Abstract
Reduced responsiveness to reward has been associated with both risk-prone and risk-averse behavior, common features of externalizing and internalizing psychopathology, respectively. Thus, evidence has suggested a potential quadratic relationship (i.e., inverted U) between reward sensitivity and risk-taking propensity. Blunted response to reward compared to loss may therefore demonstrate transdiagnostic utility as it relates to different patterns of maladaptive risk behavior. The current study sought to disentangle the relationship between risk and reward in a clinically diverse sample. In a sample of 210 adults (aged 18–30), the RewP (an ERP indexing differentiation between rewards and losses) was measured during a monetary guessing game, and risk-taking propensity was measured with a behavioral task (i.e., BART) that simulates real-world risk taking. Participants also completed clinical assessments to assess for lifetime psychopathology. Results indicated that there was no linear association between the RewP and risk-taking propensity; however, there was a significant quadratic relationship. Thus, a reduced sensitivity to reward receipt was associated with both risk-prone and risk-averse behavior. There was also a significant quadratic relationship between the RewP and money won during the BART, indicating that being too risk prone or risk averse is disadvantageous and leads to missed reward. Overall, these findings suggested that blunted neural differentiation between gains and losses may contribute to deficits in effectively weighing reward and loss and result in maladaptive risk-taking behavior. These findings support continued examination of reward dysfunction dimensionally in order to better characterize behavioral profiles implicated in clinical phenotypes.
Keywords: EEG/ERP, psychopathology, reward positivity, risk taking
1 |. INTRODUCTION
Often, the pursuit of rewarding outcomes requires the assumption of some risk: asking for a date conjures the promise of romance but also the specter of rejection. Unsurprisingly, individuals differ in their risk-taking propensity, which is based largely upon the weight they accord to rewards and risks (Byrnes, Miller, & Schafer, 1999; Figner & Weber, 2011). Taking too many or too few risks, however, is associated with adverse outcomes; excessive risk taking may lead to harm without the benefit of increasing rewards, while being too risk averse may lead to missed opportunities for reward (Byrnes, 2011; Furby & Beyth-Marom, 1992; Smoski et al., 2008).
Although individual differences in risk and reward are closely interconnected, these constructs are frequently examined separately and, moreover, in separate and distinct populations. For instance, risk taking is often viewed as a core feature of externalizing psychopathology and is commonly studied in populations such as alcohol/substance use disorders (Colder & O’Connor, 2002; Gorka, Liu, Klein, Daughters, & Shankman, 2015) and attention-deficit hyperactivity disorder (ADHD; Drechsler, Rizzo, & Steinhausen, 2007; Matthies, Philipsen, & Svaldi, 2012), whereas research on risk-taking behavior in internalizing populations is much less common (Auerbach, Abela, & Ho, 2007; Chapman et al., 2007; Charpentier, Aylward, Roiser, & Robinson, 2016; Smoski et al., 2008). On the other hand, deficits in reward processing are frequently implicated across both internalizing and externalizing disorders, but very rarely do samples include individuals across both classes of disorders. More importantly, studies have yet to elucidate how individual differences in reward reactivity and risk-taking propensity interact to influence symptoms and/or behavioral outcomes (Ait Oumeziane & Foti, 2016; Baskin-Sommers & Foti, 2015). As such, examining these features transdiagnostically may allow for a better understanding of psychopathology. This line of work is also consistent with the National Institute of Mental Health’s (NIMH) Research Domain Criteria (RDoC) initiative, which emphasizes a need for further research focused on identifying transdiagnostic constructs that reflect core mechanisms of psychopathology (Kozak & Cuthbert, 2016; Zalta & Shankman, 2016).
Converging evidence suggests that aberrant reward processing may play an important role in individuals’ propensities for risk taking. Both reward reactivity and risk taking are mediated by activity of the mesolimbic dopaminergic (DA) system (Schultz, 2002; Treadway, Buckholtz, & Cowan, 2012; Zald, Cowan, & Riccardi, 2008). The ventral striatum is a key region in this system, receiving DA projections from the ventral tegmental area (Nestler & Carlezon, 2006). Notably, increased ventral striatal activation has been observed during the processing of monetary reward (Breiter & Rosen, 1999; Knutson, Adams, Fong, & Hommer, 2001) and while making risky decisions (Christopoulos, Tobler, & Bossaerts, 2009; Matthews, Simmons, Lane, & Paulus, 2004). Furthermore, individual differences in reward responsivity have been theorized to directly influence risk-taking behaviors such as substance abuse. According to Blum et al.’s reward deficiency syndrome (RDS) hypothesis (Blum, Cull, Braverman, & Comings, 1996), deficits in dopamine neurotransmission and hyporeactivity to rewards may contribute to impulsive behaviors as individuals with RDS seek to compensate for insufficient natural reinforcement via risk taking as a means of activating the DA system.
At the same time, hyporeactive reward responding (and a hypoactive DA system) may also contribute to less risk-taking propensity. A core feature of some internalizing disorders, such as depression, is blunted reward reactivity (Der-Avakian & Markou, 2012; Keedwell, Andrew, Williams, Brammer, & Phillips, 2005). Numerous neuroimaging studies have demonstrated dysfunction of the DA reward system in both depressed individuals (Dunlop & Nemeroff, 2007; Nestler & Carlezon, 2006; Tremblay et al., 2005) and individuals vulnerable to depression (Gotlib et al., 2010; Nestler & Carlezon, 2006; Tremblay et al., 2005). Yet, rather than increased risk-taking propensity, evidence suggests that individuals with internalizing psychopathology tend to be risk averse (Auerbach et al., 2007; Chapman et al., 2007; Charpentier et al., 2016; Smoski et al., 2008). As such, it appears that reward deficits may influence both types of dysfunctional risk taking; however, to date, this issue has remained largely unexplored, as few studies have linked these two constructs dimensionally (Ait Oumeziane & Foti, 2016; Baskin-Sommers & Foti, 2015). Furthermore, many studies have relied on self-report measures to assess reward sensitivity and risk behavior, which are susceptible to inaccurate or biased reporting. Psychophysiological and behavioral measures can provide important insight into these constructs, by reflecting neural processes and simulating real-world behaviors (Ait Oumeziane & Foti, 2016; Crowley, Wu, Crutcher, & Bailey, 2009; Fein & Chang, 2008).
To this end, one well-validated and reliable way to measure individual differences in reward sensitivity is the feedback negativity (FN), an ERP that is sensitive to rewards relative to nonrewards (Carlson, Foti, Mujica-Parodi, Harmon-Jones, & Hajcak, 2011; Miltner, Braun, & Coles, 1997; Weinberg, Luhmann, Bress, & Hajcak, 2012; Weinberg, Riesel, & Proudfit, 2014; although see Ullsperger, Danielmeier, & Jocham, 2014). The FN peaks frontocentrally between 200 and 300 ms following the presentation of feedback (Holroyd & Coles, 2002; Miltner et al., 1997). Recent evidence has suggested that the FN is driven by variation in a reward-related positivity (i.e., the RewP; Proudfit, 2015) that is more positive for rewards than nonrewards (Carlson et al., 2011; Foti, Carlson, Sauder, & Proudfit, 2014; Weinberg et al., 2012, 2014). Therefore, in the current study, the RewP is used to represent individuals’ responses to reward receipt as the difference between gains and losses. The RewP is specifically noted in the RDoC positive valence system as an indicator of the hedonic impact of rewards (i.e., “liking”; Baskin-Sommers & Foti, 2015; Proudfit, 2015). As such, simple guessing tasks typically used to generate the RewP (as in the current study) are listed by the NIMH as an approved paradigm for examining the RDoC construct of “initial responsiveness to reward attainment.”
Individual differences in the RewP relate to individual differences in reward sensitivity: a larger RewP is associated with both higher self-reported reward sensitivity (Bress, Smith, Foti, Klein, & Hajcak, 2012) and behavioral measures of reward-driven response biases (Bress & Hajcak, 2013). In clinical populations, a blunted RewP has been associated with depression (Foti & Hajcak, 2009; Liu, Wang, Shang, Shen, & Li, 2014; Nelson, Perlman, Klein, Kotov, & Hajcak, 2016; Weinberg, Liu, & Shankman, 2016) and vulnerability to depression (Bress et al., 2012; Foti, Kotov, Klein, & Hajcak, 2011; Kujawa, Proudfit, & Klein, 2014; Weinberg, Liu, Hajcak, & Shankman, 2015). Studies have also shown a blunted RewP in individuals high in broad externalizing traits (Bernat, Nelson, Steele, Gehring, & Patrick, 2011; although see Ait Oumeziane & Foti, 2016) as well as in substance abusing samples (Baker, Stockwell, Barnes, Haesevoets, & Holroyd, 2016; Baker, Stockwell, Barnes, & Holroyd, 2011) and problem gamblers (Hewig et al., 2010; Oberg, Christie, & Tata, 2011).
Prior research has utilized different methodologies for the assessment of risk-taking propensity, including both self-report (Colder & O’Connor, 2002) and behavioral measures (MacPherson, Magidson, Reynolds, Kahler, & Lejuez, 2010). Behavioral measures of risk-taking propensity have many advantages over self-report as they are not influenced by recall or social desirability biases (Harrison, Young, Butow, Salkeld, & Solomon, 2005). One frequently used behavioral measure of risk-taking propensity is the balloon analogue risk task (BART; Lejuez et al., 2002), a laboratory-based behavioral task designed to capture the multifaceted nature of risk-taking propensity by simulating real-world risk taking. The BART models financial risk taking over time, such that a certain amount of risk taking is beneficial, leading to positive outcomes (i.e., reward); however, too much risk taking becomes detrimental. The BART has demonstrated good construct validity (Hunt, Hopko, Bare, Lejuez, & Robinson, 2005; Lejuez, 2005; Lejuez et al., 2003) and has predicted real-world risk behaviors such as substance use and gambling (Lejuez et al., 2003, 2002), becoming the gold standard behavioral measure for investigating individual differences in risk-taking propensity.
Although the RewP and BART have been shown to be sensitive measures of reward responsivity and risk taking, respectively, very few studies to date have assessed whether neural response to reward relative to loss relates to behavioral risk taking. In a sample of individuals with alcohol dependence, Fein and Chang (2008) found a blunted neural response to negative feedback during the BART to be associated with greater family history density of alcohol problems. In another study, Crowley and colleagues (2009) divided a sample of children at risk for substance use disorders into high risk-takers and low risk-takers and assessed the RewP during the BART, finding that low-risk males had greater differentiation between gains and losses than high-risk males. More recently, Yau, Potenza, Mayes, and Crowley (2015) found that, during the BART, adolescents at risk for problematic Internet use exhibited blunted ERPs to negative and positive feedback relative to adolescents not at risk.
While these studies provide preliminary support for a link between reward reactivity and risk-taking propensity, they have largely focused on characterizing specific psychiatric populations. Thus, there is still a need to directly test the association between the RewP and risk-taking propensity, particularly in representative samples with a wide range of both internalizing and externalizing symptoms and personality traits, as it is unclear how individual differences in response to reward versus loss and risk-taking propensity relate to each other more generally, across multiple psychopathologies. Importantly, while the current literature has independently implicated blunted reward sensitivity in both depressive and alcohol/substance use populations, these disorders are characterized by quite opposite patterns of maladaptive risk behavior (i.e., excessive risk aversion and risk proneness in depression and substance use, respectively). Given this background, one might predict that there is actually a quadratic, or U-shaped, association between reward sensitivity and risk-taking propensity. That is, blunted reward sensitivity may characterize individuals on both poles of risk-taking propensity, with “normative” reward sensitivity only characterizing those with normative risk-taking propensity. The extant literature has been inherently limited in its ability to examine such potential nonlinear, quadratic associations, as a focus on specific psychopathologies naturally truncates the range of a construct’s dimension.
In sum, consistent with a transdiagnostic perspective and the RDoC framework, we aimed to use two well-validated measures, the RewP and BART, to examine the manner in which reward and risk are related. Critically, the current study included clinical assessments in a diverse community sample, allowing us to examine this association across a dimension. Given the extant literature finding reward deficits in both risk-prone and risk-averse individuals, we tested both linear and nonlinear models using behavioral risk-taking propensity (i.e., BART) to predict neural differentiation between gains and losses (i.e., RewP). As these constructs are viewed as broader individual difference factors, we sought to examine associations between the RewP and BART independent of psychiatric diagnosis, although diagnoses were examined as covariates. We hypothesized that there would be a significant quadratic association between the RewP and risk-taking propensity, such that blunted response to reward relative to loss would be linked to both risk-prone and risk-averse behavior.
2 |. METHOD
2.1 |. Participants and procedure
The sample included 210 individuals drawn from a larger family study on emotional risk factors for psychopathology (see Gorka, Lieberman, Shankman, & Phan, 2017; Weinberg et al., 2015). For the purposes of the larger study, participants were required to be between the ages of 18 and 30 and have at least one full biological sibling within the same age range who was eligible to enroll. The sample therefore consisted of adult, biological sibling pairs. A typical sibling design would include a proband and their unaffected (i.e., “healthy”) sibling. However, in the current study, in line with the RDoC initiative, we did not make categorical designations of healthy versus unhealthy siblings. Neither member of the sibling dyad was therefore required to meet a categorical diagnosis for any psychiatric disorder; thus, the sample comprises sibling pairs where both, neither, or one sibling(s) report internalizing and/or externalizing symptoms. All individuals were recruited from the community via advertisements designed to capture a wide range of internalizing and externalizing psychopathology (e.g., substance use, depression, anxiety), as well as healthy controls. In order to ensure ability to provide consent and minimize potential data confounds, participants were excluded if they had a lifetime or family history of a psychotic or bipolar disorder, were unable to read or write English, had a history of head trauma with loss of consciousness, or were left-handed. The final sample was 58.6% female and was racially diverse (47.4% Caucasian American, 23.9% Hispanic, 9.6% African American, 12.9% Asian, 2.4% Middle Eastern, 1.0% other, and 2.8% mixed race), well-educated (54.1% had completed some college education; 21.5% had completed 4 years of college), and relatively young (age M = 22.47, SD = 3.04). Sample characteristics are provided in Table 1.
TABLE 1.
Descriptive statistics and associations between demographics, predictor variables, the reward positivity (RewP), and risk-taking propensity
| Mean (SD)/% | 1. | 2. | 3. | 4. | 5. | 6 | 7. | 8. | |
|---|---|---|---|---|---|---|---|---|---|
| 1. Gender (% female) | 58.6% | ||||||||
| 2. Age | 22.47 (3.04) | 0.07 | |||||||
| 3. Risk-taking propensity (BART) | 1759.42a (301.85) | −0.27*** | 0.02 | ||||||
| 4. Total money won in cents (BART) | 801.40a (117.82) | −0.11 | −0.01 | 0.46*** | |||||
| 5. Total balloon explosions (BART) | 13.80a (2.43) | −0.28*** | 0.03 | 0.94*** | 0.23*** | ||||
| 6. Δ RewP | −3.77a (3.01) | −0.14* | −0.02 | −0.04 | −0.16* | 0.01 | |||
| 7. Lifetime dx of SUD (% hx) | 21.9%a | −0.13* | 0.14* | 0.06 | 0.07 | 0.05 | −0.09 | ||
| 8. Lifetime dx of AUD (% hx) | 28.9%a | −0.20** | 0.24*** | 0.06 | 0.05 | 0.08 | 0.04 | 0.38*** | |
| 9. Lifetime dx of MDD (% hx) | 30.9%a | 0.15* | 0.13 | −0.11 | 0.02 | −0.13 | 0.06 | 0.19** | 0.09 |
Note. N = 210. dx =diagnosis; hx =history.
To account for shared genetic variance in variable, reported descriptives reflect the mean/SD averaged across sibling pairs.
p < 0.001;
p < 0.01;
p < 0.05.
All participants provided written informed consent after review of the protocol, and all procedures were approved by the University of Illinois–Chicago Institutional Review Board. As part of the study protocol, participants completed a set of laboratory tasks, a battery of questionnaires, and a semistructured clinical interview. Laboratory tasks and questionnaires were administered in a counterbalanced order to eliminate potential order effects. Participants received cash as payment for participation.
2.2 |. Psychopathology
Lifetime diagnoses of depression, alcohol use disorder, and substance use disorder were assessed using the Structured Clinical Interview for DSM-5 (SCID; First, Williams, Karg, & Spitzer, 2015). To ensure valid scoring of psychopathology, raters were trained by (a) viewing the SCID-101 training videos (SCID-101, 1998), (b) observing two to three SCID interviews with an experienced rater, and (c) administering three SCID interviews supervised by a licensed clinical psychologist or advanced rater (in which the supervisor and trainee demonstrated 100% diagnostic agreement). Diagnoses demonstrated high test-retest reliability (Shankman et al., 2017).
During the clinical interview, continuous measures of current alcohol use were obtained using a timeline follow-back procedure (Sobell & Sobell, 1992). Participants were asked to indicate number of drinks consumed each day over the past 30 days. For the current study, average number of drinks per week and number of binge episodes were used to examine the BART’s association with real-world risk behavior in the sample. Binge episodes were defined as consuming ≥5 standard drinks for men or ≥4 standard drinks for women within a 2-hr period (National Institute on Alcohol Abuse and Alcoholism, 2004).
2.3 |. Risk-taking propensity
The BART auto pump (Pleskac, Wallsten, Wang, & Lejuez, 2008), a modified version of the original BART (Lejuez et al., 2002), was used to assess risk-taking propensity. Participants were presented with 30 computerized balloons, one at a time. They were instructed to inflate each balloon by typing the total number of desired “pumps” between 1 and 128. For each pump, they received 1 cent and, thus, the higher the number of entered pumps, the greater the amount of potential earnings. Participants were also told that the balloons might explode at any given pump, but they were unaware of the actual explosion point for each balloon. If the participant typed in a number that exceeded the balloon’s explosion point, the balloon on the screen popped and the participant did not receive any money for that balloon. If the number they entered did not exceed the explosion point, the balloon on the screen inflated and the money they earned was deposited into their “bank account.” The explosion point for each balloon was derived such that, within each sequence of 10 balloons, the average explosion point was on pump 64. For all trials, the explosion point for the previous balloon was displayed in the left-hand corner of the screen along with the balloon number (e.g., 10 of 30). Participants were told that the amount of their prize money at the end of the session was dependent on the amount of money they accumulated throughout the task. Total number of pumps, across all 30 balloons, was used as an index of risk-taking propensity (Daughters, Gorka, Matusiewicz, & Anderson, 2013). As an alternative measure of BART performance, total money won was also examined (i.e., total cash accumulated from unpopped balloons; Killgore, Kamimori, & Balkin, 2011; Skeel, Neudecker, Pilarski, & Pytlak, 2007).
2.4 |. Response to reward receipt
The reward task was a simple guessing task previously used in other studies (Foti et al., 2014; Weinberg et al., 2015). The task consisted of 60 trials, presented in three blocks of 20. At the beginning of each trial, participants were presented with an image of two doors and were instructed to choose one door by clicking the left or right mouse button. The doors remained on the screen until the participant responded. Next, a fixation mark (+) appeared for 1,000 ms, and feedback was presented on the screen for 2,000 ms. Participants were told that they could either win $0.50 or lose $0.25 on each trial. A win was indicated by a green “↑,” and a loss was indicated by a red “↓.” Next, a fixation mark appeared for 1,500 ms and was followed by the message “Click for the next round,” which remained on the screen until the participant responded and the next trial began. Across the task, 30 win and 30 loss trials were presented in a random order.
2.5 |. Psychophysiological recording, data reduction, and analysis of reward task
During the doors task, continuous EEG recordings were collected using an elastic cap and the ActiveTwo BioSemi system (BioSemi, Amsterdam, Netherlands). Sixty-four electrodes were used, based on the 10–20 system, as well as two electrodes on the right and left mastoids. Electrooculogram (EOG) generated from eye movements and eyeblinks was recorded using four facial electrodes: horizontal eye movements were measured via two electrodes located approximately 1 cm outside the outer edge of the right and left eyes. Vertical eye movements and blinks were measured via one electrode placed approximately 1 cm below the left eye and electrode FP1. The data were digitized at a sampling rate of 1,024 Hz, using a low-pass fifth order sinc filter with −3 dB cutoff point at 208 Hz. Each active electrode was measured online with respect to a common mode sense (CMS) active electrode, located between PO3 and POz, producing a monopolar (non-differential) channel. CMS forms a feedback loop with a paired driven right leg (DRL) electrode, located between POz and PO4, reducing the potential of the participants and increasing the common mode rejection rate. Offline, all data were analyzed in Brain Vision Analyzer and were referenced to the average of the left and right mastoids and band-pass filtered with low and high cutoffs of 0.1 and 30 Hz, respectively.
Semiautomatic procedures were employed to detect and reject artifacts, including a modification of Gratton, Coles, and Donchin (1983) to correct for eyeblink and ocular artifacts. The criteria applied were a voltage step of more than 50.0 μV between sample points, a voltage difference of 300.0 μV within a trial, and a maximum voltage difference of less than 0.50 μV within 100-ms intervals. Visual inspection of the data was then conducted to detect and reject any remaining artifacts.
The EEG was segmented into 1,200-ms windows for each trial, beginning 200 ms before each response onset and continuing for 1,000 ms following feedback. A 200-ms window from −200 to 0 ms prior to feedback onset served as the baseline. The RewP appears maximal around 300 ms at frontocentral sites; therefore, the time window-scored response to rewards and nonrewards was scored as the average activity at electrode site FCz, between 220 and 360 ms on gain and loss trials, respectively. For each participant, a loss minus win score was then calculated to represent the RewP. Difference scores have previously been used in RewP studies to isolate neural sensitivity to outcome valence independent of the source of the variance (i.e., rewards or nonrewards; Foti et al., 2011; Kujawa et al., 2014). In addition, the RewP, scored as a difference measure, has been related to behavioral and self-report measures of reward sensitivity and reward-related neural activity using fMRI (Bress & Hajcak, 2013; Carlson et al., 2011; Foti et al., 2014). More negative values for the difference score indicate greater differentiation between wins and losses (i.e., increased reward sensitivity). This scoring method is limited to some extent by the fact that it does not allow for investigating the individual contributions of responses to gains and losses. However, individual scoring of gain and loss responses can be misleading in that it can indicate an ERP component where one does not exist (Luck, 2005).
2.6 |. Data analysis plan
Analyses were conducted in SPSS v.23. Multilevel mixed models were used to test the bivariate associations between variables and the association between risk-taking propensity and the RewP. Multilevel mixed models are ideal for the current analyses as participants were nested within families, and multilevel mixed models account for the shared variance between sibling pairs.
The model used restricted maximum likelihood estimation and an unstructured covariance matrix. Age and gender were included as covariates. To rule out the confounding effects of psychiatric diagnoses associated with aberrant reward responding, the analysis was run a second time, adding lifetime diagnoses of major depressive disorder (MDD), illicit substance use disorder (SUD), and alcohol use disorder (AUD) as covariates. BART total pumps and BART total pumps squared (i.e., the quadratic effect) were specified as fixed effects to predict neural response to loss minus rewards (i.e., the RewP). Thus, Level 1 of the statistical model was specified as follows: RewP = β0 + β1(age) + β2(sex) + β3(MDD) + β4(SUD) + β5(AUD) +β6(BART) + β7(BART)2 + ε. Level 2 of the model nests the effects specified in the Level 1 equation within sibling pairs.1
3 |. RESULTS
3.1 |. Participant characteristics
Table 1 presents demographic variables, means for risk-taking propensity on the BART, and neural response to rewards, and associations among these variables (taking into account the nested structure of the data). Significant positive associations between BART indices and self-reported alcohol use suggest external validity of BART for real-world risk behavior. Specifically, average number of drinks over the past 30 days was associated with increased risk-taking propensity (β = 0.17, p = 0.04) and marginally associated with number of explosions heard (β = 0.13, p = 0.07). Similarly, number of binge episodes over the past 30 days was marginally associated with increased risk-taking propensity (β = 0.13, p = 0.06) and significantly associated with number of explosions (β = 0.15, p = 0.03). Men displayed greater risk-taking propensity on the BART (M = 1894.90, SD = 367.22) than women (M = 1648.84, SD = 428.88). Men were also more likely to have met criteria for AUD (N = 34, 39%) but not SUD (N = 20, 23%) than women (N = 25, 20%, and N = 17, 14%, respectively). Men (N = 18, 21%) and women (N = 41, 34%) did not differ in their rates of lifetime MDD. Having a lifetime diagnosis of one disorder was related to the likelihood of having another (see associations reported in Table 1). Twenty-one individuals met lifetime criteria for both MDD and AUD (10%), 18 met criteria for lifetime MDD and SUD (8.6%), and 24 individuals met criteria for both AUD and SUD (11.4%).
As shown in Table 1, risk-taking propensity (i.e., total number of pumps during the BART) did not differ by age or lifetime diagnosis, nor did risk-taking propensity and neural response to rewards significantly relate to one another at the bivariate level. The RewP was significantly negatively associated with total amount of money won during the task, such that greater differentiation between gains and losses was associated with more money won (β = −0.16, p < 0.05).
3.2 |. Risk taking and money won during BART
Risk-taking propensity was significantly and positively associated with total amount of money won in the task (b = 0.46, p < 0.001). We followed this bivariate analysis with a multilevel mixed model controlling for the linear association and found that there was also a significant quadratic association evident, b = −68.56, t(205) = −4.54, p < 0.001, such that both risk-averse and risk-prone individuals won less money overall than individuals in the middle of the distribution of risk-taking propensity.
3.3 |. Association between neural response to rewards, loss, and risk-taking propensity
In predicting the magnitude of the RewP in the multivariate model, there was no main effect of age, b = −0.01, t(202.20) = −0.12, p = 0.90. Gender was significantly associated with neural response to rewards, b = −1.16, t(203.30) = −2.07, p < 0.05, such that women had a larger RewP (M = −4.04, SD = 4.12) compared to men (M = −2.95, SD = 3.59). As was the case with the bivariate analysis, there was no significant linear association between the RewP and risk-taking propensity (i.e., total number of pumps), b = 0.05, t(204.24) = 0.15, p = 0.88. However, there was a significant quadratic association, b = 0.53, t(204.24) = 3.33, p = 0.001, such that a blunted neural response to reward receipt was related to both high and low levels of risk-taking propensity.
Similar results were obtained when controlling for psychiatric diagnoses. There were no main effects of age, b = −0.04, t(200.87) = −0.41, p = 0.68, lifetime diagnosis of MDD, b = 1.11, t(201.98) = 1.83, p = 0.07, lifetime diagnosis of SUD, b = −1.23, t(201.99) = −1.68, p = 0.10, or lifetime diagnosis of AUD, b = 0.74, t(201.91) = 1.40, p = 0.26. Gender was significantly associated with neural response to rewards, b = −1.29, t(200.29) = −2.22, p < 0.05, in that women had a larger RewP (M = −4.04, SD = 4.12) compared to men (M = −2.95, SD = 3.59). Consistent with above, there was no linear association between the RewP and risk-taking propensity, b = 0.07, t(200.18) = 0.22, p = 0.83; however, there was a significant quadratic association, b = 0.54, t(201.68) = 3.39, p = 0.001 (see Figure 1 for scatterplot depicting this association), such that a blunted neural response to rewards was related to both high and low levels of risk-taking propensity.2
FIGURE 1.
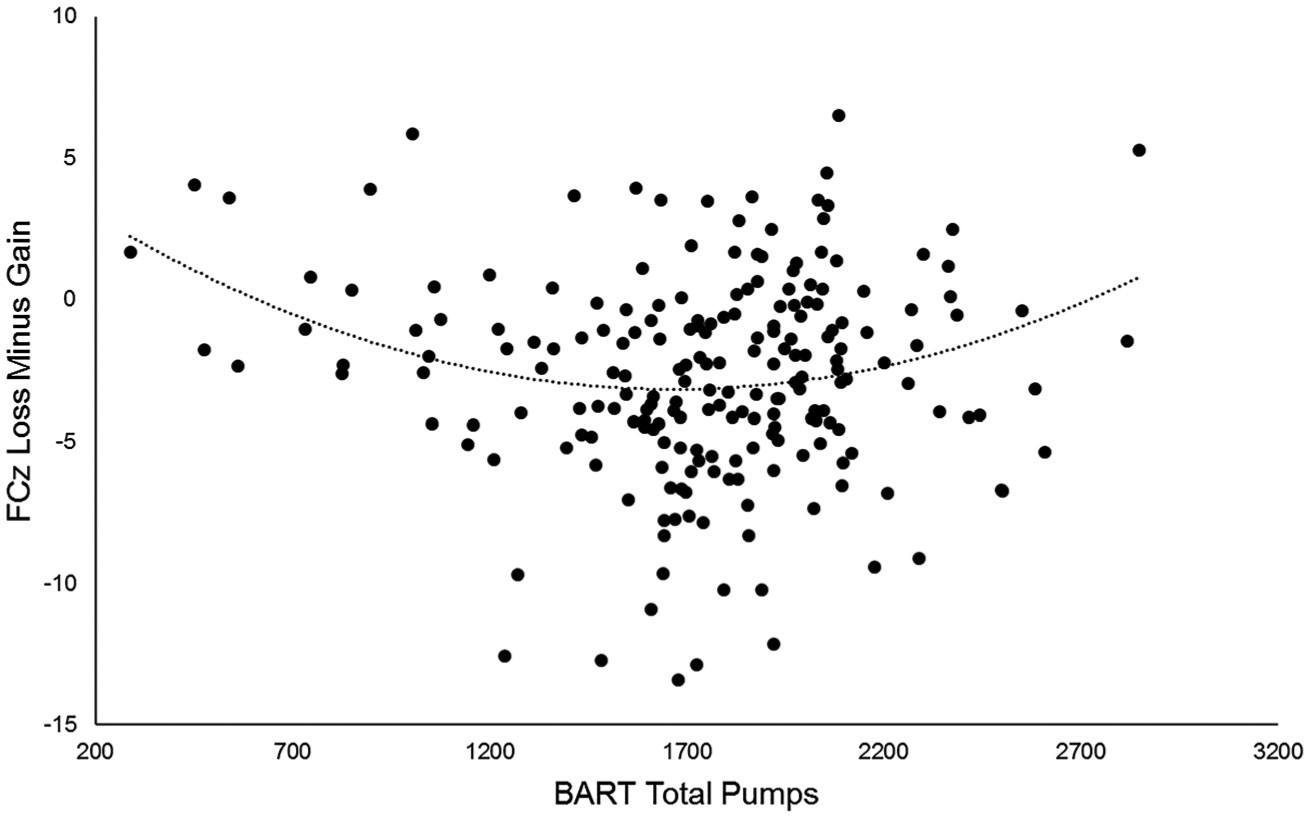
Scatterplots depicting the curvilinear association between risk-taking propensity measured through the BART task on the x axis and neural response to rewards compared to nonrewards (loss minus gain) on the y axis
For presentation purposes of the ERPs, the sample was split into the bottom 25% of risk propensity on the BART (risk-averse), the middle 50% of risk propensity on the BART (controls), and the top 25% of risk propensity on the BART (risk-prone). Figure 2 displays grand-averaged response-locked ERPs at FCz for each of these groups. Topographic maps are also presented in Figure 3 for each group. These maps depict voltage differences (in μV) across the scalp for nonreward minus reward responses in the time window of the RewP.
FIGURE 2.
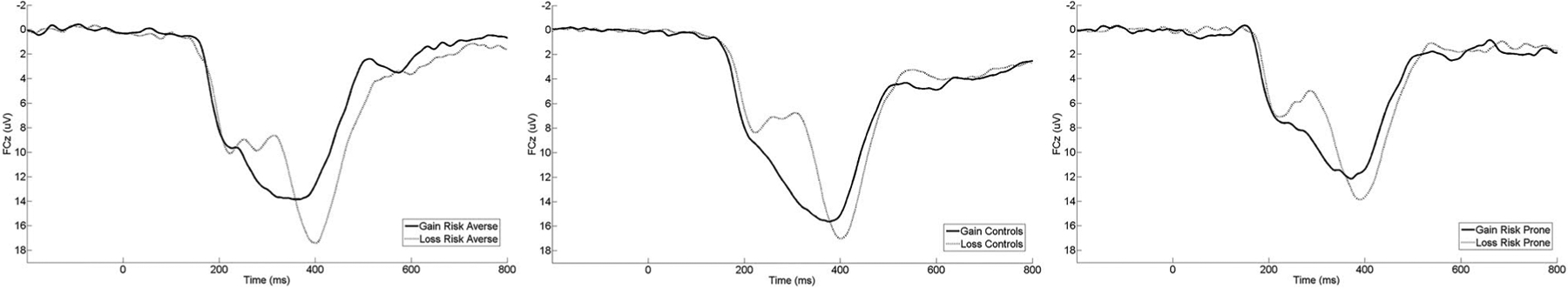
Stimulus-locked ERP waveforms at electrode site FCz for reward and nonreward trials separately. For presentation purposes, subjects were divided into the following groups: risk-averse (bottom 25% on the BART), controls (middle 50% on the BART), and risk-prone (top 25% on the BART). For each panel, feedback onset occurred at 0 ms. Per ERP convention, negative voltages are plotted up
FIGURE 3.
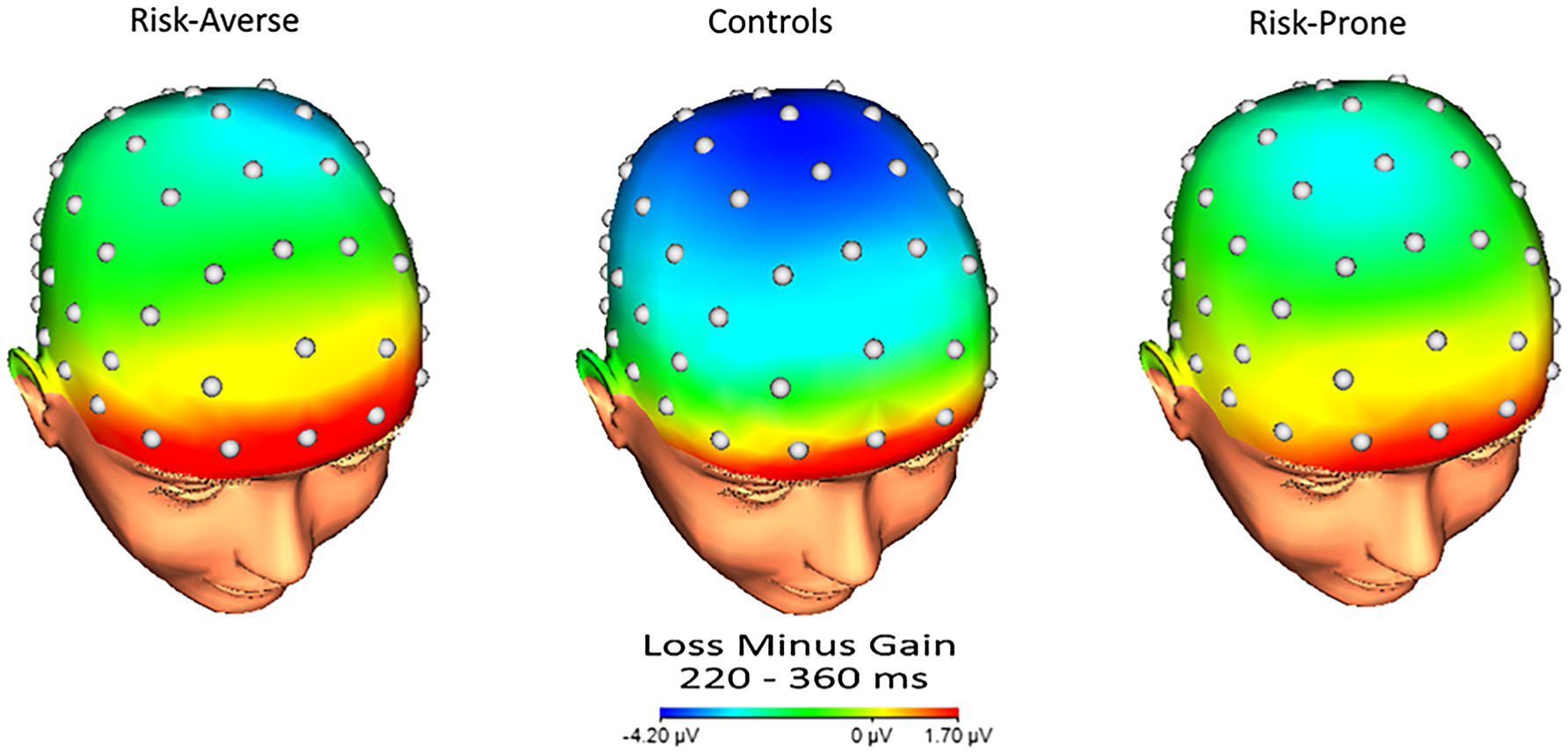
Scalp topographies representing the average neural response to nonrewards minus rewards. For presentation purposes, subjects were divided into the following groups: risk-averse (bottom 25% on the BART; left), controls (middle 50% on the BART; center), and risk-prone (top 25% on the BART; right)
4 |. DISCUSSION
Previous research has implicated aberrant reward processing in individuals demonstrating maladaptive risk-taking behavior, finding reduced reward responsivity in varied psychopathologies associated with risk proneness and risk aversion (Baskin-Sommers & Foti, 2015; Foti et al., 2014; Proudfit, 2015). However, to date, no study has examined the nature of this association across a clinically diverse population that includes individuals both high and low in both internalizing and/or externalizing symptoms. Thus, questions about how reduced sensitivity to reward (relative to loss) may influence differential patterns of risk taking (e.g., too much or too little risk taking) have remained largely unanswered. The current study aimed to disentangle the nature of the relationship between these two constructs by testing both linear and nonlinear models on the association between neural response to reward versus loss (i.e., RewP) and behavioral measures of risk-taking propensity (i.e., BART performance). Results indicated that there was no linear association between the RewP and risk-taking propensity; however, there was a significant quadratic relationship, such that both those who were risk prone and those who were risk averse displayed decreased neural differentiation between losses and gains. This result was also independent of several psychiatric diagnoses that relate to blunted reward processing (MDD, AUD, SUD). Overall, these findings provide evidence that blunted neural responsivity to reward relative to loss may contribute to deficits in the ability to effectively weigh reward and loss and subsequently relate to maladaptive risk-taking behavior.
The findings also highlight that taking far too many and far too few risks are both maladaptive. Specifically, the current study found a quadratic association between risk-taking propensity and money won during the BART, such that individuals in the middle of the risk-taking propensity distribution won the most money, while those nearer to the poles of the distribution won less money in the task. This indicates that overly cautious and risky behavior both led to poorer outcomes (i.e., less financial gain/reward), consistent with prior findings (Lejuez et al., 2002). The RewP was also significantly correlated with money won in the task, such that a greater differentiation between gain and loss (i.e., more negative RewP) was associated with winning more money. These results highlight that a certain amount of risk taking is necessary in order to maximize potential reward, as too little risk taking results in missed reward. However, when too many risks are taken, individuals also lose out on reward, as the consequences of the risks they take diminish their potential earnings. In other words, both risk-prone and risk-averse individuals demonstrate an inability to maximize rewards, and this deficit may relate to a reduced responsiveness to the receipt of rewards.
The results of the current study are consistent with extant literature finding deficient reward processing in both risk-prone and risk-averse individuals. For instance, blunted response to reward versus loss has been found across internalizing and externalizing psychopathologies, including depression (Foti & Hajcak, 2009; Liu et al., 2014; Weinberg et al., 2015), substance abuse (Baker et al., 2016, 2011), and problem gambling (Hewig et al., 2010; Oberg et al., 2011). Yet, despite a seemingly common feature of reduced reward responsivity, these disorders are also characterized by a number of features that are discrepant across internalizing and externalizing psychopathologies. Notably, while externalizing psychopathology is often linked with excessive risk taking (Bechara & Damasio, 2002; Stautz & Cooper, 2013; Zuckerman, 2007), avoidance of risk has been implicated in internalizing psychopathology (Auerbach et al., 2007; Chapman et al., 2007; Smoski et al., 2008). Internalizing and externalizing pathologies may thus both be marked by dysfunction within neurobiological systems (e.g., mesolimbic DA system) that contribute to appropriate valuation of rewards, yet there appear to be quite opposite consequences for risk behavior; for those with internalizing psychopathology, lackluster rewards may be seen as not worth the risk it takes to obtain them, whereas for those with externalizing psychopathology, more risks may be taken in order to compensate for insufficient natural reinforcement. In light of the current findings, reduced response to reward over loss may be a transdiagnostic construct that relates to generally maladaptive risk behavior, which may be characterized by either excessive or insufficient risk taking. These opposite patterns likely relate to deficits in appropriately weighting reward and risk, yet most studies that have attempted to clarify this relation in a controlled, systematic fashion have done so in attempts to differentiate groups and better understand specific psychopathologies (Crowley et al., 2009; Fein & Chang, 2008; Yau et al., 2015). This is the first study, to our knowledge, that has found both risk aversion and risk proneness, in the same sample, to relate to blunted neural responding to reward compared to loss, regardless of psychiatric diagnosis.
Adopting a dimensional approach toward defining risk-taking propensity and neural differentiation between gains and losses may help to clarify the transdiagnostic utility of these constructs and inform our understanding of psychopathology. Initial work examining the interrelationships of reward responding and externalizing traits has demonstrated that different biobehavioral profiles (e.g., low RewP amplitude and low impulsivity) can predict depressive symptoms (Ait Oumeziane & Foti, 2016). Further study of the interactions among neural and behavioral systems as they relate to symptoms may help to explain how clinically distinct psychopathologies arise from similar core features, such as reduced response to reward compared to loss. In considering blunted response to reward versus loss as a transdiagnostic mechanism of psychopathology, it may be useful to further examine factors (e.g., salience of drug-related cues) that contribute to distinct psychopathologies (e.g., MDD vs. AUD) from this common core reward deficit.
Furthermore, a transdiagnostic perspective on these constructs may also help improve our understanding of comorbidity, as “pure” cases of psychopathology are uncommon (Kessler, Chiu, Demler, & Walters, 2005; Kessler et al., 1997). For instance, among those with a history of lifetime major depressive disorder, 40.3% have a history of alcohol use disorder, 17.2% of drug use disorder, and 30.0% of nicotine dependence (Hasin, Goodwin, Stinson, & Grant, 2005). Reward abnormalities are seen across these disorders (Baskin-Sommers & Foti, 2015; Foti et al., 2014; Proudfit, 2015), yet our present understanding is limited as few studies have systematically contrasted these disorders in examining reward dysfunction (Baskin-Sommers & Foti, 2015). As such, a narrow focus on reward dysfunction and risk behaviors in these samples has limited applicability to clinical practice. The current study aimed to provide an initial integration of reward responsivity and risk-taking constructs across the internalizing/externalizing dimension; however, further work is warranted to better characterize biobehavioral profiles as they link to clinical presentation, such as examining other factors that may interact with reward dysfunction. For instance, the significance of environmental contexts (such as environments lacking sources of drug-free reward) and their impact on reward dysfunction and psychopathology should be examined in future lines of research; careful study of the presence or absence of factors in individuals’ environments and their contributions to comorbidity is likely to help continue to disentangle the complex associations between aberrant reward responding and psychopathology.
The current study has several limitations. First, the RewP was not collected during the BART. Simultaneous recording of electrophysiological and behavioral data would likely be informative in linking neural response to reward as arising directly from decision making involving risk. The utilization of the RewP as an index of reward sensitivity is also a limitation as there is some controversy in the field as to whether the negative deflection in the loss-minus-gain waveform represents response to positive versus negative outcomes (e.g., monetary gain or loss; Kimura, Kimura, & Iwaki, 2016; Proudfit, 2015; Ullsperger et al., 2014). Future work should aim to include other indices of reward responding to continue to reliably characterize this quadratic relationship between reward sensitivity and risk behavior. The BART is also limited in its utility as an index of risk-taking propensity. Although the BART has been found to correlate with real-world risk behaviors such as substance use and gambling (Lejuez et al., 2003, 2002), the risks taken during the task specifically affect monetary accrual or loss over time; however, a single risky decision taken in the real world can have significant negative consequences (e.g., deciding to drive while impaired). Thus, risk aversion in such cases may, indeed, be adaptive. The results of the current study are therefore limited in their scope, and future work should continue to examine how reward responsivity relates to other domains of risk behavior. Additionally, the current study excluded individuals with bipolar disorder. Given the associations between bipolar disorder and elevated response to reward (e.g., Nusslock et al., 2012), this exclusion may have limited the range of reward responsiveness in our study. Future work to understand the link between reward responsivity and risk-taking propensity would likely benefit by expanding the sample to include these individuals.
In sum, the results of the current study aimed to delineate the relationship between risk taking and reward responsivity, ultimately indicating an inverted U-shaped association such that blunted neural differentiation between rewards and losses was associated with both risk-averse and risk-prone behavior. This examination of a transdiagnostic association between reward responding and risk behavior aligns with the NIMH’s RDoC mission to identify core mechanisms of psychopathology. Importantly, it demonstrates that deficits in reward processing may map onto opposite patterns of risk-taking behavior, both of which are maladaptive in nature. As risk-averse and risk-prone behavior are associated with varied psychopathological profiles, further work should seek to better characterize the association between these constructs across psychopathology. Continued focus on a dimensional perspective of how reward dysfunction is implicated across different behavioral profiles, such as maladaptive risk taking, may help refine clinical phenotypes and ultimately aid in creating targeted treatment strategies.
ACKNOWLEDGEMENTS
This work was supported by National Institute of Mental Health grant R01 MH098093 (PI: S.A.S.). All authors report no conflicts of interest.
Funding information
National Institute of Mental Health grant (R01 MH098093) (to S.A.S.)
Footnotes
Intraclass correlations (ICC) examining the associations among siblings for the main variables suggested that risk-taking propensity was familial (ICC = 0.37, p = 0.02), but the RewP was not (ICC = 0.21, p = 0.16). ICCs for the alternative measures of risk-taking propensity from the BART indicated a familial association for the number of balloon explosions (ICC = 0.45, p < 0.01), but not total money accrued (ICC = 0.14, p = 0.24).
Recent studies have suggested that difference waveforms can be problematic, and alternative approaches using residualized scores (e.g., gain covarying out the variance due to loss) may be preferred (Meyer, Lerner, De Los Reyes, Laird, & Hajcak, 1997). We therefore conducted the same analyses as above using residualized scores instead of difference scores and found the same results (i.e., there were significant quadratic—but not linear—effects of risk-taking propensity in predicting gain and loss separately).
REFERENCES
- Ait Oumeziane B, & Foti D (2016). Reward-related neural dysfunction across depression and impulsivity: A dimensional approach. Psychophysiology, 53(8), 1174–1184. 10.1111/psyp.12672 [DOI] [PubMed] [Google Scholar]
- Auerbach RP, Abela JRZ, & Ho MR (2007). Responding to symptoms of depression and anxiety: Emotion regulation, neuroticism, and engagement in risky behaviors. Behaviour Research and Therapy, 45(9), 2182–2191. 10.1016/j.brat.2006.11.002 [DOI] [PubMed] [Google Scholar]
- Baker TE, Stockwell T, Barnes G, Haesevoets R, & Holroyd CB (2016). Reward sensitivity of ACC as an intermediate phenotype between DRD4–521T and substance misuse. Journal of Cognitive Neuroscience, 28(3), 460–471. 10.1162/jocn_a_00905 [DOI] [PubMed] [Google Scholar]
- Baker TE, Stockwell T, Barnes G, & Holroyd CB (2011). Individual differences in substance dependence: At the intersection of brain, behaviour and cognition. Addiction Biology, 16(3), 458–466. 10.1111/j.1369-1600.2010.00243.x [DOI] [PubMed] [Google Scholar]
- Baskin-Sommers AR, & Foti D (2015). Abnormal reward functioning across substance use disorders and major depressive disorder: Considering reward as a transdiagnostic mechanism. International Journal of Psychophysiology, 98(2), 227–239. 10.1016/j.ijpsycho.2015.01.011 [DOI] [PubMed] [Google Scholar]
- Bechara A, & Damasio H (2002). Decision-making and addiction (Part I): Impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia, 40, 1675–1689. 10.1016/S0028-3932(02)00015-5 [DOI] [PubMed] [Google Scholar]
- Bernat EM, Nelson LD, Steele VR, Gehring WJ, & Patrick CJ (2011). Externalizing psychopathology and gain–loss feedback in a simulated gambling task: Dissociable components of brain response revealed by time-frequency analysis. Journal of Abnormal Psychology, 120(2), 352–364. 10.1037/a0022124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K, Cull JG, Braverman ER, & Comings DE (1996). Reward deficiency syndrome. American Scientist, 84, 132–145. [Google Scholar]
- Breiter HC, & Rosen BR (1999). Functional magnetic resonance imaging of brain reward circuitry in the human. Annals of the New York Academy of Sciences, 877, 523–547. 10.1111/j.1749-6632.1999.tb09287.x. [DOI] [PubMed] [Google Scholar]
- Bress JN, & Hajcak G (2013). Self-report and behavioral measures of reward sensitivity predict the feedback negativity. Psychophysiology, 50(7), 610–616. 10.1111/psyp.12053 [DOI] [PubMed] [Google Scholar]
- Bress JN, Smith E, Foti D, Klein DN, & Hajcak G (2012). Neural response to reward and depressive symptoms in late childhood to early adolescence. Biological Psychology, 89(1), 156–162. 10.1016/j.biopsycho.2011.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes JP (2011). The nature and development of decision-making: A self-regulation model. London, UK: Psychology Press. [Google Scholar]
- Byrnes JP, Miller DC, & Schafer WD (1999). Gender differences in risk taking: A meta-analysis. Psychological Bulletin, 125(3), 367–383. 10.1037//0033-2909.125.3.367 [DOI] [Google Scholar]
- Carlson JM, Foti D, Mujica-Parodi LR, Harmon-Jones E, & Hajcak G (2011). Ventral striatal and medial prefrontal BOLD activation is correlated with reward-related electrocortical activity: A combined ERP and fMRI study. NeuroImage, 57(4), 1608–1616. 10.1016/j.neuroimage.2011.05.037 [DOI] [PubMed] [Google Scholar]
- Chapman AL, Lynch TR, Rosenthal MZ, Cheavens JS, Smoski MJ, & Krishnan KRR (2007). Risk aversion among depressed older adults with obsessive compulsive personality disorder. Cognitive Therapy and Research, 31(2), 161–174. 10.1007/s10608-006-9114-x [DOI] [Google Scholar]
- Charpentier CJ, Aylward J, Roiser JP, & Robinson OJ (2016). Enhanced risk aversion, but not loss aversion, in unmedicated pathological anxiety. Biological Psychiatry, 81(12), 1014–1022. 10.1016/j.biopsych.2016.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopoulos GI, Tobler PN, & Bossaerts P (2009). Neural correlates of value, risk, and risk aversion contributing to decision making under risk. Journal of Neuroscience, 29(40), 12574–12583. 10.1523/JNEUROSCI.2614-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colder CR, & O’Connor R (2002). Attention bias and disinhibited behavior as predictors of alcohol use and enhancement reasons for drinking. Psychology of Addictive Behaviors, 16(4), 325–332. 10.1037//0893-164X.16.4.325 [DOI] [PubMed] [Google Scholar]
- Crowley MJ, Wu J, Crutcher C, & Bailey CA (2009). Risk-taking and the feedback negativity response to loss among at-risk adolescents. Developmental Neuroscience, 31(1–2), 137–148. 10.1159/000207501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughters SB, Gorka SM, Matusiewicz A, & Anderson K (2013). Gender specific effect of psychological stress and cortisol reactivity on adolescent risk taking. Journal of Abnormal Child Psychology, 41(5), 749–758. 10.1007/s10802-013-9713-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Avakian A, & Markou A (2012). The neurobiology of anhedonia and other reward-related deficits. Trends in Neurosciences, 35(1), 68–77. 10.1016/j.tins.2011.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drechsler R, Rizzo P, & Steinhausen HC (2007). Decision-making on an explicit risk-taking task in preadolescents with attention-deficit/hyperactivity disorder. Journal of Neural Transmission, 115(2), 201–209. 10.1007/s00702-007-0814-5 [DOI] [PubMed] [Google Scholar]
- Dunlop BW, & Nemeroff CB (2007). The role of dopamine in the pathophysiology of depression. Archives of General Psychiatry, 64(3), 327–337. 10.1001/archpsyc.64.3.327 [DOI] [PubMed] [Google Scholar]
- Fein G, & Chang M (2008). Smaller feedback ERN amplitudes during the BART are associated with a greater family history density of alcohol problems in treatment-naïve alcoholics. Drug and Alcohol Dependence, 92(1–3), 141–148. 10.1016/j.drugalcdep.2007.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figner B, & Weber EU (2011). Who takes risks when and why?: Determinants of risk taking. Current Directions in Psychological Science, 20(4), 211–216. 10.1177/0963721411415790 [DOI] [Google Scholar]
- First MB, Williams JBW, Karg RS, & Spitzer RL (2015). Structured Clinical Interview for DSM-5: Research version. Arlington, VA: American Psychiatric Association. [Google Scholar]
- Foti D, Carlson JM, Sauder CL, & Proudfit GH (2014). Reward dysfunction in major depression: Multimodal neuroimaging evidence for refining the melancholic phenotype. NeuroImage, 101, 50–58. 10.1016/j.neuroimage.2014.06.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, & Hajcak G (2009). Depression and reduced sensitivity to non-rewards versus rewards: Evidence from event-related potentials. Biological Psychology, 81(1), 1–8. 10.1016/j.biopsycho.2008.12.004 [DOI] [PubMed] [Google Scholar]
- Foti D, Kotov R, Klein DN, & Hajcak G (2011). Abnormal neural sensitivity to monetary gains versus losses among adolescents at risk for depression. Journal of Abnormal Child Psychology, 39(7), 913–924. 10.1007/s10802-011-9503-9 [DOI] [PubMed] [Google Scholar]
- Furby L, & Beyth-Marom R (1992). Risk taking in adolescence: A decision-making perspective. Developmental Review, 12(1), 1–44. 10.1016/0273-2297(92)90002-J [DOI] [Google Scholar]
- Gorka SM, Lieberman L, Shankman SA, & Phan KL (2017). Startle potentiation to uncertain threat as a psychophysiological indicator of fear-based psychopathology: An examination across multiple internalizing disorders. Journal of Abnormal Psychology, 126(1), 8–18. 10.1037/abn0000233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, Liu H, Klein D, Daughters SB, & Shankman SA (2015). Is risk-taking propensity a familial vulnerability factor for alcohol use? An examination in two independent samples. Journal of Psychiatric Research, 68, 54–60. 10.1016/j.jpsychires.2015.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Hamilton JP, Cooney RE, Singh MK, Henry ML, & Joormann J (2010). Neural processing of reward and loss in girls at risk for major depression. Archives of General Psychiatry, 67(4), 380–387. 10.1001/archgenpsychiatry.2010.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MGH, & Donchin E (1983). A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology, 55(4), 468–484. 10.1016/0013-4694(83)90135-9 [DOI] [PubMed] [Google Scholar]
- Harrison JD, Young JM, Butow P, Salkeld G, & Solomon MJ (2005). Is it worth the risk? A systematic review of instruments that measure risk propensity for use in the health setting. Social Science & Medicine, 60(6), 1385–1396. 10.1016/j.socscimed.2004.07.006 [DOI] [PubMed] [Google Scholar]
- Hasin DS, Goodwin RD, Stinson FS, & Grant BF (2005). Epidemiology of major depressive disorder: Results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Archives of General Psychiatry, 62(10), 1097–1106. 10.1001/archpsyc.62.10.1097 [DOI] [PubMed] [Google Scholar]
- Hewig J, Kretschmer N, Trippe RH, Hecht H, Coles MGH, Holroyd CB, & Miltner WHR (2010). Hypersensitivity to reward in problem gamblers. Biological Psychiatry, 67(8), 781–783. 10.1016/j.biopsych.2009.11.009 [DOI] [PubMed] [Google Scholar]
- Holroyd CB, & Coles MGH (2002). The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychological Review, 109(4), 679–709. 10.1037//0033-295x.109.4.679 [DOI] [PubMed] [Google Scholar]
- Hunt MK, Hopko DR, Bare R, Lejuez CW, & Robinson EV (2005). Construct validity of the balloon analog risk task (BART): Associations with psychopathy and impulsivity. Assessment, 12, 416–428. 10.1177/1073191105278740 [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SCR, Brammer MJ, & Phillips ML (2005). The neural correlates of anhedonia in major depressive disorder. Biological Psychiatry, 58(11), 843–853. 10.1016/j.biopsych.2005.05.019 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, & Walters EE (2005). Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62(6), 617–627. 10.1001/archpsyc.62.6.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Crum RM, Warner LA, Nelson CB, Schulenberg J, & Anthony JC (1997). Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Archives of General Psychiatry, 54(4), 313–321. 10.1001/archpsyc.1997.01830160031005 [DOI] [PubMed] [Google Scholar]
- Killgore WDS, Kamimori GH, & Balkin TJ (2011). Caffeine protects against increased risk-taking propensity during severe sleep deprivation. Journal of Sleep Research, 20(3), 395–403. 10.1111/j.1365-2869.2010.00893.x [DOI] [PubMed] [Google Scholar]
- Kimura K, Kimura M, & Iwaki S (2016). Temporal prediction modulates the evaluative processing of “good” action feedback: An electrophysiological study. Psychophysiology, 53, 1552–1559. 10.1111/psyp.12697 [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, & Hommer D (2001). Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience, 21, 1–5. 10.1523/JNEUROSCI.21-16-j0002.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak MJ, & Cuthbert BN (2016). The NIMH Research Domain Criteria Initiative: Background, issues, and pragmatics. Psychophysiology, 53, 286–297. 10.1111/psyp.12518 [DOI] [PubMed] [Google Scholar]
- Kujawa A, Proudfit GH, & Klein DN (2014). Neural reactivity to rewards and losses in offspring of mothers and fathers with histories of depressive and anxiety disorders. Journal of Abnormal Psychology, 123(2), 287–297. 10.1037/a0036285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejuez CW (2005). Evaluation of behavioral measures of risk taking propensity with inner city adolescents. Behaviour Research and Therapy, 43(2), 215–228. 10.1016/j.brat.2003.12.007 [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Aklin WM, Jones HA, Richards JB, Strong DR, Kahler CW, & Read JP (2003). The balloon analogue risk task (BART) differentiates smokers and nonsmokers. Experimental and Clinical Psychopharmacology, 11, 26–33. 10.1037/1064-1297.11.1.26 [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, … Brown RA (2002). Evaluation of a behavioral measure of risk taking: The balloon analogue risk task (BART). Journal of Experimental Psychology: Applied, 8(2), 75–84. 10.1037//1076-898x.8.2.75 [DOI] [PubMed] [Google Scholar]
- Liu W-H, Wang L-Z, Shang H-R, Shen Y, & Li Z (2014). The influence of anhedonia on feedback negativity in major depressive disorder. Neuropsychologia, 53, 213–220. 10.1016/j.neuropsychologia.2013.11.023 [DOI] [PubMed] [Google Scholar]
- Luck SJ (2005). Ten simple rules for designing and interpreting ERP experiments. In Handy TC (Ed.), Event-related potentials: A methods handbook (pp. 17–32). Cambridge, MA: MIT Press. [Google Scholar]
- MacPherson L, Magidson JF, Reynolds EK, Kahler CW, & Lejuez CW (2010). Changes in sensation seeking and risk-taking propensity predict increases in alcohol use among early adolescents. Alcoholism: Clinical and Experimental Research, 34(8), 1400–1408. 10.1111/j.1530-0277.2010.01223.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews SC, Simmons AN, Lane SD, & Paulus MP (2004). Selective activation of the nucleus accumbens during risk-taking decision making. NeuroReport, 15(13), 2123–2127. 10.1097/00001756-200409150-00025 [DOI] [PubMed] [Google Scholar]
- Matthies S, Philipsen A, & Svaldi J (2012). Risky decision making in adults with ADHD. Journal of Behavior Therapy and Experimental Psychiatry, 43(3), 938–946. 10.1016/j.jbtep.2012.02.002 [DOI] [PubMed] [Google Scholar]
- Meyer A, Lerner MD, De Los Reyes A, Laird RD, & Hajcak G (2017). Considering ERP difference scores as individual difference measures: Issues with subtraction and alternative approaches. Psychophysiology, 54, 114–122. 10.1111/psyp.12664 [DOI] [PubMed] [Google Scholar]
- Miltner W, Braun CH, & Coles M (1997). Event-related brain potentials following incorrect feedback in a time-estimation task: Evidence for a “generic” neural system for error detection. Journal of Cognitive Neuroscience, 9(6), 788–798. 10.1162/jocn.1997.9.6.788 [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. (2004, Winter). National Institute of Alcohol Abuse and Alcoholism Council approves definition of binge drinking. NIAAA Newsletter, 3. Retrieved from https://pubs.niaaa.nih.gov/publications/Newsletter/winter2004/Newsletter_Number3.pdf [Google Scholar]
- Nelson BD, Perlman G, Klein DN, Kotov R, & Hajcak G (2016). Blunted neural response to rewards as a prospective predictor of the development of depression in adolescent girls. American Journal of Psychiatry, 173(12), 1223–1230. 10.1176/appi.ajp.2016.15121524 [DOI] [PubMed] [Google Scholar]
- Nestler EJ, & Carlezon WA Jr (2006). The mesolimbic dopamine reward circuit in depression. Biological Psychiatry, 59(12), 1151–1159. 10.1016/j.biopsych.2005.09.018 [DOI] [PubMed] [Google Scholar]
- Nusslock R, Almeida JR, Forbes EE, Versace A, Frank E, LaBarbara EJ, … Phillips ML (2012). Waiting to win: Elevated striatal and orbitofrontal cortical activity during reward anticipation in euthymic bipolar disorder adults. Bipolar Disorders, 14(3), 249–260. 10.1111/j.1399-5618.2012.01012.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberg SAK, Christie GJ, & Tata MS (2011). Problem gamblers exhibit reward hypersensitivity in medial frontal cortex during gambling. Neuropsychologia, 49(13), 3768–3775. 10.1016/j.neuropsychologia.2011.09.037 [DOI] [PubMed] [Google Scholar]
- Pleskac TJ, Wallsten TS, Wang P, & Lejuez CW (2008). Development of an automatic response mode to improve the clinical utility of sequential risk-taking tasks. Experimental and Clinical Psychopharmacology, 16, 555–564. 10.1037/a0014245 [DOI] [PubMed] [Google Scholar]
- Proudfit GH (2015). The reward positivity: From basic research on reward to a biomarker for depression. Psychophysiology, 52(4), 449–459. 10.1111/psyp.12370 [DOI] [PubMed] [Google Scholar]
- Schultz W (2002). Getting formal with dopamine and reward. Neuron, 36, 241–263. 10.1016/S0896-6273(02)00967-4 [DOI] [PubMed] [Google Scholar]
- Shankman SA, Funkhouser CJ, Klein DN, Davila J, Lerner D, & Hee D (2017). Reliability and validity of severity dimensions of psychopathology assessed using the Structured Clinical Interview for DSM-5 (SCID). International Journal of Methods in Psychiatric Research, e1590. 10.1002/mpr.1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeel RL, Neudecker J, Pilarski C, & Pytlak K (2007). The utility of personality variables and behaviorally-based measures in the prediction of risk-taking behavior. Personality and Individual Differences, 43(1), 203–214. 10.1016/j.paid.2006.11.025 [DOI] [Google Scholar]
- Smoski MJ, Lynch TR, Rosenthal MZ, Cheavens JS, Chapman AL, & Krishnan RR (2008). Decision-making and risk aversion among depressive adults. Journal of Behavior Therapy and Experimental Psychiatry, 39(4), 567–576. 10.1016/j.jbtep.2008.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, & Sobell MB (1992). Timeline follow-back: A technique for assessing self-reported ethanol consumption. In Allen J, & Litten RZ (Eds.), Measuring alcohol consumption: Psychosocial and biological methods (pp. 41–72). Totowa, NJ: Humana Press. [Google Scholar]
- Stautz K, & Cooper A (2013). Impulsivity-related personality traits and adolescent alcohol use: A meta-analytic review. Clinical Psychology Review, 33(4), 574–592. 10.1016/j.cpr.2013.03.003 [DOI] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, & Cowan RL (2012). Dopaminergic mechanisms of individual differences in human effort-based decision-making. Journal of Neuroscience, 32(18), 6170–6176. 10.1523/jneurosci.6459-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay LK, Naranjo CA, Graham SJ, Herrmann N, Mayberg HS, Hevenor S, & Busto UE (2005). Functional neuro-anatomical substrates of altered reward processing in major depressive disorder revealed by a dopaminergic probe. Archives of General Psychiatry, 62(11), 1228–1236. 10.1001/archpsyc.62.11.1228 [DOI] [PubMed] [Google Scholar]
- Ullsperger M, Danielmeier C, & Jocham G (2014). Neurophysiology of performance monitoring and adaptive behavior. Physiological Reviews, 94, 35–79. 10.1152/physrev.00041.2012 [DOI] [PubMed] [Google Scholar]
- Weinberg A, Liu H, Hajcak G, & Shankman SA (2015). Blunted neural response to rewards as a vulnerability factor for depression: Results from a family study. Journal of Abnormal Psychology, 124(4), 878–889. 10.1037/abn0000081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Liu H, & Shankman SA (2016). Blunted neural response to errors as a trait marker of melancholic depression. Biological Psychology, 113, 100–107. 10.1016/j.biopsycho.2015.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Luhmann CC, Bress JN, & Hajcak G (2012). Better late than never? The effect of feedback delay on ERP indices of reward processing. Cognitive, Affective, & Behavioral Neuroscience, 12(4), 671–677. 10.3758/s13415-012-0104-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Riesel A, & Proudfit GH (2014). Show me the money: The impact of actual rewards and losses on the feedback negativity. Brain and Cognition, 87, 134–139. 10.1016/j.bandc.2014.03.015 [DOI] [PubMed] [Google Scholar]
- Yau YHC, Potenza MN, Mayes LC, & Crowley MJ (2015). Blunted feedback processing during risk-taking in adolescents with features of problematic Internet use. Addictive Behaviors, 45, 156–163. 10.1016/j.addbeh.2015.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH, Cowan RL, & Riccardi P (2008). Midbrain dopamine receptor availability is inversely associated with novelty-seeking traits in humans. Journal of Neuroscience, 28(53), 14372–14378. 10.1523/jneurosci.2423-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalta AK, & Shankman SA (2016). Conducting psychopathology prevention research in the RDoC era. Clinical Psychology Science and Practice, 23, 94–104. 10.1111/cpsp.12144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M (2007). Sensation seeking and substance use and abuse: Smoking, drinking, and drugs. In Zuckerman IM (Ed.), Sensation seeking and risky behavior (pp. 107–143). Washington, DC: American Psychological Association. 10.1037/11555-004 [DOI] [Google Scholar]


