Summary
Production of the high‐value carotenoid astaxanthin, which is widely used in food and feed due to its strong antioxidant activity and colour, is less efficient in cereals than in model plants. Here, we report a new strategy for expressing β‐carotene ketolase and hydroxylase genes from algae, yeasts and flowering plants in the whole seed using a seed‐specific bidirectional promoter. Engineered maize events were backcrossed to inbred maize lines with yellow endosperm to generate progenies that accumulate astaxanthin from 47.76 to 111.82 mg/kg DW in seeds, and the maximum level is approximately sixfold higher than those in previous reports (16.2–16.8 mg/kg DW) in cereals. A feeding trial with laying hens indicated that they could take up astaxanthin from the maize and accumulate it in egg yolks (12.10–14.15 mg/kg) without affecting egg production and quality, as observed using astaxanthin from Haematococcus pluvialis. Storage stability evaluation analysis showed that the optimal conditions for long‐term storage of astaxanthin‐rich maize are at 4 °C in the dark. This study shows that co‐expressing of functional genes driven by seed‐specific bidirectional promoter could dramatically boost astaxanthin biosynthesis in every parts of kernel including embryo, aleurone layer and starch endosperm other than previous reports in the starch endosperm only. And the staple crop maize could serve as a cost‐effective plant factory for reliably producing astaxanthin.
Keywords: astaxanthin, maize, laying hens, metabolic engineering, bidirectional promoter, multigene expression
Introduction
Astaxanthin, a lipid‐soluble carotenoid in the xanthophyll class, is synthesized primarily by various marine microalgae and rarely reported in higher plants. Despite astaxanthin accumulation in the carapace and flesh of marine animals, such as lobster, shrimp, krill, crabs, salmon, red snapper and rainbow trout, animals are unable to synthesize it de novo and must obtain it from astaxanthin‐containing algae (Higuera‐Ciapara et al., 2006; Kurnia et al., 2015). In other animals, such as poultry and birds, astaxanthin‐containing feed can dye their feathers, skin or eggs with attractive colours. Astaxanthin contains 13 conjugated double bonds with an alternating polar–nonpolar–polar structure (McNulty et al., 2008) which allows it to span cellular and organelle membranes and thus exhibit stronger antioxidant activity against lipid peroxidation than zeaxanthin and lutein (Hussein et al., 2006; Rao et al., 2015). Astaxanthin can significantly reduce oxidative damage to DNA, proteins and lipids, decrease inflammation and enhance the immune response in humans. Astaxanthin has health‐promoting effects for the prevention and treatment of numerous diseases related to ageing in humans and animals, including cardiovascular disease and age‐related cognitive and memory dysfunction (Fassett and Coombes, 2011; Goswami et al., 2010).
Currently, astaxanthin is widely used in feed, foods, nutraceuticals, pharmaceuticals and cosmetics (Ambati et al., 2014). Chemical synthesis and extraction from the microalga Haematococcus pluvialis are the main methods used to manufacture nature astaxanthin (Koller et al., 2014). Astaxanthin derived from H. pluvialis can account for up to 4%–5% of the dry weight (DW) of the alga (Wayama et al., 2013) and is used in supplements for human consumption and for colouring fish and poultry feeds (Shah et al., 2016). However, microalgae‐derived astaxanthin contributed to less than 1% of commercial production due to its high costs and technological barriers (Ambati et al., 2019; Koller et al., 2014; Olaizola, 2003). Synthetic astaxanthin, consisting of the stereoisomers (3S, 3′S), (3R, 3′S) and (3R, 3′R) at a ratio of 1 : 2 : 1, exhibits 20‐fold lower antioxidant capacity compared to astaxanthin from H. pluvialis (Koller et al., 2014; Lorenz and Cysewski, 2000; Megdal et al., 2009). The stereoisomer of astaxanthin extracted from H. pluvialis is the natural form (3S, 3′S) and the most valuable stereoisomer (Shah et al., 2016). Meanwhile, synthetic astaxanthin exhibits different stereochemistry and may be contaminated with by‐products, which makes it less suitable for direct consumption by humans (Shah et al., 2016). The cost of synthetic astaxanthin for the salmon feed industry is $2500 per kg, and the annual market value has reached $200 million. The overall animal feed market for astaxanthin was $300 million in 2009 and has been predicted to increase to $800 million by 2020. The nutraceutical market for astaxanthin is expected to also increase dramatically, reaching $300 million by 2020 (Ambati et al., 2019).
To develop an efficient and low‐cost approach to producing astaxanthin with high bioactivity, plants have been employed by various research teams, as astaxanthin may accumulate in plant plastids (Farré et al., 2016; Hasunuma et al., 2008; Huang et al., 2012, 2013; Mann et al., 2000; Park et al., 2017; Xie et al., 2019; Zhong et al., 2011; Zhu et al., 2018). The bkt (β‐carotene ketolase) and bhy (β‐carotene hydroxylase) genes from algae have been widely applied in various plant metabolic engineering contexts for astaxanthin production. Tobacco was the first plant used for astaxanthin production, through expression of the HpCrtO gene (a β‐carotene ketolase from H. pluvialis) integrated into its genome (Mann et al., 2000). Using chloroplastic metabolic engineering to express CrtW and CrtZ in tobacco leaves led to the accumulation of up to 5.44 mg/g DW of astaxanthin (Hasunuma et al., 2008). A breakthrough study of tomato plants co‐expressing Crbkt from Chlamydomonas reinhardtii and Hpbhy from H. pluvialis reported the production of 16.1 mg/g DW astaxanthin in tomato fruits (Huang et al., 2013). However, the high‐water contents of fresh tobacco leaves and tomato fruits limit their storage duration in large‐scale production. Maize is one of the three main staple crops worldwide, making it a cost‐effective platform for the manufacture of astaxanthin in seed. Transgenic maize harbouring Crbkt and BrCrtZ that was generated through particle delivery system‐mediated transformation produced 16.77 mg/kg DW astaxanthin in its endosperm (Farré et al., 2016). Recently, the variety aSTARice was reported to accumulate 16.23 mg/kg DW astaxanthin in the rice endosperm after four synthetic genes related to carotene biosynthesis were introduced (Zhu et al., 2018).
In this study, we selected maize seed as a platform for the manufacture of astaxanthin through modification of its carotene biosynthesis pathway. Using the regulated ‘source‐flux‐sink’ strategy by simultaneously providing sufficient phytoene as substrates, adjusting metabolic knot to enforce β‐carotene pathway and choosing enzymes of three origins: the phytoene synthase gene (ZmPSY1) from maize and the phytoene desaturase gene (PaCrtI) from Pantoea ananatis were combined with three pairs of β‐carotene hydroxylase genes and β‐carotene ketolase genes from a flowering plant, an alga and a yeast respectively. All these measures dramatically broaden the metabolic flux to synthesis astaxanthin in maize seeds to generate diverse astaxanthin‐rich maize events. Also, the bidirectional promoter facilitates to generate compact vectors in size and make synchronism of the paired genes’ expression. A feeding trial with laying hens indicated that the astaxanthin‐rich maize seed could be used as a reliable source of astaxanthin as H. pluvialis for the feed industry.
Results
Construct design for astaxanthin biosynthesis in maize kernels
According to the biosynthesis pathway of astaxanthin (Figure 1), lycopene is an important node in the precursor supply chain for astaxanthin biosynthesis, and there are several biosynthetic pathways for converting β‐carotene or zeaxanthin to astaxanthin through catalysis by various enzymes in astaxanthin‐producing organisms. Thus, the ‘source‐flux‐sink’ strategy for high‐yield production of astaxanthin in maize is carried out as follows: First, precursor source broadening, to increase the supply of the upstream precursor lycopene to support astaxanthin biosynthesis, all constructs (Figures 2a and 3a) contained the phytoene synthase gene (ZmPSY1) from maize and phytoene desaturase gene (PaCrtI) from P. ananatis, which catalyses the reactions from geranylgeranyl diphosphate to phytoene and then lycopene respectively (Figure 1, in green). Second, adjusting the lycopene flux diversion, the expression of LCY‐e which catalyses the reaction of lycopene to α‐carotene was knocked down by RNAi to ensure that the β‐carotene pathway was favoured (Figure 1, in grey). Finally, astaxanthin sink enhancement, to enhance the throughput of the reactions converting β‐carotene or zeaxanthin into astaxanthin, we overexpressed various combinations of β‐carotene ketolase and β‐carotene hydroxylase genes in the seed (Figure 1, in red and box with dotted outline). In total, eight genes were used, including ZmPSY1 from the Zea mays inbred line B73; PaCrtI from P. ananatis; CrBKT from C. reinhardtii; HpCrtZ from H. pluvialis; XdCrtS and XdCrtR from Xanthophyllomyces dendrorhous; AdCBFD1 and AdHBFD1 from Adonis aestivalis. All genes were codon optimized for maize, except ZmPSY1 and then fused with a transit peptide from maize ribulose bisphosphate carboxylase small subunit 1 (ZmSSU1) for plastid targeting. Each pair of genes was fused to both ends of a seed‐specific bidirectional promoter (Liu et al., 2018a) forming two frames as a module, and different modules were then combined to form different constructs (Figures 2a and 3a). The bidirectional promoter is the intergenic region of a divergent gene pair that mostly co‐expressed and usually involved in the same pathway (Krom and Ramakrishna, 2008). One bidirectional promoter directed two genes expression simultaneously could reduce the construct size which could facilitate complex metabolic engineering (Liu et al., 2018b).
Figure 1.

Schematic representation of reconstructed astaxanthin biosynthesis pathways in maize seed. Green indicates the enhancement of early precursor biosynthesis due to the expression of ZmPSY1 and PaCRTI. Grey represents a competitive metabolic pathway that converts lycopene into lutein; we aimed to limit lycopene conversion to lutein by down‐regulating the expression of LCY‐e. Red shows our attempt to produce astaxanthin through the elongation of the β‐carotene biosynthesis pathway. The box with the black dotted outline contains the details of the strategy highlighted in red. Hydroxylases (CBFD/P450/CrtZ) and ketolases (HBFD/BKT/P450) were employed to convert the β‐rings of β‐carotene into 3‐hydroxy‐4‐keto‐β‐rings. GGPS, geranylgeranyl pyrophosphate synthase; LCY‐e, lycopene ɛ‐cyclase; LCY‐b, lycopene β‐cyclase; ABA, abscisic acid.
Figure 2.

Development of astaxanthin‐rich maize. (a) Schematic diagram of the constructs pBDEN‐CP‐BZ and pBDEN‐CP‐BZ‐RNAi, functional genes and sense (lcy‐e) and anti‐sense (lcy‐e′) fragments of ZmLCY‐e directed by the seed‐specific bidirectional promoter PR5SGPA. (b–g) Engineered astaxanthin‐rich maize seeds at different developmental stages; (b–d) seeds derived from pBDEN‐CP‐BZ; (e–g) seeds derived from pBDEN‐CP‐BZ‐RNAi; (b, e) at 20 days after pollination (DAP); (c, f) at 30 DAP; (d, g) mature dry seeds. (h–l) High‐performance liquid chromatography (HPLC) analysis of astaxanthin in dried astaxanthin‐rich maize seeds; (h) a mixture of all‐E, 9Z and 13Z astaxanthin standards; (i) the transgenic event LX68‐1, derived from pBDEN‐CP‐BZ; (j) the transgenic event LX71‐2, derived from pBDEN‐CP‐BZ‐RNAi; (k) the yellow endosperm inbred line Z58 used as the recurrent parent; (l) quantitative analysis of isomers and total astaxanthin. all‐E, all‐trans geometric isomer; 9Z, cis‐9 geometric isomer; 13Z, cis‐13 geometric isomer. All data were derived from three technical repetitions.
Figure 3.

Evaluation of backcrossed astaxanthin‐rich maize. (a–d) BC3F1 seeds from event LX71‐2 crossed with inbred line Z58 (a), C7‐2 (b), NM28 (c) and XF28 (d), respectively. (e) Quantitative analysis of isomers and total astaxanthin in backcrossed astaxanthin‐rich maize seeds. All data were derived from three technical repetitions.
Generation and characterization of astaxanthin‐rich maize germplasm
According to the design described above and the literature, we chose the modules CP (PaCrtI::BDEN::ZmPSY1), BZ (CrBKT::BDEN::HpCrtZ) and LCYERNAi to form two constructs, pBDEN‐CP‐BZ and pBDEN‐CP‐BZ‐LCYERNAi. The Agrobacterium‐mediated transformation was employed to introduce the constructs into Hi‐II maize, generating stable transgenic maize events. Polymerase chain reaction (PCR) and reverse transcription PCR (RT‐PCR) were carried out using genomic DNA and cDNA from the transgenic events as templates respectively. The results confirmed that the functional genes and sequences for the hairpin of the LCY‐e RNAi construct were successfully integrated into the genome and transcribed as expected (Figure S1). As Hi‐II, like other maize varieties with white endosperm, contains multiple copies of carotenoid cleavage dioxygenase 1 (CCD1) genes, which encode enzymes catalysing carotenoids (lycopene, β‐carotene and zeaxanthin, also the right precursor of astaxanthin) degradation and interfering these substances accumulation in endosperm (Tan et al., 2017; Vogel et al., 2008), all T0 plants were crossed to the inbred line Z58 (yellow endosperm, only low copy CCD1 genes and thus sufficient carotenoids could be accumulated and converted to astaxanthin efficiently in it) to generate T1 seeds. Two developmental stages (at 20 and 30 days after pollination [DAP]) of T1 seeds derived from the pBDEN‐CP‐BZ (Figure 2b–d) and pBDEN‐CP‐BZ‐RNAi (Figure 2e–g) constructs were reddish‐orange in colour due to pigment accumulation throughout the seed.
To analyse the composition of the pigment produced, high‐performance liquid chromatography (HPLC) and mass spectrometer (MS) were used to analyse the dry mature reddish‐orange T1 seeds. According to astaxanthin standards, both constructs derived transgenic maize successfully produced astaxanthin in seeds (Figures 2h,i and S2). The astaxanthin level in event LX68‐1, derived from pBDEN‐CP‐BZ, was 73.65 mg/kg DW, and the levels in events LX71‐1, LX71‐2 and LX71‐8, which were all derived from pBDEN‐CP‐BZ‐RNAi, were 46.76, 52.40 and 58.67 mg/kg DW, respectively (Figure 2h,i). The isomer composition of astaxanthin in transgenic maize included all‐E, 9Z and 13Z, which is similar to that in H. pluvialis. The all‐E form of astaxanthin was the dominant component in events LX71‐1, LX71‐2 and LX71‐8, whereas the content of 9Z astaxanthin (42.49 mg/kg DW) was higher than that of the all‐E type (20.38 mg/kg DW) in event LX68‐1 (Figure 2i). Because the total content of astaxanthin (46.76–73.65 mg/kg DW) in these engineered maize events is higher than those produced in previous reports of astaxanthin metabolic engineering in cereals (0.2–16.8 mg/kg DW; Bai et al., 2017; Farré et al., 2016; Zhu et al., 2018), we designated these engineered maize events as astaxanthin‐rich maize.
Backcross breeding for high‐yielding astaxanthin‐rich maize
It has been reported that colours of dark or light yellowish maize germplasms are due to differing contents of β‐carotene or zeaxanthin in the endosperm. To test whether yellow endosperm maize germplasm affected the accumulation of astaxanthin and screen the best recurrent parents for astaxanthin‐rich maize breeding, we gathered 19 elite inbred lines with yellow endosperm as female parents to cross with event LX71‐2. Based on qualitative analysis of seed colour and quantification of astaxanthin content, four inbred lines—Z58, C7‐2, NM28 and NF28, which were the parents of the hybrid maize varieties ZD958 and ND28, respectively, were chosen as suitable recurrent parents. On the other hand, ZD958 and ND28 are the most widely cultivated hybrid maize varieties in China for many years because their stable and higher yields and the purpose of using them as background is to make most high‐yielding astaxanthin‐rich maize. BC3F1 seeds from the four backcrossed inbred lines exhibited a distinct orange‐red colour (Figure 3a–d). PCR analysis of these lines’ genomes confirmed that the four transgenes and LCYERNAi were stably integrated (Figure S1). Quantification of astaxanthin using HPLC revealed that 64.11, 48.54, 52.22 and 42.46 mg/kg DW astaxanthin were present in backcrossed Z58, C7‐2, NM28 and NF28, respectively (Figure 3e). These four inbred lines could be used as parents for improved ZD958 and ND28 hybrid maize varieties after further backcrossing.
Exploration of new ketolase and hydroxylase genes for astaxanthin biosynthesis
To explore novel ketolase and hydroxylase genes for high‐level production of astaxanthin, we first applied the ketolase and hydroxylase genes, AdCBFD1 and AdHBFD1 from Adonis aestivalis (Cunningham and Gantt, 2011), which could naturally covert carotene into astaxanthin in higher flowering plants, for plant metabolic engineering of astaxanthin biosynthesis. We also tested the efficiency of astaxanthin biosynthesis using ketolase and hydroxylase combinations from a microalga, a yeast and a higher flowering plant. For those purposes, we synthesized AdCBFD1, AdHBFD1, XdCrtS and XdCrtR with codon optimization for maize and fused each gene to a maize plastid targeting peptide, SSU1, along with previously synthesized PaCrtI, HpCrtZ and CrBKT, and ZmPSY1 cloned from maize, resulting in eight genes directed by four seed‐specific bidirectional P2BDEN promoters (Liu et al., 2018a) in four frames: CP (Pa C rtI::2BDEN::Zm P SY1), CH (Ad C BFD1::2BDEN:: Ad H BFD1), BZ (Cr B KT::2BDEN::HpCrt Z ) and SR (XdCrt S ::2BDEN:: XdCrt R ). Seven constructs were derived from stacking these frames (Figure 4a). We conducted Agrobacterium‐mediated transformation of Hi‐II hybrid maize with these seven constructs to generate transgenic maize, and all T0 plants were crossed with the inbred line Z58 to generate T1 seeds. PCR analysis confirmed the presence of the transgenes for six constructs in the corresponding transgenic maize events, but the construct p2BDEN‐CP‐BZ‐CH failed to generate young shoots from bialaphos‐resistant callus (Figure S3).
Figure 4.
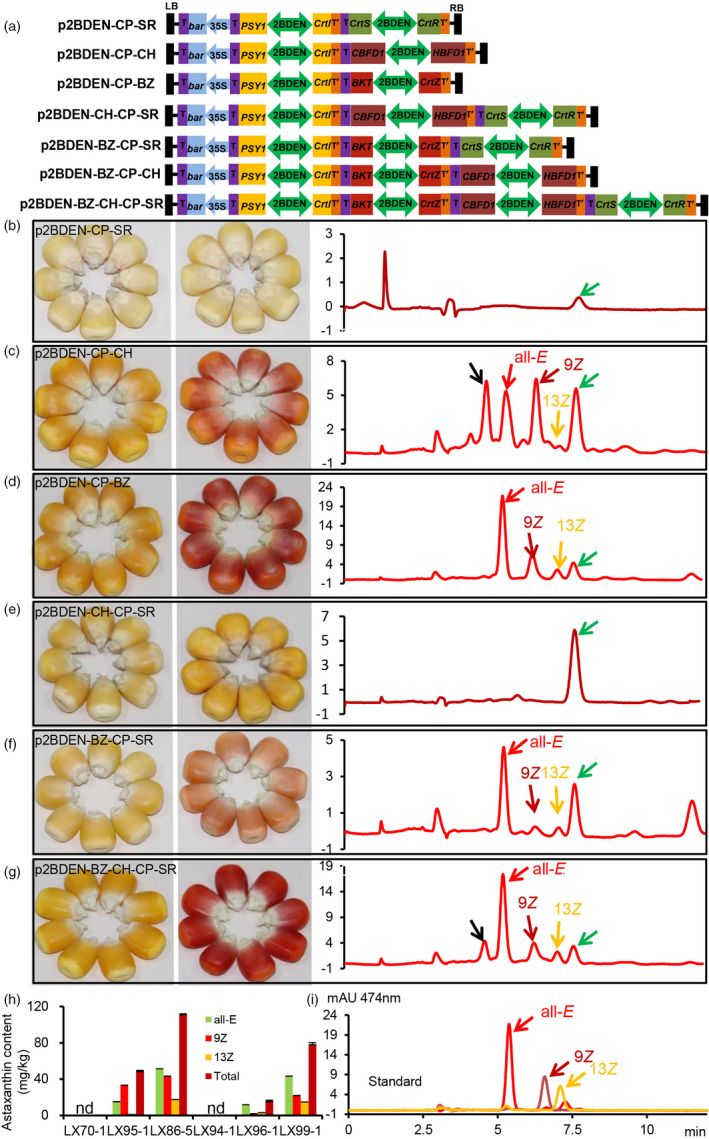
Analysis of F1 progenies of astaxanthin‐rich maize generated using new ketolase and hydroxylase gene pairs. (a) Schematic diagram of constructs. (b–g) Transgenic maize seeds derived from each type of constructs. (b, e) transgenic maize samples derived from ‘SR’ and ‘CH+SR’ showing no astaxanthin peak; (c) samples derived from ‘CH’ showing a unique unidentified peak (black arrow) and three astaxanthin peaks; (d, f) samples derived from ‘BZ’ and ‘BZ + SR’ showing the same peaks as astaxanthin isomer standards; (g) samples derived from ‘BZ + CH + SR’ containing all peaks that appear in maize derived from other constructs. The peak indicated with a green arrow was a common unknown peak in all samples. (h) Quantitative analysis of isomers and total astaxanthin. (i) A mixture of all‐E, 9Z and 13Z astaxanthin standards. nd, not detected. All data were derived from three technical repeats.
According to the colour of transgenic maize seed derived from these constructs (Figure 4b–g), five out of six constructs supported successful pigment accumulation (Figure 4c–g), with the exception of p2BDEN‐CP‐SR (Figure 4b). HPLC analysis showed that the events derived from p2BDEN‐CP‐CH (Figure 4c), p2BDEN‐CP‐BZ (Figure 4d), p2BDEN‐BZ‐CP‐SR (Figure 4f) and p2BDEN‐BZ‐CH‐CP‐SR (Figure 4g) biosynthesized the all‐E, 9Z and 13Z isomers of astaxanthin in transgenic maize seeds, whereas the p2BDEN‐CP‐SR‐ and p2BDEN‐CP‐CH‐SR‐derived events failed to produce astaxanthin (Figure 4b,e). Quantitative analysis showed that the contents of astaxanthin in transgenic maize harbouring CH and BZ were 49.23 and 111.82 mg/kg DW, respectively (Figure 4c,d,h). However, when SR was co‐expressed with CH (p2BDEN‐CP‐CH‐SR), no astaxanthin biosynthesis was detected in the resulting maize (Figure 4b,h); when SR was co‐expressed with BZ (p2BDEN‐BZ‐CH‐SR), the content of astaxanthin decreased from 111.82 mg/kg DW to 16.28 mg/kg DW (Figure 4f,h); and when SR, CH and BZ were expressed simultaneously (p2BDEN‐BZ‐CH‐CP‐SR), the content of astaxanthin was 78.70 mg/kg DW (Figure 4g,h). These results indicate that SR from yeast alone could not drive the biosynthesis of astaxanthin in maize, whereas both CH from a flowering plant and BZ from an alga could. In addition, SR supports the conversion of β‐carotene to astaxanthin in yeast but might have played a negative role in astaxanthin biosynthesis in maize, whereas CH and BZ clearly played positive roles.
Functional evaluation of astaxanthin from astaxanthin‐rich maize through a feeding trial with laying hens
To evaluate the bioactivity of maize‐derived astaxanthin, we used astaxanthin‐rich maize in place of non‐transgenic maize in feed for laying hens. First, to determine the optimal amount of astaxanthin in the feed, we added powdered H. pluvialis containing 2.84% astaxanthin to obtain feed with different contents of astaxanthin (0, 5, 10, 15, 30 and 150 mg/kg). The yolk colour transitioned from yellow to red with increasing astaxanthin content in the feed (Figure 5a). We selected 30 mg astaxanthin per kg feed as the optimal concentration for astaxanthin‐added feed, mainly based on the cost of adding astaxanthin and the degree of redness of the egg yolk. Meanwhile, based on the reddening of the yolk (Figure 5b), as measured using brightness (L*), red (a*) and yellow (b*) values (Figure S4a), and the content of astaxanthin in the yolk (Figure S4b), it appears that that yolk astaxanthin content gradually increased in laying hens provided feed containing 30 mg/kg astaxanthin, with the content stabilizing after 8 days and then gradually falling to zero at 14 days after the astaxanthin supply was stopped (Figures 5b and S4). Additionally, we used astaxanthin‐rich maize (hybrid maize variety ND28, derived from NM28*LX71‐2 and NF28*LX71‐2; Figure S5) to replace all non‐transgenic maize in the laying hens’ feed (Table 1). Thus, the new feed contained 24.32 mg/kg astaxanthin, whereas the control feed contained no astaxanthin. Algal powder (with an astaxanthin content of 2.84%) was added to another batch of feed such that it had the same astaxanthin content as astaxanthin‐rich maize feed. As shown in Figure 5c, the yolks from hens fed astaxanthin‐rich maize and algal powder‐fortified feed were red, whereas yellow yolks were observed in the control feed group. HPLC analysis showed that laying hens could take up astaxanthin from the feed and accumulate it into the egg yolks at week 4 (algal powder‐fortified feed group, 13.67 mg/kg; astaxanthin‐rich maize group, 14.15 mg/kg) and week 8 (algal powder‐fortified feed group, 12.10 mg/kg; astaxanthin‐rich maize group, 13.40 mg/kg; Figure 5d). Moreover, astaxanthin isomers found in the yolks matched those of the corresponding astaxanthin sources. However, compared to the isomer compositions in astaxanthin‐rich maize and algal powder, yolks from both groups contained increased levels of the 13Z astaxanthin isomer, which indicates that laying hens could convert other isomers of astaxanthin to 13Z (Figure 5d).
Figure 5.

Functional evaluation of astaxanthin from astaxanthin‐rich maize based on a feeding trial with laying hens. (a) Screening of the optimal amount of astaxanthin to add via the addition of Haematococcus pluvialis algal powder containing 2.84% astaxanthin to laying hen feed. A0, no addition; A5–A150, feeds with final astaxanthin contents of 5, 10, 15, 30 and 150 mg/kg, respectively. (b) Profile of accumulated astaxanthin in egg yolks produced by laying hens provided feed containing astaxanthin (24.32 mg/kg) from day 0 to day 14. (c) Egg yolks from hens in different groups. C, provided feed containing no astaxanthin; A, provided feed containing astaxanthin from algal powder; T, provided feed containing astaxanthin from transgenic maize. (d) Quantitative analysis of isomers and total astaxanthin contents in egg yolks at weeks 4 and 8 in the three treatments.
Table 1.
Composition of experimental diets for laying hens
| Ingredient | Control group | Astaxanthin group | Transgenic maize group |
|---|---|---|---|
| Corn (%) | 63.00 | 63.00 | |
| Astaxanthin corn (%) | 63.00 | ||
| Soybean meal (%) | 26.30 | 26.30 | 26.30 |
| Limestone (%) | 8.90 | 8.90 | 8.90 |
| Premix * (%) | 0.65 | 0.564 | 0.65 |
| CaHPO4 (%) | 1.00 | 1.00 | 1.00 |
| DL‐Met (%) | 0.15 | 0.15 | 0.15 |
| Astaxanthin (2.84%; %) | 0.086 | ||
| Total | 100.00 | 100.00 | 100.00 |
| Nutrient level † | |||
| ME (MJ/kg) | 11.17 | 11.17 | 11.17 |
| Astaxanthin (mg/kg) | 0.00 | 24.32 | 24.32 |
| Crude protein (%) | 16.50 | 16.50 | 16.50 |
| Calcium (%) | 3.36 | 3.36 | 3.36 |
| Non‐phytate phosphorus (%) | 0.34 | 0.34 | 0.34 |
| Lysine (%) | 0.86 | 0.86 | 0.86 |
| Methionine (%) | 0.42 | 0.42 | 0.42 |
| Met + Cys (%) | 0.72 | 0.72 | 0.72 |
| Lys/Met (%) | 2.06 | 2.06 | 2.06 |
Premix provides the following per kg of diet: VA 12 500 IU, VD3 425 IU, VE 15 IU, VK 2 mg, VB1 0.98 mg, VB2 8.5 mg, VB6 8 mg, D‐pantothenic acid 50 mg, niacin 32.5 mg, biotin 2 mg, folic acid 5 mg, VB12 5 mg, Cu (as copper sulphate) 8 mg, I (as potassium iodide) 1 mg, Fe (as ferrous sulphate) 60 mg, Se (as sodium selenite) 0.3 mg, Mn (as manganese sulphate) 65 mg, Zn (as zinc sulphate) 66 mg, phytase 500 mg, NaCl 3 g, choline 0.5 g, ethoxyquinoline ethoxyquin 100 mg.
Nutritional indicators except for metabolic energy and non‐phytate phosphorus were measured. ME and AP were calculated values, and others were measured values.
Meanwhile, to determine the effects of astaxanthin‐rich maize on the production performance of laying hens and egg quality, feed intake was measured biweekly, egg production was measured daily, health status was checked daily and egg quality was determined at weeks 4 and 8. Dietary supplementation of astaxanthin did not affect egg production, daily egg mass, egg weight or egg ratio (P > 0.05), although a significant difference was found in the daily feed intake of hens (P < 0.05; Table S1). Astaxanthin‐rich maize had no effects on egg shape, shell strength, albumen height, Haugh unit score, yolk percentage, shell percentage or albumen percentage based on comparisons between hens consuming the control diet and those consuming the astaxanthin‐added or astaxanthin‐rich maize diet (Table S2). The values for each egg quality characteristic were similar between the control and test groups (P > 0.05), and all eggs were graded as grade AA quality.
Taken together, consumption of astaxanthin‐rich maize as well as astaxanthin in H. pluvialis algal powder enhances astaxanthin incorporation into eggs without adverse effects on the production performance of laying hens or egg quality.
Evaluation of storage stability of astaxanthin‐rich maize
Astaxanthin is highly sensitive to oxygen, temperature and light, and previous research showed that storage temperature and air exposure affected the stability of astaxanthin from H. pluvialis (Ahmed et al., 2015). Therefore, it is necessary to test the stability of astaxanthin in astaxanthin‐rich maize to facilitate its future application. We divided the event LX71‐2‐derived BC3F1 seeds Z58*LX71‐2, C7‐2*LX71‐2, NM28*LX71‐2 and NF28*LX71‐2 into four batches, which were stored in the dark at room temperature, 4, −20 °C or −80 °C. HPLC quantitative analysis showed that the astaxanthin contents in Z58*LX71‐2 seeds were 45.74, 74.81, 89.98 and 99.99 mg/kg DW after 7 months of storage at room temperature, 4, −20 and −80 °C, respectively (Figure 6). Thus, the astaxanthin contents in Z58*LX71‐2 seeds were negatively correlated with storage temperature. Similar trends were observed in C7‐2*LX71‐2, NM28*LX71‐2 and NF28*LX71‐2 seeds (Figure 6). These results suggest that low temperatures maintain the stability of astaxanthin in maize kernels. However, from an economic perspective and for the practicality of large‐scale storage, 4 °C is the optimal temperature.
Figure 6.

Evaluation of storage stability of astaxanthin in transgenic maize. The event LX71‐2‐derived BC3F1 seeds were divided into four batches, which were stored in the dark at different temperature. HPLC was employed to analyse the content of astaxanthin after 7 months. Z58, C7‐2, NM28 and NF28 were four inbred maize lines; RT, room temperature. All data were derived from three technical repetitions.
Discussion
Production of high‐value astaxanthin in heterologous hosts is a popular aim of metabolic engineering, and plants are an optimal platform due to the low cost, ease in scaling up, lack of safety concerns and abundant native enzymes for the astaxanthin biosynthesis pathway (Giddings et al., 2000; Hirschberg, 2001; Sharma and Sharma, 2009). To date, tobacco, Arabidopsis, tomato, potato, carrot, lettuce, maize and rice have been employed for the metabolic engineering of astaxanthin biosynthesis (Campbell et al., 2015; Farré et al., 2016; Harada et al., 2014; Hasunuma et al., 2008; Huang et al., 2013; Jayaraj et al., 2008; Zhong et al., 2011; Zhu et al., 2018). Generally speaking, astaxanthin accumulation levels in engineered dicotyledons—namely, 5.44 mg/g DW in tobacco leaves (Hasunuma et al., 2008) and 16.1 mg/g DW in tomato fruits (Huang et al., 2013)—have been higher than those in monocotyledon cereals [e.g. 16.2 mg/kg DW in rice seeds (Zhu et al., 2018) and 16.8 mg/kg DW in maize seeds (Farré et al., 2016)]. The high‐water contents of fresh tobacco leaves and tomato fruits limit their storage duration in large‐scale production; therefore, in this study, we selected maize seeds as a platform for astaxanthin production. At harvest, the engineered maize seeds accumulated high levels of astaxanthin (47.76–111.82 mg/kg DW), approximately sixfold higher than those in previous reports (16.2–16.8 mg/kg DW) using rice and maize (Farré et al., 2016; Zhu et al., 2018). Our T1 transgenic maize accumulated lower levels of astaxanthin in white endosperm germplasm than in backcrossed BC1F1 yellow endosperm germplasm (Figure S6), indicating that the lack of a native β‐carotene supply may be a limiting factor for the production of high astaxanthin levels. Our engineered maize seeds could accumulate higher levels of astaxanthin than transgenic maize seeds in a previous report due to differing promoter characteristics. We employed a seed‐specific bidirectional promoter that can direct gene pairs to be expressed throughout the seeds, including in the embryo, aleurone layer and endosperm (Liu et al., 2018a) whereas an endosperm‐specific promoter was used in the previous report that did not boost astaxanthin yields in the aleurone layer and embryo (Farré et al., 2016). The aleurone layer and embryo in maize are important biologically and nutritionally, as endosperm cells undergo programmed cell death during seed maturation (Becraft and Yi, 2010). The colour of the aleurone layer and embryo in engineered maize is darker than that of the endosperm (Figure 2c,f), indicating that the aleurone layer and embryo may account for an important proportion of the total astaxanthin content in engineered maize.
Previously, most β‐carotene hydroxylase and ketolase genes used for metabolic engineering of astaxanthin in plants originated from algae. In this study, for the first time, the β‐carotene hydroxylase and ketolase genes AdCBFD1 and AdHBFD1 from the flowering plant Adonis aestivalis (Cunningham and Gantt, 2005, 2011) were employed in astaxanthin plant engineering research. A previous study comparatively analysed the activities of various algal carotenoid ketolases (Zhong et al., 2011); by contrast, we comparatively evaluated the effects of co‐expressing hydroxylase and ketolase gene pairs from an alga, a flowering plant and a yeast in astaxanthin‐rich engineered maize. We speculated that XdCrtS and XdCrtR, which belong to the P450 group, employ a unique pathway to convert β‐carotene to astaxanthin (Figure 1) that is less efficient but still competes for precursors, thus hampering astaxanthin production by AdCBFD1/AdHBFD1 or CrBKT/HpCrtZ. AdCBFD1/AdHBFD1 also participates in a unique pathway, but their 4‐keto‐β‐ring intermediate brings two pathways together (AdCBFD1/AdHBFD1 and CrBKT/HpCrtZ) (Figure 1), as evidenced by the negative effect of XdCrtS/XdCrtR being weakened in engineered maize events derived from the construct p2BDEN‐BZ‐CH‐CP‐SR (Figure 4g,n,o). Unfortunately, we failed to generate transgenic maize plants despite producing a bialaphos‐resistant callus from the construct p2BDEN‐BZ‐CP‐CH (Figure S3). Furthermore, β‐carotene is a precursor for the synthesis of the hormone abscisic acid (ABA; Figure 1), which plays roles in seed germination, embryo development and regeneration from callus (e.g. 26.4 μg/L ABA in a regeneration medium; Frame et al., 2002; Hirschberg, 2001). The dramatic increase in astaxanthin content in the callus reduced the synthesis of ABA and potentially had a negative impact on regeneration from the callus (Figure S3). Therefore, to generate a higher level of astaxanthin in engineered maize, expressing CrBKT/HpCrtZ throughout the seed and co‐expressing AdCBFD1/AdHBFD1 only in the endosperm may be the optimal strategy.
Synthetic astaxanthin and natural astaxanthin from algae have been commonly applied; however, astaxanthin from various engineered plants has seldom been tested in feed or other potential applications. Rainbow trout and Kampachi (Seriola rivoliana) fed on biofortified maize and soybean could accumulate astaxanthin in the flesh (Breitenbach et al., 2016; Park et al., 2017). The biofortified maize also used to feed laying hens to trace the fate of astaxanthin from maize after consumption, that is a new optical angle for astaxanthin nutriology. (Moreno et al., 2016). The minimum astaxanthin content in trout feed is 30 mg/kg (Kurnia et al., 2015), which is higher than that of previously reported engineered maize (16.8 mg/kg) and therefore requires extraction and enrichment (Breitenbach et al., 2016), whereas our astaxanthin‐rich maize (111.82 mg/kg; Figure 4d,h) could be directly applied to trout feed by replacing 40% of the carbohydrates in the feed with maize. Except fish feed, feed for poultry, pig, flamingo, aquarium fish, red and brown pets need astaxanthin and carbohydrates, and astaxanthin as the most valuable component takes the 10%–20% of salmon feed costs (Baker et al., 2002). The synthetic astaxanthin contributed to more than 97% of commercial market due to its relative low production costs ($1000/kg; Milledge, 2011; Schmidt et al., 2011). Microalgae‐derived astaxanthin contributed to less than 1% of commercial production due to its high production costs ($3000/kg), high market price ($7000/kg) and technological barriers (Ambati et al., 2019; Koller et al., 2014; Schmidt et al., 2011). However, the advantage of maize‐based astaxanthin in feeds verses synthetic / other natural sources is obvious. Take the laying hens feeding trials in this study for instance, the costs of feeds could divided into three parts: maize, astaxanthin and other nutrients. The costs of maize and astaxanthin of feeds for our astaxanthin‐rich maize are just maize, while for synthetic / other natural sources astaxanthin are maize plus the production costs of astaxanthin. This is the most convenient, economical and attractive application of our astaxanthin‐rich maize in the feed industry in the future. In this study, astaxanthin‐rich maize was directly applied to chicken feed (Figure 5) and laying hens successfully accumulated astaxanthin in the egg yolk (Figure 5c,d). Interestingly, some volunteer tasters could distinguish between the yolks and could not accept the fishy smell of the red‐yolk eggs from the algal‐derived feed group. Eggs from the astaxanthin‐rich maize group and control feed group had no such smell. Meanwhile, another interesting observation was that the colour of the algal powder changed quickly when its packaging bag was opened and stored at 4 °C, whereas the astaxanthin‐rich maize retained most of its astaxanthin when stored at 4 °C for 7 months (Figure 6).
In conclusion, we successfully elongated the β‐carotene biosynthesis pathway to obtain much higher astaxanthin levels in maize seeds than have been achieved previously in cereals. Ketolase and hydroxylase genes from a flowering plant were used as novel gene resources and coupled with a gene pair from algae for astaxanthin metabolic engineering in maize. Our astaxanthin‐rich maize, which has a high astaxanthin content, stable storage quality, similar isomer composition to that in H. pluvialis, and strong colourant capacity, could serve as a cost‐effective plant factory for the reliable production of astaxanthin in a staple crop.
Methods
Synthesis of foreign genes and expression vector construction
The medium vectors pBDEN‐mtp and p2BDEN‐mtp were derived from the binary vector pCAMBIA3301. A reversed 35S poly(A) terminator was located at the 5′ end of the seed‐specific bidirectional promoter PR5SGPA/P2R5SGPA, and its 3′ end was followed by a NOS terminator. Two restriction enzyme sites, EcoRI and XbaI, were fused to the 3′ end of 35S poly(A), and another two sites, PstI and BamHI, were added to the intergenic region between 35S poly(A) and PR5SGPA/P2R5SGPA; between PR5SGPA/P2R5SGPA and NOS were HindIII/NcoI, and SpeI was located at the 3′ end of NOS. Excluding ZmPSY1 (AY773475.1), the other seven foreign genes, PaCrtI (D90087.2), CrBKT (AY860820.1), HpCrtZ (AY187011.1), XdCrtS (HG939455.2), XdCrtR (LN554258.1), AdHBFD1 (DQ902555.1) and AdCBFD1 (AY644757.1), were synthesized by Convenience Biology (Changzhou, China) with a maize plastid‐transit peptide fused to the 5’ end of each gene. PstI/BamHI and HindIII/NcoI were added to each open frame that was used to transfer the synthesized genes to pBDEN‐mtp or p2BDEN‐mtp. XbaI and SpeI are isocaudomers, and therefore, EcoRI/XbaI and EcoRI/SpeI can be used to stack multiple cassettes to form expression constructs.
Generation of transgenic maize
Agrobacterium tumefaciens‐mediated transformation was employed to generate transgenic maize, as described previously (Frame et al., 2002). Immature embryos were isolated from Hi‐II maize ears at 9–12 DAP and treated with 5% sodium hypochlorite solution for 30 min. The immature embryos (length, 1.0–2.0 mm) were washed twice with liquid infection medium. Agrobacterium tumefaciens harbouring the expression vector was co‐cultured with 1% acetosyringone until its optical density at 550 nm reached 0.3–0.4. Immature embryos were transferred to Agrobacterium tumefaciens liquid culture for 5 min in the dark. Embryos were then transferred to infection medium and selection medium with 3 mg/L bialaphos for selection of bialaphos‐resistant calli. Regenerated transgenic T0 plants were identified using PCR and seed colour. Given that Hi‐II is a white endosperm hybrid cultivar, the yellow endosperm inbred line Z58 was used as the female parent and as recurrent parents for the production of F1 seeds and backcrossed progeny.
DNA/RNA preparation and quantitative RT‐PCR
Genomic DNA was isolated from transgenic maize leaves using cetrimonium bromide. Total RNA was extracted from 20‐DAP seeds using an RNA extraction kit from TransGen Biotech (Beijing, China). One‐step gDNA Removal and cDNA Synthesis SuperMix (TransGen Biotech) was used to synthesize first‐strand cDNA. The quality of cDNA was determined with primers targeting the maize ZmActin1 gene, which was also used as the internal normalization factor in maize. Primers used for quantitative RT‐PCR and PCR are listed in Tables S3 and S4.
Experimental design and diets
A total of 270 Hy‐Line Brown hens (28 weeks old) were randomly allocated to three dietary treatment groups (six replicates/treatment and 15 hens/replicate). The control group was fed non‐transgenic corn, treatment group A was fed the basal diet supplemented with astaxanthin powder (H. pluvialis algal powder containing 2.84% astaxanthin purchased from Alphy Biotech Co., Ltd., Yunnan, China), and treatment group T was fed astaxanthin‐rich maize with the same total astaxanthin content as in treatment A. Hen feeding formulations and the astaxanthin concentrations of the experimental diets are presented in Table 1. Hens were housed in wire cages (length × width × height, 57 × 47 × 47 cm) in an environmentally controlled room maintained at 26 °C and 45% relative humidity with a 16 : 8 light/dark schedule. Water and feed were provided ad libitum during 1 week of pre‐feeding and for 8 weeks in the experimental period.
Production performance
Feed intake was measured biweekly, whereas egg production was measured daily and the health status of each group of chickens was checked daily. During the test period, total egg weight and egg production were measured on a daily basis, and the number of soft, broken or abnormal eggs (various deformities, oversized and ultra‐small) was determined to calculate the average egg weight excluding such eggs. The feed conversion ratio was calculated as feed consumption (g) divided by the egg mass (g). The number of eggs produced was calculated over intervals of 2 weeks. Egg production, egg weight (EW), daily egg mass [total EW (g)/(number of hens × number of days)], daily feed intake [feed consumption (g)/(number of hens × number of days)], feed consumption over the whole test period and egg ratio (feed/egg) were also calculated.
Egg quality
Egg production was calculated as the total number of eggs divided by the total number of days and number of hens. A total of 18 eggs (three from each replicate) from each treatment were sampled on weeks 4 and 8 to determine egg quality. The eggs were analysed using an egg analyser (ORKA Food Technology Ltd., Herzliya, Israel) to measure Haugh units and yolk colour. An egg force reader (ORKA Food Technology Ltd.) was used to measure shell strength. Shell thickness was measured using an electronic micrometre. An analytical balance (BSA224S‐CW; Sartorius, Göttigen, Germany) was used to measure specific egg weight, yolk weight and shell weight. Egg yolk colour was measured via a spectrophotometric method using a Konica Minolta Chroma Meter (Tokyo, Japan). The results are expressed as values of L*, a* and b* according to the International Colorimetric System (CIE units, CIELAB). The parameter L* characterizes lightness (0, black; 100, white), a* describes changes in wavelength in the red spectral region with a maximum value of +120 for a bright red colour and a minimum value of −80 for a blue‐green colour, and b* represents the transition from yellow (+120) to blue‐purple (−80; Dvořák et al., 2005). All measurements were performed three times, and the final values were calculated as the average of the three measured values.
Astaxanthin extraction and quantitative HPLC analysis
Engineered maize dry seeds were crushed into powder. Astaxanthin was extracted from a mixture of 1.0 g of maize powder and 5.0 mL of methanol/tetrahydrofuran (50 : 50, v/v) in a 15.0‐mL tube, which was vortexed for 5 min. Then, the tubes were placed in a thermostatic bath at 60 °C for 20 min. After the addition of 5.0 mL ethyl acetate, samples were vortexed for 1 min and then centrifuged at 3220 g for 10 min at room temperature. The upper phase was collected and filtered with 0.45‐μm filters prior to HPLC analysis. Astaxanthin was extracted from the diets and egg yolks using the 2327–2009 fine‐tuned extraction conditions, with some modifications. Briefly, extracted astaxanthin was eluted isocratically with methanol and methyl tert‐butyl ether (15 : 81) using ultra‐pure water at a flow rate of 1 mL/min in an Agilent Eclipse plus C30 reverse‐phase column (5 μm, 4.6 × 250 mm; Santa Clara, CA) and an HPLC system (LC‐10AD vp pumps, SIL‐10Ai auto‐injector and an SPD‐10 AV vp UV–vis detector; Shimadzu, Kyoto, Japan). The column temperature was set to 30 °C. The mobile phase was sonicated at room temperature for 15 min before its use. Chromatographic peaks were identified through comparisons with the retention time of standard astaxanthin. To validate the results, sample extracts were spiked with standard astaxanthin to confirm that sample peaks appeared in the chromatogram at the same time as peaks due to the standard. Astaxanthin standards (all‐E CAS, 472‐61‐7; 9Z CAS, 113085‐04‐4; 13Z CAS, 113085‐05‐5) were purchased from Sigma‐Aldrich (St. Louis, MO). Astaxanthin enrichment was calculated as follows: astaxanthin content (μg/g) × fresh yolk weight as a proportion of total weight (g) divided by egg weight.
Statistical analysis
Data were pre‐processed with Microsoft Excel, and SPSS 23.0 statistical software (SPSS Inc., Chicago, IL) was used for data analysis. Data were transformed to obtain a normal distribution and then analysed using one‐way analysis of variance. Duncan's method was used for multiple comparisons, with P < 0.05 taken to indicate significant differences. The results were expressed as the means and standard deviations.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
XQ.L., H.W., QY.Z., JM.Z. and RM.C. designed the experiments. XQ.L., XH.M., H.W., RD.N., CH.T., LL.L. and SZ.L., performed experiments. XQ.L., XH.M., H.W., RD.N., WZ.Y., and XJ.Z., YL.F. and QY.Z. analysed and interpreted the data. XQ.L., H.W., RD.N and RM.C wrote the manuscript with input from all authors.
Supporting information
Figure S1 Integration and expression of foreign genes.
Figure S2 Astaxanthin was identified and confirmed by MS.
Figure S3 Integration of foreign genes and bialaphos‐resistant callus.
Figure S4 Effects of astaxanthin‐added feed on egg yolks.
Figure S5 Preparation of the hybrid maize variety ND28 for the laying hen feeding trial.
Figure S6 Effects of germplasm on astaxanthin engineering.
Table S1 Effect of astaxanthin‐rich maize on production performance of laying hens
Table S2 The effect of astaxanthin‐rich maize on egg quality of layer
Table S3 Primers used in PCR
Table S4 Primers used in RT‐qPCR
Acknowledgements
We appreciate discussion and useful suggestion provided by Prof. Bin Yao, Haiyang Wang, Zhengkui Zhou and Dr. Qingshi Meng and Huoqing Huang. This work was supported by the National Special Program for GMO Development of China (grant number 2016ZX08003‐002).
Liu, X. , Ma, X. , Wang, H. , Li, S. , Yang, W. , Nugroho, R. D. , Luo, L. , Zhou, X. , Tang, C. , Fan, Y. , Zhao, Q. , Zhang, J. and Chen, R. (2021) Metabolic engineering of astaxanthin‐rich maize and its use in the production of biofortified eggs. Plant Biotechnol. J., 10.1111/pbi.13593
Contributor Information
Junmin Zhang, Email: zhangjunmin@caas.cn.
Rumei Chen, Email: chenrumei@caas.cn.
References
- Ahmed, F. , Li, Y. , Fanning, K. , Netzel, M. and Schenk, P.M. (2015) Effect of drying, storage temperature and air exposure on astaxanthin stability from Haematococcus pluvialis. Food Res. Int. 74, 231–236. [DOI] [PubMed] [Google Scholar]
- Ambati, R.R. , Gogisetty, D. , Aswathanarayana, R.G. , Ravi, S. , Bikkina, P.N. , Bo, L. and Yuepeng, S. (2019) Industrial potential of carotenoid pigments from microalgae: current trends and future prospects. Crit. Rev. Food Sci. Nutr. 59(12), 1880–1902. [DOI] [PubMed] [Google Scholar]
- Ambati, R.R. , Phang, S.‐M. , Ravi, S. and Aswathanarayana, G.R. (2014) Astaxanthin: sources, extraction, stability, biological activities and its commercial applications—a review. Marine Drugs, 12, 128–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai, C. , Berman, J. , Farre, G. , Capell, T. , Sandmann, G. , Christou, P. and Zhu, C. (2017) Reconstruction of the astaxanthin biosynthesis pathway in rice endosperm reveals a metabolic bottleneck at the level of endogenous β‐carotene hydroxylase activity. Transgenic Res. 26, 13–23. [DOI] [PubMed] [Google Scholar]
- Baker, R.T.M. , Pfeiffer, A.M. , Schöner, F.J. and Smith‐Lemmon, L. (2002) Pigmenting efficacy of astaxanthin and canthaxanthin in fresh‐water reared Atlantic salmon, Salmo salar. Anim. Feed Sci. Technol. 99, 97–106. [Google Scholar]
- Becraft, P.W. and Yi, G. (2010) Regulation of aleurone development in cereal grains. J. Exp. Bot. 62, 1669–1675. [DOI] [PubMed] [Google Scholar]
- Breitenbach, J. , Nogueira, M. , Farré, G. , Zhu, C. , Capell, T. , Christou, P. , Fleck, G. et al. (2016) Engineered maize as a source of astaxanthin: processing and application as fish feed. Transgenic Res. 25, 785–793. [DOI] [PubMed] [Google Scholar]
- Campbell, R. , Morris, W.L. , Mortimer, C.L. , Misawa, N. , Ducreux, L.J.M. , Morris, J.A. , Hedley, P.E. et al. (2015) Optimising ketocarotenoid production in potato tubers: effect of genetic background, transgene combinations and environment. Plant Sci. 234, 27–37. [DOI] [PubMed] [Google Scholar]
- Cunningham, F.X. Jr. and Gantt, E. (2005) A study in scarlet: enzymes of ketocarotenoid biosynthesis in the flowers of Adonis aestivalis. Plant J. 41, 478–492. [DOI] [PubMed] [Google Scholar]
- Cunningham, F.X. and Gantt, E. (2011) Elucidation of the pathway to astaxanthin in the flowers of Adonis aestivalis. Plant Cell, 23, 3055–3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvořák, P. , Kunová, J. , Straková, E. , Suchý, P. and Kunová, V. (2005) Changes in the colour and the acidity number of egg yolk upon irradiation. Eur. Food Res. Technol. 221, 348–350. [Google Scholar]
- Farré, G. , Perez‐Fons, L. , Decourcelle, M. , Breitenbach, J. , Hem, S. , Zhu, C. , Capell, T. et al. (2016) Metabolic engineering of astaxanthin biosynthesis in maize endosperm and characterization of a prototype high oil hybrid. Transgenic Res. 25, 477–489. [DOI] [PubMed] [Google Scholar]
- Fassett, R.G. and Coombes, J.S. (2011) Astaxanthin: a potential therapeutic agent in cardiovascular disease. Mar. Drugs, 9, 447–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame, B.R. , Shou, H. , Chikwamba, R.K. , Zhang, Z. , Xiang, C. , Fonger, T.M. , Pegg, S.E.K. et al. (2002) Agrobacterium tumefaciens‐mediated transformation of maize embryos using a standard binary vector system. Plant Physiol. 129, 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giddings, G. , Allison, G. , Brooks, D. and Carter, A. (2000) Transgenic plants as factories for biopharmaceuticals. Nat. Biotechnol. 18, 1151–1155. [DOI] [PubMed] [Google Scholar]
- Goswami, G. , Chaudhuri, S. and Dutta, D. (2010) The present perspective of astaxanthin with reference to biosynthesis and pharmacological importance. World J. Microbiol. Biotechnol. 26, 1925–1939. [Google Scholar]
- Harada, H. , Maoka, T. , Osawa, A. , Hattan, J.‐I. , Kanamoto, H. , Shindo, K. , Otomatsu, T. et al. (2014) Construction of transplastomic lettuce (Lactuca sativa) dominantly producing astaxanthin fatty acid esters and detailed chemical analysis of generated carotenoids. Transgenic Res. 23, 303–315. [DOI] [PubMed] [Google Scholar]
- Hasunuma, T. , Miyazawa, S.‐I. , Yoshimura, S. , Shinzaki, Y. , Tomizawa, K.‐I. , Shindo, K. , Choi, S.‐K. et al. (2008) Biosynthesis of astaxanthin in tobacco leaves by transplastomic engineering. Plant J. 55, 857–868. [DOI] [PubMed] [Google Scholar]
- Higuera‐Ciapara, I. , Félix‐Valenzuela, L. and Goycoolea, F.M. (2006) Astaxanthin: a review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 46, 185–196. [DOI] [PubMed] [Google Scholar]
- Hirschberg, J. (2001) Carotenoid biosynthesis in flowering plants. Curr. Opin. Plant Biol. 4, 210–218. [DOI] [PubMed] [Google Scholar]
- Huang, J. , Zhong, Y. , Sandmann, G. , Liu, J. and Chen, F. (2012) Cloning and selection of carotenoid ketolase genes for the engineering of high‐yield astaxanthin in plants. Planta, 236, 691–699. [DOI] [PubMed] [Google Scholar]
- Huang, J.‐C. , Zhong, Y.‐J. , Liu, J. , Sandmann, G. and Chen, F. (2013) Metabolic engineering of tomato for high‐yield production of astaxanthin. Metab. Eng. 17, 59–67. [DOI] [PubMed] [Google Scholar]
- Hussein, G. , Sankawa, U. , Goto, H. , Matsumoto, K. and Watanabe, H. (2006) Astaxanthin, a carotenoid with potential in human health and nutrition. J. Nat. Prod. 69, 443–449. [DOI] [PubMed] [Google Scholar]
- Jayaraj, J. , Devlin, R. and Punja, Z. (2008) Metabolic engineering of novel ketocarotenoid production in carrot plants. Transgenic Res. 17, 489–501. [DOI] [PubMed] [Google Scholar]
- Koller, M. , Muhr, A. and Braunegg, G. (2014) Microalgae as versatile cellular factories for valued products. Algal Res. 6, 52–63. [Google Scholar]
- Krom, N. and Ramakrishna, W. (2008) Comparative analysis of divergent and convergent gene pairs and their expression patterns in rice, Arabidopsis, and %3cem%3ePopulus%3c/em>. Plant Physiol. 147, 1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurnia, A. , Satoh, S. , Haga, Y. , Kudo, H. , Nakada, M. , Matsumura, H. , Watanabe, Y. et al. (2015) Muscle coloration of rainbow trout with astaxanthin sources from marine bacteria and synthetic astaxanthin. J. Aquacult. Res. Dev. 6, 337. [Google Scholar]
- Liu, X. , Li, S. , Yang, W. , Mu, B. , Jiao, Y. , Zhou, X. , Zhang, C. et al. (2018a) Synthesis of seed‐specific bidirectional promoters for metabolic engineering of anthocyanin‐rich maize. Plant Cell Physiol. 59, 1942–1955. [DOI] [PubMed] [Google Scholar]
- Liu, X. , Yang, W. , Mu, B. , Li, S. , Li, Y. , Zhou, X. , Zhang, C. et al. (2018b) Engineering of ‘Purple Embryo Maize’ with a multigene expression system derived from a bidirectional promoter and self‐cleaving 2A peptides. Plant Biotechnol. J. 16, 1107–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz, R.T. and Cysewski, G.R. (2000) Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Trends Biotechnol. 18, 160–167. [DOI] [PubMed] [Google Scholar]
- Mann, V. , Harker, M. , Pecker, I. and Hirschberg, J. (2000) Metabolic engineering of astaxanthin production in tobacco flowers. Nat. Biotechnol. 18, 888–892. [DOI] [PubMed] [Google Scholar]
- McNulty, H. , Jacob, R.F. and Mason, R.P. (2008) Biologic activity of carotenoids related to distinct membrane physicochemical interactions. Am. J. Cardiol. 101, S20–S29. [DOI] [PubMed] [Google Scholar]
- Megdal, P.A. , Craft, N.A. and Handelman, G.J. (2009) A simplified method to distinguish farmed (Salmo salar) from wild salmon: fatty acid ratios versus astaxanthin chiral isomers. Lipids, 44, 569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milledge, J.J. (2011) Commercial application of microalgae other than as biofuels: a brief review. Rev. Environ. Sci. Bio/Technol. 10, 31–41. [Google Scholar]
- Moreno, J.A. , Díaz‐Gómez, J. , Nogareda, C. , Angulo, E. , Sandmann, G. , Portero‐Otin, M. , Serrano, J.C.E. et al. (2016) The distribution of carotenoids in hens fed on biofortified maize is influenced by feed composition, absorption, resource allocation and storage. Sci. Rep. 6, 35346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaizola, M. (2003) Commercial development of microalgal biotechnology: from the test tube to the marketplace. Biomol. Eng. 20, 459–466. [DOI] [PubMed] [Google Scholar]
- Park, H. , Weier, S. , Razvi, F. , Peña, P.A. , Sims, N.A. , Lowell, J. , Hungate, C. et al. (2017) Towards the development of a sustainable soya bean‐based feedstock for aquaculture. Plant Biotechnol. J. 15, 227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao, A.R. , Sarada, R. , Shylaja, M.D. and Ravishankar, G.A. (2015) Evaluation of hepatoprotective and antioxidant activity of astaxanthin and astaxanthin esters from microalga‐Haematococcus pluvialis . J. Food Sci. Technol. 52, 6703–6710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, I. , Schewe, H. , Gassel, S. , Jin, C. , Buckingham, J. , Hümbelin, M. , Sandmann, G. et al. (2011) Biotechnological production of astaxanthin with Phaffia rhodozyma/Xanthophyllomyces dendrorhous. Appl. Microbiol. Biotechnol. 89, 555–571. [DOI] [PubMed] [Google Scholar]
- Shah, M.M. , Liang, Y. , Cheng, J.J. and Daroch, M. (2016) Astaxanthin‐producing green microalga Haematococcus pluvialis: from single cell to high value commercial products. Front. Plant Sci. 7, 531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, A.K. and Sharma, M.K. (2009) Plants as bioreactors: recent developments and emerging opportunities. Biotechnol. Adv. 27, 811–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, B.‐C. , Guan, J.‐C. , Ding, S. , Wu, S. , Saunders, J.W. , Koch, K.E. and McCarty, D.R. (2017) Structure and origin of the white cap locus and its role in evolution of grain color in maize. Genetics, 206, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel, J.T. , Tan, B.‐C. , McCarty, D.R. and Klee, H.J. (2008) The carotenoid cleavage dioxygenase 1 enzyme has broad substrate specificity, cleaving multiple carotenoids at two different bond positions. J. Biol. Chem. 283(17), 11364–11373. [DOI] [PubMed] [Google Scholar]
- Wayama, M. , Ota, S. , Matsuura, H. , Nango, N. , Hirata, A. and Kawano, S. (2013) Three‐dimensional ultrastructural study of oil and astaxanthin accumulation during encystment in the green alga Haematococcus pluvialis . PLoS One 8, e53618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, L. , Cahoon, E.B. , Zhang, Y. and Ciftci, O.N. (2019) Extraction of astaxanthin from engineered Camelina sativa seed using ethanol‐modified supercritical carbon dioxide. J. Supercrit. Fluids, 143, 171–178. [Google Scholar]
- Zhong, Y.J. , Huang, J.C. , Liu, J. , Li, Y. , Jiang, Y. , Xu, Z.F. , Sandmann, G. et al. (2011) Functional characterization of various algal carotenoid ketolases reveals that ketolating zeaxanthin efficiently is essential for high production of astaxanthin in transgenic Arabidopsis. J. Exp. Bot. 62, 3659–3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Q. , Zeng, D. , Yu, S. , Cui, C. , Li, J. , Li, H. , Chen, J. et al. (2018) From golden rice to aSTARice: bioengineering astaxanthin biosynthesis in rice endosperm. Mol. Plant, 11, 1440–1448. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Integration and expression of foreign genes.
Figure S2 Astaxanthin was identified and confirmed by MS.
Figure S3 Integration of foreign genes and bialaphos‐resistant callus.
Figure S4 Effects of astaxanthin‐added feed on egg yolks.
Figure S5 Preparation of the hybrid maize variety ND28 for the laying hen feeding trial.
Figure S6 Effects of germplasm on astaxanthin engineering.
Table S1 Effect of astaxanthin‐rich maize on production performance of laying hens
Table S2 The effect of astaxanthin‐rich maize on egg quality of layer
Table S3 Primers used in PCR
Table S4 Primers used in RT‐qPCR


