Abstract
Background
A variety of neurosensory symptoms including tinnitus have been associated with COVID-19 infection. While most cases of tinnitus are associated with hearing loss, here we report a case of severe tinnitus following COVID-19 infection with normal thresholds through 8000 Hz.
Case report
A 49-year-old male presented with new onset severe tinnitus following COVID-19 infection. Tinnitus was bilateral, constant and nonpulsatile. Audiometric evaluation revealed normal threshold through 8000 Hz, with mild hearing loss at 16,000 Hz. Conservative measures including masking strategies failed to mitigate symptoms. A trial of gabapentin 300 mg twice per day improved tinnitus with no notable side effects.
Conclusion
This patient may represent a subpopulation of patients who suffer from severe tinnitus following COVID-19 infection in the setting of largely normal hearing. The pathophysiology may be distinct from the more common hearing loss associated tinnitus and perhaps neuromodulators may play a larger role in mitigating tinnitus in this patient subset.
Keywords: Tinnitus, Hearing loss, COVID-19 infection, Gabapentin, Neuromodulators
1. Introduction
Tinnitus affects up to 50 million adults in the United States, with 16 million experiencing chronic or frequent tinnitus [1]. Tinnitus is subjective and can have detrimental effects on mental health and quality of life [2]. Although majority of tinnitus cases are associated with hearing loss, up to 10% of cases occur in patients with no changes in hearing sensitivity on standard audiometric evaluation [3]. The current index patient and others suffering from COVID-19 infections brought forth two questions. First, does COVID-19 cause tinnitus? This important question can only be answered through careful data driven analysis and not anecdotal reports or case series. Second, is the pathophysiology of tinnitus related to COVID-19 different than that related to hearing loss? If so, then should we treat it differently?
The impact of COVID-19 infection on neurosensory systems is not well-understood; however, COVID-19 infection has been hypothesized to result in neurosensory dysfunction, most commonly in olfactory and gustatory alterations in up to 30% of affected patients [4], [5]. Tinnitus, vertigo, and sensorineural hearing loss occurring after COVID-19 infections have been reported; however, correlation and causation have not been determined [6]. Early studies reported new onset tinnitus after COVID-19 infection typically in conjunction with hearing loss [7], [8], [9]. Proposed mechanisms for audio-vestibular disorders coinciding with COVID-19 infection include direct cochlear and labyrinthine injury from persistent inflammatory cytokines within the inner ear, concomitant onset of an autoimmune response, direct viral invasion to nerves via ACE receptors, and ototoxicity of drugs used in COVID-19 patients such as hydroxychloroquine and macrolides [10], [11], [12]. In the present study, we report a unique case of severe, new onset tinnitus in a COVID-19 patient without significant hearing loss, which was successfully treated with gabapentin.
2. Index case
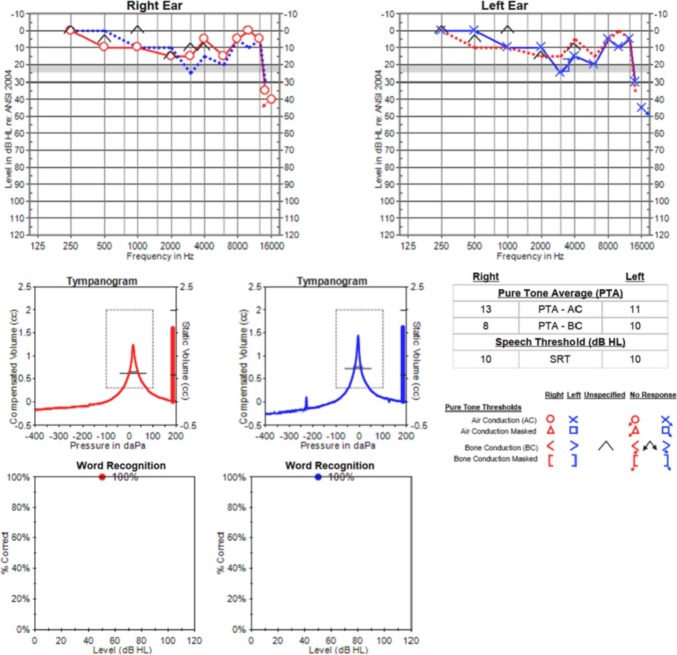
A 49-year-old male with past medical history of hyperlipidemia, and laboratory-confirmed COVID-19 infection three months prior, presented to our clinic with tinnitus. The patient had a mild COVID-19 course and did not require hospitalization. His tinnitus developed shortly after his COVID-19 infection, and was bilateral, constant, and nonpulsatile. Specifically, he described a “high-pitched buzzing sound,” which was constant throughout the day and most severe at night. He had no history of tinnitus prior to this incident. Audiometric testing demonstrated normal thresholds through 8000 Hz, with mild hearing loss above 12.5 kHz (Fig. 1 ). Tympanometry revealed normal tympanic membrane movement (type A), and word recognition scores using recorded isophonemic stimuli (20-word lists) were 100% bilaterally.
Fig. 1.
Audiometric testing revealed normal hearing thresholds through 8000 Hz, with mild bilateral hearing loss above 12.5 kHz. Tympanometry was normal, and word recognition scores were excellent bilaterally.
Initial treatment involved conservative measures such as white noise masking. Despite the conservative treatment strategies, education and time the tinnitus was persistent and significantly bothersome to the patient. Consequently, he was started on gabapentin 300 mg daily, which was tolerated well for two weeks and then titrated up to 300 mg twice per day. Upon titrating to the more frequent dosing schedule, the patient's tinnitus symptoms significantly improved within two weeks on starting the medication, and while he continued to have tinnitus, it had reduced to an acceptable level. He did not experience any adverse side effects from the medication. His tinnitus continued to be improved for a one month follow up length.
3. Discussion
The patient highlighted in this study may represent a unique group of individuals presenting with new-onset severe tinnitus symptoms out of proportion with audiometric findings following a COVID-19 infection. Despite time, education and conservative treatments the persistent tinnitus severely impacted his quality of life. A trial of gabapentin was successful in reducing the severity of his tinnitus symptoms to an acceptable level.
While a correlation between COVID-19 infection and the onset of tinnitus has been explored previously, no appropriately constructed studies have determined an association between the two events [9], [13]. Psychological triggers of tinnitus, particularly those exacerbated by the pandemic such as loneliness, poor sleep, depression and anxiety, may play a role in inciting or worsening tinnitus [14], [15]. A subset of patients with pre-existing tinnitus (32%) experienced worsening symptoms during the pandemic regardless of COVID-19 infection status [7], [16]. In a cross-sectional, multicenter study of individuals with COVID-19 infection, Beukes et al. reported exacerbated tinnitus symptoms in 40% of patients, no change in 54% and improved tinnitus in 6%; however, only 0.2% (7/3103) reported new onset tinnitus after COVID-19 infection [7].
Tinnitus in the normal or mild hearing loss population has been previously reported, however, patients with normal hearing or mild hearing loss typically have milder tinnitus symptoms compared to those with more significant degrees of hearing loss [17], [18]. Interestingly, our patient with severe tinnitus does not follow this pattern; rather, this patient exhibits normal hearing thresholds through 8000 Hz, with mild hearing loss above 12.5 kHz. Although measurable hearing loss is typically present in 90% of tinnitus cases, recent studies discussing new-onset tinnitus following a COVID-19 infection may suggest that rates of tinnitus in patients with normal or mild hearing loss may be higher in this population. Ozecelik Korkmaz et al. reported that 7 out of 10 (70%) patients diagnosed with COVID-19 infection cited new onset tinnitus without new subjective hearing loss [19]. Similarly, Murno et al. reported 3 of 8 (38%) patients experienced new onset tinnitus in the setting of subjectively stable hearing after diagnosis of COVID-19 [20]. Both studies, however, were limited by subjective hearing data, as neither reported audiometric testing results. One study reporting audiometric testing noted that two patients experiencing persistent tinnitus symptoms after COVID-19 infection demonstrated mild transient declines in pure tone thresholds with no subjective hearing loss, which may suggest that cochlear changes associated with hearing loss were not persistent in these cases [21].
While current research has no definitive explanation for patients who develop severe tinnitus without associated hearing loss or with mild hearing loss, several hypotheses are worth exploring. First, comorbid psychosocial factors, such as depression, may play a significant role in the development of or exacerbation of underlying tinnitus [22]. This may play a larger role more recently as social stressors may have been exaggerated by the pandemic. Alternatively, pathways associated with sensory gating, or the suppression of non-novel input, have been implicated in tinnitus development [23]. More specifically, normal hearing or mild hearing loss adults with tinnitus have been speculated to have greater difficulty in suppressing incoming electrophysiologic inputs; this may provide a mechanism for the development of tinnitus in the absence of significant cochlear injury, however, further work to demonstrate this association is required. The specific patient population with normal hearing or mild hearing loss with severe tinnitus may represent a unique patient population with a different underlying cause of tinnitus. While gabapentin is not routinely used for the treatment of tinnitus, as it has not been identified to consistently improve symptoms [24], it is possible that neuromodulators may be more effective in subsets of tinnitus patient populations. Further research into the association of COVID-19 infection and the development of tinnitus in the normal hearing patient population is warranted, particularly with support from objective audiologic and tinnitus measures.
4. Conclusions
While a direct association between COVID-19 infection and new onset tinnitus has yet to be established, our case may represent a subpopulation of patients who suffer from severe tinnitus following COVID-19 infection in the setting of largely normal hearing. The pathophysiology may be distinct from the more common hearing loss associated tinnitus and perhaps neuromodulators may play a larger role in mitigating tinnitus in this patient subset. Further investigation into this subset of the COVID-19 patient population is warranted.
Financial material & support
No funding or other support was required for this study.
Institutional review board approval
Mayo IRB: 21-005146
Declaration of competing interest
GSD: None
AMN: Research funding from Cochlear Americas
GV: None
MLC: Research funding from Cochlear Americas
BAN: None
CLWD: Consultant for Cochlear Corporation, Advanced Bionics, Envoy Medical
References
- 1.Shargorodsky J., Curhan G.C., Farwell W.R. Prevalence and characteristics of tinnitus among US adults. Am J Med. 2010;123(8):711–718. doi: 10.1016/j.amjmed.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Langguth B., Kreuzer P.M., Kleinjung T., De Ridder D. Tinnitus: causes and clinical management. Lancet Neurol. 2013;12(9):920–930. doi: 10.1016/S1474-4422(13)70160-1. [DOI] [PubMed] [Google Scholar]
- 3.McCormack A., Edmondson-Jones M., Somerset S., Hall D. A systematic review of the reporting of tinnitus prevalence and severity. Hear Res. 2016;337:70–79. doi: 10.1016/j.heares.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Hu J., Jolkkonen J., Zhao C. Neurotropism of SARS-CoV-2 and its neuropathological alterations: similarities with other coronaviruses. Neurosci Biobehav Rev. 2020;119:184–193. doi: 10.1016/j.neubiorev.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luis M.E., Hipolito-Fernandes D., Mota C., et al. A review of neuro-ophthalmological manifestations of human coronavirus infection. Eye Brain. 2020;12:129–137. doi: 10.2147/EB.S268828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fancello V., Hatzopoulos S., Corazzi V., et al. SARS-CoV-2 (COVID-19) and audio-vestibular disorders. Int J Immunopathol Pharmacol. 2021;35 doi: 10.1177/20587384211027373. [20587384211027373] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beukes E.W., Baguley D.M., Jacquemin L., et al. Changes in tinnitus experiences during the COVID-19 pandemic. Front Public Health. 2020;8 doi: 10.3389/fpubh.2020.592878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viola P., Ralli M., Pisani D., et al. Tinnitus and equilibrium disorders in COVID-19 patients: preliminary results. Eur Arch Otorhinolaryngol. 2021;278(10):3725–3730. doi: 10.1007/s00405-020-06440-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wichova H., Miller M.E., Derebery M.J. Otologic manifestations after COVID-19 vaccination: the house ear clinic experience. Otol Neurotol. 2021 doi: 10.1097/MAO.0000000000003275. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alsowaida Y.S., Almulhim A.S., Oh M., Erstad B., Abraham I. Sensorineural hearing loss with macrolide antibiotics exposure: a meta-analysis of the association. Int J Pharm Pract. 2021;29(1):21–28. doi: 10.1111/ijpp.12670. [DOI] [PubMed] [Google Scholar]
- 11.Pawlowski K.S., Si E., Wright C.G., Koulich E., Hosseini K., Roland P.S. Ototoxicity of topical azithromycin solutions in the Guinea pig. Arch Otolaryngol Head Neck Surg. 2010;136(5):481–487. doi: 10.1001/archoto.2010.54. [DOI] [PubMed] [Google Scholar]
- 12.Saniasiaya J., Kulasegarah J. Auditory cinchonism in COVID era. Ear Nose Throat J. 2020;99(9):597–598. doi: 10.1177/0145561320947255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beukes E., Ulep A.J., Eubank T., Manchaiah V. The impact of COVID-19 and the pandemic on tinnitus: a systematic review. J Clin Med. 2021;10(13) doi: 10.3390/jcm10132763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xia L., He G., Feng Y., et al. COVID-19 associated anxiety enhances tinnitus. PLoS One. 2021;16(2) doi: 10.1371/journal.pone.0246328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belli H., Belli S., Oktay M.F., Ural C. Psychopathological dimensions of tinnitus and psychopharmacologic approaches in its treatment. Gen Hosp Psychiatry. 2012;34(3):282–289. doi: 10.1016/j.genhosppsych.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Micarelli A., Granito I., Carlino P., Micarelli B., Alessandrini M. Self-perceived general and ear-nose-throat symptoms related to the COVID-19 outbreak: a survey study during quarantine in Italy. J Int Med Res. 2020;48(10) doi: 10.1177/0300060520961276. [300060520961276] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waechter S. Association between hearing status and tinnitus distress. Acta Otolaryngol. 2021;141(4):381–385. doi: 10.1080/00016489.2021.1876919. [DOI] [PubMed] [Google Scholar]
- 18.Mahafza N., Zhao F., El Refaie A., Chen F. A comparison of the severity of tinnitus in patients with and without hearing loss using the tinnitus functional index (TFI) Int J Audiol. 2021;60(3):220–226. doi: 10.1080/14992027.2020.1804081. [DOI] [PubMed] [Google Scholar]
- 19.Ozcelik Korkmaz M., Egilmez O.K., Ozcelik M.A., Guven M. Otolaryngological manifestations of hospitalised patients with confirmed COVID-19 infection. Eur Arch Otorhinolaryngol. 2021;278(5):1675–1685. doi: 10.1007/s00405-020-06396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munro K.J., Uus K., Almufarrij I., Chaudhuri N., Yioe V. Persistent self-reported changes in hearing and tinnitus in post-hospitalisation COVID-19 cases. Int J Audiol. 2020;59(12):889–890. doi: 10.1080/14992027.2020.1798519. [DOI] [PubMed] [Google Scholar]
- 21.Gallus R., Melis A., Rizzo D., et al. Audiovestibular symptoms and sequelae in COVID-19 patients. J Vestib Res. 2021 doi: 10.3233/VES-201505. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Choi J., Lee C.H., Kim S.Y. Association of tinnitus with depression in a normal hearing population. Medicina (Kaunas) 2021;57(2) doi: 10.3390/medicina57020114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell J., Bean C., LaBrec A. Normal hearing young adults with mild tinnitus: reduced inhibition as measured through sensory gating. Audiol Res. 2018;8(2):214. doi: 10.4081/audiores.2018.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tavares M.P., Bahmad F., Jr. Analysis of Gabapentin’s efficacy in tinnitus treatment: a systematic review. Ann Otol Rhinol Laryngol. 2021 doi: 10.1177/00034894211018921. [34894211018921], [Epub ahead of print] [DOI] [PubMed] [Google Scholar]