Abstract
Objective:
The purpose of this trial is to evaluate the novel use of an empirically supported treatment for sleep problems for people with residual insomnia disorder following ED treatment.
Method:
Participants (N = 6) will complete a single-case multiple baseline study using Brief Behavioral Treatment for Insomnia (Buysse et al., Archives of Internal Medicine, 171, 2011, 887–895; Troxel et al., Behavioral Sleep Medicine, 10, 2012, 266–279). Participants will complete pre- and post-treatment evaluations of insomnia severity, sleep efficiency, daytime fatigue, ED symptoms, depressive symptoms, and anxiety symptoms. Throughout treatment, participants will complete daily diaries of sleep indices (sleep latency, wake after sleep onset, total sleep time, and sleep efficiency).
Results:
The primary outcome will be treatment effects on insomnia severity, measured by the Insomnia Severity Index. Secondary outcomes include sleep efficiency and daytime fatigue. Exploratory outcomes include ED-related impairment and symptoms, anxiety symptoms, and depression symptoms. We will provide subject-level graphs of sleep indices and ED symptoms throughout treatment. Additionally, treatment effects will be examined at one- and three-month follow-up.
Discussion:
Although insomnia treatments have been evaluated in other psychiatric disorders, there has yet to be a study examining behavioral interventions for insomnia in EDs. Results of this study will inform the development and application of interventions for residual insomnia symptoms in this population.
Keywords: behavioral therapy, eating disorders, insomnia disorder, multiple baseline design, single-case experimental design
Insomnia disorder is a condition characterized by difficulty falling asleep, staying asleep, or waking early in the morning, despite adequate opportunities to sleep. There is increasing evidence that insomnia disorder is common in eating disorder (ED) populations (Allison, Spaeth, & Hopkins, 2016; Padez-Vieira & Afonso, 2016) and sleep disturbances may bidirectionally impact ED, mood, and anxiety symptoms (Linnaranta et al., 2020).
Although insomnia treatments have been tested in major depressive disorder (Cunningham & Shapiro, 2018; Taylor & Pruiksma, 2014), anxiety disorders (Cox & Olatunji, 2020; Taylor & Pruiksma, 2014), and post-traumatic stress disorder (Taylor & Pruiksma, 2014), there has yet to be a study examining insomnia treatment for people with eating pathology. This protocol proposes a novel application of a brief treatment for insomnia disorder in a sample of people who have completed a guided self-help cognitive-behavioral treatment for an ED.
1 |. WHY INTERVENE ON SLEEP PROBLEMS IN PEOPLE WITH EDS
Sleep problems are common in people with EDs. In one transdiagnostic ED sample, 50% of respondents reported symptoms of insomnia (KyungRan et al., 2010). Another study examining college students with EDs found 21.7% of participants reported moderate to severe insomnia symptoms (Goel et al., 2020). Anorexia nervosa has been associated with increased sleep disturbances, poorer sleep quality, and lower sleep efficiency and bulimia nervosa has been associated with greater symptoms of insomnia, parasomnias, and hypersomnolence (Asaad Abdou et al., 2018). Similarly, adults with binge-eating disorder report poorer sleep quality compared to weight-matched controls (Kenny, Wijk, Singleton, & Carter, 2018).
Furthermore, there are high rates of co-occurrence between insomnia disorder and conditions common to EDs, such as mood and anxiety disorders. Insomnia is a significant predictor of the onset of depression and anxiety (Hertenstein et al., 2019). Bidirectional effects have been observed between insomnia symptoms and internalizing symptoms, particularly in young adults (Kortesoja et al., 2020). Consequently, treating insomnia symptoms has the potential to reduce sleep-related distress, as well as symptoms of EDs and internalizing symptoms.
2 |. TREATING RESIDUAL INSOMNIA SYMPTOMS FOLLOWING ED TREATMENT
There is evidence suggesting the importance of treating residual insomnia disorder following treatment of other mental health conditions. Although cognitive-behavioral therapy (CBT) for anxiety and depression may reduce sleep problems, many people still have significant insomnia symptoms following CBT. For example, in one study of CBT for generalized anxiety disorder and panic disorder, 63% of people had clinically significant insomnia symptoms at post-test; moreover, 33% of participants still met diagnostic criteria for insomnia disorder (Cousineau et al., 2016). Similarly, two studies found that 10–26% of people fully remitted from depression after treatment still reported clinically significant insomnia symptoms (Carney, Segal, Edinger, & Krystal, 2007; Mason & Harvey, 2014). It is possible that although CBT may reduce the rumination or worry that contributes to sleep disturbances, it does not adequately target maintaining behavioral processes (e.g., extended time in bed) that result in residual insomnia after treatment.
This is important because residual insomnia symptoms increase relapse risk for depression and anxiety (e.g., Chen et al., 2017; Manber et al., 2008; Ohayon & Roth, 2003), as well as smoking (Short et al., 2017), cannabis (Babson, Boden, Harris, Stickle, & Bonn-Miller, 2013), and alcohol use (Brower, 2003; Brower, Aldrich, Robinson, Zucker, & Greden, 2001). Although changes in sleep disturbances during ED treatment have not yet been examined, one study did find that poor sleep at intake predicted higher ED severity after 6 months of treatment (Lombardo, Battagliese, Venezia, & Salvemini, 2015).
3 |. THE TIRED STUDY: TREATING INSOMNIA IN PEOPLE RECOVERING FROM EATING DISORDERS
The American Academy of Sleep Medicine recommends cognitive-behavioral approaches as first-line treatments for insomnia disorder (Schutte-Rodin, Broch, Buysse, Dorsey, & Sateia, 2008). CBT-I has moderate-to-large short-term effects on insomnia severity and sleep efficiency (van Straten et al., 2018), with effects persisting at three, six, and 12 months post-treatment (Van Der Zweerde, Bisdounis, Kyle, Lancee, & Van Straten, 2019) and small effects on depressive symptoms (Ho, Chan, Lo, & Leung, 2020).
For this study, participants will complete brief behavioral treatment for insomnia (BBTI) (Buysse et al., 2011; Troxel, Germain, & Buysse, 2012), which is an empirically supported treatment for insomnia disorder. BBTI, while similar to CBT-I, differs in a few components that make it suitable as a follow-up to ED treatment. Although both treatments involve psychoeducation and implementing stimulus control and sleep restriction, BBTI maintains a focus on behavioral processes. By contrast, CBT-I includes cognitive mechanisms, such as thought restructuring, which are covered in ED treatments, such as CBT-E (Fairburn, 2008). Consequently, BBTI has a shorter treatment duration (four sessions over four weeks) and shorter session lengths (20–75 min). Finally, BBTI was developed to be administered by providers who are not sleep medicine experts and uses a standardized treatment manual, making it a highly accessible treatment option for ED providers—the majority of whom are not trained in behavioral sleep medicine.
Although BBTI has shown excellent promise in treating internalizing disorders, EDs provide additional levels of complexity over these other conditions in terms of behavioral/circadian disruptions resulting from dysregulated eating behaviors and altered hypothalamic neuropeptides (e.g., orexin) that impact both sleep and eating behaviors. Consequently, it is important to demonstrate the efficacy of this intervention in an ED sample prior to the implementation of larger-scale clinical trials. Thus, we will use a single-case multiple baseline design (MBD), which is optimal when an intervention reversal (e.g., A-B-A design) would be difficult to use (Kazdin, 2011). It is likely that once BBTI is administered, participants will learn skills about sleep hygiene that would make them unable to return to true “untreated” status (carryover effects; Barlow, Nock, & Hersen, 2008). Similarly, although the sleep prescription could be withheld during a reversal phase, it is unknown if participants would comply with returning to their former behaviors, particularly if the intervention reduces insomnia.
MBD allows each subject to act as their own “control” by providing data during a baseline phase and intervention phase, which makes it methodologically superior to standard case study designs. Fewer participants are required to evaluate treatment effects, which is an advantage when evaluating new treatments.
In a MBD, when subjects achieve symptom stability during the baseline phase, they begin the intervention phase. Causal inferences of treatment effectiveness can be inferred if changes in the targeted behavior occur only after applying the intervention. Although MBDs allow researchers to maximize external and internal validity for small samples, these designs are not used frequently in ED research, despite calls for their implementation (De Young & Bottera, 2018). Consequently, this study provides an innovative and methodologically rigorous step forward in single-case design research in EDs.
The primary outcome is treatment effects on insomnia severity. Additional outcomes include sleep efficiency, daytime fatigue, ED-related impairment and behaviors, anxiety symptoms, and depression symptoms. We predict the following:
H1.Insomnia severity will significantly decrease over the course of treatment and be maintained at follow-up surveys.
H2.Sleep efficiency will increase and daytime fatigue will decrease from pre-to-post treatment and be maintained at follow-up surveys.
Exploratory:
We will additionally examine levels of ED-related impairment, ED behaviors, and symptoms of depression and anxiety at pre-treatment, post-treatment, and one- and three-month follow-ups. We expect that the insomnia treatment will have transdiagnostic effects across all participants, however, we do not have specific predictions about if participants will respond differentially depending on the type of ED behaviors that they were endorsing during or after the gsh-CBT treatment.
4 |. METHODS
4.1 |. Participants and recruitment
We will recruit six individuals with residual insomnia disorder following a guided self-help Cognitive-Behavior Therapy for Eating Disorders (gsh-CBT) protocol. Potential participants will complete the Insomnia Severity Index at the conclusion of gsh-CBT. People who score a 10 or higher (indicating probable insomnia disorder) will be invited to participate and insomnia disorder diagnosis will be confirmed using the Structured Clinical Interview for Sleep Disorders-Revised (SCISD-R). As part of the consenting process, participants will give permission for their data to be included in publication with their identity blinded.
4.1.1 |. Feasibility
Previous research suggested that approximately 21.75% of college students with EDs have clinically significant insomnia symptoms (Goel et al., 2020). Based on our current enrollment rate for the parent trial, we expect to be able to enroll as many as 24 students in the trial, exceeding our proposed sample size of six.
4.2 |. Inclusion and exclusion criteria
Inclusion criteria for the parent protocol (gsh-CBT) requires participants to be university students over the age of 18 with an ED without low weight (BN, BED, or OSFED). Participants in the parent protocol are excluded for reporting any of the following: (a) significant psychiatric comorbidity that interferes with treatment (e.g., severe obsessive–compulsive disorder); (b) medical instability at intake; (c) moderate to severe suicidal ideation; (d) a body mass index <19.0 kg/m2; or (e) medical conditions that affect eating behavior or body weight, such as Type I diabetes mellitus. Participants will be included regardless of level of ED pathology at completion of gsh-CBT.
We will exclude participants with conditions for whom BBTI would be contraindicated due to concerns about the effects of sleep restriction, such as bipolar disorder, seizure disorders, narcolepsy, and untreated obstructive sleep apnea (Troxel et al., 2012). We will not exclude individuals who are currently taking sleep medications or prescribed stimulant medications; however, participants’ medication dosages must be steady for the past 6 weeks. We will ask participants to report any changes in medication status during enrollment.
4.3 |. Instruments
4.3.1 |. Sleep measures
Flinders Fatigue Scale (Gradisar et al., 2007)
The Flinders Fatigue Scale (FFS) is a seven-item measure used to assess daytime fatigue resulting from insomnia. The FFS has shown sensitivity to changes resulting from insomnia treatment, discriminant validity from measures capturing daytime sleepiness, and excellent internal reliability (Gradisar et al., 2007). The FFS will be used to examine the secondary outcome of daytime fatigue.
Insomnia Severity Index (Bastien, Vallières, & Morin, 2001)
The Insomnia Severity Index (ISI) is a seven-item measure used to assess insomnia severity and impairment over the past month. We will use a modified version to assess past week insomnia symptoms. Psychometric studies have found adequate reliability and validity for this measure (Bastien et al., 2001). Receiver operator curve analysis has identified a score of 10 as an optimal cutoff for detecting insomnia in a community sample (Morin, Belleville, Bélanger, & Ivers, 2011). The ISI will be used for screening purposes to identify participants with insomnia symptoms at the end of gsh-CBT and during the baseline to establish insomnia severity stability.
Structured clinical interview for DSM 5 sleep disorders-revised (Pruiksma et al., 2019)
The SCISD-R is a semi-structured interview for assessing DSM-5 sleep disorders. The SCISD-R has shown excellent interrater reliability for diagnosing insomnia disorder (Pruiksma et al., 2019).
Sleep diaries
Participants will complete daily sleep diaries, indicating the time they got in bed for the evening, sleep onset latency, how long they were awake total after initial sleep onset (wake after sleep onset; WASO), the time they woke up in the morning, the time they got out of bed in the morning, sleep quality, caffeine use and napping behaviors (frequency, duration) for the previous day. Time-in-bed (TIB) will be derived from the participant-reported time of getting in and out of bed. We will calculate total sleep time (TST) by using total amount of sleep from time of sleep initiation to reported morning wakeup time minus WASO. Finally, we will calculate sleep efficiency by dividing TST by TIB and multiplying by 100.
We will use the PiLR mobile application to deliver sleep diaries every morning at 7 a.m. Participants will have 24 hr to complete each survey.
Wearable sleep tracker measurement
Participants will wear medical-grade actigraphy watches (Centrepoint Insight Watch by ActiGraph) continuously during the study. Research comparing previous ActiGraph devices to polysomnography (the clinical benchmark for sleep measurements) found comparable performance in measuring total sleep time (Quante et al., 2018). The sleep tracker data will be used as an external validator of participant-reported sleep indices.
4.3.2 |. Psychopathology measures
Clinical impairment assessment (Bohn & Fairburn, 2008)
The clinical impairment assessment (CIA) is a 16-item measure used for assessing the impact of ED symptoms on psychosocial outcomes with higher scores indicating greater impairment (Reas, Stedal, Dahlgren, & Rø, 2016). The CIA has shown excellent discriminant validity in clinical and community samples (Jenkins, 2013).
Eating pathology symptoms inventory (Forbush et al., 2013)
The eating pathology symptoms inventory (EPSI) is a 45-item measure that assesses ED symptoms over the past month. For the purposes of this study, we will use a weekly adaptation of the measure. The monthly version of the EPSI demonstrated excellent convergent and discriminant validity (Forbush et al., 2013). The EPSI will be administered weekly during the treatment phase and graphed to show change in ED behaviors. For this study, scores on the Binge Eating, Excessive Exercise, Restricting, and Purging subscales will be used as exploratory outcomes.
Inventory of depression and anxiety symptoms-II (IDAS-II): General Depression Subscale (Watson & O’Hara, 2017)
The General Depression Subscale of the IDAS-II consists of 20-questions with higher scores indicating greater depression symptoms. The IDAS-II has demonstrated good internal consistency and temporal stability (Watson et al., 2012).
Generalized anxiety disorder-7 (Spitzer, Kroenke, Williams, & Löwe, 2006)
The generalized anxiety disorder-7 (GAD-7) is a seven-item measure used for assessing symptoms of generalized anxiety disorder with higher scores indicating greater symptoms. The GAD-7 has demonstrated good construct and convergent validity (Spitzer et al., 2006).
4.3.3 |. Other measures
Demographics Questionnaire
We will use the demographics questionnaire administered at intake from the parent trial to collect information about age, gender, race, ethnicity, and education.
Treatment Evaluation Questionnaire
This measure was developed by our team to assess treatment acceptability. Participants will use a Likert-scale to rate treatment acceptability, satisfaction with improvements in sleep, and plans to implement strategies in the future.
4.4 |. Intervention protocol
Participants will complete a 1-hr assessment session, during which they will complete baseline questionnaires (Table 1). The first author will administer the SCISD-R to confirm the diagnosis of insomnia disorder and rule out other sleep disorders. The participant will be oriented to the sleep diaries and provided with the wearable sleep tracker.
TABLE 1.
Questionnaire administration schedule
| Timepoint | Measures |
|---|---|
| Baseline assessment |
|
| Weekly baseline phase (3–5 weeks) |
|
| Session 1 |
|
| Session 2 |
|
| Session 3 |
|
| Session 4 |
|
| EOT surveys |
|
| Follow-up surveys |
|
Abbreviations: CIA, clinical impairment assessment; EPSI, eating pathology symptoms inventory; FFS, Flinders Fatigue Scale; GAD-7, generalized anxiety disorder-7; ISI, Insomnia Severity Index; IDAS-II, Inventory of depression and anxiety symptoms-II.
Multiple baseline across individuals design (MBD)
Participants will be randomized to one of two triads, which will be examined separately. Participants will complete a minimum baseline of 3 weeks, during which they will complete the ISI and EPSI weekly. ISI scores will be plotted and visually inspected. Participants who demonstrate insomnia severity stability, as indicated by similar scores on the ISI across the baseline period or an increasing severity trend (i.e., a slope greater than or equal to 0), will be moved to the intervention phase. For practical reasons, we will move all participants to the intervention phase after 5 weeks, even if stability is not achieved.
The four sessions of BBTI are described in Table 2. Although typically providers deliver BBTI via two in-person sessions and two phone sessions, for the purposes of this trial, which is being initiated during the COVID-19 pandemic, all study procedures will be conducted using a secured telehealth platform. The University of Kansas Instituitional Review Board has reviewed and approved this study.
TABLE 2.
Brief behavioral treatment for insomnia (BBTI) session outlines
| Session | Length | Topic |
|---|---|---|
| 1 | 45–75 min |
|
| 2 | 20 min |
|
| 3 | 30 min |
|
| 4 | 20 min |
|
Following completion of BBTI, participants will complete end-of-treatment surveys to evaluate progress on sleep, ED, and other psychopathology indices, as well as acceptability of treatment. They will be emailed follow-up surveys at one- and three-months post-BBTI completion to evaluate stability of treatment effects (Table 1).
4.5 |. Data analysis
We will follow best practices for reporting single-case research using the Single-Case Reporting guideline In BEhavioural interventions checklist (SCRIBE; Tate et al., 2016). For the primary outcome (H1), we will calculate between-case standardized mean differences on insomnia severity to compute treatment effect size and perform visual analysis on subject-level data using the Conservative Dual-Criterion method (Fisher, Kelley, & Lomas, 2003). We will perform simulation modeling analysis, which will use level, trend, and auto-correlation factors at baseline to simulate thousands of models based on each case (Borckardt & Nash, 2014). This will allow us to evaluate the significance of changes in insomnia severity at the subject-level. The use of the baseline stability period prior to intervention will allow us to ensure that it is the application of the insomnia intervention, rather than continuing, or delayed, effects of gsh-CBT on sleep outcomes.
For the secondary outcomes (H2), we will report and graph subject-level data at the week-level from the sleep diaries and use the objective sleep data from the sleep trackers as external validators. We will report between-case standardized mean differences using the sleep diaries and FFS to evaluate effects on sleep efficiency and daytime fatigue, respectively.
For the exploratory analyses, we will examine stability in a composite measure of ED behaviors (i.e., binge eating, excessive exercise, restricting, and purging) during the baseline period. If the behavioral data show stability or an increasing trend, then we can conclude that effects during the treatment phase are not likely due to delayed or continuing effects of gsh-CBT (Figure 1a). In this case, we will calculate standardized mean differences for effect sizes, perform visual analysis, and perform simulation analysis to examine significance of treatment effects on ED behaviors. We also will report pre-post scores of ED-related impairment, anxiety (GAD-7), and depression (IDAS-II General Depression Subscale) symptoms. If the participants do not show stability or an increasing trend on ED behaviors during the baseline phase, then we will still perform analyses, with the caveat that we cannot conclude that the effects are due to the gsh-CBT, BBTI, or a combination of the two (Figure 1b).
FIGURE 1.
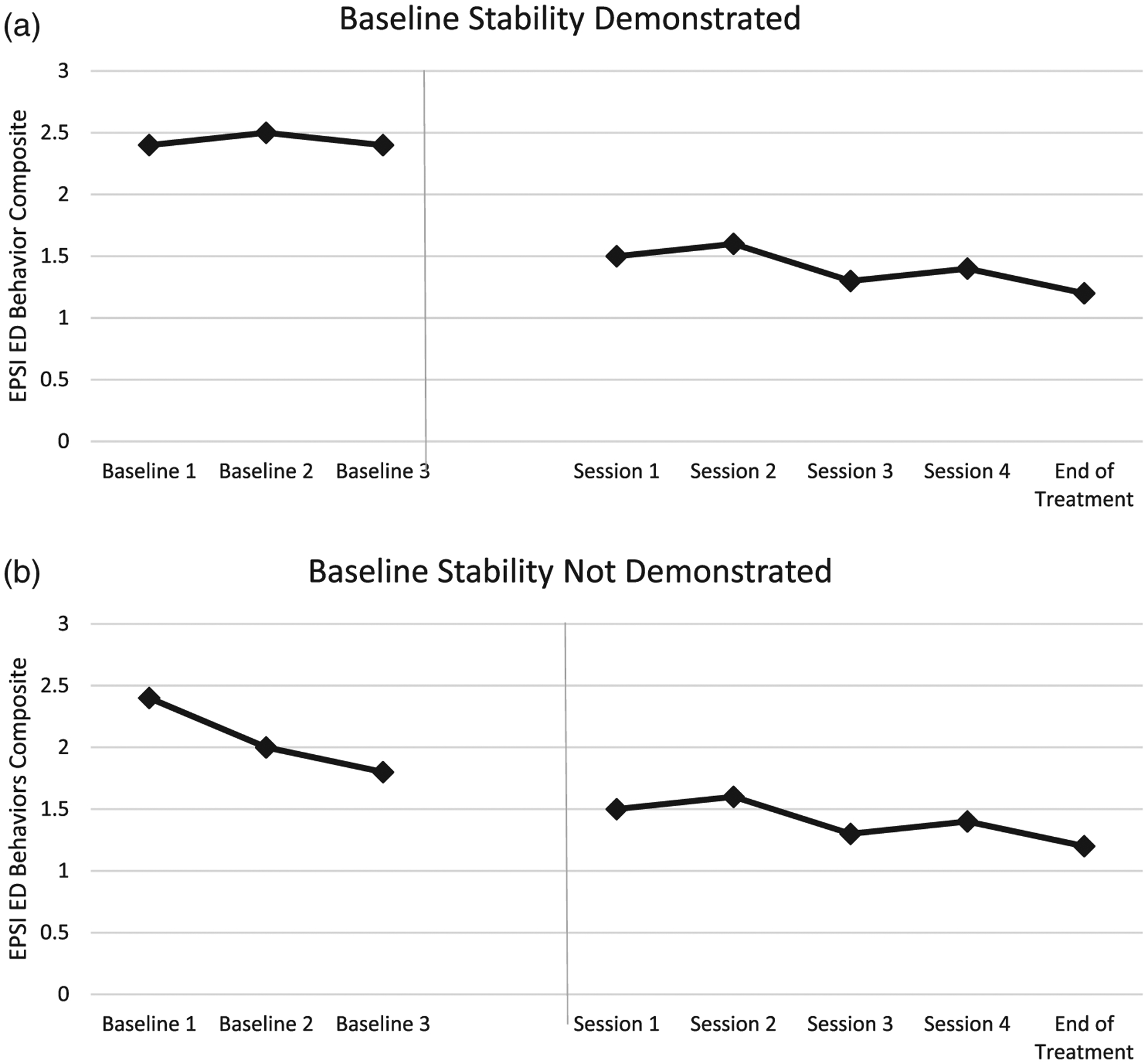
(a) Stability demonstrated in the composite eating disorder (ED) behavior variable during baseline, therefore, brief behavioral treatment for insomnia (BBTI)-specific effects on ED symptoms can be inferred. (b) Stability not demonstrated in the composite ED behavior variable during baseline, therefore BBTI-specific effects on ED symptoms cannot be inferred
Participant adherence will be determined by calculating the percentage of completed sleep diaries. Finally, we will include data on the participants’ quantitative treatment evaluation ratings.
5 |. CONCLUSION
Given the prevalence of insomnia symptoms in ED populations, interventions that target sleep disturbances have great potential value. To date, there has been no published research applying an evidence-based insomnia treatment protocol for an ED population. This project will evaluate the efficacy, acceptability, and feasibility of BBTI for individuals with residual insomnia disorder following completion of guided self-help CBT for EDs. Results from this study will inform the future implementation of protocols treating sleep problems in ED populations.
ACKNOWLEDGMENTS
The proposed research is supported by the University of Kansas Research Excellence Initiative (REI) Research Accelerator grant award (KTF) and the Postdoctoral Grant Writing Incentive (KAC). KAC is supported by a CTSA grant from NCATS awarded to Frontiers: University of Kansas Clinical and Translational Science Institute (# TL1TR002368). The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the University of Kansas, NIH, or NCATS.
Funding information
National Center for Advancing Translational Sciences, Grant/Award Number: TL1TR002368; University of Kansas, Grant/Award Numbers: Postdoctoral Grant Writing Initiative, Research Excellence Initiative
Footnotes
CONFLICT OF INTEREST
None.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- Allison KC, Spaeth A, & Hopkins CM (2016). Sleep and eating disorders. Current Psychiatry Reports, 18(10), 92. 10.1007/s11920-016-0728-8 [DOI] [PubMed] [Google Scholar]
- Asaad Abdou T, Esawy HI, Abdel Razek Mohamed G, Hussein Ahmed H, Elhabiby MM, Khalil SA, & El-Hawary YA (2018). Sleep profile in anorexia and bulimia nervosa female patients. Sleep Medicine, 48, 113–116. 10.1016/j.sleep.2018.03.032 [DOI] [PubMed] [Google Scholar]
- Babson KA, Boden MT, Harris AH, Stickle TR, & Bonn-Miller MO (2013). Poor sleep quality as a risk factor for lapse following a cannabis quit attempt. Journal of Substance Abuse Treatment, 44(4), 438–443. [DOI] [PubMed] [Google Scholar]
- Barlow D, Nock M, & Hersen M (2008). Single case experimental designs: Strategies for studying behavior change (3rd ed.). Boston, MA: Pearson Education, Inc. [Google Scholar]
- Bastien CH, Vallières A, & Morin CM (2001). Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Medicine, 2(4), 297–307. 10.1016/S1389-9457(00)00065-4 [DOI] [PubMed] [Google Scholar]
- Bohn K (2008). The clinical impairment assessment questionnaire (CIA 3.0). In Fairburn CG (Ed.), Cognitive Behavioral Therapy and Eating Disorder. New York, NY: Guilford Press. [Google Scholar]
- Borckardt JJ, & Nash MR (2014). Simulation modelling analysis for small sets of single-subject data collected over time. Neuropsychological Rehabilitation, 24(3–4), 492–506. 10.1080/09602011.2014.895390 [DOI] [PubMed] [Google Scholar]
- Brower KJ (2003). Insomnia, alcoholism and relapse. Sleep Medicine Reviews, 7(6), 523–539. [DOI] [PubMed] [Google Scholar]
- Brower KJ, Aldrich MS, Robinson EA, Zucker RA, & Greden JF (2001). Insomnia, self-medication, and relapse to alcoholism. American Journal of Psychiatry, 158(3), 399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Germain A, Moul DE, Franzen PL, Brar LK, Fletcher ME, … Reynolds CF (2011). Efficacy of brief behavioral treatment for chronic insomnia in older adults. Archives of Internal Medicine, 171(10), 887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney CE, Segal ZV, Edinger JD, & Krystal AD (2007). A comparison of rates of residual insomnia symptoms following pharmacotherapy or cognitive-behavioral therapy for major depressive disorder. The Journal of Clinical Psychiatry, 68(2), 254–260. 10.4088/JCP.v68n0211 [DOI] [PubMed] [Google Scholar]
- Chen P-J, Huang CL-C, Weng S-F, Wu M-P, Ho C-H, Wang J-J, … Hsu Y-W (2017). Relapse insomnia increases greater risk of anxiety and depression: Evidence from a population-based 4-year cohort study. Sleep Medicine, 38, 122–129. 10.1016/j.sleep.2017.07.016 [DOI] [PubMed] [Google Scholar]
- Cousineau H, Marchand A, Bouchard S, Bélanger C, Gosselin P, Langlois F, … Belleville G (2016). Insomnia symptoms following treatment for comorbid panic disorder with agoraphobia and generalized anxiety disorder. The Journal of Nervous and Mental Disease, 204 (4), 267–273. 10.1097/NMD.0000000000000466 [DOI] [PubMed] [Google Scholar]
- Cox RC, & Olatunji BO (2020). Sleep in the anxiety-related disorders: A meta-analysis of subjective and objective research. Sleep Medicine Reviews, 51, 101282. 10.1016/j.smrv.2020.101282 [DOI] [PubMed] [Google Scholar]
- Cunningham JEA, & Shapiro CM (2018). Cognitive Behavioural therapy for insomnia (CBT-I) to treat depression: A systematic review. Journal of Psychosomatic Research, 106, 1–12. 10.1016/j.jpsychores.2017.12.012 [DOI] [PubMed] [Google Scholar]
- De Young KP, & Bottera AR (2018). A summary of reporting guidelines and evaluation domains for using single-case experimental designs and recommendations for the study of eating disorders. International Journal of Eating Disorders, 51(7), 617–628. 10.1002/eat.22887 [DOI] [PubMed] [Google Scholar]
- Fairburn CG (2008). Cognitive behavior therapy and eating disorders. New York, NY: Guilford Press. [Google Scholar]
- Fisher WW, Kelley ME, & Lomas JE (2003). Visual aids and structured criteria for improving visual inspection and interpretation of single-case designs. Journal of Applied Behavior Analysis, 36(3), 387–406. 10.1901/jaba.2003.36-387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbush KT, Wildes JE, Pollack LO, Dunbar D, Luo J, Patterson K, … Watson D (2013). Development and validation of the eating pathology symptoms inventory (EPSI). Psychological Assessment, 25(3), 859–878. 10.1037/a0032639 [DOI] [PubMed] [Google Scholar]
- Goel NJ, Sadeh-Sharvit S, Trockel M, Flatt RE, Fitzsimmons-Craft EE, Balantekin KN, … Taylor CB (2020). Depression and anxiety mediate the relationship between insomnia and eating disorders in college women. Journal of American College Health, 0(0), 1–6. 10.1080/07448481.2019.1710152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradisar M, Lack L, Richards H, Harris J, Gallasch J, Boundy M, &Johnston A (2007). The Flinders fatigue scale: Preliminary psychometric properties and clinical sensitivity of a new scale for measuring daytime fatigue associated with insomnia. Journal of Clinical Sleep Medicine: JCSM: Official Publication of the American Academy of Sleep Medicine, 3(7), 722–728. [PMC free article] [PubMed] [Google Scholar]
- Hertenstein E, Feige B, Gmeiner T, Kienzler C, Spiegelhalder K, Johann A, … Baglioni C (2019). Insomnia as a predictor of mental disorders: A systematic review and meta-analysis. Sleep Medicine Reviews, 43, 96–105. 10.1016/j.smrv.2018.10.006 [DOI] [PubMed] [Google Scholar]
- Ho FY-Y, Chan CS, Lo W-Y, & Leung JC-Y (2020). The effect of self-help cognitive behavioral therapy for insomnia on depressive symptoms: An updated meta-analysis of randomized controlled trials. Journal of Affective Disorders, 265, 287–304. 10.1016/j.jad.2020.01.062 [DOI] [PubMed] [Google Scholar]
- Jenkins PE (2013). Psychometric validation of the clinical impairment assessment in a UKeating disorder service. Eating Behaviors, 14(2), 241–243. 10.1016/j.eatbeh.2012.12.001 [DOI] [PubMed] [Google Scholar]
- Kazdin AE (2011). Single-case research designs: Methods for clinical and applied settings (2nd ed.). New York, NY: Oxford University Press. [Google Scholar]
- Kenny TE, Wijk MV, Singleton C, & Carter JC (2018). An examination of the relationship between binge eating disorder and insomnia symptoms. European Eating Disorders Review, 26(3), 186–196. 10.1002/erv.2587 [DOI] [PubMed] [Google Scholar]
- Kortesoja L, Vainikainen M-P, Hotulainen R, Rimpelä A, Dobewall H, Lindfors P, … Merikanto I (2020). Bidirectional relationship of sleep with emotional and behavioral difficulties: A five-year follow-up of finnish adolescents. Journal of Youth and Adolescence, 49(6), 1277–1291. 10.1007/s10964-020-01203-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- KyungRan K, YoungChul J, MiYeon S, NamKoong K, JoonKi K, & JungHyun L (2010). Sleep disturbance in women with eating disorder: Prevalence and clinical characteristics. Psychiatry Research, 176(1), 88–90. [DOI] [PubMed] [Google Scholar]
- Linnaranta O, Bourguignon C, Crescenzi O, Sibthorpe D, Buyukkurt A, Steiger H, & Storch K-F (2020). Late and instable sleep phasing is associated with irregular eating patterns in eating disorders. Annals of Behavioral Medicine, 54, 680–690. 10.1093/abm/kaaa012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo C, Battagliese G, Venezia C, & Salvemini V (2015). Persistence of poor sleep predicts the severity of the clinical condition after 6months of standard treatment in patients with eating disorders. Eating Behaviors, 18, 16–19. 10.1016/j.eatbeh.2015.03.003 [DOI] [PubMed] [Google Scholar]
- Manber R, Edinger JD, Gress JL, Pedro-Salcedo MGS, Kuo TF, & Kalista T (2008). Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep, 31(4), 489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason EC, & Harvey AG (2014). Insomnia before and after treatment for anxiety and depression. Journal of Affective Disorders, 168, 415–421. 10.1016/j.jad.2014.07.020 [DOI] [PubMed] [Google Scholar]
- Morin CM, Belleville G, Bélanger L, & Ivers H (2011). The insomnia severity index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep, 34(5), 601–608. 10.1093/sleep/34.5.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon MM, & Roth T (2003). Place of chronic insomnia in the course of depressive and anxiety disorders. Journal of Psychiatric Research, 37 (1), 9–15. 10.1016/S0022-3956(02)00052-3 [DOI] [PubMed] [Google Scholar]
- Padez-Vieira F, & Afonso P (2016). Sleep disturbances in anorexia nervosa. Advances in Eating Disorders, 4(2), 176–188. 10.1080/21662630.2016.1175958 [DOI] [Google Scholar]
- Pruiksma KE, Wilkerson A, Dietch JR, Pinkston S, Dolan M, & Taylor DJ (2019). User’s manual for the Structure Clinical Interview for DSM-5 Sleep Disorders-Revised (SCISD-R). Retrieved from http://insomnia.arizona.edu/SCISD
- Quante M, Kaplan ER, Cailler M, Rueschman M, Wang R, Weng J, … Redline S (2018). Actigraphy-based sleep estimation in adolescents and adults: A comparison with polysomnography using two scoring algorithms. Nature and Science of Sleep, 10, 13–20. 10.2147/NSS.S151085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reas D, Stedal K, Dahlgren CL, & Rø Ø (2016). Impairment due to eating disorder pathology: Identifying the cut-off score on the clinical impairment assessment in a clinical and community sample. International Journal of Eating Disorders, 49(6), 635–638. 10.1002/eat.22517 [DOI] [PubMed] [Google Scholar]
- Schutte-Rodin S, Broch L, Buysse D, Dorsey C, & Sateia M (2008). Clinical guideline for the evaluation and Management of Chronic Insomnia in adults. Journal of Clinical Sleep Medicine, 04(05), 487–504. 10.5664/jcsm.27286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short NA, Mathes BM, Gibby B, Oglesby ME, Zvolensky MJ, & Schmidt NB (2017). Insomnia symptoms as a risk factor for cessation failure following smoking cessation treatment. Addiction Research & Theory, 25(1), 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Kroenke K, Williams JB, & Löwe B (2006). A brief measure for assessing generalized anxiety disorder: The GAD-7. Archives of Internal Medicine, 166(10), 1092–1097. [DOI] [PubMed] [Google Scholar]
- Tate RL, Perdices M, Rosenkoetter U, Shadish W, Vohra S, Barlow DH, … Wilson B (2016). The single-case reporting guideline in BEhavioural interventions (SCRIBE) 2016 statement. Physical Therapy, 96(7), e1–e10. 10.2522/ptj.2016.96.7.e1 [DOI] [PubMed] [Google Scholar]
- Taylor DJ, & Pruiksma KE (2014). Cognitive and behavioural therapy for insomnia (CBT-I) in psychiatric populations: A systematic review. International Review of Psychiatry, 26(2), 205–213. 10.3109/09540261.2014.902808 [DOI] [PubMed] [Google Scholar]
- Troxel WM, Germain A, & Buysse DJ (2012). Clinical Management of Insomnia with brief behavioral treatment (BBTI). Behavioral Sleep Medicine, 10(4), 266–279. 10.1080/15402002.2011.607200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Zweerde T, Bisdounis L, Kyle S, Lancee J, & Van Straten A (2019). Long-term effects of cognitive behavioral therapy for insomnia: A meta-analysis. Sleep Medicine Reviews, 48, 101208. [DOI] [PubMed] [Google Scholar]
- van Straten A, van der Zweerde T, Kleiboer A, Cuijpers P, Morin CM, & Lancee J (2018). Cognitive and behavioral therapies in the treatment of insomnia: A meta-analysis. Sleep Medicine Reviews, 38, 3–16. 10.1016/j.smrv.2017.02.001 [DOI] [PubMed] [Google Scholar]
- Watson D, & O’Hara MW (2017). Development of the IDAS and IDAS-II. In Understanding the Emotional Disorders. New York, NY: Oxford University Press. Retrieved from https://www.oxfordclinicalpsych.com/view/10.1093/med:psych/9780199301096.001.0001/med-9780199301096-chapter-2 [Google Scholar]
- Watson D, O’Hara MW, Naragon-Gainey K, Koffel E, Chmielewski M, Kotov R, … Ruggero CJ (2012). Development and validation of new anxiety and bipolar symptom scales for an expanded version of the IDAS (the IDAS-II). Assessment, 19(4), 399–420. 10.1177/1073191112449857 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


