Abstract
There has been no effective biomarker for small cell lung cancer (SCLC) patients with first-line immune checkpoint inhibitors (ICIs) treatment. The predictive value of neuron-specific enolase (NSE) in this cohort remains unclear.
The medical records of 254 consecutive SCLC patients receiving programmed cell death receptor-1/programmed cell death-ligand 1 (PD-1/PD-L1) inhibitors were compiled from January 2015 to October 2020 in Chinese PLA General Hospital. Survival analysis was performed to explore the prognostic role of NSE at baseline and 3 weeks post treatment.
One hundred two advanced SCLC patients treated with first-line PD-1/PD-L1 inhibitors were enrolled in this study. Normal baseline NSE levels were correlated with significantly prolonged progression-free survival (PFS, median: 8.7 vs 4.7 months, P = .006) and overall survival (OS, median: 23.8 vs 15.2 months, P = .014) compared with elevated baseline NSE levels, so as for normal NSE levels at 3 weeks with prolonged PFS (median PFS: 8.4 vs 4.5 months, P = .0002) and OS (median OS: 23.3 vs 7.4 months, P < .0001). Intriguingly, elevated NSE levels at 3 weeks were associated with shorter PFS (median PFS: 4.5 vs 5.8 months, P = .04) and OS (median OS: 5.5 vs 14.7 months, P < .0001) compared with normal NSE levels in the elevated baseline NSE subgroup. Most subgroup analyses stratified by clinical characteristics confirmed the prognostic value of baseline NSE level.
Elevated NSE levels at baseline and 3 weeks were associated with worse prognosis in advanced SCLC patients receiving first-line ICIs treatment. NSE level might be applied as a useful prognostic tool for SCLC patients with immunotherapy.
Keywords: first-line, immune checkpoint inhibitor, neuron-specific enolase, prognosis, small cell lung cancer
1. Introduction
Lung cancer is still the leading cause of cancer-related morbidity and mortality worldwide.[1] Small cell lung cancer (SCLC) constitutes a relatively uncommon type (approximately 15%) of lung cancer, characterized by vigorous growth, early metastasis and dismal prognosis.[2,3] SCLC can be stratified into limited disease (LD) and extensive disease (ED) according to the Veteran's Administration Lung Cancer Study Group Staging System, which account for about one-third and two-thirds, respectively.[2,4]
Unlike non-small lung cancer (NSCLC), the options for treating SCLC remain limited. Surgical approach can be proposed in only a small fraction (5%) of SCLC patients, which present with early stage.[5,6] Other unrespectable patients have to receive chemotherapy with or without radiotherapy.[7–9] Despite highly sensitive to traditional chemotherapy and radiotherapy, most patients relapse after several months. The median overall survival (OS) is approximately 10 to 12 months for ED-SCLC and 15 to 20 months for LD-SCLC, respectively.[10–12] As the driver genes of SCLC are still unclear, there have been few advances in the treatment of SCLC until the application of immune checkpoint inhibitors (ICIs) in SCLC patients.
Programmed cell death receptor-1/programmed cell death-ligand 1 (PD-1/PD-L1) inhibitors, as the representation of ICIs, have been demonstrated to improve the prognosis of NSCLC, melanoma, head and neck cancers and other malignancies.[13–16] In recent years, several clinical trials, including IMpower133, CASPIAN and ECOG-ACRIN EA5161, have shown the first-line PD-1/PD-L1 inhibitors treatment could significantly improve progression-free survival (PFS) and OS compared with chemotherapy alone in ED-SCLC.[17–19] These trials demonstrated the patients with ED-SCLC could benefit fromPD-1/PD-L1 inhibitors. Despite the promising results, the high expenditure and potential risk of immunotherapy cannot be neglected. Hence, it is of crucial importance to explore biomarkers to identify SCLC patients getting benefit from PD-1/PD-L1 inhibitors.
PD-L1 expression and tumor mutational burden (TMB) are commonly used biomarkers for patients with PD-1/PD-L1 inhibitors treatment.[20] However, previous studies indicated that only about 10% of SCLC patients were positive with a PD-L1 expression when the cutoff value was 1%.[21,22] In the CheckMate-032 study, the PD-L1 expression was not related to the response of SCLC patients.[19] Although a previous study indicated that TMB might be a biomarker for SCLC treated with nivolumab,[23] the prognostic role of TMB remains unclear in SCLC patients with ICIs treatment. Summarily, up to now, there have been no effective biomarkers that could guide the application of PD-1/PD-L1 inhibitors in SCLC patients. Hence, there is an urgent need to explore effective biomarkers in clinical practice.
Neuron-specific enolase (NSE) is an important neuroendocrine tumor marker routinely used for diagnosis and therapeutic monitoring in SCLC patients. However, the prognostic role of NSE in SCLC remains controversial in previous studies.[24] In addition, there has been no study evaluating the prognostic value of NSE level in SCLC patients treated with first-line PD-1/PD-L1 inhibitors. A previous study indicated that approximately 30% of SCLC patients had a normal NSE level at diagnosis.[25] In this study, we investigated whether NSE level could serve as an effective biomarker to identify SCLC patients getting benefit from the ICIs plus chemotherapy in first-line treatment, which would be easily attainable and cost-effective.
2. Methods
2.1. Study design and patients
This retrospective study was conducted in the First Medical Center of Chinese PLA General Hospital in the real clinical practice setting. The ethical approval was waived as it was a retrospective study without patients’ privacy information. The medical records of 254 consecutive SCLC patients (Stage IIB-IV) receiving PD-1/PD-L1 inhibitors were compiled from January 1, 2015 to October 31, 2020. Among these patients, 102 patients met the including criteria:
-
1.
patients were pathologically diagnosed as SCLC;
-
2.
PD-1/PD-L1 inhibitors combined with chemotherapy were used at first-line treatment;
-
3.
at least 2 cycles of PD-1/PD-L1 inhibitors treatment (generally 6 weeks);
-
4.
serum NSE was measured at baseline (around 5 days);
-
5.
tumor assessment was performed at baseline and 3 weeks later.
2.2. Data collection
Serum NSE levels at baseline and 3 weeks were measured with Access NSE test kits (Roche, Inc, Switzerland) of E601 Immunoassay System with the normal upper limit of 24 ng/mL. Patients’ characteristics at baseline including age, gender, Eastern Cooperative Oncology Group Performance Status (ECOG PS), stage, smoking history and the presence of brain, liver, and bone metastasis were recorded. Treatment response was evaluated every 6 to 8 weeks by 2 investigators (YH and ZZ) independently according to Response Evaluation Criteria in Solid Tumors criteria version 1.1,[26] including complete response, partial response, stable disease (SD), and progressive disease (PD). PFS was referred to the interval time from the start of PD-1/PD-L1 inhibitors until PD, death, or the last follow-up (censored). OS was referred to the interval time from PD-1/PD-L1 inhibitors initiation until death or the last follow-up (censored). All patients were followed up by counseling telephone and searching electronic medical records with the cut-off date of March 20, 2021.
2.3. Statistical analysis
Statistical analysis was performed with IBM SPSS 23.0, and graphs were drawn with GraphPad Prism 8.0. The cohort was divided into 2 groups according to NSE level with a cutoff value of 24 ng/mL. Categorical variables were compared by the Chi-Squared test. Survival estimates were calculated by the Kaplan–Meier method, and group differences were compared by log-rank test. Univariate and multivariate analyses were applied for identifying independent variables. Hazard ratio (HR) with its 95% confidence interval (CI) was determined by Cox proportional hazard regression model. All statistical tests were two-sided, and P values <.05 were considered as statistically significant.
3. Results
3.1. Patient characteristics
A total of 102 advanced SCLC with first-line ICIs treatment were included in this study. The detailed information was displayed in Table 1, the median age was 60 years with a range of 32 to 82 years; about 90% were male and 80% had a smoking history; most of the patients (96%) had an ECOG PS of 0 to 1; 75 patients (73.5%) had extensive-stage disease (ED), and 66 patients (64.7%) received PD-1 inhibitors; the presence of brain metastasis, liver metastasis, and bone metastasis accounted for 21.6%, 23.5% and 28.4%, respectively; more than half of patients (59.8%) were evaluated as partial response, 31.4% were SD, and 8.8% were PD; 52.9% of patients had elevated NSE levels (NSE ≥ 24ng/mL) at baseline, and 22.5% still had elevated NSE levels at 3 weeks after the first ICIs treatment. The median level of baseline NSE was 29.5 ng/mL with a range of 5.9 to 1333.0 ng/mL. The median follow-up time was 19.2 months with 95%CI of 13.7 to 24.7 months.
Table 1.
Characteristics of patients with advanced SCLC.
| Characteristics | No. of patients (n = 102) | Percentage (%) |
| Age (yr), median (range) | 60 (32–82) | |
| <60 | 50 | 49.0 |
| ≥60 | 52 | 51.0 |
| Sex | ||
| Male | 90 | 88.2 |
| Female | 12 | 11.8 |
| ECOG PS | ||
| 0–1 | 96 | 94.1 |
| ≥2 | 6 | 5.9 |
| Stage | ||
| LD | 27 | 26.5 |
| ED | 75 | 73.5 |
| Smoking history | ||
| Never smoke | 21 | 20.6 |
| Smoke | 81 | 79.4 |
| ICIs | ||
| PD-1 inhibitors | 66 | 64.7 |
| PD-L1 inhibitors | 36 | 35.3 |
| Brain metastasis | ||
| Yes | 22 | 21.6 |
| No | 80 | 78.4 |
| Liver metastasis | ||
| Yes | 24 | 23.5 |
| No | 78 | 76.5 |
| Bone metastasis | ||
| Yes | 29 | 28.4 |
| No | 73 | 71.6 |
| Best response | ||
| PR | 61 | 59.8 |
| SD | 32 | 31.4 |
| PD | 9 | 8.8 |
| NSE at baseline (ng/mL) | ||
| Median (range) | 29.5 (5.9–1333.0) | |
| Normal (<24) | 48 | 47.1 |
| Elevated (≥24) | 54 | 52.9 |
| NSE levels at 3 weeks (ng/mL) | ||
| Median (range) | 15.0 (5.9–694.1) | |
| Normal (<24) | 70 | 68.6 |
| Elevated (≥24) | 23 | 22.5 |
| Unknown | 9 | 8.8 |
3.2. Univariate and multivariate analysis of progression-free survival and overall survival
In terms of PFS, the univariate analysis indicated that ECOG PS ≥2, the presence of bone metastasis and elevated baseline NSE levels were correlated with shorter PFS with all P < .05, and the multivariate analysis showed that ECOG PS ≥2 (HR: 2.66; 95%CI, 1.13–6.24; P = .025), bone metastasis (HR: 2.75; 95% CI, 1.62–4.67; P < .001) and elevated baseline NSE levels (HR: 1.93; 95%CI, 1.18–3.17; P = .009) were independently associated with worse PFS (Table 2). In terms of OS, the univariate analysis showed that ECOG PS 0–1, LD, PD-1 inhibitors, liver metastasis, bone metastasis and normal baseline NSE levels were correlated with better OS with all P < .05, and multivariate analysis demonstrated that ECOG PS ≥ 2 (HR: 6.06; 95% CI, 1.99–18.44; P = .002), liver metastasis (HR: 2.92; 95% CI, 1.30–6.59; P = .01), bone metastasis (HR: 4.59; 95%CI, 2.06–10.22; P < .001) and elevated NSE levels (HR: 2.41; 95% CI, 1.14–5.10; P = .021) were independent risk factors for OS (Table 2). In summary, elevated baseline NSE level was an independent risk factor for PFS (HR: 1.93) and OS (HR: 2.41) with all P < .05.
Table 2.
Univariate and Multivariate Analysis for PFS and OS.
| PFS | OS | ||||||||
| Variable | Category | Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | ||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95%CI) | P value | HR (95% CI) | P value | ||
| Age (yr) | ≥60 vs < 60 | 1.12 (0.70, 1.80) | .63 | 1.24 (0.66, 2.32) | .507 | ||||
| Sex | Female vs Male | 1.26 (0.63, 2.55) | .52 | 0.34 (0.08, 1.40) | .133 | ||||
| Smoking history | Yes vs No | 0.58 (0.33, 1.03) | .06 | 1.73 (0.67, 4.42) | .255 | ||||
| Agent | PD-L1 inhibitors vs PD-1 inhibitors | 1.29 (0.79, 2.10) | .31 | 1.97 (1.01, 3.84) | .048 | 2.01 (0.95, 4.23) | .066 | ||
| Stage | ED vs LD | 1.05 (0.61, 1.79) | .87 | 3.49 (1.24, 9.84) | .018 | 1.33 (0.41, 4.33) | .639 | ||
| ECOG PS | ≥ 2 vs 0–1 | 2.74 (1.17, 6.39) | .02 | 2.66 (1.13, 6.24) | 0.025 | 6.29 (2.59, 15.30) | <.001 | 6.06 (1.99, 18.44) | .002 |
| Brain metastasis | Yes vs No | 1.13 (0.64, 1.97) | .68 | 1.84 (0.91, 3.71) | .089 | ||||
| Liver metastasis | Yes vs No | 1.57 (0.92, 2.69) | .10 | 4.62 (2.43, 8.80) | <.001 | 2.92 (1.30, 6.59) | .01 | ||
| Bone metastasis | Yes vs No | 2.86 (1.70, 4.80) | <.001 | 2.75 (1.62, 4.67) | <0.001 | 5.53 (2.86, 10.68) | <.001 | 4.59 (2.06, 10.22) | <.001 |
| Baseline NSE (ng/mL) | ≥24 vs <24 | 1.95 (1.20, 3.16) | .007 | 1.93 (1.18, 3.17) | 0.009 | 2.19 (1.15, 4.16) | .017 | 2.41 (1.14, 5.10) | .021 |
3.3. Association of neuron-specific enolase levels at baseline and 3 weeks with progression-free survival and overall survival
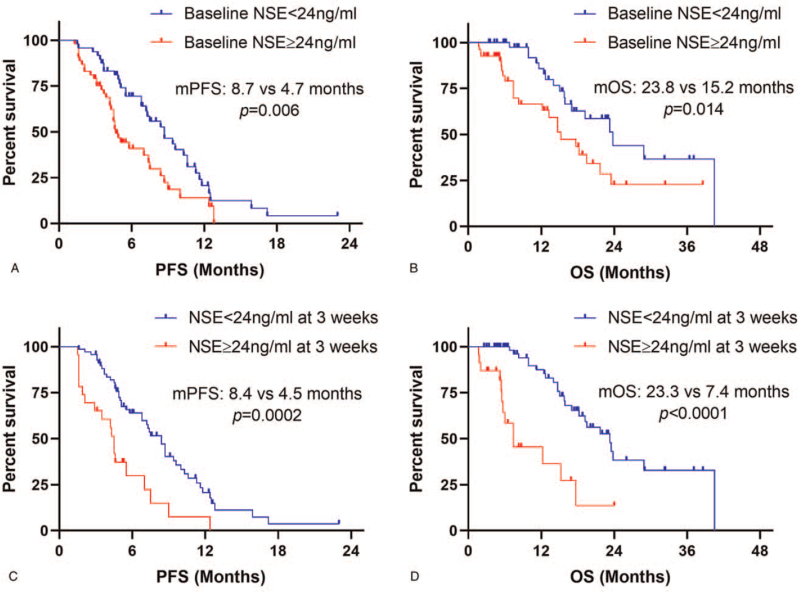
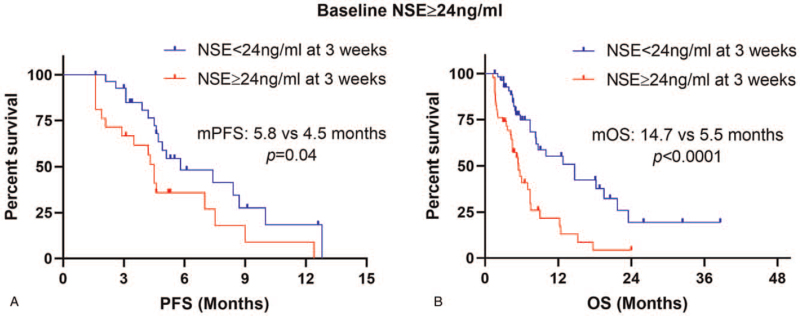
A total of 102 patients were included for baseline NSE analysis. There was no statistically significant difference in patients’ characteristics between the normal baseline NSE group and elevated NSE group with all P > .05 (Table 3). The results of Kaplan–Meier survival curves showed that normal baseline NSE levels were correlated with significantly prolonged PFS (median PFS: 8.7 vs 4.7 months, P = .006) and OS (median OS: 23.8 vs 15.2 months, P = .014) compared with elevated baseline NSE levels (Fig. 1a - b). After 3 weeks’ ICIs treatment, 93 patients (91.2%) examined the blood tests of NSE levels. Kaplan–Meier survival curves demonstrated that normal NSE levels at 3 weeks were also correlated with significantly prolonged PFS (median PFS: 8.4 vs 4.5 months, P = .0002) and OS (median OS: 23.3 vs 7.4 months, P < .0001) compared with elevated NSE levels (Fig. 1c-d). Further, we conducted subgroup analysis in patients with elevated baseline NSE levels. The results showed that patients with elevated NSE levels at 3 weeks had shorter PFS (median PFS: 4.5 vs 5.8 months, P = .04) and OS (median OS: 5.5 vs 14.7 months, P < .0001) than those with normal NSE levels in the elevated baseline NSE subgroup (Fig. 2a-b).
Table 3.
The differences of patients’ characteristics between normal and elevated baseline NSE levels.
| Baseline NSE levels | ||||
| Characteristics | Normal | Elevated | X 2 | P value |
| Age (yr) | ||||
| <60 | 28 | 22 | 3.15 | 0.11 |
| ≥60 | 20 | 32 | ||
| Sex | ||||
| Male | 40 | 50 | 2.10 | 0.22 |
| Female | 8 | 4 | ||
| ECOG PS | ||||
| 0–1 | 47 | 49 | 2.36 | 0.21 |
| ≥ 2 | 1 | 5 | ||
| Stage | ||||
| LD | 16 | 11 | 2.19 | 0.18 |
| ED | 32 | 43 | ||
| Smoke | ||||
| Never smoke | 9 | 12 | 0.19 | 0.81 |
| Smoke | 39 | 42 | ||
| ICIs | ||||
| PD-1 inhibitors | 34 | 32 | 1.49 | 0.30 |
| PD-L1 inhibitors | 14 | 22 | ||
| Brain metastasis | ||||
| Yes | 11 | 11 | 0.10 | 0.81 |
| No | 37 | 43 | ||
| Liver metastasis | ||||
| Yes | 8 | 16 | 2.37 | 0.16 |
| No | 40 | 38 | ||
| Bone metastasis | ||||
| Yes | 9 | 20 | 4.18 | 0.05 |
| No | 39 | 34 | ||
Figure 1.
NSE levels at baseline and 3 weeks associated with PFS and OS in SCLC patients receiving first-line PD-1/PD-L1 inhibitors. NSE levels at baseline were associated with PFS (a) and OS (b). NSE levels at 3 weeks were associated with PFS (c) and OS (d). NSE = neuron-specific enolase, PFS = progression-free survival, OS = overall survival.
Figure 2.
NSE levels at 3 weeks associated with PFS (a) and OS (b) in elevated baseline NSE subgroup. NSE = neuron-specific enolase, PFS = progression-free survival, OS = overall survival.
3.4. Subgroup analysis of association between baseline neuron-specific enolase and survival time
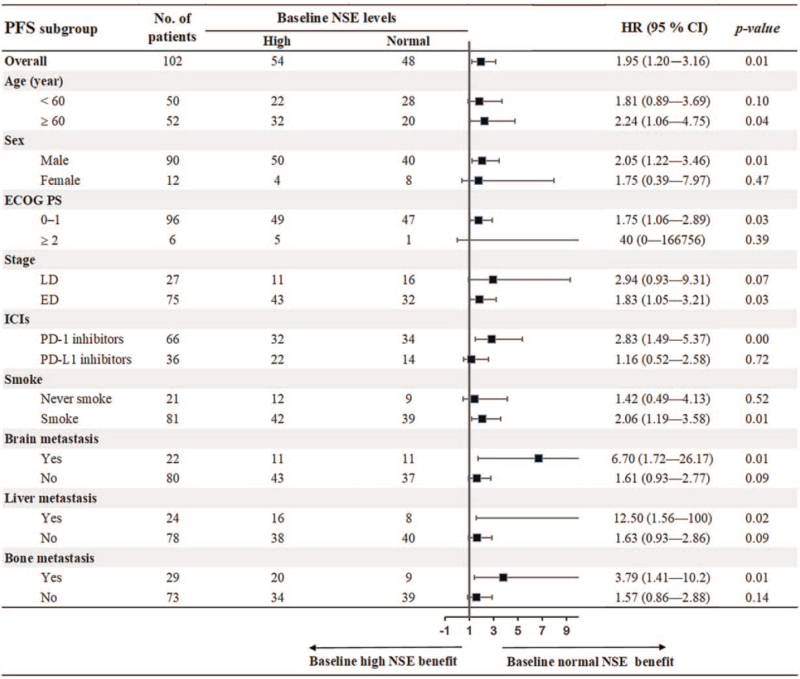
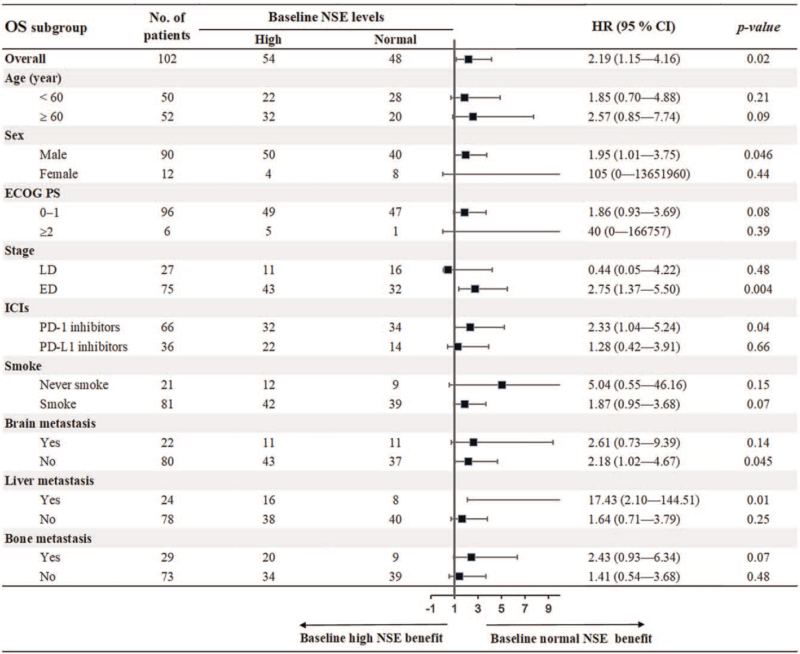
To further evaluate the prognostic value of baseline NSE, we conducted subgroup analysis stratified by patients’ characteristics. As shown in Figure 3, the results demonstrated that baseline normal NSE was associated with prolonged PFS compared with baseline elevated NSE in most of subgroups, including age ≥60 years, male, ECOG PS 0–1, ED, PD-1 inhibitors, smokers, and the presence of brain, liver, and bone metastasis with all P < .05. For OS, the results showed that baseline normal NSE was correlated with better OS in subgroups of male, ED, PD-1 inhibitors, no brain metastasis and the presence of liver metastasis with all P < .05 (Fig. 4).
Figure 3.
Subgroup analysis of association between baseline NSE and PFS. NSE = neuron-specific enolase, PFS = progression-free survival.
Figure 4.
Subgroup analysis of association between baseline NSE and OS. NSE = neuron-specific enolase, OS = overall survival.
4. Discussion
The treatment advances for SCLC patients were stagnant for nearly 3 decades until the application of PD-1/PD-L1 inhibitors brought new hope to advanced SCLC patients.[27] However, not all SCLC patients could get benefit from the first-line ICIs treatment. Disappointingly, there has been no effective biomarker to identify SCLC patients likely to get benefit from ICIs. The prognostic role of PD-L1 expression and TMB in SCLC patients receiving ICIs treatment remains unclear. Therefore, it is urgently needed to explore additional prognostic biomarkers for these patients in the clinical practice.
The detection of serum tumor markers is routinely used in the clinic, which is more convenient and affordable compared with PD-L1 expression and TMB. As a relatively specific tumor marker for SCLC patients, NSE was reported previously to be an independent prognostic indicator for OS in both LD-SCLC patients and ED-SCLC patients.[28,29] However, there has been no research investigating its prognostic value in SCLC patients with first-line ICIs treatment. Hence, we conducted this study to determine whether NSE level could predict the prognosis of SCLC patients with first-line ICIs treatment.
Our data showed that elevated NSE level at baseline was correlated with worse PFS and OS in SCLC patients with first-line ICIs treatment. Previous studies also indicated that high NSE level was correlated with shorter OS in SCLC patients receiving traditional chemotherapy with or without radiotherapy.[28–34] Although the definite underlying mechanisms of the relationship between high NSE level and poor prognosis remain unclear, an experimental study demonstrated that the knockdown of NSE restrained the migration and proliferation of SCLC cells with downregulated pro-metastatic gene vascular endothelial growth factor and upregulated metastasis suppressor genes.[35] We speculated that there might be a difference in the tumor immune microenvironment or transcriptome between SCLC patients with elevated NSE levels and normal NSE levels. Therefore, related research needs to be conducted in the future to figure out the mechanisms for the relationship between high NSE levels and poor prognosis. Multivariate analysis of OS also demonstrated that elevated NSE level was an independent risk factor in addition to ECOG PS ≥2, liver metastasis, and bone metastasis. Optimization-based method or propensity score matching was intended to be used like our previous study to balance the baseline covariates between the elevated NSE group and normal NSE group.[36,37] However, as the baseline covariates were comparable between the 2 groups, these methods were waived. Taken together, elevated NSE level at baseline was associated with dismal prognosis of SCLC patients with first-line ICIs treatment.
We explored the predictive value of baseline NSE level by subgroup analysis. PFS and OS between baseline NSE high group and normal group were statistically significant different in the PD-1 subgroup, but not for the PD-L1 subgroup. Several reasons should be taken into consideration. Firstly, for the retrospective nature, it is inevitable to have selective bias. Secondly, the sample size of the PD-L1 subgroup was relatively small, which only have 36 patients. Lastly, anti-tumor mechanisms of PD-1 inhibitor and PD-L1 inhibitor were different, which may lead to the different anti-tumor effect.
We also investigated the prognostic role of the NSE level at 3 weeks post initial treatment. Most patients had a decreased NSE level at 3 weeks than the baseline value. Elevated NSE level at 3 weeks was also correlated with shorter PFS and OS compared with normal NSE level. Intriguingly, we wondered if there was a survival difference between patients with high NSE level at 3 weeks and patients with normal NSE level at 3 weeks in the baseline elevated NSE subgroup. Surprisingly, there was a significant difference in PFS and OS between these 2 groups, suggesting the strong prognostic power of NSE level in the SCLC patients. We did not perform the survival analysis in the baseline NSE normal subgroup, as there was only one patient who had an elevated NSE level at 3 weeks in the baseline NSE normal group. Summarily, the NSE levels at 3 weeks were also correlated with worse PFS and OS, and the patients with elevated baseline NSE level could still benefit from the ICIs treatment if serum NSE decreased to normal level at 3 weeks.
To our knowledge, this is the first study to provide survival data of SCLC patients receiving first-line ICIs treatment in the real world and thoroughly examine the prognostic role of NSE level. However, there remain some limitations in this study. Firstly, for a single-center retrospective study with limited cases, these results need to be validated in the multi-center prospective study. Secondly, the cutoff value of NSE level in survival analysis was set at 24 ng/mL (normal upper limit of the reference range), which might be not optimal. Last but not least, related research still needs to be performed in the future to explain the definite reasons for the relationship between high NSE levels and poor prognosis. However, we proposed a simple and effective tool for physicians to guide the application of first-line PD-1/PD-L1 inhibitors in advanced SCLC patients.
5. Conclusions
Our study showed that elevated NSE levels at baseline and 3 weeks were correlated with worse clinical outcomes in advanced SCLC patients receiving PD-1/PD-L1 inhibitors at first-line treatment. NSE level might serve as a useful prognostic factor for patients with immunotherapy.
Acknowledgments
Thanks for Zhiyue Huang's professional statistical consultation.
Author contributions
Conceptualization: Lingling Li, Zhibo Zhang.
Data curation: Yi Hu.
Formal analysis: Lingling Li, Zhibo Zhang.
Funding acquisition: Yi Hu.
Investigation: Yi Hu.
Methodology: Lingling Li.
Project administration: Yi Hu.
Resources: Yi Hu.
Software: Lingling Li, Zhibo Zhang.
Supervision: Yi Hu.
Visualization: Lingling Li, Zhibo Zhang.
Writing – original draft: Lingling Li.
Writing – review & editing: Zhibo Zhang, Yi Hu.
Footnotes
Abbreviations: CI = confidence interval, ECOG PS = Eastern Cooperative Oncology Group Performance Status, ED = extensive disease, HR = hazard ratio, ICIs = immune checkpoint inhibitors, LD = limited disease, NSCLC = non-small cell lung cancer, NSE = neuron-specific enolase, OS = overall survival, PD = progressive disease, PD-1 = programmed cell death receptor-1, PD-L1 = programmed cell death-ligand 1, PFS = progression-free survival, SCLC = small cell lung cancer, TMB = tumor mutational burden.
How to cite this article: Li L, Zhang Z, Hu Y. Neuron - specific enolase predicts the prognosis in advanced small cell lung cancer patients treated with first-line PD-1/PD-L1 inhibitors. Medicine. 2021;100:36(e27029).
This work was supported by the Military Health Special Research Project Under Grant 20BJZ37.
Informed consent was obtained from all individual participants included in the study.
All the authors and participants agree to publish the data.
The ethics approval was waived, as this is a retrospective study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the national research committee and with the 1964 Helsinki declaration and its later amendments.
The authors have no conflicts of interests to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
ECOG PS = Eastern Cooperative Oncology Group Performance Status, ED = extensive disease, ICI = immune checkpoint inhibitor, LD = limited disease, NSE = neuron-specific enolase, PD = progressive disease, PD-1 = programmed cell death-1, PD-L1 = programmed cell death-ligand 1, PR = partial response, SD = steady disease.
CI = confidence interval, ECOG PS = Eastern Cooperative Oncology Group Performance Status, ED = extensive disease, HR = hazard ratio, ICI = immune checkpoint inhibitor, LD = limited disease, NSE = neuron-specific enolase, PD-1 = programmed cell death-1, PD-L1 = programmed cell death-ligand 1.
CI = confidence interval, ECOG PS = Eastern Cooperative Oncology Group Performance Status, ED = extensive disease, HR = hazard ratio, ICI = immune checkpoint inhibitor, LD = limited disease, NSE = neuron-specific enolase, PD-1 = programmed cell death-1, PD-L1 = programmed cell death-ligand 1.
References
- [1].Bray F, Ferlay J, Soerjomataram I, Siegel R, Torre L, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [2].Oronsky B, Reid T, Oronsky A, Carter C. What's new in SCLC? A review. Neoplasia 2017;19:842–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Simon GR, Turrisi A. Management of small cell lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). CHEST8308 2007;132:324s–39s. [DOI] [PubMed] [Google Scholar]
- [4].Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 2006;24:4539–44. [DOI] [PubMed] [Google Scholar]
- [5].Yu JB, Decker RH, Detterbeck FC, Wilson LD. Surveillance epidemiology and end results evaluation of the role of surgery for stage I small cell lung cancer. J Thorac Oncol 2010;5:215–9. [DOI] [PubMed] [Google Scholar]
- [6].Shepherd FA, Crowley J, Van Houtte P, et al. The International Association for the Study of Lung Cancer lung cancer staging project: proposals regarding the clinical staging of small cell lung cancer in the forthcoming (seventh) edition of the tumor, node, metastasis classification for lung cancer. J Thorac Oncol 2007;2:1067–77. [DOI] [PubMed] [Google Scholar]
- [7].Pietanza MC, Byers LA, Minna JD, Rudin CM. Small cell lung cancer: will recent progress lead to improved outcomes? Clin Cancer Res 2015;21:2244–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Früh M, De Ruysscher D, Popat S, Crinò L, Peters S, Felip E. Small-cell lung cancer (SCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24: Suppl 6: vi99–105. [DOI] [PubMed] [Google Scholar]
- [9].Rudin CM, Ismaila N, Hann CL, et al. Treatment of small-cell lung cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians Guideline. J Clin Oncol 2015;33:4106–11. [DOI] [PubMed] [Google Scholar]
- [10].Bernhardt EB, Jalal SI. Small cell lung cancer. Cancer Treat Res 2016;170:301–22. [DOI] [PubMed] [Google Scholar]
- [11].Farago A, Keane F. Current standards for clinical management of small cell lung cancer. Translat Lung Cancer Res 2018;7:69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rossi A, Di Maio M, Chiodini P, et al. Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: the COCIS meta-analysis of individual patient data. J Clin Oncol 2012;30:1692–8. [DOI] [PubMed] [Google Scholar]
- [13].Brahmer J, Reckamp K, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Eng J Med 2015;373:123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Eng J Med 2015;373:1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bagchi S, Yuan R, Engleman E. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Ann Rev Pathol 2021;16:223–49. [DOI] [PubMed] [Google Scholar]
- [16].Mok T, Wu Y, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet (London, England) 2019;393:1819–30. [DOI] [PubMed] [Google Scholar]
- [17].Horn L, Mansfield A, Szczęsna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Eng J Med 2018;379:2220–9. [DOI] [PubMed] [Google Scholar]
- [18].Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet (London, England) 2019;394:1929–39. [DOI] [PubMed] [Google Scholar]
- [19].Ready N, Ott P, Hellmann M, et al. Nivolumab monotherapy and nivolumab plus ipilimumab in recurrent small cell lung cancer: results from the checkmate 032 randomized cohort. J Thoracic Oncol 2020;15:426–35. [DOI] [PubMed] [Google Scholar]
- [20].Memmott R, Wolfe A, Carbone D, Williams T. Predictors of response, progression free survival, and overall survival in lung cancer patients treated with immune checkpoint inhibitors. J Thorac Oncol 2021;doi:10.1016/j.jtho.2021.03.017. [DOI] [PubMed] [Google Scholar]
- [21].Zhao X, Kallakury B, Chahine J, et al. Surgical resection of SCLC: prognostic factors and the tumor microenvironment. J Thorac Oncol 2019;14:914–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Carvajal-Hausdorf D, Altan M, Velcheti V, et al. Expression and clinical significance of PD-L1, B7-H3, B7-H4 and TILs in human small cell lung Cancer (SCLC). J Immuno Therapy Cancer 2019;7:65.doi:10.1186/s40425-019-0540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hellmann MD, Callahan MK, Awad MM, et al. Tumor mutational burden and efficacy of nivolumab monotherapy and in combination with ipilimumab in small-cell lung cancer. Cancer Cell 2018;33:853–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tian Z, Liang C, Zhang Z, et al. Prognostic value of neuron-specific enolase for small cell lung cancer: a systematic review and meta-analysis. World J Surg Oncol 2020;18:116.doi:10.1186/s12957-020-01894-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Quoix E, Purohit A, Faller-Beau M, Moreau L, Oster JP, Pauli G. Comparative prognostic value of lactate dehydrogenase and neuron-specific enolase in small-cell lung cancer patients treated with platinum-based chemotherapy. Lung Cancer 2000;30:127–34. [DOI] [PubMed] [Google Scholar]
- [26].Eisenhauer E, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- [27].Facchinetti F, Di Maio M, Tiseo M. Adding PD-1/PD-L1 inhibitors to chemotherapy for the first-line treatment of extensive stage small cell lung cancer (SCLC): a meta-analysis of randomized trials. Cancers6126 2020;12: doi:10.3390/cancers12092645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zhou M, Wang Z, Yao Y, Zhou H, Liu M, Sun J. Neuron-specific enolase and response to initial therapy are important prognostic factors in patients with small cell lung cancer. Clin Transl Oncol 2017;19:865–73. [DOI] [PubMed] [Google Scholar]
- [29].Liu X, Zhang W, Yin W, et al. The prognostic value of the serum neuron specific enolase and lactate dehydrogenase in small cell lung cancer patients receiving first-line platinum-based chemotherapy. Medicine (Baltimore) 2017;96:e8258.doi:10.1097/md.0000000000008258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhang C, Jia Y, Jia Y, Zhang X, Li K. Prognostic and predictive value of plasma D-dimer levels in patients with small-cell lung cancer. Int J Clin Oncol 2018;23:1070–5. [DOI] [PubMed] [Google Scholar]
- [31].Jiang X, Mei X, Wu H, Chen X. D-dimer level is related to the prognosis of patients with small cell lung cancer. Ann Translat Med 2017;5:394.doi:10.21037/atm.2017.07.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wojcik E, Tarapacz J, Rychlik U, et al. Human epididymis protein 4 (HE4) in patients with small-cell lung cancer. Clin Laboratory 2016;62:1625–32. [DOI] [PubMed] [Google Scholar]
- [33].van der Gaast A, van Putten WL, Oosterom R, Cozijnsen M, Hoekstra R, Splinter TA. Prognostic value of serum thymidine kinase, tissue polypeptide antigen and neuron specific enolase in patients with small cell lung cancer. Br J Cancer 1991;64:369–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Fan S, Zhao G, An G. High pretreatment plasma D-dimer levels are associated with shorter overall survival in patients with small cell lung cancer. J Int Med Res 2019;47:215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Liu X, Liu S, Fu J, et al. Knockdown of neuron-specific enolase suppresses the proliferation and migration of NCI-H209 cells. Oncol Lett 2019;18:4809–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zhang Z, Zhang F, Yuan F, et al. Pretreatment hemoglobin level as a predictor to evaluate the efficacy of immune checkpoint inhibitors in patients with advanced non-small cell lung cancer. Therapeutic Adv Med Oncol 2020;12:1758835920970049.doi:10.1177/1758835920970049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhang Z, Yuan F, Chen R, et al. Dynamics of serum tumor markers can srve as a prognostic biomarker for Chinese advanced non-small cell lung cancer patients treated with immune checkpoint inhibitors. Frontiers Immunol 2020;11:1173.doi:10.3389/fimmu.2020.01173. [DOI] [PMC free article] [PubMed] [Google Scholar]