Abstract
Introduction:
Sleep, sedentary behavior, and moderate-to-vigorous physical activity (MVPA) are altered in pregnancy and may affect pregnancy health; however, how these behaviors are associated with each other is unclear.
Methods:
Pregnant women (N = 120) completed the Pittsburgh Sleep Quality Index and wore an activPAL3 micro and ActiGraph GT3X for 7 days in each trimester to assess sleep, sedentary behavior, and MVPA, respectively. Latent trajectories described patterns of sleep duration, efficiency, and quality as well as sedentary behavior and MVPA. Multinomial logistic regression examined associations of sleep patterns with sedentary behavior and MVPA patterns and, in exploratory analyses, with adverse pregnancy outcomes.
Results:
Trajectories were identified for sleep duration (consistently short, 20.7% of sample; consistently adequate, 79.3%), efficiency (consistently low, 17.5%; consistently high, 82.5%), and quality (consistently poor, 15.1%; worsening, 23.5%; and consistently good, 61.5%). Compared with those in more optimal sleep groups, women in the short duration, low efficiency, and worsening quality groups had lower odds of being in the moderate and/or high sedentary behavior group (odds ratio range, 0.21–0.31; 95% confidence interval range, 0.09–0.65). Women in the worsening quality group had greater odds of being in the low MVPA group (odds ratio, 2.51; 95% confidence interval, 1.18–5.38). Trends were observed with women in less optimal sleep groups having greater odds of adverse pregnancy outcomes and lower odds of excessive gestational weight gain.
Conclusions:
Less optimal sleep patterns in pregnancy are associated with less sedentary behavior and MVPA; additional research is needed to confirm associations between sleep and pregnancy outcomes.
Poor sleep is an extremely common complaint during pregnancy, with nearly 80% of pregnant women self-reporting poor sleep across pregnancy trimesters (Mindell, Cook, & Nikolovski, 2015), compared with 35%–52% of nonpregnant women (Asghari, Farhadi, Kamrava, Ghalehbaghi, & Nojomi, 2012; Beaudreau et al., 2012; Ko, Chang, & Chen, 2010). The high prevalence of sleep complaints in pregnancy is particularly concerning given the known associations of poor sleep with cardiovascular and metabolic outcomes in the general adult population (Cappuccio, Cooper, D’Elia, Strazzullo, & Miller, 2011; Doyle et al., 2019; Larcher, Benhamou, Pepin, & Borel, 2015).
Moderate-to-vigorous physical activity (MVPA) and sedentary behavior are two additional lifestyle behaviors that are altered in pregnancy. Despite the well-known health benefits of MVPA in pregnant women (2018 Physical Activity Guidelines Advisory Committee, 2018), more than three-fourths of pregnant women do not meet the aerobic physical activity guidelines (Evenson & Wen, 2011) and MVPA levels in pregnancy seem to decrease as pregnancy progresses (Evenson & Wen, 2011). Sedentary behavior, which is defined as any waking behavior characterized by an energy expenditure of 1.5 or more metabolic equivalents while in a sitting, reclining, or lying posture (Tremblay et al., 2017) is emerging as an independent risk factor for cardiovascular disease and diabetes in nonpregnant populations (Biswas et al., 2015; Thorp, Owen, Neuhaus, & Dunstan, 2011). Epidemiological evidence indicates that young adults including pregnant women spend the majority of their waking hours (approximately 60%) engaged in sedentary behaviors (Fazzi, Saunders, Linton, Norman, & Reynolds, 2017; Hawkins, Kim, Gabriel, Rockette-Wagner, & Chasan-Taber, 2017), and sedentary behavior may increase across pregnancy trimesters (Di Fabio, Blomme, Smith, Welk, & Campbell, 2015; Hawkins et al., 2017).
Sleep, MVPA, and sedentary behavior are three lifestyle behaviors that are altered during pregnancy. There is some evidence in nonpregnant populations that poor sleep is associated with less subsequent physical activity (Baron, Reid, & Zee, 2013; Bromley, Booth, Kilkus, Imperial, & Penev, 2012; Lambiase, Gabriel, Kuller, & Matthews, 2013). However, whether poor sleep is associated with adverse MVPA and sedentary behavior patterns in pregnancy is unknown. It is critical to better understand the associations of sleep, MVPA, and sedentary behavior in pregnancy because they are modifiable behaviors that have been previously associated with pregnancy outcomes (Davenport et al., 2018; Ding et al., 2014; Fazzi et al., 2017). Further, given that increasing time in one of these lifestyle behaviors will inherently lead to decreased time in another behavior, existing research that assesses one behavior in isolation cannot fully understand the true association of that behavior with health outcomes.
Although several studies have assessed self-reported sleep longitudinally across pregnancy trimesters (Christian, Carroll, Porter, & Hall, 2019; Tomfohr, Buliga, Letourneau, Campbell, & Giesbrecht, 2015; Tsai, Lee, Lin, & Lee, 2016, 2017), the existing literature examining sleep in pregnancy is largely limited by a single assessment of sleep. Further, little is known about the longitudinal associations of sleep with other objectively measured lifestyle behaviors during this unique period. Finally, how sleep patterns in pregnancy are related to adverse pregnancy outcomes and gestational weight gain (GWG) is unclear. To address the limitations in the literature, the aims of this study were to examine the 1) changes and patterns in self-reported sleep duration, efficiency, and quality across pregnancy trimesters, 2) associations of these sleep dimensions with accelerometer-measured sedentary behavior and MVPA across pregnancy trimesters, and 3) associations of these sleep dimensions with adverse pregnancy outcomes as exploratory analyses. We hypothesized that sleep duration, efficiency, and quality would be lowest in the third trimester of pregnancy compared with the first and second trimesters. Further, we hypothesized longer sleep duration and higher sleep efficiency and quality would be associated with more optimal sedentary behavior and MVPA patterns and a lower risk of adverse pregnancy outcomes and excessive GWG.
Methods
Design, Setting, and Participants
Data for this project were obtained from two parallel prospective cohort studies of pregnant women, the Monitoring Movement and Health study (MoM Health; Pittsburgh, PA) and the Pregnancy Activity Monitoring Study (PRAMS; Iowa City, IA). The primary objective of both studies was to use best practice methods to characterize patterns of objectively measured sedentary behavior and MVPA across pregnancy trimesters, as well as examine associations of sedentary behavior and MVPA patterns with adverse pregnancy outcomes and excessive GWG. The MoM Health study was conducted between March 2017 and June 2019; PRAMS was conducted between July 2018 and December 2019. In both studies, participants had three study visits that occurred during the first (8–13 weeks), second (20–22 weeks), and third (32–34 weeks) trimesters of pregnancy. Study visits took place at collaborating prenatal clinics or affiliated research centers. All participants provided written informed consent. The University of Pittsburgh and the University of Iowa Institutional Review Boards approved all research procedures.
Participants were recruited during their first trimester using media advertisements, information tables, research registries, and referrals from other research studies or prenatal care providers. Women were 8–13 weeks pregnant by self-report, between 18 and 45 years old, and planned to receive prenatal care and give birth at a University of Pittsburgh Medical Center facility or a University of Iowa Health Care facility. Women were excluded if they were currently using antihypertensive or glucose-lowering medications, had a serious medical condition or one that severely limited ambulation, or were participating in another research study that intervened upon physical activity. A total of 140 women were recruited from the University of Pittsburgh (n = 120) and the University of Iowa (n = 20). Participant characteristics by study site are reported in Supplemental Table 1. For the current analyses, women were included if they had at least one assessment of sleep, sedentary behavior, and MVPA and had complete data from electronic health records, resulting in a final sample of 120 for analysis (Pittsburgh n = 100; Iowa n = 20).
Exposures
Sleep parameters were assessed at each study visit using the Pittsburgh Sleep Quality Index (PSQI), a widely used and validated 19-question self-reported survey that assesses sleep disturbances in adults (Buysse, Reynolds, Monk, Berman, & Kupfer, 1989). For the current study, sleep dimensions derived from this survey included sleep duration, sleep efficiency, and sleep quality. Sleep duration (hours) was obtained by asking “During the past month, how many hours of actual sleep did you get at night?” Sleep efficiency (%) was calculated as the number of hours slept divided by number of hours in bed × 100. The number of hours in bed was assessed by calculating the duration of time between when the participant reported usually going to bed at night and the time they reported getting up in the morning. The PSQI also provides a global sleep quality score (range, 0–21), which consists of seven components (duration of sleep, sleep disturbance, sleep latency, daytime dysfunction owing to sleepiness, sleep efficiency, overall sleep quality, and medication for sleep), with higher scores indicating worse sleep quality. A global score of greater than 5 indicates poor sleep quality.
Outcomes
Sedentary behavior and MVPA were assessed at each study visit using two objective monitors. Two monitors were deemed necessary to meet best practice standards for measuring sedentary behavior (activPAL3 micro) (Edwardson et al., 2017; Gibbs, Hergenroeder, Katzmarzyk, Lee, & Jakicic, 2015; Kozey-Keadle, Libertine, Lyden, Staudenmayer, & Freedson, 2011) and MVPA (ActiGraph GT3X) (Tudor-Locke, Camhi, & Troiano, 2012). Participants were instructed to wear both monitors for 7 days, complete a monitor wear log denoting any nonwear and sleep periods, and return the monitors and log using postage-paid mail.
The total time spent in sedentary behavior was assessed using the activPAL3. The device was affixed to the anterior thigh using a transparent, waterproof dressing (Edwardson et al., 2017). Participants were instructed to wear the device 24 hours per day, with removal only when swimming. Event-type data were exported using PALtechnologies software (v.7.2.38) and nonwear periods and sleep time were removed using participant logs (Barone Gibbs & Kline, 2018; Edwardson et al., 2017). Total time spent in MVPA was assessed using the ActiGraph GT3X triaxial accelerometer. Participants were instructed to wear the monitor on a waist belt during all waking hours, except during water activities. To account for changing anatomy across pregnancy, pictures adapted from a previously published validation study (Connolly, Coe, Kendrick, Bassett, & Thompson, 2011) were provided to facilitate correct positioning of the device directly above the right knee and below the abdomen. Using ActiLife software v6.12.2 and 1-minute epochs, the Choi algorithm defined valid wear time. Epochs with 2,690 or more counts per minute were summed to quantify daily MVPA (Sasaki, John, & Freedson, 2011). For both sedentary behavior and MVPA, data were quantified within each day and averaged, with 4 or more days with 10 or more hours considered valid (Matthews, Hagstromer, Pober, & Bowles, 2012). Sedentary behavior and MVPA were reported as the mean percent of time per day to account for differences in wear time.
After participants delivered their babies, adverse pregnancy outcomes and GWG were abstracted from electronic health records independently by two research personnel at each study site (a study investigator and trained research personnel). Adverse pregnancy outcomes included a physician’s diagnosis of gestational hypertension (n = 18), preeclampsia (n = 7), gestational diabetes (n = 5), or fetal growth restriction (n = 4) during prenatal care and/or a preterm birth (defined as gestational age at delivery <37 weeks; n = 6). Owing to the limited number of cases for each adverse pregnancy outcome, we combined all adverse pregnancy outcomes into a composite measure (n = 27; 2.5% of total sample) and also created a measure of hypertensive disorders of pregnancy (gestational hypertension and preeclampsia; n = 20; 16.8% of total sample). Self-reported prepregnancy weight and measured weight at delivery were also abstracted and used to calculate GWG. Excessive GWG was categorized using the 2009 Institute of Medicine GWG guidelines, with prepregnancy BMI category inversely related to the threshold defining excessive weight gain (Institute of Medicine and National Research Council, 2009).
Covariates
During the first trimester study visit, participants self-reported demographic information and prior medical history. Gestational age at delivery was abstracted from medical records. Height was measured with shoes removed using a stadiometer.
Statistical Analysis
Latent trajectories (PROC TRAJ in SAS) were created to identify subgroups of participants following similar patterns for sleep duration, sleep efficiency, and sleep quality (separate models for each) across pregnancy trimesters. Women with at least one PSQI assessment were assigned to a trajectory group, with a missing at random assumption for those missing data at up to two time points (n = 4). Optimal trajectories were chosen using the Bayesian information criterion, a maximum proportion of posterior probabilities of more than 70%, and clinical meaning-fulness of trajectory groups. Given the relatively small sample size, up to a three-group solution was considered. Trajectory groups were also created using the proportion of time spent in sedentary behavior and MVPA using the same approach as described for the sleep parameters (Barone Gibbs et al., 2020).
Participant characteristics were described using means and frequencies overall and stratified by sleep duration, sleep efficiency, and sleep quality trajectory groups using paired t tests and one-way analysis of variance for continuous variables and χ2 or Fisher’s exact tests for categorical variables, as appropriate. Unadjusted repeated measures analysis of variance were used to examine differences in sleep parameters, sedentary behavior, and MVPA across trimesters. Associations of sleep trajectories (independent variables) with sedentary behavior and MVPA trajectories (dependent variables) were examined in separate models using multinomial logistic regression. In exploratory analyses, logistic regression was used to examine associations of the sleep trajectories (independent variables) with all adverse pregnancy outcomes (composite measure), hypertensive disorders of pregnancy, and excessive GWG (dependent variables) in separate models. All models were adjusted for study site (Pittsburgh/Iowa), age, race (Black/non-Black), parity (primipara/multipara) and prepregnancy BMI. The excessive GWG model was additionally adjusted for gestational age at delivery.
Results
As seen in Figure 1A, two patterns of sleep duration were identified from trajectory analysis, with groups reflecting consistently short sleep duration (approximately 5 hours of sleep/night, 20.7% of sample) and consistently adequate sleep duration (approximately 7.5 hours of sleep/night; 79.3% of sample). Compared with those in the short sleep duration group, those in the adequate sleep duration group had higher levels of education and lower prepregnancy BMIs and were more likely to be in the moderate or high sedentary behavior trajectory group (Table 1). For sleep efficiency, two patterns were identified with groups reflecting consistently low sleep efficiency (approximately 65% sleep efficiency; 17.5% of sample) and consistently high sleep efficiency (approximately 90% sleep efficiency; 82.5% of sample; Figure 1B). For sleep quality, three patterns were identified with groups reflecting consistently poor sleep quality (PSQI global score of approximately 11, 15.1% of sample), worsening sleep quality (PSQI global score of approximately 6 in first the trimester to approximately 9 in the third trimester, 23.5% of sample), and good sleep quality (PSQI global score approximately 5; 61.5% study sample; Figure 1C). Participant characteristics stratified by sleep efficiency and sleep quality groups can be found in Supplemental Tables 2 and 3, respectively.
Figure 1.
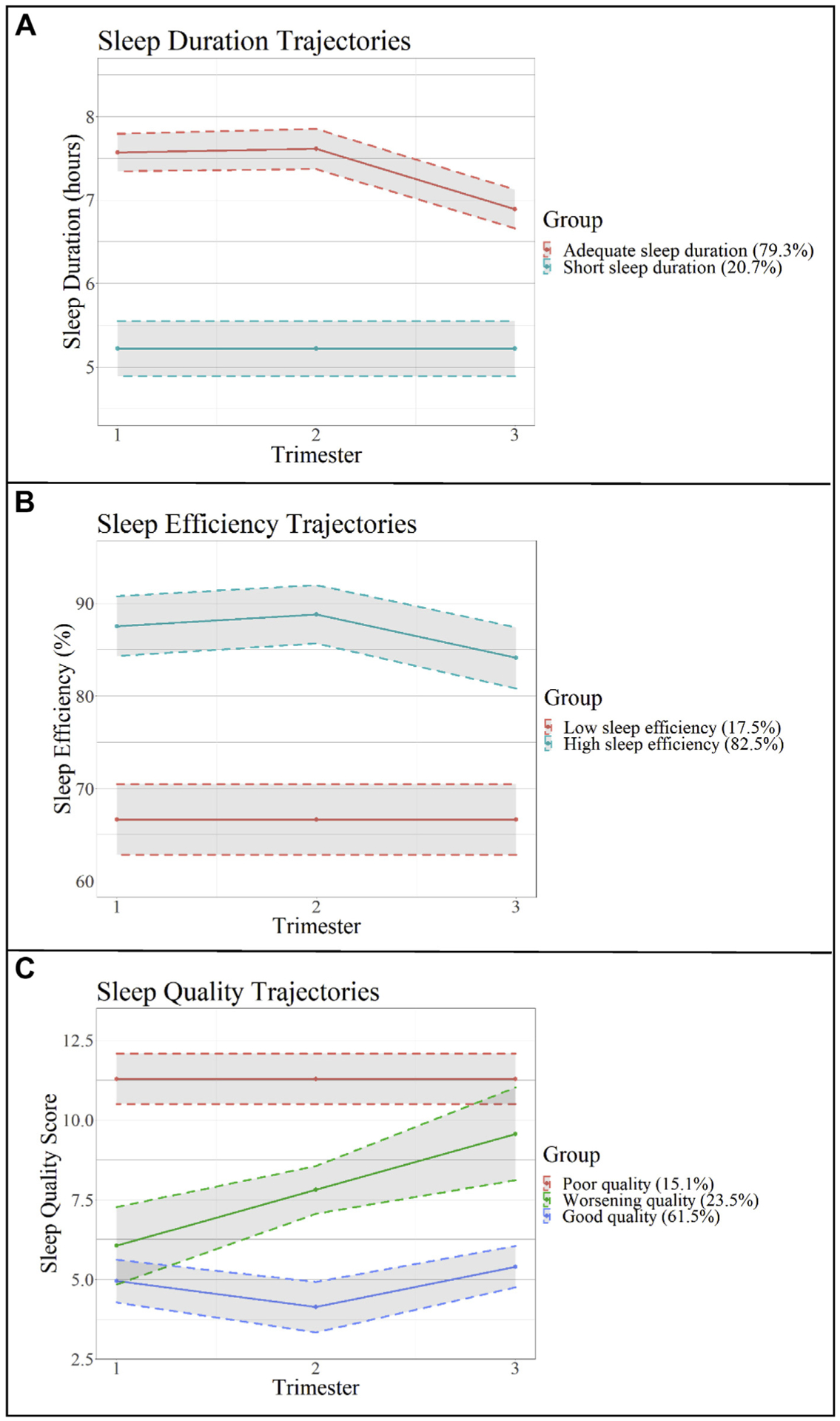
Sleep duration, efficiency, and quality trajectories across pregnancy trimesters. Sleep trajectory groups created using latent trajectories.
Table 1.
Participant Characteristics Overall and Stratified by Sleep Duration Trajectory Groups*
| Participant Characteristics | Overall (N = 120) | Short Sleep Duration (n = 24) | Adequate Sleep Duration (n = 96) | p Value |
|---|---|---|---|---|
| Age, years | 31.1 ± 4.7 | 31.4 ± 4.9 | 31.0 ± 4.7 | .716 |
| Race | .206 | |||
| Black | 18 (15.0) | 6 (25.0) | 12 (12.5) | |
| White | 94 (78.3) | 16 (66.7) | 78 (81.3) | |
| Other | 8 (6.7) | 2 (8.3) | 6 (6.3) | |
| Education | <.001 | |||
| High school or less | 12 (10.0) | 4 (16.7) | 8 (8.3) | |
| Some college/associate degree | 27 (22.5) | 13 (54.2) | 14 (14.6) | |
| Bachelor’s degree | 31 (25.8) | 1 (4.2) | 30 (31.3) | |
| Graduate degree | 50 (41.7) | 6 (25.0) | 44 (45.8) | |
| Parity | .064 | |||
| Primipara | 50 (41.7) | 6 (25.0) | 44 (45.8) | |
| Multipara | 70 (58.3) | 18 (75.0) | 52 (54.2) | |
| Pregnancy history† | ||||
| Gestational hypertension | 5 (6.9) | 0 (0.0) | 5 (9.3) | .319 |
| Preeclampsia | 7 (9.9) | 1 (5.9) | 6 (11.1) | .999 |
| Preterm birth | 8 (11.1) | 2 (11.1) | 6 (11.1) | .999 |
| Gestational diabetes | 5 (6.9) | 0 (0.0) | 5 (9.3) | .322 |
| Prepregnancy BMI, kg/m2 | 26.8 ± 6.7 | 30.2 ± 8.8 | 25.9 ± 5.8 | .030 |
| Prepregnancy BMI category | .052 | |||
| Underweight (<18.5 kg/m2) | 5 (4.2) | 1 (4.2) | 4 (4.2) | |
| Normal (18.5–24.9 kg/m2) | 52 (43.3) | 7 (29.2) | 45 (46.9) | |
| Overweight (25.0–29.9 kg/m2) | 30 (25.0) | 4 (16.7) | 26 (27.1) | |
| Obese (≥30.0 kg/m2) | 33 (27.5) | 12 (50.0) | 21 (21.9) | |
| Gestational age at delivery, weeks | 39.1 ± 4.7 | 39.0 ± 1.1 | 39.0 ± 1.8 | .959 |
| SED trajectory groups | .003 | |||
| Low | 25 (20.8) | 11 (45.8) | 14 (14.6) | |
| Moderate | 43 (35.8) | 5 (20.8) | 38 (39.6) | |
| High | 52 (43.3) | 8 (33.3) | 44 (45.8) | |
| MVPA trajectory groups | .111 | |||
| Low | 35 (29.2) | 11 (45.8) | 24 (25.0) | |
| Moderate | 59 (49.2) | 8 (33.3) | 51 (53.1) | |
| High | 26 (21.7) | 5 (20.8) | 21 (21.9) | |
| Pregnancy outcomes | ||||
| All APOs‡ | 27 (22.5) | 5 (20.8) | 22 (22.9) | .827 |
| Hypertensive disorders | 20 (16.8) | 3 (13.0) | 17 (17.7) | .761 |
| Excessive GWG | 60 (50.9) | 14 (60.9) | 46 (48.4) | .284 |
Abbreviations: APO, adverse pregnancy outcomes; BMI, body mass index; GWG, gestational weight gain; MVPA, moderate-to-vigorous-intensity physical activity; SED, sedentary behavior.
Values are mean ± standard deviation or number (%).
Bolded values are statistically significant (p < .05).
Sleep duration groups created using latent trajectories.
Among women with previous pregnancy and delivery (n = 72).
APOs include gestational hypertension, preeclampsia, gestational diabetes, fetal growth restriction, and preterm delivery.
We identified three patterns of sedentary behavior from trajectory analysis, with groups reflecting consistently low (approximately 50% of waking day; 20.8% of sample), moderate (approximately 63% of waking day; 35.8% of sample), or high (approximately 75% of waking day; 43.3% of sample) sedentary behavior. Similarly, three patterns of MVPA were identified from trajectory analysis, with groups reflecting consistently low (approximately 2% of waking day; 29.2% of sample), moderate (approximately 4% of waking day; 49.2% of sample), and high (approximately 6% of waking day; 21.7% of sample; Table 1) MVPA.
Sleep, sedentary behavior, and MVPA measures differed across pregnancy trimesters (Table 2). Sleep duration and sleep efficiency were lower, and sleep quality scores (higher scores indicate worse sleep quality) and the percentage of participants with poor sleep quality were higher in the third trimester compared with the first and second trimesters. Sedentary behavior was higher in the first trimester compared with the second and third trimesters, and MVPA was lower in the third trimester compared with the first and second trimesters. Notably, the relative differences in sedentary behavior were smaller than the observed differences for MVPA.
Table 2.
Sleep, SED, and MVPA Across Pregnancy Trimesters
| Sleep, SED, and MVPA Measures | Pregnancy Trimesters | p Value | ||
|---|---|---|---|---|
| First | Second | Third | ||
| PSQI sleep measures | ||||
| Sleep duration, mean hours/day | 7.1 ± 1.4‡ | 7.1 ± 1.5‡ | 6.5 ± 1.2*,† | <.001 |
| Sleep efficiency, %§ | 84.9 ± 13.4‡ | 85.0 ± 12.4‡ | 80.8 ± 12.6*,† | .006 |
| Global score, score‖ | 6.1 ± 3.0‡ | 6.0 ± 3.3‡ | 7.3 ± 3.5*,† | <.001 |
| Poor sleep quality,¶ | 58 (48.7)‡ | 56 (49.6)‡ | 75 (66.4)*,† | <.001 |
| ActivPAL3 micro measures | ||||
| SED, % time/day | 65.0 ± 10.4‡,† | 63.0 ± 9.8* | 63.3 ± 10.1* | .029 |
| Total waking wear time, hours/day | 15.0 ± 1.0 | 15.2 ± 1.1 | 15.0 ± 1.0 | .239 |
| Valid wear days, days | 6.8 ± 1.4‡ | 6.4 ± 2.1 | 6.2 ± 2.3* | .029 |
| ActiGraph GT3X measures | ||||
| MVPA, % time/day | 3.8 ± 1.9‡ | 3.6 ± 1.8‡ | 3.0 ± 1.7*,† | <.001 |
| Total waking wear time, hours/day | 14.7 ± 1.2 | 14.6 ± 1.2 | 14.6 ± 1.5 | .567 |
| Valid wear days, mean days | 7.5 ± 1.4† | 7.2 ± 1.1* | 7.2 ± 0.9 | .087 |
Abbreviations: MVPA, moderate-to-vigorous-intensity physical activity; PSQI, Pittsburgh Sleep Quality Index; SED, sedentary behavior.
Values are number (%) or mean ± standard deviation.
Bolded values are statistically significant (p < .05).
Indicates a significant difference from trimester 1 (p < .05).
Indicates a significant difference from trimester 2 (p < .05).
Indicates a significant difference from trimester 3 (p < .05).
Possible score range 0%–100%, higher scores indicate better sleep efficiency.
Possible score range 0–21, higher scores indicate poorer sleep quality.
Defined as PSQI global score of >5.
When the examining associations of sleep trajectory groups with sedentary behavior and MVPA trajectory groups (Table 3), we found that, compared with the adequate sleep duration group, those in the short sleep duration group were less likely to be in the moderate (odds ratio [OR], 0.21; 95% confidence interval [CI], 0.10–0.45) or high (OR, 0.23; 95% CI, 0.11–0.45) sedentary behavior group. Compared with those in the high sleep efficiency group, those in the low sleep efficiency group were less likely to be in the moderate (OR, 0.21; 95% CI, 0.09–0.50) and high (OR, 0.31; 95% CI, 0.15–0.65) sedentary behavior groups. Compared with women with good sleep quality, women in the worsening sleep quality group were less likely to be in the high sedentary behavior group (OR, 0.25; 95% CI, 0.12–0.52), and were more likely to be in the low MVPA group (OR, 2.51; 95% CI, 1.18–5.38).
Table 3.
Associations of Sleep Trajectories With SED and MVPA Trajectories Across Pregnancy Trimesters
| Sleep Trajectory Groups* | SED Trajectory Groups | MVPA Trajectory Group | ||||
|---|---|---|---|---|---|---|
| Low | Moderate | High | Low | Moderate | High | |
| Or (95% CI) | Or (95% CI) | Or (95% CI) | Or (95% CI) | Or (95% CI) | Or (95% CI) | |
| Sleep duration | ||||||
| Short | Reference | 0.21 (0.10–0.45) | 0.23 (0.11–0.45) | 1.88 (0.87–4.08) | 0.62 (0.29–1.33) | Reference |
| Adequate | Reference | Reference | Reference | Reference | Reference | Reference |
| Sleep efficiency | ||||||
| Low | Reference | 0.21 (0.09–0.50) | 0.31 (0.15–0.65) | 1.05 (0.48–2.29) | 0.44 (0.21–0.91) | Reference |
| High | Reference | Reference | Reference | Reference | Reference | Reference |
| Sleep quality | ||||||
| Good | Reference | Reference | Reference | Reference | Reference | Reference |
| Worsening | Reference | 0.85 (0.44–1.67) | 0.25 (0.12–0.52) | 2.51 (1.18–5.38) | 0.92 (0.46–1.86) | Reference |
| Poor | Reference | 0.39 (0.15–1.02) | 0.72 (0.32–1.61) | 1.73 (0.72–4.13) | 0.71 (0.31–1.59) | Reference |
Abbreviations: CI, confidence interval; MVPA, moderate-to-vigorous-intensity physical activity; OR, odds ratio; SED, sedentary behavior.
Bolded values are statistically significant (p < .05).
Models adjusted for site, age, race (Black vs. non-Black), parity (primipara vs. multipara), and prepregnancy body mass index (continuous). Bolded values are statistically significant, p < .05.
Sleep trajectory groups created using latent trajectories.
As seen in Table 4, women in the worsening sleep quality group had lower odds of excessive GWG compared with those in the good sleep quality group (OR, 0.33; 95% CI, 0.13–0.89). Those in the short sleep duration and low sleep efficiency groups had greater odds of all adverse pregnancy outcomes (OR, 1.44; 95% CI, 0.44–4.76; OR, 1.93; 95% CI, 0.48–8.36, respectively) and greater odds of hypertensive disorders of pregnancy (OR, 1.58; 95% CI, 0.38–6.66; OR, 1.79; 95% CI, 0.34–9.52, respectively), although the 95% CIs were wide and these associations were not statistically significant. Similar nonsignificant trends were observed for the sleep quality trajectory groups, with those in the worsening and poor sleep quality groups having greater odds of adverse pregnancy outcomes and hypertensive disorders of pregnancy. Similar findings were observed with additional adjustment for sedentary behavior and MVPA trajectory groups (Supplemental Table 4).
Table 4.
Associations of Sleep Measures With Adverse Pregnancy Outcomes
| Sleep trajectory groups* | All APOs (n = 27) | Hypertensive Disorders (n = 20) | Excessive GWG (n = 60) |
|---|---|---|---|
| Or (95% CI) | Or (95% CI) | Or (95% CI) | |
| Sleep duration | |||
| Short | 1.44 (0.44–4.76) | 1.58 (0.38–6.66) | 0.92 (0.33–2.56) |
| Adequate | Reference | Reference | Reference |
| Sleep efficiency | |||
| Low | 1.93 (0.48–8.36) | 1.79 (0.34–9.52) | 0.52 (0.17–1.63) |
| High | Reference | Reference | Reference |
| Sleep quality | |||
| Good | Reference | Reference | Reference |
| Worsening | 1.40 (0.46–4.23) | 1.04 (0.30–3.62) | 0.33 (0.13–0.89) |
| Poor | 1.49 (0.38–5.76) | 1.33 (0.29–6.09) | 0.45 (0.14–1.46) |
Abbreviations: CI, confidence interval; APO, adverse pregnancy outcomes; GWG, gestational weight gain; OR, odds ratio.
Models adjusted for site, age, race (Black vs. non-Black), parity (primipara vs. multipara), prepregnancy body mass index (continuous), and gestational age at delivery (excessive gestational weight gain model only).
Bolded values are statistically significant (p < .05).
Sleep trajectory groups created using latent trajectories.
Discussion
In this observational cohort study of pregnant women, we characterized patterns of self-reported sleep duration, efficiency, and quality across pregnancy trimesters and related these patterns with sedentary behavior, MVPA, and adverse pregnancy outcomes. We identified two distinct patterns for sleep duration and sleep efficiency and three distinct patterns for sleep quality. These sleep trajectories were associated with both sedentary behavior and MVPA across pregnancy trimesters. The associations with sedentary behavior were contrary to our hypothesis, with women in the short sleep duration, low sleep efficiency, and worsening sleep quality groups being less likely to engage in high sedentary behavior. However, consistent with our hypothesis, women in the worsening sleep quality group engaged in less MVPA. Finally, nonsignificant trends were observed between sleep trajectories and adverse pregnancy outcomes where women in the short sleep duration, low sleep efficiency, and poor sleep quality groups had greater odds of all adverse pregnancy outcomes and hypertensive disorders of pregnancy, consistent with our hypothesis. Unexpectedly, less optimal sleep trajectory groups had lower odds of excessive GWG. Together, these findings demonstrate there is variation in lifestyle behaviors across pregnancy trimesters; multiple dimensions of sleep are associated with sedentary behavior and MVPA; and additional research is warranted in larger sample sizes to further examine the potential role of sleep in adverse pregnancy outcomes.
Our findings demonstrating changes in sleep duration, efficiency, and quality across pregnancy trimesters are largely consistent with the existing literature (Christian et al., 2019; Mindell et al., 2015; Sedov, Cameron, Madigan, & Tomfohr-Madsen, 2018). For example, Mindell et al. (2015) characterized sleep patterns across all pregnancy months using a cross-sectional Internet-based survey. The authors found that sleep duration decreased from 7.6 hours within the first 2 months of pregnancy to 6.9 hours per night in the final 2 months of pregnancy, which is similar to sleep duration reported in the present study in the first and third trimesters (7.1 and 6.5 hours, respectively). Mindell et al. (2015) also found the number and duration of nightly wakings significantly increased across pregnancy months, indicating lower sleep efficiency later in pregnancy. A meta-analysis examining sleep quality in pregnancy reported significant differences in mean PSQI scores (indicator of sleep quality) in the second versus third trimesters of pregnancy (6.4; 95% CI, 5.4–7.3 vs. 8.1; 95% CI, 6.8–9.3; p = .003), similar to the findings in the present study (Sedov et al., 2018). This study contributes to the literature by demonstrating the importance of examining distinct patterns of sleep across pregnancy. The majority of women in this study had healthy sleep patterns; however, a subset experienced adverse changes to sleep patterns, and these individuals may be at the greatest risk for pregnancy complications. Our findings illustrate that simply reporting average changes in sleep metrics across pregnancy trimesters may not identify those at highest risk.
Unexpectedly, we found that women in the less optimal sleep trajectory groups were less likely to be in the moderate and/or high sedentary behavior trajectory groups. A recent systematic review and meta-analysis in the general adult population found that high levels of sedentary behavior are associated with a greater risk of insomnia and sleep disturbance (Yang, Shin, Li, & An, 2017). Notably, no associations were observed between sedentary behavior and sleep quality, and the results were conflicting for sedentary behavior and sleep efficiency in this systematic review. The associations between sedentary behavior and various dimensions of sleep in the general population remain mixed, and even less is known about these associations during pregnancy. One potential explanation for our study findings is that low levels of sedentary behavior and less optimal sleep patterns may have a common cause as women with high levels of stress and responsibilities may have limited time to engage in restful behaviors (i.e., sedentary behavior and sleep).
Consistent with our hypothesis, we found that women with worsening sleep quality (vs. good quality) were more likely to be in the low MVPA group. In nonpregnant populations, higher levels of MVPA have beneficial associations with a variety of sleep dimensions (Kredlow, Capozzoli, Hearon, Calkins, & Otto, 2015; Lambiase et al., 2013). However, fewer observational studies have examined associations of physical activity and sleep during pregnancy, with inconsistent findings (Borodulin et al., 2010; Hawkins et al., 2019; Loprinzi, Loprinzi, & Cardinal, 2012). For example, Hawkins et al. (2019) found self-reported household and caregiving physical activities were associated with higher odds of poor sleep quality, while occupational activity was associated with lower odds of poor sleep quality among pregnant women (26.5 ± 6.9 weeks gestation). A meta-analysis of randomized controlled trials concluded there was an association between regular exercise and enhanced sleep quality in pregnant women (OR, 6.2; 95% CI, 2.0–19.1) (Yang et al., 2020). It is difficult to draw comparisons between our studies and others given differences in study design, assessment methods, classification of variables, and analytic approach. However, our study provides additional evidence of a beneficial association between sleep parameters and MVPA in pregnancy.
In exploratory analyses, we observed trends between less optimal sleep trajectory groups and greater odds of adverse pregnancy outcomes, including hypertensive disorders of pregnancy. Although the majority of research on sleep and adverse pregnancy outcomes has focused on sleep-disordered breathing, there is evidence that short sleep duration is associated with an increased risk of adverse maternal and fetal outcomes, including increased risk of gestational hypertension (Williams et al., 2010), gestational diabetes (Facco et al., 2017; Herring et al., 2014), and preterm delivery (Warland, Dorrian, Morrison, & O’Brien, 2018). Little is known about the associations of sleep efficiency and quality with pregnancy outcomes, and findings are inconsistent (Warland et al., 2018). Given that short sleep duration and other indicators of disturbed sleep lead to oxidative stress, increased sympathetic activity, inflammation, endothelial dysfunction, and insulin resistance, all of which could contribute to adverse vascular and metabolic outcomes (Anothaisintawee, Reutrakul, Van Cauter, & Thakkinstian, 2016; Izci-Balserak & Pien, 2010; Lavie, 2009; Reutrakul & Van Cauter, 2014), it is biologically plausible that suboptimal patterns of sleep could contribute to risk of adverse pregnancy outcomes.
We also observed associations between worsening sleep quality and lower odds of excessive GWG. Although there are limited studies examining associations of sleep and GWG (Gay, Richoux, Beebe, & Lee, 2017), others have argued the importance for appropriate sleep hygiene to help regulate energy balance and optimize weight gain in pregnancy (Ferraro, Chaput, Gruslin, & Adamo, 2014). There is a clear need for additional research with larger sample sizes to further explore associations of various sleep dimensions in pregnancy with maternal and fetal outcomes, including excessive GWG.
This study had multiple strengths, including repeated assessments of sleep in all trimesters of pregnancy; concurrent assessment of sedentary behavior and MPVA using best practice assessment methods (Gibbs et al., 2015; Kozey-Keadle et al., 2011; Tudor-Locke et al., 2012); the use of latent trajectories to describe patterns of sleep, sedentary behavior, and MVPA in pregnancy; and linkage with adverse pregnancy outcomes. Despite these strengths, a number of limitations must be noted. First, this study relied on self-reported sleep, which is weakly correlated with objective measures of sleep (e.g., polysomnography) in the general adult population (Matthews et al., 2018) and among pregnant women (Herring et al., 2013). It is, therefore, plausible that objective assessment of sleep may have resulted in different associations than those observed in the present study. However, the PSQI is a widely used and validated survey (Buysse et al., 1989) commonly administered in pregnant samples (Qiu et al., 2016). Furthermore, the use of a self-reported instrument provides information on perceptions of sleep that are not captured through objective assessments and have been found to have stronger associations with negative postpartum outcomes compared with objective assessments (Coo, Milgrom, & Trinder, 2014). Notably, we used the PSQI to assess sleep duration, efficiency, and quality, and did not assess other sleep metrics that researchers have calculated using this instrument, such as night and daytime disturbances factor (Skouteris, Wertheim, Germano, Paxton, & Milgrom, 2009); future research could use these additional metrics. Second, this study did not include assessments of sleep-disordered breathing or restless legs syndrome, which are associated with adverse pregnancy outcomes (Ding et al., 2014; Gupta et al., 2016; Luque-Fernandez, Bain, Gelaye, Redline, & Williams, 2013; Pamidi et al., 2014; Warland et al., 2018). Third, this was a relatively small sample with few adverse pregnancy outcomes; thus, the study was likely underpowered to detect associations with these events. However, these associations were examined for exploratory purposes and provide preliminary evidence that poor sleep may be associated with an increased risk of adverse pregnancy outcomes. As a result of the relatively small sample, we were also unable to examine associations by race. This is a study limitation because there is evidence that Black women report poorer sleep and also exhibit greater inflammatory responses to sleep disturbance than White women, which may contribute to racial disparities in birth outcomes (Blair, Porter, Leblebicioglu, & Christian, 2015). For this reason, associations of sleep with adverse pregnancy outcomes may have been biased toward the null owing to our small sample of Black women. Larger studies are needed to assess race differences in the associations between sleep, lifestyle behaviors, and adverse pregnancy outcomes. Finally, although there is evidence that sleep disturbance in pregnancy is associated with cesarean birth (Lee & Gay, 2004), we chose to focus on adverse pregnancy outcomes with long-term health consequences for mother and/or baby.
This study found that poor sleep is common in pregnancy, various dimensions of sleep are adversely altered in the third trimester, and these dimensions of sleep are associated with sedentary behavior and MVPA patterns across pregnancy trimesters.
Implications for Practice And/or Policy
There is a clear need for larger, fully powered studies—including both objective and self-reported assessment of sleep, sedentary behavior, and MVPA during each trimester of pregnancy—to better understand the dynamic interplay of these lifestyle behaviors, including how sleep and activity patterns are associated with adverse pregnancy outcomes. This information could inform behavioral guidelines and recommendations on optimal lifestyle patterns to promote pregnancy health.
Supplementary Material
Acknowledgments
We would like to thank the women who participated in this research study.
The MoM Health Study was supported by the American Heart Association under Grant 17GRNT3340016 with research registry and recruitment support from the University of Pittsburgh Clinical and Translational Science Institute (NIH UL1TR000005). PRAMS was supported in part by the University of Iowa Institute for Clinical and Translational Science (NIH UL1TR002537).
Author Descriptions
Kara M. Whitaker, PhD, MPH, is an Assistant Professor in the Department of Health and Human Physiology at the University of Iowa. Her research focuses on physical activity and sedentary behavior epidemiology, with emphasis in women during pregnancy and postpartum.
Dong Zhang, PhD, is a Postdoctoral Researcher in the Department of Health and Human Physiology at the University of Iowa.
Christopher E. Kline, PhD, is an Assistant Professor in the Department of Health and Human Development at the University of Pittsburgh. His research focuses on the intersection of exercise and sleep on health outcomes.
Janet Catov, PhD, is an Associate Professor in the Department of Obstetrics, Gynecology, and Reproductive Sciences and the Department of Epidemiology. Her research examines the relationship between cardiovascular risk factors and preterm birth.
Bethany Barone Gibbs, PhD, is an Associate Professor in the Department of Health and Human Development at the University of Pittsburgh. She is an epidemiologist whose research focuses on the prevention and treatment of cardiometabolic disease through healthy lifestyle behaviors.
Footnotes
Supplementary Data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.whi.2021.02.003.
References
- 2018 Physical Activity Guidelines Advisory Committee. (2018). Physical Activity Guidelines Advisory Committee Scientific Report. Washington, DC: Department of Health and Human Services. [Google Scholar]
- Anothaisintawee T, Reutrakul S, Van Cauter E, & Thakkinstian A (2016). Sleep disturbances compared to traditional risk factors for diabetes development: Systematic review and meta-analysis. Sleep Medicine Reviews, 30, 11–24. [DOI] [PubMed] [Google Scholar]
- Asghari A, Farhadi M, Kamrava SK, Ghalehbaghi B, & Nojomi M (2012). Subjective sleep quality in urban population. Archive of Iranian Medicine, 15(2), 95–98. [PubMed] [Google Scholar]
- Baron KG, Reid KJ, & Zee PC (2013). Exercise to improve sleep in insomnia: Exploration of the bidirectional effects. Journal of Clinical Sleep Medicine, 9(8), 819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone Gibbs B, Jones MA, Jakicic JM, Jeyebalan A, Whitaker KM, & Catov JM (2020). Prospective associations of prenatal sedentary behavior and physical activity trajectories with adverse pregnancy outcomes. Circulation, 141(Suppl 1), MP13. [Google Scholar]
- Barone Gibbs B, & Kline CE (2018). When does sedentary behavior become sleep? A proposed framework for classifying activity during sleep-wake transitions. International Journal of Behavioral Nutrition and Physical Activity, 15(1), 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudreau SA, Spira AP, Stewart A, Kezirian EJ, Lui LY, Ensrud K, & … Study of Osteoporotic, F. (2012). Validation of the Pittsburgh Sleep Quality Index and the Epworth Sleepiness Scale in older black and white women. Sleep Medicine, 13(1), 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas A, Oh PI, Faulkner GE, Bajaj RR, Silver MA, Mitchell MS, & Alter DA (2015). Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: A systematic review and meta-analysis. Annals of Internal Medicine, 162(2), 123–132. [DOI] [PubMed] [Google Scholar]
- Blair LM, Porter K, Leblebicioglu B, & Christian LM (2015). Poor sleep quality and associated inflammation predict preterm birth: Heightened risk among African Americans. Sleep, 38(8), 1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borodulin K, Evenson KR, Monda K, Wen F, Herring AH, & Dole N (2010). Physical activity and sleep among pregnant women. Paediatric and Perinatal Epidemiology, 24(1), 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromley LE, Booth JN 3rd, Kilkus JM, Imperial JG, & Penev PD (2012). Sleep restriction decreases the physical activity of adults at risk for type 2 diabetes. Sleep, 35(7), 977–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, & Kupfer DJ (1989). The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. [DOI] [PubMed] [Google Scholar]
- Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, & Miller MA (2011). Sleep duration predicts cardiovascular outcomes: A systematic review and meta-analysis of prospective studies. European Heart Journal, 32(12), 1484–1492. [DOI] [PubMed] [Google Scholar]
- Christian LM, Carroll JE, Porter K, & Hall MH (2019). Sleep quality across pregnancy and postpartum: Effects of parity and race. Sleep Health, 5(4), 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly CP, Coe DP, Kendrick JM, Bassett DR Jr., & Thompson DL (2011). Accuracy of physical activity monitors in pregnant women. Medicine and Science in Sports and Exercise, 43(6), 1100–1105. [DOI] [PubMed] [Google Scholar]
- Coo S, Milgrom J, & Trinder J (2014). Mood and objective and subjective measures of sleep during late pregnancy and the postpartum period. Behavioral Sleep Medicine, 12(4), 317–330. [DOI] [PubMed] [Google Scholar]
- Davenport MH, Ruchat SM, Poitras VJ, Jaramillo Garcia A, Gray CE, Barrowman N, … Mottola MF (2018). Prenatal exercise for the prevention of gestational diabetes mellitus and hypertensive disorders of pregnancy: A systematic review and meta-analysis. British Journal of Sports Medicine, 52(21), 1367–1375. [DOI] [PubMed] [Google Scholar]
- Di Fabio DR, Blomme CK, Smith KM, Welk GJ, & Campbell CG (2015). Adherence to physical activity guidelines in mid-pregnancy does not reduce sedentary time: An observational study. International Journal of Behavioral Nutrition and Physical Activity, 12, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding XX, Wu YL, Xu SJ, Zhang SF, Jia XM, Zhu RP, … Tao FB (2014). A systematic review and quantitative assessment of sleep-disordered breathing during pregnancy and perinatal outcomes. Sleep Breathing, 18(4), 703–713. [DOI] [PubMed] [Google Scholar]
- Doyle CY, Ruiz JM, Taylor DJ, Smyth JW, Flores M, Dietch JR, … Uchino BN (2019). Associations between objective sleep and ambulatory blood pressure in a community sample. Psychosomatic Medicine, 81(6), 545–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwardson CL, Winkler EAH, Bodicoat DH, Yates T, Davies MJ, Dunstan DW, & Healy GN (2017). Considerations when using the activPAL monitor in field-based research with adult populations. Journal of Sport and Health Science, 6(2), 162–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenson KR, & Wen F (2011). Prevalence and correlates of objectively measured physical activity and sedentary behavior among US pregnant women. Preventive Medicine, 53(1–2), 39–43. [DOI] [PubMed] [Google Scholar]
- Facco FL, Grobman WA, Reid KJ, Parker CB, Hunter SM, Silver RM, & Zee PC (2017). Objectively measured short sleep duration and later sleep midpoint in pregnancy are associated with a higher risk of gestational diabetes. American Journal of Obstetrics and Gynecology, 217(4), 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazzi C, Saunders DH, Linton K, Norman JE, & Reynolds RM (2017). Sedentary behaviours during pregnancy: A systematic review. International Journal of Behavioral Nutrition and Physical Activity, 14(1), 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro ZM, Chaput JP, Gruslin A, & Adamo KB (2014). The potential value of sleep hygiene for a healthy pregnancy: A brief review. ISRN Family Medicine, 2014, 928293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay CL, Richoux SE, Beebe KR, & Lee KA (2017). Sleep disruption and duration in late pregnancy is associated with excess gestational weight gain among overweight and obese women. Birth, 44(2), 173–180. [DOI] [PubMed] [Google Scholar]
- Gibbs BB, Hergenroeder AL, Katzmarzyk PT, Lee IM, & Jakicic JM (2015). Definition, measurement, and health risks associated with sedentary behavior. Medicine and Science in Sports and Exercise, 47(6), 1295–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R, Dhyani M, Kendzerska T, Pandi-Perumal SR, BaHammam AS, Srivanitchapoom P, … Hallett M (2016). Restless legs syndrome and pregnancy: Prevalence, possible pathophysiological mechanisms and treatment. Acta Neurologica Scandinavica, 133(5), 320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins M, Kim Y, Gabriel KP, Rockette-Wagner BJ, & Chasan-Taber L (2017). Sedentary behavior patterns in non-pregnant and pregnant women. Preventive Medicine Reports, 6, 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins M, Marcus B, Pekow P, Rosal MC, Tucker KL, Spencer RMC, & Chasan-Taber L (2019). Physical activity and sleep quality and duration during pregnancy among Hispanic women: Estudio PARTO. Behavioral Sleep Medicine, 17(6), 804–817. [DOI] [PubMed] [Google Scholar]
- Herring SJ, Foster GD, Pien GW, Massa K, Nelson DB, Gehrman PR, & Davey A (2013). Do pregnant women accurately report sleep time? A comparison between self-reported and objective measures of sleep duration in pregnancy among a sample of urban mothers. Sleep Breathing, 17(4), 1323–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring SJ, Nelson DB, Pien GW, Homko C, Goetzl LM, Davey A, & Foster GD (2014). Objectively measured sleep duration and hyperglycemia in pregnancy. Sleep Medicine, 15(1), 51–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine and National Research Council. (2009). Weight gain during pregnancy: Reexamining the guidelines. Washington, DC: Author. [Google Scholar]
- Izci-Balserak B, & Pien GW (2010). Sleep-disordered breathing and pregnancy: Potential mechanisms and evidence for maternal and fetal morbidity. Current Opinion in Pulmonary Medicine, 16(6), 574–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko SH, Chang SC, & Chen CH (2010). A comparative study of sleep quality between pregnant and nonpregnant Taiwanese women. Journal of Nursing Scholarship, 42(1), 23–30. [DOI] [PubMed] [Google Scholar]
- Kozey-Keadle S, Libertine A, Lyden K, Staudenmayer J, & Freedson PS (2011). Validation of wearable monitors for assessing sedentary behavior. Medicine and Science in Sports and Exercise, 43(8), 1561–1567. [DOI] [PubMed] [Google Scholar]
- Kredlow MA, Capozzoli MC, Hearon BA, Calkins AW, & Otto MW (2015). The effects of physical activity on sleep: A meta-analytic review. Journal of Behavioral Medicine, 38(3), 427–449. [DOI] [PubMed] [Google Scholar]
- Lambiase MJ, Gabriel KP, Kuller LH, & Matthews KA (2013). Temporal relationships between physical activity and sleep in older women. Medicine and Science in Sports and Exercise, 45(12), 2362–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larcher S, Benhamou PY, Pepin JL, & Borel AL (2015). Sleep habits and diabetes. Diabetes Metabolism, 41(4), 263–271. [DOI] [PubMed] [Google Scholar]
- Lavie L (2009). Oxidative stress–A unifying paradigm in obstructive sleep apnea and comorbidities. Progress in Cardiovascular Diseases, 51(4), 303–312. [DOI] [PubMed] [Google Scholar]
- Lee KA, & Gay CL (2004). Sleep in late pregnancy predicts length of labor and type of delivery. American Journal of Obstetrics and Gynecology, 191(6), 2041–2046. [DOI] [PubMed] [Google Scholar]
- Loprinzi PD, Loprinzi KL, & Cardinal BJ (2012). The relationship between physical activity and sleep among pregnant women. Mental Health and Physical Activity, 5, 22–27. [Google Scholar]
- Luque-Fernandez MA, Bain PA, Gelaye B, Redline S, & Williams MA (2013). Sleep-disordered breathing and gestational diabetes mellitus: A meta-analysis of 9,795 participants enrolled in epidemiological observational studies. Diabetes Care, 36(10), 3353–3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews CE, Hagstromer M, Pober DM, & Bowles HR (2012). Best practices for using physical activity monitors in population-based research. Medicine and Science in Sports and Exercise, 44(1 Suppl 1), S68–S76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews KA, Patel SR, Pantesco EJ, Buysse DJ, Kamarck TW, Lee L, & Hall MH (2018). Similarities and differences in estimates of sleep duration by polysomnography, actigraphy, diary, and self-reported habitual sleep in a community sample. Sleep Health, 4(1), 96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindell JA, Cook RA, & Nikolovski J (2015). Sleep patterns and sleep disturbances across pregnancy. Sleep Medicine, 16(4), 483–488. [DOI] [PubMed] [Google Scholar]
- Pamidi S, Pinto LM, Marc I, Benedetti A, Schwartzman K, & Kimoff RJ (2014). Maternal sleep-disordered breathing and adverse pregnancy outcomes: A systematic review and metaanalysis. American Journal of Obstetrics and Gynecology, 210(1), 52. [DOI] [PubMed] [Google Scholar]
- Qiu C, Gelaye B, Zhong QY, Enquobahrie DA, Frederick IO, & Williams MA (2016). Construct validity and factor structure of the Pittsburgh Sleep Quality Index among pregnant women in a Pacific-Northwest cohort. Sleep Breathing, 20(1), 293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reutrakul S, & Van Cauter E (2014). Interactions between sleep, circadian function, and glucose metabolism: Implications for risk and severity of diabetes. Annals of the New York Academy of Science, 1311, 151–173. [DOI] [PubMed] [Google Scholar]
- Sasaki JE, John D, & Freedson PS (2011). Validation and comparison of ActiGraph activity monitors. Journal of Science and Medicine in Sport, 14(5), 411–416. [DOI] [PubMed] [Google Scholar]
- Sedov ID, Cameron EE, Madigan S, & Tomfohr-Madsen LM (2018). Sleep quality during pregnancy: A meta-analysis. Sleep Medicine Reviews, 38, 168–176. [DOI] [PubMed] [Google Scholar]
- Skouteris H, Wertheim EH, Germano C, Paxton SJ, & Milgrom J (2009). Assessing sleep during pregnancy: A study across two time points examining the Pittsburgh Sleep Quality Index and associations with depressive symptoms. Womens Health Issues, 19(1), 45–51. [DOI] [PubMed] [Google Scholar]
- Thorp AA, Owen N, Neuhaus M, & Dunstan DW (2011). Sedentary behaviors and subsequent health outcomes in adults a systematic review of longitudinal studies, 1996–2011. American Journal of Preventive Medicine, 41(2), 207–215. [DOI] [PubMed] [Google Scholar]
- Tomfohr LM, Buliga E, Letourneau NL, Campbell TS, & Giesbrecht GF (2015). Trajectories of sleep quality and associations with mood during the perinatal period. Sleep, 38(8), 1237–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay MS, Aubert S, Barnes JD, Saunders TJ, Carson V, Latimer-Cheung AE, … Participants, S. T. C. P. (2017). Sedentary Behavior Research Network (SBRN) - Terminology Consensus Project process and outcome. International Journal of Behavioral Nutrition and Physical Activity, 14(1), 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SY, Lee PL, Lin JW, & Lee CN (2016). Cross-sectional and longitudinal associations between sleep and health-related quality of life in pregnant women: A prospective observational study. International Journal of Nursing Studies, 56, 45–53. [DOI] [PubMed] [Google Scholar]
- Tsai SY, Lee PL, Lin JW, & Lee CN (2017). Persistent and new-onset daytime sleepiness in pregnant women: A prospective observational cohort study. International Journal of Nursing Studies, 66, 1–6. [DOI] [PubMed] [Google Scholar]
- Tudor-Locke C, Camhi SM, & Troiano RP (2012). A catalog of rules, variables, and definitions applied to accelerometer data in the National Health and Nutrition Examination Survey, 2003–2006. Preventing Chronic Disease, 9, E113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warland J, Dorrian J, Morrison JL, & O’Brien LM (2018). Maternal sleep during pregnancy and poor fetal outcomes: A scoping review of the literature with meta-analysis. Sleep Medicine Reviews, 41, 197–219. [DOI] [PubMed] [Google Scholar]
- Williams MA, Miller RS, Qiu C, Cripe SM, Gelaye B, & Enquobahrie D (2010). Associations of early pregnancy sleep duration with trimester-specific blood pressures and hypertensive disorders in pregnancy. Sleep, 33(10), 1363–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SY, Lan SJ, Yen YY, Hsieh YP, Kung PT, & Lan SH (2020). Effects of exercise on sleep quality in pregnant women: A systematic review and meta-analysis of randomized controlled trials. Asian Nursing Research, 14(1), 1–10. [DOI] [PubMed] [Google Scholar]
- Yang Y, Shin JC, Li D, & An R (2017). Sedentary Behavior and Sleep Problems: A Systematic Review and Meta-Analysis. International Journal of Behavioral Medicine, 24(4), 481–492. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


