320-1440 | 1 (1) | |
| 1320 | 2 (2) | |
| 1440 | 2 (2) |
Lung Shielding
Lung shielding techniques were not reported in 23% of studies evaluated (Table 4). In 26%, authors explicitly stated that no pulmonary shielding was used. In 42% of studies the lungs were shielded to a set mean dose, most commonly 800cGy (range 400cGy – prescription dose). Other studies reported pulmonary shielding by technique rather than dose limit, including use of 5-7 HVL blocks for a single treatment (3%), use of the patient’s arms (3%), or use of bolus, compensators, attenuators, or other unspecified custom blocks (8%).
Table 4.
Lung shielding.
| Technique or Dose | N (%) |
|---|---|
| Shielding not reported | 27 (23) |
| No shielding | 31 (26) |
| 400cGy mean | 1 (1) |
| 500cGy mean | 1 (1) |
| 600cGy mean | 2 (2) |
| 730cGy mean | 1 (1) |
| 750cGy mean | 2 (2) |
| 800cGy mean | 11 (9) |
| 820cGy mean | 1 (1) |
| 850cGy mean | 1 (1) |
| 900cGy mean | 8 (7) |
| 1000cGy mean | 10 (8) |
| 1050cGy mean | 4 (3) |
| 1100cGy mean | 2 (2) |
| 1200cGy mean | 4 (3) |
| Limit to prescription dose | 2 (2) |
| 10% shielding | 1 (1) |
| 40% shielding | 1 (1) |
| 50% shielding | 1 (1) |
| Bolus/compensators/attenuators/custom blocks | 10 (8) |
| 5 HVL single treatment | 1 (1) |
| 6 HVL single treatment | 2 (2) |
| 7 HVL single treatment | 1 (1) |
| Arms | 3 (2) |
Pulmonary Toxicity
In studies where this was reported, PT occurred in 0-71% of patients treated with TBI, and IPS in 1-60%. Late PT occurred in 3-48% of patients treated with TBI and late IPS in 14-16%. Mortality from PT ranged from 0-61% and mortality from IPS ranged from 0-50%.
Risk Factors for Pulmonary Toxicity
Fifty-three studies reported risk factors for PT (45%). Of these, the most frequently reported were GVHD (26%), older age (20%), increasing dose rate (13%), cytomegalovirus (8%), single fraction TBI (7%), and impaired pre-transplant pulmonary function tests (6%) (Table 5).
Table 5.
Risk factors for pulmonary toxicity and IPS.
| Risk Factors for Pulmonary Toxicity | N (%) |
|---|---|
| GVHD | 14 (26) |
| Increasing age | 9 (20) |
| Dose rate | 7 (13) |
| CMV | 4 (8) |
| Single fraction TBI | 4 (8) |
| Impaired pre-transplant PFTs | 3 (6) |
| Receipt of TBI | 3 (6) |
| MTX | 2 (4) |
| Performance status | 2 (4) |
| Donor type | 2 (4) |
| Lung dose/Lack of Shielding | 2 (4) |
| Prior chemotherapy | 1 (2) |
| >6 months from diagnosis to transplant | 1 (2) |
| Prior radiation | 1 (2) |
| T lymphocyte depletion | 1 (2) |
| Infection | 1 (2) |
| Non-CR at transplant | 1 (2) |
| Co-60 based TBI | 1 (2) |
| Diagnosis | 1 (2) |
| Cyclosporine | 1 (2) |
| Granulocyte infusion | 1 (2) |
| Number of prior regimens | 1 (2) |
| Graft failure | 1 (2) |
| Year of BMT | 1 (2) |
| AP-PA fields | 1 (2) |
| Body weight | 1 (2) |
| Prone position | 1 (2) |
| Risk Factors for IPS | |
| Lung dose/Lack of Shielding | 2 (10) |
| Dose rate | 2 (10) |
| Receipt of TBI | 2 (10) |
| Diagnosis | 2 (10) |
| Myeloablative conditioning | 1 (5) |
| CY dose | 1 (5) |
| Anemia | 1 (5) |
| CMV | 1 (5) |
| Impaired pre-transplant PFTs | 1 (5) |
| Parotitis | 1 (5) |
| Single fraction TBI | 1 (5) |
GVHD, graft versus host disease; TBI, total body irradiation; MTX, methotrexate; CR, complete response; BMT, bone marrow transplant; AP-PA, anterio-posterior posterior-anterior; IPS, idiopathic pulmonary syndrome; CY, cyclophosphamide; CMV, cytomegalovirus.
Risk Factors for IPS
Twenty-one studies reported risk factors for IPS. The most common risk factors were increased lung dose, increasing dose rate, receipt of a TBI containing regimen, and diagnosis (Table 3). Increased lung dose was associated with increased risk of toxicity in two studies (evidence level III). Increasing dose rate was associated with increased risk of pulmonary toxicity in two studies (evidence level III). Receipt of a TBI-containing regimen was associated with an increased risk of toxicity in two studies (evidence level III). Diagnosis was associated with increased risk of toxicity in two studies (evidence level III).
Age Specific Considerations
Of the studies evaluated, 14 (12%) reported toxicity outcomes by patient age or stratified between adult and pediatric patients. Four of these studies found an increasing risk of PT with increasing age, one of which determined a cutoff of >20 years old (19, 20, 37, 43). Eighteen studies evaluated pediatric patients only, one of which demonstrated an increasing risk of IPS with dose rate >15cGy/min and one of which found an increased risk associated with chronic GVHD (69, 75).
Discussion
In this study, we perform a critical review of existing literature defining and reporting the incidence of PT in patients who receive hematopoietic stem cell transplant with a myeloablative, TBI-based regimen. In our review, we find that rates of IPS may be as high as 60% with mortality as high as 50%. However, there are significant limitations in the existing literature defining and providing high-level evidence of risk factors for IPS.
Defining IPS
Idiopathic pulmonary toxicity following TBI-based myeloablative transplant is thought to be due to direct injury to type II alveolar epithelial and endothelial cells from cells of lymphoid and myeloid origin as well as by inflammatory stimulators including TNF-α, lipo-polysaccharide, and reactive oxygen species (128–131). In mouse models of IPS, pulmonary toxicity due to host monocytes and donor T cells in the lungs occurs within the first 2 weeks of transplant (128). Increased cytotoxic T lymphocytes result in parenchymal damage and reduced compliance, total lung capacity, and increased wet and dry lung weights. In its advanced stages, IPS is characterized by cellular proliferation and matrix accumulation (132).
A standard clinical definition of IPS has been proposed (133). Criteria include evidence of widespread alveolar injury as evidenced by chest x-ray (CXR) or computed tomography (CT) scan, signs and symptoms of pneumonia, or abnormal pulmonary physiology, absence of active lower respiratory tract infection as diagnosed by BAL, transbronchial biopsy if feasible, and ideally a second confirmatory test to rule out infection. In this report, authors recommended against histopathologic definitions including “interstitial pneumonitis” citing concerns for accuracy. An expanded definition was proposed by the American Thoracic Society in which additional viral, fungal, and bacterial studies as well as evaluation of extra-pulmonary etiologies were recommended (10).
The studies evaluated for this critical review utilize multiple definitions of PT and IPS. The majority did not distinguish between idiopathic and non-idiopathic pulmonary complications. Of those that did, many relied on a definition of “interstitial pneumonitis” and less than half documented radiographic or other criteria supporting the diagnosis. Given that many studies had limited diagnostic workup, the true incidence of IPS secondary to an inflammatory-mediated process may not be accurately reported. In addition, varying definitions of IPS limit comparisons of the incidence between treatment regimens and evaluation of risk factors for IPS specifically as compared to other infectious pulmonary toxicity.
The working group publications do not make recommendations regarding definitions of acute and late toxicity, which is also reflected in the limited reporting and lack of consensus seen in the studies evaluated. In general, idiopathic pulmonary toxicity is thought to occur early after transplant, historically reported between six to seven weeks, although more recently as early as 19 days (133–135). In their studies, Kim et al. and Gao et al. restricted pulmonary events to those occurring within 90-100 days of transplant based on previous data demonstrating a maximal risk for IPS within the first 100 days of transplantation (77, 110). However, Nagasawa et al. evaluated pulmonary toxicities occurring 3 months or more after HSCT (hematopoietic stem cell transplant) (75). They found a 16% (4/25) incidence of late non-infectious pulmonary toxicities for patients receiving a TBI-based myeloablative regimen, suggesting that late pulmonary complications may occur frequently.
In newer studies using intensity modulated techniques, pulmonary toxicity has been preliminarily evaluated. However, definitions in these publications are also variable. In their study, Shinde et al. rigorously defined radiation pneumonitis as greater than or equal to grade 3 pneumonitis not attributable to infection, graft versus host disease, or disease progression as assessed by standard institutional post-HCT protocols including bronchoscopy (136). Others have reported only rates of any pulmonary toxicity without specifying workup or etiology (137, 138).
Recommendations for Definition
We recommend routine adoption of the diagnostic workup and definition of IPS proposed by the American Thoracic Society. Based on the available literature and previously used definitions we suggest a cutoff of early toxicity within 90 days of transplant and encourage continued patient follow-up for late treatment-related toxicity.
Grading IPS
Toxicity grading scales were not commented on in the working group publications and infrequently utilized in the papers studied for this review. Seven studies relied on CTCAE definitions and two utilized Radiation Therapy Oncology Group (RTOG) definitions (47, 55, 73, 81–83). Five defined toxicity with an individualized scale, one of which utilized the extent of imaging changes on CXR (41, 44, 52, 97). In studies without a grading scale, pulmonary toxicity is most often reported as present and resolved or a cause of mortality. Notably, no patient reported outcomes (PRO) were utilized in these studies, although PRO surveillance using the PRO-CTCAE has been shown to be feasible in the transplant setting (139). Studies using 3D treatment planning have relied on multiple grading scales including CTCAE and Bearman Toxicity Scale for bone marrow transplantation (136–138, 140).
Recommendations for Grading
There is little data to suggest benefit to one grading scale over another. However, given the availability of both provider and patient-reported outcomes, authors encourage consideration of the CTCAE for future toxicity reporting.
Risk Factors for IPS
The studies reporting risk factors for IPS were, in general, lower levels of evidence. Limitations of these publications included heterogeneous diagnoses for which patients may have received previous chemotherapy and/or radiation, wide age ranges, multiple conditioning regimens, approaches to GVHD prophylaxis, and a variety of donor sources which all impact the risk of complications and could not be controlled for in evaluation of risk factors for PT or IPS.
The details of TBI and cytotoxic chemotherapy were not uniformly reported and limit the ability to evaluate safety and efficacy between varying chemotherapy regimens, doses, dose rates, beam arrangements, and shielding techniques. Cytotoxic chemotherapy may result in pneumonitis without the addition of radiation, and few studies provided the regimen and doses of chemotherapy delivered in conjunction with TBI (141). In addition, all studies are limited by the accuracy of true lung dose assessment. The majority of TBI patients are treated with either right and left lateral or AP-PA fields without 3D treatment planning. Details of the techniques have been previously described (51, 142). It is difficult to assess the limiting lung dose, due particularly to inaccuracies in the largely inhomogeneous dose distribution within the lung resulting from the single-point dose calculation model generally used (143, 144). The CT based treatment planning simulation showed a highly inhomogeneous dose distribution from TBI delivery to different organs. The greatest variation in radiation dose in the lung was as much as 32% above that prescribed (145). Therefore, the exact correlation of the single point dose and lung pneumonitis from the reported studies will have to be carefully considered.
In spite of these limitations, some risk features were seen in multiple studies which may inform radiation planning parameters. Dose rate was found to be significantly associated with development of IPS in several non-randomized studies (Table 6). Abugideiri et al. evaluated 129 pediatric patients who underwent TBI-based myeloablative conditioning at dose rates from 2.6cGy/min to 20.9cGy/min and found a statistically significant association of dose rate with IPS on multivariate analysis (p=0.002) (69). Gao et al. evaluated 202 patients with acute leukemia at a dose rate from 8.7cGy/min to 19.2cGy/min. Patients treated with high dose rates, defined as >15cGy/min, had a 29% incidence of IPS as compared to 10% in those patients treated with lower dose rates (p<0.01). In a retrospective study of 92 patients with hematolymphoid malignancies treated with 900-1200cGy fractionated TBI, a trend towards decreased toxicity was seen in those treated at a lower dose rate, defined as <6cGy/min (p=0.07) (110). In their analysis, Barrett et al. found that dose rate had an effect on PT only at total lung doses of >900cGy without an effect at lower total doses (80).
Table 6.
Summary of key literature reports on the effect of dose rate and lung dose.
| First author, publication year | N | Prescribed dose (Gy)/fx | Lung dose (Gy) | Dose rate (cGy/min) | Findings |
|---|---|---|---|---|---|
| Barrett, 1982 (80) | 402* | 7.5-10.5/1 | 1-12 | 2.5-46 | Dose rate associated with incidence of PT only for lung dose ≥9 Gy. |
| Bortin, 1982 (95) | 176* | ≥8 | NR | 2.3-30 | Dose rate ≤5.7 cGy/min associated with lower risk of PT 30% vs. 6%) |
| Weiner, 1986 (19) | 932^ | 10/1 or 12/5-6 | 5.6-12.8 | 2-108 | Dose rate significantly correlated with risk of PT only in those receiving MTX after transplantation. |
| Ozsahin, 1996 (43) | 186^ | 10/1 or 12/6 BID | 8 or 9 | 2.6-16.9 | PT incidence was significantly higher in the high dose rate patients – 56% (> 9cGy/min) vs. 20% (≤4.8 cGy/min). |
| Corvo, 1999 (89) | 93^ | 12/6 BID | 10.8 or 12 | 2.5-15 | PT incidence was correlated with higher dose rate – 33% (>6 cGy/min) vs. 12% (<6 cGy/min). |
| Carruthers, 2004 (53) | 84^ | 12/6 | NR | 7.5 and 15 | A higher dose rate associated with a higher risk of PT - 43% (15 cGy/min) vs. 13% (7.5 cGy/min). |
| Abugideiri, 2016 (69) | 129± | 10.5-14 (1.5-2 Gy/fx) | ≤ 10 | 5.6-20.9 | TBI dose rate ≥15 cGy/min significantly increased incidence of PT [HR 4.85] and IPS [HR 4.94]. |
| Kim, 2018 (17) | 92^ | 9-12/3-4 daily | 5-10% attenuation | 4.2-17.3 | Reducing the dose rate decreased the risk of PT - 74.1% (≥6 cGy/min) vs. 43.5% (<6 cGy/min). |
| Gao, 2019 (77) | 202^ | 13.2/8 BID | None | 8.6-19.2 | IPS in 29% (>15cGy/min) vs 10% (≤15cGy/min). |
| Petersen, 1992 (87) | 36^ | 12 or 16/6 BID, 17/7 BID | Prescription | 8 | PT or IPS in 50% of patients receiving 17Gy as compared to 15% after 16Gy. |
| Sampath, 2005 (91) | 1090* | Up to 15.6 | Up to 15.6 | 3-41 | Lung dose was associated with PT in patients receiving 1 fx/day – 2.3% if ≤6 Gy to lungs. |
| Soule, 2007 (57) | 181≠ | 12 or 13.6 (1.5-1.7 Gy/fx bid) | 6 to >13.6 | 12 | Lung dose reduction should be employed primarily to decrease mortality from PT in high-risk patients. |
| Weschler, 1990 (30) | 43^ | 6/4 BID (TLI) or 12/6 BID (TBI) | 6 or 12 | 15-18 | IPS occurred in 26% without lung shielding as compared to 0% with partial lung shielding. |
bid, twice a day; fx, fraction; HR, hazard ratio; IP, interstitial pneumonitis; IPS, idiopathic pneumonia syndrome; MTX, methotrexate; NR, not reported; PT, pulmonary toxicity; TBI, total body irradiation; TLI, total lymphoid irradiation; *ages not specified; ^adults and children; ±children; ≠adults and adolescents.
Higher lung dose has also been found to be associated with IPS at a range of dose rates (Table 7). Weshler et al. evaluated 44 patients with malignant disorders treated with TBI containing myeloablative transplants (30). Their first 23 patients were treated to 1200cGy with fractionated TBI without lung shielding at a dose rate of 15-18cGy/min and found a 26% risk of IPS. The remainder were treated with a 50% transmission lung block at a dose rate of 15cGy/min with no IPS. Petersen et al. performed a phase I dose escalation trial utilizing TBI given at a rate of 8cGy/min in 200cGy fractions twice daily from 1200-1700cGy without lung shielding. They found 50% risk of PT at a dose of 1700cGy as compared to 15% after 1600cGy (87). Sampath et al. similarly evaluated 20 articles to develop a multivariate logistic regression to determine dosimetric and chemotherapeutic factors influencing the incidence of IPS (91). In their analysis, a conditioning regimen of 1200cGy fractionated TBI resulted in an incidence of IPS of 11% as compared to 2.3% with 50% lung shielding (p<0.05) without any effect from dose rate.
Table 7.
Summary of key literature reports on the effect of lung dose.
| First author, publication year | N | Prescribed dose (Gy)/fx | Lung dose (Gy) | Dose rate (cGy/min) | Dose rate finding |
|---|---|---|---|---|---|
| Petersen, 1992 (87) | 36^ | 12 or 16/6 BID, 17/7 BID | Prescription | 8 | PT or IPS in 50% of patients receiving 17Gy as compared to 15% after 16Gy. |
| Sampath, 2005 (91) | 1090* | Up to 15.6 | Up to 15.6 | 3-41 | Lung dose was associated with PT in patients receiving 1 fx/day – 2.3% if ≤6 Gy to lungs. |
| Soule, 2007 (57) | 181≠ | 12 or 13.6 (1.5-1.7 Gy/fx bid) | 6 to >13.6 | 12 | Lung dose reduction should be employed primarily to decrease mortality from PT in high-risk patients. |
| Weschler, 1990 (30) | 43^ | 6/4 BID (TLI) or 12/6 BID (TBI) | 6 or 12 | 15-18 | IPS occurred in 26% without lung shielding as compared to 0% with partial lung shielding. |
bid, twice a day; fx, fraction; IP, interstitial pneumonitis; IPS, idiopathic pneumonia syndrome; NR, not reported; PT, pulmonary toxicity; TLI: total lymphoid irradiation; *ages not specified; ^ adults and children ≠adults and adolescents.
New advancements using CT guided intensity modulated TBI or TMI or TMLI allow 3D lung dose distribution to be calculated with high precision (146, 147). Reported rates of pneumonitis are low in spite of dose rates up to 200cGy/min (136, 148). Clinically, a mean lung dose of 800cGy or less has still been associated with decreased risk, suggesting need for further study using 3D planning to understand the relationship between dose rate, mean lung dose, and IPS (136).
There are limited data regarding differences in pulmonary risk and modifications to treatment planning that should be made based on patient age. In many of the studies evaluated, increasing age was found to be a risk factor for pulmonary toxicity. This may be due to worse pre-transplant pulmonary function, which has been found to be a risk factor for IPS (79). However, pediatric patients remain at risk of pulmonary toxicity. In a study of only pediatric patients, increasing dose rate was found to be a risk factor for IPS (69). Similarly, increasing mean lung dose, while not correlated with risk of IPS in any study of only pediatric patients, was correlated with reduced survival (100). TBI using intensity modulated techniques has been reported in children and young adults without any early evidence of increased risks of toxicity (149). However, longer follow up, larger patient numbers, and more comprehensive dosimetric evaluation is needed in order to obtain pediatric-specific planning parameters.
Recommendations for Dose Rate and Shielding
Risk factors for true IPS remain poorly defined given limitations in definitions, workup, and reporting of TBI parameters. A dose rate of ≤15cGy/min and a mean lung dose ≤600cGy using traditional planning techniques is supported by the literature.
Future Directions
As more centers adopt 3D image guided intensity modulated treatment planning for TBI to reduce the lung dose, quantitative knowledge of how dose distribution and lung volume coverage may be correlated with IPS should emerge. Consequently, standard methods of evaluating and reporting pulmonary toxicity will become even more critical. Efforts to adopt the definition and workup for IPS proposed by the NIH and American Thoracic Society are needed in addition to further studies of differences in risk factors and clinical outcomes between pediatric and adult patients and a greater understanding of the contribution of specific chemotherapy regimens to the overall risk. More comprehensive and reliable reporting of TBI dosimetric parameters will provide greater understanding of the range of treatment and planning techniques and their relationship to IPS.
Author Contributions
JV, SH, and NE contributed to study design, data analysis, manuscript writing, and manuscript review. C-HH contributed to manuscript review and data presentation. KD, PR, JK, and LC contributed to manuscript review. All authors contributed to the article and approved the submitted version.
Funding
The work was partially supported by the National Institutes of Health under R01CA154491 (SH).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.708906/full#supplementary-material
References
- 1.Barrington-Trimis JL, Cockburn M, Metayer C, Gauderman JW, Wiemels J, McKean-Cowdin R. Trends in Childhood Leukemia Incidence Over Two Decades From 1992 to 2013. Int J Cancer (2017) 140:1000–08. 10.1002/ijc.30487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dores GM, Devesa SS, Curtis RE, Linet MS, Morton LM. Acute Leukemia Incidence and Patient Survival Among Children and Adults in the United States, 2001-2007. Blood (2012) 119:34–43. 10.1182/blood-2011-04-347872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bunin N, Aplenc R, Kamani N, Shaw K, Cnaan A, Simms S. Randomized Trial of Busulfan vs Total Body Irradiation Containing Conditioning Regimens for Children With Acute Lymphoblastic Leukemia: A Pediatric Blood and Marrow Transplant Consortium Study. Bone Marrow Transplant (2003) 32:543–48. 10.1038/sj.bmt.1704198 [DOI] [PubMed] [Google Scholar]
- 4.Davies SM, Ramsay NK, Klein JP, Weisdorf DJ, Bolwell B, Cahn JY, et al. Comparison of Preparative Regimens in Transplants for Children With Acute Lymphoblastic Leukemia. J Clin Oncol (2000) 18:340–47. 10.1200/JCO.2000.18.2.340 [DOI] [PubMed] [Google Scholar]
- 5.Eapen M, Raetz E, Zhang MJ, Muehlenbein C, Devidas M, Abshire T, et al. Outcomes After HLA-Matched Sibling Transplantation or Chemotherapy in Children With B-Precursor Acute Lymphoblastic Leukemia in a Second Remission: A Collaborative Study of the Children’s Oncology Group and the Center for International Blood and Marrow Tr. Blood (2006) 107:4961–97. 10.1182/blood-2005-12-4942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott BL, Pasquini MC, Logan BR, Wu J, Devine SM, Porter DL, et al. Myeloablative Versus Reduced-Intensity Hematopoietic Cell Transplantation for Acute Myeloid Leukemia and Myelodysplastic Syndromes. J Clin Oncol (2017) 35:1154–61. 10.1200/JCO.2016.70.7091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohty M, Labopin M, Volin L, Gratwohl A, Soci G. Reduced-Intensity Versus Conventional Myeloablative Conditioning Allogeneic Stem Cell Transplantation for Patients With Acute Lymphoblastic Leukemia: A Retrospective Study From the European Group for Blood and Marrow Transplantation. Blood (2010) 116:4439–43. 10.1182/blood-2010-02-266551 [DOI] [PubMed] [Google Scholar]
- 8.D’Souza A, Lee S, Zhu X, Pasquini M. Current Use and Trends in Hematopoietic Cell Transplantation in the United States. Biol Blood Marrow Transplant (2017) 23:1417–21. 10.1016/j.bbmt.2017.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lichter AS, Tutschka PJ, Wharam MD, Elfenbein GJ, Sensenbrenner LL, Saral R, et al. The Use of Fractionated Radiotherapy as Preparation for Allogeneic Bone Marrow Transplantation. Transplant Proc (1979) 11:1492–94. [PubMed] [Google Scholar]
- 10.Panoskaltsis-Mortari A, Griese M, Madtes D, Belperio JA, Haddad IY, Folz RJ, et al. An Official American Thoracic Society Research Statement: Noninfectious Lung Injury After Hematopoietic Stem Cell Transplantation: Idiopathic Pneumonia Syndrome. Am J Respir Crit Care Med (2011) 183:1262–79. 10.1164/rccm.2007-413ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shankar G, Cohen DA. Idiopathic Pneumonia Syndrome After Bone Marrow Transplantation: The Role of Pre-Transplant Radiation Conditioning and Local Cytokine Dysregulation in Promoting Lung Inflammation and Fibrosis. Int J Exp Pathol (2001) 82:101–13. 10.1111/j.1365-2613.2001.iep182.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas ED, Clift RA, Hersman J, Sanders JE, Stewart P, Buckner CD, et al. Marrow Transplantation for Acute Nonlymphoblastic Leukemia in First Remission Using Fractionated or Single-Dose Irradiation. IJROBP (1982) 8:817–21. 10.1016/0360-3016(82)90083-9 [DOI] [PubMed] [Google Scholar]
- 13.Thomas ED, Buckner CD, Clift RA, Fefer A, Johnson FL, Neiman PE, et al. Marrow Transplantation for Acute Nonlymphoblastic Leukemia in First Remission. N Engl J Med (1979) 301:597–99. 10.1056/NEJM197909133011109 [DOI] [PubMed] [Google Scholar]
- 14.Torres JL, Bross DS, Chef LW, Wharam MD, Santos GW, Order SE, et al. Risk Factors in Interstitial Pneumonitis Following Allogenic Bone Marrow Transplantation. IJROBP (1982) 8:1031–37. 10.1016/0360-3016(82)90579-X [DOI] [PubMed] [Google Scholar]
- 15.Shank B, Chu FC, Dinsmore R, Kapoor N, Kirkpatrick D, Teitelbaum H, et al. Hyperfractionated Total Body Irradiation for Bone Marrow Transplantation Results in Seventy Leukemia Patients With Allogeneic Transplants. IJROBP (1983) 9:1607–11. 10.1016/0360-3016(83)90412-1 [DOI] [PubMed] [Google Scholar]
- 16.Parkman R, Rappeport JM, Hellman S, Lipton J, Smith B, Geha R, et al. Busulfan and Total Body Irradiation as Antihematopoietic Stem Cell Agents in the Preparation of Patients With Congenital Bone Marrow Disorders for Allogenic Bone Marrow Transplantation. Blood (1984) 64:852–7. 10.1182/blood.V64.4.852.bloodjournal644852 [DOI] [PubMed] [Google Scholar]
- 17.Kim TH, Rybka WB, Lehnert S, Podgorsak EB, Freeman CR. Interstitial Pneumonitis Following Total Body Irradiation for Bone Marrow Transplantation Using Two Different Dose Rates. Int J Radiat Oncol Biol Phys (1985) 11:1285–91. 10.1016/0360-3016(85)90243-3 [DOI] [PubMed] [Google Scholar]
- 18.Sullivan KM, Meyers JD, Flournoy N, Rainer S, Thomas ED. Early and Late Interstitial Pneumonia Following Human Bone Marrow Transplantation. Int J Cell Cloning (1986) S:107–21. 10.1002/stem.5530040712 [DOI] [PubMed] [Google Scholar]
- 19.Weiner RS, Bortin MM, Gale RP, Gluckman E, Kay HE, Kolb HJ, et al. Interstitial Pneumonitis After Bone Marrow Transplantation. Ann Internal Med (1986) 104:168–75. 10.7326/0003-4819-104-2-168 [DOI] [PubMed] [Google Scholar]
- 20.Pecego R, Hill R, Appelbaum FR, Bruckner AD, Fefer A, Thomas ED. Interstitial Pneumonitis Following Autologous Bone Marrow Transplantation. Transplantation (1986) 42:515–17. 10.1097/00007890-198611000-00015 [DOI] [PubMed] [Google Scholar]
- 21.Deeg HJ, Sullivan KM, Buckner CD, Storb R, Appelbaum FR, Clift RA, et al. Marrow Transplantation for Acute Nonlymphoblastic Leukemia in First Remission: Toxicity and Long-Term Follow-Up of Patients Conditioned With Single Dose or Fractionated Total Body Irradiation. Bone Marrow Transplant (1986) 1:151–7. [PubMed] [Google Scholar]
- 22.Cordonnier C, Bernaudin JF, Bierling P, Huet Y, Vernant JP. Pulmonary Complications Occurring After Allogeneic Bone Marrow Transplantation. A Study of 130 Consecutive Transplanted Patients. Cancer (1986) 58:1047–54. [DOI] [PubMed] [Google Scholar]
- 23.Dinsmore R, Kirkpatrick D, Flomenberg N, Gulati S, Kapoor N, Brochstein J, et al. Allogeneic Bone Marrow Transplantation for Patients With Acute Nonlymphocytic Leukemia. Blood (1984) 63:649–56. 10.1182/blood.V63.3.649.649 [DOI] [PubMed] [Google Scholar]
- 24.Dinsmore R, Kirkpatrick D, Flomenberg N, Gulati S, Kapoor N, Shank B, et al. Allogeneic Bone Marrow Transplantation for Patients With Acute Lymphoblastic Leukemia. Blood (1983) 62:381–8. 10.1182/blood.V62.2.381.381 [DOI] [PubMed] [Google Scholar]
- 25.Barrett A, Depledge MH, Powles RL. Interstitial Pneumonitis Following Bone Marrow Transplantation After Low Dose Rate Total Body Irradiation. IJROBP (1983) 9:1029–33. 10.1016/0360-3016(83)90393-0 [DOI] [PubMed] [Google Scholar]
- 26.Sutedja TG, Apperley JF, Hughes JM, Aber VR, Kennedy HG, Nunn P, et al. Pulmonary Function After Bone Marrow Transplantation for Chronic Myeloid Leukaemia. Thorax (1988) 43:163–9. 10.1136/thx.43.3.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas ED, Buckner CD, Banaji M, Clift RA, Fefer A, Flournoy N, et al. One Hundred Patients With Acute Leukemia Treated by Chemotherapy, Total Body Irradiation, and Allogeneic Marrow Transplantation. Blood (1977) 49:511–33. 10.1182/blood.V49.4.511.511 [DOI] [PubMed] [Google Scholar]
- 28.Wingard JR, Mellits ED, Sostrin MB, Chen DY, Burns WH, Santos GW, et al. Interstitial Pneumonitis After Allogeneic Bone Marrow Transplantation. Med (United States) (1988) 67:175–86. 10.1097/00005792-198805000-00004 [DOI] [PubMed] [Google Scholar]
- 29.Kim TH, McGlave PB, Ramsay N, Woods W, Bostrom B, Vercellotti G, et al. Comparison of Two Total Body Irradiation Regimens in Allogeneic Bone Marrow Transplantation for Acute Non-Lymphoblastic Leukemia in First Remission. IJORBP (1990) 19:889–97. 10.1016/0360-3016(90)90009-9 [DOI] [PubMed] [Google Scholar]
- 30.Weshler Z, Breuer R, Or R, Naparstek E, Pfeffer MR, Lowental E, et al. Interstitial Pneumonitis After Total Body Irradiation: Effect of Partial Lung Shielding. BJHaem (1990) 74:61–4. 10.1111/j.1365-2141.1990.tb02538.x [DOI] [PubMed] [Google Scholar]
- 31.Socie G, Devergie A, Girinisky T, Reiffers J, Vernant JP, Le Bourgeois JP, et al. Influence of the Fractionation of Total Body Irradiation on Complications and Relapse Rate for Chronic Myelogenous Leukemia. The Group d’Etude Des Greffes De Moelle Osseus (GEGBM). IJORBP (1991) 20:397–404. 10.1016/0360-3016(91)90048-9 [DOI] [PubMed] [Google Scholar]
- 32.Blaise D, Maraninchi D, Archimbaud E, Reiffers J, Devergie A, Jouet JP, et al. Allogeneic Bone Marrow Transplantation for Acute Myeloid Leukemia in First Remission: A Randomized Trial of a Busulfan-Cytoxan Versus Cytoxan-Total Body Irradiation as Preparative Regimen: A Report From the Groupe d’Etudes De La Greffe De Moelle Osseuse. Blood (1992) 79:2578–82. 10.1182/blood.V79.10.2578.2578 [DOI] [PubMed] [Google Scholar]
- 33.Gonga NK, Morgan G, Downs K, Atkinson K, Biggs J. Lung Dose Rate and Interstitial Pneumonitis in Total Body Irradiation for Bone Marrow Transplantation. Australas Radiol (1992) 36:317–20. 10.1111/j.1440-1673.1992.tb03208.x [DOI] [PubMed] [Google Scholar]
- 34.Labar B, Bogdanic V, Nemet D, Mrsic M, Vrtar M, Grgic-Markulin L, et al. Total Body Irradiation With or Without Lung Shielding for Allogeneic Bone Marrow Transplantation. Bone Marrow Transplant (1992) 9:343–7. [PubMed] [Google Scholar]
- 35.Ozsahin M, Pne F, Touboul E, et al. Total-Body Irradiation Before Bone Marrow Transplantation. Results of Two Randomized Instantaneous Dose Rates in 157 Patients. Cancer (1992) 69:2853–65. [DOI] [PubMed] [Google Scholar]
- 36.Appelbaum FR, Meyers JD, Fefer A, et al. Nonbacterial Nonfungal Pneumonia Following Marrow Transplantation in 100 Identical Twins. Transplantation (1982) 33:265–8. 10.1097/00007890-198203000-00011 [DOI] [PubMed] [Google Scholar]
- 37.Granena A, Carreras E, Rozman C, Salgado C, Sierra J, Algara M, et al. Interstitial Pneumonitis After BMT: 15 Years Experience in a Single Institution. Bone Marrow Transplant (1993) 11:453–8. [PubMed] [Google Scholar]
- 38.Sutton L, Kuentz M, Cordonnier C, Blaise D, Devergie A, Guyotat D, et al. Allogeneic Bone Marrow Transplantation for Adult Acute Lymphoblastic Leukemia in First Complete Remission: Factors Predictive of Transplant-Related Mortality and Influence of Total Body Irradiation Modalities. Bone Marrow Transplant (1993) 12:583–9. [PubMed] [Google Scholar]
- 39.Valls A, Algara M, Marrugat J, Carreras E, Sierra J, Graena A. Risk Factors for Early Mortality in Allogeneic Bone Marrow Transplantation. A Multivariate Analysis on 174 Leukaemia Patients. Eur J Cancer (1993) 29:1523–8. 10.1016/0959-8049(93)90287-P [DOI] [PubMed] [Google Scholar]
- 40.Clift RA, Buckner CD, Thomas ED, Bryant E, Anasetti C, Bensinger WI, et al. Marrow Transplantation for Chronic Myeloid Leukemia: A Randomized Study Comparing Cyclophosphamide and Total Body Irradiation With Busulfan and Cyclophosphamide. Blood (1994) 84:2036–43. 10.1182/blood.V84.6.2036.bloodjournal8462036 [DOI] [PubMed] [Google Scholar]
- 41.Demirer T, Petersen FB, Appelbaum FR, Barnett TA, Sanders J, Deeg HJ, et al. Allogeneic Marrow Transplantation Following Cyclophosphamide and Escalating Doses of Hyperfractionated Total Body Irradiation in Patients With Advanced Lymphoid Malignancies: A Phase I II Trial. Int J Radiat Oncol Biol Phys (1995) 32:1103–9. 10.1016/0360-3016(95)00115-F [DOI] [PubMed] [Google Scholar]
- 42.Morgan TL, Falk PM, Kogut N, Shah KH, Tome M, Kagan AR. A Comparison of Single-Dose and Fractionated Total-Body Irradiation on the Development of Pneumonitis Following Bone Marrow Transplantation. IJROBP (1996) 36:61–6. 10.1016/S0360-3016(96)00246-5 [DOI] [PubMed] [Google Scholar]
- 43.Ozsahin M, Belkacmi Y, Pène F, Laporte J, Rio B, Leblond V, et al. Interstitial Pneumonitis Following Autologous Bone-Marrow Transplantation Conditioned With Cyclophosphamide and Total-Body Irradiation. IJROBP (1996) 34:71–7. 10.1016/0360-3016(95)02063-2 [DOI] [PubMed] [Google Scholar]
- 44.Kantrow SP, Hackman RC, Boeckh M, Myerson D, Crawford SW. Idiopathic Pneumonia Syndrome: Changing Spectrum of Lung Injury After Marrow Transplantation. Transplantation (1997) 63:1079–86. 10.1097/00007890-199704270-00006 [DOI] [PubMed] [Google Scholar]
- 45.Abraham R, Chen C, Tsang R, Simpson D, Murray C, Davidson M, et al. Intensification of the Stem Cell Transplant Induction Regimen Results in Increased Treatment-Related Mortality Without Improved Outcome in Multiple Myeloma. Bone Marrow Transplant (1999) 24:1291–7. 10.1038/sj.bmt.1702060 [DOI] [PubMed] [Google Scholar]
- 46.Girinsky T, Benhamou E, Bourhis JH, Dhermain F, Guillot-Valls D, Ganansia V, et al. Prospective Randomized Comparison of Single-Dose Versus Hyperfractionated Total-Body Irradiation in Patients With Hematologic Malignancies. J Clin Oncol (2000) 18:981–6. 10.1200/JCO.2000.18.5.981 [DOI] [PubMed] [Google Scholar]
- 47.Sobecks RM, Daugherty CK, Hallahan DE, Laport GF, Wagner ND, Larson RA. A Dose Escalation Study of Total Body Irradiation Followed by High-Dose Etoposide and Allogeneic Blood Stem Cell Transplantation for the Treatment of Advanced Hematologic Malignancies. Bone Marrow Transplant (2000) 25:807–13. 10.1038/sj.bmt.1702230 [DOI] [PubMed] [Google Scholar]
- 48.Bieri S, Helg C, Chapuis B, Miralbell R. Total Body Irradiation Before Allogeneic Bone Marrow Transplantation: Is More Dose Better? Int J Radiat Oncol Biol Phys (2001) 49:1071–7. 10.1016/S0360-3016(00)01491-7 [DOI] [PubMed] [Google Scholar]
- 49.Chen CI, Abraham R, Tsang R, Crump M, Keating A, Stewart AK. Radiation-Associated Pneumonitis Following Autologous Stem Cell Transplantation: Predictive Factors, Disease Characteristics and Treatment Outcomes. Bone Marrow Transplant (2001) 27:177–82. 10.1038/sj.bmt.1702771 [DOI] [PubMed] [Google Scholar]
- 50.Gopal R, Ha CS, Tucker SL, Khouri IF, Giralt SA, Gajewski JL, et al. Comparison of Two Total Body Irradiation Fractionation Regimens With Respect to Acute and Late Pulmonary Toxicity. Cancer (2001) 92:1949–58. [DOI] [PubMed] [Google Scholar]
- 51.Thomas O, Mahé MA. Long-Term Complications of Total Body Irradiation in Adults. Int J Radiat Oncol Biol Phys (2001) 49:125–31. 10.1016/S0360-3016(00)01373-0 [DOI] [PubMed] [Google Scholar]
- 52.Lohr F, Wenz F, Schraube P, Flentje M, Haas R, Zierhut D, et al. Lethal Pulmonary Toxicity After Autologous Bone Marrow Transplantation/Peripheral Blood Stem Cell Transplantation for Hematological Malignancies. Radiotherapy Oncol (1998) 48:45–1. 10.1016/S0167-8140(98)00045-0 [DOI] [PubMed] [Google Scholar]
- 53.Carruthers SA, Wallington MM. Total Body Irradiation and Pneumonitis Risk: A Review of Outcomes. (2004) 90:2080–4. 10.1038/sj.bjc.6601751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Savani BN, Srinivasan R. Chronic GVHD and Pretransplantation Abnormalities in Pulmonary Function Are the Main Determinants Predicting Worsening Pulmonary Function in Long-Term Survivors After Stem Cell Transplantation. Biol Blood Marrow Transplant (2006) 12:1261–9. 10.1016/j.bbmt.2006.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kornguth DG, Mahajan A, Woo S, Chan KW, Antolak J, Ha CS. Fludarabine Allows Dose Reduction for Total Body Irradiation in Pediatric Hematopoietic Stem Cell Transplantation. Int J Radiat Oncol Biol Phys (2007) 68:1140–4. 10.1016/j.ijrobp.2007.01.003 [DOI] [PubMed] [Google Scholar]
- 56.Soejima T, Hirota S, Tsujino K, Yoden E, Fujii O, Ichimiya Y, et al. Total Body Irradiation Followed by Bone Marrow Transplantation: Comparison of Once-Daily and Twice-Daily Fractionation Regimens. Radiat Med - Med Imaging Radiat Oncol (2007) 25:402–6. 10.1007/s11604-007-0157-z [DOI] [PubMed] [Google Scholar]
- 57.Soule BP, Simone NL, Savani BN, Ning H, Albert PS, Barrett AJ, et al. Pulmonary Function Following Total Body Irradiation (With or Without Lung Shielding) and Allogeneic Peripheral Blood Stem Cell Transplant. Bone Marrow Transplant (2007) 40:573–8. 10.1038/sj.bmt.1705771 [DOI] [PubMed] [Google Scholar]
- 58.Kennedy-Nasser AA, Bollard CM, Myers GD, Leung KS, Gottschalk S, Zhang Y, et al. Comparable Outcome of Alternative Donor and Matched Sibling Donor Hematopoietic Stem Cell Transplant for Children With Acute Lymphoblastic Leukemia in First or Second Remission Using Alemtuzumab in a Myeloablative Conditioning Regimen. Biol Blood Marrow Transplant (2008) 14:1245–52. 10.1016/j.bbmt.2008.08.010 [DOI] [PubMed] [Google Scholar]
- 59.Neiman PE, Thomas ED, Reeves WC, Ray CG, Sale G, Lerner KG, et al. Opportunistic Infection and Interstitial Pneumonia Following Marrow Transplantation for Aplastic Anemia and Hematologic Malignancy. Transplant Proc (1976) 8:663–7. [PubMed] [Google Scholar]
- 60.Fujimaki K, Tanaka M, Takasaki H, Hyo R, Kawano T, Sakai R, et al. Thiotepa/Cyclophosphamide/TBI as a Conditioning Regimen for Allogeneic Hematopoietic Stem Cell Transplantation in Patients Aged 50 Years and Over. Internal Med (2008) 47:379–83. 10.2169/internalmedicine.47.0598 [DOI] [PubMed] [Google Scholar]
- 61.Buckner CD, Clift RA, Thomas D, Sanders JE, Stewart PS, Storb R, et al. Allogeneic Marrow Transplantation for Acute Non-Lymphoblastic Leukemia in Relapse Using Fractionated Total Body Irradiation. Leukemia Res (1982) 6:389–94. 10.1016/0145-2126(82)90102-3 [DOI] [PubMed] [Google Scholar]
- 62.Wang HX, Yan HM, Wang ZD, Xue M, Liu J, Guo ZK. Haploidentical Hematopoietic Stem Cell Transplantation in Hematologic Malignancies With G-CSF Mobilized Bone Marrow Plus Peripheral Blood Stem Cells Grafts Without T Cell Depletion: A Single Center Report of 29 Cases. Leukemia Lymphoma (2012) 53:654–9. 10.3109/10428194.2011.624225 [DOI] [PubMed] [Google Scholar]
- 63.Li DZ, Kong PY, Sun JG, Wang XX, Li GH, Zhou YB, et al. Comparison of Total Body Irradiation Before and After Chemotherapy in Pretreatment for Hematopoietic Stem Cell Transplantation. Cancer Biother Radiopharm (2012) 27:119–23. 10.1089/cbr.2011.1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bhatnagar B, Rapoport AP, Fang HB, Ilyas C, Marangoz D, Akbulut V, et al. Single Center Experience With Total Body Irradiation and Melphalan (TBI-MEL) Myeloablative Conditioning Regimen for Allogeneic Stem Cell Transplantation (SCT) in Patients With Refractory Hematologic Malignancies. Ann Hematol (2014) 93:653–60. 10.1007/s00277-013-1908-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Park J, Choi EK, Kim JH, Lee SW, Song SY, Yoon SM, et al. Effects of Total Body Irradiation-Based Conditioning on Allogeneic Stem Cell Transplantation for Pediatric Acute Leukemia: A Single-Institution Study. Br J Cancer (2014) 32:198–207. 10.3857/roj.2014.32.3.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Motohashi K, Fujisawa S, Onizuka M, Kako S, Sakaida E, Shono K, et al. Effects of the Order of Administration of Total Body Irradiation and Cyclophosphamide on the Outcome of Allogeneic Hematopoietic Cell Transplantation. Blood (2014) 124:3900. 10.1182/blood.V124.21.3900.3900 [DOI] [Google Scholar]
- 67.Peters C, Matthes-Martin S, Poetschger U, Schrappe M, Schrauder A. Stem-Cell Transplantation in Children With Acute Lymphoblastic Leukemia:a Prospective International Multicenter Trial Comparing Sibling Donors With Matched Unrelated Donors-the ALL-SCT-BFM-2003 Trial. J Clin Oncol (2015) 33:1265–74. 10.1200/JCO.2014.58.9747 [DOI] [PubMed] [Google Scholar]
- 68.Madden LM, Ngwube AI, Shenoy S, Druley TE, Hayashi RJ. Late Toxicity of a Novel Allogeneic Stem Cell Transplant Using Single Fraction Total Body Irradiation for Hematologic Malignancies in Children. J Pediatr Hematol/Oncol (2015) 37:e94–101. 10.1097/MPH.0000000000000272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abugideiri M, Nanda RH, Butker C, Zhang C, Kim S, Chiang KY, et al. Factors Influencing Pulmonary Toxicity in Children Undergoing Allogeneic Hematopoietic Stem Cell Transplantation in the Setting of Total Body Irradiation-Based Myeloablative Conditioning. IJROBP (2016) 94:349–59. 10.1016/j.ijrobp.2015.10.054 [DOI] [PubMed] [Google Scholar]
- 70.Chiang Y, Tsai CH, Kuo SH, Liu CY, Yao M, Li CC, et al. Reduced Incidence of Interstitial Pneumonitis After Allogeneic Hematopoietic Stem Cell Transplantation Using a Modified Technique of Total Body Irradiation. Sci Rep (2016) 6:36730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ishibashi N, Maebayashi T, Sakaguchi M, Aizawa T, Saito T, Tanaka Y. Prospective Study of Total Body Irradiation as Pretreatment for Hematopoietic Stem Cell Transplantation. Int J Radiat Oncol Biol Phys (2014) 90:S682–83. 10.1016/j.ijrobp.2014.05.2006 [DOI] [Google Scholar]
- 72.Ujaimi RK, Isfahanian N, Russa DJ, Samant R, Bredeson C, Genest P. Pulmonary Toxicity Following Total Body Irradiation for Acute Lymphoblastic Leukaemia: The Ottawa Hospital Cancer Centre (TOHCC) Experience. J Radiother Pract (2016) 15:54–60. 10.1017/S1460396915000497 [DOI] [Google Scholar]
- 73.Stephens SJ, Thomas S, Rizzieri DA, Horwitz ME, Chao NJ, Engemann AM, et al. Myeloablative Conditioning With Total Body Irradiation for AML: Balancing Survival and Pulmonary Toxicity. Adv Radiat Oncol (2016) 1:272–80. 10.1016/j.adro.2016.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Byun HK, Yoon HI, Cho JH, Kim HJ, Cho Y, Suh CO. Factors Associated With Pulmonary Toxicity After Myeloablative Conditioning Using Total Body Irradiation. Int J Radiat Oncol Biol Phys (2017) 99:E179. 10.1016/j.ijrobp.2017.06.1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nagasawa N, Mitsuiki N, Aoki Y, Ono T, Isoda T, Imai K, et al. Effect of Reduced-Intensity Conditioning and the Risk of Late-Onset Non-Infectious Pulmonary Complications in Pediatric Patients. Eur J Haematol (2017) 99:525–31. 10.1111/ejh.12967 [DOI] [PubMed] [Google Scholar]
- 76.Gooptu M, Kim HT, Ho VT, Alyea EP, Koreth J, Armand P, et al. A Comparison of the Myeloablative Conditioning Regimen Fludarabine/Busulfan With Cyclophosphamide/Total Body Irradiation, for Allogeneic Stem Cell Transplantation in the Modern Era: A Cohort Analysis. Biol Blood Marrow Transplant (2018) 24:1733–40. 10.1016/j.bbmt.2018.03.011 [DOI] [PubMed] [Google Scholar]
- 77.Gao RW, Weisdorf DJ, DeFor TE, Ehler E, Dusenbery KE. Influence of Total Body Irradiation Dose Rate on Idiopathic Pneumonia Syndrome in Acute Leukemia Patients Undergoing Allogeneic Hematopoietic Cell Transplantation. Int J Radiat Oncol Biol Phys (2019) 103:180–9. 10.1016/j.ijrobp.2018.09.002 [DOI] [PubMed] [Google Scholar]
- 78.Sabloff M, Chhabra S, Wang T, Fretham C, Kekre N, Abraham A, et al. Comparison of High Doses of Total Body Irradiation in Myeloablative Conditioning Before Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant (2019) 25:2398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wenger DS, Triplette M, Crothers K, Cheng GS, Hill JA, Milano F, et al. Incidence, Risk Factors, and Outcomes of Idiopathic Pneumonia Syndrome After Allogeneic Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant (2020) 26:413–20. 10.1016/j.bbmt.2019.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barrett A. Total Body Irradiation (TBI) Before Bone Marrow Transplantation in Leukaemia: A Co-Operative Study From the European Group for Bone Marrow Transplantation. Br J Radiol (1982) 55:562–7. 10.1259/0007-1285-55-656-562 [DOI] [PubMed] [Google Scholar]
- 81.Oya N, Sasai K, Tachiiri S, Sakamoto T, Nagata Y, Okada T, et al. Influence of Radiation Dose Rate and Lung Dose on Interstitial Pneumonitis After Fractionated Total Body Irradiation: Acute Parotitis may Predict Interstitial Pneumonitis. Int J Hematol (2006) 83:86–91. 10.1532/IJH97.05046 [DOI] [PubMed] [Google Scholar]
- 82.Kelsey CR, Horwitz ME, Chino JP, Craciunescu O, Steffey B, Folz RJ, et al. Severe Pulmonary Toxicity After Myeloablative Conditioning Using Total Body Irradiation: An Assessment of Risk Factors. Int J Radiat Oncol Biol Phys (2011) 81:812–8. 10.1016/j.ijrobp.2010.06.058 [DOI] [PubMed] [Google Scholar]
- 83.De Felice F, Grapulin L, Musio D, Pomponi J, DI Felice C, Iori AP, et al. Treatment Complications and Long-Term Outcomes of Total Body Irradiation in Patients With Acute Lymphoblastic Leukemia: A Single Institute Experience. Anticancer Res (2016) 36:4859–64. 10.21873/anticanres.11049 [DOI] [PubMed] [Google Scholar]
- 84.Brochstein JA, Kernan NA, Groshen S, Cirrincione C, Shank B, Emanuel D, et al. Allogeneic Bone Marrow Transplantation After Hyperfractionated Total-Body Irradiation and Cyclophosphamide in Children With Acute Leukemia. N Engl J Med (1987) 37:1618–24. 10.1056/NEJM198712243172602 [DOI] [PubMed] [Google Scholar]
- 85.Clift RA. Allogeneic Marrow Transplantation Using Fractionated Total Body Irradiation in Patients With Acute Lymphoblastic Leukemia in Relapse. Leukemia Res (1982) 6:401–7. 10.1016/0145-2126(82)90104-7 [DOI] [PubMed] [Google Scholar]
- 86.Resbeut M, Cowen D, Blaise D, Gluckman E, Cosset JM, Rio B, et al. Fractionated or Single-Dose Total Body Irradiation in 171 Acute Myeloblastic Leukemias in First Complete Remission: Is There a Best Choice? Int J Radiat Oncol Biol Phys (1995) 3:509–17. 10.1016/0360-3016(94)00446-R [DOI] [PubMed] [Google Scholar]
- 87.Petersen FB, Deeg HJ, Buckner CD, Appelbaum FR, Storb R, Clift RA, et al. Marrow Transplantation Following Escalating Doses of Fractionated Total Body Irradiation and Cyclophosphamide-a Phase I Trial. Int J Radiat Oncol Biol Phys (1992) 23:1027–32. 10.1016/0360-3016(92)90909-2 [DOI] [PubMed] [Google Scholar]
- 88.Ringdn O, Ruutu T, Remberger M, Nikoskelainen J, Volin L, Vindelov L, et al. A Randomized Trial Comparing Busulfan With Total Body Irradiation as Conditioning in Allogeneic Marrow Transplant Recipients With Leukemia: A Report From the Nordic Bone Marrow Transplantation Group. Blood (1994) 83:2723–30. 10.1182/blood.V83.9.2723.bloodjournal8392723 [DOI] [PubMed] [Google Scholar]
- 89.Corvo R, Paoli G, Barra S. Total Body Irradiation Correlates With Chronic Graft Versus Host Disease and Affects Prognosis of Patients With Acute Lymphoblastic Leukemia Receiving an HLA Identical Allogeneic Bone Marrow Transplant. Int J Radiat Oncol Biol Phys (1999) 43:497–503. 10.1016/S0360-3016(98)00441-6 [DOI] [PubMed] [Google Scholar]
- 90.Huisman C, van der Straaten HM. Pulmonary Complications After T-Cell-Depleted Allogeneic Stem Cell Transplantation: Low Incidence and Strong Association With Acute Graft-Versus-Host Disease. Bone Marrow Transplant (2006) 38:561–6. 10.1038/sj.bmt.1705484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sampath S, Schultheiss TE, Wong J. Dose Response and Factors Related to Interstitial Pneumonitis After Bone Marrow Transplant. Int J Radiat Oncol Biol Phys (2005) 63:876–84. 10.1016/j.ijrobp.2005.02.032 [DOI] [PubMed] [Google Scholar]
- 92.Thomas ED, Herman EC, Greenough WB, Hager EB, Cannon JH, Sahler OD, et al. Irradiation and Marrow Infusion in Leukemia. Arch Internal Med (1961) 107:95–111. 10.1001/archinte.1961.03620060029006 [DOI] [PubMed] [Google Scholar]
- 93.Blume KG, Beutler E, Bross KJ, Chillar RK, Ellington OB, Fahey JL, et al. Bone-Marrow Ablation and Allogeneic Marrow Transplantation in Acute Leukemia. N Engl J Med (1980) 302:1041–6. 10.1056/NEJM198005083021901 [DOI] [PubMed] [Google Scholar]
- 94.Winston DJ, Gale RP, Meyer DV, Young LS. Infectious Complications of Human Bone Marrow Transplantation. Medicine (1979) 58:1–31. 10.1097/00005792-197901000-00001 [DOI] [PubMed] [Google Scholar]
- 95.Bortin MN, Kay HEM, Gale RP, Rimm AA. Factors Associated With Interstitial Pneumonitis After Bone Marrow Transplantation for Acute Leukaemia. Lancet (1982) 20:437–9. 10.1016/S0140-6736(82)91633-6 [DOI] [PubMed] [Google Scholar]
- 96.Cosset JM, Baume D, Pico JL, Shank B, Girinski T, Benhamou E, et al. Single Dose Versus Hyperfractionated Total Body Irradiation Before Allogeneic Bone Marrow Transplantation: A Non-Randomized Comparative Study of 54 Patients at the Institut Gustave-Roussy. Radiother Oncol (1989) 174:92–104. 10.1016/0167-8140(89)90129-1 [DOI] [PubMed] [Google Scholar]
- 97.Bearman SI, Appelbaum FR, Buckner CD, Petersen FB, Fisher LD, Clift RA, et al. Regimen-Related Toxicity in Patients Undergoing Bone Marrow Transplantation. J Clin Oncol (1988) 6:1562–8. 10.1200/JCO.1988.6.10.1562 [DOI] [PubMed] [Google Scholar]
- 98.Volpe AD, Ferreri AJM, Annaloro C, Mangili P, Rosso A, Calandrino R, et al. Lethal Pulmonary Complications Significantly Correlate With Individually Assessed Mean Lung Dose in Patients With Hematologic Malignancies Treated With Total Body Irradiation. Int J Radiat Oncol Biol Phys (2002) 52:483–8. 10.1016/S0360-3016(01)02589-5 [DOI] [PubMed] [Google Scholar]
- 99.Gore EM, Lawton CA, Ash RC, Lipchik RJ. Pulmonary Function Changes in Long-Term Survivors of Bone Marrow Transplantation. Int J Radiat Oncol Biol Phys (1996) 36:67–75. 10.1016/S0360-3016(96)00123-X [DOI] [PubMed] [Google Scholar]
- 100.Esiashvili N, Lu X, Ulin K, Laurie F, Kessel S, Kalapurakal JA, et al. Higher Reported Lung Dose Received During Total Body Irradiation for Allogeneic Hematopoietic Stem Cell Transplantation in Children With Acute Lymphoblastic Leukemia Is Associated With Inferior Survival: A Report From the Children’s Oncology Group. Int J Radiat Oncol Biol Phys (2019) 104:513–21. 10.1016/j.ijrobp.2019.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Neiman P, Wasserman PB, Wentworth BB, Kao GF, Lerner KG, Storb R, et al. Interstitial Pneumonia and Cytomegalovirus Infection as Complications of Human Marrow Transplantation. Transplantation (1973) 15:478–85. 10.1097/00007890-197305000-00011 [DOI] [PubMed] [Google Scholar]
- 102.Clift RA, Buckner CD, Appelbaum FR, Bryant E, Bearman SI, Petersen FB, et al. Allogeneic Marrow Transplantation in Patients With Chronic Myeloid Leukemia in the Chronic Phase: A Randomized Trial of Two Irradiation Regimens. Blood (1991) 77:1660–5. 10.1182/blood.V77.8.1660.bloodjournal7781660 [DOI] [PubMed] [Google Scholar]
- 103.Le Bourgeois J, Vernant J, Thiellet A. Unusual Incidence of Localized Pneumonitis After Total Body Irradiation for Bone Marrow Transplantation. Exp Hematol (1984) S:12–23. [Google Scholar]
- 104.Ozsahin M, Schwartz LH, Pene F, Touboul E, Schlienger M, Laugier A. Is Body Weight a Risk Factor of Interstitial Pneumonitis After Bone Marrow Transplantation? Bone Marrow Transplant (1992) 10:97. [PubMed] [Google Scholar]
- 105.Gluckman E, Devergie A, Dutreix A, Dutreix J, Boiron M, Bernard J. Total Body Irradiation in Bone Marrow Transplantation. Hopital Saint-Louis Results. Pathologie Biologie (1979) 27:349–52. [PubMed] [Google Scholar]
- 106.Speck B, Cornu P, Nissen C, Gratwohl A, Sartorius J. The Basel Experience With Total Body Irradiation for Conditioning Patients With Acute Leukemia for Allogeneic Bone Marrow Transplantation. Pathologie Biologie (1979) 27:353–5. [PubMed] [Google Scholar]
- 107.Brunvand MW, Bensinger WI, Soll E, Weaver CH, Rowley SD, Appelbaum FR, et al. High-Dose Fractionated Total-Body Irradiation, Etoposide and Cyclophosphamide for Treatment of Malignant Lymphoma: Comparison of Autologous Bone Marrow and Peripheral Blood Stem Cells. Bone Marrow Transplant (1996) 18:131–41. [PubMed] [Google Scholar]
- 108.Neiman PE, Reeves W, Ray G, Flournoy N, Lerner KG, Sale GE, et al. A Prospective Analysis of Interstitial Pneumonia and Opportunistic Viral Infection Among Recipients of Allogeneic Bone Marrow Grafts. J Infect Dis (1977) 136:754–67. 10.1093/infdis/136.6.754 [DOI] [PubMed] [Google Scholar]
- 109.Singh AK, Karimpour SE, Savani BN, Guion P, Hope AJ, Mansueti JR, et al. Pretransplant Pulmonary Function Tests Predict Risk of Mortality Following Fractionated Total Body Irradiation and Allogeneic Peripheral Blood Stem Cell Transplant. Int J Radiat Oncol Biol Phys (2006) 66:520–7. 10.1016/j.ijrobp.2006.05.023 [DOI] [PubMed] [Google Scholar]
- 110.Kim DY, Kim IH, Yoon SS, Kang HJ, Shin HY, Kang HC. Effect of Dose Rate on Pulmonary Toxicity in Patients With Hematolymphoid Malignancies Undergoing Total Body Irradiation. Radiat Oncol (2018) 13:180. 10.1186/s13014-018-1116-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Faraci M, Barra S, Cohen A, Lanino E, Grisolia F, Miano M, et al. Very Late Nonfatal Consequences of Fractionated TBI in Children Undergoing Bone Marrow Transplant. Int J Radiat Oncol Biol Phys (2005) 63:1568–75. 10.1016/j.ijrobp.2005.04.031 [DOI] [PubMed] [Google Scholar]
- 112.Bredeson C, Le-Rademacher J, Zhu X, Burkart J, Kato K, Armstrong E, et al. Improved Survival With Intravenous Busulfan (IV BU) Compared to Total Body Irradiation (TBI)-Based Myeloablative Conditioning Regimens: A Cibmtr Prospective Study. Biol Blood Marrow Transplant (2013) 19:S110–1. 10.1016/j.bbmt.2012.11.030 [DOI] [Google Scholar]
- 113.Lucchini G, Labopin M, Beohou E, Dalissier A, Dalle JH, Cornish J, et al. Impact of Conditioning Regimen on Outcomes for Children With Acute Myeloid Leukemia Undergoing Transplantation in First Complete Remission. An Analysis on Behalf of the Pediatric Disease Working Party of the European Group for Blood and Marrow Transplant. Biol Blood Marrow Transplant (2017) 23:467–74. 10.1016/j.bbmt.2016.11.022 [DOI] [PubMed] [Google Scholar]
- 114.Holter-Chakrabarty JL, Pierson N, Zhang MJ, Zhu X, Akpek G, Aljurf MD, et al. The Sequence of Cyclophosphamide and Myeloablative Total Body Irradiation in Hematopoietic Cell Transplantation for Patients With Acute Leukemia. Biol Blood Marrow Transplant (2015) 21:1251–7. 10.1016/j.bbmt.2015.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kebriaei P, Anasetti C, Zhang MJ, Wang HL, Aldoss I, de Lima M, et al. Intravenous Busulfan Compared With Total Body Irradiation Pretransplant Conditioning for Adults With Acute Lymphoblastic Leukemia. Biol Blood marrow Transplant (2018) 24:726–33. 10.1016/j.bbmt.2017.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Arai Y, Aoki K, Takeda J, Kondo T, Eto T, Ota S, et al. Clinical Significance of High-Dose Cytarabine Added to Cyclophosphamide/Total-Body Irradiation in Bone Marrow or Peripheral Blood Stem Cell Transplantation for Myeloid Malignancy. J Hematol Oncol (2015) 8:102. 10.1186/s13045-015-0201-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Linsenmeier C, Thoennessen D, Negretti L, Bourquin JP, Streller T, Lütolf UM, et al. Total Body Irradiation (TBI) in Pediatric Patients: A Single-Center Experience After 30 Years of Low-Dose Rate Irradiation. Strahlentherapie und Onkologie (2010) 186:614–20. 10.1007/s00066-010-2089-2 [DOI] [PubMed] [Google Scholar]
- 118.Freycon F, Casagranda L, Trombert-Paviot B. The Impact of Severe Late-Effects After 12 Gy Fractionated Total Body Irradiation and Allogeneic Stem Cell Transplantation for Childhood Leukemia (1988-2010). Pediatr Hematol Oncol (2019) 36:86–102. 10.1080/08880018.2019.1591549 [DOI] [PubMed] [Google Scholar]
- 119.Harden SV, Routsis DS, Geater AR, Thomas SJ, Coles C, Taylor PJ, et al. Total Body Irradiation Using a Modified Standing Technique: A Single Institution 7 Year Experience. Br J Radiol (2001) 74:1041–7. 10.1259/bjr.74.887.741041 [DOI] [PubMed] [Google Scholar]
- 120.Veys P, Wynn RF, Ahn KW, Samarasinghe S, He W, Bonney D, et al. Impact of Immune Modulation With in Vivo T-Cell Depletion and Myleoablative Total Body Irradiation Conditioning on Outcomes After Unrelated Donor Transplantation for Childhood Acute Lymphoblastic Leukemia. Blood (2012) 119:6155–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sakellari I, Gavriilaki E, Chatziioannou K, Papathanasiou M, Mallouri D, Batsis I, et al. Long-Term Outcomes of Total Body Irradiation Plus Cyclophosphamide Versus Busulfan Plus Cyclophosphamide as Conditioning Regimen for Acute Lymphoblastic Leukemia: A Comparative Study. Ann Hematol (2018) 97:1987–94. 10.1007/s00277-018-3383-9 [DOI] [PubMed] [Google Scholar]
- 122.Tseng YD, Stevenson PA, Cassaday RD, Cowan A, Till BG, Shadman M, et al. Total Body Irradiation Is Safe and Similarly Effective as Chemotherapy-Only Conditioning in Autologous Stem Cell Transplantation for Mantle Cell Lymphoma. Biol Blood Marrow Transplant (2018) 24:282–7. 10.1016/j.bbmt.2017.10.029 [DOI] [PubMed] [Google Scholar]
- 123.Yoshimi A, Nannya Y, Sakata-Yanagimoto M, Oshima K, Takahashi T, Kanda Y, et al. A Myeloablative Conditioning Regimen for Patients With Impaired Cardiac Function Undergoing Allogeneic Stem Cell Transplantation: Reduced Cyclophosphamide Combined With Etoposide and Total Body Irradiation. Am J Hematol (2008) 83:635–9. 10.1002/ajh.21208 [DOI] [PubMed] [Google Scholar]
- 124.Küenkele A, Engelhard M, Hauffa BP, Mellies U, Müntjes C, Hüer C, et al. Long-Term Follow-Up of Pediatric Patients Receiving Total Body Irradiation Before Bone Marrow Transplantation. Pediatr Blood Cancer (2013) 60:1792–7. 10.1002/pbc.24702 [DOI] [PubMed] [Google Scholar]
- 125.Saglio F, Zecca M, Pagliara D, Giorgiani G, Balduzzi A, Calore E, et al. Occurrence of Long-Term Effects After Hematopoietic Stem Cell Transplantation in Children Affected by Acute Leukemia Receiving Either Busulfan or Total Body Irradiation: Results of an AIEOP (Associazione Italiana Ematologia Oncologia Pediatrica) Retrospe. Bone Marrow Transplant (2020) 55:1918–27. 10.1038/s41409-020-0806-8 [DOI] [PubMed] [Google Scholar]
- 126.Belkacemi Y, Labopin M, Giebel S, Miszyk L, Loganadane G, Michallet M, et al. Fractionated-TBI Schedules Prior to Allograft: Study From the Acute Leukemia Working Party (EBMT). Radiother Oncol (2017) 123:S17–8. 10.1016/S0167-8140(17)30486-3 [DOI] [Google Scholar]
- 127.Litzow MR, Prez WS, Klein JP, Bolwell BJ, Camitta B, Copelan EA, et al. Comparison of Outcome Following Allogeneic Bone Marrow Transplantation With Cyclophosphamide-Total Body Irradiation Versus Busulphan-Cyclophosphamide Conditioning Regimens for Acute Myelogenous Leukaemia in First Remission. Br J Haematol (2002) 119:1115–24. 10.1046/j.1365-2141.2002.03973.x [DOI] [PubMed] [Google Scholar]
- 128.Panoskaltsis-Mortari A, Taylor PA, Yaeger TM. The Criticla Early Proinflammatory Events Associated With Idiopathic Pneumonia Syndrome in Irradiated Murine Allogeneic Recipients Are Due to Donor T Cell Infusion and Potentiated by Cyclophosphamide. J Clin Investigations (1997) 100:1015–27. 10.1172/JCI119612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cooke KR, Kobzik L, Martin TR, Brewer J, Delmonte J, Jr, Crawford JM, et al. An Experimental Model of Idiopathic Pneumonia Syndrome After Bone Marrow Transplantation: 1. The Roles of Minor H Antigens and Endotoxin. Blood (1996) 88:3230–39. 10.1182/blood.V88.8.3230.bloodjournal8883230 [DOI] [PubMed] [Google Scholar]
- 130.Janin A, Deschaumes C, Daneshpouy M, Estaquier J, Micic-Polianski J, Rajagopalan-Levasseur P, et al. CD95 Engagement Induces Disseminated Endothelial Cell Apoptosis in Vivo: Immunopathologic Implications. Blood (2002) 99:2940–7. 10.1182/blood.V99.8.2940 [DOI] [PubMed] [Google Scholar]
- 131.Panoskaltsis-Mortari A, Hermanson JR, Haddad IY, Wangensteen OD, Blazar BR. Intercellular Adhesion Molecule-I (ICAM-I, CD54) Deficiency Segregates the Unique Pathophysiological Requirements for Generating Idiopathic Pneumonia Syndrome (IPS) Versus Graft-Versus-Host Disease Following Allogeneic Murine Bone Marrow Transplantation. Biol Blood Marrow Transplant (2001) 7:368–77. 10.1053/bbmt.2001.v7.pm11529486 [DOI] [PubMed] [Google Scholar]
- 132.Goldstein RH. Control of Type I Collagen Formation in the Lung. Am J Physiol (1991) 261:L29–40. 10.1152/ajplung.1991.261.2.L29 [DOI] [PubMed] [Google Scholar]
- 133.Clark JG, Hansen JA, Hertz MI, Parkman R, Jensen L, Peavy HH. Idiopathic Pneumonia Syndrome After Bone Marrow Transplantation. Am Rev Respir Dis (1993) 147:1601–06. 10.1164/ajrccm/147.6_Pt_1.1601 [DOI] [PubMed] [Google Scholar]
- 134.Fukuda T, Hackman RC, Guthrie KA, Sandmaier BM, Boeckh M, Maris MB, et al. Risks and Outcomes of Idiopathic Pneumonia Syndrome After Nonmyeloablative and Conventional Conditioning Regimens for Allogeneic Hematopoietic Stem Cell Transplantation. Blood (2003) 102:2777–85. 10.1182/blood-2003-05-1597 [DOI] [PubMed] [Google Scholar]
- 135.Keates-Baleeiro J, Moore P, Koyama T, Manes B, Calder C, Frangoul H. Incidence and Outcome of Idiopathic Pneumonia Syndrome in Pediatric Stem Cell Transplant Recipients. Bone Marrow Transplant (2006) 38:285–9. 10.1038/sj.bmt.1705436 [DOI] [PubMed] [Google Scholar]
- 136.Shinde A, Yang D, Frankel P, Liu A, Han C, Del Vecchio B, et al. Radiation-Related Toxicities Using Organ Sparing Total Marrow Irradiation Transplant Conditioning Regimens. Int J Radiat Oncol Biol Phys (2019) 105:1025–33. 10.1016/j.ijrobp.2019.08.010 [DOI] [PubMed] [Google Scholar]
- 137.Stein A, Palmer J, Tasi NC, Malki MM, Aldoss I, Ali H, et al. Phase I Trial of Total Marrow and Lymphoid Irradiation Transplantation Conditioning in Patients Wtih Relapsed/Refractory Acute Leukemia. Biol Blood Marrow Transplant (2017) 23:618–24. 10.1016/j.bbmt.2017.01.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wong J, Forman S, Somlo G, Rosenthal J, Liu A, Schultheiss T, et al. Dose Escalation of Total Marrow Irradiation With Concurrent Chemotherapy in Patients With Advanced Acute Leukemia Undergoing Allogeneic Hematopoietic Cell Transplantation. Int J Radiat Oncol Biol Phys (2013) 84:148–56. 10.1016/j.ijrobp.2012.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wood WA, Deal AM, Abernethy A, Basch E, Battaglini C, Kim YH, et al. Feasibility of Frequent Patient-Reported Outcome Surveillance in Patients Undergoing Hematopoietic Cell Transplantation. Blood Marrow Transplant (2013) 19:450–59. 10.1016/j.bbmt.2012.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rosenthal J, Wong J, Stein A, Qian D, Hitt D, Naeem H, et al. Phase 1/2 Trial of Total Marrow and Lymph Node Irradiation to Augment Reduced-Intensity Transplantation for Advanced Hematologic Malignancies. Blood (2010) 117:309–15. 10.1182/blood-2010-06-288357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wong R, Rondon G, Saliiba RM, Shannon VR, Giralt SA, Champlin RE, et al. Idiopathic Pneumonia Syndrome After High-Dose Chemotherapy and Autologous Hematopoietic Stem Cell Transplantation for High-Risk Breast Cancer. Bone Marrow Transplant (2003) 31:1157–63. 10.1038/sj.bmt.1704141 [DOI] [PubMed] [Google Scholar]
- 142.Dusenbery KE, Gerbi BJ. Total Body Irradiation Conditioning Regimens in Stem Cell Transplantation. In: Levitt SH, Purdy JA, editors. Technical Basis of Radiation Therapy. Medical Radiology (Radiation Oncology). Berlin, Heidelberg: Springer; (2006). [Google Scholar]
- 143.Briot E, Dutreix A, Bridier A. Dosimetry for Total Body Irradiation. Radiother Oncol (1990) 18:16–29. 10.1016/0167-8140(90)90175-V [DOI] [PubMed] [Google Scholar]
- 144.Planskoy B, Bedford AM, Davis FM, Tapper PD, Loverock LT. Physical Aspects of Total-Body Irradiation at the Middlesex Hospital (UCL Group of Hospitals), London 1988-1993: I. Phantom Measurements and Planning Methods. Phys Med Biol (1996) 41:2307–26. 10.1088/0031-9155/41/11/005 [DOI] [PubMed] [Google Scholar]
- 145.Hui SK, Das RK, Thomadsen B, Henderson D. CT-Based Analysis of Dose Homogeneity in Total Body Irradiation Using Lateral Beam. J Appl Clin Med Phys (2004) 5:71–9. 10.1120/jacmp.2022.25311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hui SK, Kapatoes J, Fowler J, Henderson D, Olivera G, Manon RR, et al. Feasibility Study of Helical Tomotherapy for Total Body or Total Marrow Irradiation. Med Phys (2005) 32:3214–24. 10.1118/1.2044428 [DOI] [PubMed] [Google Scholar]
- 147.Dandapani SV, Wong JYC. Modern Total Body Irradiation (Tbi): Intensity-Modulated Radiation Treatment (IMRT). In: Wong J, Hui S, editors. Total Marrow Irradiation. Cham: Springer; (2020). [Google Scholar]
- 148.Sarradin V, Simon L, Huynh A, Gilhods J, Filleron T, Izar F. Total Body Irradiation Using Helical Tomotherapy: Treatment Technique, Dosimetric Results, and Initial Clinical Experience. Cancer Radiother (2018) 22:17–24. 10.1016/j.canrad.2017.06.014 [DOI] [PubMed] [Google Scholar]
- 149.Gruen A, Ebell W, Wlodarczyk W, Neumann O, Kuehl JS, Stromberger C, et al. Total Body Irradiation Using Helical Tomotherapy in Children and Young Adults Undergoing Stem Cell Transplantation. Radiat Oncol (2013) 8:92. 10.1186/1748-717X-8-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.