Abstract
Objective:
To determine the pregnancy and neonatal outcomes of women who recovered from coronavirus disease 2019 (COVID-19) that developed in early pregnancy.
Methods:
This case series analyzed five pregnant women (26–33 years) whom recovered from COVID-19 which were developed in early pregnancy (6–27 weeks) and admitted at the Wuhan Union Hospital from January 15, 2020 to April 30, 2020. The clinical manifestation, laboratory examinations, treatment, pregnancy outcomes, maternal and neonatal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) throat swab reverse transcription polymerase chain reaction test results, and SARS-CoV-2 antibody test results in neonates were reviewed. The placental pathology, placental angiotensin-converting enzyme 2 expression were studied by hematoxylin-eosin and immunohistochemistry staining, SARS-CoV-2 presence was examined by QT-PCR. We also followed up the infants at 3–6 months.
Results:
Three pregnant women were diagnosed with COVID-19 in early pregnancy (Cases 1–3), and two were serum immunoglobulin G positive asymptomatic cases (Cases 4 and 5). Cases 1–3 showed complete recovery after severe COVID-19. Case 3 was infected at 6 weeks of gestation during the first trimester and had induced medical abortion at 12 weeks of gestation. All neonates had no pneumonia, SARS-CoV-2 mRNA reverse transcription polymerase chain reaction and serum immunoglobulin M were negative, and immunoglobulin G were positive. All placental samples were negative for SARS-CoV-2 in the nucleic acid test. Placental pathology showed chronic ischemia changes. ACE-2 expressed in both placenta and decidua. The follow-up showed that the infants were healthy and asymptomatic at 3–6 months.
Conclusion:
No adverse outcomes was observed in our case series. However, systemic inflammatory responses to SARS-CoV-2 infection may cause placental injury. At the time of delivery after recovery from COVID-19, no SARS-CoV-2 positive results was found in the placenta in this case series.
Keywords: Coronavirus infections, COVID-19, SARS-CoV-2, Pregnancy outcome, Placenta, Vertical transmission
Introduction
The coronavirus disease 2019 (COVID-19) was prevalent in Wuhan since December 2019, which has also affected pregnant women.1 In Wuhan, these patients accounted for 0.24% of all reported COVID-19 cases.2 Most of them had the mild form of the disease, and have successful delivery, there were no maternal deaths. Most research on pregnancy outcomes has focused on women who were infected in the third trimester and peripartum.2–4 While there are few studies on the outcome of pregnant women complicated by previous COVID-19 that developed in the first and second trimesters, as well post-recovery period. The present study aimed to determine the pregnancy and neonatal outcomes of women who recovered from COVID-19 that occurred in early pregnancy and to analyze the placental pathological changes to explore the effect of the previous severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
Material and methods
Cases
This study was approved by the Medical Ethics Committee of the Union Hospital, TongJi Medical College of Huazhong University of Science and Technology (2020, No. 0144). Written informed consent was obtained from the patients before enrolment and during data collection. This case series was performed at Wuhan Union Hospital, Hubei Province, China, from January 15, 2020 to April 30, 2020. A total of five cases were analyzed in this study. We included three fully-recovered pregnant women with a confirmed diagnosis of COVID-19 in the first or second trimester (<28 weeks’ gestation). The COVID-19 recovery criteria were defined according to the World Health Organization interim guidance criteria.5 Two asymptomatic cases with positive immunoglobulin G (IgG) at the time of delivery were also included. Cases 4 and 5 developed mild cold symptoms at 26 and 27 weeks, but they did not seek medical attention. Their symptoms improved after 1 week. We reviewed the patients’ medical records, laboratory results, computed tomography (CT) imaging results, treatment course, pregnancy outcomes, neonatal throat swab test results, and antibody test results. The placental pathology, placental angiotensin-converting enzyme 2 (ACE2) expression, and SARS-CoV-2 presence were also studied. We also followed up the infants at 3–6 months.
Tissue collection
The entire placentas from cases (case 1, 2, 3, 4) were collected and saved in formaldehyde solution.
Hematoxylin-eosin stained
The placenta was inspected by sectioning at 1–2 cm intervals looking for irregularities and procured tissue was fixed in 10% formalin for hematoxylin for 24–48 h, and then routinely processed under standard biosafety measures. Hematoxylin-eosin stained sections were prepared, and slides were examined by two pathologists (Bangxing Huang & Xiu Nie).
Immunohistochemistry analysis
To detect the distribution of ACE2 in placenta, immunohistochemistry staining was carried out, using antibody ACE2 from Abcam (catalog no. ab15348; dilution 1:200; Cambridge, MA). Immunohistochemistry was performed according to manufacturers’ protocols on a Dako Link 48 automated stainer. Whole 4-μm tissue sections were de-waxed, rehydrated in an ethanol gradient, and rinsed with phosphate-buffered saline. Following pressure cooker-mediated antigen retrieval in citrate buffer (pH 6.0) for 1 min and 30 s, the endogenous peroxidase activity and non-specific binding were blocked using 1% H2O2 and 1% bovine serum albumin for 20 min at room temperature. The slides were subsequently incubated with antibody ACE2 from Abcam (catalog no. ab15348; dilution 1:200) overnight at 4 °C. Immunodetections were performed using the DaKo EnVision Kit (DakoCytomation, Denmark), and Diaminobenzidine (DakoCytomation) was used as the chromogen. Negative controls (primary antibodies replaced by phosphate-buffered saline buffer) were conducted to exclude the possibility of non-specific staining.
ACE2 evaluation criteria: the area quartering method was 1, 2, 3, and 4 scores, and the staining intensity was 1 (weak), 2 (medium), and 3 (strong), respectively. The total scores were area scores multiply staining intensity scores.
Real-time PCR analysis
Real-time reverse transcription-polymerase chain reaction (RT-PCR) assay for SARS-CoV-2 infection Fourteen 5-mm-thick formalin-fixed, paraffin-embedded sections were used for RNA extraction using an AmoyDx FFPE RNA extraction kit (Amoy Diagnostics, Xiamen, China). SARS-CoV-2 RNA was detected using a real-time multiplex RT-PCR kit (Liferiver Biotechnology, Shanghai, China), which detects the SARS-CoV-2 RdRp gene, E gene, and N gene simultaneously. RT-PCR was performed on an Mx3000 P real-time PCR system (Agilent Technologies, Santa Clara, CA, USA). According to the manufacturer's protocol, a threshold cycle (Ct) of 43 for all three genes, or the RdRp and E genes, or the RdRp and N genes indicated the presence of SARS-CoV-2.
Results
Early pregnancy infections were noted in three women who were infected at 6–27 weeks of gestation and who recovered after treatment for pneumonia. Before delivery, CT imaging showed the resolution of pneumonia. Throat swab SARS-CoV-2 tests were performed twice, which showed negative results. Only the SARS-CoV-2 IgG antibody test was positive. Case 1 delivered at 40 weeks of gestation after administration of dinoprostone suppository, which improved cervical ripening and oxytocin augmentation of uterine contraction. Case 2 had premature rupture of membranes at 35 weeks with a transverse presentation and delivered via cesarean section. Case 3 was infected with the SARS-CoV-2 at 6 weeks of gestation and underwent an induced medical abortion at 12 weeks after administration of mifepristone and misoprostol.
Additionally, in two asymptomatic cases (Cases 4 and 5) who had serum SARS-CoV-2 IgG positivity on routine screening before delivery, their chest CT scan and nucleic acid test results were unremarkable.
COVID-19 during pregnancy
Following the World Health Organization interim guidance criteria,5 three cases were diagnosed with COVID-19, including Case 1 who was 24+1 weeks of gestation and Case 2 who was 26+3 weeks of gestation when they were diagnosed with COVID-19. They were previously healthy. Both presented with fever, fatigue, and dry cough. The descriptions are listed in Table 1. Case 1 had respiratory distress and dyspnea. Case 2 presented with stuffy nose and respiratory distress. Case 3 had no dyspnea. All three cases had moderate anemia. Case 2 had lymphopenia (0.54 × 109/L), leukopenia (3.21 × 109/L), and neutropenia (1.65 × 109/L). Case 3 presented with moderate leukopenia. Cases 1, 2, and 3 had elevated infection indexes, including C-reactive protein and interleukin 6 levels. They all had decreased albumin and total protein levels, and two cases had elevated liver enzyme, alanine aminotransferase, and aspartate aminotransferase levels (Table 2). Cases 1 and 3 had positive nucleic acid tests. Case 2 had two consecutive negative nucleic acid tests; however, she presented with viral pneumonia. On readmission after 70 days, SARS-CoV-2 IgG was positive confirming the presence of a previous SARS-CoV-2 infection. Case 1 had a severe type of COVID-19 and was admitted for 12 days. Cases 2 and 3 had mild infections and were treated for 6 and 10 days as inpatients, respectively. According to chest CT imaging, they all had viral pneumonia. CT imaging of Case 1 showed multiple, membranous, ground-glass-patterns or dense shadows surrounding the parenchyma of both lungs. The pulmonary lesions were improved after treatment. Cases 2 and 3 showed similar chest CT findings (Fig. 1). All cases received oxygen therapy using a nasal cannula. Case 1 had severe dyspnea, fatigue, and desaturations (oxygen saturation, 90% on room air). Cases 2 and 3 had moderate distress (oxygen saturation, 95%–96%) (Table 1).
Table 1.
Clinical characteristics of cases.
| Pregnant women complicated with COVID-19 before 28 weeks and had recovered before delivery | Pregnant women without COVID-19 history, only IgG+ before delivery | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Items | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 |
| Age (years) | 26 | 33 | 33 | 30 | 26 |
| Gestational age when infected (weeks) | 24+1 | 26+3 | 6 | 27 | 26 |
| Previous pregnancy history | G0 | G2P0A1 | G8P1 previous cesarean section | G0 | G0 |
| Pregnancy complications | No | Threatened abortion | No | No | No |
| Symptoms | Pneumonia-related manifestations | Pneumonia-related manifestations | Pneumonia-related manifestations | Cold symptoms | Cold symptoms |
| Fever days and temperature | 37.3–38.5 °C 10 days | 38.2 °C 3 days | 38.5 °C 7 days | No | No |
| Fatigue | Yes | Yes | Yes | Yes | Yes |
| Cough | Yes | Yes | Yes | No | No |
| Shortness of breath | Yes | Yes | No | No | No |
| SpO2 without oxygen inhalation (%) | 90 | 94 | 95 | 95 | 96 |
| Oxygen administration | Nasal cannula | Nasal cannula | Nasal cannula | No | No |
| Anti-virus medicine | Abby dole, Recombinant human interferon a-2b | Oseltamivir | No | No | No |
| Antibiotics | Yes | Yes | Yes | No | No |
| Hospitalization (days) | 12 | 6 | 10 | No | No |
| Complications | Elavated liver enzyme | Lymphopenia | Elavated liver enzyme | No | No |
A: Abortion; COVID-19: Coronavirus disease 2019; G: Gravida; IgG: Immunoglobulin G; P: Para; SpO2: Oxygen saturation.
Table 2.
Infection and liver function index.
| Items | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| C-reactive protein (mg/L) | 6.90 | 31.88 | 26.80 |
| Interleukin 6 (pg/mL ) | 67.98 | NA | 7.49 |
| Albumin (g/L) | 27.9 | 31.4 | 31.4 |
| Total protein (g/L) | 55.8 | 57.1 | 57.6 |
| Alanine aminotransferase (U/L) | 510 | 14 | 104 |
| Aspartate aminotransferase (U/L) | 246 | 21 | 38 |
NA: Not available.
Figure 1.
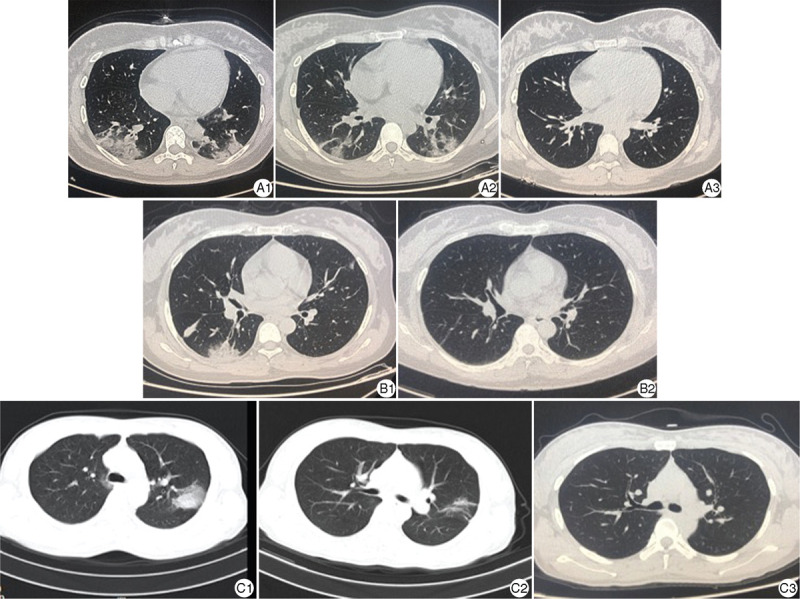
Chest CT image of the cases. A1, B1, C1 were the CT scanning images of pregnant women (Case 1–3) complicated with COVID-19. Multiple or scattered membranous glass density shadows/dense porphyry shadows surrounding the parenchyma of both lungs could be seen at the onset of COVID-19, which indicated the possibility of viral pneumonia. A2, C2 were the CT image of Case 1 and Case 3 which showed lesions in lung were improved after being treated for 7 days. A3, B2, C3 were the CT image of Case 1–3 at the time before delivery, no obvious abnormality was observed. COVID-19: Coronavirus disease 2019; CT: Computed tomography.
Case 1 received antiviral treatment, interferon (recombinant human interferon a-2b 5 million IU daily), atomization inhalation for 7 days, oral umifenovir tablets (200 mg three times daily for 7 days), and azithromycin (0.25 g two times daily for 3 days). Polyene phosphatidyl choline (458 mg three times daily) and reduced glutathione (1.8 g daily) were used. All cases were given an antibiotic treatment (Table 3) They were discharged in a stable condition. Follow-up CT scan showed the improvement of pulmonary lesions, and the SARS-CoV-2 throat swab test showed two consecutive negative results. Repeat antibody testing before delivery showed positive serum IgG and negative immunoglobulin M (IgM) antibodies in all three cases (see Table 3).
Table 3.
Maternal characteristics, pregnancy outcomes, and placenta test results.
| Items | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 |
|---|---|---|---|---|---|
| Interval from the onset of COVID-19 to delivery (days) | 121 | 76 | 52 | 84 | 98 |
| Gestational weeks at delivery (weeks) | 40 | 35 | 12 | 38+4 | 40 |
| Delivery mode | Vaginal delivery | CS | Induced abortion | CS | Vaginal delivery |
| Fetal Weight (grams) | 3650 | 2800 | – | 3200 | 3750 |
| Apgar scores at 1 min and 5 min | 8–9 | 7–9 | – | 8–9 | 8–9 |
| Neonates | |||||
| Neonatal throat swab test for SARS-CoV-2 | Negative | Negative | – | Negative | Negative |
| SARS-CoV-2 IgG in umbilical cord blood | Positive | Positive | – | Positive | Positive |
| SARS-CoV-2 IgM in umbilical cord blood | Negative | Negative | – | Negative | Negative |
| Placenta | |||||
| RT-PCR of SARS-CoV-2 | Negative | Negative | Negative | Negative | – |
| ACE2 expression scores | |||||
| Placenta | 1 | 2 | 6 | 3 | – |
| Decidua | 4 | 4 | 1 | 1 | – |
| Pathological expression | Multiple placental calcifications and dense small villi with increased syncytial knots | Placental chronic infarct was seen at the maternal side | Part villous edema and fibrosis | Massive infarct and calcification | |
ACE2: Angiotensin-converting enzyme 2; COVID-19: Coronavirus disease 2019; CS: Cesarean section; IgM: Immunoglobulin M; IgG: Immunoglobulin G; RT-PCR: Reverse transcription polymerase chain reaction; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; –: Not applicable.
Pregnancy outcome
The interval period from discharge to delivery was 52–121 days for all cases. Case 1 was admitted at 40 weeks, with a Bishop Score of 6. After dinoprostone and oxytocin administration, a 3650 g male newborn was delivered with an Apgar score of 8 at 1 min and 9 at 5 min. Epidural labor analgesia was administered when the cervix was 3 cm dilated. Case 2 had premature rupture of membranes at 35 weeks with a transverse presentation and delivered via cesarean section to a 2800 g female baby. Apgar score was 7 at 1 min and 9 at 5 min.
The neonates had no pneumonia, negative COVID-19 throat swab nucleic acid test results, and serum IgG positivity. Neonate 1 was roomed-in and was breastfed. At the 1-month follow-up, neonate 1 was healthy and asymptomatic. Neonate 2 was transferred to neonatal intensive care unit due to preterm birth. The neonate remained asymptomatic during admission and was discharged after a week following two consecutive negative nucleic acid test results. IgG was positive. At 3–6 months after birth, the infants were healthy.
Cases 4 and 5 delivered healthy, full-term babies. Both of them showed negative SARS-CoV-2 throat swab nucleic acid test results and serum IgG positivity (Table 3).
Placental examination
The placental pathology performance of Cases 1 to 4 showed varying pathological changes. In general, the placentas of pregnant women with COVID-19 show more calcification, increased syncytial, fibrous deposition, and other chronic ischemic manifestations. No definite thrombosis was found in these cases. No obvious chorioamnionitis or virus inclusion body formation was observed in all cases. The umbilical cord and amnion membranes were normal (Fig. 2). Immunohistochemical staining of ACE2 showed that both the placenta and the decidual membrane expression, which were located in the membrane and cytoplasm of the syncytiotrophoblast and decidual cells. ACE2 expression was stronger in the early pregnancy (Fig. 3). Placental SARS-CoV-2 nucleic acid test results of all the cases were negative (Table 1).
Figure 2.
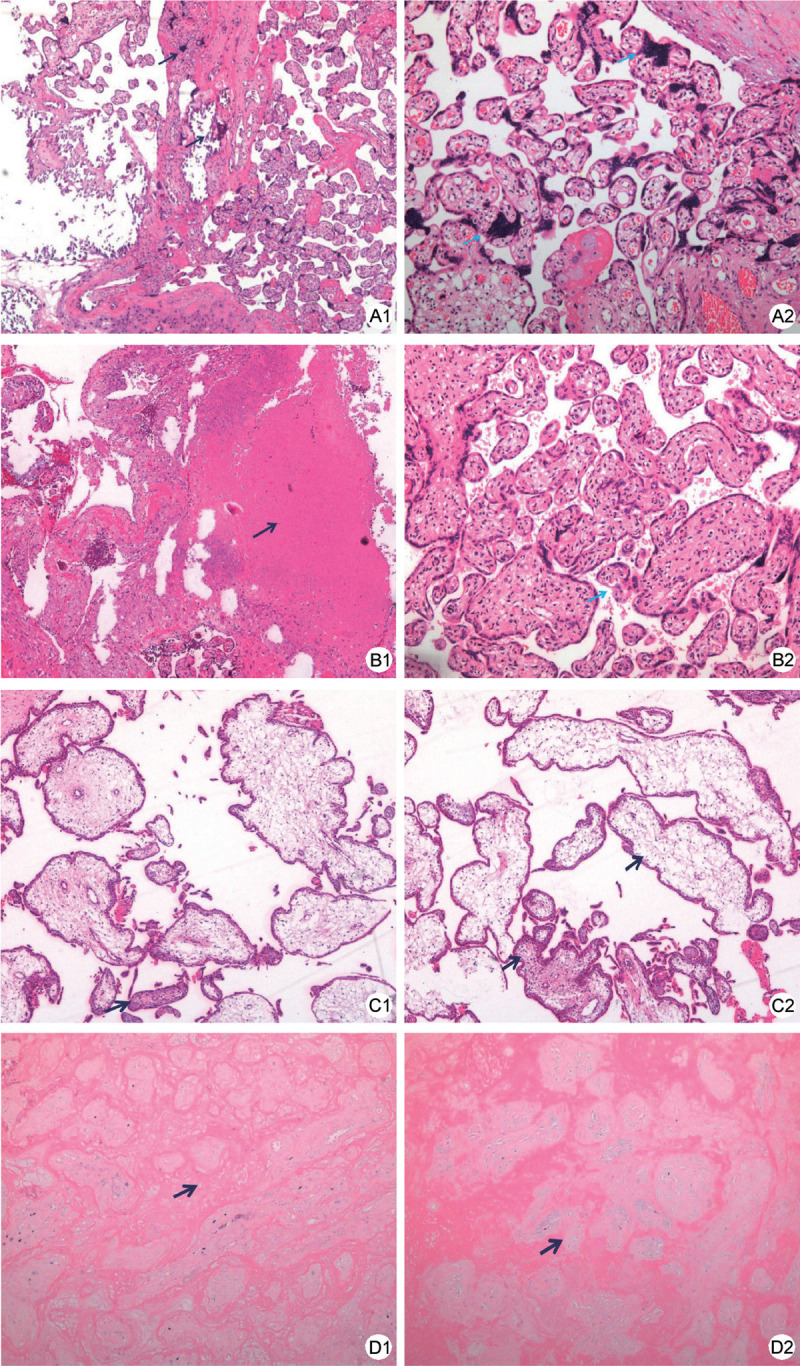
Pathological findings of placental tissue. A1, A2 Pregnant woman (Case 1) of 24 weeks complicated with COVID-19, and delivered at 40 weeks. Placental chronic ischemia changes was shown: presence of multiple placental calcifications (black arrow) and dense small villi with increased syncytial knots (blue arrow). (A1 HE, ×40; A2 HE, ×100). B1 Pregnant woman (Case 2) of 26 weeks complicated with COVID-19, and delivered at 35 weeks. Placental chronic infarct was seen at the maternal side (black arrow) (HE, ×40). In the middle part of the placenta, normal villi structure could be seen (B2). In the middle part of the placenta, normal villi structure could be seen (blue arrow) (HE, ×100). C1, C2 Pregnant woman (Case 3) of 6 weeks complicated with COVID-19, and the pregnancy was terminated at 12 weeks. Part of the villous was edema and fibrosis (black arrow) (HE, ×40). No definite thrombosis was found. D1, D2 Pregnant women (Case 4) of 38 weeks who was serum IgG positive asymptomatic case. Chronic placental ischemia infarction could be seen (black arrow) (D1 HE, ×40, D2 HE, ×100). COVID-19: Coronavirus disease 2019; HE: Hematoxylin-eosin staining; IgG: Immunoglobulin G.
Figure 3.
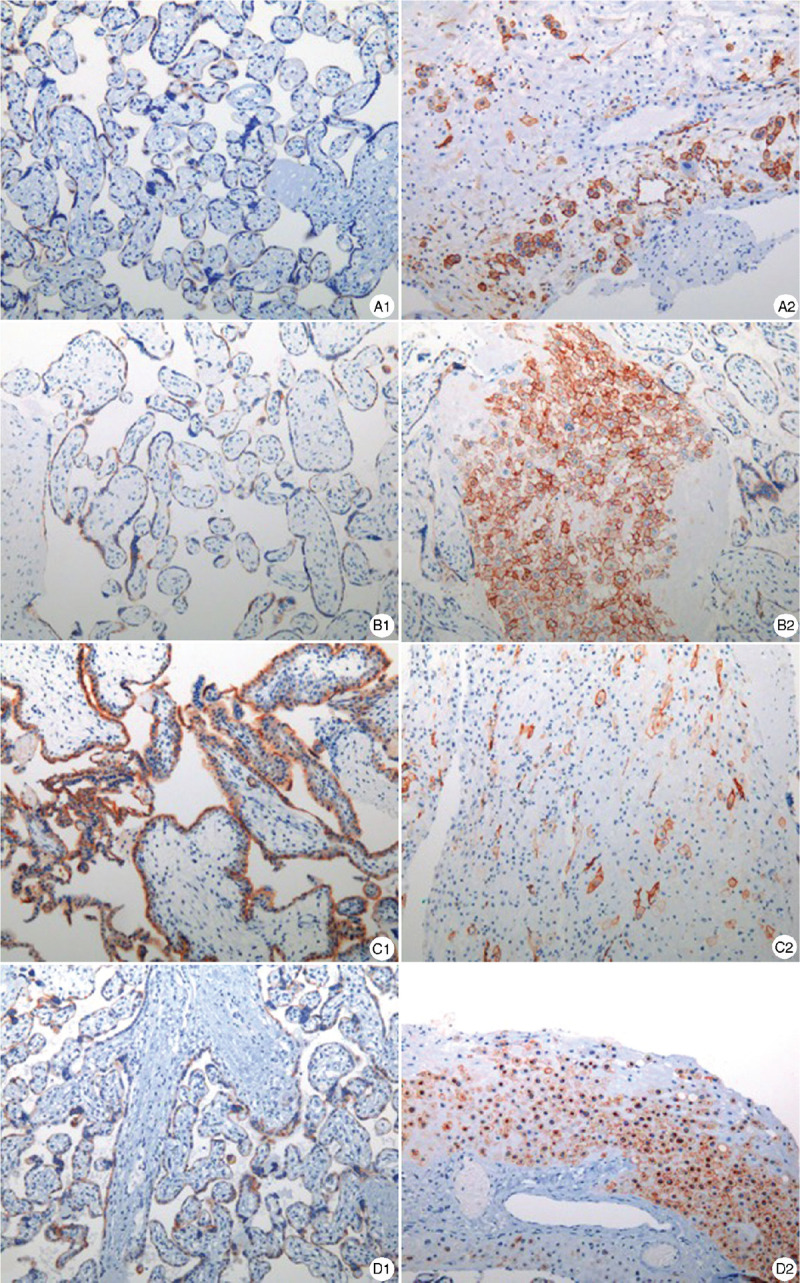
Representative ACE2 immunohistochemical staining in placenta. ACE2 was positive in both villi and decidua, which was mainly expressed in membrane and cytoplasm. A1, B1, C1 and D1 show placental ACE2 expression in Case 1–4. A2, B2, C2 and D2 show ACE2 expression in decidua in Case 1–4. It expressed stronger in 12 weeks (Case 3). (Immunohistochemical staining, ×100). ACE2: Angiotensin-converting enzyme 2.
Discussion
The current cases series study showed that pregnant women infected with SARS-CoV-2 in the early pregnancy could recover with a relatively good outcome. All neonates had no pneumonia, the nucleic acid test and serum IgM were negative, but IgG were positive. All placental samples were negative for SARS-CoV-2 nucleic acid test. Placental pathology showed chronic ischemia changes, for example the increased villous syncytic knot, fibrinoid necrotic villi, and increased and calcified villous stromal fibers. At 3–6 months after birth, the babys’ health were known through telephone follow-up.
According to the data of 118 COVID-19 cases in pregnant women in Wuhan,2 64% of the cases occurred during late pregnancy, and the rate of severe COVID-19 cases in pregnant women was 8%, which was lower than the average level in the general population (15.7%).6 There were no deaths reported. This was consistent with our study, wherein even severe COVID-19 cases improved after receiving proper treatment (Case 1).
Among the three confirmed patients, two showed elevated liver enzymes, but all of them had increased pro-inflammatory factors. The liver might be affected by the systematic inflammatory situation. Finally, after the administration of antiviral therapy and liver supplements, the symptoms of these cases were improved.
None of the patients showed significant fetal malformations or fetal or placental virus positive findings. Even Case 3, who had artificial abortion, the fetus had normal crown-rump length at 12 weeks, which was consistent with the gestational age.
Placental pathology of the cases suggests chronic placental injury. Calcification of a normal full-term placenta usually occurs at the edge of the placenta, covering no more than 10% of the placenta's surface area. However, the calcification area in case 1 was large and irregular, which was different from the scattered calcification in termed placenta, which suggested chronic ischemia injury. Especially, in Case 2, massive perivillous fibrin deposition and large agglomeration of necrotic villi suggested that placental infarction was developed. These may be secondary to the hypoxic and inflammatory condition observed in COVID-19 cases. Hence, pregnancy complicated with COVID-19 may decrease maternal blood perfusion into the villi, hindering maternal-fetal exchange.2 Although placental pathological changes were noted, the placental potency may be enough to overcome the villi impairment, so the outcomes were relatively ideal. However, extremely severe placental infarction may result in placental abruption and fetal growth restriction.7 If pregnant women have already complicated with placental dysfunction (preeclampsia, fetal growth restriction, and so on), COVID-19 may severely affect the placenta and fetus, and result in adverse pregnancy outcomes. Recently, a case of a pregnant woman in the second trimester with symptomatic COVID-19 complicated by severe preeclampsia and placental abruption was reported. SARS-CoV-2 localized predominantly to syncytiotrophoblast cells at the maternal-fetal interface of the placenta was found. Histological examination of the placenta revealed a dense macrophage infiltrate, which demonstrates SARS-CoV-2 invasion of the placenta, highlighting the potential for severe morbidity among pregnant women with COVID-19.8
These studies indicated that pregnancy with COVID-19 during the first and second trimesters may result in full recovery allowing the continuation of pregnancy to term with good maternal and fetal outcomes. For infected pregnant women who had critical conditions or pregnant women who were already complicated by other comorbidities, SARS-CoV-2 infection is a great threat to pregnancy and may lead to adverse outcomes.
ACE2 is a functional receptor for SARS-CoV-2 infection.9 Theoretically, ACE2 provides the opportunity for SARS-CoV-2 infection. SARS-CoV-2 binds to ACE2 receptor via the S protein, then enters the cells.10,11 The available evidence suggests that ACE2 is widely expressed in the female reproductive system and the maternal-fetal interface, which includes the stromal and perivascular cells of decidua, cytotrophoblasts, and syncytiotrophoblasts.9,12 Our results demonstrated that ACE2 was expressed in both the placenta and decidua, which seemed to be stronger in the early pregnancy. However, we found no SARS-CoV-2 nucleic acids in all the placental samples. But whether a placental infection occurred at the time of COVID-19 in the early pregnancy cannot be ruled out completely.
Many studies had tried to determine whether there is clear evidence of intrauterine infection in pregnant women with COVID-19. Although the majority of mothers had a mild form of the disease without serious complications, sporadic severe maternal morbidity as a result of COVID-19 and perinatal deaths were reported. Vertical transmission of COVID-19 could not be excluded.2,4,13,14 Evidence of intrauterine infection has recently been accumulating. Two different studies reported that both SARS-CoV-2 IgM and IgG were detected in newborns of infected mothers, which raise the question of intrauterine infection possibility.15,16 However, positive IgM test result could not be regarded as the definite evidence of vertical transmission.17 Lamouroux et al.18 reported three positive cases of SARS-CoV-2 infection among 11 placental or membrane swabs sent following the delivery in women with moderate-to-severe COVID-19. None of the infants tested positive for SARS-CoV-2 on days 1 to 5 of life, and none demonstrated COVID-19 symptoms. While there were no clinical signs of vertical transmission, they postulated the presence of a possible intrapartum viral exposure. For infants delivered vaginally, viral contamination may be obtained from room air, vaginal secretions, maternal blood, or amniotic fluid. Wang et al.19 reported a case of positive SARS-CoV-2 throat swab test in a newborn at 36 h after delivery. The possibility of airborne transmission after delivery cannot be excluded. Recently, researchers in France reported a neonate born to a mother infected in the last trimester and presented with neurological compromise. The presence of vertical transmission was demonstrated by maternal and neonatal viremia, positive placental immunohistochemistry results of SARS-CoV-2, and placental inflammation, following placental infection.20
High viral loads of SARS-CoV-2 is necessary for vertical transmission. The placental villi may provide more opportunities for SARS-CoV-2 to enter the fetal blood, allowing the virus to infect the trophoblast cells. SARS-CoV-2 may then pass through the placental villous lobules to enter the fetal capillaries and infect the fetus. According to some reports, the lungs and intestine may be major viral target organs of SARS-CoV-2.21 Studies have shown from fetal to neonate phase, ACE2 may have a high expression in the lungs.22 This places neonate at a high risk for SARS-CoV-2 infection. Thus, obstetricians should pay more attention to pregnant women with COVID-19, amniotic fluid and placenta should be collected to determine the presence of SARS-CoV-2 infection, and neonatal conditions should be closely monitored.
In clinical studies, most of the neonates were negative of SARS-CoV-2.2,4 However, for pregnant women who had viremia, the possibility of intrauterine infection still remains. Further studies on the risk factors for intrauterine infection are warranted, and we should explore the treatment approaches to reduce the risk of intrauterine infection in pregnancy.
Our study still has several limitations. Only five cases were enrolled, which allowed us to follow the outcomes of COVID-19 from early pregnancy to delivery. Therefore, our results may not be generalizable to other populations. Further studies are needed. Second, fetal and maternal outcomes were only observed until 3–6 months after birth, which provides limited information. Further follow-up is important to assess the long-term effects of maternal COVID-19 on the infant.
In conclusion, no adverse fetal outcomes were observed during the neonatal follow-up in the infants born to mothers infected with SARS-CoV-2 in early pregnancy in the current study. COVID-19 during pregnancy may lead to a systemic inflammatory response and hypoxia, resulting in elevated liver enzyme levels and placental insufficiency. The placenta has a potential reserve capacity allowing pregnancy to continue to term. At the time of delivery after recovery, no SARS-CoV-2 positive results were found in the placenta. However, for infected pregnant women with critical conditions and pregnant women already complicated by other comorbidities, SARS-CoV-2 infection may disrupt the placental barrier or lead to severe placental dysfunction and adverse outcomes.
Funding
This work was funded by the National Natural Science Foundation of China (81771605) and the General Project of Hubei Provincial Health and Health Commission (WJ2017M093).
Author Contributions
Yin Zhao and Li Zou conceived the study. Bangxing Huang and Xiu Nie performed the placental study. Hui Ma carried out the computed tomography imaging data collection. You Shang collected the clinical data. Yin Zhao wrote the first draft. Xiu Nie and Li Zou reviewed the article.
Conflicts of Interest
None.
References
- [1].Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382(8):727–733. doi:10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chen L, Li Q, Zheng D, et al. Clinical characteristics of pregnant women with Covid-19 in Wuhan, China. N Engl J Med 2020;382(25):e100. doi:10.1056/NEJMc2009226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sutton D, Fuchs K, D’Alton M, et al. Universal screening for SARS-CoV-2 in women admitted for delivery. N Engl J Med 2020;382(22):2163–2164. doi:10.1056/NEJMc2009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Juan J, Gil MM, Rong Z, et al. Effect of coronavirus disease 2019 (COVID-19) on maternal, perinatal and neonatal outcome: systematic review. Ultrasound Obstet Gynecol 2020;56(1):15–27. doi:10.1002/uog.22088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. World Health Organization. Clinical Management of Severe Acute Respiratory Infection When Novel Coronavirus (2019-nCoV) Infection is Suspected: Interim Guidance, 28 January 2020. Available at: https://apps.who.int/iris/handle/10665/330893. Accessed April 2020. [Google Scholar]
- [6].Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382(18):1708–1720. doi:10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Laurini R, Laurin J, Marsal K. Placental histology and fetal blood flow in intrauterine growth retardation. Acta Obstet Gynecol Scand 1994;73(7):529–534. doi:10.3109/00016349409006268. [DOI] [PubMed] [Google Scholar]
- [8].Hosier H, Farhadian SF, Morotti RA, et al. SARS-CoV-2 infection of the placenta. J Clin Invest 2020;130(9):4947–4953. doi:10.1172/JCI139569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ge XY, Li JL, Yang XL, et al. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature 2013;503(7477):535–538. doi:10.1038/nature12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Li Y, Zhou W, Yang L, et al. Physiological and pathological regulation of ACE2, the SARS-CoV-2 receptor. Pharmacol Res 2020;157:104833. doi:10.1016/j.phrs.2020.104833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kai H, Kai M. Interactions of coronaviruses with ACE2, angiotensin II, and RAS inhibitors-lessons from available evidence and insights into COVID-19. Hypertens Res 2020;43(7):648–654. doi:10.1038/s41440-020-0455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jing Y, Run-Qian L, Hao-Ran W, et al. Potential influence of COVID-19/ACE2 on the female reproductive system. Mol Hum Reprod 2020;26(6):367–373. doi:10.1093/molehr/gaaa030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yan J, Guo J, Fan C, et al. Coronavirus disease 2019 in pregnant women: a report based on 116 cases. Am J Obstet Gynecol 2020;223(1):111.e1–111.e14. doi:10.1016/j.ajog.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zaigham M, Andersson O. Maternal and perinatal outcomes with COVID-19: a systematic review of 108 pregnancies. Acta Obstet Gynecol Scand 2020;99(7):823–829. doi:10.1111/aogs.13867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dong L, Tian J, He S, et al. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA 2020;323(18):1846–1848. doi:10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zeng H, Xu C, Fan J, et al. Antibodies in infants born to mothers with COVID-19 pneumonia. JAMA 2020;323(18):1848–1849. doi:10.1001/jama.2020.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kimberlin DW, Stagno S. Can SARS-CoV-2 infection be acquired in utero?: more definitive evidence is needed. JAMA 2020;323(18):1788–1789. doi:10.1001/jama.2020.4868. [DOI] [PubMed] [Google Scholar]
- [18].Lamouroux A, Attie-Bitach T, Martinovic J, et al. Evidence for and against vertical transmission for severe acute respiratory syndrome coronavirus 2. Am J Obstet Gynecol 2020;223(1):91.e1–91.e4. doi:10.1016/j.ajog.2020.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wang S, Guo L, Chen L, et al. A case report of neonatal 2019 coronavirus disease in China. Clin Infect Dis 2020;71(15):853–857. doi:10.1093/cid/ciaa225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Vivanti AJ, Vauloup-Fellous C, Prevot S, et al. Transplacental transmission of SARS-CoV-2 infection. Nat Commun 2020;11(1):3572. doi:10.1038/s41467-020-17436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lamers MM, Beumer J, van der Vaart J, et al. SARS-CoV-2 productively infects human gut enterocytes. Science 2020;369(6499):50–54. doi:10.1126/science.abc1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Li M, Chen L, Zhang J, et al. The SARS-CoV-2 receptor ACE2 expression of maternal-fetal interface and fetal organs by single-cell transcriptome study. PLoS One 2020;15(4):e0230295. doi:10.1371/journal.pone.0230295. [DOI] [PMC free article] [PubMed] [Google Scholar]


