A. Introduction
Host defense from infection depends upon a complex integrated system of physical barriers (e.g., skin, stomach acid, and mucociliary clearance), innate immunity (e.g., phagocytic cells, natural killer cells, complement) and adaptive immunity (B and T lymphocytes). An individual may have deficiencies of one or more components of host defense, but no individual is defenseless. Because each functional compartment of the immune system plays a specialized role in host defense, defects in specific functions lead to increased susceptibility to specific pathogens. The key to understanding the susceptibility of a particular patient is to understand the specific host defense defects of that patient. This chapter will briefly review the components of host defense and the types of infections that are most likely to occur with specific defects in those defense mechanisms. Following that will be descriptions of the infections that occur in patients with a variety of primary and secondary immune deficiency disorders, with the intent of providing illustrative examples.
B. Overview of Host Defenses
Host defense depends upon physical barriers, as well as the immune system. In conceptual terms, the components of the immune system can be divided into two compartments – innate and adaptive – with fundamentally different modes of action (Table 1). Innate host defense mechanisms are rapid (minutes to hours), depend upon patterned responses to pathogens (e.g., by phagocytic cells, complement) and do not improve with repeated exposure to one or many pathogens. In contrast, adaptive immune mechanisms are slow (days), depend upon very specific responses to individual antigens (e.g., by B and T lymphocytes), and improve with repeated exposure to an individual antigen. Successfully integrated and functioning together, physical barriers and the components of innate and adaptive immunity form a critical homeostatic mechanism necessary for the host’s defense against infection and the generation of normal inflammatory responses (1, 2).
Table 1.
Components of the Immune System
| Feature | Innate immunity | Adaptive immunity |
|---|---|---|
| Cells | Neutrophils Monocytes/macrophages Natural killer cells |
B lymphocytes T lymphocytes |
| Receptors | Expressed by all cells of a particular type (e.g., macrophages) Recognize broad classes of pathogens |
Clonal distribution on individual cells Highly specific |
| Soluble factors | Complement Mannose-binding lectin Chemokines Cytokines (including IL-1, TNF-α) |
Antibody Cytokines (including IL-2, IL-4, IL-5, IL-6, IL-10) |
| Change with repeated exposure to antigen | No | Yes (clonal expansion, memory lymphocytes) |
Physical Barriers
The initial defenses against infection are provided by physical and chemical barriers (3). These include the tight junctions between epithelial cells of the skin; a protective barrier of mucus that traps microorganisms on mucosal surfaces and then is swept out of the respiratory tract by cilia and from the gastrointestinal tract by peristalsis; lysozyme in saliva and tears; acid in the stomach; antimicrobial peptides such as defensins; surfactant proteins that can opsonize microorganims for easier phagocytosis; and mechanical flushing of the gastrointestinal and urinary tracts. In addition, microbial pathogens must compete for space and nutrients with the normal microbiota on the skin and mucosal surfaces. Defects of physical barriers (e.g., disruption of the skin by burns or a vascular catheter; reduced mucociliary clearance because sedation is needed to keep a patient comfortable on mechanical ventilation; and obstruction of urinary drainage by a renal stone) are not the subject of this chapter, though they are common causes for increased susceptibility to infection.
Innate immunity
The components of the innate immune system (neutrophils, macrophages, natural killer or NK cells, and complement) recognize foreign antigens by receptors encoded by intact germline genes (e.g., toll-like receptors and mannose binding lectin) (4). These receptors bind to pathogen-associated molecular patterns (PAMPs) that are shared by many microorganisms (e.g., bacterial lipopolysaccharide). For example, the macrophage mannose receptor binds specific sugar molecules found on the surface of many bacteria and viruses. A family of transmembrane receptors called Toll-like receptors (TLR) have specifity for a variety of PAMPs (5). Binding to these receptors triggers a signalling cascade with induction of transcription factors and activation of pro-inflammatory genes. One particularly important role for TLRs is to trigger macrophage responses to bacterial lipopolysacharide (LPS) (6). Bacterial LPS in body fluids is bound by the circulating LPS-binding protein, and this complex then binds to CD14 on the macrophage surface. When the LPS/LPS-binding protein/CD14 complex binds to TLR-4, the transcription factor NF-κB is translocated into the nucleus where it activates genes involved in host defense such as tumor necrosis factor-alpha (TNF-α) and inducible nitric oxide synthetase (7). The receptors for PAMPs are displayed non-clonally on cells of the innate immune system. That is, all neutrophils display the same set of PAMP receptors, whereas all NK cells display a another set of PAMP receptors. Repeated exposure to an antigen does not alter the innate immune response to that antigen.
Phagocytes
Phagocytic cells ingest foreign antigens and microorganisms (8). Although many phagocytic cells are mobile and can move from the bloodstream through tissues to the site of microbial invasion or inflammation, other phagocytic cells are fixed in the sinusoids of the bloodstream and the lymphatic system where they clear microorganisms and other particulate matter from the circulation. A variety of cells possess phagocytic activity, but neutrophils, monocytes and macrophages are the most critical to the function of the immune system. Monocytes and macrophages can also present antigen to lymphoid cells and secrete a variety of proinflammatory substances (including cytokines and complement components). These cells thus play an important role in the generation of innate and adaptive immune responses, in addition to their role in phagocytosis.
To function properly, all phagocytic cells must attach to a substrate (adherence), move through tissues toward the site of microbial invasion (chemotaxis), attach and ingest microbes (phagocytosis), and finally kill them (intracellular killing). The adherence of phagocytic cells is mediated by a family of cell surface glycoproteins (integrins including CR3, LFA-1, and p150, 95) and is enhanced by a number of soluble mediators (including C5a, thromboxane A2, leukotrienes, and platelet-activating factor).
The directed movement of phagocytic cells toward a chemical stimulus is termed chemotaxis. Phagocytic cells sense chemical gradients across their length, and then move in the direction of the higher concentration (i.e., the source of the chemotactic stimulus). A variety of substances act as chemoattractants (including C5a which is produced by activation of the complement system, some bacterial peptides, prostaglandins, and monocyte- and lymphocyte-derived cytokines). Once phagocytic cells reach the site of infection, they ingest the microbes. The process is facilitated if the microbes have been coated (opsinized) with IgG antibody and/or the larger cleavage product of the third component of complement, C3b, as phagocytic cells have surface receptors for IgG and C3b.
The process of intracellular killing begins soon after the phagosome is internalized. Both primary (azurophilic) and secondary (specific) granules can fuse with the phagosome, and a number of antimicrobial substances are thereby introduced into the phagosome. These substances include lysozyme, lactoferrin, acid hydrolases, and cationic proteins. Perhaps the most important killing mechanism, however, is the myeloperoxidase-H2O2-halide system. After ingestion of microorganisms, molecular oxygen is reduced to superoxide by a series of reactions involving nicotinamide-adenine dinucleotide phosphate (NADPH, reduced form) oxidase. The superoxide, in turn, undergoes further reactions, leading to the generation of reduced oxygen derivatives such as hydrogen peroxide and hydroxyl radicals. Myeloperoxidase catalyzes the reaction of hydrogen peroxide with chloride to create hypochlorite ions. The net effect of these toxic derivatives of reduced molecular oxygen is to kill microorganisms within the phagocytic vacuole.
Complement System
The complement system is composed of about two dozen serum proteins that, when functioning in an ordered and integrated fashion, mediate a variety of defensive and inflammatory reactions (9, 10). The majority of the biologically significant effects of the complement system are mediated by the third component (C3) and the terminal components (C5 through C9). To subserve their biologic functions, however, C3 and C5 through C9 must first be activated via either the classical, the alternative or the mannan-binding lectin (MBL) complement pathway.
In the classical complement pathway, antigen-antibody complexes composed of either IgG or IgM activate the first component of complement (C1). Activation of the alternative complement pathway, in contrast, can occur in the absence of specific antibody if there is a “non-mammalian” cell surface. A third activation pathway, the MBL pathway, uses a molecule homologous to C1q to trigger the complement cascade. MBL binds to mannose residues on microbial surfaces, but does not bind mannose on host cells because it is blocked by sialic acid. Activation of any of these pathways leads to the proteolytic cleavage of C3 into C3a and C3b. The activation of C3 represents an amplification step because hundreds of C3 molecules can be cleaved by a single C3-convertase. C3a is released into the fluid phase, where it can act as an anaphylatoxin, releasing the tight junctions between vascular endothelial cells thus facilitating the movement of inflammatory cells from the bloodstream to an infected tissue. C3b binds covalently to the surface of the activating cell or to the immunoglobulins of the activating immune complex, thereby acting as an opsonin or combining with either of the C3-convertases to create a C5-convertase. Activation of C5 creates a small cleavage product, C5a, which is released into the fluid phase where it can act as an anaphylatoxin and a chemotaxin. C5b can combine with native C6, and thereby initiate the formation of a membrane attack complex (a multimolecular assembly of C5b, C6, C7, C8, and C9) which is inserted into cell membranes and is responsible for the cytolytic and bactericidal actions of complement.
Natural killer cells
Natural killer (NK) cells are derived from the common lymphoid progenitor cell (11, 12). However, unlike other lymphocytes, NK cells have invariant receptors that are not expressed clonally. One type of receptor binds a variety of cell surface carbohydrates, and is able to activate the NK cell. A second type of receptor binds MHC class I alleles, and has inhibitory activity. NK cells can kill targets that express a net excess of activating vs. inhibitory signals. This can occur, for example, if a viral-infected host cell has decreased expression of MHC class I molecules. NK cells also have receptors for the Fc portion of IgG (FcγR), so they can bind to host cells expressing viral or tumor antigens to which IgG antibodies have attached. Once an NK cell has attached to a target, it can release cytotoxic granules that penetrate the target cell and induce programmed cell death (apoptosis). The cytotoxic actvity of NK cells can be enhanced by prior exposure to interferons and the macrophage-derived cytokine IL-12.
Adaptive Immunity
The cells of the adaptive immune system (B and T lymphocytes) recognize antigen via receptors assembled from rearranged gene segments, and each lymphocyte expresses a unique antigen receptor (13). Repeated exposure to an antigen selects those cells with the highest affinity receptors for that antigen, induces proliferation of that clonal population, and differentiation into effector and long-lived memory cells. The net effect is to increase the kinetics and magnitude of the response to subsequent exposures of the same antigen.
B lymphocytes
Each B lymphocyte has a unique antigenic specificity, marked by the immunoglobulin receptor on its cell membrane. When antigen binds to the immunoglobulin (antibody) expressed on the surface of one of the B lymphocytes, that cell proliferates to form a clone of progeny cells with identical antibody specificity. These cells then differentiate into plasma cells that secrete immunoglobulins (IgM, IgG, IgA, IgE, or IgD). Most antigens are T-cell dependent, that is, optimal B-cell differentiation into plasma cells requires the presence of T-lymphocyte helper cells. There are a few antigens, however, including such clinically important ones as bacterial capsular polysaccharides, that are T independent and able to trigger terminal B-cell differentiation even in the absence of T lymphocytes. In all cases, CD4 helper T lymphocytes (TH) are important modulators of B-cell function, influencing the degree, duration, and quality (affinity and class distribution) of the antibody response.
The five major classes of immunoglobulins are IgG, IgM, IgA, IgE, and IgD. Each class has unique structural and functional characteristics. Depending on the class, immunoglobulins function in host defense by opsonization of foreign microorganisms, activation of serum complement, neutralization of toxins and viruses, and inhibition of microbial attachment to mucosal surfaces. IgM is the first immunoglobulin produced in an immune response and is the most efficient activator of complement. IgG is the predominant serum immunoglobulin, is actively transported across the placenta, possesses opsonic activity, and activates complement. IgA, which is the major immunoglobulin secreted onto mucosal surfaces, is largely silent as an inflammatory mediator, but can prevent microbial adherence and penetration across the mucosal surface, and clears and disposes of antigens. IgE is a mediator of allergic disease. By means of interactions with mast cells and eosinophils, IgE also can play a role in host defense against parasitic infections. Most IgD is expressed on the surface of naïve B-lymphocytes, though limited amounts are secreted. It has no known role in host defense.
T lymphocytes
T lymphocytes are the effectors for cell-mediated immunity. They also serve as important regulators of both the humoral and cell-mediated immune systems and modulate the activities of nonlymphoid cells such as monocytes. Like B lymphocytes, each T-lymphocyte has a unique antigenic specificity. The diverse effector and regulatory functions of T lymphocytes are carried out by distinct lymphocyte subpopulations. CD4 T lymphocytes carry out immunoregulatory functions by the release of cytokines, some of which stimulate B-lymphocyte (IL-2, IL-4, IL-5) and T-lymphocyte (IL-2, IL-4) proliferation and differentiation, activation of monocytes (interferon-gamma), and proliferation of hematopoietic precursors of lymphoid and nonlymphoid cells (IL-3). Some lymphokines preferentially stimulate secretion of IgG1; others lead to the secretion of IgA and IgE. When CD4 TH lymphocytes proliferate, they differentiate into a variety of effector cells, termed TH1, TH2, TH17, TFH and Treg cells. Although the factors determining the differentiation of each of these types of effector cells have not been fully elucidated, the functions are relatively clearly understood. The TH1 cell secretes cytokines (IL-2, interferon-gamma, and tumor necrosis factor–alpha) that stimulate cell-mediated immune responses such as activation of macrophage bactericidal function, delayed-type hypersensitivity, and cytotoxicity. The TH2 cell secretes cytokines (IL-4, IL-5, IL-6, and IL-10) that drive B-cell proliferation and differentiation, resulting in antibody synthesis. These TH subsets are not mutually exclusive, but most infectious pathogens induce a response that is predominantly TH1 or TH2. In addition, there is cross-regulation of TH1 and TH2 cells. The TH1 cytokine IFN- gamma downregulates TH2 cells, whereas the TH2 cytokine IL-10 downregulates TH1 cells.
TH17 cells secrete cytokines (IL-17 and Il-22) that promote neutrophil accumulation, changes in barrier function, and inflammation. They are probably critically important in the host response to extracellular bacteria. TFH cells reside in the lymph nodes and spleen where they trigger B- cell activation, leading to germinal center formation and the production of antibody. Treg cells secrete cytokines (TGF-β and Il-10) that suppress immune responses and inflammation. Cytotoxic T cells (TC) express CD8 on their surface, not CD4. TC can kill target cells such as virus-infected host cells, tumor cells, or the cells of a histoincompatible tissue graft. TC cells reversibly bind to their targets by means of the T-cell antigen receptor as well as several other cell surface molecules.
Specific immune defects predispose to specific types of infections
Because each functional compartment of the immune system plays a specialized role in host defense, infections with certain microorganisms characteristically are found in association with specific types of immunodeficiency (Table 2). For example, patients with abnormalities of cell-mediated immunity characteristically develop pneumocystis pneumonia, disseminated fungal infections, mucocutaneous candidiasis, chronic or disseminated viral infections, and severe mycobacterial disease. Patients with defects of antibody or complement more often have infections with pyogenic encapsulated bacteria. Patients with phagocytic defects develop bacterial and fungal infections of the skin and reticuloendothelial system. These distinctions may be blurred, however, because the host’s defense against any given microorganism depends on the successful integration of all components of the immune system. Thus, a rare patient with an antibody deficiency can develop pneumocystis pneumonia or chronic enteroviral meningitis, whereas patients with deficiencies of cell-mediated immunity can develop pyogenic bacterial infections. Recurrent infections at a single anatomic site should always prompt consideration of other predisposing conditions such as ciliary dyskinesia, cystic fibrosis, or bronchial obstruction. The key to understanding the susceptibility of a particular compromised host is to understand the immune defects of that host. These are most easily illustrated by the primary immunodeficiency diseases in which a single gene disorder causes one change in immune function (Table 3). Other disorders which predispose the host to develop infection often are due to multiple factors. For example, cancer chemotherapy can cause neutropenia and mucositis, each of which will increase the host’s susceptibility to infection.
Table 2.
Patterns of Illness Associated with Primary Immunodeficiency Diseases
| Disorder | Illnesses | |
|---|---|---|
| Infection | Other | |
| Antibody | Sinopulmonary (pyogenic bacteria, viruses) | Autoimmune disease (auto antibodies, inflammatory bowel disease) |
| Gastrointestinal (enterovirus, giardia) | ||
| Cell-mediated immunity | Wide range of microorganisms, including opportunistic pathogens | |
| Pneumonia, (pyogenic bacteria, Pneumocystis jirovecii, viruses) | ||
| Gastrointestinal (viruses) | ||
| Skin, mucous membranes (fungi) | ||
| Complement | Sepsis and other blood-borne (streptococci, pneumococci, neisseria) | Autoimmune disease (systemic lupus erythematosus, glomerulonephritis) |
| Phagocytes | Skin, reticuloendothelial system (staphylococcus, enteric bacteria, fungi, mycobacteria) | |
Table 3.
Illustrative Primary Immunodeficiency Diseases
| Disorder of host defense | Disease |
|---|---|
| Antibody | X-linked agammaglobulinemia |
| Common variable immunodeficiency* | |
| Selective IgA deficiency | |
| Wiskott-Aldrich syndrome* | |
| Antibody and cell-mediated immunity | Severe combined immunodeficiency |
| Cell-mediated immunity | Chronic mucocutaneous candidiasis |
| Phagocytes | Congenital neutropenia |
| Chronic granulomatous disease | |
| Congenital asplenia (Ivemark syndrome) | |
| Leukocyte adhesion deficiency | |
| Chediak-Higashi syndrome | |
| Complement | Classical pathway (C1q,r,s; C4; C2; C3 deficiency) |
| Alternative pathway (factor D, factor I, factor H, properdin) | |
| Mannan-binding lectin pathway | |
| Terminal components (C5, C6, C7, C8, C9) |
May have associated defects of cell-mediated immunity
C. Primary Immunodeficiency Diseases
Disorders of Antibody
X-linked agammaglobulinemia
X-linked agammaglobulinemia (X-LA) is the prototypic disorder of humoral immunity that best illustrates the role of antibody in host defense. Male patients with this disease have no B lymphocytes and severe panhypogammaglobulinemia, but all other components of the immune system are normal. Boys with X-LA are protected by transplacentally acquired maternal IgG for the first 3 to 4 months of life. Thereafter, chronic and recurrent infections are the predominant clinical manifestation of X-LA. Otitis media, pneumonia, diarrhea, and sinusitis occur most often, usually in combination. S. pneumoniae, H. influenzae, and S. aureus are the most frequently identified bacterial pathogens, but nontypeable, unencapsulated H. influenzae, Salmonella, Pseudomonas, and Mycoplasma infections occur with increased frequency (14). Infections are not limited to mucosal surfaces, as bacterial meningitis, sepsis, and osteomyelitis occur in as many as 10% to 15% of untreated patients. Enterovirus infections are a particularly difficult clinical problem in patients with X-LA. This group of viruses (coxsackie, enteric cytopathogenic human orphan (ECHO), and polio viruses) tends to cause chronic diarrhea, hepatitis, pneumonitis, and meningoencephalitis in patients with X-LA. The peculiar susceptibility to enteroviruses is perhaps best illustrated by the fact that these children are at risk of developing a chronic infection after receiving a live poliovirus vaccine or even being exposed to someone who was recently immunized (15). In an agammaglobulinemic host, viral replication can continue long enough for there to be reversion to wild-type virus with the subsequent development of paralytic poliomyelitis. In some instances, enterovirus infections take the form of a dermatomyositis-like syndrome consisting of rash, edema of subcutaneous tissues, and muscle weakness (16). Enterovirus infections often are fatal in patients with X-LA (17). There is also an unexplained susceptibility to chronic skin infections caused by Helicobacter and Campylobacter (18).
Common variable immunodeficiency
Common variable immunodeficiency (CVID) is a heterogeneous group of disorders that is characterized by hypogammaglobulinemia and impaired antibody responses. Additional immunologic abnormalities such as T-cell dysfunction and autoimmune diseases are expressed variably. Most patients do not manifest symptoms until after the first decade of life, but some patients present in early childhood or infancy. It has become increasingly apparent that the clinical phenotype of CVID can be the result of a wide variety of immunologic abnormalities. For example, genetic analyses have identified mutations of Btk (the gene causing XLA), SH2D1A (the gene causing the X-linked lymphoproliferative syndrome) and ICOS (the “inducible stimulator” on activated T cells) among small numbers of individuals previously identified as having CVID (19). It is likely that such analyses will help to define subgroups of CVID patients who differ in presentation and outcome, and perhaps lead to novel therapies. Because the one common abnormality of immune function in CVID is antibody deficieny, it is not surprising that the most frequent infections in CVID are similar to those seen in X-LA (20, 21). Chronic or recurrent pneumonia, bronchitis, and/or sinusitis occur in the majority of patients, and some eventually develop chronic pulmonary dysfunction. Most of the identified respiratory tract pathogens are encapsulated bacteria. In contrast to patients with X-LA, disease of the gastrointestinal tract occurs with almost equal frequency as disease of the respiratory tract in patients with CVID. As many as 30% to 60% of patients with CVID have chronic diarrhea. An infectious agent is identified in approximately one-half of these patients, but many of the others have autoimmune/ inflammatory bowel diseases. The most frequently documented gastrointestinal pathogen is Giardia lamblia. Bacterial overgrowth of the small bowel is an important cause of chronic diarrhea in patients with CVID; enteroviruses are less of a problem.
Selective IgA deficiency
Selective IgA deficiency is diagnosed by convention when a patient has a serum IgA level less than 7 mg/dL with normal levels of other immunoglobulin classes, normal serum antibody responses, and normal cell-mediated immunity. The majority of patients with IgA deficiency lack both serum and secretory IgA, but rare cases occur in which there is a deficiency of secretory but not serum IgA. Unlike the other major serum immunoglobulin classes, IgA is largely silent as a mediator of inflammatory responses, but IgA provides an antimicrobial defense by inhibiting microbial adherence, and neutralizing viruses and toxins. Some patients with selective IgA deficiency are more susceptible to infection, although disagreement exists about the relative risk of infection that IgA deficiency imposes on the host (22, 23). Among patients referred to tertiary care centers for evaluation of recurrent sinopulmonary infections, the incidence of IgA deficiency is significantly higher compared with that of the general population. However, many apparently asymptomatic IgA deficient individuals have been identified by population-based screening (24). As might be expected by its role as the predominant secretory immunoglobulin, the most common infections in IgA-deficient patients occur on mucosal surfaces. Otitis media, sinusitis, bronchitis, pneumonia, and diarrhea are common; meningitis and bacterial sepsis are rare. The second major target for infections in IgA-deficient patients is the gastrointestinal tract. Chronic diarrhea is often idiopathic; Giardia is the most frequently identified microbial pathogen.
Disorders of antibody and cell-mediated immunity
Severe combined immunodeficiency (SCID) causes absence or near-absence of humoral and cell-mediated adaptive immunity, but all components of the innate immune system are intact (25). This heterogenous group of disorders is almost always caused by defects intrinsic to the T lymphocyte (e.g., mutations in cytokine receptor genes) but affects both cellular and humoral immunity because of the essential role of CD4 T cells in controlling virtually every aspect of adaptive immunity. For example, the absence of CD4 T lymphocytes interferes with the growth and differentiation of B lymphocytes, as well as the growth and differentiation of T cells. Affected children have severe deficiencies of all T lymphocyte subsets and have virtually no T-lymphocyte function. They may or may not have normal numbers of B lymphocytes in the peripheral blood, but those B cells do not differentiate into plasma cells and the children do not make antibody responses to vaccines or infections. Infants with SCID almost always become symptomatic within the first months of life. Unless the immunodeficiency is treated, most die from infections within the first year of life. These children are susceptible to virtually any microbial pathogen (26). Just as in patients with X-LA, they are susceptible to infection by encapsulated bacteria and enteroviruses. However, they are also susceptible to a much wider array of viruses. Pathogens as diverse as adenovirus, rotavirus, cytomegalovirus (CMV), varicella-zoster virus (VZV) and respiratory syncytial virus (RSV) can cause chronic or fatal infections. Fungal infections (e.g., aspergillosis and candidiasis) are problematic because this group of patients lack the CD4 TH lymphocyte production of interferon-gamma that is responsible for improving the intracellular killing of phagocytic cells. Patients with SCID also can be infected with opportunistic pathogens such as Pneumocystis jirovecii, Mycobacterium avium intracellulare, and even Mycobacterium bovis Bacille Calmette-Guerin (BCG) from immunization. Curative treatment of most of these infections requires definitive treatment of the underlying immunodeficiency by bone marrow transplantation.
Disorders of phagocytes
Chronic granulomatous disease is a disorder of intracellular killing that is caused by defects in the NADPH oxidase-dependent respiratory burst system of phagocytic cells. Neutrophils and monocytes of affected individuals are able to follow chemotactic signals and ingest microbial pathogens. Once ingested, organisms such as the pneumococcus or group A Streptococcus are killed efficiently because those bacteria produce hydrogen peroxide and thus compensate for the lack of respiratory burst. However, catalse-producing microbes are not killed. This leads to susceptibility to a restricted group of microorganisms including Staphylococcus aureus (S. aureus), Burkholderia cepacia, Serratia marcescens and other gram-negative rods, Nocardia, Aspergillus, and Mycobacteria. The most frequent sites of infection are the lungs, lymph nodes, skin, perianal area and gingivae (27–29). Phagocytes with live intracellular organisms may travel to reticuloendothelial tissues such as liver and spleen where micro or occasionally large abcesses occur. Typically, patients develop granulomas at the site of infections as an increasing number of phagocytes and T lymphocytes are drawn to the area of chronic infection. Aspergillus infections of the lung have historically had a dismal prognosis, and the infections spread from lung to overlying ribs to the vertebrae. Fortunately, outcomes have dramatically improved with the relatively recent use of interferon-gamma to increase the killing capacity of phagocytes via a non-NADPH-dependent pathway combined with the use of non-nephrotoxic orally administered antifungals such as itraconazole and posaconazole.
The first gene to be identified as a cause of congenital neutropenia was elastase 2 (30), but there are at least 15 gene defects now known to be causative. Patients present early in life with cellulitis, perirectal abcesses, stomatitis and gingivitis. Pneumonia, sepsis, and meningitis can also occur (31). As expected, the risk of infection varies inversely with the neutrophil count, and the highest risk is with absolute neutrophil counts less than 500/mm3. Infections are caused by S. aureus; gram-negative rods including Klebsiella, Pseudomonas, and Escherichia coli (E. coli), and rarely fungi. This group of organisms causes disease in these patients because of their prevalence on the skin and gastrointestinal tract; but not related to the presence or absence of catalase.
Disorders of complement
Diminished C3 activation via the classical pathway can be caused by an autosomal deficiency of C1q, C1r, C1s, C4, C2 or C3. Each of these disorders is associated with sepsis and other bloodstream infections (32–34). The risk is highest for individuals with C3 deficiency since they are unable to mount complement effector function via the classical, the alternative or the MBL pathway. The most common pathogens are Streptococcus pneumoniae (S. pneumoniae), Haemophilus influenzae (H. influenzae), gram negative Enterobacteriaceae, Neisseria meningitidis (N. meningitidis) and staphylococci. Individuals with these deficiencies also have a propensity to develop immune complex mediated diseases such as systemic lupus erythematosus (SLE) and glomerulonephritis, at least in part because the inability to bind C3b to circulating IgG and IgM-containing immune complexes impairs their clearance from the bloodstream.
Diminished activation of the terminal complement components/membrane attack complex can be caused by an autosomal deficiency of C5, C6, C7, C8 or C9. Individuals with any of these disorders have a markedly increased risk for neisserial infections – including meningococcemia, meningococcal meningitis and disseminated gonococcal infections (32, 33). Despite the fact that C5a is an important chemoattractant, only a single C5a deficient patient has been reported to have symptoms consistent with defective chemotaxis – recurrent infections of the skin and subcutaneous abscesses. The propensity to develop systemic neisserial infections is so great with these complement deficiencies, that 1 in 7 patients with non-epidemic invasive meningococcal infection will be found to have a terminal complement component deficiency. The chances increase to almost 1 in 3 for patients with more than one episode of invasive meningoccal disease.
Diminished activation of the MBL pathway, caused by MBL deficiency, increases the susceptibility of children under the age of 2 years to acute respiratory tract infections, as assessed in population-based studies (35, 36). No studies have yet reported information about the microbial pathogens seen in such children. Similar studies in adults have failed to show any correlation with risk for infection or death from infection.
Diminished activation of the alternative pathway can be caused by deficiencies of factor D, factor I, factor H or properdin. The latter, a disease with X-linked inheritance, is the most common defect of the alternative pathway, but all of these disorders are very rare (or at least rarely diagnosed). Patients with properdin deficiency have a propensity to develop meningococcal meningitis, and invasive S. pneumoniae infections to a lesser degree (32, 33). Patients with factor D deficiency present in childhood with systemic infections, usually caused by N eisseria or S. pneumoniae. Those with factor I deficiency consume so much C3 that their presentation is identical to that of patients with C3 deficiency, developing invasive infections caused by S. pneumoniae, H. influenzae, Enterobacteriaceae, N. meningitidis and staphylococci. Those with factor H deficiency appear to be most susceptibile to autoimmune/chronic inflammatory diseases (especially hemolytic uremic syndrome), but also to meningococcal infections.
Disorders of NK cells
Natural killer cell deficiency results in susceptibility to chronic and severe viral infections caused by herpes simplex virus, varicella-zoster virus, cytomegalovirus, Epstein-Barr virus, and papillomavirus infections (37). Patients die at a young age as a direct consequence of these infections or from viral-induced malignancy.
Other disorders of innate immunity
The TLR-associated adaptor protein Myd88 and the kinase IRAK-4 are required for transducing the signal of each of the TLRs to the nucleus with subsequent synthesis of inflammatory cytokines. Although mutations of either Myd88 or IRAK-4 knocks out all TLR function, affected patients have a very limited clinical susceptiblity of recurrent life-threatening bacterial infections, particularly invasive pneumococcal disease, and bacterial infections of the upper respiratory tract and skin. The susceptibility to infection seems to wane with age, as there are virtually no invasive bacterial infections in patients over the age of 14. More surprising, these patients do not have severe viral, parasitic or fungal infections. The other striking feature of Myd88/IRAK-4 deficiency is that patients have an impaired ability to mount an inflammatory response, so that it is typical to see little or no fever, leukocytosis or elevation in CRP level even with invasive bacterial infections (38).
The spleen is a phagocytic filter which efficiently removes microorganisms from the blood. Asplenia may be caused by congenital malformation or surgery, and functional asplenia is most often caused by vaso-occlusive events in patients with sickle cell disease. Regardless of the etiology, the lack of splenic function causes susceptibility to pneumococcal sepsis, which can progress rapidly and be fatal in up to 50% of cases (39, 40).
Chronic mucocutaneous candidiasis can be caused by a variety of genetic defects of innate immunity including IL-17 and IL-17 receptor mutations, gain of function STAT1 mutations (that cause an increase in interferon production with secondary inhibiton of TH17 development), Dectin -1 deficiency (a receptor on phagocytes that recognizes beta-glucans on fungal cell walls) and CARD9 deficiency (a signal transducer of the Dectin-1 signal) (41).
D. Secondary Immunodeficiencies
Secondary immunodeficiencies are those that are related to another illness or condition or occur as a result of treatment for such a condition. In this section, we review secondary immunodeficiencies and other compromises in host defenses that result because of treatment with a variety of agents. The consequences of treatment for malignancy, of prevention and treatment of allograft rejection, and of the treatment for rheumatologic and autoimmune diseases will be reviewed. The degree of immunodeficiency associated with various immunosuppressive agents used to treat a variety of conditions depends on the underlying condition, the doses of single agents, and drug combinations that may act synergistically.
Treatment-related
Corticosteroids are used to treat a variety of diseases because of their anti-inflammatory and immunosuppressive properties (42, 43). They have many effects on innate and acquired immunity. Corticosteroids impair trafficking of neutrophils and monocytes to sites of inflammation and inhibit macrophage and neutrophil phagocytic and microbicidal function (44, 45). They inhibit the production of almost all known cytokines (46). Corticosteroids markedly reduce the number of circulating dendritic and T cells and affect antigen presentation by impairing the effector functions of macrophages and dendritic cells (47–50). Their effects on the immune system are dose-dependent. At doses < 2 mg/kg, T lymphocytes numbers are slightly reduced (CD4+ > CD8+). Higher doses, > 2 mg/kg, result in suppression of lymphocyte activation and suppression of antibody production by B cells. Hence, corticosteroids predispose to infection in a dose-dependent manner (51, 52). The risk of infection is also determined by the underlying disorder and concomitant treatment with immunosuppressive agents. Viral (mainly herpesvirus), bacterial, and fungal (Candida) infections are encountered with greater frequency in patients treated with corticosteroids—up to 40 times greater (45). Pneumonia caused by P. jirovecii is the main opportunistic infection that occurs in patients treated with these agents. Reactivation of tuberculosis is also a potential complication of corticosteroid therapy.
Therapeutic interventions for neoplasia
When treating malignancy, the goal is to target mitotically active tumor cells as specifically as possible. However, in addition to destroying malignant cells, normal cells that are rapidly dividing will be affected by cytotoxic antineoplastic agents (chemotherapy). The primary non-malignant cells affected include bone marrow cells and cells of the gastrointestinal mucosa. Therefore, the main chemotherapy-associated toxicities contributing to infectious risk are myelosuppression and mucositis (53). Neutropenia is usually an unavoidable consequence of the treatment of malignancy and significantly increases a patient’s risk of infection (54). This risk increases with severity of neutropenia—the highest risk of infection being associated with an absolute neutrophil count < 100 cells/mm3 (55). In addition, chemotherapy results in chemotactic and phagocytic defects in neutrophils, further increasing the risk of severe infection (56, 57). The source of infection in the majority of patients is the patient’s endogenous microbiota that is enabled to translocate across mucocutaneous barriers secondary to chemotherapy-induced mucosal injury of the oral cavity and intestinal epithelium and due to indwelling vascular and urinary catheters (53). The course of mucositis after standard or high dose chemotherapy parallels that of neutropenia. The onset of mucositis occurs at the nadir of the neutrophil count and resolves with count recovery.
The constellation of defects in host defense, neutropenia, mucositis, and indwelling catheters, predispose the patient to bacterial, fungal, and viral (mainly herpes simplex) infections. In the 1980s, bacterial bloodstream infections were most often caused by gram negative bacteria, such as Pseudomonas sp. (58). Subsequently, a large study of 22,631 episodes of bacteremia occuring in 2,340 patients with underlying malignancy revealed that Gram-positive bacteria were the most prevalent pathogens in neutropenic and non-neutropenic patients (59). This shift from the previous predominance of Gram-negative organisms was attributed to empiric antibiotic regimens targeted to Gram negative organisms, common use of long-term indwelling vascular catheters and prophylactic antibiotics, such as fluoroquinolones. In recent years, studies of the epidemiology of bacterial infections in neutropenic cancer patients have revealed a trend back to Gram-negative bacteria and the emergence of highly resistant strains (60, 61). The risk of invasive fungal infection with Candida species or molds, such as Aspergillus, increases with the severity and duration of neutropenia (62, 63).
Lymphocyte depletion can occur as a complication of cytotoxic antineoplastic therapy (64, 65). Some cancers, such as Hodgkin’s lymphoma, are associated with lymphocyte dysfunction, however, significant T cell immunodeficiency is usually uncommon prior to initiation of cytotoxic therapy (66). Agents such as cyclophosphamide, administered as a single agent at a high intensity dose or as part of a multiagent dose intensive regimen, can cause profound depletion of the lymphocyte populations and predispose patients to opportunistic infections. Humoral immunity tends to be relatively spared from the effects of short courses of chemotherapy because of the long half-life of previously secreted IgG antibodies.
A variety of cytotoxic antineoplastic agents are used in combination to treat various malignancies. These agents are classified based on the mechanism by which they inhibit cell proliferation (Table 4). They all cause myelosuppression and most cause some degree of mucositis.
Table 4.
Host Immune Deficits and Infections Associated with Immunosuppressive and Chemotherapeutic Agents
| Agent Class | Effects on Immune System | Associated Pathogens |
|---|---|---|
| Corticosteroids | Decrease chemotactic activity to sites of inflammation; Inhibition of phagocytic, microbicidal, and T cell functions | Herpes virus, Candida sp. bacteria, P. jirovecii |
| Cytotoxic Drugs: methotrexate, f-fluorouracil (antimetabolites) cyclophosphamide, chlorambucil, melphalan (alkylating agents), doxorubicin, daunorubicin, idarubicin, mitoxantrone (anthracyclines), vincristine, vinblastine, cisplatin, bleomycin | Suppress bone marrow and significantly reduce counts at high doses; neutropenia; some cause lymphopenia, some cause significant mucositis | Bacteria (gram positive and negative), Candida, invasive mold infection with prolonged neutropenia |
| Purine analogs (eg. fludarabine, cladribine, pentostatin) | Neutropenia, lymphopenia, ± hypogammaglobulinemia | Encapsulated, gram positive, gram negative bacteria Herpesviruses (HSV, VZV, CMV), mycobacteria, Candida, Aspergillus, Cryptococcus |
| Azathioprine | Inhibits B and T cell proliferation, decreased antibody production, myelosuppresssion | Bacterial infections (leukopenia), herpes zoster, CMV, JC virus (PML*) hepatitis virus B and C exacerbation, P. jirovecii, Nocardia |
| Cyclosporine, tacrolimus | Inhibit production of IL-2 and other cytokines by CD4- positive T cells | CMV, EBV PTLD, JC virus (PML), BK polyomavirus nephropathy, hepatitis C virus reactivation |
| Mycophenolate mofetil | Inhibits B and T cell proliferation; decreased antibody production; leukopenia | Herpesviruses (HSV, VZV, CMV), hepatitis C virus and B virus reactivation, JC virus (PML), Candida mucocutaneous disease, P. jirovecii, Cryptococcus, Aspergillus, Mucor |
| Rapamycin (sirolimus) Everolimus | Inhibition of T cell activation and proliferation; inhibition of antibody production | Herpesviruses (HSV, VZV, CMV), Pneumocystis jiroveci pneumonia, EBV-associated PTLD**, BK polyomavirus nephropathy, JC virus (PML), Tuberculous and non-tuberculous mycobacterial infections |
| Other: Phenytoin | IgA deficiency or hypogammaglobulinemia | Bacteria (including encapsulated bacteria), respiratory viruses, enteroviruses, Giardia |
PML, progressive multifocal leukoencephalopathy;
PTLD, post-transplant lymphoproliferative disease
The antimetabolite antineoplastic agents include methotrexate, fluorouracil, and gemcitabine. Methotrexate is an inhibitor of dihydrofolate reductase that interferes with the synthesis of purine nucleotides and hence, with DNA synthesis, repair, and cellular replication. The major side effects of treatment with methotrexate include myelosuppression, causing significant neutropenia, and a dose-dependent, ulcerative mucositis (67, 68). High dose methotrexate (> 20 mg/kg) used in cancer therapy causes profound bone marrow suppression that also depresses primary and secondary cellular and humoral immune responses (69). As expected, opportunistic infections that arise in the setting of compromised T cell function, such as those caused by P. jirovecii, CMV, Cryptococcus, Histoplasmosa capsulatum (H. capsulatum), Nocardia sp., and varicella zoster virus have been reported (70–74). Many of these patients were also receiving corticosteroids. Long-term treatment with methotrexate may place one at risk for EBV-associated lymphoma (75, 76). The risk of infection with low dose methotrexate is not well established, but is lower given that the lymphocyte subsets and in vitro T cell mitogen responses are unaffected. The other commonly used antimetabolites are fluorouracil and gemcitabine. These are antimetabolites of the pyrimidine analog type and are cell cycle specific (S phase) in inhibiting DNA synthesis. Both agents can cause significant myelosuppression and mucositis.
The alkylating agents, cyclophosphamide, chlorambucil, and melphalan, induce cytotoxic effects by chemically modifying nucleotides, cross-linking DNA or RNA, and inhibiting protein synthesis. Depending on the dose and duration of treatment, treatment with alkylating agents can result in significant bone marrow suppression with a decline in neutrophil and T and B lymphocyte counts (64, 65). The tendency for alkylating agents to cause lymphopenia is enhanced by co-administration of corticosteroids. These cumulative negative effects on cellular host defense predispose recipients of alkylating agents to a variety of infections, including routine bacterial infections that cause pneumonia, sepsis, or urinary tract infection and opportunistic infections caused by P. jirovecii, fungi, Nocardia, VZV, and M. tuberculosis (64, 77, 78). Patients who are neutropenic or are treated concomitantly with high doses of glucocorticoids have an enhanced risk of infection (77, 78).
The anthracyclines, doxorubicin, daunorubicin, and idarubicin, are cytotoxic antineoplastic agents that are used to treat a variety of malignancies. They cause cytotoxicity by intercalating between DNA base pairs and by inhibiting topoisomerase II, resulting in inhibition of RNA and DNA synthesis. Neutropenia and mucositis are reported in a significant number of patients, depending on the agent used.
A variety of other classes of anti-neoplastic cytotoxic agents, including the vinca alkaloids, platinum compounds, taxanes, glycopeptides antibiotics, and topoisomerase inhibitors (Table 4) have similar effects to various degrees on the bone marrow and mucosa. Patients treated with these agents are primarily at risk for bacterial and candidal infections. This risk increases with the duration and depth of neutropenia and with the severity of mucositis (53, 79).
Purine analogs inhibit DNA synthesis and are used to treat a variety of hematologic malignancies. These agents induce severe immunosuppression, affecting multiple lineages of host defense: T and B lymphocytes, neutrophils, and monocytes (80). After treatment with purine analogs, a profound T cell lymphopenia, especially affecting CD4 cells, develops in 2–3 months and can persist for several years (80). Many patients develop neutropenia and a depletion of monocytes. Some patients may become hypogammaglobulinemic. Hence, a broad spectrum of infections is encountered in patients treated with purine analogs: bacterial infections (staphylococcal, streptococcal, gram negative rods, Listeria, Nocardia, Legionella, mycobacteria), opportunistic viral infections (herpes simplex, herpes zoster, CMV, EBV), and opportunistic fungal infections (P. jirovecii, Candida, and Aspergillus). Bacterial, fungal, and HSV infections occur early after treatment in the setting of neutropenia. Opportunistic infections, associated with depressed cell-mediated immunity, occur later after treatment. The type and stage of the underlying disorder, prior anti-neoplastic therapy, and concurrent treatment with steroids significantly influence the incidence of infectious complications. Listeria, P. jirovecii, and CMV infections occur more frequently in those treated concomitantly with corticosteroids.
Anti-lymphocyte antibody therapies
Anti-lymphocyte monoclonal antibody therapies belong to the larger group of therapies known as biologic immune response modulators (Tables 5 and 6). Compared to traditional therapies, these biologic agents do not cause global immunosuppression, because they selectively target cells and pathways. However, these agents do have secondary unintended effects on immune function that can compromise host defenses and lead to serious infections. Rituximab is a chimeric murine/human monoclonal antibody and ofatumumab is a human monoclonal antibody directed against CD20 antigen on B lymphocytes (81; Table 5). Binding to CD20 results in complement and/or antibody-dependent cellular cytotoxicity, with depletion of B lymphocytes except plasma cells. Rituximab is approved for the treatment of CD20-positive B-cell non-Hodgkin’s lymphoma. In addition, this agent is approved for the treatment of autoimmune disorders such as rheumatoid arthritis, Wegener’s granulomatosis, and microscopic polyangiitis. Ofatumumab is approved for the treatment of CLL. Immunologic effects of these CD20 directed cytolytic antibodies include B cell depletion for 6–9 months or longer and possibly hypogammablobulinemia. In addition to bacterial infections causing bronchitis, sinusitis, and pneumonia, a variety of viral infections, either new or reactivated and some severe and potentially fatal, have been reported with use of these agents: HSV, VZV, CMV, parvovirus B19, hepatitis B and C, enterovirus, JC virus, and West Nile virus (81) These infections may be delayed, occurring up to a year after treatment. For both of these agents, the FDA has issued boxed warnings for progressive multifocal leukoencephalopthy, PML, possibly resulting in death (82, 83) and for hepatitis B virus reactivation resulting in fulminant hepatitis, hepatic failure, and possibly death. Persistent and severe hypogammaglobulinemia has been reported in rare patients who have been treated with rituximab, especially if give in multiple cycles (84). These patients are at risk for the same infections (encapsulated bacteria and some viruses) as those seen in patients with X-linked agammaglobulinemia or common variable immunodeficiency, and are given intravenous immunoglobulin to prevent these infections (85).
Table 5.
Biologic Immune Response Modulators Targeting Lymphocytes
| Immune Response Modulator |
Agent(s) | Target Cell | Mechanism(s) | Immunologic Effects |
Reported Infectious Complications |
Treatment Indications |
|---|---|---|---|---|---|---|
| Anti-thymocyte globulin | ATG: Thymoglobulin (rabbit) Atgam (equine) |
T lymphocyte |
|
|
Herpes virus infections, particularly CMV | Organ transplant rejection Graft versus host disease prophylaxis Aplastic anemia |
| Monoclonal antibodies to T cells | OKT3 | T lymphocyte | Binds to TCR-CD3 complex on T cells and blocks T cell proliferation and function | Profound T cell lymphopenia and poor function | Herpesviruses (HSV CMV EBV-associated LPD*) Pneumocystis jirovecii Listeria Mycobacteria Nocardia Toxoplasmosis |
Acute organ transplant rejection |
| Basiliximab | T lymphocyte | Binds to IL-2 receptor alpha chain (CD25), inhibiting lymphocyte activation | Impairment of antigen-specific cytotoxic T cell response | Incidence of infections not increased when added to dual immunosuppression regimens (steroids/cyclosporine) | Organ transplant rejection Graft versus host disease |
|
| Monoclonal antibody to B cells | Rituximab Ofatumumab |
B cell from pre-B cell to pre-plasma cell stage | B cell death by complement-, cell mediated-, and antibody- dependent cellular cytotoxicity |
|
Hepatitis B virus Hepatitis C virus JC virus (PML**) Herpesviruses (HSV, VZV, CMV) Parvovirus B19 West Nile virus Enteroviral encephalitis If hypogammaglobulinemia severe: Viral and bacterial sinusitis and pneumonia |
B cell non-Hodgkin’s lymphoma CLL Autoimmune disorders: RA, Wegener’s granulomatosis, microscopic polyangiitis |
| Belimumab | B lymphocytes | Inhibits the binding of soluble human B lymphocyte stimulator protein (BLyS) to its receptors on B cells |
|
Cellulitis Pneumonia JC virus (PML) CMV pneumonia Coccidiodomycosis |
Systemic lupus erythematosus | |
| Monoclonal antibody to T and B cells | Alemtuzumab (Campath and Lemtrada) | T and B lymphocytes, plus monocytes, macrophages, and natural killer cells | Antibody-dependent and complement-mediated cell lysis after binding to CD52 | Profound and prolonged depletion of T and B lymphocytes, natural killer cells, and monocytes | Bacterial sepsis, pneumonia Herpesviruses (HSV, CMV, VZV EBV-associated LPD*) Hepatitis B virus Hepatitis C virus Adenovirus JC virus PML IFI§: aspergillosis, mucormycosis, histoplasmosis, cryptococcosis, pneumocystosis Nocardia Mycobacteria Toxoplasmosis |
|
| Fusion proteins disrupting T cell costimulation | Abatacept | T lymphocyte | Binds to CD80 and CD86 antigen presenting cells | Suppresses T cell activation | Bacterial pneumonia, Cellulitis, urinary tract infection | Refractory rheumatoid arthritis Juvenile idiopathic arthritis |
LPD, lymphoproliferative disease;
PML, progressive multifocal leukoencephalopathy,
IFI: invasive fungal infection
Table 6.
Biologic Immune Response Modulators Targeting Cytokines and other Immune Mediators
| Immune Response Modulator | Agent | Targeted Immune Mediator | Mechanism(s) | Immunologic Effects | Reported Infectious Complications | Treatment Indications |
|---|---|---|---|---|---|---|
| Anti-cytokine therapies | Anakinra | IL-1 receptor | Recombinant human IL-1 receptor antagonist protein, IL-1Ra, competitively binds to IL-1 receptor | Inhibits immune and pro-inflammatory actions of IL-1 | Increased risk of serious bacterial infection with doses ≥ 100 mg/day (cellulitis, pneumonia) | -Moderate to severe rheumatoid arthritis - NOMID* - TRAPS** |
| Tocilizumab | IL-6 receptor | Competitively blocks interaction of IL-6 with its receptor | Interferes with proliferation and differentiation of T cells and terminal differentiation of B cells | Bacterial pneumonia, cellulitis, sepsis Herpes zoster virus Tuberculous and nontuberculous mycobacteria Pneumocystis jirovecii pneumonia Invasive fungal infections |
-Moderate to severe rheumatoid arthritis -- Juvenile rheumatoid and idiopathic arthritis (JIA) | |
| Infliximab (chimeric human mouse anti-TNFα mAb) Adalimumab (human anti-TNFα mAb) Golimumab (human anti-TNFα mAb) Etanercept (soluble TNFα receptor fusion protein) Certolizumab (pegol-pegylated Fab fragment of human mAb) |
TNF | Bind to TNF-α | Impairment of differentiation of monocytes to macrophages, macrophage and phagosome activation, recruitment of neutrophils and macrophages, formation and maintenance of granulomas | Active and latent tuberculosis Bacterial pneumonia Herpes zoster virus Tuberculosis Non-tuberculous mycobacteria Listeria Legionella Nocardia Hepatitis B virus Hepatitis C virus Invasive fungal infections |
-Rheumatoid arthritis -Psoriatic arthritis -Seronegative spondyloarthropathies -Inflammatory bowel disease -Sarcoidosis |
|
| Inhibitors of leukocyte migration | Natalizumab | Alpha 4 integrin | Blocks integrin association with vascular receptors, limiting adhesion and transmigration of leukocytes from vasculature into tissues | Reduction of specific inflammatory cell populations in target tissues | PML HSV and VZV encephalitis and meningitis | -Multiple sclerosis -Crohn’s disease Chronic moderate to severe plaque psoriasis |
| Fingolimod | Sphingosine phosphate 1, 3, 4, and 5 receptors | Fingolimod-phosphate binds to sphingosine phosphate 1, 3, 4, and 5 receptors | Blocks lymphocyte egress from lymph nodes | PML Cryptococcal meningitis | Multiple sclerosis Disseminated primary herpes zoster virus Herpes simplex encephalitis |
|
| JAK Inhibitor | Tofacitinib | Janus associated kinase (JAK) inhibitor | Inhibition of JAK prevents the phosphorylation and activation of Signal Transducers and Activators of Transcription (STATs) | -Prevents cytokine and growth factor mediated gene expression and intracellular activity of immune cells -Reduces circulating NK cells and serum immunoglobulin levels |
Bacterial pneumonia and cellulitis TB Herpes zoster EBV PTLD (in renal transplant) Cryptococcus PCP CMV BK virus |
Moderate to severe RA for patients with inadequate response to or intolerant of methotrexate |
| Monoclonal antibodies to complement | Eculizumab | C5 | Binds complement protein C5, preventing cleavage into C5a and C5b | Inhibits terminal complement activation | Bacteremia/sepsis: Meningococcal infections (Neisseria meningitidis) S. pneumoniae H. influenzae |
-Atypical hemolytic uremic syndrome -Paroxysmal nocturnal hemoglobinuria |
Neonatal Onset Multisystem Inflammatory Disease
Tumor necrosis receptor-1 associated periodic syndrome
Alemtuzumab is a humanized monoclonal IgG1 antibody directed against the CD52 cell surface glycoprotein approved as therapy for B-cell chronic lymphocytic leukemia (CLL) and peripheral and cutaneous T cell lymphomas (Table 5). It was also used as a conditioning agent in hematopoietic stem cell transplantation, for induction of immunosuppression or treatment of acute rejection in solid organ transplantation, for rhematoid arthritis, and for the prevention of graft-versus-host-disease (86). Lemtrada is used for the treatment of relapsing and remitting multiple sclerosis (87). Both of these agents are not commercially available and can only be obtained through the manufacturer via a restricted distribution program because of the risk of autoimmunity, cytopenias, infusion reactions, serious infections, and malignancies. The monoclonal antibody binds to CD52 antigen on the surface of malignant lymphocytes and causes cell lysis through complement activation and antibody-dependent cell-mediated toxicity (88). CD52 is also expressed on the surface of non-malignant T- and B-lymphocytes, monocytes, macrophages, natural killer cells, some granulocytes and normal bone marrow cells; therefore, cell destruction is not restricted to the malignant cell and significant impairment in cellular host defenses can occur. Profound and long-lasting depletion of mature B- and T-lymphocytes, natural killer cells, and monocytes occurs after treatment with alemtuzumab (89). Treated patients develop a profound lymphopenia by 1–2 weeks after initiation of treatment that may persist for over 1 year (90). Neutropenia (0.5 × 109/l) occurs in one-third of pateints around 4 weeks of therapy, but usually recovers in 2 to 3 weeks (91). As a consequence, the infections encountered are non-opportunistic and opportunistic. The incidence of infectious complications has been noted to range from 35 to 65%. However, the majority of studies reporting these data include patients with lymphoproliferative disorders who were pretreated with other agents, such as purine analogues, rituximab, and alkylating agents (92).
In a recent study reporting on infectious complications associated with alemtuzumab use for lymphoproliferative disorders, non-opportunistic bacterial infections causing sepsis, pneumonia, and catheter-related bacteremia were commonly encountered (86). Fifty-six percent of patients developed an opportunistic infection during the study period. Herpesvirus infections (HSV, VZV, CMV) were most common. CMV reactivation with resulting viremia is a well-described complication of therapy with alemtuzumab with a reported incidence as high as 50% (93, 94). Other opportunistic infections reported with alemtuzumab treatment include adenovirus infection, PML, invasive pulmonary aspergillosis, disseminated histoplasmosis and cryptococcosis, pneumocystosis, tuberculosis, cerebral toxoplasmosis, and disseminated acanthamebiasis (86). CMV reactivation and invasive aspergillosis appear to be the most commonly reported opportunistic infections in the setting of lymphoproliferative disease.
Immunosuppressive therapy for the prevention and treatment of allograft rejection after organ transplantation
Maintenance immunosuppressive therapy is administered to organ transplant recipients to help prevent acute rejection. The maintenance regimen usually consists of a combination of immunosuppressive agents with different mechanisms of action. Currently, most transplant centers use a regimen consisting of prednisone, an anti-metabolite [azathioprine or mycophenolate mofetil (MMF), mycophenolic acid (MPA)], and a calcineurin inhibitor (cyclosporine or tacrolimus). For renal transplantation, if a reduced dose or delayed introduction of a calcineurin inhibitor strategy is employed for induction immunosuppression, an antilymphocyte antibody treatment, such as rATG, basiliximab or alemtuzumab can be added to the regimen. The level of immunosuppression is gradually decreased over time to lower the risk of infection and malignancy while maintaining sufficient suppression to prevent organ rejection.
Antimetabolites
Azathioprine is a precursor of 6-mercaptopurine that inhibits purine biosynthesis and hence, DNA, RNA, and protein synthesis (Table 4). The effects of azathioprine include a decrease in circulating T and B lymphocytes, decrease in immunoglobulin production, diminished IL-2 secretion, and myelosuppression (95). Leukopenia is the most serious side effect of azathioprine. It is approved for the prevention of rejection in renal transplant recipients and for the treatment of rheumatoid arthritis. Infections reported in patients taking azathioprine are bacterial infections in the setting of leukopenia, herpes zoster, exacerbation of hepatitis B and C virus infections and opportunistic infections such as PML caused by JC virus, Nocardia, P. jirovecii pneumonia, and CMV viremia (96, 97).
Azathioprine has been used to prevent allograft rejection since the early 1980s. Several large trials comparing azathioprine to MMF have shown that MMF is superior to azathioprine in reducing the number of episodes of transplant rejection in heart, kidney, and liver transplant recipients (98–100). As a result, most transplant centers have switched to using MMF as part of their immunosuppressive regimen. MMF interferes with the de novo synthesis of purine nucleotides and in this way inhibits primarily T cell proliferation. In addition, MMF inhibits B cell proliferation and results in decreased antibody production (101, 102). Because of its potent inhibition of lymphocyte proliferation, treatment with MMF predisposes to infections associated with depressed cell-mediated immunity, such as herpes simplex, herpes zoster, and CMV (Table 4). A higher incidence of tissue invasive CMV disease has been reported in renal and heart transplant recipients treated with MMF particularly in those patients receiving > 2 g of MMF per day (98, 103). However, clinical trials of liver and lung transplant recipients receiving MMF failed to show an increased incidence in CMV infection or disease (104, 105). Interestingly, mycophenolate exhibits an anti-microbial effect against P. jirovecii (106). Renal transplant patients taking MMF had no episodes of Pneumocystis in a randomized trial comparing MMF to azathioprine for the prevention of acute rejection (99). Heart transplant patients receiving MMF had a higher rate of acute cholestatic hepatitis due to hepatitis C virus. No effect of MMF on bacterial infections in organ transplant recipients has been documented. Mycophenolate is also employed as a potential steroid -sparing agent in the treatment of a variety of autoimmune diseases.
Cyclosporine and tacrolimus
Organ allograft survival has improved significantly since the introduction of cyclosporine in the 1980s and tacrolimus in the 1990s. In addition, these agents are becoming increasingly popular for the treatment of a variety of rheumatic diseases. Cyclosporine is an 11 amino acid cyclic peptide and tacrolimus is a macrolide antibiotic. They bind to intracellular proteins called immunophilins--cyclosporine binds to cyclophilins and tacrolimus to FK binding proteins. The complex between drug and immunophilin inhibits calcineurin, a calcium and calmodulin dependent phosphatase. Hence, these agents are commonly referred to as calcineurin inhibitors. This inhibition of calcineurin results in prevention of translocation of a family of transcription factors, nuclear factor of activated T cells or NFAT, into the nucleus. As a consequence, transcription of a variety of cytokine genes involved in T cell activation is inhibited. The calcineurin inhibitors primarily affect T-helper cells, although some inhibition of T-suppressor and T-cytotoxic cells may occur.
Over the past 2 decades, calcineurin-inhibitors have become the cornerstone of immunosuppressive therapy in the organ transplant population. These agents are usually combined with corticosteroids and MMF. A large European randomized multicenter trial comparing the efficacy of tacrolimus plus low dose corticosteroids versus a conventional multidrug cyclosporine-based regimen (corticosteroids plus azathioprine) to prevent allograft rejection in liver transplant recipients revealed a similar incidence of infection in patients receiving the tacrolimus or cyclosporine-based regimens (107). The incidence of sepsis was approximately 20% in both groups and the incidence of CMV infection ranged from 15 to 25% with a lower incidence in the tacrolimus treated patients. Despite an immunosuppressive effect that is estimated to be 36 to 100 times more potent than that of cyclosporine A, tacrolimus has been associated with fewer cytomegalovirus infections compared with cyclosporine A-containing regimens (107, 108). This is likely due to the fact that the incidence of rejection is lower with tacrolimus compared to cyclosporine. Hence, the requirement for additional immunosuppression is lower (107, 109).
Other viral infections such as EBV, hepatitis C virus, and polyomavirus have been linked to treatment with the calcineurin inhibitors. However, none of these infections are linked with a particular agent per se but likely arise as a result of the cumulative effect of immunosuppression. The more intense the immunosuppressive regimen, the more likely a patient may acquire or reactivate one of these infections. Anti T cell antibody therapy is the most significant component of the immunosuppressive regimen contributing to the risk of CMV infection and EBV-related PTLD (110–112).
Patients on potent immunosuppressive regimens are at risk for fungal infections. The majority of these infections are caused by Candida and Aspergillus species. Candida infections often arise in the setting of neutropenia and compromised mucocutaneous barriers. Susceptibility to Aspergillus infections is influenced by the type and intensity of immunosuppressive regimens. High dose steroids and OKT3 monoclonal antibody therapy are known to confer an increased risk for invasive aspergillosis (113). Interestingly, the calcineurin inhibitors possess in vitro activity against Aspergillus species (114). Because invasive aspergillosis continues to occur in patients treated with these agents, the immunosuppressant effects of multidrug regimens predominate over the antifungal effects in vivo. There is evidence in animal models and in humans that calcineurin inhibitors may alter the pathogenesis of aspergillus infection with less dissemination (114, 115). Similar observations have been made for cryptococcal infection in organ transplant recipients (116).
Sirolmius (rapamycin) and everolimus are macrolide antibiotics used to prevent rejection in organ transplant patients. The drugs bind intracellularly to the FK binding protein-12 (FKBP-12). This complex binds to and inhibits a key regulatory kinase, mammalian target of rapamycin, mTOR, which regulates translation of mRNA required for cell division. A result of this interaction is the inhibition of T lymphocyte activation and proliferation and an inhibition of antibody production. Patients treated with rapamycin are particularly at increased risk for infections with intracellular pathogens (Table 4). They probably are protected from extracelluar pathogens because of continued production of antibody from pre-existing plasma cells. Interestingly, several studies have revealed that treatment with sirolimus and everolimus are associated with a decreased incidence of CMV infection. One study demonstrated a lower incidence of CMV infection in renal transplant recipients treated with rapamycin, MMF, and corticosteroids compared to cyclosporine replacing rapamycin in the same regimen (117). Two reviews of mTOR-inhibitor-containing regimens for the prevention of rejection in organ transplant recipients revealed that patients treated with these regimens had a lower incidence of CMV infection compared to regimens that lacked an mTOR-inhibitor (118, 119). Rapamycin possesses potent in vitro antifungal activity that translates into a beneficial clinical effect (120).
OKT3 (muromonab-CD3) is a murine IgG2a monoclonal antibody that binds the CD3-epsilon chain of the T cell receptor-CD3 complex on T cells (Table 5). OKT3 has been used for induction immunosuppressive therapy and for the treatment of acute or steroid resistant allograft rejection in transplant recipients. In vivo, OKT3 reacts with most peripheral blood T cells and T cells in tissues and causes a rapid and profound decrease in lymphocytes (121). T cells are not detectable between 2 to 7 days after administration, but reappear rapidly and reach pre-treatment levels within a week after termination of treatment. The antibody also causes T cell receptor modulation that interferes with T cell activation. Patients treated with OKT3 are at a significant risk for infectious complications, especially herpesvirus (HSV and CMV) infections that require functioning cytotoxic T cells for control of infection. In a prospective study that investigated risk factors of CMV disease in renal transplant recipients, treatment with OKT3 increased the risk of CMV disease by five fold in CMV-seropositive transplant patients (122). OKT3 administration is also associated with an increased risk of PTLD that in most transplant patients is EBV associated (112). The impairment of T-cell cytotoxic function allows for the proliferation and transformation of EBV-infected B lymphocytes. The risk of transformation is highest when OKT3 is utilized for the treatment of rejection (123). Other infections related to depressed T cell function induced by OKT3 include fungal infections, such as aspergillosis, cryptococcosis, and infections caused by P. jirovecii, Listeria, mycobacteria, Nocardia, Toxoplasma gondii (Orthoclone OKT3 product information page 1–19). Routine bacterial infections causing pneumonia and sepsis are also encountered.
Anti-thymocyte globulin (ATG) is a polyclonal antibody preparation of rabbit or equine origin that is used for the prevention or treatment of rejection in renal transplant recipients in conjunction with other immunosuppressive therapy. In addition, ATG has also been used in the field of hematologic malignancies to treat moderate or severe aplastic anemia, as part of conditioning regimens prior to bone marrow transplantation, or for the prevention of graft-versus-host disease (GVHD). The exact mechanism by which ATG causes immunosuppression is not known but is likely similar to the mechanism employed by OKT3. ATG acts on a variety of T cell antigens resulting in depletion of thymus-dependent lymphocytes and suppression of T cell activation (Product Information ATGAM(R) IV injection, 2005; 334). Rabbit ATG also contains antibodies against natural killer cell markers as well as against CD20, a B cell marker. Lymphopenia can persist for a year or more with rabbit ATG (124, 125). Severe infections can develop in patients treated with ATG, including infections caused by bacteria and organisms that depend on cell-mediated immunity for prevention or control of infection. For example, as with OKT3, ATG has been identified as a risk factor for CMV infection. However, in recent studies of ATG, a lower incidence of CMV infection is attributed to more effective antiviral prophylaxis (124).
Monoclonal antibodies that more specifically target the immune system have been developed. Basiliximab binds to the α chain of the IL-2 receptor, preventing T cell proliferation (CD25; Table 5). A reduction in allograft rejections has been demonstrated in kidney, heart, liver, lung, and kidney-pancreas transplant recipients treated with this agent (126, 127). A significantly lower incidence of herpes simplex virus infection in transplant recipients treated with basiliximab, cyclosporine, and corticosteroids compared to placebo, cyclosporine, and coricosteroids was attributed to the greater use of OKT3 and corticosteroids for rejection in the placebo group (128). Otherwise, significant differences in the incidence of bacterial, viral, and fungal infections in patients treated with IL-2 receptor monoclonal antibodies have not been demonstrated (126, 129).
Clinical trials have revealed that alemtuzumab (Campath) is efficacious for the prevention or treatment of acute allograft rejection in organ transplant recipients (131, 132, 133). A study of a large cohort of organ transplant recipients who received alemtuzumab for induction therapy or for the treatment of rejection reported a 10% incidence of opportunistic infections (130). CMV disease and esophageal candidiasis were the most common opportunistic infections. Other infections included, BK polyomavirus infection, Epstein Barr Virus-associated post transplant lymphoproliferative disorder (PTLD), invasive mold infections (aspergillosis, mucormycosis, pseudoallescheriosis), nocardiosis, tuberculous and non-tuberculous mycobacterial infections and toxoplasmosis (Table 5). Campath is no longer commmercially available, but is available via the Campath Distribution Program.
Prevention and treatment of graft versus host disease in hematopoietic cell transplantation
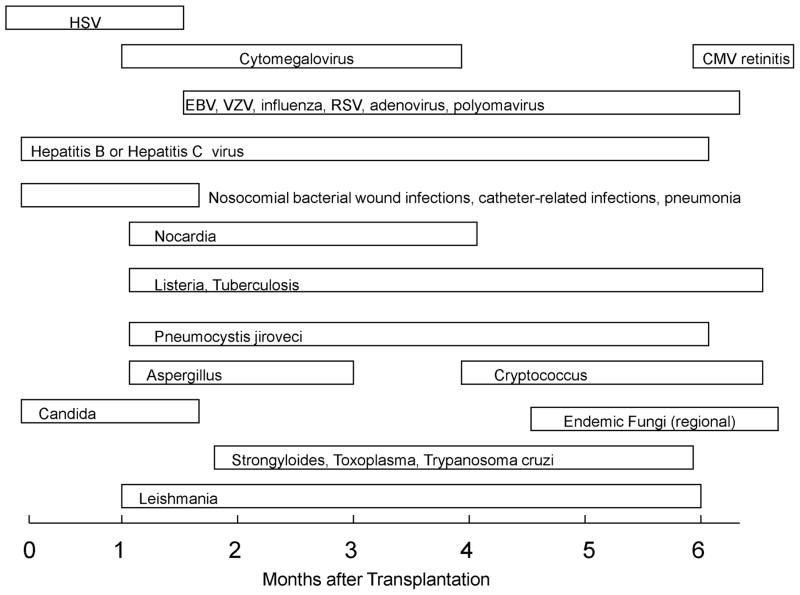
The occurrence of GVHD post-transplantation remains the most important factor influencing outcome following allogeneic blood and marrow transplantation (131). Acute GVHD is common in recipients with matched unrelated and with haploidentical related donors. The agents reviewed above for the prevention and treatment of allograft rejection in organ transplant recipients are also used for the prevention and treatment of GVHD in allogeneic blood and marrow transplant recipients. The most common prophylactic regimen in use at many transplant centers is a combination of methotrexate and cyclosporine for myeloablative conditioning regimens. Cyclosporine and mycophenolate mofetil is a standard regimen used with reduced intensity conditioning. Anti-thymocyte globulin can been included in the regimen with unrelated donors. Tacrolimus and sirolimus are alternative agents used if contraindications or toxicities are encountered. Corticosteroids are the first line agents for the treatment of acute GVHD. Second line agents for steroid non-responders possibly include tacrolimus, sirolimus, MMF, ATG, OKT3, etanercept, rituximab, and alemtuzumab. In addition to the enhanced susceptibility to infectious complications imposed by the immunosuppressive therapy to treat or prevent GVHD, GVHD itself also contributes to the risk of infection. GVHD of the skin and gut causes impairment of the mucocutaneous barrier. Chronic GVHD contributes to the immunocompromised state as it affects the persistence of defects in cell-mediated and humoral immunity and reticuloendothelial system function in the post-engraftment period (132, 133). Hence, patients with chronic GVHD remain at risk for a variety of opportunistic infections including fungal (invasive aspergillosis or other mold infection) and viral (CMV, VZV, EBV) infections and infections caused by P jirovecii (134). In addition, hypogammaglobulinemia that occurs in patients with GVHD predisposes these patients to infections with encapsulated bacteria, H influenzae and S. pneumoniae (134, 135).
Agents for the treatment of rheumatic diseases
Many of the agents used for the treatment of malignancies and for the prevention of allograft rejection are also used for the treatment of a variety of rheumatic and autoimmune diseases. Cyclophosphamide, an alkylating agent, and one of the most potent immunosuppressive agents available, is a first-line agent used for the treatment of severe, organ-threatening manifestations of systemic vasculitides and for severe immune-complex mediated manifestations of lupus. For flare ups of these diseases, moderate to high dose corticosteroids are used to induce remissions. After disease control is achieved, often maintenance therapy with less toxic agents, such as methotrexate, azathioprine, MMF, or leflunomide, is initiated.
With the continued progress in the field of molecular immunology, new treatments that target specific immune cells or mediators have been developed for the treatment of rheumatic diseases. These biologic immune response modulators have been designed to interfere with cytokine function, to inhibit T cell activation, and to deplete B cells with the goal of downregulating the proinflammatory responses that underlie the clinical manifestations of rheumatic diseases. The degree of immunosuppression is not as broad as that caused by traditional immunosuppressive drugs, however, the impairment of host defenses, especially if combined with other immunosppressive medications, can result in serious infections. Tables 5 and 6 group examples of biologic immune response modulators by their immune system target, describes their mechanisms of action, their treatment indications, and reported infectious complications. A systematic review and meta-analysis of serious infections in rheumatoid arthritis patients treated with biological drugs in 106 randomized trials revealed that standard, high-dose, and combination biological durgs (with or without traditional disease-modifying antirheumatic drugs, DMARDs, such as methotrexate) are associated with more serious infections than traditional DMARDs (129).
Tumor necrosis factor (TNF) antagonists such as etanercept, infliximab, and adalimumab are used to treat moderate to severe rheumatoid arthritis, Crohn’s disease, and other inflammatory syndromes (Table 2). Treatment with these agents is associated with an increased risk of serious infection. Binding of TNF to the TNF receptor stimulates release of inflammatory cytokines and expression of chemokines and endothelial adhesion molecules (7, 136). Inhibition of these effects results in a decrease in migration of inflammatory cells to sites of infection, and hence, a decrease in granuloma maintenance and formation (137). Experiments of TNF blockade in animal models have revealed the importance of TNF in the control of infections caused by intracellular pathogens or those maintained in a latent state by cell-mediated immunity (138–141). These pathogens include: M. tuberculosis, M. avium, M. bovis, Aspergillus fumigatus, P. jirovecii, Histoplasma capsulatum, Cryptococcus neoformans, C. albicans, Listeria monocytogenes, and Toxoplasma gondii (142).
Etanercept, a soluble receptor antagonist, binds to TNF in the serum to inhibit its function. Infliximab has a broader spectrum of activity than etanercept and also predisposes to a higher risk of infection than etanercept (143). This is likely linked to the mechanisms by which infliximab versus etanercept inhibit TNF activity-- a monoclonal antibody, infliximab, has a higher affinity for TNF than etanercept, a soluble TNF receptor fusion protein (144). Monoclonal antibodies such as infliximab and adalimumab bind to TNF in the serum and, in addition, to TNF bound to the cell surface. In the FDA Adverse Event Reporting System, tuberculosis, histoplasmosis, coccidioidiomycosis and listeriosis occurred 2 to 10 fold more often in patients treated with infliximab compared to etanercept (143). M. tuberculosis remains the most commonly reported infection associated with anti-TNF inhibitors therapy and is more often associated with infliximab treatment (145). Tuberculosis in patients treated with TNF inhibitors is frequently atypical in presentation and appears as disseminated and/or extrapulmonary disease. A variety of fungal infections that are kept in check by granuloma formation have also been reported in these patients (see Table 4), the most common of which are disseminated histoplasmosis, cryptococcosis, coccidioidiomycosis, and aspergillosis (146–149). In addition to tubercuolosis and invasive fungal infections, hepatitis B virus has been reported to reactivate and hepatitis C virus infection has progressed while patients are treated with TNF inhibitors (150, 151).
Cell surface receptor antagonists, such as anakinra, are biologically inactive proteins that compete with cytokines for binding to their membrane receptors. Anakinra is a recombinant IL-1 receptor antagonist protein (IL-1Ra) that binds to the IL-1R preventing the activity of IL-1α and IL-1β. Analysis of studies have revealed that IL-1 inhibitors are less effective than TNF inhibitors for the treatment of rheumatoid arthritis (152). However, they may be more effective for the treatment of autoinflammatory conditions such as Neonatal Onset Multisystem Inflammatory Disease (NOMID) and Tumor necrosis receptor-1 associated periodic syndrome (TRAPS) (153). Anakinra was associated with an increased risk of bacterial infections (e.g. cellulitis, pneumonia), especially at high doses, compared to placebo in rheumatoid arthritis studies although the results were not statistically significant when adjusted for underlying comorbidities (154). Treatment with anakinra may lead to reactivation of tuberculosis or other atypical or opportunistic infections, but these have not been frequently reported.
IL-6 is a cytokine involved in T and B cell differentiation and in the inflammatory response. Tocilizumab is a humanized monoclonal antibody to the IL-6 receptor that competitively blocks the interaction of IL-6 with its receptor interfering with B and T cell differentiation. Tocilizumab treatment studies of patients with rheumatoid arthritis have revealed that the incidence of serious infections is about 3.67 per 100 patient-years and is influenced by disease duration, advanced age, and underyling lung disease (155). Serious and fatal infections can occur especially in patients treated concomitantly with other immunosuppressive medications such as steroids or methotrexate. The most common infections are bacterial pneumonia, sepsis, or cellulitis. However, herpes zoster, Pneumocystis jirovecii pneumonia, and non-tuberculous mycobacterial infections also occur (155, 156). New and reactivation of latent tuberculosis infections and invasive fungal infections, such as aspergillosis, cryptococcosis, and candidiasis, have been reported.
Belimumab is a monoclonal antibody that inhibits the binding of BLyS, B lymphocyte stimulator protein, to B lymphocytes, inhibiting their survival and differentiation into plasma cells. This biologic agent is approved for the treatment of systemic lupus erythematosus. Serious and fatal infections have been reported during treatment with belimumab with bacterial pneumonia, cellulits, and urinary tract infections being most common. Reported opportunistic infections include JC virus-associated progressive multifocal leukoencephalopathy (PML), CMV pneumonia, and coccidioidomycosis (157, 158).
Abatacept is a fusion protein of the extracellular domain of cytotoxic T lymphocyte-antigen 4 (CTLA-4) linked to a modified Fc portion of IgG1 that is used to treat rheumatoid arthritis. It inhibits T cell lymphocyte activation by binding to CD80 and CD86 on antigen presenting cells, blocking the required co-stimulatory interaction between CD28 on T lymphocytes (159). A safety analysis of 8 clinical trials evaluating abatacept for the treatment of rheumatoid arthritis revealed that there was no difference in infection rate between those receiving abatacept versus placebo (160). The most frequent infections include bacterial pneumonia, cellulitis, and urinary tract infections. Opportunitistic infections are not common unless abatacept is combined with other immunosuppressive agents such as TNF inhibitors.
Natalizumab is a recombinant, humanized monoclonal antibody against the alpha-4 subunit of integrin molecules. Integrins are important for adhesion and migration of leukocytes from the vasculature into inflamed tissue. Natalizumab blocks this process, hindering inflammatory cell migration into the gastrointestinal tract and the central nervous system and providing relief from symptoms due Crohn’s disease and multiple sclerosis. Natalizumab decreases the CD4+/CD8+ T cell ratio in the cerebrospinal fluid, predisposing to JC virus-associated PML (161). Postmarketing reports have identified patients treated with natalizumab who developed central nervous system herpesvirus infections caused by HSV-1, HSV-2, and VZV (162). Some of these infections, which included meningitis, encephalitis, and meningomyelitis, occurred in patients who were not receiving any other type of immunsuppressive therapy besides natalizumab.
Fingolimod is another biologic immune response modifier used to treat relapsing multiple sclerosis. Fingolimod phosphate is a sphingosine analogue that binds to the sphingosine-1-phosphate receptors and blocks lymphocytes’ ability to egress from lymph nodes. Two large controlled trials demonstrated that fingolimod significantly reduces the relapse rate of multiple sclerosis, however at the risk of serious infection (163, 164). The following opportunistic infections have been reported during treatment with fingolimod: PML, cryptococcal meningitis, disseminated primary herpes zoster virus infection, and herpes simplex encephalitis (Table 6).
The Janus kinase (JAK) enzymes are a group of intracellular tyrosine kinases that are found mainly in hematopoietic cells. After cytokines bind to their cognate receptors, JAKs activate the intracellular transcription factors known as signal transducers and activators of transcription (STATs) by phosphorylation. Then, STATs directly modulate gene expression in the nucleus. Tofacitinib is a JAK inhibitor that is used to treat moderately to severely active rheumatoid arthiritis with an inadequate response to methotrexate. Patients treated with tofacitinib are at increased risk for serious infections because of the inhibition of cytokine and growth factor mediated gene expression and downstream immune cell activity. Serious infections most often develop in patients concomitantly treated with other immunosuppressive drugs such as methotrexate or corticosteroids. The most common serious sites of infection reported include pneumonia, cellulitis, and urinary tract infections (165). Pulmonary and extrapulmonary active tuberculosis, local or disseminated cryptococcosis and pneumocystosis, esophageal candidiasis, multidermatomal herpes zoster, cytomegalovirus, and BK virus infections have been reported in patients treated with tofacitinib. In clinical trials, increased rates of herpes zoster have been observed in patients treated with tofacitinib compared to placebo (166).
Eculizumab is a humanized monoclonal antibody that binds C5 complement to inhibit terminal complement activation. Patients treated with eculizumab are at risk for life-threatening N. meningitidis infection and for infection with ecapsulated bacteria such as S. pneumoniae and H. influenzae. It is used for the treatment of atypical hemolytic uremic syndrome and paroxysmal nocturnal hemoglobonuria (167)
Other Drugs
Phenytoin
This commonly used anti-convulsant causes a reversible decrease in the serum IgA level of approximately 20% of treated patients (168, 169). In 5% of cases, there is a severe deficiency of IgA, and in a smaller percentage, phenytoin can cause severe panhypogammaglobulinemia. These effects are usually, but not always reversible. Similar effects have been reported, though much less often, with other anti-convulsants including valproic acid (170). Affected individuals have the same propensity to develop infection as those with IgA deficiency or hypogammaglobulinemia.
Solid Organ Transplantation
The population of patients receiving organ transplants to restore vital organ functions and prolong life continues to grow. Immunosuppressive regimens that suppress T cell immune function are employed to prevent organ rejection and maintain long-term allograft function (171). The immunosuppressive regimens employed in all forms of organ transplantation are similar with cyclosporine or tacrolimus providing the cornerstone of maintenance anti-rejection treatment, along with an anti-metabolite, such as MMF, and possibly a low dose corticosteroid. Hence, the types, pattern, and timetable of infections encountered are similar in all forms of organ transplantation. The risk of infection is primarily determined by the intensity of exposure to potential pathogens and the patient’s net state of immunosuppression (172, 173). The main determinants of the net state of immunosuppression are: the dose, duration, and temporal sequence of the immunosuppressive agents and the presence or absence of infection with immunomodulating viruses (CMV, EBV, Hepatitis B or C and HIV) (172). The infectious risks encountered by organ transplant recipients can be divided into three phases: the first month after transplantation, 1 to 6 months after transplantation, and greater than 6 months after transplantation. These phases are determined by the degree of immunosuppression expected during these time periods (Figure 1) (174). These phases are dynamic and can be altered by routine antimicrobial prophylaxis (for Pneumocystis, CMV, and hepatitis B virus, for example) and by the requirement to change or intensify immunosuppressive therapy for the prevention or treatment of rejection (172, 175).
Figure 1. Timeline of Infections after Solid Organ Transplantation.
Alterations in the timeline occur as a result of antimicrobial prophylaxis or in the presence of excessive immunosuppression or intense epidemiologic exposure to a potential pathogens. HSV denotes herpes simplex virus, EBV, Epstein-Barr virus, VZV, varicella-zoster virus, RSV, respiratory syncytial virus.
The First Month after Transplantation
During the first month after transplantation, the consequences of immunosuppressive therapy have not yet taken effect, therefore, there is usually a notable absence of opportunistic infections during this time. Infections present in the allograft recipient prior to transplantation, infections transmitted via a contaminated allograft, and infections encountered in a post-operative setting are encountered. Undetected or incompletely treated infections occurring in the pre-transplant setting may manifest or exacerbate in the first month after transplantation. Pneumonia and bloodstream infections related to vascular access devices are common in pre-transplant recipients. Infections specific to different transplanted organs may occur; for example, peritonitis in a patient who received a liver transplant for end stage liver disease. Infections transmitted via a contaminated allograft are rare, but do occur, and are frequent amongst lung transplant recipients (176). These allograft-transmitted infections are usually caused by a variety of bacteria from a donor who was not known to be bacteremic at the time of organ harvest (177). More unusual viral infections such as rabies, lymphocytic choriomeningitis virus, and West Nile virus, have been transmitted via transplanted organs (178–181). Transmission of tuberculosis, Trypanosomea cruzi, and Toxoplasma gondii has been reported with a higher incidence in endemic areas (182–186). To prevent transmission of infection via the allograft, extensive screening of donors is performed and includes blood, urine, and sputum cultures; serologic tests for HIV, HTLV, CMV, hepatitis C, hepatitis B, EBV, VZV, and Toxoplasma (heart); PPD skin testing,; and Trypanosoma cruzi serology for donors from endemic areas (174).
The majority (> 90 %) of infections encountered in the first month after transplant are those related to the surgical procedure and are the same as those that occur in other postoperative patients. These infections result from the breakdown of normal mucocutanous barriers and the presence of devitalized tissue and fluid collections at the operative site. Bacterial and candidal infections of surgical wounds, pneumonia, urinary tract infections and infections related to vascular access devices, stents, and drainage catheters are very common. Any type of leak related to the surgical procedure that may form a biloma, urinoma, seroma, lymphocoele or hematoma can readily become secondarily infected with bacteria or Candida in the post-transplant setting (187).
One to Six Months after Transplantation
The period from 1 to 6 months after transplantation is the critical time period after transplantation during which infections unique to these immunocompromised hosts most often arise (172, 173). During this time period, the depth of immunosuppression is at its greatest and may be further enhanced by the presence of infection with immunomodulating viruses. CMV is a key player amongst these viruses and has been demonstrated to have multiple mechanisms by which it can induce further immunosuppression (188). Other herpes viruses, such as EBV, HHV-6, HHV-7, and HBV, HCV and HIV, if present, also are immunomodulating (189–193).
The cellular immune system is the primary arm of host defense that is impaired, therefore, these patients acquire opportunistic infections that require an intact cell-mediated immune response to prevent or control the infection. CMV infection is the most important opportunistic infection occurring in the organ transplant population given its direct and indirect effects on the organ transplant recipient (194). The direct effects include the clinical manifestations of CMV infection such as viremia that often is accompanied by the CMV syndrome or end organ disease, such as gastritis, colitis, pneumonitis, or hepatitis (195). The indirect effects of CMV infection include the virus’ ability to modulate the immune system contributing to the net state of immunosuppression, its contribution to oncogenesis, and its role in allograft injury and rejection (194). Other herpesviruses such as VZV can reactivate and present as shingles or disseminated infection (196). Parvovirus B19 can be acquired by the usual respiratory route, from the transplanted organ, or the virus can reactivate from a latent state (197). The most common manifestations of parvovirus B19 infection in renal transplant recipients are anemia with reticulocytopenia and pancytopenia. Intracellular bacterial pathogens such as Nocardia, Listeria, Legionella and tuberculosis can cause infection during this time period (185). In addition, patients are susceptible to infection with invasive molds, such as Aspergillus, and later in this time period, to infection with Cryptococcus and with the geographically restricted, endemic mycoses, Histoplasmsa capsulatum, Coccidioides immitis, and Blastomyces dermatitidis (198–201). Patients are at risk for infection with P. jirovecii, but this risk is minimized with antibiotic prophylaxis (202). The occurrence of parasitic infections (strongyloidiasis, toxoplasmosis, leishmaniasis, trypanosomiasis) depends on prior exposure of the donor or recipient to these pathogens in endemic areas (203).
The polyomaviruses, BK and JC, may reactivate during this time causing nephropathy particularly in renal transplant recipients or PML in any of the organ transplant recipients (204, 205). Respiratory viral infections such as influenza, RSV, parainfluenza, adenovirus, can cause serious infections in an organ transplant recipient (206–208). Infections, often at this time caused by antibiotic-resistant bacteria (vancomycin resistant enterococci (VRE), methicillin resistant S. aureus (MRSA), gram negative rods) and azole-resistant Candida species, linger on from the early post-operative period in the setting of persistent drains and catheters (209–211).
Greater than Six Months after Transplantation
The susceptibility to infections in this time period after transplantation depends on the presence or absence of a well functioning allograft and/or of chronic or progressive infection with CMV, EBV or the hepatitis viruses, B or C (172, 173). Patients with a well-functioning allograft on minimal maintenance immunosuppressive therapy are at risk for the usual community acquired infections, primarily respiratory. Patients maintained on higher doses of immunosuppressive agents because of recurrent or chronic rejection remain at risk for opportunistic infections with the pathogens described above. Those patients chronically infected with the immunomodulating viruses remain at significant risk for secondary infections as well as for the virus-associated malignancies (193). Those infected with the hepatitis C or B viruses are at risk for hepatocellular carcinoma. Chronic EBV infection can result in PTLD with its protean indolent or fulminant manifestations (212). Papillomavirus infection can result in squamous cell carcinoma of the skin that may be difficult to control and treat, resulting in metastases and significant morbidity and mortality (213).
Hematopoietic Cell Transplantation
The implementation of bone marrow transplantation in the early 1970’s revolutionized the treatment of hematologic malignancies (214). The successes of this treatment, however, have been counterbalanced by the significant morbidity and mortality associated with the transplantation of allogeneic or autologous bone marrow, peripheral blood stem cells or umbilical cord blood cells. These negative consequences are the result of the toxicities associated with the preparative regimen given prior to infusion of the hematopoietic cells and the result of GVHD, the major risk of hematopoietic cell transplantation (215). The goal of the preparative regimen is two fold: to eradicate the disease for which the transplant is being performed and to prevent rejection of the graft. The primary toxicities associated with the preparative regimen, usually consisting of total body irradiation plus a chemotherapeutic agent such as cyclophosphamide, are myelotoxicity and mucositis. Given the variable time frames during which these toxicities are manifested, the type of infection for which a particular patient is at risk is determined by the duration elapsed since transplant: the pre-engraftment period (from hematopoietic cell infusion to about 30 days), the immediate post-engraftment period (from engraftment to day 100), and the late post-engraftment period (after day 100); (Figures 2 and 3) (216, 217).
Figure 2.
Timeline of Infections after Autologous Hematopoietic Cell Transplantation.
Figure 3. Timeline of Opportunistic Infections after Allogeneic Hematopoietic Cell Transplantation.
PTLD, post-transplant lymphoproliferative disease; TB, tuberculosis; NTM, non-tuberculous mycobacteria; GVHD, graft versus host disease; RES, reticuloendothelial system.
The major risk factors for infection during the pre-engraftment period include: mucocutaneous damage, neutropenia with the loss of neutrophil phagocytic ability, and organ dysfunction related to the preparative regimen. These defects predispose the patient primarily to bacterial and candida infections. In the earlier era of bone marrow transplantation, bacterial infections caused by gram negative rods, such as Pseudomonas sp. and Enterobacteriaceae, were most prominent with bacteremia and pneumonia being the most common manifestations of infection (58). Subsequently with the use of antibiotic prophylaxis and the employment of broad spectrum antibiotics with gram negative rod activity for neutropenic fever, the spectrum of bacterial infections had switched from gram negative rods to gram-positive organisms, including coagulase-negative staphylococci, S. aureus, viridans streptococci, S. pneumoniae, enterococci, including VRE strains, and Corynebacterium species (59, 218, 219). In recent years, a trend back to gram negative bacterial infections has occurred; unfortunately, too often these bacteria are highly resistant to antimicrobial therapy (41, 60).
The factors predisposing to bacterial infection also predispose patients in the pre-engraftment period to infections with Candida species. In addition to neutropenia and mucocutaneous damage, broad-spectrum antibiotic use, organ dysfunction, and heavy density yeast colonization are risk factors for invasive candidiasis (220). As with bacterial infections, in some centers, there has been a shift in the spectrum of Candida species that cause invasive candidiasis due to the use of fluconazole prophylaxis (221, 222). The spectrum has switched from fluconazole susceptible strains, such as C. albicans, C. parapsilosis and C. tropicalis, to strains with partial or full resistance to fluconazole, such as C. glabrata and C. krusei.
Mold infections caused by Aspergillus, Zygomycetes or agents of hyalohyphomycosis (Fusarium, Scedosporium) or phaeohyphomycosis (Curvularia, Alternaria, Bipolaris) are not as common during the pre-engraftment phase (223). However, these infections can occur in patients who have delayed engraftment, and hence, prolonged neutropenia, in those whose disease process or its treatment have resulted in prolonged neutropenia, or those who had a mold infection at some point prior to transplantation. In addition to delayed engraftment, allogeneic transplantation and positive pre-transplant CMV serology have been identified as risk factors (223, 224). Mold infections primarily manifest in the lungs, sinuses, and skin.
Human herpes simplex virus is the most common virus causing infection in the pre-engraftment period in the absence of anti-viral prophylaxis. Virus reactivation occurs in more than 70% of seropositive patients with comparable rates in allogeneic and autologous transplant recipients (225). The most common manifestation of herpes reactivation is severe mucositis, but end organ disease, such as esophagitis, tracheobronchitis, and pneumonitis, can occur. Prophylaxis with acyclovir or valacylovir has significantly reduced the incidence of HSV infections in transplant recipients (226).
Respiratory virus infections can play a prominent role in the pre-engraftment phase and their incidence varies with the pattern of infection in the community and with community outbreaks. The most common respiratory viruses include RSV, the parainfluenza viruses, influenza A and B viruses, and rhinoviruses (126, 227). RSV, parainfluenza, and influenza A virus infections have been reported to cause outbreaks, severe pneumonitis, and fatal outcomes during epidemics in the community (228–230). The human metapneumoviruses are another group of respiratory pathogens that has emerged in the last 10 years in this patient population (231, 232).
The spectrum of infections encountered in the immediate post-engraftment period (from engraftment to day 100) is expanded because, at this point after transplantation, significant cellular immune dysfunction comes into play as a result of the preparative regimen and myeloablation. The degree of the genetic relatedness of the donor inversely correlates with the risk of acute GVHD and hence, with the risk of infection (215, 232). Patients who receive autologous grafts are at significantly decreased risk of opportunistic infection compared to those who receive an allogeneic source of hematopoietic cells (Figures 2 and 3). For patients who have received an allogeneic transplant, acute GVHD and therapy for this condition and the mucocutaneous damage caused by GVHD enhance the depth of immunosuppression (Figure 3).
Patients with HLA-matched related donors share more minor HLA antigens than unrelated donors who are matched at all major HLA loci; hence, the risk of GVHD is substantially greater with HLA matched unrelated donors. Haploidentical transplants in which the donor and recipient share half of the major HLA loci are at significant risk of serious viral and fungal infections, given the associated risk of GVHD (215). The risk of GVHD with mismatched donors can be reduced by T cell lymphocyte depletion of the donor hematopoietic stem cell product. Despite a decreased incidence of GVHD, studies have revealed a significantly higher incidence of severe CMV or life-threatenting or fatal Aspergillus infections in patients who received a T cell depleted marrow (233) because of the slower recovery of T lymphocytes. The source of the hematopoietic cells, bone marrow versus peripheral blood versus umbilical cord, may influence the risk of infection (215). A recent study revealed that the risk of serious infections in children receiving umbilical cord blood grafts was comparable to that of children receiving unmanipulated marrow and lower than recipients of a T cell-depleted stem cell source (234).
The bacterial and candidal infections encountered in the pre-engraftment period continue to occur in the post-engraftment phase. In addition, opportunistic infections, such as Legionella and Listeria monocytogenes may occur. The incidence of P. jirovecii is very low (<1%) in the setting of prophylaxis against this infection. Mold infections, especially those caused by Aspergillus spp., play a prominent role and their incidence is associated with acute GVHD and prednisone therapy (doses > 1 mg/kg per day). Hence, the incidence of mold infections is significantly higher in allogeneic transplant recipients than in autologous recipients (5 to 30% versus 1 to 5%) (224, 235). As noted in the pre-engraftment section, less common mold infections, such as those caused by Fusarium or the Zygomycetes can occur, especially in the setting of continued neutropenia.
Because of deficient CMV-specific T cell immunity, CMV infection occurs during this time period with a median onset of 8 weeks after transplantation. The incidence of early CMV infection has decreased as a result of molecular monitoring for this infection and preemptive therapy. Late CMV infections (> 3 months after transplantation) continue to occur in those patients with a persistent defect in CMV-specific T-cell immunity due to chronic GVHD and its treatment. In the absence of antiviral prophylaxis, CMV seropositive allogeneic transplant recipients have a 70–80% risk of reactivating the CMV (236). In contrast, only 40% of CMV seropositive autologous hematopoietic cell recipients reactivate CMV and less than 5% develop disease (355). Other herpes virus infections, such as HHV-6, can manifest during this time. Respiratory virus infections continue to occur. Other less common viral infections, such as adenovirus, are encountered during this time period. Reactivation of adenovirus occurs in greater than 80% of autologous and allogeneic transplant recipients (237). Disease, such as pneumonitis, colitis, nephritis, cystitis, occurs in less than 2 % with GVHD a primary risk factor. A higher incidence of infection and disease is encountered in children (238).
With the exception of toxoplasmosis, the occurrence of parasitic infections in this patient population requires specific exposures to particular parasitic pathogens, such as Strongyloides, Leishmania, Trypanosoma or Cryptosporidium. Toxoplasmosis can reactivate in severely immunosuppressed, seropositive, usually allogeneic, transplant patients and cause localized or disseminated disease.
Mycobacterial infections are rare in the hematopoietic cell transplant population in non-endemic areas and occur more commonly in allogeneic than autologous recipients. Infections caused by M. tuberculosis or the non-tuberculous mycobacteria (NTM) arise due to reactivation or to a new exposure (239, 240). As expected, with M. tuberculosis infection, pneumonia is most common; however, extrapulmonary disease, such as catheter-related bloodstream infections, soft tissue and bone and joint infections are more common manifestations of NTM infections (M. fortuitum, M. chelonae, M. abscessus, M. avium complex).
Infections occurring in the last post-engraftment period are typically seen in allogeneic transplant recipients who have chronic GVHD. By this time after transplantation, the immune system of autologous recipients has begun to reconstitute and most do not experience opportunistic infections. Chronic GVHD and its treatment result in chronic mucocutaneous damage and immunodeficiency that continues to predispose patients with this condition to opportunistic infections in the late post-engraftment period. The immune deficits that characterize chronic GVHD include: cellular immune deficiencies, humoral immune dysfunction, hyposplenism, decreased opsonization, and diminished reticuloendothelial cell function (132, 133). Because of this enhanced, prolonged immune deficiency, many of the infections that occur in the pre-engraftment and immediate post-engraftment periods continue to occur during this time. However, because of the additional defects in humoral immunity associated with chronic GVHD, patients during this phase are predisposed to serious infections caused by encapsulated bacteria, such as S. pneumoniae, H. influenzae, N. meningitidis.
Patients with chronic GVHD may lose their immunity to several viruses that may play a prominent role during this period. VZV can reactivate and cause cutaneous, central nervous system (CNS) or disseminated disease. This infection occurs in up to 90% of children during the first year after transplant (241). In adults, the infection usually occurs 6 to 9 months after transplantation and can present as a cutaneous, CNS, or disseminated infection (242). EBV is another viral infection to which patients with chronic GVHD lose immunity. The median time to onset of infection is 3 to 5 months after transplant. Infection is common, however, disease is rare. The disease manifestations include a mononucleosis-like syndrome with fever and neutropenia, aplastic anemia, oral hairy leukoplakia, and PTLD. The patients at highest risk for PTLD include those patients who have received an allogeneic transplant from a matched unrelated, mismatched or T-cell depleted donor, have chronic GVHD and have received anti-lymphocyte antibody therapy for GVHD prophylaxis (243). Patients may also lose B-cell specific immunity to the measles, mumps, rubella, parvovirus B-19, and the polyomavirus, JC and BK viruses (244). Examples of disease attributed to infection with these viruses in hematopoietic cell recipients include parvovirus B-19 induced severe anemia, late onset hemorrhagic cystitis caused by BK virus, and PML caused by JC virus. Reactivation of hepatitis B or C viruses occurs in up to 70% of patients, but, rarely, is disease associated with this reactivation (245, 246).
Nonmyeloablative conditioning regimens
In order to offer hematopoietic cell transplantation as a treatment option to patients who are elderly or have significant co-morbidities, non-myeloablative or reduced intensity conditioning regimens have been recently introduced (215). These regimens are designed to reduce treatment-related toxicities such as mucositis and neutropenia, but to preserve the graft-versus-leukemia effect. Many regimens include a purine analog such as fludarabine, and an alkylating agent, such as cyclophosphamide with or without an anti-T cell monoclonal antibody such as alemtuzumab. Despite the “reduced intensity” regimen, the literature reveals that because of the potent immunosuppressing regimens utilized and because of the occurrence of GVHD after nonmyeloablative BMT, the risk for opportunistic infections remains significant (247). Studies reporting on the incidence of infectious complications in patients who have received a nonmyeloablative hematopoietic cell transplant are difficult to compare because of the variety of conditioning and GVHD prophylaxis regimens used. In addition, the studies have included patients with a wide variety of underlying illnesses, prior therapies, and anti-prophylaxis regimens, all of which are determinants of the frequency and types of infections encountered (247). Studies of immune reconstitution after nonmyeloablative conditioning regimens have revealed that peripheral T and B cell subsets were similar during the 12 months after transplant or slightly depressed late after transplant (> 90 days) compared to myeloablative regimens (248, 249). Hence, these studies showed a similar rate of bacterial and CMV infections (248) or an increased rate of bacterial and fungal infections late post-transplant (> 90 days) (249). Another study in which fludarabine and cyclophosphamide composed the nonmyeloablative conditioning regimen, found similar frequencies of CMV and invasive fungal infections compared to infection frequencies in patients who received a myeloablative regimen (247). Overall, the data reveal that opportunistic infections, especially those caused by CMV and Aspergillus spp., occur and are common after nonmyeloablative hematopoietic cell transplantation (215, 250–252). The frequency and type of infections is variable and determined by underyling illness, prior therapies, and the conditioning regimen (247).
Human Immunodeficiency Virus Infection and Acquired Immunodeficiency Disease
Infection with the human immunodeficiency virus (HIV-1) causes a chronic progressive immunodeficiency. Initially, it was thought that this immunodeficiency arose from the exclusive infection of CD4+ T cell lymphocytes with subsequent cell death resulting in significant CD4+ T cell depletion. However, with time, it was discovered that this virus is capable of infecting a range of cells, including monocytes, follicular dendritic cells, epidermal Langerhans cells (253), alveolar macrophages, and cells within the central nervous system. In the early stages of infection, the high rate of viral replication in CD4+ T cells results in a major increase in the daily production of CD4+ cells that is balanced by a similar rate of CD4+ cell destruction (254). Over time, a gradual decrease in cellular and humoral immune functions occurs. Accompanying this decline in CD4+ T cell counts, investigators have characterized a wide array of functional abnormalities of the immune system. These abnormalities include: a decrease in responsiveness to mitogens and antigens, decreased proliferative responses to certain pathogens, decreased cytotoxic and natural killer cell activity, and decreased humoral immune responses. In concert, these abnormalities produce a state of general activation and disordered regulation of the cellular and humoral immune responses (255).
A unique example of dysregulation of the immune response in HIV-infected patients is the immune reconstitution inflammatory syndrome (IRIS) (255). This syndrome occurs in patients with AIDS (CD4 count often < 100 cells/mm3) who have a pre-existing infectious process that worsens paradoxically after initiation of highly active antiretroviral therapy (HAART). The IRIS has been most frequently reported with herpes zoster, M. tuberculosis, M. avium complex, CMV, and Cryptococcus infections (256, 257). The IRIS likely arises from an interaction of an exuberant, recovering immune response following HAART with the residual antigenic burden and the host genetic susceptibility to such a process.
HIV-1 infection is divided into stages: primary infection with seroconversion, clinical latency, early symptomatic disease, and acquired immunodeficiency syndrome (AIDS). The infectious complications that occur in patients infected with the HIV-1 virus correlate with the level of CD4+ cell counts. After the resolution of primary infection, patients enter the phase of clinical latency when they are primarily asymptomatic if the peripheral CD4+ T cell count remains above 500/mm3. As the CD4 count declines below 500/mm3, patients begin to develop constitutional symptoms (258, 259). These symptoms are often accompanied by infections caused by reactivating herpes viruses, such as herpes zoster virus, herpes simplex virus, or EBV virus (oral hairy leukoplakia), or mucosal infection cause by Candida spp. (thrush, vaginitis). Additional infectious complications that occur when the CD4 count ranges from 200–500/mm3 include pneumonia caused by pneumococci and other bacteria, reactivation of pulmonary tuberculosis, and cervical and anal dysplasia or cancer attributed to human papillomavirus infection. Other, less common syndromes that occur during this time and are associated with viral reactivation, include EBV-associated B cell lymphoma and HHV-8-associated Kaposi’s sarcoma.
Late stage disease and an AIDS defining state occur when the CD4 count falls to < 200/mm3. The median time from the onset of this severe immunosuppression to an AIDS-defining diagnosis is 12 to 18 months in persons not receiving anti-retroviral therapy. This phase is characterized by the development of opportunistic infections, tumors, wasting, and neurologic complications. The most common opportunistic infection encountered during this time period, in the absence of antibiotic prophylaxis, is pneumonia caused by P. jirovecii. Disseminated endemic mycoses, such as histoplasmosis and coccidioidiomycosis and extrapulmonary tuberculosis are encountered. PML caused by reactivation of the polyomavirus, JC, is a less common diagnosis. With further decline in the CD4 count, a broader array of opportunistic infections occurs. CD4 counts below 100/mm3 are associated with toxoplasmosis encephalitis, cryptococcal meningitis, candidal esophagitis, chronic diarrheal illnesses caused by Cryptosporidium or microsporidia, and disseminated HSV infection. Disseminated M. avium complex and CMV infections are two of the main opportunistic infections usually encountered with severe immunosuppression, when the CD4 counts falls below 50/mm3. The function of T lymphocytes (260) and the risk of opportunistic infections (261) decreases with reduction of viremia by anti-retroviral therapy, even when the CD4 counts are not restored to normal.
Other Immunomodulating Viruses
Epstein-Barr virus can cause a variety of immunologic perturbations. This virus infects human B lymphocytes and drives their proliferation until it is controlled by T lymphocytes. (The activated T lymphocytes are the “atypical lymphocytes” seen in the peripheral blood of patients with acute EBV infection.) Despite the fact that numbers of peripheral blood lymphocytes increase, it is well known that patients with acute infectious mononucleosis have depressed cell-mediated immunity and can become anergic. The mechanism for this became apparent when it was discovered that the EBV gene BCRF1 shares extensive sequence homology with the human Interleukin 10 (IL-10) gene, and that EBV-infected B lymphocytes produce IL-10 (262). This cytokine has two roles in immunoregulation – it is a growth and differentiation factor for B lymphocytes, and it is a negative regulator of TH1 T lymphocyte activity. These are the cells responsible for secreting cytokines such as IL-2 and interferon-gamma that are needed for delayed hypersensitivity reactions and cell-mediated immune responses. Therefore, during and sometimes for months following an acute EBV infection, individuals may be anergic and have difficulty responding to other viral and intracellular bacterial (e.g., mycobacteria) pathogens (263). EBV can cause hypogammaglobulinemia and pancytopenia. These effects are usually, but not always transient. Rarely among otherwise normal individuals but frequently among people with a rare immunodeficiency, the X-linked lymphoproliferative syndrome (X-LP), EBV infection can cause chronic infection, pancytopenia, severe hypogammaglobulinemia, hemophagocytic syndrome, a lymphoproliferative syndrome or B cell lymphoma (264, 265). EBV infection is invariably fatal in people with X-LP syndrome.
Measles
Much of the morbidity and mortality from measles is due to secondary infections, particularly diarrhea, pneumonia and reactivation of tuberculosis (266). Some of the predisposition to secondary infections is due to damage of mucosal barriers, but measles suppresses cell-mediated immune function for weeks following infection. During that period, people have decreased delayed-type hypersensitivity skin test responses (267), in vitro natural killer cell function and in vitro T-lymphoproliferative responses to mitogens. Similar changes, but of smaller magnitude, follow immunization with measles vaccine. The Th2 response predominates in children recovering from measles, inhibits Th1 responses, and increases susceptibility to intracellular pathogens (268)
Immunodeficiency Associated with Hematologic Malignancies
In addition to the effects of chemotherapy, cancers can predispose the host to develop infections if the cancer spreads to the bone marrow and/or lymph nodes, and thereby reduces the number of normal hematopoeitic cells. This is particularly true for lymphoid malignancies, such as leukemia and lymphoma.
Chronic lymphocytic leukemia (CLL)
CLL patients are at increased risk for infection because the leukemia cells can replace normal lymphocytes in the bone marrow and lymph nodes, and because of adverse effects of the drugs used to treat the disease. Defects of humoral immunity are common, and the problem appears to be exacerbated by the use of rituximab (269). Sometimes humoral immune deficiency is accompanied by hypogammaglobulinemia, but some CLL patients have oligoclonal or monoclonal gammopathies that lead to the unusual combination of normal or elevated immunoglobulin levels with deficiency of antibody responses. Evaluation of humoral immune function in these patients requires an immunofixation electrophoresis to test for gammopathy, and measurement of antibody responses to T-dependent and T-independent vaccine antigens. Affected individuals are at increased risk for upper and lower respiratory tract infections caused by encapsulated bacteria (54). Prophylactic infusions of pooled human gamma globulin (IVIG) have been shown to be cost effective, though they may not alter the long-term prognosis (270). Neutropenia and deficiencies of T cell number and function can occur in CLL, though most likely as a consequence of chemotherapy. The types of infections can be predicted by the specific chemotherapeutic drugs that are being used in an individual patient.
Multiple myeloma
This B-cell malignancy causes effects similar to those seen with CLL (271). The malignant B cell clone can displace other B cells, thus leading to humoral immune deficiency, even in the face of an elevated gamma globulin fraction. Myeloma cells secrete transforming growth factor-β which suppresses the inflammatory responses of monocytes and macrophages, and decreases the production of IgG and IgM antibodies by non-malignant B cells. Myeloma cells can also secrete vascular endothelial growth factor which has deleterious effects on dendritic cell differentiation and function, thus decreasing antigen presentation for adaptive immunity. Responses to pneumococcal vaccine are sub-optimal. Patients with multiple myeloma have an increased risk for infection caused by pneumococci and other encapsulated bacteria. More widespread immunologic dysfunction is likely to be the result of chemotherapy and not a direct effect of the myeloma.
Metabolic Diseases
Diabetes mellitus
Both type 1 and type 2 diabetes mellitus can increase an individual’s risk for infections because of reduced blood supply and denervation of peripheral tissues. It also appears that poor glycemic control is associated with impaired neutrophil function. Neutrophils from diabetics have decreased expression of adhesion molecules, as well as impaired in vitro adhesion and chemotaxis (272). Phagocytosis appears to be normal, but bactericidal function is impaired. Adaptive immune function is normal. Finally, diabetics have increased nasal colonization with S. aureus and may have increased binding between Candida and epithelial cells of the oral mucosa and vagina. These defects lead to predictable patterns of infections. Diabetics are at increased risk for developing infections of the lower respiratory tract, urinary tract, skin and mucous membranes (273–275). They are also at increased risk for recurrences of these infections. Infections caused by S. aureus, gram-negative rods and M. tuberculosis occur at increased frequency, and infections caused by S. pneumoniae and influenza virus cause increased morbidity and mortality (276). Diabetics are at increased risk for developing cellulitis and necrotizing fasciitis, most often due to polymicrobial infections caused by combinations of gram negative rods and anaerobes, but also due to S. aureus, or group A streptococci (277). There are also several specific types of infections and specific pathogens that occur much more often in diabetics than other people. These include rhinocerebral mucormycosis, necrotizing otitis externa associated with P. aeruginosa, emphysematous cystitis, and emphysematous cholecystitis. The latter may be a secondary effect of diabetic gastrointestinal dysmotility.
Protein-losing enteropathy
Loss of protein across the gastrointestinal mucosa can occur in association with chronic inflammatory diseases (e.g., Crohn’s colitis, celiac disease, and systemic lupus erythematosus). Though IgG levels may fall well below the normal range, affected individuals usually continue to produce normal antibody responses, and do not have trouble with infections. There are two exceptions to this generalization. People who are treated with chronic immunosuppression may have reduced production of antibody in association with increased loss of immunoglobulin through the stool. This rarely is severe enough to cause a predisposition to develop infections. (The effects of immunosuppression on T cell function are similar to those encountered by organ transplant recipients taking the same drugs at the same doses.) Protein-losing enteropathy can also be associated with lymphatic obstruction or intestinal lymphangiectasia, in which case there is concomitant reduction in T lymphocyte numbers (278). There can be sufficient loss of T lymphocytes to cause anergy, thus compromising the diagnostic utility of the delayed-type hypersensitivity skin tests. In very rare cases, the loss of T cells can be so severe that it causes a severe combined immunodeficiency.
Nephrotic syndrome
It would be expected that the consequences of protein loss through the urinary tract would be similar to those seen with protein loss through the gastrointestinal tract. That is, that most patients would have reduced levels of serum immunoglobulins with normal or near normal antibody production and no significant predisposition to infection. However, there is one glaring exception to this generalization, the propensity of children and less often adults with nephrotic syndrome to develop primary or spontaneous pneumococcal peritonitis (279, 280). The explanation for this association is not known.
Asplenia
Individuals can have congenital asplenia as an isolated finding or in association with dextroposition of the heart, lungs and abdominal viscera (Ivemark syndrome). The spleen can be surgically removed for trauma. There can be functional asplenia from splenic infarcts, most often caused by sickle cell disease, but sometimes caused by portal hypertension or infiltration by malignant cells. Asplenia is associated with a reduction in serum IgM, impaired IgM antibody reponses to polysaccharide vaccines (281), and a reduction in the capacity for phagocytosis of blood-borne microorganisms. The overall incidence of sepsis among asplenic individuals is 4.25% and the mortality rate is 2.52%, which represents an approximately 200-fold increase above the risks among the general population (282). The risk of infection is greater among children less than 5 years old, and in the first few years after surgical splenectomy. Infections are most often caused by encapsulated bacteria. The most frequent pathogen is S. pneumoniae, but N. meningitidis, E. coli, H. influenzae type b and S. aureus are important pathogens, as are Babesia spp. and malaria.
Effects of Age
Infants and young children
The neonate and very young infant have increased susceptibility to infection for a variety of reasons (283). First, though the repertoire of the adaptive immune system is generated during fetal life, there is little clonal selection and terminal differentiation to generate effector T lymphocytes and antibody-secreting B lymphocytes until the child has been exposed to antigens. That is, adaptive immunity depends in large part upon previous exposure to pathogens via immunization or infection. (The mother provides some compensation for the lack of antibody as maternal IgG antibodies are transported across the placenta, and provide protection to the baby for up to 6 months.) In addition, T cells from the fetus and young infant have decreased cytokine production and moderate reductions in cytotoxic function compared to adult T cells. Neonates and young children are usually anergic to a panel of delayed-type hypersensitivity skin tests, which limits their effectiveness as screening tests for deficiencies of cell-mediated immune function or mycobacterial infections (284, 285). The major defect of humoral immunity is that until children are 18–24 months old, they are generally unable to produce antibody in response to T-independent antigens (including clinically important bacterial capsular polysaccharides such as those produced by pathogenic pneumococci and H. influenzae) (286, 287). Before the development of polysaccharide-protein conjugate vaccines that activate B cells in a T-dependent manner, children under 2 years of age were at high risk for developing invasive infections (sepsis and meningitis) caused by these organisms. It was most remarkable that even after recovering from sepsis and/or meningitis, such infants did not produce protective levels of IgG antibody to the infecting pathogen. Young infants and children are still at increased risk for developing infections to pneumococcal serotypes not contained in the polysaccharide-protein conjugate vaccine. The NK cells of neonates have reduced cytotoxic activity compared to that of adults (288). Multiple functions of phagocytic cells, including adhesion and chemotaxis are reduced, but, bacterial killing appears to be at or very near adult normal levels. The levels of complement components are generally reduced by approximately 50% in comparison to normal adults (289). The effector function of both phagocytes and the classical pathway of complement are further reduced by the absence of IgG antibodies to relevant pathogens. This is compounded in premature babies who do not receive the full complement of maternal IgG antibodies, most of which are transferred during the last trimester of pregnancy.
Newborn babies are exposed to vaginal microbiota during delivery, and to the same microbiota prior to delivery if there has been premature rupture of the amniotic sac. If colonized by this microbiota, the combination of defects in the newborn makes them more susceptible to develop blood borne infections that can be widely disseminated. In this host, infections by bacteria including group B streptococci, E. coli and other Enterobacteriaceae, and Listeria (290), as well as HSV (291) and enteroviruses, (292) are particularly problematic.
Aging
It is difficult to clearly define the effects of aging on susceptibility to infection because of confounding comorbidities. Nevertheless, it is apparent that there are age-related deficiencies of adaptive immunity (293–295). Thymic involution leads to the decreased production of naïve T cells by age 40 years, and this is coupled with an age-related decline in the replicative potential of memory T cells. There also is a shift toward a TH2 T-cell response, and a relative decrease in the CD4:CD8 ratio. Serum immunoglobulin levels do not decline, but both primary and secondary antibody responses are impaired relative to those of younger individuals. For example, it has been estimated that influenza vaccine can prevent infection in 70–90% of people less than 65 years old (296), but only 30–40% of people who are older (297). Similarly, the immunogenicity of pneumococcal polysaccharide vaccines is reduced in individuals over the age of 65 years, compared to the populations of younger subjects (302, 303). Innate immune function appears to be stable with aging. Based upon the diminished T and B cell function of the elderly, it should not be surprising that they have an increased incidence and severity of pneumococcal, RSV (298) and influenza virus pneumonia, group B streptococcal bacteremia and sepsis, tuberculosis and herpes zoster.
Surgery or Trauma
Major trauma triggers a massive inflammatory response that is due to widespread activation of monocytes and macrophages by necrotic tissue (299). A consequence of this inflammatory state is depression of T and B cell function. Macrophages produce prostaglandins, such as prostagladin E2 (PGE2) that is a powerful immune suppressant. PGE2 inhibits T cell division, IL-2 production, IL-2 receptor expression, and affects the quality of antibody synthesis by B lymphocytes. In addition, PGE2 induces a TH2 lymphocyte response that is immunosuppressive in that immunosuppressive cytokines such as IL-4 and IL-10 are produced. This suppression of the adaptive immune response predisposes trauma patients to serious infections.
In patients who have experienced severe burns, the immune system undergoes a similar evolution in response to injury. Severe burns induce activation of an inflammatory cascade that contributes to the development of subsequent immunosuppression and increased susceptibility to infection and multiple organ system failure. The mechanisms by which burns cause immunosuppression have not been completely elucidated. However, macrophages likely play a major role in post-burn immunosuppression as their productive capacity for inflammatory mediators (e.g. prostaglandins, nitric oxide, TNF-α, IL-6 and other cytokines) is significantly increased (300). A downstream consequence of this inflammatory state is that patients with extensive burns exhibit decreased T and B lymphocyte function and impaired function of circulating leukocytes and complement (301, 302). Despite recent advances in the care of burn patients, overwhelming infection remains the leading cause of death from serious burn injury. In patients with burns affecting over 40% of the total body surface area, 75% of all deaths are attributed to sepsis from infection of the burn wound or other infections complications and/or inhalation injury (105). In addition to immune system dysfunction, the burn destroys the physical barrier to infection enabling tissue invasion by bacterial pathogens and possible systemic dissemination. High density (up to 105 colonies of bacteria per gram of tissue) bacterial colonization of burn eschar occurs. Colonization with P. aeruginosa is most common but colonization with other bacteria also occurs (e.g. E. coli, Acinetobacter, S. aureus) (303). P. aeruginosa skin infection in burn victims remains a serious complication associated with a very high mortality despite aggressive antibiotic therapy (304).
Chronic disease
Systemic lupus erythematosis
Infections are a major cause of morbidity and mortality in patients with SLE. Infections account for the majority of deaths among SLE patients in developing countries, and are the first or second most common cause of death in developing countries (305). Primary defects in innate and adaptive immunity that occur in SLE plus defects resulting from immunosuppressive therapy account for the high incidence of infections in this patient population. Immune defects in almost every component of the immune system have been reported. This impairment is not universal. The reported immune dysfunctions of the innate immune system include: inherited complement deficiencies, decreased levels of complement proteins and reduced numbers of complement receptors, abnormalities of chemotaxis, phagocytosis, and oxidative metabolism of polymorphonuclear leukocytes, monocytes, and macrophages. These immune abnormalities are more pronounced during periods of increased disease activity.
The adaptive immune system can also be compromised in patients with SLE. This appears to occur in the setting of enhanced disease activity and as a result of immunosuppressive therapy. During periods of exacerbation, patients with SLE have decreased levels of T cells and T-helper cell responses to viral antigens, toxoids, and allogeneic cells are diminished (306). T cell and other immune functions, as described above, are further impaired by corticosteroid therapy. Patients who are treated with cyclophosphamide and/or plasmapheresis because of serious disease manifestations unresponsive to corticosteroid therapy are at significant risk of developing fatal opportunistic infections.
Common bacteria, both gram positive and gram negative, are responsible for most infections in patients who have SLE (305). Some infections can have more severe manifestations if inherited deficiencies of complement or splenic dysfunction are present. Infections with encapsulated bacteria (S. pneumoniae, N. meningitidis) can cause meningitis and sepsis. Salmonella infections, such as bacteremia, occur in patients who have similar immune system deficiencies. Infection with Listeria monocytogenes, causing sepsis and meningitis, has been reported in patients receiving high doses of corticosteroids. Other opportunistic infections occur in patients who are treated with high dose corticosteroids and/or additional immunosuppressive therapy such as cyclophosphamide. Opportunistic infection with Nocardia, M. tuberculosis, atypical mycobacteria, VZV, CMV, P. jirovecii, C. albicans, Cryptococcus neoformans, Aspergillus, Strongyloides stercoralis, and Toxoplasma gondii has been reported in lupus patients.
Chronic active hepatitis and cirrhosis
Infections are a major complication and a major cause of death in advanced liver disease (313). The most common infections are bacterial and include spontaneous bacterial peritonitis, pneumonia, bacteremia, urinary tract infections, and endocarditis. Many immunologic abnormalities have been detected in patients with cirrhosis. Many of these studies have been performed in patients with alcohol induced liver disease. Low levels of complement and decompensated alcoholic cirrhosis have been associated with an increased risk of infections and mortality (307). The liver is the primary site of C3 synthesis; therefore, in the presence of severe liver failure, opsonization of bacteria may be impaired. The reticuloendothelial system is an important filtering system for bloodborne pathogens. However, because of impaired macrophage activation and mobilization in the presence of cirrhosis, this filtering may be impaired (308). In addition, portal-systemic shunting occurs in cirrhosis allowing portal blood to reach the systemic circulation without passing through the reticuloendothelial system. This is a suspected etiology of some bacteremias in patients with cirrhosis.
Patients with cirrhosis frequently demonstrate anergy and fail to respond to vaccination suggesting delayed hypersensitivity and other impaired T cell dependent functions (309). Recent studies of vaccination with influenza vaccine demonstrated that patients with more advanced liver disease had significantly lower post-immunization levels of IFN-γ that is due to decreased lymphocyte responsiveness to specific antigen in advanced liver disease (310). This decreased lymphocyte responsiveness may be due to the effects of hepatitis C virus on dendritic cells, antigen-presenting cells that are essential for the development of an effective immune response (311). Hepatitis C virus binds to dendritic cells, replicates to a low level in these cells and impairs their maturation. The result is an impaired ability of dendritic cells to stimulate alloreactive T cells. Similar findings have been reported in patients with chronic hepatitis B virus infection (312).
End stage renal disease
Bacterial infections are the second most common cause of death in the end stage renal disease (ESRD) population (313). Death rates from sepsis are 100 to 300 fold higher in ESRD compared to the general population. The following immunologic abnormalities have been reported in patients with ESRD: decreased granulocyte and macrophage phagocytic function, reduced killing capacity of neutrophils, lower antibody titers and inability to maintain adequate antibody titers over time post-vaccination, and impaired T cell-mediated immunity (314–318). A recent study of patients with ESRD maintained on hemodialysis found a reduction of naïve and central memory T cells that may in part contribute to the increased predisposition to infection and the diminished response to vaccination in the ESRD population (318). This reduction in lymphocytes may result from apoptosis induced by uremia (319).
E. Screening for Suspected Immunodeficiency
Although immune system dysfunction can be suspected by the clinician after careful review of the history and physical exam, specific diagnoses of primary immunodeficiency are rarely evident without the use of the laboratory (320). Similarly, one may be able to suspect the most likely secondary immunomodulating consequences of a drug, infection or other illness. However, the specific host defect and its severity may vary widely among different patients. Based upon information provided in this chapter, the types of infections, the drugs being used, and other symptoms should help to focus the laboratory workup on specific parts of the immune system (Table 2). For example, patients with antibody deficiency typically have sinopulmonary infections caused by encapsulated bacteria and viruses as a prominent presenting feature. Deficiency of cell-mediated immunity predisposes individuals to develop infections caused by opportunistic pathogens such as P. jirovecii and other fungi, bacteria of low virulence, and a variety of viruses. Abnormalities of phagocytic function should be suspected when patients have recurrent skin infections or visceral abscesses, whereas patients with complement deficiency most often present with bacterial sepsis or immune complex-mediated diseases. Screening tests should be guided by the clinical features of the patient with the aim of identifying possible primary and secondary immunodeficiency, and defining the relevant defect(s) in host defense.
Examination of the Peripheral Blood Smear
A complete blood count, together with blood smear examination, is an inexpensive and readily available test that provides important diagnostic information relating to a number of immunodeficiency states (Table 6). Neutropenia most often occurs secondary to immunosuppressive drugs, infection, malnutrition, autoimmunity, but may be a primary problem (congenital or cyclic neutropenia). In contrast, persistent neutrophilia is characteristic of leukocyte adhesion molecule deficiency, and abnormal cytoplasmic granules may be seen in the peripheral blood smear of patients with Chediak-Higashi Syndrome.
The blood is predominantly a “T cell organ”, i.e., the majority (50–70%) of peripheral blood lymphocytes are T cells whereas only 5–15% are B cells. Therefore, lymphopenia is often a presenting feature of T cell or combined immunodeficiency disorders such as severe combined immunodeficiency disease or DiGeorge Syndrome (321). Pediatricians and specialists in infectious diseases are often so intent on looking for abnormal neutrophil counts and the presence of elevated numbers of young neutrophils (e.g., bands), that they fail to note this important abnormality when it is present.
Thrombocytopenia may occur as a secondary manifestation of immunodeficiency, but is often a presenting manifestation of the Wiskott-Aldrich Syndrome (322). A unique finding in the latter group of patients is an abnormally small platelet volume, a measurement that is easily made by automated blood counters.
Examination of red blood cell morphology can yield clues about splenic function. Howell-Jolly bodies may be visible in peripheral blood in cases of splenic dysfunction or asplenia. However, the converse is not always true and the absence of Howell-Jolly bodies does not guarantee that splenic function is normal.
Evaluation of Humoral Immunity
Measurement of serum immunoglobulin levels is an important screening test to detect immunodeficiency for three reasons 1) More than 80% of patients with primary disorders of immunity will have abnormalities of serum immunoglobulins; 2) Immunoglobulin measurements yield indirect information about several disparate aspects of the immune system because immunoglobulin synthesis requires the coordinated function of B lymphocytes, T lymphocytes and monocytes; and 3) The measurement of serum immunoglobulin levels is readily available, highly reliable and relatively inexpensive. The initial screening test for humoral immune function is the quantitative measurement of serum immunoglobulins (320). Neither serum protein electrophoresis nor immunoelectrophoresis is sufficiently sensitive or quantitative to be useful for this purpose. Instead, quantitative measurements of serum IgG, IgA and IgM should be used as that will identify patients with panhypogammaglobulinemia as well as those with deficiencies of an individual class of immunoglobulins, such as selective IgA deficiency. Interpretation of results must be made in view of the marked variations in normal immunoglobulin levels with age. Therefore, age-related normal values must always be used for comparison.
A clue to immunodeficiency may be a low normal IgG level in an individual with recurrent infections. In such cases, it is critical to assess antibody function in addition to immunoglobulin levels. Antibody levels should be measured in response to T-dependent (e.g., tetanus toxoid or influenza virus vaccines), and T-independent (e.g., pneumococcal polysaccharide) vaccines. Two caveats for the latter group of vaccines are that pneumococcal polysaccharide/protein conjugate vaccines are not useful for this purpose because they are T-dependent, and that children under the age of l8–24 months generally cannot respond to T-independent antigens whether presented by immunization or natural infection. As an alternative, T-independent antibody responses can be assessed by quantitating isoagglutinin titers, as the ABO blood group antigens are polysaccharides. However, the value of measuring anti-isoagglutinin antibodies is also limited in children less than 2 years of age. It is also important to assess responses to new antigens as well as recall antigens, as some acquired (e.g., common variable immunodeficiency) and secondary (rituximab therapy) immunodeficiency diseases may not effect previously generated memory cells. Meningococcal and hepatitis A vaccines are useful for this purpose as many people have not had previous immunization or exposure to these antigens. Live vaccines (varicella-zoster, measles, mumps, rubella or BCG) should never be used for diagnostic testing, as immunodeficient patients can sometimes develop serious and potentially life-threatening infections from vaccine-strain microorganisms.
The role for IgG subclass measurements is controversial. There are four subclasses of IgG, and selective deficiencies of each of these have been described. However, the significance of an IgG subclass deficiency in the presence of normal antibody responses to protein and polysaccharide antigens is not known. Therefore, it is probably best to rely upon antibody measurements and not IgG subclass levels.
Evaluation of Cell-Mediated Immunity
Testing for defects of cell-mediated immunity is relatively difficult because of the lack of good screening tests. Lymphopenia is suggestive of T-lymphocyte deficiency because T lymphocytes comprise the majority (50–70%) of peripheral blood mononuclear cells. However, lymphopenia is not always present in patients with T lymphocyte functional defects. Similarly, the lack of a thymus silhouette on chest x-ray is rarely helpful in the evaluation of T lymphocyte disorders because the thymus may involute following stress and give the appearance of thymic hypoplasia.
Indirect information about T cell function may be obtained by flow cytometric enumeration of peripheral blood T lymphocytes with appropriate monoclonal antibodies (anti-CD3 for total T cells, anti-CD4 for T-helper cells, anti-CD8 for T-cytotoxic cells). Patients with severe combined immunodeficiency and DiGeorge Syndrome generally have decreased numbers of both CD4+ and CD8+ T lymphocytes. In contrast, patients infected with HIV have decreased T lymphocyte numbers because there are decreased numbers of CD4+ lymphocytes.
Delayed type hypersensitivity (DTH) skin testing with a panel of antigens (323) generally should not be used to screen for defects of cell-mediated immunity because there are significant limitations to this testing: (i) Prior exposure to antigen is a prerequisite. (ii) Normal patients may have transient depression of delayed-type hypersensitivity with acute viral infections such as infectious mononucleosis; poor nutrition; or stress. In fact, the incidence of anergy among hospitalized patients is significantly higher than what one might suppose based upon their admitting diagnoses and the lack of infections with opportunistic pathogens. (iii) A positive skin test to some antigens does not insure that the patient has normal cell-mediated immunity to all antigens (e.g., patients with chronic mucocutaneous candidiasis have a limited defect in which cell-mediated immunity is generally intact except for their response to candida). (iv) There currently are only 3 antigen preparations available in the United States that have been standardized and licensed for DTH testing (candida, mumps and PPD) (324), and the latter preparation is not useful for anergy testing since most people should be expected to be negative.) (v) Normal children under the age of l2 months frequently are unresponsive to all of the antigens in the panel. (vi) False negatives can easily result from incorrect application of antigens, which is dependent on characteristics of the patient’s skin as well as the skill of the operator. DTH skin tests are, therefore, generally not helpful for evaluation of suspected T-lymphocyte abnormalities, especially those that present early in life (e.g., severe combined immunodeficiency or DiGeorge Syndrome). Instead, the preferred method is measurement of in vitro T cell proliferation and/or cytokine production in response to mitogens and soluble antigens (320). An example of the latter are the commercial tests for TB that depend upon interferon-gamma release after exposure to PPD (QuantiFERON®–TB Gold In-Tube test and T-SPOT®.TB test). When specimens are sent to an off-site reference laboratory for testing, they should always be accompanied by a specimen from a normal individual to control for the effects of shipment on the results.
Evaluation of the Complement System
Most of the genetically determined deficiencies of complement can be detected with the total serum hemolytic complement (CH50) assay (325). Since this assay depends on the functional integrity of the classical complement pathway (C1 through C9), a severe deficiency of any of these components leads to a marked reduction or absence of total hemolytic complement activity. Alternative pathway deficiencies (e.g., factor H, factor I and properdin) are extremely rare; they may be suspected if the CH50 is in the low range of normal and the serum C3 level is low. The screening test for alternative pathway abnormalities is the AH50. The screening test for abnormalities of the mannose-binding pathway is measurement of the MBL level. The final identification of the specific complement component that is deficient usually rests on both functional and immunochemical tests, and highly specific assays have been developed for each individual complement component. These tests are all commercially available at reference laboratories.
Evaluation of Phagocytic Cells
Evaluation of phagocytic cells usually entails assessment of both their number and their function. Disorders, such as congenital agranulocytosis or cyclic neutropenia, which are characterized by a deficiency in phagocytic cell number, can be easily detected by using a white blood cell count and differential. Beyond that, assessment of phagocytic cell function is relatively specialized because it depends upon a variety of in vitro assays including measurement of directed cell motility (chemotaxis), ingestion (phagocytosis) and intracellular killing (bactericidal activity) (27). The most common of the phagocyte function disorders, chronic granulomatous disease, can be identified by the nitroblue tetrazolium (NBT) dye test or the dihydrorhodamine dye flow cytometry assay (326), either of which measures the oxidative metabolic response of neutrophils and monocytes. This is another test for which patient specimens sent to an off- site reference laboratory for testing should always be accompanied by a specimen from a normal individual.
F. Summary
Immunocompromised hosts have an increased susceptibility to infections for a wide variety of reasons. As a result, it is not correct, nor is it particularly useful, for the clinician to consider all immunocompromised hosts to have the same susceptibility to infection. This chapter should lay the groundwork for understanding why an individual patient may have an increased susceptibility to specific types of pathogens. Some of the defects in host defense are due to the effects of the environment (e.g., exposure to immunosuppressive drugs or viruses) and other defects are due to genetic traits (e.g., primary immunodeficiency diseases). We are likely to learn much more about both of these processes as data from the human genome project enables us to identify genetic variants (polymorphisms) that make some individuals more susceptible to a toxic effect of a drug or to have some variation (but not complete defect) in a host defense mechanism.
The identification of specific host defense defects in an individual patient should lead to a more efficient strategy for identification and management of specific pathogens. Subsequent chapters of this book will elaborate on the specific pathogens and syndromes peculiar to the expanding group of immunocompromised hosts.
Contributor Information
Lesia K. Dropulic, The National Institutes of Health, National Institute of Allergy and Infectious Diseases, Division of Intramural Research, Bethesda, MD
Howard M. Lederman, Departments of Pediatrics, Medicine and Pathology, The Johns Hopkins University School of Medicine, Baltimore, MD 21287-3923
References
- 1.Parkin J, Cohen B. An overview of the immune system. Lancet. 2001;357:1777–1789. doi: 10.1016/S0140-6736(00)04904-7. [DOI] [PubMed] [Google Scholar]
- 2.Chaplin DD. Overview of the immune response. J Allergy Clin Immunol. 2010;125(Suppl 2):S3–S23. doi: 10.1016/j.jaci.2009.12.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodarzi H, Trowbridge J, Gallo RL. Innate immunity: a cutaneous perspective. Clin Rev Allergy Immunol. 2007;33:15–26. doi: 10.1007/s12016-007-0037-4. [DOI] [PubMed] [Google Scholar]
- 4.Turvey SE, Broide D. Innate immunity. J Allergy Clin Immunol. 2010;125:S24–S32. doi: 10.1016/j.jaci.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumagai Y, Akira S. Identification and functions of pattern-recognition receptors. J Allergy Clin Immunol. 2010;125:985–992. doi: 10.1016/j.jaci.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 6.Medzhitov R, Janeway C., Jr The Toll receptor family and microbial recognition. Trends Microbiol. 2000;8:452–456. doi: 10.1016/s0966-842x(00)01845-x. [DOI] [PubMed] [Google Scholar]
- 7.Beutler B, Cerami A. The biology of cachectin/TNF--a primary mediator of the host response. Annu Rev Immunol. 1989;7:625–655. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- 8.Aderem A. Phagocytosis and the inflammatory response. J Infect Dis. 2003;187(Suppl 2):S340–S345. doi: 10.1086/374747. [DOI] [PubMed] [Google Scholar]
- 9.Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344:1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 10.Walport MJ. Complement. Second of two parts. N Engl J Med. 2001;344:1140–1144. doi: 10.1056/NEJM200104123441506. [DOI] [PubMed] [Google Scholar]
- 11.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 12.Min-Oo G, Kamimura Y, Hendricks DW, Nabekura T, Lanier LL. Natural killer cells: walking three paths down memory lane. Trends Immunol. 2013;34:251–258. doi: 10.1016/j.it.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonilla FA, Oettgen HC. Adaptive immunity. J Allergy Clin Immunol. 2010;125(Suppl 2):S33–S40. doi: 10.1016/j.jaci.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 14.Wisplinghoff H, Seifert H, Wenzel RP, Edmond MB. Current trends in the epidemiology of nosocomial bloodstream infections in patients with hematological malignancies and solid neoplasms in hospitals in the United States. Clin Infect Dis. 2003;36:1103–1110. doi: 10.1086/374339. [DOI] [PubMed] [Google Scholar]
- 15.Hidalgo S, García Erro M, Cisterna D, Freire MC. Paralytic poliomyelitis caused by a vaccine-derived polio virus in an antibody-deficient Argentinean child. Pediatr Infect Dis J. 2003;22:570–572. [PubMed] [Google Scholar]
- 16.Quartier P, Foray S, Casanova JL, Hau-Rainsard I, Blanche S, Fischer A. Enteroviral meningoencephalitis in X-linked agammaglobulinemia: intensive immunoglobulin therapy and sequential viral detection in cerebrospinal fluid by polymerase chain reaction. Rev Infect Dis. 1987;9:334–356. doi: 10.1097/00006454-200011000-00020. [DOI] [PubMed] [Google Scholar]
- 17.McKinney RE, Jr, Katz SL, Wilfert CM. Chronic enteroviral meningoencephalitis in agammaglobulinemic patients. Rev Infect Dis. 1987;9:334–356. doi: 10.1093/clinids/9.2.334. [DOI] [PubMed] [Google Scholar]
- 18.Yel L. Selective IgA deficiency. J Clin Immunol. 2010;30:10–16. doi: 10.1007/s10875-009-9357-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orange JS, Glessner JT, Resnick E, Sullivan KE, Lucas M, Ferry B, Kim CE, Hou C, Wang F, Chiavacci R, Kugathasan S, Sleasman JW, Baldassano R, Perez EE, Chapel H, Cunningham-Rundles C, Hakonarson H. Genome-wide association identifies diverse causes of common variable immunodeficiency. J Allergy Clin Immunol. 2011;127:1360–7. e6. doi: 10.1016/j.jaci.2011.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cunningham-Rundles C, Maglione PJ. Common variable immunodeficiency. J Allergy Clin Immunol. 2012;129:1425–141197. doi: 10.1016/j.jaci.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 21.Plebani A, Soresina A, Rondelli R, Amato GM, Azzari C, Cardinale F, Cazzola G, Consolini R, De Mattia D, Dell’Erba G, Duse M, Fiorini M, Martino S, Martire B, Masi M, Monafo V, Moschese V, Notarangelo LD, Orlandi P, Panei P, Pession A, Pietrogrande MC, Pignata C, Quinti I, Ragno V, Rossi P, Sciotto A, Stabile A Italian Pediatric Group for XLA-AIEOP. Clinical, immunological, and molecular analysis in a large cohort of patients with X-linked agammaglobulinemia: an Italian multicenter study. Clin Immunol. 2002;104:221–230. doi: 10.1006/clim.2002.5241. [DOI] [PubMed] [Google Scholar]
- 22.Jorgensen GH, Gardulf A, Sigurdsson MI, Sigurdardottir ST, Thorsteinsdottir I, Gudmundsson S, Hammarström L, Ludviksson BR. Clinical symptoms in adults with selective IgA deficiency: a case-control study. J Clin Immunol. 2013;33:742–747. doi: 10.1007/s10875-012-9858-x. [DOI] [PubMed] [Google Scholar]
- 23.Aytekin C, Tuygun N, Gokce S, Dogu F, Ikinciogullari A. Selective IgA deficiency: clinical and laboratory features of 118 children in Turkey. J Clin Immunol. 2012;32:961–966. doi: 10.1007/s10875-012-9702-3. [DOI] [PubMed] [Google Scholar]
- 24.Ram S, Lewis LA, Rice PA. Infections of people with complement deficiencies and patients who have undergone splenectomy. Clin Microbiol Rev. 2010;23:740–780. doi: 10.1128/CMR.00048-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chinn IK, Shearer WT. Severe Combined Immunodeficiency Disorders. Immunol Allergy Clin North Am. 2015;35:671–694. doi: 10.1016/j.iac.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Stephan JL, Vlekova V, Le Deist F, Blanche S, Donadieu J, De Saint-Basile G, Durandy A, Griscelli C, Fischer A. Severe combined immunodeficiency: a retrospective single-center study of clinical presentation and outcome in 117 patients. J Pediatr. 1993;123:564–572. doi: 10.1016/s0022-3476(05)80951-5. [DOI] [PubMed] [Google Scholar]
- 27.Marciano BE, Spalding C, Fitzgerald A, Mann D, Brown T, Osgood S, Yockey L, Darnell DN, Barnhart L, Daub J, Boris L, Rump AP, Anderson VL, Haney C, Kuhns DB, Rosenzweig SD, Kelly C, Zelazny A, Mason T, DeRavin SS, Kang E, Gallin JI, Malech HL, Olivier KN, Uzel G, Freeman AF, Heller T, Zerbe CS, Holland SM. Common severe infections in chronic granulomatous disease. Clin Infect Dis. 2015;60:1176–1183. doi: 10.1093/cid/ciu1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winkelstein JA, Marino MC, Lederman HM, Jones SM, Sullivan K, Burks AW, Conley ME, Cunningham-Rundles C, Ochs HD. X-linked agammaglobulinemia: report on a United States registry of 201 patients. Medicine (Baltimore) 2006;85:193–202. doi: 10.1097/01.md.0000229482.27398.ad. [DOI] [PubMed] [Google Scholar]
- 29.Winkelstein JA, Marino MC, Johnston RB, Jr, Boyle J, Curnutte J, Gallin JI, Malech HL, Holland SM, Ochs H, Quie P, Buckley RH, Foster CB, Chanock SJ, Dickler H. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine (Baltimore) 2000;79:155–169. doi: 10.1097/00005792-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Skokowa J, Germeshausen M, Zeidler C, Welte K. Severe congenital neutropenia: inheritance and pathophysiology. Curr Opin Hematol. 2007;14:22–28. doi: 10.1097/00062752-200701000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Fioredda F, Calvillo M, Burlando O, Riccardi F, Caviglia I, Tucci F, Bonanomi S, Ghilardi R, Martire B, Farruggia P, Mastrodicasa E, Barone A, Castagnola E, Dufour C. Infectious complications in children with severe congenital, autoimmune or idiopathic neutropenia: a retrospective study from the Italian Neutropenia Registry. Pediatr Infect Dis J. 2013;32:410–412. doi: 10.1097/INF.0b013e3182814b5a. [DOI] [PubMed] [Google Scholar]
- 32.Figueroa JE, Densen P. Infectious diseases associated with complement deficiencies. Clin Microbiol Rev. 1991;4:359–395. doi: 10.1128/cmr.4.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ross SC, Densen P. Complement deficiency states and infection: epidemiology, pathogenesis and consequences of neisserial and other infections in an immune deficiency. Medicine (Baltimore) 1984;63:243–273. [PubMed] [Google Scholar]
- 34.Orange JS. Natural killer cell deficiency. J Allergy Clin Immunol. 2013;132:515–525. doi: 10.1016/j.jaci.2013.07.020. quiz 526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cedzynski M, Szemraj J, Swierzko AS, Bak-Romaniszyn L, Banasik M, Zeman K, Kilpatrick DC. Mannan-binding lectin insufficiency in children with recurrent infections of the respiratory system. Clin Exp Immunol. 2004;136:304–311. doi: 10.1111/j.1365-2249.2004.02453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koch A, Melbye M, Sørensen P, Homøe P, Madsen HO, Mølbak K, Hansen CH, Andersen LH, Hahn GW, Garred P. Acute respiratory tract infections and mannose-binding lectin insufficiency during early childhood. JAMA. 2001;285:1316–1321. doi: 10.1001/jama.285.10.1316. [DOI] [PubMed] [Google Scholar]
- 37.Picard C, et al. Clinical features and outcome of patients with IRAK-4 and MyD88 deficiency. Medicine (Baltimore) 2010;89:403–42. doi: 10.1097/MD.0b013e3181fd8ec3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ram S, Lewis LA, Rice PA. Infections of people with complement deficiencies and patients who have undergone splenectomy. Clin Microbiol Rev. 2010;23:740–780. doi: 10.1128/CMR.00048-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states. Lancet. 2011;378:86–97. doi: 10.1016/S0140-6736(10)61493-6. [DOI] [PubMed] [Google Scholar]
- 40.Pichard DC, Freeman AF, Cowen EW. Primary immunodeficiency update: part II. Syndromes associated with mucocutaneous candidiasis and noninfectious cutaneous manifestations. J Am Acad Dermatol. 2015;73:367–381. doi: 10.1016/j.jaad.2015.01.055. quiz 381–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trecarichi EM, Tumbarello M. Antimicrobial-resistant Gram-negative bacteria in febrile neutropenic patients with cancer: current epidemiology and clinical impact. Curr Opin Infect Dis. 2014;27:200–210. doi: 10.1097/QCO.0000000000000038. [DOI] [PubMed] [Google Scholar]
- 42.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Engl J Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 43.Scheinman RI, Cogswell PC, Lofquist AK, Baldwin AS., Jr Role of transcriptional activation of I kappa B alpha in mediation of immunosuppression by glucocorticoids. Science. 1995;270:283–286. doi: 10.1126/science.270.5234.283. [DOI] [PubMed] [Google Scholar]
- 44.Boumpas DT, Chrousos GP, Wilder RL, Cupps TR, Balow JE. Glucocorticoid therapy for immune-mediated diseases: basic and clinical correlates. Ann Intern Med. 1993;119:1198–1208. doi: 10.7326/0003-4819-119-12-199312150-00007. [DOI] [PubMed] [Google Scholar]
- 45.Fauci AS, Dale DC, Balow JE. Glucocorticosteroid therapy: mechanisms of action and clinical considerations. Ann Intern Med. 1976;84:304–315. doi: 10.7326/0003-4819-84-3-304. [DOI] [PubMed] [Google Scholar]
- 46.Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: inhibition of NF-κβ activation through induction of Iκβa. Science. 1995;270:286–290. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- 47.Balow JE, Rosenthal AS. Glucocorticoid suppression of macrophage migration inhibitory factor. J Exp Med. 1973;137:1031–1041. doi: 10.1084/jem.137.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paliogianni F, Ahuja SS, Balow JP, Balow JE, Boumpas DT. Novel mechanism for inhibition of human T cells by glucocorticoids. Glucocorticoids inhibit signal transduction through IL-2 receptor. J Immunol. 1993;151:4081–4089. [PubMed] [Google Scholar]
- 49.Rinehart JJ, Balcerzak SP, Sagone AL, LoBuglio AF. Effects of glucocorticoids on human monocyte function. J Clin Invest. 1974;54:1337–1343. doi: 10.1172/JCI107880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shodell M, Shah K, Siegal FP. Circulating human plasmacytoid dendritic cells are highly sensitive to corticosteroid administration. Lupus. 2003;12:222–230. doi: 10.1191/0961203303lu362xx. [DOI] [PubMed] [Google Scholar]
- 51.Ginzler E, Diamond H, Kaplan D, Weiner M, Schlesinger M, Seleznick M. Computer analysis of factors influencing frequency of infection in systemic lupus erythematosus. Arthritis Rheum. 1978;21:37–44. doi: 10.1002/art.1780210107. [DOI] [PubMed] [Google Scholar]
- 52.Stuck AE, Minder CE, Frey FJ. Risk of infectious complications in patients taking glucocorticosteroids. Rev Infect Dis. 1989;11:954–963. doi: 10.1093/clinids/11.6.954. [DOI] [PubMed] [Google Scholar]
- 53.Khan SA, Wingard JR. Infection and mucosal injury in cancer treatment. J Natl Cancer Inst Monogr. 2001;29:31–36. doi: 10.1093/oxfordjournals.jncimonographs.a003437. [DOI] [PubMed] [Google Scholar]
- 54.Wadhwa PD, Morrison VA. Infectious complications of chronic lymphocytic leukemia. Semin Oncol. 2006;33:240–249. doi: 10.1053/j.seminoncol.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 55.Bodey GP, Buckley M, Sathe YS, Freireich EJ. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med. 1966;64:328–340. doi: 10.7326/0003-4819-64-2-328. [DOI] [PubMed] [Google Scholar]
- 56.Humphreys JM, Stringer RE, Hart CA, Edwards SW. Effect of cytotoxic drugs on mature neutrophil function in the presence and absence of granulocyte-macrophage colony-stimulating factor. Br J Haematol. 1993;84:316–321. doi: 10.1111/j.1365-2141.1993.tb03071.x. [DOI] [PubMed] [Google Scholar]
- 57.Mendonça MAO, Cunha FQ, Murta EFC, Tavares-Murta BM. Failure of neutrophil chemotactic function in breast cancer patients treated with chemotherapy. Cancer Chemother Pharmacol. 2006;57:663–670. doi: 10.1007/s00280-005-0086-4. [DOI] [PubMed] [Google Scholar]
- 58.Bodey GP, Jadeja L, Elting L. Pseudomonas bacteremia. Retrospective analysis of 410 episodes. Arch Intern Med. 1985;145:1621–1629. doi: 10.1001/archinte.145.9.1621. [DOI] [PubMed] [Google Scholar]
- 59.Wisplinghoff H, Seifert H, Wenzel RP, Edmond MB. Current trends in the epidemiology of nosocomial bloodstream infections in patients with hematological malignancies and solid neoplasms in hospitals in the United States. Clin Infect Dis. 2003;36:1103–1110. doi: 10.1086/374339. [DOI] [PubMed] [Google Scholar]
- 60.Perez F, Adachi J, Bonomo RA. Antibiotic-resistant gram-negative bacterial infections in patients with cancer. Clin Infect Dis. 2014;59(Suppl 5):S335–S339. doi: 10.1093/cid/ciu612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cohen JA, Coles AJ, Arnold DL, Confavreux C, Fox EJ, Hartung HP, Havrdova E, Selmaj KW, Weiner HL, Fisher E, Brinar VV, Giovannoni G, Stojanovic M, Ertik BI, Lake SL, Margolin DH, Panzara MA, Compston DA. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet. 2012;380:1819–1828. doi: 10.1016/S0140-6736(12)61769-3. [DOI] [PubMed] [Google Scholar]
- 62.Chung JW, Lee SO, Choi SH, Woo JH, Ryu J, Kim YS, Kim NJ. Risk factors and outcome for breakthrough candidaemia in patients with cancer. Mycoses. 2006;49:114–118. doi: 10.1111/j.1439-0507.2006.01198.x. [DOI] [PubMed] [Google Scholar]
- 63.Dvorak CC, Steinbach WJ, Brown JMY, Agarwal R. Risks and outcomes of invasive fungal infections in pediatric patients undergoing allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2005;36:621–629. doi: 10.1038/sj.bmt.1705113. [DOI] [PubMed] [Google Scholar]
- 64.Mackall CL. T-cell immunodeficiency following cytotoxic antineoplastic therapy: a review. Stem Cells. 2000;18:10–18. doi: 10.1634/stemcells.18-1-10. [DOI] [PubMed] [Google Scholar]
- 65.Mackall CL, Fleisher TA, Brown MR, Magrath IT, Shad AT, Horowitz ME, Wexler LH, Adde MA, McClure LL, Gress RE. Lymphocyte depletion during treatment with intensive chemotherapy for cancer. Blood. 1994;84:2221–2228. [PubMed] [Google Scholar]
- 66.Holm G, Mellstedt H, Björkholm M, Johansson B, Killander D, Sundblad R, Söderberg G. Lymphocyte abnormalities in untreated patients with Hodgkin’s disease. Cancer. 1976;37:751–762. doi: 10.1002/1097-0142(197602)37:2<751::aid-cncr2820370223>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 67.Gutierrez-Ureña S, Molina JF, García CO, Cuéllar ML, Espinoza LR. Pancytopenia secondary to methotrexate therapy in rheumatoid arthritis. Arthritis Rheum. 1996;39:272–276. doi: 10.1002/art.1780390214. [DOI] [PubMed] [Google Scholar]
- 68.Ortiz Z, Shea B, Suarez-Almazor ME, Moher D, Wells GA, Tugwell P. The efficacy of folic acid and folinic acid in reducing methotrexate gastrointestinal toxicity in rheumatoid arthritis. A meta-analysis of randomized controlled trials. J Rheumatol. 1998;25:36–43. [PubMed] [Google Scholar]
- 69.Segal BH, Sneller MC. Infectious complications of immunosuppressive therapy in patients with rheumatic diseases. Rheum Dis Clin North Am. 1997;23:219–237. doi: 10.1016/s0889-857x(05)70327-6. [DOI] [PubMed] [Google Scholar]
- 70.Antonelli MA, Moreland LW, Brick JE. Herpes zoster in patients with rheumatoid arthritis treated with weekly, low-dose methotrexate. Am J Med. 1991;90:295–298. [PubMed] [Google Scholar]
- 71.Arunkumar P, Crook T, Ballard J. Disseminated histoplasmosis presenting as pancytopenia in a methotrexate-treated patient. Am J Hematol. 2004;77:86–87. doi: 10.1002/ajh.20058. [DOI] [PubMed] [Google Scholar]
- 72.Kanik KS, Cash JM. Does methotrexate increase the risk of infection or malignancy? Rheum Dis Clin North Am. 1997;23:955–967. doi: 10.1016/s0889-857x(05)70368-9. [DOI] [PubMed] [Google Scholar]
- 73.Keegan JM, Byrd JW. Nocardiosis associated with low dose methotrexate for rheumatoid arthritis. J Rheumatol. 1988;15:1585–1586. [PubMed] [Google Scholar]
- 74.Lang B, Riegel W, Peters T, Peter HH. Low dose methotrexate therapy for rheumatoid arthritis complicated by pancytopenia and Pneumocystis carinii pneumonia. J Rheumatol. 1991;18:1257–1259. [PubMed] [Google Scholar]
- 75.Kamel OW, van de Rijn M, Weiss LM, Del Zoppo GJ, Hench PK, Robbins BA, Montgomery PG, Warnke RA, Dorfman RF. Brief report: reversible lymphomas associated with Epstein-Barr virus occurring during methotrexate therapy for rheumatoid arthritis and dermatomyositis. N Engl J Med. 1993;328:1317–1321. doi: 10.1056/NEJM199305063281806. [DOI] [PubMed] [Google Scholar]
- 76.Maruani A, Wierzbicka E, Machet MC, Abdallah-Lotf M, de Muret A, Machet L. Reversal of multifocal cutaneous lymphoproliferative disease associated with Epstein-Barr virus after withdrawal of methotrexate therapy for rheumatoid arthritis. J Am Acad Dermatol. 2007;57(suppl 5):S69–S71. doi: 10.1016/j.jaad.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 77.Gourley MF, Austin HA, III, Scott D, Yarboro CH, Vaughan EM, Muir J, Boumpas DT, Klippel JH, Balow JE, Steinberg AD. Methylprednisolone and cyclophosphamide, alone or in combination, in patients with lupus nephritis. A randomized, controlled trial. Ann Intern Med. 1996;125:549–557. doi: 10.7326/0003-4819-125-7-199610010-00003. [DOI] [PubMed] [Google Scholar]
- 78.Pryor BD, Bologna SG, Kahl LE. Risk factors for serious infection during treatment with cyclophosphamide and high-dose corticosteroids for systemic lupus erythematosus. Arthritis Rheum. 1996;39:1475–1482. doi: 10.1002/art.1780390906. [DOI] [PubMed] [Google Scholar]
- 79.Viscoli C, Varnier O, Machetti M. Infections in patients with febrile neutropenia: epidemiology, microbiology, and risk stratification. Clin Infect Dis. 2005;40(Suppl 4):S240–S245. doi: 10.1086/427329. [DOI] [PubMed] [Google Scholar]
- 80.Cheson BD. Infectious and immunosuppressive complications of purine analog therapy. J Clin Oncol. 1995;13:2431–2448. doi: 10.1200/JCO.1995.13.9.2431. [DOI] [PubMed] [Google Scholar]
- 81.Aksoy S, Harputluoglu H, Kilickap S, Dede DS, Dizdar O, Altundag K, Barista K. Rituximab-related viral infections in lymphoma patients. Leuk Lymphoma. 2007;48:1307–1312. doi: 10.1080/10428190701411441. [DOI] [PubMed] [Google Scholar]
- 82.Calabrese LH, Molloy ES, Huang D, Ransohoff RM. Progressive multifocal leukoencephalopathy in rheumatic diseases: evolving clinical and pathologic patterns of disease. Arthritis Rheum. 2007;56:2116–2128. doi: 10.1002/art.22657. [DOI] [PubMed] [Google Scholar]
- 83.Pelosini M, Focosi D, Rita F, Galimberti S, Caracciolo F, Benedetti E, Papineschi F, Petrini M. Progressive multifocal leukoencephalopathy: report of three cases in HIV-negative hematological patients and review of the literature. Ann Hematol. 2008;87:405–412. doi: 10.1007/s00277-007-0411-6. [DOI] [PubMed] [Google Scholar]
- 84.Roberts DM, Jones RB, Smith RM, Alberici F, Kumaratne DS, Burns S, Jayne DR. Rituximab-associated hypogammaglobulinemia: incidence, predictors and outcomes in patients with multi-system autoimmune disease. J Autoimmun. 2015;57:60–65. doi: 10.1016/j.jaut.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 85.Barmettler S, Price C. Continuing IgG replacement therapy for hypogammaglobulinemia after rituximab-for how long? J Allergy Clin Immunol. 2015;136:1407–1409. doi: 10.1016/j.jaci.2015.06.035. [DOI] [PubMed] [Google Scholar]
- 86.Martin SI, Marty FM, Fiumara K, Treon SP, Gribben JG, Baden LR. Infectious complications associated with alemtuzumab use for lymphoproliferative disorders. Clin Infect Dis. 2006;43:16–24. doi: 10.1086/504811. [DOI] [PubMed] [Google Scholar]
- 87.Andrassy J, Hoffmann VS, Rentsch M, Stangl M, Habicht A, Meiser B, Fischereder M, Jauch KW, Guba M. Is cytomegalovirus prophylaxis dispensable in patients receiving an mTOR inhibitor-based immunosuppression? a systematic review and meta-analysis. Transplantation. 2012;94:1208–1217. doi: 10.1097/TP.0b013e3182708e56. [DOI] [PubMed] [Google Scholar]
- 88.James LC, Hale G, Waldmann H, Bloomer AC. 1.9 A structure of the therapeutic antibody CAMPATH-1H fab in complex with a synthetic peptide antigen. J Mol Biol. 1999;289:293–301. doi: 10.1006/jmbi.1999.2750. [DOI] [PubMed] [Google Scholar]
- 89.Lundin J, Porwit-MacDonald A, Rossmann ED, Karlsson C, Edman P, Rezvany MR, Kimby E, Osterborg A, Mellstedt H. Cellular immune reconstitution after subcutaneous alemtuzumab (anti-CD52 monoclonal antibody, CAMPATH-1H) treatment as first-line therapy for B-cell chronic lymphocytic leukaemia. Leukemia. 2004;18:484–490. doi: 10.1038/sj.leu.2403258. [DOI] [PubMed] [Google Scholar]
- 90.Rawstron AC, Kennedy B, Moreton P, Dickinson AJ, Cullen MJ, Richards SJ, Jack AS, Hillmen P. Early prediction of outcome and response to alemtuzumab therapy in chronic lymphocytic leukemia. Blood. 2004;103:2027–2031. doi: 10.1182/blood-2002-10-3270. [DOI] [PubMed] [Google Scholar]
- 91.Keating M, Coutré S, Rai K, Osterborg A, Faderl S, Kennedy B, Kipps T, Bodey G, Byrd JC, Rosen S, Dearden C, Dyer MJ, Hillmen P. Management guidelines for use of alemtuzumab in B-cell chronic lymphocytic leukemia. Clin Lymphoma. 2004;4:220–227. doi: 10.3816/clm.2004.n.002. [DOI] [PubMed] [Google Scholar]
- 92.Thursky KA, Worth LJ, Seymour JF, Miles Prince H, Slavin MA. Spectrum of infection, risk and recommendations for prophylaxis and screening among patients with lymphoproliferative disorders treated with alemtuzumab. Br J Haematol. 2006;132:3–12. doi: 10.1111/j.1365-2141.2005.05789.x. [DOI] [PubMed] [Google Scholar]
- 93.Ng AP, Worth L, Chen L, Seymour JF, Prince HM, Slavin M, Thursky K. Cytomegalovirus DNAemia and disease: incidence, natural history and management in setting other than allogeneic stem cell transplantation. Haematologica. 2005;90:1672–1679. [PubMed] [Google Scholar]
- 94.Yoon J-W, Gollapudi S, Pahl MV, Vaziri ND. Naïve and central memory T-cell lymphopenia in end-stage renal disease. Kidney Int. 2006;70:371–376. doi: 10.1038/sj.ki.5001550. [DOI] [PubMed] [Google Scholar]
- 95.Marder W, McCune WJ. Advances in immunosuppressive therapy. Semin Respir Crit Care Med. 2007;28:398–417. doi: 10.1055/s-2007-985612. [DOI] [PubMed] [Google Scholar]
- 96.David-Neto E, da Fonseca JA, de Paula FJ, Nahas WC, Sabbaga E, Ianhez LE. Is azathioprine harmful to chronic viral hepatitis in renal transplantation? A long-term study on azathioprine withdrawal. Transplant Proc. 1999;31:1149–1150. doi: 10.1016/s0041-1345(98)01941-1. [DOI] [PubMed] [Google Scholar]
- 97.Sullivan KM, Witherspoon RP, Storb R, Weiden P, Flournoy N, Dahlberg S, Deeg HJ, Sanders JE, Doney KC, Appelbaum FR, McCuffin R, McDonald GB, Meyers J, Schubert MM, Gauvreau J, Shulman HM, Sale GE, Anasetti C, Loughran TP, Strom S, Nims J, Thomas ED. Prednisone and azathioprine compared with prednisone and placebo for treatment of chronic graft-v-host disease: prognostic influence of prolonged thrombocytopenia after allogeneic marrow transplantation. Blood. 1988;72:546–554. [PubMed] [Google Scholar]
- 98.Eisen HJ, Kobashigawa J, Keogh A, Bourge R, Renlund D, Mentzer R, Alderman E, Valantine H, Dureau G, Mancini D, Mamelok R, Gordon R, Wang W, Mehra M, Constanzo MR, Hummel M, Johnson J Mycophenolate Mofetil Cardiac Study Investigators. Three-year results of a randomized, double-blind, controlled trial of mycophenolate mofetil versus azathioprine in cardiac transplant recipients. J Heart Lung Transplant. 2005;24:517–525. doi: 10.1016/j.healun.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 99.Sollinger HW U.S. Renal Transplant Mycophenolate Mofetil Study Group. Mycophenolate mofetil for the prevention of acute rejection in primary cadaveric renal allograft recipients. Transplantation. 1995;60:225–232. doi: 10.1097/00007890-199508000-00003. [DOI] [PubMed] [Google Scholar]
- 100.Wiesner R, Rabkin J, Klintmalm G, McDiarmid S, Langnas A, Punch J, McMaster P, Kalayoglu M, Levy G, Freeman R, Bismuth H, Neuhaus P, Mamelok R, Wang W. A randomized double-blind comparative study of mycophenolate mofetil and azathioprine in combination with cyclosporine and corticosteroids in primary liver transplant recipients. Liver Transpl. 2001;7:442–450. doi: 10.1053/jlts.2001.23356. [DOI] [PubMed] [Google Scholar]
- 101.Corales R, Chua J, Mawhorter S, Young JB, Starling R, Tomford JW, McCarthy P, Braun WE, Smedira N, Hobbs R, Haas G, Pelegrin D, Majercik M, Hoercher K, Cook D, Avery RK. Significant post-transplant hypogammaglobulinemia in six heart transplant recipients: an emerging clinical phenomenon? Transpl Infect Dis. 2000;2:133–139. doi: 10.1034/j.1399-3062.2000.020306.x. [DOI] [PubMed] [Google Scholar]
- 102.Hutchinson P, Jose M, Atkins RC, Holdsworth SR. Ex vivo lymphocyte proliferative function is severely inhibited in renal transplant patients on mycophenolate mofetil treatment. Transpl Immunol. 2004;13:55–61. doi: 10.1016/j.trim.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 103.Sarmiento JM, Dockrell DH, Schwab TR, Munn SR, Paya CV. Mycophenolate mofetil increases cytomegalovirus invasive organ disease in renal transplant patients. Clin Transplant. 2000;14:136–138. doi: 10.1034/j.1399-0012.2000.140206.x. [DOI] [PubMed] [Google Scholar]
- 104.Jain AB, Hamad I, Rakela J, Dodson F, Kramer D, Demetris J, McMichael J, Starzl TE, Fung JJ. A prospective randomized trial of tacrolimus and prednisone versus tacrolimus, prednisone, and mycophenolate mofetil in primary adult liver transplant recipients: an interim report. Transplantation. 1998;66:1395–1398. doi: 10.1097/00007890-199811270-00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Church D, Elsayed D, Reid O, Winston B, Lindsay R. Burn wound infections. Clin Microbiol Rev. 2006;19:403–434. doi: 10.1128/CMR.19.2.403-434.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Oz HS, Hughes WT. Novel anti-Pneumocystis carinii effects of the immunosuppressant mycophenolate mofetil in contrast to provocative effects of tacrolimus, sirolimus, and dexamethasone. J Infect Dis. 1997;175:901–904. doi: 10.1086/513988. [DOI] [PubMed] [Google Scholar]
- 107.Neuhaus P, Pichlmayr R, Williams R. Randomised trial comparing tacrolimus (FK506) and cyclosporine in prevention of liver allograft rejection. Lancet. 1994;344:423–428. [PubMed] [Google Scholar]
- 108.Singh N, Mieles L, Yu VL, Starzl TE. Decreased incidence of viral infections in liver transplant recipients. Possible effects of FK506? Dig Dis Sci. 1994;39:15–18. doi: 10.1007/BF02090054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.The US Multicenter FK506 Liver Study Group. A comparison of tacrolimus (FK 506) and cyclosporine for immunosuppression in liver transplantation. The U.S. Multicenter FK506 Liver Study Group. N Engl J Med. 1994;331:1110–1115. doi: 10.1056/NEJM199410273311702. [DOI] [PubMed] [Google Scholar]
- 110.Andreone P, Gramenzi A, Lorenzini S, Biselli M, Cursaro C, Pileri S, Bernardi M. Posttransplantation lymphoproliferative disorders. Arch Intern Med. 2003;163:1997–2004. doi: 10.1001/archinte.163.17.1997. [DOI] [PubMed] [Google Scholar]
- 111.Bustami RT, Ojo AO, Wolfe RA, Merion RM, Bennett WM, McDiarmid SV, Leichtman AB, Held PJ, Port FK. Immunosuppression and the risk of post-transplant malignancy among cadaveric first kidney transplant recipients. Am J Transplant. 2004;4:87–93. doi: 10.1046/j.1600-6135.2003.00274.x. [DOI] [PubMed] [Google Scholar]
- 112.Swinnen LJ, Costanzo-Nordin MR, Fisher SG, O’Sullivan EJ, Johnson MR, Heroux AL, Dizikes GJ, Pifarre R, Fisher RI. Increased incidence of lymphoproliferative disorder after immunosuppression with the monoclonal antibody OKT3 in cardiac-transplant recipients. N Engl J Med. 1990;323:1723–1728. doi: 10.1056/NEJM199012203232502. [DOI] [PubMed] [Google Scholar]
- 113.Singh N, Avery RK, Munoz P, Pruett TL, Alexander B, Jacobs R, Tollemar JG, Dominguez EA, Yu CM, Paterson DL, Husain S, Kusne S, Linden P. Trends in risk profiles for and mortality associated with invasive aspergillosis among liver transplant recipients. Clin Infect Dis. 2003;36:46–52. doi: 10.1086/345441. [DOI] [PubMed] [Google Scholar]
- 114.Singh N. Infectious complications in organ transplant recipients with the use of calcineurin-inhibitor agent-based immunosuppressive regimens. Curr Opin Infect Dis. 2005;18:342–345. doi: 10.1097/01.qco.0000172698.52408.be. [DOI] [PubMed] [Google Scholar]
- 115.High KP. The antimicrobial activities of cyclosporine, FK506, and rapamycin. Transplantation. 1994;57:1689–1700. [PubMed] [Google Scholar]
- 116.Singh N, Alexander BD, Lortholary O, Dromer F, Gupta KL, John GT, del Busto R, Klintmalm GB, Somani J, Lyon GM, Pursell K, Stosor V, Munoz P, Limaye AP, Kalil AC, Pruett TL, Garcia-Diaz J, Humar A, Houston S, House AA, Wray D, Orloff S, Dowdy LA, Fisher RA, Heitman J, Wagener MM, Husain S Cryptococcal Collaborative Transplant Study Group. Cryptococcus neoformans in organ transplant recipients: impact of calcineurin-inhibitor agents on mortality. J Infect Dis. 2007;195:756–764. doi: 10.1086/511438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kreis H, Cisterne JM, Land W, Wramner L, Squifflet JP, Abramowicz D, Campistol JM, Morales JM, Grinyo JM, Mourad G, Berthoux FC, Brattström C, Lebranchu Y, Vialtel P. Sirolimus in association with mycophenolate mofetil induction for the prevention of acute graft rejection in renal allograft recipients. Transplantation. 2000;69:1252–1260. doi: 10.1097/00007890-200004150-00009. [DOI] [PubMed] [Google Scholar]
- 118.Nashan B, Gaston R, Emery V, Säemann MD, Mueller NJ, Couzi L, Dantal J, Shihab F, Mulgaonkar S, Seun Kim Y, Brennan DC. Review of cytomegalovirus infection findings with mammalian target of rapamycin inhibitor-based immunosuppressive therapy in de novo renal transplant recipients. Transplantation. 2012;93:1075–1085. doi: 10.1097/TP.0b013e31824810e6. [DOI] [PubMed] [Google Scholar]
- 119.Benjamin D, Colombi M, Moroni C, Hall MN. Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat Rev Drug Discov. 2011;10:868–880. doi: 10.1038/nrd3531. [DOI] [PubMed] [Google Scholar]
- 120.Issa NC, Fishman JA. Infectious complications of antilymphocyte therapies in solid organ transplantation. Clin Infect Dis. 2009;48:772–786. doi: 10.1086/597089. [DOI] [PubMed] [Google Scholar]
- 121.Bonnefoy-Berard N, Revillard JP. Mechanisms of immunosuppression induced by antithymocyte globulins and OKT3. J Heart Lung Transplant. 1996;15:435–442. [PubMed] [Google Scholar]
- 122.Hibberd PL, Tolkoff-Rubin NE, Cosimi AB, Schooley RT, Isaacson D, Doran M, Delvecchio A, Delmonico FL, Auchincloss H, Jr, Rubin RH. Symptomatic cytomegalovirus disease in the cytomegalovirus antibody seropositive renal transplant recipient treated with OKT3. Transplantation. 1992;53:68–72. doi: 10.1097/00007890-199201000-00013. [DOI] [PubMed] [Google Scholar]
- 123.Caillard S, Dharnidharka V, Agodoa L, Bohen E, Abbott K. Posttransplant lymphoproliferative disorders after renal transplantation in the United States in era of modern immunosuppression. Transplantation. 2005;80:1233–1243. doi: 10.1097/01.tp.0000179639.98338.39. [DOI] [PubMed] [Google Scholar]
- 124.Brennan DC, Daller JA, Lake KD, Cibrik D, Del Castillo D Thymoglobulin Induction Study Group. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med. 2006;355:1967–1977. doi: 10.1056/NEJMoa060068. [DOI] [PubMed] [Google Scholar]
- 125.Brennan DC, Flavin K, Lowell JA, Howard TK, Shenoy S, Burgess S, Dolan S, Kano JM, Mahon M, Schnitzler MA, Woodward R, Irish W, Singer GG. A randomized, double-blinded comparison of Thymoglobulin versus Atgam for induction immunosuppressive therapy in adult renal transplant recipients. Transplantation. 1999;7:1011–1018. doi: 10.1097/00007890-199904150-00013. [DOI] [PubMed] [Google Scholar]
- 126.Husain S, Singh N. The impact of novel immunosuppressive agents on infections in organ transplant recipients and the interactions of these agents with antimicrobials. Clin Infect Dis. 2002;35:53–61. doi: 10.1086/340867. [DOI] [PubMed] [Google Scholar]
- 127.Ramirez CB, Marino IR. The role of basiliximab induction therapy in organ transplantation. Expert Opin Biol Ther. 2007;7:137–148. doi: 10.1517/14712598.7.1.137. [DOI] [PubMed] [Google Scholar]
- 128.Kahan BD, Rajagopalan PR, Hall M. Reduction of the occurrence of acute cellular rejection among renal allograft recipients treated with basiliximab, a chimeric anti-interleukin-2-receptor monoclonal antibody. United States Simulect Renal Study Group. Transplantation. 1999;67:276–284. doi: 10.1097/00007890-199901270-00016. [DOI] [PubMed] [Google Scholar]
- 129.Singh JA, Cameron C, Noorbaloochi S, Cullis T, Tucker M, Christensen R, Ghogomu ET, Coyle D, Clifford T, Tugwell P, Wells GA. Risk of serious infection in biological treatment of patients with rheumatoid arthritis: a systematic review and meta-analysis. Lancet. 2015;386:258–265. doi: 10.1016/S0140-6736(14)61704-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Peleg AY, Husain S, Kwak EJ, Silveira FP, Ndirangu M, Tran J, Shutt KA, Shapiro R, Thai N, Abu-Elmagd K, McCurry KR, Marcos A, Paterson DL. Opportunistic infections in 547 organ transplant recipients receiving alemtuzumab, a humanized monoclonal CD-52 antibody. Clin Infect Dis. 2007;44:204–212. doi: 10.1086/510388. [DOI] [PubMed] [Google Scholar]
- 131.Sakoda Y, Hashimoto D, Asakura S, Takeuchi K, Harada M, Tanimoto M, Teshima T. Donor-derived thymic-dependent T cells cause chronic graft-versus-host disease. Blood. 2007;109:1756–1764. doi: 10.1182/blood-2006-08-042853. [DOI] [PubMed] [Google Scholar]
- 132.Lum LG, Seigneuret MC, Storb RF, Witherspoon RP, Thomas ED. In vitro regulation of immunoglobulin synthesis after marrow transplantation. I. T-cell and B-cell deficiencies in patients with and without chronic graft-versus-host disease. Blood. 1981;58:431–439. [PubMed] [Google Scholar]
- 133.Saxon A, McIntyre RE, Stevens RH, Gale RP. Lymphocyte dysfunction in chronic graft-versus-host disease. Blood. 1981;58:746–751. [PubMed] [Google Scholar]
- 134.Guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. Recommendations of the CDC, the Infectious Diseases Society of America, and the American Society of Blood and Marrow Transplantation. MMWR. 2000 Oct 20;49(RR10):1–128. doi: 10.1016/S1083-8791(00)70002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Perreault C, Giasson M, Gyger M, Belanger R, David M, Bonny Y, Boileau J, Barcelo R, Moquin JP. Serum immunoglobulin levels following allogeneic bone marrow transplantation. Blut. 1985;51:137–142. doi: 10.1007/BF00320027. [DOI] [PubMed] [Google Scholar]
- 136.Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411–452. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- 137.Algood HM, Lin PL, Flynn JL. Tumor necrosis factor and chemokine interactions in the formation and maintenance of granulomas in tuberculosis. Clin Infect Dis. 2005;41(Suppl 3):S189–S193. doi: 10.1086/429994. [DOI] [PubMed] [Google Scholar]
- 138.Allendoerfer R, Deepe GS., Jr Blockade of endogenous TNF-alpha exacerbates primary and secondary pulmonary histoplasmosis by differential mechanisms. J Immunol. 1998;160:6072–6082. [PubMed] [Google Scholar]
- 139.Flynn JL, Goldstein MM, Chan J, Triebold KJ, Pfeffer K, Lowenstein CJ, Schreiber R, Mak TW, Bloom BR. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 140.Huffnagle GB, Toews GB, Burdick MD, Boyd MB, McAllister KS, McDonald RA, Kunkel SL, Strieter RM. Afferent phase production of TNF-alpha is required for the development of protective T cell immunity to Cryptococcus neoformans. J Immunol. 1996;157:4529–4536. [PubMed] [Google Scholar]
- 141.Mehrad B, Strieter RM, Standiford TJ. Role of TNF-alpha in pulmonary host defense in murine invasive aspergillosis. J Immunol. 1999;162:1633–1640. [PubMed] [Google Scholar]
- 142.Kaur N, Mahl TC. Pneumocystis jirovecii (carinii) pneumonia after infliximab therapy: a review of 84 cases. Dig Dis Sci. 2007;52:1481–1484. doi: 10.1007/s10620-006-9250-x. [DOI] [PubMed] [Google Scholar]
- 143.Wallis RS, Broder M, Wong J, Lee A, Hoq L. Reactivation of latent granulomatous infections by infliximab. Clin Infect Dis. 2005;41(Suppl 3):S194–S198. doi: 10.1086/429996. [DOI] [PubMed] [Google Scholar]
- 144.Ehlers S. Tumor necrosis factor and its blockade in granulomatous infections: differential modes of action of infliximab and etanercept? Clin Infect Dis. 2005;41(Suppl 3):S199–S203. doi: 10.1086/429998. [DOI] [PubMed] [Google Scholar]
- 145.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, Siegel JN, Braun MM. Tuberculosis associated with infliximab, a tumor necrosis factor α-neutralizing agent. N Engl J Med. 2001;345:1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 146.Bergstrom L, Yocum DE, Ampel NM, Villanueva I, Lisse J, Gluck O, Tesser J, Posever J, Miller M, Araujo J, Kageyama DM, Berry M, Karl L, Yung CM. Increased risk of coccidioidiomycosis in patients treated with tumor necrosis factor alpha antagonists. Arthritis Rheum. 2004;50:1959–1966. doi: 10.1002/art.20454. [DOI] [PubMed] [Google Scholar]
- 147.Lee J-H, Slifman NR, Gershon SK, Edwards ET, Schwieterman WD, Siegel JN, Wise RP, Brown SL, Udall JN, Jr, Braun MM. Life-threatening histoplasmosis complicating immunotherapy with tumor necrosis factor α antagonists infliximab and etanercept. Arthritis Rheum. 2002;46:2565–2570. doi: 10.1002/art.10583. [DOI] [PubMed] [Google Scholar]
- 148.True DG, Penmetcha M, Peckham SJ. Disseminated cryptococcal infection in rheumatoid arthritis treated with methotrexate and infliximab. J Rheumatol. 2002;29:1561–1563. [PubMed] [Google Scholar]
- 149.Warris A, Bjørneklett A, Gaustad P. Invasive pulmonary aspergillosis associated with infliximab therapy. N Engl J Med. 2001;344:1099–1100. [PubMed] [Google Scholar]
- 150.Nard FD, Todoerti M, Grosso V, Monti S, Breda S, Rossi S, Montecucco C, Caporali R. Risk of hepatitis B virus reactivation in rheumatoid arthritis patients undergoing biologic treatment: extending perspective from old to newer drugs. World J Hepatol. 2015;7:344–361. doi: 10.4254/wjh.v7.i3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Murdaca G, Spanò F, Contatore M, Guastalla A, Penza E, Magnani O, Puppo F. Infection risk associated with anti-TNF-α agents: a review. Expert Opin Drug Saf. 2015;14:571–582. doi: 10.1517/14740338.2015.1009036. [DOI] [PubMed] [Google Scholar]
- 152.Siebert S, Tsoukas A, Robertson J, McInnes I. Cytokines as therapeutic targets in rheumatoid arthritis and other inflammatory diseases. Pharmacol Rev. 2015;67:280–309. doi: 10.1124/pr.114.009639. [DOI] [PubMed] [Google Scholar]
- 153.Goldbach-Mansky R, Dailey NJ, Canna SW, Gelabert A, Jones J, Rubin BI, Kim HJ, Brewer C, Zalewski C, Wiggs E, Hill S, Turner ML, Karp BI, Aksentijevich I, Pucino F, Penzak SR, Haverkamp MH, Stein L, Adams BS, Moore TL, Fuhlbrigge RC, Shaham B, Jarvis JN, O’Neil K, Vehe RK, Beitz LO, Gardner G, Hannan WP, Warren RW, Horn W, Cole JL, Paul SM, Hawkins PN, Pham TH, Snyder C, Wesley RA, Hoffmann SC, Holland SM, Butman JA, Kastner DL. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition. N Engl J Med. 2006;355:581–592. doi: 10.1056/NEJMoa055137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Salliot C, Dougados M, Gossec L. Risk of serious infections during rituximab, abatacept and anakinra treatments for rheumatoid arthritis: meta-analyses of randomised placebo-controlled trials. Ann Rheum Dis. 2009;68:25–32. doi: 10.1136/ard.2007.083188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yamamoto K, Goto H, Hirao K, Nakajima A, Origasa H, Tanaka K, Tomobe M, Totsuka K. Longterm safety of tocilizumab: results from 3 years of follow-up post-marketing surveillance of 5573 patients with rheumatoid arthritis in Japan. J Rheumatol. 2015;42:1368–1375. doi: 10.3899/jrheum.141210. [DOI] [PubMed] [Google Scholar]
- 156.Curtis JR, Perez-Gutthann S, Suissa S, Napalkov P, Singh N, Thompson L, Porter-Brown B Actemra Pharmacoepidemiology Board. Tocilizumab in rheumatoid arthritis: a case study of safety evaluations of a large postmarketing data set from multiple data sources. Semin Arthritis Rheum. 2015;44:381–388. doi: 10.1016/j.semarthrit.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 157.Merrill JT, Ginzler EM, Wallace DJ, McKay JD, Lisse JR, Aranow C, Wellborne FR, Burnette M, Condemi J, Zhong ZJ, Pineda L, Klein J, Freimuth WW LBSL02/99 Study Group. Long-term safety profile of belimumab plus standard therapy in patients with systemic lupus erythematosus. Arthritis Rheum. 2012;64:3364–3373. doi: 10.1002/art.34564. [DOI] [PubMed] [Google Scholar]
- 158.Henegar CE, Eudy AM, Kharat V, Hill DD, Bennett D, Haight B. Progressive multifocal leukoencephalopathy in patients with systemic lupus erythematosus: a systematic literature review. Lupus. 2016 Jan 6; doi: 10.1177/0961203315622819. pii: 0961203315622819 Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 159.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 160.Weinblatt ME, Moreland LW, Westhovens R, Cohen RB, Kelly SM, Khan N, Pappu R, Delaet I, Luo A, Gujrathi S, Hochberg MC. Safety of abatacept administered intravenously in treatment of rheumatoid arthritis: integrated analyses of up to 8 years of treatment from the abatacept clinical trial program. J Rheumatol. 2013;40:787–797. doi: 10.3899/jrheum.120906. [DOI] [PubMed] [Google Scholar]
- 161.Stüve O, Marra CM, Bar-Or A, Niino M, Cravens PD, Cepok S, Frohman EM, Phillips JT, Arendt G, Jerome KR, Cook L, Grand’Maison F, Hemmer B, Monson NL, Racke MK. Altered CD4+/CD8+ T-cell ratios in cerebrospinal fluid of natalizumab-treated patients with multiple sclerosis. Arch Neurol. 2006;63:1383–1387. doi: 10.1001/archneur.63.10.1383. [DOI] [PubMed] [Google Scholar]
- 162.Fine AJ, Sorbello A, Kortepeter C, Scarazzini L. Central nervous system herpes simplex and varicella zoster virus infections in natalizumab-treated patients. Clin Infect Dis. 2013;57:849–852. doi: 10.1093/cid/cit376. [DOI] [PubMed] [Google Scholar]
- 163.Kappos L, Radue EW, O’Connor P, Polman C, Hohlfeld R, Calabresi P, Selmaj K, Agoropoulou C, Leyk M, Zhang-Auberson L, Burtin P FREEDOMS Study Group. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362:387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- 164.Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, Pelletier J, Capra R, Gallo P, Izquierdo G, Tiel-Wilck K, de Vera A, Jin J, Stites T, Wu S, Aradhye S, Kappos L TRANSFORMS Study Group. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362:402–415. doi: 10.1056/NEJMoa0907839. [DOI] [PubMed] [Google Scholar]
- 165.Cohen S, Radominski SC, Gomez-Reino JJ, Wang L, Krishnaswami S, Wood SP, Soma K, Nduaka CI, Kwok K, Valdez H, Benda B, Riese R. Analysis of infections and all-cause mortality in phase II, phase III, and long-term extension studies of tofacitinib in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66:2924–2937. doi: 10.1002/art.38779. [DOI] [PubMed] [Google Scholar]
- 166.Winthrop KL, Yamanaka H, Valdez H, Mortensen E, Chew R, Krishnaswami S, Kawabata T, Riese R. Herpes zoster and tofacitinib therapy in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66:2675–2684. doi: 10.1002/art.38745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hillmen P, Young NS, Schubert J, Brodsky RA, Socié G, Muus P, Röth A, Szer J, Elebute MO, Nakamura R, Browne P, Risitano AM, Hill A, Schrezenmeier H, Fu C-L, Maciejewski J, Rollins SA, Mojcik CF, Rother RP, Luzzatto L. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2006;355:1233–1243. doi: 10.1056/NEJMoa061648. [DOI] [PubMed] [Google Scholar]
- 168.Braconier JH. Reversible total IgA deficiency associated with phenytoin treatment. Scand J Infect Dis. 1999;31:515–516. doi: 10.1080/00365549950164102. [DOI] [PubMed] [Google Scholar]
- 169.Seager J, Jamison DL, Wilson J, Hayward AR, Soothill JF. IgA deficiency, epilepsy, and phenytoin treatment. Lancet. 1975;2:632–635. doi: 10.1016/s0140-6736(75)90115-4. [DOI] [PubMed] [Google Scholar]
- 170.Ranua J, Luoma K, Auvinen A, Peltola J, Haapala AM, Raitanen J, Isojärvi J. Serum IgA, IgG, and IgM concentrations in patients with epilepsy and matched controls: a cohort-based cross-sectional study. Epilepsy Behav. 2005;6:191–195. doi: 10.1016/j.yebeh.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 171.Meier-Kriesche HU, Li S, Gruessner RW, Fung JJ, Bustami RT, Barr ML, Leichtman AB. Immunosuppression: evolution in practice and trends, 1994–2004. Am J Transplant. 2006;6(5p2):1111–1131. doi: 10.1111/j.1600-6143.2006.01270.x. [DOI] [PubMed] [Google Scholar]
- 172.Fishman JA, Rubin RH. Infection in organ-transplant recipients. N Engl J Med. 1998;338:1741–1751. doi: 10.1056/NEJM199806113382407. [DOI] [PubMed] [Google Scholar]
- 173.Rubin RH. Infection in the Organ Transplant Recipient. In: Rubin RH, Young LS, editors. Clinical Approach to Infection in the Compromised Host. 4. Kluwer Academic/Plenum Publishers; New York, N. Y: 2002. pp. 573–679. [Google Scholar]
- 174.Guidelines for the Prevention and Management of Infectious Complications of Solid Organ Transplantation. Introduction to the guidelines. Am J Transplant. 2004;4(Suppl 10):6–9. [Google Scholar]
- 175.Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357:2601–2614. doi: 10.1056/NEJMra064928. [DOI] [PubMed] [Google Scholar]
- 176.Ruiz I, Gavaldà J, Monforte V, Len O, Román A, Bravo C, Ferrer A, Tenorio L, Román F, Maestre J, Molina I, Morell F, Pahissa A. Donor-to-host transmission of bacterial and fungal infections in lung transplantation. Am J Transplant. 2006;6:178–182. doi: 10.1111/j.1600-6143.2005.01145.x. [DOI] [PubMed] [Google Scholar]
- 177.Singh N. Impact of donor bacteremia on outcome in organ transplant recipients. Liver Transpl. 2002;8:975–976. doi: 10.1053/jlts.2002.0080975. [DOI] [PubMed] [Google Scholar]
- 178.Fischer SA, Graham MB, Kuehnert MJ, Kotton CN, Srinivasan A, Marty FM, Comer JA, Guarner J, Paddock CD, DeMeo DL, Shieh W-J, Erickson BR, Bandy U, DeMaria A, Jr, Davis JP, Delmonico FL, Pavlin B, Likos A, Vincent MJ, Sealy TK, Goldsmith CS, Jernigan DB, Rollin PE, Packard MM, Patel M, Rowland C, Helfand RF, Nichol ST, Fishman JA, Ksiazek T, Zaki SR. Transmission of lymphocytic choriomeningitis virus by organ transplantation. N Engl J Med. 2006;354:2235–2249. doi: 10.1056/NEJMoa053240. [DOI] [PubMed] [Google Scholar]
- 179.Kotton CN. Zoonoses in solid-organ and hematopoietic stem cell transplant recipients. Clin Infect Dis. 2007;44:857–866. doi: 10.1086/511859. [DOI] [PubMed] [Google Scholar]
- 180.Srinivasan A, Burton EC, Kuehnert MJ, Rupprecht C, Sutker WL, Ksiazek TG, Paddock CD, Guarner J, Shieh WJ, Goldsmith C, Hanlon CA, Zoretic J, Fischbach B, Niezgoda M, El-Feky WH, Orciari L, Sanchez EQ, Likos A, Klintmalm GB, Cardo D, LeDuc J, Chamberland ME, Jernigan DB, Zaki SR Rabies in Transplant Recipients Investigation Team. Transmission of rabies virus from an organ donor to four transplant recipients. N Engl J Med. 2005;352:1103–1111. doi: 10.1056/NEJMoa043018. [DOI] [PubMed] [Google Scholar]
- 181.Centers for Diseases Control and Prevention (CDC) West Nile virus infections in organ transplant recipients—New York and Pennsylvania, August-September, 2005. MMWR Morb Mortal Wkly Rep. 2005;54:1021–1023. [PubMed] [Google Scholar]
- 182.Assi MA, Rosenblatt JE, Marshall WF. Donor-transmitted toxoplasmosis in liver transplant recipients: a case report and literature review. Transpl Infect Dis. 2007;9:132–136. doi: 10.1111/j.1399-3062.2006.00187.x. [DOI] [PubMed] [Google Scholar]
- 183.Barcán L, Luna C, Clara L, Sinagra A, Valledor A, De Rissio AM, Gadano A, García MM, de Santibañes E, Riarte A. Transmission of T. cruzi infection via liver transplantation to a nonreactive recipient for Chagas’ disease. Liver Transpl. 2005;11:1112–1116. doi: 10.1002/lt.20522. [DOI] [PubMed] [Google Scholar]
- 184.Centers for Disease Control and Prevention (CDC) Chagas disease after organ transplantation—United States, 2001. MMWR Morb Mortal Wkly Rep. 2002;51:210–212. [PubMed] [Google Scholar]
- 185.Muñoz P, Rodríguez C, Bouza E. Mycobacterium tuberculosis infection in recipients of solid organ transplants. Clin Infect Dis. 2005;40:581–587. doi: 10.1086/427692. [DOI] [PubMed] [Google Scholar]
- 186.Rogers NM, Peh CA, Faull R, Pannell M, Cooper J, Russ GR. Transmission of toxoplasmosis in two renal allograft recipients receiving an organ from the same donor. Transpl Infect Dis. 2007 Jul;:1. doi: 10.1111/j.1399-3062.2007.00244.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 187.Safdar N, Said A, Lucey MR, Knechtle SJ, D’Alessandro A, Musat A, Pirsch J, McDermott J, Kalayoglu M, Maki DG. Infected bilomas in liver transplant recipients: clinical features, optimal management, and risk factors for mortality. Clin Infect Dis. 2004;39:517–525. doi: 10.1086/422644. [DOI] [PubMed] [Google Scholar]
- 188.Mocarski ES., Jr Immunomodulation by cytomegaloviruses: manipulative strategies beyond evasion. Trends Microbiol. 2002;10:332–339. doi: 10.1016/s0966-842x(02)02393-4. [DOI] [PubMed] [Google Scholar]
- 189.Boni C, Fisicaro P, Valdatta C, Amadei B, Di Vincenzo P, Giuberti T, Laccabue D, Zerbini A, Cavalli A, Missale G, Bertoletti A, Ferrari C. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol. 2001;81:4215–4225. doi: 10.1128/JVI.02844-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Guerreiro-Cacais AO, Li L, Donati D, Bejarano MT, Morgan A, Masucci MG, Hutt-Fletcher L, Levitsky V. Capacity of Epstein-Barr virus to infect monocytes and inhibit their development into dendritic cells is affected by the cell type supporting virus replication. J Gen Virol. 2004;85:2767–2778. doi: 10.1099/vir.0.80140-0. [DOI] [PubMed] [Google Scholar]
- 191.Jabs WJ, Wagner HJ, Maurmann S, Hennig H, Kreft B. Inhibition of macrophage inflammatory protein-1 alpha production by Epstein-Barr virus. Blood. 2002;99:1512–1516. doi: 10.1182/blood.v99.5.1512. [DOI] [PubMed] [Google Scholar]
- 192.Neuman MG, Benhamou JPIM, Malkiewicz IM, Akremi R, Shear NH, Asselah T, Ibrahim A, Boyer N, Martinot-Peignoux M, Jacobson-Brown P, Katz GG, Le Breton V, Le Guludec G, Suneja A, Marcellin P. Cytokines as predictors for sustained response and as markers for immunomodulation in patients with chronic hepatitis C. Clin Biochem. 2001;34:173–182. doi: 10.1016/s0009-9120(01)00212-0. [DOI] [PubMed] [Google Scholar]
- 193.Singh N. Interactions between viruses in transplant recipients. Clin Infect Dis. 2005;40:430–436. doi: 10.1086/427214. [DOI] [PubMed] [Google Scholar]
- 194.Rubin RH. The pathogenesis and clinical management of cytomegalovirus infection in the organ transplant recipient: the end of the ‘silo hypothesis’. Curr Opin Infect Dis. 2007;20:399–407. doi: 10.1097/QCO.0b013e328285a358. [DOI] [PubMed] [Google Scholar]
- 195.Pereyra F, Rubin RH. Prevention and treatment of cytomegalovirus infection in solid organ transplant recipients. Curr Opin Infect Dis. 2004;17:357–361. doi: 10.1097/01.qco.0000136933.67920.dd. [DOI] [PubMed] [Google Scholar]
- 196.Miller GG, Dummer JS. Herpes simplex and varicella zoster viruses: forgotten but not gone. Am J Transplant. 2007;7:741–747. doi: 10.1111/j.1600-6143.2006.01718.x. [DOI] [PubMed] [Google Scholar]
- 197.Waldman M, Kopp JB. Parvovirus-B19-associated complications in renal transplant recipients. Nat Clin Pract Nephrol. 2007;3:540–550. doi: 10.1038/ncpneph0609. [DOI] [PubMed] [Google Scholar]
- 198.Blair JE. Coccidioidomycosis in patients who have undergone transplantation. Ann N Y Acad Sci. 2007;1111:365–376. doi: 10.1196/annals.1406.009. [DOI] [PubMed] [Google Scholar]
- 199.Freifeld AG, Iwen PC, Lesiak BL, Gilroy RK, Stevens RB, Kalil AC. Histoplasmosis in solid organ transplant recipients at a large Midwestern university transplant center. Transpl Infect Dis. 2005;7:109–115. doi: 10.1111/j.1467-8365.2005.00105.x. [DOI] [PubMed] [Google Scholar]
- 200.Gauthier GM, Safdar N, Klein BS, Andes DR. Blastomycosis in solid organ transplant recipients. Transpl Infect Dis. 2007;9:310–317. doi: 10.1111/j.1399-3062.2007.00227.x. [DOI] [PubMed] [Google Scholar]
- 201.Silveira FP, Husain S. Fungal infections in solid organ transplantation. Med Mycol. 2007;45:305–320. doi: 10.1080/13693780701200372. [DOI] [PubMed] [Google Scholar]
- 202.Fishman JA. Prevention of infection caused by Pneumocystis carinii in transplant recipients. Clin Infect Dis. 2001;33:1397–1405. doi: 10.1086/323129. [DOI] [PubMed] [Google Scholar]
- 203.Barsoum RS. Parasitic infections in organ transplantation. Exp Clin Transplant. 2004;2:258–267. [PubMed] [Google Scholar]
- 204.Drachenberg CB, Hirsch HH, Papadimitriou JC, Gosert R, Wali RK, Munivenkatappa R, Nogueira J, Cangro CB, Haririan A, Mendley S, Ramos E. Polyomavirus BK versus JC replication and nephropathy in renal transplant recipients: a prospective evaluation. Transplantation. 2007;84:323–330. doi: 10.1097/01.tp.0000269706.59977.a5. [DOI] [PubMed] [Google Scholar]
- 205.Shitrit D, Lev N, Bar-Gil-Shitrit A, Kramer MR. Progressive multifocal leukoencephalopathy in transplant recipients. Transpl Int. 2005;11:658–665. doi: 10.1007/s00147-004-0779-3. [DOI] [PubMed] [Google Scholar]
- 206.Ison MG. Respiratory viral infections in transplant recipients. Antivir Ther. 2007;12:627–638. [PubMed] [Google Scholar]
- 207.Ison MG, Hayden FG. Viral infections in immunocompromised patients: what’s new with respiratory viruses? Curr Opin Infect Dis. 2002;15:355–367. doi: 10.1097/00001432-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 208.López-Medrano F, Aguado JM, Lizasoain M, Folgueira D, Juan RS, Díaz-Pedroche C, Lumbreras C, Morales JM, Delgado JF, Moreno-González E. Clinical implications of respiratory virus infections in solid organ transplant recipients: a prospective study. Transplantation. 2007;84:851–856. doi: 10.1097/01.tp.0000282788.70383.8b. [DOI] [PubMed] [Google Scholar]
- 209.Linares L, Cervera C, Cofán F, Ricart MJ, Esforzado N, Torregrosa V, Oppenheimer F, Campistol JM, Marco F, Moreno A. Epidemiology and outcomes of multiple antibiotic-resistant bacterial infection in renal transplantation. Transplant Proc. 2007;39:2222–2224. doi: 10.1016/j.transproceed.2007.06.061. [DOI] [PubMed] [Google Scholar]
- 210.McNeil SA, Malani PN, Chenoweth CE, Fontana RJ, Magee JC, Punch JD, Mackin ML, Kauffman CA. Vancomycin-resistant enterococcal colonization and infection in liver transplant candidates and recipients: a prospective surveillance study. Clin Infect Dis. 2006;42:195–203. doi: 10.1086/498903. [DOI] [PubMed] [Google Scholar]
- 211.Schneider CR, Buell JF, Gearhart M, Thomas M, Hanaway MJ, Rudich SM, Woodle ES. Methicillin-resistant Staphylococcus aureus infection in liver transplantation: a matched controlled study. Transplant Proc. 2005;37:1243–1244. doi: 10.1016/j.transproceed.2005.01.059. [DOI] [PubMed] [Google Scholar]
- 212.Lim WH, Russ GR, Coates PTH. Review of Epstein-Barr virus and post-transplant lymphoproliferative disorder post-solid organ transplantation. Nephrology (Carlton) 2006;11:355–366. doi: 10.1111/j.1440-1797.2006.00596.x. [DOI] [PubMed] [Google Scholar]
- 213.Turvey SE, Leo SH, Boos A, Deans GD, Prendiville J, Crawford RI, Senger C, Conley ME, Tilley P, Junker A, Janz L, Azana R, Hoang L, Morton TL. Successful approach to treatment of Helicobacter bilis infection in X-linked agammaglobulinemia. J Clin Immunol. 2012;32:1404–1408. doi: 10.1007/s10875-012-9750-8. [DOI] [PubMed] [Google Scholar]
- 214.Santos GW. Application of marrow grafts in human disease. Am J Pathol. 1971;65:653–668. [PMC free article] [PubMed] [Google Scholar]
- 215.Brown JMY. The influence of the conditions of hematopoietic cell transplantation on infectious complications. Curr Opin Infect Dis. 2005;18:346–351. doi: 10.1097/01.qco.0000172699.90525.80. [DOI] [PubMed] [Google Scholar]
- 216.Wingard JR, Hsu J, Hiemenz JW. Hematopoietic stem cell transplantation: an overview of infection risks and epidemiology. Infect Dis Clin North Am. 2010;24:257–272. doi: 10.1016/j.idc.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 217.Tomblyn M, Chiller T, Einsele H, Gress R, Sepkowitz K, Storek J, Wingard JR, Young JA, Boeckh MJ. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15:1143–1238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Castagnola E, Fontana V, Caviglia I, Caruso S, Faraci M, Fioredda F, Garrè ML, Moroni C, Conte M, Losurdo G, Scuderi F, Bandettini R, Tomà P, Viscoli C, Haupt R. A prospective study on the epidemiology of febrile episodes during chemotherapy-induced neutropenia in children with cancer or after hemopoietic stem cell transplantation. Clin Infect Dis. 2007;45:1296–1304. doi: 10.1086/522533. [DOI] [PubMed] [Google Scholar]
- 219.Klastersky J, Ameye L, Maertens J, Georgala A, Muanza F, Aoun M, Ferrant A, Rapoport B, Rolston K, Paesmans M. Bacteraemia in febrile neutropenic cancer patients. Int J Antimicrob Agents. 2007;30(Suppl 1):S51–S59. doi: 10.1016/j.ijantimicag.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 220.Pappas PG. Invasive candidiasis. Infect Dis Clin North Am. 2006;20:485–506. doi: 10.1016/j.idc.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 221.Marr KA. Invasive Candida infections: the changing epidemiology. Oncol Williston Park. 2004;18(Suppl 13):9–14. [PubMed] [Google Scholar]
- 222.Marr KA, Seidel K, White TC, Bowden RA. Candidemia in allogeneic blood and marrow transplant recipients: evolution of risk factors after the adoption of prophylactic fluconazole. J Infect Dis. 2000;181:309–316. doi: 10.1086/315193. [DOI] [PubMed] [Google Scholar]
- 223.Marr KA, Bowden RA. Fungal infections in patients undergoing blood and marrow transplantation. Transpl Infect Dis. 1999;1:237–246. doi: 10.1034/j.1399-3062.1999.010403.x. [DOI] [PubMed] [Google Scholar]
- 224.Barnes PD, Marr KA. Risks, diagnosis and outcomes of invasive fungal infections in haematopoietic stem cell transplant recipients. Br J Haematol. 2007;139:519–531. doi: 10.1111/j.1365-2141.2007.06812.x. [DOI] [PubMed] [Google Scholar]
- 225.Wingard JR. Infections in allogeneic bone marrow transplant recipients. Semin Oncol. 1993;20(Suppl 6):80–87. [PubMed] [Google Scholar]
- 226.Epstein JB, Ransier A, Sherlock CH, Spinelli JJ, Reece D. Acyclovir prophylaxis of oral herpes virus during bone marrow transplantation. Eur J Cancer B Oral Oncol. 1996;32B:158–162. doi: 10.1016/0964-1955(95)00091-7. [DOI] [PubMed] [Google Scholar]
- 227.Ljungman P, Ward KN, Crooks BN, Parker A, Martino R, Shaw PJ, Brinch L, Brune M, De La Camara R, Dekker A, Pauksen K, Russell N, Schwarer AP, Cordonnier C. Respiratory virus infections after stem cell transplantation: a prospective study from the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2001;28:479–484. doi: 10.1038/sj.bmt.1703139. [DOI] [PubMed] [Google Scholar]
- 228.Cortez KJ, Erdman DD, Peret TC, Gill VJ, Childs R, Barrett AJ, Bennett JE. Outbreak of human parainfluenza virus 3 infections in a hematopoietic stem cell transplant population. J Infect Dis. 2001;184:1093–1097. doi: 10.1086/322041. [DOI] [PubMed] [Google Scholar]
- 229.Nichols WG, Guthrie KA, Corey L, Boeckh M. Influenza infections after hematopoietic stem cell transplantation: risk factors, mortality, and the effect of antiviral therapy. Clin Infect Dis. 2004;39:1300–1306. doi: 10.1086/425004. [DOI] [PubMed] [Google Scholar]
- 230.Small TN, Casson A, Malak SF, Boulad F, Kiehn TE, Stiles J, Ushay HM, Sepkowitz KA. Respiratory syncytial virus infection following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2002;29:321–332. doi: 10.1038/sj.bmt.1703365. [DOI] [PubMed] [Google Scholar]
- 231.Englund JA, Boeckh M, Kuypers J, Nichols WG, Hackman RC, Morrow RA, Fredricks DN, Corey L. Brief communication: fatal human metapneumovirus infection in stem-cell transplant recipients. Ann Intern Med. 2006;144:344–349. doi: 10.7326/0003-4819-144-5-200603070-00010. [DOI] [PubMed] [Google Scholar]
- 232.Renaud C, Xie H, Seo S, Kuypers J, Cent A, Corey L, Leisenring W, Boeckh M, Englund JA. Mortality rates of human metapneumovirus and respiratory syncytial virus lower respiratory tract infections in hematopoietic cell transplantation recipients. Biol Blood Marrow Transplant. 2013;19:1220–1226. doi: 10.1016/j.bbmt.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.van Burik JA, Carter SL, Freifeld AG, High KP, Godder KT, Papanicolaou GA, Mendizabal AM, Wagner JE, Yanovich S, Kernan NA. Higher risk of cytomegalovirus and aspergillus infections in recipients of T cell-depleted unrelated bone marrow: analysis of infectious complications in patients treated with T cell depletion versus immunosuppressive therapy to prevent graft-versus-host disease. Biol Blood Marrow Transplant. 2007;13:1487–1498. doi: 10.1016/j.bbmt.2007.08.049. [DOI] [PubMed] [Google Scholar]
- 234.van Burik JA, Brunstein CG. Infectious complications following unrelated cord blood transplantation. Vox Sang. 2007;92:289–296. doi: 10.1111/j.1423-0410.2007.00900.x. [DOI] [PubMed] [Google Scholar]
- 235.Upton A, Marr KA. Emergence of opportunistic mould infections in the hematopoietic stem cell transplant patient. Curr Infect Dis Rep. 2006;8:434–441. doi: 10.1007/s11908-006-0017-5. [DOI] [PubMed] [Google Scholar]
- 236.Gluckman E, Traineau R, Devergie A, Esperou-Bourdeau H, Hirsch I. Prevention and treatment of CMV infection after allogeneic bone marrow transplant. Ann Hematol. 1992;64(Suppl:A):158–161. doi: 10.1007/BF01715372. [DOI] [PubMed] [Google Scholar]
- 237.Erard V, Huang ML, Ferrenberg J, Nguy L, Stevens-Ayers TL, Hackman RC, Corey L, Boeckh M. Quantitative real-time polymerase chain reaction for detection of adenovirus after T cell-replete hematopoietic cell transplantation: viral load as a marker for invasive disease. Clin Infect Dis. 2007;45:958–965. doi: 10.1086/521851. [DOI] [PubMed] [Google Scholar]
- 238.Hutspardol S, Essa M, Richardson S, Schechter T, Ali M, Krueger J, Fujii H, Egeler RM, Gassas A. Significant Transplantation-Related Mortality from Respiratory Virus Infections within the First One Hundred Days in Children after Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2015;21:1802–1807. doi: 10.1016/j.bbmt.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Cordonnier C, Martino R, Trabasso P, Held TK, Akan H, Ward MS, Fabian K, Ullmann AJ, Wulffraat N, Ljungman P, Alessandrino EP, Pretnar J, Gmür J, Varela R, Vitek A, Sica S, Rovira M European Blood and Marrow Transplant Group Infectious Diseases Working Party. Mycobacterial infection: a difficult and late diagnosis in stem cell transplant recipients. Clin Infect Dis. 2004;38:1229–1236. doi: 10.1086/383307. [DOI] [PubMed] [Google Scholar]
- 240.Doucette K, Fishman JA. Nontuberculous mycobacterial infection in hematopoietic stem cell and solid organ transplant recipients. Clin Infect Dis. 2004;38:1428–1439. doi: 10.1086/420746. [DOI] [PubMed] [Google Scholar]
- 241.Kawasaki H, Takayama J, Ohira M. Herpes zoster infection after bone marrow transplantation in children. J Pediatr. 1996;128:353–356. doi: 10.1016/s0022-3476(96)70280-9. [DOI] [PubMed] [Google Scholar]
- 242.Koc Y, Miller KB, Schenkein DP, Griffith J, Akhtar M, DesJardin J, Snydman DR. Varicella zoster virus infections following allogeneic bone marrow transplantation: frequency, risk factors, and clinical outcome. Biol Blood Marrow Transplant. 2000;6:44–49. doi: 10.1016/s1083-8791(00)70051-6. [DOI] [PubMed] [Google Scholar]
- 243.Kinch A, Oberg G, Arvidson J, Falk KI, Linde A, Pauksens K. Post-transplant lymphoproliferative disease and other Epstein-Barr virus diseases in allogeneic haematopoietic stem cell transplantation after introduction of monitoring of viral load by polymerase chain reaction. Scand J Infect Dis. 2007;39:235–244. doi: 10.1080/00365540600978906. [DOI] [PubMed] [Google Scholar]
- 244.Machado CM, Gonçalves FB, Pannuti CS, Dulley FL, de Souza VA. Measles in bone marrow transplant recipients during an outbreak in São Paulo, Brazil. Blood. 2002;99:83–87. doi: 10.1182/blood.v99.1.83. [DOI] [PubMed] [Google Scholar]
- 245.Peffault de Latour R, Lévy V, Asselah T, Marcellin P, Scieux C, Adès L, Traineau R, Devergie A, Ribaud P, Espérou H, Gluckman E, Valla D, Socié G. Long-term outcome of hepatitis C infection after bone marrow transplantation. Blood. 2004;103:1618–1624. doi: 10.1182/blood-2003-06-2145. [DOI] [PubMed] [Google Scholar]
- 246.Lubel JS, Testro AG, Angus PW. Hepatitis B virus reactivation following immunosuppressive therapy: guidelines for prevention and management. Intern Med J. 2007;37:705–712. doi: 10.1111/j.1445-5994.2007.01479.x. [DOI] [PubMed] [Google Scholar]
- 247.Narreddy S, Mellon-Reppen S, Abidi MH, Klein JL, Peres E, Heilbrun LK, Smith D, Alangaden G, Chandrasekar PH. Non-bacterial infections in allogeneic non-myeloablative stem cell transplant recipients. Transpl Infect Dis. 2007;9:3–10. doi: 10.1111/j.1399-3062.2006.00172.x. [DOI] [PubMed] [Google Scholar]
- 248.Busca A, Lovisone E, Aliberti S, Locatelli F, Serra A, Scaravaglio P, Omedè P, Rossi G, Cirillo D, Barbui A, Ghisetti V, Dall’Omo AM, Falda M. Immune reconstitution and early infectious complications following nonmyeloablative hematopoietic stem cell transplantation. Hematology. 2003;8:303–311. doi: 10.1080/10245330310001612125. [DOI] [PubMed] [Google Scholar]
- 249.Maris M, Boeckh M, Storer B, Dawson M, White K, Keng M, Sandmaier B, Maloney D, Storb R, Storek J. Immunologic recovery after hematopoietic cell transplantation with nonmyeloablative conditioning. Exp Hematol. 2003;31:941–952. doi: 10.1016/s0301-472x(03)00201-7. [DOI] [PubMed] [Google Scholar]
- 250.Hagen EA, Stern H, Porter D, Duffy K, Foley K, Luger S, Schuster SJ, Stadtmauer EA, Schuster MG. High rate of invasive fungal infections following nonmyeloablative allogeneic transplantation. Clin Infect Dis. 2003;36:9–15. doi: 10.1086/344906. [DOI] [PubMed] [Google Scholar]
- 251.Junghanss C, Boeckh M, Carter RA, Sandmaier BM, Maris MB, Maloney DG, Chauncey T, McSweeney PA, Little MT, Corey L, Storb R. Incidence and outcome of cytomegalovirus infections following nonmyeloablative compared with myeloablative allogeneic stem cell transplantation, a matched control study. Blood. 2002;99:1978–1985. doi: 10.1182/blood.v99.6.1978. [DOI] [PubMed] [Google Scholar]
- 252.Nachbaur D, Larcher C, Kircher B, Eibl G, Nussbaumer W, Gunsilius E, Haun M, Grünewald K, Gastl G. Risk for cytomegalovirus infection following reduced intensity allogeneic stem cell transplantation. Ann Hematol. 2003;82:621–627. doi: 10.1007/s00277-003-0706-1. [DOI] [PubMed] [Google Scholar]
- 253.Fauci AS, Pantaleo G, Stanley S, Weissman D. Immunopathogenic mechanisms of HIV infection. Ann Intern Med. 1996;124:654–663. doi: 10.7326/0003-4819-124-7-199604010-00006. [DOI] [PubMed] [Google Scholar]
- 254.Ho DD, Neumann AU, Perelson AS, Chen W, Leonard JM, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 255.Munier ML, Kelleher AD. Acutely dysregulated, chronically disabled by the enemy within: t-cell responses to HIV-1 infection. Immunol Cell Biol. 2007;85:6–15. doi: 10.1038/sj.icb.7100015. [DOI] [PubMed] [Google Scholar]
- 256.Murdoch DM, Venter WD, Van Rie A, Feldman C. Immune reconstitution inflammatory syndrome (IRIS): review of common infectious manifestations and treatment options. AIDS Res Ther. 2007;8:4–9. doi: 10.1186/1742-6405-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Singh N, Perfect JR. Immune reconstitution syndrome associated with opportunistic mycoses. Lancet Infect Dis. 2007;7:395–401. doi: 10.1016/S1473-3099(07)70085-3. [DOI] [PubMed] [Google Scholar]
- 258.Hanson DL, Chu SY, Farizo M, Ward JW. Distribution of CD4+ lymphocytes at diagnosis of the acquired immunodeficiency syndrome-defining and other human immunodeficiency virus-related illnesses. The adult and adolescent spectrum of HIV disease project group. Arch Intern Med. 1995;155:1537–1542. [PubMed] [Google Scholar]
- 259.Moore RD, Chaisson RE. Natural history of opportunistic disease in an HIV-infected urban clinical cohort. Ann Intern Med. 1996;124:633–642. doi: 10.7326/0003-4819-124-7-199604010-00003. [DOI] [PubMed] [Google Scholar]
- 260.Lederman HM, Williams PL, Wu JW, Evans TG, Cohn SE, McCutchan JA, Koletar SL, Hafner R, Connick E, Valentine FT, McElrath MJ, Roberts NJ, Jr, Currier JS AIDS Clinical Trials Group 889 Study Team. Incomplete immune reconstitution after initiation of highly active antiretroviral therapy in human immunodeficiency virus-infected patients with severe CD4+ cell depletion. J Infect Dis. 2003;188:1794–1803. doi: 10.1086/379900. [DOI] [PubMed] [Google Scholar]
- 261.Koletar SL, Williams PL, Wu J, McCutchan JA, Cohn SE, Murphy RL, Lederman HM, Currier JS AIDS Clinical Trials Group 362 Study Team. Long-term follow-up of HIV-infected individuals who have significant increases in CD4+ cell counts during antiretroviral therapy. Clin Infect Dis. 2004;39:1500–1506. doi: 10.1086/424882. [DOI] [PubMed] [Google Scholar]
- 262.Moore KW, Vieira P, Fiorentino DF, Trounstine ML, Khan TA, Mosmann TR. Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein-Barr virus gene BCRFI. Science. 1990;248:1230–1234. doi: 10.1126/science.2161559. [DOI] [PubMed] [Google Scholar]
- 263.Mangi RJ, Niederman JC, Kelleher JE, Jr, Dwyer JM, Evans AS, Kantor FS. Depression of cell-mediated immunity during acute infectious mononucleosis. N Engl J Med. 1974;291:1149–1153. doi: 10.1056/NEJM197411282912202. [DOI] [PubMed] [Google Scholar]
- 264.Gaspar HB, Sharifi R, Gilmour KC, Thrasher AJ. X-linked lymphoproliferative disease: clinical, diagnostic and molecular perspective. Br J Haematol. 2002;119:585–595. doi: 10.1046/j.1365-2141.2002.03851.x. [DOI] [PubMed] [Google Scholar]
- 265.Seemayer TA, Gross TG, Egeler RM, Pirruccello SJ, Davis JR, Kelly CM, Okano M, Lanyi A, Sumegi J. X-linked lymphoproliferative disease: twenty-five years after the discovery. Pediatr Res. 1995;38:471–478. doi: 10.1203/00006450-199510000-00001. [DOI] [PubMed] [Google Scholar]
- 266.Miller DL. Frequency of complications of measles. BMJ. 1964;2:75–78. doi: 10.1136/bmj.2.5401.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Tamashiro VG, Perez HH, Griffin DE. Prospective study of the magnitude and duration of changes in tuberculin reactivity during uncomplicated and complicated measles. Pediatr Infect Dis J. 1987;6:451–454. doi: 10.1097/00006454-198705000-00007. [DOI] [PubMed] [Google Scholar]
- 268.Moss WJ, Griffin DE. Measles. Lancet. 2012;379:153–164. doi: 10.1016/S0140-6736(10)62352-5. [DOI] [PubMed] [Google Scholar]
- 269.Tsiodras S, Samonis G, Keating MJ, Kontoyiannis DP. Infection and immunity in chronic lymphocytic leukemia. Mayo Clin Proc. 2000;75:1039–1054. doi: 10.4065/75.10.1039. [DOI] [PubMed] [Google Scholar]
- 270.Intravenous immunoglobulin for the prevention of infection in chronic lymphocytic leukemia: a randomized, controlled clinical trial. Cooperative group for the study of immunoglobulin in chronic lymphocytic leukemia. N Engl J Med. 1988;319:902–907. doi: 10.1056/NEJM198810063191403. No Authors. [DOI] [PubMed] [Google Scholar]
- 271.Pratt G, Goodyear O, Moss P. Immunodeficiency and immunotherapy in multiple myeloma. Br J Haematol. 2007;138:563–579. doi: 10.1111/j.1365-2141.2007.06705.x. [DOI] [PubMed] [Google Scholar]
- 272.Delamaire M, Maugendre D, Moreno M, Le Goff MC, Allannic H, Genetet B. Impaired leucocyte functions in diabetic patients. Diabet Med. 1997;14:29–34. doi: 10.1002/(SICI)1096-9136(199701)14:1<29::AID-DIA300>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 273.Calvet HM, Yoshikawa TT. Infections in diabetes. Infect Dis Clin North Am. 2001;15:407–421. doi: 10.1016/s0891-5520(05)70153-7. [DOI] [PubMed] [Google Scholar]
- 274.Joshi N, Caputo GM, Weitekamp MR, Karchmer AW. Infections in patients with diabetes mellitus. N Engl J Med. 1999;341:1906–1912. doi: 10.1056/NEJM199912163412507. [DOI] [PubMed] [Google Scholar]
- 275.Muller LM, Gorter KJ, Hak E, Goudzwaard WL, Schellevis FG, Hoepelman AI, Rutten GE. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin Infect Dis. 2005;41:281–288. doi: 10.1086/431587. [DOI] [PubMed] [Google Scholar]
- 276.Koziel H, Koziel MJ. Pulmonary complications of diabetes mellitus. Pneumonia. Infect Dis Clin North Am. 1995;9:65–96. [PubMed] [Google Scholar]
- 277.Caputo GM, Joshi N, Weitekamp MR. Foot infections in patients with diabetes. Am Fam Physician. 1997;56:195–202. [PubMed] [Google Scholar]
- 278.Lynn J, Knight AK, Kamoun M, Levinson AI. A 55-year-old man with hypogammaglobulinemia, lymphopenia, and unrelenting cutaneous warts. J Allergy Clin Immunol. 2004;114:409–414. doi: 10.1016/j.jaci.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 279.Gorensek MJ, Lebel MH, Nelson JD. Peritonitis in children with nephrotic syndrome. Pediatrics. 1988;81:849–856. [PubMed] [Google Scholar]
- 280.Hemsley C, Eykyn SJ. Pneumococcal peritonitis in previously healthy adults: case report and review. Clin Infect Dis. 1998;27:376–379. doi: 10.1086/514670. [DOI] [PubMed] [Google Scholar]
- 281.Molrine DC, Siber GR, Samra Y, Shevy DS, MacDonald K, Cieri R, Ambrosino DM. Normal IgG and impaired IgM responses to polysaccharide vaccines in asplenic patients. J Infect Dis. 1999;179:513–517. doi: 10.1086/314582. [DOI] [PubMed] [Google Scholar]
- 282.Singer DB. Postsplenectomy sepsis. Perspect Pediatr Pathol. 1973;1:285–311. [PubMed] [Google Scholar]
- 283.Pabst HF, Kreth HW. Ontogeny of the immune response as a basis of childhood disease. J Pediatr. 1980;97:519–534. doi: 10.1016/s0022-3476(80)80003-5. [DOI] [PubMed] [Google Scholar]
- 284.Kniker WT, Lesourd BM, McBryde JL, Corriel RN. Cell-mediated immunity assessed by Multitest CMI skin testing in infants and preschool children. Am J Dis Child. 1985;139:840–845. doi: 10.1001/archpedi.1985.02140100102044. [DOI] [PubMed] [Google Scholar]
- 285.Munoz AI, Limbert D. Skin reactivity to Candida and streptokinase-streptodornase antigens in normal pediatric subjects: influence of age and acute illness. J Pediatr. 1977;91:565–568. doi: 10.1016/s0022-3476(77)80503-9. [DOI] [PubMed] [Google Scholar]
- 286.Barrett DJ. Human immune responses to polysaccharide antigens: an analysis of bacterial polysaccharide vaccines in infants. Adv Pediatr. 1985;32:139–158. [PubMed] [Google Scholar]
- 287.Fothergill LD, Wright J. Influenzal meningitis: the relation of age incidence to the bactericidal power of blood against the causal organism. J Immunol. 1933;24:273–284. [Google Scholar]
- 288.Georgeson GD, Szony BJ, Streitman K, Kovács A, Kovács L, László A. Natural killer cell cytotoxicity is deficient in newborns with sepsis and recurrent infections. Eur J Pediatr. 2001;160:478–482. doi: 10.1007/s004310100773. [DOI] [PubMed] [Google Scholar]
- 289.Davis CA, Vallota EH, Forristal J. Serum complement levels in infancy: age related changes. Pediatr Res. 1979;13:1043–1046. doi: 10.1203/00006450-197909000-00019. [DOI] [PubMed] [Google Scholar]
- 290.Bizzarro MJ, Raskind C, Baltimore RS, Gallagher PG. Seventy-five years of neonatal sepsis at Yale: 1928–2003. Pediatrics. 2005;116:595–602. doi: 10.1542/peds.2005-0552. [DOI] [PubMed] [Google Scholar]
- 291.Kimberlin DW. Herpes simplex virus infections of the newborn. Semin Perinatol. 2007;31:19–25. doi: 10.1053/j.semperi.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 292.Abzug MJ, Levin MJ, Rotbart HA. Profile of enterovirus disease in the first two weeks of life. Pediatr Infect Dis J. 1993;12:820–824. doi: 10.1097/00006454-199310000-00005. [DOI] [PubMed] [Google Scholar]
- 293.Castle SC. Impact of age-related immune dysfunction on risk of infections. Z Gerontol Geriatr. 2000;33:341–349. doi: 10.1007/s003910070030. [DOI] [PubMed] [Google Scholar]
- 294.Castle SC. Clinical relevance of age-related immune dysfunction. Clin Infect Dis. 2000;31:578–585. doi: 10.1086/313947. [DOI] [PubMed] [Google Scholar]
- 295.Castle SC, Uyemura K, Fulop T, Makinodan T. Host resistance and immune responses in advanced age. Clin Geriatr Med. 2007;23:463–479. doi: 10.1016/j.cger.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Mullooly JP, Bennett MD, Hornbrook MC, Barker WH, Williams WW, Patriarca PA, Rhodes PH. Influenza vaccination programs for elderly persons: cost-effectiveness in a health maintenance organization. Ann Intern Med. 1994;121:947–952. doi: 10.7326/0003-4819-121-12-199412150-00008. [DOI] [PubMed] [Google Scholar]
- 297.Patriarca PA, Weber JA, Parker RA, Hall WN, Kendal AP, Bregman DJ, Schonberger LB. Efficacy of influenza vaccine in nursing homes: reduction in illness and complications during an influenza A (H3N2) epidemic. JAMA. 1985;253:1136–1139. [PubMed] [Google Scholar]
- 298.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352:1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 299.Mannick JA, Rodrick ML, Lederer JA. The immunologic response to injury. J Am Coll Surg. 2001;193:237–244. doi: 10.1016/s1072-7515(01)01011-0. [DOI] [PubMed] [Google Scholar]
- 300.Schwacha MG. Macrophages and post-burn immune dysfunction. Burns. 2003;29:1–14. doi: 10.1016/s0305-4179(02)00187-0. [DOI] [PubMed] [Google Scholar]
- 301.Bhat S, Milner S. Antimicrobial peptides in burns and wounds. Curr Protein Pept Sci. 2007;8:506–520. doi: 10.2174/138920307782411428. [DOI] [PubMed] [Google Scholar]
- 302.Bjerknes R, Vindenes H, Laerum OD. Altered neutrophil functions in patients with large burns. Blood Cells. 1990;16:127–141. discussion 142–143. [PubMed] [Google Scholar]
- 303.Estahbanati HK, Kashani PP, Ghanaatpisheh F. Frequency of Pseudomonas aeruginosa serotypes in burn wound infections and their resistance to antibiotics. Burns. 2002;28:340–348. doi: 10.1016/s0305-4179(02)00024-4. [DOI] [PubMed] [Google Scholar]
- 304.Tredget EE, Shankowsky HA, Rennie R, Burrell RE, Logsetty S. Pseudomonas infections in the thermally injured patient. Burns. 2004;30:3–26. doi: 10.1016/j.burns.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 305.Alarcón GS. Infections in systemic connective tissue diseases: systemic lupus erythematosus, scleroderma, and polymyositis/dermatomyositis. Infect Dis Clin North Am. 2006;20:849–875. doi: 10.1016/j.idc.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 306.Bermas BL, Petri M, Goldman D, Mittleman B, Miller MW, Stocks NI, Via CS, Shearer GM. T helper cell dysfunction in systemic lupus erythematosus (SLE): relation to disease activity. J Clin Immunol. 1994;14:169–177. doi: 10.1007/BF01533366. [DOI] [PubMed] [Google Scholar]
- 307.Homann C, Varming K, Høgåsen K, Mollnes TE, Graudal N, Thomsen AC, Garred P. Acquired C3 deficiency in patients with alcoholic cirrhosis predisposes to infection and increased mortality. Gut. 1997;40:544–549. doi: 10.1136/gut.40.4.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Rimola A, Soto R, Bory F, Arroyo V, Piera C, Rodes J. Reticuloendothelial system phagocytic activity in cirrhosis and its relation to bacterial infections and prognosis. Hepatology. 1984;4:53–58. doi: 10.1002/hep.1840040109. [DOI] [PubMed] [Google Scholar]
- 309.Schirren CA, Jung MC, Zachoval R, Diepolder H, Hoffmann R, Riethmüller G, Pape GR. Analysis of T cell activation pathways in patients with liver cirrhosis, impaired delayed hypersensitivity and other T cell-dependent functions. Clin Exp Immunol. 1997;108:144–150. doi: 10.1046/j.1365-2249.1997.d01-985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Cheong H-J, Song J-Y, Park J-W, Yeon J-E, Byun K-S, Lee C-H, Cho H-I, Kim T-G, Kim W-J. Humoral and cellular immune responses to influenza vaccine in patients with advanced cirrhosis. Vaccine. 2006;24:2417–2422. doi: 10.1016/j.vaccine.2005.11.064. [DOI] [PubMed] [Google Scholar]
- 311.Pachiadakis I, Pollara G, Chain BM, Naoumov NV. Is hepatitis C virus infection of dendritic cells a mechanism facilitating viral persistence? Lancet Infect Dis. 2005;5:296–304. doi: 10.1016/S1473-3099(05)70114-6. [DOI] [PubMed] [Google Scholar]
- 312.Arima S, Akbar SM, Michitaka K, Horiike N, Nuriya H, Kohara M, Onji M. Impaired function of antigen-presenting dendritic cells in patients with chronic hepatitis B: localization of HBV DNA and HBV RNA in blood DC by in situ hybridization. Int J Mol Med. 2003;11:169–174. [PubMed] [Google Scholar]
- 313.Sarnak MJ, Jaber BL. Mortality caused by sepsis in patients with end-stage renal disease compared with the general population. Kidney Int. 2000;58:1758–1764. doi: 10.1111/j.1523-1755.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- 314.Anding K, Gross P, Rost JM, Allgaier D, Jacobs E. The influence of uraemia and haemodialysis on neutrophil phagocytosis and antimicrobial killing. Nephrol Dial Transplant. 2003;18:2067–2073. doi: 10.1093/ndt/gfg330. [DOI] [PubMed] [Google Scholar]
- 315.Dinits-Pensy M, Forrest GN, Cross AS, Hise MK. The use of vaccines in adult patients with renal disease. Am J Kidney Dis. 2005;46:997–1011. doi: 10.1053/j.ajkd.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 316.Girndt M, Sester M, Sester U, Kaul H, Köhler H. Molecular aspects of T and B cell function in uremia. Kidney Int. 2001;59(suppl 78):S206–S211. doi: 10.1046/j.1523-1755.2001.59780206.x. [DOI] [PubMed] [Google Scholar]
- 317.Massry S, Smogorzewski M. Dysfunction of polymorphonuclear leukocytes in uremia: role of parathyroid hormone. Kidney Int Suppl. 2001;78(suppl 78):S195–S196. doi: 10.1046/j.1523-1755.2001.59780195.x. [DOI] [PubMed] [Google Scholar]
- 318.Zilliox MJ, Moss WJ, Griffin DE. Gene expression changes in peripheral blood mononuclear cells during measles virus infection. Clin Vaccine Immunol. 2007;14:918–923. doi: 10.1128/CVI.00031-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Matsumoto Y, Shinzato T, Amano I, Takai I, Kimura Y, Morita H, Miwa M, Nakane K, Yoshikai Y, Maeda K. Relationship between susceptibility to apoptosis and Fas expression in peripheral blood T cells from uremic patients: a possible mechanism for lymphopenia in chronic renal failure. Biochem Biophys Res Commun. 1995;215:98–105. doi: 10.1006/bbrc.1995.2438. [DOI] [PubMed] [Google Scholar]
- 320.Folds JD, Schmitz JL. Clinical and laboratory assessment of immunity. J Allergy Clin Immunol. 2003;111(Suppl):S702–S711. doi: 10.1067/mai.2003.122. [DOI] [PubMed] [Google Scholar]
- 321.Kobrynski LJ, Sullivan KE. Velocardiofacial syndrome, DiGeorge syndrome: the chromosome 22q11.2 deletion syndromes. Lancet. 2007;370:1443–1452. doi: 10.1016/S0140-6736(07)61601-8. [DOI] [PubMed] [Google Scholar]
- 322.Ochs HD, Thrasher AJ. The Wiskott-Aldrich syndrome. J Allergy Clin Immunol. 2006;117:725–738. doi: 10.1016/j.jaci.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 323.Anergy skin testing and tuberculosis preventive therapy for HIV-infected persons: revised recommendations. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1997;46:1–10. No authors listed. [PubMed] [Google Scholar]
- 324.Ohri LK, Manley JM, Chatterjee A, Cornish NE. Pediatric case series evaluating a standardized Candida albicans skin test product. Ann Pharmacother. 2004;38:973–977. doi: 10.1345/aph.1D518. [DOI] [PubMed] [Google Scholar]
- 325.Wen L, Atkinson JP, Giclas PC. Clinical and laboratory evaluation of complement deficiency. J Allergy Clin Immunol. 2004;113:585–593. doi: 10.1016/j.jaci.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 326.Jirapongsananuruk O, Malech HL, Kuhns DB, Niemela JE, Brown MR, Anderson-Cohen M, Fleisher TA. Diagnostic paradigm for evaluation of male patients with chronic granulomatous disease, based on the dihydrorhodamine 123 assay. J Allergy Clin Immunol. 2003;111:374–379. doi: 10.1067/mai.2003.58. [DOI] [PubMed] [Google Scholar]
- 327.Maloney DG, Smith B, Rose A. Rituximab: mechanism of action and resistance. Semin Oncol. 2002;29(Suppl 2):2–9. [PubMed] [Google Scholar]
- 328.Basu A, Ramkumar M, Tan HP, Khan A, McCauley J, Marcos A, Fung JJ, Starzl TE, Shapiro R. Reversal of acute cellular rejection after renal transplantation with Campath H-1. Transplant Proc. 2005;37:923–926. doi: 10.1016/j.transproceed.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 329.McCurry KR, Iacono A, Zeevi A, Yousem S, Girnita A, Husain S, Zaldonis D, Johnson B, Hattler BG, Starzl TE. Early outcomes in human lung transplantation with thymoglobulin or Campath-1H for recipient pretreatment followed by posttransplant tacrolimus near monotherapy. J Thorac Cardiovasc Surg. 2005;130:528–537. doi: 10.1016/j.jtcvs.2004.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Watson CJ, Bradley JA, Friend PJ, Firth J, Taylor CJ, Bradley JR, Smith KG, Thiru S, Jamieson NV, Hale G, Waldmann H, Calne R. Alemtuzumab (CAMPATH 1H) induction therapy in cadaveric kidney transplantation—efficacy and safety at five years. Am J Transplant. 2005;5:1347–1353. doi: 10.1111/j.1600-6143.2005.00822.x. [DOI] [PubMed] [Google Scholar]
- 331.Romero-Steiner S, Musher DM, Cetron MS, Pais LB, Groover JE, Fiore AE, Plikaytis BD, Carlone GM. Reduction in functional antibody activity against Streptococcus pneumoniae in vaccinated elderly individuals highly correlates with decreased IgG antibody avidity. Clin Infect Dis. 1999;29:281–288. doi: 10.1086/520200. [DOI] [PubMed] [Google Scholar]
- 332.Rubins JB, Puri AK, Loch J, Charboneau D, MacDonald R, Opstad N, Janoff EN. Magnitude, duration, quality, and function of pneumococcal vaccine responses in elderly adults. J Infect Dis. 1998;178:431–440. doi: 10.1086/515644. [DOI] [PubMed] [Google Scholar]
- 333.Johnson DH, Cunha BA. Infections in cirrhosis. Infect Dis Clin North Am. 2001;15:363–371. doi: 10.1016/s0891-5520(05)70150-1. [DOI] [PubMed] [Google Scholar]
- 334.Hardinger KL. Rabbit antithymocyte globulin induction therapy in adult renal transplantation. Pharmacotherapy. 2006;26:1771–1783. doi: 10.1592/phco.26.12.1771. [DOI] [PubMed] [Google Scholar]
- 335.Wingard JR, Chen DY, Burns WH, Fuller DJ, Braine HG, Yeager AM, Kaiser H, Burke PJ, Graham ML, Santos GW. Cytomegalovirus infection after autologous bone marrow transplantation with comparison to infection after allogeneic bone marrow transplantation. Blood. 1988;71:1432–1437. [PubMed] [Google Scholar]