Abstract
Objectives:
To measure the feasibility, reach, and potential impact of a virtual family-centered rounds (FCR) intervention in the neonatal intensive care unit.
Methods:
We conducted a randomized controlled pilot trial with a 2:1 intervention-to-control arm allocation ratio. Caregivers of intervention arm neonates were invited to participate in virtual FCR plus standard of care. We specified five feasibility objectives. We profiled intervention usage by neonatal and maternal characteristics. Exploratory outcomes included FCR caregiver attendance, length of stay, breast milk feeding at discharge, caregiver experience, and medical errors. We performed descriptive analyses to calculate proportions, means, and rates with 95% confidence intervals (CI).
Results:
We included 74 intervention and 36 control subjects. Three of the five feasibility objectives were met based on the point estimates. The recruitment and intervention uptake objectives were not achieved. Among intervention arm subjects, recruitment of a caregiver occurred for 47 (63.5%, 95% CI 51.5–74.4%) neonates. Caregiver use of the intervention occurred for 36 (48.6%, 95% CI 36.8–60.6%) neonates in the intervention arm. Feasibility objectives assessing technical issues, burden, and data collection were achieved. Among the attempted virtual encounters, 95.0% (95% CI 91.5–97.3%) had no technical issues. The survey response rate was 87.5% (95% CI 78.2–93.8%). Intervention arm neonates had 3.36 (95% CI 2.66–4.23) times the FCR caregiver attendance rate of subjects in the control arm.
Conclusion:
A randomized trial to compare virtual FCR to standard of care in neonatal subjects is feasible and has potential to improve patient and caregiver outcomes.
Keywords: telemedicine, pediatric, neonatology, patient-centered care, clinical rounds
INTRODUCTION
Family-centered rounds (FCR) are multidisciplinary bedside rounds with active engagement by the family.1 FCR benefits include improved family experience, reduced parental anxiety, improved staff teamwork, and shortened hospital stays.2–5 For these reasons, FCR is the standard of care for hospitalized children.6,7 However, since FCR includes active engagement by the family, FCR is only possible for patients whose families are at the bedside during the rounding process.
Unfortunately, barriers exist that limit family presence at the bedside during neonatal intensive care unit (NICU) rounds. Critically ill neonates may be hospitalized in a NICU for extended periods of time. Their caregivers often live far distances from the hospital and have financial constraints that limit their ability to be physically present in the NICU.8,9 These caregivers must also balance being at the bedside versus their competing home and work responsibilities. NICU parents have high rates of anxiety, depression, and post-traumatic stress disorder,10–12 and reduced parental visitation can exacerbate their depression risk.11 The emergence of the novel coronavirus disease 2019 (COVID-19) further exacerbated the infrequent NICU parent or guardian attendance at FCR.13
Telehealth is a potential solution to mitigate these challenges. HIPAA (Health Insurance Portability and Accountability Act)-compliant technology exists that permits caregivers to be virtually present at the bedside in the NICU in order to virtually participate in FCR.14 Prior pediatric and adult research suggests that virtual FCR is feasible and meaningful to patients, families, and clinicians.15,16 However, these prior studies are limited in their lack of randomization, small sample sizes, and limited outcomes measured. A rigorous and adequately powered randomized trial is needed to fill this literature gap. We therefore conducted a pilot trial to investigate areas of uncertainty about this future definitive trial.17
To optimally prepare for a future effectiveness randomized trial to compare virtual FCR to standard of care in NICU patients for the improvement in patient and family outcomes, we conducted a pilot trial to test implementation strategies and to obtain effect size estimates for exploratory outcomes. This manuscript presents how this pilot trial aimed to (1) measure the feasibility of conducting a virtual FCR trial, (2) characterize the reach of the virtual FCR intervention, and (3) assess the potential impact of the virtual FCR intervention on exploratory patient and family outcomes.
METHODS
Design, Subject Population, and Setting
We conducted a two-arm randomized controlled pilot trial. Eligible subjects were neonates (age <365 days) admitted to the NICU between March 1, 2020 and August 31, 2020. Eligible neonates had one or more non-incarcerated, English-speaking parent or guardian (referred to as “caregiver” hereafter) aged ≥ 18 years. We excluded neonates if they had restrictions placed by child protective services, including visitation restrictions or parental restricted access to patient information. We only enrolled the first-born neonate of twins or higher-level multiples that were admitted to the NICU, thereby excluding neonates with a sibling already enrolled in the study. Neonates with more than one NICU admission during the trial period were only included on their first admission.
The hospital was a 121 bed quaternary care children’s hospital in Northern California with a level IV NICU with 49 NICU beds. This hospital is the referral center for children across a 33-county region covering 65,000 square miles and serving over 1 million children. The hospital routinely receives neonatal transfers from 30 hospitals in the region and admits over 900 neonatal patients annually. During this study period, the hospital’s visitation policy permitted the mother plus one adult support person to enter the NICU.
Subject Randomization
The principal investigator generated a random allocation list in Stata and employed allocation concealment to assign eligible subjects with a 2:1 intervention-to-control arm ratio. Unequal randomization was chosen over an equal allocation, because although it requires a 12.5% increase in the total sample size to maintain precision of between arm effect size estimates, it yields a 50% larger number of subjects in the intervention arm, which provides more opportunities to explore intervention feasibility aspects.
A research assistant opened the sequentially numbered assignments to invite caregivers of subjects assigned to the intervention arm to sign up for virtual FCR. Neonates of caregivers accepting this offer were considered “recruited.” Efforts to recruit caregivers consisted of emailing or using StudyPages, a secure communication platform, to text or call. For subjects with more than one caregiver listed in the electronic health record (EHR), separate invitations were sent to each caregiver. Five attempts within the first 96 hours of admission were made per caregiver, or until the caregiver declined or accepted the invitation.
Intervention
We used Zoom (San Jose, California) as the software interface to perform virtual FCR. This secure platform meets Health Insurance Portability and Accountability Act security rules. Caregivers downloaded Zoom onto their personal computer or smart device. The NICU medical team used Zoom on a computer with a speaker and pan-tilt-zoom camera, mounted on a stand with wheels.
Virtual FCR were available for caregivers of recruited neonates to attend Monday through Friday. A research assistant texted, called, or emailed recruited caregivers through StudyPages, based on caregiver preference, each weekday morning to ask if the caregiver(s) wanted to join virtual rounds that day. The research assistant messaged the caregivers who replied “yes” with 10–20 minute warnings prior to the start of rounds for their child.
Once the medical team was at the bedside, the research assistant admitted the caregiver(s) from the Zoom waiting room to establish the real-time, bidirectional audio and visual connection. FCR proceeded in usual fashion with a neonatologist, neonatal fellow, two pediatric residents, charge nurse, bedside nurse, pharmacist, dietician, and social worker. The research assistant joined FCR virtually. The medical team decided which team members joined FCR virtually versus in person at the bedside. The pharmacist and dietician joined rounds virtually on most days. The neonatologist typically joined rounds in person, but occasionally joined virtually. Caregivers could participate in virtual FCR as much, or as little, as they chose. Caregivers additionally had the option to participate in FCR in person or to not participate in FCR. Parents and providers were given a 24/7 helpdesk number to call to report and troubleshoot any technical issues.
Control
Subjects assigned to the control arm received standard of care; they had the option to participate in FCR in person or to not participate in FCR.
Measuring Feasibility, Reach, and Exploratory Outcomes
Data collection began on March 1, 2020 and continued through September 30, 2020, which provided 1 month of data collection following the last enrolled subject. Data collection consisted of daily weekday virtual observations of FCR; caregiver surveys; and review of the EHR, incident reports, and helpdesk technical issues log. For caregiver-reported outcomes, a research assistant messaged caregivers to participate in the survey at the time nearing the neonate’s discharge. Caregivers were simultaneously offered a paper survey by the medical team. Data from one survey per subject were included; the included survey was the first completed survey. Included surveys were completed within 10 days of discharge.
Trial Feasibility:
We specified five a priori feasibility objectives (Table 1).
Table 1:
Feasibility Objectives
| Objective # | Aspect Measured | Feasibility Statement |
|---|---|---|
| 1 | Recruitment | Among intervention arm subjects, recruitment of at least one caregiver will be ≥ 80% |
| 2 | Uptake | Among intervention arm subjects, use of the intervention at least once will be ≥ 75% |
| 3 | Technical issues | Among attempted virtual FCR connections, ≥ 90% of encounters will have no technical issues |
| 4 | Time burden | Among FCR encounters attended by a caregiver, the duration of virtual FCR will be no longer than standard FCR |
| 5 | Data collection | Survey response rate will be ≥ 75% |
FCR – family-centered rounds
Intervention Reach:
Among the intervention group, subjects were categorized into two groups based on whether or not their caregiver used the intervention at least once. Data were collected in order to profile intervention usage by neonatal characteristics (estimated gestational age at birth, admission age, sex, race, ethnicity, insurance, birth weight, ventilator use, NICU disposition, NICU length of stay) and maternal characteristics (age, parity, home-to-hospital distance, internet connection, highest education).
Exploratory Outcomes:
We collected subject-level outcomes data. The primary exploratory outcome was the FCR caregiver attendance proportion, which was the number of weekday round encounters with at least one caregiver present – either virtually or in person – divided by the neonate’s total number of weekday round encounters.
Secondary exploratory outcomes included patient length of stay, breast milk feeding at discharge, caregiver experience, and medical errors and adverse events. Length of stay and breast milk feeding data were obtained from EHR. These pre-specified measures were selected as indicators of possible mechanisms of action; engagement via virtual FCR might facilitate discharge readiness, stronger caregiver motivation to provide breast milk, positive caregiver experience, and improved collaboration to mitigate safety events.
Caregiver experience was assessed using the Child Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) Survey.18 Caregiver-reported HCAHPS surveys included the communication with parent domain and the global rating domain. Consistent with the Child HCAHPS top-box scoring method,18,19 the top-box score for each item was defined as the percentage of respondents selecting the most positive response options. The bottom-box score for each item was defined as the percentage of respondents selecting the negative response options. Composite measure scores were defined as the mean of the item top- and bottom-box scores comprising the composite measure.
Medical errors and adverse events were collected using an established surveillance process20–23 that involves review of data from EHR, incident reporting system, and solicited reports. Two neonatologists, blinded to the study arm, independently categorized each event as a harmful error, a non-harmful error, a non-preventable adverse event, or an exclusion (i.e., neither a medical error nor an adverse event)23 (74.3% agreement; kappa, 0.59; 95% confidence interval [CI], 0.53 – 0.66). The neonatologists reconciled discordant classifications using discussion.
Sample Size for Statistical Precision
We aimed to include 90 subjects (60 intervention, 30 control) to provide adequate sample size to estimate feasibility objective proportions with sufficient precision that error margins were ≤0.10.24 This pilot trial was not powered to test the null hypothesis that virtual FCR is equivalent to standard of care,25–27 as that effectiveness question will be answered in our future trial. For the exploratory outcomes, this sample size allowed us to estimate intervention versus control arm differences in proportions with 95% CI whose half-width was ≤0.22 standard deviations.
Analysis
Proportions and means with 95% CI were calculated for the feasibility objectives. To characterize intervention reach among the group of neonates assigned to the intervention group, we compared data between intervention users and non-users using Pearson’s Chi-square test for categorical variables, t-test for normally distributed continuous variables, and nonparametric equality-of-medians test for non-normally distributed continuous variables. These tests were also used to compare neonatal and maternal characteristics between study arms.
For between arm effect size estimation, we analyzed participant data according to their assigned group (intervention versus control). We reported group-specific descriptive statistics and summarized effect sizes (with 95% CI) with differences in means for the normally distributed continuous variables and differences in proportions of binary variables. The length of stay outcome demonstrated right skewed distribution, thus we used the c-statistic with 95% CI, obtained by transforming Somers’ D. The c-statistic estimates the probability that a randomly chosen length of stay from the intervention arm would be less than or equal to a randomly chosen length of stay from the control arm. To account for increased exposure (opportunity) among subjects with longer NICU hospitalizations, we used Poisson regression to estimate and compare rates for the FCR caregiver attendance outcome and the medical errors and adverse events outcome, using the logarithm of the number of weekdays and length of stay, respectively, as offset terms.
All available data were analyzed; we did not use imputation for non-response or missing data. Neonates with visitation restrictions placed by child protective services after randomization allocation and subjects who were not discharged home from the NICU prior to the completion of data collection were excluded from the analysis of these secondary exploratory outcomes: length of stay, breast milk feeding, and caregiver experience. For the subjects with post-allocation visitation restrictions, we included their data for analyses up to the day that the child protective services restriction was placed. The University of California Davis Institutional Review Board approved this study with a waiver of consent to collect data from observations of FCR, EHR, incident reports, and helpdesk technical issues log. Caregivers read a consent document prior to voluntarily proceeding with the caregiver survey. This trial was registered with ClinicalTrials.gov (NCT04265677).
RESULTS
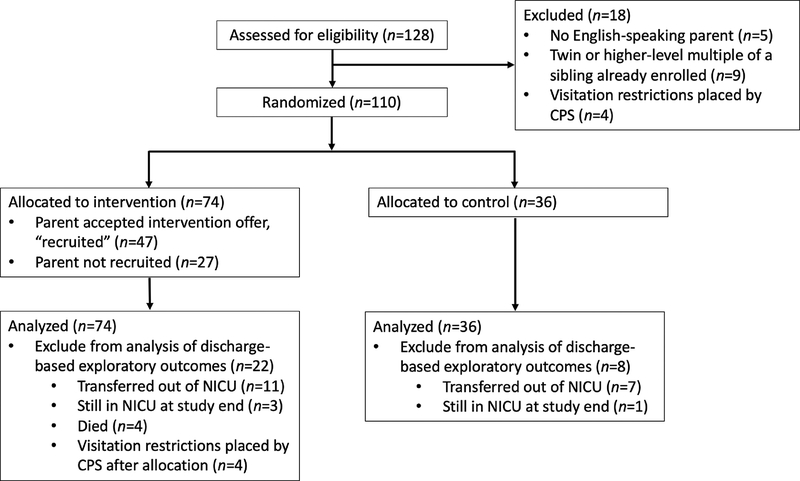
As shown in Figure 1, 110 overall subjects were included and randomly assigned in this trial (74 intervention, 36 control). The intervention and control groups were similar in regards to each measured neonatal and maternal characteristic (Table 2).
Fig. 1.
Trial Sample Size Flow Diagram
CPS – child protective services. No randomized subjects were withdrawn from the trial. Subjects with visitation restrictions placed by CPS after allocation and subjects who were not discharged home from the NICU prior to the completion of data collection were excluded from the analysis of these secondary exploratory outcomes: patient length of stay, breast milk feeding at discharge, and caregiver experience. For the subjects with post-allocation visitation restrictions, we included their data on family-centered rounds caregiver attendance up to the day that the CPS restriction was placed.
Table 2:
Neonatal and Maternal Characteristics by Intervention versus Control Group
| Intervention n=74 | Control n=36 | P a | |||
|---|---|---|---|---|---|
| Neonatal Characteristics | |||||
| Gestational Age, weeks, mean (95% CI) | 35.6 | (34.6 – 36.7) | 34.7 | (33.1 – 36.3) | 0.33 |
| Admission Age, days, median (IQR) | 0 | (0 – 1) | 0 | (0 – 0) | 0.16 |
| Sex, n (%) | 0.41 | ||||
| Female | 37 | (50.0) | 15 | (41.7) | |
| Male | 37 | (50.0) | 21 | (58.3) | |
| Race, n (%) | 0.36 | ||||
| White | 37 | (50.0) | 15 | (41.7) | |
| Asian | 8 | (10.8) | 5 | (13.9) | |
| Black | 4 | (5.4) | 3 | (8.3) | |
| Other or Multiple | 6 | (8.1) | 7 | (19.4) | |
| Unknown | 19 | (25.7) | 6 | (16.7) | |
| Ethnicity, n (%) | 0.83 | ||||
| Hispanic | 20 | (27.0) | 8 | (22.2) | |
| Not Hispanic | 51 | (68.9) | 26 | (72.2) | |
| Unknown | 3 | (4.0) | 2 | (5.6) | |
| Insurance, n (%) | 0.77 | ||||
| Public | 45 | (60.8) | 24 | (66.7) | |
| Private | 19 | (25.7) | 7 | (19.4) | |
| Other | 10 | (13.5) | 5 | (13.9) | |
| Birth Weight, kilograms, mean (95% CI) | 2.5 | (2.3 – 2.7) | 2.4 | (2.1 – 2.7) | 0.70 |
| Ventilator Use, n (%) | 12 | (16.4) | 8 | (22.9) | 0.42 |
| Disposition, n (%) | 0.50 | ||||
| Discharged Home | 56 | (75.7) | 28 | (77.8) | |
| Transfer to Other Unit or Hospital | 11 | (14.9) | 7 | (19.4) | |
| Still in NICU at End of Study | 3 | (4.0) | 1 | (2.8) | |
| Died | 4 | (5.4) | 0 | (0.0) | |
| Maternal Characteristics | |||||
| Maternal Age, mean (95% CI) | 30.3 | (28.9 – 31.7) | 29.9 | (28.1 – 31.7) | 0.74 |
| Maternal Parity, median (IQR) | 2 | (1 – 3) | 1 | (1 – 3) | 0.52 |
| Distance, miles, median (IQR) b | 39.9 | (18.7 – 78.2) | 30.6 | (16.4 – 63.4) | 0.36 |
CI – confidence interval. NICU – neonatal intensive care unit. IQR – interquartile range.
P values refer to comparisons between the intervention group and control group. Determined by using Pearson’s Chi-square test for categorical variables, t-test for normally distributed continuous variables, and nonparametric equality-of-medians test for non-normally distributed continuous variables.
Distance was the shortest driving distance from the home to the study hospital.
Trial Feasibility:
Three of the five feasibility objectives were met based on the point estimates. Objective (1) was not achieved; among intervention arm subjects, recruitment of at least one caregiver occurred for 47 (63.5%, 95% CI 51.5 – 74.4%) neonates. Objective (2) was also not achieved; caregiver use of the intervention at least once occurred for 36 (48.6%, 95% CI 36.8 – 60.6%) neonates in the intervention arm. Objective (3) was achieved; among the 258 attempted virtual FCR connections, 245 (95.0%, 95% CI 91.5 – 97.3%) encounters had no technical issues. There were three visual issues, whereby the visual connection was lost for 15–45 seconds. There were ten audio issues, due to either poor connection or loud background noises. We achieved objective (4); among FCR encounters attended by a caregiver, the mean (95% CI) duration of virtual FCR and standard rounds was 5.3 (4.9 – 5.6) and 6.4 (5.9 – 6.9) minutes, respectively. The mean (95% CI) duration of FCR with no caregiver present was 5.6 (5.4 – 6.0) minutes. Lastly, we achieved objective (5); the overall survey response rate was 87.5% (95% CI 78.2 – 93.8%). The survey response rate was 90.4% (95% CI 79.0 – 96.8) among intervention arm subjects and 82.1% (95% CI 63.1 – 93.9) among control arm subjects.
Intervention Reach:
In the post-hoc analysis of the subjects randomized to the intervention group, in comparison to the neonates whose caregivers did not use the intervention, the group with caregiver intervention use had a smaller proportion of Hispanic subjects (16.7 versus 36.8%, P=0.042) and a greater proportion of neonates whose disposition from the NICU was discharged home (94.4 versus 57.9%, P=0.002). Among the neonates whose caregiver(s) used the intervention, the median NICU length of stay was 20 (interquartile range [IQR] 9 – 32.5) days. In contrast, the median length of stay among neonates of caregivers with no intervention use was 6 (IQR 4 – 15) days. Other neonatal and maternal characteristics were similar between those with and without caregiver intervention use (Supplemental Table A).
Exploratory Outcomes:
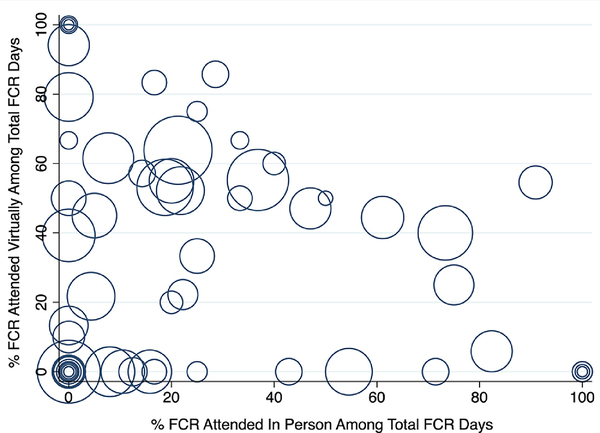
Among subjects assigned to the intervention arm, the weighted mean for the FCR caregiver attendance proportion was 53.8% (95% CI 48.9 – 59.2%). Whereas, among the control arm, the weighted mean for the FCR caregiver attendance proportion was 16.0% (95% CI 13.0 – 19.8%). The subjects in the intervention arm were estimated to have 3.36 (95% CI 2.66 – 4.23) times the FCR caregiver attendance rate of subjects in the control arm. Among the intervention group, the type of caregiver attendance consisted of 236 (56.3%) virtual encounters, 161 (38.4%) in person encounters, and 22 (5.2%) mixed encounters. Figure 2 shows a scatter plot of the type of FCR caregiver attendance for each neonate in the intervention group.
Fig. 2.
Family-Centered Rounds Caregiver Attendance Type per Neonate, Intervention Group
FCR – family-centered rounds. Each hollow circle represents one neonate, whereby the circle size is proportional to the total number of rounding encounters (i.e., opportunities for attendance). FCR encounters with mixed attendance (i.e., one caregiver virtual and one caregiver in person) were counted in the virtual attendance type numerator and the in person attendance type numerator.
As shown in Table 3, the median subject length of stay was 12 (IQR 5.5 – 29) and 20 (IQR 13.5 – 30.5) days for the intervention and control group, respectively. The probability that a randomly chosen length of stay from the control arm was at least as long as one from the intervention arm was 0.62 (95% CI 0.496 – 0.75).
Table 3:
Length of Stay and Breast Milk Feeding by Intervention versus Control Group
| Exploratory Outcome | Intervention | Control | Effect Size |
|---|---|---|---|
| Breast Milk Feeding at Discharge, % | Mean (95% CI) | Percentage Point Difference (95% CI) | |
| Any Breast Milk | 88.5 (79.8 – 97.1) | 78.6 (63.4 – 93.8) | 9.9 (−7.6 – 27.4) a |
| Exclusive Breast Milk | 30.8 (18.2 – 43.3) | 10.7 (−0.7 – 22.2) | 20.0 (3.1 – 37.0) a |
| Median (IQR) | C-statistic (95% CI) | ||
| Length of Stay, days | 12 (5.5 – 29) | 20 (13.5 – 30.5) | 0.62 (0.496 – 0.75) b |
CI – confidence interval. IQR – interquartile range.
Wald CI for the difference in proportion.
The point and CI for the C-statistic was calculated by transforming the corresponding Somers’ D point and interval estimates using the transformation C=0.5(D+1).
The between arm differences (intervention arm minus control arm) in proportion were 9.9% (95% CI −7.6 – 27.4%) and 20.0% (95% CI 3.1 – 37.0%) for receiving any breast milk and exclusive breast milk, respectively, at the time of discharge (Table 3).
Among the intervention arm, the overall medical error rate was 21.8 per 1000 patient days (95% CI 15.1 – 31.6). The overall medical error rate among the control group was 33.3 per 1000 patient days (95% CI 23.6 – 46.8). The subjects in the intervention arm were estimated to have 0.66 (95% CI 0.40 – 1.09) times the overall medical errors rate of subjects in the control arm (Table 4).
Table 4:
Medical Errors and Adverse Events by Intervention versus Control Group
| Event Type | Intervention | Control | Difference | ||
|---|---|---|---|---|---|
| # of Events | Incidence Rate (# of Events/ 1000 Patient Days) (95% CI) | # of Events | Incidence Rate (# of Events/ 1000 Patient Days) (95% CI) | Incidence Rate Ratio (95% CI) | |
| Overall Medical Errors | 28 | 21.8 (15.1 – 31.6) | 33 | 33.3 (23.6 – 46.8) | 0.66 (0.40 – 1.09) |
| Non-Harmful Errors | 17 | 13.3 (8.2 – 21.3) | 23 | 23.2 (15.4 – 34.9) | 0.57 (0.30 – 1.07) |
| Harmful Errors | 11 | 8.6 (4.8 – 15.5) | 10 | 10.1 (5.4 – 18.7) | 0.85 (0.36 – 2.00) |
| Non-Preventable Adverse Events | 8 | 6.2 (3.1 – 12.5) | 7 | 7.0 (3.4 – 14.8) | 0.88 (0.32 – 2.44) |
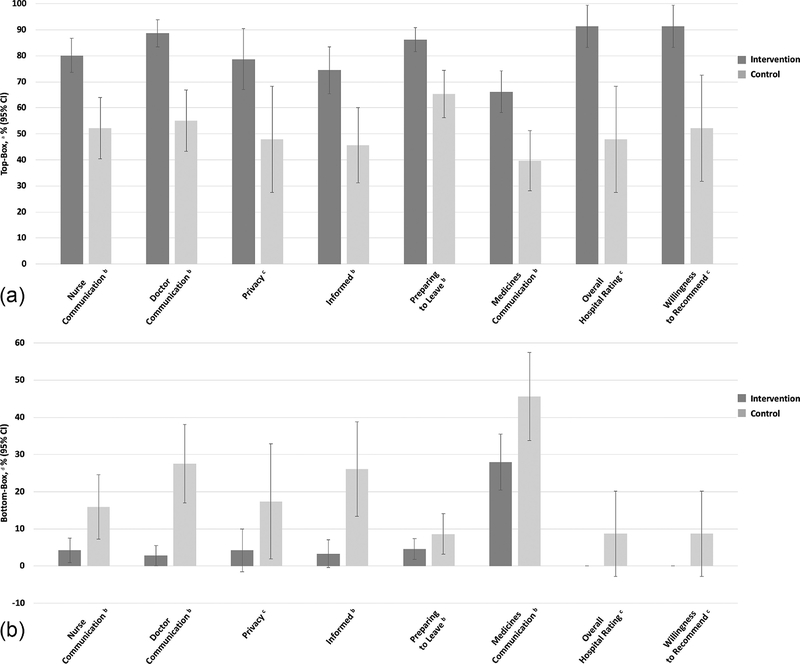
Regarding caregiver experience, the difference in HCAHPS top-box composite scores for each composite measure was higher among the intervention arm. The difference between study arms in top-box scores for overall hospital rating was 43.5% (95% CI 21.5 – 65.4%). Figures 3a and 3b present top-box and bottom-box scores by study arm. Individual HCAHPS item score data are provided in Supplemental Table B.
Fig. 3.
a. Child Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) Survey Top-Box Scores
Fig. 3. b. Child Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) Survey Bottom-Box Scores
a Top-box scores for component items were the percentage of respondents selecting the most positive response options (“always” and “yes, definitely”). Top-box scores for overall hospital rating were the percentage of respondents who selected 9 or 10 on the 11-point Likert scale. Top-box scores for willingness to recommend the hospital were the percentage of respondents who selected “definitely yes.”
b Composite measure, whereby top-box and bottom box composite scores were the mean of the item top- and bottom-box scores, respectively, within that composite measure.
c Single item measure.
d Bottom-box scores component items were the percentage of respondents selecting the negative response options (“sometimes/never” and “no”). Bottom-box scores for overall hospital rating were the percentage of respondents who selected 0–6 on the 11-point Likert scale. Bottom-box scores for willingness to recommend the hospital were the percentage of respondents who selected “definitely/probably no.”
DISCUSSION
Our pilot trial supports the feasibility of conducting a randomized trial to compare virtual FCR to standard of care in NICU patients for the improvement in patient and family outcomes. Virtual FCR is technically feasible and does not burden the medical team with increased duration of rounding times. Our high survey response rates support that data collection for the caregiver-reported outcomes is also feasible. Although use of virtual FCR among the intervention group was lower than our stated goal, caregivers of neonates assigned to the intervention arm attended FCR at relatively high rates, thereby increasing our ability to practice this family-centered standard of care.
Pilot trials serve to enhance the probability of success in the subsequent anticipated effectiveness trial.26 We therefore stated explicit feasibility objectives with a priori success criteria. Although we only achieved three of our five feasibility objectives, we conclude that a virtual FCR hypothesis testing randomized trial is feasible. Despite having lower recruitment proportions than our goal, the proportion of recruited caregivers who used the intervention was high. Furthermore, our exploratory outcomes data suggest that our virtual FCR intervention has potential to improve patient and family outcomes. Our pilot trial provided useful findings on the challenges of recruiting NICU caregivers to use our intervention; our approach proved to be insufficient. We avoided in person recruitment due to COVID-19; however, in person efforts might have been beneficial. Importantly, caregivers were infrequently at the bedside during weekday rounds, thus in person recruitment would likely need to be conducted during weekend or evening hours to be beneficial. Further work is needed to understand the contextual factors impacting the adoption of the virtual FCR intervention.
To our knowledge, no prior clinical trial has studied virtual FCR in the NICU. One pre-post study tested the feasibility of providing brief videoconferencing updates to parents of hospitalized neonates.28 These updates occurred with one clinician rather than a multidisciplinary team during FCR. Nevertheless, Epstein et al.28 demonstrated the feasibility of using real-time videoconferencing to communicate with parents of patients in the NICU. The authors concluded that this technology could potentially allow parents to be virtually involved in daily FCR; our present pilot trial supports their theory.
Prior research has examined virtual FCR in non-NICU settings.15,16 First, a non-randomized study examined the use of virtual FCR for 13 pediatric intensive care unit patients. Study parents reported that virtual FCR improved their communication with the medical team,15 which is consistent with our present trial’s HCAHPS survey data. Second, qualitative evidence from a study with adult oncology patients found that virtual rounds is perceived by clinicians to enhance the family-centeredness of care, but the perceived time burden of virtual FCR was a major barrier to adoption.16 Our present pilot trial, however, suggests that virtual FCR was not more burdensome than either standard FCR nor rounds with no caregiver present with regard to the duration of the rounding encounters.
Telehealth became especially relevant with the emergence of COVID-19. Hospital-based telehealth interventions were rapidly implemented across the nation as a means to practice social distancing and to minimize use of personal protective equipment.29,30 Virtual practices implemented in NICUs during COVID-19 have included remote video monitoring for families, virtual FCR, and virtual handoffs.31,32 The body of literature on these COVID-19 innovative care delivery systems largely consist of case studies and perspective pieces. As virtual FCR is increasingly used, these clinical interventions should be studied, and the results disseminated, in order to identify optimal implementation strategies.
This pilot trial was not designed to detect intervention efficacy. Rather, we included the exploratory outcomes to determine feasibility of data collection and to obtain empirical estimates of statistical parameters. Caution must be used when interpreting the effect sizes estimated from our pilot trial, as we might have overestimated or underestimated the true effect size.33 This limitation must be taken into consideration before generalizing these findings.
Another limitation of our study was the restriction to subjects with an English-speaking caregiver. However, the use of a homogeneous sample for this pilot trial was a necessary first step; future trials will include other languages. Our study was conducted at a level IV NICU within a teaching children’s hospital; feasibility of conducting a virtual FCR trial may differ in other settings. Furthermore, we specified five a priori feasibility objectives; however, other relevant measures of feasibility were not included. Despite these limitations, our pilot trial provides useful data to enhance the design and implementation of future virtual FCR trials.
We conclude that a randomized trial to compare virtual FCR to standard of care in NICU patients is feasible in regard to intervention technical feasibility, intervention burden on the medical team, and caregiver survey data collection. Efforts to improve recruitment of intervention arm caregivers are needed. Outcomes to measure in the future hypothesis testing trial should include FCR caregiver attendance, length of stay, breast milk feeding at discharge, caregiver experience, and medical errors and adverse events.
Supplementary Material
WHAT’S NEW.
The data from this pilot trial suggest that a randomized trial to compare virtual FCR to standard of care in NICU patients is feasible. Virtual FCR has potential to improve FCR caregiver attendance, length of stay, breast milk feeding, caregiver experience, and medical errors.
ACKNOWLEDGMENTS
Funding Sources: This work was supported by a pilot research grant from the University of California Davis Center for Healthcare Policy and Research and Center for Health Technology; the National Center for Advancing Translational Sciences, National Institutes of Health [grant number UL1 TR001860 and linked award KL2 TR001859]; and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health [grant number K23HD101550 to Dr. Rosenthal]. The content is solely the authors’ responsibility and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest Statement: The authors have no conflicts of interest to declare.
Declarations of Interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Sisterhen LL, Blaszak RT, Woods MB, Smith CE. Defining family-centered rounds. Teaching and learning in medicine. 2007;19(3):319–322. [DOI] [PubMed] [Google Scholar]
- 2.Landry M-A, Lafrenaye S, Roy M-C, Cyr C. A randomized, controlled trial of bedside versus conference-room case presentation in a pediatric intensive care unit. Pediatrics. 2007;120(2):275–280. [DOI] [PubMed] [Google Scholar]
- 3.Curley C, McEachern JE, Speroff T. A firm trial of interdisciplinary rounds on the inpatient medical wards: an intervention designed using continuous quality improvement. Medical care. 1998:AS4–AS12. [DOI] [PubMed] [Google Scholar]
- 4.Rosen P, Stenger E, Bochkoris M, Hannon MJ, Kwoh CK. Family-centered multidisciplinary rounds enhance the team approach in pediatrics. Pediatrics. 2009;123(4):e603–e608. [DOI] [PubMed] [Google Scholar]
- 5.Kuo DZ, Sisterhen LL, Sigrest TE, Biazo JM, Aitken ME, Smith CE. Family experiences and pediatric health services use associated with family-centered rounds. Pediatrics. 2012;130(2):299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Committee on Hospital Care and Institute for Patient- and Family-Centered Care. Family-centered care and the pediatrician’s role. Pediatrics. 2003;112(3 Pt 1):691. [PubMed] [Google Scholar]
- 7.Committee on Hospital Care and Institute for Patient- and Family-Centered Care. Patient-and family-centered care and the pediatrician’s role. Pediatrics. 2012;129(2):394. [DOI] [PubMed] [Google Scholar]
- 8.Ray JG, Urquia ML, Berger H, Vermeulen MJ. Maternal and neonatal separation and mortality associated with concurrent admissions to intensive care units. CMAJ. 2012;184(18):E956–E962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Safran C The collaborative edge: patient empowerment for vulnerable populations. International journal of medical informatics. 2003;69(2–3):185–190. [DOI] [PubMed] [Google Scholar]
- 10.Greene MM, Rossman B, Patra K, Kratovil AL, Janes JE, Meier PP. Depressive, anxious and perinatal post-traumatic distress in mothers of very low birth weight Infants in the NICU. Journal of developmental and behavioral pediatrics: JDBP. 2015;36(5):362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greene MM, Rossman B, Patra K, Kratovil A, Khan S, Meier PP. Maternal psychological distress and visitation to the neonatal intensive care unit. Acta Paediatrica. 2015;104(7):e306–e313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alkozei A, McMahon E, Lahav A. Stress levels and depressive symptoms in NICU mothers in the early postpartum period. The Journal of Maternal-Fetal & Neonatal Medicine. 2014;27(17):1738–1743. [DOI] [PubMed] [Google Scholar]
- 13.Mahoney AD, White RD, Velasquez A, Barrett TS, Clark RH, Ahmad KA. Impact of restrictions on parental presence in neonatal intensive care units related to coronavirus disease 2019. Journal of perinatology: official journal of the California Perinatal Association. 2020;40(Suppl 1):36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.U.S. Department of Health and Human Services. Office for Civil Rights. Notification of Enforcement Discretion for Telehealth Remote Communications During the COVID-19 Nationwide Public Health Emergency. Available at: https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html.Published 2021.Accessed February 17, 2021.
- 15.Yager PH, Clark M, Cummings BM, Noviski N. Parent participation in pediatric intensive care unit rounds via telemedicine: feasibility and impact. The journal of pediatrics. 2017;185:181–186. e183. [DOI] [PubMed] [Google Scholar]
- 16.Østervang C, Vestergaard LV, Dieperink KB, Danbjørg DB. Patient rounds with video-consulted relatives: qualitative study on possibilities and barriers from the perspective of healthcare providers. Journal of medical Internet research. 2019;21(3):e12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eldridge SM, Chan CL, Campbell MJ, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. bmj. 2016;355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toomey SL, Zaslavsky AM, Elliott MN, et al. The Development of a Pediatric Inpatient Experience of Care Measure: Child HCAHPS. Pediatrics. 2015;136(2):360–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giordano LA, Elliott MN, Goldstein E, Lehrman WG, Spencer PA. Development, implementation, and public reporting of the HCAHPS survey. Medical Care Research and Review. 2010;67(1):27–37. [DOI] [PubMed] [Google Scholar]
- 20.Bates DW, Boyle DL, Vander Vliet MB, Schneider J, Leape L. Relationship between medication errors and adverse drug events. Journal of general internal medicine. 1995;10(4):199–205. [DOI] [PubMed] [Google Scholar]
- 21.Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events: implications for prevention. Jama. 1995;274(1):29–34. [PubMed] [Google Scholar]
- 22.Kaushal R Using chart review to screen for medication errors and adverse drug events. American journal of health-system pharmacy. 2002;59(23):2323–2325. [DOI] [PubMed] [Google Scholar]
- 23.Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803–1812. [DOI] [PubMed] [Google Scholar]
- 24.Billingham SA, Whitehead AL, Julious SA. An audit of sample sizes for pilot and feasibility trials being undertaken in the United Kingdom registered in the United Kingdom Clinical Research Network database. BMC medical research methodology. 2013;13(1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kistin C, Silverstein M. Pilot studies: a critical but potentially misused component of interventional research. Jama. 2015;314(15):1561–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leon AC, Davis LL, Kraemer HC. The role and interpretation of pilot studies in clinical research. Journal of psychiatric research. 2011;45(5):626–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore CG, Carter RE, Nietert PJ, Stewart PW. Recommendations for planning pilot studies in clinical and translational research. Clinical and translational science. 2011;4(5):332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Epstein EG, Sherman J, Blackman A, Sinkin RA. Testing the feasibility of Skype and FaceTime updates with parents in the neonatal intensive care unit. American Journal of Critical Care. 2015;24(4):290–296. [DOI] [PubMed] [Google Scholar]
- 29.Hron JD, Parsons CR, Williams LA, Harper MB, Bourgeois FC. Rapid Implementation of an inpatient telehealth program during the COVID-19 pandemic. Applied Clinical Informatics. 2020;11(03):452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ong SY, Stump L, Zawalich M, et al. Inpatient Telehealth Tools to Enhance Communication and Decrease Personal Protective Equipment Consumption during Disaster Situations: A Case Study during the COVID-19 Pandemic. Applied Clinical Informatics. 2020;11(05):733–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Umoren RA, Gray MM, Handley S, et al. In-Hospital telehealth supports care for neonatal patients in strict isolation. American Journal of Perinatology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaulton J, Ziegler K, Chang E. Virtual practices transform the care delivery model in an intensive care unit during the coronavirus pandemic. Nejm Catalyst Innovations in Care Delivery. 2020. [Google Scholar]
- 33.Kraemer HC, Mintz J, Noda A, Tinklenberg J, Yesavage JA. Caution regarding the use of pilot studies to guide power calculations for study proposals. Archives of general psychiatry. 2006;63(5):484–489. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.