Abstract
Background: Pyrotinib is a novel irreversible pan-ErbB receptor tyrosine kinase inhibitor. Evidence of the efficacy of pyrotinib-based treatments for HER2-positive metastatic breast cancer (MBC) in patients exposed to lapatinib is limited.
Methods: Ninety-four patients who received pyrotinib as a third- or higher-line treatment for HER2-positive MBC were included in this retrospective study. The primary and secondary endpoints were overall survival (OS) and progression‐free survival (PFS). Propensity score matching (PSM) and inverse probability of treatment weighting (IPTW) analysis were implemented to balance important patient characteristics between groups.
Results: Thirty (31.9%) patients were pretreated with lapatinib and subsequently received pyrotinib as an anti-HER2 treatment, and 64 (68.1%) patients did not receive this treatment. The OS and PFS indicated a beneficial trend in lapatinib-naive group compared to lapatinib-treated group in either the original cohort (PFS: 9.02 vs 6.36 months, p = 0.05; OS: 20.73 vs 14.35 months, p = 0.08) or the PSM (PFS: 9.02 vs 6.08 months, p = 0.07; OS: 19.07 vs 18.00 months, p = 0.61) or IPTW (PFS: 9.90 vs 6.17 months, p = 0.05; OS: 19.53 vs 15.10 months, p = 0.08) cohorts. Subgroup analyses demonstrated lapatinib treatment-related differences in PFS in the premenopausal subgroup and the no prior trastuzumab treatment subgroup, but no significant differences were observed in OS.
Conclusion: Pyrotinib-based therapy demonstrated promising effects in HER2-positive MBC patients in a real-world study, especially in lapatinib-naive patients, and also some activity in lapatinib-treated patients.
Keywords: pyrotinib, lapatinib-treated, lapatinib-naive, HER2 breast cancer, metastases
Introduction
Among patients with metastatic breast cancer (MBC), more than 20% have HER2-positive disease (Cobleigh et al., 2020; Howlader et al., 2014). Although this subtype of breast cancer has been historically associated with poor outcomes, the development of anti-HER2-targeted therapies has notably increased the median progression-free survival (PFS) and overall survival (OS) of patients (Slamon et al., 2001; Dawood et al., 2010; Swain et al., 2013; Mendes et al., 2015; Swain et al., 2015; Jain et al., 2018; Tripathy et al., 2020). Currently, tyrosine kinase inhibitors (TKIs) are officially approved by the International and Chinese Food and Drug Administrations for HER2-positive recurrence and MBC as second- or higher-line treatments (Ryan et al., 2008; Deeks, 2017; Blair, 2018).
Four TKIs are used to treat HER2-positive MBC, namely, lapatinib, tucatinib, neratinib, and pyrotinib (Xuhong et al., 2019; Lee, 2020). All of them were pan-ErbB receptors TKIs except tucatinib, which was a single HER2-targeted TKI (Wong et al., 2009; Awada et al., 2016; Li et al., 2017; Lin et al., 2020). Clinical trial results and our previous real-world study indicated that pyrotinib plus capecitabine had significantly superior efficacy and resulted in greater PFS than lapatinib combined with capecitabine (Jiang et al., 2019; Ma et al., 2019; Chen et al., 2020; Xuhong et al., 2020; Xu et al., 2021). Pyrotinib also demonstrated promising effects in brain metastatic HER2-positive breast cancer regardless of whether patients were previously treated with trastuzumab (Ma et al., 2019; (Anwar et al., 2021). The TBCRC022 study indicated that neratinib plus capecitabine was effective in HER2-positive patients with brain metastasis of breast cancer among the lapatinib-treated group (Freedman et al., 2019). However, whether pyrotinib is effective in patients after lapatinib treatment remains controversial (Lin et al., 2020; Song et al., 2020). This study was conducted after obtaining our final follow-up data to evaluate the effectiveness of pyrotinib as a third- or higher-line treatment. The aim of the study is to report the results of pyrotinib therapy in patients with and without prior lapatinib exposure before and after propensity score matching (PSM) analysis and inverse probability of treatment weighting (IPTW) analysis, with the hope of providing evidence of the effectiveness of pyrotinib-based treatment after failure of lapatinib-treated therapy.
Methods
Patient Eligibility and Data Collection
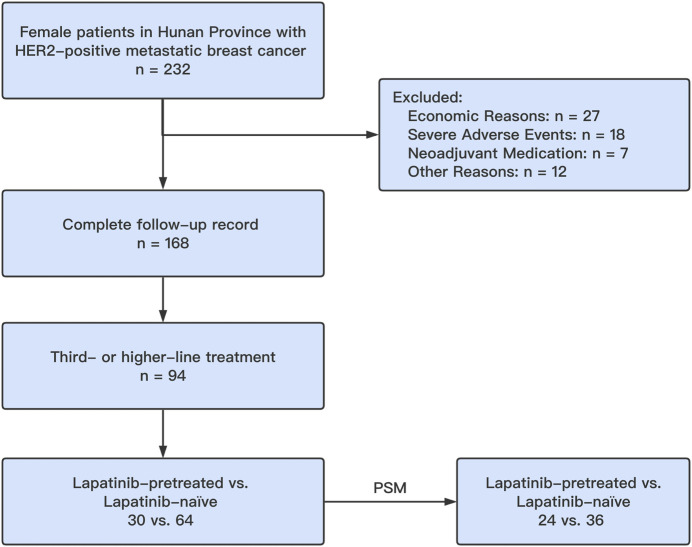
One hundred sixty-eight female patients with HER2-positive MBC were enrolled from June 2018 to August 2019. The follow-up period of the present study lasted until December 2020. Among these patients, 94 were treated with pyrotinib as a third- or higher-line treatment. Thirty (31.9%) patients had previously been treated with lapatinib and subsequently received pyrotinib-based therapy, and 64 (68.1%) patients had not been treated with lapatinib in this retrospective, multicenter, real-world study. Using PSM, a total of 60 patients (24 lapatinib-treated patients (40.0%) versus 36 lapatinib-naive patients (60.0%)) were matched, and the two groups were confirmed to have similar baseline clinical data (p > 0.05). Pyrotinib treatment was identical to that in our previous study (Geyer et al., 2006; Cameron et al., 2010; Xu et al., 2011) (Figure 1). The inclusion criteria were as follows (Cobleigh et al., 2020): confirmed HER2 positivity by immunohistochemistry (IHC) or fluorescence in situ hybridization (FISH) according to the HER2 status testing guidelines (Wolff et al., 2018; Howlader et al., 2014) stable vital signs and adequate physiological function (heart, liver, and kidney); and (Tripathy et al., 2020) a measurable lesion. The exclusion criteria were as follows (Cobleigh et al., 2020): discontinued pyrotinib treatment (Howlader et al., 2014); pyrotinib use in a neoadjuvant therapy setting (Tripathy et al., 2020); severe adverse side effects could not be controlled by dose reduction or adjuvant medication; and (Dawood et al., 2010) dropped out for other unknown reasons.
FIGURE 1.
CONSORT diagram for patient selection for the study. PSM, propensity score matching.
The pyrotinib treatment stage was defined as follows: first-line treatment was defined as the treatment of a patient with de novo stage IV breast cancer who was not treated previously with anti-HER2 medications or treatment of a patient with recurrence >12 months after discontinuation of trastuzumab. Second-line treatment was administered to patients with recurrence within 12 months of discontinuation of trastuzumab, recurrence during adjuvant therapy with trastuzumab, or progression following first-line treatment. Third- or higher-line treatment was administered to patients with progression or recurrence following second-line treatment and for whom any one of the anti-HER2 or chemotherapeutic drugs had been changed.
All patients and/or their immediate families understood and consented to participate in this study and provided written informed consent for clinical data access, scheduled follow-up, and survival analysis. The Ethics Committee of the Second Xiangya Hospital of Central South University reviewed and approved the study.
Endpoint Definition and Assessments
OS, the primary endpoint of our study, was defined as the time from enrollment until death due to any cause or the latest date the patient was known to be alive. The secondary endpoint, PFS, was defined as the time from drug administration to death or disease progression (whichever occurred first). For patients without OS/PFS events, the follow-up information was estimated by each center’s staff based on the Response Evaluation Criteria in Solid Tumours (RECIST) v1.1 criterion. Each patient underwent a 14- to 21-day clinical follow-up schedule and 2 to 3 drug cycles (6–9 weeks) of imaging follow-up (computed tomography (CT), ultrasound, magnetic resonance imaging (MRI), positron emission tomography (PET) scan, PET/CT scan, and bone scan) after the beginning of pyrotinib treatment until the primary endpoint was reached.
Propensity Score Matching and Inverse Probability of Treatment Weighting
The critical covariate (metastatic site) exhibited heterogeneity between the lapatinib-treated and lapatinib-naive groups (Table 1), possibly affecting the outcomes from a clinical perspective. To balance the heterogeneous characteristics between the two groups, we implemented PSM using the R package “MatchIt” version 4.1.0 with the following settings: 1:2 pairing, nearest-neighbor methods, and a caliper of 0.02 (Pattanayak et al., 2011). After PSM, all categories were comparable (Table 1). Inverse probability of treatment weighting-adjusted (IPTW-adjusted) survival analysis was applied to reduce the differences in baseline variables.
TABLE 1.
Characteristics of patients who received pyrotinib as third- or higher-line therapy who previously were or were not treated with lapatinib.
| Category | Before PSM | After PSM | ||||
| Lapatinib-naive | Lapatinib-treated | p-Value | Lapatinib-naive | Lapatinib-treated | p-Value | |
| No. (%) | No. (%) | No. (%) | No. (%) | |||
| Age | ||||||
| <50 | 31 (48.4) | 18 (60.0) | 0.377 | 17 (47.2) | 15 (62.5) | 0.369 |
| ≥50 | 33 (51.6) | 12 (40.0) | 19 (52.8) | 9 (37.5) | ||
| ECOG Scale | ||||||
| 0–1 | 63 (98.4) | 27 (90.0) | 0.181 | 35 (97.2) | 23 (95.8) | 1.000 |
| ≥2 | 1 (1.6) | 3 (10.0) | 1 (2.8) | 1 (4.2) | ||
| Menopausal Status | ||||||
| Postmenopausal | 30 (46.9) | 17 (56.7) | 0.507 | 17 (47.2) | 13 (54.2) | 0.792 |
| Premenopausal | 34 (53.1) | 13 (43.3) | 19 (52.8) | 11 (45.8) | ||
| HR Status | ||||||
| Positive | 37 (57.8) | 16 (53.3) | 0.853 | 20 (55.6) | 13 (54.2) | 1.000 |
| Negative | 27 (42.2) | 14 (46.7) | 16 (44.4) | 11 (45.8) | ||
| Prior trastuzumab treatment | ||||||
| No prior trastuzumab | 19 (29.7) | 5 (16.7) | 0.273 | 12 (33.3) | 4 (16.7) | 0.258 |
| Previous use of trastuzumab | 45 (70.3) | 25 (83.3) | 24 (66.7) | 20 (83.3) | ||
| Treatment type | ||||||
| Pyrotinib + Capecitabine | 45 (70.3) | 16 (53.3) | 0.102 | 24 (66.7) | 12 (50.0) | 0.330 |
| Pyrotinib + Abraxane | 12 (18.8) | 5 (16.7) | 8 (22.2) | 5 (20.8) | ||
| Pyrotinib + Trastuzumab | 2 (3.1) | 1 (3.3) | 1 (2.8) | 1 (4.2) | ||
| Pyrotinib + Others | 5 (7.8) | 8 (26.7) | 3 (8.3) | 6 (25.0) | ||
| Metastatic Site | ||||||
| Soft tissue and/or bone | 9 (14.1) | 5 (16.7) | <0.001 | 9 (25.0) | 5 (20.8) | 0.480 |
| Lung and/or liver | 44 (68.8) | 8 (26.7) | 16 (44.4) | 8 (33.3) | ||
| Brain and/or others | 11 (17.2) | 17 (56.7) | 11 (30.6) | 11 (45.8) | ||
| Total | 64 | 30 | 36 | 24 | ||
Abbreviations: PSM, propensity score matching; ECOG, Eastern Cooperative Oncology Group; HR, hormone receptor.
Statistical Analyses
Pearson’s chi-squared test or Fisher’s exact test was utilized to assess the heterogeneity of categorical variables among the lapatinib-treated and lapatinib-naive groups. Survival curves for OS and PFS were constructed using the Kaplan–Meier methodology, and the distribution was estimated using the log-rank test. Median OS times and PFS were calculated and reported. Hazard ratios (HRs) and 95% confidence intervals (CIs) for OS and PFS were computed using a univariable Cox proportional hazards regression model (using the R package “survminer”) and are presented as Forrest plots (using the R package “forestplot”). Statistical analyses and data visualization were performed using R (https://www.r-project.org/version 4.0.3) and RStudio (R-Studio Inc., Boston, United States version 1.3.1056). A p-value of less than 0.05 indicated statistical significance.
Results
Baseline Characteristics
Of 94 eligible patients, the median age of the 94 patients was 48.5 years (range 28–71 years). Ninety (95.7%) patients had an Eastern Cooperative Oncology Group (ECOG) performance status of 0–1. Most patients were treated with pyrotinib plus capecitabine and had prior trastuzumab treatment. In the lapatinib-naive cohort, 44 (68.8%) patients were with lung and/or liver metastasis, 33 (51.6%) were ≥50 years old, and 34 (53.1%) had a premenopausal status. The hormone receptor status was similar between groups. The PSM cohort showed similar but more balanced patient characteristics than those in the initial cohort. The baseline clinical features of the patients before and after PSM are summarized in Table 1. The median PFS time of the patients was 7.54 months (95% CI 6.67–10.67 months), and the median OS time was 18.67 months (95% CI 14.97–24.47 months) (Supplementary Figure S1).
Patient Outcomes After Changing Tyrosine Kinase Inhibitor Treatment
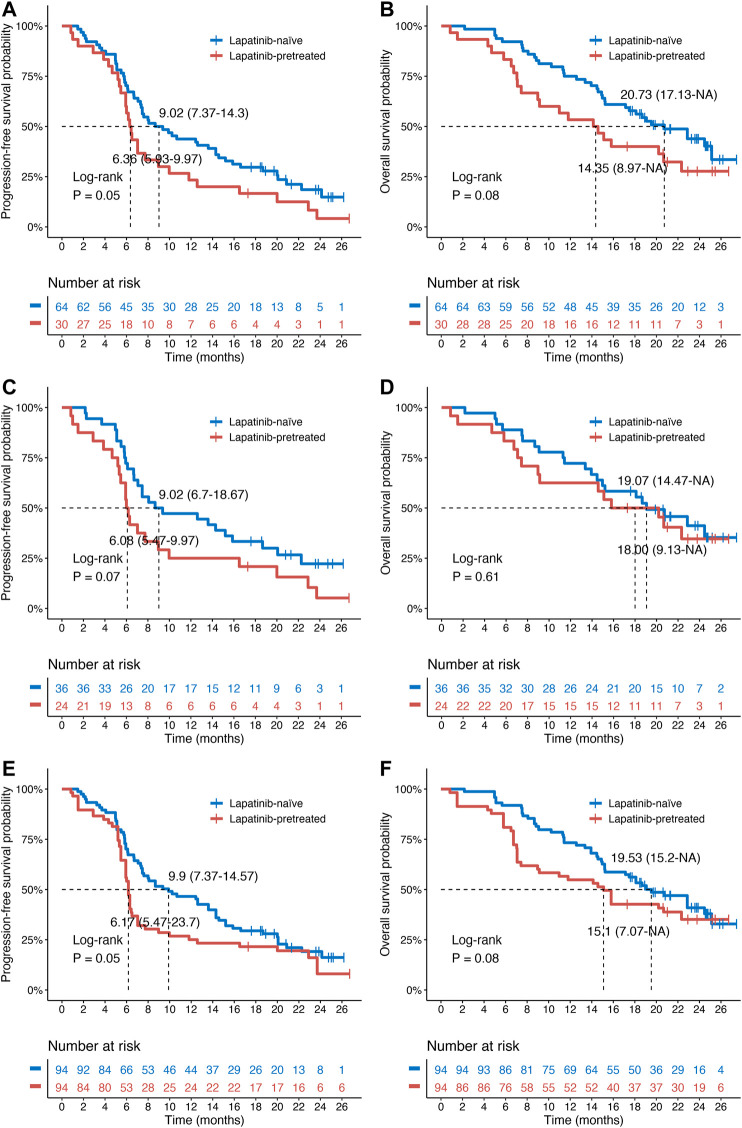
The numbers of PFS events in the lapatinib-naive group were 51/64 (before PSM) and 27/36 (after PSM), and the numbers of OS events were 36/64 and 21/36, respectively. In the lapatinib-treated group, the numbers of PFS events were 28/30 (before PSM) and 22/24 (after PSM), and the numbers of OS events were 21/30 and 15/24, respectively. Kaplan–Meier survival curves for OS and PFS were constructed to compare the survival distribution according to previous lapatinib treatment (Figure 2). The PFS and OS of the lapatinib-naive group were 43.8 and 75.0% at 12 months and 29.7% and 57.8% at 18 months, respectively. Comparatively, the PFS and OS of the lapatinib-treated group were 23.3 and 53.3% at 12 months and 16.7% and 40.0% at 18 months, respectively. The log-rank test results indicated a beneficial trend in the lapatinib-naive group compared to the lapatinib-treated group in terms of PFS (9.02 (7.37–14.30) vs 6.36 (5.93–9.97) p = 0.05, Figure 2A) and OS (20.73 (17.13–NA) vs 14.35 (8.97–NA) p = 0.08, Figure 2B). We also confirmed this finding by performing analysis between the two groups in terms of PFS (9.02 (6.70–18.67) vs 6.08 (5.47–9.97) p = 0.07, Figure 2C) and OS (19.07 (14.47–NA) vs 18.00 (9.13–NA) p = 0.61, Figure 2D) in the PSM cohort and in IPTW-adjusted cohort (PFS: 9.90 (7.37–14.57) vs 6.17 (5.47–23.70) p = 0.05, Figure 2E, and OS: 19.53 (15.20–NA) vs 15.10 (7.07–NA) p = 0.08, Figure 2F).
FIGURE 2.
Kaplan–Meier survival curves for patients with HER2-positive MBC treated with pyrotinib as a third- or higher-line treatment. (A,B) Progression-free survival (PFS)/overall survival (OS) of lapatinib-naive (n = 64) and lapatinib-treated (n = 30) patients in the original cohort. (C,D) PFS/OS of lapatinib-naive (n = 36) and lapatinib-treated (n = 24) patients in the PSM cohort. (E,F) PFS/OS of lapatinib-naive (n = 94) and lapatinib-treated (n = 94) patients in the IPTW-adjusted cohort. The p-values were determined by univariate log-rank tests. PSM, propensity score matching; IPTW, inverse probability of treatment weighting.
In the lapatinib-naive cohort, 24 patients (37.5%) achieved a partial response (PR), and two patients (3.10%) achieved a complete response (CR), resulting in an objective response rate (ORR) of 40.60%. In the lapatinib-treated cohort, 11 patients (36.70%) achieved a PR, and one patient (3.30%) achieved a CR, resulting in an ORR of 40.00%.
Subgroup Analysis
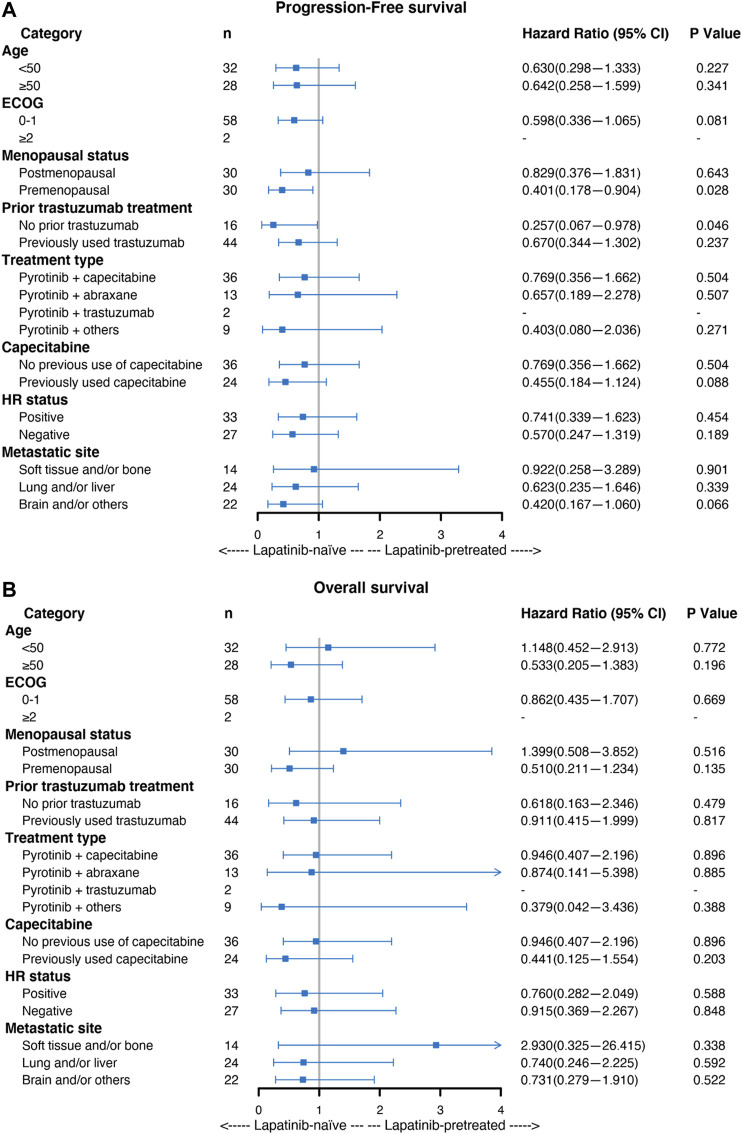
A subgroup analysis was performed to investigate the effect of previous lapatinib treatment on PFS and OS. Forest plots of the subgroup analysis are shown in Figure 3.
FIGURE 3.
Forest plot of the subgroup analysis of patients with HER2-positive MBC with regard to (A) progression-free survival (PFS) and (B) overall survival (OS). The hazard ratio (HR) and 95% confidence interval (CI) and the p-values were determined by Cox proportional hazard regression.
Univariable Cox analysis including the lapatinib-treated and lapatinib-naive groups showed similar outcomes. Most subgroups showed no significant difference in PFS (Figure 3A), except for the premenopausal subgroup (HR = 0.401, 95% CI 0.178–0.904, p = 0.028) and the subgroup without previous use of trastuzumab (HR = 0.257, 95% CI 0.067–0.978, p = 0.046). Similarly, no significant differences were found in OS in any subgroup analyses (Figure 3B).
Discussion
HER2-positive MBC has a poor prognosis and a short survival time (4). Only 13.2% of patients survive for more than 5 years if they do not receive treatments that target HER2(4). Conversely, the continuous development and widespread use of anti-HER2 drugs such as trastuzumab (Slamon et al., 2001; Burstein et al., 2007; Robert et al., 2006), pertuzumab (Baselga et al., 2012), TDM1 (Verma et al., 2012), and lapatinib (Geyer et al., 2006; Cameron et al., 2010; Xu et al., 2011) have significantly prolonged the median survival time of HER2-positive MBC patients. Moreover, China has recently authorized the use of pyrotinib for HER2-positive MBC patients.
Lapatinib and pyrotinib are both small molecule TKIs. Lapatinib reversibly inhibits HER1 and HER2, while pyrotinib inhibits HER1, HER2, and HER4 (Ma et al., 2017; Li et al., 2019). The curative effect of pyrotinib is stronger than that of lapatinib because of the conjugated double bond structure (Ma et al., 2017; Li et al., 2019). Previous randomized controlled trials on pyrotinib have not included lapatinib-treated patients, resulting in a lack of evidence to guide practice for follow-up treatment after lapatinib failure. In this study, 24 patients were considered to be lapatinib-treated after PSM analysis, which resulted in an ORR of 40.0% and median PFS of 6.08 months. Furthermore, IPTW analysis showed 6.17 months of PFS in patients who were exposed to lapatinib previously. We compared our results with those of two other real-world studies. Lin et al. (2020) and Song et al. (2020) reported median PFS times of 5.4 months (ORR 23.2%) and 7.9 months (ORR 22.2%), respectively. Our results showed a median PFS of 9.02 months (PSM analysis) and 9.90 months (IPTW analysis) in the lapatinib-naive group, which was better than that from Lin’s study (9.0 months) and Song’s study (7.2 months). The differences between the studies of Lin and Song may be due to selection bias. To minimize this bias, our study assessed the efficacy of pyrotinib by applying a PSM and IPTW approach. Additionally, our results first revealed the OS of pyrotinib-based therapy, with survival times of 19.07 and 18.00 months (PSM analysis) and 19.53 and 15.10 months (IPTW analysis) for the lapatinib-naive and lapatinib-treated groups, respectively. Therefore, our study suggested that pyrotinib is still effective in patients who have lapatinib treatment failure.
Another TKI neratinib also showed good therapeutic effects (Geyer et al., 2006; Cameron et al., 2010; Xu et al., 2011). The NALA study reported median PFS times of 8.8 months in the neratinib plus capecitabine group and 6.6 months in the lapatinib plus capecitabine group (Geyer et al., 2006; Cameron et al., 2010; Xu et al., 2011), and the NEfERT-T trial reported a PFS time of 12.9 months in the neratinib plus paclitaxel group (Geyer et al., 2006; Cameron et al., 2010; Xu et al., 2011), suggesting that the curative effect of neratinib is stronger than that of lapatinib. Among patients treated with neratinib, the PFS time was 3.1 months for those previously treated with lapatinib, and the PFS time was and 5.5 months in the lapatinib-naive cohort (Freedman et al., 2019). Thus, this finding indicated that the therapeutic effect of pyrotinib in lapatinib-naive patients has a similar beneficial trend to that of neratinib. Therefore, giving priority to pyrotinib treatment may increase survival benefits, but more detailed clinical studies are needed in the future.
Our study was retrospective, and thus, the groups could not be prospectively randomized; therefore, it was subject to limitations, including a lack of some clinical factors, such as combined treatment, and possible selection bias. The sample size should be further expanded in clinical randomized controlled studies.
In conclusion, pyrotinib-based therapy exhibited potential effects on HER2-positive MBC patients in a real-world study, regardless of whether lapatinib treatment was previously administered or not. Particularly for patients without lapatinib exposure, they seemed to benefit more from pyrotinib-based therapy, reaching a better prognosis, which still awaits more solidate verification.
Acknowledgments
The authors are grateful all of the staffs involved in this real-world study for collecting information, communicating with the patients and providing important advice in the revise process. The information of staffs is as follows: Jinhui Hu (Department of Breast Surgery, The First Hospital Hunan University of Chinese Medicine, Changsha, China), Wei Zhou (Department of Breast and Thyroid Surgery, The Affiliated ZhuZhou Hospital of Xiangya School of Medicine Central South University, Zhuzhou, China), Bo Qiu (Department of Oncology, The Affiliated ZhuZhou Hospital of Xiangya School of Medicine Central South University, Zhuzhou, China), Zhongming Wang (Department of Breast Surgery, The Third People's Hospital of Yongzhou, Yongzhou, China), Jie Weng (Department of Oncology, The First People’s Hospital of Yueyang, Yueyang, China), Mingwen Liu (Department of Breast and Thyroid Surgery, The First People’s Hospital of Xiangtan City, Xiangtan, China), Yi Li (Department of Oncology, The Third People’s Hospital of Changde, Changde, China), Tiegang Tang (Department of Oncology, Xiangtan Central Hospital, Xiangtan, China), Jianguo Wang (Department of General Surgery, Xiangtan Central Hospital, Xiangtan, China), Hui Zhang (Department of Oncology, Central Hospital of Shaoyang, Shaoyang, China), Bin Dai (Department of Breast and Thyroid Surgery, Central Hospital of Shaoyang, Shaoyang, China), Wuping Tang (Department of Breast Surgery, Shaoyang Hospital of Traditional Chinese Medicine, Shaoyang, China), Tao Wu (Department of Oncology, The First People’s Hospital of Changde, Changde, China), Maoliang Xiao (Department of Oncology, The Third Hospital of Hunan University of Chinese Medicine, Zhuzhou, China), Xiantao Li (Department of Oncology, The Central Hospital of Yiyang, Yiyang, China), Hailong Liu (Department of Internal Medicine-Oncology, The First People’s Hospital of Chenzhou, Chenzhou, China), Lai Li (Department of Breast and Thyroid Surgery, The People’s Hospital of Xiangtan County, Xiangtan, China).
Data Availability Statement
The code and datasets analyzed during the present study are available from the corresponding authors upon reasonable request.
Ethics Statement
The studies involving human participants were reviewed and approved by the Second Xiangya Hospital of Central South University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
MA and QC collected and compiled the patients’ information. DO and QC performed the literature search, data extraction, and statistical analysis and drafted the manuscript. WY and SW designed and supervised the study. Other authors administered clinical therapy to patients and obtained patient follow-up information. All authors have read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.682568/full#supplementary-material
References
- Anwar M., Chen Q., Ouyang D., Wang S., Xie N., Ouyang Q., et al. (2021). Pyrotinib Treatment in Patients with HER2-Positive Metastatic Breast Cancer and Brain Metastasis: Exploratory Final Analysis of Real-World, Multicenter Data. Clin Cancer Res. 10.1158/1078-0432.ccr-21-0474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awada A., Colomer R., Inoue K., Bondarenko I., Badwe R. A., Demetriou G., et al. (2016). Neratinib Plus Paclitaxel vs Trastuzumab Plus Paclitaxel in Previously Untreated Metastatic ERBB2-Positive Breast Cancer. JAMA Oncol. 2 (12), 1557–1564. 10.1001/jamaoncol.2016.0237 [DOI] [PubMed] [Google Scholar]
- Baselga J., Cortés J., Kim S.-B., Im S.-A., Hegg R., Im Y.-H., et al. (2012). Pertuzumab Plus Trastuzumab Plus Docetaxel for Metastatic Breast Cancer. N. Engl. J. Med. 366 (2), 109–119. 10.1056/nejmoa1113216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair H. A. (2018). Pyrotinib: First Global Approval. Drugs 78 (16), 1751–1755. 10.1007/s40265-018-0997-0 [DOI] [PubMed] [Google Scholar]
- Burstein H. J., Keshaviah A., Baron A. D., Hart R. D., Lambert-Falls R., Marcom P. K., et al. (2007). Trastuzumab Plus Vinorelbine or Taxane Chemotherapy for HER2-Overexpressing Metastatic Breast Cancer: the Trastuzumab and Vinorelbine or Taxane Study. Cancer 110 (5), 965–972. 10.1002/cncr.22885 [DOI] [PubMed] [Google Scholar]
- Cameron D., Casey M., Oliva C., Newstat B., Imwalle B., Geyer C. E. (2010). Lapatinib Plus Capecitabine in Women with HER‐2-Positive Advanced Breast Cancer: Final Survival Analysis of a Phase III Randomized Trial. The oncologist 15 (9), 924–934. 10.1634/theoncologist.2009-0181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Ouyang D., Anwar M., Xie N., Wang S., Fan P., et al. (2020). Effectiveness and Safety of Pyrotinib, and Association of Biomarker with Progression-free Survival in Patients with HER2-Positive Metastatic Breast Cancer: A Real-World, Multicentre Analysis. Front. Oncol. 10, 811. 10.3389/fonc.2020.00811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobleigh M., Yardley D. A., Brufsky A. M., Rugo H. S., Swain S. M., Kaufman P. A., et al. (2020). Baseline Characteristics, Treatment Patterns, and Outcomes in Patients with HER2-Positive Metastatic Breast Cancer by Hormone Receptor Status from SystHERs. Clin. Cancer Res. 26 (5), 1105–1113. 10.1158/1078-0432.ccr-19-2350 [DOI] [PubMed] [Google Scholar]
- Dawood S., Broglio K., Buzdar A. U., Hortobagyi G. N., Giordano S. H. (2010). Prognosis of Women with Metastatic Breast Cancer by HER2 Status and Trastuzumab Treatment: an Institutional-Based Review. Jco 28 (1), 92–98. 10.1200/jco.2008.19.9844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks E. D. (2017). Neratinib: First Global Approval. Drugs 77 (15), 1695–1704. 10.1007/s40265-017-0811-4 [DOI] [PubMed] [Google Scholar]
- Freedman R. A., Gelman R. S., Anders C. K., Melisko M. E., Parsons H. A., Cropp A. M., et al. (2019). TBCRC 022: A Phase II Trial of Neratinib and Capecitabine for Patients with Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer and Brain Metastases. J. Clin. Oncol. 37 (13), 1081–1089. 10.1200/JCO.18.01511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer C. E., Forster J., Lindquist D., Chan S., Romieu C. G., Pienkowski T., et al. (2006). Lapatinib Plus Capecitabine for HER2-Positive Advanced Breast Cancer. N. Engl. J. Med. 355 (26), 2733–2743. 10.1056/nejmoa064320 [DOI] [PubMed] [Google Scholar]
- Howlader N., Altekruse S. F., Li C. I., Chen V. W., Clarke C. A., Ries L. A., et al. (2014). US Incidence of Breast Cancer Subtypes Defined by Joint Hormone Receptor and HER2 Status. J. Natl. Cancer Inst. 106 (5). 10.1093/jnci/dju055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S., Shah A. N., Santa-Maria C. A., Siziopikou K., Rademaker A., Helenowski I., et al. (2018). Phase I Study of Alpelisib (BYL-719) and Trastuzumab Emtansine (T-DM1) in HER2-Positive Metastatic Breast Cancer (MBC) after Trastuzumab and Taxane Therapy. Breast Cancer Res. Treat. 171 (2), 371–381. 10.1007/s10549-018-4792-0 [DOI] [PubMed] [Google Scholar]
- Jiang Z., Yan M., Hu X., Zhang Q., Ouyang Q., Feng J., et al. (2019). Pyrotinib Combined with Capecitabine in Women with HER2+ Metastatic Breast Cancer Previously Treated with Trastuzumab and Taxanes: A Randomized Phase III Study. Jco 37, 1001. 10.1200/jco.2019.37.15_suppl.1001 [DOI] [Google Scholar]
- Lee A. (2020). Tucatinib: First Approval. Drugs 80 (10), 1033–1038. 10.1007/s40265-020-01340-w [DOI] [PubMed] [Google Scholar]
- Li Q., Guan X., Chen S., Yi Z., Lan B., Xing P., et al. (2019). Safety, Efficacy, and Biomarker Analysis of Pyrotinib in Combination with Capecitabine in HER2-Positive Metastatic Breast Cancer Patients: A Phase I Clinical Trial. Clin. Cancer Res. 25 (17), 5212–5220. 10.1158/1078-0432.ccr-18-4173 [DOI] [PubMed] [Google Scholar]
- Li X., Yang C., Wan H., Zhang G., Feng J., Zhang L., et al. (2017). Discovery and Development of Pyrotinib: A Novel Irreversible EGFR/HER2 Dual Tyrosine Kinase Inhibitor with Favorable Safety Profiles for the Treatment of Breast Cancer. Eur. J. Pharm. Sci. 110, 51–61. 10.1016/j.ejps.2017.01.021 [DOI] [PubMed] [Google Scholar]
- Lin Y., Lin M., Zhang J., Wang B., Tao Z., Du Y., et al. (2020). Real-World Data of Pyrotinib-Based Therapy in Metastatic HER2-Positive Breast Cancer: Promising Efficacy in Lapatinib-Treated Patients and in Brain Metastasis. Cancer Res. Treat. 52 (4), 1059–1066. 10.4143/crt.2019.633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma F., Li Q., Chen S., Zhu W., Fan Y., Wang J., et al. (2017). Phase I Study and Biomarker Analysis of Pyrotinib, a Novel Irreversible Pan-ErbB Receptor Tyrosine Kinase Inhibitor, in Patients with Human Epidermal Growth Factor Receptor 2-Positive Metastatic Breast Cancer. Jco 35 (27), 3105–3112. 10.1200/jco.2016.69.6179 [DOI] [PubMed] [Google Scholar]
- Ma F., Ouyang Q., Li W., Jiang Z., Tong Z., Liu Y., et al. (2019). Pyrotinib or Lapatinib Combined with Capecitabine in HER2-Positive Metastatic Breast Cancer with Prior Taxanes, Anthracyclines, And/or Trastuzumab: A Randomized, Phase II Study. Jco 37 (29), 2610–2619. 10.1200/jco.19.00108 [DOI] [PubMed] [Google Scholar]
- Mendes D., Alves C., Afonso N., Cardoso F., Passos-Coelho J. L., Costa L., et al. (2015). The Benefit of HER2-Targeted Therapies on Overall Survival of Patients with Metastatic HER2-Positive Breast Cancer-Aa Systematic Review. Breast Cancer Res. : BCR 17, 140. 10.1186/s13058-015-0648-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattanayak C. W., Rubin D. B., Zell E. R. (2011). Propensity Score Methods for Creating Covariate Balance in Observational Studies. Revista Española de Cardiología (English Edition) 64 (10), 897–903. 10.1016/j.rec.2011.06.008 [DOI] [PubMed] [Google Scholar]
- Robert N., Leyland-Jones B., Asmar L., Belt R., Ilegbodu D., Loesch D., et al. (2006). Randomized Phase III Study of Trastuzumab, Paclitaxel, and Carboplatin Compared with Trastuzumab and Paclitaxel in Women with HER-2-Overexpressing Metastatic Breast Cancer. Jco 24 (18), 2786–2792. 10.1200/jco.2005.04.1764 [DOI] [PubMed] [Google Scholar]
- Ryan Q., Ibrahim A., Cohen M. H., Johnson J., Ko C. w., Sridhara R., et al. (2008). FDA Drug Approval Summary: Lapatinib in Combination with Capecitabine for Previously Treated Metastatic Breast Cancer that Overexpresses HER‐2. Oncol. 13 (10), 1114–1119. 10.1634/theoncologist.2008-0816 [DOI] [PubMed] [Google Scholar]
- Saura C., Oliveira M., Feng Y.-H., Dai M.-S., Chen S.-W., Hurvitz S. A., et al. (2020). Neratinib Plus Capecitabine versus Lapatinib Plus Capecitabine in HER2-Positive Metastatic Breast Cancer Previously Treated with ≥ 2 HER2-Directed Regimens: Phase III NALA Trial. Jco 38 (27), 3138–3149. 10.1200/jco.20.00147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamon D. J., Leyland-Jones B., Shak S., Fuchs H., Paton V., Bajamonde A., et al. (2001). Use of Chemotherapy Plus a Monoclonal Antibody against HER2 for Metastatic Breast Cancer that Overexpresses HER2. N. Engl. J. Med. 344 (11), 783–792. 10.1056/nejm200103153441101 [DOI] [PubMed] [Google Scholar]
- Song G. H., Li H. P., Di L. J., Yan Y., Jiang H. F., Xu L., et al. (2020). [Efficacy and Safety of Oral Pyrotinib in HER2 Positive Metastatic Breast Cancer: Real-World Practice]. Beijing Da Xue Xue Bao Yi Xue Ban 52 (2), 254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain S. M., Baselga J., Kim S.-B., Ro J., Semiglazov V., Campone M., et al. (2015). Pertuzumab, Trastuzumab, and Docetaxel in HER2-Positive Metastatic Breast Cancer. N. Engl. J. Med. 372 (8), 724–734. 10.1056/nejmoa1413513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain S. M., Kim S.-B., Cortés J., Ro J., Semiglazov V., Campone M., et al. (2013). Pertuzumab, Trastuzumab, and Docetaxel for HER2-Positive Metastatic Breast Cancer (CLEOPATRA Study): Overall Survival Results from a Randomised, Double-Blind, Placebo-Controlled, Phase 3 Study. Lancet Oncol. 14 (6), 461–471. 10.1016/s1470-2045(13)70130-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy D., Brufsky A., Cobleigh M., Jahanzeb M., Kaufman P. A., Mason G., et al. (2020). De Novo Versus Recurrent HER2‐Positive Metastatic Breast Cancer: Patient Characteristics, Treatment, and Survival from the SystHERs Registry. Oncol. 25 (2), e214–e22. 10.1634/theoncologist.2019-0446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S., Miles D., Gianni L., Krop I. E., Welslau M., Baselga J., et al. (2012). Trastuzumab Emtansine for HER2-Positive Advanced Breast Cancer. N. Engl. J. Med. 367 (19), 1783–1791. 10.1056/nejmoa1209124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff A. C., Hammond M. E. H., Allison K. H., Harvey B. E., Mangu P. B., Bartlett J. M. S., et al. (2018). Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Arch. Pathol. Lab. Med. 142 (11), 1364–1382. 10.5858/arpa.2018-0902-sa [DOI] [PubMed] [Google Scholar]
- Wong K.-K., Fracasso P. M., Bukowski R. M., Lynch T. J., Munster P. N., Shapiro G. I., et al. (2009). A Phase I Study with Neratinib (HKI-272), an Irreversible pan ErbB Receptor Tyrosine Kinase Inhibitor, in Patients with Solid Tumors. Clin. Cancer Res. 15 (7), 2552–2558. 10.1158/1078-0432.ccr-08-1978 [DOI] [PubMed] [Google Scholar]
- Xu B.-H., Jiang Z.-F., Chua D., Shao Z.-M., Luo R.-C., Wang X.-J., et al. (2011). Lapatinib Plus Capecitabine in Treating HER2-Positive Advanced Breast Cancer: Efficacy, Safety, and Biomarker Results from Chinese Patients. Chin. J. Cancer 30 (5), 327–335. 10.5732/cjc.010.10507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B., Yan M., Ma F., Hu X., Feng J., Ouyang Q., et al. (2021). Pyrotinib Plus Capecitabine versus Lapatinib Plus Capecitabine for the Treatment of HER2-Positive Metastatic Breast Cancer (PHOEBE): a Multicentre, Open-Label, Randomised, Controlled, Phase 3 Trial. Lancet Oncol. 22 (3), 351–360. 10.1016/s1470-2045(20)30702-6 [DOI] [PubMed] [Google Scholar]
- Xuhong J., Qi X., Tang P., Fan L., Chen L., Zhang F., et al. (2020). Neoadjuvant Pyrotinib Plus Trastuzumab and Chemotherapy for Stage I-III HER2-Positive Breast Cancer: A Phase II Clinical Trial. The oncologist 25 (12), e1909–e20. 10.1002/onco.13546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuhong J. C., Qi X. W., Zhang Y., Jiang J. (2019). Mechanism, Safety and Efficacy of Three Tyrosine Kinase Inhibitors Lapatinib, Neratinib and Pyrotinib in HER2-Positive Breast Cancer. Am. J. Cancer Res. 9 (10), 2103–2119. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The code and datasets analyzed during the present study are available from the corresponding authors upon reasonable request.