Abstract
We aimed to prospectively identify the risk factors of sarcopenia in patients with cirrhosis.
Patients (n = 193) included in a discovery cohort (January 2011 and December 2014) were categorized into alcoholic (A1; n = 55) and non-alcoholic cirrhosis (NA; n = 138) groups, and those (n = 235) in a validation cohort (January 2015 to December 2019) were categorized into alcoholic (n = 92), non-alcoholic steatohepatitis-related (n = 27), and hepatitis C virus-related cirrhosis groups (n = 116). Skeletal muscle mass index (SMI) was determined using computed tomography (SMI-CT) and bioelectrical impedance analysis (SMI-BIA). Endotoxin activity (EA) was measured with an EA assay.
SMI-CT correlated with grip strength in all the groups but significantly correlated with SMI-BIA of the men in group A1 (R = 0.64, P < .0001) and both sexes in group NA (male: R = 0.44, P = .0001; female: R = 0.35, P = .003). SMI-CT inversely correlated with the EA levels of the men in group A1 (R = −0.67, P < .0001) and myostatin levels in group NA (R = −0.53, P < .0001). Lower extremity SMI had a strong negative correlation with the EA levels of the men in group A1 (R = −0.58, P < .001), whereas upper extremity SMI showed an inverse trend with EA levels (R = −0.28, P = .08). SMI-CT also inversely correlated with the EA levels in groups A2 (R = −0.52, P = .003) and N (R = −0.67, P < .0001) and myostatin levels in group C (R = −0.65, P < .0001). Moreover, SMI-CT correlated with nutritional factors, including cholinesterase (R = 0.50, P = .005), zinc (R = 0.45, P = .01), branched amino acid-to-tyrosine ratio (R = 0.39, P = .02), and triglyceride (R = 0.33, P = .03) in group N.
Sarcopenia risk factors differ among cirrhosis etiologies. Alcohol-induced, intestine-mediated peripheral endotoxemia could participate in sarcopenia development in patients with alcoholic cirrhosis.
Keywords: alcoholic cirrhosis, endotoxin, sarcopenia
1. Introduction
Patients with cirrhosis have protein-energy malnutrition and impaired carbohydrate metabolism due to reduced liver function reserve resulting in a loss of skeletal muscle mass and weakness.[1] This clinical feature is defined as sarcopenia that results from multiple interacting factors that contribute to changes in skeletal muscles, such as age-related factors, chronic inflammation, hormonal and metabolic disorders, and insufficient nutrition.[2] Sarcopenia is categorized into primary sarcopenia, which is age related, and secondary sarcopenia, which often manifests nutritional disorders, liver disease, and other factors not related to aging.[3] Sarcopenia is defined in accordance with the Japan Society of Hepatology criteria, which adopt cutoff values for muscle mass and strength that are identical to those in the Asian Working Group for Sarcopenia criteria but omit the age-related criterion and gait speed.[4] Moreover, sarcopenia has been reported as an independent risk factor of mortality in patients with cirrhosis.[5]
The prevalence of alcoholic cirrhosis has been increasing, and alcohol abuse contributes to 50% of all cirrhosis-related deaths worldwide.[6] Alcoholic cirrhosis refers to the final stage of liver disease resulting from chronic excessive consumption of alcohol. Patients with alcoholic cirrhosis have a clinical syndrome of weight loss and muscle wasting or weakness, and often have concurrent micronutrient deficiencies, as malnutrition results from chronic alcohol consumption. Ethanol exposure causes harmful pleiotropic effects on skeletal muscle function, although the specific mechanisms of the effects of ethanol remain obscure. In alcoholic cirrhosis, impaired intestinal barrier function and consequent alterations in the composition of the gut microbiota may lead to augmented gut permeability, bacterial translocation, and inflammation.[7] In addition to hyperammonemia resulting from alcoholic liver disease, gut-derived endotoxemia and cytokine-mediated effects are attributable to sarcopenia in patients with alcoholic cirrhosis.[8] Emerging evidence showed that ethanol has deleterious effects on muscle proteostasis.[7] Ethanol, or its metabolite acetaldehyde, has been shown to inhibit mammalian targets of rapamycin complex 1, which facilitate stimulation of protein synthesis via downstream signaling molecules, P70S6 kinase, and 4E binding protein 1.[9] Early abstinence is a key factor for improving survival and preventing symptoms and complications of alcoholic cirrhosis.[10]
Branched chain amino acid (BCAA), especially leucine, is a key regulator of TORC1 signaling associated with the insulin/insulin-like growth factor 1 regulatory pathway.[11] BCAA supplementation increases serum albumin levels and improves the blood Fischer's ratio (BCAA-to-aromatic amino acid ratio) in patients with cirrhosis.[12] Albumin has been shown to be an essential endotoxin-binding protein that inactivates endotoxin.[13] In addition, recent evidence has shown that BCAA supplements are expected to be useful for the prevention and treatment of sarcopenia.[14] However, the pathogenesis of sarcopenia remains poorly understood. We aimed to investigate the correlation between skeletal mass index (SMI) and clinical and laboratory variables and identify the factors associated with the development of sarcopenia among the etiologies of cirrhosis.
2. Methods
This was a retrospective observational study conducted at the Nara University Hospital in Nara, Japan. A total of 428 consecutive patients with cirrhosis treated between January 2011 and December 2019 were included in the study. Patients were divided into 2 mutually exclusive cohorts, a discovery cohort (January 2011 to December 2014, n = 193) and a validation cohort (January 2015 to December 2019, n = 235). On the basis of clinical data, including laboratory tests, medical imaging features, or liver histology, a diagnosis of liver cirrhosis was made. Skeletal muscle mass and handgrip strength were measured at admission. Clinical parameters and serum assay for endotoxin activity (EA) were evaluated in 428 patients with cirrhosis. Among the 428 patients with cirrhosis, 193 in the discovery cohort were further divided into 2 groups, those with alcoholic cirrhosis (group A1; n = 55) and those with non-alcoholic cirrhosis (group NA; n = 138). The remaining 235 patients in the validation cohort were divided into 3 groups as follows: patients with alcoholic cirrhosis (group A2; n = 92), patients with non-alcoholic steatohepatitis (NASH)-related cirrhosis (group N; n = 27), and patients with hepatitis C virus-related cirrhosis (group C; n = 116). Patients with hepatocellular carcinoma or extrahepatic cancers, infectious diseases, and concomitant liver disease (i.e., chronic hepatitis B and C infections) were excluded. The primary outcome is clinical factor related to muscle volume loss (MVL) in patients with cirrhosis. The present study protocols conformed to the principles in the 1964 Declaration of Helsinki and its later amendments. The study protocol was approved by the medical ethics committee of Nara Medical University (approval No. 0132-011-1), and written informed consent for blood sample collection was obtained from all 428 patients enrolled.
Informed consent for the use of resected tissue was obtained from all the patients, and the study protocol was approved by the ethics committee of Nara Medical University. All the study participants or their legal guardians provided informed written consent prior to study enrollment.
2.1. Diagnosis of sarcopenia
Sarcopenia was diagnosed when a patient was positive for both handgrip strength decline and MVL. MVL was calculated on the basis of the psoas muscle area on computed tomography (CT) imaging at the level of the third lumbar vertebra (L3; total bilateral psoas muscle area/height2, cm2/m2; SMI by CT [SMI-CT]). Upper and lower extremity SMIs were assessed using bioelectrical impedance analysis (bioelectrical impedance analysis [BIA]; SMI by BIA [SMI-BIA]).[4] Sarcopenia was diagnosed using the Assessment Criteria for Sarcopenia in Liver Disease (1st edition) reported by the Working Group for the Creation of Sarcopenia Assessment Criteria in the Japan Society of Hepatology.[4] The cutoff value of SMI depletion was set at CT-SMI ≤6.36 cm2/m2 for men and ≤3.92 cm2/m2 for women. The cutoff BIA-SMI value was ≤7.0 kg/m2 for men and ≤5.7 kg/m2 for women. The cutoff handgrip strength decline was <26 kg for men and <18 kg for women.
2.2. Endotoxin activity measurements
We assessed whole blood EA using the commercially available endotoxin activity assay kit (Spectral Diagnostics, Toronto, Canada).[15] EA was determined using a chromogenic substrate assay (Toxicolor LS-50-M Set; Seikagaku Corp., Tokyo, Japan).[16]
2.3. Measurement of myostatin concentrations and ammonia in human serum
Myostatin concentrations were measured in duplicate using commercially available kits (DGDF80, R&D Systems, Minneapolis, MN). The intraassay and interassay coefficients of variation were <10%. For fasting venous ammonia concentrations, blood samples were obtained in the morning after the patients had fasted overnight or for at least 6 hours and placed on ice immediately after collection. An enzymatic method was used for ammonia measurement.[17] Blood samples were analyzed within 15 minutes of collection because γ-glutamyl transferase activity level increases, which is frequently detected in cirrhotic patients with increasing enzymatic hydrolysis of glutamine, resulting in an artifactual increase in ammonia levels.[18]
2.4. Statistical analyses
Data were expressed as median ± standard deviation. Baseline patient characteristics were compared between the groups using the Student t test or Mann–Whitney U test. Normally distributed continuous data were analyzed using parametric tests, whereas categorical or continuous data that were not normally distributed were examined using nonparametric tests. In addition, categorical data were analyzed using the Fisher exact test. Correlations were calculated with the Spearman rank test. All analyses were conducted with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R 2.13.0 (R Foundation for Statistical Computing, Vienna, Austria).[19] Two-sided P < .05 was considered statistically significant.
3. Results
3.1. Baseline clinical characteristics
The baseline characteristics of the 193 patients with cirrhosis enrolled in the discovery cohort are summarized in Table 1. The patients consisted of 117 men (60.6%) with a mean age of 68 ± 10 years. Fifty-five patients with cirrhosis had alcohol intake in each causal group; 72 had hepatitis C virus; 27 had hepatitis B virus; 39 had other conditions (primary biliary cholangitis in 4, autoimmune hepatitis in 5, non-alcoholic steatohepatitis in 20, and cryptogenic cirrhosis in 10). The median follow-up period was 7.7 ± 2.4 years. The percentage of male patients was greater in group A than in group NA (P = .002). The patients in group A1 were significantly younger than those in group NA (P = .02). A higher percentage of patients with Child-Pugh's A cirrhosis was observed in group NA than in group A1 (P = .01). No significant difference in the prevalence of sarcopenia was observed between the 2 groups (P = .93). The patients with cirrhosis in the 2 groups had no significant difference in ascites grade or hepatic encephalopathy (P = .45 and P = .46, respectively). Table 2 summarizes the baseline characteristics of the 235 patients with cirrhosis enrolled in the validation cohort. Group A2 had significantly more male patients with cirrhosis than the other groups. The patients in group C were significantly older than those in the other groups. The mean body mass index (BMI) and cholinesterase (Ch-E) levels were highest in group N (27.7 kg/m2 and 213 U/L, respectively) and lowest in group C (22.7 kg/m2 and 165 U/L, respectively).
Table 1.
Clinical and demographic characteristics of patients with cirrhosis for Study 1.
| Total (n = 193) | Group A1 (n = 55) | Group NA (n = 138) | P value | |
| Gender (male/female) | 117/76 | 47/8 | 70/68 | .002 |
| Age∗ | 68 ± 10 | 66 ± 10 | 70 ± 9 | .02 |
| Child-Pugh classification (A/B/C) | 133/53/7 | 37/16/2 | 96/37/5 | .01 |
| Etiology (alcohol/HCV/HBV/others) | 55/72/27/39 | 55/0/0/0 | 0/72/27/39 | – |
| Sarcopenia/non-sarcopenia | 50/143 | 15/40 | 35/103 | .93 |
| Ascites grade 0/1/2/3 | 156/32/5/0 | 45/7/2/1 | 111/24/3/0 | .45 |
| Hepatic encephalopathy grade 0/1/2/3 | 151/27/5/0 | 43/9/3/0 | 118/18/2/0 | .46 |
| BMI (kg/m2)∗ | 23.6 ± 4.1 | 24.1 ± 5.0 | 23.5 ± 3.4 | .23 |
| PT (%)∗ | 75 ± 15 | 71 ± 16 | 80 ± 13 | .03 |
| Alb (g/dL)∗ | 3.7 ± 0.4 | 3.7 ± 0.05 | 3.7 ± 0.5 | .73 |
| T-Bil (mg/dL) | 1.0 ± 0.3 | 0.9 ± 0.3 | 1.2 ± 0.2 | .73 |
| T-Cho (mg/dL)∗ | 163 ± 45 | 164 ± 46 | 163 ± 43 | .85 |
| Ch-E (U/L)∗ | 195 ± 84 | 194 ± 79 | 197 ± 85 | .78 |
| TG (mg/dL)∗ | 91 ± 52 | 85 ± 45 | 95 ± 54 | .34 |
| BTR (μmol/L)∗ | 4.5 ± 2.1 | 4.7 ± 2.0 | 4.5 ± 1.7 | .71 |
| Ammonia (μg/dL)∗ | 45 ± 25 | 44 ± 26 | 48 ± 24 | .42 |
| EA levels∗ | 0.27 ± 0.14 | 0.29 ± 013 | 0.26 ± 013 | .20 |
Table 2.
Clinical and demographic characteristics of patients with cirrhosis for Study 2.
| N | Group A2 (n = 92) | Group N (n = 27) | Group C (n = 116) | P value |
| Gender (male/female) | 83/9 | 14/13 | 60/56 | .04 |
| Age∗ | 65 ± 10 | 70 ± 9 | 72 ± 9 | .02 |
| Child-Pugh classification (A/B/C) | 57/29/6 | 18/7/2 | 73/36/5 | .06 |
| Sarcopenia/non-sarcopenia | 32/60 | 8/19 | 37/79 | .85 |
| Sarcopenia obesity/non-sarcopenia obesity | 10/82 | 3/24 | 5/111 | .17 |
| Ascites grade 0/1/2/3 | 73/16/3/0 | 21/5/1/0 | 90/18/7/1 | .93 |
| Hepatic encephalopathy grade 0/1/2/3 | 88/3/1/0 | 25/2/0/0 | 108/8/0/0 | .87 |
| BMI (kg/m2)∗ | 23.8 ± 4.8 | 27.7 ± 7.8 | 22.7 ± 4.1 | .02 |
| PT (%)∗ | 70 ± 15 | 68 ± 13 | 72 ± 13 | .08 |
| Alb (g/dL)∗ | 3.7 ± 0.6 | 3.7 ± 0.6 | 3.6 ± 0.7 | .42 |
| T-Bil (mg/dL)∗ | 1.1 ± 0.2 | 0.9 ± 0.1 | 1.3 ± 0.2 | .73 |
| T-Cho (mg/dL)∗ | 161 ± 39 | 160 ± 37 | 149 ± 41 | .09 |
| Ch-E (U/L)∗ | 202 ± 87 | 213 ± 75 | 165 ± 83 | .04 |
| TG (mg/dL)∗ | 85 ± 45 | 93 ± 40 | 89 ± 51 | .63 |
| BTR (μmol/L)∗ | 4.6 ± 2.1 | 4.7 ± 2.0 | 4.5 ± 1.7 | .65 |
| Ammonia (μg/dL)∗ | 51 ± 31 | 49 ± 31 | 43 ± 27 | .51 |
| Zn (μg/dL)∗ | 68 ± 17 | 49 ± 22 | 64 ± 19 | .11 |
| EA levels∗ | 0.30 ± 0.11 | 0.33 ± 0.14 | 0.31 ± 0.12 | .17 |
The prevalence rates of sarcopenia were 34.7% (32/92), 29.6% (8/27), and 31.9% (37/116), whereas those of sarcopenic obesity were 10.8% (10/92), 11.1% (3/27), and 4.3% (5/116) in groups A2, N, and C, respectively. Among the different etiologies of liver cirrhosis, no significant differences were found in the prevalence rates of sarcopenia and sarcopenia obesity, ascites grade, or hepatic encephalopathy (P = .85, P = .17, P = .93, and P = .87, respectively).
3.2. Complications and mortality
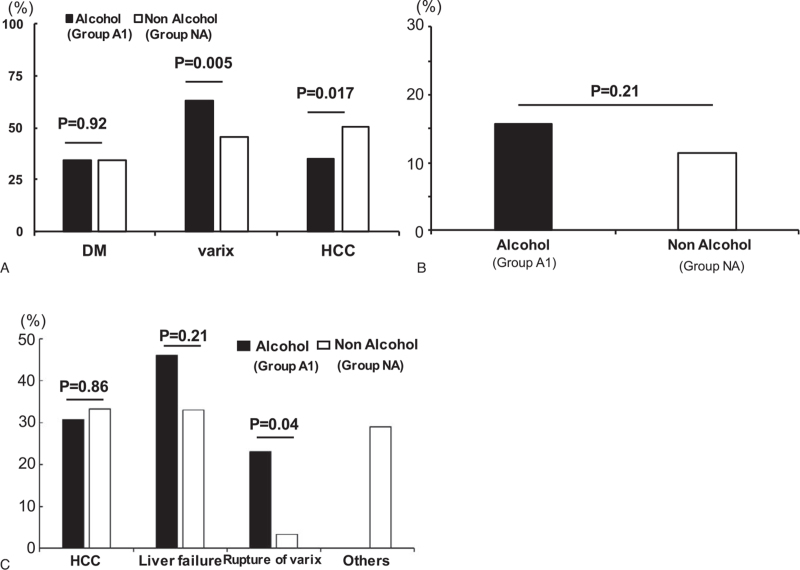
While variceal recurrence was observed more frequently in group A1 than in group NA, the occurrence of hepatocellular carcinoma was higher in group NA than in group A1 (Fig. 1A). No significant differences were observed in the liver disease-related mortality rate between the 2 groups (Fig. 1B). The mortality rate from variceal bleeding was higher in group A1 than in group NA (Fig. 1C). No significant differences in total mortality and hepatocellular carcinoma- and liver failure-related mortality rates were found between the 2 groups.
Figure 1.
Incidence rates of complications and mortality in patients with cirrhosis. Differences in the complications of (A) alcoholic and non-alcohol-related cirrhosis. Differences in (B) total mortality and (C) complication-related mortality rates between the patients with alcoholic and non-alcoholic cirrhosis.
3.3. Prevalence of sarcopenia
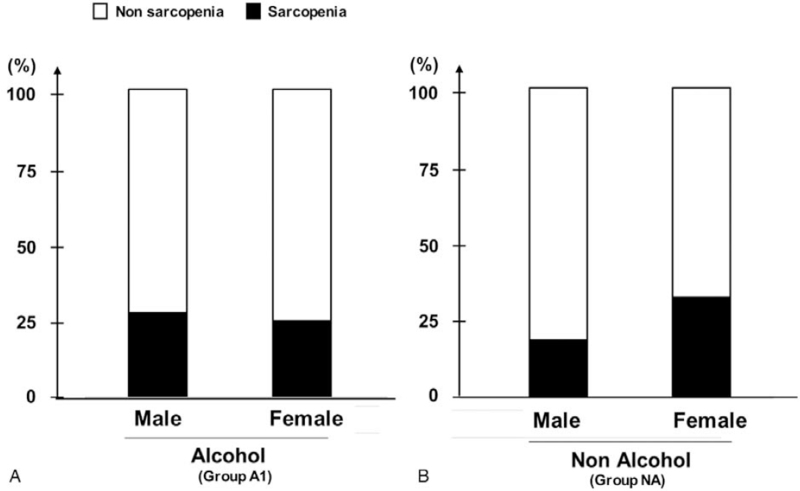
The prevalence rate of sarcopenia was 27.7% (13/47) in the males and 25.0% (2/8) in females in group A1 (Fig. 2A), and 18.6% (13/70) in the males and 32.4% (22/68) in the females in group NA (Fig. 2B). No significant difference in the prevalence rate of sarcopenia was found between the male and female patients in either group (A1: P = .86, NA: P = .1).
Figure 2.
Prevalence of sarcopenia among male and female patients with (A) alcoholic and (B) non-alcoholic cirrhosis.
3.4. Correlation of SMI by CT imaging with several indexes
SMI-CT significantly correlated with BMI (R = 0.69, P < .0001) in the male patients but not in the female patients in group A1 (R = 0.12, P = .77; Table 3). SMI-CT correlated with BMI in both the male and female patients in group NA (R = 0.66, P < .0001 and R = 0.65, P < .0001, respectively). SMI-CT significantly correlated with grip strength in the males and females in both groups A1 (R = 0.35, P = .016 and R = 0.8, P = .016, respectively) and NA (R = 0.39, P = .001 and R = 0.33, P = .02, respectively). SMI-CT correlated with SMI-BIA in the male patients (R = 0.64, P < .0001) but not in the female patients (R = 0.39, P = .33) in group A1. SMI-CT correlated with SMI-BIA in the males and females in group NA (R = 0.44, P = .0001 and R = 0.35, P = .003, respectively). SMI-CT highly correlated with total cholesterol (T-cho) and Ch-E levels in the female patients in group A1 (R = 0.71, P = .04 and R = 0.9, P = .002, respectively) and showed the same trend of correlation with branched amino acid-to-tyrosine ratio (BTR; R = 0.61, P = .09). In group NA, SMI-CT had no correlations with albumin level (male: R = 0.04, P = .75; female: R = 0.08, P = .59), BTR (male: R = 0.07, P = .78; female: R = 0.09, P = .29), and ammonia (NH3; male: R = 0.14, P = .4; female: R = 0.14, P = .3).
Table 3.
Correlation of skeletal mass index with clinical parameters in Group A and Group NA for Study 1.
| Group A1 (n = 55) | Group NA (n = 138) | |||||||
| Male | Female | Male | Female | |||||
| R | P | R | P | R | P | R | P | |
| BMI | 0.69 | <.0001 | 0.12 | .77 | 0.66 | <.0001 | 0.65 | <.0001 |
| Grip strength | 0.35 | .016 | 0.8 | .016 | 0.39 | .001 | 0.33 | .02 |
| SMI (BIA) | 0.64 | <.0001 | 0.39 | .33 | 0.44 | .0001 | 0.35 | .003 |
| PT | 0.05 | .73 | 0.68 | .13 | 0.09 | .54 | 0.14 | .31 |
| Alb | 0.08 | .62 | 0.61 | .1 | 0.04 | .75 | 0.08 | .59 |
| TG | 0.1 | .45 | 0.5 | .2 | 0.12 | .43 | 0.01 | .92 |
| T-cho | 0.09 | .86 | 0.71 | .04 | 0.09 | .56 | 0.02 | .87 |
| ChE | 0.27 | .1 | 0.9 | .002 | 0.27 | .07 | 0.03 | .84 |
| BTR | 0.09 | .65 | 0.61 | .09 | 0.07 | .78 | 0.09 | .29 |
| Ammonia | 0.06 | .71 | 0.03 | .98 | 0.14 | .4 | 0.14 | .3 |
| EA levels | −0.67 | <.0001 | −0.35 | .20 | −0.08 | .64 | −0.04 | .80 |
3.5. Correlation of SMI by CT imaging with EA and myostatin levels
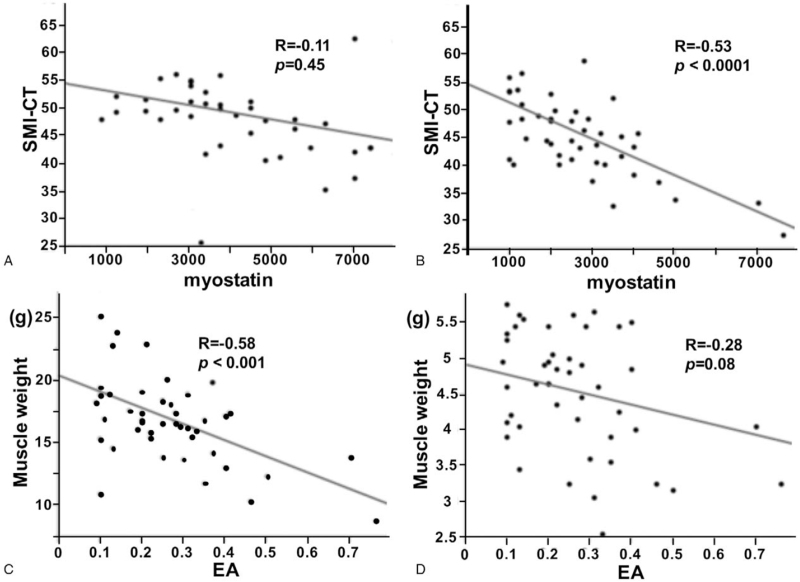
SMI-CT negatively correlated with EA levels in the male patients (R = −0.67, P < .0001) but not in the female patients (R = −0.35, P = .20) in group A1 (Table 3). However, SMI-CT did not correlate with EA levels in both the male and female patients in group NA (R = −0.08, P = .64 and R = −0.04, P = .80, respectively). Conversely, SMI-CT did not correlate with serum myostatin levels in group A1 (R = −0.11, P = .45; Fig. 3A). However, SMI-CT negatively correlated with serum myostatin levels in group NA (R = −0.53, P < .0001; Fig. 3B). Furthermore, lower extremity SMI negatively correlated with EA levels in the males in group A1 (R = −0.58, P < .001; Fig. 3C), whereas the correlation between upper extremity SMI and EA levels showed an inverse trend (R = −0.28, P = .08; Fig. 3D).
Figure 3.
Correlation of skeletal mass index measured on computed tomography (SMI-CT) with the myostatin levels of patients with (A) alcoholic and (B) non-alcoholic cirrhosis. Correlations of endotoxin activity level (EA) with (C) lower and (D) upper extremity skeletal muscle masses.
3.6. Factors associated with skeletal volume loss among cirrhosis etiologies
SMI-CT inversely correlated with EA levels in groups A2 (R = −0.52, P = .003) and N (R = −0.67, P < .0001) but not in group C (R = −0.08, P = .82; Table 4). However, SMI-CT inversely correlated with myostatin levels in group C (R = −0.65, P < .0001), but showed no correlation in group A2 (R = −0.22, P = .15) or N (R = 0.28, P = .07). Furthermore, SMI-CT moderately correlated with nutritional factors, including Ch-E (R = 0.50, P = .005) and zinc (R = 0.45, P = .01). Meanwhile, its associations with both BTR (R = 0.39, P = .02) and triglyceride levels (R = 0.33, P = .03) in group N were significant but weak.
Table 4.
Correlation of skeletal mass index with clinical parameters among etiologies of cirrhosis for Study 2.
| Group A2 (n = 92) | Group N (n = 27) | Group C (n = 116) | ||||
| R | P | R | P | R | P | |
| BMI | 0.66 | <.0001 | 0.61 | <.0001 | 0.75 | <.0001 |
| Grip strength | 0.37 | .02 | 0.31 | .03 | 0.41 | .03 |
| SMI (BIA) | 0.61 | <.0001 | 0.65 | <.0001 | 0.62 | <.0001 |
| Myostatin | −0.22 | .15 | 0.28 | .07 | −0.65 | <.0001 |
| ALB | 0.08 | .45 | 0.59 | .1 | 0.34 | .75 |
| TG | 0.12 | .41 | 0.33 | .03 | 0.08 | .73 |
| ChE | 0.37 | .01 | 0.50 | .005 | 0.15 | .35 |
| BTR | 0.21 | .11 | 0.39 | .02 | 0.10 | .65 |
| Zn | 0.17 | .40 | 0.45 | .01 | 0.09 | .76 |
| Ammonia | 0.06 | .71 | 0.03 | .98 | 0.14 | .4 |
| EA levels | −0.52 | .003 | −0.67 | <.0001 | −0.08 | .82 |
4. Discussion
We have shown that endotoxemia was significantly associated with SMI in male patients with alcoholic cirrhosis. Excessive alcohol use can lead to the development of cirrhosis that results in a decline in skeletal muscle mass, quality, and strength, accompanied by reduction in protein synthesis and anabolic resistance, or inadequate response to dietary interventions.[20] Increases in paracellular translocation of bacteria and their products (e.g., endotoxins) across the intestinal barrier can be a resulting outcome of alcohol-induced augmented gut permeability.[21] To the best of our knowledge, this is the first study to report the inverse correlation of EA levels with lower extremity skeletal muscle mass in male patients with alcoholic cirrhosis. The factors associated with sarcopenia are different among the etiologies of cirrhosis. Endotoxin is a major risk factor that may contribute to the development of sarcopenia in patients with alcoholic cirrhosis. As sex-related differences have been observed in the cutoff values of sarcopenia parameters, the prevalence of sarcopenia in patients with cirrhosis has been reported in both sexes separately. Males were more likely to be sarcopenic than their female counterparts in Japan.[4] In the present study, however, females were more likely to develop sarcopenia. Although the positive effects of estrogen on the skeletal muscle are known, all the female patients with alcoholic cirrhosis were postmenopausal. The fact that as a result of their lower estrogen levels, postmenopausal women are at increased risk of sarcopenia led to discrepancy in the risk of sarcopenia between the sexes.[22] Furthermore, a negative correlation was found between lower extremity SMI and the EA levels of the males with alcoholic cirrhosis (R = −0.58, P < .001), whereas an inverse positive correlation was found between the upper extremity SMI and EA levels (R = −0.28, P = .08) partly because of the notion that the skeletal muscle mass in the lower extremities can be easily influenced by gravity-associated edema as compared with that in the upper extremities.
Hyperammonemia has recently been recognized as a potential mediator in the liver–muscle axis, and myostatin upregulation represents the development of sarcopenia, which is linked to impaired protein synthesis. Higher serum myostatin levels correlate with lower albumin levels and lower BTRs, which reflects hyperammonemia and skeletal muscle mass loss.[23] However, in the present study, no correlation was observed between SMI-CT and NH3, or between SMI-CT and BTR in group NA, whereas a significant inverse correlation was found between SMI-CT and myostatin level. The elevation of myostatin expression level can be attributable to multiple parameters in cirrhosis. The reduction in serum testosterone and insulin-like growth factor-1 (IGF-1) levels contribute to elevated myostatin levels.[24,25] Decreased IGF-1 levels indicate an increased production of myostatin, and a reduced mTOR-mediated activation of muscle protein synthesis in cirrhosis.[26] These findings may explain the reason why no correlation was observed between SMI-CT and any of the 3 parameters (NH3, albumin, and BTR) in group NA, although the myostatin signaling pathway plays an important role in the development of sarcopenia in patients with cirrhosis. These findings reinforce our suspicion that the factors that contribute to sarcopenia development are different among cirrhosis etiologies.
Alcoholic liver disease and NASH are firmly linked to leaky gut syndrome. However, the difference in portal pressure between alcoholic and non-alcoholic cirrhosis remains disputed. Portal vein pressure is influenced by alcohol consumption in patients with alcoholic cirrhosis. Physicians persuaded patients diagnosed as having alcoholic cirrhosis to quit drinking alcohol. However, 50 (91%) of 55 patients in group A1 continued their alcohol drinking habits. These findings explain why variceal recurrence was observed more frequently in group A1 than in group NA. Furthermore, EA levels were not significantly different between the alcoholic and NASH-related cirrhosis. Endotoxin activity assay measures the endotoxin activity in the whole blood by the priming of the host's neutrophil respiratory burst activity, which is triggered by acute and chronic alcohol consumptions.[27] As the intestinal permeability is already increased in patients with cirrhosis regardless of alcohol abuse, the effects of chronic ethanol consumption and ethanol withdrawal on EA levels in patients with alcoholic cirrhosis remains obscure. The expression levels of the microbial translocation markers glycosylphosphatidyl inositol-anchored myeloid glycoprotein and lipopolysaccharide-binding protein were decreased after 6 weeks of abstinence.[28] Restoration of the functional gut barrier, probably because of the rapid turnover of the intestinal mucosa, is a result of sufficient abstinence for 3 weeks.[29] Further studies should evaluate the impacts of chronic alcohol exposure and withdrawal on intestinal permeability in patients with alcoholic cirrhosis.
Alcohol abuse impairs the gut barrier function and increases the intestinal permeability in patients with alcoholic liver disease. Alcohol withdrawal has been shown to reduce the intestinal permeability in patients with alcohol use disorder.[28] Besides alcohol abstinence, several potential therapeutic options are available to improve intestinal permeability in cirrhosis, including fecal microbiota transplantation, antibiotics, and probiotics.[30] Several clinical studies have shown that probiotics can decrease intestinal permeability.[31–33] Either bacterial infection or endotoxin administration has been shown to promote skeletal muscle proteolysis in experimental models of sepsis.[34,35] Several experimental studies have shown that intake of probiotics decreases muscle mass loss by reducing gut permeability.[36–39] Furthermore, recent evidence has emerged that endotoxemia contributes to the development of sarcopenia in patients with cirrhosis.[3,40] These findings support the hypothesis that supplementation with probiotics may have some beneficial effect on skeletal muscle growth in cirrhosis. However, further research should be undertaken to investigate the effects of probiotics on sarcopenia in patients with cirrhosis.
Several limitations of this study must be acknowledged. First, this study was a prospective single-center study with a small number of patients, especially females in group A. Second, we did not evaluate the risk factors associated with decreased grip strength in the development of sarcopenia, although SMI-CT correlated with grip strength in all the study groups. A multivariate analysis was required to confirm the factors contributing to sarcopenia in the patients with alcoholic cirrhosis. Nevertheless, EA levels were associated with lower extremity SMI in the males in group A, indicating that alcohol-induced, intestine-mediated peripheral endotoxemia plays a certain role in the development of sarcopenia in alcoholic cirrhosis, although the risk factors associated with the development of sarcopenia are different among the etiologies of cirrhosis. However, the associations between gut microbiota changes, leaky gut, and the development of sarcopenia in alcoholic cirrhosis warrants further investigation. Larger studies are required to elucidate the relationship between sarcopenia indexes and demographic, clinical, and nutritional variables in patients with alcoholic cirrhosis.
Acknowledgments
The authors would like to thank Ms. Nakai for collecting the clinical data.
Author contributions
Conceptualization: H.Y., T.A., and A.M; Methodology: K.K. (Kousuke Kaji); Validation: K.M. (Koji Murata) and H.T. (Hirotetsu Takagi); Investigation: Y.F. (Yukihisa Fujinaga), N.S, H.K, K.M. (Kei Moriya), and S.T.; Resources: M.E., A.S., K.I., H.O., H.T. (Hiroaki Takaya), Y.T., and D.K.; Data curation: Y.F. (Yuki Fujimoto), M.F., Y.S., N.N., K.K. (Koh Kitagawa), and T.O.; Formal analysis: T.I.; Writing – original draft preparation: S.S.; Writing – review and editing: T.N. and H.Y. All authors have read and approved the manuscript.
Conceptualization: Hitoshi Yoshiji.
Data curation: Koji Murata, Hiroyuki Ogawa, Takashi Inoue.
Formal analysis: Akihiko Shibamoto, Hideto Kawaratani.
Funding acquisition: Yuki Tsuji, Takahiro Ozutsumi.
Investigation: Yuki Fujimoto, Naotaka Shimozato.
Methodology: Soichi Takeda, Masahide Enomoto, Hirotetsu Takagi, Koh Kitagawa.
Project administration: Yukihisa Fujinaga, Yasuhiko Sawada.
Resources: Daisuke Kaya, Hiroaki Takaya, Akira Mitoro.
Software: Koji Ishida, Norihisa Nishimura.
Supervision: Tadashi Namisaki, Kosuke Kaji.
Validation: Masanori Furukawa, Kei Moriya.
Visualization: Takemi Akahane.
Writing – original draft: Shinya Sato.
Writing – review & editing: Tadashi Namisaki.
Footnotes
Abbreviations: BCAA = branched chain amino acid, BIA = bioelectrical impedance analysis, BMI = body mass index, BTR = branched amino acid-to-tyrosine ratio, Ch-E = cholinesterase, CT = computed tomography, EA = endotoxin activity, MVL = muscle volume loss, NASH = non-alcoholic steatohepatitis, NH3 = ammonia, SMI = skeletal muscle mass index.
How to cite this article: Sato S, Namisaki T, Murata K, Fujimoto Y, Takeda S, Enomoto M, Shibamoto A, Ishida K, Ogawa H, Takagi H, Tsuji Y, Kaya D, Fujinaga Y, Furukawa M, Inoue T, Sawada Y, Nishimura N, Kitagawa K, Ozutsumi T, Takaya H, Kaji K, Shimozato N, Kawaratani H, Moriya K, Akahane T, Mitoro A, Yoshiji H. The association between sarcopenia and endotoxin in patients with alcoholic cirrhosis. Medicine. 2021;100:36(e27212).
The authors received no financial support for the research.
Raw data were generated at Nara Medical University Hospital. Derived data supporting the findings of the study are available from the corresponding author (Tadashi Namisaki) on request.
The authors have no conflicts of interest to disclose.
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Mean ± standard error of mean, Alb = albumin, BMI = body mass index, BTR = branched chain amino acid and tyrosine ratio, Ch-E = cholinesterase, EA = endotoxin activity, Group A1 = alcoholic cirrhosis, Group NA = non-alcohol-related cirrhosis, PT = prothrombin time, T-Bil = total bilirubin, T-cho = total cholesterol, TG = triglyceride.
Mean ± standard error of mean, Alb = albumin, BMI = body mass index, BTR = branched chain amino acid and tyrosine ratio, Ch-E = cholinesterase, EA = endotoxin activity, Group A2 = alcoholic cirrhosis, Group N = NASH-related cirrhosis, Group = HCV-related cirrhosis, NASH = non-alcoholic steatohepatitis, PT = prothrombin time, T-Bil = total bilirubin, T-cho = total cholesterol, TG = triglyceride, Zn = zinc.
Alb = albumin, BIA = bioelectrical impedance analysis, BMI = body mass index, BTR = branched chain amino acid and tyrosine ratio, Ch-E = cholinesterase, EA = endotoxin activity, Group A1 = alcoholic cirrhosis, Group NA = non-alcohol-related cirrhosis, PT = prothrombin time, SMI = skeletal mass index, T-Cho = total cholesterol, TG = triglyceride.
Alb = albumin, BIA = bioelectrical impedance analysis, BMI = body mass index, BTR = branched chain amino acid and tyrosine ratio, Ch-E = cholinesterase, EA = endotoxin activity, Group A2 = alcoholic cirrhosis, Group N = NASH-related cirrhosis, Group = HCV-related cirrhosis, NASH = non-alcoholic steatohepatitis, PT = prothrombin time, SMI = skeletal mass index, TG = triglyceride, Zn = zinc.
References
- [1].Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kalyani RR, Corriere M, Ferrucci L. Age-related and disease-related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol 2014;2:819–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dasarathy S, Merli M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J Hepatol 2016;65:1232–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nishikawa H, Shiraki M, Hiramatsu A, et al. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol Res 2016;46:951–63. [DOI] [PubMed] [Google Scholar]
- [5].Kang SH, Jeong WK, Baik SK, et al. Impact of sarcopenia on prognostic value of cirrhosis: going beyond the hepatic venous pressure gradient and MELD score. J Cachexia Sarcopenia Muscle 2018;9:860–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Stein E, Cruz-Lemini M, Altamirano J, et al. Heavy daily alcohol intake at the population level predicts the weight of alcohol in cirrhosis burden worldwide. J Hepatol 2016;65:998–1005. [DOI] [PubMed] [Google Scholar]
- [7].Dasarathy J, McCullough AJ, Dasarathy S. Sarcopenia in alcoholic liver disease: clinical and molecular advances. Alcohol Clin Exp Res 2017;41:1419–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dasarathy S. Nutrition and alcoholic liver disease: effects of alcoholism on nutrition, effects of nutrition on alcoholic liver disease, and nutritional therapies for alcoholic liver disease. Clin Liver Dis 2016;20:535–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Steiner JL, Lang CH. Alcohol impairs skeletal muscle protein synthesis and mTOR signaling in a time-dependent manner following electrically stimulated muscle contraction. J Appl Physiol 19852014;117:1170–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Verrill C, Markham H, Templeton A, et al. Alcohol-related cirrhosis – early abstinence is a key factor in prognosis, even in the most severe cases. Addiction 2009;104:768–74. [DOI] [PubMed] [Google Scholar]
- [11].Narasimhan SD, Yen K, Tissenbaum HA. Converging pathways in lifespan regulation. Curr Biol 2009;19:R657–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ijichi C, Matsumura T, Tsuji T, et al. Branched-chain amino acids promote albumin synthesis in rat primary hepatocytes through the mTOR signal transduction system. Biochem Biophys Res Commun 2003;303:59–64. [DOI] [PubMed] [Google Scholar]
- [13].Gioannini TL, Zhang D, Teghanemt A, et al. An essential role for albumin in the interaction of endotoxin with lipopolysaccharide-binding protein and sCD14 and resultant cell activation. J Biol Chem 2002;277:47818–25. [DOI] [PubMed] [Google Scholar]
- [14].Martinez-Arnau FM, Fonfria-Vivas R, Cauli O. Beneficial effects of leucine supplementation on criteria for sarcopenia: a systematic review. Nutrients 2019;11: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Okura Y, Namisaki T, Sato S, et al. Proton pump inhibitor therapy does not increase serum endotoxin activity in patients with cirrhosis. Hepatol Res 2019;49:232–8. [DOI] [PubMed] [Google Scholar]
- [16].Yaroustovsky M, Plyushch M, Popov D, et al. Prognostic value of endotoxin activity assay in patients with severe sepsis after cardiac surgery. J Inflamm (Lond) 2013;10:08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ong JP, Aggarwal A, Krieger D, et al. Correlation between ammonia levels and the severity of hepatic encephalopathy. Am J Med 2003;114:188–93. [DOI] [PubMed] [Google Scholar]
- [18].da Fonseca-Wollheim F. Deamidation of glutamine by increased plasma gamma-glutamyltransferase is a source of rapid ammonia formation in blood and plasma specimens. Clin Chem 1990;36:1479–82. [PubMed] [Google Scholar]
- [19].Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013;48:452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kaji K, Takaya H, Saikawa S, et al. Rifaximin ameliorates hepatic encephalopathy and endotoxemia without affecting the gut microbiome diversity. World J Gastroenterol 2017;23:8355–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Anand AC. Nutrition and muscle in cirrhosis. J Clin Exp Hepatol 2017;7:340–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Maltais ML, Desroches J, Dionne IJ. Changes in muscle mass and strength after menopause. J Musculoskelet Neuronal Interact 2009;9:186–97. [PubMed] [Google Scholar]
- [23].Nishikawa H, Enomoto H, Ishii A, et al. Elevated serum myostatin level is associated with worse survival in patients with liver cirrhosis. J Cachexia Sarcopenia Muscle 2017;8:915–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Assy N, Pruzansky Y, Gaitini D, et al. Growth hormone-stimulated IGF-1 generation in cirrhosis reflects hepatocellular dysfunction. J Hepatol 2008;49:34–42. [DOI] [PubMed] [Google Scholar]
- [25].Zietz B, Lock G, Plach B, et al. Dysfunction of the hypothalamic–pituitary–glandular axes and relation to Child-Pugh classification in male patients with alcoholic and virus-related cirrhosis. Eur J Gastroenterol Hepatol 2003;15:495–501. [DOI] [PubMed] [Google Scholar]
- [26].Ebadi M, Bhanji RA, Mazurak VC, et al. Sarcopenia in cirrhosis: from pathogenesis to interventions. J Gastroenterol 2019;54:845–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tamai H, Kato S, Horie Y, et al. Effect of acute ethanol administration on the intestinal absorption of endotoxin in rats. Alcohol Clin Exp Res 2000;24:390–4. [PubMed] [Google Scholar]
- [28].Donnadieu-Rigole H, Pansu N, Mura T, et al. Beneficial effect of alcohol withdrawal on gut permeability and microbial translocation in patients with alcohol use disorder. Alcohol Clin Exp Res 2018;42:32–40. [DOI] [PubMed] [Google Scholar]
- [29].Leclercq S, Cani PD, Neyrinck AM, et al. Role of intestinal permeability and inflammation in the biological and behavioral control of alcohol-dependent subjects. Brain Behav Immun 2012;26:911–8. [DOI] [PubMed] [Google Scholar]
- [30].Albillos A, de Gottardi A, Rescigno M. The gut–liver axis in liver disease: pathophysiological basis for therapy. J Hepatol 2020;72:558–77. [DOI] [PubMed] [Google Scholar]
- [31].Liu ZH, Huang MJ, Zhang XW, et al. The effects of perioperative probiotic treatment on serum zonulin concentration and subsequent postoperative infectious complications after colorectal cancer surgery: a double-center and double-blind randomized clinical trial. Am J Clin Nutr 2013;97:117–26. [DOI] [PubMed] [Google Scholar]
- [32].Liu Z, Qin H, Yang Z, et al. Randomised clinical trial: the effects of perioperative probiotic treatment on barrier function and post-operative infectious complications in colorectal cancer surgery – a double-blind study. Aliment Pharmacol Ther 2011;33:50–63. [DOI] [PubMed] [Google Scholar]
- [33].Garcia Vilela E, De Lourdes De Abreu Ferrari M, Oswaldo Da Gama Torres H, et al. Influence of Saccharomyces boulardii on the intestinal permeability of patients with Crohn's disease in remission. Scand J Gastroenterol 2008;43:842–8. [DOI] [PubMed] [Google Scholar]
- [34].Chai J, Wu Y, Sheng ZZ. Role of ubiquitin-proteasome pathway in skeletal muscle wasting in rats with endotoxemia. Crit Care Med 2003;31:1802–7. [DOI] [PubMed] [Google Scholar]
- [35].Tiao G, Fagan JM, Samuels N, et al. Sepsis stimulates nonlysosomal, energy-dependent proteolysis and increases ubiquitin mRNA levels in rat skeletal muscle. J Clin Invest 1994;94:2255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ni Y, Yang X, Zheng L, et al. Lactobacillus and bifidobacterium improves physiological function and cognitive ability in aged mice by the regulation of gut microbiota. Mol Nutr Food Res 2019;63:e1900603. [DOI] [PubMed] [Google Scholar]
- [37].Obermuller B, Singer G, Kienesberger B, et al. The effects of prebiotic supplementation with OMNi-LOGiC((R)) FIBRE on fecal microbiome, fecal volatile organic compounds, and gut permeability in murine neuroblastoma-induced tumor-associated cachexia. Nutrients 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bindels LB, Neyrinck AM, Claus SP, et al. Synbiotic approach restores intestinal homeostasis and prolongs survival in leukaemic mice with cachexia. ISME J 2016;10:1456–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Uchiyama K, Wakino S, Irie J, et al. Contribution of uremic dysbiosis to insulin resistance and sarcopenia. Nephrol Dial Transplant 2020;35:1501–17. [DOI] [PubMed] [Google Scholar]
- [40].Chen HW, Dunn MA. Arresting frailty and sarcopenia in cirrhosis: future prospects. Clin Liver Dis (Hoboken) 2018;11:52–7. [DOI] [PMC free article] [PubMed] [Google Scholar]