Abstract
The use of transdermal alcohol monitors has burgeoned in recent years, now encompassing hundreds of thousands of individuals globally. A new generation of sensors promises to expand the range of applications for transdermal technology exponentially, and advances in machine learning modeling approaches offer new methods for translating the data produced by transdermal devices. This article provides 1) a review of transdermal sensor research conducted to date, including an analysis of methodological features of past studies potentially key in driving reported sensor performance; 2) updates on methodological developments likely to be transformative for the field of transdermal sensing, including the development of new-generation sensors featuring smartphone integration and rapid sampling capabilities as well as developments in machine learning analytics suited to data produced by these novel sensors and; 3) an analysis of the expanded range of applications for this new generation of sensor, together with corresponding requirements for sensor accuracy and temporal specificity. We also note questions as yet unanswered and key directions for future research.
Keywords: Alcohol, biosensor, machine learning, transdermal sensors, time series analysis
Introduction
A reliable alcohol biosensor could constitute a critical step towards helping individuals make informed decisions about their drinking and, ultimately, towards curbing problematic alcohol use (1,2). Transdermal sensors offer a uniquely non-invasive and low-cost method for assessing drinking, and thus the prospect of transdermal measurement of alcohol consumption has been met with tremendous enthusiasm (3,4). Enthusiasm increased as research emerged indicating that transdermal ankle bracelets could be used to effectively monitor alcohol abstinence within criminal justice (5) and specialized treatment contexts (6), with the use of these abstinence monitors quickly burgeoning to encompass over 800,000 individuals globally (7). Over time, however, it has become apparent that the relationship between transdermal alcohol concentration (TAC) and blood alcohol concentration (BAC) is highly complex. Thus, the task of translating transdermal sensor data into more fine grained estimates of alcohol consumption represents a considerable challenge (8).
Recent years have seen remarkable developments in both transdermal sensor technology as well as computational modeling methods, offering the potential to address some of these challenges while also expanding the range of applications for transdermal devices (9,10). Recognition of these momentous developments for transdermal technology is evident within a rapidly expanding scientific literature, with five reviews of transdermal alcohol sensors published within the past two years alone (10–14; see also 15 for a published pre-registration). Yet the extant literature tells us little about the future of transdermal alcohol sensing, a question that looms particularly large in the face of a global pandemic that has stymied much face-to-face research, driving many addiction scientists to extend beyond the laboratory and embrace tools for measuring alcohol consumption in real-world contexts. The current review aims to provide: 1) a review of transdermal sensor research conducted to date, including an analysis of methodological features of past studies potentially key in driving reported sensor performance; 2) information surrounding methodological developments likely to be transformative for the field of transdermal sensing; and 3) an analysis of the expanded range of applications for this new generation of sensor.
The Promise of Transdermal Assessment
Addiction scientists have explored a variety of different methods to assess alcohol use. For example, self-reports can be useful for understanding broad patterns of drinking. However, they have the potential to be biased by several factors including alcohol-related memory/cognitive impairment (16,17), variability in drink strengths/sizes (variable “pours”; 17,18), as well as demand characteristics of the assessment context (20). Breathalyzers have the capability to produce accurate estimates of intoxication but provide only a single estimate of intoxication at one point in time. Every test requires action by the user, and repeated tests require a wait period (21). Finally, in more recent years, microneedle arrays have come under development, devices that offer the advantage of direct measurement of alcohol content within interstitial fluid (22). However, these devices typically require application by a trained professional, involve regular disposal/re-application, and can cause skin irritation in some (23).
Above we list some of the most commonly employed existing methods of assessing alcohol consumption, along with one method based in developing technology. All of these methods are likely to gain and/or retain an important place in our toolkit of techniques for assessing drinking. At the same time, transdermal sensors have the potential to complement these tools, offering distinct advantages above and beyond extant and emerging measures. Based in research indicating that approximately 1% of ingested alcohol is diffused through the skin, transdermal sensors assess alcohol consumption by quantifying the content of alcohol contained in sweat and insensible perspiration (24,25). Thus, similar to the manner in which a breathalyzer estimates BAC by measuring the quantity of alcohol in expired air, transdermal sensors might estimate BAC by examining alcohol in water vapor emitted from the skin’s surface (24). Unlike a breathalyzer, however, transdermal alcohol sensors have the potential to measure alcohol consumption discretely and continuously, without requiring any motivated action on the part of the user. Further, unlike self-reports, transdermal assessment is based in objective measurement and, unlike methods assessing BAC in interstitial fluid, it is wholly noninvasive, low-cost, and likely to be attractive to a wide population of drinkers. In light of these advantages, it is no wonder that transdermal sensors have sparked enduring interest among addiction scientists (3,4,10).
The Challenge of Transdermal Assessment
Whereas breathalyzer readings can be translated into BAC estimates via a straightforward conversion factor (21), the relationship between transdermal readings and BAC is considerably more complex. The TAC-BAC relationship can be impacted by a variety of factors, including individual-difference factors that covary with physical properties of the skin (e.g., gender, race, age, BMI; 24,25), as well as contextual factors that affect levels of sweating (e.g., skin temperature, motion; 26). Thus, the relationship between TAC and BAC might differ across both individuals and also contexts (8,29). In addition, the process of transdermal diffusion of alcohol involves some degree of lag time, such that alcohol can be detected in the blood stream before it can be detected at the skin’s surface. Empirical studies seeking to quantify the exact degree of this lag time have produced highly variable findings, ranging from 30 minutes (30) to 5 hours (27).
While the complexities of the TAC-BAC association have been apparent, also apparent have been limitations of research methods applied to model this relationship (9,11,31). Importantly, the majority of studies seeking to capture the TAC-BAC relationship have relied on data from a single device—the AMS SCRAM™ ankle monitor (31,32). Design features of the SCRAM device, which integrates a pump to actively generate airflow across the transdermal sensor, increase its size to a bulky 6oz (see Figure 1) and reduce its TAC sampling interval to a relatively sparse 30 minutes (11). Further, the relationship between TAC and BAC can vary depending on where on the body TAC is assessed (e.g., wrist vs. ankle, 33), and the ankle positioning of SCRAM could potentially impact its temporal sensitivity to changes in BAC (31). Indeed, transdermal devices worn around the ankle consistently produce about double the TAC-BAC lag time as devices worn on the wrist, hand, or arm (27,31,see also 32).
Figure 1.

Top panel displays two new generation transdermal wrist sensors—Milo (left) and BACtrack (right). Bottom panel displays old-generation (AMS SCRAM; left) and new-generation (BACtrack Skyn prototype; right) devices to scale, side by side. Bottom panel adapted from (10).
Beyond limitations of the devices themselves, this literature is also characterized by limitations in methods applied to examine data from these devices. The range of statistical techniques employed to characterize the relationship between TAC and BAC is relatively narrow, relying on conventional statistical methods (e.g., regression) and/or applied mathematics (e.g., first principles models) (32). Our most powerful models for characterizing highly complex associations tend to be “data hungry” (34). With a mean sample size of less than 20 participants (largest objective validation study N=48) and a total of only five studies conducted in field settings, extant trials have been inadequately powered to model the TAC-BAC association across individuals and contexts (32). Such observations on the existing literature have led researchers to suggest that the inconsistent performance of transdermal BAC monitors in prior research may not reflect a failure of concept for transdermal assessment, but rather limitations of methods applied in this area (9–11,31).
A New Era for Transdermal Assessment
The past several decades have given rise to remarkable technological and analytic developments. Although substantial progress has taken place across a variety of domains—including big data analytics, miniaturization, and wireless communication—some of the more extraordinary of these developments have emerged within the field of portable computing technology (11,34,35). The use of smartphones now extends to billions of individuals globally (35). An estimated 81% of the US population now owns a smartphone, up from just 35% in 2011, with the computing capabilities of the average device constantly expanding (35). We thus find ourselves in a strange new reality in which a sizable proportion of the world’s population has at their fingertips a powerful microcomputer with far ranging capabilities. Combined with advances in data analytic methods, the technological development and burgeoning usage of smartphones has the potential to represent a game-changer for transdermal sensor technology—substantially reducing device size, reducing device cost, and also permitting the application of more computationally demanding models for real-time TAC data processing.
Leveraging these advances and spurred on by interest within the addiction research community, a new generation of transdermal alcohol sensor has recently emerged (10,11,31,36). Featuring smartphone integration and sleek designs, similar to a Fitbit, these devices are intended to appeal to large populations of voluntary users (see Figure 1). Several new-generation devices exist at varying stages of development, including BACtrack Skyn™, Smart Start BARE™, and Milo ION™.1 The mechanism for transdermal detection of alcohol varies across these newer devices—Skyn employs fuel cell based technology, whereas ION relies on enzymatic sensing (37). A key feature that unites these devices, however, is their unprecedented capability for rapid TAC sampling. More specifically, advances in wireless data transmission/storage and also transdermal sensor technology mean that new-generation sensors can sample TAC at approximately 90 times the rate of SCRAM devices, producing measurements as frequently as every 20 seconds (11). Information from relatively dense TAC time series might be used to address the challenge posed by contextual influences on the TAC-BAC relationship—a sudden spike in TAC might signal something different from a gradual rise (e.g., increase in sweating vs. increase in drinking). Further, and importantly, one of the more widespread applications of time series analysis is that of future forecasting, as time series offer information concerning not only where values currently are but also where they are going (38,39). Thus, information from the relatively dense TAC time series provided by new-generation sensors might be used to help collapse across the lag time between TAC and BAC and forecast estimates of alcohol consumption in near real-time (39).
In addition to developments in transdermal devices themselves, recent years have also seen progress in analytic methods for translating data produced by these devices (40–43). In particular, the years since 2010 have seen substantial advances in a family of computational approaches known as machine learning, leading some to christen this decade the “AI Spring” (44,45). Machine learning methods, which have long been applied to yield predictions based on raw data in time series format, differ from conventional statistical approaches in that they are first and foremost data-driven (46,47). Rather than restricting the relationship among variables within a model to a pre-determined set of structures (e.g., linear, quadratic, diffusion-equation based), as is the case with most conventional statistical approaches, machine learning models instead “learn” the shape of these relationships directly from the data itself. These models can thus cleave closely to data, reflecting nuanced relationships between variables in all their complexity—if analytic models might be considered as conforming to data like various articles of clothing conform to the human body, then linear regression would hang like a starched dress suit whereas machine learning models might hug like a leotard (see Figure 2). Machine learning approaches excel in contexts where the “true” relationship between predictor and outcome is complex/multi-dimensional, and where sufficient quantities of data exist to correctly distinguish between generalizable and spurious relationships. In technical terms, most machine learning methods have low bias (predetermined assumptions, such as expert knowledge dictating the form of the learned relationships) but have correspondingly higher variance (specificity to the particular dataset used for model fitting) (48). Thus, data must not only be sufficient in quantity for complex model fitting but must also be closely representative of the conditions under which the model will be deployed. When models are applied to new contexts, such as laboratory-created models deployed in real-world settings, rigorous evaluation is therefore essential to determine whether the models will work in the new context.
Figure 2.
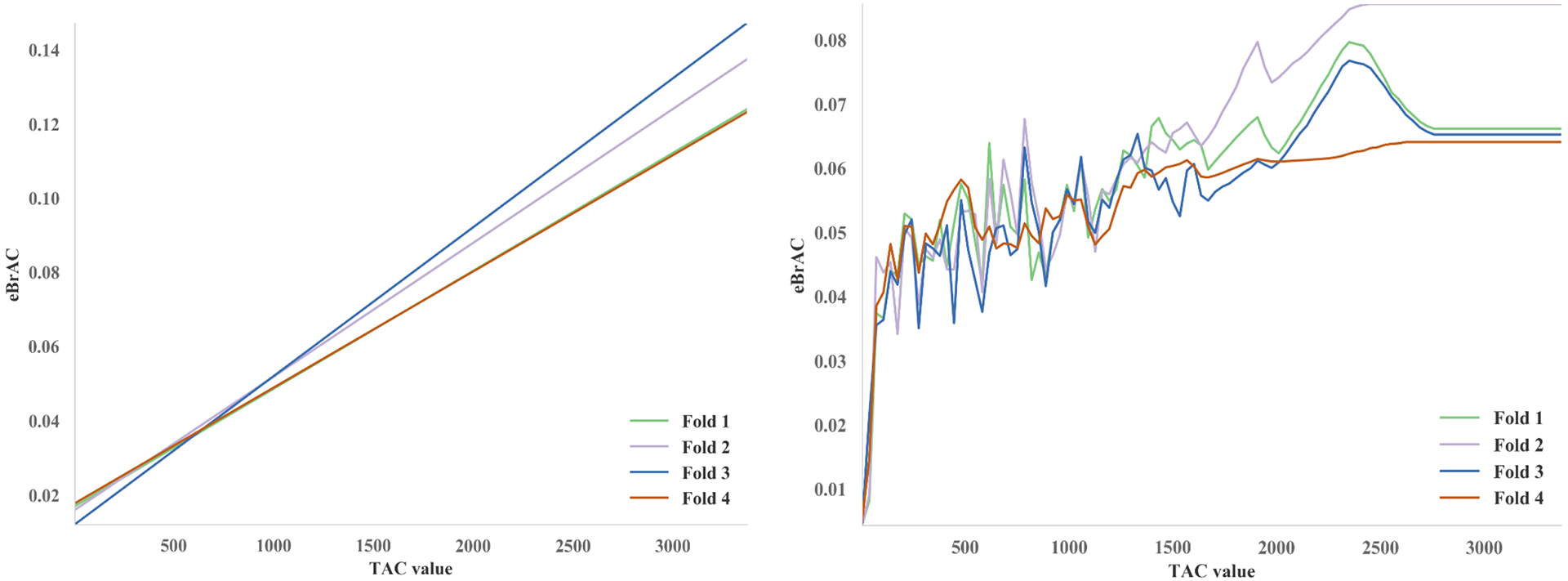
The relationship between a single TAC measurement and BAC as estimated using linear regression (left) and Extra-Trees machine-learning (right). “Fold”s refer to portions of the dataset held out for testing during four-fold cross validation. In the case of a single TAC measurement, the machine learning model predicted ~0% eBAC for TAC values close to 0, and a gradually increasing TAC–eBAC relationship for higher values (with noise due to measurement error, individual differences, and other causes).
The past decade in particular has seen advances in machine learning methods that may be especially well suited to facilitating TAC-BAC translation. These include convolutional neural networks with the capability to recognize short time series patterns of virtually any shape (40,43), methods for automated data processing with capabilities for providing fast extraction of hundreds of predictors from time series data (42), as well as methods for controlling machine learning model complexity to avoid overfitting models to data (i.e., “regularization”; 38). Innovations in a family of methods called transfer learning may also help when models need to be adapted for new contexts (49). Advances in machine learning, and neural networks especially, tend to produce inscrutable models, however. These models trade increased accuracy for decreased interpretability, which may limit their application in settings where explaining why a prediction was made is essential—for example, in legal settings where a defendant might question the evidence against them, or in places where people have a legal right to explanation of model predictions (50).
Regarding the utility of these methods for yielding interpretable transdermal indicators of drinking, the bulk of validation research is yet to be done. However, data from initial research underscores the potential incremental validity of these new tools over extant methods. In early work employing single subject experimental designs, BACtrack Skyn, Quantac Tally™,2 and Milo ION all demonstrated responsiveness to ingested alcohol (11,37). A study involving 30 participants wearing both SCRAM and Skyn in the laboratory indicated strong correlations between Skyn and BAC and also substantially reduced lag times with Skyn vs. SCRAM (31). A field study (N=10) indicated Skyn detected alcohol soon after drinking onset (~30-mins), and a 2-week feasibility study (N=12) indicated acceptability of Skyn for longer-term wear (51). Of particular relevance, in a study that represents the largest objective transdermal sensor validation study conducted to date (N=73), we applied time-series feature extraction paired with tree-based machine learning algorithms to Skyn data collected in the laboratory (9). Results revealed that highly accurate real-time estimates of BAC could be created from new-generation sensor data under controlled conditions (r=.91; mean absolute error=.01% BAC). Finally, although new-generation sensors currently comprise a limited number of devices, early work indicates promising findings with novel prototypes, suggesting the range of available devices is likely to expand (52–54). To fully gauge the accuracy level of new-generation transdermal sensors, assessment under real-world conditions in response to variable alcohol doses will be required. Nonetheless, results of these early studies indicate transdermal devices may have capabilities beyond what was previously considered feasible based on research with older methods.
Applications for New-Generation Transdermal Technology
New-generation sensors offer a uniquely passive, noninvasive, low-cost method for assessing alcohol use likely to be attractive to broad populations of drinkers, including for longer term wear. Thus, these newer devices open up a new universe of applications for transdermal technology. Table 1 lists applications of new-generation sensor technology together with requirements for accuracy, temporal specificity, and contextual dependence associated with each potential application (see Table 1 for evaluation metric definitions and application requirements).
Table 1.
Description of sensor evaluation metrics (Part 1) and potential applications for new-generation sensors according to predicted evaluation metric requirements (Part 2)
| Part 1. Description of Evaluation Metrics | |||
|---|---|---|---|
| Evaluation Metric | Definition | Level | Level Description |
| Accuracy | Congruency between transdermal estimates of alcohol use and “true” alcohol use | High | Provides estimate of actual BAC (eBAC within .01% of true BAC) |
| Mod | Provides estimate of standard drinks consumed (eBAC within .02% of true BAC) | ||
| Low | Provides estimate only of abstinence (0% BAC), low risk (0%−.08), and high-risk drinking (>.08%) | ||
| Temporal Specificity | Temporal displacement between transdermal estimates of alcohol use and “true” alcohol use | High | Provides estimates of alcohol consumption in near real-time (e.g., contemporaneous with drinking) |
| Mod | Provides retroactive estimation (e.g., after drinking episode concludes) time-locked to the minute | ||
| Low | Provides retroactive estimation (e.g., after drinking episode concludes) time-locked to the day | ||
| Contextual Dependency | Existence of (substantial) contextual effects on transdermal estimates | No | Accuracy in real-world settings is acceptable and/or similar to accuracy in controlled settings |
| Yes | No accuracy in real-world. Only accuracy in controlled contexts (e.g., lab, medical setting, etc.) | ||
| Part 2. New-Generation Transdermal Sensor Applications | ||||
|---|---|---|---|---|
| Domain | Application Description | Accuracy | TempSp | CntxD |
| Prevention | Health Tracker: Sleek device, similar to Fitbit, providing day-level record of abstinence and/or high-risk drinking episodes for longer term wear | Mod/Low | Low | No |
| Intervention | Treatment-Adherence/Relapse Tracker: Comfortable/compact device providing objective, day-level record of abstinence and/or high-risk drinking episodes for patients enrolled in MI, HR, or CM programs | Mod/Low | Low | No |
| JITAI/Relapse Prevention: Device for real-time detection of drinking episodes among those in recovery, prompting intervention and thus diminishing relapse severity/duration | Low | High | No | |
| Medical | Medical Monitor: Comfortable device for objective tracking of drinking risk level for providers treating those with medical diagnoses contraindicating higher level of alcohol consumption (e.g., diabetes, etc.) | Mod/Low | Low | No |
| BAC Monitor: Non-invasive, real-time BAC monitor for patients presenting to hospitals or medical facilities with clinically relevant intoxication | High | High | Yes | |
| Motor Vehicle | Driving Safety Monitor: Device for passive real-time BAC estimation could prompt alarms or contact a ride sharing service when BAC approaching higher levels | High/Mod | High | No |
| Research | Longitudinal Research: Comfortable device providing an objective day-level record of changes in abstinence and/or high-risk drinking over months/years | Mod/Low | Low | No |
| Laboratory BAC Monitor: Continuous, passive, real-time BAC monitor for participants in laboratory studies involving alcohol | High | High | Yes | |
| Basic Field Research: A discreet device for assessing immediate causes/consequences of alcohol consumption in real-world contexts | Mod/Low | High/Mod | No | |
| Intervention Outcome: Comfortable device providing an objective day-level record of drinking following participation in trials of novel addiction interventions | Mod/Low | Mod/Low | No | |
Part 2 displays our predictions regarding the level of accuracy and temporal specificity (low/moderate/high) required for various applications of transdermal technology. Part 2 also indicates whether the application would be possible given a pattern of context dependence characterized by accuracy only in controlled settings (yes/no).
BAC=Blood alcohol concentration; eBAC=Transdermal estimates of BAC; Mod=Moderate; TempSp=Temporal specificity; CntxD=Context dependence; MI=Motivational interviewing; HR=Harm Reduction; CM=Contingency management; JITAI=Just-in-time adaptive interventions.
Early validation research has examined new-generation sensor performance with respect to the relatively fine-grained metric of BAC, and has further been aimed at creating estimates of alcohol consumption in real-time (9). Importantly, however, new-generation sensors employing broader, category-focused drinking measures (e.g., abstinence, low risk, or high-risk drinking) and demonstrating lower levels of temporal specificity (e.g., retroactive day-level time locking) might also have a range of potential applications (see Table 1).3 For example, in the realm of prevention, a sleek sensor providing a day-level record of abstinence and/or high risk drinking events might open up new frontiers for public health initiatives by increasing awareness of consumption levels in broader populations of drinkers. In the realm of intervention, a comfortable, compact device providing objective information on drinking risk level could represent a significant asset to programs such as motivational enhancement therapy (55), harm reduction (56), and contingency management (2) which incorporate alcohol monitoring into treatment as a critical change process. Finally, in the realm of research, such devices could represent an important advance for longitudinal studies exploring patterns of alcohol use over time, providing objective measures of alcohol use risk category over the course of months or even years and thus avoiding biases associated with retrospective recall self-report measures (57).
If results from laboratory studies extend to real-world contexts (9), it is possible new-generation sensor data might ultimately be used to identify episodes of alcohol use in near real time. Such contemporaneous signals could open up far ranging possibilities for application. In particular, sensors providing automated real-time signals of drinking might forge new ground in relapse prevention by prompting intervention in real-world drinking contexts. Within the context of such treatments (e.g., Just-in-Time Adaptive Interventions; 47), transdermal sensors might be used to issue alcohol alerts to a family member, sponsor, or treatment provider when a drinking episode is detected, thus potentially decreasing severity and duration of relapse (58,59). Finally, in the context of a global pandemic that has forced many addiction scientists to push beyond the boundaries of the laboratory, such temporally precise sensors might have key research applications. Real-time alcohol biosensors could enhance basic research exploring immediate correlates and consequences of alcohol consumption in real-world contexts (12,60,61), including by triggering surveys and other data collection functions when drinking is detected.
Beyond these, a transdermal alcohol biosensor with a range of accuracy levels might enhance health and health-related research across various other domains. In the realm of motor vehicle safety, a device capable of reliably categorizing drinking into low vs higher risk levels in real-time might be used to reduce the risk of drunk driving, issuing vibrations/alarms or contacting a ride-sharing service when BACs are approaching higher levels. In the medical realm, interventions for some of the world’s most common chronic health conditions require that patients moderate their alcohol intake (e.g., diabetes, cardiovascular disorders; 53,54). A transdermal sensor providing day-level standard drink or drinking risk level estimates could help patients maintain healthy levels of drinking while also offering critical information to health care providers. Finally, in the realm of research, devices capable of providing a day-level record of standard drinks consumed could refine the precision of outcome assessments within addiction treatment trials, thus increasing statistical power for the identification of effective new addiction interventions (64).
Future Directions for New-Generation Sensors
At the current time, limited research has examined the relationship between new-generation transdermal sensor output and objective indicators of alcohol consumption, and the research that exists has been conducted mainly within the laboratory (9,11,31,37). The task of predicting alcohol consumption from transdermal sensor data under real world drinking conditions certainly represents a challenge. Importantly, however, as larger transdermal datasets accrue, additional powerful modeling tools become available, including models capable of accounting for individual and contextual influences on the TAC-BAC relationship. In particular, within the context of larger datasets, machine learning models might incorporate large numbers of features (e.g., data from temperature and motion sensors embedded within transdermal devices; individual-level factors including race, BMI, and gender) and more advanced machine learning model types (e.g., deep learning) become available. Deep learning is invaluable for achieving high accuracy in many modeling tasks (34), and may be essential for some applications of transdermal technology (e.g., informing driving advisability, some just-in-time interventions). However, many challenges remain for deep learning in this domain; current datasets lack the millions of datapoints often needed in deep learning, and further work is needed to design domain-specific models that are small and fast enough to run on devices like smartphones (as has been done in other domains, 65).
Regarding the transdermal monitors themselves, although suitable for many research functions, additional work is needed before these devices are ready for widespread application. To date the majority of new-generation sensor validation work has involved BACtrack Skyn (9,11,31,37). Earlier Skyn prototypes showed high rates of device failure, although reliability has improved substantially with newer prototypes (9,31). Current Skyn prototypes feature limited waterproofing, compatibility only with iOS devices, and limited battery life. In addition, as is typical early in the development of any novel technology, prototype sensors are currently expensive. Given that the underlying technology and materials supporting transdermal devices are not costly in-and-of themselves, prices are likely to decrease with expanding usage of transdermal sensors.
Finally, although machine learning approaches can have advantages for modeling complex associations, other frameworks can also excel in such contexts including first-principles mathematical models (27,56,57). First principles models have the advantage of requiring less data because they rely on expert knowledge, while machine learning might uncover previously-unknown relationships given sufficient data. Future research might directly compare these modeling frameworks for TAC-BAC translation.
Conclusion
Recent technological advances have led to the development of a new generation of transdermal sensor and novel tools for transdermal data processing. Additional research will be required to explore the validity of these new sensors within real-world contexts in response to variable alcohol doses in large and diverse populations of drinkers, as well as to establish the feasibility and acceptability of these devices for longer term wear. Nonetheless, early research indicates these sensors will greatly expand the range of application for biosensor technology within addiction science.
Acknowledgments
This research was supported by NIH grant R01AA025969 to Catharine Fairbairn. Our thanks to Nancy Barnett for helpful comments on an earlier version of this manuscript. C.E.F. received transdermal devices at discounted rates from BACtrack for the purposes of performing validation research. BACtrack did not have a role in conducting, evaluating, or disseminating the research. The authors have no competing interests to declare.
Footnotes
As of the time of this writing, Skyn and ION are available for research purposes, whereas BARE is still under development. Progress in this area is currently moving quickly, and the next several years are likely to see new devices entering the marketplace.
Quantac ceased business operations in 2017.
Research with old-generation sensors already demonstrates transdermal devices are capable of providing a highly accurate record of alcohol abstinence (5), and early research with new-generation sensors indicates their accuracy level is nearly double that of older devices (9). Thus, the notion that new-generation sensors might ultimately provide reasonably accurate estimates of day-level drinking risk category might be considered a conservative prediction.
References
- 1.Fairbairn CE, Kang D. Transdermal alcohol monitors: Research, applications, and future directions. In: Frings D, Albery I, editors. The Handbook of Alcohol Use and Abuse. Elsevier; in press. [Google Scholar]
- 2.Barnett NP. Alcohol sensors and their potential for improving clinical care. Addiction. 2015;110(1):1–3. [DOI] [PubMed] [Google Scholar]
- 3.Leffingwell TR, Cooney NJ, Murphy JG, Luczak S, Rosen G, Dougherty DM, et al. Continuous objective monitoring of alcohol use: Twenty-first century measurement using transdermal sensors. Alcohol Clin Exp Res. 2013;37(1):16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swift RM, Swette L. Assessment of ethanol consumption with a wearable, electronic ethanol sensor/recorder. In: Litten RZ, Allen JP, editors. Measuring alcohol consumption: Psychosocial and biochemical methods. Totowa, NJ: Humana Press; 1992. p. 189–202. [Google Scholar]
- 5.Sakai JT, Mikulich-Gilbertson SK, Long RJ, Crowley TJ. Validity of transdermal alcohol monitoring: Fixed and self-regulated dosing. Alcohol Clin Exp Res. 2006;30(1):26–33. [DOI] [PubMed] [Google Scholar]
- 6.Alessi SM, Barnett NP, Petry NM. Experiences with SCRAMx alcohol monitoring technology in 100 alcohol treatment outpatients. Drug Alcohol Depend. 2017;178:417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alcohol Monitoring Services. About SCRAM systems [Internet]. 2018. Available from: https://www.scramsystems.com/about/
- 8.Luczak SE, Ramchandani VA. Special issue on alcohol biosensors: Development, use, and state of the field. Alcohol. 2019;81:161–5. [DOI] [PubMed] [Google Scholar]
- 9.Fairbairn CE, Kang D, Bosch N. Using machine learning for real-time BAC estimation from a new-generation transdermal biosensor in the laboratory. Drug Alcohol Depend. in press; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Egmond K, Wright C JC, Livingston M, Kuntsche E. Wearable transdermal alcohol monitors: A systematic review of detection validity, relationship between transdermal and breath alcohol concentration and influencing factors. Alcohol Clin Exp Res. 2020;44(10):1918–32. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Fridberg DJ, Leeman RF, Cook RL, Porges EC. Wrist-worn alcohol biosensors: Strengths, limitations, and future directions. Alcohol Biomed J. 2019;81:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piasecki TM. Assessment of alcohol use in the natural environment. Alcohol Clin Exp Res. 2019;43(4):564–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thungon PD, Kakoti A, Ngashangva L, Goswami P. Advances in developing rapid, reliable and portable detection systems for alcohol. Biosens Bioelectron. 2017;97:83–99. [DOI] [PubMed] [Google Scholar]
- 14.Davis-Martin RE, Alessi SM, Boudreaux ED. Alcohol use disorder in the age of technology: A review of wearable biosensors in alcohol use disorder treatment. Front Psychiatry. 2021;12:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kianersi S, Luetke M, Agley J, Gassman R, Ludema C, Rosenberg M. Validation of transdermal alcohol concentration data collected using wearable alcohol monitors: A systematic review and meta-analysis. Drug Alcohol Depend. 2020;216:108304–108304. [DOI] [PubMed] [Google Scholar]
- 16.White AM. What happened? Alcohol, memory blackouts, and the brain. Alcohol Res Health. 2003;27(2):186–96. [PMC free article] [PubMed] [Google Scholar]
- 17.Kirchner TR, Sayette MA. Effects of alcohol on controlled and automatic memory processes. Exp Clin Psychopharmacol. 2003;11:167–75. [DOI] [PubMed] [Google Scholar]
- 18.Barnett NP, Wei J, Czachowski C. Measured alcohol content in college party mixed drinks. Psychol Addict Behav. 2009;23(1):152–6. [DOI] [PubMed] [Google Scholar]
- 19.Kerr WC, Patterson D, Koenen MA, Greenfield TK. Alcohol content variation of bar and restaurant drinks in Northern California. Alcohol Clin Exp Res. 2008;32(9):1623–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwarz N. Self-reports: How the questions shape the answers. Am Psychol. 1999;54(2):93–105. [Google Scholar]
- 21.Jones AW. Measuring alcohol in blood and breath for forensic purposes: A historical review. Forensic Sci Rev. 1996;8:13–44. [PubMed] [Google Scholar]
- 22.Vinu Mohan AM, Windmiller JR, Mishra RK, Wang J. Continuous minimally-invasive alcohol monitoring using microneedle sensor arrays. Biosens Bioelectron. 2017May15;91:574–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mi O, Okafor HK. Microneedles: A Potent Modern Tool in Enhancing Transdermal Drug Delivery. Curr Trends Biomed Eng Biosci. 2017;10:555776. [Google Scholar]
- 24.Swift RM. Direct measurement of alcohol and its metabolites. Addiction. 2003;98(s2):73–80. [DOI] [PubMed] [Google Scholar]
- 25.Swift RM. Transdermal alcohol measurement for estimation of blood alcohol concentration. Alcohol Clin Exp Res. 2000;24(4):422–3. [PubMed] [Google Scholar]
- 26.Hill-Kapturczak N, Roache JD, Liang Y, Karns TE, Cates SE, Dougherty DM. Accounting for sex-related differences in the estimation of breath alcohol concentrations using transdermal alcohol monitoring. Psychopharmacology (Berl). 2015;232(1):115–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marques PR, McKnight AS. Field and laboratory alcohol detection with 2 types of transdermal devices. Alcohol Clin Exp Res. 2009;33(4):703–11. [DOI] [PubMed] [Google Scholar]
- 28.Anderson JC, Hlastala MP. The kinetics of transdermal ethanol exchange. J Appl Physiol. 2006;100(2):649–55. [DOI] [PubMed] [Google Scholar]
- 29.Luczak SE, Rosen IG. Estimating BrAC from transdermal alcohol concentration data using the BrAC estimator software program. Alcohol Clin Exp Res. 2014;38(8):2243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swift RM, Martin CS, Swette L, Laconti A, Kackley N. Studies on a wearable, electronic, transdermal alcohol sensor. Alcohol Clin Exp Res. 1992;16(4):721–5. [DOI] [PubMed] [Google Scholar]
- 31.Fairbairn CE, Kang D. Temporal dynamics of transdermal alcohol concentration measured via new-generation wrist-worn biosensor. Alcohol Clin Exp Res. 2019;43(10):2060–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fairbairn CE, Bosch N. A meta-analysis of transdermal alcohol sensor validation studies. OSF [Internet]. 2021; Available from: https://osf.io/ymr3f/?view_only=283d3d3bb8c44bd1997f6766d69fad12 [Google Scholar]
- 33.Swift RM. Transdermal measurement of alcohol consumption. Addiction. 1993;88(8):1037–9. [DOI] [PubMed] [Google Scholar]
- 34.LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521(7553):436–44. [DOI] [PubMed] [Google Scholar]
- 35.Pew Research Center. Mobile Fact Sheet [Internet]. 2018. Available from: http://www.pewinternet.org/fact-sheet/mobile/
- 36.NIAAA. Wearable alcohol biosensor challenge [Internet]. 2015. Available from: https://www.nih.gov/news-events/news-releases/niaaa-selects-winners-its-wearable-alcohol-biosensor-challenge
- 37.Lansdorp B, Ramsay W, Hamid R, Strenk E. Wearable enzymatic alcohol biosensor. Sensors. 2019;19(10):2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Box GEP, Jenkins GM, Reinsel GC. Time series analysis: Forecasting and control. Englewood Cliffs, N.J.: Prentice-Hall; 1994. [Google Scholar]
- 39.Montgomery DC, Jennings CL, Kulahci M. Introduction to time series analysis and forecasting. New York: John Wiley & Sons; 2015. [Google Scholar]
- 40.Lee H, Pham P, Largman Y, Ng AY. Unsupervised feature learning for audio classification using convolutional deep belief networks. In: Advances in Neural Information Processing Systems. 2009. p. 1096–104. [Google Scholar]
- 41.Chen T, Guestrin C. Xgboost: A scalable tree boosting system. In: Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining. 2016. p. 785–94. [Google Scholar]
- 42.Christ M, Braun N, Neuffer J, Kempa-Liehr AW. Time series feature extraction on basis of scalable hypothesis tests (tsfresh–a python package). Neurocomputing. 2018;307:72–7. [Google Scholar]
- 43.Xu Y, Kong Q, Wang W, Plumbley MD. Large-scale weakly supervised audio classification using gated convolutional neural network. In: 2018 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP). IEEE; 2018. p. 121–5. [Google Scholar]
- 44.Maclure J. The new AI spring: A deflationary view. AI Soc. 2020;35:747–50. [Google Scholar]
- 45.Holzinger A, Kieseberg P, Weippl E, Tjoa AM. Current advances, trends and challenges of machine learning and knowledge extraction: from machine learning to explainable AI. In: International Cross-Domain Conference for Machine Learning and Knowledge Extraction. Springer; 2018. p. 1–8. [Google Scholar]
- 46.Dwyer DB, Falkai P, Koutsouleris N. Machine learning approaches for clinical psychology and psychiatry. Annu Rev Clin Psychol. 2018;14:91–118. [DOI] [PubMed] [Google Scholar]
- 47.Coutanche MN, Hallion LS. Machine learning for clinical psychology and clinical neuroscience. In: Wright AGC, Hallquist MN, editors. The Cambridge Handbook of Research Methods in Clinical Psychology. Cambridge, UK: Cambridge University Press; in press. [Google Scholar]
- 48.Kohavi R, Wolpert DH. Bias plus variance decomposition for zero-one loss functions. In: Proceedings of the 13th International Conference on Machine Learning. Morgan Kaufmann; 1996. p. 275–83. [Google Scholar]
- 49.Zhuang F, Qi Z, Duan K, Xi D, Zhu Y, Zhu H, et al. A comprehensive survey on transfer learning. Proc IEEE. 2020;109(1):43–76. [Google Scholar]
- 50.Vollmer N. Recital 71 EU General Data Protection Regulation (EU-GDPR) [Internet]. SecureDataService; 2020. [cited 2021 Feb 8]. Available from: http://www.privacy-regulation.eu/en/recital-71-GDPR.htm [Google Scholar]
- 51.Wang Y, Fridberg DJ, Shortell DD, Leeman RF, Barnett NP, Cook RL, et al. Wrist-worn alcohol biosensors: Applications and usability in behavioral research. Alcohol Biomed J. in press; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lawson B, Aguir K, Fiorido T, Martini-Laithier V, Bouchakour R, Burtey S, et al. Skin alcohol perspiration measurements using MOX sensors. Sens Actuators B Chem. 2019;280:306–12. [Google Scholar]
- 53.Li B, Downen RS, Dong Q, Tran N, Le Saux M, Meltzer AC, et al. A discreet wearable IoT sensor for continuous transdermal alcohol monitoring–challenges and opportunities. IEEE Sens J. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin K, Kinnamon D, Sankhala D, Muthukumar S, Prasad S. AWARE: A Wearable Awareness with Real-time Exposure, for monitoring alcohol consumption impact through Ethyl Glucuronide detection. Alcohol. 2019;81:93–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miller WR, Zweben A, DiClimente C, Rychtarik R. Motivational enhancement therapy manual: A clinical research guide for therapists treating individuals with alcohol abuse and dependence. Bethesda MD: NIAAA; 1994. [Google Scholar]
- 56.Marlatt GA, Witkiewitz K. Harm reduction approaches to alcohol use: Health promotion, prevention, and treatment. Addict Behav. 2002;27(6):867–86. [DOI] [PubMed] [Google Scholar]
- 57.Sher KJ, Grekin ER, Williams NA. The development of alcohol use disorders. Annu Rev Clin Psychol. 2005;1:493–523. [DOI] [PubMed] [Google Scholar]
- 58.Nahum-Shani I, Smith SN, Spring BJ, Collins LM, Witkiewitz K, Tewari A, et al. Just-in-time adaptive interventions (JITAIs) in mobile health: Key components and design principles for ongoing health behavior support. Ann Behav Med. 2017;52(6):446–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marlatt GA, Donovan DM. Relapse prevention: Maintenance strategies in the treatment of addictive behaviors. New York: Guilford Press; 2005. [Google Scholar]
- 60.Trull TJ, Ebner-Priemer U. Ambulatory assessment. Annu Rev Clin Psychol. 2013;9:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shiffman S. Ecological momentary assessment (EMA) in studies of substance use. Psychol Assess. 2009;21(4):486–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Howard AA, Arnsten JH, Gourevitch MN. Effect of alcohol consumption on diabetes mellitus: A systematic review. Ann Intern Med. 2004;140(3):211–9. [DOI] [PubMed] [Google Scholar]
- 63.Puddey IB, Beilin LJ. Alcohol is bad for blood pressure. Clin Exp Pharmacol Physiol. 2006;33(9):847–52. [DOI] [PubMed] [Google Scholar]
- 64.Roberts W, McKee SA. Mobile alcohol biosensors and pharmacotherapy development research. Alcohol. 2019;81:149–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sandler M, Howard A, Zhu M, Zhmoginov A, Chen L. Mobilenetv2: Inverted residuals and linear bottlenecks. In: Proceedings of the IEEE conference on computer vision and pattern recognition. 2018. p. 4510–20. [Google Scholar]
- 66.Sirlanci M, Luczak SE, Fairbairn CE, Kang D, Pan R, Yu X, et al. Estimating the distribution of random parameters in a diffusion equation forward model for a transdermal alcohol biosensor. Automatica. 2019;106:101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sirlanci M, Rosen IG, Luczak SE, Fairbairn CE, Bresin K, Kang D. Deconvolving the input to random abstract parabolic systems: A population model-based approach to estimating blood/breath alcohol concentration from transdermal alcohol biosensor data. Inverse Probl. 2018;34(12):125006. [DOI] [PMC free article] [PubMed] [Google Scholar]


