Abstract
Two hundred twenty-four Mycobacterium bovis isolates, mainly from South American countries, were typed by spoligotyping, and 41 different spoligotypes were identified. A total of 202 M. bovis isolates (90%) were grouped into 19 different clusters. The largest cluster contained 96 isolates (42.8%) on the basis of the most frequently observed spoligotype, spoligotype 34. Nineteen M. bovis isolates from humans in Argentina had spoligotypes and polymorphic GC-rich repetitive sequence (PGRS) types that represented the most common types found among isolates from cattle. All five isolates from Uruguay and three of the six isolates from Paraguay had spoligotypes that were also detected for isolates from Argentina. The spoligotypes of isolates from Brazil, Costa Rica, and Mexico and of some of the isolates from Paraguay could not be found in Argentina. A total of 154 M. bovis isolates were selected in order to compare the discriminative power of spoligotyping and restriction fragment length polymorphism (RFLP) analysis with direct repeat (DR) and PGRS probes. By spoligotyping, 31 different types were found, while AluI-digested DR probe-associated RFLP analysis identified 42 types, and RFLP analysis with the PGRS probe also detected 42 types; these were partly independent of the DR types. By combining the results obtained by spoligotyping and by RFLP analysis with the DR and PGRS probes, 88 different types were obtained. Although the differentiation of M. bovis by spoligotyping was less discriminatory than differentiation by RFLP analysis with the DR and PGRS probes, spoligotyping is easier to perform and its results are easier to interpret. Therefore, for the purpose of typing of M. bovis isolates, spoligotyping could be performed first and the isolates could be grouped into clusters and then analyzed by RFLP analysis with the DR and PGRS probes.
Bovine tuberculosis (BTB) is an infection of major importance among cattle in South America. Mycobacterium bovis, the causative agent, is also responsible for tuberculosis in other animals of agricultural importance and in humans (11, 12, 23, 24). The disease causes direct economic losses in agricultural areas and hampers the commercial exchange of animal products.
Of a population of approximately 300 million heads of cattle in Latin America and the Caribbean, 240 million live in countries in which the prevalence of BTB is greater than 0.5%. Brazil and Argentina, with a cattle population of 190 million, have prevalences of BTB that range from 3 to 6% (6), while neighboring countries, such as Paraguay and Uruguay, have prevalences estimated to be 0.25 and 0.01%, respectively (6).
There is limited epidemiological information on the impact of BTB on human health in Latin America and the Caribbean, mainly because the means of bacteriological diagnosis of human tuberculosis (TB) is generally limited to the sputum smear examination. Furthermore, samples are cultured on glycerol-containing Lowenstein-Jensen medium, on which M. bovis strains are difficult to grow, since it is the only available growth medium for M. bovis. Between 1984 and 1989, a study was performed in Santa Fe, a province of Argentina with a high prevalence of BTB. M. bovis was identified in 2.4% of human patients with TB, and 64% of these patients with M. bovis isolates were slaughterhouse or rural workers (16).
Techniques that detect the molecular epidemiology of animal and human M. bovis infections are being used as new tools for the examination of BTB transmission (1, 8, 11, 12, 17, 18, 21–24). In Spain, molecular techniques recently demonstrated that a particular multidrug-resistant M. bovis strain was responsible for a nosocomial outbreak involving at least 16 human immunodeficiency virus-positive patients (3). Comparison of the fingerprint types of these multidrug-resistant M. bovis isolates demonstrated that this strain was responsible for a nosocomial outbreak in a second hospital (20).
One of the most commonly used methods in molecular epidemiology is restriction fragment length polymorphism (RFLP) analysis with different specific probes. IS6110 is the most useful probe for determination of the molecular epidemiology of human tuberculosis, because this element is usually present in multiple copies and in different locations in the genome of Mycobacterium tuberculosis (13, 25). The level of differentiation of M. bovis isolates obtained by IS6110-associated RFLP analysis depends on the origin of the strain (24). The majority of bovine and human M. bovis isolates from Argentina harbor only a single copy of this element, significantly reducing the usefulness of IS6110-associated RFLP typing for determination of the molecular epidemiology of tuberculosis in animals (18). The IS6110 element was sufficiently sensitive for the DNA typing of isolates whose DNAs contain more than three copies of this element (4). Other repetitive elements, such as the polymorphic GC-rich repetitive sequence (PGRS) (2, 7, 19) and the direct repeat (DR) sequence (10, 14), have been used to study the epidemiology of BTB (5, 8, 18, 21, 22, 24). Results obtained from a study in Argentina indicated that RFLP analysis with the combination of DR and PGRS offers a suitable alternative for the typing of M. bovis isolates (8, 18). These results, along with the recording and tracing of cattle movements, could contribute to a better knowledge of the origins of M. bovis infections in herds from different areas in Argentina.
Recently, a rapid technique designated “spoligotyping” has been introduced as a means of determining the epidemiology of human TB (9, 15). This method is based on PCR amplification of a highly polymorphic DR locus in the M. tuberculosis complex, which contains DR sequences interspersed with variable spacer sequences, followed by a reversed line blot hybridization (15). This typing method relies on determination of the presence or absence of spacers in the in vitro-amplified DNA by hybridization to multiple synthetic spacer oligonucleotides covalently bound to a filter.
The aim of the present study was to evaluate the usefulness of spoligotyping for the differentiation of M. bovis isolates.
MATERIALS AND METHODS
Two hundred twenty-four M. bovis isolates were subjected to spoligotyping as described by Kamerbeek et al. (15). This set of strains comprised 197 isolates from cattle; 19 from humans; 1 each from a cat, a deer, and a buffalo; 2 from pigs; and an additional 2 from goats. The 19 human patients from whom M. bovis was isolated were all at the National Institute of Respiratory Diseases and were from the central region of Argentina. All patients were symptomatic and were part of a research study on the incidence of human M. bovis infection in Argentina. Of these patients, 11 were rural and slaughterhouse workers, while 3 had no contact with animals and 5 registered no occupation. The isolates from animals were from animals from different regions of Argentina (south, northeast, and Central regions and Buenos Aires Province), from three neighboring countries (Uruguay, Brazil, and Paraguay), and from two distant countries (Mexico and Costa Rica). The reference strain M. bovis AN5 originated in The Netherlands and was also included in the study.
Analysis of spoligotypes was performed with GelCompar, version 2.1, software (Applied Maths, Kortrijk, Belgium). Comparison of the spoligotypes of the test isolates with the spoligotypes of M. bovis isolates from Spain was done by using a database of spoligotypes at the School of Medicine of Zaragoza University.
A total of 154 M. bovis isolates were selected to compare the results of spoligotyping and RFLP analysis with the DR and PGRS probes. M. bovis genomic DNAs digested with AluI were used for RFLP analysis. The origins of these strains, the RFLP typing method, and the RFLP typing results have been described previously (8). The combined patterns obtained by RFLP analysis with the DR and PGRS probes were analyzed with the NTSYS-pc Numerical Taxonomy and Multivariate Analysis System computer software (Exeter Software, Setauket, N.Y.). This program draws a dendrogram and calculates a similarity coefficient (SC) between fingerprints on the basis of band positions alone. Increasing similarity results in similarity coefficient values ranging from 0 to 1.0.
Nomenclature of the strain types.
The different DNA types were arbitrarily assigned letters or numbers. The RFLP types are indicated by different numbers and/or letters. The DR and PGRS types are separated by a slash; the first letter or number denotes the DR type and the second one denotes the PGRS type. The different spoligotypes were each allocated a number.
RESULTS
Spoligotyping results.
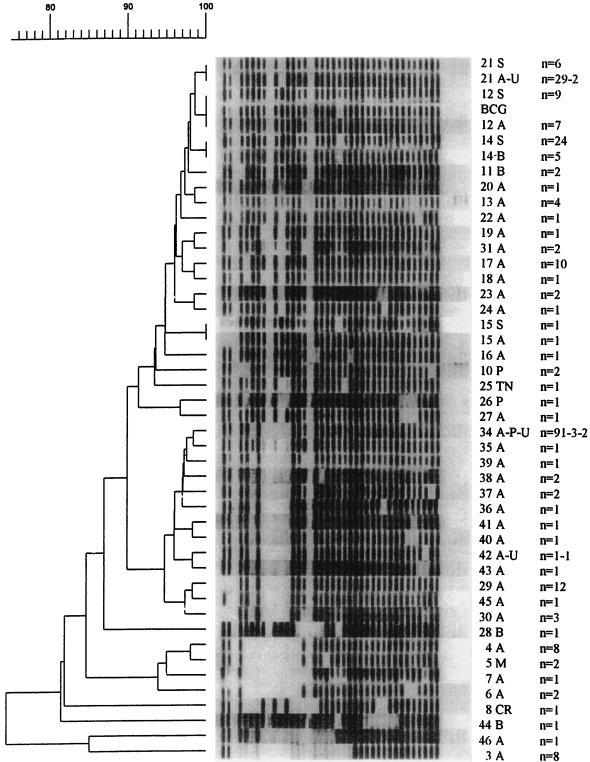
Forty-one different spoligotypes were identified among the 224 M. bovis isolates, of which 22 were unique (Fig. 1 and Table 1). The remaining 202 isolates (90%) were grouped into 19 clusters of strains sharing identical spoligotypes. One of these clusters contained 96 isolates (42.8%) and represented the most frequently observed type. It was arbitrarily designated spoligotype 34. The other major spoligotypes were spoligotype 21, observed for 31 isolates (13.8%); spoligotype 29, observed for 12 isolates (5.3%); spoligotype 17, observed for 10 isolates (4.4%); spoligotypes 3 and 4, each observed for 8 isolates (3.6%); and spoligotype 12 (identical to the spoligotype for M. bovis BCG), observed for 7 isolates (3.1%); while the remaining types grouped only five or fewer isolates (Table 1).
FIG. 1.
Dendrogram showing the relationship between 41 spoligotypes identified among 224 M. bovis isolates from South America and the 4 spoligotypes found simultaneously among isolates from South America and Spain. The designations on the right correspond to the spoligotypes that were obtained. A, Argentina; B, Brazil; CR, Costa Rica; M, Mexico; P, Paraguay; TN, The Netherlands; U, Uruguay; S, Spain; n, number of isolates. The first, second, and third numbers refer to the number of isolates for the first, second, and third countries indicated, respectively.
TABLE 1.
Number of strains of each spoligotype by origin and species
| Country and region | No. of isolates, source | No. of isolates with the following spoligotype:
|
||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 4 | 5 | 6 | 7 | 8 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 | 42 | 43 | 44 | 45 | 46 | ||
| Argentina | ||||||||||||||||||||||||||||||||||||||||||
| Bs. As. | 47, cattle | 4 | 2 | 1 | 2 | 3 | 5 | 1 | 9 | 3 | 2 | 15 | ||||||||||||||||||||||||||||||
| 1, cat | 1 | |||||||||||||||||||||||||||||||||||||||||
| 2, goats | 1 | 1 | ||||||||||||||||||||||||||||||||||||||||
| 1, deer | 1 | |||||||||||||||||||||||||||||||||||||||||
| Center | 101, cattle | 1 | 5 | 4 | 5 | 1 | 14 | 1 | 2 | 1 | 3 | 58 | 1 | 2 | 1 | 1 | 1 | |||||||||||||||||||||||||
| 19, humans | 3 | 1 | 1 | 5 | 9 | |||||||||||||||||||||||||||||||||||||
| N.E. | 26, cattle | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 3 | 8 | 2 | 1 | 1 | 1 | 1 | |||||||||||||||||||||||||||
| South | 3, cattle | 2 | 1 | |||||||||||||||||||||||||||||||||||||||
| Brazil | 6, cattle | 5 | 1 | |||||||||||||||||||||||||||||||||||||||
| 2, pigs | 2 | |||||||||||||||||||||||||||||||||||||||||
| 1, buffalo | 1 | |||||||||||||||||||||||||||||||||||||||||
| Costa Rica | 1, cattle | 1 | ||||||||||||||||||||||||||||||||||||||||
| Mexico | 2, cattle | 2 | ||||||||||||||||||||||||||||||||||||||||
| Paraguay | 6, cattle | 2 | 1 | 3 | ||||||||||||||||||||||||||||||||||||||
| The Netherlands | 1, AN5b | 1 | ||||||||||||||||||||||||||||||||||||||||
| Uruguay | 5, cattle | 2 | 2 | 1 | ||||||||||||||||||||||||||||||||||||||
| Total | 224 | 8 | 8 | 2 | 2 | 1 | 1 | 2 | 2 | 7 | 4 | 5 | 1 | 1 | 10 | 1 | 1 | 1 | 31 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 12 | 3 | 2 | 96 | 1 | 1 | 2 | 2 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 |
Bs. As., Buenos Aires province; Center, Cordoba province, center and south of Santa Fe and Entre Rios provinces; N.E., north of Santa Fe and Entre Rios provinces and Chaco, Corrientes, Formosa, and Misiones provinces; South, Patagonia region.
AN5, reference M. bovis strain.
The comparison of the spoligotypes of the M. bovis strains isolated in South America with those isolated in Spain revealed only four spoligotypes that were found in both regions (Fig. 1). The first was spoligotype 21, which was found for 5 human isolates and 26 animal isolates from Argentina and Uruguay and 6 human isolates from Spain. The second was spoligotype 12 (identical to the spoligotype for M. bovis BCG), which was found for seven bovine isolates from Argentina and one human and eight animal isolates from Spain. The third spoligotype shared by isolates from both regions was spoligotype 14, which grouped 5 bovine isolates from Brazil and 11 human and 13 animal isolates from Spain; while the fourth type, spoligotype 15, was found for 1 bovine isolate from Argentina and 1 human isolate from Spain (Fig. 1). The most frequent type found among South American isolates (spoligotype 34) was not found among isolates from Spain (Fig. 1).
All the strains from South America and Spain analyzed lacked spacers 3, 9, 16, and 39 to 43 (Fig. 1). The absence of these spacers is characteristic of M. bovis isolates from these regions. Furthermore, all these strains harbored spacers 24 and 25, while spacer 38 was present in all isolates from South America but in only some strains from Spain.
Human M. bovis isolates.
Spoligotypes were determined for the 19 human M. bovis strains isolated in the central region of Argentina, 11 of which were from rural or slaughterhouse workers (Table 1). The spoligotypes of these isolates were spoligotype 34 (nine isolates), 21 (five isolates), 3 (three isolates), 17 (one isolate), and 4 (one isolate) (Table 1 and Fig. 1). These represented the most common types among isolates from cattle and were isolated from cattle from the same geographic regions where the patients lived (Table 1).
The isolates from humans were also typed by RFLP analysis with the PGRS probe, by which all isolates had the same types found among isolates from cattle, including the predominant PGRS pattern, pattern A (8, 18). Most of the M. bovis isolates from humans had the same PGRS patterns found among isolates from cattle from central Argentina, the region from which the isolates from humans came (data not shown).
Geographical spread of M. bovis spoligotypes.
The nine isolates from Brazil, two isolates from Mexico, one isolate from Costa Rica, three isolates from Paraguay, and the AN5 strain from The Netherlands had spoligotypes not found among isolates from Argentina (Table 1). For example, spoligotypes 14, 11, 28, and 44 were found only among isolates from Brazil; spoligotype 5 was found only among isolates from Mexico, spoligotype 8 was found only for the isolate from Costa Rica, spoligotypes 10 and 26 were found only among the isolates from Paraguay, and spoligotype 25 was found only for strain AN5 from The Netherlands (Table 1). However, spoligotype 14, found among isolates from Brazil, on occasion was found in the spoligotype pattern datebase at the School of Medicine, University of Zaragoza (Fig. 1).
In addition, five of the isolates from Uruguay had spoligotypes that were also found among isolates from Argentina, four of which corresponded to the major types found among isolates from Argentina (spoligotypes 34 and 21). Only one isolate from Uruguay had an infrequently occurring spoligotype (spoligotype 42). Three isolates from Paraguay were of spoligotypes that were the most commonly observed spoligotypes among isolates from Argentina (spoligotype 34) (Table 1 and Fig. 1).
The percentages of isolates in clusters were 93.3% for the central region of Argentina, 94.2% for Buenos Aires Province, and 64% for the northeast region of Argentina.
Comparison of spoligotyping and typing by RFLP analysis with DR and PGRS probes.
The results of spoligotyping and RFLP analysis with the DR and PGRS probes were compared for 154 M. bovis isolates previously reported in an RFLP typing study (8).
Hybridization with DR with AluI digests identified 42 different patterns, and 38.9% of the isolates were clustered in the major pattern, pattern A. When these membranes were rehybridized with the PGRS probe, 42 different patterns were also identified, and some of these were not associated with pattern obtained by RFLP analysis with the DR probe. In this case, 30.5% of the isolates were grouped in the most predominant PGRS pattern, pattern A. In addition, the spoligotyping method identified 31 different types among the 154 M. bovis isolates, of which the predominant spoligotype, spoligotype 34, included 50% of the isolates (Table 2).
TABLE 2.
Results of spoligotyping and RFLP analysis with DR and PGRS probes for M. bovis isolates from different regions
| Geographic origina | Spoligotype | No. of isolates | DR and PGRS patterns (no. of isolates)b |
|---|---|---|---|
| Argentina | |||
| Bs.As. | 3 | 4 | G/A (1), G/Y (1), T/B (1), V/A (1) |
| 17 | 2 | T/B (2) | |
| 21 | 1 | E/I (1) | |
| 27 | 1 | I/J (1) | |
| 34 | 10 | A/C (2), B/A (3), B/25 (1), B/S (1), D/A (1), D/H (1), F/E (1) | |
| Center | 3 | 1 | 13/A (1) |
| 4 | 5 | G/O2 (1), G/U (4) | |
| 12 | 4 | K/7 (1), 5/01 (1), A/A (1), 18/01 (1) | |
| 17 | 4 | T/B (1), T/5 (1), T/S (1), 17/U (1) | |
| 18 | 1 | C/S (1) | |
| 21 | 13 | E/01 (1), E/02 (2), E/V (4), G/V (1), L/Q (1), L/V (1), N/C (1), Y/R2 (1), Y/V (1) | |
| 22 | 1 | I/V (1) | |
| 23 | 2 | O/D (1), O/10 (1) | |
| 24 | 1 | E/02 (1) | |
| 29 | 3 | A/A (1), 15/C (2) | |
| 34 | 57 | A/A (24), A/C (1), A/01 (3), A/P (3), A/P1 (1), A/R (4), A/W (1), A/Y (1), A/1 (5), A/3 (2), A2/A (1), B/A (1), D/C (2), G/X (1), W/M (1), 3/A (2), 6/2 (1), 14/A (1), 24/A (1), A/24 (1) | |
| 35 | 1 | A/R (1) | |
| 37 | 2 | 2/A (1), D/P (1) | |
| 39 | 1 | A/A (1) | |
| 43 | 1 | P/A (1) | |
| N.E. | 4 | 1 | G/X (1) |
| 12 | 3 | K/O1 (1), K1/19 (2) | |
| 15 | 1 | 25/19 (1) | |
| 16 | 1 | 17/21 (1) | |
| 17 | 1 | Q/S (1) | |
| 19 | 1 | A1/03 (1) | |
| 20 | 1 | L/Q (1) | |
| 21 | 3 | E/71 (2), 1/02 (1) | |
| 34 | 8 | A/A (3), A/R (1), A2/P (1), B/F (2), 3/H1 (1) | |
| 38 | 2 | J/A (1), J1/23 (1) | |
| 40 | 1 | 19/A (1) | |
| 41 | 1 | R/T (1) | |
| 42 | 1 | 3/A (1) | |
| Brazil | 14 | 5 | K/P (5) |
| 44 | 1 | 11/P (1) | |
| Costa Rica | 8 | 1 | H/D (1) |
| Mexico | 5 | 2 | G/16 (2) |
| Paraguay | 10 | 1 | 8/V (1) |
| 26 | 1 | 9/S (1) | |
| 34 | 2 | A/A (1), A/1 (1) | |
| The Netherlands | 25 | 1 | U/8 (1) |
| Total | 154 |
Bs.As., Buenos Aires Province; Center, Cordoba Province and center and south of Santa Fe and Entre Rios provinces; N.E., north of Santa Fe and Entre Rios provinces and Chaco, Corrientes, Formosa, and Misiones provinces; AN5, reference M. bovis strain.
The RFLP types are indicated with different numbers and/or letters. The DR and PGRS types are separated by a slash; the first letter or number denotes the DR type, and the second letter or number indicates the PGRS type. Isolates with the DR and PGRS patterns of group 1 isolates and with spoligotype 34 are indicated in boldface. The nomenclature was arbitrarily assigned to the different DNA types.
The majority of the isolates (28 of 31) showing the predominant DR and PGRS patterns (pattern A/A) were also grouped into the predominant spoligotype, spoligotype 34. In contrast, 75 isolates of spoligotype 34 from Argentina (10 from Buenos Aires Province, 57 from the central region, and eight from the northeast region) and 2 isolates from Paraguay were grouped in 11 different DR patterns, 20 different PGRS patterns, and 28 different combined (DR and PGRS) patterns (Table 2).
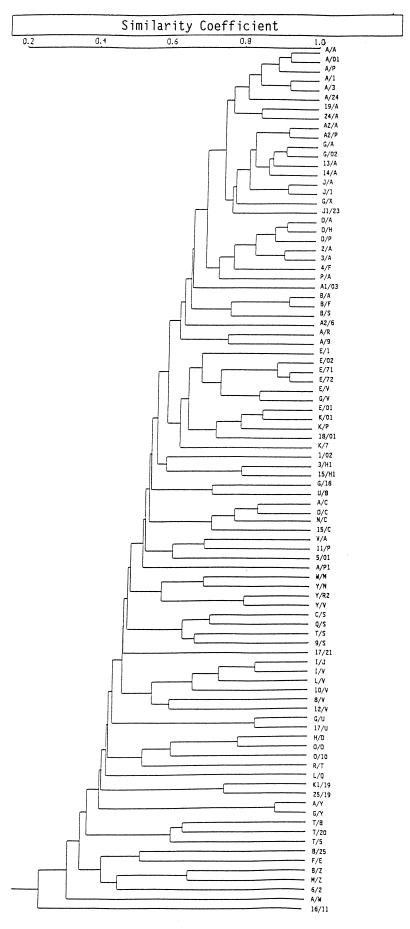
A dendrogram was used to analyze the relationship between the different combined (DR and PGRS) patterns (Fig. 2). If an SC of 0.6 was considered, two groups of patterns with SC values of 0.6 or greater could be found. Groups 1 and 2 contained patterns A/A to A/9 and E/I to K/7, respectively (Fig. 2). These patterns were clearly separated from the patterns for the other nonepidemiologically related strains, such as those from Costa Rica, Mexico, and The Netherlands (H/D, G/16, and U/8, respectively). Eighty-three percent (64 of 77) of the M. bovis isolates of the predominant spoligotype (spoligotype 34) had DR and PGRS patterns that corresponded to those of group 1 isolates (boldface in Table 2), while 64% (11 of 17) of the M. bovis isolates of a major spoligotype (spoligotype 21) had DR and PGRS patterns that corresponded to those of group 2 isolates (Table 2; Fig. 2). These results indicate that several of the isolates with the predominant spoligotypes (spoligotypes 34 and 21) had DR and PGRS patterns that were quite related, while the isolates from distant countries, such as Costa Rica, Mexico, and The Netherlands, had DR and PGRS patterns that were quite different.
FIG. 2.
Dendrogram based on computer-assisted comparison of DNA fingerprints. The types obtained by RFLP analysis are indicated with different numbers and/or letters. The types obtained by RFLP analysis with the DR and PGRS probes are separated by a slash: the first number or letter denotes the DR type, and the second one indicates the PGRS type. SC was defined in Materials and Methods.
Geographical spread of M. bovis according to combined results of spoligotyping and RFLP analysis.
By combining the results of spoligotyping and RFLP analysis with the DR and PGRS probes, a total of 88 types were obtained among the 154 M. bovis isolates (Table 2). The isolates from Brazil, Mexico, and Costa Rica, some isolates from Paraguay, and strain AN5 from The Netherlands had combined types not found among isolates from Argentina (Table 2). These combined types were exclusive to those countries. For example, spoligotype 44 and DR and PGRS pattern 11/P were found only among isolates from Brazil, spoligotype 26 and DR and PGRS pattern 9/S were found only among isolates from Paraguay, spoligotype 5 and DR and PGRS pattern G/16 were found only among isolates from Mexico, spoligotype 8 and DR and PGRS pattern H/D were found only among isolates from Costa Rica, and spoligotype 25 and DR and PGRS pattern U/8 were found only for strain AN5 from The Netherlands (Table 2).
On the other hand, a number of combined patterns were found simultaneously for isolates from different regions of Argentina and other countries. For example, the most common combined type, spoligotype 34 and DR and PGRS pattern A/A, was found among isolates from the central (24 isolates) and northeast (three isolates) regions of Argentina, as well as Paraguay (one isolate); spoligotype 34 and DR and PGRS pattern A/C were found among isolates from the central region (one isolate) and Buenos Aires Province (two isolates) of Argentina; spoligotype 34 and DR and PGRS pattern A/1 were found among isolates from the central region of Argentina (five isolates) and Paraguay (one isolate); spoligotype 34 and DR and PGRS pattern A/R were found among isolates from the central (four isolates) and northeast (one isolate) regions of Argentina; spoligotype 34 and DR and PGRS pattern B/A were found among isolates from the central region (one isolate) and Buenos Aires Province (three isolates) of Argentina; and spoligotype 17 and DR and PGRS pattern T/B were found among isolates from the central region (one isolate) and Buenos Aires Province (two isolates) of Argentina (Table 2).
DISCUSSION
In the present study, 41 different spoligotypes were found among 224 M. bovis isolates from different regions of Argentina and neighboring and distant countries. One hundred fifty-four isolates of this same set of strains were selected to compare the results of spoligotyping and RFLP analysis with the DR and PGRS probes. By spoligotyping, 31 types were obtained, whereas 42 types were obtained by RFLP analysis with both the AluI-digested DR probe and the PGRS probe. Thus, the differentiation of M. bovis by spoligotyping was less discriminatory than differentiation by RFLP analysis with the DR or PGRS probe.
The spoligotypes and PGRS types obtained for M. bovis strains from humans from the central region of Argentina were identical to those obtained for strains from cattle. Of the 19 patients infected with M. bovis, 11 were rural or slaughterhouse workers. These results indicate that cattle could be the main source of human infection in this region of Argentina. A high percentage of these patients were from rural areas and did not complete the anti-TB treatment (data not shown), indicating a risk factor for the development of resistant M. bovis strains.
Most isolates from the central region and Buenos Aires Province of Argentina were grouped into clusters by spoligotyping (93.3 and 94.2%, respectively) (Table 1). In a recent paper (8), several of these isolates were typed by RFLP analysis with DR and PGRS as probes, and the majority were grouped into clusters (69 and 53% in the central region and Buenos Aires Province of Argentina, respectively). This larger number of isolates in clusters in Argentina may indicate that these strains represent the most successful M. bovis genotypes with regard to the active transmission of BTB. These regions contain most of the dairy farms in Argentina. Dairy cattle management leads to diseases such as BTB due to the stress derived from continuous milk production and the prolonged lifetimes of dairy cows. Clustering of isolates has been described as an indication of active transmission (26). Accordingly, in those countries where BTB eradication efforts are under way, clustering of cases on the basis of DNA fingerprinting should decrease in the future. The majority of the Argentine strains (42.8%) were clustered into one major spoligotype (spoligotype 34). This spoligotype was found among isolates from different regions of Argentina, as well as isolates from Uruguay and Paraguay. When the spread of the most common combined type (spoligotyping 34 and DR and PGRS pattern A/A) was analyzed, this combination was found simultaneously among isolates from different regions of Argentina and from Paraguay. These results provide substantial evidence of the transmission of M. bovis between animals in different geographic areas in South America. This may reflect the active trade of livestock between these regions and countries. A more extensive use of these methods for the epidemiological surveillance of BTB could be helpful in examining active TB transmission in these areas.
In the present study, isolates with the same spoligotype could be differentiated by RFLP analysis with the DR and PGRS probes. The two major spoligotypes, spoligotypes 34 and 21, grouped isolates with different DR and PGRS patterns; however, most of these DR and PGRS patterns had many bands in common. The observation that the discriminatory power of RFLP analysis with the DR and PGRS probes was greater than that of spoligotyping may be explained in part by the fact that more DNA rearrangements can be expected from variations in two different repetitive DNA sequences. Isolates of spoligotype 34 had both different DR patterns and different PGRS patterns (Table 2). This means that the individual rearrangements of DR or PGRS occur more frequently than the loss of any of the 43 different spacer sequences linked to the spoligotyping filter. In addition, typing by RFLP analysis with the DR or the PGRS probe could detect initial changes in the genome earlier than the spoligotyping technique could. Thus, the latter technique can be useful for the determination of more distant relationships between isolates; e.g., M. tuberculosis strains of the Beijing family have different types by RFLP analysis with IS6110 but identical types by spoligotyping (27); consequently, the latter technique is useful for the quick detection of strains of the Beijing type.
All strains from Brazil, Mexico, and Costa Rica, as well as several of the isolates from Paraguay, had spoligotypes and combined types (spoligotype plus DR and PGRS pattern) not found among isolates from Argentina. Analysis on the basis of the results obtained with a larger number of isolates could determine whether these types are specific for these geographical regions. Furthermore, most spoligotypes for South American isolates were not found among isolates from Spain. Strains infecting animals in South America could mostly be differentiated from those infecting animals in Spain by spoligotyping. However, Cousins et al. (4) recently described the spoligotypes identified among 273 M. bovis isolates from Australia, Canada, Ireland, and Iran. Fourteen of these spoligotypes were also found among isolates from South America. The most common spoligotype found among isolates from Australia (the spoligotype for 72% of the isolates) is identical to the most common spoligotype found among isolates from Argentina, Uruguay, and Paraguay (the spoligotype for 42.8% of the isolates). Taking into account the fact that the most frequent spoligotype among isolates from Australia (4) was also found among isolates from Argentina, Uruguay, and Paraguay, it is clear that isolates from distant regions can have the same spoligotype. However, only simultaneous RFLP analyses with different probes for these isolates could elucidate whether they are different strains. In the 19th century several breeds of cattle were imported into Argentina and Australia from the United Kingdom. Thus, the finding of similarities between the M. bovis isolates from these countries may be a consequence of the importation of infected cattle in the past. This could be used as an example of the capacity of spoligotyping to detect distant relationships among M. bovis strains. For M. bovis isolates from Australia, Canada, Ireland, and Iran, the best method of differentiation was RFLP analysis with PGRS as a genetic marker (4). According to the previous investigators, the fact that the PGRS probe detected the greatest number of types could be due to the fact that this repetitive element is found in many locations of the M. bovis genome. In the present study, the numbers of different patterns obtained by RFLP analysis with the PGRS or DR probe were found to be equal. Nevertheless, it should be taken into account that the patterns obtained by RFLP analysis with the PGRS probe are difficult to analyze and that we only analyzed fragments larger than 2 kb, while Cousins et al. (4) included in their analysis all bands larger than 1.3 kb obtained by RFLP analysis.
Further studies correlating epidemiological data with fingerprinting results will be necessary to clarify the best method to be used for the typing of M. bovis isolates. In the present study, the epidemiological data were the geographic origin of the isolates, since in this region it is often difficult to determine the origin of cattle, since their management is complicated. This situation is further impaired by the high incidence of BTB and, occasionally, incomplete documentation for cattle.
Even though the spoligotyping technique is less discriminatory than RFLP analysis with the DR and PGRS probes it was able to differentiate between isolates from Argentina, Paraguay, Brazil, Costa Rica, Mexico, The Netherlands, and Spain. However, the results of spoligotyping alone do not allow us to conclude that strains with the same type are identical. A combination of the two methods (spoligotyping and typing by RFLP analysis with the DR and PGRS probes) would be necessary for the differentiation of M. bovis strains for a detailed epidemiological study.
Nevertheless, the spoligotyping method is suitable for initial screening, since it is easier to perform, its results are easier to interpret, and it leads to the fast detection and direct typing of M. bovis isolates in clinical material. All of these steps are essential for the application of typing methods in control and eradication programs. Furthermore, an improvement in the degree of differentiation of M. bovis strains by spoligotyping could presumably be obtained by using additional new spacer sequences derived from sequence analyses of M. bovis strains.
In response to the results obtained in this study, we have adopted a methodology which first consists of typing of the M. bovis isolates by spoligotyping, followed by additional typing of the strains already grouped on the basis of spoligotyping by RFLP analysis with the DR and PGRS probes.
ACKNOWLEDGMENTS
This study was supported by the Fondo Mixto de Cooperación Hispano-Argentina and CABBIO. C.M., A.C., D.V.S., and M.I.R. are fellows of the RELACTB (Red Latinoamericana y del Caribe de Tuberculosis).
We are grateful to Aide Gil for excellent technical assistance and Claudia Vallejos for secretarial assistance.
REFERENCES
- 1.Aranaz A, Liébana E, Mateos A, Dominguez L, Vidal D, Domingo M, González O, Rodríguez-Ferri E F, Bunschoten A E, van Embden J D A, Cousins D. Spacer oligonucleotide typing of Mycobacterium bovis strains from cattle and other animals: a tool for studying epidemiology of tuberculosis. J Clin Microbiol. 1996;34:2734–2740. doi: 10.1128/jcm.34.11.2734-2740.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bigi F, Romano M I, Alito A, Cataldi A. Cloning of a novel polymorphic GC rich repetitive DNA from Mycobacterium bovis. Res Microbiol. 1995;146:341–348. doi: 10.1016/0923-2508(96)81057-6. [DOI] [PubMed] [Google Scholar]
- 3.Blázquez J, Espinosa de Los Monteros L E, Samper S, Martín C, Guerrero A, Cobo J, van Embden J, Baquero F, Gómez-Mampaso E. Genetic characterization of multidrug-resistant Mycobacterium bovis strains from a hospital outbreak involving human immunodeficiency virus-positive patients. J Clin Microbiol. 1997;35:1390–1393. doi: 10.1128/jcm.35.6.1390-1393.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cousins D V, Williams S N, Liébana E, Aranaz A, Bunschoten A, van Embden J, Ellis T. Evaluation of four DNA typing techniques in epidemiological investigations of bovine tuberculosis. J Clin Microbiol. 1998;36:168–178. doi: 10.1128/jcm.36.1.168-178.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cousins D V, Williams S N, Ross B C, Ellis T M. Use of a repetitive element isolated from Mycobacterium tuberculosis in hybridization studies with Mycobacterium bovis: a new tool for epidemiological studies of bovine tuberculosis. Vet Microbiol. 1993;37:1–7. doi: 10.1016/0378-1135(93)90178-a. [DOI] [PubMed] [Google Scholar]
- 6.de Kantor I N, Ritacco V. Bovine tuberculosis in Latin America and the Caribbean: current status, control and eradication programs. Vet Microbiol. 1994;40:5–14. doi: 10.1016/0378-1135(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 7.Doran T J, Hodgson A L M, Davies J K, Radford A J. Characterisation of a highly repeated DNA sequence from Mycobacterium bovis. FEMS Microbiol Lett. 1993;111:147–152. doi: 10.1111/j.1574-6968.1993.tb06377.x. [DOI] [PubMed] [Google Scholar]
- 8.Fisanotti J C, Alito A, Bigi F, Latini O, Roxo E, Cicuta E, Zumarraga M, Cataldi A, Romano M I. Molecular epidemiology of Mycobacterium bovis isolates from South America. Vet Microbiol. 1998;60:251–257. doi: 10.1016/s0378-1135(98)00150-3. [DOI] [PubMed] [Google Scholar]
- 9.Goguet de la Salmonière Y O, Minh Li H, Torrea G, Bunschoten A, van Embden J, Gicquel B. Evaluation of spoligotyping in a study of the transmission of Mycobacterium tuberculosis. J Clin Microbiol. 1997;35:2210–2214. doi: 10.1128/jcm.35.9.2210-2214.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Groenen P M A, Bunschoten A E, van Soolingen D, van Embden J D A. Nature of DNA polymorphism in the direct repeat cluster of Mycobacterium tuberculosis: application of strain differentiation by a novel typing method. Mol Microbiol. 1993;10:1057–1065. doi: 10.1111/j.1365-2958.1993.tb00976.x. [DOI] [PubMed] [Google Scholar]
- 11.Gutiérrez M, Samper S, Gavigan J A, García-Marín J F, Martín C. Differentiation by molecular typing of Mycobacterium bovis strains causing tuberculosis in cattle and goats. J Clin Microbiol. 1995;33:2953–2956. doi: 10.1128/jcm.33.11.2953-2956.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gutiérrez M, Samper S, Jiménez M S, van Embden J D A, García Marín J F, Martín C. Identification by spoligotyping of a caprine genotype in Mycobacterium bovis strains causing human tuberculosis. J Clin Microbiol. 1997;35:3328–3330. doi: 10.1128/jcm.35.12.3328-3330.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hermans P W M, Martín C, McAdam R, Shinnick T M, Small P M. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol. 1992;31:406–409. doi: 10.1128/jcm.31.2.406-409.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hermans P W M, van Soolingen D, Bik E M, de Haas P E W, Dale J W, van Embden J D A. The insertion element IS987 from Mycobacterium bovis BCG is located in a hot-spot integration region for insertion elements in Mycobacterium tuberculosis complex strains. Infect Immun. 1991;59:2695–2705. doi: 10.1128/iai.59.8.2695-2705.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamerbeek J, Schouls L, Kolk A, van Agterveld M, van Soolingen D, Kuijper S, Bunschoten A, Molhuizen H, Shaw R, Goyal M, van Embden J D A. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol. 1997;35:907–914. doi: 10.1128/jcm.35.4.907-914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Latini M S, Latini O A, Lopez M L, Cecconi J O. Tuberculosis bovina en seres humanos. Rev Argent Torax. 1990;51:13–16. [Google Scholar]
- 17.Liébana E, Aranaz A, Dominguez L, Mateos A, González-Llamazares O, Rodriguez-Ferri E F, Domingo M, Vidal D, Cousins D. The insertion element IS6110 is a useful tool for DNA fingerprinting of Mycobacterium bovis isolates from cattles and goats in Spain. Vet Microbiol. 1997;54:223–233. doi: 10.1016/s0378-1135(96)01282-5. [DOI] [PubMed] [Google Scholar]
- 18.Romano M I, Alito A, Fisanotti J C, Bigi F, De Kantor I, Cicuta M E, Cataldi A. Comparison of different genetic markers for molecular epidemiology of bovine tuberculosis. Vet Microbiol. 1996;50:59–71. doi: 10.1016/0378-1135(95)00197-2. [DOI] [PubMed] [Google Scholar]
- 19.Ross B C, Raios K, Jackson K, Dwyer B. Molecular cloning of a highly repeated DNA element from Mycobacterium tuberculosis and its use as an epidemiological tool. J Clin Microbiol. 1992;30:942–946. doi: 10.1128/jcm.30.4.942-946.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samper S, Martín C, Pinedo A, Rivero A, Blázquez J, Baquero F, van Soolingen D, van Embden J D A. Transmission between HIV-infected patients of multidrug-resistant tuberculosis caused by Mycobacterium bovis. AIDS. 1997;11:1237–1242. doi: 10.1097/00002030-199710000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Skuce R A, Brittain D, Hughes M S, Beck L A, Neill S D. Genomic fingerprinting of Mycobacterium bovis from cattle by restriction fragment length polymorphism analysis. J Clin Microbiol. 1994;32:2387–2392. doi: 10.1128/jcm.32.10.2387-2392.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skuce R A, Brittain D, Hughes M S, Neil S D. Differentiation of Mycobacterium bovis isolates from animals by DNA typing. J Clin Microbiol. 1996;34:2469–2474. doi: 10.1128/jcm.34.10.2469-2474.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szewzyk R, Svenson S B, Hoffner S E, Bolske G, Wahlstrom H, Englund L, Engvall A, Kallenius G. Molecular epidemiological studies of Mycobacterium bovis infection in humans and animals in Sweden. J Clin Microbiol. 1995;33:3183–3185. doi: 10.1128/jcm.33.12.3183-3185.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Soolingen D, de Haas P E W, Haagsma J, Eger T, Hermans P W M, Ritacco V, Alito A, van Embden J D A. Use of various genetic markers in differentiation of Mycobacterium bovis strains from animals and humans and for studying epidemiology of bovine tuberculosis. J Clin Microbiol. 1994;32:2425–2433. doi: 10.1128/jcm.32.10.2425-2433.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Soolingen D, de Haas P E W, Hermans P W M, Groenen P M A, van Embden J D A. Comparison of various repetitive DNA elements as genetic markers for strain differentiation and epidemiology of Mycobacterium tuberculosis. J Clin Microbiol. 1993;31:1987–1995. doi: 10.1128/jcm.31.8.1987-1995.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Soolingen D, Hermans P W M, de Haas P E W, Soll D R, van Embden J D A. Occurrence and stability of insertion sequences in Mycobacterium tuberculosis complex strains: evaluation of a insertion sequence-dependent DNA polymorphism as a tool in the epidemiology of tuberculosis. J Clin Microbiol. 1991;29:2578–2586. doi: 10.1128/jcm.29.11.2578-2586.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Soolingen D, Qian L, de Haas P E W, Douglas J T, Traore H, Portaels F, Qing H Z, Enkhsaikan D, Nymadawa P, van Embden J D A. Predominance of a single genotype of Mycobacterium tuberculosis in countries of East Asia. J Clin Microbiol. 1995;33:3234–3238. doi: 10.1128/jcm.33.12.3234-3238.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]