Abstract
Autism spectrum disorder (ASD) has been associated with imbalance between excitatory and inhibitory (E/I) neurotransmission systems, as well as with neuroinflammation. Sitting at the crossroads between E/I imbalance and neuroinflammation is a class of endogenous hormones known as neurosteroids. Current literature points to dysregulated steroid metabolism and atypical neurosteroid levels in ASD as early as in utero. However, due to the complexity of neurosteroid metabolomics, including possible sex differences, the impact of neurosteroids on ASD symptomatology remains unclear. In this study, we assessed neurosteroid levels and ASD symptom severity of 21 males with ASD and 20 full-scale-IQ-matched typically developing (TD) males, all aged 18 to 39. Using liquid chromatography-tandem mass spectrometry, concentrations of allopregnanolone, cortisol, dehydroepiandrosterone, progesterone, and testosterone were measured in saliva and serum. With the exception of cortisol’s, all neurosteroids’ concentrations were found to have ASD vs. TD group differences in distribution, where one group was normally distributed and the other non-normally distributed. Serum allopregnanolone levels in males with ASD were found to negatively correlate with clinician-rated measures of restricted and repetitive behavior measures (ADOS-2 RRB and ADI-R RRSB domain scores). Additionally, lower serum allopregnanolone levels were found to predict more negative camouflaging scores, which represent greater differences in self- and clinician-rated symptom severity, of both ASD symptomatology overall and repetitive behaviors in particular. Taken together, our findings demonstrate that in adult males with ASD, decreased serum allopregnanolone levels are associated with more severe restricted and repetitive behaviors and with less insight into the severity of these behaviors.
Keywords: autism spectrum disorder, neurosteroids, allopregnanolone, liquid chromatography-mass spectrometry, repetitive behaviors, camouflaging
1. INTRODUCTION
Autism spectrum disorder (ASD) is a condition defined by deficits in social interactions, communication, stereotypic behaviors, and restricted interests (American Psychiatric Association. and American Psychiatric Association. DSM-5 Task Force., 2013). ASD is a heterogeneous condition. About 900 genes have been found to be associated with ASD; about 100 of these genes are strongly associated with the condition (Satterstrom et al., 2020). Many of these genes affect synapses, consistent with prominent hypotheses of ASD. One major hypothesis proposes that an imbalance between excitatory and inhibitory (E/I) neurotransmission systems may be a key biological factor in ASD (Rubenstein and Merzenich, 2003). Another major hypothesis of ASD suggests that the condition is a result of neuroinflammation (Sciara et al., 2020). Sitting at the crossroads between E/I imbalance and neuroinflammation is a class of endogenous hormones known as neurosteroids.
Modulators of the E/I balance include neurosteroids, which are endogenous steroid hormones that act at the central nervous system. They regulate a host of neurotransmitter receptors—most prominently the GABAA and NMDA receptors—that function in the GABAergic and glutamatergic systems (Mellon, 2007; Zorumski et al., 2019). Allopregnanolone is a potent allosteric agonist of the GABAA receptors and increases inhibitory neurotransmission (Lambert et al., 1995; Majewska et al., 1986; Pinna et al., 2000; Puia et al., 1990); it has little effect on excitatory NMDA receptors. By enhancing the hyperpolarizing Cl− currents, allopregnanolone slows the rate of recovery from desensitization (Zhu and Vicini, 1997). With an EC50 value in the high nanomolar range (Akk et al., 2007), allopregnanolone has marked anxiolytic and anti-stress effects (reviewed in Paul et al., 2020). Progesterone also potentiates the GABA response, but with considerably lower affinity, and cannot directly modulate the GABAA receptor (Callachan et al., 1987; Wu et al., 1990). Allopregnanolone, however, is a derivative of progesterone (Dong et al., 2001), and thus fluctuations in progesterone levels can have significant influence on the GABAergic system. Testosterone can also exhibit influences on the E/I balance via its metabolites. Androstanediol, a testosterone-derived neurosteroid, is a positive allosteric modulator of GABAA receptors with an EC50 of about 5 μM (Reddy and Jian, 2010). Estradiol, formed from testosterone, has been shown to negatively modulate NMDA receptors for neuroprotection against NMDA excitotoxicity, as well as enhance GABA release from GABAA receptors in gonadotropin-releasing hormone neurons (Romanò et al., 2008; Weaver et al., 1997). Dehydroepiandrosterone (DHEA), on the other hand, is a GABAA antagonist, with typical IC50 values in the high nanomolar to micromolar range (Akk et al., 2007; Imamura and Prasad, 1998). DHEA also positively modulates NMDA receptors (Maninger et al., 2009).
Current literature points to dysregulated steroid metabolism and atypical levels in ASD, although the data and collection methods—saliva, serum, or plasma—are inconsistent. Using gas chromatography–mass spectrometry and radioimmunoassay, Majewska et al. found significantly higher salivary concentrations of many steroid hormones in children with ASD compared to neurotypical controls, particularly in older children and in males (Majewska et al., 2014). These results are consistent with other findings of hyperandrogenemia in Egyptian and U.S. autistic children using immunoassay (El-Baz et al., 2014; Geier and Geier, 2007), but conflict with a radioimmunoassay study that did not find elevated levels of DHEA in serum levels of autistic children, except for those with aggression (Tordjman et al., 1995). In adults, one radioimmunoassay study found significantly lower plasma levels of DHEA sulfate (DHEA-S) with ASD when compared to controls (Strous et al., 2005). However, another immunoassay study did not find significant differences in levels of DHEA-S in adults with ASD, but did find significantly elevated levels of androstenedione (Ruta et al., 2011). Finally, in animal models, one study induced ASD-like behavior in male but not female mice by inhibiting allopregnanolone biosynthesis; subsequent administration of allopregnanolone abolished the phenotype (Ebihara et al., 2017).
Along this line of investigation, recent studies in other psychiatric conditions have administered allopregnanolone as a possible treatment. Studies have implicated allopregnanolone levels in posttraumatic stress disorder (PTSD) symptom severity, specifically in the plasma and cerebrospinal fluid of premenopausal women with PTSD (Pineles et al., 2020; Rasmusson et al., 2006), and in the cerebrospinal fluid of men with PTSD (Kim et al., 2020). To veterans with posttraumatic stress disorder, a synthetic derivative of allopregnanolone called ganaxolone was administered orally in a randomized controlled trial (Rasmusson et al., 2017). Ganaxolone was not found to outperform placebo in reducing PTSD symptom scores or increasing global well-being. However, the study may have had issues with dosing, since 23.5% of the participants on active ganaxolone had undetectable plasma trough levels at the end of the placebo-controlled trial. In contrast, for women with moderate to severe postpartum depression, single continuous infusion of allopregnanolone for 60 hours resulted in greater reduction of mean depression severity scores compared to placebo (Meltzer-Brody et al., 2018). Future investigations may account for the difference in these treatment outcomes by studying associated factors, such as dosing, sex differences, and baseline allopregnanolone levels before starting treatment.
In the context of allopregnanolone’s relevance to postpartum depression, altered levels of fetal neurosteroids in ASD are especially of interest. In amniotic fluid samples of males who later received ASD diagnoses, elevated steroidogenic activity for sex steroids and cortisol was observed, compared to samples of matched typically developing males (Baron-Cohen et al., 2015). In contrast, placental allopregnanolone insufficiency in mice has been shown to lead to male-specific cerebellar white matter abnormalities and core ASD symptoms (Vacher et al., 2019).
Due to the complexity of neurosteroid metabolomics, including possible sex differences, the impact of neurosteroids on ASD symptomatology remains unclear. This study aims to elucidate the relationships between neurosteroid levels and symptom severity in ASD, particularly in high-functioning adult males. We focused on males due to the previous literature surrounding allopregnanolone’s gender-dependent effect, and because allopregnanolone levels vary across the menstrual cycle in females (Ossewaarde et al., 2010). With liquid chromatography-tandem mass spectrometry (LC-MS/MS), concentrations of allopregnanolone, cortisol, DHEA, progesterone, and testosterone were measured in both saliva and serum, collected from typically developing adult males and from adult males with ASD. The characterization of neurosteroid concentrations and their associated symptomatology in ASD may help address the need for clinically useful biomarkers that can aid in personalized therapy and response monitoring.
2. MATERIAL AND METHODS
2.1. Participants
21 males with ASD and 20 typically developing (TD) males aged 18 to 39 were included in this study. Methodology of the study was approved by the Institutional Review Board of Stanford University. Participants with ASD were excluded for the following criteria: (a) evidence of a genetic, metabolic, or infectious etiology for their autism, on the basis of medical history, neurological history, and available laboratory testing for inborn errors of metabolism and chromosomal analysis; (b) a DSM-5 diagnosis of any severe mental disorder such as schizophrenia and bipolar disorder; (c) taking psychotropic medications that have not been stable for over a week; (d) a history of alcoholism; (e) a diagnosis of a neurological disorder with active symptoms; or (f) IQ < 70. TD participants were excluded for the following criteria: (a) a diagnosed neurological disorder with active symptoms; (b) a diagnosis of psychiatric disorders currently and in the past on the basis of a clinical psychiatric evaluation and information obtained from behavioral scale; (c) historical evidence of significant difficulty during pregnancy, labor, delivery, or immediate neonatal period, or abnormal developmental milestones as determined by neurological history; or (d) a history of alcoholism.
Our sample sizes were chosen based on previous literature. According to a recent study (Majewska et al., 2014), the effect size for allopregnanolone was found to be large when comparing salivary levels of the hormone between children with ASD and neurotypical controls. Taking a conservative approach, assuming Cohen’s d of 1, we calculated that 17 subjects in each group would be required to achieve power of 0.8 and alpha level of 0.05.
Among the 21 participants with ASD, 15 were taking at least 1 psychotropic medication, including serotonin reuptake inhibitors (N = 11), stimulants (7), atypical antipsychotics (3), non-stimulants (3), and other medications (melatonin (3), bupropion (2), and oxcarbazepine (2)). Among the 20 TD participants, one was taking melatonin and another was taking medical marijuana. To account for possible confounding, in our investigations of neurosteroid—behavior relationships in the ASD group, psychotropic medication usage was included as a binary covariate in subsequent generalized linear model (GLM) analyses (see 2.6. Statistical methods).
2.2. Clinical assessments
To confirm the diagnosis of ASD participants, the Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) and the Autism Diagnostic Interview-Revised (ADI-R) were administered by a qualified clinician. ADOS-2 is a 45-minute direct assessment involving observation of the participant’s social cues, and the ADI-R is a 3-hour interview of the participant’s parent regarding the participant’s developmental history (Lord et al., 2000; Lord et al., 1994). Both were used in order to assess each participant’s ASD symptom severity at present and in early childhood.
Neuropsychological assessments of the participants included the Stanford Binet Intelligence Scale-Fifth Edition (SB-5), Autism-Spectrum Quotient (AQ), Ritvo Autism Asperger Diagnostic Scale-Revised (RAADS-R), Social Responsiveness Scale-Second Edition (SRS-2), and Repetitive Behavior Scale-Revised (RBS-R).
The SB-5 is an intelligence test that measures five factors: knowledge, quantitative reasoning, visual-spatial processing, working memory, and fluid reasoning (Goldstein and Naglieri, 2011). The verbal and nonverbal subtests measuring these factors provide composite IQ scores: Verbal IQ, Non-verbal IQ, and Full Scale IQ. Higher scores indicate higher cognitive abilities.
The AQ assists the diagnosis of autism in adults, and it uses 50 questions to assess social skill, attention switching, attention to detail, communication, and imagination (Baron-Cohen et al., 2001). The RAADS-R also assists diagnosis of autism in adults, containing 80 questions related to language, social relatedness, circumscribed interests, and sensory-motor symptom areas (Ritvo et al., 2011). Higher scores on the AQ and RAADS-R indicate the degree to which individuals exhibit autistic traits.
The SRS-2 is a 65-item questionnaire that measures one’s ability to engage in appropriate reciprocal social behaviors. Five subscale scores are provided: social awareness, social cognition, social communication, social motivation, and restricted interests and repetitive behavior. Higher scores represent more severe behavioral symptoms (Constantino et al., 2003).
The RBS-R is a 43-item questionnaire measuring repetitive behaviors over six subscales—stereotyped, self-injurious, compulsive, ritualistic, sameness, and restricted—as well as a Global Severity Score (Lam and Aman, 2007). Higher scores indicate greater symptom severity.
2.3. Sample acquisition
For each participant, blood and saliva samples were collected at a subsequent study visit no more than 8 weeks after the neuropsychological assessments. Sample acquisition occurred between 11am and 6pm. Approximately 10 mL of blood was drawn by a qualified lab technician or nurse, and approximately 2 mL of whole unstimulated saliva was collected using a synthetic swab specifically designed for cortisol determination. Whole blood aliquots were collected into chilled heparin-treated vacutainer tubes. Blood aliquots were centrifuged, then serum fractions were transferred and aliquoted into small, code-labeled polypropylene collection vials. Saliva samples were also obtained via centrifugation and transferred into code-labeled polypropylene collection vials. All serum and saliva samples were kept at −80°C until LC-MS/MS analyses.
2.4. Neurosteroids processing and LC-MS/MS
Five neurosteroids of interest were identified for analysis: allopregnanolone, cortisol, DHEA, progesterone, and testosterone. Samples were processed by a liquid-liquid extraction method. 200 μl of each sample—serum, saliva, standard curve, or quality control—was mixed with 10 μl of the internal standard mix (100 ng/ml). Analytes were extracted by methyl tert-butyl ether (MTBE) and derivatized to run in positive mode in a LC-MS/MS system (see Supplemental Material). Separation was achieved by using a Shimadzu UFLC system and C18 columns. Quantitation was performed on a triple quadrupole MS with electron spray ionization. For each analyte, multiple reaction monitoring (MRM) transition was used with a deuterated analogue or similar-structure compound as an internal standard to perform quantitation (see Supplemental Material). The lower limit of quantitation was 10 pg/mL for all compounds.
2.5. Hypotheses
The primary hypothesis was that allopregnanolone concentrations in serum and saliva would correlate with ASD symptom severity, in terms of both socio-communicative function and restricted and repetitive behaviors.
There were two exploratory hypotheses. First, we explored whether concentrations of four other neurosteroids of interest (cortisol, DHEA, progesterone, and testosterone) would also correlate with ASD symptom severity. Then, we explored whether any neurosteroid concentrations correlated with the ASD behavior of camouflaging, which represents the difference in self- and clinician-rated symptom severity. We used and extended the methods found in previous literature (Lai et al., 2017; Schuck et al., 2019) to formulate three camouflaging metrics: of overall symptom severity, based on total AQ and ADOS-2 scores; of restricted and repetitive behaviors, based on SRS-2: RRB domain and ADOS-2: RRB domain scores; and of circumscribed interests, based on RAADS-R: Circumscribed Interest and ADOS-2: Highly Specific Interests subscale scores.
2.6. Statistical methods
Analyses were run in R version 3.5.3. Demographic and neuropsychological data were compared between ASD males and TD males with two-sided Welch’s T-test not assuming equal variances. Significance was set at P < 0.05. Variables with significant group differences were identified as possible confounders and included as covariates in subsequent GLM analyses.
With ASD males and TD males separately, the distributions of salivary and serum concentrations of each neurosteroid were characterized with Shapiro-Wilk’s test for normality. Then, between ASD males and TD males, neurosteroid concentrations were assessed for heteroscedasticity with Levene’s test. Significance for both tests was set at P < 0.05 (significance α = 0.05, two-tailed). For any neurosteroid identified to be normally distributed for each group, as well as having insignificantly different variances between groups, two-sided student’s T-test was used to compare means between groups.
Pearson’s correlations were calculated between neurosteroid salivary/serum concentrations and time of saliva/blood collection. Circadian rhythms of steroids in young adult males have been found to involve mid-day decreases in plasma concentrations of steroids closely related to allopregnanolone (Kage et al., 1982). To our knowledge, the circadian rhythm of allopregnanolone itself has not been studied.
Pearson’s correlations were also calculated between neurosteroid salivary/serum concentrations and age. With our more focused group of men ages 18–39, we sought to replicate previous findings of age-related declines in male allopregnanolone (Genazzani et al., 1998) and DHEA levels (Orentreich et al., 1992).
To investigate how allopregnanolone concentrations in males with ASD correlate with behavior, both at present and in early childhood (as assessed with the ADI-R), two-sided Spearman’s correlations were calculated with the total scores of ADOS-2, ADI-R, AQ, RAADS-R, SRS-2, and RBS-R, as well as with the repetitive behavior subscales of ADOS-2, ADI-R, RAADS-R, and SRS-2. Significance was set at P < 0.005 to correct for 10 bivariate correlations per collection method (serum or saliva).
To explore behavioral correlates of the four other neurosteroids of interest in males with ASD, Spearman’s correlations were calculated as above.
To investigate possible confounding by age of the relationships between neurosteroid concentrations and behavior, Spearman’s correlations were calculated between age and behavioral scores of interest identified in the investigations above.
To test our exploratory hypothesis about camouflaging, the method to quantify camouflaging as previously described (Schuck et al., 2019) was followed. Scores were calculated by standardizing (mean-centering and scaling) ADOS and AQ total scores, then subtracting standardized ADOS from standardized AQ: SAQ–SADOS. In an exploratory fashion, similar methods were utilized to calculate camouflaging specifically related to repetitive behaviors. Using data from the RAADS-R and the SRS-2, SRAADS-Circumscribed Interest–SADOS-Highly Specific Interests and SSRS-RRB–SADOS-RRB were also calculated. Positive camouflaging scores correspond to more severe self-reported symptoms than those observed by clinicians. Negative camouflaging scores represent how much more severe clinicians rated participants’ symptoms compared to what was self-reported.
The associations between these three camouflaging scores and neurosteroid concentrations in males with ASD were assessed with two-sided Pearson’s correlations, after any necessary log transformation to normality of the neurosteroid concentrations. Significance was set to P < 0.0167 to correct for 3 comparisons. Post-hoc GLM analyses, with log-transformed neurosteroid concentrations and possible confounders as the independent variables and camouflaging score as the dependent variable, were run to test possible confounding by medication usage (coded as a binary variable), age, and any variables with significant group differences between TD males and males with ASD.
3. RESULTS
3.1. Demographics and clinical assessments
Table 1 reports the demographic data of the participants by group, as well as the results of neuropsychological assessments. The two groups were age-matched (ASD mean 23.7 years, TD mean 27.0 years, P = 0.23) and full-scale-IQ-matched (ASD mean 100.5, TD mean 109.8, P = 0.11), but there existed a significant group difference in non-verbal IQ (P = 0.033). Because of this group difference, non-verbal IQ was included as a covariate in subsequent GLM analyses to test for confounding. As expected, males with ASD reported significantly higher symptom severity than TD males did in terms of socio-communicative function and repetitive behavior (P < 0.001 for AQ, RAADS-R, and SRS-2).
Table 1.
Participant demographics and clinical assessments
| ASD Males N=21 |
TD Males N=20 |
Welch’s T-Test P |
|
|---|---|---|---|
| Age | 23.71 ±5.05 | 26.00 ± 6.69 | 0.2267 |
| FSIQ | 100.45 ± 17.65 | 109.82 ± 17.49 | 0.1148 |
| VIQ | 103.10 ± 17.55 | 108.71 ± 16.50 | 0.3242 |
| NVIQ | 97.95 ± 18.69 | 111.06 ± 17.31 | 0.03357 |
| ADOS-2 – Total | 12.61 ±5.83 | N/A | N/A |
| ADOS-2 – RRB Domain | 3.00 ±2.14 | N/A | N/A |
| ADI-R – Total | 38.21 ±11.62 | N/A | N/A |
| ADI-R – RRSB Domain | 5.05 ± 2.88 | N/A | N/A |
| AQ – Total | 29.48 ± 7.00 | 19.20 ±8.97 | 0.0002405 |
| RAADS-R – Total | 114.43 ±40.59 | 63.20 ±43.12 | 0.0003596 |
| RAADS-R – Circumscribed Interest | 22.76 ±9.19 | 12.25 ±7.15 | 0.0002134 |
| SRS-2 – Total | 67.48 ±10.90 | 54.11 ± 10.30 | 0.0002938 |
| SRS-2 – Repetitive Behaviors | 71.67 ±12.46 | 56.16 ±9.49 | 0.00007564 |
| RBS-R – Total | 51.07 ±35.68 | N/A | N/A |
| CamouflagingAQ | −0.006 ± 0.209 | N/A | N/A |
| CamouflagingRAADs | −0.033 ±0.416 | N/A | N/A |
| CamouflagingSRS | −0.016 ±0.289 | N/A | N/A |
Note: Values reported are mean ± SD. Bolded Welch’s T-test results are P < 0.05.
Abbreviations: FSIQ = Full-scale IQ. VIQ = Verbal IQ. NVIQ = Non-verbal IQ. ADOS-2 = Autism Diagnostic Observation Schedule, Second Edition-2. ADI-R = Autism Diagnostic Interview-Revised. AQ = Autism-Scale Quotient. RAADS-R = Ritvo Autism Asperger’s Diagnostic Scale. SRS-2 = Social Responsiveness Scale. RBS-R = Repetitive Behavior Scale. CamouflagingAQ, CamouflagingRAADS, and CamouflagingSRS are described in Materials and Methods: Statistical methods.
3.2. Neurosteroid concentrations
Table 2 reports the concentrations of each neurosteroid by group. Serum allopregnanolone, DHEA, progesterone, and testosterone, as well as salivary DHEA and progesterone, were found to have group differences in the distribution of concentrations, where one group was normally distributed (Shapiro-Wilk P > 0.05) and the other non-normally distributed. Specifically, males with ASD had non-normally distributed concentrations of serum allopregnanolone (W = 0.84, P = 0.011, Figure 1), serum DHEA (W = 0.83, P = 0.006), and salivary DHEA (W = 0.82, P = 0.003), while TD males had non-normally distributed concentrations of salivary progesterone (W = 0.84, P = 0.01), serum progesterone (W = 0.84, P = 0.011), and serum testosterone (W = 0.83, P =0.005).
Table 2.
Serum and salivary concentrations of neurosteroids in ASD and TD males
| Neurosteroid | ASD Males | TD Males | Levene’s Test | ||||
|---|---|---|---|---|---|---|---|
| N | Median (IQR) S-W Test W, P |
N | Median (IQR) S-W Test W, P |
F | P | ||
| Allopregnanolone | Serum | 16 | 74.45 (26.0–91.1) W = 0.84, P = 0.01* |
17 | 99 (63.5–122.0) W = 0.97, P = 0.73 |
0.1773 | 0.6766 |
| Saliva | 17 | 5 (5.0–52.0) W = 0.69, P < 0.001* |
18 | 5 (5.0–29.1) W = 0.68, P < 0.001* |
3.7547 | 0.06126 | |
| Cortisol | Serum | 19 | 83900 (52300–92500) W = 0.77, P < 0.001* |
18 | 89950 (65450–102000) W = 0.79, P = 0.001* |
0.221 | 0.6412 |
| Saliva | 20 | 2805 (1385.0–4705.0) W = 0.91, P = 0.05 |
19 | 1810 (1295.0–3200.0) W = 0.91, P = 0.08 |
2.9578 | 0.09382 | |
| DHEA | Serum | 17 | 4030 (3230–5750) W = 0.83, P = 0.006* |
17 | 4340 (3570–6000) W = 0.93, P = 0.25 |
0.3241 | 0.5732 |
| Saliva | 18 | 23.0 (10.0–91.7) W = 0.82, P = 0.003* |
18 | 94.10 (39.1–120.5) W = 0.91, P = 0.09 |
0.0964 | 0.7581 | |
| Progesterone | Serum | 17 | 44.2 (22.9–62.1) W = 0.91, P = 0.12 |
16 | 58.3 (45.6–114.3) W = 0.84, P = 0.01* |
1.6549 | 0.2078 |
| Saliva | 11 | 114.00 (73.4–192.0) W = 0.90, P = 0.20 |
10 | 5.0 (5.0–5.0) W = 0.47, P < 0.001* |
12.078 | 0.00253 | |
| Testosterone | Serum | 17 | 3400 (1320.0–4640.0) W = 0.93, P = 0.22 |
17 | 3280 (1650.0–3790.0) W = 0.83, P = 0.005* |
0.0105 | 0.9191 |
| Saliva | 18 | 45.45 (22.2–72.0) W = 0.88, P = 0.03* |
18 | 24.15 (16.1–36.7) W = 0.88, P = 0.02* |
2.9385 | 0.09559 | |
Note: Units for all neurosteroids are pg/mL. IQR is Inter-Quartile Range, 25%–75%. S-W Test is Shapiro-Wilk test (* signifies non-normal distribution based on Shapiro-Wilk test P < 0.05). Bolded Levene’s test results are P < 0.05.
Figure 1.
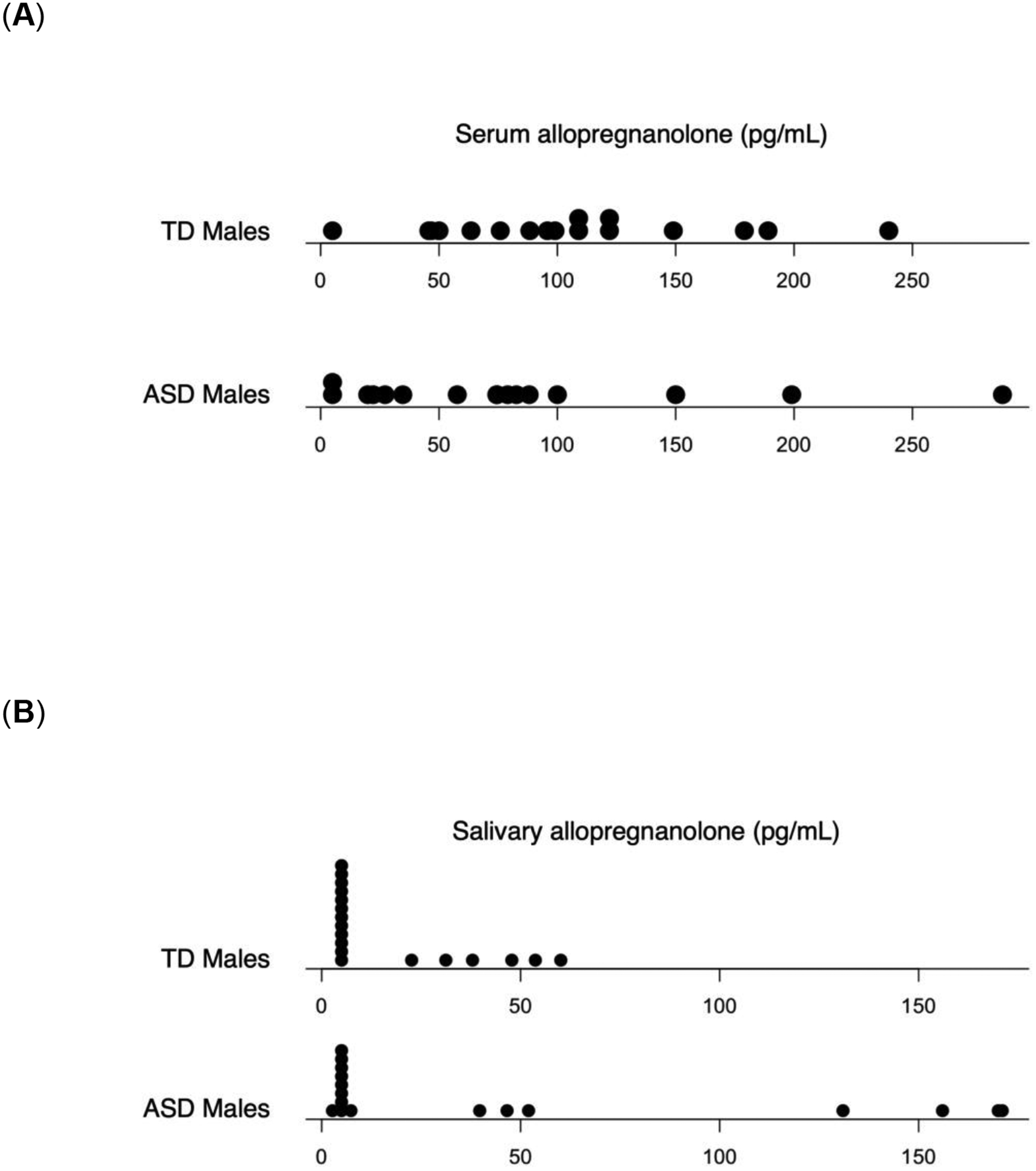
Dot plots of serum and salivary allopregnanolone (pg/mL). (A) Serum levels in TD males and ASD males, (B) Salivary levels in TD males and ASD males.
In terms of variance, salivary progesterone was the one neurosteroid whose concentrations were found to differ by group on Levene’s test (F(1,19) = 12.08, P = 0.003).
Since salivary cortisol levels were normally distributed for both groups and homoscedastic, student’s T-test was used to compare the mean salivary cortisol levels of TD males and males with ASD; no significant difference in means was found (P = 0.17).
In our investigation of the effect of circadian rhythms on neurosteroid concentrations, no significant correlation was found between the time of sample collection and any neurosteroid level, salivary or serum, across both groups (see Supplemental Material).
In our investigation of the effect of age on neurosteroid concentrations, our data replicated previous literature by demonstrating decreasing serum allopregnanolone (r = −0.39, P = 0.026) and salivary DHEA (r = −0.45, P = 0.0064) with age across both groups.
3.3. RRB correlates of allopregnanolone
Figure 2 shows scatterplots of repetitive behavior measures and serum allopregnanolone concentrations. Serum allopregnanolone levels in males with ASD had significant Spearman correlations with ADOS-2 RRB domain score (rho = −0.74, P = 0.0025) and ADI-R RRSB domain score (rho = −0.70, P = 0.0039). Exploratory analyses found that the “Highly Specific Interests” subscale of the ADOS-2 drove the correlation, being the only subscale of five within the ADOS-2 RRB domain that correlated with serum allopregnanolone (rho = −0.86, P = 0.000069). The correlation with the total score on the ADOS-2 was nearly significant (rho = −0.66, P = 0.0095) but did not meet the threshold set for multiple comparisons. Age did not correlate with any of these four behavioral scores (P > 0.05).
Figure 2.
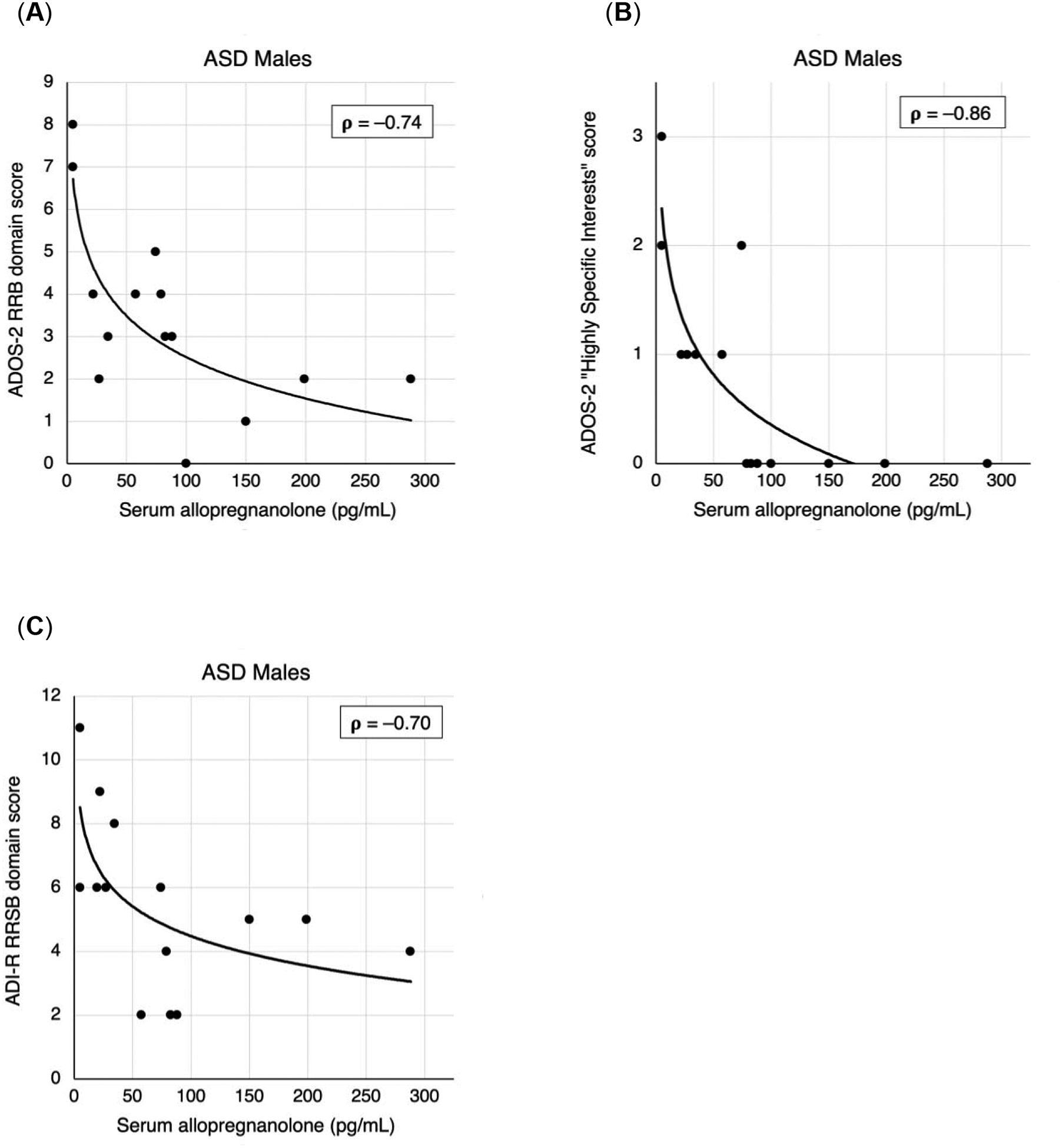
Clinician-rated measures of restricted and repetitive behavior vs. serum allopregnanolone (pg/mL) for ASD males. (A) ADOS-2 RRB domain scores, (B) ADOS-2 “Highly Specific Interests” subscale scores, (C) ADI-R RRSB domain scores. Correlation values reported are Spearman’s rho. Simple logistic trendlines are provided to illustrate correlation.
No significant correlations were found with the total scores of AQ, ADI-R, RAADS-R, SRS-2, or RBS-R, nor with the repetitive behavior subscales of RAADS-R or SRS-2 (P > 0.005 on all). Additionally, there were no significant correlations between behavioral scores and salivary allopregnanolone.
3.4. Camouflaging and allopregnanolone
One possible reason for the discordant correlation findings is that whereas the ADOS-2 and ADI-R are administered by clinicians, the AQ, RAADS-R, and SRS-2 are self-reported. In order to address this possibility, we tested our exploratory hypothesis around camouflaging, since camouflaging represents a difference in self- and clinician-rated symptom severity.
Figure 3 shows camouflaging scores plotted against serum allopregnanolone concentrations that were log-transformed to normality, all in males with ASD. Significant correlations were found between camouflaging and log-transformed serum allopregnanolone: SAQ–SADOS, representing camouflaging of a broader scope of symptoms (r = 0.75, P = 0.0021), and SRAADS-Circumscribed Interest–SADOS-Highly Specific Interests, representing camouflaging of circumscribed interests (r = 0.75, P = 0.0019). Camouflaging defined with SRS-2 was nearly significant (r = 0.58, P = 0.030) but did not meet the threshold set for multiple comparisons.
Figure 3.
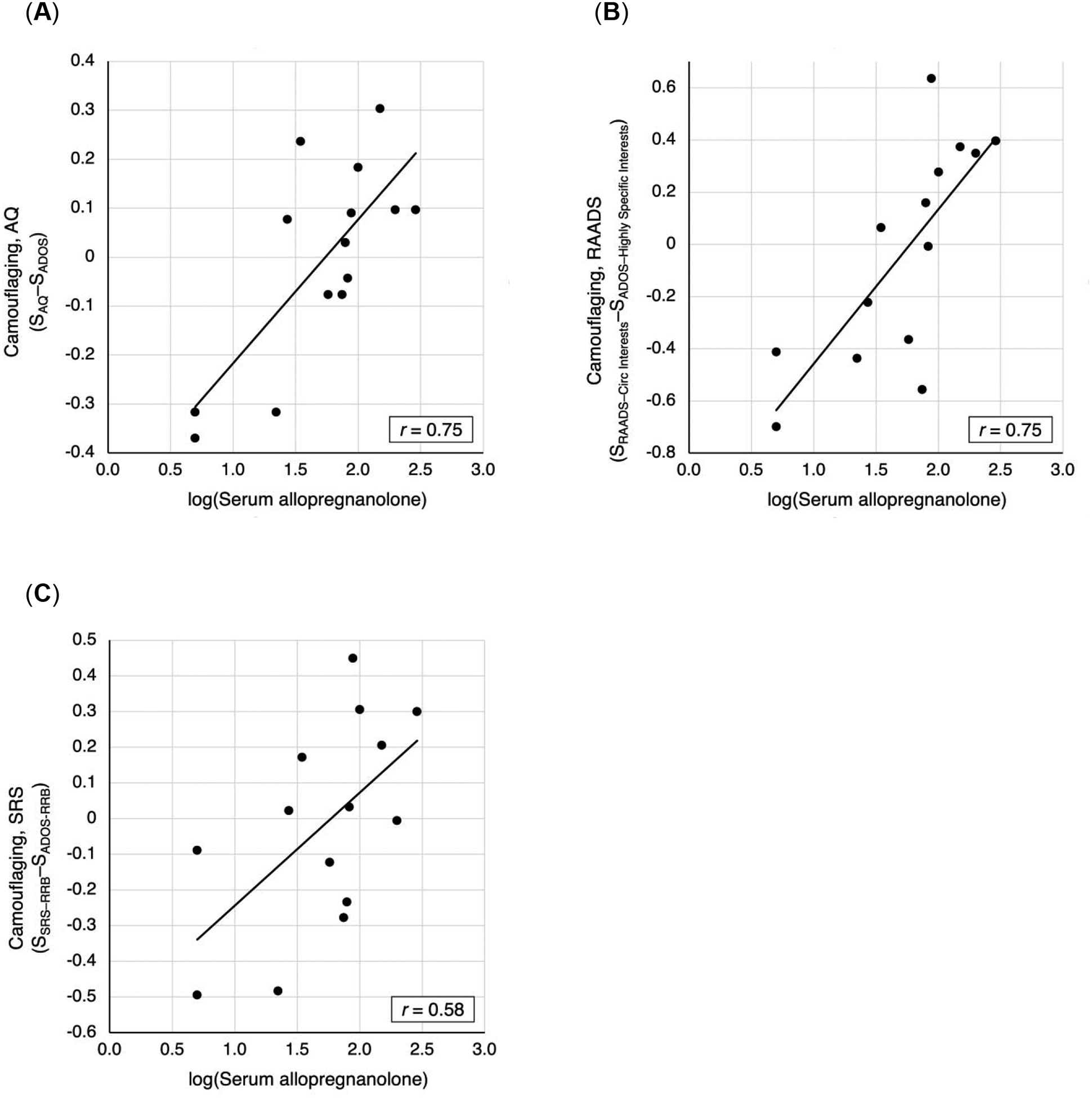
Scatterplots of camouflaging vs. log-transformed serum allopregnanolone for ASD males, with trendlines and Pearson’s correlation coefficients. (A) Camouflaging based on standardized AQ and ADOS total scores. (B) Camouflaging based on standardized RAADS: Circumscribed Interests and ADOS: Highly Specific Interests subscale scores. (C) Camouflaging based on standardized SRS: RRB and ADOS: RRB domain scores.
GLM analyses, with camouflaging scores as the dependent variables, and non-verbal IQ, medication usage, age, and log-transformed serum allopregnanolone concentrations as the independent variables, validated the significant effect of serum allopregnanolone on camouflaging while accounting for possible confounding. The GLM predicting SAQ–SADOS (adjusted R2 = 0.78, F(4,9) = 12.41, P = 0.0010) reported P = 0.049 for log-transformed serum allopregnanolone. The GLM predicting SRAADS-Circumscribed Interest–SADOS-Highly Specific Interests (adjusted R2 = 0.65, F(4,9) = 7.13, P = 0.0072) reported P = 0.012 for log-transformed serum allopregnanolone.
3.5. Other neurosteroids
Despite the significant group difference in variance in salivary progesterone levels, no significant behavioral correlates of salivary progesterone were found in males with ASD (see Supplemental Material). Additionally, no significant behavioral correlates of any of the other neurosteroids of interest were found.
4. DISCUSSION
To our knowledge, this is the first study to provide evidence that levels of a neurosteroid are correlated with ASD symptom severity in adult males. Specifically, in adult males with ASD, we found that serum concentrations of allopregnanolone, but not salivary concentrations, were non-normally distributed and right-skewed, negatively correlated with clinician-rated restricted and repetitive behavior measures, and positively correlated with camouflaging scores. Previous literature had shown the clinical significance of altered serum levels of allopregnanolone as related to pregnancy (Meltzer-Brody and Kanes, 2020): in women with depressed mood during pregnancy, when levels normally increase throughout gestation (Hellgren et al., 2014; Luisi et al., 2000); in women with depression postpartum, when levels normally return to baseline (Nappi et al., 2001; Pennell et al., 2015), and in male mice with ASD-like behaviors that had placental allopregnanolone insufficiency (Vacher et al., 2019). Our findings extend the likely clinical significance of lowered serum allopregnanolone levels into young adulthood for human males with ASD. In addition, our findings suggest that measurement of allopregnanolone in serum rather than saliva can be more clinically relevant.
Allopregnanolone is a GABAA receptor agonist (Majewska et al., 1986). Recent literature suggests that allopregnanolone exerts protective effects on the brain by inhibiting proinflammatory neuroimmune signaling (Balan et al., 2019). This protection against inflammation has been shown to be therapeutic in animal models of Alzheimer’s (Irwin and Brinton, 2014) and multiple sclerosis (Noorbakhsh et al., 2014), and it may be significant in ASD as well. Selective induction of astrocytic gliosis has been found to cause deficits in neuronal inhibition (and likely GABAergic activity) (Ortinski et al., 2010), a finding consistent with the evidence of neuroinflammation in the postmortem brain samples of individuals with ASD (Vargas et al., 2005). In children with ASD, higher plasma IL-1β and IL-6 levels predicted greater severity of stereotyped behaviors (Ashwood et al., 2011). Collectively, these findings suggest a relationship between inflammatory response, GABAergic function, and restricted and repetitive behaviors, potentially moderated by agents that modulate both the immune and GABAergic systems (e.g., allopregnanolone). Future studies that investigate the connections between GABA signaling and inflammatory processes will help better characterize the systemic biochemical changes in ASD.
In our study, participants with ASD and lower serum allopregnanolone levels demonstrated more severe restricted and repetitive behaviors, especially “highly specific interests” as measured on ADOS-2. This is a novel finding, as we are unaware of previous studies reporting biochemical correlates of restricted and repetitive behaviors in adults with ASD. Studies that administer allopregnanolone as treatment may very well assess any changes in restricted interests and perseveration, as well as inflammatory markers, to better characterize the neurosteroid’s role in complex systemic and behavioral changes.
Our group has shown previously that high-functioning females with ASD on average self-report more severe symptoms than what clinicians observe on ADOS-2 (Schuck et al., 2019). Interestingly, here we observe in males with ASD a spectrum of camouflaging that is associated with allopregnanolone. Males with lower serum allopregnanolone levels rate the severity of their repetitive behaviors and their ASD symptomatology overall with lower scores than those given by clinician’s assessment. Males with higher serum allopregnanolone levels, on the other hand, are more similar to females in how they camouflage their symptoms. This finding suggests that decreased allopregnanolone may correlate with decreased insight into symptom severity in males with ASD, even those with normal or elevated IQ. Further investigation into administering allopregnanolone may demonstrate allopregnanolone’s potential as adjuvant treatment to therapy-based interventions of restricted and repetitive behaviors. Relatedly, previous literature has shown that oral pregnenolone reduces irritability in adults with ASD (Fung et al., 2014). In addition, in typically developing adult males, the concomitant elevation in allopregnanolone levels after oral pregnenolone intake has been shown to increase functional connectivity between dorsal medial prefrontal cortex and left amygdala, and this connectivity was associated with less self-reported anxiety (Sripada et al., 2013).
We acknowledge several limitations to our study. First, our sample size was modest, and we focused our investigation on males. Future studies with more participants, and with a female cohort analyzed at a specific phase of the menstrual cycle, may help demonstrate not just differences in neurosteroid levels between ASD and TD, but also sex differences. Second, our study found behavioral correlates of neurosteroid levels only in the ASD group, since we lacked behavioral assessments of restricted and repetitive behaviors that were valid for both ASD and TD participants. However, restricted and repetitive behaviors are less common and less severe in typically developing people, and our findings help characterize a set of core ASD symptoms. Third, collection of blood and saliva samples was not at the same time of day for all participants, and the intake of food and water was not regulated prior to saliva acquisition. However, we found no correlation between neurosteroid concentrations and times of sample collections. Fourth, though our LC-MS/MS method was both sensitive and specific, the difficulty of measuring neurosteroid levels was nontrivial. Salivary allopregnanolone and progesterone were especially difficult to measure, perhaps contributing to the lack of correlation between behavioral measures and salivary levels. Salivary levels of these neurosteroids are known to be low, with mean allopregnanolone found to be about 16 pg/mL in a study of premenopausal women (Ossewaarde et al., 2011) and median progesterone found to be 29.79 pg/mL in a study of adult men (Gavrilova and Lindau, 2009). Even with blood, 3 of our 33 serum allopregnanolone levels were below the lower limit of quantitation of 10 pg/mL. These 3 values were set at 5 pg/mL (½ LLQ). Future studies with even finer methods of quantification will help demonstrate the prevalence and clinical significance of extremely low levels of allopregnanolone. Finally, our study looks at neurosteroid levels in young adulthood, well after early neurodevelopment when ASD features manifest; it does not address how altered neurosteroid metabolomics affect neurodevelopmental trajectories. However, by acknowledging that ASD behaviors and altered allopregnanolone levels persist into adulthood, our study underscores the need for investigating both treatment options and developmental mechanisms.
5. CONCLUSIONS
In conclusion, we demonstrate that decreased serum allopregnanolone levels in adult males with ASD are associated with more severe restricted and repetitive behaviors, and with less insight into the severity of these behaviors. In light of these findings and recent research showing efficacy of intravenous administration of single dose allopregnanolone in women with postpartum depression (Meltzer-Brody et al., 2018), administration of allopregnanolone may be a possible treatment strategy in treating disabling repetitive behaviors in adults with ASD.
Supplementary Material
HIGHLIGHTS.
Serum allopregnanolone levels in adult males with ASD are non-normally distributed.
Lower allopregnanolone levels correlate with more severe repetitive behaviors.
Lower allopregnanolone levels correlate with less insight into behavioral severity.
ACKNOWLEDGMENTS
We thank Harsh Gandhi and Dawn Holley for collecting blood and saliva samples, and Rachel Schuck for conducting behavioral assessments.
FUNDING AND DISCLOSURE
Research reported in this publication was supported by the National Institute of Mental Health (K08MH111750 awarded to LKF). The authors have no conflicts of interest to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
On behalf of myself and my co-authors, I report that we do not have any conflicts of interest associated with the manuscript (“Association of Serum Allopregnanolone with Restricted and Repetitive Behaviors in Adult Males with Autism”) we submitted.
REFERENCES
- Akk G, Covey DF, Evers AS, Steinbach JH, Zorumski CF, Mennerick S, 2007. Mechanisms of neurosteroid interactions with GABA(A) receptors. Pharmacol Ther 116, 35–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association., American Psychiatric Association. DSM-5 Task Force., 2013. Diagnostic and statistical manual of mental disorders : DSM-5, 5th ed.American Psychiatric Association, Washington, D.C. [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, Van de Water J, 2011. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun 25, 40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balan I, Beattie MC, O’Buckley TK, Aurelian L, Morrow AL, 2019. Endogenous Neurosteroid (3α,5α)3-Hydroxypregnan-20-one Inhibits Toll-like-4 Receptor Activation and Pro-inflammatory Signaling in Macrophages and Brain. Sci Rep 9, 1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Auyeung B, Nørgaard-Pedersen B, Hougaard DM, Abdallah MW, Melgaard L, Cohen AS, Chakrabarti B, Ruta L, Lombardo MV, 2015. Elevated fetal steroidogenic activity in autism. Mol Psychiatry 20, 369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E, 2001. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J Autism Dev Disord 31, 5–17. [DOI] [PubMed] [Google Scholar]
- Callachan H, Cottrell GA, Hather NY, Lambert JJ, Nooney JM, Peters JA, 1987. Modulation of the GABAA receptor by progesterone metabolites. Proc R Soc Lond B Biol Sci 231, 359–369. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, Metzger LM, Shoushtari CS, Splinter R, Reich W, 2003. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. J Autism Dev Disord 33, 427–433. [DOI] [PubMed] [Google Scholar]
- Dong E, Matsumoto K, Uzunova V, Sugaya I, Takahata H, Nomura H, Watanabe H, Costa E, Guidotti A, 2001. Brain 5alpha-dihydroprogesterone and allopregnanolone synthesis in a mouse model of protracted social isolation. Proc Natl Acad Sci U S A 98, 2849–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara K, Fujiwara H, Awale S, Dibwe DF, Araki R, Yabe T, Matsumoto K, 2017. Decrease in endogenous brain allopregnanolone induces autism spectrum disorder (ASD)-like behavior in mice: A novel animal model of ASD. Behav Brain Res 334, 6–15. [DOI] [PubMed] [Google Scholar]
- El-Baz F, Hamza RT, Ayad MS, Mahmoud NH, 2014. Hyperandrogenemia in male autistic children and adolescents: relation to disease severity. Int J Adolesc Med Health 26, 79–84. [DOI] [PubMed] [Google Scholar]
- Fung LK, Libove RA, Phillips J, Haddad F, Hardan AY, 2014. Brief report: an open-label study of the neurosteroid pregnenolone in adults with autism spectrum disorder. J Autism Dev Disord 44, 2971–2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilova N, Lindau ST, 2009. Salivary sex hormone measurement in a national, population-based study of older adults. J Gerontol B Psychol Sci Soc Sci 64Suppl 1, i94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier DA, Geier MR, 2007. A prospective assessment of androgen levels in patients with autistic spectrum disorders: biochemical underpinnings and suggested therapies. Neuro Endocrinol Lett 28, 565–573. [PubMed] [Google Scholar]
- Genazzani AR, Petraglia F, Bernardi F, Casarosa E, Salvestroni C, Tonetti A, Nappi RE, Luisi S, Palumbo M, Purdy RH, Luisi M, 1998. Circulating levels of allopregnanolone in humans: gender, age, and endocrine influences. J Clin Endocrinol Metab 83, 2099–2103. [DOI] [PubMed] [Google Scholar]
- Goldstein S, Naglieri JA, 2011. Encyclopedia of child behavior and development. Springer Science+Business Media, New York. [Google Scholar]
- Hellgren C, Åkerud H, Skalkidou A, Bäckström T, Sundström-Poromaa I, 2014. Low serum allopregnanolone is associated with symptoms of depression in late pregnancy. Neuropsychobiology 69, 147–153. [DOI] [PubMed] [Google Scholar]
- Imamura M, Prasad C, 1998. Modulation of GABA-gated chloride ion influx in the brain by dehydroepiandrosterone and its metabolites. Biochem Biophys Res Commun 243, 771–775. [DOI] [PubMed] [Google Scholar]
- Irwin R, Brinton R, 2014. Allopregnanolone as regenerative therapeutic for Alzheimer’s disease: Translational development and clinical promise. Progress in Neurobiology 113, 40–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kage A, Fenner A, Weber B, Schöneshöfer M, 1982. Diurnal and ultradian variations of plasma concentrations of eleven adrenal steroid hormones in human males. Klin Wochenschr 60, 659–666. [DOI] [PubMed] [Google Scholar]
- Kim BK, Fonda JR, Hauger RL, Pinna G, Anderson GM, Valovski IT, Rasmusson AM, 2020. Composite contributions of cerebrospinal fluid GABAergic neurosteroids, neuropeptide Y and interleukin-6 to PTSD symptom severity in men with PTSD. Neurobiol Stress 12, 100220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai MC, Lombardo MV, Ruigrok AN, Chakrabarti B, Auyeung B, Szatmari P, Happé F, Baron-Cohen S, Consortium MA, 2017. Quantifying and exploring camouflaging in men and women with autism. Autism 21, 690–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam KS, Aman MG, 2007. The Repetitive Behavior Scale-Revised: independent validation in individuals with autism spectrum disorders. J Autism Dev Disord 37, 855–866. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Belelli D, Hill-Venning C, Peters JA, 1995. Neurosteroids and GABAA receptor function. Trends Pharmacol Sci 16, 295–303. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, Pickles A, Rutter M, 2000. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 30, 205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A, 1994. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 24, 659–685. [DOI] [PubMed] [Google Scholar]
- Luisi S, Petraglia F, Benedetto C, Nappi RE, Bernardi F, Fadalti M, Reis FM, Luisi M, Genazzani AR, 2000. Serum allopregnanolone levels in pregnant women: changes during pregnancy, at delivery, and in hypertensive patients. J Clin Endocrinol Metab 85, 2429–2433. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM, 1986. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science 232, 1004–1007. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Hill M, Urbanowicz E, Rok-Bujko P, Bieńkowski P, Namysłowska I, Mierzejewski P, 2014. Marked elevation of adrenal steroids, especially androgens, in saliva of prepubertal autistic children. Eur Child Adolesc Psychiatry 23, 485–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH, 2009. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Front Neuroendocrinol 30, 65–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon SH, 2007. Neurosteroid regulation of central nervous system development. Pharmacol Ther 116, 107–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer-Brody S, Colquhoun H, Riesenberg R, Epperson CN, Deligiannidis KM, Rubinow DR, Li H, Sankoh AJ, Clemson C, Schacterle A, Jonas J, Kanes S, 2018. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet 392, 1058–1070. [DOI] [PubMed] [Google Scholar]
- Meltzer-Brody S, Kanes SJ, 2020. Allopregnanolone in postpartum depression: Role in pathophysiology and treatment. Neurobiol Stress 12, 100212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nappi RE, Petraglia F, Luisi S, Polatti F, Farina C, Genazzani AR, 2001. Serum allopregnanolone in women with postpartum “blues”. Obstet Gynecol 97, 77–80. [DOI] [PubMed] [Google Scholar]
- Noorbakhsh F, Baker G, Power C, 2014. Allopregnanolone and neuroinflammation: a focus on multiple sclerosis. Frontiers in Cellular Neuroscience 8, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orentreich N, Brind JL, Vogelman JH, Andres R, Baldwin H, 1992. Long-term longitudinal measurements of plasma dehydroepiandrosterone sulfate in normal men. J Clin Endocrinol Metab 75, 1002–1004. [DOI] [PubMed] [Google Scholar]
- Ortinski PI, Dong J, Mungenast A, Yue C, Takano H, Watson DJ, Haydon PG, Coulter DA, 2010. Selective induction of astrocytic gliosis generates deficits in neuronal inhibition. Nat Neurosci 13, 584–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossewaarde L, Hermans EJ, van Wingen GA, Kooijman SC, Johansson IM, Bäckström T, Fernández G, 2010. Neural mechanisms underlying changes in stress-sensitivity across the menstrual cycle. Psychoneuroendocrinology 35, 47–55. [DOI] [PubMed] [Google Scholar]
- Ossewaarde L, van Wingen GA, Kooijman SC, Bäckström T, Fernández G, Hermans EJ, 2011. Changes in functioning of mesolimbic incentive processing circuits during the premenstrual phase. Soc Cogn Affect Neurosci 6, 612–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul SM, Pinna G, Guidotti A, 2020. Allopregnanolone: From molecular pathophysiology to therapeutics. A historical perspective. Neurobiol Stress 12, 100215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennell KD, Woodin MA, Pennell PB, 2015. Quantification of neurosteroids during pregnancy using selective ion monitoring mass spectrometry. Steroids 95, 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineles SL, Nillni YI, Pinna G, Webb A, Arditte Hall KA, Fonda JR, Irvine J, King MW, Hauger RL, Resick PA, Orr SP, Rasmusson AM, 2020. Associations between PTSD-Related extinction retention deficits in women and plasma steroids that modulate brain GABA. Neurobiol Stress 13, 100225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna G, Uzunova V, Matsumoto K, Puia G, Mienville JM, Costa E, Guidotti A, 2000. Brain allopregnanolone regulates the potency of the GABA(A) receptor agonist muscimol. Neuropharmacology 39, 440–448. [DOI] [PubMed] [Google Scholar]
- Puia G, Santi MR, Vicini S, Pritchett DB, Purdy RH, Paul SM, Seeburg PH, Costa E, 1990. Neurosteroids act on recombinant human GABAA receptors. Neuron 4, 759–765. [DOI] [PubMed] [Google Scholar]
- Rasmusson AM, Marx CE, Jain S, Farfel GM, Tsai J, Sun X, Geracioti TD, Hamner MB, Lohr J, Rosse R, Summerall L, Naylor JC, Cusin C, Lang AJ, Raman R, Stein MB, 2017. A randomized controlled trial of ganaxolone in posttraumatic stress disorder. Psychopharmacology (Berl) 234, 2245–2257. [DOI] [PubMed] [Google Scholar]
- Rasmusson AM, Pinna G, Paliwal P, Weisman D, Gottschalk C, Charney D, Krystal J, Guidotti A, 2006. Decreased cerebrospinal fluid allopregnanolone levels in women with posttraumatic stress disorder. Biol Psychiatry 60, 704–713. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Jian K, 2010. The testosterone-derived neurosteroid androstanediol is a positive allosteric modulator of GABAA receptors. J Pharmacol Exp Ther 334, 1031–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritvo RA, Ritvo ER, Guthrie D, Ritvo MJ, Hufnagel DH, McMahon W, Tonge B, Mataix-Cols D, Jassi A, Attwood T, Eloff J, 2011. The Ritvo Autism Asperger Diagnostic Scale-Revised (RAADS-R): a scale to assist the diagnosis of Autism Spectrum Disorder in adults: an international validation study. J Autism Dev Disord 41, 1076–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanò N, Lee K, Abrahám IM, Jasoni CL, Herbison AE, 2008. Nonclassical estrogen modulation of presynaptic GABA terminals modulates calcium dynamics in gonadotropin-releasing hormone neurons. Endocrinology 149, 5335–5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM, 2003. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav 2, 255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruta L, Ingudomnukul E, Taylor K, Chakrabarti B, Baron-Cohen S, 2011. Increased serum androstenedione in adults with autism spectrum conditions. Psychoneuroendocrinology 36, 1154–1163. [DOI] [PubMed] [Google Scholar]
- Satterstrom FK, Kosmicki JA, Wang J, Breen MS, De Rubeis S, An JY, Peng M, Collins R, Grove J, Klei L, Stevens C, Reichert J, Mulhern MS, Artomov M, Gerges S, Sheppard B, Xu X, Bhaduri A, Norman U, Brand H, Schwartz G, Nguyen R, Guerrero EE, Dias C, Betancur C, Cook EH, Gallagher L, Gill M, Sutcliffe JS, Thurm A, Zwick ME, Børglum AD, State MW, Cicek AE, Talkowski ME, Cutler DJ, Devlin B, Sanders SJ, Roeder K, Daly MJ, Buxbaum JD, Consortium AS, Consortium i.-B., 2020. Large-Scale Exome Sequencing Study Implicates Both Developmental and Functional Changes in the Neurobiology of Autism. Cell 180, 568–584.e523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuck RK, Flores RE, Fung LK, 2019. Brief Report: Sex/Gender Differences in Symptomology and Camouflaging in Adults with Autism Spectrum Disorder. J Autism Dev Disord 49, 2597–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciara AN, Beasley B, Crawford JD, Anderson EP, Carrasco T, Zheng S, Ordway GA, Chandley MJ, 2020. Neuroinflammatory Gene Expression Alterations in Anterior Cingulate Cortical White and Gray Matter of Males With Autism Spectrum Disorder. Autism Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada RK, Marx CE, King AP, Rampton JC, Ho SS, Liberzon I, 2013. Allopregnanolone elevations following pregnenolone administration are associated with enhanced activation of emotion regulation neurocircuits. Biol Psychiatry 73, 1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strous RD, Golubchik P, Maayan R, Mozes T, Tuati-Werner D, Weizman A, Spivak B, 2005. Lowered DHEA-S plasma levels in adult individuals with autistic disorder. Eur Neuropsychopharmacol 15, 305–309. [DOI] [PubMed] [Google Scholar]
- Tordjman S, Anderson GM, McBride PA, Hertzig ME, Snow ME, Hall LM, Ferrari P, Cohen DJ, 1995. Plasma androgens in autism. J Autism Dev Disord 25, 295–304. [DOI] [PubMed] [Google Scholar]
- Vacher C-M, O’Reilly JJ, Salzbank J, Lacaille H, Bakalar D, Sebaoui-Illoul S, Liere P, Clarkson-Paredes C, Sasaki T, Sathyanesan A, Kawasawa YI, Popratiloff A, Hashimoto-Torii K, Gallo V, Schumacher M, Penn AA, 2019. Placental neurosteroids shape cerebellar development and social behaviour. bioRxiv, 730150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA, 2005. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol 57, 67–81. [DOI] [PubMed] [Google Scholar]
- Weaver CE, Park-Chung M, Gibbs TT, Farb DH, 1997. 17beta-Estradiol protects against NMDA-induced excitotoxicity by direct inhibition of NMDA receptors. Brain Res 761, 338–341. [DOI] [PubMed] [Google Scholar]
- Wu FS, Gibbs TT, Farb DH, 1990. Inverse modulation of gamma-aminobutyric acid- and glycine-induced currents by progesterone. Mol Pharmacol 37, 597–602. [PubMed] [Google Scholar]
- Zhu WJ, Vicini S, 1997. Neurosteroid prolongs GABAA channel deactivation by altering kinetics of desensitized states. J Neurosci 17, 4022–4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorumski CF, Paul SM, Covey DF, Mennerick S, 2019. Neurosteroids as novel antidepressants and anxiolytics: GABA-A receptors and beyond. Neurobiol Stress 11, 100196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


