ABSTRACT
Porcine epidemic diarrhea virus (PEDV) is a globally distributed alphacoronavirus that has reemerged lately, resulting in large economic losses. During viral infection, type I interferon (IFN-I) plays a vital role in the antiviral innate immunity. However, PEDV has evolved strategies to limit IFN-I production. To suppress virus replication, the host must activate IFN-stimulated genes and some host restriction factors to circumvent viral replication. This study observed that PEDV infection induced early growth response gene 1 (EGR1) expression in PEDV-permissive cells. EGR1 overexpression remarkably suppressed PEDV replication. In contrast, depletion of EGR1 led to a significant increase in viral replication. EGR1 suppressed PEDV replication by directly binding to the IFN-regulated antiviral (IRAV) promoter and upregulating IRAV expression. A detailed analysis revealed that IRAV interacts and colocalizes with the PEDV nucleocapsid (N) protein, inducing N protein degradation via the E3 ubiquitin ligase MARCH8 to catalyze N protein ubiquitination. Knockdown of endogenous MARCH8 significantly reversed IRAV-mediated N protein degradation. The collective findings demonstrate a new mechanism of EGR1-mediated viral restriction, in which EGR1 upregulates the expression of IRAV to degrade PEDV N protein through MARCH8.
IMPORTANCE PEDV is a highly contagious enteric coronavirus that has rapidly emerged worldwide and has caused severe economic losses. No currently available drugs or vaccines can effectively control PEDV. PEDV has evolved many strategies to limit IFN-I production. We identified EGR1 as a novel host restriction factor and demonstrated that EGR1 suppresses PEDV replication by directly binding to the IRAV promoter and upregulating the expression of IRAV, which interacts with and degrades the PEDV N protein via the E3 ubiquitin ligase MARCH8 to catalyze nucleocapsid protein ubiquitination, which adds another layer of complexity to the innate antiviral immunity of this newly identified restriction factor. A better understanding of the innate immune response to PEDV infection will aid the development of novel therapeutic targets and more effective vaccines against virus infection.
KEYWORDS: PEDV, EGR1, IRAV, nucleocapsid protein, viral replication, antiviral response
INTRODUCTION
Porcine epidemic diarrhea (PED) is caused by the PED virus (PEDV). PED is an acute and highly contagious enteric viral disease characterized by vomiting, watery diarrhea, and a high mortality rate in suckling pigs (1, 2). PED was first reported in England in 1971 and has spread to many swine-producing countries in Europe and Asia (3, 4). In 2010, a large-scale outbreak of PED occurred in China and produced a mortality rate of 80 to 100% in suckling piglets (1, 5, 6). In 2013, PED appeared and swiftly spread in swine farms in the United States. It rapidly and subsequently spread to other countries in North and South America, killed millions of pigs, and resulted in substantial economic losses (7–10). Identifying viral antagonists and investigating the mechanism of escaping innate responses is important for controlling viral epidemics and developing new targets for therapeutics.
PEDV is a single-stranded positive-sense RNA virus in the genus Alphacoronavirus, family Coronaviridae (11). The 28-kb PEDV genome contains at least seven open reading frames (ORFs), which encode two polyproteins (pp1a and pp1ab), four structural proteins (spike [S], envelope [E], membrane [M], and nucleocapsid [N]), and one accessory protein (ORF3) (12, 13). Among the structural proteins, the N protein has multiple functions in viral replication. Besides being a structural protein that protects the viral genome by forming complexes with genomic RNA, N protein plays an important role in promoting viral replication by counteracting the host's innate immunity (14, 15). PEDV N protein can induce endoplasmic reticulum (ER) stress and activate the nuclear factor-κB (NF-κB) signaling pathway by upregulating interleukin-8 (IL-8) expression to promote viral replication (16). N protein also suppresses type I interferon (IFN-I) regulatory factor 3 (IRF3)-mediated IFN-I production by interacting with TANK-binding kinase (TBK1) to block the phosphorylation and nuclear translocation of IRF3. N protein also enhances cell survival by interacting with nucleolar phosphoprotein nucleophosmin 1 (NPM1) during PEDV infection (17, 18). Therefore, N protein is important in the proliferation of viruses and may be a potential drug target for treating PEDV infection.
Early growth response gene 1 (EGR1) is a zinc finger DNA-binding protein and host transcriptional regulator that is involved in multiple cellular processes, such as cell proliferation, cell growth, differentiation, and apoptosis (19–21). EGR1 is activated by various stimuli, including growth factors, cytokines, oxygen deprivation, shear stress, and injury (22–24). Recent evidence has linked EGR1 to viral infection and immune response. EGR1 upregulation can suppress foot-and-mouth disease virus (FMDV) replication by activating the IFN-I signaling pathway (25). However, human cytomegalovirus (HCMV) can use EGR1 to facilitate virus replication and proliferation by binding EGR1 to the viral genome upstream of UL138 to promote UL138 expression (26). EGR1 can also induce proapoptotic pathways to promote Venezuelan equine encephalitis virus replication (27). The function of EGR1 in virus replication varies in different viral infections. This study observed that a robust EGR1 was upregulated during PEDV infection and EGR1 efficiently inhibited PEDV replication. Further analysis demonstrated that EGR1 could upregulate the IFN-regulated antiviral (IRAV) gene (also termed C19orf66, UPF0515, or RyDEN), utilizing the IRAV antiviral function to degrade the PEDV N protein. This study reveals a new antiviral mechanism of EGR1, in which EGR1 suppresses PEDV replication through degradation of the viral N protein.
RESULTS
EGR1 expression is upregulated by PEDV infection.
The host's innate immune system has evolved many intrinsic antiviral proteins to inhibit the distinct stages of the viral life cycle. The host cell protein EGR1 regulates virus replication and the immune response (25–27). The expression of EGR1 was investigated during PEDV infection to explore the EGR1 antiviral function. LLC-PK1 cells were infected with PEDV, and EGR1 expression was assessed using Western blotting. As expected, EGR1 expression increased in LLC-PK1 cells infected with PEDV (multiplicity of infection [MOI] of 1) at 20, 23, and 26 h postinfection (Fig. 1A). The mRNA levels of EGR1 were also upregulated in PEDV-infected LLC-PK1 cells, compared to the levels in uninfected cells (Fig. 1B). The findings indicated that PEDV infection could induce EGR1 expression.
FIG 1.
PEDV infection induces EGR1 expression. (A) LLC-PK1 cells were infected with PEDV (MOI of 1) or mock infected. Cells were harvested at the indicated times. Expression of EGR1 protein was determined by Western blotting using β-actin as the sample loading control. IB, immunoblotting. (B) EGR1 mRNA levels in the samples described for panel A were analyzed using qRT-PCR and were normalized to the expression level of GAPDH. Data are expressed as mean ± SD of triplicate samples. ***, P < 0.001 (two-tailed Student's t test).
EGR1 suppresses PEDV replication.
EGR1-FLAG plasmids or control vectors were transfected into Vero cells to evaluate the role of EGR1 during PEDV infection. The cells were incubated with equal amounts of PEDV (MOI of 0.01) at 24 h posttransfection. The cells and supernatants of the infected cells were collected at the indicated times, and the levels of viral protein expression and viral RNA replication were determined. Overexpression of EGR1 significantly decreased PEDV N protein abundance in Vero cells during PEDV infection (Fig. 2A). Viral RNA detection also revealed that increased expression of EGR1 inhibited PEDV replication (Fig. 2B). To confirm the antiviral role of EGR1 during PEDV infection, we designed and evaluated small interfering RNAs (siRNAs) targeting EGR1. Vero cells were transfected with siRNA to EGR1 and infected with PEDV. PEDV N protein and RNA levels were detected using Western blotting and quantitative real-time PCR (qRT-PCR). Knockdown of endogenous EGR1 considerably increased N protein expression and promoted viral replication during PEDV infection (Fig. 2C and D). These results demonstrated that EGR1 could suppress PEDV propagation.
FIG 2.
EGR1 restricts PEDV infection in cells. (A) Vero cells were transfected with the vector expressing EGR1 or a vector control, infected with PEDV (MOI of 0.01), and collected at the indicated times. PEDV N protein was analyzed with Western blotting using β-actin as the sample loading control. IB, immunoblotting. (B) The viral RNA levels in the culture supernatants of Vero cells treated as described for panel A were determined using qRT-PCR. (C and D) Vero cells were transfected with mEGR1 siRNA or negative-control siRNA for 24 h. The cells were infected with PEDV for 14 h or 20 h. Viral protein abundance (C) and viral RNA levels (D) were measured using Western blotting and qRT-PCR, respectively. Data are expressed as mean ± SD of triplicate samples. ***, P < 0.001 (two-tailed Student's t test).
EGR1 regulates the expression of IRAV.
Vero cells were transfected with plasmids encoding FLAG-EGR1 to increase the expression of EGR1 protein or were transfected with EGR1 siRNA to decrease the expression of EGR1 protein, to investigate the underlying mechanism of EGR1 to inhibit viral proliferation. Changing the abundance of EGR1 protein could significantly regulate IRAV protein expression (Fig. 3A). The IRAV mRNA level was also affected by EGR1 protein expression in Vero cells (Fig. 3B), LLC-PK1 cells (Fig. 3C), and 293T cells (Fig. 3D). Since EGR1 is a transcription factor that can be modulated by many genes and participates in various cellular functions (19, 21, 28), we hypothesized that EGR1 might regulate the expression of IRAV by binding to the IRAV promoter. We amplified the 1,906-bp IRAV promoter sequence and a series of truncated promoters. These were cloned into the pGL3-Basic luciferase vector. The results of the dual luciferase reporter assay showed that the boundaries of the minimal IRAV core promoter were at positions −61 to −1 (Fig. 3E).
FIG 3.
EGR1 directly controls the transcription of IRAV. (A) Vero cells were transfected with the vector expressing EGR1, vector control, mEGR1 siRNA, or negative-control siRNA. IRAV protein abundance was analyzed using Western blotting. β-Actin was used as the sample loading control. IB, immunoblotting. (B to D) The vector expressing EGR1, vector control, mEGR1 siRNA, or negative-control siRNA was transfected into Vero cells (B), LLC-PK1 cells (C), or 293T cells (D) for 24 h. IRAV RNA levels were measured using qRT-PCR. (E) 293T cells were cotransfected with a series of truncated IRAV promoter constructs (D1 to D3 and F1 to F5) and the pRL-TK-Luc Renilla luciferase reporter vector, and the dual luciferase activity was analyzed. (F) The cis-acting elements of IRAV were predicted using the JASPAR vertebrate database (http://jaspar.genereg.net). (G) Vero cells were infected with PEDV (MOI of 0.01) or mock infected and were harvested at 20 h postinfection. ZNF740, EGR1, KLF16, KLF5, KLF4, SP1, SP2, and SP3 mRNA levels were analyzed by qRT-PCR. (H) 293T cells were transfected with the pRL-TK-Luc IRAV promoter-driven luciferase vector and plasmids encoding FLAG-SP1, FLAG-SP2, FLAG-EGR1, or FLAG-ZNF740. The dual luciferase activity was analyzed. (I) 293T cells were transfected with plasmids encoding FLAG-SP1, FLAG-SP2, FLAG-EGR1, or FLAG-ZNF740. qRT-PCR was then used to measure the levels of IRAV transcription. (J) LLC-PK1 cells were transfected with FLAG-EGR1 plasmid or empty vector, and then the cells were harvested at 24 h posttransfection and processed for ChIP analysis. Chromatin-bound EGR1 was precipitated with an anti-FLAG antibody or normal rabbit IgG. The IRAV promoter sequences were amplified with qRT-PCR. Data are expressed as mean ± SD of triplicate samples. *, P < 0.05; ***, P < 0.001 (two-tailed Student′s t test).
To analyze the transcriptional regulation of IRAV, we examined the potential transcription factor binding sites of the IRAV promoter using the JASPAR vertebrate database (http://jaspar.genereg.net) (29). The IRAV core promoter region (−61 to −1 bp) contained ZNF740, KLF16, SP1, EGR1, SP2, SP3, KLF4, and KLF5 binding sites (Fig. 3F). Following this, we detected the mRNA levels of the putative transcription factors using qRT-PCR. Only EGR1 was upregulated, which was consistent with IRAV during PEDV infection (Fig. 3G). To further evaluate whether EGR1 is involved in regulating IRAV expression, the vectors expressing the putative transcription factors were transfected into 293T cells. The dual luciferase reporter assay and qRT-PCR showed that only the expression of EGR1 protein increased the IRAV mRNA levels (Fig. 3H and I). As expected, decreasing the expression of EGR1 protein using EGR1 siRNA downregulated IRAV protein expression (Fig. 3A). A chromatin immunoprecipitation (ChIP) assay was performed with FLAG-EGR1 to immunoprecipitate the IRAV core promoter region, to confirm the binding of EGR1 to the IRAV promoter. The results showed that EGR1 directly binds to the IRAV core promoter region (Fig. 3J). These data indicated that induction of IRAV by EGR1 is likely achieved through EGR1 binding to the base −61 to −1 region of the IRAV promoter.
Activation of IRAV decreases PEDV replication.
IRAV is a novel IFN-stimulated gene (ISG) that suppresses dengue virus, hepatitis C virus, Kaposi's sarcoma-associated herpesvirus, and Zika virus (ZIKV) replication in vitro (30–33). Given these antiviral functions of IRAV, we hypothesized that EGR1 might upregulate IRAV expression to decrease PEDV infection. IRAV protein and mRNA levels increased during PEDV infection (Fig. 4A and B). To assess the role of IRAV in PEDV proliferation, we designed and evaluated siRNAs targeting IRAV and transfected the IRAV-FLAG plasmids or siRNA targeting IRAV to change the abundance of IRAV protein. The cells were incubated with equal amounts of PEDV (MOI of 0.01). The qRT-PCR and 50% tissue culture infective dose (TCID50) assays revealed that overexpression of IRAV significantly decreased PEDV proliferation (Fig. 4C and D). Interfering with IRAV protein expression increased PEDV proliferation (Fig. 4E and F). PEDV N protein levels were consistently significantly lower in Vero cells that had been previously transfected with FLAG-IRAV and higher in Vero cells that had been previously transfected with siIRAV, compared with cells that had been previously transfected with the control (Fig. 4G). The viral titers in the supernatants of the Vero cells overexpressing IRAV were also lower than those in the cells that had been transfected with the empty vector (Fig. 4H). Silencing IRAV expression increased PEDV replication in LLC-PK1 cells (Fig. 4I and J). Together, these results indicated that IRAV participates in the inhibition of PEDV replication.
FIG 4.
Antiviral effect of IRAV against PEDV. (A) LLC-PK1 cells were infected with PEDV (MOI of 1) or mock infected. Cells were harvested at the indicated times. The abundance of IRAV was detected by Western blotting using β-actin as the sample loading control. IB, immunoblotting. (B) IRAV mRNA levels in the culture supernatants of LLC-PK1 cells treated as described for panel A were analyzed by qRT-PCR. The results were normalized to the GAPDH expression level. (C and D) Vero cells were transfected with the vector expressing IRAV or vector control, infected with PEDV (MOI of 0.01), and collected at the indicated times. The viral RNA levels (C) and virus titers (D) were measured by qRT-PCR and TCID50 assay, respectively. (E and F) Vero cells were transfected with negative-control (NC) or IRAV siRNA for 24 h and then infected with PEDV. The viral RNA levels (E) and virus titers (F) were measured by qRT-PCR and TCID50 assay, respectively. (G) The vector expressing IRAV, vector control, mIRAV siRNA, or negative-control siRNA was transfected into Vero cells. The cells were then infected with PEDV and collected at the indicated times. Viral protein abundance was analyzed with Western blotting. (H) PEDV titers in the culture supernatants of the Vero cells treated as described for panel G were measured as TCID50. (I and J) LLC-PK1 cells were transfected with negative-control or IRAV siRNA for 24 h. The cells were infected with PEDV. The viral protein abundance (I) and viral RNA levels (J) were measured by Western blotting and TCID50 assay, respectively. Data are expressed as mean ± SD of triplicate samples. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (two-tailed Student's t test).
IRAV interacts with PEDV N proteins.
To determine the molecular mechanisms by which IRAV suppresses PEDV replication, we performed a coimmunoprecipitation assay. PEDV N was precipitated by FLAG-IRAV (Fig. 5A), and FLAG-IRAV was precipitated by PEDV N (Fig. 5B). Treatment of the cell lysates with RNase did not affect the interaction between IRAV and PEDV N proteins (Fig. 5C), suggesting that the interaction between IRAV and PEDV N protein did not depend on RNA. Besides the coimmunoprecipitation assay, the glutathione S-transferase (GST) pulldown assay also verified the binding between IRAV and PEDV N protein. GST fused to the N protein (GST-N) bound to IRAV but GST protein did not (Fig. 5D), indicating that IRAV directly binds to PEDV N protein. Furthermore, PEDV N protein could efficiently coimmunoprecipitate with the endogenous IRAV protein (Fig. 5E). These findings suggested that IRAV and N form a complex in PEDV-infected cells. Next, we investigated the colocalization of IRAV and N by confocal microscopy in cells coexpressing IRAV-green fluorescent protein (GFP) and N-mCherry after staining with 4,6-diamidino-2-phenylindole (DAPI). IRAV and N proteins colocalized in the cytoplasm (Fig. 5F). The collective data demonstrated that IRAV coimmunoprecipitated with the PEDV N protein. Coronavirus N proteins contain three domains separated by two spacers. The main domain is the RNA-binding domain, and the remaining two domains presumably participate in protein-protein interactions (34–36). To map the essential domains of PEDV N that mediate its association with IRAV, we generated two deletion constructs of N. IRAV interacted with hemagglutinin (HA)-N-(N) (the N terminus of PEDV N spanning amino acids 1 to 278) but not with HA-N-(C) the (C terminus of PEDV N spanning amino acids 271 to 442) (Fig. 5G). This result indicated the importance of the N-terminal region in IRAV-N interaction.
FIG 5.
IRAV colocalizes and interacts with PEDV N protein. (A) 293T cells were transfected for 24 h with plasmids encoding HA-N and FLAG-IRAV or empty vectors, followed by coimmunoprecipitation with anti-FLAG binding beads. Precipitated proteins were analyzed by Western blotting. IB, immunoblotting; IP, immunoprecipitation; WCL, whole-cell lysate. (B) The interaction of IRAV with N protein was precipitated with anti-HA binding beads. (C) Interaction of IRAV with PEDV N protein after RNase treatment. (D) The GST-N and IRAV proteins were expressed in bacterial strain BL21(DE3) and purified for GST pulldown analysis. (E) Vero cells were infected with PEDV (MOI of 1) or mock infected and were harvested at 24 h postinfection. Immunoprecipitation was performed with an anti-PEDV N protein antibody, and Western blot analysis was performed with a monoclonal antibody against PEDV N protein and an anti-IRAV antibody. (F) HeLa cells were transfected with plasmids encoding IRAV-GFP and N-mCherry. Cell nuclei were stained with DAPI 24 h later. Fluorescence signals were observed using confocal immunofluorescence microscopy. Scale bars, 20 μm. (G) 293T cells were transfected with plasmids encoding FLAG-IRAV and HA-N or the indicated N mutants. Cell lysates were precipitated with anti-FLAG binding beads, and the immunocomplexes were analyzed by Western blotting.
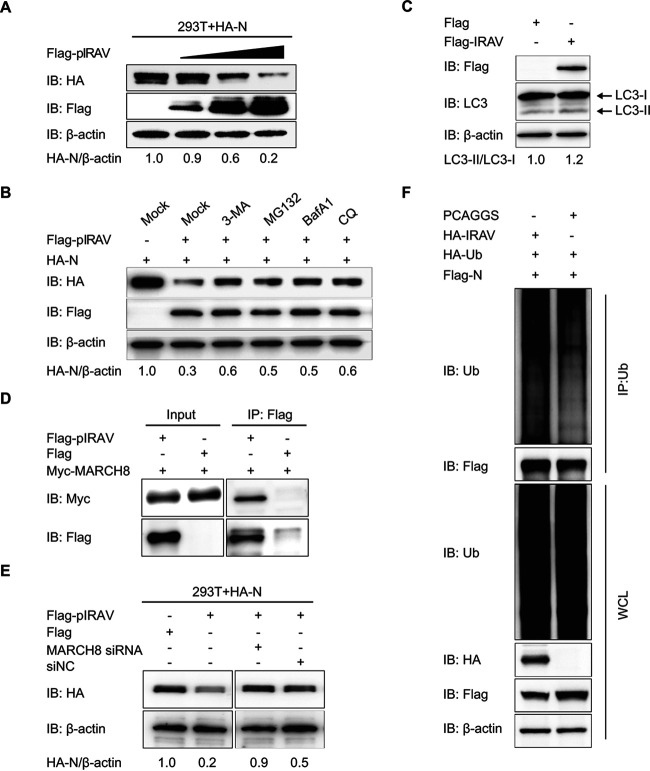
IRAV promotes PEDV N protein degradation through MARCH8 to ubiquitinate N.
Recent evidence indicated that IRAV interacts with ZIKV NS3 protein and degrades the NS3 protein to interrupt replication of ZIKV (33). That observation and the present finding that IRAV interacts with PEDV N protein led us to hypothesize that IRAV also promotes the degradation of N protein. To assess this hypothesis, we transfected 293T cells with increasing amounts of IRAV expression plasmid along with a plasmid expressing PEDV N. The amount of N protein was negatively correlated with the amount of IRAV protein (Fig. 6A). The ubiquitin-proteasome and autolysosome pathways are two major intracellular protein degradation pathways in eukaryotic cells (37). To assess the predominant degradation pathways that mediate the degradation of the PEDV N protein by IRAV, we transfected 293T cells with plasmids encoding FLAG-IRAV and HA-N. The cells were then treated with the autophagy inhibitors 3-methyladenine (3MA), bafilomycin A1 (Baf A1), or chloroquine (CQ) or the proteasome inhibitor MG132. Western blotting showed that treatment with autophagy inhibitors or proteasome inhibitors partly reversed IRAV-mediated N degradation (Fig. 6B), suggesting that IRAV promoted proteasomal and autophagic degradation of PEDV N.
FIG 6.
IRAV expression results in degradation of PEDV N protein. (A) 293T cells were transfected with plasmids encoding HA-N and increasing concentrations of FLAG-IRAV for 24 h. The cell lysates were analyzed with Western blotting using β-actin as the sample loading control. IB, immunoblotting. (B) 293T cells were transfected with plasmids encoding FLAG-IRAV and HA-N. Twenty-four hours later, the cells were treated with 3MA (0.5 mM), MG132 (5 μM), Baf A1 (0.1 μM), or CQ (10 μM). The cell lysates were then analyzed by Western blotting. (C) Vero cells were transfected for 24 h with the vector expressing FLAG-IRAV and HA-N. The cell lysates were analyzed with Western blotting. (D) 293T cells were transfected for 24 h with plasmids encoding Myc-MARCH8 and HA-N, followed by coimmunoprecipitation with anti-FLAG binding beads. Precipitated proteins were analyzed by Western blotting. (E) 293T cells were cotransfected with plasmids encoding HA-N, FLAG-IRAV, and siRNA (MARCH8 siRNA or negative-control siRNA). The cells were analyzed for protein abundance by Western blotting. (F) 293T cells were cotransfected with FLAG-N and HA-IRAV. Cell lysates were harvested 24 h posttransfection. The proteins were immunoprecipitated with anti-ubiquitin (Ub) antibody and analyzed with Western blotting. IP, immunoprecipitation; WCL, whole-cell lysate.
Microtubule-associated protein 1 light chain 3 (MAP1LC3), a hallmark of autophagy, was increased in IRAV-overexpressing Vero cells (Fig. 6C), suggesting that IRAV could increase the levels of autophagy in cells expressing N protein. In protein degradation pathways, the substrate proteins must be ubiquitinated by an E3 ubiquitin ligase. The E3 ubiquitin ligase MARCH8 is important in ubiquitin-PEDV N protein degradation by bone marrow stromal cell antigen 2 (BST2) (29). To test whether MARCH8 is involved in regulating N stability mediated by IRAV, we examined the association of MARCH8 and IRAV (Fig. 6D). We next reduced the endogenous MARCH8 expression in 293T cells overexpressing FLAG-IRAV and HA-N protein. Interestingly, we observed that the knockdown of endogenous MARCH8 significantly reversed N degradation mediated by IRAV (Fig. 6E). The overexpression of IRAV significantly increased the polyubiquitination of N protein (Fig. 6F). The collective results suggested that IRAV-MARCH8 plays a critical role in the ubiquitination of N for protein degradation.
IRAV is essential for EGR1 suppression of PEDV replication.
Since IRAV was upregulated by EGR1 (Fig. 3A) and decreased PEDV infection (Fig. 4G), IRAV might be essential for the EGR1-mediated suppression of PEDV replication. LLC-PK1 cells were transfected with FLAG-EGR1 and the siRNA targeting IRAV and then infected with PEDV at an MOI of 1. Western blotting, qRT-PCR, and TCID50 assays showed that interrupting IRAV expression effectively abolished the inhibition of PEDV replication by EGR1 (Fig. 7A to C). Similar to the results obtained with LLC-PK1 cells, IRAV knockdown also dramatically increased PEDV replication in Vero cells with ectopic expression of EGR1 (Fig. 7D to F), indicating that the ability of EGR1 to inhibit PEDV proliferation was significantly decreased when IRAV expression was interrupted. Altogether, these results suggested that EGR1 suppresses PEDV replication by regulating IRAV to degrade the virus nucleocapsid protein.
FIG 7.
EGR1 inhibits PEDV replication-dependent expression of IRAV. (A to C) LLC-PK1 cells were transfected with plasmids encoding FLAG-IRAV and IRAV siRNA; 24 h later, the cells were infected with PEDV (MOI of 1) and then collected at the indicated times. The protein abundance (A), viral RNA levels (B), and virus titers (C) were measured by Western blotting, qRT-PCR, and TCID50 assay, respectively. β-Actin was used as the sample loading control. IB, immunoblotting. (D to F) Vero cells were transfected with plasmids encoding FLAG-IRAV and IRAV siRNA; 24 h later, the cells were infected with PEDV (MOI of 0.01) and then collected at the indicated times. The lysates and virus titers were analyzed by Western blotting (D), qRT-PCR (E), and TCID50 assay (F). Data are expressed as mean ± SD of triplicate samples. *, P < 0.05; **, P < 0.01 (two-tailed Student's t test).
DISCUSSION
The innate immune response is the first line of defense against viral infections and can prevent the establishment and spread of infection. Host cells quickly activate innate immunity during virus infection to produce IFN-I and induce hundreds of ISGs, creating an antiviral host state (38, 39). PEDV is a highly virulent reemerging enteric coronavirus that causes acute diarrhea, vomiting, and high mortality rates in neonatal piglets (40). The virus has spread rapidly and has caused huge economic losses to the swine industry worldwide (5, 9). There is currently no effective treatment or vaccine for PEDV. In the present study, we identified EGR1 as an effective antiviral agent to suppress PEDV replication and we investigated the underlying molecular mechanism. Our results indicate that EGR1 upregulates IRAV expression and utilizes IRAV to degrade PEDV N protein to suppress PEDV replication.
EGR1 is a multifunctional transcription factor that regulates multiple cellular responses, including cell apoptosis, proliferation, and differentiation (19, 20, 41). Recent evidence has implicated EGR1 in the host antiviral response to regulate virus replication. EGR1 can directly induce the transcription of p53, which has been implicated in functions that play important roles in health and disease (42, 43). EGR1 can also activate IFN-I signaling to decrease the replication of FMDV (25). In this study, we investigated the function of EGR1 and demonstrated its antiviral role against PEDV. EGR1 can be upregulated upon viral infection by severe acute respiratory syndrome, mouse hepatitis virus, and Epstein-Barr virus (44–46). Consistent with the current reports, we also found that EGR1 was upregulated during PEDV infection. Overexpression of EGR1 significantly decreased the replication of PEDV, and knockdown of EGR1 considerably increased PEDV replication.
EGR1 has regulatory roles in various viral infections by regulating the transcription of downstream proteins (21). However, inhibition of PEDV replication by EGR1 remains poorly understood. In this study, we determined that EGR1 upregulated the expression of IRAV. HCMV infection induces EGR-1 expression, and EGR-1 is directly targeted for downregulation by HCMV microRNA-US22 to decrease CD34+ hematopoietic progenitor cell proliferation (41). EGR-1 can also bind to the HCMV genome upstream of UL138 to promote UL138 expression (26). We observed that EGR1 could bind to the promoter of IRAV and induce the expression of IRAV.
IRAV is a newly identified IFN-I-inducible inhibitory factor that suppresses the replication of several important viruses, including dengue virus (30), Kaposi's sarcoma-associated herpesvirus (32), ZIKV (33), and hepatitis C virus (31). Consistent with current reports, we also found that IRAV could inhibit the multiplication of PEDV, and interference with the expression of IRAV renders cells more susceptible to PEDV replication. IRAV binds to dengue virus RNA and the positive modulators polyadenylate-binding protein 1 and La motif-related ribonucleoprotein 1 to inhibit viral replication in cells (47). IRAV also colocalizes with cytoplasmic processing bodies (P bodies) to influence the processing of dengue virus RNA, leading to decreased replication of the virus (30). The observation of the interaction of IRAV with PEDV N protein supports the view that IRAV is critical to the antiviral response against PEDV through differences in the underlying mechanism.
The ubiquitin-proteasome and autolysosome pathways are two major intracellular protein degradation pathways (37, 48). IRAV mediates ZIKV NS3 protein degradation in a lysosome-dependent manner to suppress virus replication (33). Here, we show that IRAV promotes the degradation of N protein. Treatment with the autophagy inhibitors 3MA, Baf A1, and CQ and the proteasome inhibitor MG132 partly reversed N degradation mediated by IRAV, suggesting that IRAV promotes proteasomal and autophagic degradation of PEDV N. Both the ubiquitin-proteasome system and selective autophagy protein degradation pathways require an E3 ubiquitin ligase to ubiquitinate the substrate proteins (49, 50). Our previous study revealed that the E3 ubiquitin ligase MARCH8 catalyzed the ubiquitination of the PEDV N protein during BST2 degradation of N protein (29). Here, we found that the knockdown of endogenous MARCH8 significantly reversed N degradation mediated by IRAV. Our findings prove that MARCH8 plays a critical role in the ubiquitination of N for protein degradation during IRAV degradation of N protein.
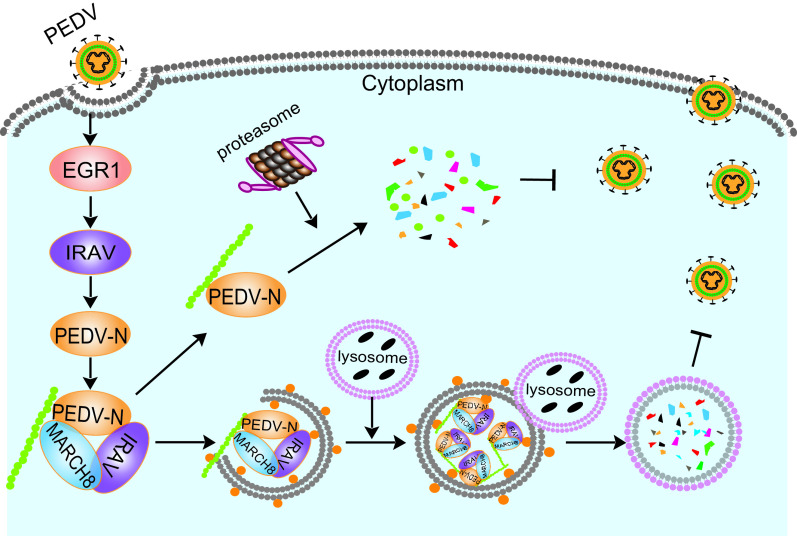
In summary, EGR1 suppresses PEDV replication. This suppression is correlated with decreased abundance of N protein. EGR1 binds to the IRAV promoter to regulate IRAV expression, and IRAV recruits the E3 ubiquitin ligase MARCH8 to catalyze the N protein and degrade the N protein (Fig. 8). Our study provides a novel antiviral mechanism of EGR1, which may offer a new therapeutic agent for PEDV infection.
FIG 8.
Proposed working model to illustrate how the EGR1-IRAV-MARCH8 axis inhibits PEDV replication. During PEDV infection, EGR1 binds to IRAV promoter to upregulate the expression of IRAV, which recruits the E3 ubiquitin ligase MARCH8 to catalyze the N protein and degrade the N protein.
MATERIALS AND METHODS
Antibodies and reagents.
Anti-EGR1 antibody (catalog no. 4154) and anti-HA antibody (catalog no. 3724) were purchased from Cell Signaling Technology. Rabbit anti-FLAG antibody (catalog no. F7425), mouse anti-Myc antibody (catalog no. M4439), and anti-FLAG M2 antibody (catalog no. F1804) were obtained from Sigma-Aldrich. Antiubiquitin antibody (catalog no. SC-8017) was obtained from Santa Cruz Biotechnology. Anti-GST antibody (catalog no. 10000-0-AP), anti-β-actin antibody (catalog no. 60008–1), anti-MARCHF8 antibody (catalog no. 14119-1-AP), horseradish peroxidase (HRP)-conjugated anti-rabbit IgG antibody (catalog no. SA00001-2), and HRP-conjugated anti-mouse IgG antibody (catalog no. SA00001-1) were purchased from Proteintech Group. Monoclonal antibodies to porcine IRAV protein and PEDV (PEDV JS-2013) N protein were prepared in our laboratory (51).
Baf A1 (catalog no. 54645) was purchased from Cell Signaling Technology. MG132 (catalog no. M7449), CQ (catalog no. PHR1258), and 3-MA (catalog no. M9281) were purchased from Sigma-Aldrich. EGR1 siRNA, IRAV siRNA, and control siRNA were designed and purchased from GenePharma (Table 1). Human MARCH8 siRNA (catalog no. sc-90432) and human ATG5 siRNA (catalog no. sc-41445) were purchased from Santa Cruz Biotechnology. The Dual-Glo luciferase assay system (catalog no. DL101-01) was purchased from Vazyme Biotech.
TABLE 1.
siRNA sequences used in this study
| Namea | Sequence (5′ to 3′) |
|---|---|
| si-pIRAV sense | GCCAUUGACAGAAGCCAACTT |
| si-pIRAV antisense | GUUGGCUUCUGUCAAUGGCTT |
| si-mIRAV sense | CCACUGUGGCCACCUGCUUTT |
| si-mIRAV antisense | AAGCAGGUGGCCACAGUGGTT |
| si-pEGR1 sense | GCACAGUGGUUUCCCAUCATT |
| si-pEGR1 antisense | UGAUGGGAAACCACUGUGCTT |
| si-mEGR1 sense | UCAUCAAACCCAGCCGAAUTT |
| si-mEGR1 antisense | AUUCGGCUGGGUUUGAUGATT |
| NC sense | UUCUCCGAACGUGUCACGUTT |
| NC antisense | ACGUGACACGUUCGGAGAATT |
NC, negative control.
Cell culture and virus.
African green monkey kidney cells (Vero cells, CCL-81; American Type Culture Collection [ATCC]) and 293T human embryonic kidney cells (CRL-11268; ATCC) were maintained in Dulbecco's modified Eagle's medium (DMEM) (catalog no. D6429; Sigma-Aldrich) supplemented with 10% fetal bovine serum (FBS) (catalog no. 10099141; Gibco). Porcine kidney cells were kindly provided by Rui Luo (Huazhong Agricultural University, Wuhan, China) and were cultured in modified Eagle’s medium (catalog no. 11095080; Invitrogen). All cells were incubated at 37°C in an atmosphere of 5% CO2.
The PEDV variant strain JS-2013 was isolated and stored in our laboratory as described previously (52). JS-2013 was propagated and titrated using Vero cells as described previously (29, 53). The viral titers were calculated using Karber's method and expressed as TCID50.
Plasmids and transfection.
The recombinant plasmids encoding EGR1 and other proteins were cloned in the p3×FLAG-CMV-7.1 vector (catalog no. P1118; Sigma-Aldrich) by homologous recombination using the ClonExpress II one-step cloning kit (catalog no. C112-02; Vazyme Biotech) as described previously (29). Transfection of 293T or Vero cells was performed using Lipofectamine 3000 reagent (catalog no. L3000015; Invitrogen) when the cells had grown to approximately 80 to 90% confluence. The siRNAs were transfected into cells using Lipofectamine RNAiMAX (catalog no. 13778150; Invitrogen) when the cells had grown to approximately 50 to 60% confluence, according to the manufacturer's instructions.
RNA extraction and qRT-PCR.
Cells were washed twice with phosphate-buffered saline (PBS) and lysed using a lysis buffer. Total RNA was extracted using an RNeasy minikit (catalog no. 74104; Qiagen) according to the manufacturer's instructions. One microgram of RNA was transcribed to cDNA using the PrimeScript RT reagent kit (catalog no. RRO47A; TaKaRa Bio). The synthesized cDNA was analyzed by qRT-PCR using SYBR Premix Ex Taq (catalog no. q711-03; Vazyme Biotech) according to the manufacturer's instructions. β-Actin and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) genes were used as the reference genes for normalization.
Western blotting.
Cells were washed with cold PBS and lysed on ice for 5 min with RIPA lysis and extraction buffer (catalog no. 89901; Thermo Fisher Scientific) containing phosphatase inhibitor cocktail (catalog no. B15001; Bimake) and protease inhibitor cocktail (catalog no. B14001; Bimake). The cell lysates were centrifuged at 12,000 × g for 10 min at 4°C and denatured for 5 min in 5× SDS-PAGE loading buffer. Proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes for Western blotting (catalog no. 10600001; GE Healthcare). The membranes were blocked for 1 h with 5% nonfat dry milk (catalog no. 232100; BD) in PBS containing 0.2% Tween 20 (0.2% PBST) (catalog no. P1379; Sigma-Aldrich) and were incubated with the primary antibody for 1 h at room temperature. The membranes were then washed with 0.1% PBST and incubated with HRP-conjugated secondary antibody for 1 h at 25°C. Proteins were detected with enhanced chemiluminescence (ECL) substrate (catalog no. 34580; Thermo Fisher Scientific).
Coimmunoprecipitation assay.
Cells were transfected with the indicated plasmids for 24 h and lysed for 30 min at 4°C with NP-40 cell lysis buffer (catalog no. FNN0021; Life Technologies) supplemented with protease inhibitor cocktail. The lysates were cleared by centrifugation and incubated for 30 min at 25°C with affinity antibodies bound to protein G Dynabeads (catalog no. 10004D; Life Technologies). The Dynabeads were washed five times with 0.02% PBST and boiled in sample buffer. The proteins were analyzed using standard immunoblotting procedures.
Dual luciferase reporter assay.
293T cells were grown in 24-well plates and transfected with the pRL-TK luciferase reporter plasmid and the indicated expression plasmid or an empty control plasmid using Lipofectamine 3000. The cells were lysed 24 h later. According to the manufacturer's protocol, firefly luciferase and Renilla luciferase activities were measured using the dual luciferase reporter assay system. Luciferase values were normalized using Renilla luciferase as the internal control. Data were obtained from three independent experiments.
GST pulldown.
The full-length PEDV N gene and IRAV gene were cloned into pCold GST plasmid (catalog no. 3372; Clontech Laboratories) or pCold TF plasmid (catalog no. 3365; Clontech Laboratories). Recombinant proteins were expressed in BL21 competent cells, and the interaction of N and IRAV proteins was analyzed with the GST protein interaction pulldown kit (catalog no. 21516; Thermo Fisher Scientific), according to the manufacturer's instructions. The proteins were eluted with reduced glutathione and analyzed by Western blotting.
ChIP assay.
LLC-PK1 cells were transfected with FLAG-EGR1 plasmid or empty vector; the cells were harvested at 24 h posttransfection and processed for ChIP analysis using the SimpleChIP enzymatic ChIP kit (catalog no. 9003; Cell Signaling Technology), according to the manufacturer's instructions. Chromatin-bound EGR1 was precipitated with anti-FLAG antibody or normal rabbit IgG, and the IRAV promoter sequences were amplified with qRT-PCR.
Fluorescence microscopy.
Cells were seeded on coverslips and transfected with IRAV-GFP and N-mCherry plasmids. Cells were fixed with 4% paraformaldehyde (catalog no. P6148; Sigma-Aldrich) for 15 min and permeabilized with 0.1% Triton X-100 (catalog no. T9284; Sigma-Aldrich) for 10 min at room temperature. After three washes with PBS, nuclei were stained with DAPI (catalog no. C1002; Beyotime Biotechnology) for 5 min at room temperature. The cells were observed by confocal immunofluorescence microscopy (Carl Zeiss).
Statistical analysis.
Data were presented as mean ± standard deviation (SD) and analyzed with the two-tailed Student's t test. GraphPad Prism 5 software (GraphPad Software) was used. P values of <0.05 were considered statistically significant.
ACKNOWLEDGMENTS
This study was supported by the National Key Research and Development Programs of China (grants 2016YFD0500103 and 2017YFC1200201), the National Natural Science Foundation of China (grant 31872478), and the Natural Science Foundation of Shanghai (grant 19ZR1469100).
Contributor Information
Guangzhi Tong, Email: gztong@shvri.ac.cn.
Tongling Shan, Email: shantongling@shvri.ac.cn.
Bryan R. G. Williams, Hudson Institute of Medical Research
REFERENCES
- 1.Sun RQ, Cai RJ, Chen YQ, Liang PS, Chen DK, Song CX. 2012. Outbreak of porcine epidemic diarrhea in suckling piglets, China. Emerg Infect Dis 18:161–163. 10.3201/eid1801.111259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun D, Wang X, Wei S, Chen J, Feng L. 2016. Epidemiology and vaccine of porcine epidemic diarrhea virus in China: a mini-review. J Vet Med Sci 78:355–363. 10.1292/jvms.15-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wood EN. 1977. An apparently new syndrome of porcine epidemic diarrhoea. Vet Rec 100:243–244. 10.1136/vr.100.12.243. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi K, Okada K, Ohshima K. 1983. An outbreak of swine diarrhea of a new-type associated with coronavirus-like particles in Japan. Nihon Juigaku Zasshi 45:829–832. 10.1292/jvms1939.45.829. [DOI] [PubMed] [Google Scholar]
- 5.Li W, Li H, Liu Y, Pan Y, Deng F, Song Y, Tang X, He Q. 2012. New variants of porcine epidemic diarrhea virus, China, 2011. Emerg Infect Dis 18:1350–1353. 10.3201/eid1808.120002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang XM, Niu BB, Yan H, Gao DS, Yang X, Chen L, Chang HT, Zhao J, Wang CQ. 2013. Genetic properties of endemic Chinese porcine epidemic diarrhea virus strains isolated since 2010. Arch Virol 158:2487–2494. 10.1007/s00705-013-1767-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Q, Li G, Stasko J, Thomas JT, Stensland WR, Pillatzki AE, Gauger PC, Schwartz KJ, Madson D, Yoon KJ, Stevenson GW, Burrough ER, Harmon KM, Main RG, Zhang J. 2014. Isolation and characterization of porcine epidemic diarrhea viruses associated with the 2013 disease outbreak among swine in the United States. J Clin Microbiol 52:234–243. 10.1128/JCM.02820-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marthaler D, Jiang Y, Otterson T, Goyal S, Rossow K, Collins J. 2013. Complete genome sequence of porcine epidemic diarrhea virus strain USA/Colorado/2013 from the United States. Genome Announc 1:e00555-13. 10.1128/genomeA.00555-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stevenson GW, Hoang H, Schwartz KJ, Burrough ER, Sun D, Madson D, Cooper VL, Pillatzki A, Gauger P, Schmitt BJ, Koster LG, Killian ML, Yoon KJ. 2013. Emergence of porcine epidemic diarrhea virus in the United States: clinical signs, lesions, and viral genomic sequences. J Vet Diagn Invest 25:649–654. 10.1177/1040638713501675. [DOI] [PubMed] [Google Scholar]
- 10.Vlasova AN, Marthaler D, Wang Q, Culhane MR, Rossow KD, Rovira A, Collins J, Saif LJ. 2014. Distinct characteristics and complex evolution of PEDV strains, North America, May 2013–February 2014. Emerg Infect Dis 20:1620–1628. 10.3201/eid2010.140491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brian DA, Baric RS. 2005. Coronavirus genome structure and replication. Curr Top Microbiol Immunol 287:1–30. 10.1007/3-540-26765-4_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duarte M, Gelfi J, Lambert P, Rasschaert D, Laude H. 1993. Genome organization of porcine epidemic diarrhoea virus. Adv Exp Med Biol 342:55–60. 10.1007/978-1-4615-2996-5_9. [DOI] [PubMed] [Google Scholar]
- 13.Kocherhans R, Bridgen A, Ackermann M, Tobler K. 2001. Completion of the porcine epidemic diarrhoea coronavirus (PEDV) genome sequence. Virus Genes 23:137–144. 10.1023/a:1011831902219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McBride R, van Zyl M, Fielding BC. 2014. The coronavirus nucleocapsid is a multifunctional protein. Viruses 6:2991–3018. 10.3390/v6082991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Q, Yoo D. 2016. Immune evasion of porcine enteric coronaviruses and viral modulation of antiviral innate signaling. Virus Res 226:128–141. 10.1016/j.virusres.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu X, Zhang H, Zhang Q, Huang Y, Dong J, Liang Y, Liu HJ, Tong D. 2013. Porcine epidemic diarrhea virus N protein prolongs S-phase cell cycle, induces endoplasmic reticulum stress, and up-regulates interleukin-8 expression. Vet Microbiol 164:212–221. 10.1016/j.vetmic.2013.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding Z, Fang L, Jing H, Zeng S, Wang D, Liu L, Zhang H, Luo R, Chen H, Xiao S. 2014. Porcine epidemic diarrhea virus nucleocapsid protein antagonizes beta interferon production by sequestering the interaction between IRF3 and TBK1. J Virol 88:8936–8945. 10.1128/JVI.00700-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi D, Shi H, Sun D, Chen J, Zhang X, Wang X, Zhang J, Ji Z, Liu J, Cao L, Zhu X, Yuan J, Dong H, Wang X, Chang T, Liu Y, Feng L. 2017. Nucleocapsid interacts with NPM1 and protects it from proteolytic cleavage, enhancing cell survival, and is involved in PEDV growth. Sci Rep 7:39700. 10.1038/srep39700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papanikolaou NA, Tillinger A, Liu X, Papavassiliou AG, Sabban EL. 2014. A systems approach identifies co-signaling molecules of early growth response 1 transcription factor in immobilization stress. BMC Syst Biol 8:100. 10.1186/s12918-014-0100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pagel JI, Deindl E. 2011. Early growth response 1: a transcription factor in the crossfire of signal transduction cascades. Indian J Biochem Biophys 48:226–235. [PubMed] [Google Scholar]
- 21.Thiel G, Cibelli G. 2002. Regulation of life and death by the zinc finger transcription factor Egr-1. J Cell Physiol 193:287–292. 10.1002/jcp.10178. [DOI] [PubMed] [Google Scholar]
- 22.Nishi H, Nishi KH, Johnson AC. 2002. Early growth response-1 gene mediates up-regulation of epidermal growth factor receptor expression during hypoxia. Cancer Res 62:827–834. [PubMed] [Google Scholar]
- 23.Sukhatme VP, Kartha S, Toback FG, Taub R, Hoover RG, Tsai-Morris CH. 1987. A novel early growth response gene rapidly induced by fibroblast, epithelial cell and lymphocyte mitogens. Oncogene Res 1:343–355. [PubMed] [Google Scholar]
- 24.Milbrandt J. 1987. A nerve growth factor-induced gene encodes a possible transcriptional regulatory factor. Science 238:797–799. 10.1126/science.3672127. [DOI] [PubMed] [Google Scholar]
- 25.Zhu Z, Du X, Li P, Zhang X, Yang F, Cao W, Tian H, Zhang K, Liu X, Zheng H. 2018. Early growth response gene-1 suppresses foot-and-mouth disease virus replication by enhancing type I interferon pathway signal transduction. Front Microbiol 9:2326. 10.3389/fmicb.2018.02326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buehler J, Carpenter E, Zeltzer S, Igarashi S, Rak M, Mikell I, Nelson JA, Goodrum F. 2019. Host signaling and EGR1 transcriptional control of human cytomegalovirus replication and latency. PLoS Pathog 15:e1008037. 10.1371/journal.ppat.1008037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baer A, Lundberg L, Swales D, Waybright N, Pinkham C, Dinman JD, Jacobs JL, Kehn-Hall K. 2016. Venezuelan equine encephalitis virus induces apoptosis through the unfolded protein response activation of EGR1. J Virol 90:3558–3572. 10.1128/JVI.02827-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silverman ES, Collins T. 1999. Pathways of Egr-1-mediated gene transcription in vascular biology. Am J Pathol 154:665–670. 10.1016/S0002-9440(10)65312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kong N, Shan T, Wang H, Jiao Y, Zuo Y, Li L, Tong W, Yu L, Jiang Y, Zhou Y, Li G, Gao F, Yu H, Zheng H, Tong G. 2020. BST2 suppresses porcine epidemic diarrhea virus replication by targeting and degrading virus nucleocapsid protein with selective autophagy. Autophagy 16:1737–1752. 10.1080/15548627.2019.1707487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balinsky CA, Schmeisser H, Wells AI, Ganesan S, Jin T, Singh K, Zoon KC. 2017. IRAV (FLJ11286), an interferon-stimulated gene with antiviral activity against dengue virus, interacts with MOV10. J Virol 91:e01606-16. 10.1128/JVI.01606-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kinast V, Plociennikowska A, Anggakusuma Bracht T, Todt D, Brown RJP, Boldanova T, Zhang Y, Bruggemann Y, Friesland M, Engelmann M, Vieyres G, Broering R, Vondran FWR, Heim MH, Sitek B, Bartenschlager R, Pietschmann T, Steinmann E. 2020. C19orf66 is an interferon-induced inhibitor of HCV replication that restricts formation of the viral replication organelle. J Hepatol 73:549–558. 10.1016/j.jhep.2020.03.047. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez W, Srivastav K, Muller M. 2019. C19ORF66 broadly escapes virus-induced endonuclease cleavage and restricts Kaposi's sarcoma-associated herpesvirus. J Virol 93:e00373-19. 10.1128/JVI.00373-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu Y, Yang X, Yao Z, Dong X, Zhang D, Hu Y, Zhang S, Lin J, Chen J, An S, Ye H, Zhang S, Qiu Z, He Z, Huang M, Wei G, Zhu X. 2020. C19orf66 interrupts Zika virus replication by inducing lysosomal degradation of viral NS3. PLoS Negl Trop Dis 14:e0008083. 10.1371/journal.pntd.0008083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masters PS. 1992. Localization of an RNA-binding domain in the nucleocapsid protein of the coronavirus mouse hepatitis virus. Arch Virol 125:141–160. 10.1007/BF01309634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parker MM, Masters PS. 1990. Sequence comparison of the N genes of five strains of the coronavirus mouse hepatitis virus suggests a three domain structure for the nucleocapsid protein. Virology 179:463–468. 10.1016/0042-6822(90)90316-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang CK, Hou MH, Chang CF, Hsiao CD, Huang TH. 2014. The SARS coronavirus nucleocapsid protein: forms and functions. Antiviral Res 103:39–50. 10.1016/j.antiviral.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mizushima N, Komatsu M. 2011. Autophagy: renovation of cells and tissues. Cell 147:728–741. 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 38.Ivashkiv LB, Donlin LT. 2014. Regulation of type I interferon responses. Nat Rev Immunol 14:36–49. 10.1038/nri3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Langel SN, Wang Q, Vlasova AN, Saif LJ. 2020. Host factors affecting generation of immunity against porcine epidemic diarrhea virus in pregnant and lactating swine and passive protection of neonates. Pathogens 9:130. 10.3390/pathogens9020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song D, Park B. 2012. Porcine epidemic diarrhoea virus: a comprehensive review of molecular epidemiology, diagnosis, and vaccines. Virus Genes 44:167–175. 10.1007/s11262-012-0713-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mikell I, Crawford LB, Hancock MH, Mitchell J, Buehler J, Goodrum F, Nelson JA. 2019. HCMV miR-US22 down-regulation of EGR-1 regulates CD34+ hematopoietic progenitor cell proliferation and viral reactivation. PLoS Pathog 15:e1007854. 10.1371/journal.ppat.1007854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fuhrman LE, Goel AK, Smith J, Shianna KV, Aballay A. 2009. Nucleolar proteins suppress Caenorhabditis elegans innate immunity by inhibiting p53/CEP-1. PLoS Genet 5:e1000657. 10.1371/journal.pgen.1000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu J, Grogan L, Nau MM, Allegra CJ, Chu E, Wright JJ. 2001. Physical interaction between p53 and primary response gene Egr-1. Int J Oncol 18:863–870. 10.3892/ijo.18.4.863. [DOI] [PubMed] [Google Scholar]
- 44.Cai Y, Liu Y, Zhang X. 2006. Induction of transcription factor Egr-1 gene expression in astrocytoma cells by murine coronavirus infection. Virology 355:152–163. 10.1016/j.virol.2006.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li SW, Wang CY, Jou YJ, Yang TC, Huang SH, Wan L, Lin YJ, Lin CW. 2016. SARS coronavirus papain-like protease induces Egr-1-dependent up-regulation of TGF-β1 via ROS/p38 MAPK/STAT3 pathway. Sci Rep 6:25754. 10.1038/srep25754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim JH, Kim WS, Park C. 2013. Epstein-Barr virus latent membrane protein 1 increases genomic instability through Egr-1-mediated up-regulation of activation-induced cytidine deaminase in B-cell lymphoma. Leuk Lymphoma 54:2035–2040. 10.3109/10428194.2013.769218. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki Y, Chin WX, Han Q, Ichiyama K, Lee CH, Eyo ZW, Ebina H, Takahashi H, Takahashi C, Tan BH, Hishiki T, Ohba K, Matsuyama T, Koyanagi Y, Tan YJ, Sawasaki T, Chu JJ, Vasudevan SG, Sano K, Yamamoto N. 2016. Characterization of RyDEN (C19orf66) as an interferon-stimulated cellular inhibitor against dengue virus replication. PLoS Pathog 12:e1005357. 10.1371/journal.ppat.1005357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mizushima N, Yoshimori T, Levine B. 2010. Methods in mammalian autophagy research. Cell 140:313–326. 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deretic V, Saitoh T, Akira S. 2013. Autophagy in infection, inflammation and immunity. Nat Rev Immunol 13:722–737. 10.1038/nri3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Komander D, Rape M. 2012. The ubiquitin code. Annu Rev Biochem 81:203–229. 10.1146/annurev-biochem-060310-170328. [DOI] [PubMed] [Google Scholar]
- 51.Pan X, Kong N, Shan T, Zheng H, Tong W, Yang S, Li G, Zhou E, Tong G. 2015. Monoclonal antibody to N protein of porcine epidemic diarrhea virus. Monoclon Antib Immunodiagn Immunother 34:51–54. 10.1089/mab.2014.0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kong N, Wu Y, Meng Q, Wang Z, Zuo Y, Pan X, Tong W, Zheng H, Li G, Yang S, Yu H, Zhou EM, Shan T, Tong G. 2016. Suppression of virulent porcine epidemic diarrhea virus proliferation by the PI3K/Akt/GSK-3α/β pathway. PLoS One 11:e0161508. 10.1371/journal.pone.0161508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kong N, Meng Q, Jiao Y, Wu Y, Zuo Y, Wang H, Sun D, Dong S, Zhai H, Tong W, Zheng H, Yu H, Tong G, Xu Y, Shan T. 2020. Identification of a novel B-cell epitope in the spike protein of porcine epidemic diarrhea virus. Virol J 17:46. 10.1186/s12985-020-01305-1. [DOI] [PMC free article] [PubMed] [Google Scholar]