Abstract
Migraine is a complex neurological disorder characterized by headache and sensory abnormalities, such as hypersensitivity to light, observed as photophobia. Whilst it is impossible to confirm that a mouse is experiencing migraine, light aversion can be used as a behavioral surrogate for the migraine symptom of photophobia. To test for light aversion, we utilize the light/dark assay to measure the time mice freely choose to spend in either a light or dark environment. The assay has been refined by introducing two critical modifications: pre-exposures to the chamber prior to running the test procedure and adjustable chamber lighting, permitting the use of a range of light intensities from 55 lux to 27,000 lux. Because the choice to spend more time in the dark is also indicative of anxiety, we also utilize a light-independent anxiety test, the open field assay, to distinguish anxiety from light-aversive behavior. Here, we describe a modified test paradigm for the light/dark and open field assays. The application of these assays is described for intraperitoneal injection of calcitonin gene-related peptide (CGRP) in two mouse strains and for optogenetic brain stimulation studies.
SUMMARY:
Rodents are not able to report migraine symptoms. Here, we describe a manageable test paradigm (light/dark and open field assays) to measure light aversion, one of the most common and bothersome symptoms in patients with migraines.
INTRODUCTION:
Migraine is a prevalent neurological disease, affecting approximately 17% of Americans1 and is the second leading cause of disability globally2,3. Patients experience headache that lasts 4-72 hours accompanied with at least one of the following symptoms: nausea and/or vomiting, or photophobia and phonophobia4. Recent advances in the development of calcitonin gene-related peptide (CGRP) antibodies that are now FDA approved have begun a new era for migraine treatment5-7. These antibodies block either CGRP or its receptor and prevent migraine symptoms in approximately 50% of migraine patients7. Within the past year, two small-molecule antagonists of the CGRP receptor have also been FDA approved for abortive treatment of migraine, and two more are in the pipeline8. Despite this therapeutic progress, mechanisms by which migraine attacks occur still remain elusive. For example, the sites of CGRP action are not known. The efficacy of therapeutic antibodies that do not appreciably cross the blood-brain barrier suggests that CGRP acts at peripheral sites, such as the meninges and/or trigeminal ganglia. However, we cannot rule out central actions at circumventricular organs, which lack a blood-brain barrier9. At least for photophobia, we think this is less likely given our results with light aversion using transgenic nestin/hRAMP1 mice in which hRAMP1 is overexpressed in the nervous tissue10. Understanding mechanisms of migraine pathophysiology will provide new avenues to the development of migraine therapeutics.
Preclinical animal models are critical to understanding disease mechanisms and the development of new drugs. However, migraine assessment in animals is challenging since animals cannot verbally report their sensations of pain. Given the fact that 80-90% of migraine patients exhibit photophobia11, light aversion is considered to be an indicator of migraine in animal models. This led to the need to develop an assay to assess light aversion in mice.
The light/dark assay contains a light zone and a dark zone. It is widely used for measuring anxiety in mice based on their spontaneous exploration of novel environments that is countered by their innate aversion to light12. Some studies set 1/3 of the chamber as the dark zone, while others set 1/2 of the chamber as the dark zone. The former setting is often used to detect anxiety13. While we initially chose equally sized light/dark chambers, we have not compared the two relative sizes. We can comment that the overall size of both chambers is not a major factor since the initial testing box14 was considerably larger than the subsequent apparatus15, yet results were essentially the same.
Two critical modifications to this light/dark assay to assess light aversion were: the testing condition and the light intensity (Figure 1). First, mice are pre-exposed to the light/dark chamber to reduce exploratory drive16 (Figure 1A). The necessity and times of pre-exposures depend on mouse strains and models. Wildtype C57BL/6J mice usually require two pre-exposures10, while only one pre-exposure for CD1 mice is sufficient17. In this manner, light-aversive behavior can be unmasked in these two mouse strains. Second, the chamber lighting has been adapted to include an adjustable range of light intensities from dim (55 lux) to bright (27,000 lux) where 55 lux is comparable to a dark overcast day, and 27,000 lux is comparable to a bright sunny day in the shade10. We have found that the required light intensity varies with the strain and genetic model. For this reason, individuals should first assess the minimum light intensity for their experimental paradigm.
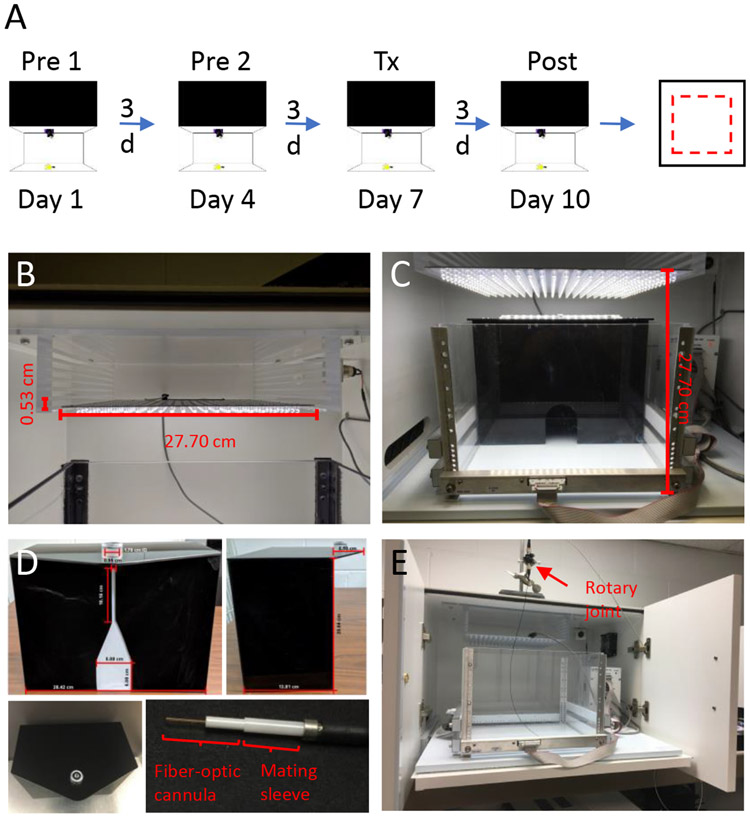
Figure 1: The light/dark assay timeline and apparatus.
(A) Timeline of the testing paradigm: After two pre-exposures to the light/dark chamber (Pre 1 and Pre 2), mice are administered CGRP (0.1 mg/kg, i.p.) followed by a post-treatment measurement (Post). At least one day after the light/dark assay, mice are given CGRP (0.1 mg/kg, i.p.) again and are run in the open field assay. Pre: pretreatment; Tx: treatment; Post: post-treatment (B) The LED panel is held at the top of the chamber by an acrylic shelf and illuminates the test area. The height of the light panel can be adjusted by using slots at different heights. (C) The light/dark chamber contains a dark insert with a small opening. A LED light panel is above the chamber. (D) Front, side, and top views of the modified dark insert. The opening in the dark insert is extended with a small slit for the movement of the patch cord (top left). The top of the dark insert extends over the light area as a triangular porch with a holder for the rotatory joint (top right and bottom left). The optic-fiber patch cord is connected to the fiber-optic cannula via a mating sleeve (bottom right). (E) The modified open field assay. The stand and clamp hold the rotatory joint. The chamber is pulled out to the front of the cubicle with the doors left open to allow the free movement of the mouse with the patch cord attached to the mouse head.
Even with these modifications to the assay, which can reveal a light-aversive phenotype, it is necessary to test anxiety-like behavior to distinguish between light aversion due to light alone versus due to anxiety. The open field assay is a traditional way to measure anxiety based on the spontaneous exploration of novel environments. It differs from the light/dark assay in that the exploratory drive is countered by the innate aversion to unprotected open spaces. Both the center and edges of the chamber are in the light, so the open field assay is a light-independent anxiety assay. Thus, the combination of the light/dark and open field assays enables us to distinguish between light aversion due to an avoidance of light versus an overall increase in anxiety.
CGRP is a multifunctional neuropeptide that regulates vasodilation, nociception, and inflammation18. It is widely expressed in the peripheral and central nervous systems. It plays an important role in migraine pathophysiology18. However, the mechanism underlying CGRP action in migraine is unclear. By utilizing the light/dark and open field assays with this modified test paradigm, we were able to identify light-aversive behavior in mice following peripheral10,16 (Figure 2) and central14-16,19 CGRP administration. In addition to neuropeptides, the identification of brain regions involved in light aversion is also important in understanding migraine pathophysiology. The posterior thalamic nuclei are an integrative brain region for pain and light processing19, and the thalamus is activated during migraine20. Thus, we targeted posterior thalamic nuclei by injecting adeno-associated virus (AAV) containing channelrhodopsin-2 (ChR2) or eYFP into this region. By combining this optogenetic approach with these two assays, we demonstrated that optical stimulation of ChR2-expressing neurons in the posterior thalamic nuclei induced light aversion19 (Figure 3). In this experiment, given the dramatic effect on the evoked light aversion in these optogenetically manipulated mice, pre-exposures to the chamber were skipped.
Figure 2: Peripheral CGRP administration evokes light aversion in bright light in two strains of wildtype mice.
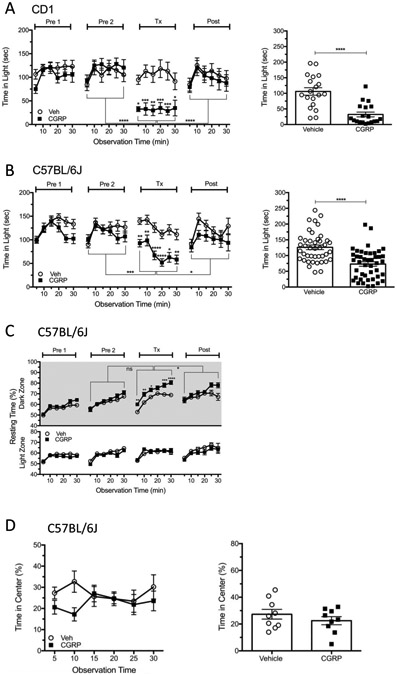
CD1 and C57/BL6J mice were tested according to the timeline described in Figure 1A. (A) The time CD1 mice spent in the light zone per 5 min interval over 30 min (27,000 lux). Time in light data is shown over time during the test (left panel) and as the average time per 5 min interval for individual mice (right panel). Comparisons were made between vehicle and CGRP at each time point, and between Tx and Pre2 or Post as indicated by brackets. (Veh, n=19; 0.1 mg/kg CGRP, n=19) (B) Time C57BL/6J mice spent in the light zone per 5 min interval over 30 min (27,000 lux). Time in light data are shown over time during the test (left panel) and as the average time per 5 min interval for individual mice (right panel) (Veh, n=42; 0.1 mg/kg CGRP, n=44). (C) The mice from panel B were also analyzed for resting behavior in the dark and light zones during the light/dark assay. (D) The mice from panel B were subsequently tested in the open field assay. The percentage of time spent in the center of the chamber per 5 min interval over 30 min after treatment with vehicle or CGRP (0.1 mg/kg, i.p.) (Veh, n=9; 0.1 mg/kg CGRP, n=9). The percentage of time in the center data is shown over time during the test (left panel) and as the average percentage of the time in the center per 5 min interval for individual mice (right panel). For all panels, mean±SEM is shown, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. This figure is modified from Mason et al. 201710.
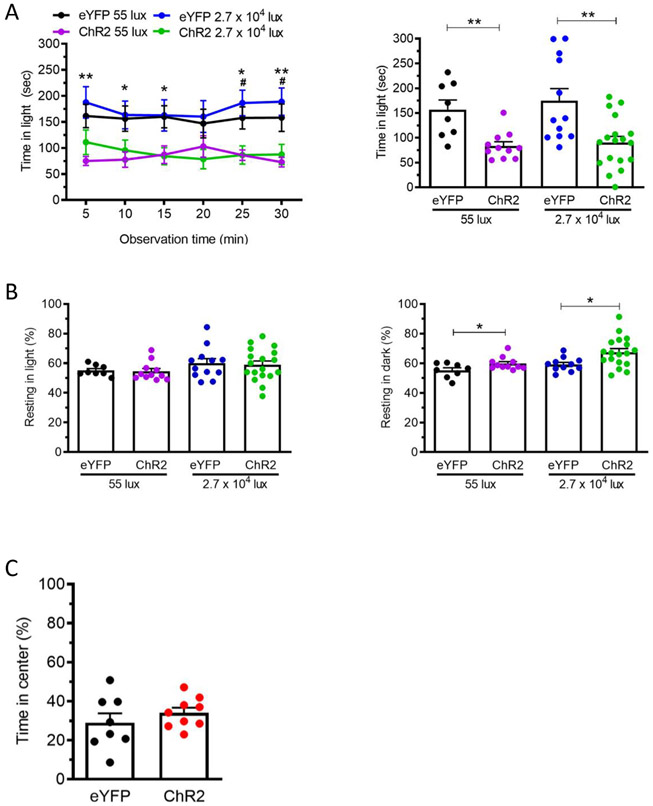
Figure 3: Optical stimulation of CaMKIIa-expressing neurons in the posterior thalamic nuclei induces light aversion in both dim and bright light.
(A) Posterior thalamic nuclei of C57BL/6J mice injected with AAV encoding either ChR2 or eYFP (at 55 lux: eYFP n = 8, ChR2 n = 11; at 27,000 lux: eYFP n = 12, ChR2 n = 18) were stimulated by blue laser (473 nm, 20 Hz, 5 ms pulse width, 10 mW/mm2 ). Left panel shows the time mice spent in the light zone per 5 min interval over 30 min at 55 or 27,000 lux. Comparisons were made between eYFP and ChR2 groups at each time point. Right panel shows the average time per 5 min interval for individual mice. (B) The mice from panel A were also analyzed for resting behavior in the light (left panel) and dark (right panel) zones during the light/dark assay. (C) The mice from panel A were subsequently tested in the open field assay. Average percentage of the time spent in the center of the open field chamber per 5 min interval over 30 min (Laser: 473 nm, 20 Hz, 5 ms pulse width, 10 mW/mm2). (eYFP n = 8, ChR2 n = 9). For all panels, mean±SEM is shown, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. This figure is modified from Sowers et al. 202019.
PROTOCOL:
Animal procedures were approved by the University of Iowa Animal Care and Use Committee and performed in compliance with the standards set by the National Institutes of Health.
1. Light/dark assay
1.1 Light/dark chamber apparatus (see Table of Materials) setup. All the equipment in this section is commercially available.
Table of Materials
| Name of Material/ Equipment | Company | Catalog Number | Comments/Description |
|---|---|---|---|
| Activity monitor | Med Assoc. Inc | Software tracking mouse behavior | |
| Customized acrylic shelf | For adjusting the height of the LED panel | ||
| Dark box insert | Med Assoc. Inc | ENV-511 | |
| DC power supply | Med Assoc. Inc | SG-500T | |
| DC regulated power supply | Med Assoc. Inc | SG-506 | |
| Fiber-optic cannula | Doric | MFC_200/240-0.22_4.5mm_ZF1.25_FLT | |
| Germicidal disposable wipes | Sani-Cloth | SKU # Q55172 | |
| Heat Sink | Wakefield | 490-6K | Connecting to LED panel |
| IR controller power cable | Med Assoc. Inc | SG-520USB-1 | |
| IR USB controller | Med Assoc. Inc | ENV-520USB | |
| Mating sleeve | Doric | SLEEVE_ZR_1.25 | |
| Modified LED light panel | Genaray Spectro | SP-E-360D | Daylight-balanced color (5600K) |
| Power supply | MEAN WELL USA | SP-320-12 | Connecting to LED panel |
| Seamless open field chamber | Med Assoc. Inc | ENV-510S | |
| Sound-attenuating cubicle | Med Assoc. Inc | ENV-022MD-027 | |
| Stand and clamp | |||
| Three 16-beam IR arrays | Med Assoc. Inc | ENV-256 |
1.1.1 On a shelf, place the sound-attenuating cubicle (interior: 59.7 x 38 x 35.6 cm in W x H x D) containing a pull-out drawer for easy access to the chamber and dark insert.
1.1.2 Connect the DC power supply and a DC-regulated power supply to the sound-attenuating cubicle.
1.1.3 Place the transparent seamless open field chamber (27.31 x 27.31 x 20.32 cm in L x W x H) on the pull-out drawer of the cubicle.
1.1.4 Place the black, infrared (IR)-transparent plastic dark insert (28.7 X 15 X 20.6 cm in L x W x H) in the open field chamber. Ensure that the chamber is divided into two zones of an equal size: a dark zone and a light zone.
1.1.5 Connect three sets of 16-beam IR arrays on the X, Y and Z axes of the open field chamber to the IR USB controller via cables.
1.1.6 Connect the IR USB controller to a computer.
1.1.7 Install the tracking software on the computer which can record and collect mouse location and activity.
1.1.8 For the light panel setup, first remove the light-emitting diode (LED) light panel (27.70 x 27.70 cm in L x W; 360 LEDs, daylight-balanced color, 5600K, 60° flood beam spread) from its original housing.
1.1.9 Assemble the light panel with the LED driver, the heat sink, and the power supply. Multiple LED light panels can be connected to one power supply, heat sink, and LED driver to achieve uniform light panel control.
1.1.10 Construct a customized acrylic platform (29.77 x 27.70 x 8.10 cm in L x W x H) comprising of 7 identical shelves at 0.53 cm intervals (Figure 1B). Permanently affix the customized acrylic shelf to the ceiling inside the cubicle above the chamber.
1.1.11 Insert the LED light panel into the slot between the bottom two shelves. Adjust the light panel to different heights (Figure 1B,C), if necessary (e.g., if using optogenetic mice. Details are discussed in Section 3).
1.1.12 Turn on the heat sink, LED driver, and power supply. Confirm that the LED driver can dictate the LED light intensity by measuring the light intensity on the chamber floor and confirm that the floor is lit evenly.
1.2 Behavioral test procedure
NOTE: Mice are housed on a 12 h light cycle. All behavioral experiments are performed during the light cycle. Mice, including both males and females, aged 10-20 weeks old, are used. In this protocol, naïve wildtype CD1 and C57BL/6J mice experience two pre-exposures to the light/dark chamber followed by exposure with treatment and a post-treatment exposure. There is a three-day interval between each exposure to allow mice to recover (Day 1, 4, 7 and 10 as described below and Figure 1A). However, CD1 mice do not require the 2nd pre-exposure and can be tested in dim light.
1.2.1 On day 1 (Pretreatment 1), turn the light/dark assay apparatus on and set the light intensity to 27,000 lux.
1.2.2 Open the tracking software and set up a new protocol. In the New Protocol setting, set the Duration to 30 min. In the New Analysis setting, set Data Bins by Duration to 300 s.
1.2.3 In the New Zone setting, choose Pre-Defined Zones. Choose 2 and then Horizontal. Check if the chamber is divided into two equal-size zones horizontally for recording.
1.2.4 Habituate mice to the testing room for 1 h prior to the testing. During habituation, keep the room light on to not disrupt the mouse’s circadian rhythm. Make sure all the equipment for the light/dark assay is turned on, allowing the mice to fully acclimate to the testing room environment.
1.2.5 Select Acquire Data. Enter mouse IDs. Start the protocol
1.2.6 Pull the drawer outside of the sound-attenuating cubicle to access the light/dark chamber and the dark insert. Gently place a mouse in the light zone of the chamber and push the drawer inside of the cubicle. Ensure that the software detects the mouse immediately and begins to record activity.
1.2.7 Wait for the recording to automatically stop after 30 min. Return the mouse to its home cage.
1.2.8 Clean the chamber and dark insert using alcohol-odor germicidal disposable wipes containing 55.0% isopropyl alcohol, 0.25% alkyl C12-18 dimethyl ethylbenzyl ammonium, and 0.25% alkyl C12-18 dimethyl benzyl ammonium chloride as anti-microbial active ingredients to eradicate any olfactory cues left by the previous mouse.
1.2.9 On day 4 (Pretreatment 2), repeat steps 1.2.1 to 1.2.8.
1.2.10 On day 7 (the treatment day), repeat step 1.2.1 and 1.2.4. After the habituation, administer mice with CGRP (0.1 mg/kg, intraperitoneal injection (i.p.)). Return mice to their home cages.
1.2.11 After 30 min, start the protocol and run the mouse in the light/dark chamber as mentioned in steps 1.2.5 to 1.2.7. The recovery time in home cages after injections can be shortened or lengthened depending on the treatment21.
1.2.12 Clean the chamber and dark insert as described in step 1.2.8.
1.2.13 On day 10 (post-treatment day), repeat steps 1.2.1 to 1.2.8. The experiment can be paused at step 1.2.13 before starting the open field assay.
2. Open field assay
2.1 The apparatus setup
2.1.1 Open field chamber setup: Use the same sound-attenuating cubicle and open field chamber used in the light/dark assay, without using the dark insert.
2.1.2 Light panel setup: Use the same setup used in the light/dark assay. Ensure that the light intensity is the same as used in the light/dark assay.
2.2 Behavioral test procedure
2.2.1 Turn the apparatus on. Set the light intensity to 27,000 lux.
2.2.2 Open the tracking software.
2.2.3 Set up a new protocol, the same as is used in the light/dark assay except for the New Zone settings. Choose 1 followed by the Center in the New Zone settings. Set the periphery as 3.97 cm from the perimeter and the center as 19.05 × 19.05 cm.
2.2.4 Habituate mice to the testing room as described in step 1.2.4.
2.2.5 Administer CGRP (0.1 mg/kg, i.p.) to the mice. Return the mice to their home cages.
2.2.6 After 30 min, start the protocol. Pull the pull-out drawer outside of the sound-attenuating cubicle and gently place a mouse in the middle of the open field chamber. Push the drawer inside of the cubicle.
2.2.7 Track behavior for 30 min. Then return mice to their home cages.
2.2.8 Clean the apparatus as described in step 1.2.8.
3. Modified light/dark assay for optogenetic mice
3.1 The apparatus setup
3.1.1 Make two modifications to the dark insert.
3.1.1.1 Modify the opening of the dark insert to 5.08 x 5.08 cm (W x H) with a small slit 0.95 x 10.16 cm (W X H) between the top and the opening of the dark insert (Figure 1D top left).
NOTE: This modification allows a mouse to go to the dark zone without difficulty when the fiber-optic cannula on the mouse head is attached to the patch cord.
3.1.1.2 Extend the top of the dark insert over the light area as a triangular porch (H=6.5 cm) (Figure 1D top right and bottom left). Cut a circular hole (D=1.7 cm) out of the porch and insert a holder into the hole to place and stabilize the rotary joint, which connects the laser and the fiber-optic patch cords (Figure 1D top left and bottom left).
NOTE: The modifications result in small change in the light intensity reaching the floor of the dark zone (17 lux with modifications vs 14 lux without modifications, measured on the back-right corner of the dark zone under 27,000 lux).
3.1.2 Insert the rotary joint into the holder on the dark insert.
3.1.3 Connect the 30.5 cm fiber-optic patch cord to the rotary joint. Confirm that the rotary joint can rotate smoothly so that the patch cord can rotate without difficulty as the mouse traverses the chamber.
3.1.4 For the rest of the setup, use the same apparatus setup as used in section 1 (light/dark assay).
3.2 Behavioral test procedure
NOTE: Unlike the wildtype mice, the optogenetic mice do not receive pre-exposures (Pretreatment 1 and 2).
3.2.1 On the test day, insert the LED light panel into the second lowest slot (28.23 cm from the floor of the camber) to allow space for connecting the patch cord. Turn the light/dark assay apparatus on and set the light intensity to 55 lux.
3.2.2 Use the same protocol setup as that in 1.2.2 and 1.2.3 except that Data Bins By Duration is set to 60 s in the New Analysis setting to be congruent with the laser stimulation protocol in Step 3.2.3.
3.2.3 Turn the laser power button on. Set the laser pulse controller to stimulate for 1 min followed by 1 min without stimulation over 30 min.
3.2.4 Habituate mice to the testing room with the light on for 1 h prior to the testing.
3.2.5 Start the protocol. Pull the pull-out drawer outside of the sound-attenuating cubicle to access the light/dark chamber and the dark insert.
3.2.6 Gently restrain the mouse and couple the optic-fiber cannula on the mouse head to the fiber-optic patch cord via a mating sleeve (Figure 1D bottom right). Place the mouse gently in the light zone and push the drawer inside of the cubicle. Make sure that the protocol will begin to record mouse behavior automatically.
3.2.7 At 1 min, switch on the pulse controller and then turn the failsafe key to ON. Make sure laser stimulation of the targeted brain region is occurring every other minute.
3.2.8 After 30 min when the protocol stops automatically, turn the failsafe key to OFF. Then turn the pulse controller off.
3.2.9 Uncouple the mouse and the fiber-optic patch cord. Return the mouse to the home cage.
3.2.10 Clean the chamber and dark insert as described in step 1.2.8.
4. Modified open field assay for optogenetic mice
4.2 The apparatus setup
4.2.1 Stabilize the rotary joint above the chamber using a stand and a clamp (Figure 1E).
4.2.2 Connect the fiber-optic patch cord with a length of 50 cm to the rotary joint. Check if the rotary joint can rotate smoothly.
4.2.3 Set the rotary joint to the appropriate height on the stand: ensure that the fiber-optic patch cord can only just reach every corner of the chamber, which will help avoid any interference with mouse movement.
4.2.4 For the rest of the setup, use the same apparatus setup as used in section 1 (light/dark assay), but without the dark insert.
4.3 Behavioral test procedure
4.3.1 Turn the light/dark assay apparatus on and set the light intensity to 55 lux.
4.3.2 Use the same protocol setup as that in the modified light/dark assay (section 3) except for the New Zone settings. Choose 1 following by Center in New Zone settings. Set the periphery as 3.97 cm from the perimeter and the center as 19.05 × 19.05 cm.
4.3.3 Turn the laser power button on. Set laser pulse controller to stimulate for 1 min followed by 1 min without stimulation over 30 min.
4.3.4 Perform habituation and the rest of the test as described in steps 3.2.4 to 3.2.10 except for two changes to step 3.2.6: place the mouse gently in the middle of the chamber instead of the light zone; keep the pull-out drawer outside of the cubicle due to the patch cord connecting to the mouse’s head.
REPRESENTATIVE RESULTS:
This behavioral test paradigm is designed to test light-aversive behavior. It can be performed using both naïve wildtype mice and optogenetic mice to investigate light aversion in real time during the stimulation of a targeted neuronal population.
This procedure has been used to study the effect of peripheral CGRP treatment in CD1 and C57BL/6J mice10,16 and optical stimulation of neurons in the posterior thalamic nuclei in C57BL/6J mice19 on light-aversive behavior. Mice, including both males and females, aged 10-20 weeks old, were used in the experiments (Figure 2A, Figure 2B-D, and Figure 3). The results revealed that i.p. injection of CGRP significantly decreased the duration of time spent in the light zone in the light/dark assay in CD1 (Figure 2A) and C57BL/6J (Figure 2B) mice, but did not affect the time mice spent in the center in the open field assay in CD1 (data not shown) and C57BL/6J mice (Figure 2D)10,16. This suggests that peripheral CGRP induces light aversion but not general anxiety. Treatment with CGRP also increased the amount of time mice rested in the dark zone but not in the light zone in both CD1 (data not shown) and C57BL/6J mice (Figure 2C).
For the optogenetic protocol, we targeted calmodulin kinase II alpha (CaMKIIa)-expressing neurons in the posterior thalamic nuclei by injecting AAV2-CaMKIIa-hChR2(E123A)-eYFP or the control virus AAV2-CaMKIIa-eYFP19. At the same time, a fiber-optic cannula was implanted in the posterior thalamic nuclei. Three weeks following injection to allow sufficient time for ChR2 expression, we performed optical stimulation of neurons in the posterior thalamic nuclei and noted a corresponding decrease in the duration mice spent in the light zone in the light/dark assay in ChR2-injected mice compared to control virus-injected mice (eYFP) (Figure 3A). There was no noted difference in the time in center in the open field assay between ChR2 and control eYFP mice (Figure 3C), indicative of a light-aversive response that was not solely driven by anxiety19. Furthermore, an increase in the resting time in the dark zone, but not in the light zone, was also noted (Figure 3B). The same results were obtained when using 55 lux and 27,000 lux (Figure 3). The 55-lux procedure was included because migraine patients are sensitive even to dim light.
DISCUSSION:
The light/dark assay is widely used to assess anxiety-like behavior12. The assay relies on the innate aversion of mice to light and their drive to explore when placed into a novel environment (light zone). However, as we report here, this assay can also be used to assess light-aversive behavior as well.
It is critical to consider the number and necessity of pre-exposures prior to testing. This depends on the mouse strain or model. For example, in our light/dark assay protocol, naïve wildtype CD1 and C57BL/6J mice are pre-exposed to the light/dark chamber twice prior to undergoing the treatment test procedure, while optogenetic mice do not undergo pre-exposure. A recent publication reported that one pre-exposure is sufficient for CD1 mice to display light aversion after i.p. CGRP administration17. Consequently, the significance of the novelty parameter will have lessened upon arrival of the treatment day10,16. Pre-exposures can unmask light-aversive phenotypes by reducing the exploratory drive and thus altering the balance between exploration and aversion. In some cases, pre-exposure is not necessary. For example, with genetically altered mice with increased CGRP receptors in the nervous system, pre-exposure was not necessary14. Likewise, with optogenetically manipulated mice, in which CaMKIIa-expressing neurons in the posterior thalamic nuclei were targeted for optical stimulation, pre-exposure was not necessary, presumably because the light-aversive response was so robust upon direct stimulation of the brain19. Thus, the number and necessity of pre-exposures to the chamber must be carefully considered when using different mouse strains or models. Indeed, overexposure of mice to the chamber may reduce exploratory behavior. This will lead to the mice preferentially occupying the dark zone, regardless of treatment, therein reducing the ability to observe a light-aversive response. Conversely, insufficient pre-exposure to the assay may lead to exploratory behavior masking potential light-aversive behavior.
A post-treatment exposure serves to identify whether a mouse has fully recovered from the CGRP injection administered 2 days prior. This is essential prior to running the open field assay or any other assay to confirm that no prolonged treatment effect is present that will affect future behavioral tests.
We opted for a 30-min protocol duration based on previous observations10. We have tested mice in the light/dark assay for 10 min15, 20 min16 and 30 min10 separately. CGRP decreased the amount of time mice spent in the light between 0-30 min, but past 30 min the control mice preferred to spend more time in the dark compared to 0-30 min, hence leading to the decision to test for 30 min. In a similar fashion, the testing duration can be adjusted with reference to the time-response curve for different mouse models. It should be noted that lengthening the exposure time to the light/dark chamber may reduce motivation to explore the light zone.
We analyzed many different parameters to assess the animal behavior. One essential feature of the light/dark assay is a measurement of the time a mouse spends in the light zone, directly reflecting light aversion. Percentage of time spent resting, the number of vertical beam breaks (to measure rearing activity) in light or dark zones, and the number of transitions between the two zones are used to assess motility. Resting time and vertical beam breaks are normalized to the time spent in each zone in order to avoid false conclusions regarding movement. We include all mice in the analyses except: mice that remain in the light zone for the entire 30 min of testing, mice that spend over 90% of time resting in total (both light and dark zones), and statistical outliers (>3 SDs from the mean). The number of mice that are excluded is generally less than 1%. For the open field assay, the percentage of time in center is the main measurement used to assess anxiety-like behavior.
In the modified light/dark assay, the positioning of the fiber-optic cannula at some brain regions can greatly restrict mouse movement and, in some instances, prevent the mouse from reaching the dark zone. Consequently, entry into the dark zone will be negatively reinforced and, after multiple attempts, the mouse may show a learnt preference for the light, even remaining in the light zone during the entire testing period. This can be rectified by modifying the size and shape of the opening in the dark insert. As an example, when fiber-optic cannulae were installed in the cerebellum of wildtype C57BL/6J mice, the mice had difficulty crossing the opening of the dark insert. After altering the width of the opening to 6.10 cm instead of 5.08 cm, the mice were able to traverse the opening freely.
A 30.5 cm fiber-optic patch cord is used in the modified light/dark assay, based on the size of the open field chamber, allowing the mouse to move freely. A shorter cord length will prevent a mouse from moving to the corners, while a longer cable may tangle and hinder movement. The length of the fiber-optic patch cord used for the modified open field assay is 50 cm. The length is not as strict as that in the light/dark assay since the height of the rotary joint can be adjusted according to the length of the fiber-optic patch cable, ensuring that the mouse is able to just reach the corners of the chamber.
Based on power analyses, 10-12 mice per group are needed for CD1 and C57BL/6J mice with i.p. CGRP, and for optogenetic C57BL/6J mice to detect significant light aversion. However, the C57BL/6J group size was considerably larger than the CD1 group size (Figure 2A,B) because the C57BL/6J mice were unresponsive to CGRP in a subset of the tests10, meaning multiple tests were conducted to account for this high variability in light-aversive behavior in these mice. Specifically, two experiments were combined for the CD1 mice and four experiments were combined for C57BL/6J mice with i.p. CGRP (Figure 2A,B)10. The reason for this variability is not known, but humans also show variability in their responses to CGRP and light. Intravenous (i.v.) injection of CGRP induced migraine attacks in around 63~75% of migraine patients, with 70~90% of patients who displayed migraine attacks exhibiting photophobia22-25. Altogether, the assay has considerable variability and in addition to the number of mice, it is essential to do at least two and preferably three fully independent experiments with different cohorts of mice.
Bedding is not required in the light/dark chamber and the experimenter is not required to pre-handle or habituate the mice. As a precautionary measure the two pre-exposure procedures serve the purpose of acclimating the mice to the olfactory and physical cues of the experimenter; however, Ueno H. et al. demonstrated that there is no difference in time in light in the light/dark assay or time in center in the open field assay between mice after repeated handling and mice with no handling26.
The open field assay can assess the contribution of anxiety towards a light-aversive phenotype. There are other well-validated anxiety-related assays, such as the elevated zero maze and the elevated plus maze27; however, the open field assay is the most procedurally relevant control to the light/dark protocol since the same testing chamber is used for both assays. Even so, an assessment of anxiety can be strengthened by utilizing multiple assays or by measuring multiple parameters in a single test given that anxiety is a complicated and multifaceted behavior. Importantly, even if there is no anxiety phenotype in the open field assay, this does not rule out an anxiety component to the light-aversive phenotype. For example, light might be triggering an anxiety response. The open field test only indicates that anxiety alone is not driving the response to light. While an anxiolytic drug, such as benzodiazepame, might be used in this assay, such an approach would have complications, e.g., anxiolytic drugs affect locomotion. Instead, we opted to use clinical anti-migraine medications, including sumatriptan, to validate the migraine-like status of the light-aversive phenotype. Sumatriptan successfully reversed CGRP-induced light aversion in both CD1 and C57BL/6J mice10.
Unlike the modified light/dark assay, the chamber on the pull-out drawer is outside of the cubicle with cubicle doors open in the modified open field assay due to the patch cord connecting to the mouse’s head. Instead of 55 lux, the room light reaches the floor of the chamber at ~1000 lux. Even though the light intensity is different, the open field assay is a light-independent test. In detail, increasing the light intensity from 55 to 27,000 lux in the open field assay resulted in a trend of a decrease in time in the center in C57BL/6J mice, suggesting that the light intensity may influence mouse behavior28. However, the difference between the control and experimental groups was not significant under neither 55 nor 27,000 lux28. Additionally, the difference in light intensity between 55 and 1000 lux is far more subtle than between 55 and 27,000 lux. Wireless optogenetics can solve this problem as there would be no patch cord, allowing the open field chamber to be pushed inside of the sound-attenuating cubicle.
In addition, the patch cord still limits mouse movement despite selecting an optimal length. In the future, wireless optogenetics will offer a non-invasive alternative to cable-based optogenetic techniques.
It should be noted that we used acute injection of CGRP, which only replicates in part the prolonged CGRP release that accompanies migraine attacks. While we injected CGRP into mice to model migraine based on the premise that plasma CGRP levels were increased29 and that i.v CGRP induced migraine attacks in migraine patients22-25,30, this will not replicate the condition in the patient where CGRP is maintained at high levels for a relatively long time (patient measurements were taken at a median 3 hours after the migraine started29), nor does it replicate chronic migraine where levels are reported to be elevated even between attacks31. Moreover, other pain-induced mediators have not been tested in our paradigm.
The Mogil group modified the elevated plus maze to measure light aversion in mice, with the closed arms being illuminated by bright light and the open arms remaining dark32. The standard elevated plus maze has often been used to detect anxiety-related behavior in animals. This assay is based on the conflict between a mouse’s innate desire to explore a novel environment and being placed in a compromising position in the open unprotected maze arms. In the modified protocol, mice are forced to select between the closed arms, which are illuminated with bright light, and the open unprotected arms, which are dark. The preference to the former suggests anxiety overrules light aversion while the preference to the latter suggests light aversion takes precedence over anxiety. The Mogil group also conducted a standard elevated plus maze to evaluate anxiety-like behavior32. The purpose is the same as conducting the open field assay in our protocol. Cacna1a mutant mice, a familial hemiplegic migraine model, showed photophobia when the closed arms were bright. In contrast, anxiety-like behavior was not detected when the standard elevated plus maze was conducted32. In rats, by using both the modified elevated plus maze and the light/dark assay, it was demonstrated that nitroglycerin (NTG) was able to induce photophobia33,34, which was rescued by sumatriptan34. In the standard elevated plus maze setting where light is absent within the closed arms, NTG induced anxiety-like behavior in rats34, suggesting that NTG-induced light aversion is accompanied with anxiety. To our knowledge, there are no publications using the light/dark assay and the modified elevated maze in the same mouse model. All in all, both the modified elevated plus maze and the light/dark assay proposed in this protocol have been demonstrated as effective measures of light-aversive behavior in mice.
We use the daylight LED panel with a daylight-balanced color (5600K), with a 60° flood beam spread, yielding no shadowing at a height of ~30 cm from the floor of chamber at either 55 lux or 27,000 lux. Other studies investigating light aversion have utilized the light/dark assay with varying modifications. For example, studies have used different light intensities for the light zone, ranging from hundreds to thousands of lux35-37; used light at different wavelengths (e.g. blue and yellow)38; or used different temperatures of light (cold and warm)39. Caution should be taken for the heat produced by the light since it can affect the temperature of the dark and light zones and interfere with the mice’s behavior, potentially causing a preference to a specific zone. Besides, it is also important to use the light with a good viewing angle to avoid shadow on the floor of the chamber. Light intensity is important for the test too. 25,000 −27,000 lux is approximately equivalent to bright daylight. By conducting the light/dark assay at such a high light intensity, it is possible to amplify the treatment effect; however, it is essential to consider the retinal damage40 and the negative effect of such a high light intensity on a mouse’s willingness to go into light. Some studies reported that mouse eyes exposed to direct light41 and mice exposed to bright light for several hours (e.g. 30,000 lux for 4 hrs42) experienced retinal damage. In the light/dark assay, there is a dark zone for the mouse to escape from the bright light if the mouse desires. In addition, previous studies found that mice in the control group (C57BL/6J mice) spent a similar amount of time in the light zone under 55, 1000 and 27,000 lux28. For CD1 mice, the control group spent about 1/3 of the time in the light under 27,000 lux10 and unpublished data had shown similar results at 55 lux. It suggests that 27,000 lux light on its own does not make CD1 and C57BL/6J mice distressed. Nonetheless, caution should be taken when opting for a higher light intensity.
Alongside differences in light setting, researchers have opted for a variety of approaches in analyzing the light/dark data. When assessing light aversion, the amount of time spent in the light zone with the light switched off (or with red light illumination of the light zone, given that mice eyes are less receptive to red light) are included in the calculation. For example, aversion index= (time in light0 lux-time in lighttest lux)/ time in light0 lux was used by the Gorin group to assess light aversion43. Here, the ‘light off’ or ‘red light’ conditions are included to confirm that the avoidance of the light zone is conditional on light being present in opposed to simple place preference. We conducted this procedure with i.p. injection of CGRP and found that mice receiving CGRP did not have a place preference with light off in the light zone, confirming that CGRP-induced aversion is light-dependent16. Lastly, the Gorin group used the time mice spent in the periphery of the light zone in the light/dark assay as a measure of anxiety36. We utilize a traditional test for anxiety, the open field assay. No matter which analysis method is chosen, it should be noted that the contribution of anxiety to light aversion cannot be ignored. This protocol attempts to partition out anxiety-like and light-aversive behavior by utilizing the light/dark and open field assays in tandem.
This protocol addresses the use of the light/dark and open field assays for the detection of light-aversive behavior in mice. This provides a useful tool to identify the mechanisms of neural circuits and brain regions driving photophobia. The test paradigm can be migraine-specific or can be expanded into other disorders involving photophobia. With respect to migraine, we have tested two other neuropeptides associated with migraine pathogenesis: pituitary adenylate cyclase-activating polypeptide (PACAP) and vasoactive intestinal peptide (VIP). PACAP and VIP were demonstrated to induce light aversion in CD1 mice17,21. In addition to migraine, photophobia is also a symptom of many other disorders, including bradyopsia, acute ocular injury or inflammation, traumatic brain syndromes, Lyme disease, albinism and cone dystrophy36. Thus, this test paradigm provides a tool to investigate mechanisms underlying photophobia-related disorders. Moreover, the pairing of optogenetic methods with conventional pharmacological approaches will undoubtedly assist in the development of novel therapeutics for photophobia-related disorders.
ACKNOWLEDGMENTS:
This work was supported by grants from the NIH NS R01 NS075599 and RF1 NS113839. The contents do not represent the views of VA or the United States Government.
Footnotes
DISCLOSURES:
The authors have no conflicts of interest to report.
REFERENCES:
- 1.Loder S, Sheikh HU & Loder E The prevalence, burden, and treatment of severe, frequent, and migraine headaches in US minority populations: statistics from National Survey studies. Headache. 55 (2), 214–228, (2015). [DOI] [PubMed] [Google Scholar]
- 2.Collaborators GBDH Global, regional, and national burden of migraine and tension-type headache, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2.16. 2016. ancet Neurology. 17 (11), 954–976, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Disease GBD, Injury I & Prevalence C Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 392 (10159), 1789–1858, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 38 (1), 1–211, (2018). [DOI] [PubMed] [Google Scholar]
- 5.Edvinsson L, Haanes KA, Warfvinge K & Krause DN CGRP as the target of new migraine therapies - successful translation from bench to clinic. Nature Reviews Neurology. 14 (6), 338–350, (2018). [DOI] [PubMed] [Google Scholar]
- 6.Rapoport AM & McAllister P The Headache Pipeline: Excitement and Uncertainty. Headache. 60 (1), 190–199, (2020). [DOI] [PubMed] [Google Scholar]
- 7.Maasumi K, Michael RL & Rapoport AM CGRP and Migraine: The Role of Blocking Calcitonin Gene-Related Peptide Ligand and Receptor in the Management of Migraine. Drugs. 78 (9), 913–928, (2018). [DOI] [PubMed] [Google Scholar]
- 8.Caronna E & Starling AJ Update on Calcitonin Gene-Related Peptide Antagonism in the Treatment of Migraine. Neurologic Clinics. 39 (1), 1–19, (2021). [DOI] [PubMed] [Google Scholar]
- 9.Eftekhari S & Edvinsson L Possible sites of action of the new calcitonin gene-related peptide receptor antagonists. Therapeutic Advances in Neurological Disorders. 3 (6), 369–378, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mason BN et al. Induction of Migraine-Like Photophobic Behavior in Mice by Both Peripheral and Central CGRP Mechanisms. Journal of Neuroscience. 37 (1), 204–216, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russell MB, Rasmussen BK, Fenger K & Olesen J Migraine without aura and migraine with aura are distinct clinical entities: A study of four hundred and eighty-four male and female migraineurs from the general population. Cephalalgia. 16 (4), 239–245, (1996). [DOI] [PubMed] [Google Scholar]
- 12.Crawley JN Exploratory behavior models of anxiety in mice. Neuroscience and Biobehavioral Reviews. 9 (1), 37–44, (1985). [DOI] [PubMed] [Google Scholar]
- 13.Crawley J & Goodwin FK Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacology, Biochemistry and Behavior. 13 (2), 167–170, (1980). [DOI] [PubMed] [Google Scholar]
- 14.Recober A et al. Role of calcitonin gene-related peptide in light-aversive behavior: implications for migraine. Journal of Neuroscience. 29 (27), 8798–8804, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Recober A, Kaiser EA, Kuburas A & Russo AF Induction of multiple photophobic behaviors in a transgenic mouse sensitized to CGRP. Neuropharmacology. 58 (1), 156–165, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaiser EA, Kuburas A, Recober A & Russo AF Modulation of CGRP-induced light aversion in wild-type mice by a 5-HT(1B/D) agonist. Journal of Neuroscience. 32 (44), 15439–15449, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuburas A et al. PACAP induces light aversion in mice by an inheritable mechanism independent of CGRP. Journal of Neuroscience. 10.1523/JNEUROSCI.2200-20.2021, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russo AF Calcitonin gene-related peptide (CGRP): a new target for migraine. Annual Review of Pharmacology and Toxicology. 55533–552, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sowers LP et al. Stimulation of Posterior Thalamic Nuclei Induces Photophobic Behavior in Mice. Headache. 60 (9), 1961–1981, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Afridi SK et al. A positron emission tomographic study in spontaneous migraine. Archives of Neurology. 62 (8), 1270–1275, (2005). [DOI] [PubMed] [Google Scholar]
- 21.Mason BN et al. Vascular actions of peripheral CGRP in migraine-like photophobia in mice. Cephalalgia. 40 (14), 1585–1604, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo S, Vollesen ALH, Olesen J & Ashina M Premonitory and nonheadache symptoms induced by CGRP and PACAP38 in patients with migraine. Pain. 157 (12), 2773–2781, (2016). [DOI] [PubMed] [Google Scholar]
- 23.Christensen CE et al. Migraine induction with calcitonin gene-related peptide in patients from erenumab trials. Journal of Headache and Pain. 19 (1), 105, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Younis S et al. Investigation of distinct molecular pathways in migraine induction using calcitonin gene-related peptide and sildenafil. Cephalalgia. 39 (14), 1776–1788, (2019). [DOI] [PubMed] [Google Scholar]
- 25.Asghar MS et al. Evidence for a vascular factor in migraine. Annals of Neurology. 69 (4), 635–645, (2011). [DOI] [PubMed] [Google Scholar]
- 26.Ueno H et al. Effects of repetitive gentle handling of male C57BL/6NCrl mice on comparative behavioural test results. Scientific Reports. 10 (1), 3509, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campos AC, Fogaca MV, Aguiar DC & Guimaraes FS Animal models of anxiety disorders and stress. Revista Brasileira De Psiquiatria. 35 S101–S111, (2013). [DOI] [PubMed] [Google Scholar]
- 28.Kuburas A, Thompson S, Artemyev NO, Kardon RH & Russo AF Photophobia and abnormally sustained pupil responses in a mouse model of bradyopsia. Investigative Ophthalmology and Visual Science. 55 (10), 6878–6885, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goadsby PJ, Edvinsson L & Ekman R Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Annals of Neurology. 28 (2), 183–187, (1990). [DOI] [PubMed] [Google Scholar]
- 30.Lassen LH et al. CGRP may play a causative role in migraine. Cephalalgia. 22 (1), 54–61, (2002). [DOI] [PubMed] [Google Scholar]
- 31.Cernuda-Morollon E et al. Interictal increase of CGRP levels in peripheral blood as a biomarker for chronic migraine. Neurology. 81 (14), 1191–1196, (2013). [DOI] [PubMed] [Google Scholar]
- 32.Chanda ML et al. Behavioral evidence for photophobia and stress-related ipsilateral head pain in transgenic Cacna1a mutant mice. Pain. 154 (8), 1254–1262, (2013). [DOI] [PubMed] [Google Scholar]
- 33.Mahmoudi J et al. Cerebrolysin attenuates hyperalgesia, photophobia, and neuroinflammation in a nitroglycerin-induced migraine model in rats. Brain Research Bulletin. 140197–204, (2018). [DOI] [PubMed] [Google Scholar]
- 34.Farajdokht F, Babri S, Karimi P & Mohaddes G Ghrelin attenuates hyperalgesia and light aversion-induced by nitroglycerin in male rats. Neuroscience Letters. 630 30–37, (2016). [DOI] [PubMed] [Google Scholar]
- 35.Jacob W et al. Endocannabinoids render exploratory behaviour largely independent of the test aversiveness: role of glutamatergic transmission. Genes, Brain, and Behavior. 8 (7), 685–698, (2009). [DOI] [PubMed] [Google Scholar]
- 36.Thiels E, Hoffman EK & Gorin MB A reliable behavioral assay for the assessment of sustained photophobia in mice. Current Eye Research. 33 (5), 483–491, (2008). [DOI] [PubMed] [Google Scholar]
- 37.Ramachandran R et al. Role of Toll-like receptor 4 signaling in mast cell-mediated migraine pain pathway. Molecular Pain. 151744806919867842, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marek V et al. Implication of Melanopsin and Trigeminal Neural Pathways in Blue Light Photosensitivity in vivo. Frontiers in Neuroscience. 13497, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Christensen SLT, Petersen S, Sorensen DB, Olesena J & Jansen-Olesen I Infusion of low dose glyceryl trinitrate has no consistent effect on burrowing behavior, running wheel activity and light sensitivity in female rats. Journal of Pharmacological and Toxicological Methods. 80 43–50, (2016). [DOI] [PubMed] [Google Scholar]
- 40.De Vera Mudry MC, Kronenberg S, Komatsu S & Aguirre GD Blinded by the light: retinal phototoxicity in the context of safety studies. Toxicologic Pathology. 41 (6), 813–825, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.White DA, Fritz JJ, Hauswirth WW, Kaushal S & Lewin AS Increased sensitivity to light-induced damage in a mouse model of autosomal dominant retinal disease. Investigative Ophthalmology and Visual Science. 48 (5), 1942–1951, (2007). [DOI] [PubMed] [Google Scholar]
- 42.Song D et al. Retinal Pre-Conditioning by CD59a Knockout Protects against Light-Induced Photoreceptor Degeneration. PloS One. 11 (11), e0166348, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matynia A et al. Light aversion and corneal mechanical sensitivity are altered by intrinscally photosensitive retinal ganglion cells in a mouse model of corneal surface damage. Experimental Eye Research. 13757–62, (2015). [DOI] [PubMed] [Google Scholar]