Abstract
To investigate the usefulness of 123I-BMIPP/201TlCl scintigraphy for evaluating the presence of myocarditis in patients with polymyositis (PM) or dermatomyositis (DM).
We performed a retrospective study of 26 patients diagnosed with new-onset active PM/DM who underwent 123I-BMIPP/201TlCl scintigraphy between 01 April 2010 and 20 March 2015. We determined the 123I-BMIPP/201TlCl ratio and grouped the patients according to presence or absence of a mismatch. We evaluated the relationship between mismatch and the laboratory and echocardiographic findings.
Mismatch was found in 13 (50%) patients. There was no statistically significant difference in age, cardiac troponin T, myoglobin, myosin light chain, aldolase levels, E wave/A wave ratio, right ventricular systolic pressure between the mismatch and non-mismatch groups. Left ventricular end-diastolic and end-systolic dimensions were significantly greater in the mismatch group (45.0 vs 42.5 mm, P = < .01 and 29.5 mm vs 25.0 mm, P < .01). Left ventricular ejection fraction was significantly lower in the mismatch group (63.5% vs 71.5%, P = .04). Significant inverse correlation (r = −0.44, P = .03) was observed between left ventricular ejection fraction and mismatch ratio.
The use of 123I-BMIPP/ 201TlCl scintigraphy may be considered for evaluating myocarditis in patients with PM/DM.
Keywords: 123I-BMIPP/201TlCl scintigraphy, dermatomyositis, mismatch, polymyositis
1. Introduction
Polymyositis (PM) and dermatomyositis (DM) are inflammatory autoimmune disease.[1] Involvement of skeletal and smooth muscles occurs throughout the body causing myopathies.[1] Cardiac involvement is an important risk factor for death in patients with PM/DM due to increased risk of cardiovascular events such as myocardial infarction.[2–4] The incidence of cardiac involvement has been reported to be between 9% and 72%.[5] Therefore, evaluation of myocardial function in patients with PM/DM is important.
In PM/DM, myocardial function is assessed by echocardiography, which evaluates for cardiac abnormality; more specifically for left ventricular and right ventricular systolic dysfunctions that have been reported in new onset PM/DM patients.[6] Left ventricular diastolic dysfunction can also occur in patients with cardiac involvement.[7] Echocardiography is used for serial monitoring of global and regional left ventricular function after ischemia,but, there are limitation for ischemic memory.[8]
Myocardial damage from past ischemic events (ischemic memory) can be evaluated using nuclear cardiac imaging that incorporates 123I-BMIPP (which reflects fatty acid metabolism) and 201TlCl (which reflects myocardial perfusion).[9]123I-BMIPP accumulates in ischemic areas are few, with delayed recovery.[8] Therefore, 123I-BMIPP single photon emission computed tomography will identify ischemic areas as low accumulation (defect) area and can be used to evaluate ischemic memory.[8] In contrast, in the ischemic environment created by vascular spasm, coronary blood flow is preserved and 201TlCl accumulation does not change. The utility of 123I-BMIPP/201TlCl mismatch for the diagnosis of coronary artery disease and as a predictor of cardiac events.[10] And the utility of 123I-BMIPP/201TlCl scintigraphy was reported in patients with dilated cardiomyopathy.[11] Despite the increased risk of myocardial damage in patients with PM/DM, there are few reports on the use of myocardial scintigraphy to identify ischemic memory in this group of patients. We hypothesize that a mismatch between 123I-BMIPP and 201TlCl uptake will be observed in patients with PM/DM. We therefore undertook a retrospective study to evaluate the hypothesis that a mismatch between 123I-BMIPP and 201TlCl uptake is seen in PM/DM, and to evaluate its usefulness in detecting myocarditis in these patients.
2. Material and methods
2.1. Patients
We checked the collagen disease patients who received the double-nuclide myocardial scintigraphy with 123I-BMIPP and 201TlCl between April 2010 and March 2015. And we selected a total of 26 consecutive patients with PM or DM who had undergone double-nuclide myocardial scintigraphy with 123I-BMIPP and 201TlCl between April 2010 and March 2015. In all cases, the diagnosis was new-onset active PM or DM, and had not received treatment prior to the study period.
2.2. Scintigraphy
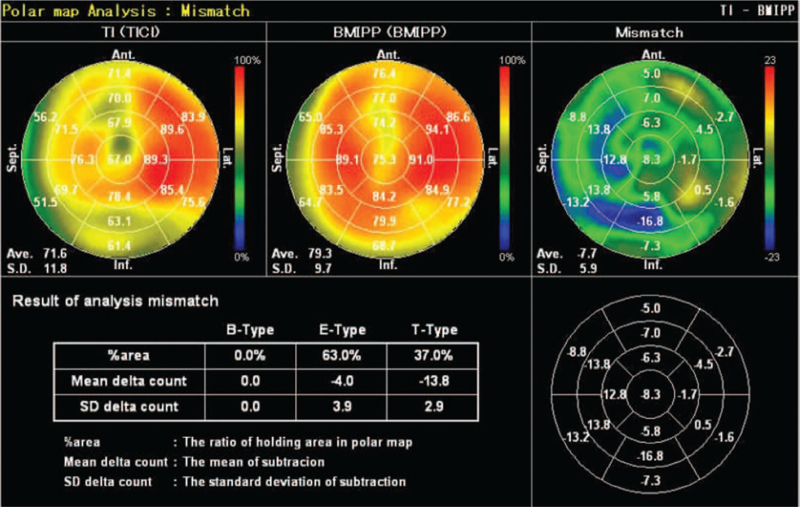
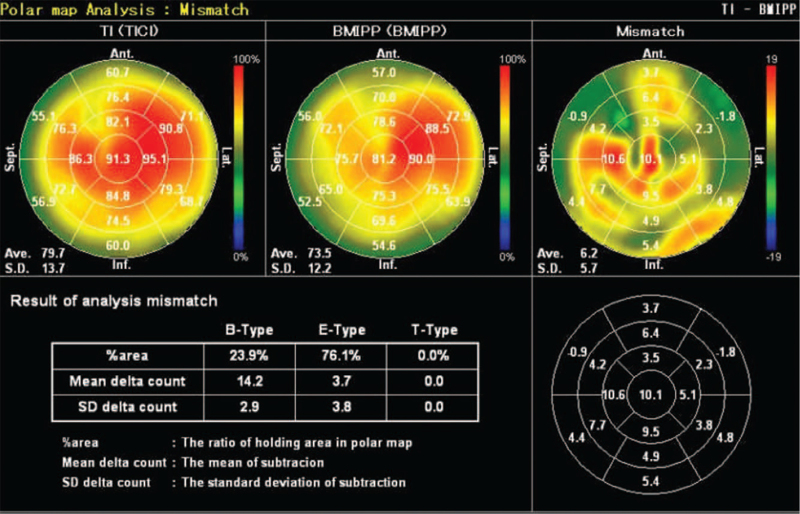
All patients underwent 123I-BMIPP/201TlCl myocardial scintigraphy. Intravenous administration of 111 to 148 MBq of 123I-BMIPP was followed by 74 MBq of 201TlCl. Accumulation of the 2 tracers was measured simultaneously during imaging: 123I-BMIPP with an ECAM (Canon Medical Systems, Otawara, Japan; imaging speed, 18 cm/min; matrix, 256 × 1024) scintillation camera, and 201TlCl with a GXA-7200 (Canon Medical Systems; imaging speed, 17.5 cm/min; matrix, 256 × 1024) scintillation camera. Risk estimation software (Heart Risk View, Nihon Medi-Physics Co. Ltd, Tokyo, Japan; AZE Co. Ltd, Tokyo, Japan) was then used to quantify the radioactivity and degree of mismatch. Mismatch was reported as the ratio of 123I-BMIPP to 201TlCl. Patients were grouped according to the presence or absence of mismatch. Representative images of absence and presence of mismatch are shown in Figures 1 and 2.
Figure 1.
123I-BMIPP/201TlCl scintigraphy images reveal no mismatch in 123I-BMIPP and 201TlCl uptake.
Figure 2.
123I-BMIPP/201TlCl scintigraphy images reveal mismatch in 123I-BMIPP and 201TlCl uptake.
2.3. Echocardiography
Of the 26 patients, 24 underwent echocardiography, and scintigraphy was performed at a median of 4.5 days (range, 1–30 days) later. The information collected include left ventricular end-diastolic dimension, left ventricular end-systolic dimension, left ventricular ejection fraction, E wave (early diastole)/A wave (peak velocity flow in late diastole caused by atrial contraction) ratio, and right ventricular systolic pressure.
2.4. Laboratory tests
The laboratory tests of serum samples and the median interval between scintigraphy and each test were as follows: cardiac troponin T assay (n = 19), 7 days (range, 2–21); myoglobin assay (n = 17), 7 days (range, 0–20); myosin light-chain assay (n = 23), 7 days (range, 1–30); aldolase assay (n = 21), 7 days (range, 0–24).
2.5. Statistical analysis
Continuous data were presented as the mean ± SD or median values. Between-group differences were analyzed by Mann–Whitney U test. The relation between left ventricular ejection fraction and mismatch ratio was determined by Spearman rank order correlation coefficient. All statistical analyses were performed with EZR software, which was developed by Jichi Medical University Saitama Medical Center (Ohmiya Medical Center) as an evolution of the R statistical software (R Foundation for Statistical Computing, version 2.13.0). A P value (2 tail) of .05 was considered to be significant.
2.6. Ethical considerations
This study was conducted with the approval of the Ethics Committee of St. Marianna University School of Medicine (2976) and used opt out.
3. Results
3.1. Patient characteristics
The patient group comprised 23 women and 3 men aged 53.3 ± 15.3 years (Table 1). None of the patients had a history of ischemic heart disease, congenital heart disease, valvular heart disease, chronic renal failure, sarcoidosis, or amyloidosis. Four patients had a history of asymptomatic hypertension. All patients satisfied the diagnostic criteria by Ministry of Health 2015 about the PM/DM. There were no patients with special antibody. There were no patients with specific outcomes.
Table 1.
Patient characteristics.
| n | P value | |
| Age (yrs) | 53.3 ± 15.3 | |
| Sex (m/f) | 3/23 | |
| Scintigraphy mismatch (%) | 26 | 2.6 ± 5.9 |
| End-diastolic dimension (mm) | 24 | 43.9 ± 3.5 |
| End-systolic dimension (mm) | 24 | 27.3 ± 3.2 |
| Ejection fraction (%) | 24 | 67.8 ± 5.7 |
| E/A | 23 | 0.9 ± 0.4 |
| DCT | 22 | 210 ± 56.4 |
| Right ventricular systolic pressure (mm Hg) | 23 | 26.2 ± 7.4 |
| Troponin T (ng/mL) | 19 | 0.09 ± 0.11 |
| Myoglobin (ng/mL) | 17 | 469.7 ± 595.4 |
| Myosin light chain (ng/mL) | 23 | 46 ± 66.6 |
| Aldolase (IU/L) | 21 | 23.1 ± 21.5 |
3.2. Scintigraphy
Uptake mismatch was observed in 13 (50%) patients. The mismatch ratio was 2.6 ± 5.9%. Upon visual inspection of the images, the distributions of 123I-BMIPP and 201TlCl appeared not to be segmental or based on coronary artery segmentation.
3.3. Echocardiographic findings
The mean left ventricular end-diastolic dimension was 43.9 ± 3.5 mm, end-systolic dimension was 27.3 ± 3.2 mm, and ejection fraction was 67.8 ± 5.7%. The E/A ratio was 0.9 ± 0.4, and right ventricular systolic pressure was 26.2 ± 7.3 mm Hg. The mean deceleration time (DCT) was 210 ± 56.4 ms.
3.4. Laboratory findings
The mean values of the laboratory tests and the number of patients showing above-normal values were as follows: cardiac troponin T concentration = 0.09 ± 0.11 ng/mL (≥0.10 ng/mL, n = 5); myoglobin concentration = 469.7 ± 595.4 ng/mL (>58 ng/mL, n = 12); myosin light-chain concentration = 46.0 ± 66.6 ng/mL (≥2.5 ng/mL, n = 22); aldolase concentration = 22.3 ± 20.9 IU/L (>6 IU/L, n = 17).
3.5. Results of 123I-BMIPP and 201TlCl
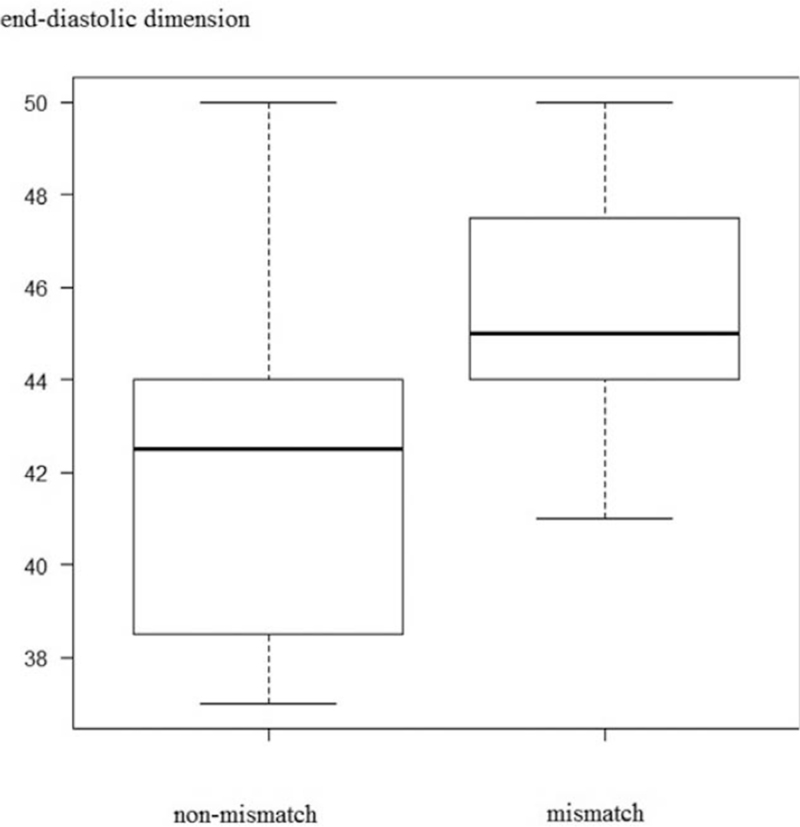
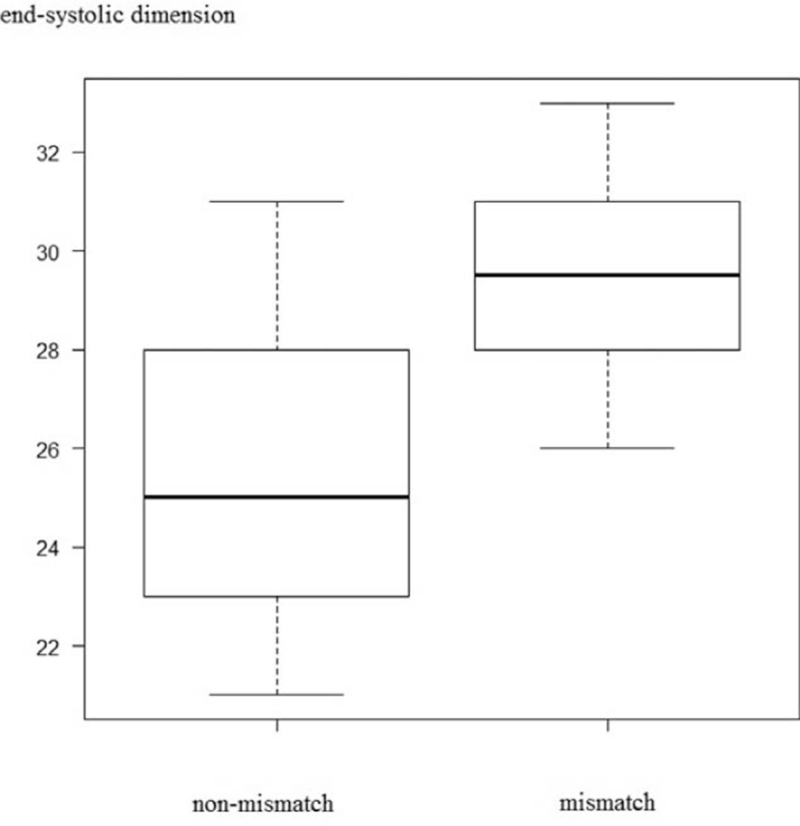
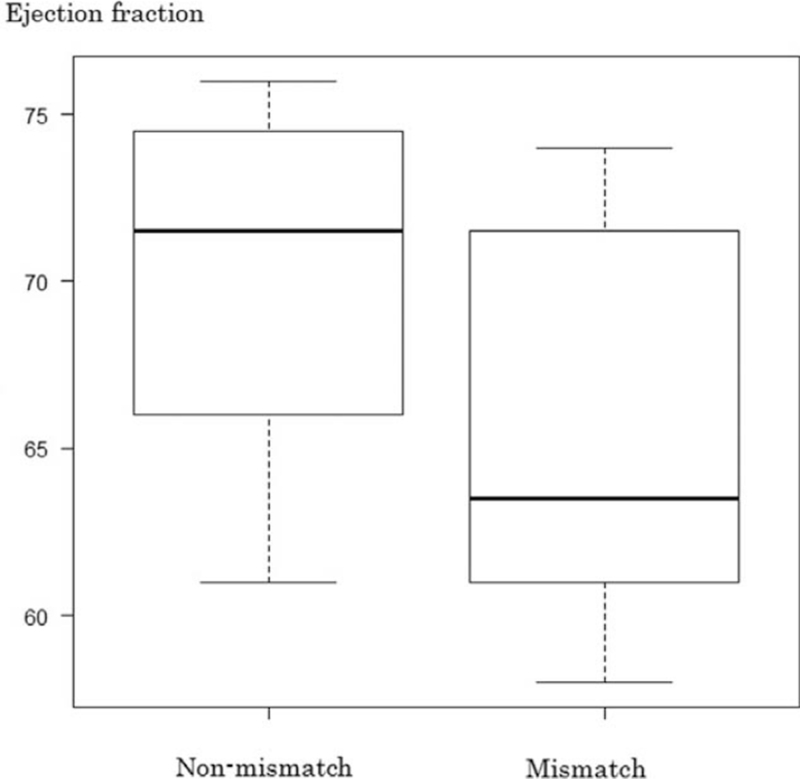
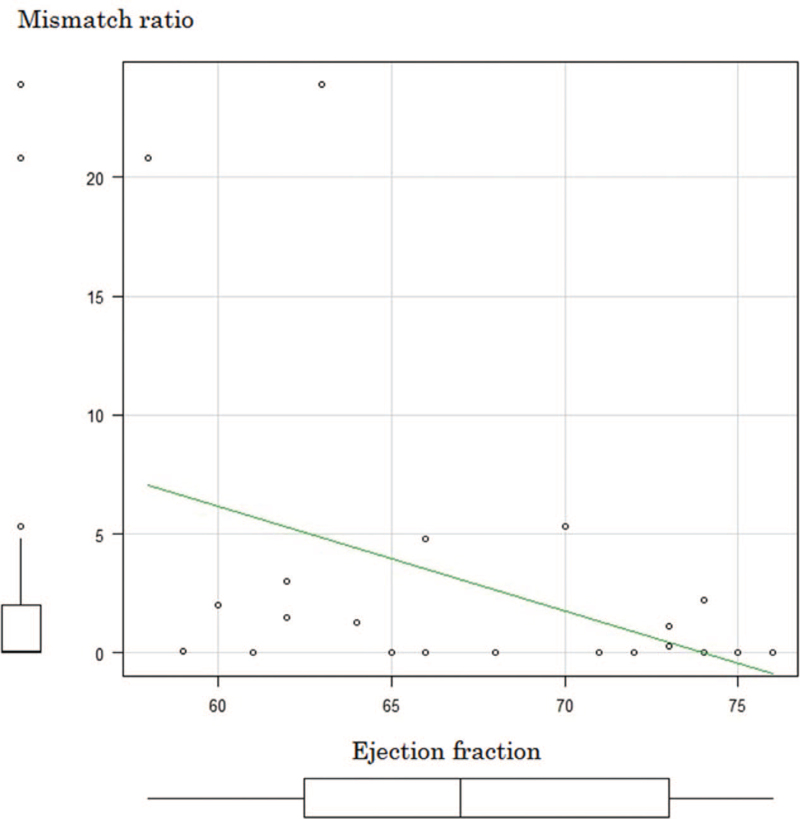
The median mismatch ratio was 0.05% (range, 0%–23.9%). The left ventricular end-diastolic and end-systolic dimensions were significantly greater in the mismatch group (45.0 mm vs 42.5 mm, P = .01 and 29.5 mm vs 25.0 mm; P < .01) (Figs. 3 and 4). The left ventricular ejection fraction was significantly lower in the mismatch group (63.5% vs 71.5%; P = .04) (Fig. 5). Significant inverse correlation (r = −0.44; P = .03) was found between left ventricular ejection fraction and the mismatch ratio (Fig. 6). The results of the 123I-BMIPP and 201TlCl mismatch analysis are shown in Table 2. No statistically significant difference was found between the mismatch and non-mismatch groups in terms of age, cardiac troponin T, myoglobin, myosin light chain, aldolase levels, E/A ratio, right ventricular systolic pressure or DCT.
Figure 3.
Cardiac end-diastolic dimensions in the non-mismatch and mismatch groups.
Figure 4.
Cardiac end-systolic dimensions in the non-mismatch and mismatch groups.
Figure 5.
Cardiac ejection fraction in the non-mismatch and mismatch groups.
Figure 6.
Scatterplot and regression line showing the correlation between left ventricular ejection fraction and mismatch ratio.
Table 2.
Results of 123I-BMIPP/201TlCl mismatch analysis.
| n | Non-mismatch group (n = 13) | Mismatch group (n = 13) | P value | |
| Age (yrs) | 26 | 58 | 51 | n.s. |
| Scintigraphic mismatch ratio (%) | 26 | 0 | 2.00 | <.01 |
| End-diastolic dimension (mm) | 24 | 42.5 | 45.0 | .01 |
| End-systolic dimension (mm) | 24 | 25.0 | 29.5 | <.01 |
| Ejection fraction (%) | 24 | 71.5 | 63.5 | .04 |
| E/A | 23 | 0.75 | 0.77 | n.s. |
| DCT | 22 | 198 | 204 | n.s |
| Right ventricular systolic pressure (mm Hg) | 23 | 24.3 | 25.1 | n.s. |
| Troponin T (ng/mL) | 19 | 0.09 | 0.11 | n.s. |
| Myoglobin (ng/mL) | 17 | 159.0 | 437.5 | n.s. |
| Myosin light chain (ng/mL) | 23 | 27.8 | 9.10 | n.s. |
| Aldolase (IU/L) | 21 | 16.0 | 10.1 | n.s. |
4. Discussion
There are some reports about the utility of 123I-BMIPP/201TlCl mismatch in the diagnosis of myocarditis. The mismatch between 123I-BMIPP and 201TlCl uptake is useful for diagnosing active cardiac sarcoidosis.[12]
In this study, 123I-BMIPP/201TlCl mismatch was observed in 50% (13/26) of the patients, the left ventricular end-diastolic and end-systolic dimensions were significantly greater in the mismatch group, and the left ventricular ejection fraction was significantly lower in the mismatch group. The mean left ventricular end-diastolic dimension of 43.9 ± 3.5 mm, end-systolic dimension of 27.3 ± 3.2 mm, and ejection fraction of 67.8 ± 5.7% are similar to that of previous reports (ventricular end-diastolic dimension/end-systolic dimensions/ejection fraction of 48.0 ± 3.82 mm/31.25 ± 3.07 mm/64.2 ± 4.76% and 47.0 ± 1.0 mm/2.83 ± 0.8 mm/60.8 ± 0.8%.[6,7] Five patients (19%) in our study showed abnormal cardiac troponin T level, which is lower than that in a previous report (41%).[13] Elevated cardiac troponin T level suggests cardiac ischemic damage and ischemic memory, which reduces cardiac function. However, the cardiac damages was small, accounting for the echocardiography and cardiac troponin T change.
The cardiac involvement in PM/DM-myocradial inflammation, degenerative change, necrosis. 46.4% patients died by heart disease in PM/DM.[5] So the cardiac damage evaluation is important. In this study, we used 123I-BMIPP/201TlCl scintigraphy as a screening, there are some abnormal value about cardiac troponin T, myoglobin, myosin light chain. But, our results should be interpreted in light of the limitations of our study, which include its retrospective design and the small number of patients, Further, we did not evaluate for myocardial function using cardiac magnetic resonance imaging. Cardiac magnetic resonance imaging is useful, but, there are side effect-especially, allergy, kidney damage. Moreover, there are sudden change risk by the load. One hand, 123I-BMIPP/201TlCl scintigraphy do not need the motion or drug load. 2D echocardiography is useful, but there are skill variation between the physician. Other hand, 123I-BMIPP/201TlCl scintigraphy do not depend on the physician skill and have the high quality of objectivity. So we used the 123I-BMIPP/201TlCl scintigraphy. But we did not identify the mismatch site. Additional research is needed in this regard. From these results, the cardiac damage evaluation is necessary.
In conclusion, we found that mismatch between 123I-BMIPP and 201TlCl uptake was useful for early detection of PM/DM-associated cardiomyopathy. A prospective clinical trial involving a large number of patients is needed to confirm the mismatch phenomenon in patients with PM/DM.
Author contributions
Data curation: Yukinori Okada, Yukiko Takakuwa.
Data analysis: Yukinori Okada, Yukiko Takakuwa.
Formal and result check: Seido Ooka, Yukihisa Ogawa.
Initial idea: Yukinori Okada, Yukiko Takakuwa.
Project administration: Yasuyuki Kobayashi, Keiichiro Yamaguchi, Yoshihiro Akashi, Kimito Kawahata.
Footnotes
Abbreviations: DCT = deceleration time, DM = dermatomyositis, PM = polymyositis.
How to cite this article: Okada Y, Takakuwa Y, Ooka S, Ogawa Y, Kawahata K, Kobayashi Y, Yamaguchi K, Akashi Y. Usefulness of 123I-BMIPP and 201TlCl nuclide scintigraphy in evaluation of myocarditis in patients with polymyositis or dermatomyositis. Medicine. 2021;100:36(e27173).
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
n = 26. Mean ± SD values are shown.
DCT = deceleration time, E/A = E wave/A wave ratio.
Median values are shown.
DCT = deceleration time, E/A = E wave/A wave ratio, n.s. = not significant.
References
- [1].Jakubaszek M, Kwitatkowska B, Maślińska M. Polymyositis and dermatomyositis as a risk of developing cancer. Reumatologia 2015;53:101–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lundberg IE. The heart in dermatomyositis and polymyositis. Rheumatology (Oxford) 2006;45: Suppl 4: iv18–21. [DOI] [PubMed] [Google Scholar]
- [3].Rai SK, Choi HK, Sayre EC, Aviña-Zubieta JA. Risk of myocardial infarction and ischemic stroke in adults with polymyositis and dermatomyositis: a general population-based study. Rheumatology (Oxford) 2016;55:461–9. [DOI] [PubMed] [Google Scholar]
- [4].Lai YT, Dai YS, Yen MF, et al. Dermatomyositis is associated with an increased risk of cardiovascular and cerebrovascular events: a Taiwanese population-based longitudinal follow-up study. Br J Dermatol 2013;168:1054–9. [DOI] [PubMed] [Google Scholar]
- [5].Zhang L, Wang GC, Ma L, Zu N. Cardiac involvement in adult polymyositis or dermatomyositis: a systematic review. Clin Cardiol 2012;35:686–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pécze NA, Balogh SA, Szilágyi S, et al. Echocardiographic abnormalities in new-onset polymyositis/dermatomyositis. J Rheumatol 2015;42:272–81. [DOI] [PubMed] [Google Scholar]
- [7].Lu Z, Wei Q, Ning Z, Qian-Zi Z, Xiao-Ming S, Guo-Chun W. Left ventricular diastolic dysfunction -- early cardiac impairment in patients with polymyositis/dermatomyositis: a tissue Doppler imaging study. J Rheumatol 2013;40:1572–9. [DOI] [PubMed] [Google Scholar]
- [8].Yoshinaga K, Naya Y, Shiga T, Suzuki N, Tamaki N. Ischaemic memory imaging using metabolic radiopharmaceuticals: overview of clinical settings and ongoing investigations. Eur J Nucl Med Mol Imaging 2014;41:384–93. [DOI] [PubMed] [Google Scholar]
- [9].Kubo A, Kinoshita F. Nuclear Medicine Notebook. 6th ed.2020;Tokyo Japan: Kanehara & Co. Ltd, 178–197. [Google Scholar]
- [10].Nakayama A, Momose M, Kondo C, Nakajima T, Kusakabe K, Hagiwara N. Ability of 201Tl and 123I-BMIPP mismatch to diagnose myocardial ischemia in patients with suspected coronary artery disease. Ann Nucl Med 2009;23:793–8. [DOI] [PubMed] [Google Scholar]
- [11].Inoue A, Fujimoto S, Yamashina S, Yamazaki J. Prediction of cardiac events in patients with dilated cardiomyopathy using 123I-BMIPP and 201Tl myocardial scintigraphy. Ann Nucl Med 2007;21:399–404. [DOI] [PubMed] [Google Scholar]
- [12].Momose M, Fukushima K, Kondo C, et al. Diagnosis and detection of myocardial injury in active cardiac sarcoidosis--significance of myocardial fatty acid metabolism and myocardial perfusion mismatch. Circ J 2015;79:2669–76. [DOI] [PubMed] [Google Scholar]
- [13].Erlacher P, Lercher A, Falkensammer J, et al. Cardiac troponin and beta-type myosin heavy chain concentrations in patients with polymyositis or dermatomyositis. Clin Chim Acta 2001;306:27–33. [DOI] [PubMed] [Google Scholar]