Abstract
Previous studies of the retrosplenial cortex (RSC) have focused on its role in navigation and memory, consistent with its well-established medial temporal connections, but recent evidence also suggests a role for this region in reward and decision-making. Because function is determined largely by anatomical connections, and to better understand the anatomy of RSC, we used tract-tracing methods to examine the anatomical connectivity between the rat RSC and frontostriatal networks (canonical reward and decision-making circuits). We find that, among frontal cortical regions, RSC bidirectionally connects most strongly with the anterior cingulate cortex, but also with an area of the central-medial orbitofrontal cortex. RSC projects to the dorsomedial striatum, and its terminal fields are virtually encompassed by the frontal-striatal projection zone, suggestive of functional convergence through the basal ganglia. This overlap is driven by anterior cingulate cortex, prelimbic cortex, and orbitofrontal cortex, all of which contribute to goal-directed decision-making, suggesting that the RSC is involved in similar processes.
Keywords: striatum, retrosplenial cortex, tract-tracing, default mode network, posteromedial cortex
Introduction
The retrosplenial cortex (RSC) in rodents occupies a large territory in the posterior medial part of the cerebral cortex. Its strong connections with medial temporal lobe areas are well-established (Sugar et al., 2011; van Groen & Wyss, 1990; van Groen & Wyss, 1992; van Groen & Wyss, 2003). The prominence of these connections has motivated theorizing on RSC function, which has, not surprisingly, focused on spatial navigation and memory (Valenstein et al., 1987; Cho & Sharp, 2001; Maguire, 2001; Harker & Whishaw, 2004; Maviel et al., 2004; Parron & Save, 2004; Keene & Bucci, 2008, 2009; Vann et al., 2009; Auger et al., 2012; Auger & Maguire, 2013; Cooper & Mizumori, 1999; Kapoodvand et al., 2018). For example, lesions in the RSC lead to deficits in performance on a water maze test (Sutherland, Wishaw, & Kolb, 1988) and to impairments in allocentric spatial memory (Pothuizen et al., 2008; Vann & Aggleton, 2002, 2004).
However, RSC likely has a broader and more integrative function than is currently appreciated. In particular, there is compelling evidence that RSC may play a crucial role in reward-guided decision-making (Hattori et al., 2019; Nelson et al., 2014; Tabuchi et al., 2005; Vedder et al., 2016; Powell et al., 2017). For example, neural population activity in the RSC encodes a persistent value, with flexible reward history encoding (Hattori et al., 2019). In addition, the RSC plays a central albeit poorly delineated role in the default mode network (DMN), a network of functionally connected brain regions associated with high activity at rest, self-projection, episodic memory, and reward monitoring (Raichle et al., 2001; Shulman et al., 1997) which has been found in humans (Raichle et al., 2001), nonhuman primates (Mantini et al., 2011), and rodents (Lu et al., 2012; Upadhyay et al., 2011). Understanding the role of RSC is particularly important because it is likely to become a major research focus in the coming years: it reaches the dorsal surface of the brain, and therefore is readily optically accessible to studies of neural activity using calcium imaging. Future work aimed at fully understanding the functions of RSC must take into account its roles in reward, decision-making, and DMN processes. However, our ability to do so is limited by a lack of integrative knowledge of the anatomical connections of the RSC.
Most studies of reward-guided decision-making implicate frontal cortico-striatal circuits (Balleine, Delgado, & Hikosaka, 2007; Burton, Nakamura, & Roesch, 2015; Gremel et al., 2016; Haber & Behrens, 2014; O’Doherty et al., 2004; Rothwell et al., 2015; Sleezer, Castagno, & Hayden, 2016; Yin, Knowlton, & Balleine, 2006; Fitoussi et al., 2018). The functions of these regions and projections are very well-studied using diverse tools, including lesions, optogenetics, electrophysiology, and pharmacology. Distinct striatal zones and their unique afferents have been implicated in different components of reward-guided decision-making. Broadly speaking, there is consensus that the ventral striatum, dorsomedial striatum, and dorsolateral striatum, along with their frontal cortical afferents, have different roles in reward-guided decision-making (Hassani, Cromwell, & Schultz, 2001; Kimchi & Laubach, 2009; Li et al., 2016; O’Hare et al., 2016; Voorn et al., 2004; Yin & Knowlton, 2004; Yin et al., 2005). One major hypothesis is that the dorsolateral striatum is involved with habits, the dorsomedial striatum is involved in goal-directed decision-making, and the ventral striatum is involved in establishing stimulus-value associations.
One way to understand the function of the RSC in reward-guided decision-making is to analyze its anatomical connections with this canonical, frontal cortico-striatal decision-making circuitry. Furthermore, the major subregions of the DMN are the RSC and portions of the frontal cortex, meaning the same cortical circuitry gives us insight into both reward-guided decision-making and DMN function. Previous studies have demonstrated that there are significant projections between the RSC and the frontal cortex and striatum in rats (Shibata, Kondo, & Naito, 2004; Shibata & Naito, 2008; van Groen & Wyss, 1990; van Groen & Wyss, 1992; van Groen & Wyss, 2003). However, the projections to the striatum in particular have not been analyzed in detail, especially in light of our current understanding of how different zones of the striatum contribute to reward-guided decision-making. Thus, in this paper, we used anatomical tract-tracing methods in rats to analyze the connections of the RSC with the striatum and frontal cortex.
Materials & Methods
Overview.
Connectivity of the RSC with the striatum and frontal cortical subregions was analyzed using anatomical tract-tracing in rats. Following surgery and perfusion, immunohistochemistry was performed and brain slices were mounted on slides. Labeling in the striatum (anterograde) and frontal cortex (anterograde and retrograde) were charted using light microscopy. Cases were registered to a standard brain for comparison and visualization.
Surgery and tissue preparation.
All procedures were approved by the Institutional Animal Care and Use Committee at the University of Minnesota. Six adult male and 5 adult female Sprague Dawley rats (weight 230–500g, Charles River) were used for these studies.
At the start of surgery, animals were anesthetized using a combination of ketamine (40–90mg/kg, IP) and xylazine (5–10mg/kg, IP). In addition, a non-steroidal anti-inflammatory drug (Carprofen, 5mg/kg, IP or SQ) and an antibiotic (Baytril, 2.5mg/kg, SQ) were administered. Saline injections were given periodically to maintain hydration. Animals were placed in a stereotaxic frame on top of a heat source. Temperature and foot withdrawal reflex were monitored throughout to ensure stable anesthesia. Lidocaine was administered at the incision site. The skull was exposed, then burr holes were made to expose the surface of the brain. The bidirectional tract-tracer fluororuby (FR, 40–50nl, 10% in 0.1M phosphate buffer, pH 7.4, Invitrogen) was injected over 10 min using a 0.5 ul Hamilton syringe. After each injection the syringe remained in situ for 10–20 minutes. The syringe was removed, and the incision was closed with sutures. Atipamezole (.1–1.0mg/kg, IP) was used to reverse the ketamine/xylazine combination. Post-operatively, Carprofen (5mg/kg, SQ) was delivered every 24 hours for 72 hours total. Targets were chosen using (Paxinos & Watson, 2014).
Animals were euthanized 10–14 days following injection with an IP injection of a lethal dose of commercial euthanasia solution (Euthasol, 0.22ml/kg), and transcardially perfused with saline followed by 4% paraformaldehyde. Brains were extracted, postfixed overnight, and cryoprotected in increasing gradients of sucrose (10%, 20%, and 30%). Serial sections of 50um were cut on a freezing microtome into cryoprotectant solution. Serial sections of 50μm were cut on a freezing microtome. One in six sections was processed free-floating for immunocytochemistry to visualize the tracer. Tissue was incubated in primary anti-FR (1:6000; Invitrogen) in 10% NGS and 0.3% Triton X-100 (Sigma-Aldrich) in PO4 for 4 nights at 4°C. After rinsing, the tissue was incubated in biotinylated secondary antibody followed by incubation with the avidin-biotin complex solution (Vectastain ABC kit, Vector Laboratories). Immunoreactivity was then visualized using standard DAB procedures. Staining was intensified by incubating the tissue for 5–15 s in a solution of 0.05% DAB tetrahydrochloride, 0.025% cobalt chloride, 0.02% nickel ammonium sulfate, and 0.01% H2O2. Sections were mounted by hand onto gel-coated slides, dehydrated, defatted in xylene, and coverslipped with Permount.
Microscopy and analysis.
Microscopy was performed using a Zeiss AxioImager M2 microscope. Three representative cases were chosen for in-depth analysis and 3D rendering on the basis of their outstanding transport and lack of contamination. Using darkfield light microscopy, brain sections and structures, the injection site, and cortical and striatal terminal fields were outlined under a 2.0, 4.0, or 10X objective with Neurolucida software (MBF Bioscience). Terminal fields were considered dense when they could be visualized at a low objective (2.0X; criterion as previously utilized in (Haber et al., 2006; Haynes & Haber, 2013; Heilbronner & Haber, 2014; Heilbronner, et al., 2018; Mailly et al., 2013). We distinguished between likely terminal fields (thin, labeled fibers containing boutons) and passing fibers (thick fibers without clear boutons). In addition, using brightfield light microscopy, retrogradely labeled cells (all within the target region) were counted using StereoInvestigator software (MBF Bioscience) under a 20X objective. This stereology software was utilized to ensure that every part of a region received equal attention and to avoid biases associated with dark staining and cell clusters. We analyzed one out of every six sections, and density was defined by total number of cells normalized by brain region size, with size defined by the sum of the areas of the brain regions across the one in six sections, rather than by volume.
For each case, stacks of coronal sections were created from chartings and scanned slides. These stacks were imported into IMOD software (Boulder Laboratory for 3D Electron Microscopy) (Kremer, Mastronarde, & McIntosh, 1996) and combined, and thus a 3D reconstruction that contained the injection sites, terminal fields, and labeled cells was created for each case separately. To merge multiple cases together, individual cases were registered in IMOD to standard rat MRI images that had been converted to IMOD files (Wisner et al., 2016). Registrations were manually checked and adjusted according to cortical and striatal landmarks.
We wanted to compare the RSC-striatal projection zone to previously established frontal-striatal projection zones. Projection zones for areas CG (anterior cingulate cortex), MO (medial orbitofrontal cortex), VOLO (ventral and lateral orbitofrontal cortex), and IL (infralimbic cortex) were drawn from Heilbronner et al. 2016. In that paper, we combined prior maps of cortico-striatal projection fields, with a focus on the projections at AP +1.1, where the frontal terminal fields appeared to be maximally segregated. The database consisted of 4 cases from IL, 10 from PL, 5 from CG, 3 from MO, and 9 from VOLO. To this database, we added projections from region M2 (3 cases) as depicted in (Reep & Corwin, 1999). Overlaps in terminal field areas at three coronal A-P positions were calculated by converting the pixels of the models in IMOD to a polygon and finding the area of the intersect using custom MatLab code.
Results
The cases included in these analyses had the bidirectional tracer fluororuby injected into the RSC. Resulting injection sites were confined to the RSC, and together covered most of its rostral-caudal length (collectively the cores of the injection sites ranged from −4.8 to −8.8 AP). We first analyzed the projections from the RSC to the striatum. Like most cortical areas, the retrosplenial cortex projects to both ipsilateral and contralateral striatum with predominance of the former (McGeorge & Faull, 1989). Therefore, we analyzed ipsilateral striatal projections, but there were similarly situated, less dense, contralateral terminal fields as well. Cases were extremely similar in their striatal projections, and thus are described here together. Fibers could be visualized exiting the injection site and traveling ventrally and rostrally toward the dorsal striatum through the cingulum bundle and the white matter directly adjacent to it. These fibers formed longitudinal bands of terminal fields that extended for nearly the entire rostral-caudal distance of the ipsilateral caudoputamen. At the most caudal levels of the caudoputamen, terminal fields were present only dorsally. Rostrally, where the caudoputamen widens in the medial-lateral dimension, terminal fields were restricted to the dorsomedial component. They frequently reached the dorsomedial boundary between the caudoputamen and the lateral ventricle (Figure 1). In all cases, both dense and diffuse projections could be observed. The dense projections were the most consistently dorsomedial, while the lighter projections extended further ventrally and laterally. However, even diffuse projections did not extend into the nucleus accumbens.
Figure 1. RSC-striatal terminal fields.
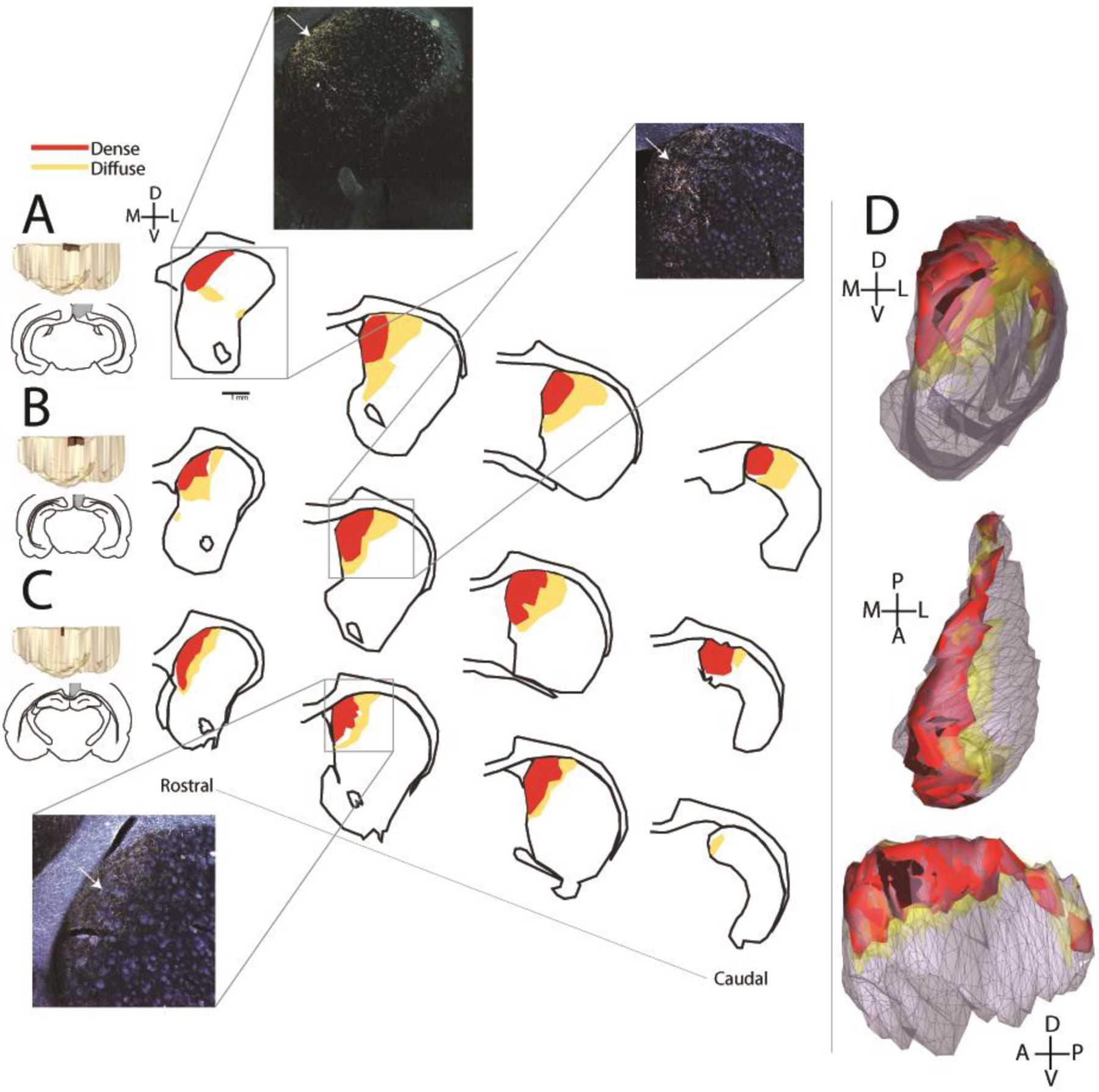
Images at far left show 3D models and outlines for the three injection sites (A-C). The coordinates of the spread of the injection sites in the anterior/ posterior direction are A. −4.8 to −8.6 AP B. −4.8 to −8.8 AP and C. −5.0 to −6.0 AP. Drawings show coronal striatal sections with terminal fields from rostral to caudal striatum. Photomicrographs show coronal darkfield images of ipsilateral striatal terminal fields. White arrows point to dense terminal fields. D. Collective model of the striatal projection zone of the RSC from coronal (top), horizontal (middle), and sagittal (bottom) views, all cases combined. Scale bar=1mm.
Next, we examined connections between RSC and different regions within the frontal cortex (Figure 2). Although there were some quantitative differences between the cases (Figure 2D), qualitatively we observed consistent bidirectional connections primarily with the dorsal anterior cingulate areas (CG1, CG2, and A33) and a specific location within the central-medial orbitofrontal cortex that sits at the border between VO and MO. Much weaker, but present, connections were observed with infralimbic cortex, prelimbic cortex, M2, and M1. Virtually no connectivity was observed with lateral orbitofrontal cortex and frontal insular regions. Thus, although there is a consistent patch of connectivity in the central-medial orbitofrontal cortex, the medial frontal cortex has, overall, much stronger connections with the RSC than the orbitofrontal cortex does. In addition, in both the medial and orbitofrontal cortices, RSC connectivity is with the central portions, rather than the ventral/dorsal or medial/lateral edges.
Figure 2. RSC-frontal projections.
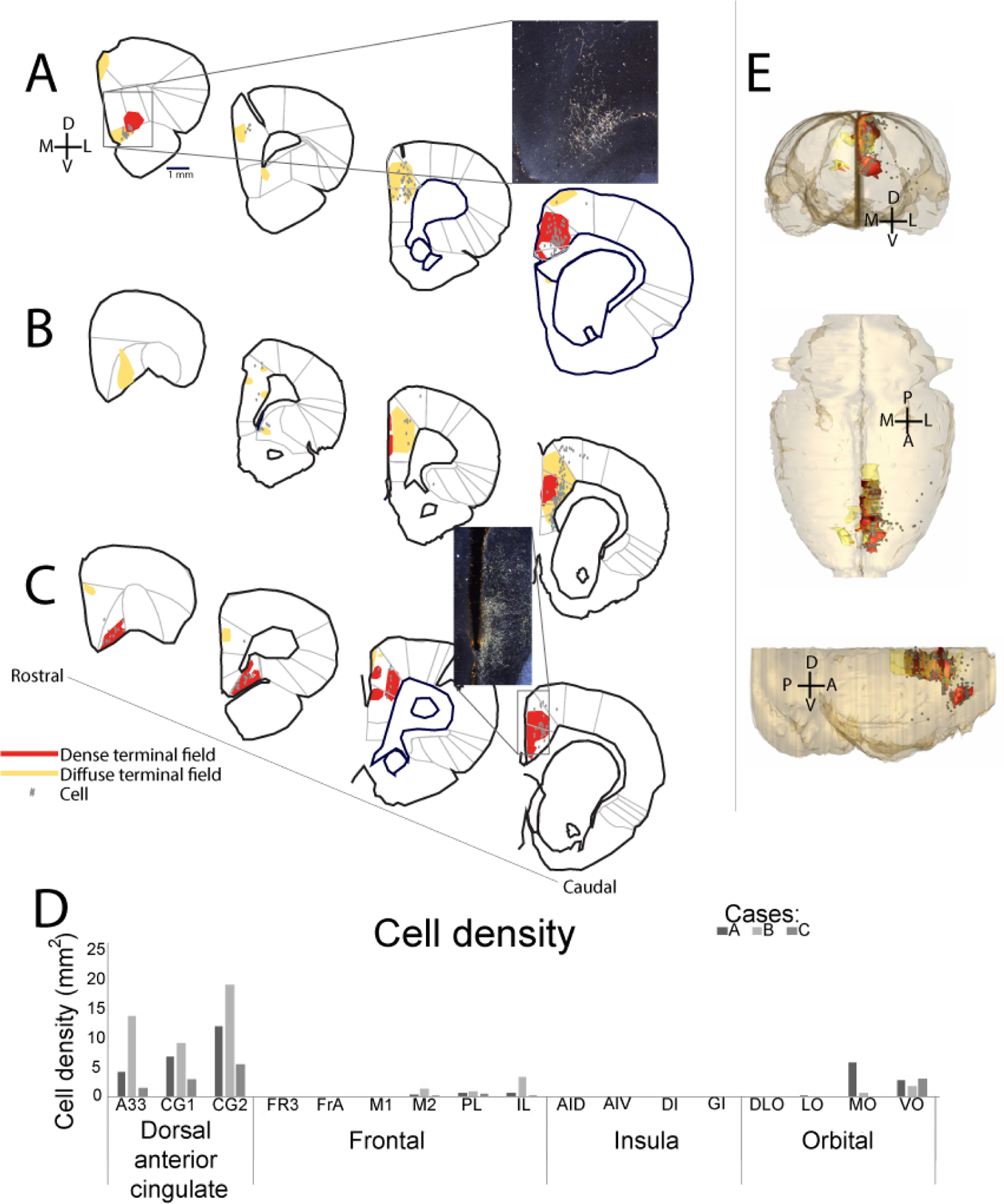
Terminal fields and labeled cells following injections into the RSC. A-C. Drawings of frontal connections (red = dense terminal fields; yellow=diffuse terminal fields; gray dots = cells; all shown for each injection site). (Each case is a different injection site, matched to Figure 1). D. Cell density from each case in frontal regions. Results are shown for each case (A-C, as above). E. Collective model of the frontal projections of the RSC from coronal (top), horizontal (middle), and sagittal (bottom) views, all cases combined. Scale bar=1mm.
We then analyzed the overlap between the striatal terminal fields from the RSC and the striatal projection zones of various frontal regions, as previously defined (Reep and Corwin 1999; Heilbronner et al., 2016) (Figure 3). At all A-P levels examined, the RSC does not occupy striatal territory isolated from frontal projections. Instead, large portions of the RSC projection zone overlap with terminal fields from frontal cortical regions (at A-P +2.3, >90%; at A-P +1.1 >95%; at A-P −0.1, >98%). At A-P +1.1, the database of frontal terminal fields is greatest, and the projection zones from these regions are maximally segregated, so we will consider this topography in detail here (although note other levels diagrammed in Figure 3). The greatest percentage of overlap is with central-medial prefrontal regions (prelimbic and anterior cingulate cortices) and central and lateral orbitofrontal regions. Smaller zones of overlap are observed between the RSC projection zones and those from M2 and medial orbitofrontal cortex. At this level and all levels, there is no overlap between the RSC projection zone and the infralimbic projection zone. Finally, although the RSC-striatal projection is not isolated from frontal-striatal projections, no frontal-striatal projection zone is as uniquely confined to the dorsomedial caudoputamen as the RSC terminal fields are.
Figure 3. Cortico-striatal overlap.
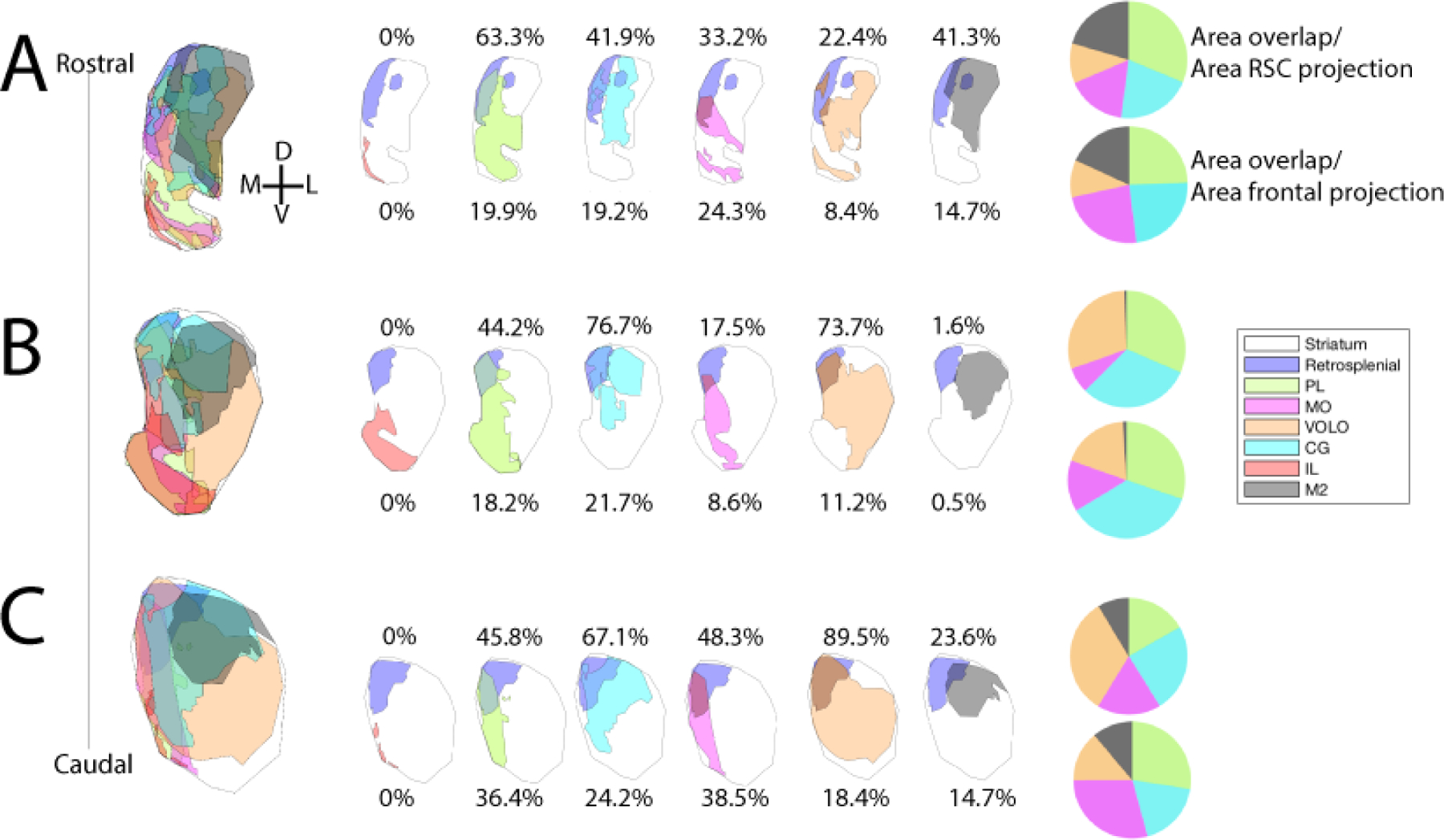
Analyses of overlap of the RSC projection zone in the striatum with select frontal area projection zones. Drawings depict the RSC projection zone (purple) at AP +2.3 (A), AP +1.1 (B), and AP −0.1 (C) compared to IL (red), PL (yellow-green), CG (light blue), MO (purple), VOLO (orange), and M2 (black) projection zones. Numbers and pie charts show the area of the RSC-striatal projection zone encapsulated within the frontal area’s projection zone, divided by the area of the RSC projection (top) or the area of the frontal projection (bottom). AP=anterior-posterior distance from bregma; IL=infralimbic cortex; PL=prelimbic cortex; CG=anterior cingulate cortex; MO=medial orbital cortex; VOLO=ventral and lateral orbital cortex.
Discussion
Here, we examined in detail the projections of the RSC to frontal cortico-basal ganglia circuits. We found that the RSC projects exclusively to the dorsomedial striatum, which also receives dense projections from dorsal anterior cingulate, ventral and lateral orbital areas, and prelimbic cortex. The RSC has bidirectional connections with dorsal anterior cingulate regions, centromedial orbital cortex, and to a lesser extent, prelimbic and infralimbic cortices. These results are in full agreement with prior anatomical work in both rats and mice (van Groen & Wyss, 1992; Cheatwood et al., 2003; van Groen & Wyss, 2003; Reep et al., 2003; Shibata et al., 2004; Shibata & Naito, 2008; Hintiryan et al., 2016; Hunnicutt et al., 2016); here, our goal was to synthesize frontal and striatal projections from a functional perspective.
While RSC has historically been known for its role in navigation and memory functions likely shared with the medial temporal lobe, it also seems to play an important role in reward-guided decision-making, a process that is most commonly associated with frontal cortico-basal ganglia regions and circuits. The dorsomedial striatum and its frontal cortical afferents are known to be involved with goal-directed decision making rather than stimulus-value or procedural learning. For example, inhibition of orbitofrontal-dorsomedial striatal neurons caused mice to be unable to shift to goal-directed action control (Gremel et al., 2016). The placement of RSC within this circuitry calls for similar manipulation studies focused on testing the role of RSC-striatal neurons in goal-directed behavior.
The RSC has mainly been studied in the context of spatial navigation and memory due in part to its strong anatomical connectivity with medial temporal lobe structures (Mitchell et al., 2018; Vann et al., 2009). The cortico-basal ganglia loops of the medial temporal lobe are both distinct and overlapping with those of the RSC. The hippocampus, for example, projects mainly to the medial nucleus accumbens, positioning it quite differently than the RSC projection (Groenewegen et al., 1987). However, very rostrally in the caudoputamen, the hippocampus projects just laterally to the lateral ventricle, similar to the rostral RSC-striatal projection zone. Similarly, entorhinal and peririhinal cortex primarily project to the nucleus accumbens, but also dorsal and medial borders of the caudoputamen, overlapping with the RSC projection zone (McGeorge & Faull, 1989; McIntyre, Kelly, & Staines, 1996). Taken together, the bulk of the medial temporal lobe structures project mainly to the nucleus accumbens, yet these structures do also project to the dorsomedial caudoputamen in such a way as to provide some overlap with the RSC projection zone there, although this overlap is not consistent across rostral vs caudal caudoputamen. Similarly, with the exception of perirhinal cortex, medial temporal lobe frontal projections are more strongly connected with ventral prefrontal cortex, rather than dorsal (Jay et al., 1989; McIntyre et al., 1996; Verwer et al., 1997; Insausti et al., 1997).
RSC-frontal projections mostly parallel the striatal projection zone overlap. Cortico-basal ganglia loops have been described as both parallel (Alexander et al., 1986; Miyachi, 2009) and integrative (Haber et al., 2006; Draganski et al., 2008; Groenewegen et al., 2016). Furthermore, tract-tracing experiments have found that connected cortical areas overlap in their striatal terminal fields (Selemon & Goldman-Rakic, 1985), creating a network with some unifying function (Selemon & Goldman-Rakic, 1988). This concept has been extended to human resting-state functional connectivity, in which distributed, correlated activity defines such functional networks as the DMN, the dorsal attention network, and the frontoparietal network (Yeo et al., 2011; Choi et al., 2012). Here, we find that, in rats, projections between the RSC and the frontal lobe roughly, but do not precisely, match the overlap in terminal fields in the striatum. Anterior cingulate cortex, for example, projects to the dorsomedial and dorsocentral striatum, overlapping considerably with the RSC striatal-projection zone, and also has strong connections with the RSC. The infralimbic cortex, by contrast, does not overlap with the RSC-striatal projection zone, and has only very weak connections with the RSC. However, the prelimbic cortex has only weak connections with the RSC, but projects strongly to the dorsomedial striatum. One possibility is that this connection serves an integrative function for decision-making processes at the level of the basal ganglia. This integrative function might relate to the RSC’s function in navigation, providing information about location to the areas that are involved in decision-making.
The human RSC is part of the larger structure of the posteromedial cortex, which is the main hub of the DMN. This network, which is more active during resting states than cognitive tasks (Greicius et al., 2003), also includes frontal and temporal regions. Mirroring the RSC-striatal anatomical connections in rats, the DMN is functionally connected with the medial caudate in humans (Choi et al., 2012). The DMN in rodents, which includes the RSC (Upadhyay et al., 2011; Lu et al., 2012), the central-medial orbitofrontal cortex, and the dorsomedial frontal cortex, seems to closely match the anatomical connectivity pattern shown here. A new theory of the role of the DMN posits that in monkeys, much of the DMN participates in cognitive shifting (Arsenault et al., 2018). Therefore, one possibility is that the RSC is involved in attentional shifting in rodents as a function of its striatal territory. As a hub of the DMN, RSC in humans is known to be impaired in various psychiatric and neurological disorders (Anderson et al., 2011; Hafkemeijer et al., 2012; Baker et al., 2014; Doucet et al., 2012; Cowdrey et al., 2012; Martino et al., 2016; Bluhm et al., 2009; Wu et al., 2016; Satyshur et al., 2018; He et al., 2018). Understanding the biology of the DMN will require a detailed, translational explication of the functional roles of the RSC, not just in navigation and memory, but also in decision-making.
Acknowledgments:
We thank Tanya Casta, Adriana Cushnie, Mark Grier, and Anish Sethi for assistance with data collection. This work was supported by the National Institute of Mental Health (R01118257); the MnDrive Brain Conditions Initiative; and the Brain & Behavior Research Foundation.
References
- Aggleton JP, & Vann SD (2004). Testing the importance of the retrosplenial navigation system: Lesion size but not strain matters: A reply to Harker and Whishaw. Neuroscience and Biobehavioral Reviews, Vol. 28, pp. 525–531. 10.1016/j.neubiorev.2004.08.003 [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, & Strick PL (1986). Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience, 9, 357–381. [DOI] [PubMed] [Google Scholar]
- Arsenault JT, Caspari N, Vandenberghe R, & Vanduffel W (2018). Attention shifts recruit the monkey default ode network. Journal of Neuroscience, 38(5), 1202–1217. 10.1523/JNEUROSCI.1111-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger SD, Mullally SL, & Maguire EA (2012). Retrosplenial cortex codes for permanent landmarks. PLoS ONE, 7(8). 10.1371/journal.pone.0043620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger SD, & Maguire EA (2013). Assessing the mechanism of response in the retrosplenial cortex of good and poor navigators. cortex, 49(10), 2904–2913. 10.1016/j.cortex.2013.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, & Hikosaka O (2007, August). The role of the dorsal striatum in reward and decision-making. Journal of Neuroscience, Vol. 27, pp. 8161–8165. 10.1523/JNEUROSCI.1554-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluhm R, Williamson P, Lanius R, Théberge J, Densmore M, Bartha R, … & Osuch E (2009). Resting state default-mode network connectivity in early depression using a seed region-of-interest analysis: Decreased connectivity with caudate nucleus. Psychiatry and clinical neurosciences, 63(6), 754–761. 10.1111/j.1440-1819.2009.02030.x [DOI] [PubMed] [Google Scholar]
- Burton AC, Nakamura K, & Roesch MR (2015, January). From ventral-medial to dorsal-lateral striatum: Neural correlates of reward-guided decision-making. Neurobiology of Learning and Memory, Vol. 117, pp. 51–59. 10.1016/j.nlm.2014.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheatwood JL, Reep RL, & Corwin JV (2003). The associative striatum: cortical and thalamic projections to the dorsocentral striatum in rats. Brain Res, 968(1), 1–14. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12644259 [DOI] [PubMed] [Google Scholar]
- Cho J, & Sharp PE (2001). Head direction, place, and movement correlates for cells in the rat retrosplenial cortex. Behavioral Neuroscience, 115(1), 3–25. 10.1037/0735-7044.115.1.3 [DOI] [PubMed] [Google Scholar]
- Choi EY, Yeo BTT, & Buckner RL (2012). The organization of the human striatum estimated by intrinsic functional connectivity. Journal of Neurophysiology, 108(8), 2242–2263. 10.1152/jn.00270.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper BG, & Mizumori SJ (1999). Retrosplenial cortex inactivation selectively impairs navigation in darkness. Neuroreport, 10(3), 625–630. [DOI] [PubMed] [Google Scholar]
- Cowdrey FA, Filippini N, Park RJ, Smith SM, & McCabe C (2014). Increased resting state functional connectivity in the default mode network in recovered anorexia nervosa. Human brain mapping, 35(2), 483–491. 10.1002/hbm.22202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet GE, Janiri D, Howard R, O’Brien M, Andrews-Hanna JR, & Frangou S (2020). Transdiagnostic and disease-specific abnormalities in the default-mode network hubs in psychiatric disorders: A meta-analysis of resting-state functional imaging studies. European Psychiatry, 63(1). 10.1192/j.eurpsy.2020.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B, Kherif F, Klöppel S, Cook PA, Alexander DC, Parker GJM, … Frackowiak RSJ (2008). Evidence for segregated and integrative connectivity patterns in the human basal ganglia. Journal of Neuroscience, 28(28), 7143–7152. 10.1523/JNEUROSCI.1486-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitoussi A, Renault P, Le Moine C, Coutureau E, Cador M, & Dellu-Hagedorn F (2018). Inter-individual differences in decision-making, flexible and goal-directed behaviors: novel insights within the prefronto-striatal networks. Brain Structure and Function, 223(2), 897–912. 10.1007/s00429-017-1530-z [DOI] [PubMed] [Google Scholar]
- Gremel CM, Chancey JH, Atwood BK, Luo G, Neve R, Ramakrishnan C, … Costa RM (2016). Endocannabinoid Modulation of Orbitostriatal Circuits Gates Habit Formation. Neuron, 90(6), 1312–1324. 10.1016/j.neuron.2016.04.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Vermeulen-Van der Zee E, Te Kortschot A, & Witter MP. (1987). Organization of the projections from the subiculum to the ventral striatum in the rat. A study using anterograde transport of Phaseolus vulgaris-leucoagglutinin. Neuroscience, 23, 103–120. [DOI] [PubMed] [Google Scholar]
- Groenewegen Henk J., Voorn P, & Scheel-Krüger J (2016). Limbic-Basal Ganglia Circuits Parallel and Integrative Aspects. 10.1007/978-3-319-42743-0_2 [DOI] [Google Scholar]
- Haber SN, & Behrens TEJ (2014, September). The Neural Network Underlying Incentive-Based Learning: Implications for Interpreting Circuit Disruptions in Psychiatric Disorders. Neuron, Vol. 83, pp. 1019–1039. 10.1016/j.neuron.2014.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Kim KS, Mailly P, & Calzavara R (2006). Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. Journal of Neuroscience, 26(32), 8368–8376. 10.1523/JNEUROSCI.0271-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harker KT, & Whishaw IQ (2004). Impaired place navigation in place and matching-to-place swimming pool tasks follows both retrosplenial cortex lesions and cingulum bundle lesions in rats. Hippocampus, 14(2), 224–231. 10.1002/hipo.10159 [DOI] [PubMed] [Google Scholar]
- Hassani OK, Cromwell HC, & Schultz W (2001). Influence of expectation of different rewards on behavior-related neuronal activity in the striatum. J Neurophysiol, 85(6), 2477–2489. Retrieved from http://www.jn.physiology.org/cgi/content/full/85/6/2477 [DOI] [PubMed] [Google Scholar]
- Hattori R, Danskin B, Babic Z, Mlynaryk N, & Komiyama T (2019). Area-Specificity and Plasticity of History-Dependent Value Coding During Learning. Cell, 177(7), 1858–1872.e15. 10.1016/j.cell.2019.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes WI, & Haber SN (2013). The organization of prefrontal-subthalamic inputs in primates provides an anatomical substrate for both functional specificity and integration: implications for Basal Ganglia models and deep brain stimulation. J Neurosci, 33(11), 4804–4814. 10.1523/JNEUROSCI.4674-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronner SR, & Haber SN (2014). Frontal cortical and subcortical projections provide a basis for segmenting the cingulum bundle: Implications for neuroimaging and psychiatric disorders. Journal of Neuroscience, 34(30), 10041–10054. 10.1523/JNEUROSCI.5459-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronner SR, Rodriguez-Romaguera J, Quirk GJ, Groenewegen HJ, & Haber SN (2016). Circuit-based corticostriatal homologies between rat and primate. Biological psychiatry, 80(7), 509–521. 10.1016/j.biopsych.2016.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronner SR, Meyer MAA, Choi EY, & Haber SN (2018). How do cortico-striatal projections impact on downstream pallidal circuitry? Brain Structure and Function, 223(6), 2809–2821. 10.1007/s00429-018-1662-9 [DOI] [PubMed] [Google Scholar]
- Hintiryan H, Foster NN, Bowman I, Bay M, Song MY, Gou L, … Dong HW (2016). The mouse cortico-striatal projectome. Nature Neuroscience, 19(8), 1100–1114. 10.1038/nn.4332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunnicutt BJ, Jongbloets BC, Birdsong WT, Gertz KJ, Zhong H, & Mao T (2016). A comprehensive excitatory input map of the striatum reveals novel functional organization. ELife, 5(November2016). 10.7554/eLife.19103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insausti R, Herrero MT, & Witter MP (1997). Entorhinal cortex of the rat: Cytoarchitectonic subdivisions and the origin and distribution of cortical efferents. Hippocampus, 7(2), 146–183. [DOI] [PubMed] [Google Scholar]
- Jay TM, Glowinski J, & Thierry AM (1989). Selectivity of the hippocampal projection to the prelimbic area of the prefrontal cortex in the rat. Brain Research, 505(2), 337–340. 10.1016/0006-8993(89)91464-9 [DOI] [PubMed] [Google Scholar]
- Kaboodvand N, Bäckman L, Nyberg L, & Salami A (2018). The retrosplenial cortex: A memory gateway between the cortical default mode network and the medial temporal lobe. Human Brain Mapping, 39(5), 2020–2034. 10.1002/hbm.23983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene CS, & Bucci DJ (2008). Neurotoxic Lesions of Retrosplenial Cortex Disrupt Signaled and Unsignaled Contextual Fear Conditioning. Behavioral Neuroscience, 122(5), 1070–1077. 10.1037/a0012895 [DOI] [PubMed] [Google Scholar]
- Keene CS, & Bucci DJ (2009). Damage to the retrosplenial cortex produces specific impairments in spatial working memory. Neurobiology of Learning and Memory, 91(4), 408–414. 10.1016/j.nlm.2008.10.009 [DOI] [PubMed] [Google Scholar]
- Kimchi EY, & Laubach M (2009). Dynamic encoding of action selection by the medial striatum. Journal of Neuroscience, 29(10), 3148–3159. 10.1523/JNEUROSCI.5206-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer JR, Mastronarde DN, & McIntosh JR (1996). Computer visualization of three-dimensional image data using IMOD. Journal of Structural Biology, 116(1), 71–76. 10.1006/jsbi.1996.0013 [DOI] [PubMed] [Google Scholar]
- Li Y, He Y, Chen M, Pu Z, Chen L, Li P, … Chen JF (2016). Optogenetic Activation of Adenosine A2A Receptor Signaling in the Dorsomedial Striatopallidal Neurons Suppresses Goal-Directed Behavior. Neuropsychopharmacology, 41(4), 1003–1013. 10.1038/npp.2015.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Zou Q, Gu H, Raichle ME, Stein EA, & Yang Y (2012). Rat brains also have a default mode network. Proceedings of the National Academy of Sciences of the United States of America, 109(10), 3979–3984. 10.1073/pnas.1200506109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire E (2001). The retrosplenial contribution to human navigation: A review of lesion and neuroimaging findings. Scandinavian Journal of Psychology, 42(3), 225–238. 10.1111/1467-9450.00233 [DOI] [PubMed] [Google Scholar]
- Mailly P, Aliane V, Groenewegen HJ, Haber SN, & Deniau JM (2013). The rat prefrontostriatal system analyzed in 3D: evidence for multiple interacting functional units. J Neurosci, 33(13), 5718–5727. 10.1523/JNEUROSCI.5248-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantini D, Gerits A, Nelissen K, Durand JB, Joly O, Simone L, … Vanduffel W (2011). Default mode of brain function in monkeys. J Neurosci, 31(36), 12954–12962. 10.1523/JNEUROSCI.2318-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maviel T, Durkin TP, Menzaghi F, & Bontempi B (2004). Sites of neocortical reorganization critical for remote spatial memory. Science, 305(5680), 96–99. 10.1126/science.1098180 [DOI] [PubMed] [Google Scholar]
- McGeorge AJ, & Faull RLM (1989). The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience, 29, 503–537. [DOI] [PubMed] [Google Scholar]
- McIntyre DC, Kelly ME, & Staines WA (1996). Efferent projections of the anterior perirhinal cortex in the rat. The Journal of Comparative Neurology, 369(2), 302–318. [DOI] [PubMed] [Google Scholar]
- Miller AP, Vedder LC, Law ML, & Smith DM (2014, August). Cues, context, and long-term memory: The role of the retrosplenial cortex in spatial cognition. Frontiers in Human Neuroscience, Vol. 8. 10.3389/fnhum.2014.00586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AS, Czajkowski R, Zhang N, Jeffery K, & Nelson AJD (2018). Retrosplenial cortex and its role in spatial cognition. Brain and Neuroscience Advances, 2, 239821281875709. 10.1177/2398212818757098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AJD, Hindley EL, Haddon JE, Vann SD, & Aggleton JP (2014). A novel role for the rat retrosplenial cortex in cognitive control. Learning & Memory, 21(2), 90–97. 10.1101/lm.032136.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, & Dolan RJ (2004). Dissociable Roles of Ventral and Dorsal Striatum in Instrumental Conditioning. Science, 304(5669), 452–454. 10.1126/science.1094285 [DOI] [PubMed] [Google Scholar]
- O’Hare JK, Ade KK, Sukharnikova T, Van Hooser SD, Palmeri ML, Yin HH, & Calakos N (2016). Pathway-Specific Striatal Substrates for Habitual Behavior. Neuron, 89(3), 472–479. 10.1016/j.neuron.2015.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parron C, & Save E (2004). Evidence for entorhinal and parietal cortices involvement in path integration in the rat. Experimental Brain Research, 159(3), 349–359. 10.1007/s00221-004-1960-8 [DOI] [PubMed] [Google Scholar]
- Paxinos G, & Watson C (2014). The Rat Brain in Stereotaxic Coordinates (7th ed.). Elsevier. [DOI] [PubMed] [Google Scholar]
- Pothuizen HHJ, Aggleton JP, & Vann SD (2008). Do rats with retrosplenial cortex lesions lack direction? European Journal of Neuroscience, 28(12), 2486–2498. 10.1111/j.1460-9568.2008.06550. [DOI] [PubMed] [Google Scholar]
- Powell AL, Nelson AJ, Hindley E, Davies M, Aggleton JP, & Vann SD (2017). The rat retrosplenial cortex as a link for frontal functions: A lesion analysis. Behavioural Brain Research, 335, 88–102. 10.1016/j.bbr.2017.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, & Shulman GL (2001). A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America, 98(2), 676–682. 10.1073/pnas.98.2.676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reep RL, & Corwin JV (1999). Topographic organization of the striatal and thalamic connections of rat medial agranular cortex. Brain Research, 841(1–2), 43–52. 10.1016/S0006-8993(99)01779-5 [DOI] [PubMed] [Google Scholar]
- Reep Roger L., Cheatwood JL, & Corwin JV. (2003). The associative striatum: Organization of cortical projections to the dorsocentral striatum in rats. The Journal of Comparative Neurology, 467(3), 271–292. 10.1002/cne.10868 [DOI] [PubMed] [Google Scholar]
- Robinson S, Poorman CE, Marder TJ, & Bucci DJ (2012). Identification of functional circuitry between retrosplenial and postrhinal cortices during fear conditioning. Journal of Neuroscience, 32(35), 12076–12086. 10.1523/JNEUROSCI.2814-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell PE, Hayton SJ, Sun GL, Fuccillo MV, Lim BK, & Malenka RC (2015). Input- and Output-Specific Regulation of Serial Order Performance by Corticostriatal Circuits. Neuron, 88(2), 345–356. 10.1016/j.neuron.2015.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, & Goldman-Rakic PS (1985). Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. J. Neurosci, 5, 776–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata H, Kondo S, & Naito J (2004). Organization of retrosplenial cortical projections to the anterior cingulate, motor, and prefrontal cortices in the rat. Neuroscience Research, 49(1), 1–11. 10.1016/j.neures.2004.01.005 [DOI] [PubMed] [Google Scholar]
- Shibata H, & Naito J (2008). Organization of anterior cingulate and frontal cortical projections to the retrosplenial cortex in the rat. The Journal of Comparative Neurology, 506(1), 30–45. 10.1002/cne.21523 [DOI] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, & Petersen SE (1997). Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. Journal of Cognitive Neuroscience, 9(5), 648–663. 10.1162/jocn.1997.9.5.648 [DOI] [PubMed] [Google Scholar]
- Sleezer BJ, Castagno MD, & Hayden BY (2016). Rule encoding in orbitofrontal cortex and striatum guides selection. Journal of Neuroscience, 36(44), 11223–11237. 10.1523/JNEUROSCI.1766-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugar J, Witter MP, van Strien NM, & Cappaert NLM (2011). The Retrosplenial Cortex: Intrinsic Connectivity and Connections with the (Para)Hippocampal Region in the Rat. An Interactive Connectome. Frontiers in Neuroinformatics, 5, 7. 10.3389/fninf.2011.00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland RJ, Wishaw IQ, & Kolb B (1988). Contributions of cingulate cortex to two forms of spatial learning and memory. Journal of Neuroscience, 8(6), 1863–1872. 10.1523/jneurosci.08-06-01863.1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi E, Furusawa AA, Hori E, Umeno K, Ono T, & Nishijo H (2005). Neural correlates to action and rewards in the rat posterior cingulate cortex. NeuroReport, 16(9), 949–953. 10.1097/00001756-200506210-00014 [DOI] [PubMed] [Google Scholar]
- Upadhyay J, Baker SJ, Chandran P, Miller L, Lee Y, Marek GJ, … Day M (2011). Default-mode-like network activation in awake rodents. PloS One, 6(11), e27839. 10.1371/journal.pone.0027839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenstein E, Bowers D, Verfaellie M, Heilman KM, Day A, & Watson RT (1987). Retrosplenial amnesia. Brain, 110(6), 1631–1646. 10.1093/brain/110.6.1631 [DOI] [PubMed] [Google Scholar]
- van Groen T, & Michael Wyss J (1990). Connections of the retrosplenial granular a cortex in the rat. The Journal of Comparative Neurology, 300(4), 593–606. 10.1002/cne.903000412 [DOI] [PubMed] [Google Scholar]
- van Groen T, & Wyss JM (1992). Connections of the retrosplenial dysgranular cortex in the rat. The Journal of Comparative Neurology, 315(2), 200–216. 10.1002/cne.903150207 [DOI] [PubMed] [Google Scholar]
- Van Groen T, & Wyss JM (2003). Connections of the retrosplenial granular b cortex in the rat. The Journal of Comparative Neurology, 463(3), 249–263. 10.1002/cne.10757 [DOI] [PubMed] [Google Scholar]
- Vann SD, & Aggleton JP (2002). Extensive cytotoxic lesions of the rat retrosplenial cortex reveal consistent deficits on tasks that tax allocentric spatial memory. Behavioral Neuroscience, 116(1), 85–94. 10.1037/0735-7044.116.1.85 [DOI] [PubMed] [Google Scholar]
- Vann SD, & Aggleton JP (2004). Testing the importance of the retrosplenial guidance system: Effects of different sized retrosplenial cortex lesions on heading direction and spatial working memory. Behavioural Brain Research, 155(1), 97–108. 10.1016/j.bbr.2004.04.005 [DOI] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP, & Maguire EA (2009). What does the retrosplenial cortex do? Nature Reviews Neuroscience, 10(11), 792–802. 10.1038/nrn2733 [DOI] [PubMed] [Google Scholar]
- Vedder LC, Miller AMP, Harrison MB, & Smith DM (2016). Retrosplenial Cortical Neurons Encode Navigational Cues, Trajectories and Reward Locations During Goal Directed Navigation. Cerebral Cortex, 27(7), 3713–3723. 10.1093/cercor/bhw192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwer RWH, Meijer RJ, Van Uum HFM, & Witter MP (1997). Collateral projections from the rat hippocampal formation to the lateral and medial prefrontal cortex. Hippocampus, 7(4), 397–402. [DOI] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJM., Groenewegen HJ, Robbins TW, & Pennartz CM. (2004). Putting a spin on the dorsal–ventral divide of the striatum. Trends in Neurosciences, 27(8), 468–474. 10.1016/j.tins.2004.06.006 [DOI] [PubMed] [Google Scholar]
- Wisner K, Odintsov B, Brozoski D, & Brozoski TJ (2016). Ratat1: A Digital Rat Brain Stereotaxic Atlas Derived from High-Resolution MRI Images Scanned in Three Dimensions. Frontiers in Systems Neuroscience, 10, 64. 10.3389/fnsys.2016.00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, … Buckner RL (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106(3), 1125–1165. 10.1152/jn.00338.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, & Knowlton BJ (2004). Contributions of striatal subregions to place and response learning. Learning and Memory, 11(4), 459–463. 10.1101/lm.81004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, & Balleine BW (2006). Inactivation of dorsolateral striatum enhances sensitivity to changes in the action-outcome contingency in instrumental conditioning. Behavioural Brain Research, 166(2), 189–196. 10.1016/j.bbr.2005.07.012 [DOI] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Knowlton BJ, & Balleine BW (2005). The role of the dorsomedial striatum in instrumental conditioning. European Journal of Neuroscience, 22(2), 513–523. 10.1111/j.1460-9568.2005.04218.x [DOI] [PubMed] [Google Scholar]


