Abstract
Hematopoietic stem cell transplantation (HCT) is a potentially curative treatment for many hematologic conditions. Despite advances in conditioning and supportive measures, however, there remain significant comorbidities that threaten survivorship. Adverse effects of stress-related biobehavioral processes—defined here as the interactions of behavioral, psychological, and socioenvironmental factors with biology—impact immune recovery and function and are particularly salient in the HCT context, given the importance of immune reconstitution for improved survivorship. However, biobehavioral processes have been underinvestigated in this vulnerable group compared with other cancer populations. Here the Biobehavioral Research Special Interest Group (SIG) of the American Society for Transplantation and Cellular Therapy provides an expert review to inform research directions explicating the biological correlates of behavioral symptoms and evaluate the impact of these on HCT outcomes. The goal of this expert review is to provide a foundation for advancing science that effectively integrates behavioral and biological processes to optimize quality of life and improve clinical outcomes for HCT recipients.
Keywords: Biobehavioral, hematopoietic cell, transplantation, survivorship, stress, outcomes
INTRODUCTION
The interactions of behavioral, psychological, and socioenvironmental factors with biological processes (summarized as "biobehavioral”) throughout the hematopoietic stem cell transplantation (HCT) trajectory significantly impact both psychological and physical morbidity and mortality [1,2]. The adverse effects of stress-related biobehavioral factors (including psychological and socioenvironmental factors) on immune function have already been well documented in cancer populations. These effects are likely to be especially salient in the HCT context, given the importance of optimal immune recovery and function in reducing complications and preventing recurrence. Although gaining recognition among the scientific communities and a burgeoning area of investigation, the intersection of biology and behavior is not well elucidated. A comprehensive understanding of the complex interplay among biobehavioral factors is essential for the development of successful interventions to mitigate complications of HCT and improve survival.
A growing body of evidence, both preclinical and clinical, suggests that stress-related psychosocial factors can affect such physiologic processes as inflammation and proteomic, genomic, and metabolomic (’omics) pathways that modulate the biological drivers of cancer progression [3]. Simultaneously, evidence for malignancy- and treatment-related biological influences on prevalent behavioral symptoms is accumulating [4]. However, it is important to note that the findings are not always straightforward or consistent, with the existence, nature, and strength of the relationships varying [5-9]. Research supports these relationships to be iterative and/or bidirectional, noting reciprocal influence between the neural and immune systems [10,11]. Therefore, thoughtful aims and methodologic rigor are paramount in deciphering the directionality of these relationships in the complex setting of HCT.
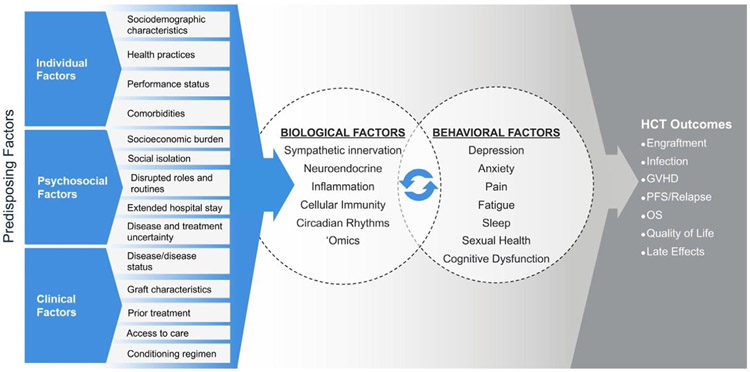
Here the Biobehavioral Research Special Interest Group (SIG) Steering Committee of the American Society for Transplantation and Cellular Therapy provides an expert review to inform research directions explicating the biological correlates of behavioral symptoms and evaluates the impact of these on HCT outcomes (Figure 1). Detailed discussion of the molecular pathways outlining these associations is beyond the scope of the current review; however, these have been described in depth both in cancer generally [12,13] and in HCT [1,2], where the reader is referred for further detail. It is also important to note that most, or at least many, biobehavioral relationships are inherently bidirectional. In addition, this review does not attempt to comprehensively address the numerous potential risk and protective factors affecting biobehavioral relationships described herein (eg, health practices, coping responses, caregiver influences). The aim of this review is to provide a foundation for advancing science that effectively integrates behavioral and biological processes to optimize quality of life (QOL) and improve clinical outcomes for HCT recipients.
Figure 1.
Biobehavioral model illustrating bidirectional relationships between biological and behavioral factors and effects of relevance to HCT throughout the trajectory from pretransplantation to long-term survivorship. It is intended not to be comprehensive but rather to provide a conceptual framework for applying biobehavioral science to the HCT setting. In brief, individual differences in sociodemographic, health, and psychosocial factors, including health practices (eg, substance use, medication adherence), as well as disease- and HCT-related factors, affect biobehavioral mechanisms and relationships explicated in this article. The article is organized by the behavioral symptoms listed here, which are among the most common and distressing for HCT recipients. Biological processes affected by HCT, including inflammation and disrupted circadian rhythms, can alter central nervous system pathways that evoke behavioral symptoms highlighted here. Psychosocial stressors and stress-related behavioral factors activate the hypothalamic-pituitary-adrenal and SNS axes as well as associated proteomic, genomic, and metabolomic pathways (elucidated in new research as ’omics technologies). The secreted products of these pathways (eg, glucocorticoids, catecholamines) and direct sympathetic innervation of the bone marrow microenvironment can modulate cell recovery following HCT and promote (or moderate) an inflammatory environment that predisposes the HCT recipient to GVHD and other HCT-associated complications. These physiologic processes have the potential to influence clinical events following HCT, including the development of infections, modulation of the activity of effector cells on residual disease, and the occurrence of acute and chronic GVHD, thereby affecting QOL and the risk for disease progression or relapse, late effects, and survival. PFS, progression-free survival; OS, overall survival.
BIOBEHAVIORAL COMORBIDITIES OF HCT
Anxiety and Depression
HCT recipients experience significant physical and psychological stressors from diagnosis through the transplantation and survivorship period, when late effects and fear of cancer recurrence are prevalent [14,15]. Therefore, it is not surprising that a significant proportion of patients report symptoms of anxiety and/or depression. Anxiety and depression symptoms are often part of the experience of what is termed “cancerrelated distress,” although distress also may include other adverse cognitive, affective, and behavioral symptoms. Among HCT survivors, 12% to 30% report clinically significant depressive symptoms [16]. Risk factors include younger age, female sex, and low socioeconomic status (SES) [16]. The prevalence of anxiety in survivors of pediatric HCT is 16% to 37% higher than that of age-matched controls and siblings [17,18]. Most studies indicate that rates of clinical anxiety and depression decline over the first year post-HCT [16,19].
These adverse psychological conditions have been shown to influence biomedical outcomes in HCT recipients. Pre-HCT depression has been associated with a higher incidence of acute graft-versus-host disease (GVHD) [20] and impaired physical function [21], and both anxiety and depression have been associated with slower engraftment [22,23]. Depression also has emerged as an important risk factor for mortality following transplantation, with estimates between a 1.13- and 3-fold increased risk of death in depressed HCT recipients when controlling for disease- and patient-related factors [20,24,25]. Conversely, increasing attention is being given to protective psychological factors in HCT; measures of optimism and psychological resilience have been associated with better health-related QOL, lower 60-day mortality, and earlier engraftment [22,26,27].
The growing body of translational psychoneuroimmunology research offers mechanistic insights into these biobehavioral relationships [1,2]. In both human and animal models, depressive symptoms or behavior are consistently associated with elevated inflammatory cytokine levels and altered immune function [28,29]. For example, higher circulating IL-6 predicts the development of depressive behavior in animal models [30] and in population-based longitudinal studies in humans [31]. Administration of proinflammatory agents such as IFN-α has been associated with an increase in depressive symptoms [30,32], whereas administration of anti-inflammatory drugs has been associated with improved mood and is used to augment treatment for depression [33]. Inflammation is implicated in many serious transplantation-related adverse events [34-36], and thus elucidation of inflammatory biobehavioral pathways are particularly salient in the HCT population.
Anxiety and other stress-related psychological states are often accompanied by increased sympathetic nervous system (SNS) activation [37], which influences the hematopoietic milieu through direct innervation of the bone marrow and affects hormonal regulation via the release of catecholamines (Figure 1) [12]. Sympathetically-mediated signaling is instrumental in stem cell migration, homing, and proliferation [38-40]. The SNS also may influence downstream genomic regulation of inflammatory processes in HCT recipients. For example, sympathetically-mediated activation of beta-adrenergic receptors stimulates the conserved transcriptional response to adversity (CTRA) gene expression profile [41]. Consisting largely of proinflammatory genes, the CTRA has been associated with both chronic stress and reduced relapse-free and overall survival following HCT [41]. Taken together, accumulating evidence indicates that SNS dysregulation may have implications for HCT mechanics at the cellular level.
There are relatively few evidence-based interventions specifically tailored to HCT patients. Behavioral interventions tested during the acute HCT recovery period include exercise, cognitive behavioral therapy (CBT), and mindfulness/stress reduction [16]. There are other modalities that have demonstrated usefulness in symptom management; however, these have generally included smaller sample sizes or less rigorous designs. For example, healing touch, light therapy, and music therapy show promise for alleviating psychological symptoms but will need to be investigated in larger efficacy trials [42-44]. In a randomized study of a palliative care intervention during HCT hospitalization, patients in the treatment arm reported decreased anxiety and depression at 2 weeks and 6 months post-HCT [45].
There have been fewer pharmacologic interventions targeting biobehavioral pathways in HCT recipients. In a phase 2 randomized study of patients undergoing HCT for multiple myeloma, propranolol was associated with reduced CTRA gene expression and up-regulation of stem cell progenitors [46]. Despite accumulating evidence that pre-HCT psychological state has important implications for outcomes, we are not aware of any published pharmacologic clinical trials targeting this critical period.
Pain
Pain, defined as a distressing experience associated with actual or potential tissue damage with sensory, emotional, cognitive, and social features [47], is common among HCT recipients and contributes to diminished QOL [48]. However, sources of pain and activated pain pathways are highly variable across HCT recipients.
Biobehavioral pathways are important in central nervous system (CNS)-mediated processes underlying pain [49], many of which are relevant in the HCT setting. Psychological stress associated with transplantation or other preexisting behavioral health factors may exacerbate the experience of pain during HCT. Anxiety and depression are associated with proinflammatory cytokines that affect CNS pathways that can enhance pain sensitivity and exacerbate pain [50].
Before HCT, treatments for underlying hematologic diseases—including platinum-based chemotherapies—predispose HCT candidates to peripheral neuropathy [51]. Psychological factors can modulate the perception of neuropathic pain by neurobiologically priming the pain response via proinflammatory and other CNS-mediated nociceptive pathways involved in stress [52]. Preexisting conditions, including visceral pain due to underlying tumors, musculoskeletal dysfunction, and mood disorders, can also contribute to pain pre- and post-HCT.
During the early post-HCT period, pain from oral mucositis is a particularly distressing HCT complication [53,54]. Later in the course of post-HCT recovery, acute and chronic GVHD and their sequelae are associated with the development of pain syndromes [55,56]. Given the etiologic proinflammatory overlap between depression or anxiety and GVHD, it is likely that crosstalk between these 2 states can affect pain perception, although this has yet to be investigated directly. Prophylactic GVHD treatments, such as calcineurin inhibitors [57,58] and corticosteroids, are also associated with pain. Corticosteroid administration is associated with the occurrence of avascular necrosis [59], presenting with severe deep-seated pain often refractory to analgesics. Because the majority of patients with active chronic GVHD are on prophylactic antimicrobials, pain syndromes as a result of antifungals (especially voriconazole) have been reported [60]. Finally, drugs administered post-HCT as maintenance therapy to decrease the risk of relapse, such as low-dose 5-azacitidine following allogeneic HCT, also can lead to painful peripheral neuropathy [61].
In addition to traditional pharmacologic methods of pain control, biobehavioral interventions including CBT, psychoeducation, mindfulness, and other mind-body approaches have synergistic pain control effects with medications [62]. This is likely secondary to nonopioid mechanisms, including autonomic reactivity and inflammation modulation [49]. Early studies indicate an effect of such interventions and mindfulness states in reducing pain among HCT patients [63-65], with one study indicating reduced narcotic use in the HCT setting with a music therapy intervention [66]. Additional work is needed to understand the molecular and signaling pathways responsible for these associations.
Fatigue
Fatigue is one of the most common and severe patient-reported symptoms among HCT recipients before, during, and after transplantation [67]. The National Comprehensive Cancer Network (NCCN) Working Group for Cancer-Related Fatigue defines fatigue as “a distressing, persistent, subjective sense of physical, emotional and/or cognitive tiredness or exhaustion that is disproportionate to recent activity and interferes with usual functioning” [68].
Fatigue tends to peak around a WBC nadir before gradually declining to pretransplantation levels within the first year post-HCT [67]. However, relative to the general population, fatigue associated with HCT remains elevated in a subset of HCT survivors for as long as 5 years post-HCT [67,69]. Among long-term survivors (ie, 6-13 years post-HCT on average), the rate of moderate to severe fatigue was 42%, which does not differ between those treated with allogeneic HCT versus recipients of autologous HCT [70].
There is mixed evidence regarding risk factors for fatigue, with many studies underpowered to detect significant risks [70]. One large study of 1188 long-term survivors identified female sex, chronic pain, and severity of chronic GVHD as risk factors for greater fatigue in recipients of allogeneic HCT. For autologous HCT recipients, younger age and chronic pain were associated with greater fatigue [70].
Although research on biological mechanisms of fatigue in HCT recipients is sparse, fatigue is associated with circulating IL-6 in HCT recipients [71-73], a finding consistent with studies of patients with solid tumors [74]. Inflammation can affect CNS pathways associated with the withdrawal and conservation of energy, thereby evoking fatigue [75]. Disruption of the hypothalamic-pituitary-adrenal axis also may affect fatigue indirectly via effects on inflammation, as well as directly via cortisol alterations [2,76]. Specifically, cortisol blocks the transcriptional activity of nuclear factor-kappa light chain enhancer of activated B cells (NF-κB) [77], which suppresses a cascade of proinflammatory cytokines, including IL-6. In other cancer samples, fatigue is associated with increased expression of NF-κB [78] as well as dysregulated hypothalamic-pituitary-adrenal axis function through flattened diurnal cortisol slopes [79-81]. Steroid use for GVHD may contribute to increased fatigue through nighttime sleep disturbance. Finally, the use of sirolimus and tacrolimus for GVHD prevention is associated with increased fatigue [82].
Pharmacologic and behavioral interventions may be beneficial in the treatment of fatigue. In a recent meta-analysis of pharmacologic trials for managing fatigue in cancer patients and HCT recipients, significant improvements in fatigue were seen for erythropoietin and methylphenidate, but not for modafinil or corticosteroids [83]. Regarding behavioral interventions, there are data to suggest that mindfulness-based approaches, exercise, and interventions to improve sleep may be helpful in HCT recipients [84]. Bright light therapy also has demonstrated efficacy in improving cancer-related fatigue [85].
Exercise is perhaps the most widely studied, recognized, and recommended intervention to manage fatigue in cancer survivors [68], with structured exercise typically more efficacious than home-based interventions [86-89]. The high levels of fatigue and inactivity in HCT recipients [90] might make it difficult for fatigued HCT recipients to start and maintain an exercise program on their own. Thus, fatigued HCT recipients should be encouraged to join a structured program that gradually increases from mild to moderate intensity over time.
Sleep Disruption
Sleep disruption includes difficulty falling asleep, staying asleep, awakening earlier than intended, and/or nonrestorative sleep [82]. Sleep disruption is a distressing and prevalent problem in HCT, with 49% of patients experiencing disrupted sleep before HCT [91]. Extended hospitalizations with nighttime disruptions, adverse side effects, and the use of corticosteroids and other medications known to disrupt sleep, contribute to high rates of sleep disturbance during the peri-HCT period; indeed, 74% of HCT recipients experience some degree of insomnia during hospitalization [92,93]. Sleep typically improves beyond the initial month after HCT, remaining relatively stable beyond day +100 [94]. However, sleep disturbance persists well beyond the acute transplantation recovery for a subset of patients; 26% of patients continue to experience sleep disorders 1 to 10 years post-HCT, including both insomnia (23%) and hypersomnia (3%) [95].
Risk factors for sleep disruption are understudied in HCT recipients. Regarding sociodemographic and clinical factors, female sex, lower annual household income, unmarried patients, worse baseline performance status, conditioning therapy with busulfan/fludarabine, and allogeneic HCT have been identified as risk factors [41,95,96]. In terms of psychological and behavioral risk factors, cancer-related distress, fear of recurrence, depression, anxiety, dysfunctional sleep cognition, and inhibitory sleep behavior have been associated with greater sleep disturbance [94,97]. In individuals with and without cancer, proinflammatory cytokines are causally linked to worse sleep, and, conversely, worse sleep can increase systemic inflammation [98,99]. In HCT recipients, higher circulating levels of IL-6 were associated with more disrupted sleep at multiple assessment points during the first 30 days [72,73] and 6 months [94] post-HCT. The development of techniques to probe the gut microbiome offers additional opportunities to explore putative biological mechanisms of sleep disturbance, as preliminary evidence suggests a relationship in cancer patients [100].
Sleep disruption is an important issue to assess and treat, given its association with worse QOL, fatigue and daytime impairment, and cognitive impairment [82,101], as well as increased risk for recurrence and poorer survival after allogeneic HCT [102]. CBT for insomnia (CBT-I) has been consistently efficacious in improving sleep in cancer patients [103]. Sedative-hypnotics have been linked to increased inpatient falls and other adverse effects [104], and thus CBT-I remains the treatment of choice for HCT recipients with sleep disruption after ruling out other contributors to sleep disruption, such as sleep apnea and restless leg syndrome [82].
Recent research also suggests an important role for circadian rhythms, with more optimally timed and consistent sleep and activity patterns associated with better recovery of QOL after HCT [105]. Interventions targeting rest and activity patterns, including behavioral approaches and bright light therapy, are also promising. A recent RCT showed improved sleep in HCT survivors using a therapeutic light box in the morning [106].
Sexual Dysfunction
Along with other biobehavioral comorbidities addressed here, sexual health has been identified as 1 of 6 major research priorities by HCT recipients, caregivers, and researchers [106]. Indeed, the National Institutes of Health (NIH) Blood and Marrow Transplant Late Effects Initiative cited sexual dysfunction as a major concern in need of interventions [16,107]. Sexual dysfunction is one of the most common late effects after HCT, with persistent problems including decreased libido, problems with erection and ejaculation, premature menopause, dyspareunia, and changes in genital tissues [108]. A prospective study indicated that sexual activity and sexual function declined after myeloablative HCT, with 80% of female and 46% of male HCT survivors reporting sexual problems, compared with 61% of female and 21% of male matched controls [109]. Although men returned to pretransplantation levels of sexual function by an average of 2 years post-HCT, women had not fully recovered by 5 years [109].
Sexual dysfunction following HCT is multifactorial and most comprehensively understood by considering biological, behavioral, and psychosocial underpinnings [110]. Biological and medical risk factors include gonadal toxicity from alkylating agents and total body irradiation, premature menopause and infertility, and effects of total body irradiation, inflammation, and chronic GVHD on genital tissues [108]. With respect to psychosocial factors, fatigue, depression, anxiety, and insomnia related to conditioning regimens, complications, and psychological stress affect sexual desire and response. Role changes and communication difficulties with a sexual partner can further compound problems [111]. It therefore follows that biobehavioral interventions to improve these factors through successful alteration of biological and psychosocial underpinnings also would subsequently improve sexual function.
A recent pilot intervention trained transplantation physicians to address the biological, psychological, and social aspects of sexual dysfunction [112]. HCT physicians provided a multimodal intervention for those one-third of patients who screened positive for sexual dysfunction with distress. The intervention (physical exam and sexual function assessment) was feasible, acceptable, and resulted in large improvements in patient-reported sexual function and an increase in sexual activity, from 67% at baseline to 93% at 6 months [113]. Further research is needed to understand the direct biobehavioral influences mediating these treatment–outcome relationships.
Cognitive Dysfunction
Cognitive dysfunction refers to a decline in neurocognitive functioning that is independent of normal aging and may include deficits in memory, attention, concentration, executive and motor function, and information processing speed [114]. Cognitive deficits are most often assessed using objective, validated neuropsychological measures in relation to samples of healthy or non-HCT case controls and population norms and/or patient reports of cognitive problems, although the 2 modalities have not correlated consistently in previous research [114]. Up to 58% of adult recipients demonstrate pretransplantation cognitive dysfunction [115]. Cognitive deficits typically worsen during the acute transplantation period but recover to pretransplantation levels [116,117]; however, up to 51% of survivors experience deficits at 1 year post-HCT, with 28% showing moderate to severe impairments [118]. In one study, up to 60% of allogeneic recipients experienced deficits at 22-82 months post-HCT, with 12% showing moderate to severe impairments [119]. Research has also identified subtle impairments in child recipients that in some cases improve (eg, visuomotor skills) and in others can become chronic (eg, verbal skills) up to 5 years post-HCT [114]. Research has identified transplantation conditioning regimens and immunosuppressive therapies, which can have direct cytotoxic effects in the brain, as risk factors for cognitive dysfunction [114]. Non-malignant primary disease (eg, sickle cell, severe combined immunodeficiency) is also a risk factor, with many patients experiencing neurocognitive dysfunction related to the disease before HCT. Aside from the biological influences of these risk factors, few studies have examined other biological correlates or mechanisms of cognitive dysfunction in HCT recipients. Several of the biobehavioral symptoms reviewed in this article, including depression, fatigue, and sleep disruption, have been associated with cognitive dysfunction following HCT and may share a common mechanism.
Research with other cancer populations suggests that inflammation may be one mechanism that contributes to cognitive dysfunction following treatment [120]. However, only one study with allogeneic HCT recipients has found that increases in circulating markers of inflammation IL-6 and soluble TNF receptor II were associated with worsening cognitive dysfunction in allogeneic HCT recipients from pre-HCT to 90 days following HCT [121]. Neuroimaging studies have suggested that changes in white matter integrity (mean diffusivity) that may result from the neurotoxicity of conditioning regimens are associated with changes in cognitive function from pretransplantation to 1 year post-transplantation [122]. Other research has identified that single-nucleotide polymorphisms that can impact DNA repair, telomere homeostasis, and blood brain barrier permeability are associated with cognitive impairments [123], suggesting other potential mechanisms for future investigation.
Cognitive dysfunction has been associated with lower physical, role, and social functioning QOL domains [115,124] and with a 10-fold increased risk of not returning to work [115,124,125]. Limited investigations of clinical outcomes have found that cognitive dysfunction predicted a higher number of serious adverse events and increased risk of readmission in the first 100 days for autologous HCT recipients age ≥50 years [126] and marginally increased risk for mortality at 1 year for allogeneic recipients [117]. Although nonpharmacologic interventions for cognitive dysfunction have not yet been implemented with HCT recipients, cognitive training interventions used with other cancer populations may be effective in this setting [121,125]. Further research is needed to understand the biological underpinnings of cognitive dysfunction specifically among HCT recipients.
CROSS-CUTTING THEMES
Although this review has focused on discrete biobehavioral symptoms of relevance to HCT clinical outcomes, it is important to note that there are shared risk factors, biobehavioral mechanisms, and research approaches relevant to multiple symptoms. In this section, we consider transdisciplinary biobehavioral factors, assessments, and clusters of symptoms and discuss their relevance to HCT.
Social Disparities
HCT outcomes are worse for underrepresented populations. Black patients have an increased risk of treatment-related mortality (TRM) and decreased chance for overall survival and disease-free survival compared with white patients [127,128]. Factors that contribute to racial differences in survival after HCT for black patients include lack of donor availability, poorer access to HCT, and worse HCT outcomes. Lower SES, regardless of race, also has a deleterious impact on HCT outcomes, including worse overall survival and increased TRM [127]. Emerging research indicates the importance of these disparities, especially socioeconomic adversity-related stress pathways, in HCT outcomes for these populations. The CTRA stress-related gene expression profile, comprising genes encoding for increased inflammation and decreased IFN and antibody synthesis [129], was more strongly expressed in HCT recipients of lower SES [130]. Increased CTRA expression was also associated with worse clinical outcomes, including greater likelihood of relapse and decreased disease-free survival among unrelated donor HCT recipients [130].
Biobehavioral Symptom Clusters
Symptoms tend to co-occur, especially fatigue, sleep disturbance, depressed mood, cognitive impairment, and pain; for example, clusters of pain, depression, and fatigue are frequent in patients with cGVHD [131]. Intersecting distressing symptoms associated with HCT likely have shared biological mechanisms, including inflammation and disrupted circadian rhythms [11]. Inflammatory cytokines released in response to development and progression of malignancies, tissue damage from conditioning therapy, pathophysiological processes involved in GVHD, and psychological stress can modulate CNS pathways associated with energy conservation, leading to biobehavioral symptoms described above [29]. Disrupted nighttime sleep, alterations in sleep timing, and inactivity during the day also may contribute to this symptom cluster [82].
These shared mechanisms have clinical relevance in that interventions targeting a single symptom, such as fatigue, also may mitigate co-occurring symptoms, such as depression and pain. In addition to pharmacologic interventions, a growing body of literature supports the efficacy of behavioral interventions, particularly CBT, mindfulness-based approaches, and exercise in alleviating multiple symptoms in cancer patients, including some trials of HCT [17]. Light therapy also may be efficacious in reducing co-occurring symptoms, including depression and fatigue, in HCT recipients [44,85,132]. Table 1 summarizes nonpharmacologic interventions that are effective across multiple symptoms and are referenced in the biobehavioral symptom sections in this review. We direct the reader to Bevans et al. [17] for a comprehensive review of the state of the science with respect to behavioral interventions targeting symptoms in HCT.
Table 1.
Cross-Cutting Nonpharmacologic Biobehavioral Interventions
| CBT [16,87,103] | CBT includes strategies to increase adaptive behavior and address unhelpful thought patterns. Exposure, cognitive restructuring, relaxation techniques, and coping skills training are common CBT-based interventions. CBT has a strong evidence base for depression and anxiety, and CBT-based interventions are effective for fatigue and pain. CBT for insomnia (CBT-I) is the gold standard treatment for insomnia. |
| Mindfulness-based interventions (MBIs) [16, 65,84,144] | MBIs increase adaptive behavior by cultivating awareness and acceptance of one’s current experience, including feelings, thoughts, and bodily sensations. Mindfulness-based stress reduction, yoga, meditation, and tai chi are MBIs that have been shown to be effective for fatigue, sleep disturbance, and depression. |
| Exercise [16, 68,84,86-89] | Exercise interventions include both aerobic and strength training approaches and have a growing evidence base for cancer-related fatigue as well as depression, anxiety, pain, and insomnia. The American College of Sports Medicine has developed materials to assist providers in prescribing exercise to cancer patients, available at https://www.exerciseismedicine.org/su.pport_page.php/moving-through-cancer/. |
| Light therapy [44,85,106,132] | Using a therapeutic light box is a known evidence-based approach to improve mood and regulate circadian rhythms. There is emerging evidence indicating benefits for sleep and fatigue in cancer patients. |
Innovative Biological Assessment Modalities
In addition to evaluating circulating cytokines and other peripheral indicators of inflammation, recent investigations have used new technologies to evaluate genomic, proteomic, and metabolomic drivers of inflammation relevant to HCT outcomes [133]. An additional biologic stress response of particular salience in biobehavioral physiology is the SNS/beta-adrenergic signaling pathway. The SNS regulates hematopoiesis, stem cell trafficking, and engraftment while also regulating multiple molecular processes that contribute to the initiation and progression of cancer [46]. In a recent randomized controlled trial (RCT) of multiple myeloma patients undergoing autologous HCT, the beta-adrenergic antagonist propranolol was effective at decreasing expression of the socioenvironmentally regulated CTRA profile [13,46]. This study demonstrates the feasibility and utility of both assessing genomic biobehavioral indicators and using targeted interventions for such.
MOVING BIOBEHAVIORAL AND CLINICAL CARE RESEARCH FORWARD
Although there is still much to learn about the biobehavioral science of symptoms in HCT, this paper details an already accumulating body of research. Patients cannot wait for the science to be fully developed before we apply what we know to improve their care. Similarly, current knowledge can clearly direct the next steps for research.
Clinical Implications
The biobehavioral complications of HCT are well defined and require standardized, routine implementation of regular screening and treatment that is integrated into care of HCT recipients from their initial evaluation for HCT throughout their long-term survivorship. Recommendations are outlined in Table 2. Given the predictable nature of these biobehavioral symptoms and related risk factors, as well as the current state of the science in evaluation and treatment, we recommend that guidelines specific to HCT recipients be developed for management of biobehavioral symptoms to ensure that this goal of routine, standardized care is accessible to all patients. Although these guidelines could be similar to or even developed from the American Society of Clinical Oncology or NCCN guidelines, the unique aspects of HCT suggest the need for modifications that identify specific time points for screening. In addition, treatment considerations need to address interactions that are of particular concern after HCT, an obvious example being GVHD.
Table 2.
Recommendations to Address Clinical Implications of Biobehavioral Complications
| Recommendation | Content Included | Timing |
|---|---|---|
| Develop guidelines for HCT symptoms screening and treatment | Identify risk factors for and prevalence of symptoms at different time points from pre-HCT to long-term survivorship. | Pre-HCT through long-term survivorship |
| Define screening recommendations using routine, standardized methods and measures. | Pre-HCT, at least monthly for the first 3 months post-HCT, then at 6 months, then annually thereafter | |
| Prepare care guidelines for managing symptoms with algorithms for increasing severity of symptoms. | As needed based on screening | |
| Identify treatment interactions with nonbiobehavioral complications and their therapy for providers. | Pre-HCT | |
| Standardize patient materials for education of all patients. | Pre-HCT, at post-HCT discharge from the transplant center, at 1 year post-HCT | |
| Identify roles of symptom specialists in the guidelines | Define when to refer to a specialist.
|
Pre-HCT through survivorship |
| Pre-HCT through long-term survivorship | ||
| Know barriers to care and strategies to overcome them | Prepare strategies to overcome disparities: lack of financial resources, health literacy issues, stigma about having “mental problems,” lack of social support to implement treatment recommendations at home, or concerns about racial and cultural differences between providers and patients. |
Pre-HCT through long-term survivorship |
Risk factors for biobehavioral symptoms can be used to create algorithms for screening while reducing patient and provider burden through targeting assessment to at-risk groups. The use of online screening tools would systematize scoring and flagging those with a need for further evaluation and intervention.
Biobehavioral interventions can be provided in a stepped-care approach, with greater specialization of interventions as symptoms become more severe or entrenched. Depending on the symptom and options for pharmacologic and nonpharmacologic treatments, algorithms can be defined for addressing patient needs.
In addition to targeting biobehavioral symptoms directly, bolstering resource and resilience factors that mitigate co-occurring symptoms may also be useful. For example, caregivers play a salient role in the HCT context, with patients who have a designated caregiver having better outcomes, including higher survival rates [134]. Interventions to improve caregiver well-being and reduce their burden merit further attention [135].
Although the biobehavioral pathways through which emotions and behaviors intersect and affect biological outcomes is still being investigated, the knowledge of screening tools and the many interventions aimed at improving these outcomes are ready for delivery as standardized HCT patient care.
Research Ready for Action
Much research activity is underway that demonstrates the potential for expanding the value of data from existing or new studies. For example, large datasets exist with both clinical and patient-reported outcome (PRO) data that can be used for secondary analyses to improve understanding of biobehavioral outcomes. Past examples include the numerous secondary analyses of the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) clinical trial 0902 [41,136-139]. Previous Center for International Blood and Marrow Transplantation Research (CIBMTR) studies have used biobehavioral variables in conjunction with existing biospecimens to evaluate mechanistic relationships for HCT clinical outcomes [130,133], with CIBMTR analyses currently underway using medical outcomes with newly acquired PROs and biospecimens [140]. Numerous secondary analyses also are using a combined dataset of 2 RCTs of long-term HCT survivors that have harmonized PRO and clinical data [141,142]. Such efforts are best supported with consistent measurement strategies. We specifically recommend the NIH-supported Patient Reported Outcomes Measurement Information System (PROMIS) measures as a unified and centralized bank of PROs that may be linked to clinical and biological data in HCT studies [143]. Several immediate next steps, as outlined in Table 3, can greatly extend the biobehavioral knowledge gain from secondary analyses of large cohort studies with clinical data, biospecimens, and/or PROs.
Table 3.
Recommendations to Address Biobehavioral Research Needs and Improve HCT Outcomes
| Recommendation | Sources and Additional Comments |
|---|---|
| Ready for action | |
| Promote use of secondary analyses for large cohort datasets | Establish centralized tracking system for locating and accessing data: |
| |
| |
| |
| Establish an HCT research community standard for symptom assessment and PRO | Harmonize measures using common data elements. Recommendation: NIH PROMIS |
| Harmonize timing of assessments. | |
| Extend assessment standards and add validated PRO to larger epidemiologic or clinical trials with longitudinal assessment regardless of targeted endpoints. | |
| Include patients and caregiver advocates on research teams. | |
| Identify studies with biospecimens and symptom measures | Identify and promote the use of biospecimens that can be assayed in conjunction with patient-reported outcomes or CTCAE measures. |
| In need of action | |
| Define standard procedures for data collection and storage | Procedures should permit interoperability across collection platforms and databases. |
| Establish a central repository for biospecimens | Requires agreement on:
|
| Biospecimens should be clinically annotated. | |
| Define a system to store and maintain centralized data | Requires structure for:
|
CTCAE indicates Common Terminology Criteria for Adverse Events.
An Integrated Vision for Biobehavioral Science in HCT
The steps outlined here will greatly extend our knowledge of biobehavioral pathways and outcomes during and after HCT. The 2020 BMT CTN State of the Science Late Effects, PRO, and Economics Committee provides an opportunity to propose biobehavioral studies that will be research priorities for the next 5 years. The biobehavioral research community should convene to agree on priority proposals for novel interventions and endpoints, as described in this article, along with the necessary clinical, PRO, and biological samples needed for those study endpoints.
Several achievements will facilitate accomplishing these visions. One underway is the integration of biobehavioral research into the existing infrastructures and organizations active in HCT research and clinical care. Another, which is more likely to follow with this integration, is improved funding opportunities for biobehavioral research. The past 5 years have seen major progress in the state of the science in biobehavioral research and the recognition of the importance of integrating that science into the medical components of patient care and research. This groundwork optimally positions us for further leaps in achieving this vision of a biobehavioral research future over the next 5 years, with resulting improved outcomes for patients.
ACKNOWLEDGMENTS
Authorship statement: E.S.C. and J.M.K. are co-senior authors. The authors have no financial or other conflicts of interest to disclose.
REFERENCES
- 1.Costanzo ES, Juckett MB, Coe CL. Biobehavioral influences on recovery following hematopoietic stem cell transplantation. Brain Behav Immun. 2013;30(suppl):S68–S74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knight JM, Lyness JM, Sahler OJZ, Liesveld JL, Moynihan JA. Psychosocial factors and hematopoietic stem cell transplantation: potential biobehavioral pathways. Psychoneuroendocrinology. 2013;38:2383–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cole SW. New challenges in psycho-oncology: neural regulation of the cancer genome. Psychooncology. 2018;27:2305–2309. [DOI] [PubMed] [Google Scholar]
- 4.Lynch Kelly D, Dickinson K, Hsiao CP, et al. Biological basis for the clustering of symptoms. Semin Oncol Nurs. 2016;32:351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang G, Orav EJ, McNamara T, Tong MY, Antin JH. Depression, cigarette smoking, and hematopoietic stem cell transplantation outcome. Cancer.. 2004;101:782–789. [DOI] [PubMed] [Google Scholar]
- 6.Akaho R, Sasaki T, Mori SI, et al. Psychological factors and survival after bone marrow transplantation in patients with leukemia. Psychiatry Clin Neurosci. 2003;57:91–96. [DOI] [PubMed] [Google Scholar]
- 7.Pillay B, Lee SJ, Katona L, Burney S, Avery S. Psychosocial factors predicting survival after allogeneic stem cell transplant. Support Care Cancer. 2014;22:2547–2555. [DOI] [PubMed] [Google Scholar]
- 8.Sato T, Konuma T, Oiwa-Monna M, et al. Does marital status affect the outcomes after allogeneic hematopoietic cell transplantation? Bone Marrow Transplant. 2018;53:774–779. [DOI] [PubMed] [Google Scholar]
- 9.Gregurek R, Labar B, Mrsić M, et al. Anxiety as a possible predictor of acute GVHD. Bone Marrow Transplant. 1996;18:585–589. [PubMed] [Google Scholar]
- 10.Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol. 2011;11:625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26:971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole SW, Nagaraja AS, Lutgendorf SK, Green PA, Sood AK. Sympathetic nervous system regulation of the tumour microenvironment. Nat Rev Cancer.. 2015;15:563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cole SW,, Sood AK. Molecular pathways: beta-adrenergic signaling in cancer. Clin Cancer Res. 2012;18:1201–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baker KS, Gurney JG, Ness KK, et al. Late effects in survivors of chronic myeloid leukemia treated with hematopoietic cell transplantation: results from the Bone Marrow Transplant Survivor Study. Blood. 2004;104:1898–1906. [DOI] [PubMed] [Google Scholar]
- 15.McQuellon RP, Duckworth KE, Campbell CR, et al. Fear of cancer recurrence, distress, depressive symptoms, and quality of life in hematopoietic stem cell transplantation patients. J Psychosoc Oncol Res Pract. 2019;1:e12. [Google Scholar]
- 16.Bevans M, El-Jawahri A, Tierney DK, et al. National Institutes of Health hematopoietic cell transplantation late effects initiative: the patient-centered outcomes working group report. Biol Blood Marrow Transplant.. 2017;23:538–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Giuseppe G, Thacker N, Schechter T, Pole JD. Anxiety, depression, and mental health-related quality of life in survivors of pediatric allogeneic hematopoietic stem cell transplantation: a systematic review. Bone Marrow Transplant. 2020;55:1240–1254. [DOI] [PubMed] [Google Scholar]
- 18.Packman W, Weber S, Wallace J, Bugescu N. Psychological effects of hematopoietic SCT on pediatric patients, siblings and parents: a review. Bone Marrow Transplant. 2010;45:1134–1146. [DOI] [PubMed] [Google Scholar]
- 19.Sun CL, Francisco L, Scott Baker K, Weisdorf DJ, Forman SJ, Bhatia S. Adverse psychological outcomes in long-term survivors of hematopoietic cell transplantation: a report from the Bone Marrow Transplant Survivor Study (BMTSS). Blood. 2011;118:4723–4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Jawahri A, Chen YB, Brazauskas R, et al. Impact of pre-transplant depression on outcomes of allogeneic and autologous hematopoietic stem cell transplantation. Cancer. 2017;123:1828–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barata A, Gonzalez BD, Sutton SK, et al. Coping strategies modify risk of depression associated with hematopoietic cell transplant symptomatology. J Health Psychol. 2018;23:1028–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knight JM, Moynihan JA, Lyness JM, et al. Peri-transplant psychosocial factors and neutrophil recovery following hematopoietic stem cell transplantation. PloS One.. 2014;9:e99778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGregor BA, Syrjala KL, Dolan ED, Langer SL, Redman M. The effect of pre-transplant distress on immune reconstitution among adult autologous hematopoietic cell transplantation patients. Brain Behav Immun. 2013;30(suppl):S142–S148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoodin F, Uberti JP, Lynch TJ, Steele P, Ratanatharathorn V. Do negative or positive emotions differentially impact mortality after adult stem cell transplant? Bone Marrow Transplant. 2006;38:255–264. [DOI] [PubMed] [Google Scholar]
- 25.Loberiza FR Jr, Rizzo JD, Bredeson CN, et al. Association of depressive syndrome and early deaths among patients after stem-cell transplantation for malignant diseases. J Clin Oncol. 2002;20:2118–2126. [DOI] [PubMed] [Google Scholar]
- 26.Amonoo HL, Massey CN, Freedman ME, et al. Psychological considerations in hematopoietic stem cell transplantation. Psychosomatics. 2019;60:331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee SJ, Loberiza FR, Rizzo JD, Soiffer RJ, Antin JH, Weeks JC. Optimistic expectations and survival after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2003;9:389–396. [DOI] [PubMed] [Google Scholar]
- 28.Köhler O, Benros ME, Nordentoft M, et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71:1381–1391. [DOI] [PubMed] [Google Scholar]
- 29.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hodes GE, Pfau ML, Leboeuf M, et al. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc Natl Acad Sci U S A.. 2014;111:16136–16141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khandaker GM, Pearson RM, Zammit S, Lewis G, Jones PB. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA Psychiatry. 2014;71:1121–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friebe A, Horn M, Schmidt F, et al. Dose-dependent development of depressive symptoms during adjuvant interferon-{alpha} treatment of patients with malignant melanoma. Psychosomatics. 2010;51:466–473. [DOI] [PubMed] [Google Scholar]
- 33.Davies KA, Cooper E, Voon V, Tibble J, Cercignani M, Harrison NA Interferon and anti-TNF therapies differentially modulate amygdala reactivity which predicts associated bidirectional changes in depressive symptoms [e-pub ahead of print]. Mol Psychiatry. doi: 10.1038/s41380-020-0790-9. Accessed March 28, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Obut F, Kasinath V, Abdi R. Post-bone marrow transplant thrombotic microangiopathy. Bone Marrow Transplant. 2016;51:891–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spitzer TR. Engraftment syndrome following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001;27:893–898. [DOI] [PubMed] [Google Scholar]
- 37.Roth WT, Doberenz S, Dietel A, et al. Sympathetic activation in broadly defined generalized anxiety disorder. J Psychiatr Res. 2008;42:205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cole SW. Human social genomics. PLoS Genet. 2014;10:e1004601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katayama Y, Battista M, Kao WM, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–421. [DOI] [PubMed] [Google Scholar]
- 40.Lucas D, Battista M, Shi PA, Isola L, Frenette PS. Mobilized hematopoietic stem cell yield depends on species-specific circadian timing. Cell Stem Cell. 2008;3:364–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knight JM, Syrjala KL, Majhail NS, et al. Patient-reported outcomes and socioeconomic status as predictors of clinical outcomes after hematopoietic stem cell transplantation: a study from the Blood and Marrow Transplant Clinical Trials Network 0902 trial. Biol Blood Marrow Transplant. 2016;22:2256–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cassileth BR, Vickers AJ, Magill LA. Music therapy for mood disturbance during hospitalization for autologous stem cell transplantation: a randomized controlled trial. Cancer. 2003;98:2723–2729. [DOI] [PubMed] [Google Scholar]
- 43.Lu DF,, Hart LK, Lutgendorf SK, Oh H, Silverman M. Effects of healing touch and relaxation therapy on adult patients undergoing hematopoietic stem cell transplant: a feasibility pilot study. Cancer Nurs. 2016;39: E1–E11. [DOI] [PubMed] [Google Scholar]
- 44.Valdimarsdottir HB, Figueiro MG, Holden W, et al. Programmed environmental illumination during autologous stem cell transplantation hospitalization for the treatment of multiple myeloma reduces severity of depression: a preliminary randomized controlled trial. Cancer Med. 2018;7:4345–4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.El-Jawahri A, Traeger L, Greer JA, et al. Effect of inpatient palliative care during hematopoietic stem-cell transplant on psychological distress 6 months after transplant: results of a randomized clinical trial. J Clin Oncol. 2017;35:3714–3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Knight JM, Rizzo JD, Hari P, et al. Propranolol inhibits molecular risk markers in HCT recipients: a phase 2 randomized controlled biomarker trial. Blood Adv. 2020;4:467–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williams ACC, Craig KD. Updating the definition of pain. Pain. 2016;157:2420–2423. [DOI] [PubMed] [Google Scholar]
- 48.O'Sullivan ML, Shelby RA, Dorfman CS, et al. The effect of pre-transplant pain and chronic disease self-efficacy on quality of life domains in the year following hematopoietic stem cell transplantation. Support Care Cancer. 2018;26:1243–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Atlas LY, al’Absi M. The neuroscience of pain: biobehavioral, developmental, and psychosocial mechanisms relevant to intervention targets. Psychosom Med. 2018;80:788–790. [DOI] [PubMed] [Google Scholar]
- 50.Matsuda M, Huh Y, Ji RR. Roles of inflammation, neurogenic inflammation, and neuroinflammation in pain. J Anesth. 2019;33:131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Windebank AJ, Grisold W. Chemotherapy-induced neuropathy. J Peripher Nerv Syst.. 2008;13:27–46. [DOI] [PubMed] [Google Scholar]
- 52.Doan L, Manders T, Wang J. Neuroplasticity underlying the comorbidity of pain and depression. Neural Plast. 2015;2015: 504691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chaudhry HM, Bruce AJ, Wolf RC, et al. The incidence and severity of oral mucositis among allogeneic hematopoietic stem cell transplantation patients: a systematic review. Biol Blood Marrow Transplant. 2016;22:605–616. [DOI] [PubMed] [Google Scholar]
- 54.Stiff P Mucositis associated with stem cell transplantation: current status and innovative approaches to management. Bone Marrow Transplant. 2001;27(suppl 2):S3–11. [DOI] [PubMed] [Google Scholar]
- 55.Bilic E, Delimar V, Desnica L, et al. High prevalence of small- and largefiber neuropathy in a prospective cohort of patients with moderate to severe chronic GvHD. Bone Marrow Transplant. 2016;51:1513–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kraus PD, Wolff D, Grauer O, et al. Muscle cramps and neuropathies in patients with allogeneic hematopoietic stem cell transplantation and graft-versus-host disease. PLoS One. 2012;7:e44922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Varma A, Loya J, Hussain MJ, et al. Calcineurin-inhibitor induced pain syndrome after stem cell transplant. Leuk Lymphoma. 2020;61:2230–2233. [DOI] [PubMed] [Google Scholar]
- 58.Prommer E Calcineurin-inhibitor pain syndrome. Clin J Pain. 2012;28:556–559. [DOI] [PubMed] [Google Scholar]
- 59.Li X, Brazauskas R, Wang Z, et al. Avascular necrosis of bone after allogeneic hematopoietic cell transplantation in children and adolescents. Biol Blood Marrow Transplant. 2014;20:587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barajas MR, McCullough KB, Merten JA, et al. Correlation of pain and fluoride concentration in allogeneic hematopoietic stem cell transplant recipients on voriconazole. Biol Blood Marrow Transplant. 2016;22:579–583. [DOI] [PubMed] [Google Scholar]
- 61.El-Cheikh J, Massoud R, Fares E, et al. Low-dose 5-azacytidine as preventive therapy for relapse of AML and MDS following allogeneic HCT. Bone Marrow Transplant. 2017;52:918–921. [DOI] [PubMed] [Google Scholar]
- 62.Astin JA, Shapiro SL, Eisenberg DM, Forys KL. Mind-body medicine: state of the science, implications for practice. J Am Board Fam Pract.. 2003;16:131–147. [DOI] [PubMed] [Google Scholar]
- 63.Somers TJ, Kelleher SA, Dorfman CS, et al. An mHealth pain coping skills training intervention for hematopoietic stem cell transplantation patients: development and pilot randomized controlled trial. JMIR Mhealth and Uhealth. 2018;6:e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zucchetti G, Candela F, Bottigelli C, et al. The power of Reiki: feasibility and efficacy of reducing pain in children with cancer undergoing hematopoietic stem cell transplantation. J Pediatr Oncol Nurs. 2019;36:361–368. [DOI] [PubMed] [Google Scholar]
- 65.Larson AG, Morris KJ, Juckett MB, et al. Mindfulness, experiential avoidance, and recovery from hematopoietic stem cell transplantation. Ann Behav Med. 2019;53:886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bates D, Bolwell B, Majhail NS, et al. Music therapy for symptom management after autologous stem cell transplantation: results from a randomized study. Biol Blood Marrow Transplant. 2017;23:1567–1572. [DOI] [PubMed] [Google Scholar]
- 67.Cohen MZ, Rozmus CL, Mendoza TR, et al. Symptoms and quality of life in diverse patients undergoing hematopoietic stem cell transplantation. J Pain Symptom Manage. 2012;44:168–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.National Comprehensive Cancer Network (NCCN). Exercising during cancer treament. Available at: https://www.nccn.org/patients/resources/life_with_cancer/exercise.aspxAccessed January 28, 2021.
- 69.Esser P, Kuba K, Mehnert A, et al. Investigating the temporal course, relevance and risk factors of fatigue over 5 years: a prospective study among patients receiving allogeneic HSCT. Bone Marrow Transplant. 2017;52:753–758. [DOI] [PubMed] [Google Scholar]
- 70.Jim HS, Sutton SK, Jacobsen PB, Martin PJ, Flowers ME, Lee SJ. Risk factors for depression and fatigue among survivors of hematopoietic cell transplantation. Cancer. 2016;122:1290–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smeland KB, Loge JH, Aass HCD, et al. Chronic fatigue is highly prevalent in survivors of autologous stem cell transplantation and associated with IL-6, neuroticism, cardiorespiratory fitness, and obesity. Bone Marrow Transplant. 2019;54:607–610. [DOI] [PubMed] [Google Scholar]
- 72.Wang XS, Shi Q, Shah ND, et al. Inflammatory markers and development of symptom burden in patients with multiple myeloma during autologous stem cell transplantation. Clin Cancer Res. 2014;20:1366–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang XS, Shi Q, Williams LA, et al. Serum interleukin-6 predicts the development of multiple symptoms at nadir of allogeneic hematopoietic stem cell transplantation. Cancer. 2008;113:2102–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bower JE. Cancer-related fatigue–mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014;11:597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chapotot F, Gronfier C, Jouny C, Muzet A, Brandenberger G. Cortisol secretion is related to electroencephalographic alertness in human subjects during daytime wakefulness. J Clin Endocrinol Metab. 1998;83:4263–4268. [DOI] [PubMed] [Google Scholar]
- 77.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids–new mechanisms for old drugs. N Engl J Med. 2005;353:1711–1723. [DOI] [PubMed] [Google Scholar]
- 78.Bower JE, Ganz PA, Irwin MR, Arevalo JMG, Cole SW. Fatigue and gene expression in human leukocytes: increased NF-κB and decreased glucocorticoid signaling in breast cancer survivors with persistent fatigue. Brain Behav Immun. 2011;25:147–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bower JE, Ganz PA, Aziz N, Olmstead R, Irwin MR, Cole SW. Inflammatory responses to psychological stress in fatigued breast cancer survivors: relationship to glucocorticoids. Brain Behav Immun. 2007;21:251–258. [DOI] [PubMed] [Google Scholar]
- 80.Bower JE, Ganz PA, Dickerson SS, Petersen L, Aziz N, Fahey JL. Diurnal cortisol rhythm and fatigue in breast cancer survivors. Psychoneuroendocrinology. 2005;30:92–100. [DOI] [PubMed] [Google Scholar]
- 81.Schmidt ME, Semik J, Haberman N, Wiskemann J, Ulrich CM, Steindorf K. Cancer-related fatigue shows a stable association with diurnal cortisol dysregulation in breast cancer patients. Brain Behav Immun. 2016;52:98–105. [DOI] [PubMed] [Google Scholar]
- 82.Jim HSL, Evans B, Jeong JM, et al. Sleep disruption in hematopoietic cell transplantation recipients: prevalence, severity, and clinical management. Biol Blood Marrow Transplant. 2014;20:1465–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tomlinson D, Robinson PD, Oberoi S, et al. Pharmacologic interventions for fatigue in cancer and transplantation: a meta-analysis. Curr Oncol. 2018;25:e152–e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Duong N, Davis H, Robinson PD, et al. Mind and body practices for fatigue reduction in patients with cancer and hematopoietic stem cell transplant recipients: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2017;120:210–216. [DOI] [PubMed] [Google Scholar]
- 85.Johnson JA, Garland SN, Carlson LE, et al. Bright light therapy improves cancer-related fatigue in cancer survivors: a randomized controlled trial. J Cancer Surviv. 2018;12:206–215. [DOI] [PubMed] [Google Scholar]
- 86.Baumann FT, Zopf EM, Nykamp E, et al. Physical activity for patients undergoing an allogeneic hematopoietic stem cell transplantation: benefits of a moderate exercise intervention. Eur J Haematol. 2011;87:148–156. [DOI] [PubMed] [Google Scholar]
- 87.Jacobsen PB, Le-Rademacher J, Jim H, et al. Exercise and stress management training prior to hematopoietic cell transplantation: Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0902. Biol Blood Marrow Transplant. 2014;20:1530–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Oberoi S, Robinson PD, Cataudella D, et al. Physical activity reduces fatigue in patients with cancer and hematopoietic stem cell transplant recipients: a systematic review and meta-analysis of randomized trials. Crit Rev Oncol Hematol. 2018;122:52–59. [DOI] [PubMed] [Google Scholar]
- 89.Wiskemann J, Dreger P, Schwerdtfeger R, et al. Effects of a partly self-administered exercise program before, during, and after allogeneic stem cell transplantation. Blood. 2011;117:2604–2613. [DOI] [PubMed] [Google Scholar]
- 90.Hacker ED, Kim I, Park C, Peters T. Real-time fatigue and free-living physical activity in hematopoietic stem cell transplantation cancer survivors and healthy controls: a preliminary examination of the temporal, dynamic relationship. Cancer Nurs. 2017;40:259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Barata A, et al. Depression and anxiety in patients before allogeneic hematopoietic cell transplantation. Presented at: 39th Annual Meeting of the European Group for Blood and Marrow Transplantation. London, UK;2013. April7–10. [Google Scholar]
- 92.Badia P, Hickey V, Flesch L, et al. Quality improvement initiative to reduce nighttime noise in a transplantation and cellular therapy unit. Biol Blood Marrow Transplant. 2019;25:1844–1850. [DOI] [PubMed] [Google Scholar]
- 93.Boonstra L, Harden K, Jarvis S, et al. Sleep disturbance in hospitalized recipients of stem cell transplantation. Clin J Oncol Nurs. 2011;15:271–276. [DOI] [PubMed] [Google Scholar]
- 94.Nelson AM, Coe CL, Juckett MB, et al. Sleep quality following hematopoietic stem cell transplantation: longitudinal trajectories and biobehavioral correlates. Bone Marrow Transplant. 2014;49:1405–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Faulhaber GA, Furlanetto TW, Astigarraga CC, Moser Filho HL, Paludo AP, Silla LMR. Association of busulfan and cyclophosphamide conditioning with sleep disorders after hematopoietic stem cell transplantation. Acta Haematol. 2010;124:125–128. [DOI] [PubMed] [Google Scholar]
- 96.D'Souza A, Millard H, Knight J, et al. Prevalence of self-reported sleep dysfunction before allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2018;53:1079–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nelson AM, Jim HSL, Small BJ, et al. Sleep disruption among cancer patients following autologous hematopoietic cell transplantation. Bone Marrow Transplant. 2018;53:307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Atzpodien J, Küchler T, Wandert T, Reitz M. Rapid deterioration in quality of life during interleukin-2- and alpha-interferon-based home therapy of renal cell carcinoma is associated with a good outcome. Br J Cancer. 2003;89:50–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Irwin MR. Depression and insomnia in cancer: prevalence, risk factors, and effects on cancer outcomes. Curr Psychiatry Rep. 2013;15:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.González-Mercado VJ,, Henderson WA, Sarkar A, et al. Changes in gut microbiome associated with co-occurring symptoms development during chemo-radiation for rectal cancer: a proof of concept study. Biol Res Nurs. 2021;23:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ghazikhanian SE, Dorfman CS, Somers TJ, et al. Cognitive problems following hematopoietic stem cell transplant: relationships with sleep, depression and fatigue. Bone Marrow Transplant. 2017;52:279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rentscher KE, Carroll JE, Juckett MB, et al. Sleep disruption, fatigue, and depression as predictors of 6-year clinical outcomes following allogeneic hematopoietic cell transplantation [e-pub ahead of print]. J Natl Cancer Inst. doi: 10.1093/jnci/djab032, accessed April 1, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Johnson JA, Rash JA, Campbell TS, et al. A systematic review and meta-analysis of randomized controlled trials of cognitive behavior therapy for insomnia (CBT-I) in cancer survivors. Sleep Med Rev. 2016;27:20–28. [DOI] [PubMed] [Google Scholar]
- 104.Kolla BP, Lovely JK, Mansukhani MP, Morgenthaler TI. Zolpidem is independently associated with increased risk of inpatient falls. J Hosp Med. 2013;8:1–6. [DOI] [PubMed] [Google Scholar]
- 105.Hoogland AI, Bulls HW, Gonzalez BD, et al. Circadian rhythmicity as a predictor of quality of life in allogeneic hematopoietic cell transplant patients. J Pain Symptom Manage. 2019;57:952–960.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wu LM, Amidi A, Valdimarsdottir H, et al. The effect of systematic light exposure on sleep in a mixed group of fatigued cancer survivors. J Clin Sleep Med. 2018;14:31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Burns LJ, Abbetti B, Arnold SD, et al. Engaging patients in setting a patient-centered outcomes research agenda in hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2018;24:1111–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Thygesen KH, Schjødt I, Jarden M. The impact of hematopoietic stem cell transplantation on sexuality: a systematic review of the literature. Bone Marrow Transplant.. 2012;47:716–724. [DOI] [PubMed] [Google Scholar]
- 109.Syrjala KL, Kurland BF, Abrams JR, Sanders JE, Heiman JR. Sexual function changes during the 5 years after high-dose treatment and hematopoietic cell transplantation for malignancy, with case-matched controls at 5 years. Blood. 2008;111:989–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li Z, Mewawalla P, Stratton P, et al. Sexual health in hematopoietic stem cell transplant recipients. Cancer. 2015;121:4124–4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yi JC, Syrjala KL. Sexuality after hematopoietic stem cell transplantation. Cancer J. 2009;15:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.El-Jawahri A, Fishman SR, Vanderklish J, et al. Pilot study of a multimodal intervention to enhance sexual function in survivors of hematopoietic stem cell transplantation. Cancer. 2018;124:2438–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Flynn KE, Lin L, Cyranowski JM, et al. Development of the NIH PROMIS® Sexual Function and Satisfaction Measures in patients with cancer. J Sex Med.. 2013;10(suppl 1):43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kelly DL, Buchbinder D, Duarte RF, et al. Neurocognitive dysfunction in hematopoietic cell transplant recipients: expert review from the Late Effects and Quality of Life Working Committee of the Center for International Blood and Marrow Transplant Research and Complications and Quality of Life Working Party of the European Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2018;24:228–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Harder H, Duivenvoorden HJ, van Gool AR, Cornelissen JJ, van den Bent MJ. Neurocognitive functions and quality of life in haematological patients receiving haematopoietic stem cell grafts: a one-year follow-up pilot study. J Clin Exp Neuropsychol. 2006;28:283–293. [DOI] [PubMed] [Google Scholar]
- 116.Syrjala KL, Artherholt SB, Kurland BF, et al. Prospective neurocognitive function over 5 years after allogeneic hematopoietic cell transplantation for cancer survivors compared with matched controls at 5 years. J Clin Oncol. 2011;29:2397–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Syrjala KL, Dikmen S, Langer SL, Roth-Roemer S, Abrams JR. Neuropsychologic changes from before transplantation to 1 year in patients receiving myeloablative allogeneic hematopoietic cell transplant. Blood. 2004;104:3386–3392. [DOI] [PubMed] [Google Scholar]
- 118.Booth-Jones M, Jacobsen PB, Ransom S, Soety E. Characteristics and correlates of cognitive functioning following bone marrow transplantation. Bone Marrow Transplant. 2005;36:695–702. [DOI] [PubMed] [Google Scholar]
- 119.Harder H, Cornelissen JJ, Van Gool AR, Duivenvoorden HJ, Eijkenboom WMH, van den Bent MJ. Cognitive functioning and quality of life in long-term adult survivors of bone marrow transplantation. Cancer. 2002;95:183–192. [DOI] [PubMed] [Google Scholar]
- 120.Lyon DE, Cohen R, Chen H, et al. Relationship of systemic cytokine concentrations to cognitive function over two years in women with early stage breast cancer. J Neuroimmunol.. 2016;301:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hoogland AI, Nelson AM, Gonzalez BD, et al. Worsening cognitive performance is associated with increases in systemic inflammation following hematopoietic cell transplantation. Brain Behav Immun. 2019;80:308–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Correa DD, Wang Y, West JD, et al. Prospective assessment of white matter integrity in adult stem cell transplant recipients. Brain Imaging Behav. 2016;10:486–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sharafeldin N, Bosworth A, Chen Y, et al. Single nucleotide polymorphisms (SNPs) associated with cognitive impairment in patients treated with hematopoietic cell transplantation (HCT): a longitudinal study. Blood. 2016;128:824.. –824. [Google Scholar]
- 124.Harder H, Van Gool AR, Duivenvoorden HJ, et al. Case-referent comparison of cognitive functions in patients receiving haematopoietic stem-cell transplantation for haematological malignancies: two-year follow-up results. Eur J Cancer.. 2007;43:2052–2059. [DOI] [PubMed] [Google Scholar]
- 125.Sharafeldin N, Bosworth A, Patel SK, et al. Cognitive functioning after hematopoietic cell transplantation for hematologic malignancy: results from a prospective longitudinal study. J Clin Oncol. 2018;36:463–475. [DOI] [PubMed] [Google Scholar]
- 126.Nawas MT, Andreadis C, Martin TG, et al. Limitation in patient-reported function is associated with inferior survival in older adults undergoing autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2019;25:1218–1224. [DOI] [PubMed] [Google Scholar]
- 127.Baker KS, Davies SM, Majhail NS, et al. Race and socioeconomic status influence outcomes of unrelated donor hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2009;15:1543–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Majhail NS, Nayyar S, Burton Santibañez ME, Murphy EA, Denzen EM. Racial disparities in hematopoietic cell transplantation in the United States. Bone Marrow Transplant. 2012;;47:1385–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cole SW. The conserved transcriptional response to adversity. Curr Opin Behav Sci. 2019;28:31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Knight JM, Rizzo JD, Logan BR, et al. Low socioeconomic status, adverse gene expression profiles, and clinical outcomes in hematopoietic stem cell transplant recipients. Clin Cancer Res. 2016;22:69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lynch Kelly D, Lyon DE, Ameringer SA, Elswick RK. Symptoms, cytokines, and quality of life in patients diagnosed with chronic graft-versus-host disease following allogeneic hematopoietic stem cell transplantation. Oncol Nurs Forum. 2015;42:265–275. [DOI] [PubMed] [Google Scholar]
- 132.Redd WH, Valdimarsdottir H, Wu LM, et al. Systematic light exposure in the treatment of cancer-related fatigue: a preliminary study. Psychooncology. 2014;23:1431–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Knight JM, Rizzo JD, Wang T, et al. Molecular correlates of socioeconomic status and clinical outcomes following hematopoietic cell transplantation for leukemia. JNCI Cancer Spectr. 2019;3:pkz073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Foster LW, McLellan LJ, Rybicki LA, Sassano DA, Hsu A, Bolwell BJ. Survival of patients who have undergone allogeneic bone marrow transplantation: the relative importance of in-hospital lay care-partner support. J Psychosoc Oncol. 2008;;22:1–20. [Google Scholar]
- 135.El-Jawahri A, Jacobs JM, Nelson AM, et al. Multimodal psychosocial intervention for family caregivers of patients undergoing hematopoietic stem cell transplantation: a randomized clinical trial. Cancer. 2020;126:1758–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jim HSL, Sutton S, Majhail NS, et al. Severity, course, and predictors of sleep disruption following hematopoietic cell transplantation: a secondary data analysis from the BMT CTN 0902 trial. Bone Marrow Transplant. 2018;53:1038–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Syrjala KL, Sutton SK, Jim HSL, et al. Cancer and treatment distress psychometric evaluation over time: A BMT CTN 0902 secondary analysis. Cancer. 2017;123:1416–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wingard JR, Wood WA, Martens M, et al. Pretransplantation exercise and hematopoietic cell transplantation survival: a secondary analysis of Blood and Marrow Transplant Clinical Trials Network (BMT CTN 0902). Biol Blood Marrow Transplant. 2017;23:161–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wood WA, Le-Rademacher J, Syrjala KL, et al. Patient-reported physical functioning predicts the success of hematopoietic cell transplantation (BMT CTN 0902). Cancer.2016;122:91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shaw BE, Brazauskas R, Millard HR, et al. Centralized patient-reported outcome data collection in transplantation is feasible and clinically meaningful. Cancer. 2017;123:4687–4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Majhail NS, Murphy E, Laud P, et al. Randomized controlled trial of individualized treatment summary and survivorship care plans for hematopoietic cell transplantation survivors. Haematologica.. 2019;104:1084–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Syrjala KL, Murphy E, Laud P, et al. Randomized controlled trial of individualized treatment summary and survivorship care plans for hematopoietic cell transplantation survivors. Haematologica.. 2018;12:560–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shaw BE, Lee SJ, Horowitz MM, Wood WA, Rizzo JD, Flynn KE. Can we agree on patient-reported outcome measures for assessing hematopoietic cell transplantation patients? A study from the CIBMTR and BMT CTN. Bone Marrow Transplant. 2016;51:1173–1179. [DOI] [PubMed] [Google Scholar]
- 144.Bauer-Wu S, Sullivan AM, Rosenbaum E, et al. Facing the challenges of hematopoietic stem cell transplantation with mindfulness meditation: a pilot study. Integr Cancer Ther.. 2008;7:62–69. [DOI] [PubMed] [Google Scholar]