Abstract
The antibody-drug conjugate (ADC) enfortumab-vedotin (EV) acts by targeting nectin-4, a protein that is nearly ubiquitously expressed in conventional urothelial cancer (UC). However, expression of nectin-4 in morphologic variants of urothelial carcinoma and non-urothelial histotypes was unknown. Immunohistochemistry for nectin-4 using was performed on 169 patients including 83 with non-muscle invasive bladder cancer and 86 patients with muscle invasive bladder cancer. Staining was scored for intensity (0–3) and extent (% positive cells) using the H-score system, where >15 was considered positive. Overall, 72/83 (87%) samples of non-muscle invasive urothelial carcinoma were positive, including 29/30 (97%) non-invasive papillary urothelial carcinomas, 7/8 (87.5%) CISs, 36/45 (80%) papillary urothelial carcinomas invading the lamina propria. Overall, 50/86 muscle invasive tumors were positive, including 15/22 (68.2%) urothelial carcinomas, 7/10 (70%) squamous cell carcinomas, 3/11 (28%) micropapillary tumors, 4/6 (66%) adenocarcinomas, 2/4 (50%) nested carcinomas, 5/8 (63%) plasmacytoid, 1/10 (10%) sarcomatoid carcinomas, and 0/15 (0%) small cell carcinomas. Whole transcriptome RNA sequencing revealed that compared to conventional urothelial carcinomas, most sarcomatoid carcinomas and all but two small cell carcinomas expressed very low levels of nectin-4 mRNA but expressed significant levels of either trop2 or ERBB2, which are the molecular targets of two other ADCs - sacituzumab gavitecan (SG; trop2) or trastuzumab deruxtecan (ERBB2/HER2). In summary, our study demonstrates that there is heterogeneity of expression of nectin-4 in morphologic variants of UC and non-urothelial histotypes, and suggests that testing expression of nectin-4 should be considered in morphologic variants or non-urothelial histotypes found to have lower expression.
Keywords: Nectin-4, urothelial carcinoma, antibody-drug conjugates
INTRODUCTION
Urothelial carcinoma is a common cancer in the United States, affecting more than 80,000 new patients and causing more than 17,000 deaths every year.1 Similar to other cancer types, recent advances in bladder cancer therapeutics include agents that target specific molecules or pathways, including FGFR3 and immune checkpoint proteins such as programmed cell death-1 (PD-1) or programmed cell death ligand-1 (PD-L1).2 Antibody-drug conjugates (ADC) are an emerging group of therapeutic agents that combine a cytotoxic agent with a monoclonal antibody as a delivery molecule.3, 4 The ADC enfortumab-vedotin (EV) is comprised of a monoclonal antibody directed against nectin-4, conjugated to the microtubule-disrupting cytotoxic agent monomethyl auristatin E (MMAE).5
The protein nectin-4, also known as poliovirus receptor-related protein (PVRL4), is an immunoglobulin-like adhesion molecule that mediates calcium-independent cell-cell adhesions.6–8 High levels of nectin-4 expression have been reported in bladder cancer samples as measured by suppression subtractive hybridization and immunohistochemistry.5 By immunohistochemistry, tissue microarrays containing 524 cases of bladder cancer were reported to be positive in 83% of cases, including relatively equal proportions of cases showing low, moderate and strong staining intensity.5 Nectin-4 expression using an anti-nectin-4 antibody clone (M22-321b41.1) was initially a protocol requirement in the phase I trial of EV. This stipulation was later amended based on high nectin-4 expression in most urothelial cancer samples (the authors reported median H-score of 290, range 0–300, 4th percentile 150, with only 5 samples with H score <150). Expression of nectin-4 in the upper urinary tract has been shown to be lower and was found in 67% of cases.9 Urothelial carcinoma is known for having multiple morphologic variants/divergent differentiations including micropapillary, nested, plasmacytoid, sarcomatoid, squamous, glandular, and non-urothelial histotypes including squamous cell carcinoma and small cell carcinoma.10, 11 The expression of nectin-4 in tumors with divergent differentiations or non-urothelial histotypes has not been reported, and could have potential implications in therapeutic responses. In this study, we used a commercially available monoclonal antibody specific for nectin-4 and investigated its expression in morphologic variants of urothelial carcinoma, small cell carcinoma and squamous cell carcinoma. Additionally, we validated the results with next generation RNA sequencing (RNAseq) of small cell, sarcomatoid and conventional urothelial carcinoma.
MATERIALS AND METHODS
Patients and Tissue Samples
With approval by the Institutional Review Board of Johns Hopkins Hospital, a retrospective review of the pathology database was performed to identify specimens with conventional and morphologic variants of urothelial carcinoma. The slides were reviewed by two expert urologic pathologists (AM and VP) and a representative section was selected and recuts obtained to perform immunohistochemical stains. Tissue microarrays were constructed with tissue from 83 patients with non-muscle invasive bladder including 8 carcinomas in situ (CIS), 30 non-invasive papillary urothelial carcinomas (pTa), and 45 papillary urothelial carcinomas with invasion in the lamina propria (pT1). The TMAs were constructed with 3 cores (2 mm in diameter each) from each tumor and one non-neoplastic core per patient when available. The study sets also included initial transurethral resection of bladder tumors (TURBTs) from 86 patients who had not received neoadjuvant chemotherapy and presented with muscle invasive bladder cancer (pT2), including 22 conventional urothelial carcinomas, 15 small cell carcinomas, 11 micropapillary urothelial carcinomas, 10 squamous cell carcinomas, 10 sarcomatoid carcinomas, 8 plasmacytoid carcinomas, 6 urothelial carcinomas with glandular differentiation, and 4 nested urothelial carcinomas.
Immunohistochemistry
Immunohistochemical staining for nectin-4 was performed on whole 5-μm-thick tissue sections from formalin fixed paraffin embedded tissue, using the Ventana Benchmark Ultra automated staining system (Ventana Medical Systems, Tucson, AZ). Slides underwent automated deparaffinization followed by antigen retrieval with CC1 solution (EDTA, pH9; Ventana) and detection was achieved using the UltraView DAB detection Kit (Ventana). The commercially available primary antibody used in this study was the rabbit monoclonal anti-recombinant human nectin-4 antibody (1:1000; EPR15613-68, Abcam). The antibody used in the EV clinical trial, clone M22-321b41.1, is not commercially available.5 Cytoplasmic and membranous staining of tumor cells was considered positive and cases were scored using the H-score system, which is the product of intensity (score, 0–3), and percentage of stained cells (0–100), as used in the initial study of nectin-4.5 Specimens were then classified as negative (0; H-score, 0–14), weak (1+; H-score, 15–99), moderate (2+; H-score, 100–199), and strong (3+; H-score, 200–300).
Gene Expression Analysis
RNA sequencing was performed on macro-dissected areas of formalin-fixed paraffin-embedded (FFPE) tissue, with a pathologist (AM) selecting the area of interest marking an H&E slide two separate areas, one for conventional urothelial carcinoma, and one for small cell carcinoma or sarcomatoid carcinoma. The slides were macrodissected to obtain tumor cells as described previously.12, 13 RNA was extracted from 10, 5 micrometers thick FFPE tissue sections using HighPure miRNA isolation kits (Roche) according to the manufacturer’s instructions, using a 3 h incubation in proteinase K. RNA purities, integrities, and concentrations were measured by the Agilent TapeStation (Agilent Technologies, Thermo Fisher Scientific) and Nanodrop ND-1000 Spectrophotometer. Whole transcriptome RNA sequencing was performed using Ion Torrent’s AmpliseqRNA platform (Thermo Fisher Scientific) and an S5XL sequencer (Thermo Fisher Scientific). Twenty nanograms of purified RNA was transcribed into cDNA using the SuperScript® VILO™ kit. Then cDNA was amplified using the Ion Ampliseq Transcriptome Human Gene Expression Core panel, followed by ligation of adapters and barcodes to amplicons and purification. Purified libraries were quantified using the Ion Library Quantification kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. Libraries were diluted to 100 pM and pooled in sets of eight. Pooled libraries were amplified on Ion SphereTM particles (ISP) using emulsion PCR and enriched on the IonChef (Thermo Fisher Scientific). Template positive ISPs were loaded into Ion 540™ chips and run on the S5XL instrument in the Genomics Core in the Department of Urology at The Johns Hopkins Greenberg Bladder Cancer Institute. Primary analysis of RNA sequencing data was performed using AmpliSeqRNA analysis plugin in the Torrent Suite Software. This plugin aligned the raw sequence reads to a human reference genome that contains 20,802 RefSeq transcripts (hg19 Ampliseq Transcriptome_ERCC_V1.fasta) using the Torrent Mapping Alignment Program (TMAP). Then the number of reads mapped per gene were counted to generate raw counts files and normalized reads per gene per million mapped reads (RPM) files. To visualize expression patterns, tumor gene expression profiles were subjected to hierarchical clustering with Cluster and TreeView, 51 or log2 normalized expression values were analyzed by Morpheus matrix visualization and analysis software (Broad Institute, Cambridge, MA).
Statistics
Statistical analysis was performed with STATA® version 13. The p values were calculated using the chi-square test. Results were reported in mean±SD. Statistical significance was considered if p≤0.05.
RESULTS
The cohort included a total of 169 patients including 83 with non-muscle invasive bladder cancer and 86 patients with muscle invasive bladder cancer. There were 131 males and 38 females (ratio 3.44:1). The average age at diagnosis was 69 years old (range 42–86). The samples from the 83 patients with non-muscle invasive bladder cancer included 8 carcinoma in-situ (CIS; pTis) cases, 30 non-invasive papillary urothelial carcinomas (pTa), and 45 papillary urothelial carcinomas with invasion in the lamina propria (pT1). The samples from the 86 patients with muscle invasive bladder cancers were grouped based on the presence of divergent differentiation of the invasive component into conventional urothelial carcinoma (n=22); small cell carcinoma (n=15); micropapillary (n=11); sarcomatoid carcinoma (n=10); squamous cell carcinoma (n=10); plasmacytoid (n=8); glandular (n=6); and nested (n=4).
Immunohistochemistry
Conventional urothelial carcinoma
Overall, 72/83 (87%) of samples of non-muscle invasive urothelial carcinoma were positive for nectin-4. Twenty-nine of 30 (97%) non-invasive papillary urothelial carcinomas were positive, including 23/29 (80%) strong, 3/29 (10%) moderate, and 3/29 (10%) weakly positive. Thirty-six of 45 (80%) papillary urothelial carcinomas invading the lamina propria were positive, including 23/36 (64%) strong, 10/36 (28%) moderate, and 3 (8%) weakly positive. Seven of 8 (87.5%) CIS samples were positive, including 3/7 (42%) strong, 2/7 (29%) moderate, and 2/7 (29%) weak. Fifteen of 22 (68.2%) muscle invasive urothelial carcinomas were positive, including 12 (80%) specimens that were strong, 1 (6.6%) moderate, and 2 (12.5%) weak. There was no significant difference in the proportion of positive cases detected by TMAs versus whole sections (87% vs. 68.2%; p=0.08).
Squamous carcinoma
There were 10 cases with at least 50% of the tumor displaying squamous differentiation, defined as morphologic evidence of cytoplasmic keratinization and intercellular bridges.11 Seven of 10 tumors (70%) were positive in the squamous component, including 4 (57%) with strong staining, 2 (28%) with moderate staining and 1 (15%) case with weak staining. All cases which were positive in the squamous component were also positive in the invasive conventional urothelial carcinoma component. Three cases were negative in both the urothelial component and the conventional urothelial carcinoma component.
Plasmacytoid
Eight cases met criteria for plasmacytoid variant, which included invasive carcinomas composed of cells that resemble plasma cells or lobular carcinoma of the breast.11, 14 There were 5 tumors positive (62.5%), including 2 with strong staining (25%), 1 case with moderate staining (12.5%) and 2 cases with weak staining (12.5%). Figure 1.
Figure 1. Immunohistochemistry for nectin-4 in conventional urothelial carcinoma, urothelial carcinoma with squamous differentiation, and plasmacytoid urothelial carcinoma.
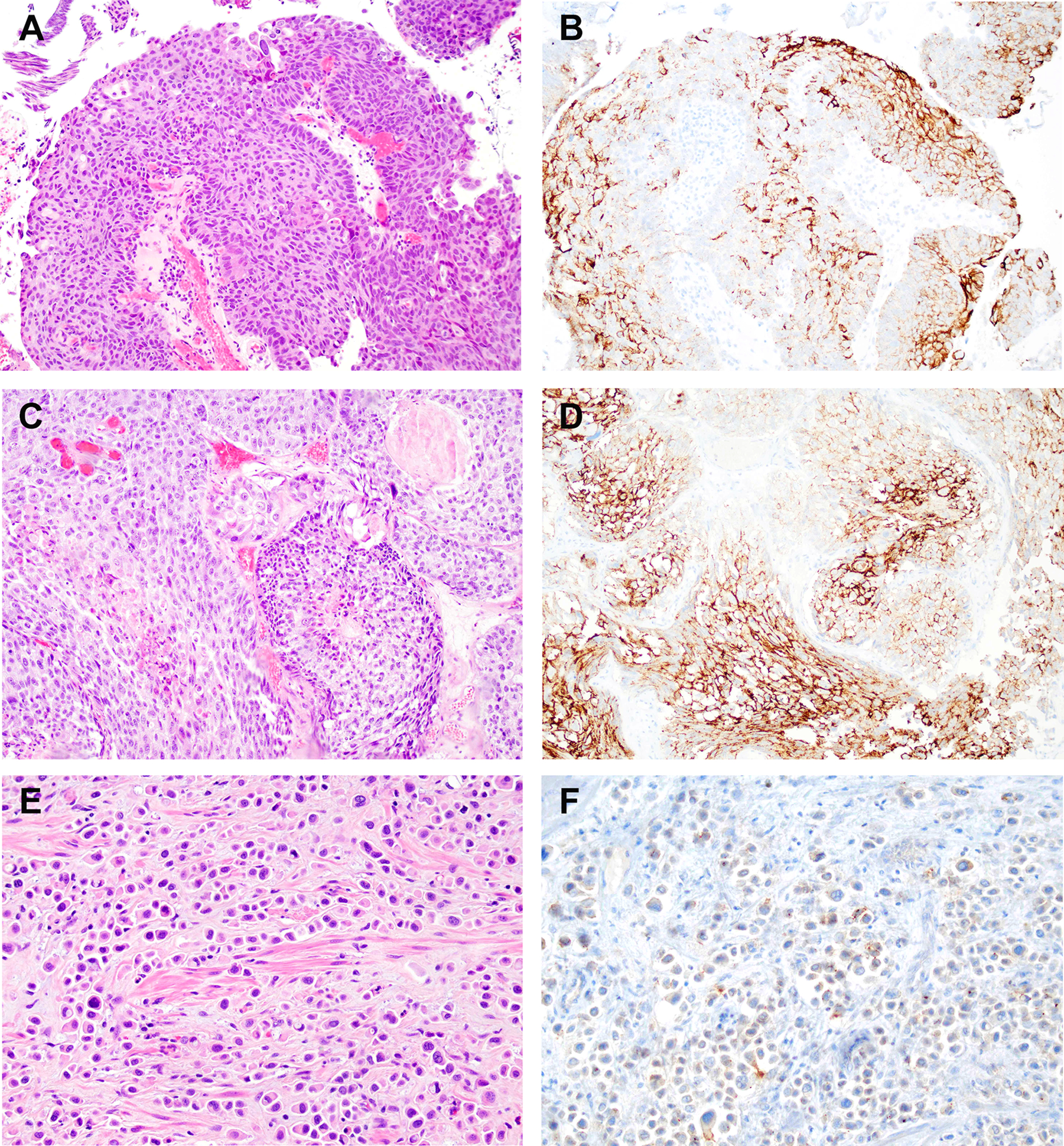
A. Hematoxylin and eosin (H&E) stained section of conventional urothelial carcinoma. B. Immunohistochemistry for nectin-4 in conventional urothelial carcinoma (same tumor as in “A”) showing strong membranous staining in the majority of tumor cells. C. H&E stained section of urothelial carcinoma with squamous differentiation. D. Immunohistochemistry for nectin-4 in same tumor as in “C” demonstrating strong cytoplasmic staining. E. H&E stained section of plasmacytoid variant of urothelial carcinoma. F. Immunohistochemistry for nectin-4 in same tumor as in “E” demonstrating weak staining in a subset of tumor cells.
Micropapillary
Diagnostic criteria for micropapillary variant included multiple nests of tumor within a single lacuna demonstrating small branching papillae or tufts without fibrovascular cores.11, 15 Three of 11 (28%) tumors were positive, all with weak but discrete staining.
Glandular
Glandular differentiation was determined by the presence of gland formation within the tumor.11, 13 Four of 6 tumors (66%) were positive including 3 (75%) strong and 1 (25%) weak.
Nested
There were 4 cases of nested urothelial carcinoma, defined as bland nests of urothelial carcinoma.11 Two tumors (50%) were positive with moderate staining. There was no conventional urothelial carcinoma in any of these cases. Figure 2.
Figure 2. Immunohistochemistry for nectin-4 in micropapillary urothelial carcinoma, urothelial carcinoma with glandular differentiation, and nested urothelial carcinoma.
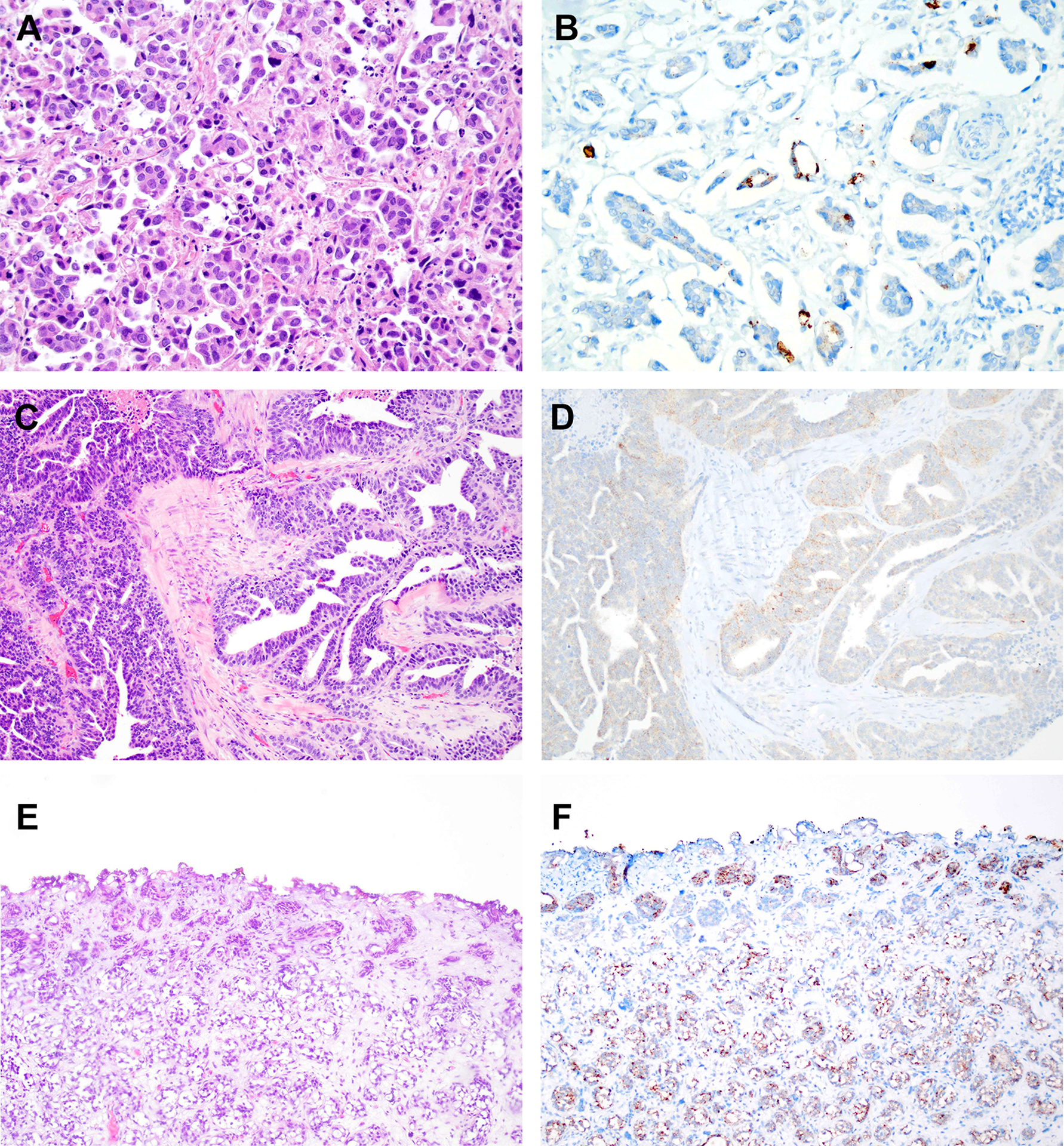
A. Hematoxylin and eosin (H&E) stained section of micropapillary urothelial carcinoma. B. Immunohistochemistry for nectin-4 in same tumor as in “A” showing strong membranous staining in a subset of tumor cells. C. H&E stained section of urothelial carcinoma with glandular differentiation. D. Immunohistochemistry for nectin-4 in same tumor as in “C” demonstrating weak cytoplasmic staining. E. H&E stained section of nested variant of urothelial carcinoma. F. Immunohistochemistry for nectin-4 in same tumor as in “E” demonstrating moderate staining in a subset of tumor cells.
Small cell carcinomas
All 15 cases of small cell carcinoma, including 9 pure small cell carcinoma and 6 mixed with conventional urothelial carcinoma, were negative. The conventional urothelial carcinoma component of cases with mixed small cell carcinoma was weakly positive in 7 of 9 cases (78%), moderately positive in 1 case (11%) and negative in 1 case (11%). Figure 3.
Figure 3. Immunohistochemistry and mRNA expression for nectin-4 in small cell carcinoma of the bladder and sarcomatoid urothelial carcinoma.
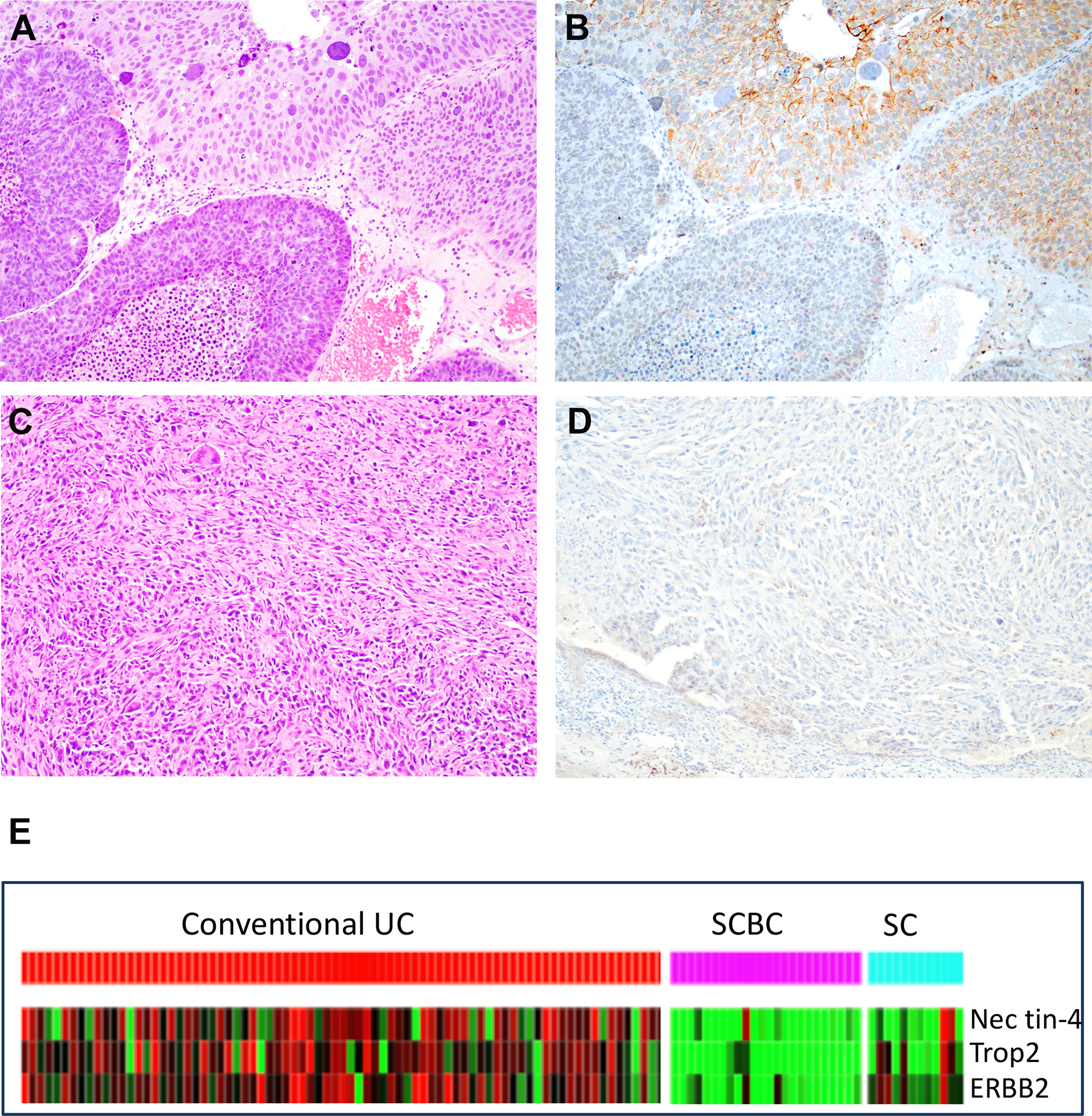
A. H&E section of conventional urothelial carcinoma with small cell carcinoma. B. Immunohistochemistry with nectin-4 in same tumor as in “A” showing strong staining in the conventional urothelial carcinoma (left, brown stain) while small cell carcinoma is negative. C. H&E stained section of sarcomatoid urothelial carcinoma. D. Immunohistochemistry for nectin-4 in same tumor as in “C” demonstrating complete absence of staining in tumor cells. E. Heat-map of next generation RNA sequencing of conventional urothelial carcinoma, small cell carcinoma of the bladder and sarcomatoid urothelial carcinoma. Red colors indicate overexpression. SC: sarcomatoid carcinoma; SCBC: small cell bladder cancer; UC: Urothelial carcinoma.
Sarcomatoid carcinomas
Nine of 10 sarcomatoid carcinomas were negative while 1 (10%) tumor was weakly positive. Figure 3.
Gene expression analysis
Whole transcriptome RNA sequencing was performed in cases of small cell carcinoma and sarcomatoid carcinoma to measure nectin-4 expression by an independent (and potentially more specific) method. These 2 groups were chosen among the different histologic variants because they expressed the lowest levels of nectin-4 protein. Compared to conventional urothelial carcinomas, most sarcomatoid carcinoma samples and all but two small cell carcinoma expressed very low levels of nectin-4 mRNA. In addition to nectin-4, we also evaluated expression of Trop2 and ERBB2, which are the molecular targets of two other ADCs - sacituzumab gavitecan (SG; trop-2) or trastuzumab deruxtecan (ERBB2/HER2).16 The results revealed that a subset of cases with very low nectin-4 expression expressed significant levels of either trop2 or ERBB2. Figure 3.
DISCUSSION
Targeted therapeutics have become the standard of care in oncology for many tumor types, but their development has lagged behind in bladder cancer.17–19 Patients with bladder cancer face challenges with treatment, given that more than half of the patients are older than 70 years with other comorbidities, and can have compromised functional status.20 ADCs are designed to target surface proteins that are highly enriched on tumor versus normal cells to enhance delivery of cytotoxic molecules to tumor cells, and reduce off-tumor toxicity. Cells that bind to some extracellular antibodies internalize them by endocytosis, which in the case of ADCs results in co-delivery of a cytotoxic “warhead.” 21
Expression of nectin-4 is essential for appropriate functional delivery of the ADC EV as it is the port of entry to tumor cells. Expression of nectin-4 by immunohistochemistry has been reported to be present in 83% of conventional urothelial carcinomas of the bladder with relatively equal proportions of strong, moderate, and weak staining.5 However, its expression in urothelial carcinomas with divergent differentiation and non-urothelial histotypes had not been reported. Furthermore, the antibody used in the study by Challita-Eid et al, was a mouse monoclonal antibody generated for the purpose of the study but the clone is not commercially available.5 In this study we used a commercially available antibody and confirmed that nectin-4 is highly expressed in conventional urothelial carcinoma. We found that its expression is highest in non-invasive carcinomas at about 90% or higher including papillary urothelial carcinomas and CIS. The rate of positivity drops to approximately 80% in urothelial carcinomas with invasion of the lamina propria, and to approximately 70% in muscle invasive urothelial carcinomas. The novel finding of this study is that nectin-4 expression is lower in muscle invasive carcinomas with morphologic variants or non-urothelial histotypes with virtually complete lack of nectin-4 protein expression in sarcomatoid carcinoma and small cell carcinomas, which was validated by low mRNA expression in these samples by RNAseq. These results have important implications for the use of EV in patients whose tumors have small cell and sarcomatoid differentiation.
Given the retrospective nature of this study that does not include information of response to therapy, it is not possible to determine whether expression of nectin-4 correlates with response to therapy by EV. Of note, the reported response rate of EV in bladder cancer patients is around 40%,22 which is much lower than the rate of positivity for nectin-4.
Recognition of morphologic variants of urothelial carcinoma continues to be a challenge in urologic surgical pathology with many reports modified after a second review by expert pathologists.23 Difficulties in the recognition of morphologic variants could explain, at least in part, the lack of significant difference in outcomes when patients with different variants are compared stage by stage at cystectomy. Some studies have identified worse outcomes in patients with histologic variants in initial biopsies because they are more commonly associated with more locally advanced disease.24–26 However, these studies were performed prior to the precision medicine era when all patients received a “standard of care” treatment with similar surgical resections and chemotherapy regimens. In this study we show that overall, carcinomas with divergent differentiation have lower expression rates of nectin-4, especially small cell carcinoma and sarcomatoid carcinomas that were uniformly negative. Based on our RNAseq results, these patients might benefit from other targeted therapies that rely on expression of trop2 (Sacituzumab gavitecan) or ERBB2 (trastuzumab).
The main limitation of this study is the lack of correlation with treatment outcomes. While expression of nectin-4 does not necessarily correlate with response to EV therapy, it is the port of cellular entry of the cytotoxic drug component MMAE, and therefore, without expression of nectin-4, there cannot be any expected response. Here we show that morphologic variants have decreased expression of nectin-4, especially small cell carcinomas and sarcomatoid carcinomas, and therefore and suggests that testing for nectin-4 expression should be considered before EV therapy in patients with these variants.
FUNDING
This work was supported by the Department of Pathology at Johns Hopkins University and by the NIH Institutional Research Training Grant T32 CA193145 to VP and SK.
Footnotes
CONFLICT OF INTEREST
All authors declare no conflict of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Carneiro BA, Meeks JJ, Kuzel TM, et al. Emerging therapeutic targets in bladder cancer. Cancer Treat Rev. 2015;41:170–178. [DOI] [PubMed] [Google Scholar]
- 3.Beck A, Reichert JM. Antibody-drug conjugates: present and future. MAbs. 2014;6:15–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mullard A. Maturing antibody-drug conjugate pipeline hits 30. Nat Rev Drug Discov. 2013;12:329–332. [DOI] [PubMed] [Google Scholar]
- 5.Challita-Eid PM, Satpayev D, Yang P, et al. Enfortumab Vedotin Antibody-Drug Conjugate Targeting Nectin-4 Is a Highly Potent Therapeutic Agent in Multiple Preclinical Cancer Models. Cancer Res. 2016;76:3003–3013. [DOI] [PubMed] [Google Scholar]
- 6.Samanta D, Almo SC. Nectin family of cell-adhesion molecules: structural and molecular aspects of function and specificity. Cell Mol Life Sci. 2015;72:645–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mori M, Rikitake Y, Mandai K, et al. Roles of nectins and nectin-like molecules in the nervous system. Adv Neurobiol. 2014;8:91–116. [DOI] [PubMed] [Google Scholar]
- 8.Rikitake Y, Mandai K, Takai Y. The role of nectins in different types of cell-cell adhesion. J Cell Sci. 2012;125:3713–3722. [DOI] [PubMed] [Google Scholar]
- 9.Tomiyama E, Fujita K, Rodriguez Pena MDC, et al. Expression of Nectin-4 and PD-L1 in Upper Tract Urothelial Carcinoma. Int J Mol Sci. 2020;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amin MB, Smith SC, Reuter VE, et al. Update for the practicing pathologist: The International Consultation On Urologic Disease-European association of urology consultation on bladder cancer. Mod Pathol. 2015;28:612–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moch H, Humphrey PA, Ulbright TM, et al. WHO classification of tumors of the urinary system and male genital organs. Lyon, France: International Agency for Research on Cancer (IARC); 2015. [Google Scholar]
- 12.Matoso A, Zhou Z, Hayama R, et al. Cell lineage-specific interactions between Men1 and Rb in neuroendocrine neoplasia. Carcinogenesis. 2008;29:620–628. [DOI] [PubMed] [Google Scholar]
- 13.Amin A, Murati-Amador B, Lombardo KA, et al. Analysis of Intestinal Metaplasia Without Dysplasia in the Urinary Bladder Reveal Only Rare Mutations Associated With Colorectal Adenocarcinoma. Appl Immunohistochem Mol Morphol. 2020;28:786–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borhan WM, Cimino-Mathews AM, Montgomery EA, et al. Immunohistochemical Differentiation of Plasmacytoid Urothelial Carcinoma From Secondary Carcinoma Involvement of the Bladder. Am J Surg Pathol. 2017;41:1570–1575. [DOI] [PubMed] [Google Scholar]
- 15.Hui Y, Lombardo KA, Quddus MR, et al. Cell Polarity Reversal Distinguishes True Micropapillary Growth From Retraction Artifact in Invasive Urothelial Carcinoma. Appl Immunohistochem Mol Morphol. 2018;26:e1–e6. [DOI] [PubMed] [Google Scholar]
- 16.Voelker R. Another Targeted Therapy for ERBB2-Positive Breast Cancer. JAMA. 2020;323:408. [DOI] [PubMed] [Google Scholar]
- 17.Mendiratta P, Grivas P. Emerging biomarkers and targeted therapies in urothelial carcinoma. Ann Transl Med. 2018;6:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fassan M, Trabulsi EJ, Gomella LG, et al. Targeted therapies in the management of metastatic bladder cancer. Biologics. 2007;1:393–406. [PMC free article] [PubMed] [Google Scholar]
- 19.Pilie PG, LoRusso PM, Yap TA. Precision Medicine: Progress, Pitfalls, and Promises. Mol Cancer Ther. 2017;16:2641–2644. [DOI] [PubMed] [Google Scholar]
- 20.Bellmunt J, Mottet N, De Santis M. Urothelial carcinoma management in elderly or unfit patients. EJC Suppl. 2016;14:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chau CH, Steeg PS, Figg WD. Antibody-drug conjugates for cancer. Lancet. 2019;394:793–804. [DOI] [PubMed] [Google Scholar]
- 22.Rosenberg J, Sridhar SS, Zhang J, et al. EV-101: A Phase I Study of Single-Agent Enfortumab Vedotin in Patients With Nectin-4-Positive Solid Tumors, Including Metastatic Urothelial Carcinoma. J Clin Oncol. 2020;38:1041–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordetsky J, Collingwood R, Lai WS, et al. Second Opinion Expert Pathology Review in Bladder Cancer: Implications for Patient Care. Int J Surg Pathol. 2018;26:12–17. [DOI] [PubMed] [Google Scholar]
- 24.Takemoto K, Teishima J, Kohada Y, et al. The Impact of Histological Variant on Oncological Outcomes in Patients With Urothelial Carcinoma of the Bladder Treated With Radical Cystectomy. Anticancer Res. 2020;40:4787–4793. [DOI] [PubMed] [Google Scholar]
- 25.Kim SP, Frank I, Cheville JC, et al. The impact of squamous and glandular differentiation on survival after radical cystectomy for urothelial carcinoma. J Urol. 2012;188:405–409. [DOI] [PubMed] [Google Scholar]
- 26.Wasco MJ, Daignault S, Zhang Y, et al. Urothelial carcinoma with divergent histologic differentiation (mixed histologic features) predicts the presence of locally advanced bladder cancer when detected at transurethral resection. Urology. 2007;70:69–74. [DOI] [PubMed] [Google Scholar]


