Abstract
Aim
To describe the nosocomial transmission of Air, multidrug-resistant, Acinetobacter baumannii, nosocomial, COVID-19 Acinetobacter baumannii (MRAB) in an open-cubicle neurology ward with low ceiling height, where MRAB isolates collected from air, commonly shared items, non-reachable high-level surfaces and patients were analysed epidemiologically and genetically by whole-genome sequencing. This is the first study to understand the genetic relatedness of air, environmental and clinical isolates of MRAB in the outbreak setting.
Findings
Of 11 highly care-dependent patients with 363 MRAB colonization days during COVID-19 pandemic, 10 (90.9%) and nine (81.8%) had cutaneous and gastrointestinal colonization, respectively. Of 160 environmental and air samples, 31 (19.4%) were MRAB-positive. The proportion of MRAB-contaminated commonly shared items was significantly lower in cohort than in non-cohort patient care (0/10, 0% vs 12/18, 66.7%; P<0.001). Air dispersal of MRAB was consistently detected during but not before diaper change in the cohort cubicle by 25-min air sampling (4/4,100% vs 0/4, 0%; P=0.029). The settle plate method revealed MRAB in two samples during diaper change. The proportion of MRAB-contaminated exhaust air grills was significantly higher when the cohort cubicle was occupied by six MRAB patients than when fewer than six patients were cared for in the cubicle (5/9, 55.6% vs 0/18, 0%; P=0.002). The proportion of MRAB-contaminated non-reachable high-level surfaces was also significantly higher when there were three or more MRAB patients in the cohort cubicle (8/31, 25.8% vs 0/24, 0%; P=0.016). Whole-genome sequencing revealed clonality of air, environment, and patients' isolates, suggestive of air dispersal of MRAB.
Conclusions
Our findings support the view that patient cohorting in enclosed cubicles with partitions and a closed door is preferred if single rooms are not available.
Keywords: Air, multidrug-resistant, Acinetobacter baumannii, nosocomial, COVID-19
Introduction
Nosocomial transmission of multidrug Acinetobacter baumannii (MRAB) has been increasingly recognized as a threat to healthcare facilities [[1], [2], [3], [4], [5], [6]]. However, the outbreak of coronavirus disease 2019 (COVID-19) has posed further challenge to the control of multidrug-resistant organisms (MDROs) in hospitals. Nosocomial outbreaks of MDROs such as carbapenem-resistant A. baumannii have been reported during the COVID-19 pandemic [[7], [8], [9], [10]]. In Hong Kong, we adopted a hospital-based approach to control COVID-19. By admitting all newly diagnosed cases to airborne infection isolation rooms (AIIRs) in public hospitals, the risk of COVID-19 outbreak in the community as well as in hospital settings are both minimized [11,12]. However, because the priority use of isolation facilities was given to patients with clinical suspicion of COVID-19, the control of MDROs may be jeopardized. Here, we described two clusters of nosocomial MRAB transmission in a medical neurology ward, a conventionally designed general ward with open cubicles during the COVID-19 pandemic. We performed thorough environmental surveillance, including air sampling in patient cubicles and surface swabbing of frequently touched areas and non-reachable surfaces at high levels, such as the exhaust air grills in the ceiling. Whole-genome sequencing was performed to understand the epidemiological relatedness of patients, air and environmental isolates of MRAB, which has important implications for infection control and prevention.
Methods
Epidemiological investigation and control of the nosocomial transmission of MRAB
We described two clusters of nosocomial transmission of MRAB in a medical neurology ward, Queen Mary Hospital, a university-affiliated hospital in which universal admission screening for multidrug-resistant organisms, including vancomycin-resistant Enterococci (VRE), carbapenem-resistant and carbapenemase-producing Enterobacteriaceae (CRE/CPE), and MRAB are in place [[13], [14], [15]]. MRAB is defined as a strain of A. baumannii demonstrating in vitro resistance to all routinely tested antimicrobial agents (including ampicillin-sulbactam, piperacillin, piperacillin-tazobactam, ticarcillin-clavulanate, cefoperazone-sulbactam, ceftazidime, ciprofloxacin, gentamicin, amikacin, tobramycin, cotrimoxazole and imipenem-cilastatin), as previously described [16]. Infection control nurses would screen microbiology laboratory reports for any new cases of MRAB and advise the wards regarding cohort nursing and environmental disinfection. Active surveillance culture (weekly plus at day 14 of hospitalization) was performed in all patients during and after the study period (until the discharge of the last patient). Contact tracing was performed for all hospitalized patients to identify any potential secondary cases. Screening specimens routinely included nasal swab, axillary and groin swabs, and rectal swab; sputum, tracheal aspirate, catheterized urine, drain fluid and wound swabs were collected if available. MRAB colonization days, defined as the number of MRAB-positive patient-days in the wards, was recorded. Cohort nursing with contact precautions and twice-daily environmental disinfection by chlorine dioxide solution 125 ppm (Tristel Solutions, NY, USA) were implemented in a designated cubicle of the ward. Staff put on personal protective equipment (PPE) outside the cubicle and removed the PPE inside the cubicle, and practised hand hygiene immediately after patient care.
Collection of environmental and air samples for MRAB culture
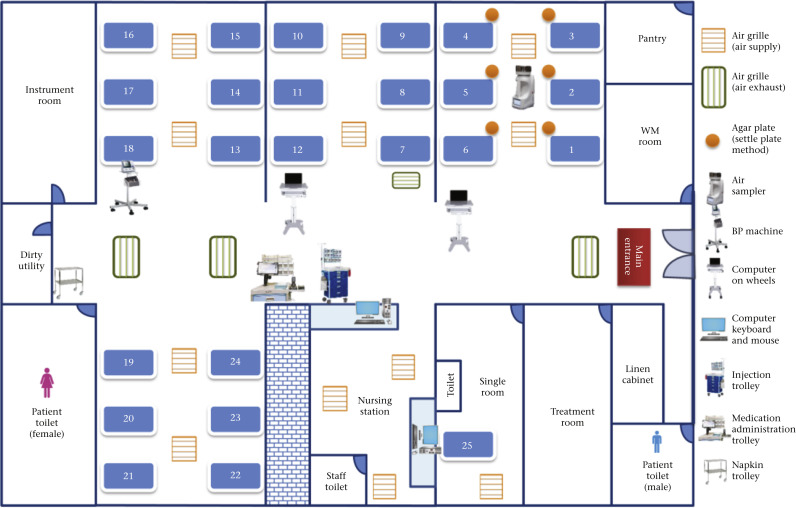
Nine frequently touched and commonly shared items or surfaces including trolley for intravenous medication administration set, cart for changing diapers, blood pressure monitoring machine and cuff, computer on wheels A and B, tablet for inpatient medication order entry, table/desk, computer keyboard, and mouse in nurse station were sampled (Figure 1 ). Three high-level non-reachable surfaces including the top surface of the vital sign monitoring screen hanging at the upper wall, walls (area of A3-paper size in landscape orientation) just below the ceiling, and exhaust air grills in the ceiling were also sampled. Flexible premoistened sterile polywipe sponge swabs of 5 × 10 cm in size (Medical Wire & Equipment, Corsham, UK) were used for environmental sampling as previously described [[17], [18], [19]].
Figure 1.
Floor plan of the medical neurology ward to illustrate the cohort area for multidrug-resistant Acinetobacter baumannii and collection of air samples and environmental samples. The ward is an open cubicle design with ceiling height of 2.2 m. The air supply was from the patient cubicle and the air exhaust was located in the corridor. The number of air changes per hour was six. Air samples were collected by the Sartorius MD8 airscan sampling device (Sartorius AG, Germany) with sterile gelatin filters. An agar plate was placed at the bedside before and during diaper changing. The items for environmental sampling are also shown.
Air samples were collected by the Sartorius MD8 airscan sampling device (Sartorius AG, Germany) with sterile gelatin filters: 80 mm in diameter and 3-μm pore size (type 17528-80-ACD) (Sartorius AG, Germany) as described previously with minor modifications [19,20]. Briefly, the air sampler was placed at the centre of the cohort cubicle for patients colonized or infected with MRAB. One thousand litres of air at a rate of 40 L/min were collected by a gelatin filter for the first 25 min before changing a diaper, and another 1000 L of air were collected by another gelatin filter for the next 25 min during changing of the diaper. In addition, MacConkey agar with meropenem (2 μg/mL) was used as settle plates [21] for 25 min and 4-h exposure, according to different manoeuvers of air sample collection (Table 1 ).
Table I.
Collection of environmental and air samples for multidrug resistant Acinetobacter baumannii (MRAB) in the medical neurology ward
| Type of samples | No. of sample | No. (%) of positive | Date (daya) of collection | No. of MRAB- positive patients during sampling | Remark |
|---|---|---|---|---|---|
| Frequently touched items by sponge swab | 9 | 3 (33.3%) | 3rd Jul 2020 (5) | 1 | Environmental sampling was initiated due to lab report of the 1st MRAB patient issued on 2nd Jul |
| 9 | 9 (100%) | 2nd Sep 2020 (66) | 1 | Associated with subsequent diagnosis of 5 MRAB patients in the same ward | |
| 9 | 0 (0%) | 11th Sep 2020 (75) | 6 | Post-disinfection sample collection as an audit of environmental cleaning | |
| Subtotal | 27 | 12 (44.4%) | |||
| Air sample by air sampler | 1 | 0 (0%) | 14th Sep 2020 (78) | 6 | Cubicle centre: 30 min before diaper change |
| 1 | 1 (100%) | 14th Sep 2020 (78) | 6 | Cubicle centre: during diaper change | |
| 1 | 0 (0%) | 17th Sep 2020 (81) | 6 | Cubicle centre: 30 min before diaper change | |
| 1 | 1 (100%) | 17th Sep 2020 (81) | 6 | Cubicle centre: during diaper change | |
| 1 | 0 (0%) | 18th Sep 2020 (82) | 6 | Cubicle centre: 30 min before diaper change | |
| 1 | 1 (100%) | 18th Sep 2020 (82) | 6 | Cubicle centre: during diaper change | |
| 1 | 0 (0%) | 23rd Sep 2020 (87) | 6 | Cubicle centre: 30 min before diaper change | |
| 1 | 1 (100%) | 23rd Sep 2020 (87) | 6 | Cubicle centre: during diaper change | |
| Subtotal | 8 | 4 (50%) | |||
| Air sample by settle plateb | 6 | 0 (0%) | 23rd Sep 2020 (87) | 6 | At beside table: 30 min before diaper change |
| 6 | 0 (0%) | 23rd Sep 2020 (87) | 6 | At bedside table: during diaper change | |
| 5 | 0 (0%) | 25th Sep 2020 (89) | 6 | At beside table: 30 min before diaper change | |
| 5 | 2 (40%) | 25th Sep 2020 (89) | 6 | At bedside table: during diaper change | |
| 6 | 0 (0%) | 30th Sep 2020 (94) | 5 | At bedside table: no patient care in daytime | |
| 14 | 0 (0%) | 30th Sep 2020 (94) | 5 | Inverted and adhered in ward ceiling for 4 h | |
| Subtotal | 42 | 2 (4.8%) | |||
| Exhaust air grills by sponge swab | 3 | 3 (100%) | Sep 14, 2020 (78) | 6 | All 3 exhaust air grills in ward were positive |
| 3 | 1 (33.3%) | 23rd Sep 2020 (87) | 6 | Close proximity to cohort cubicle was positive | |
| 3 | 1 (33.3%) | 25th Sep 2020 (89) | 6 | Close proximity to cohort cubicle was positive | |
| 3 | 1 (33.3%) | 20th Oct 2020 (114) | 3 | Close proximity to cohort cubicle was positive | |
| 3 | 0 (0%) | 23rd Oct 2020 (117) | 3 | ||
| 3 | 0 (0%) | 27th Oct 2020 (121) | 3 | ||
| 3 | 0 (0%) | 3rd Nov 2020 (128) | 2 | ||
| 3 | 0 (0%) | 17th Nov 2020 (142) | 1 | ||
| 3 | 0 (0%) | 24th Nov 2020 (149) | 1 | ||
| Subtotal | 27 | 6 (22.2%) | |||
| Non-reachable surfaces at high levels by sponge swab | 8 | 5 (62.5%) | Oct 20, 2020 (114) | 3 | Pre-disinfection |
| 8 | 2 (25%) | 20th Oct 2020 (114) | 3 | 4 h post-disinfection | |
| 8 | 1 (12.5%) | 23rd Oct 2020 (117) | 3 | Pre-disinfection | |
| 8 | 0 (0%) | 27th Oct 2020 (121) | 3 | Pre-disinfection | |
| 8 | 0 (0%) | 3rd Nov 2020 (128) | 2 | Pre-disinfection | |
| 8 | 0 (0%) | 17th Nov 2020 (142) | 1 | Pre-disinfection | |
| 8 | 0 (0%) | 24th Nov 2020 (149) | 1 | Pre-disinfection | |
| Subtotal | 56 | 8 (14.3%) |
The number of days after the first MRAB patient diagnosed in the ward. Day 1 was denoted as 29th June 2020: the date of specimen collection with positive MRAB culture.
The exposure time for settle plate was 25 minutes unless specified in the remark.
Laboratory investigation and whole-genome sequencing
Patients and environmental specimens were incubated in 2 mL of brain heart infusion enrichment broth with 10 μg/mL vancomycin (Sigma-Aldrich, St Louis, MO, USA) and 0.5 μg/mL meropenem (Hospira, Melbourne, Australia) at 35°C for 18 h. Ten microlitres of the enriched broth were subcultured on to MacConkey agar with 2 μg/mL meropenem for further incubation at 35°C for 48 h in air. Acinetobacter species were identified by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (Bruker Daltonics, Bremen, Germany). Antimicrobial susceptibility tests were performed using the Kirby–Bauer disk diffusion method according to the Clinical and Laboratory Standards Institute recommendations, or manufacturer's instructions. Whole-genome sequencing of 10 patient isolates, 11 environmental isolates and two clinical reference strains was performed. DNA extraction was performed using the Qiagen DNeasy Blood and Tissue kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Library was prepared by the Nanopore native barcoding kit (SQK-LSK109) with a modified protocol [22]. Sequencing was performed on a Nanopore MinION device using an R9.4 flow cell. De novo assembly of raw data reads was performed using Canu version 1.9 and polished with racon version 1.4.10 and medaka 1.3.2. Whole-genome sequence alignment and maximum-likelihood whole-genome phylogenetic tree were performed by the CLC Genomics Workbench version 21.3 (Qiagen, Hilden, Germany) and the IQ-Tree 1.6.12. This study was approved by the Institutional Review Board of The University of Hong Kong/Hospital Authority Hong Kong West Hospital Cluster.
Statistical analysis
χ2-test, Fisher's exact test, and t-test were used as appropriate. A P-value of <0.05 was considered statistically significant.
Results
Epidemiological investigation and control of the nosocomial transmission of MRAB
A total of 11 patients were involved in two clusters of nosocomial transmission of MRAB in the mixed-gender medical neurology ward between 29th June 2020 and 26th September 2020 (study period) (Supplementary Table S1). Cases 1 and 2 were epidemiologically linked, whereas cases 3–11 belonged to another cluster. All patients were highly care-dependent with prolonged hospitalization (median: 51 days, range: 18–158). The total and median (range) MRAB colonization days was 363 and 21 (range, 4–102), respectively. Among 11 MRAB patients, colonization rate of axilla and groin was 90.9%, whereas colonization rates in rectal and respiratory specimens (sputum or endotracheal aspirate) were both 81.8%. Seven (63.6%) of 11 MRAB patients had MRAB colonization in three or more body sites. Patients with MRAB colonization of three or more body sites had significantly higher numbers of MRAB colonization days (mean ± standard deviation) than those with two or fewer body sites (48.1 ± 29.1 vs 6.5 ± 2.3, P=0.029). A total of 158 patients were actively screened for MRAB until the discharge of the last patient on 11th February 2021. The MRAB attack rate was 7.0% (11/158 patients).
Environmental and air samples for MRAB culture
A total of 160 environmental and air samples were prospectively collected from 3rd July 2020 to 24th November 2020 (eight weeks after the diagnosis of the last MRAB patient) (Table I), including 27 (16.9%) samples from frequently touched commonly shared items or surfaces, 56 (35.0%) from high-level non-reachable surfaces, 27 (16.9%) exhaust air grills (30 × 60 cm) in the ceiling (2.2 m), and 50 (31.3%) air samples. MRAB was isolated in 31 (19.4%) samples. When there was only one unrecognized MRAB patient (case 1 and subsequently case 3) in the ward, the extent of contamination in frequently touched commonly shared items or surfaces was 33.3% (3rd July 2020) and 100% (2nd September 2020). Cohort nursing of six MRAB patients in a designated cubicle reduced contamination of commonly shared items or surfaces to zero (11th September 2020, 0/10, 0% vs 12/18 in non-cohort patient care, 66.7%; P<0.001) (Figure 1). In the cohort cubicle, dispersal of MRAB in air was consistently detected during but not before diaper change during the 25-min air-sampling time (4/4, 100% vs 0/4, 0%; P=0.029). Settle plate method only revealed MRAB in air in two (4.8%) of 42 samples, and these two positive samples were obtained during diaper change. Of 83 sponge swabs collected in exhaust air grills and high-level non-reachable surfaces, 14 (16.9%) of 83 were contaminated by MRAB, including six (22.2%) of 27 samples from exhaust air grills and eight (14.3%) of 56 samples from high-level non-reachable surfaces. The proportion of MRAB contamination in exhaust air grills was significantly higher when the cohort cubicle was full (six MRAB patients) than when it housed fewer than six patients (5/9, 55.6% vs 0/18, 0%; P=0.002). The proportion of MRAB contamination in the non-reachable surfaces was also significantly higher when the cohort cubicle housed three or more MRAB patients (8/31, 25.8% vs 0/24, 0%; P=0.016).
Whole-genome sequencing
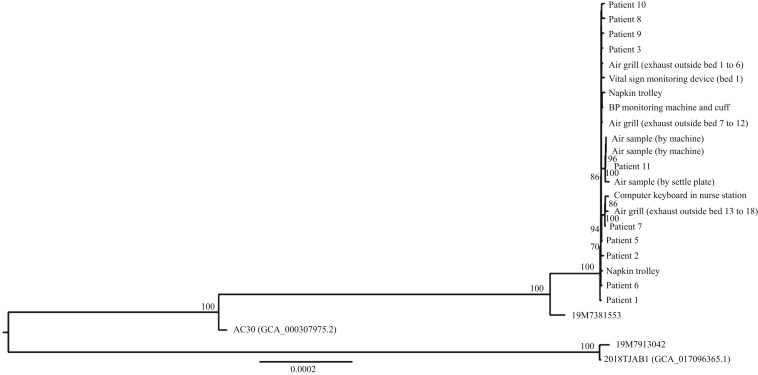
Among the 11 MRAB patients, whole-genome chromosomal DNA sequence of MRAB could be retrieved from 10 patient isolates. The maximum likelihood tree included 10 patient MRAB strains, eight environmental isolates and three air isolates. We included two other clinical MRAB reference strains isolated in 2019 and two MRAB genome sequences from GenBank as the outgroup of the tree (Figure 2 ). From the maximum likelihood tree, the sequences of the MRAB strains isolated from patients, environmental and air samples clustered together, suggesting that all patient and environmental isolates shared the same origin with epidemiological linkage.
Figure 2.
Whole-genome phylogenetic analysis of 25 multidrug-resistant Acinetobacter baumannii (MRAB) genomes showing the relationship between the patients in a medical neurology ward and the ward environment. The tree was constructed by maximum likelihood method with IQ-Tree. The 2018TJAB1 MRAB strain (GenBank assembly accession number GCA_017096365.1) was used as the root of the tree. The substitution model TPM3u+I was used.
Discussion
Air dispersal of A. baummanii was previously investigated in clinical settings (Table II ) [[22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34]], mainly focusing on the dissemination of carbapenem-susceptible and carbapenem-resistant A. baummanii in intensive care units. In contrast to the previous studies using pulse field gel electrophoresis, repetitive extragenic palindromic sequence polymerase chain reaction, and multi-locus sequence typing, this is the first study using whole-genome sequencing to understand the genetic relatedness of air, environmental and clinical isolates of MRAB in the outbreak setting.
Table II.
Literature review on air sampling for Acinetobacter baumannii in the clinical setting
| No. | Country (setting) | Study period [year of publication] | Resistant pattern | Air sampling method (by machine, M, or settle plate, SP) | No. of patientsa | No. (%) of air sample +ve for A. baumannii | Typingb [relatedness of clinical & air samples] | Ref |
|---|---|---|---|---|---|---|---|---|
| 1 | Argentina (ICU) | Jul–Sep 2003 [2008] | CSAB, CRAB | M: AF 11, T 20 min M: AF 250, T 4 min SP: NS |
NM | 4 (7.4%)/54 | PFGE [NM] | [23] |
| 2 | US (ICU) | Mar–Apr 2012 [2013] | CSAB, CRAB | SP: T 24–48 h; at head of bed ∼ 2.25 m from the floor |
53 | 12 (22.6%)/53 | PFGE [clonally related] | [24] |
| 3 | China (ward) | Jan–Jun 2011 [2014] | CRAB | M: AF 28.3, T 10 min; at ward centre & corridor ∼ 1.5 m from the floor | NM | 16 [majority of air samples were negative] | REP-PCR [NM] | [25] |
| 4 | US (ICU) | Mar–Jul 2013 [2015] | CRAB | SP: T 24 h; ∼ 90 cm from headboard & within 90 cm of roof tile | 30 | NS [positive air sample ∼ 21% of study days] | REP-PCR [4 of 6 air/clinical isolate pairs closely related] | [26] |
| 5 | US (ICU) | May–Dec 2013 [2015] | MRABc | M: AF 28.3, T 1 h; 3 feet from head of bed | 12 | 1 (8.3%)/12 | ND | [27] |
| 6 | US (ICU) | Oct 2013 to Feb 2014 [2016] | CRAB | SP: T 24 h; ∼ 2 feet from headboards & 2 feet from roof tiles | 25 | 36 (19.6%)/184 | ND | [28] |
| 7 | Thailand (ward) | Jan 2015 [2016] | CRAB | SP: T 6 h; vicinity at bedside & 1 m from the floor | 434d | 0e | NA | [29] |
| 8 | Turkey (ICU) | NM [2016] | CSAB, CRAB |
M: AF NM, T NM; from 4 defined areas in ICU & ∼ 1 m, 2 m, and 3 m from bed | NM | 26 (13.9%)/186f | REP-PCR [18 air strains clonally related to the clinical strains of 9 patients] | [30] |
| 9 | Ethiopia (ICU, OT, DR) | Dec 2015 to Apr 2016 [2017] | CSAB, CRAB | M: AF 28.3, T 5 min SP: NS |
NM | 43 (19.9%)/216 | ND | [31] |
| 10 | Iran (ICU, OT ward)g | Apr 2014 to Apr 2015 [2017] | CRAB | M: AF 10; T 4 h; ∼ 1.5 m from the floor |
NM | 7 (10.9%)/64 | ND | [32] |
| 11 | China (ICU, ward) | May–Nov 2014 [2018]h | CRAB | M: AF 28.3, T 20 min; at ward centre & corridor ∼ 1.5 m from the floor | NM | 4 (6.3%)/64 | PFGE & MLST [1 air & 1 clinical strain with same PFGE & MLST pattern] | [33] |
| 12 | Israel (ICU, ward) | Sep–Dec 2016 [2019] | CRAB | M: AF 60, T 30 min; i ∼ 1.5 m from patient's head at a height of 1 m |
10 | 22 (15.7%)/140 | ND | [34] |
AF, air flow of air collection in terms of liter per minute by air sampling machine; CRAB, carbapenem-resistant A. baumannii; CSAB, carbapenem-susceptible A. baumannii; DR, delivery room; ICU, intensive care unit; MLST, multi-locus sequence typing; MRAB, Multidrug-resistant A. baumannii; NA, not applicable; ND, not done; NM, not mentioned; NS, not specified; OT, operating theatre; PFGE, pulse field gel electrophoresis; REP-PCR, repetitive extragenic palindromic sequence polymerase chain reaction; T, time of air collection per sample using either air sampling machine or settled plate.
i Air sampling was performed continuously for 7 h for each patient.
Number of patients subjected to the collection of air samples.
Whole-genome sequencing was not performed for all studies.
Multidrug-resistant A. baumannii was found in seven (58%) of 12 patients.
The median proportion of A. baumannii positive patients was 20% (range, 18–25%) in the unit during the study period. The denominator of air sample was not specified.
At each site, two settle plates were placed and a total of four plates were placed over a 24-h period. The denominator of air sample was not specified.
Of 186 air samples, 118 samples were collected from four defined areas (entrance, centre, corridor and nurse station) in ICU areas, and 68 samples were collected from patients' bedsides.
Air samples were collected from the intensive care units, operating theatres, medical, and surgical ward in four hospitals.
The strains of A. baumannii were collected from 12th May to 5th Jun and 11th October to 15th November 2014.
Our findings have important implications in MRAB infection control. The clonal relationship between the air, environmental and clinical isolates not only confirmed the extensive contamination of MRAB during outbreak [18,35], but the presence of air dispersal of MRAB during change of diaper deserves further investigation. 90% of our patients had gastrointestinal colonization of MRAB, thus it was not unexpected to detect MRAB in air samples when diapers were being changed sequentially within a short time. Alternatively, dispersal of MRAB in air may be related to the manipulation of the bed linen, as 80% of patients also carry MRAB on the skin and 1 million skin squamous cells containing viable micro-organisms are shed daily from normal skin [36]. In addition to the detection of MRAB in the air samples, environmental contamination of non-reachable surfaces further supports air dispersal of MRAB. Therefore, to prevent extensive environmental contamination and reduce the transmission of MDROs such as MRAB [37], single-room isolation is strongly recommended.
However, the availability of single-room isolation facilities may be limited in acute hospitals, especially during the COVID-19 pandemic. In Hong Kong, patients with suspected or confirmed COVID-19 were given priority of admission to isolation facilities [11,12]. As illustrated in our case, nosocomial transmission of MRAB occurred readily in a conventionally designed general ward with low ceiling height and open cubicles, where the direction of airflow was from the patient cubicle to the corridor. The same ward design has been shown to contribute to the nosocomial outbreak of COVID-19 by possible airborne transmission from an unrecognized index patient [38]. When we performed environmental sampling of the exhaust air grills, all three exhaust air grills in the corridor were positive for MRAB. This may explain the extensive contamination of the commonly shared items and surfaces in the ward at the onset of this outbreak. Although hand hygiene of healthcare workers remained an important confounding factor for environmental contamination and nosocomial transmission of MRAB, this outbreak occurred during the COVID-19 pandemic, where hand hygiene compliance among healthcare workers increased [39].
When the MRAB patients were cohorted in a designated cubicle, the extent of air dispersal and environmental contamination of the non-reachable surfaces inside the cubicle was significantly increased with the increasing number of MRAB patients during the cohort period. Increasing the number of patients will increase the bacterial burden in the confined clinical area, as illustrated in patients with VRE colonization, where the extent of environmental contamination was associated with the number of positive body sites [40]. Chlorhexidine gluconate bathing may reduce the cutaneous colonization of MRAB [41]. In our patient cohort, the detection rates of MRAB in air and non-reachable surfaces gradually decreased after the initiation of daily chlorhexidine gluconate bathing during hospitalization for the MRAB patients.
There are several limitations to this study. We did not perform air sampling continuously during the cohort period and did not assess the extent of air dispersal of MRAB throughout the day. However, we believe diaper change is a high-risk procedure for air dispersal of MRAB. In addition, the different methods of air and environmental sampling were performed sequentially instead of simultaneously, which makes it difficult to compare the yield of different methods, especially when the number of MRAB patients decreased with time. Nevertheless, our findings emphasized the presence of extensive air dispersal of MRAB in a conventionally designed ward with low ceiling height. The lessons learned are that we may consider adding a portable high-efficiency particulate air filter to the conventionally designed open cubicle, especially when the cubicle is fully occupied by MRAB patients. More importantly, the setting of an air ventilation system with a self-contained air inflow and exhaust within a cubicle, and preferably with the door closed, should be included in the hospital renovation and redevelopment programme.
Acknowledgements
We thank our frontline staff in Department of Medicine, Queen Mary Hospital, Hospital Authority for their help in facilitating this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhin.2021.08.005.
Conflict of interest statement
All authors report no conflicts of interest relevant to this article.
Funding sources
This study was supported by the Health and Medical Research Fund (HMRF) Commissioned Research on Control of Infectious Disease (Phase IV), CID-HKU1-16, Food and Health Bureau, Hong Kong SAR Government.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Nasr P. Genetics, epidemiology, and clinical manifestations of multidrug-resistant Acinetobacter baumannii. J Hosp Infect. 2020 Jan;104(1):4–11. doi: 10.1016/j.jhin.2019.09.021. [DOI] [PubMed] [Google Scholar]
- 2.Barnaud G., Zihoune N., Ricard J.D., Hippeaux M.C., Eveillard M., Dreyfuss D. Two sequential outbreaks caused by multidrug-resistant Acinetobacter baumannii isolates producing OXA-58 or OXA-72 oxacillinase in an intensive care unit in France. J Hosp Infect. 2010 Dec;76(4):358–360. doi: 10.1016/j.jhin.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 3.Ansaldi F., Canepa P., Bassetti M., Zancolli M., Molinari M.P., Talamini A. Sequential outbreaks of multidrug-resistant Acinetobacter baumannii in intensive care units of a tertiary referral hospital in Italy: combined molecular approach for epidemiological investigation. J Hosp Infect. 2011 Oct;79(2):134–140. doi: 10.1016/j.jhin.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 4.Sengstock D.M., Thyagarajan R., Apalara J., Mira A., Chopra T., Kaye K.S. Multidrug-resistant Acinetobacter baumannii: an emerging pathogen among older adults in community hospitals and nursing homes. Clin Infect Dis. 2010 Jun 15;50(12):1611–1616. doi: 10.1086/652759. [DOI] [PubMed] [Google Scholar]
- 5.Mortensen E., Trivedi K.K., Rosenberg J., Cody S.H., Long J., Jensen B.J. Multidrug-resistant Acinetobacter baumannii infection, colonization, and transmission related to a long-term care facility providing subacute care. Infect Control Hosp Epidemiol. 2014 Apr;35(4):406–411. doi: 10.1086/675612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng V.C., Chen J.H., Poon R.W., Lee W.M., So S.Y., Wong S.C. Control of hospital endemicity of multiple-drug-resistant Acinetobacter baumannii ST457 with directly observed hand hygiene. Eur J Clin Microbiol Infect Dis. 2015 Apr;34(4):713–718. doi: 10.1007/s10096-014-2281-x. [DOI] [PubMed] [Google Scholar]
- 7.Perez S., Innes G.K., Walters M.S., Mehr J., Arias J., Greeley R. Increase in hospital-acquired carbapenem-resistant Acinetobacter baumannii infection and colonization in an acute care hospital during a surge in COVID-19 admissions – New Jersey, February–July 2020. MMWR Morb Mortal Wkly Rep. 2020 Dec 4;69(48):1827–1831. doi: 10.15585/mmwr.mm6948e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shinohara D.R., Dos Santos Saalfeld S.M., Martinez H.V., Altafini D.D., Costa B.B., Fedrigo N.H. Outbreak of endemic carbapenem-resistant Acinetobacter baumannii in a coronavirus disease 2019 (COVID-19)-specific intensive care unit. Infect Control Hosp Epidemiol. 2021 Mar 9:1–3. doi: 10.1017/ice.2021.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pascale R., Bussini L., Gaibani P., Bovo F., Fornaro G., Lombardo D. Carbapenem resistant bacteria in Intensive Care Unit during COVID-19 pandemic: multicenter before-after cross sectional study. Infect Control Hosp Epidemiol. 2021 Apr 16:1–25. doi: 10.1017/ice.2021.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai C.C., Chen S.Y., Ko W.C., Hsueh P.R. Increased antimicrobial resistance during the COVID-19 pandemic. Int J Antimicrob Agents. 2021 Apr;57(4):106324. doi: 10.1016/j.ijantimicag.2021.106324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng V.C.C., Wong S.C., Chuang V.W.M., So S.Y.C., Chen J.H.K., Sridhar S. Absence of nosocomial transmission of coronavirus disease 2019 (COVID-19) due to SARS-CoV-2 in the prepandemic phase in Hong Kong. Am J Infect Control. 2020 Aug;48(8):890–896. doi: 10.1016/j.ajic.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng V.C., Wong S.C., Tong D.W., Chuang V.W., Chen J.H., Lee L.L. Multipronged infection control strategy to achieve zero nosocomial coronavirus disease 2019 (COVID-19) cases among Hong Kong healthcare workers in the first 300 days of the pandemic. Infect Control Hosp Epidemiol. 2021 Mar 19:1–10. doi: 10.1017/ice.2021.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng V.C., Tai J.W., Chen J.H., So S.Y., Ng W.C., Hung I.F. Proactive infection control measures to prevent nosocomial transmission of vancomycin-resistant enterococci in Hong Kong. J Formos Med Assoc. 2014 Oct;113(10):734–741. doi: 10.1016/j.jfma.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Cheng V.C., Chan J.F., Wong S.C., Chen J.H., Tai J.W., Yan M.K. Proactive infection control measures to prevent nosocomial transmission of carbapenem-resistant Enterobacteriaceae in a non-endemic area. Chin Med J (Engl) 2013 Dec;126(23):4504–4509. [PubMed] [Google Scholar]
- 15.Wong S.C., Chan V.W.M., Lam G.K., AuYeung C.H., Leung E.Y., So S.Y. The use of multi-pronged screening strategy to understand the epidemiology of carbapenemase-producing Enterobacteriaceae in Hong Kong: transition from epidemic to endemic setting. Eur J Clin Microbiol Infect Dis. 2021 Sep;40(9):2017–2022. doi: 10.1007/s10096-021-04173-x. [DOI] [PubMed] [Google Scholar]
- 16.Cheng V.C., Chen J.H., So S.Y., Wong S.C., Yan M.K., Chau P.H. Use of fluoroquinolones is the single most important risk factor for the high bacterial load in patients with nasal and gastrointestinal colonization by multidrug-resistant Acinetobacter baumannii. Eur J Clin Microbiol Infect Dis. 2015 Dec;34(12):2359–2366. doi: 10.1007/s10096-015-2489-4. [DOI] [PubMed] [Google Scholar]
- 17.Cheng V.C.C., Chen J.H.K., Wong S.C.Y., Leung S.S.M., So S.Y.C., Lung D.C. Hospital outbreak of pulmonary and cutaneous zygomycosis due to contaminated linen items from substandard laundry. Clin Infect Dis. 2016 Mar 15;62(6):714–721. doi: 10.1093/cid/civ1006. [DOI] [PubMed] [Google Scholar]
- 18.Cheng V.C.C., Wong S.C., Chen J.H.K., So S.Y.C., Wong S.C.Y., Ho P.L. Control of multidrug-resistant Acinetobacter baumannii in Hong Kong: role of environmental surveillance in communal areas after a hospital outbreak. Am J Infect Control. 2018 Jan;46(1):60–66. doi: 10.1016/j.ajic.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 19.Cheng V.C., Wong S.C., Chan V.W., So S.Y., Chen J.H., Yip C.C. Air and environmental sampling for SARS-CoV-2 around hospitalized patients with coronavirus disease 2019 (COVID-19) Infect Control Hosp Epidemiol. 2020 Nov;41:1258–1265. doi: 10.1017/ice.2020.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong S.C., Yuen L.L., Chen J.H., Yuen K.Y., Cheng V.C. Infection control challenges in handling recurrent blockage of sewage pipes in isolation facility designated for COVID-19 patients. J Hosp Infect. 2021 Aug;114:187–189. doi: 10.1016/j.jhin.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng V.C., Lo W.K., Woo P.C., Chan S.B., Cheng S.W., Ho M. Polymicrobial outbreak of intermittent peritoneal dialysis peritonitis during external wall renovation at a dialysis center. Perit Dial Int. 2001 May-Jun;21(3):296–301. [PubMed] [Google Scholar]
- 22.Quick J. 2018. One-pot ligation protocol for Oxford Nanopore libraries. Protocols.io. Available at: [last accessed June 2021] [DOI] [Google Scholar]
- 23.Barbolla R.E., Centrón D., Maimone S., Rospide F., Salgueira C., Altclas J. Molecular epidemiology of Acinetobacter baumannii spread in an adult intensive care unit under an endemic setting. Am J Infect Control. 2008 Aug;36(6):444–452. doi: 10.1016/j.ajic.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 24.Munoz-Price L.S., Fajardo-Aquino Y., Arheart K.L., Cleary T., DePascale D., Pizano L. Aerosolization of Acinetobacter baumannii in a trauma ICU∗. Crit Care Med. 2013 Aug;41(8):1915–1918. doi: 10.1097/CCM.0b013e31828a39c0. [DOI] [PubMed] [Google Scholar]
- 25.Gao J., Zhao X., Bao Y., Ma R., Zhou Y., Li X. Antibiotic resistance and OXA-type carbapenemases-encoding genes in airborne Acinetobacter baumannii isolated from burn wards. Burns. 2014 Mar;40(2):295–299. doi: 10.1016/j.burns.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Shimose L.A., Doi Y., Bonomo R.A., De Pascale D., Viau R.A., Cleary T. Contamination of ambient air with Acinetobacter baumannii on consecutive inpatient days. J Clin Microbiol. 2015 Jul;53(7):2346–2348. doi: 10.1128/JCM.00198-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rock C., Harris A.D., Johnson J.K., Bischoff W.E., Thom K.A. Infrequent air contamination with Acinetobacter baumannii of air surrounding known colonized or infected patients. Infect Control Hosp Epidemiol. 2015 Jul;36(7):830–832. doi: 10.1017/ice.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimose L.A., Masuda E., Sfeir M., Berbel Caban A., Bueno M.X., dePascale D. Carbapenem-resistant Acinetobacter baumannii: concomitant contamination of air and environmental surfaces. Infect Control Hosp Epidemiol. 2016 Jul;37(7):777–781. doi: 10.1017/ice.2016.69. [DOI] [PubMed] [Google Scholar]
- 29.Apisarnthanarak A., Tantajina P., Laovachirasuwan P., Weber D.J., Singh N. Is aerosalization a problem with carbapenem-resistant Acinetobacter baumannii in a Thailand Hospital? Open Forum Infect Dis. 2016 Jun 20;3(3):ofw124. doi: 10.1093/ofid/ofw124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yakupogullari Y., Otlu B., Ersoy Y., Kuzucu C., Bayindir Y., Kayabas U. Is airborne transmission of Acinetobacter baumannii possible: a prospective molecular epidemiologic study in a tertiary care hospital. Am J Infect Control. 2016 Dec 1;44(12):1595–1599. doi: 10.1016/j.ajic.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 31.Solomon F.B., Wadilo F., Tufa E.G., Mitiku M. Extended spectrum and metalo beta-lactamase producing airborne Pseudomonas aeruginosa and Acinetobacter baumanii in restricted settings of a referral hospital: a neglected condition. Antimicrob Resist Infect Control. 2017 Oct 23;6:106. doi: 10.1186/s13756-017-0266-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shamsizadeh Z., Nikaeen M., Nasr Esfahani B., Mirhoseini S.H., Hatamzadeh M., Hassanzadeh A. Detection of antibiotic resistant Acinetobacter baumannii in various hospital environments: potential sources for transmission of Acinetobacter infections. Environ Health Prev Med. 2017 May 8;22(1):44. doi: 10.1186/s12199-017-0653-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang M., Mu Y., Li N., Zhang Z., Han S. Carbapenem-resistant Acinetobacter baumannii from air and patients of intensive care units. Pol J Microbiol. 2018;67(3):333–338. doi: 10.21307/pjm-2018-040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mousa M., Schwartz D., Carmeli Y., Nutman A. Droplet aerosol dissemination of carbapenem-resistant Acinetobacter baumannii surrounding ventilated patients. Infect Control Hosp Epidemiol. 2019 Mar;40(3):365–367. doi: 10.1017/ice.2018.335. [DOI] [PubMed] [Google Scholar]
- 35.Enoch D.A., Summers C., Brown N.M., Moore L., Gillham M.I., Burnstein R.M. Investigation and management of an outbreak of multidrug-carbapenem-resistant Acinetobacter baumannii in Cambridge, UK. J Hosp Infect. 2008 Oct;70(2):109–118. doi: 10.1016/j.jhin.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 36.Pittet D., Allegranzi B., Sax H., Dharan S., Pessoa-Silva C.L., Donaldson L. Evidence-based model for hand transmission during patient care and the role of improved practices. Lancet Infect Dis. 2006 Oct;6(10):641–652. doi: 10.1016/S1473-3099(06)70600-4. [DOI] [PubMed] [Google Scholar]
- 37.Tacconelli E., Cataldo M.A., Dancer S.J., De Angelis G., Falcone M., Frank U. ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant Gram-negative bacteria in hospitalized patients. Clin Microbiol Infect. 2014 Jan;20(Suppl 1):1–55. doi: 10.1111/1469-0691.12427. [DOI] [PubMed] [Google Scholar]
- 38.Cheng V.C., Fung K.S., Siu G.K., Wong S.C., Cheng L.S., Wong M.S. Nosocomial outbreak of COVID-19 by possible airborne transmission leading to a superspreading event. Clin Infect Dis. 2021 Apr 14 doi: 10.1093/cid/ciab313. ciab313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong S.C., AuYeung C.H., Lam G.K., Leung E.Y., Chan V.W., Yuen K.Y. Is it possible to achieve 100 percent hand hygiene compliance during the coronavirus disease 2019 (COVID-19) pandemic? J Hosp Infect. 2020 Aug;105(4):779–781. doi: 10.1016/j.jhin.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonten M.J., Hayden M.K., Nathan C., van Voorhis J., Matushek M., Slaughter S. Epidemiology of colonisation of patients and environment with vancomycin-resistant enterococci. Lancet. 1996 Dec 14;348(9042):1615–1619. doi: 10.1016/S0140-6736(96)02331-8. [DOI] [PubMed] [Google Scholar]
- 41.Fan C.Y., Lee W.T., Hsu T.C., Lee C.H., Wang S.P., Chen W.S. Effect of chlorhexidine bathing on colonization or infection with Acinetobacter baumannii: a systematic review and meta-analysis. J Hosp Infect. 2019 Nov;103(3):284–292. doi: 10.1016/j.jhin.2019.08.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.