Abstract
Aims:
Although models exist to predict amputation among people with type 2 diabetes with foot ulceration or infection, we aimed to develop a prediction model for a broader range of major adverse limb events (MALE)—including gangrene, revascularization, and amputation—among individuals with type 2 diabetes.
Methods:
In a post-hoc analysis of data from the Exenatide Study of Cardiovascular Event Lowering (EXSCEL) trial, we compared participants who experienced MALE with those who did not. A multivariable model was constructed and translated into a risk score.
Results:
Among the 14,752 participants with type 2 diabetes in EXSCEL, 3.6% experienced MALE. Characteristics associated with increased risk of MALE were peripheral artery disease (PAD) (HRadj 4.83, 95% CI 3.94–5.92), prior foot ulcer (HRadj 2.16, 95% CI 1.63–2.87), prior amputation (HRadj 2.00, 95% CI 1.53–2.64), current smoking (HRadj 2.00, 95% CI 1.54–2.61), insulin use (HRadj 1.86, 95% CI 1.52–2.27), coronary artery disease (HRadj 1.67, 95% CI 1.38–2.03), and male sex (HRadj 1.64, 95% CI 1.31–2.06). Cerebrovascular disease, former smoking, age, glycated haemoglobin, race, and neuropathy were also associated significantly with MALE after adjustment. A risk score ranging from 6-96 points was constructed, with a C-statistic of 0.822 (95% CI 0.803–0.841).
Conclusions:
The majority of MALE occurred among participants with PAD, but participants without a history of PAD also experienced MALE. A risk score with good performance was generated. Although it requires validation in an external dataset, this risk score may be valuable in identifying patients requiring more intensive care and closer follow-up.
Keywords: peripheral artery disease, gangrene, amputation, type 2 diabetes
Introduction
More than 10% of the U.S. population – 34.2 million people – had type 2 diabetes in 2018 [1]. In 2016, there were 130,000 diabetes-related lower-extremity amputations (LEA) performed in the United States [2]. Individuals with type 2 diabetes have an increased risk of requiring LEA, with an age-adjusted non-traumatic LEA yearly rate of 4.62 (95% CI 4.25–5.00) per 1,000 adults with type 2 diabetes versus 0.18 (95% CI 0.17–0.18) per 1,000 adults without type 2 diabetes [1, 3]. Type 2 diabetes patients are also at risk of developing lower-extremity gangrene and requiring lower-extremity revascularization [4]. These other, non-LEA, major adverse limb events (MALE) may require hospitalizations, be associated with complications, and negatively affect individuals’ quality of life [5-7]. This increased risk of MALE (amputation, revascularization, or gangrene) among people with type 2 diabetes is partially related to the increased frequency of peripheral artery disease (PAD) in this population. PAD affects 20% of people with type 2 diabetes versus <5% of people without and is associated with worse cardiovascular and limb-related outcomes among persons with type 2 diabetes [8-10]. Yet, MALE also occurs in individuals with type 2 diabetes who do not have PAD [11]. Currently, the risk of MALE faced by a person with type 2 diabetes is poorly defined. Most of the available risk models predict only LEA and have been derived from patients being treated for ulcerations or gangrene [12-17]. Most scores therefore ignore non-LEA limb events that matter to patients, which limits their usefulness for preventing MALE.
The Exenatide Study of Cardiovascular Event Lowering (EXSCEL) trial randomized 14,752 participants at high risk of cardiovascular events across a wide range of type 2 diabetes severity, with median glycated haemoglobin [HbA1c] of 64 mmol/mol (interquartile range [IQR] 56, 74) (8.0%, IQR 7.3, 8.9) to once-weekly exenatide (EQW) versus placebo for the prevention of cardiovascular events. Our objective was to use data from EXSCEL to 1) describe differences in baseline clinical characteristics among participants with and without MALE, 2) examine the association between baseline factors and MALE, and 3) create a risk score for the occurrence of MALE among people with type 2 diabetes.
Participants and Methods
The EXSCEL trial design and primary outcomes have been reported elsewhere; EQW did not significantly decrease the rate of major adverse cardiovascular events [18, 19]. Participants were monitored for adverse events over the course of the trial and a 90-day post-trial follow-up period. The trial was designed and overseen by an executive committee, and an independent data and safety monitoring committee performed regular safety surveillance. All participants provided written informed consent, all participating institutions obtained institutional review board approval, and the trial was registered on ClinicalTrials.gov (NCT01144338).
Study Cohort
Individuals were eligible to enrol in EXSCEL if they had type 2 diabetes with an HbA1c of 48–96 mmol/mol (6.5 to 10%). Enrolment was stratified such that roughly 70% of participants had previous cardiovascular events, including any major clinical manifestation of coronary artery disease (CAD), ischemic cerebrovascular disease (CBVD), or PAD. These major clinical manifestations included, for CAD: myocardial infarction, surgical or percutaneous revascularization, or angiographic evidence of at least 50% stenosis in at least one major epicardial artery or branch vessel; for CBVD: a prior ischemic stroke or at least 50% carotid stenosis; and for PAD: a prior PAD-related amputation, prior surgical or percutaneous lower extremity revascularization, or current intermittent claudication with an ankle-brachial index (ABI) of less than 0.90.
Key exclusion criteria included two or more episodes of severe hypoglycaemia requiring third-party assistance in the preceding year, end-stage renal disease or an estimated glomerular filtration rate < 30 mL/min/1.73 m2 of body surface area, previous treatment with a glucagon-like peptide-1 receptor agonist (GLP-1 RA), personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia type 2, and a baseline calcitonin level > 40 ng/L.
Outcomes
For the present analysis, the primary outcome of interest was a composite of MALE, which included non-traumatic amputation, gangrene, and lower-extremity revascularization (endovascular or surgical). These events were collected by the site investigator and captured on the case report form but were not adjudicated. Other lower-extremity-related outcomes such as the development of infected ulcers or Charcot foot were not included in the list of clinical events collected at each follow-up visit and therefore were unable to be included in the composite outcome. Outcomes assessment was integrated into usual clinical care visits, which occurred at 1 week, 2 months, 6 months, and every 6 months thereafter for the duration of trial enrolment (median follow-up of 3.2 years overall) [19]. Mortality was also reported at 3 years using Kaplan–Meier estimates.
Statistical Approach
Participants were categorized according to whether they did or did not experience MALE during follow-up. Risk of MALE associated with baseline demographic, comorbidity, and diabetes-related features was evaluated using univariable Cox proportional hazards models with time to MALE as an outcome and mortality used as a competing risk. Complications of diabetes such as neuropathy and diabetic eye disease were collected based on participant history, not dedicated physical examination manoeuvers.
The predictive model was a proportional hazards regression model developed using stepwise selection (alpha ≤ 0.05) from among a pool of candidate variables preselected based on clinical feasibility. Mortality was used as a competing risk throughout the covariate selection and model evaluation process. These candidate variables included demographics (age, sex, race), medical history (body mass index, smoking status [current, former, or never], CAD, PAD, CBVD, polyvascular disease [defined as ≥2 of CAD, CBVD, or PAD], estimated glomerular filtration rate, previous cardiovascular events), measures of diabetes severity (duration of type 2 diabetes, HbA1c, insulin use, previous amputation, previous foot ulceration, retinopathy, blindness, albuminuria, or neuropathy), and treatment allocation. Participants of American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, or Other races were combined into a single “Other” category for the model because of a low number of events among these participants. Aspirin and statin use were also included as candidate variables, but other medications (beta-blockers, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, and other antiplatelet agents) were not included because they have not been shown to have an effect on MALE in other trial and registry analyses. Therefore, including these medications in a model to predict MALE would run the risk of spuriously attributing risk of MALE to these medications due to unadjusted-for confounders. Discrimination of the model was evaluated using a C-statistic with a bootstrapped 95% confidence interval and compared with an optimism-corrected C-statistic to evaluate for overfitting [20]. The model’s calibration was assessed using a decile plot to compare observed with predicted risk values.
From the predictive model, a risk score was created by assigning numeric values to each covariate included in the predictive model based on magnitude of association. This predictive score was also evaluated using a C-statistic with a bootstrapped 95% confidence interval.
All statistical analyses were performed in SAS version 9.4 (SAS Institute, Cary, NC). As EXSCEL was approved by institutional review boards at each participating site, this secondary analysis did not require institutional review board approval.
Results
Baseline Characteristics and Comorbidities of Participants With MALE
Of the 14,752 participants with type 2 diabetes enrolled in EXSCEL (with average 3.2 years’ follow-up), 523 (3.55%) had one or more MALE. Those with MALE were older compared to those without MALE (median age 64 [IQR 58, 70] vs. 62 [56, 68] years, p<.0001) and less likely to be female (21.2% vs. 38.6%, p<.0001; Table 1). Participants with MALE were more likely to be current smokers or former smokers (17.2% vs. 11.5%, and 50.1% vs. 38.1%, respectively, p<.0001), and to have CAD (60.8% vs. 50.9%, p<.0001), CBVD (22.2% vs. 16.4%, p<.0001), PAD (56.4% vs. 16.4%, p<.0001), or polyvascular disease (39.6% vs. 10.9%, p<.0001). Participants with MALE also had lower estimated glomerular filtration rate (median 71 [IQR 57, 89] vs. 77 [61, 92] mL/min/1.73 m2, p<.0001). Patients with MALE without a history of PAD were more likely to have histories of CAD or CBVD but less likely to have a smoking history or to have polyvascular disease than patients with MALE with a history of PAD (Table 2).
Table 1:
Baseline demographic and medical characteristics of participants with and without MALE
| Overall (N=14,752) |
No Event (N=14,229) |
≥ 1 MALE (N=523) |
p-Value* | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 62 (56, 68) | 62 (56, 68) | 64 (58, 70) | <.0001 |
| Female | 5,603 (38.0%) | 5,492 (38.6%) | 111 (21.2%) | <.0001 |
| Race† | <.0001 | |||
| White | 11,175 (75.8%) | 10,724 (75.4%) | 451 (86.2%) | |
| Asian | 1,452 (9.8%) | 1,438 (10.1%) | 14 (2.7%) | |
| Black or African American | 878 (6.0%) | 850 (6.0%) | 28 (5.4%) | |
| American Indian or Alaska Native | 73 (0.5%) | 71 (0.5%) | 2 (0.4%) | |
| Native Hawaiian or other Pacific Islander | 35 (0.2%) | 35 (0.2%) | 0 (0.0%) | |
| Other | 1,134 (7.7%) | 1,106 (7.8%) | 28 (5.4%) | |
| BMI‡ | 32 (28, 36) | 32 (28, 36) | 32 (29, 36) | .8508 |
| Smoking status at baseline§ | <.0001 | |||
| Current | 1,721 (11.7%) | 1,631 (11.5%) | 90 (17.2%) | |
| Former | 5,791 (39.3%) | 5,529 (38.9%) | 262 (50.1%) | |
| Never | 7,233 (49.1%) | 7,062 (49.7%) | 171 (32.7%) | |
| Prior history | ||||
| Previous MI | 4,679 (31.7%) | 4,489 (31.5%) | 190 (36.3%) | .0038 |
| Previous stroke | 1,867 (12.7%) | 1,788 (12.6%) | 79 (15.1%) | .0284 |
| CAD | 7,555 (51.2%) | 7,237 (50.9%) | 318 (60.8%) | <.0001 |
| CBVD | 2,450 (16.6%) | 2,334 (16.4%) | 116 (22.2%) | <.0001 |
| Polyvascular disease (≥2 of PAD, CAD, or CBVD) | 1,760 (11.9%) | 1,553 (10.9%) | 207 (39.6%) | <.0001 |
| CBVD and PAD | 173 (1.17%) | |||
| CAD and PAD | 602 (4.08%) | |||
| CBVD and CAD | 797 (5.40%) | |||
| CBVD, PAD, and CAD | 188 (1.27%) | |||
| PAD | 2,624 (17.8%) | 2,329 (16.4%) | 295 (56.4%) | <.0001 |
| Medications | ||||
| Exenatide | 7,356 (49.9%) | 7,108 (50.0%) | 248 (47.4%) | 0.217 |
| ACE inhibitor | 7,182 (48.7%) | 6,884 (48.4%) | 298 (57.0%) | .0005 |
| Angiotensin receptor blocker | 4,606 (31.2%) | 4,456 (31.3%) | 150 (28.7%) | .2671 |
| Beta blocker | 8,211 (55.7%) | 7,868 (55.3%) | 343 (65.6%) | <.0001 |
| Aspirin | 9,380 (63.6%) | 8,993 (63.2%) | 387 (74.0%) | <.0001 |
| Clopidogrel/ticlopidine | 2,524 (17.1%) | 2,388 (16.8%) | 136 (26.0%) | <.0001 |
| Other antiplatelet | 564 (3.8%) | 537 (3.8%) | 27 (5.2%) | .0455 |
| Statin | 10,845 (73.5%) | 10,433 (73.3%) | 412 (78.8%) | .0046 |
| Laboratory measures ‖ | ||||
| eGFR (mL/min/1.73 m2) | 76 (61, 92) | 77 (61, 92) | 71 (57, 89) | <.0001 |
| eGFR ≤ 60 | 3,436 (23.4%) | 3,274 (23.1%) | 162 (31.1%) | <.0001 |
| Diabetes-related characteristics | ||||
| Type 2 diabetes duration (years) ¶ | 12 (7, 18) | 12 (7, 17) | 14 (10, 21) | <.0001 |
| HbA1c (%)# | 8.0 (7.3, 8.9) | 8.0 (7.3, 8.9) | 8.3 (7.5, 9.1) | <.0001 |
| Insulin | 6,836 (46.3%) | 6,466 (45.4%) | 370 (70.7%) | <.0001 |
| Type 2 diabetes complications | ||||
| Prior amputation | 520 (3.5%) | 401 (2.8%) | 119 (22.8%) | <.0001 |
| Prior foot ulcer | 463 (3.1%) | 366 (2.6%) | 97 (18.5%) | <.0001 |
| Retinopathy | 2,516 (17.1%) | 2,363 (16.6%) | 153 (29.3%) | <.0001 |
| Blindness | 154 (1.0%) | 149 (1.0%) | 5 (1.0%) | .8291 |
| Albuminuria | 2,356 (16.0%) | 2,217 (15.6%) | 139 (26.6%) | <.0001 |
| Neuropathy | 4,838 (32.8%) | 4,540 (31.9%) | 298 (57.0%) | <.0001 |
| Microvascular disease (≥2 of retinopathy, neuropathy, or albuminuria) | 6,832 (46.3%) | 6,482 (45.6%) | 350 (66.9%) | <.0001 |
Data are n (%) or median (IQR). ACE: angiotensin-converting enzyme; CAD: coronary artery disease; CBVD: cerebrovascular disease; eGFR: estimated glomerular filtration rate; HbA1c: glycated haemoglobin; IQR: interquartile range; MALE: major adverse limb events; MI: myocardial infarction; PAD: peripheral artery disease.
Comparing participants with and without ≥1 MALE.
Available in 14,224 participants without MALE.
Available in 14,089 participants without MALE and 513 participants with MALE.
Available in 14,222 participants without MALE.
Available in 14,184 participants without MALE and 521 participants with MALE.
Available in 14,178 participants without MALE and 521 participants with MALE.
Available in 14,155 participants without MALE and 520 participants with MALE.
Table 2:
Characteristics of patients experiencing MALE with and without PAD
| Overall (N=523) |
No PAD (N=228) |
PAD (N=295) |
|
|---|---|---|---|
| Demographics | |||
| Age (years) | 64 (58, 70) | 64 (58, 70) | 64 (58, 70) |
| Female | 111 (21.2%) | 58 (25.4%) | 53 (18.0%) |
| White race | 451 (86.2%) | 199 (87.3%) | 252 (85.4%) |
| BMI* | 32 (29, 36) | 33 (30, 37) | 32 (29, 35) |
| Smoking status at baseline | |||
| Current | 90 (17.2%) | 38 (16.7%) | 52 (17.6%) |
| Former | 262 (50.1%) | 106 (46.5%) | 156 (52.9%) |
| Never | 171 (32.7%) | 84 (36.8%) | 87 (29.5%) |
| Prior history | |||
| Previous MI | 190 (36.3%) | 96 (42.1%) | 94 (31.9%) |
| Previous stroke | 79 (15.1%) | 49 (21.5%) | 30 (10.2%) |
| CAD | 318 (60.8%) | 157 (68.9%) | 151 (54.6%) |
| CBVD | 116 (22.2%) | 58 (25.4%) | 58 (19.7%) |
| Polyvascular disease (≥2 of PAD, CAD, or CBVD) | 207 (39.6%) | 27 (11.8%) | 180 (61.0%) |
| Medications | |||
| Exenatide | 248 (47.4%) | 109 (47.8%) | 139 (47.1%) |
| ACE inhibitor | 298 (57.0%) | 135 (59.2%) | 163 (55.3%) |
| Angiotensin receptor blocker | 150 (28.7%) | 63 (27.6%) | 87 (29.5%0 |
| Beta blocker | 343 (65.6%) | 163 (71.5%) | 180 (61.0%) |
| Aspirin | 387 (74.0%) | 164 (71.9%) | 223 (75.6%) |
| Clopidogrel/ticlopidine | 136 (26.0%) | 61 (26.8%) | 75 (25.4%) |
| Other antiplatelet | 27 (5.2%) | 9 (3.9%) | 18 (6.1%) |
| Statin | 412 (78.8%) | 176 (77.2%) | 236 (80.0%) |
| Laboratory measures † | |||
| eGFR (mL/min/1.73 m2) | 71 (57, 89) | 71 (56, 88) | 71 (57, 91) |
| eGFR ≤ 60 | 162 (31.1%) | 76 (33.6%) | 86 (29.2%) |
| Diabetes-related characteristics | |||
| Type 2 diabetes duration (years)† | 14 (10, 21) | 15 (9, 21) | 14 (10, 21) |
| HbA1c (%)‡ | 8.3 (7.5, 9.1) | 8.3 (7.5, 9.2) | 8.2 (7.5, 9.1) |
| Insulin | 370 (70.7%) | 161 (70.6%) | 209 (70.8%) |
| Type 2 diabetes complications | |||
| Prior amputation | 119 (22.8%) | 5 (2.2%) | 114 (38.6%) |
| Prior foot ulcer | 97 (18.5%) | 21 (9.2%) | 76 (25.8%) |
| Retinopathy | 153 (29.3%) | 59 (25.9%) | 94 (31.9%) |
| Blindness | 5 (1.0%) | 1 (0.4%) | 4 (1.4%) |
| Albuminuria | 139 (26.6%) | 53 (23.2%) | 86 (29.2%) |
| Neuropathy | 298 (57.0%) | 109 (47.8%) | 189 (64.1%) |
Data are n (%) or median (IQR). ACE: angiotensin-converting enzyme; CAD: coronary artery disease; CBVD: cerebrovascular disease; eGFR: estimated glomerular filtration rate; HbA1c: glycated haemoglobin; IQR: interquartile range; MALE: major adverse limb events; MI: myocardial infarction; PAD: peripheral artery disease.
Available in 513 participants with MALE.
Available in 521 participants with MALE.
Available in 520 participants with MALE.
Diabetes Severity Among Participants With MALE
Participants with MALE had a longer duration of type 2 diabetes (14 [IQR 10, 21] vs. 12 [7, 17] years, p<.0001), had slightly but significantly higher HbA1c (8.3% [7.5, 9.1] vs. 8.0% [7.3, 8.9], p<.0001), and were much more likely to be treated with insulin (70.7% vs. 45.4%, p<.0001, Table 1). Participants with MALE more frequently had prior amputations (22.8% vs. 2.8%, p<.0001), prior foot ulcers (18.5% vs. 2.6%, p<.0001), retinopathy (29.3% vs. 16.6%, p<.0001), albuminuria (26.6% vs. 15.6%, p<.0001), and neuropathy (57.0% vs. 31.9%, p<.0001).
MALE Types and Associated Factors
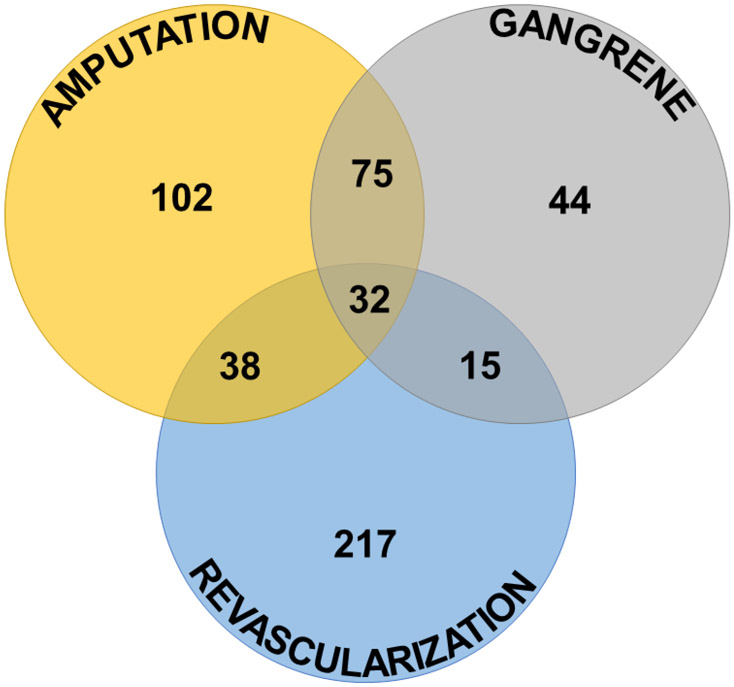
In total, there were 736 events among the 523 participants with ≥1 MALE, including 247 amputations, 166 episodes of gangrene, and 323 revascularizations (218 endovascular and 105 surgical) (Figure 1). A single event was experienced by 363 participants, two by 128 participants, and three by 32 participants. In the predictive model, the risk of having ≥1 MALE remained associated with PAD (HRadj 4.83, 95% CI 3.94–5.92), prior foot ulcer (HRadj 2.16, 95% CI 1.63–2.87), prior amputation (HRadj 2.00, 95% CI 1.53–2.64), current smoking (HRadj 2.00, 95% CI 1.54–2.61), insulin use (HRadj 1.86, 95% CI 1.52–2.27), CAD (HRadj 1.67, 95% CI 1.38–2.03), male sex (HRadj 1.64, 95% CI 1.31–2.06), former smoking (HRadj 1.41, 95% CI 1.15–1.72), and CBVD (HRadj 1.41, 95% CI 1.14–1.73) (Table 3). HbA1c (HRadj 1.24, 95% CI 1.13–1.36), neuropathy (HRadj 1.26, 95% CI 1.03–1.53), and older age were also associated significantly with MALE, although age was associated only among participants aged 65 years or younger. Asian and Other races were associated with lower risk of MALE compared with white race (HRadj 0.35, 95% CI 0.21–0.61, and HRadj 0.66, 95% CI 0.45–0.95, respectively). Assignment to the EQW treatment arm was not associated with MALE on unadjusted analysis (HR 0.90, 95% CI 0.76–1.07) or adjusted analysis (HRadj 0.86, 95% CI 0.72–1.02). The C-index for this model was 0.822 (95% CI 0.803–0.842), and it was well-calibrated (Supplemental Figure S1A). The optimism-corrected C-index was very similar (0.820, 95% CI 0.801–0.839), indicating minimal risk of overfitting.
Figure 1: Major adverse limb events (MALE) components and overlap.
Most participants experienced only one MALE (most commonly revascularization), with gangrene and amputation the most common pairing of two events.
Table 3:
Multivariable predictive model for MALE
| Covariate | HR (95% CI) | P Value |
|---|---|---|
| PAD | 4.83 (3.94–5.92) | <.0001 |
| Prior Foot Ulcer | 2.16 (1.63–2.87) | <.0001 |
| Prior Amputation | 2.00 (1.53–2.64) | <.0001 |
| Insulin | 1.86 (1.52–2.27) | <.0001 |
| Male Sex | 1.64 (1.31–2.06) | <.0001 |
| CAD | 1.67 (1.38–2.03) | <.0001 |
| Current smoker (versus never) | 2.00 (1.54–2.61) | <.0001 |
| Former smoker (versus never) | 1.41 (1.15–1.72) | |
| Age, per 10-year increase* | 1.42 (1.20–1.69) | <.0001 |
| CBVD | 1.41 (1.14–1.73) | .0015 |
| Neuropathy | 1.26 (1.03–1.53) | 0.0248 |
| Baseline HbA1c | 1.24 (1.13–1.36) | <.0001 |
| Race Group | .0002 | |
| Race=Asian (vs. White) | 0.35 (0.21–0.61) | . |
| Race=Black (vs. White) | 0.79 (0.54–1.17) | . |
| Race=Other (vs. White) | 0.66 (0.45–0.95) | . |
Table includes variables meeting model entry criterion (alpha ≤ 0.05) using stepwise selection methods. Medications including aspirin, statin, and exenatide were included as candidate variables but did not meet model entry criteria (alpha ≤ 0.05). CAD: coronary artery disease; CBVD: cerebrovascular disease; HbA1c: glycated haemoglobin; PAD: peripheral artery disease.
When age ≤65 years.
Risk Score for MALE
Using the developed predictive model, a risk score was constructed with a maximum score of 96 (Table 4). Participants with 40 points or fewer had a ≤0.8% chance of MALE at 3 years (95% CI 0.7–1.0%), increasing to 11.8% in participants with 65–69 points (95% CI 10.7–12.9%), and 64.5% in participants with 85–89 points (95% CI 58.0–71.7%, Supplemental Figure S2). The C-index for this risk score was 0.822 (95% CI 0.803–0.841), and the score was well-calibrated (Supplemental Figure S1B).
Table 4:
MALE risk score
| Age Range | Score | Sex & Race | Score | Medical History | Score | HbA1c Range | Score |
|---|---|---|---|---|---|---|---|
| <20 | 0 | Prior Amputation | 6 | <5 | 0 | ||
| 20–24 | 1 | Male | 4 | History of PAD | 14 | 5–6.99 | 4 |
| 25–29 | 3 | History of CAD | 4 | 7–8.99 | 8 | ||
| 30–34 | 4 | White | 15 | Prior Foot Ulcer | 7 | 9–10.99 | 12 |
| 35–39 | 6 | Black | 13 | History of CBV Disease | 3 | ≥11 | 16 |
| 40–44 | 7 | Asian | 6 | Neuropathy | 2 | ||
| 45–49 | 9 | Other | 11 | Medicated with Insulin | 6 | ||
| 50–54 | 10 | ||||||
| 55–59 | 12 | Current Smoker | 6 | ||||
| ≥60 | 13 | or Former Smoker | 3 | ||||
| ____ | + | ____ | + | ___ | + | ____ |
Total score is obtained by summing the category scores, with a score range from 6 to 96.
Mortality and Associated Factors
There were 765 deaths by 3 years (5.2%). The model created to predict MALE predicted mortality with a C-statistic of 0.694. Prior amputation, neuropathy, and race were no longer significant when the model was used to predict mortality (p>0.05, Supplemental Table S1).
Discussion
Overall, this analysis of EXSCEL study participants who experienced ≥1 MALE had several key findings. First, risks of MALE based on baseline clinical data could be reasonably predicted with a risk score generally considered as very good with a C-statistic of 0.822. Second, most of the factors associated with MALE in our model were non-modifiable, illustrating the importance of prevention of vascular disease. Third, atherosclerosis of other arterial beds (coronary, cerebrovascular) was predictive of MALE even in the absence of PAD. Fourth, exenatide was not associated with lower risk of MALE. These findings help contextualize MALE occurrence and related risk factors while suggesting some possible ways to further study and treat this at-risk population.
Other models exist to predict amputations among individuals with diabetes. Our model performs similarly, with a C-statistic of 0.822 that is comparable to other models that have reported C-statistics around 0.80 [13, 15, 16]. However, our model differs in several important ways. First, it is the only one to predict gangrene and revascularizations together with amputation. While amputations are certainly deeply concerning to patients, gangrene and revascularizations may also significantly negatively affect quality of life and should be included as events of interest in predictive models [4-6].
Second, all other models except the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model and a model developed by Martins-Mendes et al predict amputation among a cohort of individuals who already have foot ulcers or gangrene [14-16]. While it is helpful to know which patients are more or less likely to heal, predicting which patients will suffer limb events in the absence of current ulceration or gangrene is also valuable.
Third, the EXSCEL study cohort from which we derived our model differs from the UKPDS and Martins-Mendes cohorts in several key ways: the UKPDS amputation model was derived from a study cohort consisting of 3,642 participants with newly diagnosed type 2 diabetes and excluded individuals with severe vascular disease, recent history of myocardial infarction or CAD, heart failure, or any other systemic illness that would limit life expectancy. The Martins-Mendes cohort consisted of 644 participants without diabetic foot ulcers who were being treated at a diabetic foot outpatient clinic at the time of enrolment [11-13]. Both of these cohorts were therefore limited in different, but important, ways. In contrast, the EXSCEL study cohort was much larger and included participants with prolonged histories of type 2 diabetes with high frequencies of other comorbidities as well, characteristics that are considered to be associated with higher MALE risks.
Fourth, the data points required as input to our model are all demographic and medical history information (in addition to HbA1c) routinely available in clinical care, rather than extensive physical examination, functional testing, or imaging data as is required in most other diabetes amputation-prediction models [14-16]. While these latter features are crucial in clinical care, a model that relies on them is less flexible and broadly applicable. In this case, the model we generated may have traded some discriminatory power for increased flexibility, insofar as physical examination findings such as Charcot foot may have improved the model’s performance. However, in the model’s current form, the included risk factors can generally be obtained from structured data fields in the electronic health record (EHR), which theoretically may allow patients to be quickly and efficiently risk-assessed using an EHR-incorporated algorithm. For instance, our model might be used for risk enrichment in trial settings or to define care pathways integrated with research, especially those that rely on EHRs for cohort identification.
Beyond investigational uses, our MALE predictive model may have utility in clinical practice. Unfortunately, other than current smoking and HbA1c, the factors that contributed to MALE risk in our model were nonmodifiable, which means the clinical application of this model must be predominantly focused on aggressive treatment of medical history and comorbidity risk factors among patients at high risk of MALE. Given that PAD was the single strongest predictor of MALE in this analysis, guideline-directed optimization of PAD treatment is of paramount importance [21, 22]. Among the patients with PAD who experienced MALE in this analysis, only 75.6% were taking aspirin and 80.0% a statin (with many of those patients potentially taking low- or moderate-intensity statins). The strong association between MALE and PAD seen in this analysis was not unexpected, although the strength of association between PAD and amputation has ranged widely in previous publications, from an adjusted odds ratio of 2.11 in Lipsky et al to an HRadj for PAD of 11.4 in the UKPDS model [11, 15].
The finding that 228 limb events occurred among participants without documented PAD is a reminder that diabetic individuals are at risk for limb events regardless of whether they carry a diagnosis of PAD. For instance, some patients without PAD who experienced MALE may have had Charcot foot or, more importantly, foot infections, which are strongly connected with amputations and were not adjudicated in EXSCEL, driving home the importance of physical exam findings in fully risk-stratifying patients [4]. It is also possible that additional participants had asymptomatic PAD but were not categorized as such because participants in EXSCEL were considered to have PAD only if they had symptoms with a diagnostic ankle- or toe-brachial index or a prior amputation or revascularization. This conjecture is supported by the observation that CAD and CBVD were independently predictive of MALE in the absence of (documented) PAD. Patients with MALE without PAD were more likely to have CAD and CBVD than patients with PAD in this analysis, pointing to the likelihood of systemic atherosclerosis affecting their lower extremities even without lower extremity symptoms or prior intervention. For instance, in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial, in which 2,240 participants with type 2 diabetes and CAD underwent ABI testing, 33% of participants had low, high, or noncompressible ABIs, and 68% of participants with low ABIs were asymptomatic [23].
People with even asymptomatic PAD are at higher risk of cardiovascular and limb events than are individuals without PAD and should be treated with aspirin and statins for risk reduction – but they must be diagnosed before appropriate treatment can begin [24]. Currently, the American Diabetes Association (ADA) recommends testing for PAD among people with diabetes only if they have symptoms or absent pedal pulses [25]. In contrast, the 2016 American College of Cardiology/American Heart Association guidelines recommend ABI screening in the absence of signs or symptoms of PAD among patients who are at increased risk (age ≥ 65 years; age 50-64 years with diabetes, smoking history, hyperlipidaemia, hypertension, or family history of PAD; age <50 years with diabetes plus one other risk factor; or any individual with known atherosclerosis in another vascular bed). This latter broad categorization therefore includes a huge range of potential people [21]. Unfortunately, ABIs have limitations, especially among patients with diabetes — a recent study of 1,204 patients undergoing endovascular revascularization in the LIBERTY 360 study showed that 20.8% of the 727 patients with diabetes and 16.4% of the 462 nondiabetic patients had normal ABIs despite having severe enough PAD to require revascularization [26]. ABIs are insensitive to the microvascular disease affecting many patients with diabetes, but there is no consensus surrounding the optimal method to measure the severity of microvascular disease [27]. In recognition of the lack of randomized data supporting broad ABI screening, the American College of Cardiology/American Heart Association recommendation is only of moderate strength (IIa B-NR), but the presence of specific management guidelines that pertain to patients with PAD suggest that efforts to recognize even asymptomatic PAD may be of value.
Therefore, one possible use for a MALE-predictive model may be to identify persons who are at high risk for MALE but have not yet had PAD documented and may therefore benefit from PAD diagnostic evaluation, while also identifying high-risk patients with documented PAD who may merit more frequent foot exams and referral to a vascular specialist. Currently, the ADA recommends yearly foot examinations for prevention and early detection of limb events, with more frequent exams recommended for patients with neuropathy or prior ulceration/amputation [25]. Evidence suggests that these foot screening guidelines are followed 50% or less of the time [28-31]. The low rate of adherence to foot screening guidelines is likely due to a wide range of factors, but risk-stratifying patients for MALE would allow more targeted deployment of foot care-related resources toward patients at higher risk.
Assignment to exenatide was not associated with any increased or decreased risk of MALE in EXSCEL. While it is reassuring that no increased risk was observed (unlike with canagliflozin in the Canagliflozin Cardiovascular Assessment Study [CANVAS]) [32], a secondary analysis of liraglutide in the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) study found that patients assigned to liraglutide had a lower risk for amputation related to diabetic foot ulcers [33]. Although the LEADER analysis used as its endpoint a specific manifestation of amputation (related to diabetic foot ulcer, rather than amputation in general), one might expect to observe a benefit of exenatide on limb events in EXSCEL given data supporting a class effect with GLP-1 RAs on some CV outcomes. However, the patient cohort in LEADER was markedly different from EXSCEL insofar as the participants had higher HbA1c levels, a higher proportion had a history of foot ulcers, and a lower proportion had PAD. Therefore, it may be that the pathway leading to amputation upon which liraglutide acts is pathophysiologically distinct from lower-extremity atherosclerosis.
This analysis has several limitations. First, although the EXSCEL cohort was large, MALE were relatively uncommon, and the EXSCEL study was not powered to study the MALE endpoint. However, other diabetes cardiovascular outcomes trials have aimed to exclude individuals at risk for MALE. This also means that our risk model may be less applicable to people at very high risk of MALE. Second, information about PAD severity was missing, although we think that using only demographic and history data has led to a uniquely flexible model with potential applications to computable phenotyping. Third, the events constituting the MALE outcome were collected by site investigators but did not undergo adjudication. It is likely that this negatively affected the quality of the MALE endpoint (for instance, 44 people improbably had isolated gangrene without revascularization or amputation), but we do not see any evidence that there were systematic patterns of underreporting that would invalidate the model. Fourth, as discussed above, our risk score requires external validation, as training and validating a model within a single cohort raises the risk of overfitting the model. While our optimism-corrected C-statistic was very similar to the regular C-statistic [20], the fact remains that patients enrolled in EXSCEL may not be representative of the broader population of people with diabetes.
This post hoc analysis of the EXSCEL trial yielded a predictive score for MALE (amputation, revascularization, or gangrene) among participants with diabetes that had a C-statistic of 0.825, generally considered as very good discrimination. Factors associated with MALE in this model included age, race, gender, and smoking; CAD and CBVD; and features related to type 2 diabetes such as elevated HbA1c, insulin use, neuropathy, prior ulceration, and prior amputation. Although the majority of MALE happened among participants with PAD, a significant minority occurred among participants with no documented history of PAD. This suggests that individuals at high risk of MALE without a documented diagnosis of PAD could be usefully identified by a risk score as they may require additional evaluation to rule out asymptomatic PAD. Equally, a MALE risk score may identify individuals with documented PAD at high risk of subsequent MALE, leading to more aggressive treatment and vascular referral.
Supplementary Material
What’s new?
Although risk scores exist to predict amputation in specific subgroups of people with type 2 diabetes, no risk score exists to predict all lower extremity events among a broader cohort of people with type 2 diabetes.
Using data from the EXSCEL trial, we derived a risk score to predict gangrene, revascularization, and amputation with a C-statistic of 0.825 (95% CI 0.806–0.844).
Peripheral artery disease (PAD) was the strongest predictor of lower extremity events, but events also occurred among patients without PAD, suggesting that the threshold for screening people with type 2 diabetes for PAD may need to be lowered.
Acknowledgments:
The authors would like to thank Peter Hoffmann of the Duke Clinical Research Institute for his assistance in editing the manuscript.
Sources of Funding
EXSCEL was sponsored and funded by Amylin Pharmaceuticals Inc. (San Diego, CA), a wholly owned subsidiary of AstraZeneca (Gaithersburg, MD). E.H.W. receives support from the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH) (1R38HL143612), and J.B.B. is supported by grants from the NIH (UL1TR002489, P30DK124723).
Footnotes
Duality of interest
EHW: None
RMC: None
YL: None
JBB: contracted consulting fees, paid to his institution, and travel support from Adocia, AstraZeneca, Dance Biopharm, Eli Lilly, MannKind, NovaTarg, Novo Nordisk, Sanofi, Senseonics, vTv Therapeutics, and Zafgen as well as grant support from NovaTarg, Novo Nordisk, Sanofi, Tolerion and vTv Therapeutics. He is also a consultant to Cirius Therapeutics Inc, CSL Behring, Mellitus Health, Neurimmune AG, Pendulum Therapeutics, and Stability Health. He holds stock/options in Mellitus Health, Pendulum Therapeutics, PhaseBio, and Stability Health.
SGG: Research grant support (e.g., steering committee or data and safety monitoring committee) and/or speaker/consulting honoraria (e.g., advisory boards) from: Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, CSL Behring, Daiichi-Sankyo/American Regent, Eli Lilly, Esperion, Ferring Pharmaceuticals, GlaxoSmithKline, HLS Therapeutics, Janssen/Johnson & Johnson, Merck, Novartis, Novo Nordisk A/C, Pfizer, Regeneron, Sanofi, Servier; and salary support/honoraria from the Heart and Stroke Foundation of Ontario/University of Toronto (Polo) Chair, Canadian Heart Research Centre and MD Primer, Canadian VIGOUR Centre, Duke Clinical Research Institute, New York University Clinical Coordinating Centre, and PERFUSE Research Institute.
BK: employee of AstraZeneca.
NI: employee of AstraZeneca.
NJP: research grants from Regeneron Pharmaceuticals, Sanofi-Aventis, Boerhinger Ingelheim, Novo Nordisk, and Verily Life Sciences.
NS: consulting or speaker’s bureau for Amgen, AstraZeneca, Boehringer Ingelheim, Novo Nordisk, Eli Lilly, Pfizer and Sanofi and; research support from Boehringer Ingelheim.
RRH: grants from AstraZeneca, grants and personal fees from Bayer, Boehringer Ingelheim and Merck Sharp & Dohme Corp, personal fees from Novartis, Amgen, and Servier, and financial support from Elcelyx, GlaxoSmithKline, Janssen, and Takeda.
AFH: research funding: AstraZeneca, GlaxoSmithKline, Merck, Novartis; Consulting: AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Merck, Novartis, Pfizer.
RJM: grants from AstraZeneca and GlaxoSmithKline and personal fees from AstraZeneca and Boehringer-Ingelheim.
MRP: research support from AstraZeneca and Bayer, speaker fees from AstraZeneca, and consulting for Bayer and Thrombosis Research Institute.
WSJ: Research Grants from Agency for Healthcare Research and Quality, AstraZeneca, American Heart Association, Bristol-Myers Squibb, Doris Duke Charitable Foundation, Patient-Centered Outcomes Research Institute; Honorarium/other from American College of Radiology, Daiichi Sankyo.
References
- 1.Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. Available from https://www.cdc.gov/diabetes/library/features/diabetes-stat-report.html.Accessed 2 September 2020.
- 2.Centers for Disease Control and Prevention. Diagnosed Diabetes. Available from https://gis.cdc.gov/grasp/diabetes/DiabetesAtlas.html.Accessed 5 July 2020.
- 3.Geiss LS, Li Y, Hora I, Albright A, Rolka D, Gregg EW. Resurgence of Diabetes-Related Nontraumatic Lower-Extremity Amputation in the Young and Middle-Aged Adult U.S. Population. Diabetes Care 2019; 42: 50–54. [DOI] [PubMed] [Google Scholar]
- 4.Lin CW, Armstrong DG, Lin CH, Liu PH, Hung SY, Lee SR, et al. Nationwide trends in the epidemiology of diabetic foot complications and lower-extremity amputation over an 8-year period. BMJ Open Diabetes Res Care 2019; 7: e000795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engelhardt M, Bruijnen H, Scharmer C, Wohlgemuth WA, Willy C, Wolfle KD. Prospective 2-years follow-up quality of life study after infrageniculate bypass surgery for limb salvage: lasting improvements only in non-diabetic patients. Eur J Vasc Endovasc Surg 2008; 36: 63–70. [DOI] [PubMed] [Google Scholar]
- 6.Eckman MH, Greenfield S, Mackey WC, Wong JB, Kaplan S, Sullivan L, et al. Foot infections in diabetic patients. Decision and cost-effectiveness analyses. JAMA 1995; 273: 712–720. [PubMed] [Google Scholar]
- 7.Raspovic KM, Wukich DK. Self-reported quality of life and diabetic foot infections. J Foot Ankle Surg 2014; 53: 716–719. [DOI] [PubMed] [Google Scholar]
- 8.Eraso LH, Fukaya E, Mohler ER 3rd, Xie D, Sha D, Berger JS. Peripheral arterial disease, prevalence and cumulative risk factor profile analysis. Eur J Prev Cardiol 2014; 21: 704–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lautsch D, Wang T, Yang L, Rajpathak SN. Prevalence of established cardiovascular disease in patients with type 2 diabetes mellitus in the UK. Diabetes Ther 2019; 10: 2131–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banerjee S, Vinas A, Mohammad A, Hadidi O, Thomas R, Sarode K, et al. Significance of an abnormal ankle-brachial index in patients with established coronary artery disease with and without associated diabetes mellitus. Am J Cardiol 2014; 113: 1280–1284. [DOI] [PubMed] [Google Scholar]
- 11.Badjatiya A, Merrill P, Buse JB, Goodman SG, Katona B, Iqbal N, et al. Clinical outcomes in patients with type 2 diabetes mellitus and peripheral artery disease: results from the EXSCEL trial. Circ Cardiovasc Interv 2019; 12: e008018. [DOI] [PubMed] [Google Scholar]
- 12.Clarke PM, Gray AM, Briggs A, Farmer AJ, Fenn P, Stevens RJ, et al. ; UK Prospective Diabetes Study (UKPDS) Group. A model to estimate the lifetime health outcomes of patients with type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS no. 68). Diabetologia 2004; 47: 1747–1759. [DOI] [PubMed] [Google Scholar]
- 13.Hayes AJ, Leal J, Gray AM, Holman RR, Clarke PM. UKPDS outcomes model 2: a new version of a model to simulate lifetime health outcomes of patients with type 2 diabetes mellitus using data from the 30 year United Kingdom Prospective Diabetes Study: UKPDS 82. Diabetologia 2013; 56: 1925–1933. [DOI] [PubMed] [Google Scholar]
- 14.Martins-Mendes D, Monteiro-Soares M, Boyko EJ, Ribeiro M, Barata P, Lima J, et al. The independent contribution of diabetic foot ulcer on lower extremity amputation and mortality risk. J Diabetes Complications 2014; 28: 632–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kasbekar PU, Goel P, Jadhav SP. A decision tree analysis of diabetic foot amputation risk in Indian patients. Front Endocrinol (Lausanne) 2017; 8: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lipsky BA, Weigelt JA, Sun X, Johannes RS, Derby KG, Tabak YP. Developing and validating a risk score for lower-extremity amputation in patients hospitalized for a diabetic foot infection. Diabetes Care 2011; 34: 1695–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pickwell K, Siersma V, Kars M, Apelqvist J, Bakker K, Edmonds M, et al. Predictors of lower-extremity amputation in patients with an infected diabetic foot ulcer. Diabetes Care 2015; 38: 852–857. [DOI] [PubMed] [Google Scholar]
- 18.Holman RR, Bethel MA, George J, Sourij H, Doran Z, Keenan J, et al. Rationale and design of the EXenatide Study of Cardiovascular Event Lowering (EXSCEL) trial. Am Heart J 2016; 174: 103–110. [DOI] [PubMed] [Google Scholar]
- 19.Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2017; 377: 1228–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steyerberg EW, Harrell FE Jr., Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol 2001; 54: 774–781. [DOI] [PubMed] [Google Scholar]
- 21.Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017; 69: e71–e126. [DOI] [PubMed] [Google Scholar]
- 22.Aboyans V, Ricco JB, Bartelink MEL, Bjorck M, Brodmann M, Cohnert T, et al. ; ESC Scientific Document Group. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur Heart J 2018; 39: 763–816.28886620 [Google Scholar]
- 23.Singh PP, Abbott JD, Lombardero MS, Sutton-Tyrrell K, Woodhead G, Venkitachalam L, et al. ; Bypass Angioplasty Revascularization Investigation 2 Diabetes Study Group. The prevalence and predictors of an abnormal ankle-brachial index in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. Diabetes Care 2011; 34: 464–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diehm C, Allenberg JR, Pittrow D, Mahn M, Tepohl G, Haberl RL, et al. ; German Epidemiological Trial on Ankle Brachial Index Study Group. Mortality and vascular morbidity in older adults with asymptomatic versus symptomatic peripheral artery disease. Circulation 2009; 120: 2053–2061. [DOI] [PubMed] [Google Scholar]
- 25.American Diabetes A 11. Microvascular Complications and Foot Care: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020; 43: S135–S151. [DOI] [PubMed] [Google Scholar]
- 26.Weissler EH, Narcisse DI, Rymer JA, Armstrong EJ, Secemsky E, Gray WA, et al. Characteristics and outcomes of patients with diabetes mellitus undergoing peripheral vascular intervention for infrainguinal symptomatic peripheral artery disease. Vasc Endovascular Surg 2021; 55: 124–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Q, Rosenson RS. Systematic review of methods used for the microvascular assessment of peripheral arterial disease. Cardiovasc Drugs Ther 2018; 32: 301–310. [DOI] [PubMed] [Google Scholar]
- 28.Tapp RJ, Zimmet PZ, Harper CA, de Courten MP, Balkau B, McCarty DJ, et al. ; Australian Diabetes, Obesity, and Lifestyle Study Group. Diabetes care in an Australian population: frequency of screening examinations for eye and foot complications of diabetes. Diabetes Care 2004; 27: 688–693. [DOI] [PubMed] [Google Scholar]
- 29.Ahluwalia HK, Miller CE, Pickard SP, Mayo MS, Ahluwalia JS, Beckles GL. Prevalence and correlates of preventive care among adults with diabetes in Kansas. Diabetes Care 2000; 23: 484–489. [DOI] [PubMed] [Google Scholar]
- 30.De Berardis G, Pellegrini F, Franciosi M, Belfiglio M, Di Nardo B, Greenfield S, et al. ; QuED Study Group-Quality of Care and Outcomes in Type 2 Diabetes. Are Type 2 diabetic patients offered adequate foot care? The role of physician and patient characteristics. J Diabetes Complications 2005; 19: 319–327. [DOI] [PubMed] [Google Scholar]
- 31.Yuen HK. Factors associated with preventive care practice among adults with diabetes. Prim Care Diabetes 2012; 6: 75–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. ; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657. [DOI] [PubMed] [Google Scholar]
- 33.Dhatariya K, Bain SC, Buse JB, Simpson R, Tarnow L, Kaltoft MS, et al. ; LEADER Publication Committee on behalf of the LEADER Trial Investigators. The impact of liraglutide on diabetes-related foot ulceration and associated complications in patients with type 2 diabetes at high risk for cardiovascular events: results from the LEADER trial. Diabetes Care 2018; 41: 2229–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.