Highlights
-
•
Resurgence of cases and infant deaths due to pertussis have been observed in the past.
-
•
Countries like the UK have adopted maternal pertussis immunisation to protect infants.
-
•
Our model suggests higher infant mortality and morbidity without maternal vaccination.
-
•
Continuing the UK maternal pertussis programme is highly likely to be cost-effective.
Keywords: Pertussis, Resurgence, Maternal vaccination, Mathematical model, Economic evaluation, Public health
Abstract
Introduction
An unexpected resurgence of pertussis cases and infant deaths was observed in some countries that had switched to acellular pertussis vaccines in the primary immunisation schedule. In response to the outbreaks, maternal pertussis vaccination programmes in pregnant women have been adopted worldwide, including the USA in 2011 and the UK in 2012. Following the success of the programme in England, we evaluated the health and economic impact of stopping versus continuing the maternal pertussis immunisation to inform public health policy making.
Methods
We used a mathematical model to estimate the number of infant hospitalisations and deaths related to pertussis in England over 2019–2038. Losses in quality-adjusted life years, QALYs, were considered for infants (aged 0–2 months) who survived or died from pertussis, bereaved parents (of infants who died from pertussis), and women with pertussis (aged 20–44 years). Direct medical costs to the National Health Service included infant hospitalisations, maternal vaccinations, and disease in women. Costs and QALYs were discounted at 3.5%. Changes in the incremental cost-effectiveness ratio, ICER, were explored in sensitivity analyses.
Results
The model supports continuing the maternal pertussis immunisation programme as a cost-effective intervention at an ICER of £14,500/QALY (2.5% and 97.5%-quantile: £7,300/QALY to £32,400/QALY). Stopping versus continuing the maternal programme results in an estimated mean of 972 (range 582 to 1489) versus 308 (184 to 471) infant hospitalisations annually. Results were most sensitive to the number of hospitalisations and deaths when stopping the maternal programme. At a cost-effectiveness threshold of £30,000/QALY, the probability of the maternal programme being cost-effective was 96.2%.
Conclusion
Our findings support continuing the maternal pertussis vaccination programme as otherwise higher levels of disease activity and infant mortality are expected to return. These results have led policy makers to decide to continue the maternal programme in the UK routine immunisation schedule.
1. Introduction
The year 2012 saw a rise in pertussis (“whooping cough”) case numbers ten-fold higher than in previous years. This spike was observed across all age groups in England with the highest case-fatality rates among infants aged 0–2 months; a population too young to be vaccinated and directly protected by primary immunisation.[1]
The likely cause of this resurgence is thought to be the switch from a whole cell pertussis (wP) vaccine to an acellular pertussis (aP) vaccine in 2004.[2] Similar resurgences have been observed in other countries who also switched to aP vaccines. These countries include the USA, Australia, and Portugal.[2], [3] It is hypothesised that aP vaccines result in a shorter duration of protection against infection and disease compared to wP vaccines.[3]
As an emergency outbreak response measure to the disease resurgence, a pertussis vaccine has been offered to all pregnant women in the UK since October 2012. Pregnant women are currently advised to receive one dose of a combination diphtheria/tetanus/aP/inactivated polio (dTaP/IPV) vaccine in each pregnancy from gestational week 16.[4] Maternal pertussis immunisation is recommended by the World Health Organization (WHO) as a supplementary strategy,[5] and it has been implemented in a number of countries (including Australia, Belgium, Brazil, Mexico, Switzerland, and the USA).[4], [6]
The maternal pertussis vaccination programme has been shown to be safe and highly effective in protecting infants.[1], [4], [6], [7], [8] In England, the maternal programme reduced the number of hospitalisations and deaths among infants aged 0–2 months to similar (if not lower) levels than those reported in 1998–2009.[9], [10] This was in sharp contrast to the epidemiology observed in all other age groups, in which numbers of hospitalisations continued to rise and levelled off at a newly established level.[1]
Due to the maternal programme being initially introduced in response to the 2012 outbreak, the Joint Committee on Vaccination and Immunisation (JCVI) convened to decide whether to incorporate it as a permanent part of the UK routine immunisation schedule by 2019.[1] Given that such recommendations by JCVI require evidence of cost-effectiveness,[11] the aim of our study is to inform the review of whether it is cost-effective to make the maternal pertussis vaccination programme permanent after 2019. Our analysis is framed in terms of stopping (de-funding) versus continuing the maternal programme. This framing reflects the potential asymmetry between equal-sized gains and losses due to loss aversion and endowment effects, with dis-investment decisions of an existing programme possibly requiring larger cost savings to compensate for health losses than the amounts accepted for equivalent health gains with investment decisions.[12], [13], [14]
2. Methods
2.1. Design, setting and participants
We performed a cost-effectiveness analysis of the maternal pertussis vacation programme from the perspective of the National Health Service (NHS) in England over 20 years (2019–2038). We discounted future costs and quality-adjusted life years (QALYs) at the recommended 3.5%.[11], [15] For an overview of input parameters see Table 1.
Table 1.
Input parameters for the economic model in the base case and in (deterministic and probabilistic) sensitivity analyses.
| Parameter | Base value (min; max) | Distribution | Sources |
|---|---|---|---|
| Illness in infants | |||
| Annual number of hospitalisations of infants (aged 0–2 mo.), without maternal pertussis vaccination | 972a (500; 1,619a) | n/a |
base: Choi et al.,[2] notifications uprated to hospitalisations (1:1.2). min: conservative assumption.[7] max: Choi et al.,[2] uprated (1:2.0; see comparison in Appendix) |
| Coverage of maternal pertussis vaccination | 0.751b (0.723; 0.900) | Beta |
base: CPRD (2017). min: ImmForm (2017). max: assumption. |
| Vaccine effectiveness in infants | 0.910 (0.880; 0.940) | Beta |
base: mean VE in infants.[1] min/max: 95%-CI.[1] |
| Proportion of infant deaths (aged 0–2 mo.) annually, without maternal pertussis vaccination | 0.031 (0.017; 0.047) | Beta |
base: as observed in 2011–09/2012.[10] min: as observed in 2002–2009.[9] max: base + 50%. |
| Proportion of infant deaths (aged 0–2 mo.) annually, with maternal pertussis vaccination | 0.014 (0.007; 0.031) | Beta |
base: as observed in 2012–2017.[7] min: base −50%. max: as without the programme.[10] |
| QALY loss in (surviving) infants from disease | 0.097 (0.089; 0.106) | Normal |
base: mean value as in adults.[19] min/max: range.[19] |
| QALY loss in infants from premature death | 25.61 (20.49; 30.74) | Normal |
base: estimated from ONS (National life tables: United Kingdom), EQ-5D norms (Ara and Brazier, 2010), and discounted at 3.5%.[11], [15] min/max: base ± 20%. |
| QALY loss in parents from premature death of infant | 3.70 (0.00; 7.41) | Log-Normal |
base: estimated from ONS (National life tables: United Kingdom, and Birth characteristics in England and Wales), EQ-5D utility impact of anxiety/depression (Dolan, 1997), and discounted at 3.5%.[11], [15] min: excluded. max: base + 100%. |
| Annual number of maternities in England (informing the number of vaccine doses) | 658,000 (639,000; 687,000) | Normal |
base: mean of 2012–2017 (Office for National Statistics, Birth characteristics in England and Wales). min/max: range of 2012–2017 (Office for National Statistics, Birth characteristics in England and Wales). |
| Vaccine price per dose | £20.00 (£12.00; £22.74) | n/a (Fixed) |
base: British National Formulary, list price (tariff) in 2019. min/max: British National Formulary, indicative list price in 2012/2019. |
| Vaccine administration costs | £10.06 (£7.67; £15.00) | n/a (Fixed) |
base: NHS England, Enhanced service specifications (2019). min: NHS England, Enhanced service specifications (2012). max: base + 50%. |
| Infant hospitalisation costs, without maternal pertussis vaccination | £3,757 (£3,010; £4,510) | various; see Appendix, pages 6–8 |
base: estimated from HES (2006–2011), PICANet (2006–09/2012), and NHS Reference costs (2017–2018). min/max: base ± 20%. |
| Infant hospitalisation costs, with maternal pertussis vaccination | £4,252 (£3,400; £5,100) | various; see Appendix, pages 6–8 |
base: estimated from HES (2013–2017), PICANet (10/2012–2017), and NHS Reference costs (2017–2018). min/max: base ± 20%. |
| Illness in mothers (exploratory, largely following Van Hoek et al.[10]) | |||
| Annual number of cases in women (aged 20–44 years), without maternal pertussis vaccination | 3,920 | n/a (Fixed) |
base: estimated from infant pertussis incidence (2012)[10] and the female population aged 20–44 years.[16] scenario analyses: excluded. |
| Vaccine effectiveness in mothers | 0.890 | n/a (Fixed) |
base: VE in adults.[17] scenario analyses: excluded. |
| Laboratory-confirmed disease in mothers | 0.088 | n/a (Fixed) |
base: based on Van Hoek et al.[10] scenario analyses: excluded. |
| Non-confirmed mild disease in mothers | 0.200 | n/a (Fixed) |
base: based on Van Hoek et al.[10] scenario analyses: excluded. |
| QALY loss of non-confirmed mild disease in mothers | 0.036 | n/a (Fixed) |
base: QALY loss of adults.[19] scenario analyses: excluded. |
| QALY loss of laboratory-confirmed disease in mothers | 0.097 | n/a (Fixed) |
base: QALY loss of adults.[19] scenario analyses: excluded. |
| Costs of non-confirmed mild disease in mothers | £27.89 | n/a (Fixed) |
base: costs of adults,[19] inflated to 2017. scenario analyses: excluded. |
| Costs of laboratory-confirmed disease in mothers | £60.44 | n/a (Fixed) |
base: costs of adults,[19] inflated to 2017. scenario analyses: excluded. |
| Methodological parameters | |||
| start year of the analysis | 2019 (2012; 2024) | n/a (Fixed) |
base: status quo. min: start year of the programme.[1] max: base + 5 years. |
| stop year of the analysis | 2038 (2028; 2048) | n/a (Fixed) |
base: 20 years to capture variation.[2] min: 10 years short-term impact.max: 30 years to avoid methodological issues afterwards (HM Treasury, The Green book, 2018). |
| discount rate for costs and QALYs | 0.035 (0.015; 0.05) | n/a (Fixed) |
base: NICE, JCVI.[11], [15] min: NICE, JCVI.[11], [15] max: exploratory. |
CI: confidence interval, CPRD: Clinical Practice Research Datalink, EQ-5D: EuroQol Five Dimension, HES: hospital episode statistics, JCVI: Joint Committee on Vaccination and Immunisation, NHS: National Health Service, NICE: National Institute for Health and Care Excellence, NOIDS: notifications of infectious diseases, PICANet: Paediatric Intensive Care Audit Network, QALYs: quality-adjusted life years, VE: vaccine effectiveness.
: Mean value of all scenarios and over 20 years shown here only for illustration (see main text).
: For the sensitivity analysis exploring an earlier start year (2012, when the programme was introduced), we used the annualised coverage data in 2012–2017.
We focused on infants aged 0–2 months, who are the target group of the maternal pertussis programme, as they are too young to be vaccinated and directly protected by primary immunisation. They are also the most affected by the pertussis-related hospitalisations and deaths.[7], [8], [9] We also looked at laboratory-confirmed as well as non-confirmed (mild) disease in mothers, and at the health-related quality of life impact of bereaved parents of infants who died from pertussis.
2.2. Intervention scenario: Stopping the maternal pertussis vaccination
For the baseline epidemiology of the intervention scenario of stopping (de-funding) the maternal programme, we obtained the outputs of a previously developed transmission-dynamic model parameterised using age-stratified pertussis notification data from England and Wales over nearly six continuous decades (1956–2013).[2] The model was originally developed to investigate the potential cause of the pertussis outbreak in England in 2012, and it did not include the maternal programme yet. Hence, the model allows us to predict the baseline epidemiology for the intervention scenario when stopping the maternal programme, assuming a near-immediate return to higher disease levels in infants given the lower degree and duration of protection from infection by the aP vaccines.[2], [3] The model also considered the decreasing proportion of individuals protected by wP vaccines over time. We then up-scaled the model output of predicted notifications to hospitalisations given that the majority of pertussis cases in infants aged 0–2 months in England are hospitalised (unlike the older age groups),[9] and we expect that most notified cases would be admitted to the hospital. For more details on our modelling approach see Appendix, pages 2–4.
Independently, we also explored a conservative scenario without the programme of a constant number of 500 hospitalised infants annually. This scenario was informed by a published post-implementation evaluation that estimated median/mean numbers across six non-vaccination scenarios of 630/670 pertussis cases in ages 0–2 months annually for the 4-year means in 2014–2017 and a minimum of 465 pertussis cases in ages 0–2 months based on the lowest scenario that extrapolated the hospitalisation trend observed for pertussis in ages 3–11 months.[7]
For disease in mothers without the maternal programme, we adopted a similar approach to Van Hoek et al. (2016).[10] Our estimated 3,920 cases annually were derived from the incidence seen in infants in the peak month of 2012 (in August 2012 with 43.3 per 100,000 population) and the female population aged 20–44 years in England in 2017 (9,053,090).[16] We assumed 20.0% with non-confirmed mild disease and 8.8% being laboratory-confirmed moderate-to-severe disease.[10]
2.3. Comparator scenario: Continuing the maternal pertussis vaccination
For the comparator scenario of continuing the maternal vaccination programme, the number of infant hospitalisations was derived from the estimated numbers of the intervention scenario (when stopping the programme). The estimated numbers were adjusted by the estimated vaccine effectiveness (VE) in infants (0.91, 95%-CI: 0.88, 0.94)[1] and the vaccine coverage estimates from the Clinical Practice Research Datalink (CPRD). CPRD is a representative sentinel clinical dataset of general practitioner (GP) registered patients in England (see Appendix, pages 2–4).[1] In sensitivity analyses we used the estimates of the ImmForm dataset, which is a routinely collected extraction of GP records in England. However, ImmForm is likely to have been underestimating vaccine coverage before a change in the data extraction occurred in April 2016.[1]
For disease in mothers with the maternal programme, we reduced the estimated numbers without vaccination by the estimated VE in adolescents and adults (0.89, 95%-CI: 0.19, 0.99)[17] and the vaccine coverage (similar to infants; see above). We assumed the same proportions as without the programme to distinguish non-confirmed from laboratory-confirmed disease in mothers.[10]
2.4. Outcomes
The main outcome was the incremental cost-effectiveness ratio (ICER), i.e. the change in costs over the change in QALYs.[14] In general, depending on how the research question is framed, the ICER may represent the additional-costs-per-QALY-gained (here: of continuing vs. stopping the maternal programme), or the cost-savings-per-QALY-lost (of stopping vs. continuing the maternal programme). Conventionally, ICERs with positive changes in costs and QALYs need to be below the cost-effectiveness threshold of e.g. £30,000/QALY gained to be considered cost-effective. However, ICERs with negative changes in costs and QALYs need to be above the threshold to be considered cost-effective (indicating the larger cost savings needed to compensate for health losses becoming acceptable).[14]
In this analysis, the pertussis-related QALY losses were based on the estimated number of hospitalisations in infants and disease in mothers (as outlined above) as well as infant mortality. The number of infant deaths was estimated using previously reported case-fatality risks (CFRs) for hospitalised infants aged 0–2 months in England. When stopping the programme we assumed the CFR to return to levels seen in the 12 months preceding the maternal vaccination introduction (i.e., 10/2011–09/2012; 16/513 = 0.0312).[10] With a continued programme we assumed the CFR to stay at levels seen since the maternal vaccination introduction (i.e., 10/2012–12/2017; 17/1,211 = 0.0140).[7] Our assumption that when stopping the programme the infant CFR would return to levels seen in the 12 months preceding the maternal vaccination introduction is informed by (i) the continued and exclusive use of aP vaccines in England since 2004,[1], [2] (ii) the slightly higher VE against death than against disease,[1] (iii) VE being highest in the very youngest age groups (0 months) who may experience the highest CFR,[1] and (iv) the CFRs reported in other settings for the periods without a maternal vaccination programme (e.g. 4.7% in infants aged 0–2 months in Brazil).[18] Note that the share of the population benefitting from having been primed (and boosted) by wP vaccines also has been continuously decreasing, and is lower compared to at the time of the outbreak in 2012. We accounted for this in the transmission-dynamic model. In the sensitivity analysis, when stopping the programme we also considered the return of the CFR to pre-2012 levels (i.e., in 2002–2009; 29/1,695 = 0.0171).[9]
The overall QALY loss due to pertussis was informed by estimates for the QALY impact of illness episodes in surviving infants and mothers from previously published studies in England and Wales.[10], [19] We estimated the QALY loss in infants from premature mortality due to pertussis using official statistics and the population norms in England (Appendix, page 5). We also quantified the QALY loss in parents from premature infant mortality due to pertussis to capture the emotional distress from losing a child.[20] We assumed a severe impact on bereaved parents in terms of “anxiety/depression” in the first year and a moderate impact in all subsequent years over the remaining life expectancy of parents. We quantified the health loss using utility decrements and official statistics for England. Additional caregiver QALY losses (e.g. in parents) due to non-fatal infant cases have not been included. More details on the QALY estimation can be found in Appendix, page 5.
2.5. Costs
We estimated the costs of infant hospitalisations, the maternal vaccination programme, and disease in mothers.
For the costs of infant hospitalisations, we accounted for day cases, overnight stays, and infants admitted to a paediatric intensive care unit (PICU). In addition, infants admitted to the PICU may require specialist transportation (retrieval), and extracorporeal membrane oxygenation (ECMO). The underlying data on proportions and the lengths of stay were obtained for 2006–2017 from hospital episode statistics (HES) and the Paediatric Intensive Care Audit Network (PICANet). We used NHS reference costs for 2017/2018. A full list of the corresponding cost and diagnostic codes is listed in Appendix, pages 6–8.
For the costs of vaccination, we estimated the annual number of vaccine doses based on the coverage estimates of CPRD[1] and the annual number of officially recorded maternities in England. We also used the coverage estimates of ImmForm in the sensitivity analysis. We then used the NHS item-of-service payment to immunisation providers and the official list price of the pertussis vaccines, which is likely to be higher than the commercially-sensitive price at which the vaccines are available as part of the national tender (although the exact prices are unpublished and unknown to the public). Therefore, we further varied the vaccine price in a separate threshold analysis.
For the costs of disease in mothers, we considered the estimated costs of laboratory-confirmed and non-confirmed (mild) disease in adults in England (inflated to 2017).[19]
2.6. Uncertainty analyses
Two deterministic sensitivity analyses explored the parameter uncertainty by changing individual parameters in isolation. In the first deterministic sensitivity analysis, we used the range of plausible values listed in Table 1 based on the individual uncertainty of parameter estimates. In the second deterministic sensitivity analysis, we changed sensible parameters uniformly by ± 35% (simply chosen to reflect the 35% higher incidence scenario in Van Hoek et al.).[10] Results were plotted in tornado plots, which rank the parameters according to their highest-to-lowest impact on the ICER.
In probabilistic sensitivity analysis we then explored the combined uncertainty of parameters, using the distributions listed in Table 1. We used 2,000 samples to report the 95% uncertainty interval (95%-UI, i.e. 2.5%- and 97.5%-quantiles). We ran the probabilistic analysis for both the base-case scenarios and the conservative minimum scenario.[7] We aimed to facilitate interpreting results by plotting in a cost-effectiveness plane the original research question (of stopping vs. continuing the programme) and also its reverse format (of continuing vs. stopping).[14]
We also plotted the probability of interventions being cost-effective for a range of willingness-to-pay thresholds (£0-£50,000/QALY). We identified the optimal intervention based on the highest mean net benefit (under an assumed policy objective for the NHS of maximising health outcomes with limited resources).[21] Lastly, we quantified the probabilities of being cost-effective of different vaccine prices at £30,000/QALY.[11], [15]
2.7. Software
The original transmission-dynamic model was coded in MATLAB. The cost-effectiveness analysis was coded in R.
3. Results
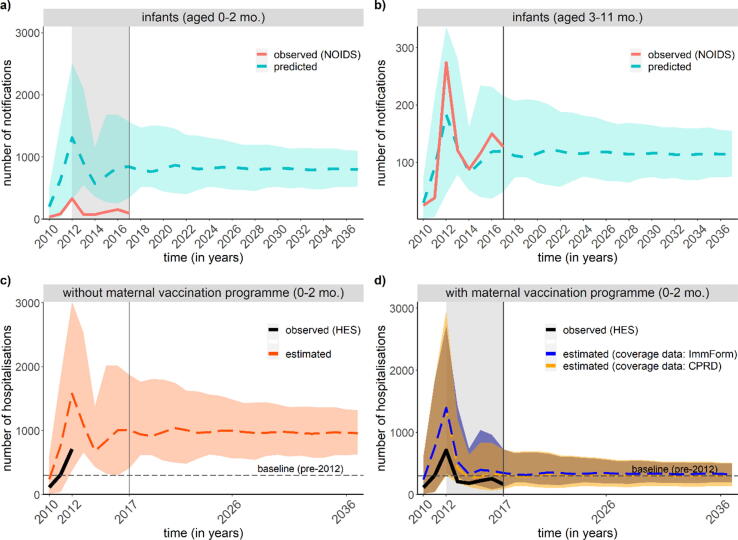
Our model estimated a mean of 972 (median: 988; range 582 to 1489) versus 308 (313; 184 to 471) annual infant hospitalisations when stopping the maternal programme versus continuing it between 2019 and 2038 (Fig. 1). The annual number of deaths predicted in infants aged 0–2 months is an estimated mean of 4.3 (1.3, 9.5) deaths and 30.3 (95%-UI: 15.1, 50.7) deaths with and without the programme, respectively. When comparing the predicted incidence of notifications for the baseline intervention scenario without maternal vaccination over time to the observed incidence the predictions in the 0–2-month age group poorly matched the observed notifications. This is to be expected as the predictions do not account for the maternal pertussis vaccination programme (Fig. 1A). In contract, the predictions in the 3–11-month age group reasonably matched the observed notifications as they are only marginally impacted by the maternal programme that aims to protect infants aged 0–2 months (Fig. 1B). Similarly, our estimated hospitalisations in infants aged 0–2 months also matched the observed number of hospitalisations, both for the period before the introduction of the maternal vaccination programme (Fig. 1C) and the period afterwards (Fig. 1D).
Fig. 1.
Comparison of observed pertussis notifications (2010–2017) and predicted notifications (2010–2037) in a) infants aged 0–2 months and b) infants aged 3–11 months; and comparison of observed pertussis hospitalisations (2010–2017) and estimated hospitalisations (2010–2037) in infants aged 0–2 months c) without the maternal vaccination programme and d) with the maternal vaccination programme.
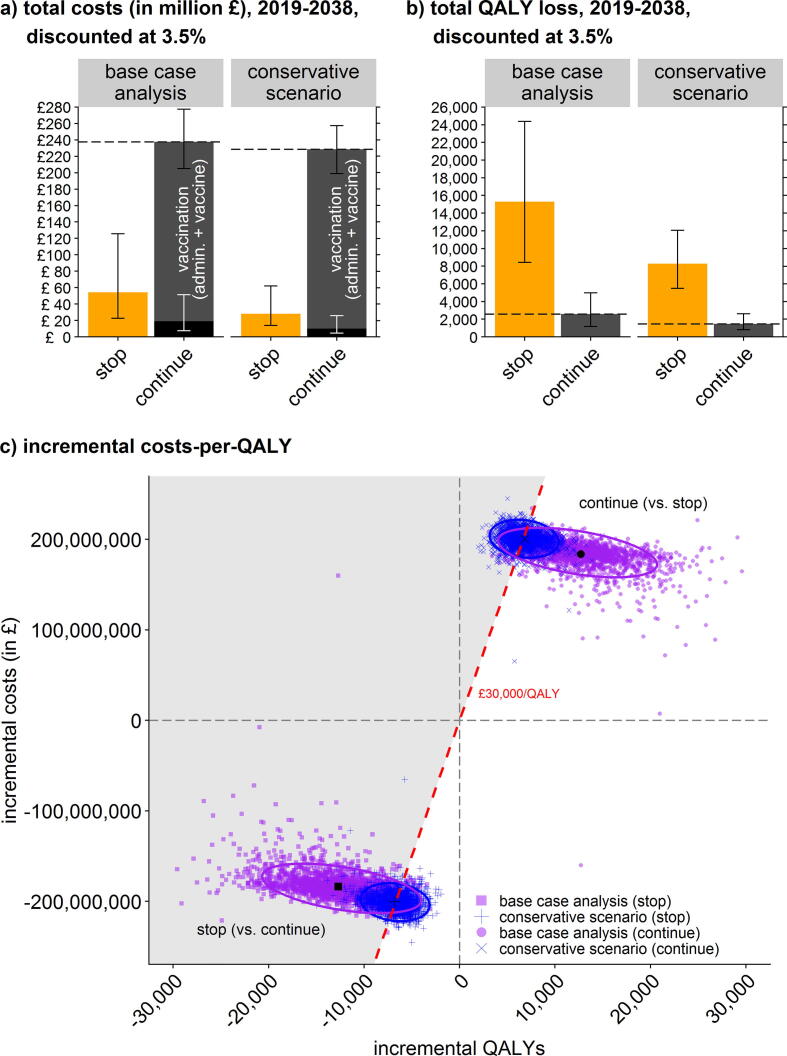
For an overview of the deterministic cost-effectiveness of stopping the maternal pertussis vaccination in England in 2019–2038 (compared to continuing the programme) see Table 2. The annual discounted costs of the programme for the NHS are estimated to be a mean of £11.9 million (95%-UI: £10.2 million, £13.9 million) versus mean costs without the programme of £2.7 million (95%-UI: £1.1 million, £6.3 million). The cost savings from stopping the programme largely reflect avoided vaccinations, but at increased costs of infant hospitalisations (Fig. 2A). The estimated annual QALY loss without the programme resulted in a mean of 765 QALYs (95%-UI: 421, 1219) vs. a loss of 130 QALYs (59, 249) with the programme; Fig. 2B). At incremental net cost savings of £9.2 million and incremental health losses of 635 QALYs, stopping the programme is expected to result in cost savings-per-QALY-lost of £14,500/QALY (95%-UI: £7,300/QALY, £32,400/QALY). This is below the incremental cost-effectiveness threshold of £30,000 (and even £20,000) per QALY lost; Fig. 2C. Stopping the programme is thus not cost-effective as the cost savings are not large enough to offset the health loss in terms of QALYs. The worst case explored in the conservative minimum scenario (i.e., n = 500) resulted in £29,400/QALY (£19,000/QALY, £52,600/QALY); Fig. 2C. Overall, the incremental costs per QALY favour continuing the programme.
Table 2.
Deterministic cost-effectiveness results of stopping vs continuing the maternal pertussis vaccination in England, 2019–2038.
| Outcomes | Base-case analysis based on transmission-dynamic model predictions. |
Conservative scenario based on 500 infant inpatients annually (worst-case scenario). |
||||
|---|---|---|---|---|---|---|
| stop programme | continue programme | incremental change | stop programme | continue programme | incremental change | |
| Costs (in £) | ||||||
| Vaccine price (at £20/dose) | 0 | 145,000,000 | −145,000,000 | 0 | 145,000,000 | −145,000,000 |
| Vaccine administration | 0 | 73,100,000 | −73,100,000 | 0 | 73,100,000 | −73,100,000 |
| Medical costs (infants aged 0–2 months) | 53,800,000 | 19,300,000 | 34,500,000 | 27,600,000 | 9,900,000 | 17,700,000 |
| Medical costs (women aged 20–44 years) | 628,000 | 208,000 | 420,000 | 628,000 | 208,000 | 420,000 |
| Total costs (discounted at 3.5%) | 54,400,000 | 238,000,000 | −184,000,000 | 28,300,000 | 229,000,000 | −200,000,000 |
| Total costs (discounted at 1.5%) | 64,400,000 | 282,000,000 | −218,000,000 | 33,500,000 | 271,000,000 | −237,000,000 |
| Total costs (undiscounted) | 73,900,000 | 323,600,000 | −250,000,000 | 38,400,000 | 311,000,000 | −272,000,000 |
| QALY losses | ||||||
| Hospitalised infants with pertussis (aged 0–2 months) | 1,400 | 440 | −950 | 710 | 230 | −490 |
| Infant deaths due to pertussis (QALY loss in infants) | 11,300 | 1,620 | −9,720 | 5,800 | 830 | −5,000 |
| Infant deaths due to pertussis (QALY loss in parents) | 1,650 | 240 | −1,420 | 850 | 120 | −730 |
| Women with pertussis (aged 20–44 years) | 910 | 300 | −610 | 910 | 300 | −610 |
| Total QALY loss (discounted at 3.5%) | 15,300 | 2,600 | −12,700 | 8,300 | 1,500 | −6,800 |
| Total QALY loss (discounted at 1.5%) | 28,000 | 4,490 | –23,500 | 14,900 | 2,500 | −12,500 |
| Total QALY loss (undiscounted) | 51,000 | 7,840 | −43,200 | 26,900 | 4,200 | –22,600 |
| Incremental costs (in £) per 1 QALY change | ||||||
| ICER (discounted at 3.5%) | n/a | n/a | 14,500 | n/a | n/a | 29,400 |
| ICER (discounted at 1.5%) | n/a | n/a | 9,200 | n/a | n/a | 19,000 |
| ICER (undiscounted) | n/a | n/a | 5,800 | n/a | n/a | 12,000 |
ICER: incremental cost-effectiveness ratio, n/a: not applicable, QALY: quality-adjusted life year.
The base-case analysis is more robust than the conservative scenario as it is based on a transmission-dynamic model exploring a wide range of plausible scenarios (Choi et al., 2016), while the conservative scenario is based on an assumed constant number of 500 infant hospitalisations annually.
Fig. 2.
Results (mean, 2.5%- and 97.5%-quantile) of stopping vs. continuing the maternal pertussis vaccination programme in England for the base-case analysis and the conservative scenario: Total costs (panel a); total QALY loss (panel b); and incremental costs-per-QALY (panel c, with unfavourable cost-effectiveness ratios >£30,000/QALY shaded in grey).
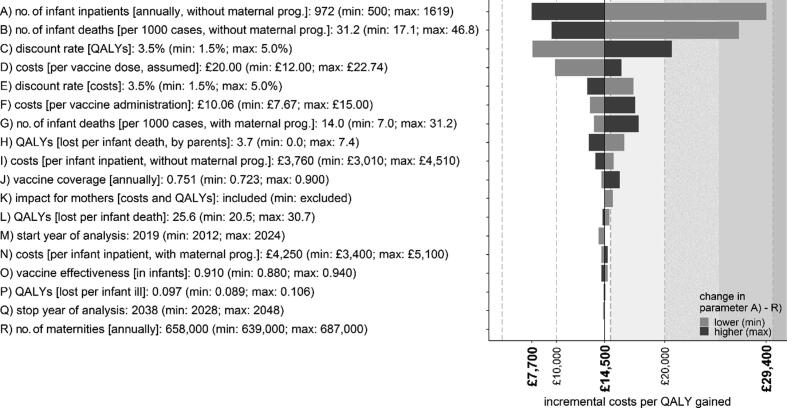
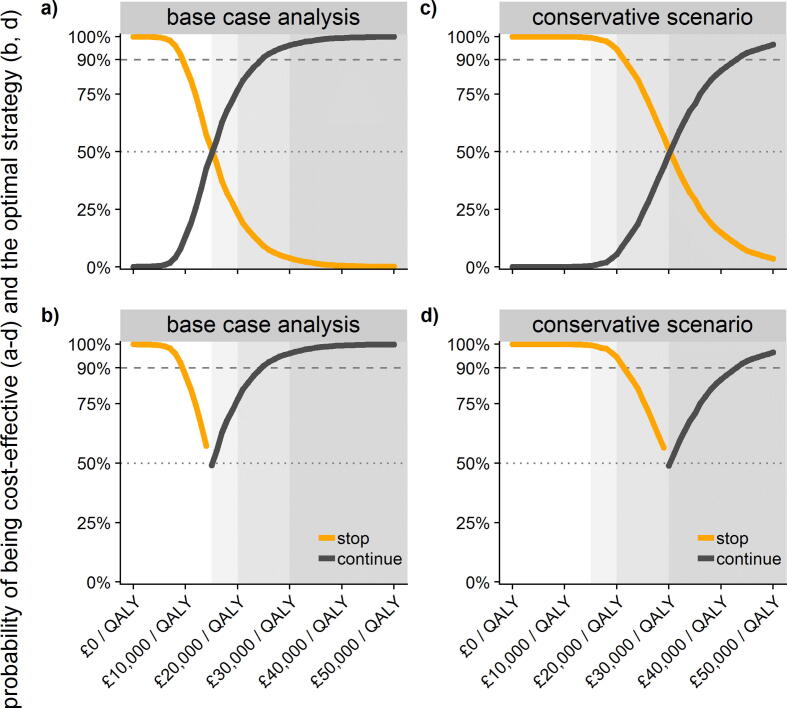
Deterministic sensitivity analysis showed that the most sensitive parameters were the number of hospitalisations and deaths when stopping the programme (Fig. 3, and Appendix, page 9). Probabilistic sensitivity analysis revealed that continuing the programme with an assumed vaccine price of £20/dose had the highest probability of being cost-effective at a cost-effectiveness threshold of £30,000/QALY (96.2%). The conservative minimum scenario (of n = 500) had a probability of being cost-effective of 48.9% at £30,000/QALY and a vaccine price of £20/dose (Fig. 4), which reached 90% at a vaccine price between £11-£12/dose (excluding administration costs; Appendix, page 10).
Fig. 3.
Results of the deterministic sensitivity analysis (tornado plot), exploring a plausible range of values.
Fig. 4.
Probability of being cost-effective at an assumed vaccine price of £20/dose (excluding administration costs) across thresholds of £0-£50,000/QALY in the base-case analysis (a-b) and the conservative minimum scenario (c-d), and of interventions being optimal in achieving the highest mean net benefit (b, d). Note: The conservative scenario is not equally likely to the scenarios underlying the base case analysis.
4. Discussion
This study analysed the cost-effectiveness of stopping the existing emergency maternal pertussis vaccination versus adopting the maternal vaccination programme as a permanent part of the national immunisation schedule. Our findings suggest that stopping the maternal programme would result in financial savings for the NHS (largely by avoiding expenditures on vaccinations), but would also result in increased expenditure on hospitalisations as well as elevated health losses from infant mortality and morbidity. Overall, the incremental costs per QALY favour continuing the routine maternal vaccination programme (£14,500/QALY), i.e. stopping the programme is unlikely to be considered cost-effective.[11], [15] Based on this study and additional evidence, the JCVI recommended to continue emergency maternal immunisation as a routine programme in the UK.[22]
Our findings are in line with previous economic evaluations of maternal immunisation strategies internationally.[7], [10], [23], [24] Similar to previous studies,[24] our results are most sensitive to the number of hospitalisations and deaths without the maternal programme. However, the actual ICER is likely to be lower than that presented here (i.e., even more favourable of continuing the programme) given that we used published list prices for the vaccines that are likely to be higher than the confidential, unpublished tender prices actually paid by the NHS.
In the future, infant cases in England may rise further due to the increasing proportion of females primed with aP vaccines since 2004 that are reaching childbearing age. The impact of the boosting effect in mothers primed by aP vaccines is still unclear. However, more rapid waning of vaccine-induced protection in aP primed children may also make them susceptible to natural infection again, with longer lasting natural immunity that may reach well into childbearing age.[2] Moreover, natural infection may also become associated with substantial morbidity and mortality. Combined with new vaccines being developed that may confer higher degrees of indirect protection again,[25] the maternal pertussis immunisation may need to be re-evaluated in the future.
There are additional reasons for continuing the programme beyond cost-effectiveness considerations, such as the duty of care towards vulnerable populations who are difficult to otherwise protect (such as infants too young to be directly protected via primary immunisation).[8], [26] We were also unable to quantify the broader (societal) implications for the reputation and future coverage of the pertussis vaccines if the programme was to be de-funded,[3] such as the possible confusion this may cause about the benefit of pertussis vaccination. The maternal pertussis vaccine being publicly funded was one of the key factors for women to accept the vaccine during pregnancy in New Zealand.[27] Furthermore, the disruption caused by a discontinued programme (even if only intermittently) on the inter-epidemic cycle of pertussis is unclear, possibly leading to long-run lower levels of uptake and making the pertussis infection prevention and control even more difficult than they have already proven to be.[1], [3], [8] Including such considerations into the appraisal of the maternal pertussis vaccination programme should further support its continuation for as long as indirect protection against infection from the infant programme is insufficiently high.
5. Strengths and limitations
The study presented here is the first to explore the heath-economic consequences of stopping the maternal pertussis vaccination programme in England after 2019. It also formed a part of the body of evidence considered by the JCVI that led it to recommend making the maternal pertussis vaccination programme permanent in the UK routine immunisation schedule.[22] Despite framing the analysis as exploring the potential disinvestment decision of stopping (de-funding) versus continuing the routine maternal vaccination programme, we applied the same rules and methods that apply to investment decisions when introducing new interventions.[11], [15] We also performed rigorous sensitivity and scenario analyses to address the various challenges and uncertainties encountered. However, the national datasets included in our analysis did not allow for addressing socio-economic gradients for disease risk and coverage, despite the adjusted coverage for the maternal programme having been an estimated 14% lower in the most deprived group in 2014–2015.[28]
Furthermore, we assumed that stopping the programme would result in a near-immediate return to higher disease levels in infants given the lower degree and duration of protection from infection afforded by the aP vaccines.[2], [3] This also reflects the rapid decrease in maternal antibody titres after delivery, hence the recommendation for mothers to receive the vaccine in each pregnancy.[8] The World Health Organization has cautioned against switching to aP vaccines in countries that have not switched yet, following outbreaks that occurred in four countries that exclusively used aP vaccines and modelling studies suggested an increased risk of death in infants too young to be vaccinated.[5] In the future, we plan to use a dynamic model to analyse the residual immunity in vaccinated mothers for subsequent pregnancies, as well as the residual indirect protection of the maternal programme for the wider population. Moreover, in response to the coronavirus disease 2019 (COVID-19) pandemic, many countries have adopted physical distancing measures, which impact coverage of routine childhood immunisation programmes as well as reduce the transmission of other infectious diseases such as pertussis. The potential impact for the maternal pertussis programme and the benefits of reduced transmission of pertussis will need to be considered in the future.
Our conservative minimum scenario was independently informed by a previously published post-implementation evaluation to illustrate the possible impact in the extreme worst case of a constant number of 500 hospitalised infants annually even without the programme.[7] Such an assumption is not necessarily reflecting the most plausible scenario or of equal likelihood to the best-fitting scenarios informing our base case. Moreover, assuming a constant number of 500 infant hospitalisations annually without the programme equates to an expected 158 hospitalisations with the programme (at an uptake of 0.751 and vaccine effectiveness of 0.910). This number is 24% lower than the mean 207 infant inpatients actually observed between 2013 and 2017.[7]
We also focused on the most important cost factors from the healthcare provider perspective, and all major QALY losses. Studies measuring the QALY loss in parents due to illness in their children are rare and mostly focus on non-fatal illness episodes, e.g. in parents of children with an illness episode of rotavirus-associated gastroenteritis.[29] Studies measuring QALY losses experienced by bereaved parents due to the premature death of their child are even rarer, although the emotional distress caused by infant deaths is well established.[20] By assuming a utility decrement for bereaved parents on solely the dimension “anxiety/depression” of the EQ-5D, we ignored any possible impact on other dimensions of quality of life as well as the impact on others (e.g. grandparents, siblings, hospital staff). We also did not account for any long-term disability in PICU survivors,[30] and our sensitivity analysis revealed a larger impact of the discount rate on QALYs than on costs. Therefore, we believe we have underestimated the magnitude of the cost-effectiveness of continuing the routine maternal programme. Had we considered any of the mentioned additional QALY losses prevented by the maternal programme the net effect would likely be to make it more cost-effective to continue.
In conclusion, our findings support continuing the maternal pertussis vaccination programme in England as a cost-effective strategy from the NHS perspective. The programme may need to be re-evaluated once new vaccines offering sufficiently high indirect protection become available.
Funding sources
This work was supported by Public Health England (PHE), which is an executive agency of the Department of Health (DH). The authors had sole responsibility for the study design, data collection, data analysis, data interpretation, and writing of the report. MJ was supported by the National Institute for Health Research (NIHR) Health Protection Research Unit in Immunisation at the London School of Hygiene and Tropical Medicine in partnership with PHE (Grant Reference Code HPRU-2012–10096; NIHR200929). FGS and MJ were also supported by the NIHR HPRU in Modelling and Health Economics, a partnership between PHE, Imperial College London and LSHTM (grant code NIHR200908). The views expressed are those of the authors and not necessarily those of the National Health Service (NHS), the NIHR, the DH, or PHE.
Declaration of Competing Interest
The Public Health England (PHE) Immunisation Department has provided post-marketing surveillance reports to Marketing Authorisation Holders which they are required to submit to the UK Licensing authority in compliance with their Risk Management Strategy. A cost recovery charge is made for these reports, which have not to date included pertussis-containing vaccines. HLB reports grants from the Healthcare Quality Improvement Partnership (HQIP) National Clinical Audit and Patient Outcomes Programme (NCAPOP) during the conduct of the study. This grant partially supports her role as the Paediatric Intensive Care Network (PICANet) Senior Statistician.
Acknowledgements
We thank Nalini Iyanger for facilitating accessing the data on paediatric intensive care of the Paediatric Intensive Care Audit Network (PICANet), and we thank the PICANet at the Universities of Leeds and Leicester for providing the data. Hospital Episode Statistics (HES) of the Health and Social Care Information Centre, ©2018, were re-used with the permission of the Health and Social Care Information Centre. All rights reserved.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2021.06.042.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Amirthalingam G., Campbell H., Ribeiro S. Sustained Effectiveness of the Maternal Pertussis Immunization Program in England 3 Years Following Introduction. Clin Infect Dis. 2016;63(suppl 4):S236–S243. doi: 10.1093/cid/ciw559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi Y.H., Campbell H., Amirthalingam G., van Hoek A.J., Miller E. Investigating the pertussis resurgence in England and Wales, and options for future control. BMC Med. 2016;14(1):121. doi: 10.1186/s12916-016-0665-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lapidot R., Gill C.J. The Pertussis resurgence: putting together the pieces of the puzzle. Trop Dis Travel Med Vaccines. 2016;2:26. doi: 10.1186/s40794-016-0043-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell H., Gupta S., Dolan G.P. Review of vaccination in pregnancy to prevent pertussis in early infancy. J Med Microbiol. 2018;67(10):1426–1456. doi: 10.1099/jmm.0.000829. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization (WHO). Revised guidance on the choice of pertussis vaccines: July 2014. Releve epidemiologique hebdomadaire 2014; 89(30): 337-40. [PubMed]
- 6.Gkentzi D., Katsakiori P., Marangos M. Maternal vaccination against pertussis: a systematic review of the recent literature. Arch Dis Child Fetal Neonatal Ed. 2017;102(5):F456–F463. doi: 10.1136/archdischild-2016-312341. [DOI] [PubMed] [Google Scholar]
- 7.Sandmann F.G., Jit M., Andrews N. Infant hospitalisations and fatalities averted by the maternal pertussis vaccination programme in England, 2012–2017: Post-implementation economic evaluation. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa165. [DOI] [PubMed] [Google Scholar]
- 8.Gopal D.P., Barber J., Toeg D. Pertussis (whooping cough) BMJ. 2019;364 doi: 10.1136/bmj.l401. [DOI] [PubMed] [Google Scholar]
- 9.Campbell H., Amirthalingam G., Andrews N. Accelerating control of pertussis in England and Wales. Emerg Infect Dis. 2012;18(1):38–47. doi: 10.3201/eid1801.110784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Hoek A.J., Campbell H., Amirthalingam G., Andrews N., Miller E. Cost-effectiveness and programmatic benefits of maternal vaccination against pertussis in England. J Infect. 2016;73(1):28–37. doi: 10.1016/j.jinf.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 11.Joint Committee on Vaccination and Immunisation (JCVI). Report from the Working Group on Uncertainty in Vaccine Evaluation and Procurement; In: Code of Practice - June 2013. 2013.
- 12.O'Brien B.J., Gertsen K., Willan A.R., Faulkner L.A. Is there a kink in consumers' threshold value for cost-effectiveness in health care? Health Econ. 2002;11(2):175–180. doi: 10.1002/hec.655. [DOI] [PubMed] [Google Scholar]
- 13.Klok R.M., Postma M.J. Four quadrants of the cost-effectiveness plane: some considerations on the south-west quadrant. Expert review of pharmacoeconomics & outcomes research. 2004;4(6):599–601. doi: 10.1586/14737167.4.6.599. [DOI] [PubMed] [Google Scholar]
- 14.Drummond M.F., Sculpher M.J., Claxton K., Stoddart G.L., Torrance G.W. Oxford University Press; 2015. Methods for the economic evaluation of health care programmes. [Google Scholar]
- 15.National Institute for Health and Care Excellence (NICE). Guide to the Methods of Technology Appraisal. London: National Institute for Health and Care Excellence (NICE); 2013. [PubMed]
- 16.Office for National Statistics (ONS). Estimates of the population for the UK, England and Wales, Scotland and Northern Ireland. 2018. https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/datasets/populationestimatesforukenglandandwalesscotlandandnorthernireland (accessed 30.04.2019.
- 17.Ward J.I., Cherry J.D., Chang S.-J. Efficacy of an Acellular Pertussis Vaccine among Adolescents and Adults. N Engl J Med. 2005;353(15):1555–1563. doi: 10.1056/NEJMoa050824. [DOI] [PubMed] [Google Scholar]
- 18.Guimarães L.M., da Costa Carneiro E.L.N., Carvalho-Costa F.A. Increasing incidence of pertussis in Brazil: a retrospective study using surveillance data. BMC Infect Dis. 2015;15(1):442. doi: 10.1186/s12879-015-1222-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Hoek A.J., Campbell H., Andrews N., Vasconcelos M., Amirthalingam G., Miller E. The burden of disease and health care use among pertussis cases in school aged children and adults in England and Wales; a patient survey. PLoS ONE. 2014;9(11) doi: 10.1371/journal.pone.0111807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song J., Floyd F.J., Seltzer M.M., Greenberg J.S., Hong J. Long-term Effects of Child Death on Parents' Health Related Quality of Life: A Dyadic Analysis. Fam Relat. 2010;59(3):269–282. doi: 10.1111/j.1741-3729.2010.00601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barton G.R., Briggs A.H., Fenwick E.A. Optimal cost-effectiveness decisions: the role of the cost-effectiveness acceptability curve (CEAC), the cost-effectiveness acceptability frontier (CEAF), and the expected value of perfection information (EVPI) Value Health. 2008;11(5):886–897. doi: 10.1111/j.1524-4733.2008.00358.x. [DOI] [PubMed] [Google Scholar]
- 22.Joint Committee on Vaccination and Immunisation (JCVI). Minute of the meeting held on 05 June 2019, Skipton House, London Road, London. 2019. https://www.gov.uk/government/groups/joint-committee-on-vaccination-and-immunisation (accessed 16/01/2020.
- 23.Atkins K.E., Fitzpatrick M.C., Galvani A.P., Townsend J.P. Cost-Effectiveness of Pertussis Vaccination During Pregnancy in the United States. Am J Epidemiol. 2016;183(12):1159–1170. doi: 10.1093/aje/kwv347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandes E.G., Rodrigues C.C.M., Sartori A.M.C., De Soarez P.C., Novaes H.M.D. Economic evaluation of adolescents and adults' pertussis vaccination: A systematic review of current strategies. Human vaccines & immunotherapeutics. 2019;15(1):14–27. doi: 10.1080/21645515.2018.1509646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Locht C. Will we have new pertussis vaccines? Vaccine. 2018;36(36):5460–5469. doi: 10.1016/j.vaccine.2017.11.055. [DOI] [PubMed] [Google Scholar]
- 26.Amirthalingam G., Gupta S., Campbell H. Pertussis immunisation and control in England and Wales, 1957 to 2012: a historical review. Euro Surveill. 2013;18(38) doi: 10.2807/1560-7917.es2013.18.38.20587. [DOI] [PubMed] [Google Scholar]
- 27.Hill L., Burrell B., Walls T. Factors influencing women's decisions about having the pertussis-containing vaccine during pregnancy. Journal of primary health care. 2018;10(1):62–67. doi: 10.1071/HC17040. [DOI] [PubMed] [Google Scholar]
- 28.Byrne L., Ward C., White J.M., Amirthalingam G., Edelstein M. Predictors of coverage of the national maternal pertussis and infant rotavirus vaccination programmes in England. Epidemiol Infect. 2018;146(2):197–206. doi: 10.1017/S0950268817002497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brisson M., Senecal M., Drolet M., Mansi J.A. Health-related quality of life lost to rotavirus-associated gastroenteritis in children and their parents: a Canadian prospective study. Pediatr Infect Dis J. 2010;29(1):73–75. doi: 10.1097/INF.0b013e3181b41506. [DOI] [PubMed] [Google Scholar]
- 30.Surridge J., Segedin E.R., Grant C.C. Pertussis requiring intensive care. Arch Dis Child. 2007;92(11):970–975. doi: 10.1136/adc.2006.114082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.