Abstract
-
•
The immune-neuroendocrine system is essential to maintain homeostasis specially during stress situations. COVID-19 infection, produce stress, and activates the immune–neuroendocrine system. During the COVID-19 pandemic, multiple studies indicate that the most vulnerable populations are older adults and patients with comorbidities including autoimmune rheumatic diseases. These patients suffer from extremely important situation that favors the inflammatory hyper response due to an inadequate reaction of the immune-neuroendocrine system. This review aims to analyze the findings of the effect of COVID-19 on the hypothalamic–pituitary–adrenal, hypothalamic–pituitary–gonadal, hypothalamic–pituitary–thyroid, hypothalamic–pituitary–prolactin axes, and central nervous system, as well as the response to this viral infection in older adults and patients with rheumatic diseases and perspectives about this subject.
Keywords: COVID-19, Immune-neuroendocrine system, Older people, Autoimmune rheumatic diseases
1. Introduction
On December 30, 2019, the Ophthalmologist Li Wenliang informed his collaborators about the existence of a new disease similar to severe acute respiratory syndrome (SARS) in Wuhan, China. The Ophthalmologist's objective was to alert his colleagues concerning this new disease, but local authorities forced him to retract this warning. Dr. Li Wenliang was infected by a patient and died due to COVID-19 on February 7, 2020, at the age of 33 [1]. This new viral infection has spread rapidly throughout the planet and, since March 11, has been recognized as a pandemic by the World Health Organization (WHO) [2,3]. As of July 7, 2021, there have been 183,934,913 confirmed cases and 3,985,022 deaths worldwide [4].
In nearly 80% of COVID-19-infected patients, the disease will be mild and restricted to the upper airways. About 20% of infected patients will develop atypical pneumonia and around 2% of these will develop a very severe disease [5]. High mortality has been observed in people over 65 years of age and with comorbidities such as diabetes mellitus, arterial hypertension, obesity, autoimmune diseases, and others [6]. At this time, biomarkers of morbimortality have been identified: lymphopenia; increased of lactic dehydrogenase (LDH); C-reactive protein; procalcitonin; D-dimer, and ferritin [7,8]. Recent studies suggest immune alterations in older people (immunosenescence), such as T-cell alterations and innate immune response, which lead to a hyperinflammatory state, known as the cytokine storm syndrome [9,10].
The immune–neuroendocrine system responds to infections, stressful events such as physical factors and chemical and psychological agents through its messengers as follows: cytokines; neuropeptides; hormones; neurotransmitters, and their receptors [[11], [12], [13]]. Similar to immunosenescence, it is possible that the immune–neuroendocrine interaction is altered with age and comorbidities that lead to severe complications of COVID-19. The purpose of this narrative review was to analyze the possible interaction of COVID-19 infection with age, the immune–neuroendocrine system, and rheumatic diseases in order to answer the following question: Why are these patients more susceptible to developing severe and devastating COVID-19?
2. COVID-19 infection and stress
When severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) reaches the oropharynx, there is an innate immune inflammatory response, and the patient develops fever, headache, sore throat, cough, malaise, myalgia, arthralgia, anosmia, ageusia, and occasional diarrhea [14]. The next stage is the invasion of the virus into the lungs, heart, and other tissues [5]. This process is permitted by ACE-2 receptors [15]. Various autopsy studies have confirmed the hyperinflammatory response and the activation of the coagulation cascade [16,17]. In relation to the immune response, a recent study shows that the degree of COVID-19 infection is inversely correlated with interferon I and directly with the overproduction of proinflammatory cytokines such as IL-1 and IL-6. This study was conducted in patients with mild/moderate, severe, and critical disease [18]. In each of these stages, the viral infection and immune/inflammatory response stimulate the stress response systems, a fundamental part of the immune–neuroendocrine system, such as the following: the hypothalamic–pituitary–adrenal (HPA) axis; the hypothalamic–pituitary–thyroid (HPT) axis, the hypothalamic–pituitary–gonadal (HPG) axis, the prolactin axis (HP-PRL), and the sympathetic and parasympathetic nervous system [19]. In fact, this type of integral response has been observed in infections of different types, such as viral, bacterial, and parasitic. Therefore, this response can be similar in patients infected with COVID-19. Viral infections engage in a very particular manner of infecting the human body, every virus has a different way of doing this; however, each mechanism ends in the relationship with the neuroendocrine system [20]. Consequently, the COVID-19 infection undoubtedly causes emotional, physical, and biological stress, leading to the to stress response systems. In fact, several studies demonstrated that approximately 60% of patients during the COVID-19 pandemic exhibited anxiety, stress, and depression [21].
3. COVID-19 and the immune–neuroendocrine system
At the beginning of the COVID-19 infection, the innate and adaptative immune systems are activated, releasing proinflammatory cytokines. Multiple evidence indicates that pro-inflammatory mediators activate the neuroendocrine system. Viral interaction has been studied for many years [20]. However, there is scarce evidence of this interaction with the COVID-19 infection. Next, we will update the interaction between viral infection and the immune–neuroendocrine system (Fig. 1 ).
Fig. 1.
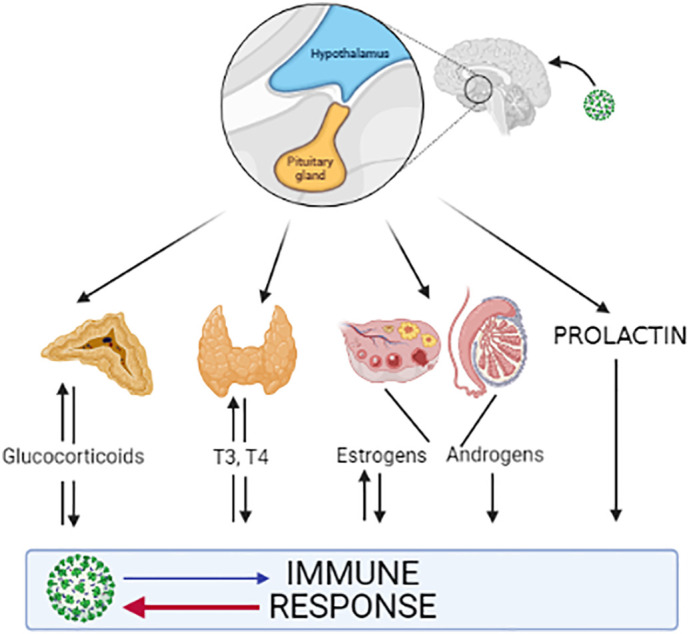
Interaction between viral infection and the immune–neuroendocrine system.
3.1. Hypothalamic–pituitary–adrenal axis
The first axis that is activated after a viral infection and stress situation is the hypothalamic–pituitary–adrenal (HPA) axis. The proinflammatory cytokines released by this viral infection cross the Blood Brain Barrier (BBB), arrive at the hypothalamus, activating neurons of the organum vasculosum of the laminae terminalis (OLVT) and of the paraventricular nucleus (PVN), stimulating the release of the corticotrophin-releasing hormone (CRH) [22], activating the anterior pituitary gland and consequently releasing the adrenocorticotropic hormone (ACTH), ending with the release of corticoids by the adrenal glands [23,25]. These corticoids yield negative feedback on immune cells, which suppress the synthesis and release of the cytokines TNF-α, IL-1β, and IL-6 [23]. Another function of glucocorticoids is to change from Th1- to Th2-type immune responses, thus counterbalancing damage to the tissues [24].
The HPA axis has receptors at every level for cytokines and it was demonstrated that this axis can also synthesize the latter [25]. Cytokines cross the BBB, which is an integral part of the immune–neuroendocrine system [26]. There are several identified ways as to how cytokines cross the BBB as follows: stimulating afferents pathways that project to the nucleus tractus solitarius, activating norepinephrine in the PVN; crossing the BBB at damaged regions; exerting a direct effect on CRH in the medium eminence, and inducing/releasing secondary messengers and crossing the BBB by active transport [24]. The BBB is injured by the TNF-α and IL-6 participating in neuroinflammation. TNF-α allows depolarization of the BBB endothelial cells and IL-6 production with the consequent stimulation of immune–neuroendocrine system [26].
A clear example of this relationship is the existing between HIV-1 and the HPA axis, in which the levels of cortisol have been observed as increased and, in several cases, adrenal insufficiency has also been observed [27]. Despite high cortisol levels, ACTH levels are low; this can indicate that some cytokines by themselves can stimulate the synthesis of glucocorticoids [28].
Anosmia and ageusia demonstrate the direct involvement of SARS-CoV-2 and cytokines on the central nervous system (CNS). In this respect, histological studies obtained on the olfactory epithelium have shown that TNF-α levels were significantly high in patients infected with COVID-19 compared to those of a control group [29]. Early studies found on tonsilitis a local production of proinflammatory cytokines and its relationship with catecholamine receptors [30]. Based on these findings, an immune–neuroendocrine interaction has been proposed. In fact, the SARS-CoV-2 virus is neurotropic [26]. COVID-19 infection alters the stress response system, and this alteration contributes to the worsening of elderly patients or those with an autoimmune disease. In fact, patients severely infected with COVID-19 exhibited significantly lower cortisol levels than severely infected non-COVID-19 patients [31]. Another study evaluated adrenocortical function in a group of patients with COVID-19 in which the study's findings suggest an inadequate response of the HPA axis [32]. In support of these findings, an autopsy study of COVID-19 patients revealed enlarged hematomas, diffuse bleeding, thrombus, necrosis, and the inflammatory infiltrate of adrenal glands. Taken this evidence together, COVID-19 has a devastating effect on the hypothalamic–pituitary–adrenal axis, that is, the principal stress response system [33].
3.2. Hypothalamic–pituitary–gonadal axis
The relation between this axis and the immune response can be sustained by certain facts, such as that the gonadotropin releasing hormone (GnRH),produced by immune cells; estrogens stimulate the immune response, and androgens inhibit this response [34]. Experimental models revealed that male rodents had a worse outcome after a Hepatitis B infection due to the immunosuppressive activity of testosterone. Another example was that after an Influenza A virus infection, female mice had a greater inflammatory response than males [35]. These examples clearly demonstrate the relation between these two systems, and this has also been studied for the SARS-COV 2 infection. It was observed that men present more complications than women, this due to the levels of androgens, particularly in the expression of a gene, TMPRSS2, whose transcription is regulated by androgen receptors [36]. With regard to ACE2 receptors and SARS-CoV-2 interaction, estrogens and androgens increase their expression [37]. It was evidenced that the hypothalamic–pituitary–gonadal axis can be directly affected by COVID-19, destroying Sertoli cells and Leydig cells, causing a worsening of spermatogenesis and a decrease in testosterone levels [38]. Several investigations have shown the involvement of the COVID-19 infection in the ovarian reserve and can affect the reproductive function of fertile women. Therefore, the latter will be monitored when COVID-19 occurs in women of reproductive age [39].
This evidence indicates the involvement of COVID-19 infection with the HPG axis. The consequence of this damage needs to be studied in the post COVID-19 stage.
3.3. Hypothalamic–pituitary–thyroid axis
The HPT axis has a clear relation with the immune system, and low levels of T3 and T4 were found in some infections [40]. An example is HIV infection where prior to the use of antiretroviral therapies, there were several patients with sub-clinical hypothyroidism and low levels of T4 [41]. During the cytokine storm for COVID-19, thyrotoxicosis and low free T3 concentrations have been described. Infection by SARS-COV-2 can destroy thyroid follicular cells and could be the reason for the thyrotoxicosis [42]. Affectation of the HPT axis can take place in two ways: by direct interaction between the SARS-CoV-2 and the thyroid glands, or by means of the hyperinflammatory response. In either situation, thyroiditis occurs. This is probably due to the high number of ACE-2 receptors that exist in the thyroid glands [43]. The description is of interest of some cases of Graves disease and thyroiditis in post-COVID-19 vaccinated patients [44,45].
3.4. Hypothalamic–pituitary–aprolactin axis
Prolactin is a lactotrophic hormone that possesses immunostimulatory properties, and it is synthetized and secreted by different cells or tissues, including immune system cells [[46], [47], [48]]. In fact, the prolactin gene in humans is located on the short arm of chromosome 6, near the major histocompatibility complex (MHC) [49].
It is well known that prolactin stimulates the innate and adaptive immune responses. Prolactin is produced by the T-lymphocytes, which are considered proinflammatory cytokines. In this regard, it was demonstrated that the hypoprolactinemic state is associated with the risk of infections and death. It has been proposed that drugs such as Domperidone/Metoclopramide (which are dopamine antagonists) can enhance PRL levels and, in this manner, the hyperprolactinemic state can increase the protective effect against COVID-19 infection [48]. In contrast, a hyperprolactinemic state has been demonstrated in systemic lupus erythematosus and other associated autoimmune diseases to increase active disease [50]. Recently, there was the first case of a COVID-19-positive patient in her third trimester of pregnancy who developed a pituitary apoplexy. This patient presented low TSH levels and high prolactin levels. It is probable that this axis is affected by COVID-19 [51].
4. COVID-19, the central nervous system, and the peripheral nervous system
The presence of fever, headache, anosmia, and early ageusia suggests an early interaction between the viral infection and the CNS. Multiple evidences suggest that SARS-CoV-2 is neurotropic, and that it possesses a neuroinvasive mechanism [52]. There is evidence that loss of smell (fifth cranial nerve) may be the gateway for SARS-CoV-2 to enter the CNS. A recent study demonstrated the presence of SARS-CoV-2 RNA and protein in distinct regions of the nasopharynx and brain [53]. One of the proposed forms of infection of the CNS is via the neural route through the olfactory bulb, which entertains direct axonal connections with the medial temporal lobe and allows it to reach the brain [52]. Another route is through the blood circulation and the ACE2 receptors, which the virus utilized to enter the cell. These receptors are known to have been detected in neurons and glial cells, as well as in the capillary endothelium, which interacts with the Spike protein of the virus, altering the blood vessels and the permeability of the cerebral BBB, affecting the nervous system and becoming an important target of COVID-19 [54].
One of the main mechanisms by which COVID-19 crosses the BBB is by means of the “Trojan Horse” strategy. During transcellular migration, viruses invade the junctions formed by the BBB endothelial cells, becoming engulfed by phagocytic cells such as macrophages. COVID-19 employs a combination of these previously mentioned mechanisms to invade the CNS [55]. Another form of COVID-19 affectation is through causing hypoxemia in neuronal tissue, due to the excessive production of mucus in the pulmonary alveoli, hindering the alveolar gas exchange, giving rise to a decrease in the arrival of oxygen to neuronal cells and ischemia [56].
Neural cells are known to lack the MHC, which renders them susceptible to viral invasion and causes pyroptosis, a type of cell death that occurs during an infection with an intracellular pathogen. Pyroptosis-induced neuroinflammation can contribute to memory loss, hypersensitivity to pain, or seizures in COVID-19 patients [56]. The most frequent neurological manifestations in infected patients include headache, dizziness, hyposmia, and hypogeusia, all of which are currently known to be associated in patients with mild-moderate COVID-19 [57,58]. There are reports in which the appearance of ischemic cerebrovascular accidents accompanied by dysarthria by COVID-19 have been mentioned, considering that this virus gives rise to a state of hypercoagulability, thus to the formation of thrombi, which contribute to the ischemic event [59]. During the infectious process, encephalopathy may occur and manifests as delirium, agitation, disorientation, paralysis, loss of consciousness, cerebral edema, coma, or epilepsy, the latter having become a commonly described manifestation and one that can occur due to hydric and electrolyte, alterations, hypoxia, or multi-organ failure [60]. It has been shown that, in COVID-19, cerebral edema of different degrees, and even fulminant edema, can occur [61]. Presently, the ways in which COVID-19 can induce demyelinating diseases such as multiple sclerosis are not known with certainty, but in the future development is expected in this regard [62].
The hypothalamus is a complex structure that has many neuronal groups and intrahypothalamic circuits that are the basis for the immuno–neuroendocrine interaction. This structure can be affected by the COVID-19 infection. The hypothalamic circuits are essential for maintaining homeostasis and the immuno–neuroendocrine response to SARS-CoV-2 [63].
5. COVID-19, immune-neuroendocrine system and age
The involvement of the immune–neuroendocrine system during the battle against SARS-CoV-2 has not been clearly described; however, we do know that the disease drastically disrupts its components, particularly in older adults. To effectively eliminate SARS-CoV-2, the immune–neuroendocrine system must recognize, alert, destroy, and clear the virus [9]. This entity causes the activation of the immune–neuroendocrine system. Dysregulation of the stress system leads to communication between the brain and the HPA axis for the adaptation of the organism [64].
The changes in this system over time are attributed to the evolution of humans due to adaptation to its environment and to the balance between aggression vs. tolerance caused by various pathogens. With regard to advanced age, the body's immuno-neuroendocrine response undergoes a progressive deterioration that is conditioned by an inefficient response to diseases [65].
Following the challenge of the immune system to fight the pathogen, it produces a series of cytokines. In fact, several cytokines were reported in the serum of COVID-19 patients, mainly proinflammatory, such as tumor necrosis factor-α (TNF α), interleukin-1 (IL-1) and IL-6, and type I interferons (IFN-α/β). Their role, in addition to contributing to the immune response against viral infection such as coronavirus, is responsible for activating the HPA axis, which releases adrenal glucocorticoids, providing negative feedback on immune cells in order to inhibit the synthesis and release of cytokines, thus protecting the host from the devastating consequences of an overactive immune response [66].
Type I Interferon (IFN-1) is essential in antiviral immunity to fight against several infections, as in the case of COVID-19 infection. In France, a study reported that COVID-19 infection is characterized by a decrease in the circulating IFN in patients of all disease-severity states, rendering us vulnerable to pathogens, which is significantly associated with a prognosis of a fatal outcome. Therefore, these authors propose that a blood IFN deficiency could be a biomarker for severe COVID-19 [18]. Agrawal [67] explained that the production of IFN-1 by dendritic cells constitutes the innate immunity of our human organism, but it is affected in older adults as the advance in age, because the immune system's capacity to express IFN-1 decreases and deteriorates. Therefore, elderly patients are more susceptible to infections and to more serious conditions compared to the young population, supporting what is observed in elderly patients with COVID-19 [68].
The activation of the HPA axis has been observed in phenomena such as immune/inflammatory processes; therefore, in patients infected with COVID-19, it can alter the HPA axis in two ways, that is, hyperactivated or hypoactivated, mainly due to the dysfunction in negative feedback between the axis and the immune system. The hyperactivation of this axis is mainly attributed to the cytokine- storm mechanism in individuals infected with SARS-CoV-2, related to lung damage and fatality. Similarly, another potential mechanism for SARS-CoV-2 for over activating this axis is the decrease in the angiotensin-converting enzyme 2 levels (ACE2). Contrariwise, the HPA is hypo-activated by TNF-α and Transforming growth factor beta (TGF-β); thus, it is possible that a certain number of cytokines that are elevated in patients with COVID-19 are involved in the hypocortisolism associated with this disease, being the main cause of depression in convalescent patients [69].
To date, it is theorized that the interaction between the Spike protein located in the SARS-CoV-2 membrane and the ACE2 receptor improves the host's tropism. Chatterjee and colleagues [70] explain that the essential part for the entry of the virus into the host cell, mainly for human respiratory epithelial cells, is the viral–receptor interaction. In elderly patients, low immunity is inversely proportional to the viral load, as well as to the increased expression of the ACE2 receptor in hematopoietic cells, cardiopulmonary tissues, macrophages, and monocytes.
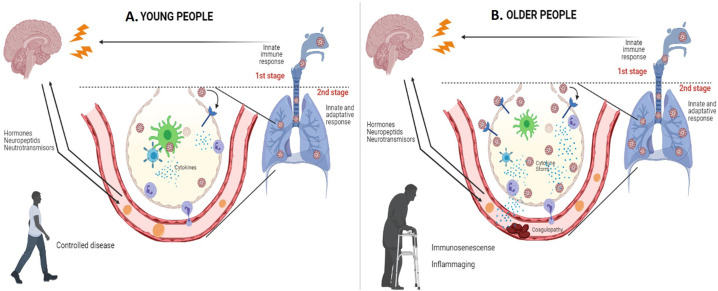
Meftahi et al. [71] propose that several factors may associate “inflame aging” with the cytokine storm in elderly patients with COVID-19; they can uncontrollably turn on the inflammatory machine in the aging, including the alteration of ACE2 receptor expression, excessive reactive oxygen species (ROS) production, senescent adipocyte activity, the alteration of autophagy and mitophagy, and immune-senescence, as well as vitamin D (VD) deficiency. Hence, older persons with severe COVID-19 infection cannot shut down their proinflammatory immune–neuroendocrine response (Fig. 2 ).
Fig. 2.
Presentation of severe COVID-19 infection, according to age group.
A. Severe COVID-19 infection in young people. B. Severe COVID-19 infection in older people.
Nevertheless, there is still much to analyze concerning the participation of the axes of the immune–neuroendocrine system in this new worldwide disease, especially in older adults and patients with rheumatic disease.
6. COVID-19, immune-neuroendocrine system, and rheumatic diseases
Patients with autoimmune rheumatic diseases (ARD) are considered as more susceptible to bacterial and viral infections, which can be related with disease activity, disease damage, comorbidities, and their treatment [72].
Patients with ARD present alterations in the axes of response to stress. Various experimental and human studies suggest that there are alterations in the axes. These alterations are observed, above all in the stages of ARD activity, in pregnancy, in infections, and under stress conditions [73]. It is characterized by decreased plasma–cortisol levels, alterations in thyroid function, a state of hyper-estrogenism, hyperprolactinemia, and dysautonomia. These abnormalities participate in the etiopathogenesis of these entities and can be modified by remission-inducing treatments [74].
With respect to patients with ARD, 30–40% may present infections of various types, which increases the immuno-neuroendocrine response, favoring a proinflammatory state [75]. A similarity of innate and adaptive immune-response alterations involving macrophages, mast cells, neutrophils, and T- and B-lymphocytes has been proposed, leading to multiorgan damage due to the release of cytokines, chemokines, and autoantibodies. It has been proposed that this similarity is due to a complex interaction between the SARS-CoV-2 virus and the patient with ARD because of molecular mimicry, among others [76].
However, there is an important difference between SARS-CoV-2 infection and ARD. Recent studies have shown that the levels of interferon alpha and beta, essential cytokines for controlling viral infection, are decreased during the SARS-CoV-2 infection. This decrease in interferon, demonstrated by lower gene expression, lower production, and decreased serum levels, especially in life-threatening COVID-19 infection, correlates directly with the viral load and inversely with the levels of proinflammatory cytokines [18]. This is partly due to the production of neutralizing the anti-interferon antibodies that are produced during this infection [77]. In contrast, interferons play a relevant role during autoimmune diseases with the overproduction of this cytokine, which participates in the proliferation and activation of cells of the immune system. During a viral infection, the patient with ARD develops an overproduction of the interferon system, which leads to a release of autoantigens that stimulate the innate and adaptive response with the production of autoantibodies, providing the ability to stimulate interferon through the dendritic cells. The increase in interferon is promoted by natural killer (NK) cells and B-lymphocytes and is regulated by monocytes. This function is poor in patients with ARD such as SLE [78].
A great amount of evidence suggests that some components of the immune–neuroendocrine system participate in the activation process of the interferon system in ARD. For example, prolactin (PRL), as a cytokine, intervenes in the proliferation and differentiation of cells of the immune system. For T-lymphocyte-stimulated PRL, the interferon regulatory factor provides the ability to adjust the prolactin immune response. Hyperprolactinemia comprises one of the important factors in the pathogenesis and course of autoimmune diseases such as SLE, rheumatoid arthritis, systemic sclerosis, and Sjogren's syndrome [79].
In a study in which the disease course was followed in 86 patients with immune-mediated inflammatory diseases, the authors included 50 cases of inflammatory arthritis (rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis). These were confirmed cases or highly suspected cases of COVID-19. The main comparison was between out-patient and in-patient management, during which 72% of patients were receiving biologic disease-modifying anti-rheumatic drugs (bDMARD) or Janus kinase (JAK) inhibitors. On comparing comorbidities such as hypertension, diabetes, or chronic obstructive pulmonary disease, these were more common in hospitalized patients than in out-patients. Also, oral glucocorticoids (29 vs. 6%), hydroxychloroquine (21 vs. 7%), and methotrexate (43 vs. 15%) were more frequently utilized in hospitalized patients, whereas biologicals (mostly TNF-blockers and anti-IL12/23 agents) were more common in out-patients. All of this could suggest a certain possible protective effect of these biologics to prevent the occurrence of a cytokine-release syndrome in patients with rheumatic diseases, with this remaining controversial [80].
On the other hand, SLE possesses similarities with COVID-19, such as the multi-organ complications of interstitial pneumonia, cytopenia, and arthralgia. Immunosuppressants employed in SLE have been investigated as an option to reduce inflammation and the development of acute respiratory distress syndrome infected with coronavirus [81]. In this sense, a recent case-based review disclosed that a previously healthy 45-year-old man developed SLE probably triggered by SARS-CoV-2 infection [82].
7. Therapeutic evidences
The use of steroids in critically ill patients during the COVID-19 pandemic was the strongest demonstration of the effect of SARS-CoV-2 on the HPA axis [83].
Accumulated evidence concerning the effect of the SARS-CoV-2 infection on the HPG axis opens the possibility of using testosterone-based hormone-replacement therapy [84]. In relation with the HPT axis, thyrotoxicosis, including the thyroid storm, are conditions described during COVID-19 infection; in these cases, the use of antithyroid drugs is indicated. Regarding to hypothyroidism prior to COVID-19, patients should continue with their therapeutic regimen [85].
The evidence of the immune–neuroendocrine axes involved is solid and opens the possibility of future trials with hormonal therapies in order to restore the homeostasis of the immune–neuroendocrine system.
8. Perspectives and conclusions
-
1.
The immune-neuroendocrine system is essential to maintain homeostasis during stress situations. Older adults, patients with autoimmune rheumatic diseases, and with other comorbidities have an altered immune–neuroendocrine system response.
-
2.
SARS-CoV-2 infection alters the mechanisms of response to stress by the direct action of the virus in the effector organs of the immune–neuroendocrine system and through a hyperinflammatory response with a devastator effect on the immune–neuroendocrine system.
-
3.
The complex interaction between COVID-19 and the immune–neuroendocrine system explains the susceptibility of older adults, patients with autoimmune rheumatic diseases, and other comorbidities to develop severe complications.
-
4.
Knowing the effect of the COVID-19 infection on the immune–neuroendocrine system and its response, is essential to propose novel treatments, to improve the survival of patients infected with COVID-19.
-
5.
The consequence of the damage caused by SARS-CoV-2 on the immune–neuroendocrine system will be the subject-of-study during the post-COVID-19 stage.
Author contributions
BLZ, IOG, MFGS, CIGM performed the literature search. GM and MPCD made the first draft of the manuscript. LJJ, GM, OLVL and MAS critically revised the manuscript and carried out major modifications. LJJ and GM equally contributed as corresponding authors. All authors read and approved the final version.
Declaration of Competing Interest
The authors declare no conflict of interest.
References
- 1.Green A. Obituary. Li Wenliang Lancet. 2020;395(10225):682. doi.10.1016/ S0140-6736(20)30382-2. [Google Scholar]
- 2.World Health Organization Director-General's opening remarks at the media briefing on COVID19 -March. 2020. http://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 [Internet]. [Cited on July, 07, 2021]. Available at.
- 3.Cucinotta D., Vanelli M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020;91(1):157–160. doi: 10.23750/abm.v91i1.9397. 10.23750/abm.v91i1.9397 Published 2020 Mar 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization WHO coronavirus disease (COVID-19) dashboard. 2021. http://covid19.who.int [Internet]. [Cited on July, 7, 2021]. Available at.
- 5.Mason R.J. Pathogenesis of COVID-19 from a cell biology perspective. Eur Respir J. 2020;55(4):2000607. doi: 10.1183/13993003.00607-2020. Published 2020 Apr 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Worldometers 2020. https://www.worldometers.info/coronavirus/coronavirus-age-sex-demographics/ [Internet]. [Cited on July, 07, 2021]. Available at.
- 7.Yan L., Zhang H.T., Goncalves J., Xiao Y., Wang M., Guo Y., et al. An interpretable mortality prediction model for COVID-19 patients. Nat Mach Intell. 2020;2:283–288. [Google Scholar]
- 8.Huang I., Pranata R., Lim M.A., Oehadian A., Alisjahbana B. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Ther Adv Respir Dis. 2020;14:1–14. doi: 10.1177/1753466620937175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mueller A.L., McNamara M.S., Sinclair D.A. Why does COVID-19 disproportionately affect older people? Aging. 2020;12(10):9959–9981. doi: 10.18632/aging.103344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fajgenbaum D.C., June C.H. Cytokine storm. N Engl J Med. 2020;383(23):2255–2273. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zefferino R., Di Gioia S., Conese M. Molecular links between endocrine, nervous and immune system during chronic stress. Brain Behav. 2021;11(2) doi: 10.1002/brb3.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Besedovsky H., Sorkin E. Network of immune-neuroendocrine interactions. Clin Exp Immunol. 1977;27:1–12. [PMC free article] [PubMed] [Google Scholar]
- 13.Besedovsky H.O., del Rey A. Immune-neuro-endocrine interactions: facts and hypotheses. Endocr Rev. 1996;17(1):64–102. doi: 10.1210/edrv-17-1-64. [DOI] [PubMed] [Google Scholar]
- 14.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39(5):405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romagnoli S., Peris A., De Gaudio A.R., Geppetti P. SARS-CoV-2 and COVID-19: from the bench to the bedside. Physiol Rev. 2020;100(4):1455–1466. doi: 10.1152/physrev.00020.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bussani R., Schneider E., Zentilin L., Collesi C., Ali H., Braga L., et al. Persistence of viral RNA, pneumocyte syncytia and thrombosis are hallmarks of advanced COVID-19 pathology. EBioMedicine. 2020;61:103104. doi: 10.1016/j.ebiom.2020.103104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calabrese F., Pezzuto F., Fortarezza F., Hofman P., Kern I., Panizo A., et al. Pulmonary pathology and COVID-19: lessons from autopsy. The experience of European pulmonary pathologists. Virchows Arch. 2020;477(3):359–372. doi: 10.1007/s00428-020-02886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hadjadj J., Yatim N., Barnabei L., Corneau A., Boussier J., Smith N., et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science (New York, NY) 2020;369(6504):718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pérez-Aguilar M.C., Rondón-Mercado R. Neuroimmunoendocrine system in health and disease. EC Microbiol. 2018;14(1):02–06. [Google Scholar]
- 20.Pearce B., Biron C., Miller A. Neuroendocrine-immune interactions during viral infections. Adv Virus Res. 2001;56:1–45. doi: 10.1016/s0065-3527(01)56036-4. [DOI] [PubMed] [Google Scholar]
- 21.Shah S.M.A., Mohammad D., Qureshi M.F.H., Abbas M.Z., Aleem S. Prevalence, psychological responses and sssociated correlates of depression, anxiety and stress in a global population, during the coronavirus disease (COVID-19) pandemic. Community Ment Health J. 2021;57(1):101–110. doi: 10.1007/s10597-020-00728-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jara L.J., Navarro C., Medina G., Vera-Lastra O., Blanco F. Immune-neuroendocrine interactions and autoimmune diseases. Clin Dev Immunol. 2006;13(2–4):109–123. doi: 10.1080/17402520600877059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsigos C., Chrousos G.P. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53(4):865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 24.Silverman M., Pearce B., Biron C., Miller A. Immune modulation of the hypothalamic-pituitary-adrenal (HPA) axis during viral infection. Viral Immunol. 2005;18(1):41–78. doi: 10.1089/vim.2005.18.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pérez A.R., Bottasso O., Savino W. The impact of infectious diseases upon neuroendocrine circuits. Neuroimmunomodulation. 2009;16:96–105. doi: 10.1159/000180264. [DOI] [PubMed] [Google Scholar]
- 26.Jara L.J., Izquierdo E., Medina G. Is the immune neuroendocrine system the connection between epipharyngitis and chronic fatigue syndrome induced by HPV vaccine? Editorial Immunol Res. 2017;65(1):5–7. doi: 10.1007/s12026-016-8854-2. [DOI] [PubMed] [Google Scholar]
- 27.Chrousos G.P., Zapanti E.D. Hypothalamic-pituitary-adrenal axis in HIV infection and disease. Endocrinol Metab Clin North Am. 2014;43(3):791–806. doi: 10.1016/j.ecl.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Zapanti E., Terzidis K., Chrousos G. Dysfunction of the hypothalamic-pituitary-adrenal axis in HIV infection and disease. Hormones (Athens) 2008;7(3):205–216. doi: 10.14310/horm.2002.1200. [DOI] [PubMed] [Google Scholar]
- 29.Torabi A., Mohammadbagheri E., Akbari Dilmaghani N., Bayat A.H., Fathi M., Vakili K., et al. Proinflammatory cytokines in the olfactory mucosa result in COVID-19 induced anosmia. ACS Chem Nerosci. 2020;11(13):1909–1913. doi: 10.1021/acschemneuro.0c00249. [DOI] [PubMed] [Google Scholar]
- 30.Ågren K., Andersson U., Nordlander B., Nord C.E., Linde A., Ernberg I., et al. Upregulated local cytokine production in recurrent tonsillitis compared with Tonsillar hypertrophy. Acta Otolaryngol. 1995;115(5):689–696. doi: 10.3109/00016489509139388. [DOI] [PubMed] [Google Scholar]
- 31.Mao Y., Xu B., Guan W., Xu D., Li F., Ren R., et al. The adrenal cortex, an underestimated site of SARS-CoV-2 infection. Front Endocrinol (Lausanne) 2021;11:593179. doi: 10.3389/fendo.2020.593179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alzahrani A.S., Mukhtar N., Aljomaiah A., Aljamei H., Bakhsh A., Alsudani N., et al. The impact of COVID-19 viral infection on the hypothalamic-pituitary-adrenal axis. Endocr Pract. 2021;27(2):83–89. doi: 10.1016/j.eprac.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freire Santana M., Borba M.G.S., Baía-da-Silva D.C., Val F., Alexandre M.A.A., Brito-Sousa J.D., et al. Case report: adrenal pathology findings in severe COVID-19: an autopsy study. Am J Trop Med Hyg. 2020;103(4):1604–1607. doi: 10.4269/ajtmh.20-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanriverdi F., Silveira L.F., MacColl G.S., Bouloux P.M. The hypothalamic-pituitary-gonadal axis: immune function and autoimmunity. J Endocrinol. 2003;176(3):293–304. doi: 10.1677/joe.0.1760293. [DOI] [PubMed] [Google Scholar]
- 35.Vom Steeg L.G., Klein S.L. Sex steroids mediate bidirectional interactions between hosts and microbes. Horm Behav. 2017;88:45–51. doi: 10.1016/j.yhbeh.2016.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moradi F., Enjezab B., Ghadiri-Anari A. The role of androgens in COVID-19. Diabetes Metab Syndr. 2020;14(6):2003–2006. doi: 10.1016/j.dsx.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen J., Jiang Q., Xia X., Liu K., Yu Z., Tao W., et al. Individual variation of the SARS-CoV-2 receptor ACE2 gene expression and regulation. Aging Cell. 2020;19(7) doi: 10.1111/acel.13168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khalili M.A., Leisegang K., Majzoub A., Finelli R., Panner Selvam M.K., Henkel R., et al. Male fertility and the COVID-19 pandemic: systematic review of the literature. World J Mens Health. 2020;38(4):506–520. doi: 10.5534/wjmh.200134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ding T., Wang T., Zhang J., Cui P., Chen Z., Zhou S., et al. Analysis of ovarian injury associated with COVID-19 disease in reproductive-aged women in Wuhan, China: an observational Study. Front Med (Lausanne) 2021;8:635255. doi: 10.3389/fmed.2021.635255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fekete C., Lechan R.M. Central regulation of hypothalamic-pituitary-thyroid axis under physiological and pathophysiological conditions. Endocr Rev. 2014;35(2):159–194. doi: 10.1210/er.2013-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parsa A.A., Bhangoo A. HIV and thyroid dysfunction. Rev Endocr Metab Disord. 2013;14(2):127–131. doi: 10.1007/s11154-013-9248-6. [DOI] [PubMed] [Google Scholar]
- 42.Caron P. Thyroid disorders and SARS-CoV-2 infection: from pathophysiological mechanism to patient management. Ann Endocrinol (Paris) 2020;81(5):507–510. doi: 10.1016/j.ando.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scappaticcio L., Pitoia F., Esposito K., Piccardo A., Trimboli P. Impact of COVID-19 on the thyroid gland: an update. Rev Endocr Metab Disord. 2020;25:1–13. doi: 10.1007/s11154-020-09615-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vera-Lastra O., Ordinola Navarro A., Cruz-Domínguez M.P., Medina G., Sánchez-Valadez T.I., Jara L.J. Two cases of Graves’ disease following SARS-CoV-2 vaccination: an autoimmune/inflammatory syndrome induced by adjuvants. Thyroid. 2021 doi: 10.1089/thy.2021.0142. doi:10.1089/thy.2021.0142. [DOI] [PubMed] [Google Scholar]
- 45.İremli B.G., Şendur S.N., Ünlütürk U. Three cases of subacute thyroiditis following SARS-CoV-2 vaccine: post-vaccination ASIA syndrome. J Clin Endocrinol Metab. 2021 doi: 10.1210/clinem/dgab373. dgab373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grattan D.R. 60 years of neuroendocrinology: the hypothalamo-prolactin axis. J Endocrinol. 2015;226(2):T101–T122. doi: 10.1530/JOE-15-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Freeman M.E., Kanyicska B., Lerant A., Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000;80(4):1523–1631. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- 48.Sen A. Repurposing prolactin as a promising immunomodulator for the treatment of COVID-19: are common antiemetics the wonder drug to fight coronavirus? Med Hypotheses. 2020;144:110208. doi: 10.1016/j.mehy.2020.110208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stevens A., Ray D., Alansari A., Hajeer A., Thomson W., Donn R., et al. Characterization of a prolactin gene polymorphism and its associations with systemic lupus erythematosus. Arthritis Rheum. 2001;44(10):2358–2366. doi: 10.1002/1529-0131(200110)44:10. [DOI] [PubMed] [Google Scholar]
- 50.Szyper-Kravitz M., Zandman-Goddard G., Lahita R.G., Shoenfeld Y. The neuroendocrine-immune interactions in systemic lupus erythematosus: a basis for understanding disease pathogenesis and complexity. Rheum Dis Clin North Am. 2005;31(1) doi: 10.1016/j.rdc.2004.10.004. 161-x. [DOI] [PubMed] [Google Scholar]
- 51.Chan J.L., Gregory K.D., Smithson S.S., Naqvi M., Mamelak A.N. Pituitary apoplexy associated with acute COVID-19 infection and pregnancy. Pituitary. 2020;23(6):716–720. doi: 10.1007/s11102-020-01080-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yachou Y., El Idrissi A., Vladimir Belapasov V., Ait Benali S. Neuroinvasion, neurotropic, and neuroinflammatory events of SARS-CoV-2: understanding the neurological manifestations in COVID-19 patients. Neurol Sci. 2020 28 July;28:1–13. doi: 10.1007/s10072-020-04575-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meinhardt J., Radke J., Dittmayer C., Franz J., Thomas C., Mothes R., et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci. 2021;24(2):168–175. doi: 10.1038/s41593-020-00758-5. [DOI] [PubMed] [Google Scholar]
- 54.Wang Lintao, Ren Zhiguang, Ma Li, Han Yanjie, Wei Wenqiang, Jiang Enshe, et al. Progress in research on SARS-Cov-2 infection causing neurological diseases and its infection mechanism. Front Neurol. 2021;11:592888. doi: 10.3389/fneur.2020.592888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Achar A., Ghosh COVID-19-associated neurological disorders: the potential route of CNS invasion and blood-brain barrier relevance. Cells. 2020 Oct 27;9(11):2360. doi: 10.3390/cells9112360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yap J.K.Y., Moriyama M., Iwasaki A. Inflammasomes and pyroptosis as therapeutic targets for COVID-19. J Immunol. 2020;205(2):307–312. doi: 10.4049/jimmunol.2000513. July 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abboud H., Abboud F.Z., Kharbouch H., Arkha Y., El Abbadi N., El Ouahabi A. COVID-19 and SARS-Cov-2 infection: pathophysiology and clinical effects on the nervous system. World Neurosurg. 2020;140:49–53. doi: 10.1016/j.wneu.2020.05.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lechien J.R., Chiesa-Estomba C.M., De Siati D.R., Horoi M., Le Bon S.D., Rodríguez A., et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277(8):2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Neha Gupta, Zhao Y.Y., Colin Evans. The stimulation of thrombosis by hypoxia. Thromb Res. 2019;181:77–83. doi: 10.1016/j.thromres.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 60.Niazkar H.R., Zibaee B., Nasimi A., Bahri N. The neurological manifestations of COVID-19: a review article. Neurol Sci. 2020;41(7):1667–1671. doi: 10.1007/s10072-020-04486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van den Enden A.J.M., van Gils L., Labout J.A.M., van der Jagt M., Moudrous W. Fulminant cerebral edema as a lethal manifestation of COVID-19. Radiol Case Rep. 2020;15(9):1705–1708. doi: 10.1016/j.radcr.2020.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Singhal A.B., Gilberto González R.G., Chwalisz B.K., Mukerji S.S. Case 26-2020: a 60-year-old woman with altered mental status and weakness on the left side. N Engl J Med. 2020;383:764–773. doi: 10.1056/NEJMcpc2004976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mussa B.M., Srivastava A., Verberne A. COVID-19 and neurological impairment: hypothalamic circuits and beyond. Viruses. 2021;13(3):498. doi: 10.3390/v13030498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pal R. COVID-19, hypothalamo-pituitary-adrenal axis and clinical implications. Endocrine. 2020;68(2):251–252. doi: 10.1007/s12020-020-02325-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Straub R.H., Besedovsky H.O. Integrated evolutionary, immunological, and neuroendocrine framework for the pathogenesis of chronic disabling inflammatory diseases. FASEB J. 2003;17(15):2176–2183. doi: 10.1096/fj.03-0433hyp. [DOI] [PubMed] [Google Scholar]
- 66.Steenblock C., Todorov V., Kanczkowski W., Eisenhofer G., Schedl A., Ma-Li Wong, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the neuroendocrine stress axis. Mol Psychiatry. 2020;25:1611–1617. doi: 10.1038/s41380-020-0758-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Agrawal A. Mechanisms and implications of age-associated impaired innate interferon secretion by dendritic cells: a mini-review. Gerontology. 2013;59(5):421–426. doi: 10.1159/000350536. [DOI] [PubMed] [Google Scholar]
- 68.Perrotta F., Corbi G., Mazzeo G., Boccia M., Aronne L., D’Agnano V., et al. COVID-19 and the elderly: insights into pathogenesis and clinical decision-making. Aging Clin Exp Res. 2020;32(8):1599–1608. doi: 10.1007/s40520-020-01631-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Raony Í., Saggioro de Figueiredo C., Pandolfo P., Giestal-de-Araujo E., Oliveira-Silva P., Savino W. Psycho-neuroendocrine-immune interactions in COVID-19: potential impacts on mental health. Front Immunol. 2020;11:1–15. doi: 10.3389/fimmu.2020.01170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chatterjee S.K., Saha S., Muñoz M.N. Molecular pathogenesis, immunopathogenesis and novel therapeutic strategy against COVID-19. Front Mol Biosci. 2020;7:1–11. doi: 10.3389/fmolb.2020.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meftahi G.H., Jangravi Z., Sahraei Bahari Z. The possible pathophysiology mechanism of cytokine storm in elderly adults with COVID 19 infection: the contribution of “inflame aging”. Inflamm Res. 2020;69:825–839. doi: 10.1007/s00011-020-01372-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stradner M.H., Dejaco C., Zwerina J., Fritsch-Stork R.D. Rheumatic musculoskeletal diseases and COVID-19 A review of the first 6 months of the pandemic. Front Med (Lausanne) 2020;7:562142. doi: 10.3389/fmed.2020.562142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hung H.M., Chen M.F., Chen C.H. Impacts of fatigue, stress, and perceived health status on women with rheumatic diseases: a comparison study. J Nurs Res. 2020;28(3) doi: 10.1097/JNR.0000000000000354. [DOI] [PubMed] [Google Scholar]
- 74.Cutolo M., Straub R.H. Sex steroids and autoimmune rheumatic diseases: state of the art. Nat Rev Rheumatol. 2020;16(11):628–644. doi: 10.1038/s41584-020-0503-4. [DOI] [PubMed] [Google Scholar]
- 75.Jara L.J., Cruz-Domínguez M.D.P., Saavedra M.A. Impact of infections in autoimmune rheumatic diseases and pregnancy. Curr Opin Rheumatol. 2019;31(5):546–552. doi: 10.1097/BOR.0000000000000636. PMID: 31169546. [DOI] [PubMed] [Google Scholar]
- 76.Liu Y., Sawalha A.H., Lu Q. COVID-19 and autoimmune diseases. Curr Opin Rheumatol. 2021;33(2):155–162. doi: 10.1097/BOR.0000000000000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bastard P., Rosen L.B., Zhang Q., Michailidis E., Hoffmann H.H., Zhang Y., et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370(6515) doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rönnblom L. The importance of the type I interferon system in autoimmunity. Clin Exp Rheumatol. 2016;34(4 Suppl 98):21–24. [PubMed] [Google Scholar]
- 79.Kokot I., Pawlik-Sobecka L., Płaczkowska S., Piwowar A. Prolactin as an immunomodulatory factor in psoriatic arthritis. Postepy Hig Med Dosw (Online) 2013;67:1265–1272. doi: 10.5604/17322693.1079893. [DOI] [PubMed] [Google Scholar]
- 80.Haberman R., Axelrad J., Chen A., Castillo R., Yan D., Izmirly P., et al. Covid-19 in immune-mediated inflammatory diseases - case series from New York. N Engl J Med. 2020;383(1):85–88. doi: 10.1056/NEJMc2009567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Spihlman A.P., Gadi N., Wu S.C., Moulton V.R. COVID-19 and systemic lupus erythematosus: focus on immune response and therapeutics. Front Immunol. 2020;11:589474. doi: 10.3389/fimmu.2020.589474. Published 2020 Oct 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gracia-Ramos A.E., Saavedra-Salinas M.A. Can the SARS-CoV-2 infection trigger systemic lupus erythematosus? A case-based review. Rheumatol Int. 2021;41(4):799–809. doi: 10.1007/s00296-021-04794-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chatterjee K., Wu C.P., Bhardwaj A., Siuba M. Steroids in COVID-19: an overview. Cleve Clin J Med. 2020:1–4. doi: 10.3949/ccjm.87a.ccc059. [DOI] [PubMed] [Google Scholar]
- 84.Auerbach J.M., Khera M. Testosterone’s role in COVID-19. J Sex Med. 2021;18(5):843–848. doi: 10.1016/j.jsxm.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Boelaert K., Visser W.E., Taylor P.N., Moran C., Léger J., Persani L. Endocrinology in the time of COVID-19: management of hyperthyroidism and hypothyroidism. Eur J Endocrinol. 2020;183(1):G33–G39. doi: 10.1530/EJE-20-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]