Abstract
Background
Coronavirus disease 2019 (COVID-19) is a disease primarily affecting the respiratory tract, however due to the nature of the pathogenesis it is able to affect the whole body. So far, no causative treatment has been found and the main strategy when dealing with COVID-19 relies on widespread vaccination programs and symptomatic treatment. Vitamin D due to its ability to modulate the immunological system has been proposed as a factor playing role in the organism response to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Therefore, we decided to perform this meta-analysis which aimed to establish a connection between vitamin D status and COVID-19 infection.
Methods
Study was designed as a systematic review and meta-analysis. PubMed, EMBASE, Web of Science, Cochrane Collaboration Databases and Scopus electronic databases were searched for relevant studies from database inception to May 10th, 2021. Mean differences (MDs) with their 95% confidence intervals (CI) were calculated.
Results
Thirteen studies providing data for 14,485 participants met the inclusion criteria. Mean vitamin D levels in SARS-CoV-2 negative patients was 17.7 ± 6.9 ng/mL compared to SARS-CoV-2 positive patients 14.1 ± 8.2 ng/mL (MD = 3.93; 95% CI 2.84–5.02; I2 = 99%; p < 0.001).
Conclusions
Low serum vitamin D levels are statistically significantly associated with the risk of COVID-19 infection. Supplementation of vitamin D especially in the deficiency risk groups is indicated.
Keywords: vitamin D, COVID-19, coronavirus disease 2019, SARS-CoV-2, systematic review, meta-analysis
Introduction
Since the outbreak of the new type of coronavirus disease called novel coronavirus disease 2019 (COVID-19) in Wuhan China in 2019 [1, 2] medical systems all over the world have been under immense pressure, resulting in a rapid increase in the cost of care [3]. The virus infects the host via angiotensin converting enzyme 2 (ACE2) [4]. Due to the fact that ACE2 expression is the highest in the respiratory tract [5] it is the respiratory symptoms that are most prominent in COVID-19, however the ACE2 is expressed in the whole body which explains the multisymptomatic nature of the disease [6]. Due to rapidly spreading nature of the disease and its ability to disorganize the healthcare systems by the increased number of patients requiring intensive care the research was focused on finding a causative treatment. Several drugs have been proposed which include, but are not limited to: hydroxychloroquine [7, 8], janus kinase 2 inhibitor Fedratinib [9] or Remdesmivir [10]. None of which had been able to demonstrate utility in the treatment of COVID-19. Therefore, the efforts were focused on the development of the vaccines and so far, there are several drugs on the market that are able to relieve some of the tension placed on the healthcare system by COVID-19 [11, 12]. However, while vaccination programs are widespread and the number of vaccinated patients grows, the underlying risk factors for the severe course of COVID-19 are still being investigated. So far, several factors were established i.e.: obesity [13], diabetes [14] and smoking [15]. The common denominator for all of these risk factors is the disturbed immunological response which may in fact be the underlying mechanism for the severe course of COVID-19. One of the most common and thoroughly examined causes of immunosuppression is vitamin D deficiency [16]. Vitamin D plays a key role the modulation of the immunological response in both autoimmune and infectious diseases [17], via multiple patterns. Among many others it modulates the maturation of macrophages [18], regulates the T-lymphocyte stimulatory function of antigen-presenting cells [19] and regulates B-lymphocyte proliferation [18]. Therefore, it comes as no surprise that in the era of COVID-19, vitamin D became an object of interest for much research worldwide in terms of preventing the severe course of the disease. We decided to perform this meta-analysis in order to establish a possible link between the levels of vitamin D and COVID-19 infections.
Methods
This trial was prepared following the recommendations of the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines [19]. Before commencing the study, analyses methods as well as inclusion and exclusion criteria to be used were agreed upon. Because of the nature of this systematic review and meta-analysis, this study was exempt review by the institutional review board.
Literature search
A systematic review was carried out using PubMed, EMBASE, Web of Science, Cochrane Collaboration Databases and Scopus electronic databases. The most recent search was performed on May 10th, 2021. Titles and abstracts were screened by two authors independently (A.G. and W.G.). All retrieved articles were reviewed by two authors (J.S. and A.G.). Any disagreement was resolved through consensus or, if necessary, by discussion with a third author (L.S.).
The search was performed using the following terms: “25-hydroxyvitamin D” OR “25(OH)D” OR “vitamin D” AND “coronavirus” OR “SARS-CoV-2” OR “COVID-19”. A manual search of references listed in reviews and reports was also performed. Only full articles in the English language were considered. All references were saved in an EndNote (End Note, Inc, Philadelphia, PA) library used to identify duplicates.
Inclusion and exclusion criteria
Studies included in this meta-analysis met the following PICOS criteria: (1) PARTICIPANTS; patients > 18 years of age, (2) INTERVENTION; SARS-CoV-2 positive patients, (3) COMPARISON; SARS-CoV-2 negative patients, (4) OUTCOMES; detailed information for vitamin D-3 levels, (5) STUDY DESIGN; randomized controlled trials, quasi-randomized or observational studies comparing cardiac arrest during and before the COVID-19 period for their effects in patients with cardiac arrest. Reviews, simulation trials, animal studies, letters, conference papers and case studies were excluded.
Data extraction
Two reviewers (L.S. and W.G.) independently assessed each article to determine which article met the inclusion criteria. Any disagreements were resolved by consensus with a third reviewer (A.G.). The following information was extracted from each included study: the first author’s name, year of publication, study design, country, sample size, age, gender, vitamin D level in SARS-CoV-2 positive and negative patients.
Quality assessment
Two reviewers (A.G. and H.K.) independently extracted individual study data and evaluated studies for risk of bias. Any disagreements were discussed and resolved in a consensus meeting with the third reviewer (M.M.). The revised tool for risk of bias in randomized trials — RoB 2 tool was used to assess the quality of randomized studies [20]. Moreover, the Robvis application was used to visualize risk of bias assessments [21].
The evaluation consisted of the following domains: confounding, participant selection, classification of interventions, deviation from interventions, missing data, outcome measurement and selection of reported results. Each domain was assessed according to the following scale: serious, moderate and low.
Statistical analysis
All statistical analysis were performed using RevMan v.5.4 (The Cochrane Collaboration, Oxford, Copenhagen, Denmark) and STATA v.16.1. (StataCorp LLC, Texas, USA). All tests were 2-sided and a p value of less than 0.05 was considered as statistically significant. To analyze dichotomous outcomes the Mantel-Haenszel method was used, and results are reported as odds ratios with a 95% confidence interval (CI) and two tailed p values. The inverse variance model with a 95% CI was used to analyze continuous outcome differences and data are reported as the mean difference (MD). Results are presented as risk ratios with 95% CI for dichotomous measures. When the continuous data were reported in the articles as the median and interquartile range, estimated means and standard deviations were calculated using the formula described by Hozo et al. [22].
Data heterogeneity was assessed using the tau-squared and I-squared statistics. Heterogeneity was detected with the chi-squared test with n – 1 degrees of freedom, which was expressed as I2 [23]. For all analysis a random model was used.
Results
Characteristics of studies included in the meta-analysis
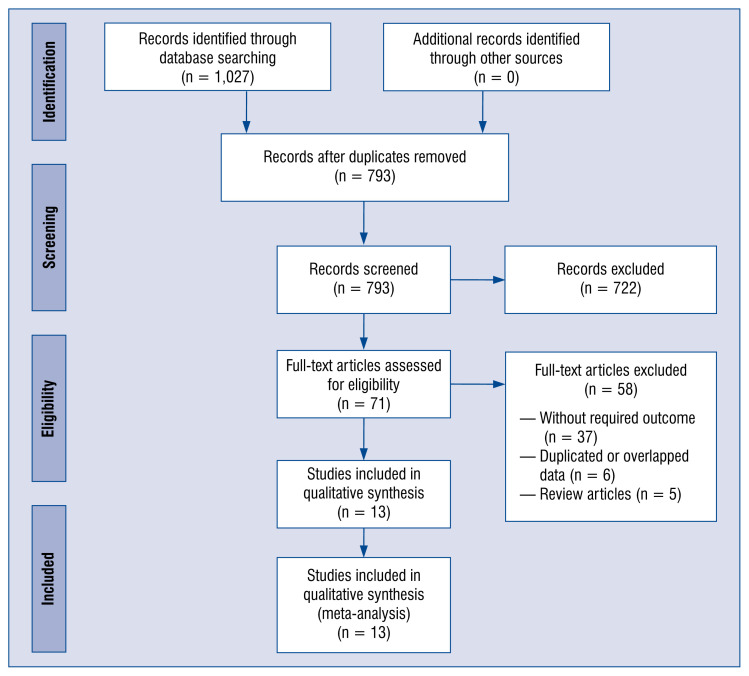
A detailed description of the process of study selection was presented in Figure 1. We found 1,027 potential citations during the search of databases. 234 articles were excluded because they were duplicates, and 722 articles were also excluded because they were unrelated studies. The remaining 71 articles were fully reviewed, and 13 studies providing data for 14,485 participants met the inclusion criteria and were included in the current meta-analysis [24–36]. The details of selected trials are summarized in Table 1. Of those trials, 3 studies were performed in United Kingdom, 2 studies in Iran, 2 in Saudi Arabia, 2 in Italy, and 1 in each of the following countries: Spain, Republic of Korea, Israel and China.
Figure 1.
Flow diagram showing stages of the database search and study selection as per Preferred Reporting Items for Systematic reviews and Meta-analysis (PRISMA) guideline.
Table 1.
Patient characteristics in included studies.
| Study | Country | Study design | SARS-CoV-2 negative group | SARS-CoV-2 positive group | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Number | Age | Sex, male | Number | Age | Sex, male | |||
| Abdollahi et al. 2020 | Iran | Case-control study | 201 | 48 ± 16.95 | Not specified | 201 | 46.34 ± 13.5 | Not specified |
| Alguwalhes et al. 2021 | Saudi Arabia | Retrospective study | 72 | 59.1 ± 16.8 | 38 (52.8%) | 150 | 55.5 ± 15.8 | 97 (64.7%) |
| Al-Daghri et al. 2021 | Saudi Arabia | Multi-center case-control study | 82 | 32 ± 13 | 41 (50.0%) | 138 | 50 ± 13 | 79 (57.2%) |
| Baktash et al. 2020 | United Kingdom | Prospective cohort study | 35 | 83.4 ± 8.1 | 15 (42.9%) | 70 | 80.2 ± 8.6 | 42 (60.0%) |
| D’Avolio et al. 2020 | Italy | Retrospective study | 80 | 72.3 ± 6.1 | 39 (48.8%) | 27 | 73.5 ± 4.6 | 19 (70.4%) |
| Hernández et al. 2020 | Spain | Retrospective case-control study | 197 | 61 ± 1.7 | 123 (62.4%) | 216 | 60.2 ± 4 | 130 (60.2%) |
| Im et al. 2020 | Republic of Korea | Prospective cohort study | 50 | 52.4 ± 20.2 | Not specified | 150 | 52.2 ± 20.7 | Not specified |
| Livingston et al. 2021 | United Kingdom | Prospective cohort study | 57 | 68.5 ± 18.1 | 19 (33.3%) | 47 | 68.6 ± 18.7 | 20 (42.6%) |
| Mardani et al. 2020 | Iran | Case-control study | 60 | 40.8 ± 15.5 | 30 (50.0%) | 63 | 43.3 ± 14.5 | 35 (55.6%) |
| Merzon et al. 2020 | Israel | Population-based study | 7,025 | 47.4 ± 0.2 | 2,849 (40.6%) | 782 | 35.6 ± 0.4 | 385 (49.2%) |
| Raisi-Estabragh et al. 2020 | United Kingdom | Prospective cohort study | 3,184 | 68.9 ± 8.7 | 1,505 (47.3%) | 1,326 | 68.1 ± 9.2 | 696 (52.5%) |
| Sulli et al. 2021 | Italy | Case-control study | 65 | 76 ± 13 | 30 (46.2%) | 65 | 76 ± 13 | 30 (46.2%) |
| Ye et al. 2020 | China | Case-control study | 80 | 41.8 ± 3.5 | 32 (40.0%) | 62 | 44.3 ± 7.8 | 23 (37.1%) |
Result of the meta-analysis
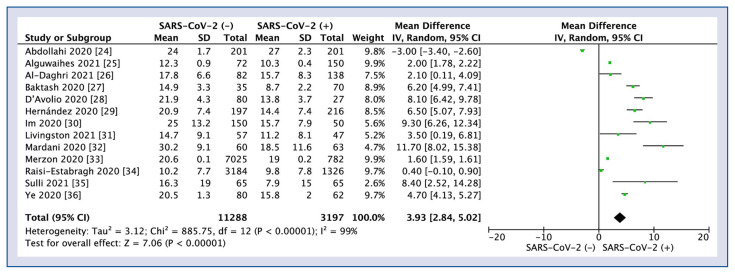
Polled analysis of all 13 studies reported vitamin D levels in the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) negative versus positive patients is shown in Figure 2. Mean vitamin D levels in SARS-CoV-2 negative patients was 17.7 ± 6.9 ng/mL compared to SARS-CoV-2 positive patients 14.1 ± 8.2 ng/mL (MD = 3.93; 95% CI 2.84–5.02; I2 = 99%; p < 0.001).
Figure 2.
Forest plot of vitamin D levels between severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) negative versus positive patients. The center of each square represents the weighted odds ratios for individual trials, and the corresponding horizontal line stands for a 95% confidence interval (CI). The diamonds represent pooled results; SD — standard deviation.
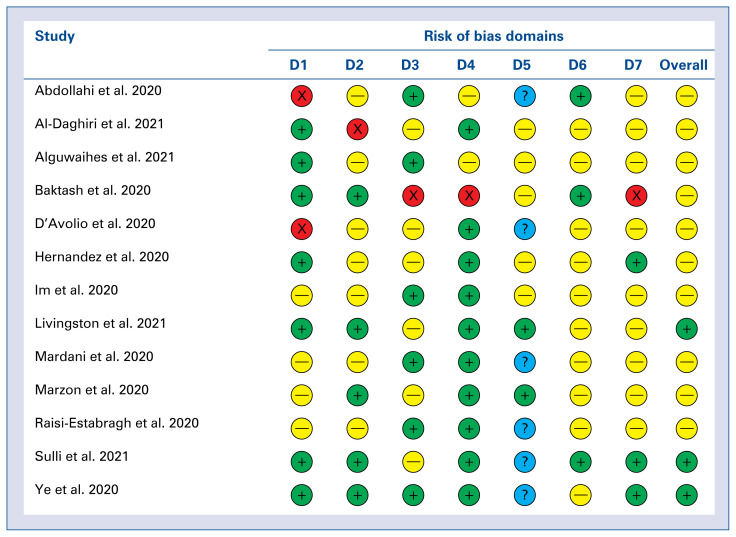
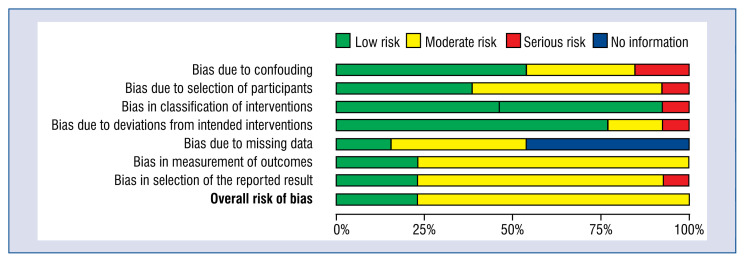
The detailed risk of bias abuts the methodological quality of the included studies that are elaborated and summarized in Figures 3 and 4.
Figure 3.
A summary table of review authors’ judgements for each risk of bias item for each study. Domains: D1 — bias due to confouding; D2 — bias due to selection of participats; D3 — bias in classification of interventions; D4 — bias due to deviations from intended interventions; D5 — bias due to missing data; D6 — bias in measurement of outcomes; D7 — bias in selection of the reported result. Judgement:
 Serious;
Serious;
 Moderate;
Moderate;
 Low;
Low;
 No information.
No information.
Figure 4.
A plot of the distribution of review authors’ judgements across studies for each risk of bias item.
Discussion
The number of reports indicating the potential role of vitamin D deficiency in the COVID-19 increases [37]. The potential role in the prevention of a severe course of COVID-19 was further strengthened by the identification of calcitriol (active form of vitamin D) as the regulator of renin-angiotensin system (RAS), of which an overactivation is associated with poor prognosis [38, 39]. Abdollahi et al. [24] found that patients who suffer from vitamin D deficiency are more vulnerable to COVID-19 infection. However, he underlines that the patients suffering from COVID-19 were more likely to be overweight or obese, while obesity is an independent risk factor for a more severe course of the disease [40] it must be noted that patients who are obese are also more likely to suffer from vitamin D deficiency [41]. Another group that suffers from the vitamin D deficiency are older patients [42] both due to the worse overall state of health and due to drugs, they take. The study by Baktash et al. [27] found that the patients who are older than 65 years and present with the COVID-19 symptoms are more likely to be vitamin D deficient, have elevated markers of cytokine release syndrome and have an increased risk of respiratory failure. However, no difference was found in terms of mortality between the patients who were deficient and those who had their vitamin D within normal ranges, indicating that in the older group the overall poor prognosis is associated with the general health status and presence of comorbidities. These findings are consistent with those achieved by D’Avolio et al. [28], who also found that vitamin D was lower in the patients positive for COVID-19, while indicating that the supplementation of vitamin D might be useful for prevention of infection.
The strategy of vitamin D supplementation as indicated by Grant et al. [43] suggests the rapid increase of vitamin D serum levels through the high supplementation for a few weeks going as high as 10,000 IU/day in order to achieve the normal range. This strategy has been used for considearable time and has proven to be safe in delaying frailty [44]. In the study by Al-Daghri et al. [26] vitamin D deficiency was only observed in the group of older patients, those with type 2 diabetes and lower density lipoprotein levels. Interestingly the author, contrary to Grant et al. [43] supports the idea of rather moderate vitamin D loading in deficient patients, not exceeding 2000 iu/day, which is supported by Bergman [45]. Alguwaihes et al. [25] provides interesting data regarding vitamin D deficiency and the risk of COVID-19 in a hospital setting. While he did not find any evidence suggesting that the risk of infection increases in deficient patients, they are, in fact, at higher risk of mortality, possibly through an unregulated inflammatory response and cytokine storm [46]. Contrary to these findings Hernandez et al. [29] found no difference in the severity of the disease when accounting for vitamin D deficiency, however he did find a higher prevalence of deficiency among hospitalized COVID-19 patients. When analyzing the nutritional status of patients suffering from COVID-19, Im et al. [30] they found that patients suffering from COVID-19 presented a higher percentage of vitamin D deficiency when compared with a control group, additionally while not statistically significant 30 out of 38 patients who suffered from respiratory distress were deficient in vitamin D. What is worth noting is that the patients who required mechanical ventilation were deficient in at least one nutrient. Therefore, it is advised to monitor and react to the nutritional status of the COVID-19 patients [47]. Mardani et al. [32], in his study, analyzed an association in the level of vitamin D and the severity of COVID-19, along with levels of ACE2 and neutrophil to lymphocyte ratio (NLR). The NLR is a useful tool to assess systemic inflammation [48] also in acute lung injury and acute respiratory distress syndrome [49] which are common findings in the severe course of COVID-19. Having found lower levels of vitamin D in COVID-19 patients, the authors concluded that the deficiency may cause an immunological imbalance, overactivation of the RAS pathway and therefore a hyperinflammation state. Raisi-Estabragh et al. [34] in her study found that vitamin D deficiency was not an independent risk factor for black, Asian and minority ethnicities and that a cascade of factors play a role rather than a single one that can be pinpointed. In a study by Ye et al. [36], he found that vitamin D deficiency increases risk of COVID-19 infection, while the supplementation of it provides protective effects against a severe course of the disease. These findings are further reinforced by Sulli et al. [35] who found that vitamin D deficiency is associated with more severe lung involvement, longer disease duration, and risk of death in elderly COVID-19 patients. A study by Livingstone et al. [31] among vitamin D deficiency indicates that social deprivation plays role in COVID-19 infection. While studies for the general population showed that social distancing is beneficial for the reduction in COVID-19 incidence rate [50], we must differentiate between social distancing and deprivation since the latter is a well-established risk factor for worsening of health outcomes [51]. Merzon et al. [33] identified vitamin D deficiency as an independent risk factor not only for COVID-19 infection, but also hospitalization, other risk factors included were being male and over the age of 50.
All of the studies measured levels of vitamin D at the moment of acute COVID-19 infection, however as previous studies showed [52], acute respiratory infection does not alter the vitamin D levels, therefore a sample on admission is representative.
Conclusions
Low serum vitamin D levels are statistically and significantly associated with the risk of COVID-19 infection. Supplementation of vitamin D especially in deficiency, risk groups are indicated.
Acknowledgments
The study was supported by the ERC Research Net and by the Polish Society of Disaster Medicine.
Footnotes
This paper was guest edited by Prof. Togay Evrin
Conflict of interest: None declared
References
- 1.Song F, Shi N, Shan F, et al. Emerging 2019 Novel Coronavirus (2019-nCoV) Pneumonia. Radiology. 2020;295(1):210–217. doi: 10.1148/radiol.2020200274. Erratum in: Radiology. 2020 Dec;297(3):E346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dzieciatkowski T, Szarpak L, Filipiak KJ, et al. COVID-19 challenge for modern medicine. Cardiol J. 2020;27(2):175–183. doi: 10.5603/CJ.a2020.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Fusco M, Shea KM, Lin J, et al. Health outcomes and economic burden of hospitalized COVID-19 patients in the United States. J Med Econ. 2021;24(1):308–317. doi: 10.1080/13696998.2021.1886109. [DOI] [PubMed] [Google Scholar]
- 4.Hamming I, Timens W, Bulthuis MLC, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sungnak W, Huang Ni, Bécavin C, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26(5):681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu J, Cao R, Xu M, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discovery. 2020;6(1) doi: 10.1038/s41421-020-0156-0.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sahraei Z, Shabani M, Shokouhi S, et al. Aminoquinolines against coronavirus disease 2019 (COVID-19): chloroquine or hydroxychloroquine. Int J Antimicrob Agents. 2020;55(4):105945. doi: 10.1016/j.ijantimicag.2020.105945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu D, Yang XO. TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib. J Microbiol Immunol Infect. 2020;53(3):368–370. doi: 10.1016/j.jmii.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szarpak Ł, Dzieciątkowski T, Jaguszewski MJ, et al. Is remdesivir important in clinical practice as a treatment of COVID-19? A study based on meta-analysis data. Pol Arch Intern Med. 2021;131(1):96–97. doi: 10.20452/pamw.15686. [DOI] [PubMed] [Google Scholar]
- 10.Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baden LR, El Sahly HM, Essink B, et al. Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho JSY, Fernando DI, Chan MY, et al. Obesity in COVID-19: a systematic review and meta-analysis. Ann Acad Med Singap. 2020;49(12):996–1008. [PubMed] [Google Scholar]
- 13.Abdi A, Jalilian M, Sarbarzeh PA, et al. Diabetes and COVID-19: a systematic review on the current evidences. Diabetes Res Clin Pract. 2020;166:108347. doi: 10.1016/j.diabres.2020.108347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu L, Chen S, Fu Y, et al. Risk factors associated with clinical outcomes in 323 coronavirus disease 2019 (COVID-19) hospitalized patients in Wuhan, China. Clin Infect Dis. 2020;71(16):2089–2098. doi: 10.1093/cid/ciaa539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palacios C, Gonzalez L. Is vitamin D deficiency a major global public health problem? J Steroid Biochem Mol Biol. 2014;144(Pt A):138–145. doi: 10.1016/j.jsbmb.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wacker M, Holick MF. Vitamin D: effects on skeletal and extraskeletal health and the need for supplementation. Nutrients. 2013;5(1):111–148. doi: 10.3390/nu5010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hewison M, Freeman L, Hughes SV, et al. Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells. J Immunol. 2003;170(11):5382–5390. doi: 10.4049/jimmunol.170.11.5382. [DOI] [PubMed] [Google Scholar]
- 18.Xu H, Soruri A, Gieseler RK, et al. 1,25-Dihydroxyvitamin D3 exerts opposing effects to IL-4 on MHC class-II antigen expression, accessory activity, and phagocytosis of human monocytes. Scand J Immunol. 1993;38(6):535–540. doi: 10.1111/j.1365-3083.1993.tb03237.x. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2021;12(1):55–61. doi: 10.1002/jrsm.1411. [DOI] [PubMed] [Google Scholar]
- 22.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdollahi A, Kamali Sarvestani H, Rafat Z, et al. The association between the level of serum 25(OH) vitamin D, obesity, and underlying diseases with the risk of developing COVID-19 infection: A case-control study of hospitalized patients in Tehran, Iran. J Med Virol. 2021;93(4):2359–2364. doi: 10.1002/jmv.26726. [DOI] [PubMed] [Google Scholar]
- 25.Alguwaihes A, Sabico S, Hasanato R, et al. Severe vitamin D deficiency is not related to SARS-CoV-2 infection but may increase mortality risk in hospitalized adults: a retrospective case–control study in an Arab Gulf country. Aging Clin Exp Res. 2021;33(5):1415–1422. doi: 10.1007/s40520-021-01831-0.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Daghri NM, Amer OE, Alotaibi NH, et al. Vitamin D status of Arab Gulf residents screened for SARS-CoV-2 and its association with COVID-19 infection: a multi-centre case-control study. J Transl Med. 2021;19(1):166. doi: 10.1186/s12967-021-02838-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baktash V, Hosack T, Patel N, et al. Vitamin D status and outcomes for hospitalised older patients with COVID-19. Postgrad Med J. 2021;97(1149):442–447. doi: 10.1136/postgradmedj-2020-138712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D’Avolio A, Avataneo V, Manca A, et al. 25-Hydroxyvitamin D concentrations are lower in patients with positive PCR for SARS-CoV-2. Nutrients. 2020;12(5) doi: 10.3390/nu12051359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hernández JL, Nan D, Fernandez-Ayala M, et al. Vitamin D Status in Hospitalized Patients with SARS-CoV-2 Infection. J Clin Endocrinol Metab. 2021;106(3):e1343–e1353. doi: 10.1210/clinem/dgaa733. [DOI] [PubMed] [Google Scholar]
- 30.Im JH, Je YS, Baek J, et al. Nutritional status of patients with COVID-19. Int J Infect Dis. 2020;100:390–393. doi: 10.1016/j.ijid.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Livingston M, Plant A, Dunmore S, et al. Detectable respiratory SARS-CoV-2 RNA is associated with low vitamin D levels and high social deprivation. Int J Clin Pract. 2021:e14166. doi: 10.1111/ijcp.14166. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mardani R, Alamdary A, Mousavi Nasab SD, et al. Association of vitamin D with the modulation of the disease severity in COVID-19. Virus Res. 2020;289:198148. doi: 10.1016/j.virusres.2020.198148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Merzon E, Tworowski D, Gorohovski A, et al. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: an Israeli population-based study. FEBS J. 2020;287(17):3693–3702. doi: 10.1111/febs.15495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raisi-Estabragh Z, McCracken C, Bethell MS, et al. Greater risk of severe COVID-19 in Black, Asian and Minority Ethnic populations is not explained by cardiometabolic, socioeconomic or behavioural factors, or by 25(OH)-vitamin D status: study of 1326 cases from the UK Biobank. J Public Health (Oxf) 2020;42(3):451–460. doi: 10.1093/pubmed/fdaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sulli A, Gotelli E, Casabella A, et al. Vitamin d and lung outcomes in elderly COVID-19 patients. Nutrients. 2021;13(3) doi: 10.3390/nu13030717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ye K, Tang F, Liao X, et al. Does serum vitamin D level affect COVID-19 infection and its severity? A case-control study. J Am Coll Nutr. 2020:1–8. doi: 10.1080/07315724.2020.1826005. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 37.Mitchell F. Vitamin-D and COVID-19: do deficient risk a poorer outcome? Lancet Diabetes Endocrinol. 2020;8(7):570. doi: 10.1016/S2213-8587(20)30183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mok C, Ng Y, Ahidjo B, et al. Calcitriol, the active form of vitamin D, is a promising candidate for COVID-19 prophylaxis. bioRxiv. 2020 doi: 10.1101/2020.06.21.162396.. [DOI] [Google Scholar]
- 39.Martineau AR, Forouhi NG. Vitamin D for COVID-19: a case to answer? Lancet Diabetes Endocrinol. 2020;8(9):735–736. doi: 10.1016/S2213-8587(20)30268-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petrakis D, Margină D, Tsarouhas K, et al. Obesity: a risk factor for increased COVID19 prevalence, severity and lethality (review) Mol Med Rep. 2020;22(1):9–19. doi: 10.3892/mmr.2020.11127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vranić L, Mikolašević I, Milić S. Vitamin D deficiency: consequence or cause of obesity? Medicina (Kaunas) 2019;55(9) doi: 10.3390/medicina55090541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kweder H, Eidi H. Vitamin D deficiency in elderly: Risk factors and drugs impact on vitamin D status. Avicenna J Med. 2018;8(4):139–146. doi: 10.4103/ajm.AJM_20_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grant WB, Lahore H, McDonnell SL, et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12(4) doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bacon CJ, Gamble GD, Horne AM, et al. High-dose oral vitamin D3 supplementation in the elderly. Osteoporos Int. 2009;20(8):1407–1415. doi: 10.1007/s00198-008-0814-9. [DOI] [PubMed] [Google Scholar]
- 45.Bergman P. The link between vitamin D and COVID-19: distinguishing facts from fiction. J Intern Med. 2021;289(1):131–133. doi: 10.1111/joim.13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Daneshkhah A, Agrawal V, Eshein A, et al. Evidence for possible association of vitamin D status with cytokine storm and unregulated inflammation in COVID-19 patients. Aging Clin Exp Res. 2020;32(10):2141–2158. doi: 10.1007/s40520-020-01677-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mehta S. Nutritional status and COVID-19: an opportunity for lasting change? Clin Med (Lond) 2020;20(3):270–273. doi: 10.7861/clinmed.2020-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martins EC, Silveira Ld, Viegas K, et al. Neutrophil-lymphocyte ratio in the early diagnosis of sepsis in an intensive care unit: a case-control study. Rev Bras Ter Intensiva. 2019;31(1):64–70. doi: 10.5935/0103-507X.20190010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Y, Ju M, Chen C, et al. Neutrophil-to-lymphocyte ratio as a prognostic marker in acute respiratory distress syndrome patients: a retrospective study. J Thorac Dis. 2018;10(1):273–282. doi: 10.21037/jtd.2017.12.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.VoPham T, Weaver MD, Hart JE, et al. Effect of social distancing on COVID-19 incidence and mortality in the US. medRxiv. 2020 doi: 10.1101/2020.06.10.20127589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Charlton J, Rudisill C, Bhattarai N, et al. Impact of deprivation on occurrence, outcomes and health care costs of people with multiple morbidity. J Health Serv Res Policy. 2013;18(4):215–223. doi: 10.1177/1355819613493772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haugen J, Chandyo RK, Ulak M, et al. 25-hydroxy-vitamin D concentration is not affected by severe or non-severe pneumonia, or inflammation, in young children. Nutrients. 2017;9(1) doi: 10.3390/nu9010052. [DOI] [PMC free article] [PubMed] [Google Scholar]