Abstract
Wearable technologies hold great promise for disease diagnosis and patient care. Despite the flourishing research activities in this field, only a handful of wearable devices have been commercialized and cleared for medical usage. The successful translation of current proof-of-concept prototypes will require extensive in-human testing. There is a lag between current standards and operation protocols to guide the responsible and ethical conduct of researchers in such in-human studies and the rapid development of the field. This essay presents relevant ethical concerns in early-stage human research from a researcher’s perspective.
Keywords: wearable technology, ethics, human research
Table of Content
Wearable technologies hold great promise for disease diagnosis and patient care. Despite the flourishing research activities in this field, only a handful of wearable devices have been commercialized and cleared for medical usage. The successful translation of current proof-of-concept prototypes will require extensive in-human testing. This essay presents relevant ethical concerns in early-stage human research from a researcher’s perspective.
1. Introduction
Driven by the promise of revolutionizing healthcare, the field of wearable technology has evolved rapidly into a broad, multidisciplinary topic in the past few years. Advances in microfabrication of silicon electronics and the development of soft electronic materials have enabled the seamless integration of sensing technologies with skin.[1] A plethora of studies have expanded the capability to access and analyze biofluids for broader applications of continuous disease monitoring.[2,3] The development of low-energy, self-powered systems makes continuous and autonomous operation for extended times possible.[4]
At the same time, commercial wearable technologies have also expanded from consumer health wearables towards wearable medical technology as fitness tracker giants like Apple Watch and Fitbit received FDA clearance for their ECG features. Accelerated by the shortage of medical resources and the need for telemedicine tools amid the pandemic, FDA also granted Emergency Use Authorizations (EUA) to several remote or wearable patient monitoring devices such as VitalPatch and VSMS ECG Patch (G Medical) to aid the remote monitoring of patients.[5]
The forced adoption of telemedicine during the extended lockdown period and the recent breakthrough in wearable technology will fuel the shift of the healthcare paradigm to virtual and voluntary at-home monitoring and diagnosis of diseases in a foreseeable future. Still, only a handful of wearable technologies have been successfully commercialized and adopted for clinical decision-making currently.[6] Solutions proposed at the bench side to address on-body operational challenges of wearable technologies will eventually need to be validated in humans and clinical studies before their translation into practice.
Similar to all emerging technologies, the lack of an overarching framework to guide wearable technology researchers in practice poses a barrier to the recruitment of subjects and the design of proper human research to collect meaningful data. Undoubtedly, wearable research involving human participants is guided by the three major principles of the Belmont Report, namely, respect of persons, beneficence, and justice. Researchers could also learn and draw parallels from past experiences on clinical trials involving new medical technologies when considering whether a study is ethical. For instance, Emanuel et al. proposed seven key evaluation requirements: (1) scientific/societal value of the research; (2) scientific validity; (3) fair subject selection; (4) risk-benefit analysis; (5) involvement of Institutional Review Board; (6) informed consent and (7) respect for participants.[7] While these broad frameworks apply to human research in general; wearable technology poses unique challenges beyond past case studies of medical technologies. The vast amount of multimodal, real-time data collected during human research instigate a new set of concerns on data privacy and security. The multidisciplinary nature of the field also makes the identification of a particular set of principles or a use case for ethical guidance difficult. Ethical considerations for the development or application of wearable technology for generic fitness tracking may differ from those for medical-grade wearable technology. Although Institutional Review Boards (IRBs) are the major stakeholder in protecting the rights and welfare of human subjects, IRB members may fall short of covering all ethical issues revolved around a new wearable technology due to the lack of experience and expertise.[8] Wearable researchers, on the other hand, are more familiar with a new technology and the potential risks involved. Therefore, the research community also shares the onus of identifying and addressing ethical concerns of human research and safeguarding the welfare of participants.
In this essay, we briefly discuss ethical considerations and challenges specific to the wearable research community with close reference to the current technological advancements and their potential applications. In their course of experimental design and subject recruitment, wearable researchers could play a role in addressing various ethical considerations, including reliability and validity of a device, risk assessment, subject selection and exclusion, data privacy and security as well as informed consent (Figure 1). While this essay is by no means an exhaustive discussion of all potential ethical concerns, we hope to provide better insight for investigators in various domains and different stages of wearable technology development.
Figure 1.
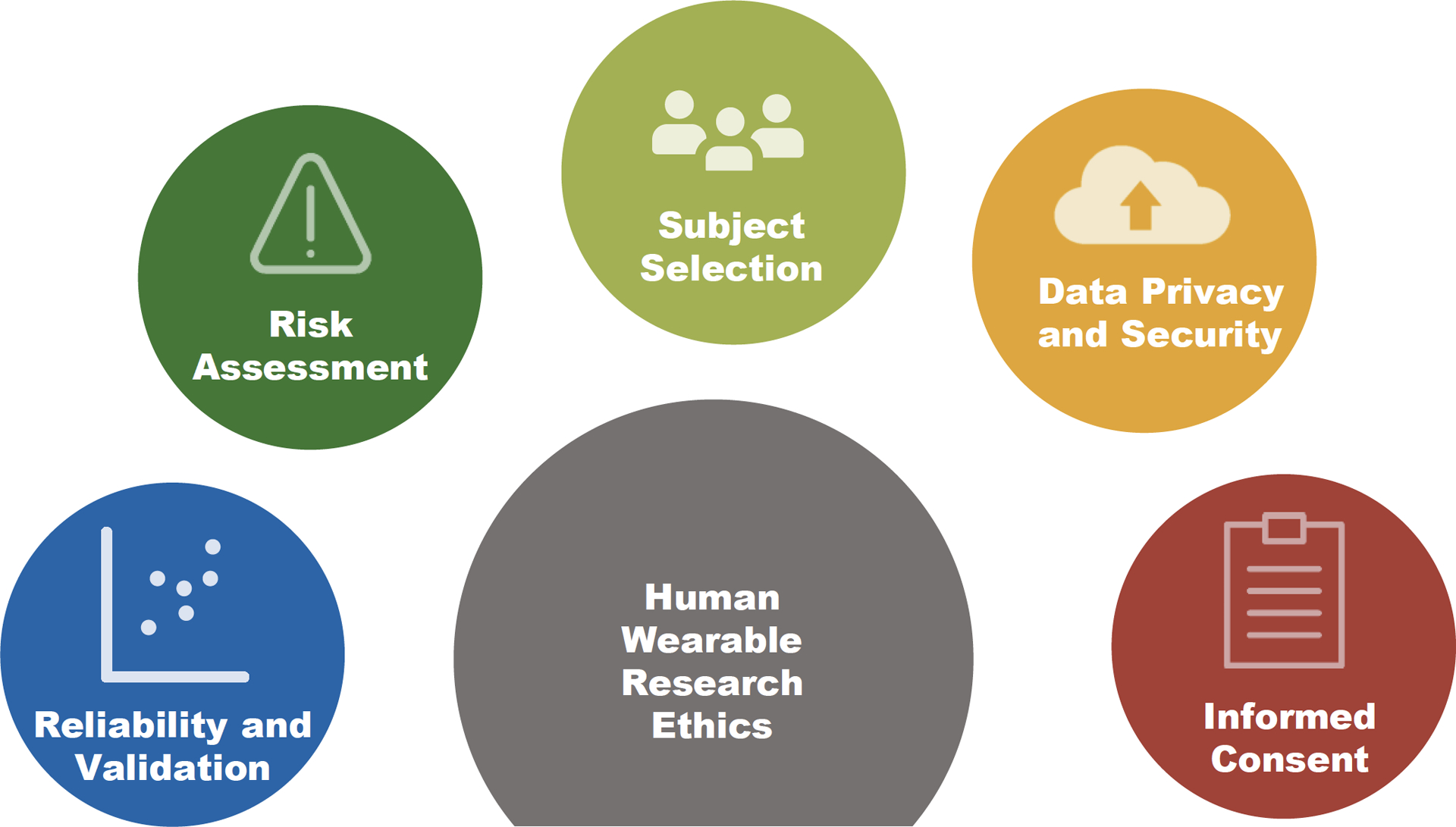
Ethical considerations and challenges of using new wearable technologies in human research.
2. Reliability and Validity
To resolve challenges faced by conventional wearable systems such as the mechanical mismatch between the skin and rigid electronics during motion, increasing efforts have been invested in the synthesis of novel stretchable materials and their integration in skin-interfaced wearable sensors wearable and mountable devices.[1,9] Soft material innovation and smart structural engineering in the past decade have enabled the development of epidermal sensing systems for monitoring physical activities and physiological signals, such as pressure, skin temperature, pulse oximetry, as well as chemical and biochemical analytes in biofluids such as sweat, saliva, and tear.[10] In the meantime, the dynamic working environment that a wearable physical or chemical sensor faces during on-body operation still introduces additional complexity and uncertainty into the real-time collection of accurate physiological information. For example, skin temperature sensors that rely on electrical behavior changes of the materials against temperature can easily be influenced by the mechanical strain.[11] Skin temperature variation inadvertently affects the performance of potentiometric sensors and enzymatic sensors.[12,13] In addition to motion artifacts, photoplethysmography (PPG) based wearable sensors may have reduced accuracy in subjects with darker tones.[14,15] Although various soft epidermal systems under research have demonstrated the intimate and unobtrusive integration of such system on the skin,[16] the technological limitations of visible light-based PPG are seldom discussed and assessed in both commercially available rigid substrate wearable devices and soft electronics research. Many factors present on the skin may affect the absorption of light differently; darker skin tones, tattoos, the presence of arm hair, sweat, body mass could all influence PPG accuracy and compromise PPG-related health outcome analysis.
Inaccurate data collected during human research due to insufficient device validation is ineffective at best. These data could also potentially exert unintended harm if they are incorporated in closed-loop body computing systems and result in incorrect health conclusions or trigger unintended intervention to the physiological environment.[17] Therefore, the onus is on researchers engaged in developing novel sensing strategies on-the-skin to account for the dynamic changes in environmental and operational factors during human research and validate the veracity of a newly developed sensor against potential influences. One common strategy adopted by several research groups is the cross-validation of sensor response with laboratory gold standard (Figure 2a and b).[18–21] Others cross-reference the data collected on-body with those collected ex vivo to identify any potential interference caused by the on-body operation.[22] Recently, various in-situ calibration mechanisms have also been introduced to account for the dynamic changes and improve sensor accuracy.[13,23,24] In conjunction with ex-situ and in-vitro validation of the sensor, many investigators of wearable chemical sensors may also opt to evaluate the relationship/correlation between serum and biomarkers present in alternative biofluid source, considering the potential influences from biofluid secretion rate and mechanism.[25,26] It is important to recognize that even if the results may not lead forward the translation of a technology (i.e., in the case of a weak or insignificant correlation), these studies still contain important information for the entire research community to evaluate the clinical significance of certain biomarkers and steer the research focus in a different direction. The appropriate and responsible reporting of validation data, as well as disclosure of uncertainty, are not only essential to ensure that results from human research are of scientific and societal significance but also the safety of participants.
Figure 2.
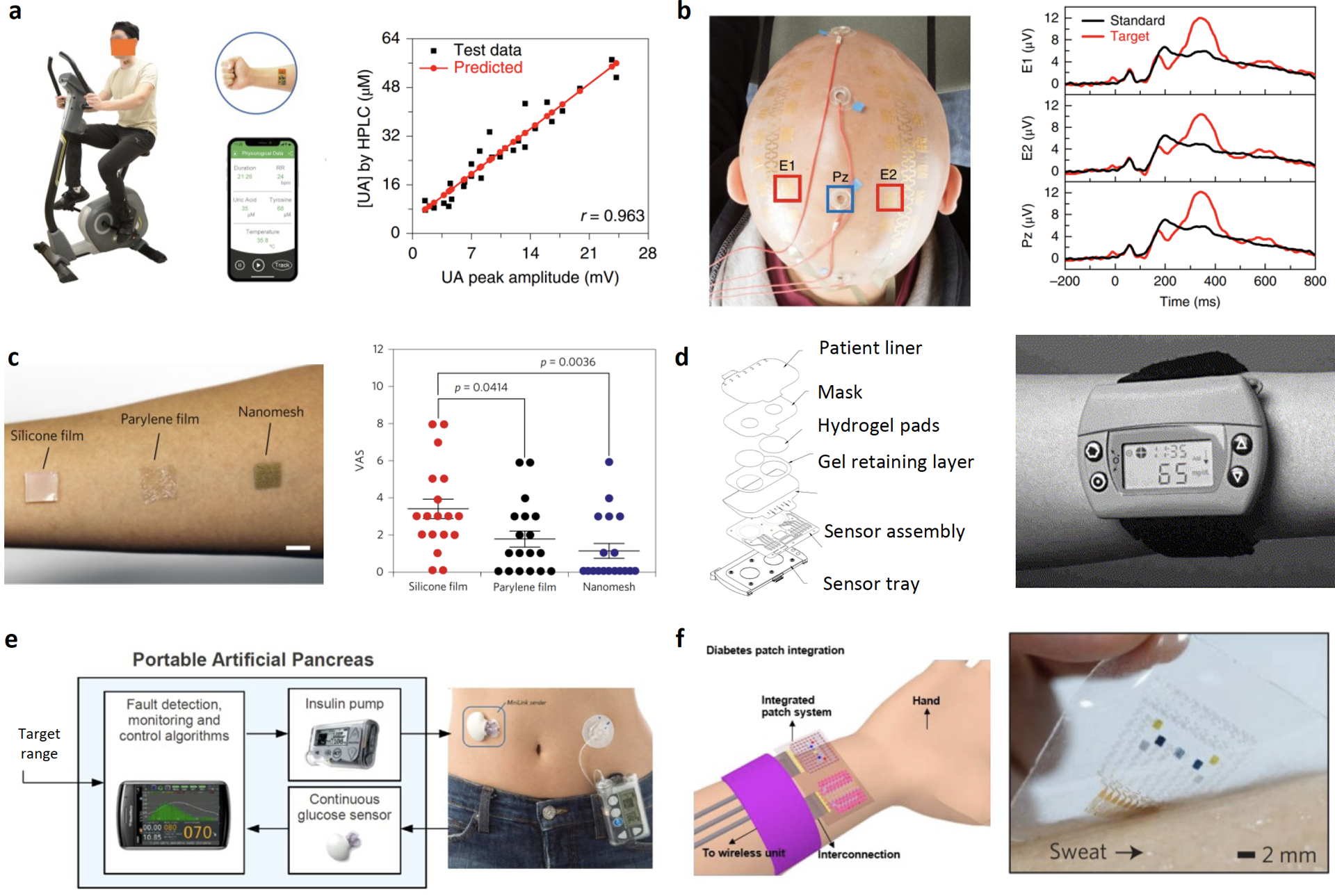
Examples of investigational wearable technologies in human studies (a-d), commercial digital therapeutics (e), and conceptualized closed loop systems (f). a) Cross validation of a wearable sweat uric acid sensor’s responses in raw sweat samples using HPLC analysis. Reproduced with permission.[30] Copyright 2020 Springer Nature. b) Performance comparison of epidermal electroencephalography (EEG) electrodes (E1 and E2) and conventional EEG cup (Pz) electrodes in recording P3 event-related potential values. Reproduced with permission.[21] Copyright 2019 Springer Nature. c) Biocompatibility test of conventional polymeric films and a newly developed gas-permeable nanomesh conductors: participants reported on feelings of discomfort based on a visual analogue scale (VAS) of 0–10 while the films were attached to the skin for a week. Reproduced with permission.[31] Copyright 2017 Springer Nature. d) Glucose monitoring by reverse iontophoresis using GlucoWatch. Reproduced with permission.[70] Copyright 2001 Elsevier. e) Illustration of a wearable sensor-augmented insulin pump which measures interstitial glucose levels and calculates appropriate insulin to be delivered in real-time and photograph of Medtronic’s MiniMed Paradigm REAL-Time which combines insulin delivery with continuous glucose monitoring (CGM) system. Reproduced with permission.[71] Copyright 2017 Elsevier. f) An integrated insulin delivery system consists of sweat glucose sensors and a thermoresponsive microneedle for diabetes therapy. Reproduced with permission.[41] Copyright 2016 Springer Nature.
In addition to the common reliability and accuracy issues faced by new sensing technologies, a unique challenge to wearable sensing devices is participants’ constant access to the sensing data. False positives as a result of inaccurate sensor reading may cause unnecessary anxiety, and the nature of wearable devices with frequent measurements and accessible data may exacerbate this emotional stress and confusion. For wearable sensing devices that target for day-to-day usage/evaluation in participants, efforts should also be devoted towards identifying the right way and appropriate frequency of presenting accurate data to the participants.
3. Risk Assessment
Although ‘non-invasiveness’ has been one of the key driving forces for the development of wearable devices for biomarkers monitoring; researchers should not overlook any physical or chemical risks associated with the operation of wearable technology in human research. Common risks associated with the on-body evaluation of wearable technologies include skin irritation, electrical shock, radiation exposure, chemical exposure and infection.
Often, epidermal devices built on conventional polymeric substrates, such as polydimethylsiloxane (PDMS), polyethylene terephthalate (PET), and polyimide (PI), are not gas permeable.[27,28] In some use case scenario this property is leveraged to prevent evaporation of sweat and facilitate the retention of volatile organic components within the skin device interface;[29, 30] on the other hand, this may also lead to skin irritation and introduce discomfort when such devices are worn for a long time. Sometimes, other choices of breathable, inflammation-free design of epidermal electronics may be available for longer-term human study (Figure 2c).[27,31] Researchers should take skin irritation and the length of study into consideration when designing human studies to minimize the risk and discomfort of participants.
Mountable devices like smart mouth guard,[32] earpieces[24] or glasses[33] warrant a closer examination of potential hazards due to chemical exposure because they are placed close to body cavities with weaker barriers of defense even though they are still considered “non-invasive” by many. In the case of a mouth guard, not only is the sensor/electrode exposed to the oral cavity but also other electronic components such as the printed circuit board (PCB).[34] The biocompatibility of individual components should be considered because even minute details like the choice of PCB solder may lead to accidental ingestion of toxic heavy metal (e.g. lead) during human studies. Additional precautions should be taken to encapsulate potential harmful components or replacing components with more biocompatible alternatives before researchers embark on device evaluation in human studies.
Soft electronics that are designed for direct contact with the ocular cavity[35,36] and open wounds[37,38] are typically associated with more risks when evaluated in vivo. In addition to biocompatibility and device design ergonomics concerns, an important factor to consider in order to meet the principle of nonmaleficence is the sterilization of devices to minimize risks of infection.[39,40] Sensible steps to take before human research include the in vitro cytotoxicity screening of materials and the testing in preclinical animal models.[41–43] In these two cases, ethical considerations relevant to animal research and the choice of animal models with modest translational distance (characterized by the number and size of inferential leaps from animals to humans[44]) are important.
Wearable transdermal sensors in the form of microneedles are minimally invasive because of the small dimensions of the needles. Although reports show that recovery of skin barrier function can be as fast as a few hours after micropore creation,[45] the application of wearable transdermal device introduces additional risks of infections as unclosed microchannels may promote microcirculation of bacteria.[46] Standard operation protocols that ensure the implementation of good clinical practice prior to the application of microneedle patches are essential in minimizing the influx of exogenous microbiomes from surroundings. Confounding factors such as random movements, natural variations in skin texture, manual application pressure may introduce additional compression or shear stress that could potentially result in the failure and fracture of hollow microneedles. Moreover, microneedle materials or residual chemicals from microneedle processing methods could introduce additional risks of skin irritation. Various mechanical and biophysical characterization methods could be conducted in vitro and in vivo to evaluate potential hazards and assess the safety (skin irritation) of new devices.[47,48]
In addition to performing sensing and monitoring tasks, many wearable technologies developed in the lab also involve certain intervention capabilities where built-in actuators are triggered to deliver electrical/thermal stimulation or, in some cases, active drug components. GlucoWatch’s reverse iontophoresis (RI) might be the earliest demonstration of such types of intervention to facilitate the access and concentration of biofluids or biomarkers (Figure 2d).[49] RI applies a mild current between two electrodes to induce ion migration across the skin and extracts interstitial fluids due to electro-osmotic flow. One reason for the later retraction of this device from the market is the reported skin irritations due to the application of current.[50] Similarly, skin irritation is also associated with the long-term operation of epidermal iontophoretic devices that rely on the application of mild current to deliver sweat-stimulating drugs to trigger the local secretion of sweat under sedentary conditions.[2,51] Risks of skin irritation due to electrical shock and chemical build-up can be controlled and minimized by reducing current density, the time of application, appropriate buffering recipe and switching of cathode/anode to maintain local pH.[52] Other examples of intervention technologies are most commonly found in next-generation closed-loop systems where continuous monitoring of biomarkers is coupled with actuators that can be triggered when the level of a biomarker fluctuates beyond desirable levels.[17,41] In addition to performing and disclosing electrical safety risk assessment, researchers should also consider biochemical risks such as allergic reaction when an intervention technology is designed to deliver active drug components to subjects. Extra caution should be taken to address potential drug interaction when the subjects are taking additional medications.
While all wearable devices with wireless communication capabilities expose subjects to radiofrequency radiation, devices employing high-power communication technologies such as Wi-Fi to transfer large datasets are more susceptible to radiation risks. Although high-power devices like smartphones are generally regulated by specific absorption rate (SAR) testing and there is currently no clear evidence on the risks of low-level radiation;[53] wearable devices are clearly placed in closer proximity to the human body for longer periods of time. Risks associated with chronic exposure to low-intensity radiation are currently unknown. In addition, researchers should also be cautious of the cumulative effects of low-intensity radiation by operating multiple high-power wearable/portable devices in parallel.[54]
4. Fair subject selection and exclusion
Human research studies in this emerging field mostly fall into the category of first-in-human (FIH) or early-stage human trials. Experiments are designed based on information from limited literature sources or animal studies that predict a participant’s safety can be adequately protected with certain assumptions. Along with the objectives of scientific validity and societal value, experimental designs of human trials should clearly identify risks of harm to the subjects and outline all possible precautionary or intervention steps during the study to minimize risks and prevent harm. Selecting subjects who can make well-informed choices about research participation and from whom scientifically relevant data with minimal risks is a critical step.
Apt and fair subject selection may pose considerable challenges for FIH trials. For wearable medical technologies targeted at various vulnerable populations (patients with specific disease conditions), substantially more risks are involved as compared to the participation of healthy subjects. The evaluation of wearable sweat sensors typically requires subjects to perform mid- to high-intensity physical exercise. Human studies dealing with the non-invasive monitoring and management of chronic diseases such as metabolic syndrome or diabetes may require the recruitment of subjects with pre-existing medical conditions. Subjects who are physically inactive may find typical cycle ergometer exercise protocol designed for sweat collection (e.g. timed trial with constant workload or graded workload) more physically demanding. Potential risks and exercise-induced emergencies (e.g. bronchoconstriction, anaphylaxis, heat-illness) should be identified with appropriate standard operating procedures outlined prior to the recruitment of subjects to safeguard vulnerable populations.
Human studies may also aim to intentionally trigger a transient physiological or psychological abnormality in subjects (e.g. stress[55] and fatigue[56] experiments). Under the oversight of IRB, researchers are responsible for weighing the potential scientific value against the susceptibility to risk for certain groups of individuals (e.g. pregnant women, students) and determining the appropriate exclusion criteria of a study. As the ultimate goal of most wearable technologies is to monitor or diagnose a user’s health conditions, researchers may occasionally encounter incidental findings (e.g. abnormalities in the data collected from a participant) in the course of human research. A detailed framework for addressing and managing incidental findings during human research can be found elsewhere.[57]
Investigators should also make concerted efforts in recruiting individuals of various backgrounds in order to conduct scientifically and ethically sound research. The main goal of early-stage human research in wearable technology is to validate and translate a novel technological breakthrough to a viable prototype that could potentially benefit the largest population. Therefore, potential risks/benefits and device validity should be evaluated across different groups to minimize subject selection biases or inadvertent exclusion-by-design.
Wearable exoskeletons that are designed to restore or enhance human strength and agility hold great promise in rehabilitation. However, the device size and weight of wearable exoskeletons impose certain weight, height restrictions on the user/subject.[58] Commercial exoskeleton providers tend to impose rigid inclusion criteria from a cost perspective by investing on one-size-fits-all prototypes. As a result, children and individuals who are obese (which could be common for disabled individuals with sedentary lifestyles) may be denied access to such technologies due to exclusion by design. Women from certain ethnic groups with lower average height also tend to be “underweight” based on the user selection criteria of most commercial exoskeletons. Wearable exoskeleton research could potentially tackle this discrimination in marginalized communities by understanding and reflecting on the exclusion criteria and improve the inclusivity of a device from the design stage. Researchers share the responsibility to identify potential biases and dismantle any disparities caused by an inappropriate device or human study designs from the start. Incomplete metrics obtained in validation studies that lack diversity may also cause unintended consequences by reinforcing existing disparities in healthcare.[59]
5. Data Privacy and Security
The integration of a plethora of sensors on soft epidermal systems has enabled the passive collection of temporal information of a wide range of behavioral and biometric data. Real-time, continuous transmission of the information collected to other devices or cloud storage for post-processing can be achieved with various wireless communication technologies such as Near Field Communication (NFC), Bluetooth, Zigbee, and Wi-Fi.[14] Information collected and transmitted through current wearable technologies ranges from a simple heartbeat to the geographical location of a user and his medical conditions. While data sharing presents its unique advantage to personalized and adaptive health interventions, the vast amount of private identifiable information associated with human research raises serious concern over the privacy and data confidentiality of participants. A recent survey on digital consumer health revealed that the use of consumer health wearable devices has decreased from 33% in 2018 to just 18% in 2019.[60] Participants of human studies involving pervasive sensing technologies also cited data privacy and confidentiality as a major concern.[61] Therefore, investigators need to think ahead of research and incorporate ethical and regulatory considerations of data privacy and security early in the research design.
At times, data anonymization via distortion or removal of identifying features is introduced in research protocol to protect personal data. However, the effectiveness of such approaches against personal identity theft is still questionable.[62] Depending on the nature of the human study (population-level or personalized medicine), requirements on the extent of personal information gathered may differ. Controversies over COVID-19 tracing with mobile health and wearable technologies manifest the risks and potential conflicts associated with personal data in large scale data-rich human research. The decentralized contact tracing app promoted by Google and Apple allows anonymized pairing between infected people and their close contacts on their phones; on the other hand, the centralized tracing method traces contacts with a health authority-owned database by collecting personal information with mobile phone apps, wearable dongle or other surveillance methods. Although advocates of centralized tracing cited epidemiological benefits as health authorities can monitor the disease’s spread, concerns over intensive surveillance and intrusion of privacy stalled the adoption of centralized tracing in many countries.[63] Some biometric information collected with wearable technology may fall in the grey zone when it comes to regulatory compliance of data protection laws like General Data Protection Regulation (GDPR) and Health Insurance Portability and Accountability Act (HIPAA). While the ethical, legal, and social concerns in data-driven human studies may require collaborative efforts from IRB-related stakeholders, security experts and legal and regulatory expertise to outline case-specific data management and storage protocols,[61] on an individual research level, investigators can also address this trust deficit crisis by being forthcoming with how data is collected and used.
6. Informed consent
Informed consent is an ethical, regulatory, and legal requirement in human research that allows researchers to communicate the potential benefits and risks of the study to the participants. However, an informed consent document can be lengthy and contain technical jargons that are hard for potential research participants to comprehend. To practice respect for persons and to minimize information asymmetry, the information about the human study must be conveyed in a simple language to ensure adequate understanding. Additional methods such as video and in-person demonstration may facilitate comprehension during the consent process. [64] Adaptions of the informed consent may be necessary to account for varying degrees of educational literacy, cognitive ability, and clinical status in potential participants.[65] In an informed consent document, potential risks and the purpose of the study should be clearly communicated for participants to make informed decision. Another important point to take note of and clarify in the informed consent for the wearable research community is the issue of data ownership and secondary use of data. In addition to the sensor and wireless communication technology development, a sizeable number of studies focus on software development and data analysis through machine learning. [66,67] Research groups with limited hardware development expertise may opt for commercially available consumer health or medical health wearable devices to collect large scale human data.[68] In such cases, end-user licensing agreement of the commercial device may complicate the issue of data ownership and usage. For example, Fitbit users may be unintentionally sharing their information with third parties when they sign up for an account.[69] Researchers should inform participants of potential secondary usage of data as stated in the privacy policy documents of commercial devices.
While both medical grade and consumer health grade wearable technologies are available on the market, the fine distinctions between these two device categories tend to cause confusion among the general public. A user’s misconception over the information collected by a wellness device may also be exacerbated by commercial advertisements’ choice of wording and the implied benefits. Therefore, an informed consent should explicitly state if the purpose of the device under evaluation is to diagnose or treat a medical condition (which constitute as a medical device) or to collect information to avoid participants’ confusion and over trust of a device and its data.
7. Summary and perspective
To date, much effort has been invested in the development and prototyping of soft electronics and robust sensing technologies at the bench side. Moving forward, current wearable technologies will need to demonstrate their validity and utility in clinical or point-of-care settings with larger scale human data from longitudinal and cohort studies. As current epidermal sensing technologies mature, they are expected to integrate into more complex closed-loop systems that allow autonomous intervention for therapeutic purposes to achieve the ultimate goal of personalized disease management. Although there have been commercial products that are capable of closing the loop in disease management such as Medtronic’s sensor-augmented insulin pump therapy for diabetes management (Figure 2e); these systems are based on rigid electronics with minimally invasive monitoring techniques. Future advances in biomaterials and flexible electronics will drive the evolution of such closed-loop systems into smaller, more conformal, hassle-free prototypes that can find applications in a broader audience. For example, an integrated drug delivery system consists of graphene-based multipixel biosensors for noninvasive sweat glucose monitoring and a thermoresponsive microneedle patch (triggered by elevated glucose level) for insulin therapy was proposed (Figure 2f).[41] Still, wearable closed-loop sensor-augmented drug delivery system is in its infancy. Such prototypes have yet to be validated rigorously in vivo. In addition to a multitude of technological bottlenecks in reliable sensor reading, energy harvesting, communication, and closed-loop algorithm, challenges such as therapy effectiveness, reliability and safety will need to be answered with large-scale and in-depth animal and/or human studies.
Despite the exponential growth of the field in the past decade, we are only at the beginning of harnessing wearable technology for performance enhancement and health management. As the field progresses, more innovative solutions to current technical challenges may become available; at the same time, these technologies may also bring about unforeseen ethical concerns during human research. We believe the active engagement of the research community in the ethical discussions and protection of human welfare is instrumental in facilitating successful early-stage human trials. Clear and close communication with research oversight bodies ensures that knowledge held by the researchers can be formalized and transferred to independent regulatory oversights and close the gap between current regulatory guidelines and the rapidly evolving research landscape. The medical community’s acceptance of these non-invasive technologies and their subsequent translation to a broader audience will require the concerted efforts of the research community to conduct scientifically and ethically sound in-human validation and extensive investigation on the clinical relevance of data collected with wearable technologies.
Acknowledgements
This project was supported by the National Institutes of Health grant 5R21NR018271, the Translational Research Institute for Space Health through NASA NNX16AO69A, NASA Cooperative Agreement 80NSSC20M0167, High Impact Pilot Research Award T31IP1666 from Tobacco Related-Disease Research Program, and the Rothenberg Innovation Initiative program at California Institute of Technology.
Biographies

Jiaobing Tu received her BEng in Materials Science and Engineering from Imperial College London. She joined Dr. Wei Gao’s research group in 2018 and is currently pursuing her PhD degree in Medical Engineering at the California Institute of Technology. Her research interests include wearable electronics and biosensors.

Wei Gao is currently an Assistant Professor of Medical Engineering at the California Institute of Technology. He received his PhD in Chemical Engineering from the University of California, San Diego in 2014. He worked as a postdoctoral fellow in Electrical Engineering and Computer Sciences at the University of California, Berkeley between 2014 and 2017. His current research interests include wearable biosensors, robotics, flexible electronics, and nanomedicine.
References
- [1].Yang JC, Mun J, Kwon SY, Park S, Bao Z, Park S, Adv. Mater 2019, 31, 1904765. [DOI] [PubMed] [Google Scholar]
- [2].Heikenfeld J, Jajack A, Feldman B, Granger SW, Gaitonde S, Begtrup G, Katchman BA, Nat. Biotechnol 2019, 37, 407. [DOI] [PubMed] [Google Scholar]
- [3].Tu J, Torrente‐Rodríguez RM, Wang M, Gao W, Adv. Funct. Mater 2020, 30, 1906713. [Google Scholar]
- [4].Yu Y, Nyein HYY, Gao W, Javey A, Adv. Mater 2020, 32, 1902083. [DOI] [PubMed] [Google Scholar]
- [5].FDA Remote or Wearable Patient Monitoring Devices EUAs, https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/remote-or-wearable-patient-monitoring-devices-euas (accessed: December 2020)
- [6].Wearable technology in healthcare, Nat. Biotechnol 2019, 37, 376. [DOI] [PubMed] [Google Scholar]
- [7].Emanuel EJ, Wendler D, Grady C, JAMA 2000, 283, 2701.10819955 [Google Scholar]
- [8].Torous J, Nebeker C, J. Med. Internet. Res 2017, 19, e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Someya T, Bao Z, Malliaras GG, Nature 2016, 540, 379. [DOI] [PubMed] [Google Scholar]
- [10].Kim J, Campbell AS, de Ávila BE-F, Wang J, Nat. Biotechnol 2019, 37, 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Xu C, Yang Y, Gao W, Matter 2020, 2, 1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bandodkar AJ, Molinnus D, Mirza O, Guinovart T, Windmiller JR, Valdés-Ramírez G, Andrade FJ, Schöning MJ, Wang J, Biosens. Bioelectron 2014, 54, 603. [DOI] [PubMed] [Google Scholar]
- [13].Hong YJ, Lee H, Kim J, Lee M, Choi HJ, Hyeon T, Kim D-H, Adv. Funct. Mater 2018, 28, 1805754. [Google Scholar]
- [14].Shcherbina A, Mattsson CM, Waggott D, Salisbury H, Christle JW, Hastie T, Wheeler MT, Ashley EA, J. Pers. Med 2017, 7, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fallow BA, Tarumi T, Tanaka H, J. Clin. Monit. Comput 2013, 27, 313. [DOI] [PubMed] [Google Scholar]
- [16].Yokota T, Zalar P, Kaltenbrunner M, Jinno H, Matsuhisa N, Kitanosako H, Tachibana Y, Yukita W, Koizumi M, Someya T, Sci. Adv 2016, 2, e1501856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lin Y, Bariya M, Javey A, Adv. Funct. Mater 2021, 31, 2008087. [Google Scholar]
- [18].Koh A, Kang D, Xue Y, Lee S, Pielak RM, Kim J, Hwang T, Min S, Banks A, Bastien P, et al. , Sci. Transl. Med 2016, 8, 366ra165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Parlak O, Keene ST, Marais A, Curto VF, Salleo A, Sci. Adv 2018, 4, eaar2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bandodkar AJ, Gutruf P, Choi J, Lee K, Sekine Y, Reeder JT, Jeang WJ, Aranyosi AJ, Lee SP, Model JB, et al. , Sci. Adv 2019, 5, eaav3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tian L, Zimmerman B, Akhtar A, Yu KJ, Moore M, Wu J, Larsen RJ, Lee JW, Li J, Liu Y, et al. , Nat. Biomed. Eng 2019, 3, 194. [DOI] [PubMed] [Google Scholar]
- [22].Gao W, Emaminejad S, Nyein HYY, Challa S, Chen K, Peck A, Fahad HM, Ota H, Shiraki H, Kiriya D, et al. , Nature 2016, 529, 509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gao L, Zhang Y, Malyarchuk V, Jia L, Jang K-I, Chad Webb R, Fu H, Shi Y, Zhou G, Shi L, et al. , Nat. Commun 2014, 5, 4938. [DOI] [PubMed] [Google Scholar]
- [24].Ota H, Chao M, Gao Y, Wu E, Tai L-C, Chen K, Matsuoka Y, Iwai K, Fahad HM, Gao W, et al. , ACS Sens. 2017, 2, 990. [DOI] [PubMed] [Google Scholar]
- [25].Choi D-H, Kitchen GB, Stewart KJ, Searson PC, Sci. Rep 2020, 10, 7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Klous L, de Ruiter CJ, Scherrer S, Gerrett N, Daanen HAM, Eur. J. Appl. Physiol 2020, 121, 803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fan YJ, Li X, Kuang SY, Zhang L, Chen YH, Liu L, Zhang K, Ma SW, Liang F, Wu T, et al. , ACS Nano 2018, 12, 9326. [DOI] [PubMed] [Google Scholar]
- [28].Zhou W, Yao S, Wang H, Du Q, Ma Y, Zhu Y, ACS Nano 2020, 14, 5798. [DOI] [PubMed] [Google Scholar]
- [29].Reeder JT, Choi J, Xue Y, Gutruf P, Hanson J, Liu M, Ray T, Bandodkar AJ, Avila R, Xia W, et al. , Sci. Adv 2019, 5, eaau6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yang Y, Song Y, Bo X, Min J, Pak OS, Zhu L, Wang M, Tu J, Kogan A, Zhang H, et al. , Nat. Biotechnol 2020, 38, 217. [DOI] [PubMed] [Google Scholar]
- [31].Miyamoto A, Lee S, Cooray NF, Lee S, Mori M, Matsuhisa N, Jin H, Yoda L, Yokota T, Itoh A, et al. , Nat. Nanotech 2017, 12, 907. [DOI] [PubMed] [Google Scholar]
- [32].Kim J, Valdés-Ramírez G, Bandodkar AJ, Jia W, Martinez AG, Ramírez J, Mercier P, Wang J, Analyst 2014, 139, 1632. [DOI] [PubMed] [Google Scholar]
- [33].Sempionatto JR, Nakagawa T, Pavinatto A, Mensah ST, Imani S, Mercier P, Wang J, Lab Chip 2017, 17, 1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kim J, Imani S, de Araujo WR, Warchall J, Valdés-Ramírez G, Paixão TRLC, Mercier PP, Wang J, Biosens. Bioelectron 2015, 74, 1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Google contact lens homepage, https://blog.google/alphabet/introducing-our-smart-contact-lens/ (accessed: December 2020)
- [36].Tseng RC, Chen C-C, Hsu S-M, Chuang H-S, Sensors 2018, 18, 2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Guinovart T, Valdés‐Ramírez G, Windmiller JR, Andrade FJ, Wang J, Electroanal. 2014, 26, 1345. [Google Scholar]
- [38].Brown MS, Ashley B, Koh A, Front. Bioeng. Biotechnol 2018, 6, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].McCrudden MTC, Alkilani AZ, Courtenay AJ, McCrudden CM, McCloskey B, Walker C, Alshraiedeh N, Lutton REM, Gilmore BF, Woolfson AD, et al. , Drug Deliv. Transl. Res 2015, 5, 3. [DOI] [PubMed] [Google Scholar]
- [40].Ochoa M, Rahimi R, Zhou J, Jiang H, Yoon CK, Maddipatla D, Narakathu BB, Jain V, Oscai MM, Morken TJ, et al. , Microsyst. Nanoeng 2020, 6, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lee H, Choi TK, Lee YB, Cho HR, Ghaffari R, Wang L, Choi HJ, Chung TD, Lu N, Hyeon T, et al. , Nat. Nanotechnol 2016, 11, 566. [DOI] [PubMed] [Google Scholar]
- [42].Lee H, Song C, Hong YS, Kim MS, Cho HR, Kang T, Shin K, Choi SH, Hyeon T, Kim D-H, Sci. Adv 2017, 3, e1601314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Keum DH, Kim S-K, Koo J, Lee G-H, Jeon C, Mok JW, Mun BH, Lee KJ, Kamrani E, Joo C-K, et al. , Sci. Adv 2020, 6, eaba3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kimmelman J, Gene Transfer and the Ethics of First-in-Human Research: Lost in Translation, Cambridge University Press, 2010. [Google Scholar]
- [45].Kalluri H, Kolli CS, Banga AK, AAPS J. 2011, 13, 473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].McConville A, Hegarty C, Davis J, Medicines 2018, 5, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Park J-H, Allen MG, Prausnitz MR, Control J. Release 2005, 104, 51. [DOI] [PubMed] [Google Scholar]
- [48].Bal SM, Caussin J, Pavel S, Bouwstra JA, Eur. J. Pharm. Sci 2008, 35, 193. [DOI] [PubMed] [Google Scholar]
- [49].Potts RO, Tamada JA, Tierney MJ, Diabetes. Metab. Res. Rev 2002, 18, S49. [DOI] [PubMed] [Google Scholar]
- [50].Kim J, Campbell AS, Wang J, Talanta 2018, 177, 163. [DOI] [PubMed] [Google Scholar]
- [51].Emaminejad S, Gao W, Wu E, Davies ZA, Nyein HYY, Challa S, Ryan SP, Fahad HM, Chen K, Shahpar Z, et al. , Proc. Natl. Acad. Sci. U. S. A 2017, 114, 4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Roustit M, Blaise S, Cracowski J-L, Br. J. Clin. Pharmacol 2014, 77, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lewis D, Nature 2020, 588, 384. [DOI] [PubMed] [Google Scholar]
- [54].Ulrich CM, Demiris G, Kennedy R, Rothwell E, Nurs. Outlook 2020, 68, 720. [DOI] [PubMed] [Google Scholar]
- [55].Torrente-Rodríguez RM, Tu J, Yang Y, Min J, Wang M, Song Y, Yu Y, Xu C, Ye C, IsHak WW, et al. , Matter 2020, 2, 921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Sedighi Maman Z, Alamdar Yazdi MA, Cavuoto LA, Megahed FM, Appl. Ergon 2017, 65, 515. [DOI] [PubMed] [Google Scholar]
- [57].Wolf SM, Lawrenz FP, Nelson CA, Kahn JP, Cho MK, Clayton EW, Fletcher JG, Georgieff MK, Hammerschmidt D, Hudson K, et al. , J. Law Med. Ethics 2008, 36, 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Søraa RA, Fosch-Villaronga E, Paladyn, Paladyn 2020, 11, 217. [Google Scholar]
- [59].Colvonen PJ, DeYoung PN, Bosompra N-OA, Owens RL, Sleep 2020, 43, zsaa159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Accenture Digital Health Consumer Survey 2020, https://www.accenture.com/us-en/insights/health/why-consumer-digital-health-adoption-stalling (accessed: December 2020)
- [61].Nebeker C, Harlow J, Giacinto RE, Orozco-Linares R, Bloss CS, Weibel N, AJOB Empir. Bioeth 2017, 8, 266. [DOI] [PubMed] [Google Scholar]
- [62].Piwek L, Ellis DA, Andrews S, Joinson A, PLoS Med. 2016, 13, e1001953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Lewis D, Nature 2020, 588, 384. [DOI] [PubMed] [Google Scholar]
- [64].Ulrich CM, Demiris G, Kennedy R, Rothwell E, Nurs. Outlook 2020, 68, 720. [DOI] [PubMed] [Google Scholar]
- [65].Kadam RA, Perspect. Clin. Res 2017, 8, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Al-Turjman F, Baali I, Trans. Emerg. Telecommun. Technol 2019, e3635. [Google Scholar]
- [67].Mohr DC, Zhang M, Schueller SM, Annu. Rev. Clin. Psychol 2017, 13, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Arriba-Pérez D, Caeiro-Rodríguez M, Santos-Gago JM, Sensors 2016, 16, 1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Fitbit Legal Privacy Policy, https://www.fitbit.com/global/us/legal/privacy-policy (accessed: December 2020)
- [70].Tierney MJ, Tamada JA, Potts RO, Jovanovic L, Garg S, Cygnus Research Team, Biosens. Bioelectron 2001, 16, 621. [DOI] [PubMed] [Google Scholar]
- [71].Boiroux D, Duun-Henriksen AK., Schmidt S, Nørgaard K, Poulsen NK, Madsen H, Jørgensen JB, Control Eng. Pract 2017, 58, 332. [Google Scholar]


