Abstract
Background and aims
Vaccine Hesitancy (VH) is a relevant obstacle for the COVID-19 vaccination campaign. The aim of this study is to assess the proportion of subjects unwilling to vaccinate among patients with type 1 (T1DM) and 2 (T2DM) diabetes, exploring factors associated with VH.
Methods and results
A purposely created interview was delivered from physicians to a consecutive series of adult (>18 years) subjects with diabetes referring to the Diabetes Outpatient Clinic of Careggi Hospital, Florence, from January 1st to April 30th 2021. Out of 502 subjects enrolled, 92 were vaccine hesitant respondents (18.3%); the corresponding figure for T1DM and T2DM was 13.0% (N = 14), and 19.9% (N = 78), respectively. After adjusting for age, higher HbA1c (1.07 [1.02–1.13], p = 0.008) and triglycerides levels (1.03 [1.01–1.06], p = 0.011) were positively associated with VH, among patients with T1DM. At multivariate analysis, after adjusting for age, creatinine, and statin use, patients with T2DM affected by obesity (9.98 [4.89–9.59], p < 0.01) and with lower levels of creatinine (0.36 [0.21–0.54], p = 0.029) were more likely to refuse COVID vaccination.
Conclusions
Hesitancy toward COVID-19 vaccination among subjects with diabetes is not negligible and seems to be more prevalent in individuals with lower adherence to medical prescriptions and/or reduced concerns over their health. This suggests the need for specific interventions to increase awareness and counter prejudices on vaccines.
Keywords: Covid-19, Vaccine hesitancy, Diabetes, Obesity
Introduction
The Italian COVID-19 vaccination campaign, started on December 2020 and aimed at covering the entire population aged >16 years [1], uses two m-RNA (Comirnaty®, Pfizer/BioNTech, and mRNA-1273, Moderna) and two DNA (Vaxzevria®, AstraZeneca, and Ad26.COV2.S, J&J) vaccines [[2], [3], [4], [5]]. The first available doses were used to immunize health professionals and nursing home residents. The second phase of the campaign targeted high-risk categories [6], including part of patients with diabetes [7]. Priority in vaccination was established for subjects with type 1 diabetes, and those with type 2 diabetes treated with at least two drugs [8]. In fact, diabetes is associated with an increased risk of infection from SARS-CoV-2 [9] and poorer outcomes [9,10].
Vaccine Hesitancy [11] (VH) is a relevant obstacle for the vaccination campaign. Available surveys worldwide report a prevalence of VH for COVID-19 ranging from 14 to 38% [[12], [13], [14], [15]]. To our knowledge, only one study on VH in patients with type 2 diabetes in Italy has been published so far, reporting the results of online-delivered (Facebook) self-administered questionnaire [16].
The aim of this study, performed on a clinical-based sample, is to assess the proportion of subjects unwilling to vaccinate among patients with type 1 and 2 diabetes, exploring factors associated with VH.
Methods
Data were collected using a purposely created interview, delivered from physicians to a consecutive series of adult (>18 years) subjects with diabetes referring to the Diabetes Outpatient Clinic of Careggi Hospital, Florence, from January 1st to April 30th, 2021, who provided their informed consent. Patients not fluent in Italian and incapable of answering because of concurrent conditions (e.g., dementia) were excluded.
The survey collected demographic and anamnestic parameters and the intention to COVID-19 vaccination (see Supplementary Materials). Further clinical parameters were retrieved from clinical records.
Subjects were considered as vaccination hesitant, if they were unsure, somewhat, or extremely unlikely to get vaccinated, those with certified contraindications were excluded from the analysis.
Analyses were performed separately in Type 1 (T1DM) and Type 2 (T2DM) diabetes. Two-tailed T-tests and chi-square tests were used for assessing between-group differences for continuous and categorial variables, respectively. All variables significantly (p < 0.05) associated with VH were adjusted for age. Furthermore, a multivariate logistic regression analysis was performed, with VH as an outcome, and age, and patients' characteristics significantly associated with VH at univariate analysis as covariates. All analyses were performed using IBM SPSS Statistics 27.0.
The study protocol was approved by the local Ethical Board of Florence (Ref. 19932_OSS).
Results
Of 538 patients invited, 36 of them (12 and 26 with type 1 and 2 diabetes, respectively) refused to participate. The clinical characteristics of refusers did not differ significantly from those of enrolled subjects (data not shown).
The sample consisted of 502 subjects, of whom 108 were affected by T1DM and 394 by T2DM. The proportion of vaccine-hesitant respondents was 18.3% (N = 92); the corresponding figure for T1DM and T2DM was 13.0% (N = 14), and 19.9% (N = 78), respectively (p = 0.48 between groups). The principal characteristics of the two samples were summarized in Table 1 .
Table 1.
Predictors of coronavirus vaccine uptake intention in the two samples.
| Characteristics | Type 1 diabetes |
p | Type 2 diabetes |
p | ||
|---|---|---|---|---|---|---|
| Likely to Get Vaccinated |
Likely to Get Vaccinated |
|||||
| Yes (n = 94) | Noa (n = 14) | Yes (n = 316) | No (n = 78) | |||
| Age (years) | 53.3 ± 19.3 | 48.9 ± 20.9 | 0.46 | 71.3 ± 11.7 | 68.7 ± 10.5 | 0.061 |
| Diabetes duration (years) | 27.1 ± 16.3 | 22.0 ± 17.4 | 0.35 | 18.6 ± 12.8 | 16.0 ± 8.6 | 0.11 |
| Gender (female) | 36 (38.3) | 4 (28.6) | 0.48 | 214 (67.7) | 48 (61.5) | 0.30 |
| Education status (n, %) | ||||||
| Less than middle school | 4 (4.3) | 2 (14.3) | 106 (33.5) | 26 (33.3) | 0.21 | |
| Middle school | 12 (12.8) | 4 (28.6) | 88 (27.8) | 14 (17.9) | ||
| High school diploma | 50 (53.2) | 6 (42.9) | 76 (24.1) | 26 (33.3) | ||
| University degree | 28 (29.8) | 2 (14.3) | 0.13 | 46 (14.6) | 12 (15.4) | |
| Flu vaccination (2020/21) | 72 (76.6) | 12 (85.7) | 0.44 | 212 (67.1) | 52 (66.7) | 0.94 |
| Pneumoniae vaccination (yes) | 34 (36.,2) | 6 (42.9) | 0.63 | 118 (37.3) | 28 (35.9) | 0.81 |
| Diagnosis COVID-19 (yes) | 4 (4.3) | 0 (0.0) | 0.43 | 16 (5.1) | 4 (5.1) | 0.98 |
| Body mass index (kg/m2) | 24.3 ± 3.4 | 17.6 ± 1.3 | <0.01 | 27.2 ± 5.6 | 32.5 ± 4.3 | <0.01 |
| HbA1c (mmol/mol) | 54.5 ± 9.2 | 64.0 ± 16.7 | <0.01 | 60.0 ± 13.1 | 58.3 ± 14.1 | 0.50 |
| Creatinine (mg/dL) | 1.19 ± 1.48 | 0.76 ± 0.03 | 0.03 | 1.35 ± 1.20 | 0.96 ± 0.30 | <0.01 |
| Obesity (n, %) | 8 (8.5) | 0 (0.0) | 0.26 | 64 (20.3) | 48 (61.5) | <0.01 |
| Total cholesterol (mg/dL) | 165 ± 30 | 211 ± 12 | <0.01 | 163 ± 41 | 161 ± 44 | 0.74 |
| HDL cholesterol (mg/dL) | 62 ± 18 | 57 ± 16 | 0.48 | 50 ± 13 | 46 ± 17 | 0.09 |
| Triglycerides (mg/dL) | 86 ± 33 | 151 ± 54 | <0.01 | 135 ± 75 | 156 ± 79 | 0.07 |
| Medical history (n, %) | ||||||
| Hypertension | 38 (40.4) | 2 (14.3) | 0.059 | 192 (60.8) | 46 (60.5) | 0.97 |
| Coronary heart disease | 6 (6.4) | 0 (0.0) | 0.33 | 96 (30.4) | 22 (28.9) | 0.81 |
| Brain vascular disease | 0 (0.0) | 0 (0.0) | – | 30 (9.5) | 8 (10.5) | 0.78 |
| Chronic kidney disease | 14 (14.9) | 0 (0.0) | 0.12 | 112 (35.4) | 18 (23.7) | 0.049 |
| Peripheral artery disease | 8 (8.5) | 2 (14.3) | 0.49 | 106 (33.8) | 20 (26.3) | 0.21 |
| Malignancies | 6 (6.4) | 0 (0.0) | 28 (8.9) | 16 (20.5) | ||
| Without metastasis | 4 | 0 (0.0) | 10 (3.2) | 8 (10.5) | 0.044 | |
| With metastasis | 2 | 0 (0.0) | 0.33 | 18 (5.7) | 8 (10.5) | |
| Cirrhosis | 0 (0.0) | 0 (0.0) | – | 4 (1.3) | 0 (0.0) | 0.32 |
| Diabetic retinopathy | 26 (27.7) | 4 (28.6) | 0.94 | 70 (22.2) | 12 (15.8) | 0.22 |
| At least one DM complication | 22 (23.4) | 6 (42.9) | 0.12 | 194 (61.4) | 44 (57.9) | 0.57 |
| Therapy (n, %) | ||||||
| Statin | 50 (53.2) | 0 (0.0) | <0.01 | 236 (74.7) | 48 (61.5) | 0.02 |
| Antihypertensive | 44 (46.8) | 2 (14.3) | 0.022 | 230 (72.8) | 62 (79.5) | 0.23 |
| Antiplatelets/anticoagulants | 24 (25.5) | 4 (28.6) | 0.81 | 154 (48.7) | 32 (41.0) | 022 |
| Non-insulin glucose-lowering drugsa | 20 (21.3) | 2 (14.3) | 0.16 | 316 (100.0) | 78 (100.0) | 0.34 |
| Insulin | 94 (100.0) | 14 (100) | – | 128 (40.5) | 32 (41) | 0.93 |
Variables were expressed as mean ± standard deviations, median [interquartiles]; proportions (%).
Bold is used for statistically significant values.
Type 1 diabetes: Metformin 12 (11.1%), SGLT2i 10 (9.3%). Type 2 diabetes: Metformin 256 (65.0%), SGLT2i 88 (22.3%), GLP1RA 76 (19.3%), DPP4i 54 (13.7%), acarbose 12 (3.0%), pioglitazone 8 (2.0%), sulfonylureas 6 (1.5%).
Among subjects with T1DM, vaccination hesitant respondents had significantly lower BMI, and creatinine, and higher HbA1c, total cholesterol, and triglycerides levels; in addition, among hesitant subjects, a lower proportion of individuals receiving statins and anti-hypertensive drugs was observed (Table 1). After adjusting for age, higher HbA1c (1.07 [1.02–1.13], p = 0.008) and triglycerides levels (1.03 [1.01–1.06], p = 0.011) were positively associated with VH, whereas patients treated with antihypertensive drugs (0.12 [0.0–0.72], p = 0.021) were likely to get vaccinated.
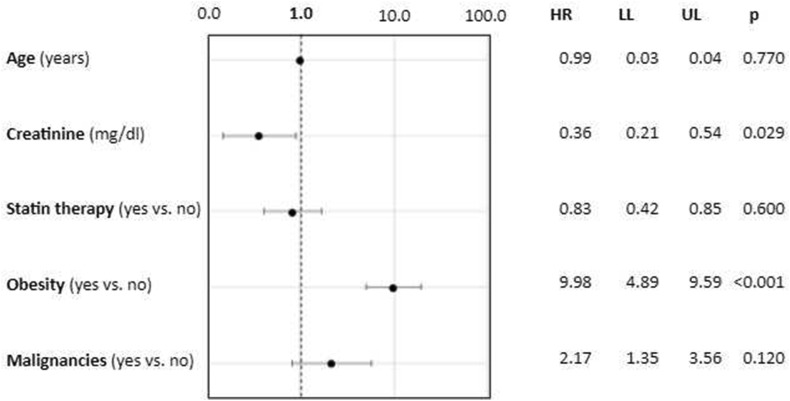
In T2DM, VH was associated with obesity and presence of malignancies; conversely, patients with renal failure and statin therapy were more likely to get vaccinated. Lower creatinine levels and higher BMI were also associated with VH. After adjusting for age, creatinine (0.49 [0.24–0.99], p = 0.048), BMI (1.17 [1.10–1.24], p < 0.01), obesity (1.07 [1.02–1.13], p < 0.01), malignancies (2.21 [1.01–4.97], p = 0.049), and statin therapy (0.57 [0.34–0.97], p = 0.037) retained statistical significance. At multivariate analysis, after adjusting for age, creatinine, and statin use, patients affected by obesity (9.98 [4.89–9.59], p < 0.01) and with lower levels of creatinine (0.36 [0.21–0.54], p = 0.029) were more likely to refuse COVID vaccination (Fig. 1 ).
Figure 1.
Predictors of vaccine hesitancy among patients with type 2 diabetes in a multivariate logistic regression analysis.
In a further analysis on the whole sample, including the type of diabetes as a covariate together with age, sex, BMI, HbA1c, creatinine, cholesterol, and triglyceride, only BMI (kg/m2) and creatinine (mg/dl) were significantly associated with hesitancy (1.17[1.08–1.26], p < 0.001, and 0.30[0.10–0.94], p = 0.04, respectively).
Discussion
The proportion of hesitant subjects among people with diabetes is not negligible, even though diabetes is a well-known risk factor for severe COVID-19 [9,10,17]. The results obtained are similar to those of a previous survey [16], collecting data from volunteers using a web-based self-reported questionnaire. In the present study, data were collected using an interview, thus reducing the chance of misunderstandings; on the other hand, the administration of the interview was performed by physicians recommending vaccination, possibly affecting patients' answers. In addition, the present study was performed on a consecutive series of patients, avoiding the biases related to self-selection typical of voluntary web-based surveys; however, the sample was composed only of patients referred to a single Clinic, unrepresentative of the general population with diabetes. Despite those differences, the present study and the previous survey [16] provided similar results, confirming the validity of the estimate of the prevalence of VH.
The exploration of determinants of VH is useful for designing specific interventions. VH was associated with poor glucose and lipid control in T1DM, and with obesity in T2DM, suggesting that hesitancy could be more prevalent in individuals with lower adherence to medical prescriptions and/or reduced concerns over their health [18]. Obesity and poor glycaemic control are major determinants of worse prognosis among patients with COVID-19 [19]; as a consequence, those with the highest risk of severe COVID-19 could be the least prone to vaccination. Further features associated with VH may have remained undetected in the present study, due to the limited sample size. Interestingly, hesitancy toward the COVID19 vaccine was not significantly associated with hesitancy toward other vaccinations.
In conclusion, hesitancy toward COVID-19 vaccination among subjects with diabetes in Italy is not negligible, suggesting the need for specific interventions to increase awareness and counter prejudices on vaccines.
Author contribution
Daniele Scoccimarro was involved in data collection, analysis and writing the manuscript. Lorenzo Panichi was involved in data collection. Benedetta Ragghianti was involved in data collection. Antonio Silverii was involved in study design. Edoardo Mannucci was involved in study design and writing the manuscript. Matteo Monami was involved in study design, analysis and writing the manuscript. All authors have read and agreed to the published version of the manuscript. Drs Scoccimarro and Monami had full access to all of the data in the study and take responsibility for the integrity and the accuracy of the data analysis.
Funding
The authors received no specific funding for this work.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgments
The authors wish to thank: Dr. Claudia Cosentino, Dr. Barbara Cresci, Dr. Ilaria Dicembrini, Dr. Stefano Giannini, Dr. Sara Liana Mariani, Dr. Silvia Minardi, Dr. Laura Pala, Dr. Maria Pieri and Dr. Valentina Vitale for their precious help in collecting data.
Handling Editor: G.P. Fadini
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.numecd.2021.09.002.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.della Salute Ministero. 2021. Piano vaccinale del commissario straordinario.https://www.governo.it/sites/governo.it/files/210313_Piano_Vaccinale_marzo_2021_1.pdf [Internet]. [cited 2021 Mar 13]. Available from: [Google Scholar]
- 2.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020 Dec 31;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021 Feb 4;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramasamy M.N., Minassian A.M., Ewer K.J., Flaxman A.L., Folegatti P.M., Owens D.R., et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2020 Dec 19;396(10267):1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson and Johnson: Sadoff J., Gray G., Vandebosch A., Cárdenas V., Shukarev G., Grinsztejn B., et al. ENSEMBLE Study Group Safety and efficacy of single-dose Ad26.COV2.S vaccine against covid-19. N Engl J Med. 2021 Apr 21 doi: 10.1056/NEJMoa2101544. Epub ahead of print. PMID: 33882225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.della Salute Ministero. 2021. Vaccinazione anti-SARS-CoV-2/COVID-19 - piano strategico. Elementi di preparazione e di implementazione della strategia vaccinale.http://www.salute.gov.it/imgs/C_17_pubblicazioni_2986_allegato.pdf [Internet]. [cited 2021 Jan 10]. Available from: [Google Scholar]
- 7.Gao Y.D., Ding M., Dong X., Zhang J.J., Kursat Azkur A., Azkur D., et al. Risk factors for severe and critically ill COVID-19 patients: a review. Allergy. 2021 Feb;76(2):428–455. doi: 10.1111/all.14657. Epub 2020 Dec 4. PMID: 33185910. [DOI] [PubMed] [Google Scholar]
- 8.Conferenza permanente per i rapporti tra lo stato, le regioni e le province autonome di Trento e Bolzano - conferenza unificata del 11/03/21. 2021. http://www.statoregioni.it/media/3364/p-1-cu-doc-regioni-11mar2021.pdf [Internet] [Google Scholar]
- 9.Bonora E., Fedeli U., Schievano E., Trombetta M., Saia M., Scroccaro G., et al. SARS-CoV-2 and COVID-19 in diabetes mellitus. Population-based study on ascertained infections, hospital admissions and mortality in an Italian region with ∼5 million inhabitants and ∼250,000 diabetic people. Nutr Metabol Cardiovasc Dis. 2021;31:2612–2618. doi: 10.1016/j.numecd.2021.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silverii G.A., Monami M., Cernigliaro A., Vigneri E., Guarnotta V., Scondotto S., et al. Are diabetes and its medications risk factors for the development of COVID-19? Data from a population-based study in Sicily. Nutr Metabol Cardiovasc Dis. 2021;31:396–398. doi: 10.1016/j.numecd.2020.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacDonald N.E., SAGE Working Group on Vaccine Hesitancy Vaccine hesitancy: definition, scope and determinants. Vaccine. 2015 Aug 14;33(34):4161–4164. doi: 10.1016/j.vaccine.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 12.Neumann-Böhme S., Varghese N.E., Sabat I., Barros P.P., Brouwer W., van Exel J., et al. Once we have it, will we use it? A European survey on willingness to be vaccinated against COVID-19. Eur J Health Econ. 2020 Jun 26:1–6. doi: 10.1007/s10198-020-01208-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peretti-Watel P., Seror V., Cortaredona S., Launay O., Raude J., Verger P., et al. A future vaccination campaign against COVID-19 at risk of vaccine hesitancy and politicisation. Lancet Infect Dis. 2020 Jul 1;20(7):769–770. doi: 10.1016/S1473-3099(20)30426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reiter P.L., Pennell M.L., Katz M.L. Acceptability of a COVID-19 vaccine among adults in the United States: how many people would get vaccinated? Vaccine. 2020 Sep 29;38(42):6500–6507. doi: 10.1016/j.vaccine.2020.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dodd R.H., Cvejic E., Bonner C., Pickles K., McCaffery K.J. Sydney health literacy lab COVID-19 group. Willingness to vaccinate against COVID-19 in Australia. Lancet Infect Dis. 2020 Jun 30 doi: 10.1016/S1473-3099(20)30559-4. S1473-3099(20)30559-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guaraldi F., Montalti M., Di Valerio Z., Mannucci E., Nreu B., Monami M., et al. Rate and Predictors of hesitancy toward SARS-CoV-2 vaccine among type 2 diabetic patients: results from an Italian survey. Vaccines. 2021 May 4;9(5):460. doi: 10.3390/vaccines9050460. PMID: 34064486; PMCID: PMC8147990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y., Ashcroft T., Chung A., Dighero I., Dozier M., Horne M., et al. Risk factors for poor outcomes in hospitalised COVID-19 patients: a systematic review and meta-analysis. J Glob Health. 2021 Mar 1;11:10001. doi: 10.7189/jogh.11.10001. PMID: 33767855; PMCID: PMC7980087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Capoccia K., Odegard P.S., Letassy N. Medication adherence with diabetes medication: a systematic review of the literature. Diabetes Educat. 2016 Feb;42(1):34–71. doi: 10.1177/0145721715619038. Epub 2015 Dec 4. PMID: 26637240. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Y., Chi J., Lv W., Wang Y. Obesity and diabetes as high-risk factors for severe coronavirus disease 2019 (covid-19) Diabetes Metab Res Rev. 2021 Feb;37(2):e3377. doi: 10.1002/dmrr.3377. Epub 2020 Jul 20. PMID: 32588943; PMCID: PMC7361201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.