This editorial refers to ‘High sensitivity C-reactive protein in chronic heart failure: patient characteristics, phenotypes and mode of death’, by P. Pellicori et al., pp. 91–100.
The role of inflammation in heart failure was initially proposed as a mechanism driving cardiac cachexia almost 30 years ago. Since then levels of inflammatory cytokines including tumour necrosis factor (TNF), interleukin (IL)-1β, and IL-6 have been found to be elevated in heart failure with reduced ejection fraction (HFrEF),1,2 (Figure 1) and TNF and IL-6 serum levels correlate with heart failure severity and prognosis.1,3 Furthermore, a prospective study showed that elevated basal cytokine levels predicted future development of heart failure.4 In light of these findings antibodies directed again TNF signalling molecules were assessed as a putative inflammatory HFrEF therapeutic target. However, TNF antagonism was proven to be detrimental rather than ameliorative.5 Since then, a broad array of biological immune-suppressing therapeutics has emerged, although none have been comprehensively explore to date in heart failure.
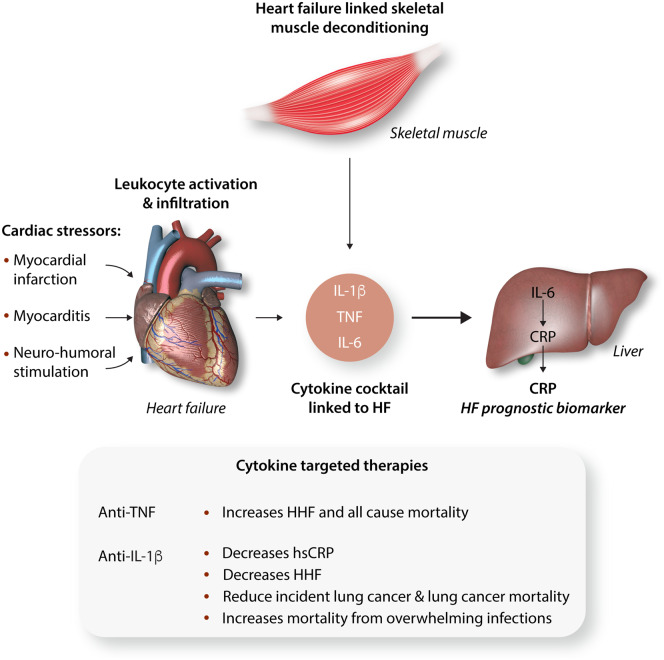
Figure 1.
The roles of cytokines in heart failure. Cardiac stressors that initiate and exacerbate cardiac dysfunction evoke leucocyte activation in the heart with cytokine production and release. The associated skeletal muscle deconditioning up-regulates the same cytokines, albeit to a lesser extent. TNF and IL-1β, through IL-6 promote CRP production in the liver. The release of this acute phase reactant appears to be a composite biomarker of the inflammatory cascade activated by heart failure and its levels predict outcome. The inset shows the targeting of cytokines with mixed effects. HF, heart failure; HHF, hospitalization from HF; hsCRP, highly sensitive C-reactive protein.
The source of circulating cytokines associated with heart failure have not been extensively explored. Nevertheless, as with other solid organs the heart possesses a discernable population of resident macrophages and dendritic cells (DCs) that provide an immune surveillance function to maintain cellular homeostasis. In response to modest insults including subclinical viral myocarditis, resident DC’s initiate immune activation to manage the infection and prevent overt heart failure. In contrast, if myocardial injury is extensive (myocardial infarction/myocarditis) and, or in response to persistent neurohumoral activation, inflammation, and the production of chemokines facilitate excessive cardiac leucocyte infiltration promoting adverse remodelling and HFrEF.6,7 Additionally, risk factors linked to heart failure with preserved ejection fraction (HFpEF) including hypertension and ageing predispose to myocardial macrophage expansion which activate fibroblasts to stimulate collagen deposition.8 In parallel, skeletal muscle fatigue/dysfunction associated with heart failure produce the same suite of cytokines within skeletal muscle as is evident in HFrEF (Figure 1).9
Interestingly, C-reactive protein (CRP), an acute phase reactant predominantly synthesized by hepatocytes, is induced by IL-6 signalling which itself is up-regulated by IL-1β and TNF signalling (Figure 1).10 As these same three cytokines associate with the pathophysiology of heart failure, CRP could be considered a composite heart failure biomarker. Pellicori et al.11 show that the index levels of highly sensitive CRP (hsCRP), at the time of presentation to a heart failure referral centre, was a robust predictor of cardiovascular (CV) and non-CV mortality over a median follow-up of 53 months. The basal hsCRP level was independent of classical heart failure risk factors including age, symptom severity, creatinine, and NT-proBNP levels. The data were analysed in quartiles and the heart failure subjects were sub-analysed according to whether they had HFrEF, HFpEF, or heart failure with mid-range ejection fractions. Interesting, the highest hsCRP quartile patients had the highest incidence of cancer and infection as their terminal disease. At the same time, the incremental increase in hsCRP paralleled CV mortality irrespective of their baseline ejection fraction.11 It is also interesting that CV mortality was lowest in the HFpEF group, although this group also has the highest incidence of non-CV mortality. This study, albeit modest in size (3756 heart failure patients), had a 48% mortality rate during the follow-up period, and it raises interesting questions and challenges as whether CRP attenuation should be directly targeted in this population.
The concept of targeting CRP has begun to be addressed in CV disease. Although direct CV therapeutic agents including β-blockade, renin–angiotensin pathway antagonists, and statins have a modest effect on reducing CRP levels, these effects in all probability stem in part from alleviating strain on the myocardium, reducing neurohumoral signalling, and via coronary plaque stabilization. More directed therapies to reduced CRP levels include blunting upstream IL-1β signalling with either an IL-1-receptor antagonist (anakinra) or with an IL-β monoclonal antibody (canakinumab). Numerous studies with functional rather than disease-outcome endpoints have been designed and undertaken to study both anakinra and canakinumab in heart failure, as documented on ClinicalTrials.gov. Although the data have yet to be extensively published both IL-1β targeting biologicals do robustly blunt CRP levels.12,13 The most extensive outcome data has recently been analysed as a prespecified exploratory endpoint to assess whether canakinumab would diminish heart failure associated hospitalization or mortality as a sub-study of the CANTOS trial.13 In CANTOS, 10 061 patients with prior myocardial infarction and a baseline hsCRP >2 mg/L, were randomized to placebo or three different doses of canakinumab therapy over a 48-month period.14 In total, 2173 of the CANTOS study patients had a diagnosis of heart failure on study entry and over the course of a median follow-up of 3.7 years; 385 of these patients had either a hospitalization for heart failure (HHF) and/or heart failure related mortality (HFM). The unadjusted hazard ratio for HHF and the composite of HHF and HFM was inversely related to the patients dose of canakinumab with the lowest incidence of outcomes in those on the highest dose of canakinumab.13 In parallel with the study by Pellicori et al.,11 the levels of baseline CRP was higher in the CANTOS HF sub-study HF patients who experienced HHF than those that did not.13
An interesting finding in the initial CANTOS study was that blocking IL-1β appeared to protect from lung cancer mortality.15 At the same time, the overall mortality in CANTOS was not improved by canakinumab due to an increased risk of sepsis. These contrary outcomes are pertinent to HF given that the Pellicori study in this issue shows that higher baseline levels of hsCRP increased the risk of infection and cancer-related deaths irrespective of the HF classification. The opposite effects of canakinumab on cancer and sepsis, should inject caution into the consideration of this therapeutic in HF, given that the HF population is ageing, and the risk of infections similarly increases with age. Further prospective studies targeting hsCRP with mortality and infection risk as an endpoints would be required before this therapeutic strategy can be embraced or considered as an additional option in the management of HF. Overall, the weight of evidence supports an important role of inflammation in the pathophysiology and prognosis of heart failure. However, directly targeting inflammation to manage heart failure remains an enigma due, in part, to systemic effects of immunosuppression.
Conflict of interest: none declared.
Funding
M.N.S. (ZIA-HL005199-10) is funded by the Division of Intramural Research of the NHLBI, NIH.
The opinions expressed in this article are not necessarily those of the Editors of Cardiovascular Research or of the European Society of Cardiology.
References
- 1.Torre-Amione G, Kapadia S, Benedict C, Oral H, Young JB, Mann DL.. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the Studies of Left Ventricular Dysfunction (SOLVD). J Am Coll Cardiol 1996;27:1201–1206. [DOI] [PubMed] [Google Scholar]
- 2.Bujak M, Dobaczewski M, Chatila K, Mendoza LH, Li N, Reddy A, Frangogiannis NG.. Interleukin-1 receptor type I signaling critically regulates infarct healing and cardiac remodeling. Am J Pathol 2008;173:57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deswal A, Petersen NJ, Feldman AM, Young JB, White BG, Mann DL.. Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone trial (VEST). Circulation 2001;103:2055–2059. [DOI] [PubMed] [Google Scholar]
- 4.Kalogeropoulos A, Georgiopoulou V, Psaty BM, Rodondi N, Smith AL, Harrison DG, Liu Y, Hoffmann U, Bauer DC, Newman AB, Kritchevsky SB, Harris TB, Butler J, Health A.. Inflammatory markers and incident heart failure risk in older adults: the Health ABC (Health, Aging, and Body Composition) study. J Am Coll Cardiol 2010;55:2129–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT, Anti T.. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation 2003;107:3133–3140. [DOI] [PubMed] [Google Scholar]
- 6.Nahrendorf M.Myeloid cell contributions to cardiovascular health and disease. Nat Med 2018;24:711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frantz S, Falcao-Pires I, Balligand JL, Bauersachs J, Brutsaert D, Ciccarelli M, Dawson D, de Windt LJ, Giacca M, Hamdani N, Hilfiker-Kleiner D, Hirsch E, Leite-Moreira A, Mayr M, Thum T, Tocchetti CG, van der Velden J, Varricchi G, Heymans S.. The innate immune system in chronic cardiomyopathy: a European Society of Cardiology (ESC) scientific statement from the Working Group on Myocardial Function of the ESC. Eur J Heart Fail 2018;20:445–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hulsmans M, Sager HB, Roh JD, Valero-Munoz M, Houstis NE, Iwamoto Y, Sun Y, Wilson RM, Wojtkiewicz G, Tricot B, Osborne MT, Hung J, Vinegoni C, Naxerova K, Sosnovik DE, Zile MR, Bradshaw AD, Liao R, Tawakol A, Weissleder R, Rosenzweig A, Swirski FK, Sam F, Nahrendorf M.. Cardiac macrophages promote diastolic dysfunction. J Exp Med 2018;215:423–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gielen S, Adams V, Mobius-Winkler S, Linke A, Erbs S, Yu J, Kempf W, Schubert A, Schuler G, Hambrecht R.. Anti-inflammatory effects of exercise training in the skeletal muscle of patients with chronic heart failure. J Am Coll Cardiol 2003;42:861–868. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka T, Narazaki M, Kishimoto T.. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol 2014;6:a016295.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pellicori P, Zhang J, Cuthbert J, Urbinati A, Shah P, Kazmi S, Clark AL, Cleland JGF.. High sensitivity C-reactive protein in chronic heart failure: patient characteristics, phenotypes and mode of death. Cardiovac Res 2020;116:91–100. [DOI] [PubMed] [Google Scholar]
- 12.Ikonomidis I, Lekakis JP, Nikolaou M, Paraskevaidis I, Andreadou I, Kaplanoglou T, Katsimbri P, Skarantavos G, Soucacos PN, Kremastinos DT.. Inhibition of interleukin-1 by anakinra improves vascular and left ventricular function in patients with rheumatoid arthritis. Circulation 2008;117:2662–2669. [DOI] [PubMed] [Google Scholar]
- 13.Everett BM, Cornel JH, Lainscak M, Anker SD, Abbate A, Thuren T, Libby P, Glynn RJ, Ridker PM.. Anti-inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation 2019;139:1289–1299. [DOI] [PubMed] [Google Scholar]
- 14.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ, Group CT.. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119.. [DOI] [PubMed] [Google Scholar]
- 15.Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ; CANTOS Trial Group. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 2017;390:1833–1842. [DOI] [PubMed] [Google Scholar]