Abstract
Stimulation of soluble guanylate cyclase (sGC) improves fetal growth at gestational day 20 in the reduced uterine perfusion pressure (RUPP) rat model of placental ischemia suggesting a role for sGC in the etiology of intrauterine growth restriction (IUGR). This study tested the hypothesis that stimulation of sGC until birth attenuates asymmetric IUGR mitigating increased cardiovascular risk in offspring. Sham or RUPP surgery was performed at gestational day 14 (G14); vehicle or the sGC stimulator Riociguat (10 mg/kg/day sc) was administered G14 until birth. Birth weight was reduced in offspring from RUPP [intrauterine growth restricted (IUGR)], sGC RUPP (sGC IUGR), and sGC Sham (sGC Control) compared with Sham (Control). Crown circumference was maintained, but abdominal circumference was reduced in IUGR and sGC IUGR compared with Control indicative of asymmetrical growth. Gestational length was prolonged in sGC RUPP, and survival at birth was reduced in sGC IUGR. Probability of survival to postnatal day 2 was also significantly reduced in IUGR and sGC IUGR versus Control and in sGC IUGR versus IUGR. At 4 mo of age, blood pressure was increased in male IUGR and sGC IUGR but not male sGC Control born with symmetrical IUGR. Global longitudinal strain was increased and stroke volume was decreased in male IUGR and sGC IUGR compared with Control. Thus late gestational stimulation of sGC does not mitigate asymmetric IUGR or increased cardiovascular risk in male sGC IUGR. Furthermore, late gestational stimulation of sGC is associated with symmetrical growth restriction in sGC Control implicating contraindications in normal pregnancy.
NEW & NOTEWORTHY The importance of the soluble guanylate cyclase-cGMP pathway in a rat model of placental ischemia differs during critical windows of development, implicating other factors may be critical mediators of impaired fetal growth in the final stages of gestation. Moreover, increased blood pressure at 4 mo of age in male intrauterine growth restriction offspring is associated with impaired cardiac function including an increase in global longitudinal strain in conjunction with a decrease in stroke volume, ejection fraction, and cardiac output.
Listen to this article’s corresponding podcast at https://ajpheart.podbean.com/e/soluble-guanylate-cyclase-intrauterine-growth-restriction-and-cardiovascular-risk/.
Keywords: blood pressure, cardiac dysfunction, intrauterine growth restriction, placental ischemia, soluble guanylate cyclase
INTRODUCTION
Low birth weight (<5.5 lbs) is a crude marker of an adverse fetal environment and intrauterine growth restriction (IUGR) (1). Numerous clinical studies identify a relationship between birth weight and blood pressure (BP). Experimental models of developmental insult that result in IUGR and low birth weight also report elevated BP in offspring (2–4). Additionally, models of IUGR report that gestational insults are associated with cardiovascular remodeling and cardiovascular dysfunction (5, 6). However, the mechanisms underlying the pathogenesis of IUGR and the development of increased BP and cardiovascular risk are not well known.
Our laboratory uses the clinically relevant reduced uterine perfusion pressure (RUPP) rodent model of placental ischemia to study the mechanisms underlying the developmental programming of increased BP and cardiovascular risk in low-birth-weight offspring (4). We previously reported that male IUGR offspring from RUPP dams exhibit a marked increase in BP by 12 and 16 wk of age (4, 7). Our laboratory has extensively investigated the etiology of increased BP in male IUGR offspring (7–12). Yet, the underlying pathogenesis of impaired fetal growth in the RUPP model and whether improvement in fetal growth is associated with mitigation of increased BP and cardiovascular risk in the IUGR offspring have not yet been clarified.
Under normal conditions, pregnancy is associated with a significant increase in guanosine 3′,5′-monophosphate (cGMP) and uterine blood flow (13, 14). Soluble guanylate cyclase (sGC) mediates the synthesis of cGMP leading to vasodilation (15). The nitric oxide (NO)-sGC-cGMP signaling pathway is impaired in pregnancies complicated by placental ischemia and preeclampsia (PE) (16). Although it is unknown if there is a deficiency in NO in the RUPP model of PE, we previously reported that maternal supplementation with l-arginine in late gestation improves BP in the RUPP (17). Moreover, placental levels of oxidative stress are increased in response to placental ischemia in the RUPP dam (18), and maternal administration of the superoxide dismutase mimetic tempol attenuates the RUPP-induced increase in BP (18). Collectively, these studies suggest that a reduction in NO bioavailability may contribute to endothelial dysfunction and the generation of oxidative stress in response to placental ischemia in the RUPP model of PE. Our laboratory previously reported that urinary, placental, and uterine artery cGMP are decreased in the RUPP model of placental ischemia in association with a reduction in fetal weight at gestation day 20 (G20) (19). We also showed that targeting the sGC pathway with a sGC stimulator, Riociguat, during late gestation restores the decrease in cGMP in association with improved uterine blood flow and placental morphology and attenuation of reduced fetal weight at G20 in the RUPP (19). Collectively, these findings implicate an important role for impaired sGC-cGMP signaling in the pathogenesis of IUGR. Although sGC stimulators are contraindicated in pregnancy, exact details as to fetal harm have not been disclosed (20). Yet, sGC stimulators can be used as a mechanistic tool to further investigate the importance of sGC-cGMP signaling in the development of asymmetric IUGR at birth and the developmental programming of increased BP and cardiovascular risk. Therefore, this study tested the hypothesis that stimulation of sGC during late gestation until birth in the RUPP model of placental ischemia and IUGR in the rat is associated with increased birth weight and attenuated asymmetrical fetal growth. Moreover, we hypothesized that improved fetal growth would be associated with a reduction in BP and cardiovascular risk in male IUGR offspring.
METHODS
Animals and Experimental Details
All experimental procedures were conducted in accordance with National Institutes of Health guidelines. All protocols were approved by the Institutional Animal Care and Use Committee at the University of Mississippi Medical Center. Four experimental groups representing five different delivery cohorts of primiparous, timed-pregnant Sprague-Dawley rats (225–250 g) were purchased from Envigo, Inc. (Indianapolis, IN). Upon arrival, all animals were placed on a 2020X diet, a breeding- and lactation-specific diet (Envigo, Inc.). Food and water were available ad libitum and rats were housed in a temperature-controlled room (23°C) with a 12:12-h light-dark cycle. Timed pregnant rats in each delivery cohort were randomly allocated to four different experimental groups: Sham or RUPP and then vehicle or sGC treated. Each delivery cohort included two to four pregnant rats per experimental group, were allowed to deliver spontaneously, and included the following: 12 vehicle-treated Sham (Sham), 14 vehicle-treated RUPP (RUPP), 15 sGC-treated Sham (sGC Sham), and 15 sGC-treated RUPP (sGC RUPP). All animals undergoing surgical procedures were anesthetized using 2–5% isoflurane by inhalation. The Sham or RUPP procedure was performed at gestational day 14 (G14) with analgesics (Rodent MD’s, Rimadyln, 2 mg/tablet, Bio-Serve, Flemington, NJ) provided before and for 3 days postsurgery (19). Completion of parturition was recorded on an hourly basis starting at day 21 of gestation (G21). Birth weight and morphometric measurements were conducted within 12 h of birth, and pups were tagged for identification. Litters were randomly culled to six pups per litter at postnatal day 2 (PN2) to ensure homogenous access to nutrition during the lactation period. Litters with less than six viable pups were cross-fostered to a dam within the same experimental group to ensure consistency of pre- and postnatal influences. Litters at PN2 were maintained as close to a 1:1 sex ratio when possible. Pups were weaned at postnatal day 21 (PN21) and then housed with same-sex littermates. Hemodynamic and cardiovascular studies were performed at 4 mo of age in male offspring. Only one male offspring per birth dam was used per study parameter with animals randomly selected and investigator(s) blinded to animal identification. Uterine artery resistance index (UARI) was measured at G20 as previously described (19) in the first three cohort of dams. To determine if serum and plasma hormones differed in vehicle and sGC Sham and RUPP dams, an additional cohort was utilized for collection of serum and plasma at G20. This cohort included: 5 Sham, 4 RUPP, 5 sGC Sham, and 4 sGC RUPP euthanized for collection of blood at G20. All animals were euthanized according to The Guide for the Care and Use of Laboratory Animals (8th ed.) and the AVMA Guidelines for the Euthanasia of Animals: 2013 Edition. Reporting of animal experiments conforms to the Animal Research: Reporting of In Vivo Experiments (ARRIVE) Guidelines (21).
Sham or Reduced Uterine Perfusion Pressure
Animals were randomly selected to undergo the reduced uterine perfusion pressure (RUPP) or Sham procedure. In brief, the RUPP procedure was performed at day 14 of gestation with a silver clip (0.203 mm) placed around the abdominal aorta directly rostral to the iliac bifurcation. A silver clip was also placed around both branches of the uterine artery (0.100 mm) to prevent a compensatory increase in flow to the uteroplacental units. The Sham procedure involved complete visualization of the uterine horns to ensure exposure to anesthesia and a comparable surgical procedure as the RUPP, all as previously described (4, 19).
Administration of the Soluble Guanylate Cyclase Stimulator (Riociguat) or Vehicle
Following the RUPP or Sham procedure, animals were randomly assigned to receive either the soluble guanylate cyclase stimulator, Riociguat (10 mg/kg/day) {methyl(4,6-diamino-2-(1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl)pyrimidin-5-yl)(methyl)carbamate, CAS No. 625115-55-1, Cat. No. G6-6188, AChemblock, Burlingame, CA} solubilized in 90% corn oil and 10% dimethyl sulfoxide or vehicle (90% corn oil and 10% DMSO) via subcutaneous injection from gestation day 14 until birth at a dose previously demonstrated by our laboratory to significantly increases production of cyclic guanosine monophosphate (cGMP) in RUPP dams at G20 (19).
Duration of Pregnancy through Completion of Parturition
The length of gestation based on the end of parturition was measured hourly starting at G21. The end of parturition was considered as delivery of the last pup in the litter followed by completion of maternal grooming and ingesting of the placenta and birth fluids.
Morphometric Measurements at Birth
Morphometric measurements were conducted in viable pups within 12 h of birth. Measurements included birth weight, crown circumference (circumferential measurement from ear to ear), abdominal circumference (circumferential measurement of the abdomen at the navel), and body length (measurement from tip of nose to anus). Viability at birth was based on active movement and respiration by a trained investigator.
Measure of Survivorship
The number of viable offspring at birth was assessed within 12 h of birth (birth); viable offspring were also determined at 24 h after birth (postnatal day 1) and at 48 h after birth (postnatal day 2). Probability of survival was calculated at postnatal day 1 and postnatal day 2 based on the number of viable offspring at birth.
Pregnancy Hormones
Serum and plasma collected at G20 from a separate cohort of RUPP or Sham experimental groups were analyzed by the Analytical and Assay Core (University of Mississippi Medical Center, Jackson, MS) to quantitate circulating levels of estrogen (BioVision, Inc.), progesterone (Crystal Chem), and prostaglandin E2 (R&D Systems), and oxytocin (Enzo Life Sciences).
Mean Arterial Pressure in Male Offspring at 4 mo of Age
Mean arterial pressure (MAP) was measured in male offspring at 4 mo of age as previously described (22). Briefly, under isoflurane anesthesia, rats were surgically implemented with a flexible catheter (PE-50) in the right carotid artery, allowed to recover for 24 h with mean arterial pressure measured in conscious, chronically instrumented rats using a data acquisition kit (DATAQ Instruments, Akron, OH).
Echocardiography in Male Offspring
Echocardiograms were performed in male offspring at 4 mo of age using a Vevo 3100 high-resolution in vivo imaging platform (Fujifilm, VisualSonics). Offspring selected for study were chosen based on similar body weight at time of study to account for differences in cardiac output and stroke volume that can occur due to a reduction in body weight and size. Rats were anesthetized (∼3% isoflurane in 2 L/min O2) and placed on a warming pad with continuous EKG measurement. Temperature and heart rate were also monitored continuously. A MX250 scan head for small animals was used to collect parasternal long-axis, parasternal short-axis, and four-chamber apical and suprasternal images. All parameters were quantified using the accompanying VevoLAB and VevoStrain analysis software. Total peripheral resistance (TPR) was calculated based on the following equation using MAP and cardiac output (CO) values per offspring: Total peripheral resistance (mmHg/ml/min) = MAP (mmHg)/CO (ml/min) with data for MAP acquired in the conscious state 24 h after measurement of CO.
Statistics
GraphPad PRISM version 8 (Graph Pad Software, San Diego, CA) was used for all statistical analysis. Comparisons among groups were performed by two-way ANOVA, followed by post hoc analysis with Tukey’s multiple comparison test if a significant interaction occurred. Data points at birth represent average per litter. To ensure diversity and that programmed influences were not representative of a litter effect, studies in male offspring at 4 mo of age involved one pup per litter per parameter selected at random. Survival probability from birth versus postnatal day 1 (PN1) and PN2 was determined by Mantel-Haenszel hazard ratio (HR) with a 95% confidence interval (CI). Correlation between duration of gestation versus viable offspring at birth was assessed using Pearson’s correlation analysis. For studies, the level of significance was set at P < 0.05. All results are presented as means ± SE.
RESULTS
Uterine Artery Resistance Index
At G20, UARI was significantly elevated in vehicle-treated RUPP (RUPP) compared with vehicle-treated Sham (Sham) (Fig. 1B). UARI was no longer significantly increased in sGC-treated RUPP (sGC RUPP) (Fig. 1B).
Figure 1.
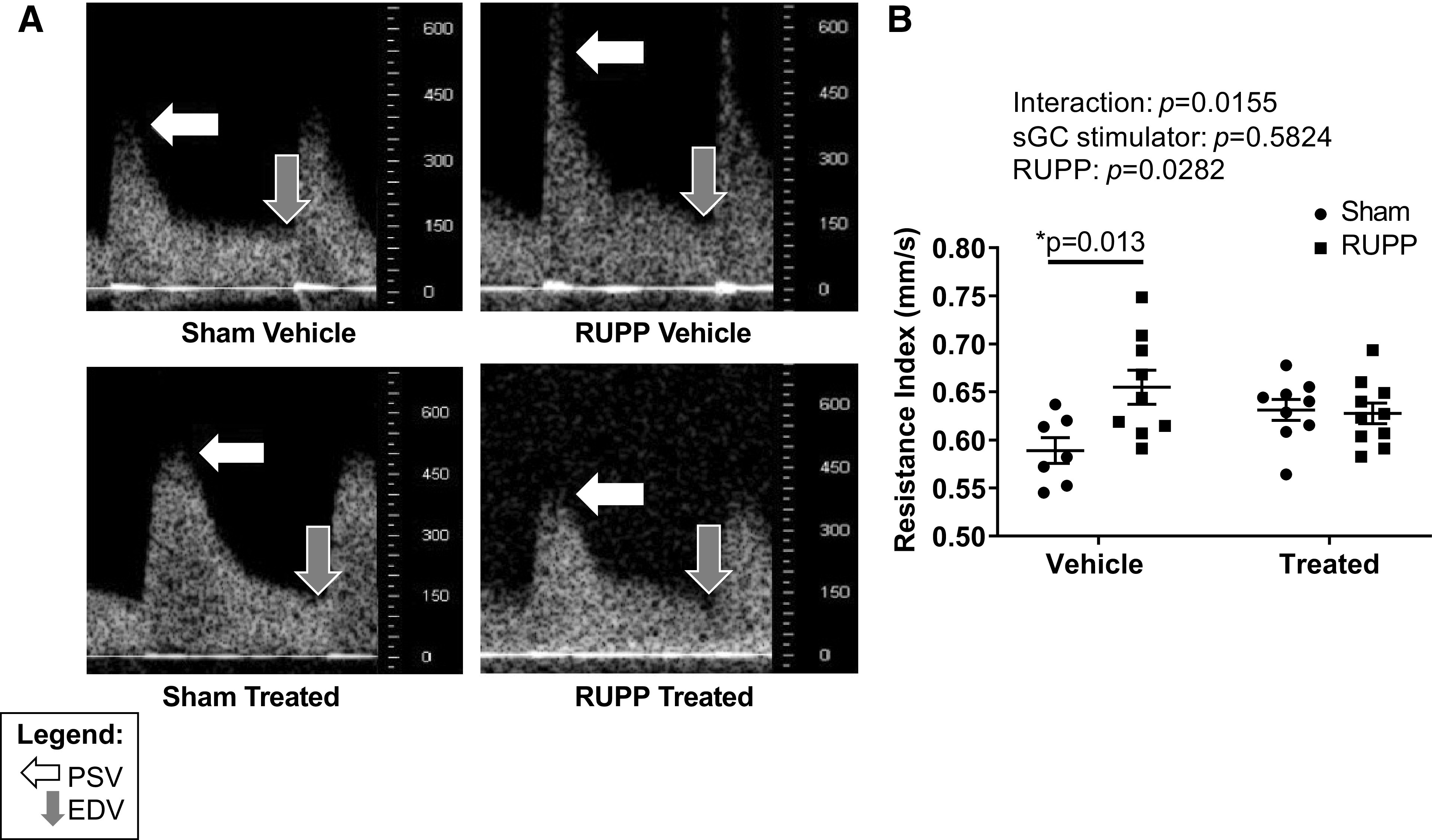
A: representative uterine artery resistance index (UARI) images at gestation day 20 (G20) in reduced uterine perfusion pressure (RUPP) and Sham-operated dams in response to vehicle or a soluble guanylate cyclase (sGC) stimulator (Riociguat 10 mg/kg/day). The white arrow indicates the point of peak systolic velocity (PSV), and the gray arrow indicates the point of end-diastolic velocity (EDV). B: UARI. UARI analysis was completed in the first 3 cohorts of dams. UARI was calculated using the following formula: UARI = (PSV – EDV)/PSV. Each scatter plot represents means ± SE; n = 7 Vehicle-treated Sham; n = 9 Vehicle-treated RUPP; n = 9 sGC-treated Sham; n = 10 sGC-treated RUPP. Two-way ANOVA with Tukey’s post hoc analysis was used for pairwise comparison. *P < 0.05, significant difference.
Morphometric Analyses
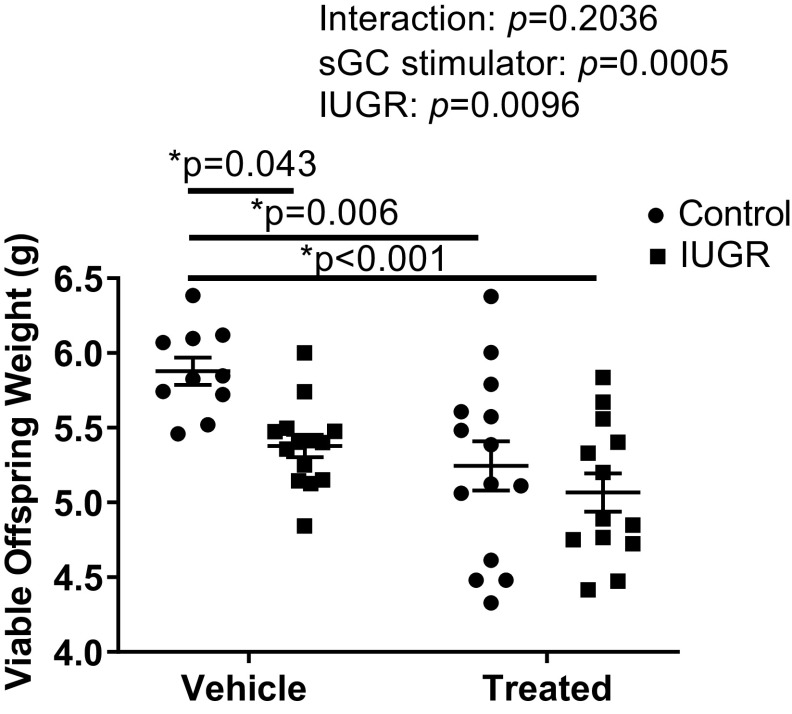
Birth weight was significantly reduced in IUGR offspring from RUPP (IUGR) and sGC IUGR offspring from sGC RUPP (sGC IUGR) compared with Control offspring from Sham (Control) (Fig. 2). Birth weight was also significantly reduced in sGC Control offspring from sGC Sham (sGC Control) compared with Control (Fig. 2). Crown circumference did not differ upon comparison of Control and IUGR offspring, regardless of maternal treatment (Fig. 3A). Abdominal circumference was significantly reduced in IUGR and sGC IUGR offspring compared with Control (Fig. 3B) indicative of asymmetrical fetal growth, but abdominal circumference was not reduced in sGC Control versus Control. Body length did not differ between groups, regardless of treatment (Fig. 3C).
Figure 2.
Average body weight of viable offspring at birth in Control and intrauterine growth-restricted (IUGR) offspring from Sham-operated and reduced uterine perfusion pressure dams treated with vehicle or a soluble guanylate cyclase (sGC) stimulator (Riociguat 10 mg/kg/day). Each data point represents the average body weight of viable offspring per dam; n = 10 Vehicle Control offspring; n = 14 Vehicle IUGR offspring; n= 14 sGC Control offspring; n = 13 sGC IUGR offspring. Scatter plots represent means ± SE. Two-way ANOVA with Tukey’s post hoc analysis was used for pairwise comparison. *P < 0.05, significant difference.
Figure 3.
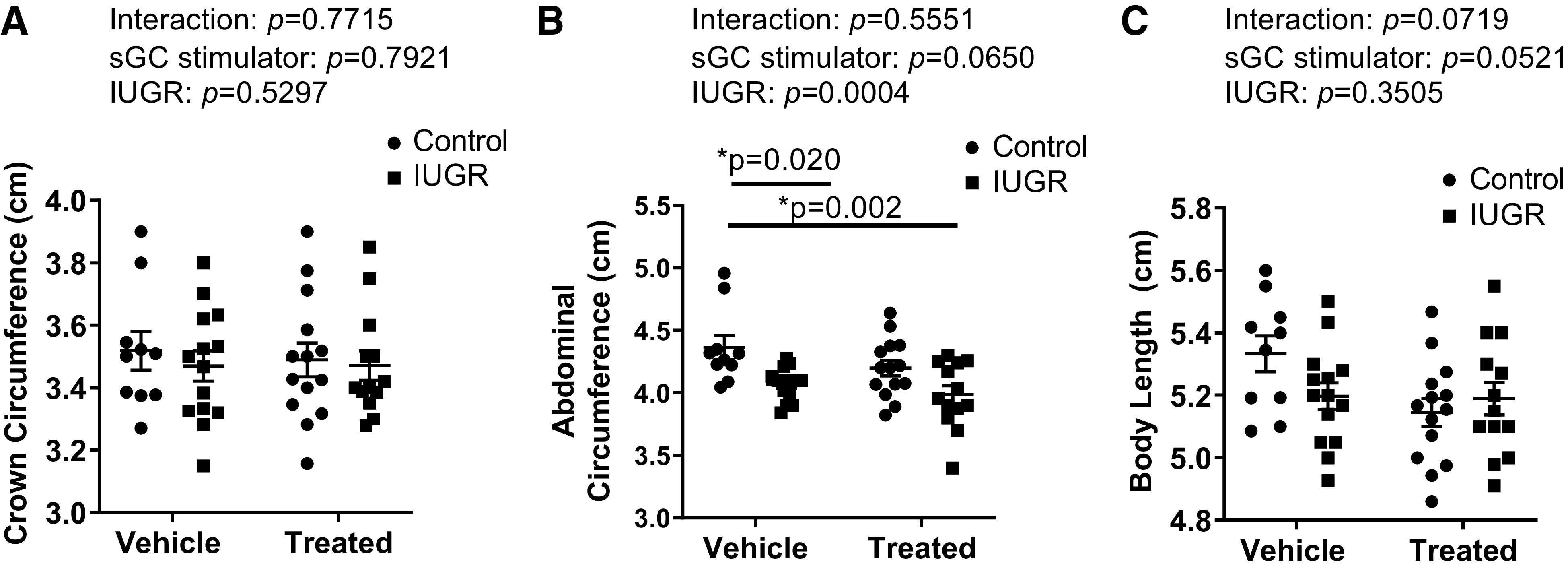
Average fetal morphometrics in viable offspring at birth in Control and intrauterine growth-restricted (IUGR) offspring from Sham-operated and reduced uterine perfusion pressure dams treated with vehicle or a soluble guanylate cyclase (sGC) stimulator (Riociguat 10 mg/kg/day). A: crown circumference. B: abdominal circumference. C: body length. Each data point represents the average per dam; n = 10 Vehicle Control offspring; n = 14 Vehicle IUGR offspring; n = 14 sGC Control offspring; n = 13 sGC IUGR offspring. Scatter plots represent means ± SE. Two-way ANOVA with Tukey’s post hoc analysis was used for pairwise comparison. *P < 0.05, significant difference.
Viability and Probability of Survival
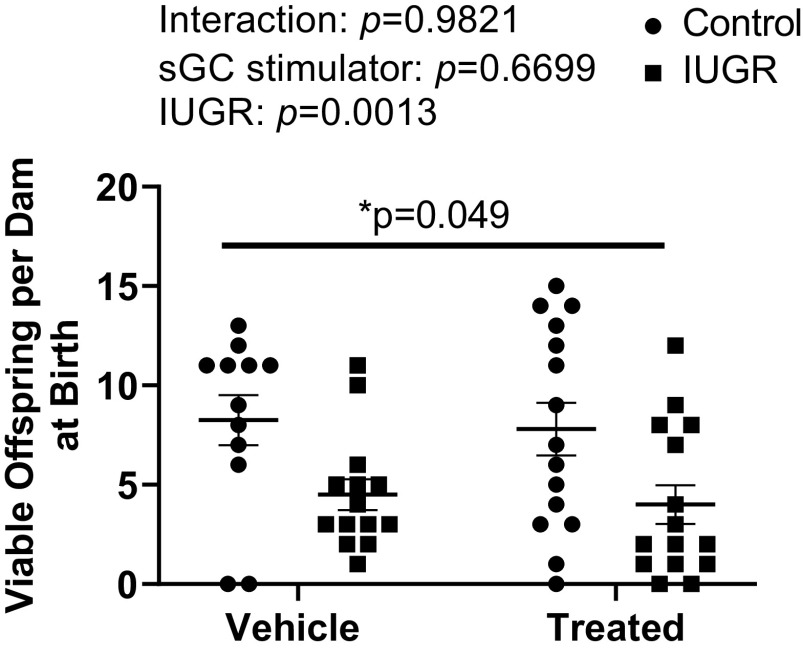
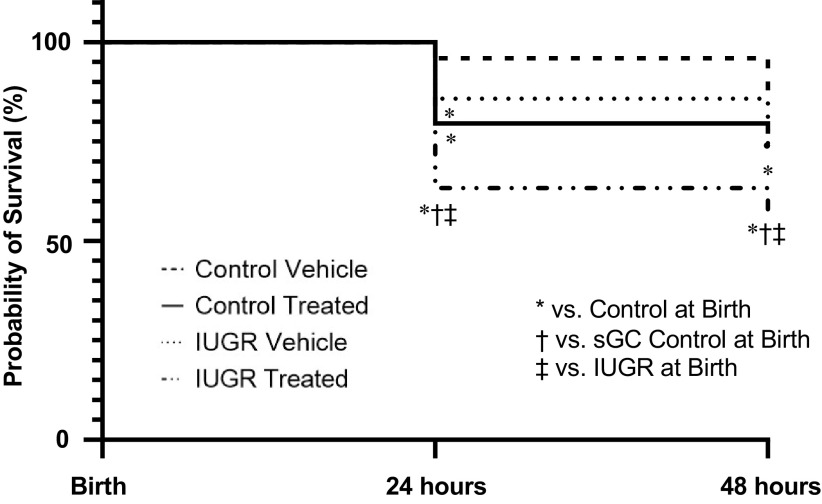
At birth, the number of viable offspring was reduced in sGC IUGR compared with Control (Fig. 4). Probability of survival at 24 h postbirth or PN1 was reduced in IUGR (86%) versus Control (98%) with a significantly higher mortality HR (P = 0.003; HR: 6.86; 95% CI: 1.96–23.99), in sGC IUGR (58%) versus Control with a significantly higher mortality HR (P < 0.001; HR 15.82; 95% CI: 6.86–36.45), and in sGC IUGR versus sGC Control (79%) with a significantly higher mortality HR (P = 0.002; HR: 2.88; CI: 1.45–5.71). Probability of survival at 24 h postbirth or PN1 was also significantly reduced in sGC IUGR versus IUGR with a significantly higher mortality HR (P < 0.001; HR: 3.90; CI: 1.79–8.49) and in sGC Control versus Control with a significantly higher mortality HR (P < 0.001; HR: 5.69; CI: 2.50–12.92) (Fig. 5).
Figure 4.
Viable offspring per dam at birth in Control and intrauterine growth-restricted (IUGR) offspring from Sham-operated and reduced uterine perfusion pressure dams treated with vehicle or a soluble guanylate cyclase (sGC) stimulator (Riociguat 10 mg/kg/day). Each data point represents the total number of viable offspring per dam; n = 12 Vehicle Control offspring; n = 14 Vehicle IUGR offspring; n = 15 sGC Control offspring; n = 15 sGC IUGR offspring. Scatter plots represent means ± SE. Two-way ANOVA with Tukey’s post hoc analysis was used for pairwise comparison. *P < 0.05, significant difference.
Figure 5.
Survival probability in Control and intrauterine growth-restricted (IUGR) offspring from Sham-operated and reduced uterine perfusion pressure dams treated with vehicle or a soluble guanylate cyclase (sGC) stimulator (Riociguat 10 mg/kg/day). Survival probability by Mantel-Haenszel Hazard Ratio and figure represented by Kaplan-Meier curve from birth versus postnatal day 1 and postnatal day 2. *P < 0.05, significant difference vs. Control at Birth; †P < 0.05, significant difference vs. sGC Control at Birth; ‡P < 0.05, significant difference vs. IUGR at Birth.
Probability of survival at 4 h postbirth or PN2 was reduced in IUGR (73%) versus Control (87%) with a significant higher mortality HR (P = 0.027; HR: 2.49; CI: 1.11–5.60), in sGC IUGR (55%) compared with Control with a significantly higher mortality HR (P < 0.001; HR: 5.35; CI: 2.59–11.06), in sGC IUGR versus sGC Control (77%) with a significantly higher mortality HR (P = 0.002; HR: 2.81; CI: 1.44–5.46), and in sGC IUGR versus IUGR with a significantly higher mortality HR (P = 0.035; HR: 2.19; CI: 1.06–4.53) (Fig. 5).
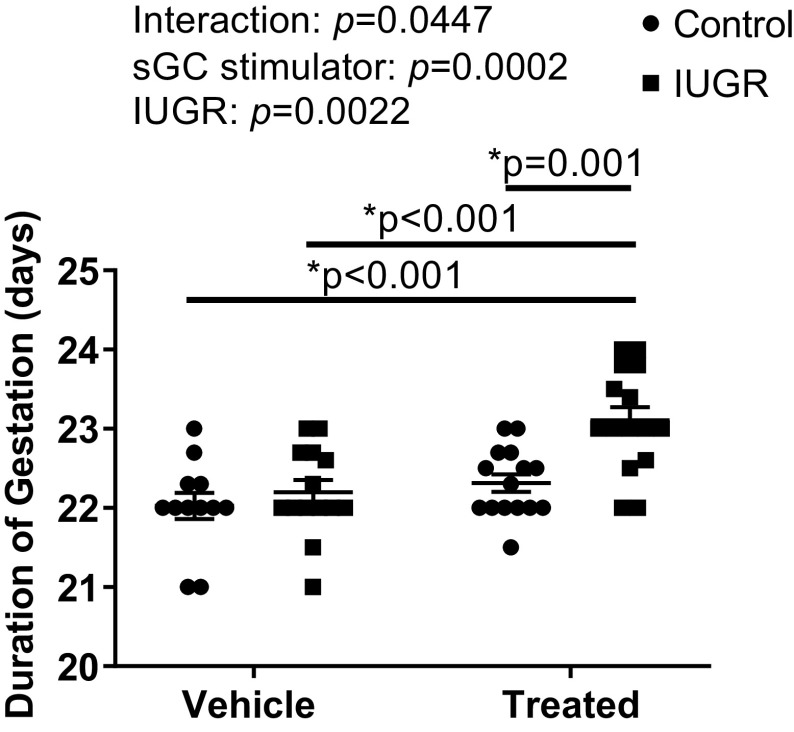
Duration of Gestation
The duration of gestation did not significantly differ upon comparison of Sham, RUPP, or sGC Sham (Fig. 6). However, the duration of gestation was significantly extended in sGC RUPP compared with Sham sGC Sham (P = 0.001) and RUPP (Fig. 6).
Figure 6.
Duration of gestation in Sham-operated and reduced uterine perfusion pressure dams treated with vehicle or a soluble guanylate cyclase (sGC) stimulator (Riociguat 10 mg/kg/day). Each data point represents the day and hour of end of parturition per dam; n = 12 Vehicle Control offspring; n = 14 Vehicle IUGR offspring; n = 15 sGC Control offspring; n = 15 sGC IUGR offspring. Scatter plots represent means ± SE. Two-way ANOVA with Tukey’s post hoc analysis was used for pairwise comparison. *P < 0.05, significant difference.
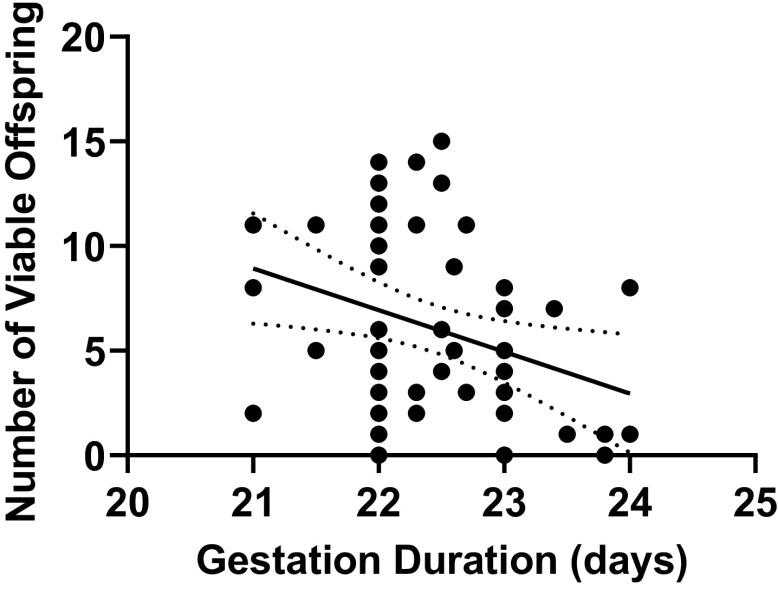
Correlations between Duration of Gestation and Viable Offspring
A significant correlation was found between the length of gestation and the number of viable offspring at birth (r = −0.312, P = 0.019) (Fig. 7).
Figure 7.
Relationship between duration of gestation and number of viable offspring from Sham-operated and reduced uterine perfusion pressure dams treated with vehicle or a soluble guanylate cyclase stimulator (Riociguat 10 mg/kg/day). Pearson’s correlation analysis: r = −0.312, P = 0.019. P < 0.05 was considered significant.
Pregnancy Hormones
Estrogen, progesterone, and prostaglandin E2 did not differ at G20 among experimental groups regardless of treatment (Fig. 8, A–C). However, oxytocin was significantly increased in RUPP compared with Sham but was not significantly elevated in sGC RUPP (Fig. 8D).
Figure 8.
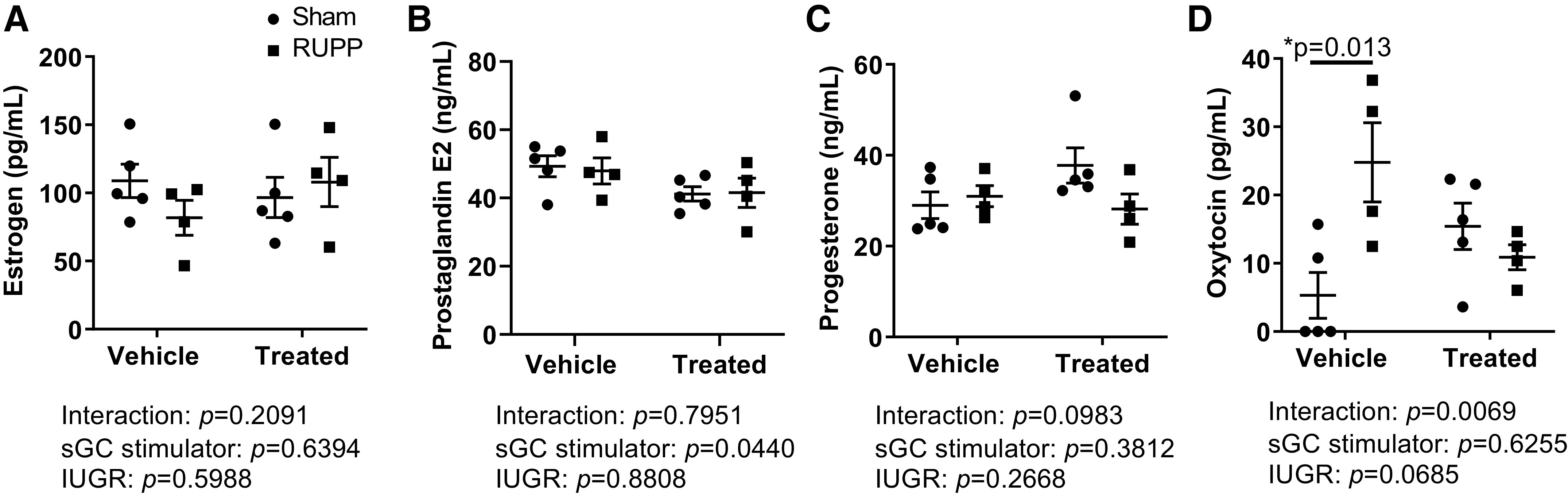
Pregnancy hormones in Sham-operated and reduced uterine perfusion pressure (RUPP) dams treated with vehicle or a soluble guanylate cyclase stimulator (Riociguat 10 mg/kg/day) at gestational day 20 in dams from a separate cohort. A: estrogen. B: prostaglandin E2. C: progesterone. D: oxytocin. Each scatter plot represents means ± SE; n = 5 vehicle-treated Sham; n = 4 vehicle-treated RUPP; n = 5 sGC-treated Sham; n = 4 sGC-treated RUPP. Two-way ANOVA with Tukey’s post hoc analysis was used for pairwise comparison. *P < 0.05, significant difference.
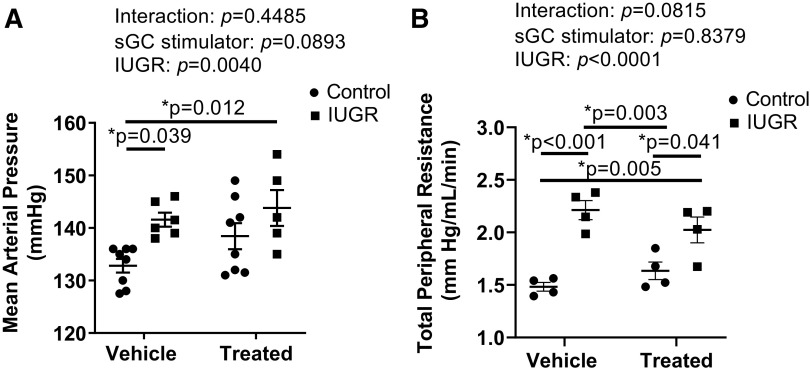
Mean Arterial Pressure and Total Peripheral Resistance in Male Offspring at 4 Mo of Age
MAP was significantly elevated in IUGR compared with Control offspring at 4 mo of age (Fig. 9A). MAP was also significantly increased in sGC IUGR compared with Control (Fig. 9A). TPR was elevated in IUGR and sGC IUGR compared with Control, and TPR was elevated in IUGR and sGC IUGR compared with sGC Control (Fig 9B).
Figure 9.
A: mean arterial pressure (MAP). B: total peripheral resistance (TPR) in Control and intrauterine growth-restricted (IUGR) offspring from Sham-operated and reduced uterine perfusion pressure dams treated with vehicle or a soluble guanylate cyclase (sGC) stimulator (Riociguat 10 mg/kg/day). Each data point for MAP represents 1 male offspring selected based on similar body weight from each litter at time of study; n = 8 Vehicle Control offspring; n = 6 Vehicle IUGR offspring; n = 8 sGC Control offspring; n = 5 sGC IUGR offspring. Each data point for TPR represents 1 male offspring selected based on similar body weight at time of study from 4 litters; n = 4 Vehicle Control offspring; n = 4 Vehicle IUGR offspring; n = 4 sGC Control offspring; n = 4 sGC IUGR offspring. Scatter plots represent means ± SE. Two-way ANOVA with Tukey’s post hoc analysis was used for pairwise comparison. *P < 0.05, significant difference.
Heart Rate and Cardiac Function in Male Offspring at 4 Mo of Age
Heart rate at 4 mo of age was significantly elevated in sGC IUGR compared with Control and sGC Control (Table 1). Cardiac function, which has not yet been assessed in this model of IUGR, was measured in a subset of male offspring at 4 mo of age. Body weight did not differ in offspring studied for cardiac function at 4 mo of age (Control: 454.50 ± 13.88 g; IUGR: 442.63 ± 2.04 g; sGC Control: 454.75 ± 11.76 g; and sGC IUGR: 452.25 ± 14.66 g). Global longitudinal strain (GLS) at 4 mo of age was elevated in IUGR and sGC IUGR compared with Control and sGC Control (Fig. 10A). Stroke volume was significantly reduced in IUGR and sGC IUGR compared with Control, and stroke volume was reduced in IUGR and sGC IUGR compared with sGC Control (Fig. 10B). Ejection fraction was decreased in IUGR and sGC IUGR compared with Control; ejection fraction was also decreased in sGC IUGR compared with sGC Control (Fig. 10C). Likewise, cardiac output at 4 mo of age was reduced in IUGR and sGC IUGR versus Control; cardiac output was also reduced in IUGR (compared with sGC Control (Fig. 10D). Fractional shortening, end-diastolic volume, end-systolic volume, and left ventricular mass did not differ among groups, regardless of treatment (Table 1).
Table 1.
Echocardiogram measurements
| Sham | sGC Sham | RUPP | sGC RUPP | P Value | |
|---|---|---|---|---|---|
| Heart rate, beats/min | 290 ± 7 | 293 ± 5 | 284 ± 6 | 322 ± 11*,† | Interaction: P = 0.0328 sGC stimulator: P = 0.1501 IUGR: P = 0.0182 |
| Fractional shortening, % | 31 ± 1 | 32 ± 1 | 29 ± 1 | 27 ± 3 | Interaction: P = 0.6496 sGC stimulator: P = 0.5993 IUGR: P = 0.0651 |
| End-diastolic volume, µL | 542 ± 6 | 534 ± 56 | 454 ± 51 | 518 ± 38 | Interaction: P = 0.4146 sGC stimulator: P = 0.5220 IUGR: P = 0.2411 |
| End-systolic volume, µL | 224 ± 7 | 247 ± 37 | 226 ± 36 | 285 ± 28 | Interaction: P = 0.5465 sGC stimulator: P = 0.1893 IUGR: P = 0.5121 |
| End-diastolic left ventricular mass, mg | 1,182 ± 37 | 1,160 ± 92 | 1,242 ± 70 | 1,351 ± 116 | Interaction: P = 0.4506 sGC stimulator: P = 0.6116 IUGR: P = 0.1613 |
| End-systolic left ventricular mass, mg | 1,248 ± 7 | 1,285 ± 114 | 1,307 ± 84 | 1,420 ± 20 | Interaction: P = 0.6990 sGC stimulator: P = 0.4477 IUGR: P = 0.3309 |
Values are means ± SE per group. Shown are echocardiogram measurements for heart rate and cardiovascular function assessment in 4-mo-old male control and intrauterine growth-restricted (IUGR) offspring from sham-operated and reduced uterine perfusion pressure (RUPP) dams treated with vehicle or a soluble guanylate cyclase (sGC) stimulator (Riociguat 10 mg/kg/day). Each value represents 1 male offspring selected based on similar body weight at time of study from 4 litters; n = 4 vehicle control offspring; n = 4 vehicle IUGR offspring; n = 4 sGC control offspring; n = 4 sGC IUGR offspring. Two-way ANOVA with Tukey’s post hoc analysis was used for pairwise comparison. *P < 0.05, significant difference between sham vs. sGC-treated RUPP. †P < 0.05, significant difference between sGC-treated sham vs. sGC-treated RUPP.
Figure 10.
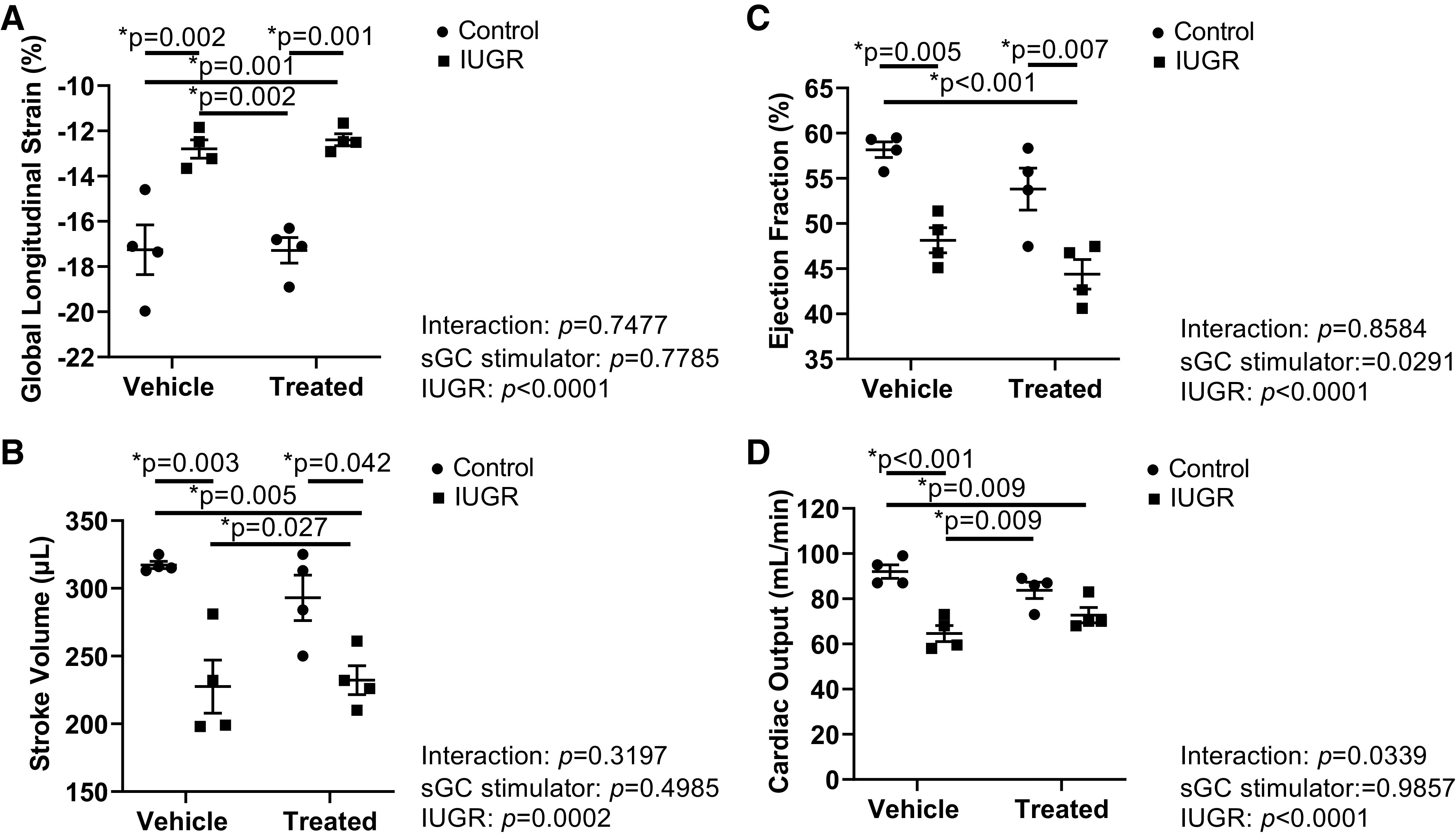
Cardiovascular function assessment in 4-mo-old male control and intrauterine growth-restricted (IUGR) offspring selected based on similar body weight at time of study from Sham-operated and reduced uterine perfusion pressure dams treated with vehicle or a soluble guanylate cyclase (sGC) stimulator (Riociguat 10 mg/kg/day). A: global longitudinal strain. B: stroke volume C: ejection fraction. D: cardiac output. Each data point represents 1 male offspring selected based on similar body weight at time of study from 4 litters; n = 4 Vehicle Control offspring; n = 4 Vehicle IUGR offspring; n = 4 sGC Control offspring; n = 4 sGC IUGR offspring. Scatter plots represent means ± SE. Two-way ANOVA with Tukey’s post hoc analysis was used for pairwise comparison. *P < 0.05, significant difference.
DISCUSSION
This study tested the hypothesis that stimulation of sGC during late gestation until birth in a rat model of placental ischemia diminished asymmetrical IUGR improving fetal growth and mitigating increased BP and cardiovascular risk in the offspring. The major findings from this study included the following: weight at birth was reduced in sGC IUGR with preservation of asymmetric growth. Birth weight was also reduced in sGC Control although symmetrical fetal growth was maintained. The number of viable offspring was significantly reduced in sGC IUGR offspring compared with Control, a decrease associated with IUGR and not sGC stimulation. Yet, the length of gestation was only significantly extended in sGC RUPP dams. The duration of gestation was significantly correlated to viability of the offspring at birth. Probability of survival from birth to PN2 was significantly reduced in IUGR and sGC IUGR compared with Control and in sGC IUGR compared with their vehicle counterparts. BP was increased at 4 mo of age in IUGR and sGC IUGR offspring that demonstrated asymmetric IUGR at birth but not Control offspring that exhibited symmetrical growth restriction at birth. Impaired indexes of cardiovascular dysfunction, a newly studied parameter in this model of IUGR induced via placental ischemia, were observed in male IUGR and sGC IUGR but not sGC Control offspring at 4 mo of age.
Our previous study demonstrated an important role for sGC-cGMP in the etiology of impaired fetal growth from to G14 to G20 in a pregnancy complicated by placental ischemia. In our previous study, stimulation of sGC from G14 to G20 was associated with a significant increase in uterine, placental, and urinary cGMP at G20 and fetal weight was not reduced in sGC RUPP compared with RUPP at G20 (19). However, data from our current study suggest that targeting the sGC-cGMP pathway from G14 to birth was not associated with improved fetal growth. Furthermore, persistence of asymmetrical growth restriction in sGC IUGR following maternal stimulation of the sGC-cGMP pathway from G14 to birth did not mitigate increased BP or cardiovascular risk in male offspring in young adulthood and was associated with an enhanced increase in morbidity in the offspring.
In our previous study, UARI was elevated at G20 in RUPP and sGC Sham, but fetal weight was only reduced at G20 in IUGR offspring from RUPP. In our current study, weight at birth was significantly reduced in IUGR, sGC IUGR, and sGC Control offspring; yet asymmetrical growth restriction was only observed in IUGR and sGC IUGR. Moreover, UARI was only increased in RUPP. Collectively, these data suggest that fetal weight at G20 is not indicative of fetal weight at birth and that UARI at G20 does not correlate to asymmetric growth restriction at birth in offspring following continued maternal exposure to stimulation of sGC. Placental ischemia induced by RUPP in the pregnant rat is associated with systemic inflammation, increased endothelin, and enhanced responsiveness to vasoconstrictors such as angiotensin II and vascular dysfunction (23). Confounding variables that develop in response to placental ischemia induced at G14 in the RUPP may contribute the development of asymmetric growth restriction in IUGR and sGC IUGR at birth.
Numerous studies report that birth weight and litter size are reduced in the RUPP model of placental ischemia (4, 24–26). In our study, birth weight of viable offspring in sGC RUPP was reduced at birth. Moreover, offspring from RUPP were born with asymmetrical IUGR, regardless of treatment. Although birth weight was reduced in sGC Control, litter size was not altered and pups were born with symmetrical growth restriction. Clinical (27, 28) and experimental (29, 30) studies indicate that birthweight influences survival. Overall, probability of survival from birth to PN2 was decreased in IUGR and sGC IUGR compared with Control. Thus these data suggest that birthweight per se is not associated with probability of survival, but that probability of survival is associated with asymmetrical fetal growth. Yet, probability of survival was further decreased in sGC IUGR compared with IUGR. These data suggest that mortality was further influenced by fetal exposure to maternal stimulation of the sGC-cGMP pathway in the RUPP. A potential confounding factor related to probability of survival in offspring from sGC RUPP may be the duration of gestation. Gestational duration was extended in sGC RUPP compared with other experimental groups by 24 h. Gestational duration was significantly correlated to viability of the offspring at birth suggesting that extension of gestation beyond the expected delivery date in sGC RUPP was detrimental to viability of the offspring after birth, which served as an additional insult in asymmetric IUGR offspring exposed to placental ischemia in utero.
Postterm pregnancy, or a pregnancy that extends beyond 42 wk of gestation, is associated with increased fetal morbidity and mortality (31). In 1975, Vorherr et al. (32) reported that prolongation of pregnancy in the primiparous Sprague-Dawley rat beyond 21–22 days induced by administration of progesterone is associated with a reduction in offspring survival. In our study, activation of the sGC-cGMP pathway from G14 to delivery was associated with a 24-h increase in the duration of gestation in sGC RUPP compared with the other experimental groups. The increase in gestational duration in sGC RUPP, which correlates to ∼13 days in the human (33), was also significantly correlated with a reduction in viable sGC IUGR offspring at birth. The cause of the increase in gestational duration in the sGC RUPP is not understood. However, prolongation of gestation could result from impaired physiological mechanisms and hormones required for parturition in rodents (34–35) including poor oxytocin elicited myometrial activity (36).
To determine possible mechanisms related to extended gestational length, we measured circulating hormones associated with the onset of labor. Oxytocin was increased RUPP compared with sGC RUPP at G20. Oxytocin is involved in the active phase of labor to increase the frequency of myometrium contractions to initiate the onset of labor (37). Serum oxytocin and uterine oxytocin receptor mRNA expression rise towards the end of pregnancy in rodents (38–42) and humans (41, 43–46). Chaud et al. (40) showed that exogenous oxytocin increases the frequency of contractions in isolated rodent uteri treated with a NO inhibitor compared with controls at day 21 of gestation. It is hypothesized that the NO pathway mediates the release of oxytocin from the supraoptic nucleus (SON) during pregnancy (47). In vitro (48) and in vivo (49) studies showed that sodium nitroprusside, a NO donor, and l-arginine, a NO precursor, inhibit SON neurons and oxytocin release. Additionally, nitro-l-arginine methyl ester, a NO synthase inhibitor, excites SON neurons. Srisawat et al. (49) also showed that NO is down-regulated during late gestation in the Sprague-Dawley rat allowing for hypersecretion of oxytocin by the posterior pituitary gland, leading to the initiation of labor. These studies suggest that NO is a major inhibitory regulator of SON neurons during pregnancy. Although sGC-cGMP controls neuronal signaling in the brain (47), whether-or-not SON control is specifically mediated by the sGC-cGMP pathway is not known.
The increase in duration of gestation could also be influenced by uterine sensitivity to oxytocin. In women with PE, oxytocin receptor expression is reduced and sensitivity to oxytocin is decreased in the uteroplacental unit (50, 51). Akahoshi et al. (52) also showed that the contractile response to oxytocin is reduced in mice with PE-like features. Yet, Gutkowska et al. (53) reported that oxytocin levels are elevated in RUPP dams at gestation day 19 compared with normal pregnant dams suggesting altered oxytocin receptor sensitivity in RUPP. Consistent with these findings, in our current study, circulating levels of oxytocin were increased in RUPP indicating a possible reduction in sensitivity to the oxytocin receptor. However, oxytocin levels were significantly reduced in sGC RUPP that completed parturition at gestation day 23 or 24 h after the other experimental groups. sGC treatment did not increase cGMP concentrations in Sham, and sGC Sham did not show changes in oxytocin or duration of gestation. Although we did not assess oxytocin receptor expression or activity, these data indicate that targeting the sGC-cGMP pathway through term can alter oxytocin concentration in RUPP dams but not Sham and, consequently, influence gestational duration and fetal survival.
Previously our laboratory reported that male but not female IUGR offspring from RUPP dams have a significant increase in BP at 12 and 16 wk of age compared with age-matched Control offspring (4, 7). In the current study, we also found that BP was elevated in IUGR and sGC IUGR at 16 wk of age. The mechanisms that contribute to increased BP in IUGR offspring have been well characterized by our laboratory and include a role for the renal nerves, renin angiotensin system, oxidative stress, and endothelin (7, 9–11, 54). The increase in MAP in our current study was also associated with an increase in TPR in male IUGR offspring at 4 mo of age. Endothelium-dependent NO-mediated vascular relaxation is inhibited in male IUGR offspring (55). Moreover, previous studies from our laboratory indicate that renal vascular resistance is also elevated (10). Collectively, these studies suggest a role for intrarenal, in addition to systemic vascular contributions to increased BP that originates in utero.
Cardiac dysfunction and increased prevalence of heart disease in women with PE is well documented (56–62). However, less is known about the impact of fetal exposure to PE on cardiac function in the IUGR offspring. Several studies indicate that PE places offspring at risk for both systolic and diastolic dysfunction (63, 64), cardiovascular remodeling (65), and impaired responses to stress (66). Risk of congenital heart disease and death from cardiovascular disease are also increased in offspring from mothers with PE (67–70). Yilgwan et al. (70) showed an eightfold greater risk of congenital heart disease was reported in offspring from women with PE with 30% of infants diagnosed by day 7 of life and 21% at day 28, as compared with 12% and 3%, respectively, in infants born from a normotensive pregnancy. Boyd et al. (68) also showed that offspring from PE pregnancies have an increased risk from cardiovascular dysfunction compared with offspring from a normotensive pregnancy, and this risk escalates in very early term and early term deliveries. Additionally, cardiac dysfunction is reported in IUGR offspring during neonatal life and childhood (71–73). Crispi et al. (71) reported that cardiac dysfunction at 3–6 yr of age occurs in association with impaired fetal growth, and is directly related to the severity of compromised fetal growth during development. These abnormalities worsen and develop into further cardiac dysfunction including a decrease in global longitudinal in IUGR pre-adolescents (74). Brain sparing as indicated by asymmetrical fetal growth may be, at least in part, responsible for worse perinatal outcomes such as cardiovascular impairments (75). Yet, studies addressing the developmental origins of cardiac dysfunction in experimental models of asymmetric IUGR are limited. A study by Xu et al. (5), using a maternal hypoxia model of IUGR in the rat, reported that male IUGR offspring at 4 mo of age have diastolic dysfunction, increased left ventricular end-diastolic pressure, reduced cardiac output, and cardiovascular remodeling in the absence of increased BP. A study by Rueda-Clausen et al. (6) found that prenatal hypoxia was associated with left ventricular hypertrophy in male IUGR but not their female IUGR littermates; yet both sexes show left ventricular diastolic dysfunction and pulmonary hypertension at 12 mo of age. We had not yet reported the effect of asymmetric fetal growth restriction on cardiac function in the RUPP model of IUGR induced by placental ischemia. In the current study, stroke volume and cardiac output were significantly reduced in male IUGR and sGC IUGR compared with Control at 4 mo of age. These data were also accompanied by a significant reduction in GLS. GLS is a measure of the deformation of the heart and can be an indication of damage and fibrosis (76). Clinically, decreases in GLS are considered abnormal (77). Reduction in GLS and ejection fraction indicate systolic dysfunction (76). Clearly, placental ischemia induced asymmetric IUGR is associated with cardiovascular harm. Thus, our findings suggest that placental insufficiency induces an increase in BP in male IUGR offspring that is associated systolic dysfunction, and that activation of the sGC pathway from G14 to birth in the RUPP dam does not improve these indices of cardiovascular risk.
Our laboratory previously reported that female IUGR do not develop an increase in BP until later in life, or one year of age (54). Whether female IUGR exhibit cardiovascular dysfunction in young adulthood before the development of increased BP is not yet known, Furthermore, whether maternal administration of a sGC stimulator induces harm in the female offspring in later life is also not known.
Perspectives
Treatment with a sGC stimulator is contraindicated pregnancy (20). The extent of fetal harm is not clarified by the manufacturer; yet, use of a sGC stimulator allowed us to investigate the importance of sGC-cGMP as a mediator of impaired fetal growth in late gestation up to birth. Although we found that targeting the sGC-cGMP pathway during G14 to G20 in a rat model of placental ischemia was associated with improved placental perfusion, placental morphology, and improved fetal growth (19), extension of maternal exposure to a sGC stimulator from G14 until birth did not provide benefit to the offspring. Prolongation of gestation in sGC RUPP and greater mortality in sGC IUGR were associated with asymmetrical fetal growth restriction and failure to mitigate increased BP and cardiovascular dysfunction in the male sGC IUGR offspring. With the use of two different study designs that included maternal administration of the same dose of Riociguat from G14 to G20 (19) and from G14 to birth, our findings suggest that activation of sGC contributes to fetal growth during critical windows of development. A limitation of our study includes whether a lower dose of Riociguat could increase sGC activity without compromising fetal weight, fetal viability, or probability of survival in IUGR offspring, and whether maternal administration of Riociguat from G14 to G20 would result in the same teratogenic effects postbirth observed in the current study when maternal administration of Riociguat was extended from G14 until birth. It is well established that homeostasis of placental function is a critical mediator of proper fetal growth. Further studies investigating the factors that contribute to impaired fetal growth at specific stages and throughout gestation are warranted to understand how potential therapeutic interventions can alleviate low birth weight and cardiovascular risk that originates during fetal life.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants R56-HL-143459 and HL-143459 with additional funding provided by NIH Grants HL-51971, P20-GM-104357, and P20-GM-121334. L. E. Coats received funding from NIH Grant T32-HL-105324.
DISCLAIMERS
The content of the manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.E.C., A.M.A., and B.T.A. conceived and designed research; L.E.C., B.A.B., D.R.B.-F., A.M.A., A.Z.R., N.B.O., and B.T.A. performed experiments; L.E.C., B.A.B., D.R.B.-F., A.M.A., N.B.O., and B.T.A. analyzed data; L.E.C., B.A.B., D.R.B.-F., A.M.A., N.B.O., and B.T.A. interpreted results of experiments; L.E.C. prepared figures; L.E.C., A.M.A., and B.T.A. drafted manuscript; L.E.C., B.A.B., D.R.B.-F., A.M.A., A.Z.R., N.B.O., and B.T.A. edited and revised manuscript; L.E.C., B.A.B., D.R.B.-F., A.M.A., A.Z.R., N.B.O., and B.T.A. approved final version of manuscript.
REFERENCES
- 1.Barker D, Osmond C. Low birth weight and hypertension. BMJ 297: 134–135, 1988. doi: 10.1136/bmj.297.6641.134-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woods LL, Ingelfinger JR, Rasch R. Modest maternal protein restriction fails to program adult hypertension in female rats. Am J Physiol Regul Integr Comp Physiol 289: R113–R1136, 2005. doi: 10.1152/ajpregu.00037.2003. [DOI] [PubMed] [Google Scholar]
- 3.Ortiz LA, Quan A, Zarzar F, Weinberg A, Baum M. Prenatal dexamethasone programs hypertension and renal injury in the rat. Hypertension 41: 328–334, 2003. doi: 10.1161/01.HYP.0000049763.51269.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexander BT. Placental insufficiency leads to development of hypertension in growth-restricted offspring. Hypertension 41: 457–462, 2003. doi: 10.1161/01.HYP.0000053448.95913.3D. [DOI] [PubMed] [Google Scholar]
- 5.Xu Y, Williams SJ, O'Brien D, Davidge ST. Hypoxia or nutrient restriction during pregnancy in rats leads to progressive cardiac remodeling and impairs postischemic recovery in adult male offspring. FASEB J 20: 1251–1253, 2006. doi: 10.1096/fj.05-4917fje. [DOI] [PubMed] [Google Scholar]
- 6.Rueda-Clausen CF, Morton JS, Dolinsky VW, Dyck JR, Davidge ST. Synergistic effects of prenatal hypoxia and postnatal high-fat diet in the development of cardiovascular pathology in young rats. Am J Physiol Regul Integr Comp Physiol 303: R418–R426, 2012. doi: 10.1152/ajpregu.00148.2012. [DOI] [PubMed] [Google Scholar]
- 7.Ojeda N, Grigore D, Yanes L, Iliescu R, Robertson E, Zhang H, Alexander B. Testosterone contributes to marked elevations in mean arterial pressure in adult male intrauterine growth restricted offspring. Am J Physiol Regul Integr Comp Physiol 292: R758–R763, 2007. doi: 10.1152/ajpregu.00311.2006. [DOI] [PubMed] [Google Scholar]
- 8.Grigore D, Ojeda NB, Robertson EB, Dawson AS, Huffman CA, Bourassa EA, Speth RC, Brosnihan KB, Alexander BT. Placental insufficiency results in temporal alterations in the renin angiotensin system in male hypertensive growth restricted offspring. Am J Physiol Regul Integr Comp Physiol 293: R804–R811, 2007. doi: 10.1152/ajpregu.00725.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexander BT, Hendon AE, Ferril G, Dwyer TM. Renal denervation abolishes hypertension in low-birth-weight offspring from pregnant rats with reduced uterine perfusion. Hypertension 45: 754–758, 2005. doi: 10.1161/01.HYP.0000153319.20340.2a. [DOI] [PubMed] [Google Scholar]
- 10.Ojeda NB, Royals TP, Black JT, Dasinger JJ, Johnson JM, Alexander BT. Enhanced sensitivity to acute angiotensin II is testosterone dependent in adult male growth-restricted offspring. Am J Physiol Regul Integr Comp Physiol 298: R1421–R1427, 2010. doi: 10.1152/ajpregu.00096.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ojeda NB, Hennington BS, Williamson DT, Hill ML, Betson NEE, Sartori-Valinotti JC, Reckelhoff JF, Royals TP, Alexander BT. Oxidative stress contributes to sex differences in blood pressure in adult growth-restricted offspring. Hypertension 60: 114–122, 2012. doi: 10.1161/HYPERTENSIONAHA.112.192955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Intapad S, Ojeda NB, Varney E, Royals TP, Alexander BT. Sex-specific effect of endothelin in the blood pressure response to acute angiotensin II in growth-restricted rats. Hypertension 66: 1260–1266, 2015. doi: 10.1161/HYPERTENSIONAHA.115.06257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Itoh H, Bird IM, Nakao K, Magness RR. Pregnancy increases soluble and particulate guanylate cyclases and decreases the clearance receptor of natriuretic peptides in ovine uterine, but not systemic, arteries. Endocrinology 139: 3329–3341, 1998. doi: 10.1210/endo.139.7.6093. [DOI] [PubMed] [Google Scholar]
- 14.Conrad KP, Kerchner LJ, Mosher MD. Plasma and 24-h NOx and cGMP during normal pregnancy and preeclampsia in women on a reduced NOx diet. Am J Physiol Renal Physiol 277: F48–F57, 1999. doi: 10.1152/ajprenal.1999.277.1.F48. [DOI] [PubMed] [Google Scholar]
- 15.Stasch JP, Pacher P, Evgenov OV. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation 123: 2263–2273, 2011. doi: 10.1161/CIRCULATIONAHA.110.981738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao Q, Tang J, Li N, Zhou X, Zhu X, Li W, Liu B, Feng X, Tao J, Han B, Zhang H, Sun M, Xu Z. New conception for the development of hypertension in preeclampsia. Oncotarget 7: 78387–78395, 2016. doi: 10.18632/oncotarget.13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alexander BT, Llinas MT, Kruckeberg WC, Granger JP. L-arginine attenuates hypertension in pregnant rats with reduced uterine perfusion pressure. Hypertension 43: 832–836, 2004. doi: 10.1161/01.HYP.0000119192.32360.a9. [DOI] [PubMed] [Google Scholar]
- 18.Sedeek M, Gilbert JS, LaMarca BB, Sholook M, Chandler DL, Wang Y, Granger JP. Role of reactive oxygen species in hypertension produced by reduced uterine perfusion in pregnant rats. Am J Hypertens 21: 1152–1156, 2008. doi: 10.1038/ajh.2008.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coats LE, Bamrick-Fernandez DR, Ariatti AM, Bakrania BA, Rawls AZ, Ojeda NB, Alexander BT. Stimulation of soluble guanylate cyclase diminishes intrauterine growth restriction in a rat model of placental ischemia. Am J Physiol Regul Integr Comp Physiol 320: R149–R161, 2021. doi: 10.1152/ajpregu.00234.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riociguat Pregnancy and Breastfeeding Warnings. 2010. Reference ID: 3501667 NDA 204819. Adempas Bayer; 24 Apr 2014. [Google Scholar]
- 21.Leary S, Underwood W, Anthony R, Cartner S, Corey D, Grandin T, Greenacre C, Gwaltney-Brant S, McCrackin M, Meyer R. AVMA Guidelines for the Euthanasia of Enimals: 2013 Edition. Schaumburg, IL: American Veterinary Medical Association, 2013. [Google Scholar]
- 22.Davis GK, Newsome AD, Cole AB, Ojeda NB, Alexander BT. Chronic estrogen supplementation prevents the increase in blood pressure in female intrauterine growth-restricted offspring at 12 months of age. Hypertension 73: 1128–1136, 2019. doi: 10.1161/HYPERTENSIONAHA.118.12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Granger JP, Spradley FT, Bakrania BA. The endothelin system: a critical player in the pathophysiology of preeclampsia. Curr Hypertens Rep 20: 32, 2018. doi: 10.1007/s11906-018-0828-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Younes ST, Maeda KJ, Sasser JM, Ryan MJ. The glucagon-like peptide 1 receptor agonist liraglutide attenuates placental ischemia-induced hypertension. Am J Physiol Heart Circ Physiol 318: H72–H77, 2020. doi: 10.1152/ajpheart.00486.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amaral LM, Faulkner JL, Elfarra J, Cornelius DC, Cunningham MW, Ibrahim T, Vaka VR, McKenzie JM, LaMarca B. Continued investigation into 17-OHPC: results from the preclinical rupp rat model of preeclampsia. Hypertension 70: 1250–1255, 2017. doi: 10.1161/HYPERTENSIONAHA.117.09969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faulkner JL, Cornelius DC, Amaral LM, Harmon AC, CunninghamMW , Jr, Darby MM, Ibrahim T, Thomas DS, Herse F, Wallukat G, Deschend R, Lamarca B. Vitamin D supplementation improves pathophysiology in a rat model of preeclampsia. Am J Physiol Regul Integr Comp Physiol 310: R346–R354, 2016. doi: 10.1152/ajpregu.00388.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watkins WJ, Kotecha SL, Kotecha S. All-cause mortality of low birthweight infants in infancy, childhood, and adolescence: population study of England and Wales. PLoS Med 13: e1002018, 2016. doi: 10.1371/journal.pmed.1002018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vilanova CS, Hirakata VN, de Souza Buriol VC, Nunes M, Goldani MZ, da Silva CH. The relationship between the different low birth weight strata of newborns with infant mortality and the influence of the main health determinants in the extreme south of Brazil. Popul Health Metr 17: 1–12, 2019. doi: 10.1186/s12963-018-0181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fushima T, Sekimoto A, Minato T, Ito T, Oe Y, Kisu K, Sato E, Funamoto K, Hayase T, Kimura Y, Ito S, Sato H, Takahashi N. Reduced uterine perfusion pressure (RUPP) model of preeclampsia in mice. PLoS One 11: e0155426, 2016. doi: 10.1371/journal.pone.0155426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cabrera RA, Lin X, Campbell JM, Moeser AJ, Odle J. Influence of birth order, birth weight, colostrum and serum immunoglobulin G on neonatal piglet survival. J Anim Sci Biotechnol 3: 42–10, 2012. doi: 10.1186/2049-1891-3-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galal M, Symonds I, Murray H, Petraglia F, Smith R. Postterm pregnancy. Facts Views Vis Obgyn 4: 175–187, 2012. [PMC free article] [PubMed] [Google Scholar]
- 32.Vorherr H. Placental insufficiency and postmaturity. Eur J Obstet Gynecol Reprod Biol 5: 109–122, 1975. doi: 10.1016/0028-2243(75)90136-7. [DOI] [PubMed] [Google Scholar]
- 33.Agoston D. How to translate time? The temporal aspect of human and rodent biology. Front Neurol 8: 92, 2017. doi: 10.3389/fneur.2017.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar P, Magon N. Hormones in pregnancy. Niger Med J 53: 179–183, 2012. pdoi: 10.4103/0300-1652.107549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liff I, Adeli S, Goldfarb IT, Sawyer MR, Phillippe M. Modulation of IL10 and its receptor subunits in normal and progesterone-prolonged gestation in the mouse. Reprod Sci 27: 555–560, 2020. doi: 10.1007/s43032-019-00022-7. [DOI] [PubMed] [Google Scholar]
- 36.Arrowsmith S, Quenby S, Weeks A, Burdyga T, Wray S. Poor spontaneous and oxytocin-stimulated contractility in human myometrium from postdates pregnancies. PLoS One 7: e36787, 2012. doi: 10.1371/journal.pone.0036787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borrow A, Cameron N. The role of oxytocin in mating and pregnancy. Horm Behav 61: 266–276, 2012. doi: 10.1016/j.yhbeh.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 38.Seymour AJ, Scott V, Augustine RA, Bouwer GT, Campbell RE, Brown CH. Development of an excitatory kisspeptin projection to the oxytocin system in late pregnancy. J Physiol 595: 825–838, 2017. doi: 10.1113/JP273051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshida M, Takayanagi Y, Ichino-Yamashita A, Sato K, Sugimoto Y, Kimura T, Nishimori K. Functional hierarchy of uterotonics required for successful parturition in mice. Endocrinology 160: 2800–2810, 2019. doi: 10.1210/en.2019-00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaud M, Franchi A, de Astrada M, Gimeno M. Role of nitric oxide on oxytocin-evoked contractions and prostaglandin synthesis in isolated pregnant rat uterus. Prostaglandins Leukot Essent Fatty Acids 57: 323–329, 1997. doi: 10.1016/S0952-3278(97)90551-2. [DOI] [PubMed] [Google Scholar]
- 41.Alotaibi M. The response of rat and human uterus to oxytocin from different gestational stages in vitro. Gen Physiol Biophys 36: 75–82, 2017. doi: 10.4149/gpb_2016022. [DOI] [PubMed] [Google Scholar]
- 42.Arthur P, Taggart MJ, Zielnik B, Wong S, Mitchell BF. Relationship between gene expression and function of uterotonic systems in the rat during gestation, uterine activation and both term and preterm labour. J Physiol 586: 6063–6076, 2008. doi: 10.1113/jphysiol.2008.164004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uvnäs-Moberg K, Ekström-Bergström A, Berg M, Buckley S, Pajalic Z, Hadjigeorgiou E, Kotłowska A, Lengler L, Kielbratowska B, Leon-Larios F, Magistretti CM, Downe S, Lindström B, Dencker A. Maternal plasma levels of oxytocin during physiological childbirth–a systematic review with implications for uterine contractions and central actions of oxytocin. BMC Pregnancy Childbirth 19: 285, 2019. 2019. doi: 10.1186/s12884-019-2365-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yulia A, Johnson MR. Myometrial oxytocin receptor expression and intracellular pathways. Minerva Ginecol 66: 267–280, 2014. [PubMed] [Google Scholar]
- 45.Arrowsmith S, Wray S. Oxytocin: its mechanism of action and receptor signalling in the myometrium. J Neuroendocrinol 26: 356–369, 2014. doi: 10.1111/jne.12154. [DOI] [PubMed] [Google Scholar]
- 46.Prevost M, Zelkowitz P, Tulandi T, Hayton B, Feeley N, Carter CS, Joseph L, Pournajafi-Nazarloo H, Yong Ping E, Abenhaim H, Gold I. Oxytocin in pregnancy and the postpartum: relations to labor and its management. Front Public Health 2: 1, 2014. doi: 10.3389/fpubh.2014.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kadekaro M. Nitric oxide modulation of the hypothalamo-neurohypophyseal system. Braz J Med Biol Res 37: 441–450, 2004. doi: 10.1590/S0100-879X2004000400001. [DOI] [PubMed] [Google Scholar]
- 48.Liu QS, Jia YS, Ju G. Nitric oxide inhibits neuronal activity in the supraoptic nucleus of the rat hypothalamic slices. Brain Res Bull 43: 121–125, 1997. doi: 10.1016/S0361-9230(96)00209-2. [DOI] [PubMed] [Google Scholar]
- 49.Srisawat R, Ludwig M, Bull PM, Douglas AJ, Russell JA, Leng G. Nitric oxide and the oxytocin system in pregnancy. J Neurosci 20: 6721–6727, 2000. doi: 10.1523/JNEUROSCI.20-17-06721.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Szczepanska-Sadowska E, Cudnoch-Jedrzejewska A, Wsol A. The role of oxytocin and vasopressin in the pathophysiology of heart failure in pregnancy and in fetal and neonatal life. Am J Physiol Heart Circ Physiol 318: H639–H651, 2020. doi: 10.1152/ajpheart.00484.2019. [DOI] [PubMed] [Google Scholar]
- 51.Fan X, Xu T, Ding H, Li H, Yang Y, He Y, Tang J, Liu Y, Chen X, Chen J, Tao J, Xu Z, Gao Q. DNA methylation‐reprogrammed oxytocin receptor underlies insensitivity to oxytocin in pre‐eclamptic placental vasculature. J Cell Mol Med 23: 4118–4126, 2019. doi: 10.1111/jcmm.14299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Akahoshi N, Handa H, Takemoto R, Kamata S, Yoshida M, Onaka T, Ishii I. Preeclampsia-like features and partial lactation failure in mice lacking cystathionine γ-lyase: an animal model of cystathioninuria. IJMS 20: 3507, 2019. doi: 10.3390/ijms20143507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gutkowska J, Granger JP, Lamarca BB, Danalache BA, Wang D, Jankowski M. Changes in cardiac structure in hypertension produced by placental ischemia in pregnant rats: effect of tumor necrosis factor blockade. J Hypertens 29: 1203–1212, 2011. doi: 10.1097/HJH.0b013e3283468392. [DOI] [PubMed] [Google Scholar]
- 54.Intapad S, Tull FL, Brown AD, Dasinger JH, Ojeda NB, Fahling JM, Alexander BT. Renal denervation abolishes the age-dependent increase in blood pressure in female intrauterine growth-restricted rats at 12 months of age. Hypertension 61: 828–834, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Payne JA, Alexander BT, Khalil RA. Reduced endothelial vascular relaxation in growth-restricted offspring of pregnant rats with reduced uterine perfusion. Hypertension 42: 768–774, 2003. doi: 10.1161/01.HYP.0000084990.88147.0C. [DOI] [PubMed] [Google Scholar]
- 56.Melchiorre K, Sutherland G, Liberati M, Thilaganathan B. Preeclampsia is associated with persistent postpartum cardiovascular impairment. Hypertension 58: 709–715, 2011. doi: 10.1161/HYPERTENSIONAHA.111.176537. [DOI] [PubMed] [Google Scholar]
- 57.Mogos MF, Piano MR, McFarlin BL, Salemi JL, Liese KL. Heart failure in pregnant women: a concern across the pregnancy continuum. Circ Heart Fail 11: e004005, 2018. doi: 10.1161/circheartfailure.117.004005. [DOI] [PubMed] [Google Scholar]
- 58.Leon LJ, McCarthy FP, Direk K, Gonzalez-Izquierdo A, Prieto-Merino D, Casas JP, Chappell L. Preeclampsia and cardiovascular disease in a large UK pregnancy cohort of linked electronic health records: a CALIBER study. Circulation 140: 1050–1060, 2019. doi: 10.1161/CIRCULATIONAHA.118.038080. [DOI] [PubMed] [Google Scholar]
- 59.Bellamy L, Casas J, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ 335: 974, 2007. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bokslag A, Teunissen PW, Franssen C, van Kesteren F, Kamp O, Ganzevoort W, Paulus WJ, de Groot CJ. Effect of early-onset preeclampsia on cardiovascular risk in the fifth decade of life. Am J Ostet Gynecol 216: 523, 2017. doi: 10.1016/j.ajog.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 61.Shahul S, Ramadan H, Nizamuddin J, Mueller A, Patel V, Dreixler J, Tung A, Lang RM, Weinert L, Nasim R, Chinthala S, Rana S. Activin A and late postpartum cardiac dysfunction among women with hypertensive disorders of pregnancy. Hypertension 72: 188–193, 2018. doi: 10.1161/HYPERTENSIONAHA.118.10888. [DOI] [PubMed] [Google Scholar]
- 62.Wu P, Haththotuwa R, Kwok CS, Babu A, Kotronias RA, Rushton C, Zaman A, Fryer AF, Kadam U, Chew-Graham CA. Preeclampsia and future cardiovascular health: a systematic review and meta-analysis. Circ Cardiovas Qual Outcomes 10: e003497, 2017. doi: 10.1161/circoutcomes.116.003497. [DOI] [PubMed] [Google Scholar]
- 63.Tapp RJ, Hughes AD, Kähönen M, Wong TY, Witt N, Lehtimäki T, Hutri-Kähönen N, Sahota P, Juonala M, Raitakari OT. Cardiometabolic health among adult offspring of hypertensive pregnancies: the cardiovascular risk in young Finns study. J Am Heart Assoc 7: e006284, 2018. doi: 10.1161/jaha.117.006284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jayet PY, Rimoldi SF, Stuber T, Salmòn CS, Hutter D, Rexhaj E, Thalmann S, Schwab M, Turini P, Sartori-Cucchia C, Nicod P, Villena M, Allemann Y, Scherrer U, Sartori C. Pulmonary and systemic vascular dysfunction in young offspring of mothers with preeclampsia. Circulation 122: 488–494, 2010. doi: 10.1161/CIRCULATIONAHA.110.941203. [DOI] [PubMed] [Google Scholar]
- 65.Crispi F, Bijnens B, Figueras F, Bartrons J, Eixarch E, Le Noble F, Ahmed A, Gratacós E. Fetal growth restriction results in remodeled and less efficient hearts in children. Circulation 121: 2427–2436, 2010. doi: 10.1161/CIRCULATIONAHA.110.937995. [DOI] [PubMed] [Google Scholar]
- 66.Burchert H, Lewandowske AJ. Preterm birth is a novel, independent risk factor for altered cardiac remondeling and early heart failure: is it time for a new cardiomyopahty. Curr Treat Options Cardiovasc Med 21: 8, 2019. doi: 10.1007/s11936-019-0712-9. [DOI] [PubMed] [Google Scholar]
- 67.Auger N, Fraser WD, Healy-Profitós J, Arbour L. Association between preeclampsia and congenital heart defects. JAMA 314: 1588–1598, 2015. doi: 10.1001/jama.2015.12505. [DOI] [PubMed] [Google Scholar]
- 68.Boyd HA, Basit S, Behrens I, Leirgul E, Bundgaard H, Wohlfahrt J, Melbye M, Øyen N. Association between fetal congenital heart defects and maternal risk of hypertensive disorders of pregnancy in the same pregnancy and across pregnancies. Circulation 136: 39–48, 2017. doi: 10.1161/CIRCULATIONAHA.116.024600. [DOI] [PubMed] [Google Scholar]
- 69.Brodwall K, Leirgul E, Greve G, Vollset SE, Holmstrøm H, Tell GS, Øyen N. Possible common etiology behind maternal preeclampsia and congenital heart defects in the child: a cardiovascular diseases in Norway Project Study. Paediatr Perinat Epidemiol 30: 76–85, 2016. doi: 10.1111/ppe.12252. [DOI] [PubMed] [Google Scholar]
- 70.Yilgwan CS, Pam VC, Ige OO, Golit WN, Anzaku S, Imade GE, Yilgwan G, Mutihir JT, Sagay AS, Odili A, Zoakah AI, Bode-Thomas F, Simon MA. Profile of congenital heart disease in infants born following exposure to preeclampsia. PLoS One 15: e0229987, 2020. doi: 10.1371/journal.pone.0229987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Crispi F, Figueras F, Cruz-Lemini M, Bartrons J, Bijnens B, Gratacos E. Cardiovascular programming in children born small for gestational age and relationship with prenatal signs of severity. Am J Obstet Gynecol 207: 121, 2012. doi: 10.1016/j.ajog.2012.05.01. [DOI] [PubMed] [Google Scholar]
- 72.Darby JRT, Varcoe TJ, Orgeig S, Morrison JL. Cardiorespiratory consequences of intrauterine growth restriction: Influence of timing, severity and duration of hypoxaemia. Theriogenology 150: 84–95, 2020. doi: 10.1016/j.theriogenology.2020.01.080. [DOI] [PubMed] [Google Scholar]
- 73.Cruz‐Lemini M, Crispi F, Valenzuela‐Alcaraz B, Figueras F, Sitges M, Bijnens B, Gratacós E. Fetal cardiovascular remodeling persists at 6 months in infants with intrauterine growth restriction. Ultrasound Obstet Gynecol 48: 349–356, 2016. doi: 10.1002/uog.15767. [DOI] [PubMed] [Google Scholar]
- 74.Sarvari SI, Rodriguez-Lopez M, Nunez-Garcia M, Sitges M, Sepulveda-Martinez A, Camara O, Butakoff C, Gratacos E, Bijnens B, Crispi F. Persistence of cardiac remodeling in preadolescents with fetal growth restriction. Circ Cardiovasc Imaging 10: e005270, 2017. doi: 10.1161/CIRCIMAGING.116.005270. [DOI] [PubMed] [Google Scholar]
- 75.Barouki R, Gluckman PD, Grandjean P, Hanson M, Heindel JJ. Developmental origins of non-communicable disease: implications for research and public health. Environ Health 11: 42, 2012. doi: 10.1186/1476-069X-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cameli M, Mondillo S, Righini F, Lisi M, Dokollari A, Lindqvist P, Maccherini M, Henein M. Left ventricular deformation and myocardial fibrosis in patients with advanced heart failure requiring transplantation. J Card Fail 22: 901–907, 2016. doi: 10.1016/j.cardfail.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 77.Yang H, Wright L, Negishi T, Negishi K, Liu J, Marwick T. Research to practice: assessment of left ventricular global longitudinal strain for surveillance of cancer chemotherapeutic-related cardiac dysfunction. JACC Cardiovasc Imaging 11: 1196–1201, 2018. doi: 10.1016/j.jcmg.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]