Abstract
Since the outbreak of the novel coronavirus in Wuhan, China, as obstetricians, we also face great challenges. We need to identify pregnant patients with 2019 coronavirus disease infection timely, and give them appropriate treatment in order to obtain a good maternal and infant prognosis. Here, we would like to share a case and provide some suggestions on how to screen, diagnose and treat pregnant women with 2019 coronavirus disease infection during the outbreak.
Keywords: COVID-19, Severe acute respiratory syndrome coronavirus 2, Obstetrics, Pregnancy
Since the novel coronavirus has been prevailing in Wuhan, China from late December 2019,1 approximately 40 000 people have been diagnosed with 2019 coronavirus disease (COVID-19) infection in Wuhan where is the worst-hit area in China until February 16th 2020, the Obstetrics Department in many hospitals of Hubei Province has received suspected and confirmed cases of pregnancy complicated with COVID-19. COVID-19 occurs in women of all gestational ages and puerperium stage. The main symptoms characterized by fever, fatigue, dry cough, shortness of breath. Normal or decreased white blood cells count in peripheral blood, and decreased lymphocyte count. Diarrhea is the primary manifestation in some cases, followed by fever and respiratory symptoms. Computed tomography (CT) scan of the lung shows viral pneumonia. All people are susceptible to the virus. Pregnant women have significantly increased inflammatory response to respiratory system infection, and the disease progresses rapidly, which tends to evolve into severe complications, so far, the descriptive data is not yet available. Anyhow, coronaviruses have the potential to cause severe maternal or perinatal adverse outcomes.2,3 In order to ensure the safety of mother and fetus, we should screen for COVID-19 more strictly in Wuhan. The challenges for obstetricians and the countermeasures of COVID-19 epidemic were described in the current report.
Pregnant women living in Wuhan have more concerns about COVID-19. In the context of the outbreak of COVID-19 in Wuhan, the incidence of pregnant women with COVID-19 is also significantly higher than other regions. Especially in the early period of the epidemic, obstetricians did not fully understand 2019 novel coronavirus (2019-nCoV) or COVID-19. There were three cases in the Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, who delivered by cesarean section during January 17th to January 23rd. They showed postoperative fever and dry cough. The CT scanning of viral pneumonia, nucleic acid positive results confirmed the diagnosis of COVID-19 and the patients were transferred to the designated hospital for treatment. The mothers and neonates have good prognosis. After the closure of Wuhan on January 24, with the increasing understanding of COVID-19, we strengthened the screening and treatment of pregnancy with COVID-19. Strict temperature screening for pregnant women, asking related symptoms and examination result is the effective way to detect pregnancy complicated with COVID-19 in the early stage, which can effectively avoid possible nosocomial contact and ensure the safety of pregnant women and fetuses.
Here, we introduced a case of a pregnant woman in the third trimester complicated COVID-19 to detail the procedure of screening, diagnosis and treatment. With the effective therapy, the prognosis for the mother and newborn is good.
Case report
A 27-year-old pregnant woman, gravida 1, para 0, the gestation weeks was 36 weeks and 5 days. She presented to the Wuhan Union Hospital with fever, cough, and shortness of breath. She had been ill for 9 days when she was admitted, she was treated with azithromycin (0.5 mg/day, p.o.) and oseltamivir (75 mg, bid, p.o.) for 5 days in the outpatient. However, her symptoms had been getting worse, after activity, she felt obvious chest tightness. The fever was retained, the temperature waved between 37.5 °C and 38.9 °C. She felt the fetal movement was as usual. She had no medical history of note: she did not go to the Wuhan Huanan seafood wholesale market, and did not contact any COVID-19 patient.
On examination, the temperature was 37.8 °C, her heart rate was 133 bpm, respiratory rate was 25 breaths/min, SpO2 92%–93% (non oxygen inhalation). The fetal heart rate was 165 bpm. Irregular uterine contraction could be touched. The non-stress test of the fetus was normal, except the baseline of the fetal heart was a little higher, the fetal movement and fetal heart acceleration were monitored which meant the fetus was well.4
A full blood count found a haemoglobin concentration of 111 g/dL and a leucocytosis count (3.66 × 109/L, normal range: (3.5–9.5) × 109/L), lymphocyte was (1.05 × 109/L) which was decreased according to the normal range of (1.1–3.2) × 109/L. The level of C-reactive protein (CRP) was a little higher than normal 10.63 mg/L. Coagulation profile, and serum biochemical test including renal and liver function, and creatine were normal. A CT of the patient's lung showed bilateral multiple lobular and subsegmental areas of consolidation and bilateral ground-glass opacities (Fig. 1). B-ultrasound examination of the fetus showed that the depth of amniotic fluid was 2.0 cm which indicated oligohydramnios and cervical length was 3.7 cm.
Figure 1.
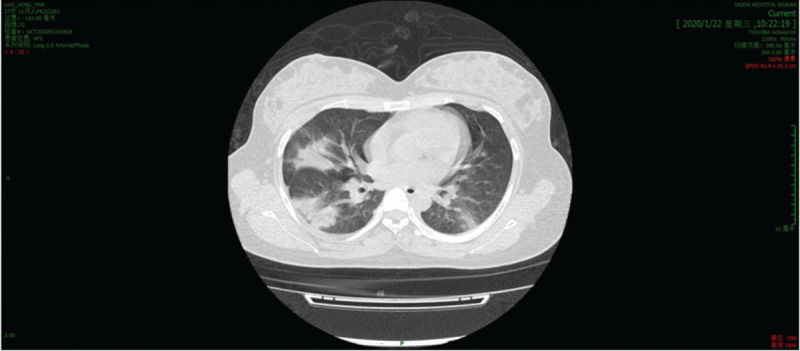
CT scan of the patient's lung showed bilateral multiple lobular and subsegmental areas of consolidation and bilateral ground-glass opacities. CT: Computed tomography.
After consultation with obstetrician, infection disease physician and neonatal pediatrician, the termination of pregnancy and cesarean section was decided. The patient delivered a 3 100 g male infant with Apgar scores of 8 and 9, at 1 and 5 minutes, respectively. The infant did not have respiratory distress syndrome and had an uneventful neonatal course. The infant did not treat with antiviral therapy and raised by the specialist isolatedly.
After delivery, the patient experienced 2 days fever, the SpO2 was 86% without oxygen supplying and maintained at 96% under 3 L/min oxygen condition. On the first day after operation, the highest temperature was 37.9 °C. Anti-inflammatory (moxifloxacin, 400 mg/day, intravenously) and antivirotic (ganciclovir, 500 mg/day, intravenously) were administered after surgery, her chest distress, cough, breathlessness symptoms were alleviated. On the second day after delivery, the temperature returned to normal.
On the 4th day after delivery, throat swabs of both the mother's and the neonate's were obtained. Quantitative polymerase chain reaction of 2019-nCoV of the mother's specimen was positive, while the neonate's throat swab test was negative. Physical examination of the newborn was normal: the weight was 3.3 kg, body temperature was 36.6 °C, the breath was stable, frequency was 45 breaths/min, heart rate was 130 bmp, yellow skin could be seen on the face and chest, no rash and bleeding point, clear breath sound in both lungs, no rale, rhythm, strong heart sound, no murmur. The abdomen was flat and soft, and the bowel sounds were normal. There was no edema in the limbs. On February 6th, the negative 2019-nCoV result of the patient indicated she was recovered.
According to the 5th edition of new coronavirus pneumonia prevention and control program from the National Health Commission of the People's Republic of China5 and the clinical experience, the countermeasures for pregnancy with COVID-19 was summarized.
Treatment of pregnant women and suspected patients with fever in the outpatient
The pregnant woman who has fever or respiratory symptoms should be treated in the hospital where the fever clinic, isolation ward, and the obstetric wards are available. The pregnant woman should undergo pre-diagnosis triage first. In Wuhan, the pregnant woman whose body temperature of infrared measurement is more than 37.3 °C or having respiratory symptoms should be immediately starts the routine protection, and guided to the designated fever clinic by the specialized personnel. More active examinations such as CT scanning of chest should be taken to screen COVID-19.
Diagnosis procedures
The diagnosis depends on the epidemiological and clinical characters according to edition of new coronavirus pneumonia prevention and control program from the National Health Commission of the People's Republic of China.5 Epidemiology history in Hubei province plus two pieces of clinical manifestations indicated suspected pregnant women with COVID-19. The suspected patients should undergo lung imaging examination, the image findings of pneumonia indicated clinical diagnostic patient. The suspected patient tested positive for pathogens could be regarded as confirmed pregnant women with COVID-19. Auxiliary examinations should be applied, besides blood routine test, the level of CRP should be tested, CRP often rises. Pathogens, for example, mycoplasma pneumoniae, chlamydia pneumoniae, respiratory syncytial virus, adenovirus, coxsackie virus, influenza A virus, influenza B virus should be screened. 2019-nCoV nucleic acid test should be applied immediately. It should be noted that even if the etiology of common respiratory infection pathogen is positive, COVID-19 cannot be excluded. After informed consent, chest CT scanning should be performed (fully explain to the patient the necessity of CT scanning and proper abdominal protection should be applied). The typical manifestations: multiple small plaque shadows and interstitial changes in the early stage, multiple ground glass shadows and infiltration shadows in both lungs after progression, pulmonary consolidation and pleural effusion in severe cases.
The suspectable or confirmed pregnant women with COVID-19 should be admitted to the isolated wards. Public health authorities should be notified immediately. All staff should wear protective items before entry to the room: gown, N95 mask, eye protection, gloves, and so on. The obstetrician should be asked to screen the condition of fetus.6 First of all, urgent and critical obstetric conditions should be excluded. After 28 weeks of pregnancy, nonstress test and ultrasonography should be applied.2
Treatment
COVID-19 develops rapidly, so it is recommended that pregnant women with COVID-19 should be admitted to the isolated wards and treated.7 The optimal admission place is designated hospital with effective isolation wards. If possible, labor and delivery (including operative delivery or cesarean section) should be managed in a designated negative pressure isolation room. Multiple disciplinary team including infectious disease specialist, obstetricians, specialist in the intensive care units should be organized to treat pregnant women with COVID-19. Critically patients should be admitted to intensive care units directly. Principles of pregnant women with COVID-19 treatment refer to the 5th edition of new coronavirus pneumonia prevention and control program from the National Health Commission of the People's Republic of China.4 During the treatment, the fetus should also be monitored routinely. If the patients get worse, the safety of pregnant women should be the first priority. The diagnosis and treatment should not be delayed for fear of affecting the fetus. The choice of medicine should be taken into account the interests of the fetus. Once diagnosed, antiviral medicine of Food and Drug Administration (FDA) C is recommended, try to avoid the use of FDA D medicine. Lopinavir/litonavir (200 mg/50 mg per pill) two pills, bid. The incidence of birth defects caused by lopinavir was reported to be comparable to that of the general population.8 Ribavirin 500 mg every 12 hour/every 8 hours (q12 h/q8 h) intravenous drip could be used during postpartum period. Interferon spray can be used for pregnant women in the second and third trimester. The choice of antibiotics should be based on the needs of the disease,9 the first choice of FDA B is prior.
According to the new coronavirus pneumonia prevention and control program from the National Health Commission of the People's Republic of China (5th edition), the patients could be classified to four types depends on the severity of COVID-19: mild, the patient has only mild clinical manifestations and no radiographic manifestations of pneumonia; common, the patient has fever, respiratory symptoms, and pneumonia imaging findings; severe, the patient has any one of the following criteria, (1) respiratory distress, respiratory rate increase ≥30 breaths/min, (2) in resting state, oxygen saturation is no more than 93%, (3) partial arterial oxygen pressure/fraction of inspired oxygen ≤300 mm Hg (1 mm Hg = 0.133 kPa); critical, the patient has any one of the following criteria, (1) respiratory failure, (2) shock, (3) concomitant with other organ failure. The critical patients should be admitted to intensive care unit in designated medical institutions as soon as possible.
The termination of the pregnancy
Timing of pregnancy termination
The timing of termination of pregnancy should be judged by the gestational week, the severity of COVID-19 and the condition of the fetus. The decision of pregnancy termination needs the complete discussion by the multiple disciplinary team. If gestational week <28 weeks, the treatment is mainly depended on infectious diseases department. During 28–32 weeks, after aggressive treatment, if the disease is controlled, the pregnancy could be continued. Once the disease progressed rapidly, the pregnancy should be terminated. After 32 weeks, especially after 34 weeks, fetal survival rate is high, termination of the pregnancy should be considered.
Mode of pregnancy termination
Pregnant woman with mild COVID-19, the cervix is ripe, and has already been in labor may choose the vagina deliver. Indications for cesarean section: (1) severe pneumonia with unsatisfactory therapy effect; (2) various emergency obstetric complications and fetal distress; (3) being in labor but cannot deliver in a short time. Epidural or general anesthesia is suitable.
Key points during cesarean section
During the cesarean section, the blood oxygen saturation should be closely monitored. Puncture and catheterization of the radial artery for monitoring is recommended. After fetal delivery, uterine contractile medicine such as oxytocin should be used to prevent postpartum hemorrhage as soon as possible. Prostaglandin F2α should be used with caution in case of heart failure. Pay attention to liquid balance, so as not to increase the patient cardiopulmonary burden. After delivery, the patients should be transferred back to the isolated ward and be treated with COVID-19.
Protecting the neonates
To reduce perinatal exposure risk, the neonate should have early clamping of the umbilical cord, they should be cleaned off the maternal blood and amniotic fluid as soon as possible.5 The neonate should be isolated from mother for 14 days. Before the mother recovered, no breast feeding.10 Due to high fever and hypoxemia in pregnant women, the risk of fetal distress and premature delivery is increased.6 Before delivery, transferring neonates to neonatal intensive care unit should be prepared. In the process of transferring, the newborns should be protected according to the isolation protective measures.
Confirmed COVID-19 or close contacting with COVID-19 patient occurred during the puerperal period
The women during postpartum period have COVID-19, or close contact with COVID-19 patient, should be isolated from the neonates, and forbidden to breastfeed.
Transferring for fever, suspected, and confirmed pregnant women with COVID-19
Local designated hospital without the ability to rescue high-risk pregnant women and premature newborns, or the pregnant women are severe or critically case. The patient should be transferred directly to a designated hospital at a higher level.
Isolation protection and avoid nosocomial infection
All obstetricians and staffs should be trained of infection control. Staffs in contact with the patient should be actively monitored for fever and other symptoms to avoid cross-infection. Guide the health pregnant women to stay at home as much as possible, reduce the risk of contact with patients. Educate pregnant women to carry out appropriate exercise and pay attention to healthy diet, and seek psychological guidance when necessary.
Funding
None.
Conflicts of Interest
None.
References
- [1].Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395(10223):497–506. doi:10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wong SF, Chow KM, Leung TN, et al. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol 2004;191(1):292–297. doi:10.1016/j.ajog.2003.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Alfaraj SHA-TJ, Memish ZA. Middle East respiratory syndrome coronavirus (MERS-CoV) infection during pregnancy: report of two cases & review of the literature. J Microbiol Immunol Infect 2019;52(3):501–503. doi:10.1016/j.jmii.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Liston R, Sawchuck D, Young D. No.197b-fetal health surveillance: intrapartum consensus guideline. J Obstet Gynaecol Can 2018;40(4):e298–e322. doi:10.1016/j.jogc.2018.02.011. [DOI] [PubMed] [Google Scholar]
- [5]. National Health Commission of the People's Republic of China. New Coronavirus Pneumonia Prevention and Control Program (5th ed.) (In Chinese). 2020. Available at: http://www.nhc.gov.cn/xcs/zhengcwj/202002/d4b895337e19445f8d728fcaf1e3e13a/files/ab6bec7f93e64e7f998d802991203cd6.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Maxwell C, McGeer A, Tai KFY, et al. No. 225-management guidelines for obstetric patients and neonates born to mothers with suspected or probable severe acute respiratory syndrome (SARS). J Obstet Gynaecol Can 2017;39(8):e130–e137. doi:10.1016/j.jogc.2017.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. World Health Organization. Clinical Management of Severe Acute Respiratory Infection When Novel Coronavirus (nCoV) Infection is Suspected: Interim Guidance. 2020. Available at: https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. [Google Scholar]
- [8].United States Public Health Service Task Force. Perinatal HIV Guidelines Working Group Members. Public Health Service Task Force recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV-1 transmission in the United States (revised November 3, 2000). HIV Clin Trials 2001;2(1):56–91. doi:10.1310/3ENW-TR0F-UQ0B-GWKD. [DOI] [PubMed] [Google Scholar]
- [9].Patel VM, Schwartz RA, Lambert WC. Topical antibiotics in pregnancy: a review of safety profiles. Dermatol Ther 2019;32(4):e12951. doi:10.1111/dth.12951. [DOI] [PubMed] [Google Scholar]
- [10].Huijun Chen, Juanjuan Guo, Chen Wang, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet 2020;395(10226):809–815. 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


